Abstract
Background
Vaccination seems to be the most effective way to prevent and control the spread of COVID-19, a disease that has adversely impacted the lives of over 7 billion people across the globe. Vaccine hesitancy represents an important threat to combat infectious diseases worldwide. This study aims to inspect the COVID-19 vaccine acceptance rate worldwide and the regional variation of the acceptance rates among the general population and healthcare workers across different territories of the world. In addition, it compares the vaccine acceptance rates between the pre- and post-vaccine approval periods.
Method
A comprehensive systematic review was conducted using PRISMA statements. After quality evaluation, the data from eligible studies were analyzed using the random effect model. Q-test and statistics were used to search for heterogeneity. The publication bias was assessed by using Egger’s test and funnel plot.
Results
The combined COVID-19 vaccine acceptance rate among the general population and healthcare workers (n = 1,581,562) was estimated at 62.79% (95% CI: 58.98–66.60). The acceptance rate substantially decreased from 66.29% (95% CI: 61.24–71.35) to 56.69% (95% CI: 48.68–64.71) among the general population from the pre-to post-vaccine approval periods but remained almost constant at 58.25% (95% CI: 46.52–69.97) among healthcare workers. The acceptance rates also varied in different regions of the world. The highest acceptance rate was found in the South-East Asia region at 70.18% (95% CI: 58.12–82.25) and the lowest was found in African Region at 39.51% (95% CI: 23.42–55.59).
Conclusion
Low COVID-19 vaccine acceptance rate might be a massive barrier to controlling the pandemic. More research is needed to address the responsible factors influencing the low global rate of COVID-19 vaccine acceptance. Integrated global efforts are required to remove the barriers.
Keywords: COVID-19, Global vaccination, Vaccine intention, Vaccine acceptance rate, Vaccine hesitancy
COVID-19; Global vaccination; Vaccine intention; Vaccine acceptance rate; Vaccine hesitancy.
1. Introduction
1.1. Background
At the end of the year 2019, the world experienced a new global threat posed by a novel coronavirus in Wuhan, China that spread rapidly around the world within a month [1, 2]. Afterward, this threat was labeled a pandemic by WHO [3]. As of now, this virus infected at least 543, 972, 975 and killed at least 6,340,008 people worldwide [4]. The pandemic also has had a disastrous impact on the mental health of people throughout the world due to fear, uncertainty, isolation, school closure, etc [5, 6]. Along with the public health crisis, the pandemic has had a devastating effect on the global economy. This pandemic resulted in at least a 2.9 percent loss of gross domestic product (GDP) in most of the major economies over 2020 [7]. Unfortunately, the virus continues to evolve [8]. Since no globally acceptable treatment has been developed to fight off the lethal infectious disease, vaccines are touted to be the most effective approach to prevent and control COVID-19. Before introducing a vaccine, people's knowledge, perception, and attitude toward vaccines come first to mind as it impacts vaccination efforts. After the clinical development of a vaccine, probably the most significant challenge is the acceptance and distribution of the vaccine. Vaccine acceptance study helps governments make immunization programs successful by mass distributing the vaccine. Therefore, many studies have been carried out to understand the knowledge about the COVID-19 vaccines, the prevalence of the vaccines’ acceptance, or vaccination intention which help to implement effective strategies to improve the vaccine coverage rate. Per the studies already conducted it has been observed that vaccine hesitancy has become a serious and growing concern worldwide despite overwhelming evidence of the importance of vaccines [9, 10]. This is remarkably influencing the rate at which immunization was expected to go through. Due to an alarming figure of vaccine hesitancy across the world, WHO announced this as one of the top ten threats to global health [9].
Several studies observed low vaccine acceptance rates (less than 40%) in the UK [11, 12], USA [13, 14], Jordan [15, 16], Kuwait [15], Hong Kong [17], Turkey [18], Congo [19], and Egypt [20]. Multiple pieces of research also reported high acceptance rates in several countries. For instance, a narrative review by Sallam et al. (2022) found that in 42 countries/territories across the world COVID-19 vaccine acceptance rates ranged between 13% and 59% [21]. A study in China reported that 91.30% of people would accept COVID-19 vaccination after the vaccine becomes available [22]. Another study in China showed that 64.01% of university students indicated their willingness to participate in COVID-19 vaccine trials [23]. A high level of acceptance for COVID-19 vaccines was found in Greece among health professionals [24]. A high intention to take vaccines among nurses and general people (60% and 66% respectively) has been observed in the USA [25, 26]. Although 61.16% of people in Bangladesh are willing to take COVID-19 vaccines, a large proportion of them (64.86%) would delay the vaccination until the vaccine’s efficacy and safety are substantiated [27]. Therefore, it is obvious that vaccine hesitancy is a common phenomenon globally with high variability.
1.2. Theoretical framework
It is obvious that the COVID-19 pandemic is not a crisis for a specific region or country, this is a global disaster. There is no alternative to combined global efforts to fight off the calamity. This is such a global challenge that nobody wins until everyone wins [28]. COVID-19 vaccines are proven to be effective and safe [29]. So, a significant portion of people across the globe must get vaccinated against COVID-19 to achieve so-called herd immunity. The WHO is working relentlessly to make sure every nation in the world gets the COVID-19 vaccines equitably through its COVAX program [28]. However, according to numerous surveys, a considerable section of the population worldwide would refuse or be unsure about receiving a COVID-19 vaccine if it were made available to them [30, 31, 32]. Fake news and misreporting on the safety and efficacy of vaccinations can spread quickly in the age of social media and online information, causing an "infodemic" and increasing both anti-vax movements and vaccine reluctance [33]. We have seen a deluge of misinformation disseminated even from political leaders and celebrities during the pandemic, which is also fueling the infodemic flame [34]. COVID-19 vaccination hesitancy is especially common among marginalized communities, which have been adversely impacted by the pandemic [35]. To increase COVID-19 vaccine uptake globally, it is of paramount importance to identify the people who are not willing to accept COVID-19 vaccines, inspect trends in vaccine hesitancy (regional/racial/ethnic), and report them to national and global leaders. A systematic review (meta-analysis) is one of the best ways to synthesize global data and investigate global phenomena. Such a study on the COVID-19 vaccine acceptance/hesitancy would be a great step forward to increase vaccine uptake. This motivated us to conduct a global meta-analysis on COVID-19 vaccine acceptance.
Prior to this study, only a few systematic reviews had been done related to vaccine acceptance with limited coverage. A meta-analysis of 46 studies inspected gender-wise differences in vaccination intention [36]. Another meta-analysis of 30 studies observed country-wise vaccination intention [37]. It was a remarkable success for medical science when the U.S Food and Drug Administration (FDA) approved the first COVID-19 vaccine for emergency use. On December 11, 2020, the Pfizer-BioNTech COVID-19 Vaccine was made available for 16 years or older individuals under Emergency Use Authorization (EUA) [38]. A COVID-19 vaccine approval by FDA might affect the vaccine acceptance rate. This study seeks answers to the following questions by conducting a systematic review of the existing studies on vaccine acceptance:
-
(a)
What is the rate of COVID-19 vaccine acceptance (willing to take) globally?
-
(b)
What are the acceptance rates across different regions of the world and do the rates differ by region?
-
(c)
Is there any difference in the acceptance rate between healthcare workers and general people?
-
(d)
Is there any difference in the acceptance rate between pre-time (before Dec. 11, 2020) and post-time (from Dec. 11, 2020, onward) of vaccine approval?
We are optimistic that the findings will help policymakers and global healthcare administrations in understanding COVID-19 vaccine hesitancy across different countries or territories. They can then design special intervention programs to make marginalized people aware of the severity of COVID-19 and the safety and efficacy of the COVID-19 vaccines. Thus this study can contribute to increasing COVID-19 vaccine acceptance and uptake of vaccines. The subsequent sections of the article will provide details of the methods (search strategy and selection criteria, inclusion/exclusion criteria, statistical analysis), results, discussion, and limitations of the study.
2. Methods
This systematic review and meta-analysis followed the Systematic Reviews and Meta-Analysis (PRISMA) statements [39] and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) checklists [40].
2.1. Search strategy and selection criteria
In this study, an Endnote (version X.8) library was created to list articles and remove replicas. A comprehensive systematic review was conducted using a systematic methodology (Figure 1) for the assessment of global acceptance rates of COVID-19 vaccination through the searches of PubMed/MEDLINE, Web of Science and GOOGLE SCHOLAR databases. The keywords used in the systematic searches were: “COVID-19 vaccine”, “Vaccination against SARS-CoV-2” “intention”, “general population, “healthcare workers”, “refusal”, “hesitancy”, “hesitance”, “hesitation’”, “acceptance”, “willingness”, “motivation”, “confidence”, “uptake”, “attitude”, “emotion”, “opinion’, “belief”, “trust”, “doubts”, “rejection”, “disapproval”. More specifically, we have used different combinations of those keywords, for instance:
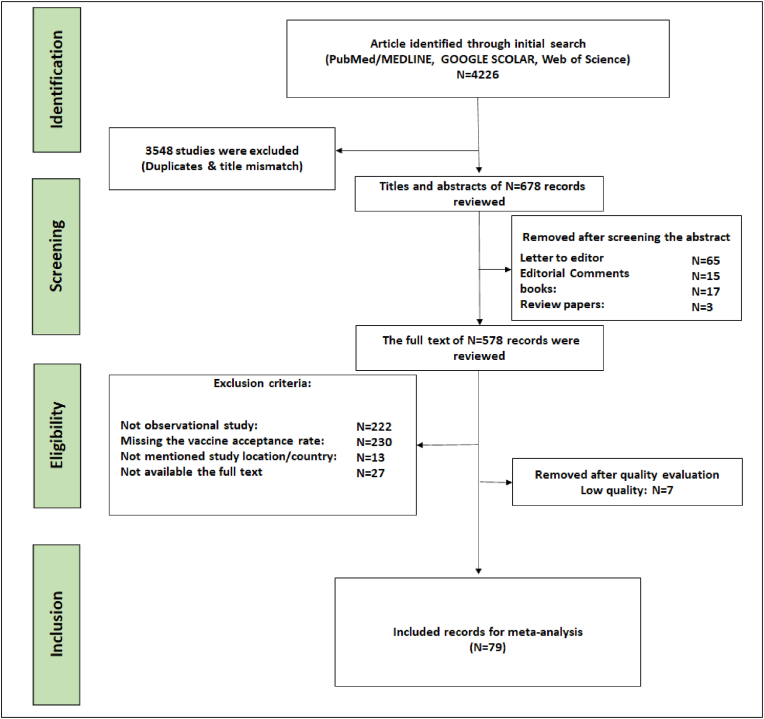
Figure 1.
Flow diagram describing the selection of the studies by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2009) guidelines.
(((COVID-19∗vaccine∗general∗population [Title/Abstract]) OR (COVID-19∗vaccine∗ hesitancy [Title/Abstract]) OR (COVID-19∗ vaccine acceptance [Title/Abstract])) OR (COVID-19∗ vaccine∗ attitude∗[Title/Abstract])) OR (COVID-19∗Vaccine∗ refusal∗ [Title/Abstract]) OR (COVID-19∗ vaccine∗ willingness∗[Title/Abstract])).
The search was conducted on April 25, 2021. The studies that were published only in English and met the predefined inclusion criteria were considered. The reference list of the included articles was inspected and cross-checked for maximizing the articles that met the specified inclusion criteria. The preprint articles published on Medrxiv, PsyArXiv, bioRxiv, arXiv, and SSRN servers were also incorporated into the study.
2.2. Inclusion/exclusion criteria
Studies were considered for final analysis if they met the following inclusion criteria: (1) studies measured the COVID-19 vaccine acceptance rate for the general population or healthcare workers; (2) studies published in English; (3) study design such as study site, the sample size must be reported; (4) studies with available full text. Studies were removed from the list if followed the following criteria: (1) studies did not specify the target population, the study site, as well as sample size; (2) studies did not use original data; (3) articles did not report valid estimates of the acceptance rates of the COVID-19 vaccines; (4) duplicate sources; (5) studies with unclear methods; (6) articles that their full text was not available; (7) not observational study; (8) Low-quality articles; (9) interventional studies, case reports, reviews articles, letters to the editor, correspondences, opinions, and comments.
2.3. Quality evaluation
The quality of the included studies needs to be evaluated before analysis. We evaluated the quality of included studies using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [[5], [42]] statements by two independent researchers (AM and SM) along with the help of a third one (SH) in case of any disagreement. With a total of 32 sections/scales, the STROBE checklist can be constructed into six general sections such as title, abstract, introduction, methods, results, and discussion. Those checklists are usually used to assess the study quality in a sense of methodological aspects including sampling and data collection approaches, study population, study design, statistical methods, and so on. The study quality evaluation scores varied between 1 to 32. The cut-off point was considered 14, which means studies with STROBE-score below 14 were removed from the list [5].
2.4. Screening and extraction
The data were extracted by three independent authors (SH, SM, and AM), with the presence of fourth reviews if necessary (MM). The first three authors screened all the articles that met the inclusion criteria and extracted data using a standardized form. The information that was extracted from the chosen articles included the title of the article, name of the first author, year of publication, study location (country), name of the authors, sampling method, study duration, study sample size, and COVID-19 vaccine acceptance rate.
2.5. Statistical analysis
The meta-analysis of COVID-19 vaccine acceptance rates was carried out using the statistical software STATA 16. The significance of the hypothesis was tested using the z statistic (level of significance p < 0.05). The heterogeneity tests were considered with a 5% level of significance to measure the homogeneity of studies. Due to significant heterogeneity, the random-effects model was used to estimate the pooled acceptance rates of the COVID-19 vaccine with 95% confidence intervals and the relative weight for each study. All the results of the meta-analysis were displayed in forest plots. The potential publication bias was inspected by using the funnel plot/Egger’s test. The subgroup analysis based on study location/territory, target population, study duration, and estimation methods was conducted to observe the COVID-19 vaccine acceptance rates from different stratifications and identify the source of heterogeneity. The study was divided into two different groups based on the target population: (i) the general population, and (ii) Healthcare workers. Here, university students were considered in the general population and medical students/Nurses were considered healthcare workers. The studies were also further classified into two groups: studies during the pre-time of COVID-19 vaccine approval (before 11 December 2020) and studies during the post-time of COVID-19 vaccine approval (from 11 December 2020). All the countries in the world which are members of the UN were classified into six territories namely the African Region, Eastern Mediterranean Region, European Region, Region of the Americas, South-East Asia Region, and Western Pacific Region by following the WHO’s regional classifications [43] to examine the regional disparities of COVID-19 vaccine acceptance. The significance of the difference between pre-time and post-time of COVID-19 vaccine approval was tested using a t-test.
3. Results
During the systematic review and meta-analysis, the PRISMA guidelines were followed for collecting and reviewing the articles in this study. In the beginning, 3548 articles were removed from initially identified 4226 articles that were unrelated to the topics or duplicate records after screening the titles and abstracts. Then, 578 articles were identified for examining the full texts and 494 records were skipped because of not having the relevant information. The rest of the articles were reviewed or checked for quality and 7 studies were excluded due to their low quality. After the quality evaluation, 79 studies were selected for the meta-synthesis. The Flow Diagram shows the article searching and selecting processes (Figure 1).
3.1. Study characteristics
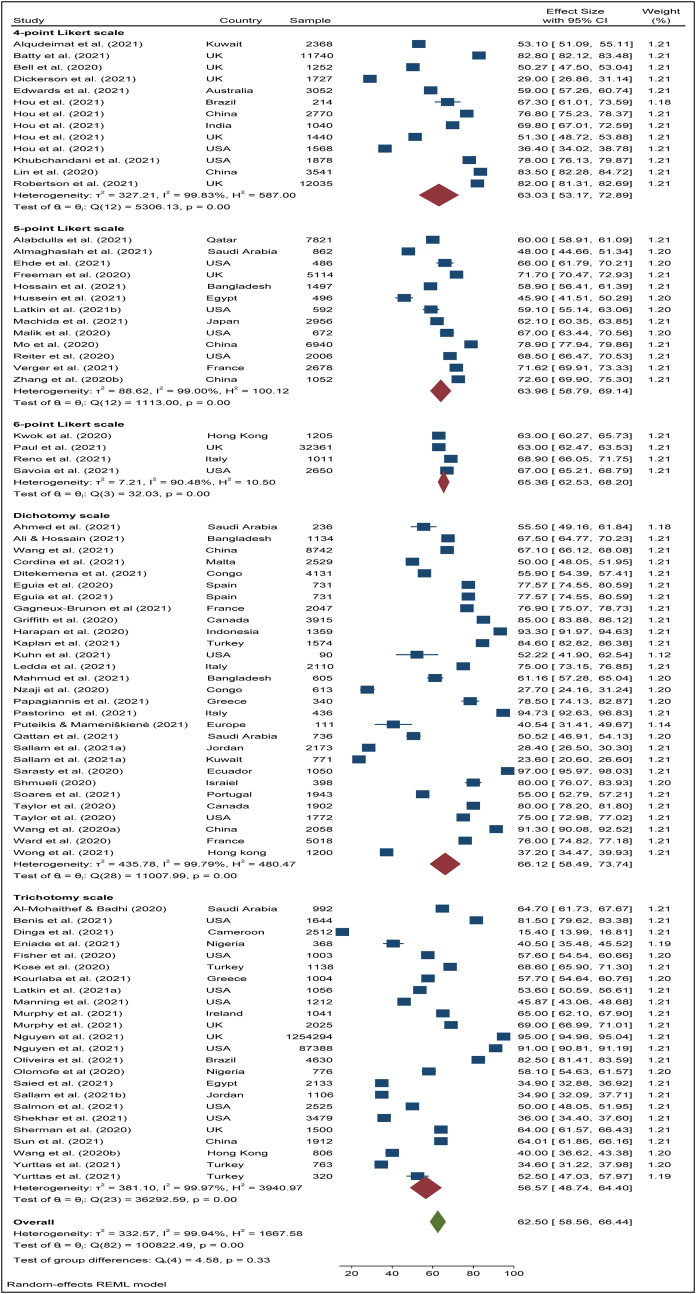
The characteristics of the selected 79 studies [1, 9, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 22, 23, 24, 25, 26, 27, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104] are presented in Table 1. Among them, 742 studies were single-country studies while four studies were conducted in two different countries, providing eight prevalence estimates. Besides, one study was conducted in five different countries and provided five estimates. Therefore, a total of 87 prevalence estimates of vaccine acceptance were used in the final analysis. In addition, this research covers studies from 31 countries. Of them, 13 studies were taken from the USA, nine from the UK, six from China, four from each of the countries: Saudi Arabia, Italy, and France, and three from each of the countries: Bangladesh, Spain, Turkey, Hong Kong, and two from each of the countries: Australia, Brazil, Canada, Egypt, Greece, Jordan, and Nigeria. One study was taken from each of the countries: Cameroon, Ecuador, England, Europe (whole), Indonesia, Ireland, Israel, Japan, Kuwait, Portugal, and Qatar. Most of the studies were cross-sectional and did not mention any sampling method. However, only 12 studies described the sampling procedure. Among them 4 studies used “Snowball sampling”, 2 studies “Stratified random sampling”, another two studies applied “Quota sampling”, one study used “Bootstrap resampling”, another one used “Commercial survey sampling”, another one applied “Multistage stratified sampling” and another one “PPS sampling”. One-third (33%) of the studies applied the “Dichotomy scale” for estimating the prevalence estimates of COVID-19 vaccine acceptance, while 28% used the “Trichotomy scale”, 15% used the “4-point Likert scale”, and another 15% used “5-point Likert scale”. Also, the “6-point Likert scale” appeared in only 5% of studies and the rest 5% of studies did not mention any measurement scale. Healthcare workers were the target population for 20.51% (16/78) of studies and for the rest of 79.49% (62/78) studies general population was the target population. The average study quality score or STROBE score was 21.74 (median: 21, range 15–30).
Table 1.
Summary characteristics of the selected Studies.
| Author name | Study Type | Sampling method | Country | Study duration | Response rate % | Male % | Measurement | Sample | Target population | Study quality | Acceptance rate % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahmed et al. (2021) | Cross-sectional | N. A. | Saudi Arabia | N. A | 37 | 29.4 | Dichotomy scale | 236 | Healthcare workers | 17 | 55.5 (49.16–61.84) |
| Alabdulla et al. (2021) | Cross-sectional | N. A. | Qatar | October 15 - November 15, 2020 | N. A. | 59.43 | 5-point Likert scale | 7821 | General Population | 19 | 60 (58.91–61.09) |
| Ali & Hossain (2021) | Cross-sectional | N. A. | Bangladesh | January 18 - January 31, 2021 | 100 | 59.2 | Dichotomy scale | 1134 | General Population | 23 | 67.5 (64.77–70.23) |
| Almaghaslah et al. (2021) | Cross-sectional | N. A. | Saudi Arabia | January 15 - February 07, 2021 | N. A. | 45.5 | 5-point Likert scale | 862 | General Population | 20 | 48 (44.66–51.34) |
| Al-Mohaithef & Badhi (2020) | Cross-sectional | Snowball sampling | Saudi Arabia | N. A. | N. A. | 34.17 | Trichotomy scale | 992 | General Population | 19 | 64.7 (61.73–67.67) |
| Alqudeimat et al. (2021) | Cross-sectional | Snowball sampling | Kuwait | 26-Aug-20 | N. A. | 31.8 | 4-point Likert scale | 2368 | General Population | 27 | 53.1 (51.09–55.11) |
| Batty et al. (2021) | Cohort Study | N. A. | UK | November–December, 2020 | 62 | 43 | 4-point Likert scale | 11740 | General Population | 21 | 82.8 (82.12–83.48) |
| Bell et al. (2020) | Cross-sectional | N. A. | UK | April 19 - May 11, 2020 | N. A. | 5 | 4-point Likert scale | 1252 | General Population | 15 | 50.27 (47.5–53.04) |
| Benis et al. (2021) | Cross-sectional | N. A. | USA | December 10 - December 24, 2020 | N. A. | 53.9 | Trichotomy scale | 1644 | General Population | 25 | 81.5 (79.62–83.38) |
| Borriello et al. (2021) | Cross-sectional | N. A. | Australia | March 27 - March 31, 2020 | 99.3 | 49.3 | N. A. | 2136 | General Population | 16 | 86.03 (84.56–87.5) |
| Cordina et al. (2021) | Cross-sectional | N. A. | Malta | Octoer 26 - November 26, 2020 | N. A. | 26.4 | Dichotomy scale | 2529 | General Population | 16 | 50 (48.05–51.95) |
| Dickerson et al. (2021) | Cohort study | N. A. | UK | October 29 - December 9, 2020 | 31 | 6.5 | 4-point Likert scale | 1727 | General Population | 21 | 29 (26.86–31.14) |
| Dinga et al. (2021) | Cross-sectional | N. A. | Cameroon | May–August, 2020 | N. A. | 45.1 | Trichotomy scale | 2512 | General Population | 20 | 15.4 (13.99–16.81) |
| Ditekemena et al. (2021) | Cross-sectional | N. A. | Congo | August 24 - September 8, 2020 | 99.3 | 31.6 | Dichotomy scale | 4131 | General Population | 21 | 55.9 (54.39–57.41) |
| Edwards et al. (2021) | Longitudinal | N. A. | Australia | Apr-20 | 7.8 | N. A. | 4-point Likert scale | 3052 | General Population | 22 | 59 (57.26–60.74) |
| Eguia et al. (2020) | Cross-sectional | N. A. | Spain | September 10 - November 23, 2020 | 72.95 | 44.04 | Dichotomy scale | 731 | General Population | 21 | 77.57 (74.55–80.59) |
| Eguia et al. (2021) | Cross-sectional | N. A. | Spain | September 10 - November 23, 2020 | N. A. | 45.05 | Dichotomy scale | 731 | Healthcare workers | 22 | 77.57 (74.55–80.59) |
| Ehde et al. (2021) | Cross-sectional | N. A. | USA | April 10 - May 6, 2020 | 93.1 | 17.3 | 5-point Likert scale | 486 | General Population | 23 | 66 (61.79–70.21) |
| Eniade et al. (2021) | Cross-sectional | N. A. | Nigeria | December, 2020 | N. A. | 41.1 | Trichotomy scale | 368 | General Population | 20 | 40.5 (35.48–45.52) |
| Fisher et al. (2020) | Cross-sectional | N. A. | USA | April 16 - April 20, 2020 | N. A. | 64 | Trichotomy scale | 1003 | General Population | 24 | 57.6 (54.54–60.66) |
| Freeman et al. (2020) | Cross-sectional | Quota sampling | UK | September 24 - October 17, 2020 | N. A. | 50 | 5-point Likert scale | 5114 | General Population | 25 | 71.7 (70.47–72.93) |
| Gagneux-Brunon et al (2021) | Cross-sectional | N. A. | France | March 26 - July 2, 2020 | 74 | 26 | Dichotomy scale | 2047 | Healthcare workers | 28 | 76.9 (75.07–78.73) |
| Griffith et al. (2020) | Cross-sectional | N. A. | Canada | December 10 - December 23, 2020 | N. A. | N. A. | Dichotomy scale | 3915 | General Population | 19 | 85 (83.88–86.12) |
| Harapan et al. (2020) | Cross-sectional | Snowball sampling | Indonesia | March 25 - April 6, 2020 | N. A. | 34.3 | Dichotomy scale | 1359 | General Population | 25 | 93.3 (91.97–94.63) |
| Hossain et al. (2021) | Cross-sectional | PPS sampling | Bangladesh | N. A. | 100 | 53 | 5-point Likert scale | 1497 | General Population | 15 | 58.9 (56.41–61.39) |
| Hou et al. (2021) | Cross-sectional | N. A. | Brazil | June 13 - July 31, 2020 | 54.6 | N.A. | 4-point Likert scale | 214 | General Population | 16 | 67.3 (61.01–73.59) |
| Hou et al. (2021) | Cross-sectional | N. A. | China | June 13 - July 31, 2020 | 54.6 | N.A. | 4-point Likert scale | 2770 | General Population | 16 | 76.8 (75.23–78.37) |
| Hou et al. (2021) | Cross-sectional | N. A. | India | June 13 - July 31, 2020 | 54.6 | N.A. | 4-point Likert scale | 1040 | General Population | 16 | 69.8 (67.01–72.59) |
| Hou et al. (2021) | Cross-sectional | N. A. | UK | June 13 - July 31, 2020 | 54.6 | N.A. | 4-point Likert scale | 1440 | General Population | 16 | 51.3 (48.72–53.88) |
| Hou et al. (2021) | Cross-sectional | N. A. | USA | June 13 - July 31, 2020 | 54.6 | N.A. | 4-point Likert scale | 1568 | General Population | 16 | 36.4 (34.02–38.78) |
| Hussein et al. (2021) | Cross-sectional | N. A. | Egypt | December 1 - January 1, 2021 | 99.2 | 34.9 | 5-point Likert scale | 496 | Healthcare workers | 25 | 45.9 (41.51–50.29) |
| Iacoella et al. (2021) | Cross-sectional | N. A. | Italy | February 1 - February 15, 2021 | 100 | 75.9 | N. A. | 112 | General Population | 16 | 64.3 (55.43–73.17) |
| Kaplan et al. (2021) | Cross-sectional | N. A. | Turkey | December, 2020 | N. A. | 41.2 | Dichotomy scale | 1574 | Healthcare workers | 28 | 84.6 (82.82–86.38) |
| Khubchandani et al. (2021) | Cross-sectional | N. A. | USA | June, 2020 | N. A. | 48 | 4-point Likert scale | 1878 | General Population | 23 | 78 (76.13–79.87) |
| Kose et al. (2020) | Cross-sectional | N. A. | Turkey | September 17- September 20, 2020 | N. A. | 27.5 | Trichotomy scale | 1138 | Healthcare workers | 24 | 68.6 (65.9–71.3) |
| Kourlaba et al. (2021) | Cross-sectional | N. A. | Greece | April 28- May 3, 2020 | 38.66 | 49 | Trichotomy scale | 1004 | General Population | 20 | 57.7 (54.64–60.76) |
| Kuhn et al. (2021) | Cross-sectional | N. A. | USA | December 2020–January 2021 | 65.8 | 41 | Dichotomy scale | 90 | General Population | 19 | 52.22 (41.9–62.54) |
| Kwok et al. (2020) | Cross-sectional | N. A. | Hong Kong | March 16 - April 29, 2020 | N. A. | N. A. | 6-point Likert scale | 1205 | Healthcare workers | 23 | 63 (60.27–65.73) |
| Latkin et al. (2021a) | Cross-sectional | N. A. | USA | May 14 - May 18, 2020 | 24.1 | 29.9 | Trichotomy scale | 1056 | General Population | 22 | 53.6 (50.59–56.61) |
| Latkin et al. (2021b) | Cross-sectional | N. A. | USA | July, 2020 | N.A. | 43.9 | 5-point Likert scale | 592 | General Population | 19 | 59.1 (55.14–63.06) |
| Ledda et al. (2021) | Cross-sectional | N. A. | Italy | January–December, 2020 | 99 | 48 | Dichotomy scale | 2110 | Healthcare workers | 20 | 75 (73.15–76.85) |
| Lin et al. (2020) | Cross-sectional | N. A. | China | May 1 - May 9, 2020 | N. A. | 48.1 | 4-point Likert scale | 3541 | General Population | 26 | 83.5 (82.28–84.72) |
| Machida et al. (2021) | Cross-sectional | N. A. | Japan | January 14 - January 18, 2021 | 98.5 | 49.32 | 5-point Likert scale | 2956 | General Population | 20 | 62.1 (60.35–63.85) |
| Mahmud et al. (2021) | Cross-sectional | N. A. | Bangladesh | January 30 - February 06, 2020 | N. A. | 62.15 | Dichotomy scale | 605 | General Population | 30 | 61.16 (57.28–65.04) |
| Malik et al. (2020) | Cross-sectional | Bootstrap resampling | USA | May, 2020 | N. A. | 43 | 5-point Likert scale | 672 | General Population | 19 | 67 (63.44–70.56) |
| Manning et al. (2021) | Cross-sectional | N. A. | USA | August 10 - September 14, 2020 | 84.9 | 10.89 | Trichotomy scale | 1212 | Healthcare workers | 17 | 45.87 (43.06–48.68) |
| Mo et al. (2020) | Cross-sectional | N. A. | China | November 1 - November 28, 2020 | 72.3 | 36.4 | 5-point Likert scale | 6940 | General Population | 23 | 78.9 (77.94–79.86) |
| Murphy et al. (2021) | Cross-sectional | N. A. | Ireland | March 31 - April 05, 2020 | N. A. | 48.2 | Trichotomy scale | 1041 | General Population | 27 | 65 (62.1–67.9) |
| Murphy et al. (2021) | Cross-sectional | N. A. | UK | March 23 - March 28, 2020 | N. A. | 48.3 | Trichotomy scale | 2025 | General Population | 27 | 69 (66.99–71.01) |
| Nguyen et al. (2021) | Cohort study | N. A. | UK | March 24, 2020–February 16, 2021 | 28.33 | 46.34 | Trichotomy scale | 1254294 | General Population | 28 | 95 (94.96–95.04) |
| Nguyen et al. (2021) | Cohort study | N. A. | USA | March 24, 2020–February 16, 2021 | 23.6 | 45.35 | Trichotomy scale | 87388 | General Population | 28 | 91 (90.81–91.19) |
| Nzaji et al. (2020) | Cross-sectional | N. A. | Congo | March–April 30, 2020 | N. A. | 50.9 | Dichotomy scale | 613 | Healthcare workers | 22 | 27.7 (24.16–31.24) |
| Oliveira et al. (2021) | Cross-sectional | Multistage stratified sampling | Brazil | October 19 - October 30, 2020 | 90.78 | 80.2 | Trichotomy scale | 4630 | General Population | 21 | 82.5 (81.41–83.59) |
| Olomofe et al (2020) | Cross-sectional | N. A. | Nigeria | N. A | N. A. | 58.1 | Trichotomy scale | 776 | General Population | 25 | 58.1 (54.63–61.57) |
| Papagiannis et al. (2021) | Cross-sectional | N. A. | Greece | December 15 - December 22, 2020 | N. A. | 50 | Dichotomy scale | 340 | Healthcare workers | 20 | 78.5 (74.13–82.87) |
| Pastorino et al. (2021) | Cross-sectional | N. A. | Italy | June 8 - July 12, 2020 | 78 | 29.59 | Dichotomy scale | 436 | General Population | 22 | 94.73 (92.63–96.83) |
| Paul et al. (2021) | Cross-sectional | N. A. | UK | September 7 - October 5, 2020 | 71.57 | 49.4 | 6-point Likert scale | 32361 | General Population | 18 | 63 (62.47–63.53) |
| Puteikis & Mameniškienė (2021) | Cross-sectional | N. A. | Europe | December 7 - December 31, 2020 | 100 | 39.6 | Dichotomy scale | 111 | General Population | 23 | 40.54 (31.41–49.67) |
| Qattan et al. (2021) | Cross-sectional | N. A. | Saudi Arabia | December 8 - December 14, 2020 | 91.44 | 60.18 | Dichotomy scale | 736 | Healthcare workers | 20 | 50.52 (46.91–54.13) |
| Reiter et al. (2020) | Cross-sectional | N. A. | USA | May, 2020 | N. A. | 43 | 5-point Likert scale | 2006 | General Population | 20 | 68.5 (66.47–70.53) |
| Reno et al. (2021) | Cross-sectional | N. A. | Italy | January 19 - January 26, 2021 | 100 | 44.8 | 6-point Likert scale | 1011 | General Population | 25 | 68.9 (66.05–71.75) |
| Robertson et al. (2021) | Cross-sectional | N. A. | UK | November 24 - December 1, 2020 | 62 | 46.8 | 4-point Likert scale | 12035 | General Population | 28 | 82 (81.31–82.69) |
| Saied et al. (2021) | Cross-sectional | N. A. | Egypt | January, 2021 | N. A. | 34.8 | Trichotomy scale | 2133 | Healthcare workers | 24 | 34.9 (32.88–36.92) |
| Sallam et al. (2021a) | Cross-sectional | N. A. | Jordan | December 14 - December 18, 2020 | N. A. | 30.6 | Dichotomy scale | 2173 | General Population | 26 | 28.4 (26.5–30.3) |
| Sallam et al. (2021a) | Cross-sectional | N. A. | Kuwait | December 14 - December 18, 2020 | N. A. | 30.6 | Dichotomy scale | 771 | General Population | 23 | 23.6 (20.6–26.6) |
| Sallam et al. (2021b) | Cross-sectional | N. A. | Jordan | January 19 - January 23, 2021 | 96.2 | 27.5 | Trichotomy scale | 1106 | General Population | 22 | 34.9 (32.09–37.71) |
| Salmon et al. (2021) | Panel survey | N. A. | USA | November 25 - December 07, 2020 | N. A. | 48.2 | Trichotomy scale | 2525 | General Population | 20 | 50 (48.05–51.95) |
| Sarasty et al. (2020) | Cross-sectional | N. A. | Ecuador | April 2 - April 7, 2020 | N. A. | 61 | Dichotomy scale | 1050 | General Population | 21 | 97 (95.97–98.03) |
| Savoia et al. (2021) | Cross-sectional | N. A. | USA | December 13 - December 23, 2020 | 84 | 53.5 | 6-point Likert scale | 2650 | General Population | 18 | 67 (65.21–68.79) |
| Schwarzinger et al. (2021) | Cross-sectional | N. A. | France | June 22 - July 3, 2020 | 97.1 | 48.9 | N. A. | 1942 | General Population | 20 | 71.2 (69.19–73.21) |
| Shekhar et al. (2021) | Cross-sectional | Snowball sampling | USA | October 7 - November 9, 2020 | 85.26 | 25 | Trichotomy scale | 3479 | Healthcare workers | 19 | 36 (34.4–37.6) |
| Sherman et al. (2020) | Cross-sectional | Quota sampling | UK | July 14 - July 17, 2020 | 98 | 49 | Dichotomy scale | 1500 | General Population | 24 | 64 (61.57–66.43) |
| Shmueli (2020) | cross-sectional | N.A | Israiel | May 24- June 24, 2020 | N. A. | 40 | Dichotomy scale | 398 | General Population | 21 | 80 (76.07–83.93) |
| Soares et al. (2021) | Cross-sectional | N. A. | Portugal | September 2020–January 2021 | N. A. | 28.89 | Dichotomy scale | 1943 | General Population | 27 | 55 (52.79–57.21) |
| Sun et al. (2021) | Cross-sectional | N. A. | China | March–April, 2020 | 100 | 30.23 | Trichotomy scale | 1912 | General Population | 21 | 64.01 (61.86–66.16) |
| Taylor et al. (2020) | Cross-sectional | Commercial survey sampling | Canada | May, 2020 | N. A. | 57 | Dichotomy scale | 1902 | General Population | 16 | 80 (78.2–81.8) |
| Taylor et al. (2020) | Cross-sectional | Commercial survey sampling | USA | May, 2020 | N. A. | 57 | Dichotomy scale | 1772 | General Population | 19 | 75 (72.98–77.02) |
| Tulloch et al. (2021) | Cross-sectional | N. A. | England | January 21 - January 29, 2021 | 53 | N. A. | N. A. | 46 | General Population | 20 | 52.4 (37.97–66.83) |
| Verger et al. (2021) | Cross-sectional | N. A. | France | October–November, 2020 | 100 | 30.75 | 5-point Likert scale | 2678 | Healthcare workers | 24 | 71.62 (69.91–73.33) |
| Wang et al. (2020a) | Cross-sectional | Stratified random sampling | China | March, 2020 | N. A. | 45.8 | Dichotomy scale | 2058 | General Population | 28 | 91.3 (90.08–92.52) |
| Wang et al. (2020b) | Cross-sectional | N. A. | Hong Kong | February–March, 2020 | 5.18 | 19.3 | Trichotomy scale | 806 | Healthcare workers | 15 | 40 (36.62–43.38) |
| Wang et al. (2021) | Cross-sectional | N. A. | China | January 10 - January 22, 2021 | 99.6 | 33.6 | Dichotomy scale | 8742 | General Population | 19 | 67.1 (66.12–68.08) |
| Ward et al. (2020) | Cross-sectional | Stratified random sampling | France | April, 2020 | N. A. | N. A. | Dichotomy scale | 5018 | General Population | 26 | 76 (74.82–77.18) |
| Wong et al. (2021) | Cross-sectional | N. A. | Hong kong | July 27 - August 27, 2020 | 55 | 28.7 | Dichotomy scale | 1200 | General Population | 20 | 37.2 (34.47–39.93) |
| Yurttas et al. (2021) | Cross-sectional | N. A. | Turkey | January 4 - January 13, 2021 | 100 | 33.81 | Trichotomy scale | 763 | General Population | 26 | 34.6 (31.22–37.98) |
| Yurttas et al. (2021) | Cross-sectional | N. A. | Turkey | January 4 - January 13, 2021 | 22.85 | 27.5 | Trichotomy scale | 320 | Healthcare workers | 26 | 52.5 (47.03–57.97) |
| Zhang et al. (2020b) | Cross-sectional | N. A. | China | September 1 - September 7, 2020 | 77.4 | 37.5 | 5-point Likert scale | 1052 | General Population | 22 | 72.6 (69.9–75.3) |
PPS, Probability proportional to size; N.A., Not available; UK, United Kingdom; USA, United States of America.
3.2. Statistical heterogeneity and publication bias
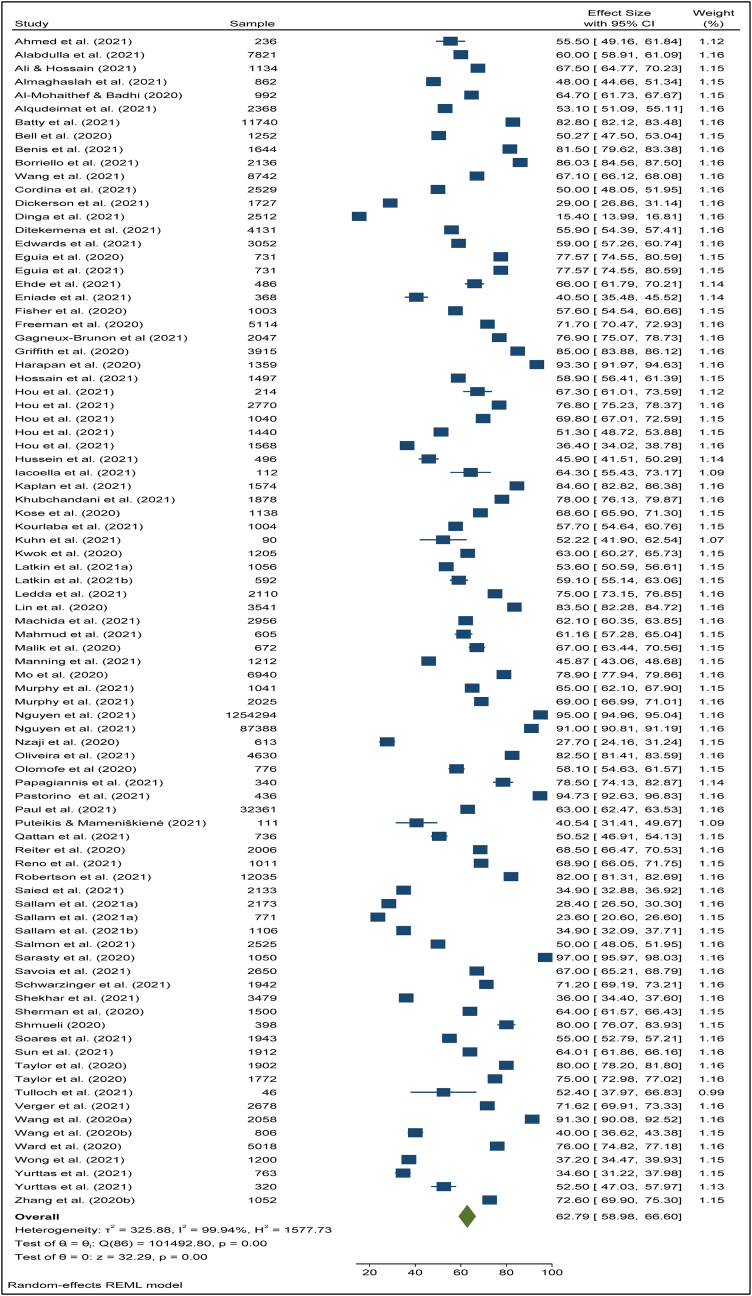
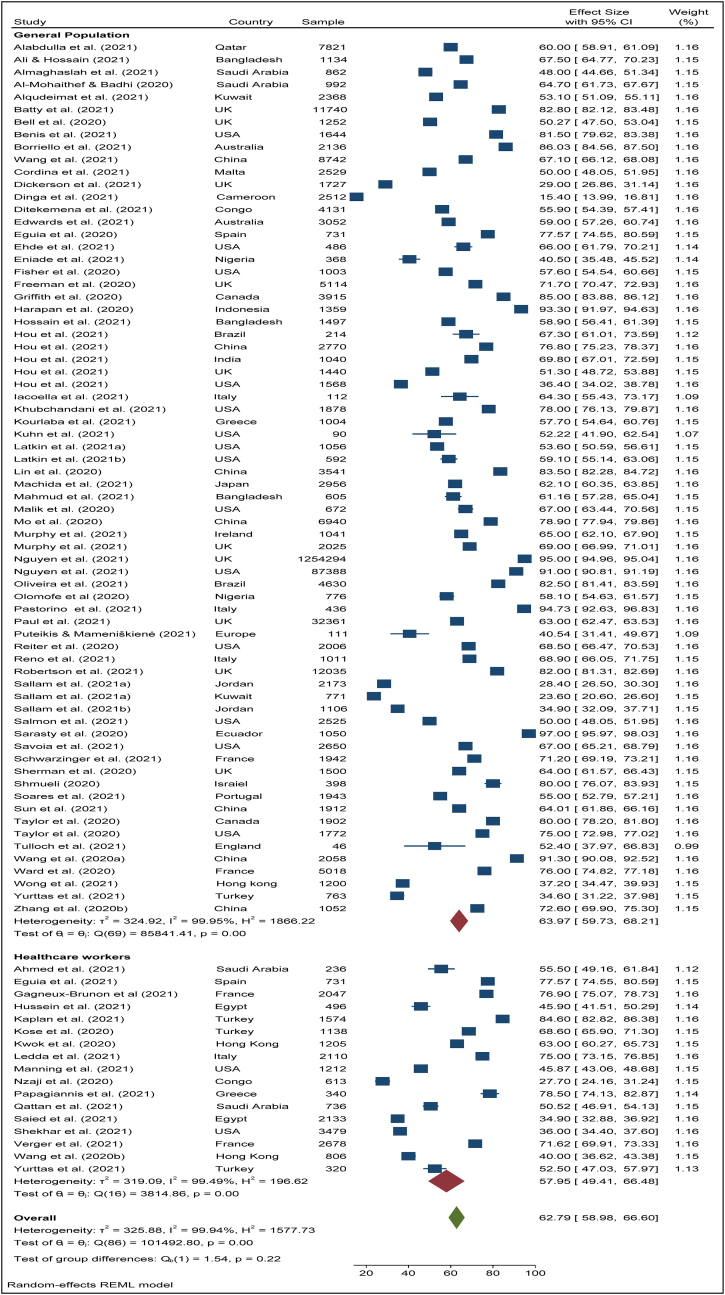
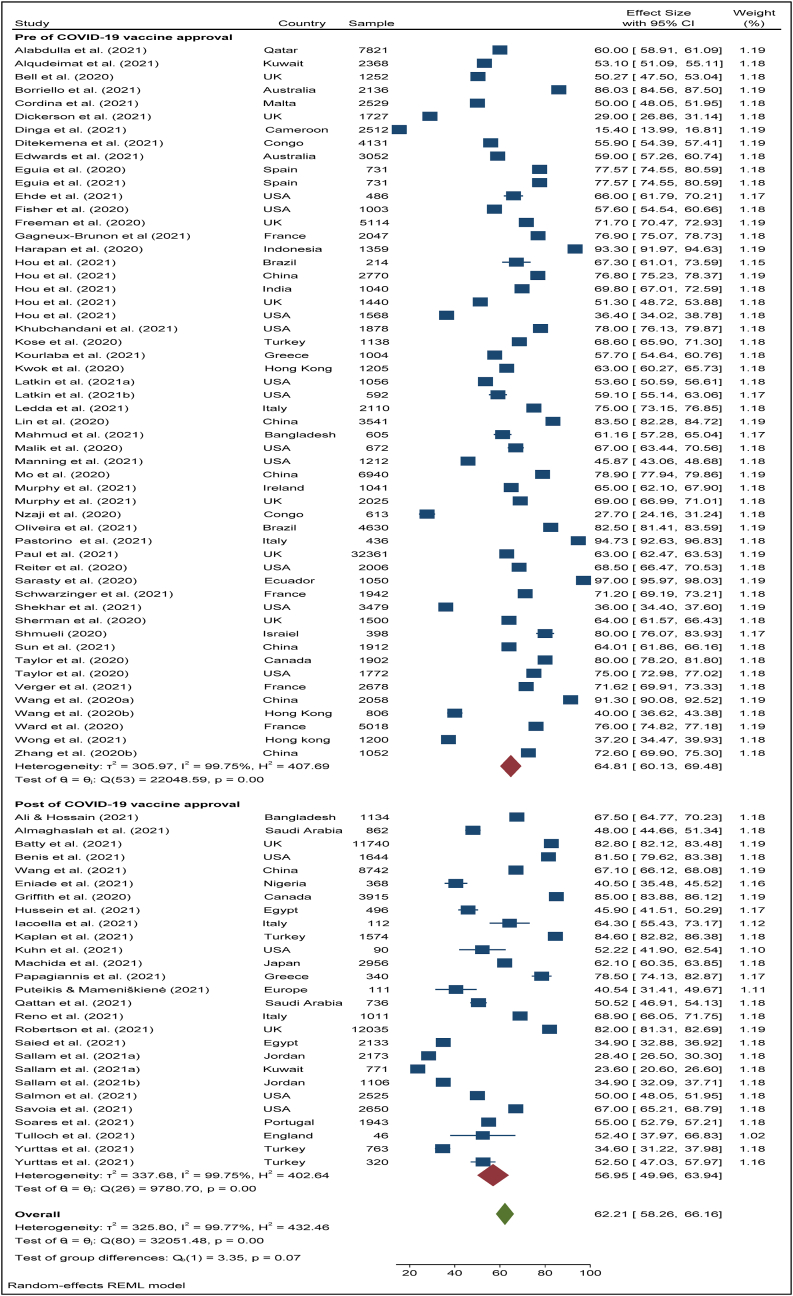
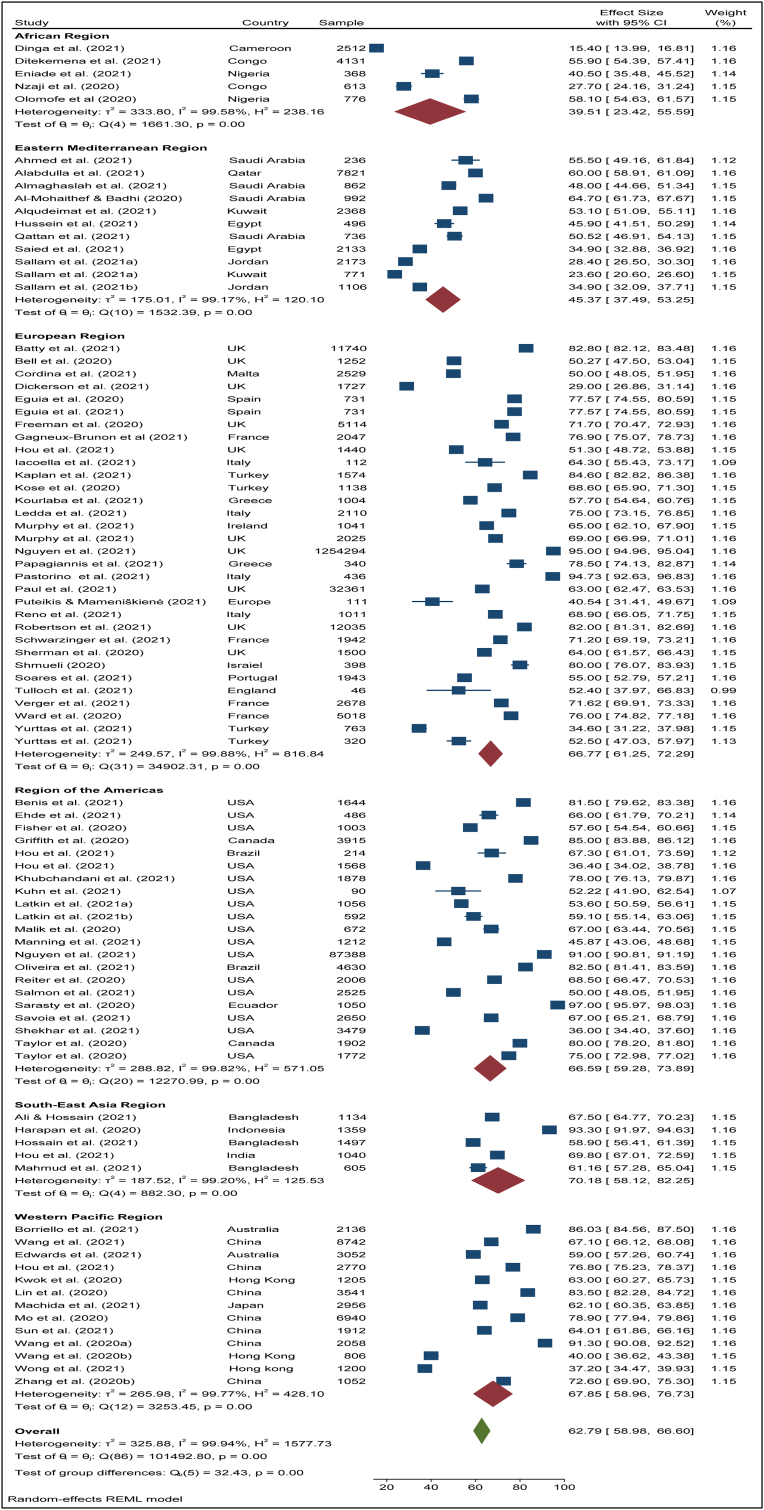
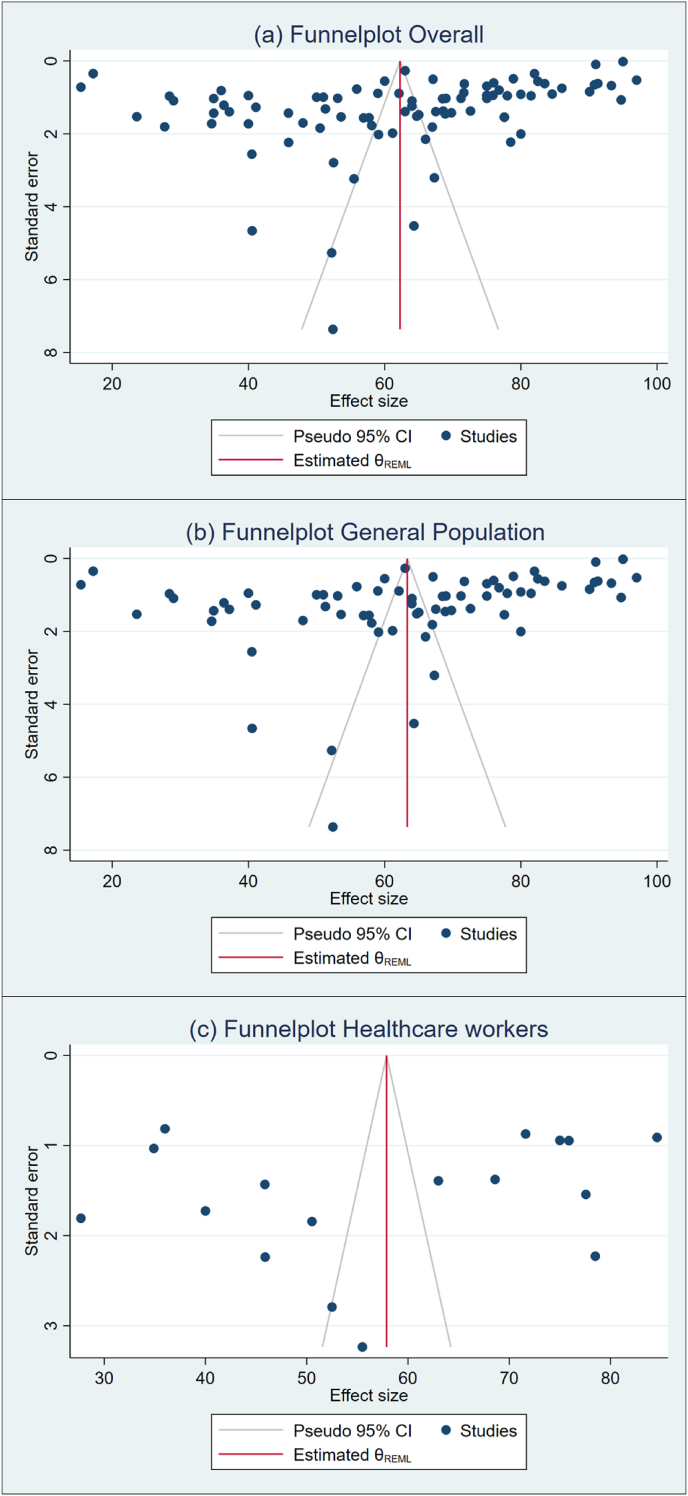
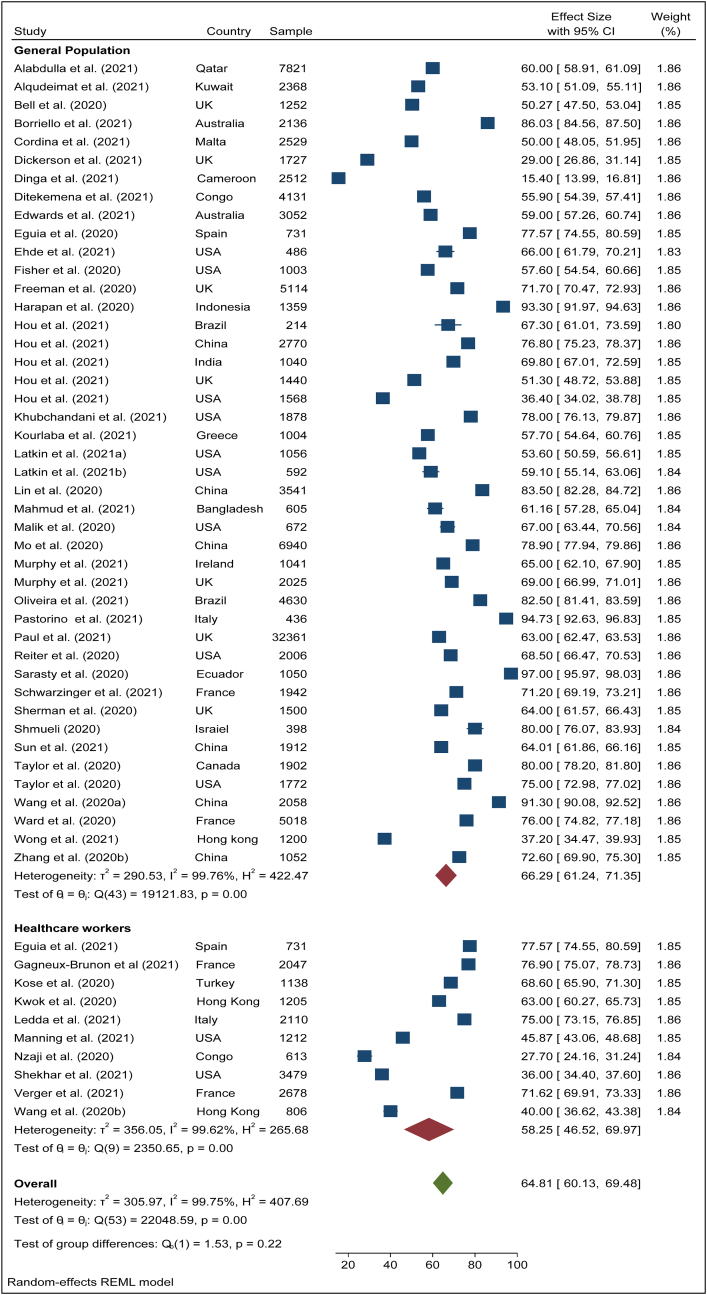
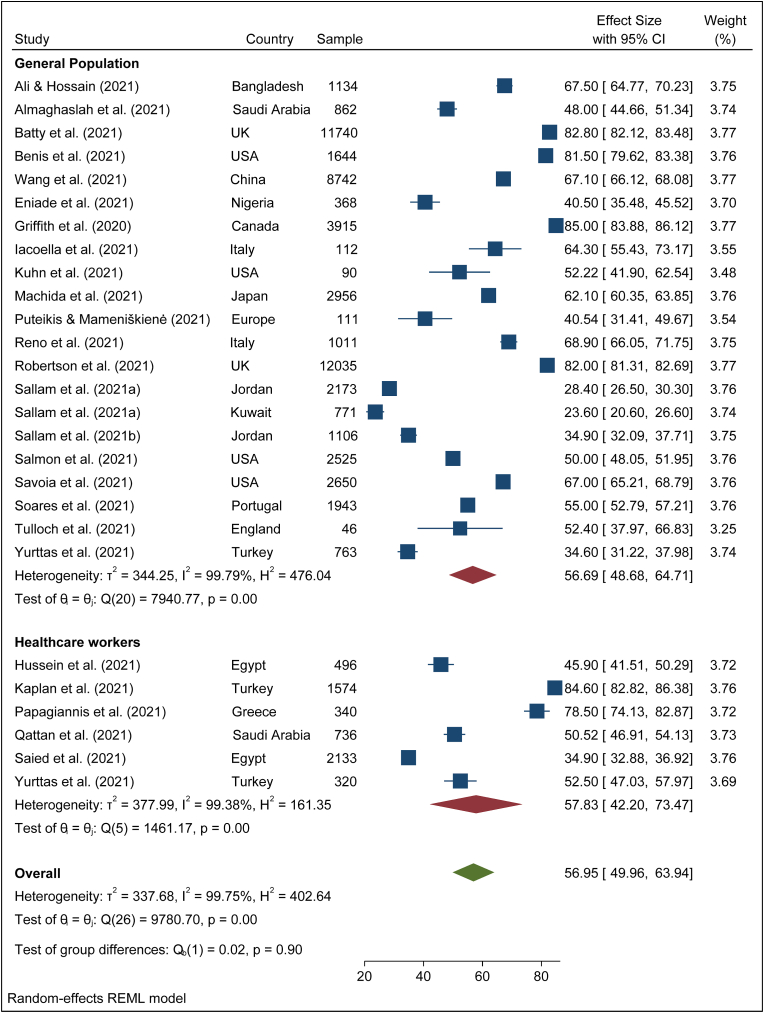
To search for the heterogeneity of the included studies, Q-test and (%) statistical tools were used. Here, we obtained for overall COVID-19 vaccine acceptance rate (Q (87) = 101492, P < 0.05) = 99.94% (Figure 2) which indicates there is a significant heterogeneity among the selected studies. The subgroup analysis and Q-test with significant p-values indicates that target population type (healthcare worker: Q (16) = 3814, P < 0.05; and general population: Q (69) = 85841, P < 0.05) (Figure 3), study duration (Pre-vaccine approval: Q (53) = 22048, P < 0.05; post-vaccine approval: Q (26) = 9780, P < 0.05) (Figure 4) are two of the sources of heterogeneity. The Q-test also reveals that the study’s attributes “region” (Figure 5) and “prevalence estimation method” (Figure 6) are also the source of heterogeneity. Due to significant heterogeneity among the selected studies, the random-effect model was applied to data analysis. The Funnel plot and Eggers’s test indicate the presence of significant publication bias (Z = -2.95, P = 0.003) in all the included studies (Figure 7a). Although, the Funnel plot and Eggers’s test indicate the presence of significant publication bias (Z = -2.89, P = 0.002) in the studies among the general population (Figure 7b), no publication bias (Z = -0.87, P = 0.54) was found in the studies among healthcare workers (Figure 7c).
Figure 2.
Overall COVID-19 vaccine acceptance rate.
Figure 3.
Pooled acceptance rate of COVID-19 vaccine among general population and healthcare workers.
Figure 4.
The pooled acceptance rate of COVID-19 vaccine in pre- and post-time of COVID-19 vaccine approval.
Figure 5.
The pooled acceptance rate of COVID-19 vaccine across different territory of the world.
Figure 6.
The pooled acceptance rate of COVID-19 vaccine across different estimation methods.
Figure 9.
Funnel plot for included studies across different target population.
3.3. COVID-19 vaccine acceptance rate
The pooled COVID-19 vaccine acceptance rate worldwide was 62.79% (95% CI: 58.98–66.60) and it was estimated from 78 included studies [1, 9, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 22, 23, 24, 25, 26, 27, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104] among the general population and health care workers/health professionals with a combined sample size of 1,581,562 (Figure 2). The pooled acceptance rate for the COVID-19 vaccine acceptance among the general population all over the world was estimated at 63.97% (95% CI: 59.73–68.21) with a sample size of 1,559,708 whereas the pooled acceptance rate among healthcare workers with a sample size of 21,854 was found to be 57.59% (95% CI: 49.41–66.48) (Figure 3).
3.4. Acceptance rate of COVID-19 vaccines during the pre- and post-vaccine approval periods
The overall vaccine acceptance rate among the general population and healthcare workers was estimated at 64.81% (95% CI: 60.13–69.48) in the pre-time of COVID-19 vaccine approval (Figure 4). During the post time of COVID-19 vaccine approval, the pooled vaccine acceptance rate among the general population and healthcare workers was found 56.59% (95% CI: 49,96–63.94) (Figure 4). During the pre-time of COVID-19 vaccine approval, the vaccine acceptance rate among the general population was 66.29% (95% CI: 61.24–71.35) while during the post-time of COVID-19 vaccine approval among them it was 56.69% (95% CI: 48.68–64.71) (Figures 8 and 9). Among healthcare workers, the acceptance rate was 58.25% (95% CI: 46.52–69.97) during pre-time of vaccine approval while during post-time of vaccine approval it was 57.83% (95% CI:42.20–73.47) (Figures 8 and 9).
Figure 7.
Pre COVID-19 vaccine approval acceptance rate among general population and healthcare workers.
Figure 8.
Post COVID-19 vaccine approval acceptance rate among general population and healthcare workers.
3.5. Regional disparities
We inspected the prevalence of COVID-19 vaccine acceptance in different territories of the world. These regions were defined following the WHO’s division of its member countries by region. The region-wise COVID-19 vaccine acceptance rate is presented in Figure 5 and described in detail below.
3.5.1. African Region
In total, we included 5 studies from this region with a sample size of 8,400 and estimated the acceptance rate of COVID-19 vaccines as 39.51% (95% CI: 23.42–55.59) (Figure 5). In this territory, the highest acceptance rate was 58.10% (95% CI: 54.63–61.57) in Nigeria [82] and the lowest acceptance rate was found at 15.40% (95% CI: 13.99–16.81) in Cameroon [9]. The overall COVID-19 vaccine acceptance rate among the general population and healthcare workers in this territory respectively was 42.46% (95% CI: 23.10–61.82) and 27.70% (95% CI: 24.16–31.24) (Table 2).
Table 2.
Regional disparities of acceptance of COVID-19 vaccine among general population and healthcare workers.
| Territory | Healthcare worker |
General population |
||
|---|---|---|---|---|
| Acceptance rate % (95% CI) | (, Q (df.)) P-value | Acceptance rate % (95% CI) | (, Q (df.)) P-value | |
| African Region | 27.70 (24.16–31.24)∗ | 42.46 (23.10–61.82) | (= 99.68, Q (3) = 1640.68) P-value < 0.001 | |
| Eastern Mediterranean Region | 46.43 (37.73–55.12) | (= 95.51, Q (3) = 86.30) P-value < 0.001 | 44.68 (32.88–56.48) | (= 99.81, Q (7) = 7241.10) P-value < 0.001 |
| European Region | 73.33 (66.90–79.75) | (= 98.34, Q (7) = 216.91) P-value < 0.001 | 64.58 (57.69–71.46) | (= 99.91, Q (23) = 32.539.23) P-value < 0.001 |
| Region of the Americas | 40.86 (31.19–50.54) | (= 97.22, Q (1) = 35.93) P-value < 0.001 | 69.33 (59.88–74.74) | (= 99.79, Q (18) = 7210.29) P-value < 0.001 |
| South-East Asia Region | - | - | 70.18 (58.12–82.25) | (= 99.20, Q (4) = 882.30) P-value < 0.001 |
| Western Pacific Region | 51.52 (28.98–74.06) | (= 99.07, Q (1) = 107.69) P-value < 0.001 | 70.80 (61.81–79.79) | (= 99.76, Q (10) = 2773.69) P-value < 0.001 |
refers that only one study was included in this subgroup; - refers that no study was considered in this subgroup; CI, Confidence Interval
3.5.2. Eastern Mediterranean Region
A total of 12 studies with a sample size of 19,694 estimated a pooled COVID-19 vaccine acceptance rate of 45.37% (95% CI: 37.49–53.25) in this region (Figure 5). In this territory, the highest acceptance rate was found at 64.70% (95% CI: 61.73–67.67) in Saudi Arabia [48] and the lowest acceptance rate was found at 23.60% (95% CI: 20.60–26.60) in Kuwait [15]. The acceptance rate of COVID-19 vaccines in this region was 46.34% (95% CI: 37.73–55.12) among healthcare workers and 44.64% (95% CI: 32.88–56.48) (Table 2) among the general population (Table 2).
3.5.3. European Region
A large number of studies (32 studies with a sample size of 1,351,511) were found in this territory where the overall COVID-19 vaccine acceptance rate was found to be 66.77% (95% CI: 61.25–72.29) (Figure 5). In this region, the highest acceptance rate was 95.00% (95% CI: 94.96–95.04) in the UK [80] and the lowest acceptance rate was 29.00% (95% CI: 26.86–31.14) also in the UK [12]. Among healthcare workers, the acceptance rate of COVID-19 vaccines was 73.33% (95% CI: 66.90–79.75) in this territory and among the general population, it was 64.58% (95% CI: 57.69–71.46) (Table 2).
3.5.4. Region of the Americas
A total of 21 studies with a sample size of 121,732 were found in this territory. The overall COVID-19 vaccine acceptance rate was found at 66.59% (95% CI: 59.28–73.89) (Figure 5). The highest acceptance rate was observed to be 97.00% (95% CI: 95.97–98.04) in Ecuador [92] and the lowest acceptance rate was 36.00% (95% CI: 34.41–37.59) in the USA [14]. In this territory, 40.86% (95% CI: 31.19–50.54) of the healthcare workers showed a willingness to accept COVID-19 vaccines, and 69.33% (95% CI: 59.88–74.74) of the general population agreed to accept COVID-19 vaccines (Table 2).
3.5.5. South-East Asia Region
The pooled COVID-19 vaccine acceptance rate in this region was estimated as 70.18% (95% CI: 58.12–82.25) (Figure 5) from five studies with a sample size of 5,635. The highest acceptance rate was seen at 93.30% (95% CI: 91.97–94.62) in Indonesia [63] and the lowest acceptance rate was 70.18% (95% CI: 58.12–82.25) in Bangladesh [64]. All the studies we found in this territory by systematic searching were among the general population (Table 2).
3.5.6. Western Pacific Region
The overall COVID-19 vaccine acceptance rate was found as 67.85% (95% CI: 58.96–76.73) (Figure 5) in this territory from 13 studies with a sample size of 38,370. The acceptance rate was highest at 91.30% (95% CI: 90.08–92.52) in China [22] and lowest at 37.20% (95% CI: 34.47–39.93) in Hong Kong [17]. The COVID-19 vaccine acceptance rate among healthcare workers and the general population were estimated respectively at 51.52% (95% CI: 28.98–74.06) and 70.80% (95% CI: 61.81–7979) (Table 2).
4. Discussion
Vaccine hesitancy is a long-standing problem that poses a significant challenge to public health, as evidenced by certain communicable diseases [105] such as Measles, pertussis, and SARS outbreaks. Vaccine acceptance can be a barrier in worldwide attempts to mitigate the detrimental effects of a pandemic on public health and can restrict the power of health systems to control the COVID-19 pandemic. Thus, by accounting for the regional disparities, estimating the COVID-19 vaccine acceptance rate can be effective in designing the action plan to improve vaccine acceptance and battle against the COVID-19 pandemic.
The results of our systematic review and meta-analysis include 1,581,562 respondents. Among them, 1,559,708 were general population and 21,854 were healthcare workers that were pulled from 78 study samples across 32 countries. Results show that the COVID-19 vaccine acceptance rate estimated combinedly among the general population and healthcare workers at post time of vaccine approval (56.59%) was considerably lower than the acceptance rate (64.81%) before the vaccine approval (Figure 4). During the pre-approval time, the general population showed a higher vaccine acceptance rate (66.29%) compared to the post-approval time (56.69%) (Figure 8, Figure 9). It was inspected in the previous two systematic reviews [37, 106] that in many regions, the COVID-19 vaccine acceptance rates were decreasing over time. This declining trend in the COVID-19 vaccine acceptance rate was also observed among the general population in a longitudinal study in China [107]. These are mixed findings because there is also evidence that the acceptance rate is increasing [21]. Researchers [27, 37, 106, 107] agreed that the misinformation about COVID-19, uncertainty about the vaccine efficacy and safety, inconvenience of vaccination, and faster development of COVID-19 vaccines might lead to a decline in vaccine acceptance rate. It is also evident that after vaccine approval, a large group of people is not willing to take the COVID-19 vaccines due to vaccine side effects which created widespread concern [108, 109, 110].
Considerable geographical disparities in vaccine acceptance were found in this study. A comparatively higher acceptance rate was found in the South-East Asia Region (70.18%%), followed by the Western Pacific Region (67.85%), the European Region (66.77%), the Region of the Americas (66.59%), Eastern Mediterranean Region (45.37%), and African Region (39.51%). In a previous study, a very high COVID-19 vaccine acceptance rate was found in the South-East Asia region (90%) by considering only 3 studies in this region [37].
In the African region, the prevalence of vaccine acceptance among the general population was estimated at 42.46% and among healthcare workers was observed at 27.70% (Table 2). An early awareness, perceptions, and practices survey study on COVID-19 in North-Central Nigeria recorded an acceptance rate of only 29%, highlighting the need for further research to accurately depict COVID-19 vaccine hesitancy in Africa [111]. A study found that a lack of desire to receive the vaccine in Cameroon is concerning and several measures need to implement to reduce the COVID-19 vaccine hesitancy to gain herd immunity among both general populations and healthcare workers [9, 112]. However, we found a lack of studies on vaccine acceptance in this region (only five). To generalize the result to the entire region, further studies are required.
In the Eastern Mediterranean Region, 46.43% of the healthcare workers and 44.68% of the general population showed a willingness to accept COVID-19 vaccines (Table 2). A cross-sectional study in 10 Countries in Eastern Mediterranean Region showed a 58% of vaccine acceptance rate among healthcare workers [113]. In this region, the highest acceptance rate was found in Saudi Arabia [48] and the lowest acceptance rate was found in Kuwait [15]. Low vaccination rates in Kuwait may be attributed to the region's prevalent acceptance of conspiratorial ideas, which has resulted in a pessimistic mindset against vaccination [114].
In Europe, both the highest and lowest acceptance rates were found in the UK [12, 80]. Such COVID-19 vaccine hesitancy trends matched those seen in a previous study, which found comparatively higher rates of vaccine hesitancy in the UK [50, 90]. Furthermore, many studies found disparities in the vaccine acceptance rate among adults, healthcare workers, students, etc. in Europe which is also partly investigated in our studies [115, 116, 117].
In American Regions, the COVID-19 vaccine acceptance rate was 69.33% among the general population and 40.86% among healthcare workers (Table 2). Ecuador [92] reported the highest acceptance rate and the lowest acceptance rate was found in the USA [14] in this territory. Ecuador and Brazil reported more than 70% of vaccine acceptance rates observed in some previous studies which are consistent with our studies as Ecuador reported the highest rate [118]. The refusal of a safe, reliable, and widely available COVID-19 vaccine has been a major issue since the beginning in the USA which might be the reason for the lowest acceptance rate in the USA, but the scenario has changed recently [119]. The lower COVID-19 vaccine acceptance rate (33.5%) among healthcare workers in American regions was found in previous studies [120] which is also consistent with our studies.
In South-East Asia Region, no studies were found among healthcare workers. All the studies showed an acceptance rate of 70.18% among the general population (Table 2). The highest acceptance rate was found in Indonesia [63] and the lowest acceptance rate was found in Bangladesh [64]. The lack of confidence in vaccine safety and efficacy, the healthcare system, and management in Bangladesh might be the reasons for the lower vaccine acceptance rate [27].
In the Western Pacific region, around 71% of the general population and 51.52% of healthcare workers intended to accept COVID-19 vaccines (Table 2). China [22] reported the highest acceptance rate, whereas, the lowest acceptance rate was found in Hong Kong [17]. A study found that almost 90% of respondents in China showed a willingness to take vaccines [118] which is consistent with our research.
4.1. Strengths
To our knowledge, this is the first comprehensive study that covered all the territories of the world to investigate COVID-19 vaccine acceptance and found a large number of studies through systematic search. In this study, we estimated the vaccine acceptance rates for several possible subgroups. We also inspected the COVID-19 vaccine approval effect on the vaccine acceptance rate worldwide among healthcare workers and general populations.
4.2. Limitations
The study has a few limitations. First, it’s important to keep in mind that many of the experiments used in this study asked people if they planned to have the vaccine before it was available. Second, diverse approaches to expressing willingness to consider COVID-19 vaccines in multiple trials posed a significant constraint. Third, in the African and South-East Asian regions, we found only five studies each. It is unwise to generalize the findings to an entire region based on only five studies’ data. Fourth, since some studies were done within the same country, some of the included studies might consider the same population. Fifth, since some of the documents were not written in English, we may have overlooked them in the final report. Last but not least, the studies did not consider any heterogeneous effect (such as age variation, sex variation, and so on) on the vaccine acceptance rate.
5. Conclusions
This systematic review and meta-analysis come up with a timely and comprehensive synthesis of the existing literature highlighting the low COVID-19 vaccine acceptance rate among both general people and healthcare workers. The study also detects regional disparities in COVID-19 vaccine acceptance rates. African region and Eastern Mediterranean region showed substantially low acceptance rates. However, very few relevant studies were found in the African and South-East Asian regions making it difficult to generalize the results to the entire regions. A lesser acceptance rate was observed among healthcare workers compared to general people and the vaccine acceptance rate considerably reduced among the general population after vaccine approval (11 December 2020). Such studies will help to implement intervention programs to reduce vaccination hesitancy and increase uptake among the vulnerable group or in regions with a low acceptance rate. The authors of this study highly recommend that more research be undertaken across all regions of the world to determine the factors that might cause COVID-19 vaccination aversion.
Declarations
Author contribution statement
Sultan Mahmud; Md Mohsin: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Sorif Hossain: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Md Mynul Islam; Abdul Muyeed: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
None.
References
- 1.Lin Y., Hu Z., Zhao Q., Alias H., Danaee M., Wong L.P. Understanding COVID-19 vaccine demand and hesitancy: a nationwide online survey in China. PLoS Neglected Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Azzam N., Elsalem L., Gombedza F. A cross-sectional study to determine factors affecting dental and medical students’ preference for virtual learning during the COVID-19 outbreak. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keni R., Alexander A., Nayak P.G., Mudgal J., Nandakumar K. COVID-19: emergence, spread, possible treatments, and global burden. Front. Public Health. 2020;8 doi: 10.3389/fpubh.2020.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID Live Update: 161,162,658 Cases and 3,347,560 Deaths from the Coronavirus - Worldometer, (n.d.). https://www.worldometers.info/coronavirus/(accessed May 13, 2021).
- 5.Mahmud S., Hossain S., Muyeed A., Islam M.M., Mohsin Md. The global prevalence of depression, anxiety, stress, and, insomnia and its changes among health professionals during COVID-19 pandemic: a rapid systematic review and meta-analysis. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e07393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahmud S., Mohsin M., Dewan Md.N., Muyeed A. The global prevalence of depression, anxiety, stress, and insomnia among general population during COVID-19 pandemic: a systematic review and meta-analysis. Trends in Psychology. 2022:1–28. [Google Scholar]
- 7.Hiscott J., Alexandridi M., Muscolini M., Tassone E., Palermo E., Soultsioti M., Zevini A. The global impact of the coronavirus pandemic. Cytokine Growth Factor Rev. 2020;53:1–9. doi: 10.1016/j.cytogfr.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohsin M., Mahmud S. Omicron SARS-CoV-2 variant of concern: a review on its transmissibility, immune evasion, reinfection, and severity. Medicine (Baltim.) 2022;101 doi: 10.1097/MD.0000000000029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinga J.N., Sinda L.K., Titanji V.P.K. Assessment of vaccine hesitancy to a COVID-19 vaccine in Cameroonian adults and its global implication. Vaccines. 2021;9:175. doi: 10.3390/vaccines9020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marti M., de Cola M., MacDonald N.E., Dumolard L., Duclos P. Assessments of global drivers of vaccine hesitancy in 2014—looking beyond safety concerns. PLoS One. 2017;12 doi: 10.1371/journal.pone.0172310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batty G.D., Deary I.J., Fawns-Ritchie C., Gale C.R., Altschul D. Pre-pandemic cognitive function and COVID-19 vaccine hesitancy: cohort study. Brain, Behavior, and Immunity. 2021:100–105. doi: 10.1016/j.bbi.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickerson J., Lockyer B., Moss R.H., Endacott C., Kelly B., Bridges S., Crossley K.L., Bryant M., Sheldon T.A., Wright J., Pickett K.E., McEachan R.R.C. Bradford institute for health research COVID-19 scientific advisory group, COVID-19 vaccine hesitancy in an ethnically diverse community: descriptive findings from the born in bradford study. Wellcome Open Res. 2021;6:23. doi: 10.12688/wellcomeopenres.16576.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou Z., Tong Y., Du F., Lu L., Zhao S., Yu K., Piatek S.J., Larson H.J., Lin L. Assessing COVID-19 vaccine hesitancy, confidence and public engagement: a global social listening study. Confid. Public Engagem. Glob. Soc. List. Study. 2021;23(6):e27632. doi: 10.2196/27632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shekhar R., Sheikh A.B., Upadhyay S., Singh M., Kottewar S., Mir H., Barrett E., Pal S. COVID-19 vaccine acceptance among health care workers in the United States. Vaccines. 2021;9:119. doi: 10.3390/vaccines9020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sallam M., Dababseh D., Eid H., Al-Mahzoum K., Al-Haidar A., Taim D., Yaseen A., Ababneh N.A., Bakri F.G., Mahafzah A. High rates of COVID-19 vaccine hesitancy and its association with conspiracy beliefs: a study in Jordan and Kuwait among other arab countries. Vaccines. 2021;9:42. doi: 10.3390/vaccines9010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sallam M., Dababseh D., Eid H., Hasan H., Taim D., Al-Mahzoum K., Al-Haidar A., Yaseen A., Ababneh N.A., Assaf A., Bakri F.G., Matar S., Mahafzah A. Low COVID-19 vaccine acceptance is correlated with conspiracy beliefs among university students in Jordan. Int. J. Environ. Res. Publ. Health. 2021;18:2407. doi: 10.3390/ijerph18052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong M.C., Wong E.L., Huang J., Cheung A.W., Law K., Chong M.K., Ng R.W., Lai C.K., Boon S.S., Lau J.T. Acceptance of the COVID-19 vaccine based on the health belief model: a population-based survey in Hong Kong. Vaccine. 2021;39:1148–1156. doi: 10.1016/j.vaccine.2020.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yurttas B., Poyraz B.C., Sut N., Ozdede A., Oztas M., Uğurlu S., Tabak F., Hamuryudan V., Seyahi E. Willingness to get the COVID-19 vaccine among patients with rheumatic diseases, healthcare workers and general population in Turkey: a web-based survey. Rheumatol. Int. 2021;41:1105–1114. doi: 10.1007/s00296-021-04841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabamba Nzaji M., Kabamba Ngombe L., Ngoie Mwamba G., Banza Ndala D.B., Mbidi Miema J., Luhata Lungoyo C., Lora Mwimba B., Cikomola Mwana Bene A., Mukamba Musenga E. Acceptability of vaccination against COVID-19 among healthcare workers in the democratic republic of the Congo, pragmatic obs. Res. 2020;11:103–109. doi: 10.2147/POR.S271096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saied S.M., Saied E.M., Kabbash I.A., Abdo S.A.E.-F. Vaccine hesitancy: beliefs and barriers associated with COVID-19 vaccination among Egyptian medical students. J. Med. Virol. 2021;n/a doi: 10.1002/jmv.26910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallam M., Al-Sanafi M., Sallam M. A global map of COVID-19 vaccine acceptance rates per country: an updated concise narrative review. J. Multidiscip. Healthc. 2022;15:21–45. doi: 10.2147/JMDH.S347669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J., Jing R., Lai X., Zhang H., Lyu Y., Knoll M.D., Fang H. Acceptance of COVID-19 vaccination during the COVID-19 pandemic in China. Vaccines. 2020;8:482. doi: 10.3390/vaccines8030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun S., Lin D., Operario D. Interest in COVID-19 vaccine trials participation among young adults in China: willingness, reasons for hesitancy, and demographic and psychosocial determinants. Prev. Med. Rep. 2021;22 doi: 10.1016/j.pmedr.2021.101350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papagiannis D., Rachiotis G., Malli F., Papathanasiou I.V., Kotsiou O., Fradelos E.C., Giannakopoulos K., Gourgoulianis K.I. Acceptability of COVID-19 vaccination among Greek health professionals. Vaccines. 2021;9:200. doi: 10.3390/vaccines9030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manning M.L., Gerolamo A.M., Marino M.A., Hanson-Zalot M.E., Pogorzelska-Maziarz M. COVID-19 vaccination readiness among nurse faculty and student nurses. Nurs. Outlook. 2021;69(4):565–573. doi: 10.1016/j.outlook.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehde D.M., Roberts M.K., Herring T.E., Alschuler K.N. Willingness to obtain COVID-19 vaccination in adults with multiple sclerosis in the United States, Mult. Scler. Relat. Disord. 2021;49 doi: 10.1016/j.msard.2021.102788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahmud S., Mohsin M., Khan I.A., Mian A.U., Zaman M.A. Knowledge, beliefs, attitudes and perceived risk about COVID-19 vaccine and determinants of COVID-19 vaccine acceptance in Bangladesh. PLoS One. 2021;16 doi: 10.1371/journal.pone.0257096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO, COVAX, Work. Glob. Equitable Access COVID-19 Vaccines. (n.d.). https://www.who.int/initiatives/act-accelerator/covax (accessed October 24, 2021).
- 29.Mohsin M., Mahmud S., Mian A.U., Hasan P., Muyeed A., Ali Md.T., Ahmed F.F., Islam A., Rahman M.M., Islam M., Rahaman Khan M.H., Rahman M.S. Side effects of COVID-19 vaccines and perceptions about COVID-19 and its vaccines in Bangladesh. Vaccine: X. 2022;12:100207. doi: 10.1016/j.jvacx.2022.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neumann-Böhme S., Varghese N.E., Sabat I., Barros P.P., Brouwer W., van Exel J., Schreyögg J., Stargardt T. Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. Eur. J. Health Econ. 2020;21:977–982. doi: 10.1007/s10198-020-01208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Megget K. Even covid-19 can’t kill the anti-vaccination movement. BMJ. 2020;369:m2184. doi: 10.1136/bmj.m2184. [DOI] [PubMed] [Google Scholar]
- 32.Mello M.M., Silverman R.D., Omer S.B. Ensuring uptake of vaccines against SARS-CoV-2. N. Engl. J. Med. 2020;383:1296–1299. doi: 10.1056/NEJMp2020926. [DOI] [PubMed] [Google Scholar]
- 33.Carrieri V., Madio L., Principe F. Vaccine hesitancy and (fake) news: quasi-experimental evidence from Italy. Health Econ. 2019;28:1377–1382. doi: 10.1002/hec.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gruzd A., Mai P. Going viral: how a single tweet spawned a COVID-19 conspiracy theory on Twitter. Big Data Soc. 2020;7 [Google Scholar]
- 35.Forman R., Shah S., Jeurissen P., Jit M., Mossialos E. COVID-19 vaccine challenges: what have we learned so far and what remains to be done? Health Pol. 2021;125:553–567. doi: 10.1016/j.healthpol.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zintel S., Flock C., Arbogast A.L., Forster A., von Wagner C., Sieverding M. Social Science Research Network; Rochester, NY: 2021. Gender Differences in the Intention to Get Vaccinated against COVID-19 - a Systematic Review and Meta-Analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines. 2021;9:160. doi: 10.3390/vaccines9020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O. of the Commissioner . 2021. FDA Approves First COVID-19 Vaccine, FDA.https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine accessed October 24, 2021. [Google Scholar]
- 39.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. For the meta-analysis of observational studies in Epidemiology (MOOSE) group, meta-analysis of observational studies in EpidemiologyA proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 42.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group T.P. Preferred reporting Items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 43.Countries overview | World Health Organization, (n.d.). https://www.who.int/countries (accessed May 24, 2021).
- 44.Ahmed G., Almoosa Z., Mohamed D., Rapal J., Minguez O., Abu Khurma I., Alnems A., Al Mutair A. Healthcare provider attitudes toward the newly developed COVID-19 vaccine: cross-sectional study. Nurs. Rep. 2021;11:187–194. doi: 10.3390/nursrep11010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alabdulla M., Reagu S.M., Al-Khal A., Elzain M., Jones R.M. COVID-19 vaccine hesitancy and attitudes in Qatar: a national cross-sectional survey of a migrant-majority population, Influenza Other Respir. Viruses. 2021;15:361–370. doi: 10.1111/irv.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ali M., Hossain A. What is the extent of COVID-19 vaccine hesitancy in Bangladesh?: a cross-sectional rapid national survey. MJ Open. 2021;11:e050303. doi: 10.1136/bmjopen-2021-050303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almaghaslah D., Alsayari A., Kandasamy G., Vasudevan R. COVID-19 vaccine hesitancy among young adults in Saudi Arabia: a cross-sectional web-based study. Vaccines. 2021;9:330. doi: 10.3390/vaccines9040330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Mohaithef M., Padhi B.K. Determinants of COVID-19 vaccine acceptance in Saudi Arabia: a web-based national survey. J. Multidiscip. Healthc. 2020;13:1657–1663. doi: 10.2147/JMDH.S276771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alqudeimat Y., Alenezi D., AlHajri B., Alfouzan H., Almokhaizeem Z., Altamimi S., Almansouri W., Alzalzalah S., Ziyab A.H. Acceptance of a COVID-19 vaccine and its related determinants among the general adult population in Kuwait. Med. Princ. Pract. 2021;30:262–271. doi: 10.1159/000514636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bell S., Clarke R., Mounier-Jack S., Walker J.L., Paterson P. Parents’ and guardians’ views on the acceptability of a future COVID-19 vaccine: a multi-methods study in England. Vaccine. 2020;38:7789–7798. doi: 10.1016/j.vaccine.2020.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benis A., Seidmann A., Ashkenazi S. Reasons for taking the COVID-19 vaccine by US social media users. Vaccines. 2021;9:315. doi: 10.3390/vaccines9040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borriello A., Master D., Pellegrini A., Rose J.M. Preferences for a COVID-19 vaccine in Australia. Vaccine. 2021;39:473–479. doi: 10.1016/j.vaccine.2020.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.B W.C.,H., T Z., H L., B L., L C., M X., J L., H Z., S Z., Y W., N H., J D., Yq L., Qb L., F C. Vaccination willingness, vaccine hesitancy, and estimated coverage at the first round of COVID-19 vaccination in China: a national cross-sectional study. Vaccine. 2021;39:2833–2842. doi: 10.1016/j.vaccine.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cordina M., Lauri M.A., Lauri J., Cordina M., Lauri M.A., Lauri J. Attitudes towards COVID-19 vaccination, vaccine hesitancy and intention to take the vaccine. Pharm. Pract. Granada. 2021;19 doi: 10.18549/PharmPract.2021.1.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ditekemena J.D., Nkamba D.M., Mutwadi A., Mavoko H.M., Siewe Fodjo J.N., Luhata C., Obimpeh M., Van Hees S., Nachega J.B., Colebunders R. COVID-19 vaccine acceptance in the democratic republic of Congo: a cross-sectional survey. Vaccines. 2021;9:153. doi: 10.3390/vaccines9020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edwards B., Biddle N., Gray M., Sollis K. COVID-19 vaccine hesitancy and resistance: correlates in a nationally representative longitudinal survey of the Australian population. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eguia H., Vinciarelli F., Bosque-Prous M., Kristensen T., Saigí-Rubió F. Spain’s hesitation at the gates of a COVID-19 vaccine. Vaccines. 2021;9:170. doi: 10.3390/vaccines9020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eniade O.D., Olarinmoye A., Otovwe A., Akintunde F.E., Okedare O.O., Aniyeloye A.O. Willingness to accept COVID-19 vaccine and its determinants among Nigeria citizens: a web-based cross-sectional study. J. Adv. Med. Med. Res. 2021:13–22. [Google Scholar]
- 59.Fisher K.A., Bloomstone S.J., Walder J., Crawford S., Fouayzi H., Mazor K.M. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of US adults. Ann. Intern. Med. 2020;173:964–973. doi: 10.7326/M20-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freeman D., Loe B.S., Chadwick A., Vaccari C., Waite F., Rosebrock L., Jenner L., Petit A., Lewandowsky S., Vanderslott S., Innocenti S., Larkin M., Giubilini A., Yu L.-M., McShane H., Pollard A.J., Lambe S. COVID-19 vaccine hesitancy in the UK: the Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol. Med. 2020:1–15. doi: 10.1017/S0033291720005188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gagneux-Brunon A., Detoc M., Bruel S., Tardy B., Rozaire O., Frappe P., Botelho-Nevers E. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: a cross-sectional survey. J. Hosp. Infect. 2021;108:168–173. doi: 10.1016/j.jhin.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Griffith J., Marani H., Monkman H. COVID-19 vaccine hesitancy in Canada: content analysis of tweets using the theoretical domains framework. J. Med. Internet Res. 2021;23 doi: 10.2196/26874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harapan H., Wagner A.L., Yufika A., Winardi W., Anwar S., Gan A.K., Setiawan A.M., Rajamoorthy Y., Sofyan H., Mudatsir M. Acceptance of a COVID-19 vaccine in southeast Asia: a cross-sectional study in Indonesia. Front. Public Health. 2020;8 doi: 10.3389/fpubh.2020.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hossain M.B., Alam M.Z., Islam M.S., Sultan S., Faysal M.M., Rima S., Hossain M.A., Al Mamun A. Health belief, planned behavior, or psychological antecedents: what predicts COVID-19 vaccine hesitancy better among the Bangladeshi adults? medRxiv. 2021 doi: 10.3389/fpubh.2021.711066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hussein A.A.M., Galal I., Makhlouf N.A., Makhlouf H.A., Abd-Elaal H.K., Kholief K.M., Saad M.M., Abdellah D.A. A national survey of potential acceptance of COVID-19 vaccines in healthcare workers in Egypt. medRxiv. 2021 [Google Scholar]
- 66.Iacoella C., Ralli M., Maggiolini A., Arcangeli A., Ercoli L. Acceptance of COVID-19 vaccine among persons experiencing homelessness in the City of Rome, Italy. Eur. Rev. Med. Pharmacol. Sci. 2021;25:3132–3135. doi: 10.26355/eurrev_202104_25568. [DOI] [PubMed] [Google Scholar]
- 67.Kaplan A.K., Sahin M.K., Parildar H., Guvenc I.A. The willingness to accept the COVID-19 vaccine and affecting factors among healthcare professionals: a cross-sectional study in Turkey. Int. J. Clin. Pract. 2021 doi: 10.1111/ijcp.14226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khubchandani J., Sharma S., Price J.H., Wiblishauser M.J., Sharma M., Webb F.J. COVID-19 vaccination hesitancy in the United States: a rapid national assessment. J. Community Health. 2021;46:270–277. doi: 10.1007/s10900-020-00958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kose S., Mandiracioglu A., Sahin S., Kaynar T., Karbus O., Ozbel Y. Vaccine hesitancy of the COVID-19 by health care personnel. Int. J. Clin. Pract. 2021;75 [Google Scholar]
- 70.Kourlaba G., Kourkouni E., Maistreli S., Tsopela C.-G., Molocha N.-M., Triantafyllou C., Koniordou M., Kopsidas I., Chorianopoulou E., Maroudi-Manta S., Filippou D., Zaoutis T.E. Willingness of Greek general population to get a COVID-19 vaccine. Glob. Health Res. Policy. 2021;6 doi: 10.1186/s41256-021-00188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuhn R., Henwood B., Lawton A., Kleva M., Murali K., Gelberg L., King C. 2021. COVID-19 Vaccine Access and Attitudes Among People Experiencing Homelessness from Pilot mobile Phone Survey in Los Angeles, CA, MedRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwok K.O., Li K.-K., Wei W.I., Tang A., Wong S.Y.S., Lee S.S. Influenza vaccine uptake, COVID-19 vaccination intention and vaccine hesitancy among nurses: a survey. Int. J. Nurs. Stud. 2021;114 doi: 10.1016/j.ijnurstu.2020.103854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Latkin C., Dayton L.A., Yi G., Konstantopoulos A., Park J., Maulsby C., Kong X. COVID-19 vaccine intentions in the United States, a social-ecological framework. Vaccine. 2021;39:2288–2294. doi: 10.1016/j.vaccine.2021.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Latkin C.A., Dayton L., Yi G., Colon B., Kong X. Mask usage, social distancing, racial, and gender correlates of COVID-19 vaccine intentions among adults in the US. PLoS One. 2021;16 doi: 10.1371/journal.pone.0246970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ledda C., Costantino C., Cuccia M., Maltezou H.C., Rapisarda V. Attitudes of healthcare personnel towards vaccinations before and during the COVID-19 pandemic. Int. J. Environ. Res. Publ. Health. 2021;18:2703. doi: 10.3390/ijerph18052703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Machida M., Nakamura I., Kojima T., Saito R., Nakaya T., Hanibuchi T., Takamiya T., Odagiri Y., Fukushima N., Kikuchi H., Amagasa S., Watanabe H., Inoue S. Acceptance of a COVID-19 vaccine in Japan during the COVID-19 pandemic. Vaccines. 2021;9:210. doi: 10.3390/vaccines9030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malik A.A., McFadden S.M., Elharake J., Omer S.B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mo P.K., Luo S., Wang S., Zhao J., Zhang G., Li L., Li L., Xie L., Lau J.T. Intention to receive the COVID-19 vaccination in China: application of the diffusion of innovations theory and the moderating role of openness to experience. Vaccines. 2021;9:129. doi: 10.3390/vaccines9020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murphy J., Vallières F., Bentall R.P., Shevlin M., McBride O., Hartman T.K., McKay R., Bennett K., Mason L., Gibson-Miller J., Levita L., Martinez A.P., Stocks T.V.A., Karatzias T., Hyland P. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat. Commun. 2021;12:29. doi: 10.1038/s41467-020-20226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nguyen L.H., Joshi A.D., Drew D.A., Merino J., Ma W., Lo C.-H., Kwon S., Wang K., Graham M.S., Polidori L., Menni C., Sudre C.H., Anyane-Yeboa A., Astley C.M., Warner E.T., Hu C.Y., Selvachandran S., Davies R., Nash D., Franks P.W., Wolf J., Ourselin S., Steves C.J., Spector T.D., Chan A.T. On behalf of the C. Consortium, Racial and ethnic differences in COVID-19 vaccine hesitancy and uptake. medRxiv. 2021 [Google Scholar]
- 81.de Oliveira B.L.C.A., Campos M.A.G., Queiroz R.C. de S., de Souza B.F., dos Santos A.M., da Silva A.A.M. Prevalence and factors associated with covid-19 vaccine hesitancy in Maranhão, Brazil, Rev. Saude Publica. 2021;55:12. doi: 10.11606/s1518-8787.2021055003417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Olomofe C.O., Soyemi V.K., Udomah B.F., Owolabi A.O., Ajumuka E.E., Igbokwe C.M., Ashaolu U.O., Adeyemi A.O., Aremu-Kasumu Y.B., Dada O.F. Predictors of uptake of a potential covid-19 vaccine among Nigerian adults. medRxiv. 2021 [Google Scholar]
- 83.Pastorino R., Villani L., Mariani M., Ricciardi W., Graffigna G., Boccia S. Impact of COVID-19 pandemic on flu and COVID-19 vaccination intentions among university students. Vaccines. 2021;9:70. doi: 10.3390/vaccines9020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paul E., Steptoe A., Fancourt D. Attitudes towards vaccines and intention to vaccinate against COVID-19: implications for public health communications. Lancet Reg. Health-Eur. 2021;1 doi: 10.1016/j.lanepe.2020.100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Puteikis K., Mameniškienė R. Factors associated with COVID-19 vaccine hesitancy among people with epilepsy in Lithuania. Int. J. Environ. Res. Publ. Health. 2021;18:4374. doi: 10.3390/ijerph18084374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qattan A.M.N., Alshareef N., Alsharqi O., Al Rahahleh N., Chirwa G.C., Al-Hanawi M.K. Acceptability of a COVID-19 vaccine among healthcare workers in the kingdom of Saudi Arabia. Front. Med. 2021;8 doi: 10.3389/fmed.2021.644300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reiter P.L., Pennell M.L., Katz M.L. Acceptability of a COVID-19 vaccine among adults in the United States: how many people would get vaccinated? Vaccine. 2020;38:6500–6507. doi: 10.1016/j.vaccine.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang C., Han B., Zhao T., Liu H., Liu B., Chen L., Xie M., Liu J., Zheng H., Zhang S., Wang Y., Huang N., Du J., Liu Y.-Q., Lu Q.-B., Cui F. Vaccination willingness, vaccine hesitancy, and estimated coverage at the first round of COVID-19 vaccination in China: a national cross-sectional study. Vaccine. 2021;39:2833–2842. doi: 10.1016/j.vaccine.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reno C., Maietti E., Fantini M.P., Savoia E., Manzoli L., Montalti M., Gori D. Enhancing COVID-19 vaccines acceptance: results from a survey on vaccine hesitancy in northern Italy. Vaccines. 2021;9:378. doi: 10.3390/vaccines9040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robertson E., Reeve K.S., Niedzwiedz C.L., Moore J., Blake M., Green M., Katikireddi S.V., Benzeval M.J. Predictors of COVID-19 vaccine hesitancy in the UK household longitudinal study. Brain Behav. Immun. 2021;94:41–50. doi: 10.1016/j.bbi.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Salmon D.A., Dudley M.Z., Brewer J., Kan L., Gerber J.E., Budigan H., Proveaux T.M., Bernier R., Rimal R., Schwartz B. COVID-19 vaccination attitudes, values and intentions among United States adults prior to emergency use authorization. Vaccine. 2021;39:2698–2711. doi: 10.1016/j.vaccine.2021.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sarasty O., Carpio C.E., Hudson D., Guerrero-Ochoa P.A., Borja I. The demand for a COVID-19 vaccine in Ecuador. Vaccine. 2020;38:8090–8098. doi: 10.1016/j.vaccine.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Savoia E., Piltch-Loeb R., Goldberg B., Miller-Idriss C., Hughes B., Montrond A., Kayyem J., Testa M. Predictors of COVID-19 Vaccine Hesitancy: Socio-Demographics. Vaccines. 2021;9:767. doi: 10.3390/vaccines9070767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schwarzinger M., Watson V., Arwidson P., Alla F., Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health. 2021;6 doi: 10.1016/S2468-2667(21)00012-8. e210–e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sherman S.M., Smith L.E., Sim J., Amlôt R., Cutts M., Dasch H., Rubin G.J., Sevdalis N. COVID-19 vaccination intention in the UK: results from the COVID-19 vaccination acceptability study (CoVAccS), a nationally representative cross-sectional survey. Hum. Vaccines Immunother. 2020:1–10. doi: 10.1080/21645515.2020.1846397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shmueli L. Predicting intention to receive COVID-19 vaccine among the general population using the health belief model and the theory of planned behavior model. BMC Publ. Health. 2021;21:1–13. doi: 10.1186/s12889-021-10816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Soares P., Rocha J.V., Moniz M., Gama A., Laires P.A., Pedro A.R., Dias S., Leite A., Nunes C. Factors associated with COVID-19 vaccine hesitancy. Vaccines. 2021;9:300. doi: 10.3390/vaccines9030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taylor S., Landry C.A., Paluszek M.M., Groenewoud R., Rachor G.S., Asmundson G.J. A proactive approach for managing COVID-19: the importance of understanding the motivational roots of vaccination hesitancy for SARS-CoV2. Front. Psychol. 2020;11:2890. doi: 10.3389/fpsyg.2020.575950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tulloch J.S., Lawrenson K., Gordon A.L., Ghebrehewet S., Ashton M., Peddie S., Parvulescu P. COVID-19 vaccine hesitancy in care home staff: a survey of Liverpool care homes. medRxiv. 2021 doi: 10.1016/j.vaccine.2023.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Verger P., Scronias D., Dauby N., Adedzi K.A., Gobert C., Bergeat M., Gagneur A., Dubé E. Attitudes of healthcare workers towards COVID-19 vaccination: a survey in France and French-speaking parts of Belgium and Canada, 2020. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.3.2002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang K., Wong E.L.Y., Ho K.F., Cheung A.W.L., Chan E.Y.Y., Yeoh E.K., Wong S.Y.S. Intention of nurses to accept coronavirus disease 2019 vaccination and change of intention to accept seasonal influenza vaccination during the coronavirus disease 2019 pandemic: a cross-sectional survey. Vaccine. 2020;38:7049–7056. doi: 10.1016/j.vaccine.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ward J.K., Alleaume C., Peretti-Watel P., Peretti-Watel P., Seror V., Cortaredona S., Launay O., Raude J., Verger P., Beck F., Legleye S., L’Haridon O., Ward J. The French public’s attitudes to a future COVID-19 vaccine: the politicization of a public health issue. Soc. Sci. Med. 2020;265 doi: 10.1016/j.socscimed.2020.113414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang K.C., Fang Y., Cao H., Chen H., Hu T., Chen Y.Q., Zhou X., Wang Z. Parental acceptability of COVID-19 vaccination for children under the age of 18 Years: cross-sectional online survey. JMIR Pediatr. Parent. 2020;3 doi: 10.2196/24827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qunaibi E.A., Helmy M., Basheti I., Sultan I. A high rate of COVID-19 vaccine hesitancy among arabs: results of a large-scale survey. MedRxiv. 2021 doi: 10.7554/eLife.68038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Phadke V.K., Bednarczyk R.A., Salmon D.A., Omer S.B. Association between vaccine refusal and vaccine-preventable diseases in the United States: a review of Measles and pertussis. JAMA. 2016;315:1149–1158. doi: 10.1001/jama.2016.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Robinson E., Jones A., Lesser I., Daly M. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39:2024–2034. doi: 10.1016/j.vaccine.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang J., Lu X., Lai X., Lyu Y., Zhang H., Fenghuang Y., Jing R., Li L., Yu W., Fang H. The changing acceptance of COVID-19 vaccination in different epidemic phases in China: a longitudinal study. Vaccines. 2021;9:191. doi: 10.3390/vaccines9030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lucia V.C., Kelekar A., Afonso N.M. COVID-19 vaccine hesitancy among medical students. J. Public Health. 2021;43:445–449. doi: 10.1093/pubmed/fdaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sudharsanan N., Favaretti C., Hachaturyan V., Bärnighausen T., Vandormael A. The effect of framing and communicating COVID-19 vaccine side-effect risks on vaccine intentions for adults in the UK and the USA: a structured summary of a study protocol for a randomized controlled trial. Trials. 2021;22:592. doi: 10.1186/s13063-021-05484-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saeed B.Q., Al-Shahrabi R., Alhaj S.S., Alkokhardi Z.M., Adrees A.O. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int. J. Infect. Dis. 2021;111:219–226. doi: 10.1016/j.ijid.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reuben R.C., Danladi M.M.A., Saleh D.A., Ejembi P.E. Knowledge, attitudes and practices towards COVID-19: an epidemiological survey in North-Central Nigeria. J. Community Health. 2021;46:457–470. doi: 10.1007/s10900-020-00881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nachega J.B., Sam-Agudu N.A., Masekela R., van der Zalm M.M., Nsanzimana S., Condo J., Ntoumi F., Rabie H., Kruger M., Wiysonge C.S., Ditekemena J.D., Chirimwami R.B., Ntakwinja M., Mukwege D.M., Noormahomed E., Paleker M., Mahomed H., Tamfum J.-J.M., Zumla A., Suleman F. Addressing challenges to rolling out COVID-19 vaccines in African countries. Lancet Global Health. 2021 doi: 10.1016/S2214-109X(21)00097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Elhadi Y.A.M., Mehanna A., Adebisi Y.A., Essar M.Y., Saeh H.M.E., Alnahari S.A., Alenezi O.H., Chbib D.E., Yahya Z., Ahmed E., Ahmad S., Uakkas S., Sabahelzain M.M., Alyamani B.A., Nemat A., Lucero-Prisno D.E., Zaghloul A. Intention of healthcare workers to receive COVID-19 vaccine: a cross-sectional survey in 10 countries in eastern mediterranean region. medRxiv. 2021 [Google Scholar]
- 114.Sallam M., Dababseh D., Yaseen A., Al-Haidar A., Ababneh N.A., Bakri F.G., Mahafzah A. Conspiracy beliefs are associated with lower knowledge and higher anxiety levels regarding COVID-19 among students at the university of Jordan. Int. J. Environ. Res. Publ. Health. 2020;17:4915. doi: 10.3390/ijerph17144915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dzieciolowska S., Hamel D., Gadio S., Dionne M., Gagnon D., Robitaille L., Cook E., Caron I., Talib A., Parkes L., Dubé È., Longtin Y. Covid-19 vaccine acceptance, hesitancy and refusal among Canadian healthcare workers: a multicenter survey. Am. J. Infect. Control. 2021;49(9):1152–1157. doi: 10.1016/j.ajic.2021.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hall V.J., Foulkes S., Saei A., Andrews N., Oguti B., Charlett A., Wellington E., Stowe J., Gillson N., Atti A., Islam J., Karagiannis I., Munro K., Khawam J., Chand M.A., Brown C.S., Ramsay M., Lopez-Bernal J., Hopkins S., Andrews N., Atti A., Aziz H., Brooks T., Brown C., Camero D., Carr C., Chand M., Charlett A., Crawford H., Cole M., Conneely J., D’Arcangelo S., Ellis J., Evans S., Foulkes S., Gillson N., Gopal R., Hall L., Hall V., Harrington P., Hopkins S., Hewson J., Hoschler K., Ironmonger D., Islam J., Kall M., Karagiannis I., Kay O., Khawam J., King E., Kirwan P., Kyffin R., Lackenby A., Lattimore M., Linley E., Lopez-Bernal J., Mabey L., McGregor R., Miah S., Monk E., Munro K., Naheed Z., Nissr A., O’Connell A., Oguti B., Okafor H., Organ S., Osbourne J., Otter A., Patel M., Platt S., Pople D., Potts K., Ramsay M., Robotham J., Rokadiya S., Rowe C., Saei A., Sebbage G., Semper A., Shrotri M., Simmons R., Soriano A., Staves P., Taylor S., Taylor A., Tengbe A., Tonge S., Vusirikala A., Wallace S., Wellington E., Zambon M., Corrigan D., Sartaj M., Cromey L., Campbell S., Braithwaite K., Price L., Haahr L., Stewart S., Lacey E., Partridge L., Stevens G., Ellis Y., Hodgson H., Norman C., Lacey E., Larru B., Mcwilliam S., Roynon A., Northfield J., Winchester S., Cieciwa P., Pai A., Bakker P., Loughrey C., Watt A., Adair F., Hawkins A., Grant A., Temple-Purcell R., Howard J., Slawson N., Subudhi C., Davies S., Bexley A., Penn R., Wong N., Boyd G., Rajgopal A., Arenas-Pinto A., Matthews R., Whileman A., Laugharne R., Ledger J., Barnes T., Jones C., Osuji N., Chitalia N., Bailey T., Akhtar S., Harrison G., Horne S., Walker N., Agwuh K., Maxwell V., Graves J., Williams S., O’Kelly A., Ridley P., Cowley A., Johnstone H., Swift P., Democratis J., Meda M., Brake S., Gunn J., Selassi A., Hams S., Irvine V., Chandrasekaran B., Forsyth C., Radmore J., Thomas C., Brown K., Roberts S., Burns P., Gajee K., Lewis T., Byrne T., Sanderson F., Knight S., Macnaughton E., Burton B., Smith H., Chaudhuri R., Aeron-Thomas J., Hollinshead K., Shorten R., Swan A., Shorten R., Favager C., Murira J., Baillon S., Hamer S., Shah A., Russell J., Brennan D., Dave A., Chawla A., Westwell F., Adeboyeku D., Papineni P., Pegg C., Williams M., Ahmad S., Horsley A., Gabriel C., Pagget K., Cieciwa P., Maloney G., Ashcroft J., Del Rosario I., Crosby-Nwaobi R., Flanagan D., Dhasmana D., Fowler S., Cameron E., Prentice L., Sinclair C., Irvine V., Bateman V., McLelland-Brooks K., Ho A., Murphy M., Cochrane A., Gibson A., Patel M., Black K., Tempeton K., Donaldson S., Coke L., Elumogo N., Elliott J., Padgett D., Cross A., Mirfenderesky M., Joyce S., Sinanovic I., Howard M., Lewis T., Cowling P., Brazil M., Hanna E., Abdelrazik A., Brand S., Sheridan E., Wadams B., Lloyd A., Mouland J., Giles J., Pottinger G., Coles H., Joseph M., Lee M., Orr S., Chenoweth H., Browne D., Auckland C., Lear R., Mahungu T., Rodger A., Warren S., Brooking D., Pai S., Druyeh R., Smith E., Stone S., Meisner S., Delgado D., Underhill E., Keen L., Aga M., Domingos P., Gormley S., Kerrison C., Birch S., DeSilva T., Allsop L., Ambalkar S., Beekes M., Jose S., Tomlinson J., Painter S., Price C., Pepperell J., James K., Trinick T., Moore L., Day J., Boulos A., Knox I., Defever E., McCracken D., Brown K., Gray K., Houston A., Planche T., Pritchard Jones R., Wycherley D., Bennett S., Marrs J., Nimako K., Stewart B., Bain S., Kalakonda N., Khanduri S., Ashby A., Holden M., Mahabir N., Harwood J., Payne B., Court K., White N., Longfellow R., Hughes L., Green M., Halkes M., Mercer P., Roebuck A., Wilson-Davies E., Gallego L., Lazarus R., Aldridge N., Berry L., Game F., Reynolds T., Holmes C., Wiselka M., Higham A., Booth M., Duff C., Alderton J., Hilton D., Powell J., Jackson A., Plant A., Ahmed N., Chin T., Qazzafi M., Moody A., Tilley R., Donaghy T., O’Kane M., Shipman K., Sierra R., Parmar C., Mills G., Harvey D., Huang Y., Birch J., Robinson L., Board S., Broadley A., Laven C., Todd N., Eyre D., Jeffery K., Dunachie S., Duncan C., Klenerman P., Turtle L., Baxendale H., Heeney J. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.MacDougall D.M., Halperin B.A., MacKinnon-Cameron D., Li L., McNeil S.A., Langley J.M., Halperin S.A. The challenge of vaccinating adults: attitudes and beliefs of the Canadian public and healthcare providers. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-009062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lazarus J.V., Ratzan S.C., Palayew A., Gostin L.O., Larson H.J., Rabin K., Kimball S., El-Mohandes A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2021;27:225–228. doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Engber D. 2021. America Is Now in the Hands of the Vaccine-Hesitant, the Atlantic. https://www.theatlantic.com/health/archive/2021/03/america-is-now-in-the-hands-of-the-vaccine-hesitant/618352/ (accessed May 17, 2021) [Google Scholar]
- 120.Gadoth A., Halbrook M., Martin-Blais R., Gray A., Tobin N.H., Ferbas K.G., Aldrovandi G.M., Rimoin A.W. Assessment of COVID-19 vaccine acceptance among healthcare workers in Los Angeles. medRxiv. 2020 doi: 10.7326/M20-7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.