Abstract
Medicinal plant microRNAs (miRNAs) are an endogenous class of small RNA central to the posttranscriptional regulation of gene expression. Biosynthetic research has shown that the mature miRNAs in medicinal plants can be produced from either the standard messenger RNA splicing mechanism or the pre-ribosomal RNA splicing process. The medicinal plant miRNA function is separated into two levels: (1) the cross-kingdom level, which is the regulation of disease-related genes in animal cells by oral intake, and (2) the intra-kingdom level, which is the participation of metabolism, development, and stress adaptation in homologous or heterologous plants. Increasing research continues to enrich the biosynthesis and function of medicinal plant miRNAs. In this review, peer-reviewed papers on medicinal plant miRNAs published on the Web of Science were discussed, covering a total of 78 species. The feasibility of the emerging role of medicinal plant miRNAs in regulating animal gene function was critically evaluated. Staged progress in intra-kingdom miRNA research has only been found in a few medicinal plants, which may be mainly inhibited by their long growth cycle, high demand for growth environment, immature genetic transformation, and difficult RNA extraction. The present review clarifies the research significance, opportunities, and challenges of medicinal plant miRNAs in drug development and agricultural production. The discussion of the latest results furthers the understanding of medicinal plant miRNAs and helps the rational design of the corresponding miRNA/target genes functional modules.
Keywords: medicinal plant, miRNA, synthesis pathway, biological function
1. Introduction
Since time immemorial, medicinal plants have fully demonstrated their therapeutic potential [1]. Medicinal plants from Asteraceae and Lamiaceae have significant effects in the treatment of cardiovascular-related diseases, including antioxidative and vasodilative activities [2]. In 2003, many descriptions of medicinal plants were used to prevent and treat severe acute respiratory syndrome (SARS) [3,4]. The clinical trials showed that prescriptions containing Ephedra sinica, Glycyrrhiza uralensis, and Rehmannia glutinosa reduce the severe symptoms of new coronavirus COVID-19 patients [5]. Although the contribution of medicinal plants to the treatment of human diseases is obvious, their cultivation area is not as wide as that of staple crops. Lacking in resources, degradation of the environment, and irregular cultivation patterns still challenge the production of medicinal plants [6]. Therefore, studies of medicinal plants have also centered on breeding high-yielding varieties with adaptability to environmental stress conditions.
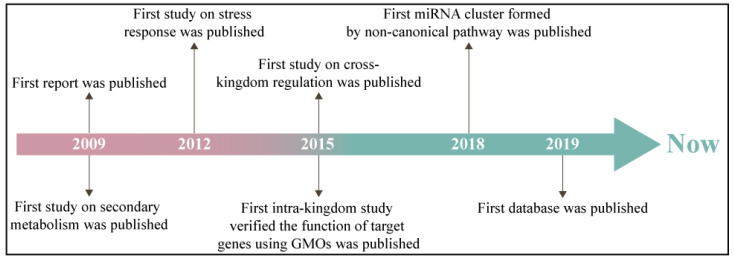
MiRNAs, a type of small non-coding RNAs (usually 20–24 nts), have been defined as crucial post-transcriptional gene regulators [7]. Plant miRNAs play important roles in growth and development, primary and secondary metabolism, response to environmental challenges, and exogenous regulation [8]. Following the publication of the first report in 2009, research on miRNA in medicinal plants has been increasing (Figure 1) [9]. The function of medicinal plant miRNAs is divided into cross- and intra-kingdom groups. The cross-kingdom studies of medicinal plant miRNAs concentrate on the major human disease treatments [10]. Six Ocimum basilicum miRNAs involving miR160, 414 and 869.1 were found to modulate 26 human malignancy-related target genes [11]. Aba-miRNA-9497 in Atropa belladonna was highly homologous to Homo sapiens miRNA-378, which both targeted the 3′-untranslated region (3′-UTR) of the mRNA encoding the neurologically relevant, zinc-finger transcription factor ZNF-691 [12]. The TRAF2 gene, a potential target of miRNAs in Bacopa monnieri, is the upstream signaling factor in the cancer pathway [13]. In the intra-kingdom, miRNA research on medicinal plants is mainly focused on the synthetic and metabolic pathways of medicinal secondary metabolites. In an endangered medicinal plant Picrorhiza kurroa, miR-4995, miR-5532, and miR-5368 participated in terpenoid biosynthesis and culture growth conditions [14]. Target genes of hop (Humulus lupulus) miRNAs responding to viral infection belonged to prenylflavonoid biosynthesis, and growth and development [15]. The comparative genomics approach identified 45 Aquilegia coerulea miRNAs that target genes involved in metabolism and stress responses [9]. As of yet, the mature research system on miRNAs in medicinal plants has not been established.
Figure 1.
Historical process of miRNA research in medicinal plants. GMOs—genetically modified organisms.
In light of the irreplaceable therapeutic value of medicinal plants and the diverse roles of miRNAs, the biosynthetic pathways and functions of medicinal plant miRNAs are comprehensively classified in this article. The criteria for document search were as follows: (1) database, Web of Science Core Collection; (2) edition, SCI-EXPANDED-1980-present; (3) searched field, All Fields; (4) searched word pairs, medicinal plant + microRNA, herb + microRNA, traditional Chinese medicine + microRNA, and herbaceous plant + microRNA (the “+” means another search line); (5) document types, article; (6) language, English; and (7) searched date, 5 September 2022. A manual search was also used to retrieve relevant papers from the results of the automatic search. The papers not included in Journal Citation Reports (JCR) were deleted, and the remaining papers were selected in this study. MiRNAs in a few medicinal plants with remarkable pharmacological effects have made breakthroughs. Research on other medicinal plant miRNAs is just beginning, but their potential applications have already emerged. Overall, this review summarizes the characteristics of the discipline development of miRNAs in medicinal plants, facilitates the establishment of a complete cross- or intra-kingdom miRNA/target gene module verification system, and discusses the possible challenges of systematic research in the future.
2. Biosynthetic Pathways of Medicinal Plant miRNAs
Eukaryotic miRNAs are synthesized based on both canonical and non-canonical mechanisms [16,17]. MiRNAs in medicinal plants were primarily generated from the canonical miRNA-generating pathway, which refers to the dicer-dependent pathway. For the non-canonical pathway, different miRNA origins were recognized in introns [18], rRNAs [18,19], snoRNAs [20], endogenous siRNAs [21], and tRNAs [22]. The atypical miRNAs generated through the precursor rRNA (pre-rRNA) splicing process have been found in medicinal plants [18,23].
2.1. Characteristics of the Canonical Pathway
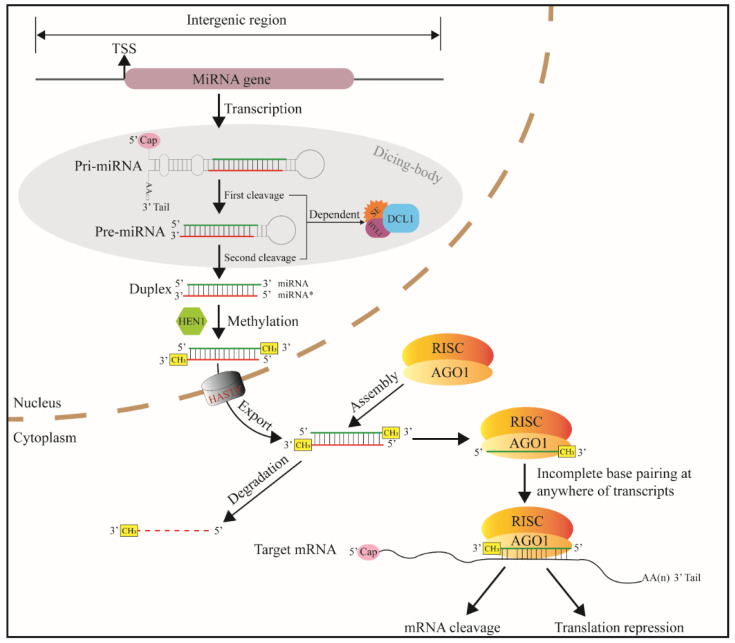
The core promoter region of the miRNA gene has TATA-box and transcription start site (TSS). The TSSs of miRNA genes are mainly located in intergenic regions, introns and reverse complementary sequences of coding sequences [17]. In the nucleus, after transcribed, primary-miRNA (pri-miRNA) having 3′ tail and 5′ cap is formed. One or several internal stem loops are folded from single pri-miRNAs [16]. The length of plant pri-miRNA hairpins is heterogeneous, ranging from approximately hundreds to even thousands of bases [22]. Pri-miRNA hairpins become precursor miRNAs (pre-miRNAs or MIRNAs) when modified by ribonuclease enzyme Dicer-like 1 enzyme (DCL1) [19]. This process is called the first cleavage (Figure 2). The stem-loop of plant MIRNAs has a wider range in length (70 to 350 nts) than that of animal MIRNAs (65 to 70 nts) [24]. In the second cleavage, MIRNA is cleaved into miRNA: miRNA * duplexes (21 to 24 bp) [25]. The two-step cleavage takes place in subnuclear regions called Dicing-bodies [26,27,28].
Figure 2.
Canonical miRNA-generating pathway in plant kingdom. TSS—transcription start site, RISC—RNA-induced silencing complex, AGO1—argonaute protein, DCL1—Dicer-like 1, SE—C2H2-zinc finger protein serrate, HYL1—Hyponastic leaves 1, and miRNA*—passenger strand of the miRNA: miRNA* duplex.
According to the length of the stem, plant MIRNA hairpins produce one or several duplexes [29]. The duplexes are then methylated with Hua enhancer1 (HEN1) at 3′ ends [30]. The methylation enhances plant miRNA stability by preventing non-template 3′-polymerization that accelerates miRNA turnover [31]. An exportin5 (Exp5) homolog transports the duplex from the nucleus into the cytoplasm. The miRNA* strand (passenger strand) is then degraded, while the mature single-strand miRNA (guide strand) is reserved. MiRNA is carried with Argonautes (AGOs, mainly AGO1), the core protein for RNA-induced silencing (RISC) complex [32,33]. MiRNA guides the RISC complex to mRNA strand via almost complete base complementation, and the RISC endonucleolytic cleavage the mRNA [7]. After binding to target mRNA, a few plant miRNAs do not perform cleavage function but reduce translation efficiency [34,35,36].
Plant miRNA target sites can be found anywhere in transcripts, for instance, 5′-UTRs, open reading frames (ORFs), 3′-UTRs, and noncoding transcripts. This finding suggests that all RNA environments are equally suitable for miRNA regulation in plants. Although animal miRNAs conservatively bind in the 3′-UTRs, the number of targets of a given plant miRNA is generally less than that of a given animal miRNA by at least an order of magnitude. This phenomenon is due to the low base complementary requirement of animal miRNAs to the target sites [37].
2.2. Discovery of the rRNA-Derived Non-Canonical Pathway
The transcript of pre-rRNA gene RN45s in eukaryotes contains 18S, 5.8S, and 28S rRNA regions with two internal transcribed spacers (ITS1 and 2). The sequence of rRNA regions is conserved and is retained completely during the subsequent rRNA splicing process. The ITSs contain more species-specific nucleotide sequences that are used as phylogenetic markers [38]. The first pre-rRNA-derived miRNA (miR-712) was found in the murine ITS2 region, in which the mature body was modified without the conventional dicer-dependent manners. The miR-712 alleviated atherosclerosis via regulating the tissue inhibitor of metalloproteinase 3 (TIMP3) [39].
There are also rRNA-derived miRNAs found in Papaver somniferum and Lonicera japonica. A clustered P. somniferum miRNA site was identified in a long polycistronic pre-rRNA region. Five of these clustered miRNAs were species conserved, which MIRNA sequences were highly homologous to 13 plant species [18]. This result suggests that the clustered P. somniferum miRNA site may not be located in ITS regions. Interestingly, such clustered miRNAs showed similar conserved pattern is rare in plants [40,41], but common in animals [42,43,44,45]. The silencing mechanism of pre-rRNA-generating miRNA remains unclear in medicinal plants, reports have shown their functional importance. Oral administration of honeysuckle decoction (HD) prevented influenza A viruses (IAVs) infection and decreased H5N1-induced mouse death due to the rRNA-derived MIR2911 (precursor of miR2911) in HD [23]. The molecular mechanism of medicinal plant-derived non-canonical miRNAs still has extensive worthwhile space for exploration.
3. Functional Research Progress of Medicinal Plant miRNAs
3.1. Cross-Kingdom Regulation
MiRNAs biosynthesized from medicinal plants can act as botanicals to regulate health-related processes. Oral plant-based diets will enable the cross-kingdom transfer of miRNAs in medicinal plants into mammalian circulation. In Table 1, miRNAs of eight medicinal plants have been predicted or experimentally proven to be bioactive factors for human disease treatment.
Table 1.
Summary of medicinal plant miRNA studies of different species.
| Latin Name | Aim Pathway | Methods of Target Functional Identification | References | ||
|---|---|---|---|---|---|
| Prediction | Indirect Verification | GMOs/GMCs Direct Validation | |||
| Cross-kingdom regulation | |||||
| Lonicera japonica | Replication of influenza A virus | − | − | √ | [23] |
| Lonicera japonica | Replication of COVID-19 | − | − | √ | [46] |
| Camptotheca acuminata | Breast cancer, leukemia and lung cancer | √ | − | − | [47] |
| Gastrodia elata | Homo sapiens A20 gene | − | − | √ | [48] |
| Ocimum basilicum | Rheumatoid arthritis and diabetes mellitus | √ | − | − | [11] |
| Lonicera japonica | Tumor proliferation | − | − | √ | [49] |
| Atropa belladonna | Central nervous system toxicity | − | √ | − | [12] |
| Panax ginseng | Cancers, immune diseases, and neurological disorders | √ | − | − | [50] |
| Viscum album | Cancers | √ | − | − | [51] |
| Ocimum basilicum | Cardiomyopathy, HIV, Alzheimer’s diseases and cancers | √ | − | − | [52] |
| Bacopa monnieri | NF-kB and MAPK pathways | √ | − | − | [13] |
| Aucklandia lapp, Rhodiola crenulata, and Taraxacum mongolicum | Stability assessment of miRNAs during decoction preparation | − | − | − | [53] |
| Ten medicinal plants | MiRNAs were detected in mammalian blood and tissues | √ | − | − | [54] |
| Viscum album | Stability assessment of miRNAs during decoction preparation | − | − | − | [55] |
| Intra-kingdom secondary metabolism | |||||
| Pogostemon cablin | Synthesis of sesquiterpenes | − | − | √ | [56] |
| Papaver somniferum | Benzylisoquinoline alkaloid synthesis | − | √ | − | [57] |
| Artemisia annua | Artemisinin synthesis | √ | − | − | [58] |
| Euphorbia kansui | Terpenoid biosynthesis | √ | − | − | [59] |
| Glycyrrhiza | Glycyrrhizic acid synthesis | √ | − | − | [60] |
| Salvia miltiorrhiza | Tanshinone synthesis and biomass | − | − | √ | [61] |
| Salvia miltiorrhiza | Synthesis of salvianolic acid | − | − | √ | [62] |
| Podophyllum hexandrum | Podophylloxin synthesis | − | √ | − | [63] |
| Taxus | Taxol, phenylpropanoid, and flavonoid biosynthesis | − | − | √ | [64] |
| Ginkgo biloba | Terpene trilactone synthesis | − | √ | − | [65] |
| Desmodium styracifolium | Schaftoside biosynthesis | √ | − | − | [66] |
| Camellia sinensis | Catechin, theanine and caffeine synthesis | √ | − | − | [67] |
| Salvia miltiorrhiza | Tanshinone, salvianolic acid, and biomass | − | − | √ | [68] |
| Catharanthus roseus | Terpenoid indole alkaloids | − | √ | − | [69] |
| Hippophae rhamnoides | Lipid synthesis | − | √ | − | [70] |
| Artemisia annua | Artemisinin synthesis | − | √ | − | [71] |
| Salvia miltiorrhiza | Phenolic acid synthesis | − | √ | − | [72] |
| Camellia sinensis | Catechin synthesis | − | √ | − | [73] |
| Picrorhiza kurroa | Terpenoid synthesis | − | √ | − | [14] |
| Dendrobium nobile | Synthesis of dendrobine | √ | − | − | [74] |
| Digitalis purpurea | Cardiac glycoside biosynthesis | − | √ | − | [75] |
| Panax notoginseng | Synthesis of triterpenoid saponins | − | √ | − | [76] |
| Lycoris aurea | Alkaloid synthesis | − | √ | − | [77] |
| Acacia | Lignin and flavonoid synthesis | √ | − | − | [78] |
| Murraya koenigii | Flavonoid and terpenoid synthesis | √ | − | − | [79] |
| Catharanthus roseus | Secondary metabolism | √ | − | − | [80] |
| Salvia sclarea | Phenylpropanoids and terpenoids synthesis | √ | − | − | [81] |
| Zingiber officinalis | Gingerol synthesis | √ | − | − | [82] |
| Ocimum basilicum | Secondary metabolism | √ | − | − | [83] |
| Taxus chinensis | Taxoid synthesis | √ | − | − | [40] |
| Ferula gummosa | Synthesis of ferulide | − | √ | − | [84] |
| Lycium chinense | Lycopene synthesis | − | √ | − | [85] |
| Salvia miltiorrhiza | Biosynthesis of tanshinones | − | √ | − | [86] |
| Xanthium strumarium | Terpenoid biosynthesis | √ | − | − | [87] |
| Salvia miltiorrhiza | Phenolic synthesis | − | √ | − | [88] |
| Azadirachta indica | Secondary metabolism | √ | − | − | [89] |
| Withania somnifera | Withanolide synthesis | − | √ | − | [90] |
| Mentha | Essential oil biosynthesis | √ | − | − | [91] |
| Salvia miltiorrhiza | Tanshinone Synthesis | − | − | √ | [61] |
| Artemisia annua | Artemisinin synthesis | √ | − | − | [92] |
| Vinca minor | Synthesis of terpenoid indole alkaloids | √ | − | − | [93] |
| Curcuma longa | Curcumin biosynthesis | √ | − | − | [94] |
| Podophyllum hexandrum | Podophyllotoxin synthesis | − | √ | − | [95] |
| Podophyllum hexandrum | Podophyllotoxin synthesis | − | √ | − | [96] |
| Persicaria minor | Terpenoid and GLV synthesis | − | √ | − | [97] |
| Gleditsia sinensis | Synthesis of monoterpenes and alkaloids | √ | − | − | [98] |
| Glycyrrhiza uralensis | Secondary metabolism | √ | − | − | [99] |
| Capsicum annuum | Anthocyanin synthesis | √ | − | − | [100] |
| Brassica oleracea | Secondary metabolism | − | √ | − | [101] |
| Persicaria minor | Terpenoid and GLV synthesis | − | √ | − | [102] |
| Dryopteris fragrans | Terpenoid synthesis | − | √ | − | [103] |
| Echinacea purpurea | Anthocyanin biosynthesis | √ | − | − | [104] |
| Intra-kingdom growth and development | |||||
| Papaver somniferum | Root, stem, leaf and young capsule prior to flowering tissues | − | √ | − | [57] |
| Panax ginseng | Flower buds, leaves, and lateral roots | √ | − | − | [105] |
| Lycium barbarum | Different fruit stages | − | √ | − | [106] |
| Lonicera japonica | Flower buds, leaves, and stems of 21 cultivated varieties | − | − | − | [107] |
| Lycopersicon esculentum and Lycium chinense | Shoot and fruit of grafted tomato | √ | − | − | [108] |
| Ginkgo biloba | Roots, stems, leaves, microstrobilus, and ovulate strobilus | − | √ | − | [65] |
| Camellia sinensis | Buds, different development stages of leaves and stems | √ | − | − | [67] |
| Dendrobium officinale | Flower, root, leaf and stem | √ | − | − | [109] |
| Panax notoginseng | Root with various biomasses | − | √ | − | [110] |
| Carthamus tinctorius | Seed, leaf, and petal | − | − | − | [111] |
| Panax ginseng | Roots, stems, leaves and flowers | √ | − | − | [112] |
| Panax notoginseng | Roots, stems, and leaves of 1-, 2-, and 3-year-old seedlings | − | √ | − | [76] |
| Gynostemma pentaphyllum | three stages of developmental stem-to-rhizome transition | − | √ | − | [113] |
| Hypericum perforatum | Flower parts | √ | − | − | [114] |
| Pinellia ternate | Leaves, stalks and tubers | − | − | − | [115] |
| Lonicera japonica | Flowers including 2 varieties of honeysuckle at 2 locations | − | √ | − | [116] |
| Ginkgo biloba | Epiphyllous ovule leaves and normal leaves | − | √ | − | [117] |
| Elettaria cardamomum | Cultivar and wild cardamom genotypes | − | √ | − | [118] |
| Ginkgo biloba | Mature ovules (pollination stage) and leaves of female trees | √ | − | − | [119] |
| Ginkgo biloba | Cambial structure | − | − | − | [120] |
| Passifora edulis | Inter-tissue and inter-varietal | √ | − | − | [121] |
| Ginkgo biloba | Female and male leaves | √ | − | − | [122] |
| Dendrobium officinale | Conventional and micropropagated plants | √ | − | − | [123] |
| Polygonatum odoratum | Leaves and roots of CC and FC seedlings | √ | − | − | [124] |
| Bletilla striata | Leaves, roots, and tubers | − | √ | − | [125] |
| Intra-kingdom stress responses | |||||
| Halostachys caspica | Salt stress | − | √ | − | [126] |
| Cicer arietinum | Ascochyta blight | − | √ | − | [127] |
| Salvia miltiorrhiza | Salt stress | − | − | − | [6] |
| Astragalus Membranaceus | Cold stress | − | √ | − | [128] |
| Zingiber officinale and Curcuma amada | Bacterial wilt | − | √ | − | [129] |
| Dendrobium huoshanense | Drought stress | − | √ | − | [130] |
| Macleaya cordata | Drought stress | − | √ | − | [131] |
| Digitalis purpurea | Cold and dehydration stresses | − | √ | − | [75] |
| Humulus lupulus | CBCVd | − | √ | − | [15] |
| Panax ginseng | High ambient temperature | − | √ | − | [132] |
| Aquilaria sinensis | Wound treatment | − | − | − | [133] |
| Panax ginseng | Dehydration and heat stresses | − | √ | − | [134] |
| Ziziphus jujuba | Jujube witches’-broom | − | √ | − | [135] |
| Polygonatum odoratum | Consecutive monoculture problem | √ | − | − | [124] |
| Pogostemon cablin | Consecutive monoculture problem | − | √ | − | [136] |
| Other research functioning in intra-kingdom | |||||
| Eucommia ulmoides | First report | − | √ | − | [137] |
| Taxus | First report | − | √ | − | [138] |
| Lotus japonicus | First report | √ | − | − | [139] |
| Humulus lupulus | First report | − | √ | − | [140] |
| Persicaria minor | First report | √ | − | − | [141] |
| Gymnema sylvestre | First report | √ | − | − | [142] |
| Rehmannia glutinosa | First report | √ | − | − | [143] |
| 29 medicinal plants | Database | √ | − | − | [144] |
| Papaver somniferum | Non-classical miRNA | √ | − | − | [18] |
| Hypericum | Evolutionary analysis | √ | − | − | [145] |
| Pinellia pedatisecta | Evolutionary analysis | − | − | − | [146] |
| Aquilegia coerulea | Evolutionary analysis | √ | − | − | [9] |
| Elettaria cardamomum | Evolutionary analysis | − | √ | − | [118] |
CBCVd—citrus bark cracking viroid, CC—consecutive cropping, FC—first cropping, GLV—green leaf volatile, GMCs—genetically modified cells, HIV—human immunodeficiency virus, and MAPK—mitogen-activated protein kinase. Indirect verification: studies that miRNA-target gene modules have not been functionally validated at the transgenic level, but the suppression relationship between their miRNAs and target genes has been verified using qRT-PCR, RACE, degradome sequencing, northern blot, β-glucuronidase reporter gene staining (GUS), and/or transient luciferase signal system.
3.1.1. Exceptional Stability
Unlike RNAs degraded during drug processing, medicinal plant miRNAs functional in cross-kingdom regulation are robust during soaking, boiling and homogenization processes. These miRNAs survive even under adversely stable animal systemic circulation, such as extreme pH (simulated gastric juice at pH 1.2), bowel movements, and ribonuclease (RNase) treatment [147]. A significant increase in an atypical rRNA-derived MIR2911 in both blood and urine was first discovered in mice that were fed with a diet of honeysuckle for several days [23]. Thousands of miRNAs derived from 10 medicinal plants were transferred to human blood cells and tissues following oral herbal decoctions (Table 1) [54].
Research showed that the stability of exogenous miRNAs during drug preparations was associated with the protection of plant macromolecules [55]. Through mammalian dietary uptake, miRNAs self-assembled into exosomes and were transported into the circulation and target tissues or cells [148,149,150]. Furthermore, there are several other factors to enhance the stability of medicinal plant miRNAs in mammals: (1) the 2′-O-methylations protect plant miRNAs avoiding degradation of exonucleolytic digestion and uridylation [7,31]; (2) high G, C content, sturdy structure and absence of RNases digestion motifs of miRNAs guarantee the stability [151]; (3) the miRNA-binding proteins like argonaute proteins (AGOs) [152] and nucleophosmin 1 [153] prevent circulating miRNAs from decay; and (4) medicinal plant metabolites create an environment for miRNAs to inhibit RNase activity [154].
The above conditions ensure the reliability of medicinal plant miRNAs in clinic treatment. However, doubts remain. A few researchers claim that plant-derived miRNAs are almost undetectable in plant-fed animal bodies [155,156]. There are five reasons for this phenomenon: (1) the diversity of sequences, structures and binding proteins are the fundamental reasons for the differences in the stability and biological activity of medicinal plant miRNAs [157]; (2) medicinal plant miRNA can effectively inhibit the function of target mammalian mRNA only when it has more than 100 copies per cell [158]; (3) the protective methylated structure adds difficulty to the identification of medicinal plant miRNAs; (4) medicinal plant miRNAs may not be detected in plasma or tissues when an herbal diet is fed for a short time and in small doses; and (5) health condition of the digestive system directly affects the absorption efficiency of medicinal plant miRNAs [159]. Accordingly, standardized plant-specific exogenous miRNA detection technology and experimental design are the prerequisites to increase the accuracy of cross-kingdom medicinal plant miRNA research [160].
3.1.2. Targeting Genes Associated with Major Diseases
The cross-kingdom function of medicinal plant miRNAs has been experimentally verified to be unexpectedly strong (Table 2). In addition to inhibiting H5N1 and H7N9 viral activity in vitro and in vivo, MIR2911 also suppressed the replication of H1N1 by decreasing H1N1-encoded PB2 and NS1 protein expression [23]. In 2019, the sudden outbreak of COVID-19, of which the causative virus is the syndrome coronavirus 2 (SARS-CoV-2), challenges the safety of human life to date [161]. MIR2911 absorbed by COVID-19 patients can promptly and effectively inhibit SARS-CoV-2 replication via binding 179 candidate target sites in the SARS-CoV-2 transcriptome [46]. In cancer treatment, the miR2911 strongly bound and down-regulated the expression of TGF-β1, retarding the colon cancer process with an increase in T lymphocyte infiltration in mice [49].
Table 2.
Names of medicinal plant miRNAs in cross-kingdom and secondary metabolism regulations.
| Name of miRNA | Aim Pathway |
|---|---|
| Cross-kingdom | |
| • MIR2911 | COVID-19, influenza A virus, and tumor proliferation |
| • Gas-miR01, and 02 | Anti-inflammatory |
| • MiR414 | Alzheimer’s diseases, diabetes, hypoganglionosis, and inflammatory bowel diseases |
| • Oba-miR156f, and 156t | Bile duct carcinoma, lung cancer, and osteoarthritis |
| • Oba-miR160g | Lung cancer, nephronophthisis, and retinitis pigmentosa |
| • Oba-miR482a | Breast cancer, gastric cancer, and ovarian cancer |
| • MiR869.1 | Alzheimer’s diseases, cataracts, and diabetes mellitus |
| • Bmn-miR156, 167h, 172d, and 396g | Immune responses |
| • MiR166 | Glioblastoma, papillary thyroid carcinoma, and secretory breast carcinomas |
| • Cac-miR-29c-5p | Breast cancer, and ovarian cancer |
| • Cac-miR-4723-3p | Prostate cancer, and renal cancer |
| • Cac-miR-548d-3p, 5653, 5780d, and 7009-3p | Tumor proliferation, ovarian clear cell adenocarcinoma, breast cancer, and lung cancer |
| • MiR10206, 5059, 5073, 5272, 6135, oba-miR531, and aba-miRNA-9497 | Tumor proliferation, psoriasis, Alzheimer’s disease, epilepsy syndromes, immune responses, retinitis pigmentosa, and central nervous system toxicity |
| Secondary metabolism | |
| • MiR5298b, and 8154 | Phenylpropanoid |
| • Smi-miR396b, and miR408 | Salvianolic acid |
| • MiR5298b, and 8154 | Taxol |
| • MiR160b, ath-MIR160b and smi-miR396b | Tanshinone |
| • MiR156 | Sesquiterpene |
| • MiR035, 1168.2, 1438, 156b, 170, 172i, 1858,1873, 2275, 2673a, 2910, 2919, 396b, 408, 5015, 5021, 5658, 828b, 829.1, 8291, f10132-akr, ain-miR1533c, ain-miR156, ain-miR157, cro-miR397a, cro-miR828a, Cs-miR156, mko-miR159b-3p, mko-miR167c-5p, mko-miR168b, mko-miR5082, mko-miR858, mko-miR8610.1, smi-miR12112, smi-miR397, smi-miR396b, and smi-miR408 | Phenolic compounds |
| • MiR_116, _1194, _1276, _15, _1508, _1900, _2141, _2596, _334, _853, 1134, 1533, 160, 164, 167a, 167b, 171, 172, 172d-3p, 2919, 396a, 398f/g, -4995, 5563-x, 5021, 5658, 6435, 838, ain-miR1525, dfr-miR156b, dfr-miR160a, mko-miR156, mko-miR167a, mko-miR396c, mko-miR396g-5p, mko-miR5082, mko-miR827b, mko-miR858, novel-m0022-5p, pmi-miR6300, pmi-miR6173, pmi-miR530, and pmi-Nov_13 | Terpenoid |
| • MiR159, 159a, 166, 171, 172, 2673a, 390, 396, 858, cro-miR160, EY064998, EY082442, EY107691, EY57163, leaf-miR-477, leaf-miR530, root-miR159, root-miR5140, mko-miR159b-3p, mko-miR5082, mko-miR858, mko-miR8610.1, novel miR_218, novel miR_2432, novel miR2642, novel miR_2924, novel miR_457, and novel miR_853 | Esters |
| • MiR2673a, 396, cro-miR160, pso-miR13, pso-miR2161, and pso-miR408 | Alkaloids |
| • MiR156, 5298b, 8154, and novel_miR_47 | Saponins |
| • MiR5072, MIR1446-x, and MIR394-y | Quinone |
| • MiR156, 414, 5015b, and 5021 | Essential oil |
| • NovelmiRNA-191, novelmiRNA-23, and novelmiRNA-58 | Triacylglycerols |
| • Pmi-miR396b and pmi-Nov_12 | Green leaf volatile |
| • MIR845-y | Steroid |
| • MiR5021 | Strictosidine |
Bold, miRNAs that have completed functional verification at the level of GMOs or GMCs. Citations refer to Table 1.
The NF-kB protein family in animals is a well-known anti-inflammatory and immune regulator. Gastrodia elata (GE) is a precious herbal medicine, in which miRNAs have been identified through the Illumina platform. Cell transfection showed that Gas-miR01 and Gas-miR02 of GE prominently restrained the accumulation of Homo sapiens A20 protein driven by NF-kB [48]. In addition to MIR2911 and Gas-miR01/02, the cross-kingdom functional exploration of medicinal plant miRNAs remains at the stage of computer prediction.
Site accessibility, low free energy, and base-pairing between the “seed” region of miRNA and target gene are features generally used for the computational recognition of heterogenous targets [162]. Xie (2017) predicted human target genes of miRNAs in Viscum album combined with four frequently used animal target prediction algorithms (TargetScan, miRanda, PITA, and RNAhybrid) [51]. Korean ginseng (Panax ginseng) has been commonly and efficiently used as medicine for thousands of years. Numerous disease-related target genes of Korean ginseng miRNAs were found combining RNAhybrid, miRanda, and TargetScan [50]. The medicinal plant happy tree (Camptotheca acuminata) is a deciduous tree having anticancer properties. A total of 152 human target genes associated with prominent types of cancers were predicted to be regulated by 14 highly stable putative novel miRNAs in the happy tree (Table 1) [47]. Although, the therapeutic role of a few medicinal plant miRNAs in mammalian major diseases has been well demonstrated [23,163], the potential mining of most medicinal plant miRNAs involved in human health regulation is still at an early stage.
3.2. Intra-Kingdom Regulation
In addition to miRNAs, the unique secondary metabolites of medicinal plants also exhibit abundant pharmacological activities. In the medicinal plant kingdom, a large number of miRNAs have been found to participate in the biosynthesis of secondary metabolites [61]. Consistently, the literature statistics in the present study showed that research on medicinal miRNAs revolved around the secondary metabolite synthesis pathway, followed by the growth, development, and environmental stress response pathways (Table 1 and Table 2).
3.2.1. Secondary Metabolism
The synthesis process of secondary metabolites in medicinal plants is complicated [164]. The transgenic plant lines are important experimental materials for the selected miRNAs and target genes functional studies. In patchouli (Pogostemon cablin), miR156-targeted squamosa promoter binding protein-like (SPL) transcription factor plays a crucial role in the patchouli oil (largely composed of sesquiterpenes) accumulation. Yu et al. (2015) demonstrated the regulatory effect of the miR156-SPL-PTS (patchoulol synthase) module on patchouli oil production by testing SPL10 and MIR156 transgenic patchouli lines [56]. The miR408 also acts in the regulation of secondary metabolism. In the medicinal model plant Salvia miltiorrhiza, recombinant laccase LAC3 was verified to be the target of Sm-miR408 using 5′-rapid amplification of cDNA ends (5′-RACE). The contents of salvianolic acid B (SalB) and rosmarinic acid (RA) were induced in both miR408-lacked and SmLAC3-overexpressed transgenic S. miltiorrhiza lines (Table 2) [62].
Cultivation of transgenic hairy roots is a common method for miRNA functional research on secondary metabolites in S. miltiorrhiza. miR396 is conserved and plays various roles in plants. MiR396b-overexpressing S. miltiorrhiza hairy roots repressed the hairy root growth and salvianolic acid concentration, but induced the tanshinone accumulation [68]. Further verification indicated that SmGRFs, SmHDT1, and SmMYB37/4 were targets of S. miltiorrhiza miR396b, which mediated the gibberellin signaling pathways and consequentially resulted in phenotype variation [68]. Conversely, the ath-miR160a overexpressed S. miltiorrhiza hairy roots down-regulated the expression levels of ARF10, 16, and 17, inhibited the biosynthesis of tanshinones, and increased hairy root biomass (Table 1 and Table 2) [61]. These results reflect the complexity of the effects of miRNAs on the mechanism of tanshinone synthesis.
The overexpression of miR8154 and miR5298b in the Taxus cell line upregulated the major enzyme genes related to taxol, phenylpropane, and flavonoid synthesis [64]. In Table 1, there are many other studies that combined high-throughput sequencing and bioinformatics to identify medicinal plant miRNAs and predict target pathways.
3.2.2. Growth and Development
Studies have exhibited that the spatiotemporal specificity expression of medicinal plant miRNAs plays a pivotal role in growth and development [165]. Zeng et al. (2015) identified the miRNA levels in Lycium barbarum seedlings at four developmental stages (S1-S4) using Illumina HiSeqTM 2000 platform [106]. Functional prediction of differentially expressed miRNAs revealed the characteristics of fruit ripening miRNA-mediated mechanism. The Hypericum perforatum flowers shared highly conserved miRNAs and these miRNAs potentially target functional genes involved in stress response, flower development, and plant reproduction [114]. The production and degradation of secondary metabolites are usually organ- and tissue-specific, and their accumulations and compositions change during plant germination, development and aging [166]. The tissue-specific expressing characteristic of a total of 232 miRNAs containing four tissues in Opium poppy was comprehensively performed using miRNA microarray technology [57]. Target gene functional prediction of these miRNAs revealed that some miRNAs might be involved in quinoline alkaloids (BIA) biosynthesis, including pso-miR13, pso-miR2161 and pso-miR408 (Table 2). In Ginkgo biloba (the “living fossil” in plant), 3314 miRNAs were identified from five organs using northern blot, quantitative real-time PCR (qRT-PCR), RACE, and degradome sequencing. Among them, four conserved miRNAs and five novel miRNAs might participate in terpene trilactones (TTL) biosynthesis pathways by targeting 12 predicted TTL biosynthesis genes (Table 1) [65].
A study of honeysuckle selected the suitable reference miRNA genes for the quantification of target miRNA expression through tissue- and variety-specific qRT-PCR [107]. Data of cycling threshold (Ct) value ranges of qRT-PCR and algorithms from GeNorm, NormFinder, and RefFinder were employed. Two stable honeysuckle miRNA reference genes (u534122 and u3868172) were found in three tissues of 21 cultivars from 16 origins.
3.2.3. Environmental Stress Response
Under biotic or abiotic stresses, medicinal plants develop numerous miRNA-regulate mechanisms to adapt to environmental challenges (Table 1). The plant sequence-conserved miR408 participates in various stress responses. The β-glucuronidase staining results of transgenic tobacco lines expressing Sm-MIR408pro::GUS revealed that Sm-MIR408 is a positive response factor to salt stress. Further study showed that tobacco lines expressing Sm-MIR408 increased the seed germination rate and decreased the accumulation of reactive oxygen species [6]. The biotic stress ascochyta blight (AB) limits chickpea (Cicer arietinum) production worldwide. Chickpea seedlings involved two susceptible genotypes, two resistant genotypes, and an introgression line containing two AB-resistance quantitative trait loci were treated infected with or without AB at two time points. The differentially expressed gene analysis combined with miRNA and mRNA transcriptome from these genotypes totally evaluated 12 miRNA-mRNA regulatory modules [127]. In an economic medicinal tree Aquilaria sinensis, expression pattern analysis indicated that eight miRNAs were wound-responsive during the recovery process after wound treatment. One miRNA was identified to be the miRNA * of asi-miR408 in A. sinensis, but with the accumulation greatly exceeding that of asi-miR408, suggested that it may have a biological function [133].
3.2.4. Other Fields
Additionally, research on medicinal plant miRNAs also refers to the evolutionary characteristics and database development, etc. (Table 1). Cardamom (Elettaria cardamomum) has been used as ayurvedic medicine for a long history. There are 1168 and 1025 unique potential targets of miRNAs found in wild and cultivated cardamom, respectively [118]. Many compounds of the genus Hypericum have therapeutic potential, and the miRNA expression profile of five Hypericum species was characterized in silico. The miR168 was identified only in H. perforatum and H. kalmianum with one highly conserved target gene (protein AGO1-like) [145]. In 2019, the first medicinal plant miRNA database, MepmiRDB (http://mepmirdb.cn/mepmirdb/index.html, accessed on 16 April 2018), was published, consisting of miRNA information on sequences, expression levels and regulatory networks from 29 species [144].
4. Conclusions
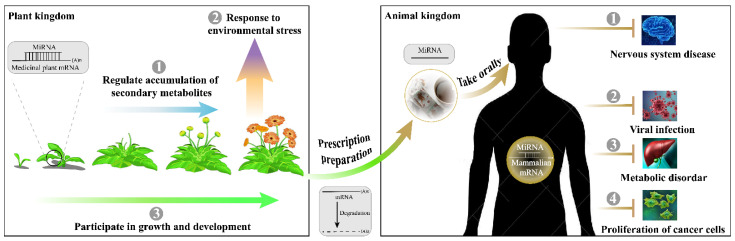
Natural drugs of medicinal plants are less toxic, have fewer side effects, and are cheaper and more available to patients around the world [167]. These pharmacologically active components are characterized by multiple molecular types, although most studies have focused on secondary metabolites at the metabolic level. As more reports unraveled the role and significance of medicinal plant miRNAs in tumor proliferation and virus replication, it is evident that they must be novel regulators of human disease at the post-transcriptional level (Figure 3). Initial research indicated that medicinal plant-derived miRNAs in a stable form can resist not only the preparation process, but the digestion and circulation process, ensuring their disease regulatory activity [47]. Nanoparticles mainly contain plant miRNAs, lipids, and proteins that have been demonstrated as natural therapeutical drugs for cancers and inflammatory diseases [168]. However, some reports related to the cross-kingdom function of miRNAs in medicinal plants remain only software predictions, the conclusions of which lack credibility. The differences between individuals also complicate the verification of cross-kingdom functionality. To prevent the question of authenticity in the academic community, here are four suggestions for future cross-kingdom research of medicinal plant miRNA. First, the stability of medicinal plant miRNAs needs to be systematically validated in vivo and in vitro. Second, reproducible validation of plant-miRNA/animal-target modules in human disease treatment should be carried out on candidate miRNAs that exist in at least 100 copies per cell. Third, multidimensional observation should be arranged for the effects of miRNAs on mice and patients with different health levels. Finally, it is also important to assess potential risks before clinical treatment, as a single miRNA can target multiple target genes.
Figure 3.
Multifunctional role of miRNAs in medicinal plants.
Before the discovery of cross-kingdom functions, medicinal plant miRNAs have shown important regulatory capabilities in the synthesis and modification of metabolites, seedling development, and stress adaptation in the plant kingdom (Figure 3). Methods of intra-kingdom identification and expression analysis of medicinal plant miRNAs mainly include direct cloning, expressed sequence tags analysis and transcriptome sequencing. Bioinformatics, degradome sequencing, qRT-PCR, 5’-RACE, northern blot, transient luciferase, and GUS technologies are used to determine target genes. Unfortunately, after the prediction or indirect verification of the miRNA-target gene modules in medicinal plants, many studies have not conducted in-depth functional research. Only about 10% of studies have reached phenotypic conclusions by developing transgenic seedlings, tissues, or cells. There are probably three reasons: (1) researchers pay far less attention to medicinal plants than to food crops, resulting in many medicinal plants not yet established as efficient genetic transformation systems; (2) the quality of medicinal plants is strongly dependent on the edaphic and climatic environments, which severely restrict their large-scale cultivation; and (3) natural secondary metabolites like terpenes, flavonoids, alkaloids, phenols, etc. which are easier to extract and preserve than miRNAs, leading the researchers to focus on their pharmacological effects first. Nonetheless, the intra-kingdom functional validation of medicinal plant miRNAs has entered a new era. The technology of genome assembly in non-model plants is becoming mature. In the last three years, more than 100 articles (over 60%) have been published on genomic information about medicinal plants [169]. Some medicinal plants have published two or more genome versions (e.g., Panax notoginseng [170,171,172,173,174], Andrographis paniculata [175,176], and Gastrodia elata [177,178]), which have significantly supported the miRNA research. The research progress of medicinal plant miRNAs will develop rapidly in the next few years. With the enrichment of the cross-kingdom function of miRNAs derived from medicinal plants, their intra-kingdom influence will also be given enough attention. More and more in-depth experiments will be used to provide molecular biology evidence of the predicted candidate miRNA/target gene modules.
Since miRNAs were discovered in medicinal plants, studies of their biogenesis and mechanism of action have achieved several milestones (Figure 1). Their function extended from the plant kingdom to the animal kingdom (Figure 3). Despite the promising data thus far, more efforts are necessary to understand the mechanisms. As summarized above, the application potential of medicinal plant miRNAs is enormous, both in terms of improving the yield and quality of source species and in terms of molecular regulation in the treatment of human diseases.
Author Contributions
Conceptualization, M.S. and S.X.; formal analysis, Y.M.; software, J.L.; investigation, Y.G.; data curation, W.Z.; writing—original draft preparation, visualization, writing—review and editing, M.S.; project administration, supervision, J.W.; funding acquisition, J.W. and M.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Research and development program in key areas of Guangdong Province (No. 2021B0707010010); Crop Research Institute, Guangdong Academy of Agricultural Sciences/Guangdong Provincial Key Laboratory of Crop Genetic Improvement Open Research Fund (202101).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Akindele A.J., Sowemimo A., Agunbiade F.O., Sofidiya M.O., Awodele O., Ade–Ademilua O., Orabueze I., Ishola I.O., Ayolabi C.I., Salu O.B. Bioprospecting for Anti–COVID–19 Interventions from African Medicinal Plants: A Review. Nat. Prod. Commun. 2022;17:1934578X221096968. doi: 10.1177/1934578X221096968. [DOI] [Google Scholar]
- 2.Michel J., Abd Rani N.Z., Husain K. A review on the potential use of medicinal plants from Asteraceae and Lamiaceae plant family in cardiovascular diseases. Front. Pharmacol. 2020;11:852. doi: 10.3389/fphar.2020.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J., Manheimer E., Shi Y., Gluud C. Chinese herbal medicine for severe acute respiratory syndrome: A systematic review and meta–analysis. J. Altern. Complem. Med. 2004;10:1041–1051. doi: 10.1089/acm.2004.10.1041. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . SARS: Clinical Trials on Treatment using a Combination of Traditional Chinese Medicine and Western Medicine. WHO; Geneva, Switzerland: 2004. Report of the WHO International Expert Meeting to review and analyse clinical reports on combination treatment for SARS, Beijing, China, 8–10 October 2003. [Google Scholar]
- 5.Xu J., Zhang Y. Traditional Chinese medicine treatment of COVID–19. Complement. Ther. Clin. 2020;39:101165. doi: 10.1016/j.ctcp.2020.101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo X., Niu J., Cao X. Heterologous expression of Salvia miltiorrhiza microRNA408 enhances tolerance to salt stress in Nicotiana benthamiana. Int. J. Mol. Sci. 2018;19:3985. doi: 10.3390/ijms19123985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voinnet O. Origin, biogenesis, and activity of plant microRNAs. Cell. 2009;136:669–687. doi: 10.1016/j.cell.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y., Kuang Z., Wang Y., Li L., Yang X. MicroRNA annotation in plants: Current status and challenges. Brief. Bioinform. 2021;22:bbab075. doi: 10.1093/bib/bbab075. [DOI] [PubMed] [Google Scholar]
- 9.Puzey J.R., Kramer E.M. Identification of conserved Aquilegia coerulea microRNAs and their targets. Gene. 2009;448:46–56. doi: 10.1016/j.gene.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Li D., Yang J., Yang Y., Liu J., Li H., Li R., Cao C., Shi L., Wu W., He K. A timely review of cross–kingdom regulation of plant–derived microRNAs. Front. Genet. 2021;12:613197. doi: 10.3389/fgene.2021.613197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel M., Patel S., Mangukia N., Patel S., Mankad A., Pandya H., Rawal R. Ocimum basilicum miRNOME revisited: A cross kingdom approach. Genomics. 2019;111:772–785. doi: 10.1016/j.ygeno.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Avsar B., Zhao Y., Li W., Lukiw W.J. Atropa belladonna expresses a microRNA (aba–miRNA–9497) highly homologous to Homo sapiens miRNA–378 (hsa–miRNA–378); both miRNAs target the 3′–Untranslated region (3′–UTR) of the mRNA encoding the neurologically relevant, zinc–finger transcription factor ZNF–691. Cell. Mol. Neurobiol. 2020;40:179–188. doi: 10.1007/s10571-019-00729-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gadhavi H., Patel M., Mangukia N., Shah K., Bhadresha K., Patel S.K., Rawal R.M., Pandya H.A. Transcriptome–wide miRNA identification of Bacopa monnieri: A cross–kingdom approach. Plant Signal. Behav. 2020;15:1699265. doi: 10.1080/15592324.2019.1699265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vashisht I., Mishra P., Pal T., Chanumolu S., Singh T.R., Chauhan R.S. Mining NGS transcriptomes for miRNAs and dissecting their role in regulating growth, development, and secondary metabolites production in different organs of a medicinal herb, Picrorhiza kurroa. Planta. 2015;241:1255–1268. doi: 10.1007/s00425-015-2255-y. [DOI] [PubMed] [Google Scholar]
- 15.Mishra A.K., Duraisamy G.S., Matoušek J., Radisek S., Javornik B., Jakse J. Identification and characterization of microRNAs in Humulus lupulus using high–throughput sequencing and their response to Citrus bark cracking viroid (CBCVd) infection. BMC Genom. 2016;17:919. doi: 10.1186/s12864-016-3271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J.-S., Lai E.C. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol. Cell. 2011;43:892–903. doi: 10.1016/j.molcel.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ha M.J., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Bio. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 18.Davoodi Mastakani F., Pagheh G., Rashidi Monfared S., Shams–Bakhsh M. Identification and expression analysis of a microRNA cluster derived from pre–ribosomal RNA in Papaver somniferum L. and Papaver bracteatum L. PLoS ONE. 2018;13:e0199673. doi: 10.1371/journal.pone.0199673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lafontaine D.L. Noncoding RNAs in eukaryotic ribosome biogenesis and function. Nat. Struct. Mol. Biol. 2015;22:11–19. doi: 10.1038/nsmb.2939. [DOI] [PubMed] [Google Scholar]
- 20.Saraiya A.A., Wang C.C. SnoRNA, a novel precursor of microRNA in Giardia lamblia. PLoS Pathog. 2008;4:e1000224. doi: 10.1371/journal.ppat.1000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyoshi K., Miyoshi T., Hartig J.V., Siomi H., Siomi M.C. Molecular mechanisms that funnel RNA precursors into endogenous small–interfering RNA and microRNA biogenesis pathways in Drosophila. RNA. 2010;16:506–515. doi: 10.1261/rna.1952110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogerd H.P., Karnowski H.W., Cai X., Shin J., Pohlers M., Cullen B.R. A mammalian herpesvirus uses noncanonical expression and processing mechanisms to generate viral microRNAs. Mol. Cell. 2010;37:135–142. doi: 10.1016/j.molcel.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Z., Li X., Liu J., Dong L., Chen Q., Liu J., Kong H., Zhang Q., Qi X., Hou D. Honeysuckle–encoded atypical microRNA2911 directly targets influenza A viruses. Cell Res. 2015;25:39–49. doi: 10.1038/cr.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhoades M.W., Reinhart B.J., Lim L.P., Burge C.B., Bartel B., Bartel D.P. Prediction of plant microRNA targets. Cell. 2002;110:513–520. doi: 10.1016/S0092-8674(02)00863-2. [DOI] [PubMed] [Google Scholar]
- 25.Kurihara Y., Watanabe Y. Arabidopsis micro–RNA biogenesis through Dicer–like 1 protein functions. Proc. Natl. Acad. Sci. USA. 2004;101:12753–12758. doi: 10.1073/pnas.0403115101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park M.Y., Wu G., Gonzalez–Sulser A., Vaucheret H., Poethig R.S. Nuclear processing and export of microRNAs in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2005;102:3691–3696. doi: 10.1073/pnas.0405570102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang Y., Spector D.L. Identification of nuclear dicing bodies containing proteins for microRNA biogenesis in living Arabidopsis plants. Curr. Biol. 2007;17:818–823. doi: 10.1016/j.cub.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song L., Han M.-H., Lesicka J., Fedoroff N. Arabidopsis primary microRNA processing proteins HYL1 and DCL1 define a nuclear body distinct from the Cajal body. Proc. Natl. Acad. Sci. USA. 2007;104:5437–5442. doi: 10.1073/pnas.0701061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Axtell M.J., Westholm J.O., Lai E.C. Vive la différence: Biogenesis and evolution of microRNAs in plants and animals. Genome Biol. 2011;12:221. doi: 10.1186/gb-2011-12-4-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu B., Yang Z., Li J., Minakhina S., Yang M., Padgett R.W., Steward R., Chen X. Methylation as a crucial step in plant microRNA biogenesis. Science. 2005;307:932–935. doi: 10.1126/science.1107130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J., Yang Z., Yu B., Liu J., Chen X. Methylation protects miRNAs and siRNAs from a 3′–end uridylation activity in Arabidopsis. Curr. Biol. 2005;15:1501–1507. doi: 10.1016/j.cub.2005.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumberger N., Baulcombe D. Arabidopsis ARGONAUTE1 is an RNA slicer that selectively recruits microRNAs and short interfering RNAs. Proc. Natl. Acad. Sci. USA. 2005;102:11928–11933. doi: 10.1073/pnas.0505461102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi Y., Denli A.M., Hannon G.J. Biochemical specialization within Arabidopsis RNA silencing pathways. Mol. Cell. 2005;19:421–428. doi: 10.1016/j.molcel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Brodersen P., Sakvarelidze–Achard L., Bruun–Rasmussen M., Dunoyer P., Yamamoto Y.Y., Sieburth L., Voinnet O. Widespread translational inhibition by plant miRNAs and siRNAs. Science. 2008;320:1185–1190. doi: 10.1126/science.1159151. [DOI] [PubMed] [Google Scholar]
- 35.Chen X. A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science. 2004;303:2022–2025. doi: 10.1126/science.1088060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gandikota M., Birkenbihl R.P., Höhmann S., Cardon G.H., Saedler H., Huijser P. The miRNA156/157 recognition element in the 3′ UTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. Plant J. 2007;49:683–693. doi: 10.1111/j.1365-313X.2006.02983.x. [DOI] [PubMed] [Google Scholar]
- 37.Bartel D.P. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schultz J., Wolf M. ITS2 sequence–structure analysis in phylogenetics: A how–to manual for molecular systematics. Mol. Phylogenet. Evol. 2009;52:520–523. doi: 10.1016/j.ympev.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Son D.J., Kumar S., Takabe W., Woo Kim C., Ni C.-W., Alberts-Grill N., Jang I.-H., Kim S., Kim W., Won Kang S. The atypical mechanosensitive microRNA–712 derived from pre–ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat. Commun. 2013;4:3000. doi: 10.1038/ncomms4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiu D., Pan X., Wilson I.W., Li F., Liu M., Teng W., Zhang B. High throughput sequencing technology reveals that the taxoid elicitor methyl jasmonate regulates microRNA expression in Chinese yew (Taxus chinensis) Gene. 2009;436:37–44. doi: 10.1016/j.gene.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Xie F., Frazier T.P., Zhang B. Identification and characterization of microRNAs and their targets in the bioenergy plant switchgrass (Panicum virgatum) Planta. 2010;232:417–434. doi: 10.1007/s00425-010-1182-1. [DOI] [PubMed] [Google Scholar]
- 42.Lai E.C., Tomancak P., Williams R.W., Rubin G.M. Computational identification of Drosophila microRNA genes. Genome Biol. 2003;4:R42. doi: 10.1186/gb-2003-4-7-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanzer A., Amemiya C.T., Kim C.B., Stadler P.F. Evolution of microRNAs located within Hox gene clusters. J. Exp. Zool. Part B. 2005;304:75–85. doi: 10.1002/jez.b.21021. [DOI] [PubMed] [Google Scholar]
- 44.Kameswaran V., Bramswig N.C., McKenna L.B., Penn M., Schug J., Hand N.J., Chen Y., Choi I., Vourekas A., Won K.-J. Epigenetic regulation of the DLK1–MEG3 microRNA cluster in human type 2 diabetic islets. Cell Metab. 2014;19:135–145. doi: 10.1016/j.cmet.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sluijter J.P., Van Rooij E. Exosomal microRNA clusters are important for the therapeutic effect of cardiac progenitor cells. Circ. Res. 2015;116:219–221. doi: 10.1161/CIRCRESAHA.114.305673. [DOI] [PubMed] [Google Scholar]
- 46.Zhou L., Zhou Z., Jiang X., Zheng Y., Chen X., Fu Z., Xiao G., Zhang C., Zhang L., Yi Y. Absorbed plant MIR2911 in honeysuckle decoction inhibits SARS–CoV–2 replication and accelerates the negative conversion of infected patients. Cell Discov. 2020;6:1–4. doi: 10.1038/s41421-020-00197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar D., Kumar S., Ayachit G., Bhairappanavar S.B., Ansari A., Sharma P., Soni S., Das J. Cross–kingdom regulation of putative miRNAs derived from happy tree in cancer pathway: A systems biology approach. Int. J. Mol. Sci. 2017;18:1191. doi: 10.3390/ijms18061191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia C., Zhou H., Xu X., Jiang T., Li S., Wang D., Nie Z., Sheng Q. Identification and investigation of miRNAs from Gastrodia elata blume and their potential function. Front. Pharmacol. 2020;11:542405. doi: 10.3389/fphar.2020.542405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu C., Xu M., Yan L., Wang Y., Zhou Z., Wang S., Sun Y., Zhang J., Dong L. Honeysuckle–derived microRNA2911 inhibits tumor growth by targeting TGF–β1. Chin. Med. 2021;16:54. doi: 10.1186/s13020-021-00453-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Peng M., Chen Y., Wang W., He Z., Yang Z., Lin Z., Gong M., Yin Y., Zeng Y. Analysis of Panax ginseng miRNAs and their target prediction based on high–throughput sequencing. Planta Med. 2019;85:1168–1176. doi: 10.1055/a-0989-7302. [DOI] [PubMed] [Google Scholar]
- 51.Xie W., Adolf J., Melzig M.F. Identification of Viscum album L. miRNAs and prediction of their medicinal values. PLoS ONE. 2017;12:e0187776. doi: 10.1371/journal.pone.0187776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel M., Mangukia N., Jha N., Gadhavi H., Shah K., Patel S., Mankad A., Pandya H., Rawal R. Computational identification of miRNA and their cross kingdom targets from expressed sequence tags of Ocimum basilicum. Mol. Biol. Rep. 2019;46:2979–2995. doi: 10.1007/s11033-019-04759-x. [DOI] [PubMed] [Google Scholar]
- 53.Li X., Liang Z., Du J., Wang Z., Mei S., Li Z., Zhao Y., Zhao D., Ma Y., Ye J. Herbal decoctosome is a novel form of medicine. Sci. China Life Sci. 2019;62:333–348. doi: 10.1007/s11427-018-9508-0. [DOI] [PubMed] [Google Scholar]
- 54.Huang F., Du J., Liang Z., Xu Z., Xu J., Zhao Y., Lin Y., Mei S., He Q., Zhu J. Large–scale analysis of small RNAs derived from traditional Chinese herbs in human tissues. Sci. China Life Sci. 2019;62:321–332. doi: 10.1007/s11427-018-9323-5. [DOI] [PubMed] [Google Scholar]
- 55.Xie W., Melzig M.F. The stability of medicinal plant microRNAs in the herb preparation process. Molecules. 2018;23:919. doi: 10.3390/molecules23040919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu Z., Wang L., Zhao B., Shan C., Zhang Y., Chen D., Chen X. Progressive regulation of sesquiterpene biosynthesis in Arabidopsis and Patchouli (Pogostemon cablin) by the miR156–targeted SPL transcription factors. Mol. Plant. 2015;8:98–110. doi: 10.1016/j.molp.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Boke H., Ozhuner E., Turktas M., Parmaksiz I., Ozcan S., Unver T. Regulation of the alkaloid biosynthesis by miRNA in Opium poppy. Plant Biotechnol. J. 2015;13:409–420. doi: 10.1111/pbi.12346. [DOI] [PubMed] [Google Scholar]
- 58.Pani A., Mahapatra R.K., Behera N., Naik P.K. Computational identification of sweet wormwood (Artemisia annua) microRNA and their mRNA targets. Genom. Proteom. Bioinf. 2011;9:200–210. doi: 10.1016/S1672-0229(11)60023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li P., Tian Z., Zhang Q., Zhang Y., Wang M., Fang X., Shi W., Cai X. MicroRNAome profile of Euphorbia kansui in response to Methyl Jasmonate. Int. J. Mol. Sci. 2019;20:1267. doi: 10.3390/ijms20061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y., Chen C., Xie Z., Xu J., Wu B., Wang W. Integrated analysis of mRNA and microRNA elucidates the regulation of glycyrrhizic acid biosynthesis in Glycyrrhiza uralensis Fisch. Int. J. Mol. Sci. 2020;21:3101. doi: 10.3390/ijms21093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang H., Chen H., Hou Z., Xu L., Jin W., Liang Z. Overexpression of Ath–MIR160b increased the biomass while reduced the content of tanshinones in Salvia miltiorrhiza hairy roots by targeting ARFs genes. Plant Cell Tiss. Org. 2020;142:327–338. doi: 10.1007/s11240-020-01865-8. [DOI] [Google Scholar]
- 62.Zou H., Guo X., Yang R., Wang S., Li L., Niu J., Wang D., Cao X. MiR408–SmLAC3 module participates in salvianolic acid b synthesis in Salvia miltiorrhiza. Int. J. Mol. Sci. 2021;22:7541. doi: 10.3390/ijms22147541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hazra S., Bhattacharyya D., Chattopadhyay S. Methyl jasmonate regulates podophyllotoxin accumulation in Podophyllum hexandrum by altering the ROS–responsive podophyllotoxin pathway gene expression additionally through the down regulation of few interfering miRNAs. Front. Plant Sci. 2017;8:164. doi: 10.3389/fpls.2017.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang M., Dong Y., Nie L., Lu M., Fu C., Yu L. High–throughput sequencing reveals miRNA effects on the primary and secondary production properties in long–term subcultured Taxus cells. Front. Plant Sci. 2015;6:604. doi: 10.3389/fpls.2015.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ye J., Zhang X., Tan J., Xu F., Cheng S., Chen Z., Zhang W., Liao Y. Global identification of Ginkgo biloba microRNAs and insight into their role in metabolism regulatory network of terpene trilactones by high–throughput sequencing and degradome analysis. Ind. Crop. Prod. 2020;148:112289. [Google Scholar]
- 66.Wang Z., Gong H., Xu X., Wei X., Wang Y., Zeng S. Transcriptome and small RNAome facilitate to study schaftoside in Desmodium styracifolium Merr. Ind. Crop. Prod. 2020;149:112352. [Google Scholar]
- 67.Zhao L., Chen C., Wang Y., Shen J., Ding Z. Conserved microRNA act boldly during sprout development and quality formation in Pingyang Tezaocha (Camellia sinensis) Front. Genet. 2019;10:237. doi: 10.3389/fgene.2019.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng X., Li H., Chen M., Zhang J., Tan R., Zhao S., Wang Z. smi–miR396b targeted SmGRFs, SmHDT1, and SmMYB37/4 synergistically regulates cell growth and active ingredient accumulation in Salvia miltiorrhiza hairy roots. Plant Cell Rep. 2020;39:1263–1283. doi: 10.1007/s00299-020-02562-8. [DOI] [PubMed] [Google Scholar]
- 69.Shen E.M., Singh S.K., Ghosh J.S., Patra B., Paul P., Yuan L., Pattanaik S. The miRNAome of Catharanthus roseus: Identification, expression analysis, and potential roles of microRNAs in regulation of terpenoid indole alkaloid biosynthesis. Sci. Rep. 2017;7:srep43027. doi: 10.1038/srep43027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ding J., Ruan C., Guan Y., Krishna P. Identification of microRNAs involved in lipid biosynthesis and seed size in developing sea buckthorn seeds using high–throughput sequencing. Sci. Rep. 2018;8:4022. doi: 10.1038/s41598-018-22464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khan S., Ali A., Saifi M., Saxena P., Ahlawat S., Abdin M.Z. Identification and the potential involvement of miRNAs in the regulation of artemisinin biosynthesis in A. annua. Sci. Rep. 2020;10:13614. doi: 10.1038/s41598-020-69707-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li C., Li D., Li J., Shao F., Lu S. Characterization of the polyphenol oxidase gene family reveals a novel microRNA involved in posttranscriptional regulation of PPOs in Salvia miltiorrhiza. Sci. Rep. 2017;7:44622. doi: 10.1038/srep44622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fan K., Fan D., Ding Z., Su Y., Wang X. Cs–miR156 is involved in the nitrogen form regulation of catechins accumulation in tea plant (Camellia sinensis L.) Plant Physiol. Bioch. 2015;97:350–360. doi: 10.1016/j.plaphy.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 74.Qian X., Zhu J., Yuan Q., Jia Q., Jin H., Han J., Sarsaiya S., Jin L., Chen J., Guo L. Illumina sequencing reveals conserved and novel microRNAs of Dendrobium nobile protocorm involved in synthesizing dendrobine, a potential nanodrug. J. Biomed. Nanotechnol. 2021;17:416–425. doi: 10.1166/jbn.2021.3036. [DOI] [PubMed] [Google Scholar]
- 75.Wu B., Li Y., Yan H., Ma Y., Luo H., Yuan L., Chen S., Lu S. Comprehensive transcriptome analysis reveals novel genes involved in cardiac glycoside biosynthesis and mlncRNAs associated with secondary metabolism and stress response in Digitalis purpurea. BMC Genom. 2012;13:15. doi: 10.1186/1471-2164-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei R., Qiu D., Wilson I.W., Zhao H., Lu S., Miao J., Feng S., Bai L., Wu Q., Tu D. Identification of novel and conserved microRNAs in Panax notoginseng roots by high–throughput sequencing. BMC Genom. 2015;16:1–10. doi: 10.1186/s12864-015-2010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu S., Jiang Y., Wang N., Xia B., Jiang Y., Li X., Zhang Z., Li Y., Wang R. Identification and differential regulation of microRNAs in response to methyl jasmonate treatment in Lycoris aurea by deep sequencing. BMC Genom. 2016;17:1–15. doi: 10.1186/s12864-016-2645-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong M.M., Cannon C.H., Wickneswari R. Identification of lignin genes and regulatory sequences involved in secondary cell wall formation in Acacia auriculiformis and Acacia mangium via de novo transcriptome sequencing. BMC Genom. 2011;12:342. doi: 10.1186/1471-2164-12-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gutiérrez–García C., Ahmed S.S., Ramalingam S., Selvaraj D., Srivastava A., Paul S., Sharma A. Identification of microRNAs from medicinal plant Murraya koenigii by high–throughput sequencing and their functional implications in secondary metabolite biosynthesis. Plants. 2021;11:46. doi: 10.3390/plants11010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prakash P., Ghosliya D., Gupta V. Identification of conserved and novel microRNAs in Catharanthus roseus by deep sequencing and computational prediction of their potential targets. Gene. 2015;554:181–195. doi: 10.1016/j.gene.2014.10.046. [DOI] [PubMed] [Google Scholar]
- 81.Legrand S., Valot N., Nicolé F., Moja S., Baudino S., Jullien F., Magnard J.-L., Caissard J.-C., Legendre L. One–step identification of conserved miRNAs, their targets, potential transcription factors and effector genes of complete secondary metabolism pathways after 454 pyrosequencing of calyx cDNAs from the Labiate Salvia sclarea L. Gene. 2010;450:55–62. doi: 10.1016/j.gene.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 82.Singh N., Srivastava S., Sharma A. Identification and analysis of miRNAs and their targets in ginger using bioinformatics approach. Gene. 2016;575:570–576. doi: 10.1016/j.gene.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 83.Singh N., Sharma A. In–silico identification of miRNAs and their regulating target functions in Ocimum basilicum. Gene. 2014;552:277–282. doi: 10.1016/j.gene.2014.09.040. [DOI] [PubMed] [Google Scholar]
- 84.Najafabadi A.S., Naghavi M.R. Mining Ferula gummosa transcriptome to identify miRNAs involved in the regulation and biosynthesis of terpenes. Gene. 2018;645:41–47. doi: 10.1016/j.gene.2017.12.035. [DOI] [PubMed] [Google Scholar]
- 85.Khaldun A., Huang W., Liao S., Lv H., Wang Y. Identification of microRNAs and target genes in the fruit and shoot tip of Lycium chinense: A traditional Chinese medicinal plant. PLoS ONE. 2015;10:e0116334. doi: 10.1371/journal.pone.0116334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu X., Jiang Q., Ma X., Ying Q., Shen B., Qian Y., Song H., Wang H. Deep sequencing identifies tissue–specific microRNAs and their target genes involving in the biosynthesis of tanshinones in Salvia miltiorrhiza. PLoS ONE. 2014;9:e111679. doi: 10.1371/journal.pone.0111679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fan R., Li Y., Li C., Zhang Y. Differential microRNA analysis of glandular trichomes and young leaves in Xanthium strumarium L. reveals their putative roles in regulating terpenoid biosynthesis. PLoS ONE. 2015;10:e0139002. doi: 10.1371/journal.pone.0139002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li C., Li D., Zhou H., Li J., Lu S. Analysis of the laccase gene family and miR397–/miR408–mediated posttranscriptional regulation in Salvia miltiorrhiza. PeerJ. 2019;7:e7605. doi: 10.7717/peerj.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rajakani R., Prakash P., Ghosliya D., Soni R., Singh A., Gupta V. Azadirachta indica microRNAs: Genome–wide identification, target transcript prediction, and expression analyses. Appl. Biochem. Biotech. 2021;193:1924–1944. doi: 10.1007/s12010-021-03500-4. [DOI] [PubMed] [Google Scholar]
- 90.Srivastava S., Singh R., Srivastava G., Sharma A. Comparative study of withanolide biosynthesis–related miRNAs in root and leaf tissues of Withania somnifera. Appl. Biochem. Biotech. 2018;185:1145–1159. doi: 10.1007/s12010-018-2702-x. [DOI] [PubMed] [Google Scholar]
- 91.Singh N., Srivastava S., Shasany A.K., Sharma A. Identification of miRNAs and their targets involved in the secondary metabolic pathways of Mentha spp. Comput. Biol. Chem. 2016;64:154–162. doi: 10.1016/j.compbiolchem.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 92.Pérez–Quintero Á.L., Sablok G., Tatarinova T.V., Conesa A., Kuo J., López C. Mining of miRNAs and potential targets from gene oriented clusters of transcripts sequences of the anti–malarial plant, Artemisia annua. Biotechnol. Lett. 2012;34:737–745. doi: 10.1007/s10529-011-0808-0. [DOI] [PubMed] [Google Scholar]
- 93.Verma P., Singh N., Khan S.A., Mathur A.K., Sharma A., Jamal F. TIAs pathway genes and associated miRNA identification in Vinca minor: Supporting aspidosperma and eburnamine alkaloids linkage via transcriptomic analysis. Physiol. Mol. Biol. Plants. 2020;26:1695–1711. doi: 10.1007/s12298-020-00842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Singh N., Sharma A. Turmeric (Curcuma longa): miRNAs and their regulating targets are involved in development and secondary metabolite pathways. CR. Biol. 2017;340:481–491. doi: 10.1016/j.crvi.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 95.Kumar P., Padhan J.K., Kumar A., Chauhan R.S. Transcriptomes of Podophyllum hexandrum unravel candidate miRNAs and their association with the biosynthesis of secondary metabolites. J. Plant Biochem. Biot. 2018;27:46–54. doi: 10.1007/s13562-017-0414-x. [DOI] [Google Scholar]
- 96.Biswas S., Hazra S., Chattopadhyay S. Deep sequencing unravels methyl jasmonate responsive novel miRNAs in Podophyllum hexandrum. J. Plant Biochem. Biot. 2021;31:511–523. doi: 10.1007/s13562-021-00698-6. [DOI] [Google Scholar]
- 97.Samad A.F.A., Nazaruddin N., Jani J., Ismail I. Identification and analysis of microRNAs responsive to abscisic acid and methyl jasmonate treatments in Persicaria minor. Sains Malays. 2020;49:1245–1272. doi: 10.17576/jsm-2020-4906-04. [DOI] [Google Scholar]
- 98.Yang Y., Wang J., Wang C., Chen H., Liu Y., Wang Y., Gao W. Comprehensive Identification and profiling of miRNAs involved in terpenoid synthesis of Gleditsia sinensis Lam. Forests. 2022;13:108. doi: 10.3390/f13010108. [DOI] [Google Scholar]
- 99.Rostami Azar A., Maroufi A. Identification of long non–coding RNA transcripts in Glycyrrhiza uralensis. Iran. J. Biotechnol. 2022;20:88–97. doi: 10.30498/ijb.2021.205469.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou Y., Mumtaz M.A., Zhang Y., Yang Z., Hao Y., Shu H., Zhu J., Bao W., Cheng S., Zhu G. Response of anthocyanin biosynthesis to light by strand–specific transcriptome and miRNA analysis in Capsicum annuum. BMC Plant Biol. 2022;22:79. doi: 10.1186/s12870-021-03423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chowdhury M.R., Bahadur R.P., Basak J. Genome–wide prediction of cauliflower miRNAs and lncRNAs and their roles in post–transcriptional gene regulation. Planta. 2021;254:72. doi: 10.1007/s00425-021-03689-y. [DOI] [PubMed] [Google Scholar]
- 102.Samad A., Rahnamaie-Tajadod R., Sajad M., Jani J., Murad A., Noor N., Ismail I. Regulation of terpenoid biosynthesis by miRNA in Persicaria minor induced by Fusarium oxysporum. BMC Genom. 2019;20:586. doi: 10.1186/s12864-019-5954-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Song C., Guan Y., Zhang D., Tang X., Chang Y. Integrated mRNA and miRNA Transcriptome Analysis Suggests a Regulatory Network for UV–B-Controlled Terpenoid Synthesis in Fragrant Woodfern (Dryopteris fragrans) Int. J. Mol. Sci. 2022;23:5708. doi: 10.3390/ijms23105708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu H., Noda N., Mikami R., Kang X., Akita Y. Insertion of a novel transposable element disrupts the function of an anthocyanin biosynthesis-related gene in Echinacea purpurea. Sci. Hortic-Amsterdam. 2021;282:110021. doi: 10.1016/j.scienta.2021.110021. [DOI] [Google Scholar]
- 105.Mathiyalagan R., Subramaniyam S., Natarajan S., Kim Y.J., Sun M.S., Kim S.Y., Kim Y.-J., Yang D.C. Insilico profiling of microRNAs in Korean ginseng (Panax ginseng Meyer) J. Ginseng Res. 2013;37:227. doi: 10.5142/jgr.2013.37.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zeng S., Liu Y., Pan L., Hayward A., Wang Y. Identification and characterization of miRNAs in ripening fruit of Lycium barbarum L. using high–throughput sequencing. Front. Plant Sci. 2015;6:778. doi: 10.3389/fpls.2015.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Y., Liu J., Wang X., Liu S., Wang G., Zhou J., Yuan Y., Chen T., Jiang C., Zha L. Validation of suitable reference genes for assessing gene expression of microRNAs in Lonicera japonica. Front. Plant Sci. 2016;7:1101. doi: 10.3389/fpls.2016.01101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Khaldun A., Huang W., Lv H., Liao S., Zeng S., Wang Y. Comparative profiling of miRNAs and target gene identification in distant–grafting between tomato and lycium (Goji Berry) Front. Plant Sci. 2016;7:1475. doi: 10.3389/fpls.2016.01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meng Y., Yu D., Xue J., Lu J., Feng S., Shen C., Wang H. A transcriptome–wide, organ–specific regulatory map of Dendrobium officinale, an important traditional Chinese orchid herb. Sci. Rep. 2016;6:18864. doi: 10.1038/srep18864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zheng Y., Chen K., Xu Z., Liao P., Zhang X., Liu L., Wei K., Liu D., Li Y.-F., Sunkar R. Small RNA profiles from Panax notoginseng roots differing in sizes reveal correlation between miR156 abundances and root biomass levels. Sci. Rep. 2017;7:9418. doi: 10.1038/s41598-017-09670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li H., Dong Y., Sun Y., Zhu E., Yang J., Liu X., Xue P., Xiao Y., Yang S., Wu J. Investigation of the microRNAs in safflower seed, leaf, and petal by high–throughput sequencing. Planta. 2011;233:611–619. doi: 10.1007/s00425-010-1327-2. [DOI] [PubMed] [Google Scholar]
- 112.Li C., Zhu Y., Guo X., Sun C., Luo H., Song J., Li Y., Wang L., Qian J., Chen S. Transcriptome analysis reveals ginsenosides biosynthetic genes, microRNAs and simple sequence repeats in Panax ginseng C. A. Meyer. BMC Genom. 2013;14:245. doi: 10.1186/1471-2164-14-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang Q., Liu S., Han X., Ma J., Deng W., Wang X., Guo H., Xia X. Integrated transcriptome and miRNA analysis uncovers molecular regulators of aerial stem–to–rhizome transition in the medical herb Gynostemma pentaphyllum. BMC Genom. 2019;20:865. doi: 10.1186/s12864-019-6250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Galla G., Volpato M., Sharbel T.F., Barcaccia G. Computational identification of conserved microRNAs and their putative targets in the Hypericum perforatum L. flower transcriptome. Plant Reprod. 2013;26:209–229. doi: 10.1007/s00497-013-0227-6. [DOI] [PubMed] [Google Scholar]
- 115.Xu T., Wang B., Liu X., Feng R., Dong M., Chen J. Microarray–based identification of conserved microRNAs from Pinellia ternata. Gene. 2012;493:267–272. doi: 10.1016/j.gene.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 116.Liu J., Yuan Y., Wang Y., Jiang C., Chen T., Zhu F., Zhao Y., Zhou J., Huang L. Regulation of fatty acid and flavonoid biosynthesis by miRNAs in Lonicera japonica. RSC Adv. 2017;7:35426–35437. doi: 10.1039/C7RA05800D. [DOI] [Google Scholar]
- 117.Zhang Q., Li J., Sang Y., Xing S., Wu Q., Liu X. Identification and characterization of microRNAs in Ginkgo biloba var. epiphylla Mak. PLoS ONE. 2015;10:e0127184. doi: 10.1371/journal.pone.0127184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nadiya F., Anjali N., Thomas J., Gangaprasad A., Sabu K. Deep sequencing identified potential miRNAs involved in defence response, stress and plant growth characteristics of wild genotypes of cardamom. BMC Plant Biol. 2019;21:3–14. doi: 10.1111/plb.12888. [DOI] [PubMed] [Google Scholar]
- 119.Wang L., Zhao J., Luo K., Cui J., He Q., Xia X., Lu Z., Li W., Jin B. Deep sequencing discovery and profiling of conserved and novel miRNAs in the ovule of Ginkgo biloba. Trees. 2016;30:1557–1567. doi: 10.1007/s00468-016-1389-2. [DOI] [Google Scholar]
- 120.Cui J., Zhao J., Zhao J., Xu H., Wang L., Jin B. Cytological and miRNA expression changes during the vascular cambial transition from the dormant stage to the active stage in Ginkgo biloba L. Trees. 2016;30:2177–2188. doi: 10.1007/s00468-016-1443-0. [DOI] [Google Scholar]
- 121.Paul S., de la Fuente–Jiménez J.L., Manriquez C.G., Sharma A. Identification, characterization and expression analysis of passion fruit (Passiflora edulis) microRNAs. 3 Biotech. 2020;10:25. doi: 10.1007/s13205-019-2000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang L., Zhao J., Zhang M., Li W., Luo K., Lu Z., Zhang C., Jin B. Identification and characterization of microRNA expression in Ginkgo biloba L. leaves. Tree Genet. Genomes. 2015;11:76. doi: 10.1007/s11295-015-0897-3. [DOI] [Google Scholar]
- 123.Yang Z., Yang D., Ding X., Gao Y., Li D., Xu T. MicroRNA expression profiles in conventional and micropropagated Dendrobium officinale. Genes Genom. 2015;37:315–325. doi: 10.1007/s13258-014-0257-y. [DOI] [Google Scholar]
- 124.Chen Y., Liu Z., Tu N., Hu Y., Jin C., Luo Y., Liu A., Zhang X. Integrated transcriptome and microRNA profiles analysis reveals molecular mechanisms underlying the consecutive monoculture problem of Polygonatum odoratum. Cell. Mol. Biol. 2020;66:47–52. doi: 10.14715/2020.66.2.7. [DOI] [PubMed] [Google Scholar]
- 125.Ma X., Tang K., Tang Z., Dong A., Xiao H., Meng Y., Wang P. An organ-specific transcriptomic atlas of the medicinal plant Bletilla striata: Protein-coding genes, microRNAs, and regulatory networks. Plant Genome. 2022;15:e20210. doi: 10.1002/tpg2.20210. [DOI] [PubMed] [Google Scholar]
- 126.Yang R., Zeng Y., Yi X., Zhao L., Zhang Y. Small RNA deep sequencing reveals the important role of microRNAs in the halophyte Halostachys caspica. Plant Biotechnol. J. 2015;13:395–408. doi: 10.1111/pbi.12337. [DOI] [PubMed] [Google Scholar]
- 127.Garg V., Khan A.W., Kudapa H., Kale S.M., Chitikineni A., Qiwei S., Sharma M., Li C., Zhang B., Xin L. Integrated transcriptome, small RNA and degradome sequencing approaches provide insights into Ascochyta blight resistance in chickpea. Plant Biotechnol. J. 2019;17:914–931. doi: 10.1111/pbi.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abla M., Sun H., Li Z., Wei C., Gao F., Zhou Y., Feng J. Identification of miRNAs and their response to cold stress in Astragalus membranaceus. Biomolecules. 2019;9:182. doi: 10.3390/biom9050182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Snigdha M., Prasath D. Transcriptomic analysis to reveal the differentially expressed miRNA targets and their miRNAs in response to Ralstonia solanacearum in ginger species. BMC Plant Biol. 2021;21:355. doi: 10.1186/s12870-021-03108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y., Dai J., Chen R., Song C., Wei P., Cai Y., Wang Y., Han B. miRNA–based drought regulation in the important medicinal plant Dendrobium huoshanense. J. Plant Growth Regul. 2022;41:1099–1108. doi: 10.1007/s00344-021-10366-7. [DOI] [Google Scholar]
- 131.Yu L., Zhou L., Liu W., Huang P., Jiang R., Tang Z., Cheng P., Zeng J. Identification of drought resistant miRNA in Macleaya cordata by high–throughput sequencing. Arch. Biochem. Biophys. 2020;684:108300. doi: 10.1016/j.abb.2020.108300. [DOI] [PubMed] [Google Scholar]
- 132.Jung I., Kang H., Kim J.U., Chang H., Kim S., Jung W. The mRNA and miRNA transcriptomic landscape of Panax ginseng under the high ambient temperature. BMC Syst. Biol. 2018;12:117–130. doi: 10.1186/s12918-018-0548-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gao Z.-H., Wei J.-H., Yang Y., Zhang Z., Xiong H.-Y., Zhao W.-T. Identification of conserved and novel microRNAs in Aquilaria sinensis based on small RNA sequencing and transcriptome sequence data. Gene. 2012;505:167–175. doi: 10.1016/j.gene.2012.03.072. [DOI] [PubMed] [Google Scholar]
- 134.Wu B., Wang M., Ma Y., Yuan L., Lu S. High–throughput sequencing and characterization of the small RNA transcriptome reveal features of novel and conserved microRNAs in Panax ginseng. PLoS ONE. 2012:e44385. doi: 10.1371/journal.pone.0044385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shao F., Zhang Q., Liu H., Lu S., Qiu D. Genome–wide identification and analysis of microRNAs involved in witches’–broom phytoplasma response in Ziziphus jujuba. PLoS ONE. 2016;11:e0166099. doi: 10.1371/journal.pone.0166099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yan W., Cao S., Wu Y., Ye Z., Zhang C., Yao G., Yu J., Yang D., Zhang J. Integrated analysis of physiological, mRNA sequencing, and miRNA sequencing data reveals a specific mechanism for the response to continuous cropping obstacles in Pogostemon cablin Roots. Front. Plant Sci. 2022;13:853110. doi: 10.3389/fpls.2022.853110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang L., Du H., Wuyun T.-N. Genome–wide identification of microRNAs and their targets in the leaves and fruits of Eucommia ulmoides using high–throughput sequencing. Front. Plant Sci. 2016;7:1632. doi: 10.3389/fpls.2016.01632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hao D.C., Yang L., Xiao P.G., Liu M. Identification of Taxus microRNAs and their targets with high-throughput sequencing and degradome analysis. Physiol. Plant. 2012;146:388–403. doi: 10.1111/j.1399-3054.2012.01668.x. [DOI] [PubMed] [Google Scholar]
- 139.Hu J., Zhang H., Ding Y. Identification of conserved microRNAs and their targets in the model legume Lotus japonicus. J. Biotechnol. 2013;164:520–524. doi: 10.1016/j.jbiotec.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 140.Mishra A.K., Duraisamy G.S., Týcová A., Matoušek J. Computational exploration of microRNAs from expressed sequence tags of Humulus lupulus, target predictions and expression analysis. Comput. Biol. Chem. 2015;59:131–141. doi: 10.1016/j.compbiolchem.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 141.Samad A.F.A., Nazaruddin N., Murad A.M.A., Jani J., Zainal Z., Ismail I. Deep sequencing and in silico analysis of small RNA library reveals novel miRNA from leaf Persicaria minor transcriptome. 3 Biotech. 2018;8:1–9. doi: 10.1007/s13205-018-1164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kalariya K.A., Meena R.P., Saran P.L., Manivel P. Identification of microRNAs from transcriptome data in gurmar (Gymnema sylvestre) Hortic. Environ. Biote. 2019;60:383–397. doi: 10.1007/s13580-019-00135-7. [DOI] [Google Scholar]
- 143.Yang Y., Chen X., Chen J., Xu H., Li J., Zhang Z. Identification of novel and conserved microRNAs in Rehmannia glutinosa L. by Solexa sequencing. Plant Mol. Biol. Rep. 2011;29:986–996. doi: 10.1007/s11105-011-0293-6. [DOI] [Google Scholar]
- 144.Yu D., Lu J., Shao W., Ma X., Xie T., Ito H., Wang T., Xu M., Wang H., Meng Y. MepmiRDB: A medicinal plant microRNA database. Database. 2019;2019:baz070. doi: 10.1093/database/baz070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Petijová L., Jurčacková Z., Čellárová E. Computational screening of miRNAs and their targets in leaves of Hypericum spp. by transcriptome–mining: A pilot study. Planta. 2020;251:49. doi: 10.1007/s00425-020-03342-0. [DOI] [PubMed] [Google Scholar]
- 146.Wang B., Dong M., Chen W., Liu X., Feng R., Xu T. Microarray identification of conserved microRNAs in Pinellia pedatisecta. Gene. 2012;498:36–40. doi: 10.1016/j.gene.2012.01.075. [DOI] [PubMed] [Google Scholar]
- 147.Philip A., Ferro V.A., Tate R.J. Determination of the potential bioavailability of plant microRNAs using a simulated human digestion process. Mol. Nutr. Food Res. 2015;59:1962–1972. doi: 10.1002/mnfr.201500137. [DOI] [PubMed] [Google Scholar]
- 148.Zhang L., Hou D., Chen X., Li D., Zhu L., Zhang Y., Li J., Bian Z., Liang X., Cai X. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: Evidence of cross–kingdom regulation by microRNA. Cell Res. 2012;22:107–126. doi: 10.1038/cr.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao C., Sun X., Li L. Biogenesis and function of extracellular miRNAs. ExRNA. 2019;1:1–9. doi: 10.1186/s41544-019-0039-4. [DOI] [Google Scholar]
- 150.Zhang S., Hong Z. Mobile RNAs—the magical elf traveling between plant and the associated organisms. ExRNA. 2019;1:1–6. doi: 10.1186/s41544-019-0007-z. [DOI] [Google Scholar]
- 151.Yang J., Elbaz–Younes I., Primo C., Murungi D., Hirschi K.D. Intestinal permeability, digestive stability and oral bioavailability of dietary small RNAs. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-28207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Winter J., Diederichs S. Argonaute proteins regulate microRNA stability: Increased microRNA abundance by argonaute proteins is due to microRNA stabilization. RNA Biol. 2011;8:1149–1157. doi: 10.4161/rna.8.6.17665. [DOI] [PubMed] [Google Scholar]
- 153.Wang K., Zhang S., Weber J., Baxter D., Galas D.J. Export of microRNAs and microRNA–protective protein by mammalian cells. Nucleic Acids Res. 2010;38:7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vora J., Patel S., Sinha S., Sharma S., Srivastava A., Chhabria M., Shrivastava N. Molecular docking, QSAR and ADMET based mining of natural compounds against prime targets of HIV. J. Biomol. Struct. Dyn. 2019;37:131–146. doi: 10.1080/07391102.2017.1420489. [DOI] [PubMed] [Google Scholar]
- 155.Masood M., Everett C.P., Chan S.Y., Snow J.W. Negligible uptake and transfer of diet–derived pollen microRNAs in adult honey bees. RNA Biol. 2016;13:109–118. doi: 10.1080/15476286.2015.1128063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dickinson B., Zhang Y., Petrick J.S., Heck G., Ivashuta S., Marshall W.S. Lack of detectable oral bioavailability of plant microRNAs after feeding in mice. Nat. Biotechnol. 2013;31:965–967. doi: 10.1038/nbt.2737. [DOI] [PubMed] [Google Scholar]
- 157.Zhang S., Sang X., Hou D., Chen J., Gu H., Zhang Y., Li J., Yang D., Zhu H., Yang X. Plant–derived RNAi therapeutics: A strategic inhibitor of HBsAg. Biomaterials. 2019;210:83–93. doi: 10.1016/j.biomaterials.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 158.Brown B.D., Venneri M.A., Zingale A., Sergi L.S., Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat. Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- 159.Xie W., Weng A., Melzig M.F. MicroRNAs as new bioactive components in medicinal plants. Planta Med. 2016;82:1153–1162. doi: 10.1055/s-0042-108450. [DOI] [PubMed] [Google Scholar]
- 160.Liang H., Zhang S., Fu Z., Wang Y., Wang N., Liu Y., Zhao C., Wu J., Hu Y., Zhang J. Effective detection and quantification of dietetically absorbed plant microRNAs in human plasma. J. Nutr. Biochem. 2015;26:505–512. doi: 10.1016/j.jnutbio.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 161.Harapan H., Itoh N., Yufika A., Winardi W., Keam S., Te H., Megawati D., Hayati Z., Wagner A.L., Mudatsir M. Coronavirus disease 2019 (COVID-19): A literature review. J. Infect. Public Health. 2020;13:667–673. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Peterson S.M., Thompson J.A., Ufkin M.L., Sathyanarayana P., Liaw L., Congdon C.B. Common features of microRNA target prediction tools. Front. Genet. 2014;5:23. doi: 10.3389/fgene.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chin A.R., Fong M.Y., Somlo G., Wu J., Swiderski P., Wu X., Wang S.E. Cross–kingdom inhibition of breast cancer growth by plant miR159. Cell Res. 2016;26:217–228. doi: 10.1038/cr.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sun R., Wang Q., Ma J., He Q., Zhang B. Differentiated expression of microRNAs may regulate genotype–dependent traits in cotton. Gene. 2014;547:233–238. doi: 10.1016/j.gene.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 165.Liu S., Yang C., Wu L., Cai H., Li H., Xu M. The peu-miR160a−PeARF17. 1/PeARF17. 2 module participates in the adventitious root development of poplar. Plant Biotechnol. J. 2020;18:457–469. doi: 10.1111/pbi.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gershenzon J., McConkey M.E., Croteau R.B. Regulation of monoterpene accumulation in leaves of peppermint. Plant Physiol. 2000;122:205–214. doi: 10.1104/pp.122.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nguyen M., Osipo C. Targeting breast cancer stem cells using naturally occurring phytoestrogens. Int. J. Mol. Sci. 2022;23:6813. doi: 10.3390/ijms23126813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang H., Li Y., Liu Y., Liu H., Wang H., Jin W., Zhang Y., Zhang C., Xu D. Role of plant microRNA in cross–species regulatory networks of humans. BMC Syst. Biol. 2016;10:60. doi: 10.1186/s12918-016-0292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cheng Q., Ouyang Y., Tang Z., Lao C., Zhang Y., Cheng C., Zhou H. Review on the development and applications of medicinal plant genomes. Front. Plant Sci. 2021;12:791219. doi: 10.3389/fpls.2021.791219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen W., Kui L., Zhang G., Zhu S., Zhang J., Wang X., Yang M., Huang H., Liu Y., Wang Y. Whole-genome sequencing and analysis of the Chinese herbal plant Panax notoginseng. Mol. Plant. 2017;10:899–902. doi: 10.1016/j.molp.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 171.Zhang D., Li W., Xia E., Zhang Q., Liu Y., Zhang Y., Tong Y., Zhao Y., Niu Y., Xu J. The medicinal herb Panax notoginseng genome provides insights into ginsenoside biosynthesis and genome evolution. Mol. Plant. 2017;10:903–907. doi: 10.1016/j.molp.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 172.Fan G., Liu X., Sun S., Shi C., Du X., Han K., Yang B., Fu Y., Liu M., Seim I. The chromosome level genome and genome-wide association study for the agronomic traits of Panax notoginseng. Iscience. 2020;23:101538. doi: 10.1016/j.isci.2020.101538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jiang Z., Tu L., Yang W., Zhang Y., Hu T., Ma B., Lu Y., Cui X., Gao J., Wu X. The chromosome-level reference genome assembly for Panax notoginseng and insights into ginsenoside biosynthesis. Plant Commun. 2021;2:100113. doi: 10.1016/j.xplc.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yang Z., Liu G., Zhang G., Yan J., Dong Y., Lu Y., Fan W., Hao B., Lin Y., Li Y. The chromosome–scale high–quality genome assembly of Panax notoginseng provides insight into dencichine biosynthesis. Plant Biotechnol. J. 2021;19:869–871. doi: 10.1111/pbi.13558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sun W., Leng L., Yin Q., Xu M., Huang M., Xu Z., Zhang Y., Yao H., Wang C., Xiong C. The genome of the medicinal plant Andrographis paniculata provides insight into the biosynthesis of the bioactive diterpenoid neoandrographolide. Plant J. 2019;97:841–857. doi: 10.1111/tpj.14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liang Y., Chen S., Wei K., Yang Z., Duan S., Du Y., Qu P., Miao J., Chen W., Dong Y. Chromosome level genome assembly of Andrographis paniculata. Front. Genet. 2020;11:701. doi: 10.3389/fgene.2020.00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yuan Y., Jin X., Liu J., Zhao X., Zhou J., Wang X., Wang D., Lai C., Xu W., Huang J. The Gastrodia elata genome provides insights into plant adaptation to heterotrophy. Nat. Commun. 2018;9:1615. doi: 10.1038/s41467-018-03423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen S., Wang X., Wang Y., Zhang G., Song W., Dong X., Arnold M., Wang W., Miao J., Chen W. Improved de novo assembly of the achlorophyllous orchid Gastrodia elata. Front. Genet. 2020;11:580568. doi: 10.3389/fgene.2020.580568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.