Abstract
Spinal cord injury (SCI) is a devastating neurological state causing physical disability, psychological stress and financial burden. SCI global rate is estimated between 250,000 and 500,000 individuals every year, of which 60% of victims are young, healthy males between 15 and 35 years. A variety of pathological conditions such as neuroinflammation, mitochondrial dysfunction, apoptosis, glial scar formation, blood-spinal cord barrier disruption, and angiogenesis disruption occur after SCI leading to a limitation in recovery. MicroRNAs (miRs) are endogenous and non-coding RNAs consisting of 22 nucleotides that regulate 60% of all human genes and involve several normal physiological processes and pathological conditions. miR-21 is among the most highly expressed miRs and its expression has been shown to increase one day after SCI and this elevation is sustained up to 28 days after injury. Overexpression of miR-21 exerts many protective effects against SCI by inhibiting neuroinflammation, improving blood-spinal cord barrier function, regulating angiogenesis, and controlling glial scar formation. It also exhibits anti-apoptotic effects in SCI by down-regulating the expression of PTEN, Spry2, and PDCD4. This review provides a novel therapeutic perspective for miR-21 in SCI.
Keywords: Spinal cord injury, MicroRNA-21, Anti-inflammatory, Anti-apoptotic, Angiogenesis, Neural stem cells
Background
Spinal cord injury (SCI) is a destructive neurological state causing dysfunction in the primary motor, sensory and autonomic neural system affecting prevalently (60%) 15–35 years old healthy males (van Den Hauwe et al. 2020). It is estimated to involve 250,000–500,000 individuals every year worldwide (Anjum et al. 2020), and the costs for each patient can arrive at 3 million dollars in a whole lifetime perspective (Katoh et al. 2019). So far, there is no treatment available due to SCI complex physiopathology. A better understanding of SCI at the molecular level can reveal recovery mechanisms and discover some biological therapeutics potentially promising to confront SCI.
MicroRNAs (miRs) are small nucleic acids involved in post-transcriptional regulatory mechanisms (Rastegar-Moghaddam et al. 2022a). These biomarkers are expressed in all body tissues with a higher level in the central nervous system (CNS) (Rastegar-Moghaddam et al. 2022b). Around 70% of known miRs are expressed in CNS (Kou et al. 2020). miRs play critical roles in several cellular and molecular mechanisms, including angiogenesis, energy-providing, neuronal differentiation, maturation, and survival (Rastegar-Moghaddam et al. 2022b; Tonacci et al. 2019).
miR-21 is one of the most expressed miRs. It contains 22 nucleotides encoded by a sequence located within the Vacuole Membrane Protein-1 (VMP-1) gene on chromosome 17 (Jenike and Halushka 2021; Surina et al. 2021). It is expressed widely in various body tissues, including the spinal cord and its expression is altered following SCI (Liu et al. 2018; Chung et al. 2020). Several studies demonstrated that miR-21 has many protective roles against SCI by decreasing apoptosis and increasing neuron survival and can be considered a potential potent therapeutics (Kang et al. 2019; Zhang et al. 2019; Lv et al. 2020; Wang et al. 2021).
Pathophysiology of SCI
Following SCI, neurons and glial cells die due to various factors, including ionic imbalance and glutamate excitotoxicity, proinflammatory cytokines release, free radical production, ATP depletion, and apoptosis (Alizadeh et al. 2019; Samandari et al. 2019; Anjum et al. 2020). A hallmark of SCI is vascular and angiogenesis disruption, resulting in reduced oxygen delivery and mitochondrial dysfunction. Subsequently, ATP depletion, calcium overload, excitotoxicity, and oxidative stress exacerbate injury (Vasiliadis et al. 2014; Scholpa et al. 2017; Anjum et al. 2020). Neurons depend highly on ATP for ion exchange and maintaining electrochemical and energy homeostasis. Because energy demand in neurons is several times greater than in other cells, they are more vulnerable to ATP depletion (Mohammadipour et al. 2020; Malvandi et al. 2021). Inflammation is another phenomenon associated with SCI, which increases neural damage by causing edema, apoptosis, and reactive gliosis (Rong et al. 2019; Anjum et al. 2020). The local expression of interleukin (IL)-1, IL-6, IL-8, and tumor necrosis factor (TNF)-α increases following SCI (Rong et al. 2019; Slota and Booth 2019; Lv et al. 2020). In addition, the augment in the expression of apoptotic proteins such as Bax, caspase-3, and caspase-9, phosphatase and tensin homolog (PTEN), and programmed cell death protein 4 (PDCD4) increase neuronal death (Kang et al. 2019; Hausott and Klimaschewski 2019).
Anti-neuroinflammatory effects
Although miR-21 has some inflammatory functions, it appears as predominantly anti-inflammatory miR in the nervous system and could effectively modulate neuroinflammation (Gaudet et al. 2018; Slota and Booth 2019). Indeed, suppression of miR-21 promotes IL-1β, IL-6, TNF-α (Table 1), and receptor activator of nuclear factor kappa-Β ligand (RANKL), leading to severe inflammation (Zhou et al. 2018). Conversely, miR-21 declines inflammatory factors such as IL-1β, IL-6, IL-8, TNF-α, and endothelial nitric oxide synthase (eNOS), and it enhances the anti-inflammatory cytokine IL-10 (Slota and Booth 2019; Lv et al. 2020).
Table 1.
The biological effects and main related mechanisms of miR-21
| Biological effects | Main mechanisms | References |
|---|---|---|
| Anti-inflammation |
• Reduces IL-1β, IL-6, IL-8, TNF-α, eNOS • Downregulates CCL3 |
Lv et al. (2020) Liu et al. (2020) |
| Anti-apoptotic |
• Reduces Bax/Bcl-2, and Caspase-3 and Caspase-9, and PTEN protein expressions • Reduces PDCD4 |
Hu et.al. (2013) Zhang et al. (2019) |
| Anti-glial scar formation |
• Modulates astrocytes’ secretion, proliferation, and apoptosis • Modulates PI3K/Akt/mTOR • Reduces astrocytes hypertrophy in the SCI |
Liu et al. (2018) Liu et al. (2018) Bhalala et al. (2012) |
| Angiogenesis modulation |
• Inhibits TIMP3 and promotes MMP2 and MMP9 • Promotes expression of Ang-1, Tie-2, and VEGF •Increases MMP-13 and p-ERK1/2 • Promotes the survival, migration and tube formation of endothelial cells |
Hu et al. (2016) Ge et al. (2014) Ma et al. (2020) Hu et al. (2016) |
| Neuroregeneration modulation |
• Promotes neural differentiation of NSPCs • Enhances the expression of cyclin D1 in NSPCs • Activates AKT/GSK-3β signaling Pathway • Modulates Wnt/β-catenin signaling pathway |
Gao et al. (2016) Song et al. (2021) Gao et al. (2016) Zhang et al. (2018) |
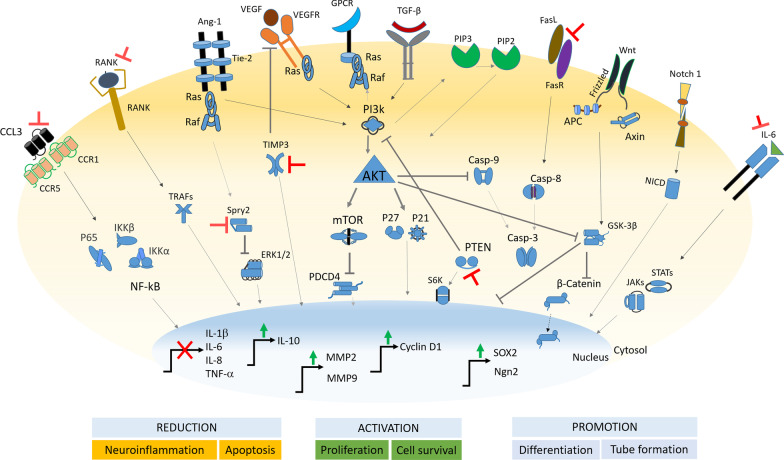
An experiment executed in a rat model of SCI showed that miR-21 overexpression could reduce the expression of IL-1β, IL-6, IL-8, and TNF-α (Lv et al. 2020). In the neonatal rat ischemia model, miR-21 showed repressor activity on proinflammatory C–C motif chemokine ligand 3 (CCL3), favoring neuroprotection (Liu et al. 2020). CCL3 and its receptors are induced after SCI and contribute to progressive tissue damage and functional impairment during secondary injury (Pelisch et al. 2020). CCL3 also activates the nuclear factor kappa B (NF-κB) signaling pathway (Fig. 1), which is a hallmark of inflammation (Mohammadipour et al. 2021). miR-21 negatively regulates CCL3, repressing -in turn- IKKα/β and p65 phosphorylation, disrupting the NF-κB signaling pathway (Liu et al. 2020). miR-21 also suppresses different target components of the toll-like receptor (TLR)/MyD88/NF-κB and JAK-STAT pathways (Slota and Booth 2019).
Fig. 1.
miR-21 global molecular mechanism of action leading to functional effects on neural cells’ status and function. ⊥ Represents inhibition, → shows induction/promotion of activity
Therefore, miR-21 overexpression seems to play a fundamental role in decreasing secondary injury after SCI by reducing the expression of inflammatory factors and suppressing inflammation.
Glial scar formation
miR-21 has a protective effect on SCI by controlling glial scar formation. Glial scar is believed to play a dual role in the pathological process of SCI (Yang et al. 2020). Although glial scar has some protective roles, they also have many detrimental effects after SCI. Glial scar is the most crucial inhibitor factor to neuroregeneration after SCI (Leal-Filho et al. 2011) and is a significant limitation in improving outcomes (Bhalala et al. 2012). In the uninjured spinal cord, miR-21 expression is neither silenced nor overexpressed in astrocytes, which indicates that this miR is not essential for maintaining astrocyte homeostasis. However, its expression rate increases sharply after SCI and reaches its maximum five weeks after injury (Bhalala et al. 2012). miR-21 has been found to act in astrocytes to control their functions and regulate the astrocytic size and glial scar formation after SCI (Table 1), interacting with bone morphogenetic protein (BMP) and JAK-STAT signaling pathways (Bhalala et al. 2012). This miR modulates astrocytes’ secretion, proliferation, and apoptosis (Fig. 1) to promote recovery through transforming growth factor (TGF)-β-mediated targeting of the phosphoinositide-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway (Liu et al. 2018). Overexpression of miR-21 reduces astrocyte hypertrophy in the traumatic SCI, whereas conversely, suppression of this miR induces astrocyte hypertrophy (Bhalala et al. 2012; Su et al. 2019).
Angiogenesis
Immediately after SCI, damage to spinal cord microvascular endothelial cells (SCMECs) occurs and not only disrupts the blood-spinal cord barrier (BSCB) but also results in disrupted angiogenesis and reduced blood supply (Zhong et al. 2020; Jin et al. 2021). Angiogenesis is necessary for axonal regeneration by facilitating tissue remodeling and survival. Although due to endogenous angiogenesis, blood vessel density transiently increases within two weeks after SCI but it is insufficient (Yao et al. 2021). Vascular endothelial growth factor (VEGF) and metalloproteinases (MMPs), especially MMP-2, are among the pro-angiogenic mediators and play fundamental roles in preserving vascular integrity, regulating basal muscle capillarization, and microvascular remodeling (Vasiliadis et al. 2014).
miR-21 is a potential pro-angiogenic factor and it is reported that decreased level of this miR inhibits angiogenesis in a rat model of SCI (Hu et al. 2016). Overexpression of miR-21 promotes the expression of angiogenesis-related molecules, including angiopoietin-1 (Ang-1), Tie-2 (receptor of Ang-1), and vascular endothelial growth factor (VEGF) (Fig. 1) in the injured neural tissues (Ge et al. 2014). In addition, this miR promotes angiogenesis by increasing the expression of MMP-13 and p-ERK1/2 (Ma et al. 2020). Overexpression of miR-21 also promotes the survival, migration and tube formation of endothelial cells by inhibiting tissue inhibitor of metalloproteinase-3 (TIMP3) expression and promoting MMP2 and MMP9 expression (Hu et al. 2016) (Table 1).
Neuroregeneration
Neural stem/progenitor cells (NSPCs) are essential for nerve regeneration after SCI (Wang et al. 2018). The main issue related to NSPCs is their poor proliferation rate and low differentiation efficiency into neurons, and if their proliferation and differentiation are promoted, it can have beneficial effects on the treatment of SCI. Besides endogenous NSPCs, the therapeutic effect of stem cell transplantation in SCI has been considered in recent studies, and it has been shown that miR-21-containing stem cell-derived exosomes promote the protective effects of stem cell transplantation against SCI, whereas miR-21 deficiency in stem cells did not exert such benefits (Ji et al. 2019). miR-21 overexpression has been found to promote neural differentiation of neural stem/precursor cells (NSPCs) (Gao et al. 2016). Overexpression of this miR enhances the expression of cyclin D1 in NSPCs (Fig. 1), a protein that has a role in the differentiation and survival of NSPCs (Song et al. 2021). A previous study showed that cyclin D1 expression is enhanced by Notch1, which promotes spinal NSPCs proliferation (Wang et al. 2018). Overexpression of this miR also positively regulates proliferation and neural differentiation of NSPCs by activating protein kinase B (AKT) and glycogen synthase kinase-3 beta (GSK-3β) signaling pathways.
Conversely, knocking down miR-21 reduces neural differentiation in NSPCs by preventing cyclin D1 expression and blocking AKT/GSK-3β (Table 1) (Gao et al. 2016). In addition to mentioned pathways, the Wnt/β-catenin pathway has exhibited an important role in regulating NSPCs fate and activation of this pathway has been demonstrated to promote NSPCs proliferation and differentiation (Zhang et al. 2018). It has been found that miR-21 can enhance the proliferation of neural stem cells and their differentiation into neurons and reduce stem cells’ differentiation into astrocytes via the Wnt/β-catenin signaling pathway (Zhang et al. 2018).
The exercise and miR-21 after SCI
Exercise can be considered a non-invasive therapy for SCI due to its benefits for stabilizing rhythmic firing patterns of spinal motoneurons, maintaining the muscle mass of paralyzed limbs (Ning et al. 2014) and possibly bone mass (Sutor et al.2022) to improve functional recovery. Several studies have shown the beneficial effects of physical activity on SCI (Vasiliadis et al. 2014; Ying et al. 2021; Nash et al. 2022). Physical activity after SCI reduces inflammation (Donia et al. 2019), neuronal and glial apoptosis (Jung et al. 2014), and increases angiogenesis (Vasiliadis et al. 2014) and cell survival (Li et al. 2020), leading to improved recovery. Short-time physical activity also led to a significant increase in miR-21 expression, at the spinal cord level, after SCI (Ning et al. 2014). Li et al. recently showed that exercise after SCI increases miR-21 expression and decreases PDCD4 levels, leading to reduced apoptotic cell number (Li et al. 2020). Since miR-21 reduces inflammation and apoptosis and increases cell survival and angiogenesis after SCI, exercise can improve recovery by overexpressing this miR.
Exercising, regardless of the type, enhances the expression, and therefore the circulating levels, of miR-21 (Horak et al. 2018) and particularly the fraction associated with extracellular vesicles (Siqueira et al. 2021). However, there is little knowledge about the effect of exercise on the expression of this miR in the nervous system. There are reports about the exercise-induced expression of miR-21 in the endothelial compartment with an intensity- (Wahl et al. 2016) and volume-dependent manner (Kilian et al. 2016).
Exercise after SCI alters gene expression leading to increased spinal cord plasticity and recovery of spinal reflexes (Mendell et al. 2001; Ying et al. 2005; Côté et al. 2011), and passive hindlimb exercise after SCI was shown to attenuate the SCI-induced increase in miR-199a-3p, a negative regulator of miR-21, with concurrent upregulation of miR-21 (Liu et al. 2012). The dynamic equilibrium between miR-21 and miR-199a-3p is responsible for the regulation of mTOR and PTEN (Hu et al. 2013), which in turn has been shown to drive axon growth in vitro and in vivo (Kar et al. 2021). While the literature is poor in this field, there are reports about the exercise-dependent miR-21-related effects on traumatic brain injury (TBI). As miR-21 is involved in the signaling pathways of inflammation, neuronal apoptosis, reactive gliosis, disruption of the blood–brain barrier, and angiogenesis in TBI (i.e., it is considered a marker and therapeutic target in TBI (Martinez and Pepolow 2017)), its induction by exercise, in the nervous system, can improve the recovery process (Ji et al. 2018). Further, in mice models of TBI, spontaneous wheel running enhanced hippocampal expression of miR-21 is associated with improved recovery (Bao et al. 2014).
Further, miR-21 is expressed by the skeletal muscle during exercise (D’Souza et al. 2018). Muscle-derived miR-21 may act at the neuromuscular plaque, exerting effects in motoneurons and backward at the spinal cord level.
Conclusions and future perspectives
Paralleling the evidence can reveal the protective potential for miR-21 against the burden of SCI through suppressing the inflammatory milieu in neural cells, apoptosis inhibition, improving angiogenesis, and synapsis protection (Fig. 1). It seems that miR-21 activates in response to the injury but declined in the following. The discovery of regulatory elements effective on miR-21 seems crucial and requires more effort.
SCI usually occurs accidentally, and there is an acute trauma period, potentially a crucial time to manage the pathology in a better condition. Current understanding showed favorable effects of miR-21 overexpression in a preclinical setting. Considering recent successful RNA-based therapeutics worldwide, it is worth exploring clinically. In this context, RNA-containing particles can be delivered using a direct administration approach.
The role of miR-21 in axon regeneration is unknown and should be more explicit to be helpful for a (pre)clinical investigation; however, the use of miR-21 as a neuroprotective along with other factors is suggested, for instance, inside a nanostructured scaffold can be mounted on the injury site-during the acute phase- to cure the consequences of the incident.
Notably, exercise, a therapeutic strategy commonly applied in the rehabilitation path of SCI patients, affects miR-21 expression at the nervous system levels. Therefore, it would be possible to enhance this effect by optimizing the rehabilitation program.
Last but not least, miR-21 is a crucial regulator of inflammatory conditions favoring metabolic resolution of the inflammatory milieu in the neural context. However, the effector target(s) and the microenvironment where miR-21 will be involved can revert the goal. The preclinical studies addressing efficacy and safety will essentially answer this doubt.
Acknowledgements
We express our sincere gratitude for the Vice Chancellor’s support for Research, Mashhad University of Medical Sciences, Iran. AMM received support from funds from the Italian health ministry, Ricerca Corrente program, to IRCCS Istituto Ortopedico Galeazzi.
Abbreviations
- ACT
Activating protein kinase B
- Ang-1
Angiopoietin-1
- BMP
Bone morphogenetic protein
- CCL3
C–C motif chemokine ligand 3
- CNS
Central nervous system
- eNOS
Endothelial nitric oxide synthase
- GSK-3β
Glycogen synthase kinase-3 beta
- IL
Interleukin
- miR
MicroRNA
- MMPs
Metalloproteinases
- mTOR
Mammalian target of rapamycin
- NF-κB
Nuclear factor kappa B
- NSPCs
Neural stem/progenitor cells
- PDCD4
Programmed cell death protein 4
- PI3K
Phosphoinositide-3-kinase
- PTEN
Phosphatase and tensin homolog
- RANKL
Receptor activator of nuclear factor kappa-Β ligand
- SCI
Spinal cord injury
- SCMECs
Spinal cord microvascular endothelial cells
- TBI
Traumatic brain injury
- TGF
Transforming growth factor
- TIMP3
Tissue inhibitor of metalloproteinase-3
- TLR
Toll-like receptor
- TNF
Tumor necrosis factor
- VEGF
Vascular endothelial growth factor
- VMP-1
Vacuole membrane protein-1
Author contributions
SHR, SE, GL, and AE contributed to the drafting of the manuscript. AMM and AM contributed to overall conceptual design, drafting and final edits and approval. All authors read and approved the final manuscript.
Funding
This work was supported by Mashhad University of medical sciences and the Italian health ministry, the Ricerca Corrente program, to IRCCS Istituto Ortopedico Galeazzi.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;22(10):282. doi: 10.3389/fneur.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum A, Yazid MD, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A, Ismail OHR, Athi Kumar RK, Lokanathan Y. Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci. 2020;21(20):7533. doi: 10.3390/ijms21207533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao TH, Miao W, Han JH, Yin M, Yan Y, Wang WW, Zhu YH. Spontaneous running wheel improves cognitive functions of mouse associated with miRNA expressional alteration in hippocampus following traumatic brain injury. J Mol Neurosci. 2014;54(4):622–629. doi: 10.1007/s12031-014-0344-1. [DOI] [PubMed] [Google Scholar]
- Bhalala OG, Pan L, Sahni V, McGuire TL, Gruner K, Tourtellotte WG, Kessler JA. microRNA-21 regulates astrocytic response following spinal cord injury. J Neurosci. 2012;32(50):17935–17947. doi: 10.1523/JNEUROSCI.3860-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Chung WH, Do SH, Lee JH, Kim HY. Up-regulation of Micrornas-21 and -223 in a Sprague-Dawley Rat model of traumatic spinal cord injury. Brain Sci. 2020;10(3):141. doi: 10.3390/brainsci10030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté MP, Azzam GA, Lemay MA, Zhukareva V, Houlé JD. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J Neurotrauma. 2011;28:299–309. doi: 10.1089/neu.2010.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza RF, Woodhead JST, Zeng N, Blenkiron C, Merry TL, Cameron-Smith D, Mitchell CJ. Circulatory exosomal miRNA following intense exercise is unrelated to muscle and plasma miRNA abundances. Am J Physiol Endocrinol Metab. 2018;315(4):E723–E733. doi: 10.1152/ajpendo.00138.2018. [DOI] [PubMed] [Google Scholar]
- Donia SA, Allison DJ, Gammage KL, Ditor DS. The effects of acute aerobic exercise on mood and inflammation in individuals with multiple sclerosis and incomplete spinal cord injury. NeuroRehabilitation. 2019;45(1):117–124. doi: 10.3233/NRE-192773. [DOI] [PubMed] [Google Scholar]
- Gao X, Li X, Qian C, Li F, Zhang Y, Dang L, Xiao X, Liu F, Li H, Zhang X. miR-21 functions oppositely in proliferation and differentiation of neural stem/precursor cells via regulating AKT and GSK-3β. Cell Mol Biol (noisy-Le-Grand) 2016;62(12):144–149. doi: 10.14715/cmb/2016.62.12.24. [DOI] [PubMed] [Google Scholar]
- Gaudet AD, Fonken LK, Watkins LR, Nelson RJ, Popovich PG. MicroRNAs: roles in regulating neuroinflammation. Neuroscientist. 2018;24(3):221–245. doi: 10.1177/1073858417721150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge XT, Lei P, Wang HC, Zhang AL, Han ZL, Chen X, Li SH, Jiang RC, Kang CS, Zhang JN. miR-21 improves the neurological outcome after traumatic brain injury in rats. Sci Rep. 2014;24(4):6718. doi: 10.1038/srep06718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausott B, Klimaschewski L. Sprouty2—a novel therapeutic target in the nervous system? Mol Neurobiol. 2019;56:3897–3903. doi: 10.1007/s12035-018-1338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak M, Zlamal F, Iliev R, Kucera J, Cacek J, Svobodova L, Hlavonova Z, Kalina T, Slaby O, Bienertova-Vasku J. Exercise-induced circulating microRNA changes in athletes in various training scenarios. PLoS ONE. 2018;13(1):e0191060. doi: 10.1371/journal.pone.0191060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Ni S, Cao Y, Zhang T, Wu T, Yin X, Lang Y, Lu H. The angiogenic effect of microRNA-21 targeting TIMP3 through the regulation of MMP2 and MMP9. PLoS ONE. 2016;11(2):e0149537. doi: 10.1371/journal.pone.0149537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JZ, Huang JH, Zeng L, Wang G, Cao M, Lu HB. Anti-apoptotic effect of microRNA-21 after contusion spinal cord injury in rats. J Neurotrauma. 2013;30(15):1349–1360. doi: 10.1089/neu.2012.2748.PMID:23647386;PMCID:PMC3727528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenike AE, Halushka MK. miR-21: a non-specific biomarker of all maladies. Biomark Res. 2021;9(1):18. doi: 10.1186/s40364-021-00272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Jiao J, Cheng C, Shao J. MicroRNA-21 in the pathogenesis of traumatic brain injury. Neurochem Res. 2018;43(10):1863–1868. doi: 10.1007/s11064-018-2602-z. [DOI] [PubMed] [Google Scholar]
- Ji W, Jiang W, Li M, Li J, Li Z. miR-21 deficiency contributes to the impaired protective effects of obese rat mesenchymal stem cell-derived exosomes against spinal cord injury. Biochimie. 2019;167:171–178. doi: 10.1016/j.biochi.2019.10.002. [DOI] [PubMed] [Google Scholar]
- Jin LY, Li J, Wang KF, Xia WW, Zhu ZQ, Wang CR, Li XF, Liu HY. Blood-spinal cord barrier in spinal cord injury: a review. J Neurotrauma. 2021;38(9):1203–1224. doi: 10.1089/neu.2020.7413. [DOI] [PubMed] [Google Scholar]
- Jung SY, Kim DY, Yune TY, Shin DH, Baek SB, Kim CJ. Treadmill exercise reduces spinal cord injury-induced apoptosis by activating the PI3K/Akt pathway in rats. Exp Ther Med. 2014;7(3):587–593. doi: 10.3892/etm.2013.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Li Z, Zhi Z, Wang S, Xu G. miR-21 derived from the exosomes of MSCs regulates the death and differentiation of neurons in patients with spinal cord injury. Gene Ther. 2019;26(12):491–503. doi: 10.1038/s41434-019-0101-8. [DOI] [PubMed] [Google Scholar]
- Kar AN, Lee SJ, Sahoo PK, Thames E, Yoo S, Houle JD, Twiss JL. MicroRNAs 21 and 199a-3p Regulate Axon Growth Potential through Modulation of Pten and mTor mRNAs. eNeuro. 2021;8(4). 10.1523/ENEURO.0155-21.2021. (PMID: 34326064; PMCID: PMC8362682). [DOI] [PMC free article] [PubMed]
- Katoh H, Yokota K, Fehlings MG. Regeneration of spinal cord connectivity through stem cell transplantation and biomaterial scaffolds. Front Cell Neurosci. 2019;6(13):248. doi: 10.3389/fncel.2019.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian Y, Wehmeier UF, Wahl P, Mester J, Hilberg T, Sperlich B. Acute response of circulating vascular regulating MicroRNAs during and after high-intensity and high-volume cycling in children. Front Physiol. 2016;7:92. doi: 10.3389/fphys.2016.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou X, Chen D, Chen N. The regulation of microRNAs in Alzheimer’s disease. Front Neurol. 2020;11:288. doi: 10.3389/fneur.2020.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal-Filho MB. Spinal cord injury: from inflammation to glial scar. Surg Neurol Int. 2011;2:112. doi: 10.4103/2152-7806.83732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Jiang WT, Li J, Ji WC. Exercise protects against spinal cord injury through miR-21-mediated suppression of PDCD4. Am J Transl Res. 2020;12(9):5708–5718. [PMC free article] [PubMed] [Google Scholar]
- Liu G, Detloff MR, Miller KN, Santi L, Houlé JD. Exercise modulates microRNAs that affect the PTEN/mTOR pathway in rats after spinal cord injury. Exp Neurol. 2012;233:447–456. doi: 10.1016/j.expneurol.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wang W, Wang S, Xie W, Li H, Ning B. microRNA-21 regulates astrocytic reaction post-acute phase of spinal cord injury through modulating TGF-β signaling. Aging (albany NY) 2018;10(6):1474–1488. doi: 10.18632/aging.101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang S, Huang Y, Sun L. miR-21 protects neonatal rats from hypoxic-ischemic brain damage by targeting CCL3. Apoptosis. 2020;25(3–4):275–289. doi: 10.1007/s10495-020-01596-3. [DOI] [PubMed] [Google Scholar]
- Lv X, Liang J, Wang Z. miR-21–5p reduces apoptosis and inflammation in rats with spinal cord injury through PI3K/AKT pathway. Panminerva Med. 2020 doi: 10.23736/S0031-0808.20.03974-9. [DOI] [PubMed] [Google Scholar]
- Ma S, Zhang A, Li X, Zhang S, Liu S, Zhao H, Wu S, Chen L, Ma C, Zhao H. miR-21-5p regulates extracellular matrix degradation and angiogenesis in TMJOA by targeting Spry1. Arthritis Res Ther. 2020;22(1):99. doi: 10.1186/s13075-020-2145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvandi AM, Shahba S, Mohammadipour A, Rastegar-Moghaddam SH, Abudayyak M. Cell and molecular toxicity of lanthanum nanoparticles: are there possible risks to humans? Nanotoxicology. 2021;15(7):951–972. doi: 10.1080/17435390.2021.1940340. [DOI] [PubMed] [Google Scholar]
- Martinez B, Peplow PV. MicroRNAs as diagnostic markers and therapeutic targets for traumatic brain injury. Neural Regen Res. 2017;12(11):1749–1761. doi: 10.4103/1673-5374.219025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell LM, Munson JB, Arvanian VL. Neurotrophins and synaptic plasticity in the mammalian spinal cord. J Physiol. 2001;533:91–97. doi: 10.1111/j.1469-7793.2001.0091b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadipour A, Abudayyak M. Hippocampal toxicity of metal base nanoparticles. Is there a relationship between nanoparticles and psychiatric disorders? Rev Environ Health. 2021 doi: 10.1515/reveh-2021-0006. [DOI] [PubMed] [Google Scholar]
- Mohammadipour A, Haghir H, Ebrahimzadeh BA. A link between nanoparticles and Parkinson’s disease. Which nanoparticles are most harmful? Rev Environ Health. 2020;35(4):545–556. doi: 10.1515/reveh-2020-0043. [DOI] [PubMed] [Google Scholar]
- Nash MS, Farkas GJ, Tiozzo E, Gater DR. Exercise to mitigate cardiometabolic disorders after spinal cord injury. Curr Opin Pharmacol. 2022;62:4–11. doi: 10.1016/j.coph.2021.10.004. [DOI] [PubMed] [Google Scholar]
- Ning B, Gao L, Liu RH, Liu Y, Zhang NS, Chen ZY. microRNAs in spinal cord injury: potential roles and therapeutic implications. Int J Biol Sci. 2014;10(9):997–1006. doi: 10.7150/ijbs.9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelisch N, Rosas Almanza J, Stehlik KE, Aperi BV, Kroner A. CCL3 contributes to secondary damage after spinal cord injury. J Neuroinflamm. 2020;17(1):362. doi: 10.1186/s12974-020-02037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastegar-Moghaddama SH, Ebrahimzadeh-Bideskan A, Shahba S, Malvandi AM, Mohammadipour A. Roles of the miR-155 in neuroinflammation and neurological disorders: a potent biological and therapeutic target. Cell Mol Neurobiol. 2022 doi: 10.1007/s10571-022-01200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastegar-Moghaddamb SH, Ebrahimzadeh-Bideskan A, Shahba S, Malvandi AM, Mohammadipour A. MicroRNA-22: a novel and potent biological therapeutics in neurological disorders. Mol Neurobiol. 2022;59(5):2694–2701. doi: 10.1007/s12035-022-02769-8. [DOI] [PubMed] [Google Scholar]
- Rong Y, Liu W, Wang J, Fan J, Luo Y, Li L, Kong F, Chen J, Tang P, Cai W. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019;10(5):340. doi: 10.1038/s41419-019-1571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samandari R, Hassanpour-Ezatti M, Fakhri S, Abbaszadeh F, Jorjani M. Sex differences and role of gonadal hormones on glutamate levelafter spinal cord injury in rats: a microdialysis study. Basic Clin Neurosci. 2019;10(3):225–234. doi: 10.32598/bcn.9.10.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholpa NE, Schnellmann RG. Mitochondrial-Based therapeutics for the treatment of spinal cord injury: mitochondrial biogenesis as a potential pharmacological target. J Pharmacol Exp Ther. 2017;363(3):303–313. doi: 10.1124/jpet.117.244806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira IR, Palazzo RP, Cechinel LR. Circulating extracellular vesicles delivering beneficial cargo as key players in exercise effects. Free Radic Biol Med. 2021;172:273–285. doi: 10.1016/j.freeradbiomed.2021.06.007. [DOI] [PubMed] [Google Scholar]
- Slota JA, Booth SA. MicroRNAs in neuroinflammation: implications in disease pathogenesis, biomarker discovery and therapeutic applications. Non-Coding RNA. 2019;5(2):35. doi: 10.3390/ncrna5020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Tian X, Schekman R. Extracellular vesicles from neurons promote neural induction of stem cells through cyclin D1. J Cell Biol. 2021;220(9):e202101075. doi: 10.1083/jcb.202101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Chen Z, Du H, Liu R, Wang W, Li H, Ning B. Silencing miR-21 induces polarization of astrocytes to the A2 phenotype and improves the formation of synapses by targeting glypican 6 via the signal transducer and activator of transcription-3 pathway after acute ischemic spinal cord injury. FASEB J. 2019;33(10):10859–10871. doi: 10.1096/fj.201900743R. [DOI] [PubMed] [Google Scholar]
- Surina S, Fontanella RA, Scisciola L, Marfella R, Paolisso G, Barbieri M. miR-21 in human cardiomyopathies. Front Cardiovasc Med. 2021;27(8):767064. doi: 10.3389/fcvm.2021.767064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutor TW, Kura J, Mattingly AJ, Otzel DM, Yarrow JF. The effects of exercise and activity-based physical therapy on bone after spinal cord injury. Int J Mol Sci. 2022;23(2):608. doi: 10.3390/ijms23020608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonacci A, Bagnato G, Pandolfo G, Billeci L, Sansone F, Conte R, Gangemi S. MicroRNA cross-involvement in autism spectrum disorders and atopic dermatitis: a literature review. J Clin Med. 2019;8(1):88. doi: 10.3390/jcm8010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van DenHauwe L, Sundgren PC, Flanders AE. Spinal trauma and spinal cord injury (SCI) In: Hodler J, Kubik-Huch RA, von Schulthess GK, editors. Diseases of the brain, head and neck, spine 2020–2023: diagnostic imaging (Chapter 19) Cham: Springer; 2020. [Google Scholar]
- Vasiliadis AV, Zafeiridis A, Dipla K, Galanis N, Chatzidimitriou D, Kyparos A, Nikolaidis MG, Vrabas IS. Circulating angiogenic biomolecules at rest and in response to upper-limb exercise in individuals with spinal cord injury. J Spinal Cord Med. 2014;37(2):226–232. doi: 10.1179/2045772313Y.0000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl P, Wehmeier UF, Jansen FJ, Kilian Y, Bloch W, Werner N, Mester J, Hilberg T. Acute effects of different exercise protocols on the circulating vascular microRNAs -16, -21, and -126 in TrainedSubjects. Front Physiol. 2016 doi: 10.3389/fphys.2016.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zhao C, Li J, Li Y, Liu Y, Dong H, Wang D, Zhao B, Zhang X, Wang S, Cui F, Li H, He X, Qin J. Notch1 promotes mouse spinal neural stem and progenitor cells proliferation via p-p38-pax6 induced cyclin D1 activation. Exp Cell Res. 2018;373(1–2):80–90. doi: 10.1016/j.yexcr.2018.09.025. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhao Y, Tang Y, Li F, Chen X. The role of lncRNA-MEG/miR-21–5p/PDCD4 axis in spinal cord injury. Am J Transl Res. 2021;13(2):646–658. [PMC free article] [PubMed] [Google Scholar]
- Yang T, Dai Y, Chen G, Cui S. Dissecting the dual role of the glial scar and scar-forming astrocytes in spinal cord injury. Front Cell Neurosci. 2020;3(14):78. doi: 10.3389/fncel.2020.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Cao X, Yu B. Revascularization after traumatic spinal cord injury. Front Physiol. 2021;30(12):631500. doi: 10.3389/fphys.2021.631500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Edgerton VR, Gómez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005;193:411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Ying X, Xie Q, Yu X, Li S, Wu Q, Chen X, Yue J, Zhou K, Tu W, Jiang S. Water treadmill training protects the integrity of the blood-spinal cord barrier following SCI via the BDNF/TrkB-CREB signalling pathway. Neurochem Int. 2021;143:104945. doi: 10.1016/j.neuint.2020.104945. [DOI] [PubMed] [Google Scholar]
- Zhang WM, Zhang ZR, Yang XT, Zhang YG, Gao YS. Overexpression of miR-21 promotes neural stem cell proliferation and neural differentiation via the Wnt/β-catenin signaling pathway in vitro. Mol Med Rep. 2018;17(1):330–335. doi: 10.3892/mmr.2017.7856. [DOI] [PubMed] [Google Scholar]
- Zhang T, Ni S, Luo Z, Lang Y, Hu J, Lu H. The protective effect of microRNA-21 in neurons after spinal cord injury. Spinal Cord. 2019;57(2):141–149. doi: 10.1038/s41393-018-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong D, Cao Y, Li CJ, Li M, Rong ZJ, Jiang L, Guo Z, Lu HB, Hu JZ. Neural stem cell-derived exosomes facilitate spinal cord functional recovery after injury by promoting angiogenesis. Exp Biol Med (maywood) 2020;245(1):54–65. doi: 10.1177/1535370219895491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Su L, Duan X, Chen X, Hays A, Upadhyayula S, Shivde J, Wang H, Li Y, Huang D, Liang S. MicroRNA-21 down-regulates inflammation and inhibits periodontitis. Mol Immunol. 2018;101:608–614. doi: 10.1016/j.molimm.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.