Abstract
The messenger RNA (mRNA) vaccines for COVID-19, Pfizer-BioNTech and Moderna, were authorized in the US on an emergency basis in December of 2020. The rapid distribution of these therapeutics around the country and the world led to millions of people being vaccinated in a short time span, an action that decreased hospitalization and death but also heightened the concerns about adverse effects and drug-vaccine interactions. The COVID-19 mRNA vaccines are of particular interest as they form the vanguard of a range of other mRNA therapeutics that are currently in the development pipeline, focusing both on infectious diseases as well as oncological applications. The Vaccine Adverse Event Reporting System (VAERS) has gained additional attention during the COVID-19 pandemic, specifically regarding the rollout of mRNA therapeutics. However, for VAERS, absence of a reporting platform for drug-vaccine interactions left these events poorly defined. For example, chemotherapy, anticonvulsants, and antimalarials were documented to interfere with the mRNA vaccines, but much less is known about the other drugs that could interact with these therapeutics, causing adverse events or decreased efficacy. In addition, SARS-CoV-2 exploitation of host cytochrome P450 enzymes, reported in COVID-19 critical illness, highlights viral interference with drug metabolism. For example, patients with severe psychiatric illness (SPI) in treatment with clozapine often displayed elevated drug levels, emphasizing drug-vaccine interaction.
Keywords: LNP, psychotropic drugs, cell-cell fusion, PEGylated lipids, DSPC, ionizable lipids, cholesterol analogs
1 Introduction
In 2021, the Centers for Disease Control and Prevention prioritized vaccination for mentally ill individuals as psychiatric illness was added to the list of COVID-19 risk factors (Mazereel et al., 2021). Currently, there are very few studies on mRNA vaccine efficacy in patients with SPI in treatment with psychotropic drugs. However, increased breakthrough infections and limited vaccine responses were reported by a recent epidemiological study on veterans with SPI, highlighting possible drug-vaccine interaction (Nishimi et al., 2022). This study is in line with earlier data, showing that, in general, patients with SPI exhibit suboptimal vaccine effectiveness, a phenomenon also documented in the geriatric population (Solomon et al., 1970; Hussar et al., 1971; Della Bella et al., 2007; Derhovanessian and Pawelec, 2012; Wang et al., 2016). Indeed, immunological similarities, but also differences, exist between the SPI patients and older individuals. For example, persons with SPI exhibit a shorter-than-average lifespan and high comorbidity with age-related diseases, implicating premature cellular senescence in this pathology (Lindqvist et al., 2015; Lee et al., 2021; Pousa et al., 2021). In addition, SPIs were associated with lower counts of regulatory T cells (Tregs) that are often reversed by the treatment with psychotropic drugs (Hwang et al., 2009; Laursen, 2011; Papanastasiou et al., 2011; Kelly et al., 2018; Solana et al., 2018; Corsi-Zuelli et al., 2021). On the other hand, unlike older individuals, SPI patients display an increased number of natural killer cell (NKC) that are unaffected by the psychotropic drugs, probably explaining the low prevalence of malignancy as well as COVID-19 critical illness in this population (Yovel et al., 2000; Bao et al., 2021; Tarantino et al., 2021). Indeed, immune malfunction may account for both limited vaccine responses and protection from COVID-19 critical illness in medicated SPI patients (Nishimi et al., 2022), (Sfera et al., 2021; Nemani et al., 2022). For example, upregulated NKCs may promptly eliminate not only virus-infected but also mRNA-transfected cells, disrupting translation at the ribosomal level as well as antibody production (Arai et al., 1983; Brieva et al., 1984; Mason et al., 1988). In addition, psychotropic drugs’ anti-inflammatory and immunosuppressant actions may protect against virus-induced “cytokine storm” but at the same time lower immune reactivity necessary for adequate vaccine responses (Gobin et al., 2014; Baumeister et al., 2016; Wei and Hui, 2022).
2 Messenger RNA vaccines
The novel mRNA COVID-19 vaccines were inspired by the similarity between extracellular vesicles (EVs) and liposomes, a characteristic exploited in the treatment of hereditary transthyretin-mediated amyloidosis, a therapy comprised of small interfering ribonucleic acids (siRNAs) embedded in LNPs (Antimisiaris et al., 2018; Urits et al., 2020). Replacing siRNA content with mRNA led to the concept of LNP therapeutics encoding for the SARS-CoV-2 spike (S) protein to elicit neutralizing antibodies against it (Manjunath et al., 2005; Suzuki and Ishihara, 2021). Compared to other methods of exogenous nucleic acid introduction into cells, such as viral vectors, LNPs are better tolerated, although their transfection efficacy is less robust (Settanni et al., 2022).
To effectively deliver the synthetic mRNA to host ribosomes, LNPs must avoid several obstacles, including hydrolysis by extracellular RNases, activation of intracellular immune sensors, and degradation by the enzymes of the endosomal lysosomal system (ELS) (Cullis and Hope, 2017) (Sahay et al., 2013; Maugeri et al., 2019). Modifying and hiding mRNAs in LNPs can overcome the first two barriers, while ionizable lipids SM-102 (Moderna) and ALC-0315 (Pfizer BioNTech) may conquer the last one (Hou et al., 2021).
The mRNA-containing LNPs are comprised of four lipids: 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), PEG, an alternative cholesterol, and ionizable lipids SM-102 or ALC-0315 (Benne et al., 2018; Aldosari et al., 2021). The SM-102, ALC-0315, and the alternative cholesterol are proprietary molecules and have not been revealed up to the present time. However, interrogating siRNA platforms, it is reasonable to conclude that ionizable lipids may resemble DLin-MC3-DMA and that a phytosterol may replace the cholesterol (Tam et al., 2013; Xia et al., 2020).
LNPs enter cells by endocytosis or phagocytosis (immune cell uptake) (Birge et al., 2016) (Battistelli and Falcieri, 2020). Entry by the endocytic pathway (EP) can take place via clathrin-dependent or independent routes. Regardless of the ingress modality, LNPs travel from the early to late endosomes and can withstand an environmental pH of 5.5 or higher (Paliwal et al., 2015; Baranov et al., 2019). As exposure to the lysosomal pH of 4.5–5.0, could degrade the LNPs, endosomal-lysosomal system (ELS) exit must take place in the late endosomes (Paliwal et al., 2015). However, as late endosomes can also release their cargo via EVs, LNPs may be expulsed into the extracellular space instead of the cytosol (Gurung et al., 2021). Indeed, studies with split green fluorescence proteins (GFPs) have found that endosomal escape in general is an inefficient process as only about 2% of ELS content reaches the cytosol (Teo et al., 2021). This ratio can be increased with the help of negatively charged phospholipids, such as phosphatidylserine (PS) or analogs. For example, externalized PS (ePS) on ELS membranes generates an electrostatic imbalance between the cationic lipids and anionic phospholipids, enabling LNP to escape (Brock et al., 2019; ur Rehman et al., 2013; Wojnilowicz et al., 2019).
Taken together, the successful delivery of LNP to the host translation machinery depends on overcoming several key obstacles. A major bottleneck that must be successfully negotiated to ensure cargo delivery involves LNP lysosomal evasion as well as the premature expulsion into the extracellular compartment.
2.1 The lipid nanoparticles in the cytosol and potential drug-interactions
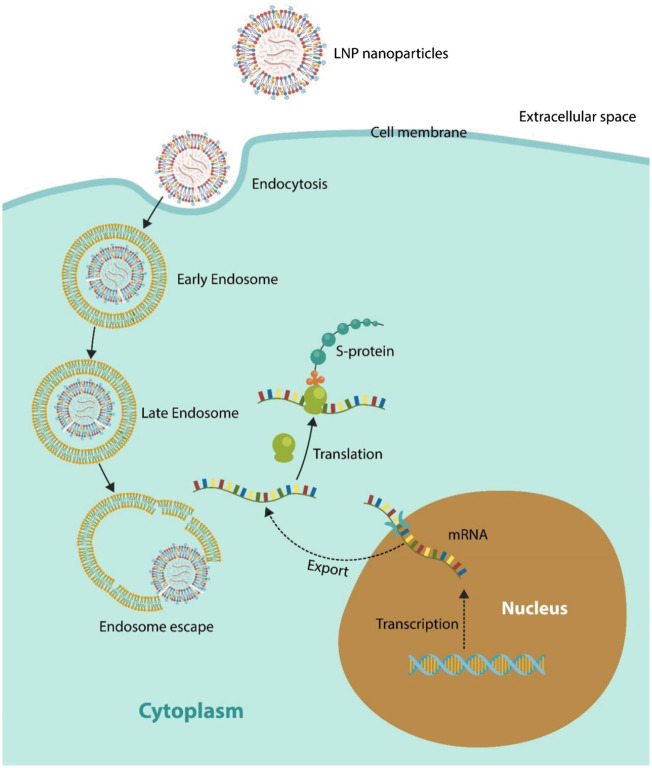
There is a paucity of studies discussing the fate of LNP-mRNA in the cytosol. It is generally assumed that once released from the liposome, mRNA can find its way to the ribosomes where the S antigen is translated (Wu and Li, 2021) (Figure 1). However, the modified vaccine mRNA may be perceived by the cell as defective or damaged, holding back translation by ribosomal stalling (Baker and Coller, 2006; Karamyshev and Karamysheva, 2018; Chandrasekaran et al., 2019; Kow et al., 2021; Brest et al., 2022; Deb et al., 2021). Interestingly, several psychotropic drugs, including aripiprazole, clozapine, and lithium, were demonstrated to alter ribosomal function and protein synthesis, highlighting possible interference with the vaccine mRNA (Bayraktar et al., 2021; Liu et al., 2022).
FIGURE 1.
LNPs enter host cells via endocytosis or phagocytosis (immune cell endocytosis). LNP is trafficked through the ELS, traveling from early to late endosomes. Progressing from late endosome to lysosomes would risk LNP degradation by the hydrolyzing enzymes; therefore, ELS escape must take place in late endosome. However, late endosomes may expulse their cargo into the extracellular compartment via EVs (not shown). This is a major hurdle that LNPs must negotiate. Under ideal circumstances, ribosomes translate the exogenous mRNA into the S protein. For this to occur, it must be assumed that the human translation machinery does not differentiate between endogenous (nucleus-derived) and exogenous mRNA.
Single molecule tracking studies have found that cytosolic mRNA can access cellular cytoskeleton and to travel throughout the cytosol from where it can diffuse into cytoplasmic organelles, including the nucleus (Yamagishi et al., 2013) (Vargas et al., 2005) (Fusco et al., 2003) (Siwaszek et al., 2014). For example, a recent study found that upon entering the host nuclear compartment, Pfizer BioNTech mRNA could be retrotranscribed into DNA by the long interspersed nuclear element-1 (LINE-1), emphasizing vaccine-genome interactions (Zhang et al., 2021; Aldén et al., 2022). Interestingly, upregulated LINE-1, a marker of SPI, can be lowered by psychotropic drugs (via DNA methylation), likely reducing, or averting the transcription of RNA to DNA (Houtepen et al., 2016; Doyle et al., 2017) (Table 1).
TABLE 1.
Psychotropic drugs compound some LNP effects, altering cell entry, endosomal release, and exit of mRNA vaccines and their responses.
EP, endosomal pathway; LNP, lipid nanoparticle; ApoE, apolipoprotein E; PEG, polyethylene glycol; DSPC, 1,2-distearoyl-sn-glycero-3-phosphocholine.
In the following sections, we take a closer look at the four LNP lipids and their interaction with psychotropic drugs.
2.1.1 PEGylated lipids
PEGylated lipids extend the duration of mRNA action and facilitate LNP endocytosis, while lowering aggregation and opsonization during the circulation (Yang and Shen, 2006; Li et al., 2014). However, despite these advantages, PEGylation raises the so-called “PEG dilemma”: prolongation of both LNP uptake and ELS escape, risking vaccine-mRNA degradation by the lysosomal enzymes (Fang et al., 2017). Given that the LNP composition is proprietary, it is unknown how the mRNA-based vaccines overcome the “PEG dilemma” however, linking PEG oxygen to the head group of SM-102 or ALC-0315 is a previously documented solution (Park et al., 2021). In addition, CHARMM-GUI membrane builder (http://www.charmm-gui.org/input/membrane), an in-silico lipid simulation platform, highlights PEG oxygen bond as the likely overcomer of the “PEG dilemma” (Lee et al., 2019).
2.2 Psychotropic drugs and PEGylated lipids
Several classes of psychotropic drugs, including phenothiazines were demonstrated to inhibit the EP by binding to adaptor protein 2 (AP2), a cell membrane protein that plays a crucial role in clathrin-mediated endocytosis (CME) (Hussar et al., 1971; Inoue et al., 2007; Kovtun et al., 2020). As CME is a major LNP intake mechanism, psychotropic drugs-inhibited AP2 likely disrupt vaccine transfection (Wang et al., 1993; Chang et al., 2014). Moreover, psychotropic medications were demonstrated to accumulate in lysosomes (lysosomotropism) and increase the ELS pH, likely delaying LNP escape thus, lowering the vaccine efficacy (Daniel, 2003; Canfrán-Duque et al., 2016). Along this line, the antipsychotic drug pimozide was shown to disrupt the LNP exit from ELS, emphasizing a drug-vaccine interaction that can inactivate the mRNA vaccines (Popova et al., 2013; Meyer et al., 2021). Interestingly, PEGylated liposomes were previously shown to induce cytochrome P450, especially CYP3A1, CYP2C6, and CYP1A2, causing accelerated blood clearance (ABC), a phenomenon that reduces the efficacy of PEGylated nanocarriers, emphasizing a less discussed LNP weakness (Su et al., 2018; Liu et al., 2020).
2.2.2 1,2-distearoyl-sn-glycero-3-phosphocholine
DSPC is a non-pyrogenic, neutral phospholipid that plays a key role in cell apoptosis and immune regulation (Chaurio et al., 2009). DSPC was added to the LNP to prevent immune detection by the cytosolic sensors, including toll-like receptors (TLRs) and retinoic acid-inducible gene I (RIG-I) (Zhang et al., 2022). DSPC plays a major role in stealthy LNP entry into the cells without alerting the host immune defenses. This is accomplished by altering the lipid asymmetry of plasma membranes, mimicking ePS, a global immunosuppressive signal (Birge et al., 2016). In addition, DSPC increases regulatory T cells (Tregs), further lowering host immune surveillance (Lin et al., 2006; Benne et al., 2020). Tregs upregulation is a double-edged sword as these lymphocytes can lower both virus-mediated inflammation (cytokine storm) and vaccine-evoked neutralizing antibodies, emphasizing that antiviral and pro-viral actions are highly intertwined (Batista-Duharte et al., 2021; Galván-Peña et al., 2021).
2.3 Potential interaction with psychotropic drugs
Psychotropic drugs may lower the robustness of vaccine responses by direct mechanisms, interaction with LNPs, or indirectly by the anti-inflammatory and anti-immunogenic properties of these agents (Baumeister et al., 2016), (Stapel et al., 2018; Lin et al., 2022). For example, the immunosuppressant properties of clozapine, haloperidol, risperidone, and antidepressant drugs are well-established, emphasizing likely interference with the vaccine-associated immunogenicity (Gobin et al., 2014) (Leykin et al., 1997; May et al., 2019; Ponsford et al., 2019). Indeed, biophysical studies show that antipsychotic drugs can insert themselves between the lipid molecules of plasma membrane, triggering anti-inflammatory responses that can impair vaccine efficacy (Jutila et al., 2001; Al-Amin et al., 2013; May et al., 2019; Pandurangi and Buckley, 2020). Moreover, leukopenia and decreased immunoglobulins, well-established properties of psychotropic drugs, may directly lower vaccine-elicited neutralizing antibodies (Ponsford et al., 2019) (Sherman et al., 1986; Goldsmith, 2002; Lozano et al., 2015). Interestingly, chlorpromazine, was found to also inhibit mRNA expression in human thymocytes, likely disrupting vaccine efficacy at the translation level (Schleuning et al., 1989) (Ficarra et al., 2016). Vaccine effectiveness can be further decreased by antipsychotic drugs-upregulated Tregs, an established defense mechanism against autoimmunity (Kelly et al., 2018) (Himmerich et al., 2010).
2.3.3 Ionizable lipids
Ionizable lipids added to the LNP, SM-102 and ALC-0315, are pH-sensitive molecules, positively charged in an acidic environment and neutral at physiological pH (Paloncýová et al., 2021). This characteristic supports protonation, an event that facilitates LNP escape from the late endosomes (Maugeri et al., 2019), (Gao and Huang, 1996; Han et al., 2021).
Ionizable lipids likely contain synthetic polyamines as amine groups accumulate in the ELS, increasing membrane permeability that in turn promotes LNP transport into the cytosol (Figure 1) (Soulet et al., 2004; Goldman et al., 2009; Jiang et al., 2020). In addition, as polyamines play a key role in mRNA translation and stability, they may be key components of SM-102/ALC-0315 lipids (Li et al., 2017). Among the polyamines, spermine has demonstrated superior cellular uptake and endosomal escape ability, suggesting that LNPs may contain this molecule (Ding et al., 2021). In addition, spermine was shown to increase vaccine efficacy by upregulating autophagy in human T cells and enhancing antigen responses (Merkley et al., 2018; Alsaleh et al., 2020).
Novel studies attributed antipsychotic properties to spermine, while its dysfunction was associated with the pathogenesis of SPI, particularly suicidal behavior (Squassina et al., 2013; Yadav et al., 2018). Moreover, as spermine plays a major role in male and female reproductive physiology, disruption of this polyamine may contribute to infertility and decreased birth rates (Lefèvre et al., 2011). Indeed, epidemiological studies from several countries have reported lower 2021 natality rates compared to the previous year, as demonstrated by Italy (−9.1%), Spain (−8.4%), Portugal (−6.6%), and New York (−19.8%) that might reflect dysfunctional polyamine signaling (Aassve et al., 2021; McLaren et al., 2021). Although it is difficult to trace the source of any potential infertility to mRNA vaccines as dysfunctional polyamines were also documented in SARS-CoV-2 infection and several psychiatric disorders, it is important to investigate these biomolecules further (Fiori and Turecki, 2008; Zhao et al., 2008; Gross and Turecki, 2013; Bourgin et al., 2021; Firpo et al., 2021).
2.4 Potential interference with psychotropic drugs
Several psychotropic drugs were shown to alter the integrity of membrane phospholipids, suggesting possible interference with the LNP ingress and ELS escape (Daniel, 2003). In addition, psychotropic drugs were demonstrated to alkalinize the ELS that in turn could disrupt the pH-dependent polyamines (Canfrán-Duque et al., 2016). Moreover, accumulating evidence suggests that polyamines, including putrescine, spermidine, and spermine, are not only involved in the pathogenesis of SPI but are also modulated by the antipsychotic drugs, suggesting possible interference with the mRNA vaccines (Squassina et al., 2013) (Fiori and Turecki, 2008). For example, spermidine, a spermine derivative, was found protective of the GABAergic and dopaminergic systems, suggesting that LNPs may interfere with this signaling (Yadav et al., 2018). This is significant as dopamine is not only involved in psychiatric disorders but is also an established fertility promoter, and dopamine agonists are frequently prescribed as part of assisted reproduction technology (ART) (Heiczman and Tóth, 1995; Tang et al., 2016).
2.4.1 Cholesterol analog
The cholesterol analog utilized in LNP is likely a phytosterol, as these molecules display high transfection capability by binding to apolipoprotein E (ApoE), followed by rapid endocytosis (Eygeris et al., 2020; Sebastiani et al., 2021). However, phytosterols have a major disadvantage as they suppress phagocytosis, probably limiting the LNP uptake in immune cells and therefore, mRNA translation (Yuan et al., 2019; Guo et al., 2022). In addition, unlike cholesterol, phytosterols cross the blood–brain barrier (BBB) and accumulate in the brain where their oxidation may precipitate the development of neurodegenerative disorders (Gamba et al., 2015; Sharma and Tan, 2021).
Several sterols, including desmosterol, were associated with both major depressive disorder and antidepressant medication, possibly accounting for the rare post-vaccination psychiatric symptoms recorded in VAERs (Cenik et al., 2017) (Balasubramanian et al., 2022; Chen et al., 2022). Moreover, cholesterol and other sterols can interact directly with dopamine transporters (DAT), possibly accounting for the post-vaccination dyskinesia noted in some patients with Parkinson’s disease (Sharma and Tan, 2021) (Jones et al., 2012; Erro et al., 2021).
2.5 Potential interference with psychotropic drugs
Several psychotropic drugs, including clozapine, olanzapine, haloperidol, and imipramine, were shown to up-regulate ApoE, a cholesterol transporter disrupted in SPI, suggesting possible interference with LNP transfection (Barroso et al., 2015; Dean et al., 2003; Digney et al., 2005; Miatmoko et al., 2021; Vik-Mo et al., 2009). As psychotropic medications upregulate the ATP-binding cassette transporter A1 (ABCA1), increasing cholesterol egress from cells, a process that may compromise vaccine efficacy by flushing LNPs into the extracellular compartment prior to mRNA release (Luquain-Costaz et al., 2020). In addition, several psychotropic drugs, including aripiprazole, haloperidol, and trazodone, were reported to increase the levels of cholesterol precursor, desmosterol, that in turn upregulates the expression of cholesterol efflux genes, likely removing LNPs from cells prematurely (Korade et al., 2017).
3 Limitations
This study has potential limitations. Firstly, it refers to a new technology within the clinical standard of care, which even though has a considerable body of literature in the scientific and preparatory phases, it is still developing the breadth of scientific observations from a clinical perspective. Secondly, it is likely that some of the potential drug-immunization interactions in the latest pandemics might be masked by the vaccine escape properties attributed to newly emerging SARS-CoV-2 variants, and as such an even more careful approach of the subject would be required to distinguish these compounding factors of lower than anticipated immune efficacy. Thirdly, it is presumed that some of the above observed interactions would have similarities in the future provision of mRNA therapeutics for non-communicable diseases, such as different cancer types. However, this remains a working hypothesis that requires further testing.
4 Discussion and conclusion
We opine that more studies are needed to assess the interaction between the major classes of psychotropic drugs, including antipsychotics, antidepressants, and mood stabilizers with the mRNA therapeutics. As the polyethylene glycol (PEG) component of lipid nanoparticles (LNPs) increases the permeability of BBB for a short interval, we anticipate that LNPs will be rapidly adopted by neuropsychiatry as vehicles for drug transport and delivery to the select CNS networks. For this reason, it is important to develop a VAERS-like system for recording the interaction of psychotropic drugs with current and future mRNA therapeutics.
Up to date, the exact LNP composition has not been released therefore, we analyze earlier data and virtual screening research, attempting to “fill-in” the blanks. For this reason, our assumptions and evidence may seem circumstantial, however, we believe, provide a foundation worth of further investigation.
LNPs are crucial for transporting exogenous mRNA to the host translational machinery where the S antigen is synthesized, eliciting neutralizing antibodies. The four LNP lipids guide the mRNA-loaded particle through the maze of extra and intracellular compartments, releasing its cargo into the cytosol. However, several bottlenecks on this journey, including ingress failure, delayed ELS escape, or premature expulsion from cells, may lower vaccine efficacy.
Treatment with psychotropic drugs may decrease the mRNA vaccine effectiveness by lowering inflammation/immunogenicity, inhibiting virus/LNP endocytosis, delaying ELS escape, or directly downregulating neutralizing antibodies.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/WHO, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/WHO.
References
- Aassve A., Cavalli N., Mencarini L., Plach S., Sanders S. (2021). Early assessment of the relationship between the COVID-19 pandemic and births in high-income countries. Proc. Natl. Acad. Sci. U. S. A. 118 (36), e2105709118. 10.1073/pnas.2105709118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Amin M. M., Nasir Uddin M. M., Mahmud Reza H. (2013). Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin. Psychopharmacol. Neurosci. 11 (3), 144–151. Epub 2013 Dec 24. PMID: 24465251. 10.9758/cpn.2013.11.3.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldén M., Olofsson Falla F., Yang D., Barghouth M., Luan C., Rasmussen M., et al. (2022). Intracellular reverse transcription of pfizer BioNTech COVID-19 mRNA vaccine BNT162b2 in vitro in human liver cell line. Curr. Issues Mol. Biol. 44 (3), 1115–1126. 10.3390/cimb44030073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldosari B. N., Alfagih I. M., Almurshedi A. S. (2021). Lipid nanoparticles as delivery systems for RNA-based vaccines. Pharmaceutics 13 (2), 206. 10.3390/pharmaceutics13020206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaleh G., Panse I., Swadling L., Zhang H., Richter F. C., Meyer A., et al. (2020). Autophagy in T cells from aged donors is maintained by spermidine and correlates with function and vaccine responses. Elife 9, e57950. 10.7554/eLife.57950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antimisiaris S. G., Mourtas S., Marazioti A. (2018). Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics 10 (4), 218. 10.3390/pharmaceutics10040218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S., Yamamoto H., Itoh K., Kumagai K. (1983). Suppressive effect of human natural killer cells on pokeweed mitogen-induced B cell differentiation. J. Immunol. 131 (2), 651–657. PMID: 6223088. [PubMed] [Google Scholar]
- Baker K. E., Coller J. (2006). The many routes to regulating mRNA translation. Genome Biol. 7 (12), 332. PMID: 17176455; PMCID: PMC1794424. 10.1186/gb-2006-7-12-332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian I., Faheem A., Padhy S. K., Menon V. (2022). Psychiatric adverse reactions to COVID-19 vaccines: A rapid review of published case reports. Asian J. Psychiatr. 71, 103129. 10.1016/j.ajp.2022.103129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao C., Tao X., Cui W., Hao Y., Zheng S., Yi B., et al. (2021). Natural killer cells associated with SARS-CoV-2 viral RNA shedding, antibody response and mortality in COVID-19 patients. Exp. Hematol. Oncol. 10, 5. 10.1186/s40164-021-00199-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranov M. V., Olea R. A., van den Bogaart G. (2019). Chasing uptake: Super-resolution microscopy in endocytosis and phagocytosis. Trends Cell Biol. 29 (9), 727–739. 10.1016/j.tcb.2019.05.006 [DOI] [PubMed] [Google Scholar]
- Barroso R. P., Basso L. G., Costa-Filho A. J. (2015). Interactions of the antimalarial amodiaquine with lipid model membranes. Chem. Phys. Lipids 186, 68–78. 10.1016/j.chemphyslip.2014.12.003 [DOI] [PubMed] [Google Scholar]
- Batista-Duharte A., Pera A., Aliño S. F., Solana R. (2021). Regulatory T cells and vaccine effectiveness in older adults. Challenges and prospects. Int. Immunopharmacol. 96, 107761. 10.1016/j.intimp.2021.107761 [DOI] [PubMed] [Google Scholar]
- Battistelli M., Falcieri E. (2020). Apoptotic bodies: Particular extracellular vesicles involved in intercellular communication. Biol. (Basel) 9 (1), 21. 10.3390/biology9010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D., Ciufolini S., Mondelli V. (2016). Effects of psychotropic drugs on inflammation: consequence or mediator of therapeutic effects in psychiatric treatment? Psychopharmacol. Berl. 233 (9), 1575–1589. 10.1007/s00213-015-4044-5 [DOI] [PubMed] [Google Scholar]
- Bayraktar İ., Yalçın N., Demirkan K. (2021). The potential interaction between COVID-19 vaccines and clozapine: A novel approach for clinical trials. Int. J. Clin. Pract. 75 (8), e14441. PMID: 34289643; PMCID: PMC8420459. 10.1111/ijcp.14441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne N., Leboux R. J. T., Glandrup M., van Duijn J., Lozano Vigario F., Neustrup M. A., et al. (2020). Atomic force microscopy measurements of anionic liposomes reveal the effect of liposomal rigidity on antigen-specific regulatory T cell responses. J. Control. Release 318, 246–255. 10.1016/j.jconrel.2019.12.003 [DOI] [PubMed] [Google Scholar]
- Benne N., van Duijn J., Lozano Vigario F., Leboux R. J. T., van Veelen P., Kuiper J., et al. (2018). Anionic 1, 2-distearoyl-sn-glycero-3-phosphoglycerol (DSPG) liposomes induce antigen-specific regulatory T cells and prevent atherosclerosis in mice. J. Control. Release 291, 135–146. 10.1016/j.jconrel.2018.10.028 [DOI] [PubMed] [Google Scholar]
- Birge R. B., Boeltz S., Kumar S., Carlson J., Wanderley J., Calianese D., et al. (2016). Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 23 (6), 962–978. 10.1038/cdd.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgin M., Derosa L., Silva C. A. C., Goubet A. G., Dubuisson A., Danlos F. X., et al. (2021). Circulating acetylated polyamines correlate with Covid-19 severity in cancer patients. Aging (Albany NY) 13 (17), 20860–20885. 10.18632/aging.203525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brest P., Mograbi B., Hofman P., Milano G. (2022). COVID-19 vaccination and cancer immunotherapy: should they stick together? Br. J. Cancer 126 (1), 1–3. 10.1038/s41416-021-01618-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieva J. A., Targan S., Stevens R. H. (1984). NK and T cell subsets regulate antibody production by human in vivo antigen-induced lymphoblastoid B cells. J. Immunol. 132 (2), 611–615. PMID: 6228592. [PubMed] [Google Scholar]
- Brock D. J., Kondow-McConaghy H. M., Hager E. C., Pellois J. P. (2019). Endosomal escape and cytosolic penetration of macromolecules mediated by synthetic delivery agents. Bioconjug. Chem. 30 (2), 293–304. 10.1021/acs.bioconjchem.8b00799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfrán-Duque A., Barrio L. C., Lerma M., de la Peña G., Serna J., Pastor O., et al. (2016). First-Generation antipsychotic haloperidol alters the functionality of the late endosomal/lysosomal compartment in vitro . Int. J. Mol. Sci. 17 (3), 404. 10.3390/ijms17030404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenik B., Cenik C., Snyder M. P., Brown E. S. (2017). Plasma sterols and depressive symptom severity in a population-based cohort. PLoS One 12 (9), e0184382. 10.1371/journal.pone.0184382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran V., Juszkiewicz S., Choi J., Puglisi J. D., Brown A., Shao S., et al. (2019). Mechanism of ribosome stalling during translation of a poly(A) tail. Nat. Struct. Mol. Biol. 26 (12), 1132–1140. 10.1038/s41594-019-0331-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Wu M., Yuan F. (2014). Role of specific endocytic pathways in electrotransfection of cells. Mol. Ther. Methods Clin. Dev. 1, 14058. 10.1038/mtm.2014.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurio R. A., Janko C., Muñoz L. E., Frey B., Herrmann M., Gaipl U. S. (2009). Phospholipids: key players in apoptosis and immune regulation. Molecules 14 (12), 4892–4914. 10.3390/molecules14124892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Aruldass A. R., Cardinal R. N. (2022). Mental health outcomes after SARS-CoV-2 vaccination in the United States: A national cross-sectional study. J. Affect. Disord. 298, 396–399. 10.1016/j.jad.2021.10.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi-Zuelli F., Deakin B., de Lima M. H. F., Qureshi O., Barnes N. M., Upthegrove R., et al. (2021). T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav. Immun. Health 17, 100330. 10.1016/j.bbih.2021.100330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis P. R., Hope M. J. (2017). Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 25 (7), 1467–1475. 10.1016/j.ymthe.2017.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel W. A. (2003). Mechanisms of cellular distribution of psychotropic drugs. Significance for drug action and interactions. Prog. Neuropsychopharmacol. Biol. Psychiatry 27 (1), 65–73. PMID: 12551728. 10.1016/s0278-5846(02)00317-2 [DOI] [PubMed] [Google Scholar]
- Dean B., Laws S. M., Hone E., Taddei K., Scarr E., Thomas E. A., et al. (2003). Increased levels of apolipoprotein E in the frontal cortex of subjects with schizophrenia. Biol. Psychiatry 54 (6), 616–622. 10.1016/s0006-3223(03)00075-1 [DOI] [PubMed] [Google Scholar]
- Deb S., Arrighi S. (2021). Potential effects of COVID-19 on cytochrome P450-mediated drug metabolism and disposition in infected patients. Eur. J. Drug Metab. Pharmacokinet. 46 (2), 185–203. 10.1007/s13318-020-00668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Bella S., Bierti L., Presicce P., Arienti R., Valenti M., Saresella M., et al. (2007). Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin. Immunol. 122 (2), 220–228. 10.1016/j.clim.2006.09.012 [DOI] [PubMed] [Google Scholar]
- Derhovanessian E., Pawelec G. (2012). Vaccination in the elderly. Microb. Biotechnol. 5 (2), 226–232. 10.1111/j.1751-7915.2011.00283.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digney A., Keriakous D., Scarr E., Thomas E., Dean B. (2005). Differential changes in apolipoprotein E in schizophrenia and bipolar I disorder. Biol. Psychiatry 57 (7), 711–715. 10.1016/j.biopsych.2004.12.028 [DOI] [PubMed] [Google Scholar]
- Ding F., Zhang H., Cui J., Li Q., Yang C. (2021). Boosting ionizable lipid nanoparticle-mediated in vivo mRNA delivery through optimization of lipid amine-head groups. Biomater. Sci. 9 (22), 7534–7546. 10.1039/d1bm00866h [DOI] [PubMed] [Google Scholar]
- Doyle G. A., Crist R. C., Karatas E. T., Hammond M. J., Ewing A. D., Ferraro T. N., et al. (2017). Analysis of LINE-1 elements in DNA from postmortem brains of individuals with schizophrenia. Neuropsychopharmacology 42 (13), 2602–2611. 10.1038/npp.2017.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erro R., Buonomo A. R., Barone P., Pellecchia M. T. (2021). Severe dyskinesia after administration of SARS-CoV2 mRNA vaccine in Parkinson's disease. Mov. Disord. 36 (10), 2219. 10.1002/mds.28772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eygeris Y., Patel S., Jozic A., Sahay G. (2020). Deconvoluting lipid nanoparticle structure for messenger RNA delivery. Nano Lett. 20 (6), 4543–4549. 10.1021/acs.nanolett.0c01386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Xue J., Gao S., Lu A., Yang D., Jiang H., et al. (2017). Cleavable PEGylation: a strategy for overcoming the "PEG dilemma" in efficient drug delivery. Drug Deliv. 24, 22–32. 10.1080/10717544.2017.1388451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficarra S., Russo A., Barreca D., Giunta E., Galtieri A., Tellone E. (2016). Short-Term effects of chlorpromazine on oxidative stress in erythrocyte functionality: Activation of metabolism and membrane perturbation. Oxid. Med. Cell. Longev. 2016, 2394130. 10.1155/2016/2394130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori L. M., Turecki G. (2008). Implication of the polyamine system in mental disorders. J. Psychiatry Neurosci. 33 (2), 102–110. PMID: 18330456; PMCID: PMC2265312. [PMC free article] [PubMed] [Google Scholar]
- Firpo M. R., Mastrodomenico V., Hawkins G. M., Prot M., Levillayer L., Gallagher T., et al. (2021). Targeting polyamines inhibits coronavirus infection by reducing cellular attachment and entry. ACS Infect. Dis. 7 (6), 1423–1432. 10.1021/acsinfecdis.0c00491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco D., Accornero N., Lavoie B., Shenoy S. M., Blanchard J. M., Singer R. H., et al. (2003). Single mRNA molecules demonstrate probabilistic movement in living mammalian cells. Curr. Biol. 13 (2), 161–167. 10.1016/s0960-9822(02)01436-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván-Peña S., Leon J., Chowdhary K., Michelson D. A., Vijaykumar B., Yang L., et al. (2021). Profound Treg perturbations correlate with COVID-19 severity. Proc. Natl. Acad. Sci. U. S. A. 118 (37), e2111315118. 10.1073/pnas.2111315118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamba P., Testa G., Gargiulo S., Staurenghi E., Poli G., Leonarduzzi G. (2015). Oxidized cholesterol as the driving force behind the development of Alzheimer's disease. Front. Aging Neurosci. 7, 119. 10.3389/fnagi.2015.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Huang L. (1996). Potentiation of cationic liposome-mediated gene delivery by polycations. Biochemistry 35 (3), 1027–1036. 10.1021/bi952436a [DOI] [PubMed] [Google Scholar]
- Gobin V., Van Steendam K., Denys D., Deforce D. (2014). Selective serotonin reuptake inhibitors as a novel class of immunosuppressants. Int. Immunopharmacol. 20 (1), 148–156. 10.1016/j.intimp.2014.02.030 [DOI] [PubMed] [Google Scholar]
- Goldman S. D., Funk R. S., Rajewski R. A., Krise J. P. (2009). Mechanisms of amine accumulation in, and egress from, lysosomes. Bioanalysis 1 (8), 1445–1459. 10.4155/bio.09.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith S. K. (2002). Haloperidol reduces IgG immunoreactivity in the rat brain. Int. J. Neuropsychopharmacol. 5 (4), 309–313. 10.1017/s146114570200305x [DOI] [PubMed] [Google Scholar]
- Gross J. A., Turecki G. (2013). Suicide and the polyamine system. CNS Neurol. Disord. Drug Targets 12 (7), 980–988. 10.2174/18715273113129990095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S. J., Ma C. G., Hu Y. Y., Bai G., Song Z. J., Cao X. Q. (2022). Solid lipid nanoparticles for phytosterols delivery: The acyl chain number of the glyceride matrix affects the arrangement, stability, and release. Food Chem. 394, 133412. 10.1016/j.foodchem.2022.133412 [DOI] [PubMed] [Google Scholar]
- Gurung S., Perocheau D., Touramanidou L., Baruteau J. (2021). The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 19, 47. 10.1186/s12964-021-00730-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Zhang H., Butowska K., Swingle K. L., Alameh M. G., Weissman D., et al. (2021). An ionizable lipid toolbox for RNA delivery. Nat. Commun. 12, 7233. 10.1038/s41467-021-27493-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiczman A., Tóth M. (1995). Effect of chlorpromazine on the synthesis of neutral lipids and phospholipids from [3H]glycerol in the primordial human placenta. Placenta 16 (4), 347–358. 10.1016/0143-4004(95)90092-6 [DOI] [PubMed] [Google Scholar]
- Himmerich H., Milenović S., Fulda S., Plümäkers B., Sheldrick A. J., Michel T. M., et al. (2010). Regulatory T cells increased while IL-1β decreased during antidepressant therapy. J. Psychiatr. Res. 44 (15), 1052–1057. 10.1016/j.jpsychires.2010.03.005 [DOI] [PubMed] [Google Scholar]
- Hou X., Zaks T., Langer R., Dong Y. (2021). Lipid nanoparticles for mRNA delivery. Nat. Rev. Mat. 6, 1078–1094. 10.1038/s41578-021-00358-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtepen L. C., van Bergen A. H., Vinkers C. H., Boks M. P. (2016). DNA methylation signatures of mood stabilizers and antipsychotics in bipolar disorder. Epigenomics 8 (2), 197–208. 10.2217/epi.15.98 [DOI] [PubMed] [Google Scholar]
- Hussar A. E., Cradle J. L., Beiser S. M. (1971). A study of the immunologic and allergic responsiveness of chronic schizophrenics. Br. J. Psychiatry 118 (542), 91–92. 10.1192/bjp.118.542.91 [DOI] [PubMed] [Google Scholar]
- Hwang K. A., Kim H. R., Kang I. (2009). Aging and human CD4(+) regulatory T cells. Mech. Ageing Dev. 130 (8), 509–517. 10.1016/j.mad.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y., Tanaka N., Tanaka Y., Inoue S., Morita K., Zhuang M., et al. (2007). Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J. Virol. 81 (16), 8722–8729. 10.1128/JVI.00253-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Lu Q., Wang Y., Xu E., Ho A., Singh P., et al. (2020). Quantitating endosomal escape of a library of polymers for mRNA delivery. Nano Lett. 20 (2), 1117–1123. 10.1021/acs.nanolett.9b04426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. T., Zhen J., Reith M. E. (2012). Importance of cholesterol in dopamine transporter function. J. Neurochem. 123 (5), 700–715. 10.1111/jnc.12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutila A., Söderlund T., Pakkanen A. L., Huttunen M., Kinnunen P. K. (2001). Comparison of the effects of clozapine, chlorpromazine, and haloperidol on membrane lateral heterogeneity. Chem. Phys. Lipids 112 (2), 151–163. 10.1016/s0009-3084(01)00175-x [DOI] [PubMed] [Google Scholar]
- Karamyshev A. L., Karamysheva Z. N. (2018). Lost in translation: Ribosome-associated mRNA and protein quality controls. Front. Genet. 9, 431. 10.3389/fgene.2018.00431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. L., Li X., Kilday C., Feldman S., Clark S., Liu F., et al. (2018). Increased circulating regulatory T cells in medicated people with schizophrenia. Psychiatry Res. 269, 517–523. 10.1016/j.psychres.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Ž., Liu W., Warren E. B., Armstrong K., Porter N. A., Konradi C. (2017). Effect of psychotropic drug treatment on sterol metabolism. Schizophr. Res. 187, 74–81. Epub 2017 Feb 12. PMID: 28202290; PMCID: PMC555446. 10.1016/j.schres.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun O., Dickson V. K., Kelly B. T., Owen D. J., Briggs J. A. G. (2020). Architecture of the AP2/clathrin coat on the membranes of clathrin-coated vesicles. Sci. Adv. 6 (30), eaba8381. 10.1126/sciadv.aba8381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow C. S., Hasan S. S. (2021). Potential interactions between COVID-19 vaccines and antiepileptic drugs. Seizure 86, 80–81. 10.1016/j.seizure.2021.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen T. M. (2011). Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr. Res. 131 (1-3), 101–104. 10.1016/j.schres.2011.06.008 [DOI] [PubMed] [Google Scholar]
- Lee J., Patel D. S., Ståhle J., Park S. J., Kern N. R., Kim S.., et al. (2019). CHARMM-GUI membrane builder for complex biological membrane simulations with glycolipids and lipoglycans. J. Chem. Theory Comput. 15 (1), 775–786. 10.1021/acs.jctc.8b01066 [DOI] [PubMed] [Google Scholar]
- Lee S., Yu Y., Trimpert J., Benthani F., Mairhofer M., Richter-Pechanska P.., et al. (2021). Virus-induced senescence is a driver and therapeutic target in COVID-19. Nature 599 (7884), 283–289. 10.1038/s41586-021-03995-1 [DOI] [PubMed] [Google Scholar]
- Lefèvre P. L., Palin M. F., Murphy B. D. (2011). Polyamines on the reproductive landscape. Endocr. Rev. 32 (5), 694–712. Epub 2011 Jul 26. PMID: 21791568. 10.1210/er.2011-0012 [DOI] [PubMed] [Google Scholar]
- Leykin I., Mayer R., Shinitzky M. (1997). Short and long-term immunosuppressive effects of clozapine and haloperidol. Immunopharmacology 37 (1), 75–86. 10.1016/s0162-3109(97)00037-4 [DOI] [PubMed] [Google Scholar]
- Li J., He Y., Wang W., Wu C., Hong C., Hammond P. T. (2017). Polyamine-mediated stoichiometric assembly of ribonucleoproteins for enhanced mRNA delivery. Angew. Chem. Int. Ed. Engl. 56 (44), 13709–13712. 10.1002/anie.201707466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Kröger M., Liu W. K. (2014). Endocytosis of PEGylated nanoparticles accompanied by structural and free energy changes of the grafted polyethylene glycol. Biomaterials 35 (30), 8467–8478. 10.1016/j.biomaterials.2014.06.032 [DOI] [PubMed] [Google Scholar]
- Lin D. Y., Gu Y., Wheeler B., Young H., Holloway S., Sunny S. K., et al. (2022). Effectiveness of covid-19 vaccines over a 9-month period in North Carolina. N. Engl. J. Med. 386 (10), 933–941. 10.1056/NEJMoa2117128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. C., Blanchette C. D., Ratto T. V., Longo M. L. (2006). Lipid asymmetry in DLPC/DSPC-supported lipid bilayers: a combined AFM and fluorescence microscopy study. Biophys. J. 90 (1), 228–237. 10.1529/biophysj.105.067066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D., Epel E. S., Mellon S. H., Penninx B. W., Révész D., Verhoeven J. E., et al. (2015). Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neurosci. Biobehav. Rev. 55, 333–364. 10.1016/j.neubiorev.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Chu Y., Liu H., Su Y., Zhang Q., Jiao J., et al. (2020). Accelerated blood clearance of nanoemulsions modified with PEG-cholesterol and PEG-phospholipid derivatives in rats: The effect of PEG-lipid linkages and PEG molecular weights. Mol. Pharm. 17 (4), 1059–1070. 10.1021/acs.molpharmaceut.9b00770 [DOI] [PubMed] [Google Scholar]
- Liu Z. S. J., Truong T. T. T., Bortolasci C. C., Spolding B., Panizzutti B., Swinton C., et al. (2022). Effects of psychotropic drugs on ribosomal genes and protein synthesis. Int. J. Mol. Sci. 23 (13), 7180. 10.3390/ijms23137180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R., Marin R., Santacruz M. J., Pascual A. (2015). Selective immunoglobulin M deficiency among clozapine-treated patients: A nested case-control study. Prim. Care Companion CNS Disord. 17 (4). 10.4088/PCC.15m01782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquain-Costaz C., Kockx M., Anastasius M., Chow V., Kontush A., Jessup W., et al. (2020). Increased ABCA1 (ATP-Binding cassette transporter a1)-specific cholesterol efflux capacity in schizophrenia. Arterioscler. Thromb. Vasc. Biol. 40 (11), 2728–2737. 10.1161/ATVBAHA.120.314847 [DOI] [PubMed] [Google Scholar]
- Manjunath K., Reddy J. S., Venkateswarlu V. (2005). Solid lipid nanoparticles as drug delivery systems. Methods Find. Exp. Clin. Pharmacol. 27 (2), 127–144. 10.1358/mf.2005.27.2.876286 [DOI] [PubMed] [Google Scholar]
- Mason P. D., Weetman A. P., Sissons J. G., Borysiewicz L. K. (1988). Suppressive role of NK cells in pokeweed mitogen-induced immunoglobulin synthesis: effect of depletion/enrichment of leu 11b+ cells. Immunology 65 (1), 113–118. PMID: 3053423; PMCID: PMC1385028. [PMC free article] [PubMed] [Google Scholar]
- Maugeri M., Nawaz M., Papadimitriou A., Angerfors A., Camponeschi A., Na M., et al. (2019). Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat. Commun. 10, 4333. 10.1038/s41467-019-12275-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M., Beauchemin M., Vary C., Barlow D., Houseknecht K. L. (2019). The antipsychotic medication, risperidone, causes global immunosuppression in healthy mice. PLoS One 14 (6), e0218937. 10.1371/journal.pone.0218937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazereel V., Van Assche K., Detraux J., De Hert M. (2021). COVID-19 vaccination for people with severe mental illness: why, what, and how? Lancet Psychiatry 8 (5), 444–450. 10.1016/S2215-0366(20)30564-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren R. A., Jr, Trejo F. E., Blitz M. J., Bianco A., Limaye M., Brustman L., et al. (2021). COVID-related "lockdowns" and birth rates in New York. Am. J. Obstet. Gynecol. MFM 3 (6), 100476. 10.1016/j.ajogmf.2021.100476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkley S. D., Chock C. J., Yang X. O., Harris J., Castillo E. F. (2018). Modulating T cell responses via autophagy: The intrinsic influence controlling the function of both antigen-presenting cells and T cells. Front. Immunol. 9, 2914. 10.3389/fimmu.2018.02914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer N., Henkel L., Linder B., Zielke S., Tascher G., Trautmann S., et al. (2021). Autophagy activation, lipotoxicity and lysosomal membrane permeabilization synergize to promote pimozide- and loperamide-induced glioma cell death. Autophagy 17 (11), 3424–3443. 10.1080/15548627.2021.1874208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miatmoko A., Nurjannah I., Nehru N. F., Rosita N., Hendradi E., Sari R., et al. (2021). Interactions of primaquine and chloroquine with PEGylated phosphatidylcholine liposomes. Sci. Rep. 11 (1), 12420. 10.1038/s41598-021-91866-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani K., Williams S. Z., Olfson M., Leckman-Westin E., Finnerty M., Kammer J., et al. (2022). Association between the use of psychotropic medications and the risk of COVID-19 infection among long-term inpatients with serious mental illness in a New York state-wide psychiatric hospital system. JAMA Netw. Open 5 (5), e2210743. 10.1001/jamanetworkopen.2022.10743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimi K., Neylan T. C., Bertenthal D., Seal K. H., O'Donovan A. (2022). Association of psychiatric disorders with incidence of SARS-CoV-2 breakthrough infection among vaccinated adults. JAMA Netw. Open 5 (4), e227287. 10.1001/jamanetworkopen.2022.7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal S. R., Paliwal R., Vyas S. P. (2015). A review of mechanistic insight and application of pH-sensitive liposomes in drug delivery. Drug Deliv. 22 (3), 231–242. 10.3109/10717544.2014.882469 [DOI] [PubMed] [Google Scholar]
- Paloncýová M., Čechová P., Šrejber M., Kührová P., Otyepka M. (2021). Role of ionizable lipids in SARS-CoV-2 vaccines as revealed by molecular dynamics simulations: From membrane structure to interaction with mRNA fragments. J. Phys. Chem. Lett. 12 (45), 11199–11205. 10.1021/acs.jpclett.1c03109 [DOI] [PubMed] [Google Scholar]
- Pandurangi A. K., Buckley P. F. (2020). Inflammation, antipsychotic drugs, and evidence for effectiveness of anti-inflammatory agents in schizophrenia. Curr. Top. Behav. Neurosci. 44, 227–244. 10.1007/7854_2019_91 [DOI] [PubMed] [Google Scholar]
- Papanastasiou E., Gaughran F., Smith S. (2011). Schizophrenia as segmental progeria. J. R. Soc. Med. 104 (11), 475–484. 10.1258/jrsm.2011.110051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Choi Y. K., Kim S., Lee J., Im W. (2021). CHARMM-GUI membrane builder for lipid nanoparticles with ionizable cationic lipids and PEGylated lipids. J. Chem. Inf. Model. 61 (10), 5192–5202. 10.1021/acs.jcim.1c00770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsford M. J., Pecoraro A., Jolles S. (2019). Clozapine-associated secondary antibody deficiency. Curr. Opin. Allergy Clin. Immunol. 19 (6), 553–562. PMID: 31567398. 10.1097/ACI.0000000000000592 [DOI] [PubMed] [Google Scholar]
- Popova N. V., Deyev I. E., Petrenko A. G. (2013). Clathrin-mediated endocytosis and adaptor proteins. Acta Naturae 5, 62–73. 10.32607/20758251-2013-5-3-62-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pousa P., Souza R., Melo P., Correa B., Mendonça T., Simões-E-Silva A., et al. (2021). Telomere shortening and psychiatric disorders: A systematic review. Cells 10, 1423. 10.3390/cells10061423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay G., Querbes W., Alabi C., Eltoukhy A., Sarkar S., Zurenko C., et al. (2013). Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat. Biotechnol. 31 (7), 653–658. 10.1038/nbt.2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleuning M. J., Duggan A., Reem G. H. (1989). Inhibition by chlorpromazine of lymphokine-specific mRNA expression in human thymocytes. Eur. J. Immunol. 19 (8), 1491–1495. 10.1002/eji.1830190822 [DOI] [PubMed] [Google Scholar]
- Sebastiani F., Yanez Arteta M., Lerche M., Porcar L., Lang C., Bragg R. A., et al. (2021). Apolipoprotein E binding drives structural and compositional rearrangement of mRNA-containing lipid nanoparticles. ACS Nano 15 (4), 6709–6722. 10.1021/acsnano.0c10064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settanni G., Brill W., Haas H., Schmid F. (2022). pH-dependent behavior of ionizable cationic lipids in mRNA-carrying lipoplexes investigated by molecular dynamics simulations. Macromol. Rapid Commun. 43 (12), e2100683. 10.1002/marc.202100683 [DOI] [PubMed] [Google Scholar]
- Sfera A., Osorio C., Afzaal J., Del Campo Z-M., Kozlakidis Z. “COVID-19: A catalyst for novel psychiatric paradigms may 2021,” in In book: Biotechnology to combat COVID-19 (London: Intechopen; ). 2021. 10.5772/intechopen.96940 [DOI] [Google Scholar]
- Sharma N., Tan M. A. (2021). An SSA. Phytosterols: Potential metabolic modulators in neurodegenerative diseases. Int. J. Mol. Sci. 22 (22), 12255. 10.3390/ijms222212255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M. A., Linthicum D. S., Bolger M. B. (1986). Haloperidol binding to monoclonal antibodies: conformational analysis and relationships to D-2 receptor binding. Mol. Pharmacol. 29 (6), 589–598. PMID: 2423865. [PubMed] [Google Scholar]
- Siwaszek A., Ukleja M., Dziembowski A. (2014). Proteins involved in the degradation of cytoplasmic mRNA in the major eukaryotic model systems. RNA Biol. 11 (9), 1122–1136. PMID: 25483043; PMCID: PMC4615280. 10.4161/rna.34406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solana C., Pereira D., Tarazona R. (2018). Early senescence and leukocyte telomere shortening in SCHIZOPHRENIA: A role for cytomegalovirus infection? Brain Sci. 8 (10), 188. 10.3390/brainsci8100188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon G. F., Rubbo S. D., Batchelder E. (1970). Secondary immune response to tetanus toxoid in psychiatric patients. J. Psychiatr. Res. 7 (3), 201–207. 10.1016/0022-3956(70)90007-5 [DOI] [PubMed] [Google Scholar]
- Soulet D., Gagnon B., Rivest S., Audette M., Poulin R. (2004). A fluorescent probe of polyamine transport accumulates into intracellular acidic vesicles via a two-step mechanism. J. Biol. Chem. 279 (47), 49355–49366. 10.1074/jbc.M401287200 [DOI] [PubMed] [Google Scholar]
- Squassina A., Manchia M., Chillotti C., Deiana V., Congiu D., Paribello F., et al. (2013). Differential effect of lithium on spermidine/spermine N1-acetyltransferase expression in suicidal behaviour. Int. J. Neuropsychopharmacol. 16 (10), 2209–2218. 10.1017/S1461145713000655 [DOI] [PubMed] [Google Scholar]
- Stapel B., Sieve I., Falk C. S., Bleich S., Hilfiker-Kleiner D., Kahl K. G. (2018). Second generation atypical antipsychotics olanzapine and aripiprazole reduce expression and secretion of inflammatory cytokines in human immune cells. J. Psychiatr. Res. 105, 95–102. 10.1016/j.jpsychires.2018.08.017 [DOI] [PubMed] [Google Scholar]
- Su Y., Liu M., Liang K., Liu X., Song Y., Deng Y. (2018). Evaluating the accelerated blood clearance phenomenon of PEGylated nanoemulsions in rats by intraperitoneal administration. AAPS PharmSciTech 19 (7), 3210–3218. 10.1208/s12249-018-1120-2 [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Ishihara H. (2021). Difference in the lipid nanoparticle technology employed in three approved siRNA (Patisiran) and mRNA (COVID-19 vaccine) drugs. Drug Metab. Pharmacokinet. 41, 100424. 10.1016/j.dmpk.2021.100424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam Y. Y., Chen S., Cullis P. R. (2013). Advances in lipid nanoparticles for siRNA delivery. Pharmaceutics 5 (3), 498–507. 10.3390/pharmaceutics5030498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Mourad S., Zhai S. D., Hart R. J. (2016). Dopamine agonists for preventing ovarian hyperstimulation syndrome. Cochrane Database Syst. Rev. 11 (11), CD008605. 10.1002/14651858.CD008605.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantino N., Leboyer M., Bouleau A., Hamdani N., Richard J. R., Boukouaci W.., et al. (2021). Natural killer cells in first-episode psychosis: an innate immune signature? Mol. Psychiatry 26 (9), 5297–5306. 10.1038/s41380-020-01008-7 [DOI] [PubMed] [Google Scholar]
- Teo S. L. Y., Rennick J. J., Yuen D., Al-Wassiti H., Johnston A. P. R., Pouton C. W. (2021). Unravelling cytosolic delivery of cell penetrating peptides with a quantitative endosomal escape assay. Nat. Commun. 12, 3721. 10.1038/s41467-021-23997-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- ur Rehman Z., Hoekstra D., Zuhorn I. S. (2013). Mechanism of polyplex- and lipoplex-mediated delivery of nucleic acids: real-time visualization of transient membrane destabilization without endosomal lysis. ACS Nano 7 (5), 3767–3777. 10.1021/nn3049494 [DOI] [PubMed] [Google Scholar]
- Urits I., Swanson D., Swett M. C., Patel A., Berardino K., Amgalan A., et al. (2020). A review of patisiran (ONPATTRO®) for the treatment of polyneuropathy in people with hereditary transthyretin amyloidosis. Neurol. Ther. 9 (2), 301–315. 10.1007/s40120-020-00208-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas D. Y., Raj A., Marras S. A., Kramer F. R., Tyagi S. (2005). Mechanism of mRNA transport in the nucleus. Proc. Natl. Acad. Sci. U. S. A. 102 (47), 17008–17013. 10.1073/pnas.0505580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vik-Mo A. O., Fernø J., Skrede S., Steen V. M. (2009). Psychotropic drugs up-regulate the expression of cholesterol transport proteins including ApoE in cultured human CNS- and liver cells. BMC Pharmacol. 9, 10. 10.1186/1471-2210-9-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Rothberg K. G., Anderson R. G. (1993). Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123, 1107–1117. 10.1083/jcb.123.5.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yu L., Zhou H., Zhou Z., Zhu H., Li Y., et al. (2016). Serologic and molecular characteristics of hepatitis B virus infection in vaccinated schizophrenia patients in China. J. Infect. Dev. Ctries. 10 (4), 427–431. 10.3855/jidc.7377 [DOI] [PubMed] [Google Scholar]
- Wei Jiao, Hui Ai-Min. (2022). The paradigm shift in treatment from Covid-19 to oncology with mRNA vaccines. Cancer Treat. Rev. 107, 102405. 10.1016/j.ctrv.2022.102405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnilowicz M., Glab A., Bertucci A., Caruso F., Cavalieri F. (2019). Super-resolution imaging of proton sponge-triggered rupture of endosomes and cytosolic release of small interfering RNA. ACS Nano 13 (1), 187–202. 10.1021/acsnano.8b05151 [DOI] [PubMed] [Google Scholar]
- Wu Z., Li T. (2021). Nanoparticle-mediated cytoplasmic delivery of messenger RNA vaccines: Challenges and future perspectives. Pharm. Res. 38 (3), 473–478. 10.1007/s11095-021-03015-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Mihai C., Griffith J. P., Hou S., Esposito A. A., Ketova T., et al. (2020). Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 11 (1), 983. 10.1038/s41467-020-14527-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M., Parle M., Jindal D. K., Sharma N. (2018). Potential effect of spermidine on GABA, dopamine, acetylcholinesterase, oxidative stress and proinflammatory cytokines to diminish ketamine-induced psychotic symptoms in rats. Biomed. Pharmacother. 98, 207–213. 10.1016/j.biopha.2017.12.016 [DOI] [PubMed] [Google Scholar]
- Yamagishi M., Shirasaki Y., Funatsu T. (2013). Single-molecule tracking of mRNA in living cells. Methods Mol. Biol. 950, 153–167. PMID: 23086875. 10.1007/978-1-62703-137-0_10 [DOI] [PubMed] [Google Scholar]
- Yang J., Shen M. H. (2006). Polyethylene glycol-mediated cell fusion. Methods Mol. Biol. 325, 59–66. PMID: 16761719. 10.1385/1-59745-005-7:59 [DOI] [PubMed] [Google Scholar]
- Yovel G., Sirota P., Mazeh D., Shakhar G., Rosenne E., Ben-Eliyahu S. (2000). Higher natural killer cell activity in schizophrenic patients: the impact of serum factors, medication, and smoking. Brain Behav. Immun. 14 (3), 153–169. 10.1006/brbi.1999.0574 [DOI] [PubMed] [Google Scholar]
- Yuan L., Zhang F., Shen M., Jia S., Xie J. (2019). Phytosterols suppress phagocytosis and inhibit inflammatory mediators via ERK pathway on LPS-triggered inflammatory responses in RAW264.7 macrophages and the correlation with their structure. Foods 8 (11), 582. 10.3390/foods8110582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Han X., Alameh M. G., Shepherd S. J., Padilla M. S., Xue L., et al. (2022). Rational design of anti-inflammatory lipid nanoparticles for mRNA delivery. J. Biomed. Mat. Res. A 110 (5), 1101–1108. 10.1002/jbm.a.37356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Richards A., Barrasa M. I., Hughes S. H., Young R. A., Jaenisch R. (2021). Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc. Natl. Acad. Sci. U. S. A. 118 (21), e2105968118. 10.1073/pnas.2105968118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. C., Chi Y. J., Yu Y. S., Liu J. L., Su R. W., Ma X. H., et al. (2008). Polyamines are essential in embryo implantation: expression and function of polyamine-related genes in mouse uterus during peri-implantation period. Endocrinology 149 (5), 2325–2332. 10.1210/en.2007-1420 [DOI] [PubMed] [Google Scholar]