Abstract
The introduction of effective vaccines in December 2020 marked a significant step forward in the global response to COVID-19. Given concerns with access, acceptability, and hesitancy across Africa, there is a need to describe the current status of vaccine uptake in the continent. An exploratory study was undertaken to investigate these aspects, current challenges, and lessons learnt across Africa to provide future direction. Senior personnel across 14 African countries completed a self-administered questionnaire, with a descriptive analysis of the data. Vaccine roll-out commenced in March 2021 in most countries. COVID-19 vaccination coverage varied from low in Cameroon and Tanzania and up to 39.85% full coverage in Botswana at the end of 2021; that is, all doses advocated by initial protocols versus the total population, with rates increasing to 58.4% in Botswana by the end of June 2022. The greatest increase in people being fully vaccinated was observed in Uganda (20.4% increase), Botswana (18.5% increase), and Zambia (17.9% increase). Most vaccines were obtained through WHO-COVAX agreements. Initially, vaccination was prioritised for healthcare workers (HCWs), the elderly, adults with co-morbidities, and other at-risk groups, with countries now commencing vaccination among children and administering booster doses. Challenges included irregular supply and considerable hesitancy arising from misinformation fuelled by social media activities. Overall, there was fair to reasonable access to vaccination across countries, enhanced by government initiatives. Vaccine hesitancy must be addressed with context-specific interventions, including proactive programmes among HCWs, medical journalists, and the public.
Keywords: COVID-19, vaccination, hesitancy, availability, challenges, African countries, policy implications, social media
1. Introduction
In March 2020, SARS-CoV-2, the virus that caused the coronavirus disease of 2019 (COVID-19), was declared a pandemic by the World Health Organisation (WHO) [1], and by late June 2022, there were 540 million confirmed cases of COVID-19 globally, with over 6.3 million deaths recorded [2].
The focus among countries and continents, certainly initially, was the introduction of public health policies to try and slow down the spread of the virus, with its subsequent impact on morbidity and mortality, in the absence of proven treatments and vaccines to treat COVID-19 [3,4]. These policies included lockdown measures incorporating the closure of educational establishments and borders, promoting hand hygiene, social distancing and the wearing of personal protective equipment (PPE) as well as quarantining measures [5,6,7,8,9,10,11,12,13]. However, there was variable implementation and adherence to the recommended preventative measures across countries, which adversely affected the subsequent prevalence and mortality rates [14,15,16,17,18].
Several re-purposed medicines were proposed for the prevention and management of patients with COVID-19 in the absence of effective vaccines. These included hydroxychloroquine, lopinavir/ritonavir, ivermectin, remdesivir, and steroids [19,20,21,22,23,24], with their endorsement resulting in appreciably increased utilisation, especially hydroxychloroquine, fuelled by social media and other activities [16,20,25,26,27,28]. This surge was despite limited evidence regarding their effectiveness, apart from dexamethasone, initially and in subsequent studies, with their overuse increasing morbidity, mortality, and costs [16,26,29,30,31,32,33,34,35,36,37]. These concerns resulted in calls across countries to enhance the evidence base of treatments before they were routinely recommended, thereby minimising the potential for misinformation [28,38,39,40,41].
Alongside this, the unintended consequences of lockdown and social distancing measures, including limited access to healthcare services, were considerable, especially in low- and middle-income countries (LMICs), including African countries [13,42,43,44,45,46,47,48,49,50,51,52,53]. The unintended consequences also included increased morbidity and mortality from reduced routine vaccinations among children in Africa [54,55,56,57].
Consequently, there was an appreciable need for effective vaccines to limit the spread of the virus. Numerous published studies have demonstrated the effectiveness of COVID-19 vaccines in reducing the impact of COVID-19 across countries, including reducing mortality, especially for patients at risk of severe disease [58,59,60,61]. These effectiveness rates resulted in a generally high acceptance of COVID-19 vaccines when available across countries [62], with booster campaigns introduced to tackle new variants and the waning of vaccine effectiveness over time [60,63,64]. However, there have been concerns with the vaccines across countries increasing hesitancy [65].
High acceptance rates (up to 88.8% acceptance with a 95% effectiveness rate) for COVID-19 vaccines were seen in a study by Bono et al., (2021) involving LMICs, including five African countries [66], although they were lower (61%) in the pooled study of Norhayati et al., (2022) [67]. Kanyanda et al., (2021) also generally identified high acceptance rates for the vaccine across sub-Saharan Africa, although they were lower in Mali (64.5%) [68]. Norhayati et al., (2022) also showed an acceptance rate of only 53% among the 15 African countries in their systematic review [67]. However, high acceptance rates were seen among the public in Nigeria, ranging from 74.5% to 85.3% of those surveyed [69,70,71], although they were lower in the study by Tobin et al., (2021) at 50.2% [72]. The major reasons for the non-acceptance of COVID-19 vaccines in Nigeria included concerns with the robustness of the published clinical trials, including the length of the follow-up when first rolled-out and the age of the included patients in the trials [69,71].
However, as with the increasing administration of COVID-19 vaccines, concerns regarding some of the rare adverse effects of the vaccines have contributed to vaccine hesitancy [73,74,75,76,77], with vaccine hesitancy defined as ‘a delay in acceptance or refusal of vaccination, despite the availability of vaccination services’ [78,79]. These concerns have resulted in increased hesitancy towards the COVID-19 vaccines across countries, including African countries [65,80,81,82,83]. Across Africa, studies have documented that between 32–37% of adults would not accept the vaccine, with hesitancy rates influenced by age, education, source of information, income and/or employment status, and the potential for increased infection [84,85,86,87]. Variable acceptance rates were also seen among African countries in the study of Sallam et al., (2022) [80], with variable hesitancy between 21% to 84.6% of those surveyed also seen in Cameroon, Ghana, Kenya, South Africa, Zimbabwe, and Zambia [82,86,88,89,90,91,92]. Whilst there have been challenges with vaccine hesitancy in Zimbabwe when COVID-19 vaccines were first made available, this was reduced with national and local community engagement programmes [93].
In Tanzania, the Health Minister in early 2021 stated that the country would not partake in vaccination campaigns as they were not satisfied with the safety of the vaccines, relying on traditional and household herbs and medicines for prevention and treatment [94]. Whilst this situation changed later in the year, appreciable hesitancy remained [95]. Alongside this, there have also been concerns with hesitancy among healthcare workers (HCWs), including healthcare professionals (HCPs), and students across Africa [96,97,98].
COVID-19 vaccine hesitancy is a key issue to address, with vaccine hesitancy already in 2019 identified by the WHO as one of the top ten global threats to public health [99,100]. Overall, a considerable number of deaths could have been averted if target vaccination rates had been achieved, especially among low- and middle-income countries, including African countries [101]. As mentioned, key attributes among those hesitant to COVID-19 vaccines include age, level of education, income and/ or employment status, and locality [84,86,87,102,103,104,105]. Religious beliefs and political issues are also key areas influencing hesitancy across Africa [106]. Identifying key reasons regarding vaccine hesitancy is important among African countries given the documented effectiveness of the vaccines, their high rates of infectious diseases, as well as high rates of antimicrobial resistance (AMR) exacerbated by excessive use of antibiotics to treat patients with COVID-19 [93,107,108,109,110,111,112,113].
Identified concerns to address include confidence surrounding the vaccines, including their effectiveness and potential safety issues, as well as addressing complacency issues incorporating beliefs of a low risk of catching COVID-19 and a low disease severity if COVID-19 is caught [65,114,115,116,117,118]. Enhancing access (convenience) and instigating robust communication programmes adjusted to the socio-demographics of the target population (context) are also important to address misinformation and disinformation promulgated via social media [103,114,119,120]. Addressing COVID-19 vaccine hesitancy is also important for the acceptance of other vaccines, as well as helping to address high AMR rates across Africa [111,121,122].
Other important challenges affecting the availability and use of COVID-19 vaccines include the availability of supplies and trained HCWs, including HCPs, to administer the vaccines once available [123].
Consequently, there is a need to build on these studies. This includes documenting key issues regarding COVID-19 vaccines across Africa, including their acceptance and challenges. Subsequently, documenting key activities that can be undertaken by governments and HCPs to address hesitancy to improve future vaccination rates for this and future pandemics.
2. Materials and Methods
2.1. Study Design
A mixed methods approach was adopted. This is similar to other Pan-African projects undertaken by the co-authors to document and debate key issues surrounding both non-infectious diseases and infectious diseases, as well as general areas, to provide future guidance [10,15,26,124,125,126,127,128,129,130]. The first stage comprised a narrative review of the literature regarding the effectiveness and safety of current vaccines for COVID-19, along with acceptance rates and hesitancy across Africa and the reasons for this. As mentioned, hesitancy was defined as ‘a delay in acceptance or refusal of vaccination, despite the availability of vaccination services’ [78,79,131]. The principal objective was to derive key discussion points for the second stage of the research. This was not a systematic review since the principal aim of this paper was to document the current situation regarding the vaccines, including vaccine hesitancy and the challenges among sub-Saharan African countries to provide future direction. The literature review was largely based on the considerable knowledge of the senior-level co-authors. This included individual country studies documenting current vaccination and hesitancy rates known to the co-authors from each country, as well as Pan-African and Global studies discussing similar issues. We adopted this approach before when discussing key activities and their future implications across countries and continents including Africa, with the deliberations based on the considerable knowledge and experience of the senior-level co-authors [125,126,127,128,129,130,132,133].
The second part of the study comprised an explorative questionnaire survey among senior-level government, HCP, and academic personnel from a range of African countries. The countries were purposefully selected based on the availability and knowledge of the senior-level co-authors to address the key issues and objectives of the paper. An analytical framework approach was used alongside a pragmatic paradigm aimed at providing future guidance, including for future pandemics [134,135,136,137]. The participating countries (Table 1) provided a range of economic status (Gross Domestic Product (GDP)/capita) [138], population size [139], and geographies, as well as current infection and mortality rates [2], to meet study objectives. We are aware though that there can be concerns with reporting mortality rates, including definitions [140,141,142].
Table 1.
Current population size, GDP/capita, and COVID-19 infection rates among participating African countries.
| Country | Population Size (Thousands) | GDP/Capita (US$) | Accumulated Infection Rate (Thousands) | Accumulated Mortality Rate (Thousands) |
|---|---|---|---|---|
| Botswana | 2351.63 | 6711.0 | 325.5 | 2.77 |
| Cameroon | 26,545.86 | 1499.4 | 121.0 | 1.93 |
| Egypt | 102,260.0 | 4028.4 | 515.3 | 1.93 |
| Eswatini | 1160.16 | 3415.5 | 73.3 | 1.42 |
| Ghana | 31,072.94 | 2328.5 | 168.5 | 1.46 |
| Kenya | 53,771.30 | 1838.2 | 338.1 | 5.67 |
| Malawi | 19,129.95 | 625.3 | 87.7 | 2.67 |
| Nigeria | 206,139.60 | 2097.1 | 263.1 | 3.15 |
| South Africa | 59,308.69 | 5090.7 | 4010.2 | 102.1 |
| Sudan | 48,892.81 | 595 | 63.2 | 4.96 |
| Tanzania | 61,498.44 | 1136 | 38.7 | 0.84 |
| Uganda | 47,123.53 | 858 | 168.7 | 3.63 |
| Zambia | 18,383.96 | 1050.9 | 332.5 | 4.02 |
| Zimbabwe | 14,862.92 | 1128.2 | 256.6 | 5.59 |
2.2. Questionnaire Design and Analysis
The key questions posed to participating countries following a narrative review of the literature included the following:
Did your country have a dedicated COVID-19 vaccine rollout programme? Was this in the public sector, private sector, or both, and were any specific age groups covered?
Which COVID-19 vaccines were made available and how were the costs covered for each (e.g., NGOs)?
What is the current coverage rate (different doses if known)?
What is being done to ensure access to COVID-19 vaccines, and how would you describe the acceptance (willingness) of the population to COVID-19 vaccinations?
What is the extent of any misinformation about the vaccine (if known), and how is misinformation being spread (e.g., social media)?
What are the challenges with COVID-19 vaccinations in addition to the above, and what are potential ways forward or measures being undertaken by national authorities and other key stakeholders to mitigate against these challenges?
Within each country, the co-authors collated the replies, which were subsequently reviewed and collated by the principal author (OO). The findings were then fed back to each country for clarification to enhance their accuracy. A common basis was used to compare vaccine findings across countries, building on country-specific information [143,144,145]. The final responses were subsequently analysed using thematic analysis techniques [10,146].
Common themes from the responses were identified and discussed with the co-authors to provide future guidance [10]. The findings were subsequently summarised into key themes and challenges faced by participating countries. Potential ways forward were broken down into the four Es where pertinent, namely ‘Education, Engineering, Economics and Enforcement’ [147,148], in order to consolidate approaches. We have used this methodology before when consolidating potential approaches and activities across disease areas to improve the use of medicine [124,127,149,150]. ‘Education’ includes disseminating information to key stakeholder groups and developing guidelines or formularies [151,152,153,154]. ‘Engineering’ includes organisational or managerial interventions such as instigating and monitoring prescribing targets and quality targets [148,150]. ‘Economics’ include financial incentives to key stakeholder groups and ‘Enforcement’ includes regulations by law including the banning of self-purchasing of antibiotics without a prescription [148,155,156].
Two timescales were employed to assess changes over time as more knowledge became available regarding the effectiveness and safety of the vaccines including boosters. These were up to the end of December 2021 and up to the end of June 2022.
2.3. Ethical Considerations
No ethical approval was sought for this study as no human subjects were involved. In addition, the co-authors, who were very knowledgeable in their country concerning these matters, voluntarily provided the information, which are typically available in the public domain. This mirrors similar studies conducted by the co-authors across Africa and wider, involving general subjects as well as both infectious and non-infectious diseases, and is in accordance with institutional guidance [10,15,26,124,125,126,127,129,157,158].
3. Results
We will first document the initial sources of COVID-19 vaccines across Africa before discussing initial and subsequent coverage rates as well as key issues surrounding access, hesitancy and challenges.
3.1. Vaccine Sources and Deployment
Vaccine roll-out commenced in a number of African countries in the first quarter of 2021, with Egypt the first African country among those studied to commence vaccination on 24 January 2021, followed by South Africa on 17 February 2021 and Zimbabwe on 18 February 2021. Other countries studied, apart from Tanzania, introduced their COVID-19 vaccines between March and April 2021 [94]. Most of the countries had dedicated vaccine roll-out programmes involving both the public and private sectors (Supplementary Table S1).
The sources of vaccines among the countries were typically from donations by multilateral agencies, non-governmental organisations, and higher income economies, including the UK and USA, with some countries, including South Africa, entering into bilateral agreements (Supplementary Table S1). Agencies and other organisations included the COVAX-WHO initiative, the African Union Vaccine Acquisition Trust (AVAT) and GAVI, the Vaccine Alliance, and the Serum Institute of India. COVAX is co-led by the Coalition for Epidemic Preparedness Innovations (CEPI), GAVI, the Vaccine Alliance, and the WHO, alongside a key delivery partner, UNICEF [159]. Typically, vaccines from multiple sources were administered across Africa (Supplementary Table S1).
3.2. Vaccination Coverage
As of 31 December 2021, vaccination coverage in the studied countries varied from very low rates in Tanzania and Nigeria, with higher rates reported in Botswana, Egypt, Eswatini, and South Africa (Table 2 and Table 3). Most of the studied African countries deployed vaccination programmes in phases, prioritising HCWs, followed by elderly patients and patients with co-morbidities at high risk of severe COVID-19 disease, hospitalisation, or death should they be infected with SARS-CoV-2 (Supplementary Table S2). A number of these countries also commenced the vaccination of children of certain age groups and began administering booster doses to the adult population by the end of December 2021 (Supplementary Table S2).
Table 2.
COVID-19 vaccination coverage across African countries as of 31 December 2021 and 30 June 2022.
| Country | Vaccination Coverage—31 December 2021—% of the Total Population | Vaccination Coverage—30 June 2022—% of the Total Population | ||
|---|---|---|---|---|
| Full (Completed) | Partial | Full (Completed) | Partial | |
| Botswana | 39.9 | 5.2 | 58.4 | 7.1 |
| Cameroon | 2.4 | 0.6 | 4.5 | 1.3 |
| Egypt | 20.9 | 11.5 | 34.1 | 12.0 |
| Eswatini | 25.4 | 2.7 | 28.7 | 5.7 |
| Ghana | 7.1 | 10.5 | 21.9 | 9.9 |
| Kenya | 6.8 | 4.4 | 17.6 | 6.3 |
| Malawi | 3.5 | 3.96 | 7.63 | 3.05 |
| Nigeria | 2.1 | 2.7 | 11.4 | 5.6 |
| South Africa | 26.7 | 5.2 | 32.1 | 5.0 |
| Sudan | 3.1 | 3.7 | 10.4 | NA |
| Tanzania | 2.3 | 0.7 | 11.8 | 2.2 |
| Uganda | 3.9 | 18.8 | 24.3 | 11.1 |
| Zambia | 6.6 | NA | 24.5 | 35.0 |
| Zimbabwe | 19.6 | 6.2 | 28.8 | 10.6 |
| Africa | 8.9 | 4.9 | 18.4 | 5.2 |
| World | 49.2 | 8.5 | 60.7 | 5.5 |
Table 3.
Number of vaccine doses utilised by the countries as of 31 December 2021 and 30 June 2022.
| Countries | Doses as of 31 December 2021 | Doses as of 30 June 2022 |
|---|---|---|
| Botswana | NA | 2.73 million |
| Cameroon | 1.02 million | 1.85 million |
| Egypt | 57.49 million | 91.45 million |
| Eswatini | 404,374 | 684,176 |
| Ghana | 7.76 million | 18.24 million |
| Kenya | 10.12 million | 18.54 million |
| Malawi | 1.8 million | 3.17 million |
| Nigeria | 14.84 million | 55.47 million |
| South Africa | 27.97 million | 36.82 million |
| Sudan | 3.64 million | NA |
| Tanzania | 2.43 million | 12.07 million |
| Uganda | 12.09 million | 21.76 million |
| Zambia | 1.73 million | 7.2 million |
| Zimbabwe | 7.26 million | 11.97 million |
| Africa | 303.51 million | 550.21 million |
| World | 9.18 billion | 12.1 billion |
The most widely administered vaccines by the end of June 2022 across Afria were Johnson & Johnson (30.3% of the total number administered), Pfizer BioNtech (19.1%), Sinopharm (14.2%), Oxford AstraZeneca (13.7%), Sinovac (7.9%), and Moderna (5.5%) [143].
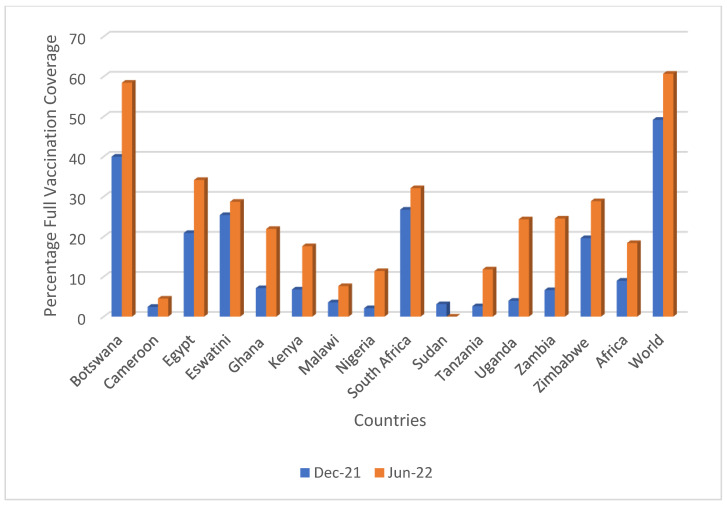
By the end of June 2022, COVID-19 vaccination coverage had increased across Africa, with an overall 18.4% full vaccination coverage, up from 8.9% at the end of December 2021. This was considerably lower though than the global rate of 60.7%, up from 49.2% in December 2021. However, again, appreciable variation was observed across the various countries (Table 2 and Table 3), with the greatest increases seen in Uganda (20.4%), Botswana (18.5%), Zambia (17.9%), and Ghana (14.8%) and with varied increase in coverage seen in the other studied countries (Table 2). Figure 1 depicts the overall increase in the administration of COVID-19 vaccines by the end of June 2022. The lowest coverage rate among the studied countries was recorded in Cameroon, with only 4.5% of the population being fully vaccinated by mid-2022.
Figure 1.
Changes in full vaccination coverage between December 2021 and June 2022. NB: DEC-21-F = fully vaccinated by protocol as of 31 December 2021, JUN-22-F = fully vaccinated by the protocol as of 30 June 2022. Based on [143,144,145,160].
3.3. Access, Hesitancy, and Challenges with the COVID-19 Vaccine Roll-Out
Table 4 summarises the levels of access, acceptance, hesitancy, and challenges with the COVID-19 vaccine roll-out across Africa, which builds on Pan-African and other African studies [66,67,68,69,70,71,72,80,81,82,83,84,85,86]. Access was generally well facilitated by the different measures and initiatives among the various African governments; however, there were concerns in some African countries, including the variable availability of the different vaccines.
Table 4.
Levels of access, acceptance, hesitancy, and challenges with COVID-19 vaccines across Africa.
| Country | Access, Acceptance, Hesitancy and Challenges |
|---|---|
| Botswana [161,162] |
Access:
|
| Cameroon [82,143,144] |
Access:
|
| Egypt [85,163,164,165,166] |
Access:
Vaccine hesitancy has been reported in many studies across Egypt due to a number of reasons. These include:
|
| Eswatini [167,168] |
Access:
|
| Ghana [15,92,169,170,171,172,173,174] |
Access:
|
| Kenya [120,175,176,177,178,179] |
Access:
|
| Malawi [180,181,182,183] |
Access:
|
| Nigeria [184,185,186,187,188] |
Access:
|
| South Africa [90,189,190,191,192] |
Access:
|
| Sudan [193,194,195] |
Access:
|
| Tanzania |
Access:
|
| Uganda [68,81,196] |
Access:
|
| Zambia [89,131,197] |
Access:
|
| Zimbabwe [93,106,109,175,198] |
Access:
|
NB: AEFI = adverse events following immunization; HCPs = healthcare professionals; HCW = healthcare workers; PHCs: Primary healthcare centres.
There were also concerns with the level of acceptance for the vaccine among a number of the studied countries, with subsequent high rates of vaccine hesitancy in some of these. In Cameroon, poor acceptance levels were observed, and Egypt and Eswatini initially presented with low levels of acceptance; however, there were considerable improvements over time.
Misinformation concerning the side-effects of the vaccine coupled with other key issues, including fertility and other conspiracy theories, were widely circulated on social media platforms. This resulted in issues of trust and high hesitancy rates among some of the studied countries. Addressing these and other highlighted challenges will be key to improving vaccination rates in Africa going forward.
3.4. Lessons Learnt and Ways Forward
A considerable number of lessons learned and ways forward to improve future vaccination rates were identified across countries. These are summarised in Table 5 and include increasing trust in governments and other key stakeholder groups, including HCPs, which has been eroded with increasing vaccine hesitancy rates [199]. This involves reducing doubts about the vaccines, including COVID-19 vaccines, among HCWs including HCPs [175,199,200,201], through social media and other channels, with social media playing an increasing role in promulgating misinformation [120,202]. Trusted politicians endorsing COVID-19 vaccines can also reduce hesitancy [203].
Table 5.
Key activities to improve vaccination rates in current and future pandemics.
| Key Activities | Ways Forward |
|---|---|
| Research activities |
|
| Education | |
| Healthcare professionals (HCPs) |
|
| Medical journalists and other key influencers |
|
| General Public |
|
| Communication strategies |
|
| Engineering | |
| Access and availability [216] |
|
| Economics | |
| Patient Incentives | |
| Local production |
|
| Financial support | |
| Enforcement | |
| Compulsory vaccination [225,226] |
|
| Health system |
|
NB: HCPs = healthcare professionals.
4. Discussion
We believe this is the first study to comprehensively review current African vaccination and hesitancy rates alongside current challenges, as well as propose potential ways forward given current concerns. This is particularly important in Africa with high existing rates of infectious diseases as well as high rates of AMR [111,121,130]. The overuse of antibiotics in treating patients with COVID-19 has further enhanced AMR rates, which urgently need to be addressed to reduce future morbidity, mortality, and costs [112,113,124,227]. The majority of African countries started their roll-out of COVID-19 vaccines in early 2021, in many cases with vaccines donated as part of COVAX, GAVI, AVAT, or bilateral agreements for specific countries (Supplementary Table S1). Tanzania was the last of the African countries to initiate its vaccine roll-out due to denial initially [94]. However, some of the COVID-19 vaccines donated were near their expiration date, creating additional challenges.
There was appreciable growth in vaccination rates in a number of the African countries surveyed between the end of 2021 and mid-2022, enhanced by greater availability and access, with Uganda, Botswana, and Zambia recording the greatest increase in vaccination coverage rates (Table 2, Figure 1). However, coverage rates continued to remain relatively low in some of the African countries surveyed, including Cameroon, Nigeria, Sudan, and Tanzania (Table 1), exacerbated by high hesitancy rates fuelled by misinformation (Table 4). These low rates impacted the global coverage rate of 60.7% in mid-year of 2022, which is below the target of 70% set by the WHO [228]. Botswana and Egypt continued to have high coverage rates among the African countries surveyed, enhanced by pro-active activities by regional and national governments (Table 2 and Table 4). The multi-faceted activities they undertook provide guidance to other African countries, including measures used to ensure availability among the general population.
There are still concerns with low vaccination rates of children in a number of the surveyed countries, with only 7% of doses administered among 23 African countries by the end of June 2022 to children and adolescents younger than 18 years of age [229]. This low vaccination rate may have been exacerbated by supply challenges as well as concerns about the safety of the vaccines among children. We will be following this up in future studies, as concerns with vaccinating children with the COVID-19 vaccines may negatively impact routine immunisation programmes among children, which were already severely compromised by the pandemic [54,55,230].
Hesitancy continued to be a concern across Africa. This is fuelled by the level of misinformation including false claims about its side-effects, the disease mainly targeting rich urban populations, it being the disease of the west, it likely to be only a mild disease among Africans, it interfering with fertility, and the ‘mark of the beast’, exacerbated by social media activities [120,215] (Table 4).
Several activities were identified to improve vaccination rates during the current and future pandemics (Table 5). These include the greater involvement of African countries in basic research and clinical research, epidemiology, as well as improving current pharmacovigilance activities, which are a concern across Africa [205,206,231]. In addition, these include improving the education of HCPs during undergraduate training and post-qualification using a variety of hybrid approaches, with hybrid learning here to stay post-pandemic [10]. Community pharmacists can also play a key role in improving vaccination rates. This is because they are often the first point of contact between patients and HCPs, particularly in rural areas and where there are high patient co-payments [208,209,210]. Alongside this, governments should make the vaccines easily available to reduce travelling times and associated costs, which could negatively impact uptake. The government and key HCPs also need to actively engage in social media activities [120]. This is because misinformation can rapidly circulate via social media, with significant implications for trust in governments as well as the prevention and management of COVID-19 if this continues [20,120,175,215]. Compulsory vaccination of certain groups has been instigated in some countries to reduce transmission rates. However, before doing this, governments need to carefully consider legal, ethical, and other major issues [225,226].
We will continue to monitor key areas across Africa regarding current vaccination rates and challenges, including continued hesitancy. This will enable African countries to continue to learn from each other. As a result, the impact of the virus can be reduced, which includes the unintended consequences of measures that were initially implemented to contain the pandemic. In addition, the continued high inappropriate use of antibiotics across Africa needs to be reduced, building on current national action plans to reduce AMR [124,130].
5. Conclusions
It is widely acknowledged that the introduction of COVID-19 vaccines was a significant step forward across countries to reduce the morbidity, mortality, and costs associated with the virus. This includes the unintended consequences of public health measures instigated to reduce its spread and impact. There were appreciable variations in vaccine coverage and hesitancy across Africa. This was fuelled by critical issues, including access, irregular supply, and the level of misinformation circulating within communities and on various media platforms. Key among these included was misinformation fuelled by social media. A number of activities were identified to address this situation across Africa, which included instigating improved pharmacovigilance activities and addressing the negative impact of social media, which will be followed up in future studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines10091553/s1, Table S1: COVID-19 vaccination deployment across African countries: commencement, rollout Programmes, and vaccine sources, Table S2: COVID-19 vaccination coverage across African countries (up to the end of 2021). Refs [232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274] in Supplementary Tables.
Author Contributions
Conception/design: O.O.O., B.G., J.O.F., S.M., E.T.T., A.A.J., S.M.C. and J.C.M. (Johanna C. Meyer); acquisition and analysis: O.O.O., B.G., J.O.F., S.M., A.O.A., A.F.Y.-O., S.O.O., M.R.O., M.S., W.M.R., A.M.G., N.M., T.Z., A.C.K., O.O.M., D.K., A.M., I.C., F.K., T.T., A.A., E.M., M.O, S.O., D.N.A.A., I.A.S., D.A., A.A.A., M.P.M., A.E., M.E.A., P.O., L.L.N., J.C.M. (Julius C. Mwita), G.M.R., J.K., C.E., R.T.M., I.M.-W. and J.C.M. (Johanna C. Meyer); interpretation of the data: O.O.O., B.G., J.O.F., S.M., A.O.A., A.F.Y.-O., S.O.O., M.R.O., M.S., W.M.R., A.M.G., N.M., T.Z., A.C.K., O.O.M., D.K., A.M., I.C., F.K., T.T., A.A., E.M., M.O., S.O., D.N.A.A., I.A.S., D.A., A.A.A., M.P.M., A.E., M.E.A., P.O., L.L.N., J.C.M. (Julius C. Mwita), G.M.R., J.K., C.E., R.T.M., I.M.-W., S.M.C. and J.C.M. (Johanna C. Meyer); drafting the work: O.O.O., B.G., J.O.F. and J.C.M. (Johanna C. Meyer); critically revising the paper: all authors; accuracy of the data appropriately collected and included in the analysis as well as resolving queries: O.O.O., B.G., J.O.F., S.M., A.O.A., A.F.Y.-O., S.O.O., M.R.O., M.S., W.M.R., A.M.G., N.M., T.Z., A.C.K., O.O.M., D.K., A.M., I.C., F.K., T.T., A.A., E.M., M.O., S.O., D.N.A.A., I.A.S., D.A., A.A.A., M.P.M., A.E., M.E.A., P.O., L.L.N., J.C.M. (Julius C. Mwita), G.M.R., J.K., C.E., R.T.M., I.M.-W., S.M.C. and J.C.M. (Johanna C. Meyer) Project management: O.O.O., B.G., J.O.F. and J.C.M. (Johanna C. Meyer). Final approval: all authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
No ethical approval was sought for this study as no human subjects were involved. In addition, the co-authors, who were very knowledgeable in their country concerning these matters, voluntarily provided the information, which is available in the public domain in some settings.
Data Availability Statement
Additional data can be obtained on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. However, a number of the co-authors are employed by National Health Services or Ministries of Health, or are advisers to Ministries of Health, the WHO or other leading Infectious Disease Groups.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bedford J., Enria D., Giesecke J., Heymann D.L., Ihekweazu C., Kobinger G., Lane H.C., Memish Z., Oh M.-D., Sall A.A., et al. COVID-19: Towards controlling of a pandemic. Lancet. 2020;395:1015–1018. doi: 10.1016/S0140-6736(20)30673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization WHO COVID-19 Dashboard. 2021. [(accessed on 1 August 2022)]. Available online: https://covid19.who.int.
- 3.Hopman J., Allegranzi B., Mehtar S. Managing COVID-19 in Low- and Middle-Income Countries. JAMA. 2020;323:1549. doi: 10.1001/jama.2020.4169. [DOI] [PubMed] [Google Scholar]
- 4.Choi A.J., Hean A.C., Lee J.K., Tran N.D., Lin T.K., Apollonio D.E. A Retrospective Global Assessment of Factors Associated With COVID-19 Policies and Health Outcomes. Front. Public Health. 2022;10:843445. doi: 10.3389/fpubh.2022.843445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nussbaumer-Streit B., Mayr V., Dobrescu A.I., Chapman A., Persad E., Klerings I., Wagner G., Siebert U., Ledinger D., Zachariah C., et al. Quarantine alone or in combination with other public health measures to control COVID-19: A rapid review. Cochrane Database Syst. Rev. 2020;9:cd013574. doi: 10.1002/14651858.CD013574.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang K.H.D. Movement control as an effective measure against COVID-19 spread in Malaysia: An overview. J. Public Health. 2022;30:583–586. doi: 10.1007/s10389-020-01316-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng Y., Li Z., Chua Y.X., Chaw W.L., Zhao Z., Er B., Pung R., Chiew C.J., Lye D., Heng D., et al. Evaluation of the Effectiveness of Surveillance and Containment Measures for the First 100 Patients with COVID-19 in Singapore—January 2–February 29, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:307–311. doi: 10.15585/mmwr.mm6911e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keni R., Alexander A., Nayak P.G., Mudgal J., Nandakumar K. COVID-19: Emergence, Spread, Possible Treatments, and Global Burden. Front. Public Health. 2020;8:216. doi: 10.3389/fpubh.2020.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talic S., Shah S., Wild H., Gasevic D., Maharaj A., Ademi Z., Li X., Xu W., Mesa-Eguiagaray I., Rostron J., et al. Effectiveness of public health measures in reducing the incidence of COVID-19, SARS-CoV-2 transmission, and COVID-19 mortality: Systematic review and meta-analysis. BMJ. 2021;375:e068302. doi: 10.1136/bmj-2021-068302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etando A., Amu A.A., Haque M., Schellack N., Kurdi A., Alrasheedy A.A., Timoney A., Mwita J.C., Rwegerera G.M., Patrick O., et al. Challenges and Innovations Brought about by the COVID-19 Pandemic Regarding Medical and Pharmacy Education Especially in Africa and Implications for the Future. Healthcare. 2021;9:1722. doi: 10.3390/healthcare9121722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiraef M.A., Friesen P., Feddern L., Weiss M.A. Did border closures slow SARS-CoV-2? Sci. Rep. 2022;12:1709. doi: 10.1038/s41598-022-05482-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng V.C., Wong S.C., Chuang V.W., So S.Y., Chen J.H., Sridhar S., To K.K.-W., Chan J.F.-W., Hung I.F.-N., Ho P.-L., et al. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J. Infect. 2020;81:107–114. doi: 10.1016/j.jinf.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Congressional Research Services Global Economic Effects of COVID-19—Updated 10 November 2021. [(accessed on 2 August 2022)]. Available online: https://sgp.fas.org/crs/row/R46270.pdf.
- 14.Verma B.K., Verma M., Msc V.K.V., Abdullah R.B., Nath D.C., Khan H.T.A., Verma A., Vishwakarma R.K., Verma V. Global lockdown: An effective safeguard in responding to the threat of COVID -19. J. Eval. Clin. Pract. 2020;26:1592–1598. doi: 10.1111/jep.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogunleye O.O., Basu D., Mueller D., Sneddon J., Seaton R.A., Yinka-Ogunleye A.F., Wamboga J., Miljković N., Mwita J.C., Rwegerera G.M., et al. Response to the Novel Corona Virus (COVID-19) Pandemic Across Africa: Successes, Challenges, and Implications for the Future. Front. Pharmacol. 2020;11:1205. doi: 10.3389/fphar.2020.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godman B., Haque M., Islam S., Iqbal S., Urmi U.L., Kamal Z.M., Shuvo S.A., Rahman A., Kamal M., Haque M., et al. Rapid Assessment of Price Instability and Paucity of Medicines and Protection for COVID-19 Across Asia: Findings and Public Health Implications for the Future. Front. Public Health. 2020;8:585832. doi: 10.3389/fpubh.2020.585832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan D.K.C., Zhang C.-Q., Weman-Josefsson K. Why people failed to adhere to COVID-19 preventive behaviors? Perspectives from an integrated behavior change model. Infect. Control Hosp. Epidemiol. 2020;42:375–376. doi: 10.1017/ice.2020.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin A.T., Owusu-Boaitey N., Pugh S., Fosdick B.K., Zwi A.B., Malani A., Soman S., Besançon L., Kashnitsky I., Ganesh S., et al. Assessing the burden of COVID-19 in developing countries: Systematic review, meta-analysis and public policy implications. BMJ Glob. Health. 2022;7:e008477. doi: 10.1136/bmjgh-2022-008477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V., Abdool Karim Q., Alejandria M.M., García C.H., Kieny M., Malekzadeh R., et al. Repurposed Antiviral Drugs for Covid-19—Interim WHO Solidarity Trial Results. N. Engl. J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schellack N., Strydom M., Pepper M.S., Herd C.L., Hendricks C.L., Bronkhorst E., Meyer J.C., Padayachee N., Bangalee V., Truter I., et al. Social Media and COVID-19—Perceptions and Public Deceptions of Ivermectin, Colchicine and Hydroxychloroquine: Lessons for Future Pandemics. Antibiotics. 2022;11:445. doi: 10.3390/antibiotics11040445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryant A., Lawrie T.A., Dowswell T., Fordham E.J., Mitchell S., Hill S.R., Tham T.C. Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-analysis, and Trial Sequential Analysis to Inform Clinical Guidelines. Am. J. Ther. 2021;28:e434–e460. doi: 10.1097/MJT.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uzunova K., Filipova E., Pavlova V., Vekov T. Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2. Biomed. Pharmacother. 2020;131:110668. doi: 10.1016/j.biopha.2020.110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abubakar A.R., Sani I.H., Godman B., Kumar S., Islam S., Jahan I., Haque M. Systematic Review on the Therapeutic Options for COVID-19: Clinical Evidence of Drug Efficacy and Implications. Infect. Drug Resist. 2020;13:4673–4695. doi: 10.2147/IDR.S289037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medhi B., Sarma P., Bhattacharyya A., Kaur H., Prajapat M., Prakash A., Kumar S., Bansal S., Kirubakaran R., Reddy D., et al. Efficacy and safety of steroid therapy in COVID-19: A rapid systematic review and Meta-analysis. Indian J. Pharmacol. 2020;52:535–550. doi: 10.4103/ijp.ijp_1146_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sulis G., Batomen B., Kotwani A., Pai M., Gandra S. Sales of antibiotics and hydroxychloroquine in India during the COVID-19 epidemic: An interrupted time series analysis. PLoS Med. 2021;18:e1003682. doi: 10.1371/journal.pmed.1003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sefah I.A., Ogunleye O.O., Essah D.O., Opanga S.A., Butt N., Wamaitha A., Guantai A.N., Chikowe I., Khuluza F., Kibuule D., et al. Rapid Assessment of the Potential Paucity and Price Increases for Suggested Medicines and Protection Equipment for COVID-19 Across Developing Countries With a Particular Focus on Africa and the Implications. Front. Pharmacol. 2021;11:588106. doi: 10.3389/fphar.2020.588106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charan J., Dutta S., Kaur R., Bhardwaj P., Ambwani S., Godman B., Jha P., Sukhija S., Venkatesh S., Lugova H., et al. Demand of COVID-19 medicines without prescription among community pharmacies in Jodhpur, India: Findings and implications. J. Fam. Med. Prim. Care. 2022;11:503. doi: 10.4103/jfmpc.jfmpc_1250_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haque M., Gowere M., Nusrat N., Chowdhury K., Godman B. The response to COVID 19 across countries and the implications for future pandemics. Bangladesh J. Med Sci. 2021;20:7–14. doi: 10.3329/bjms.v20i5.55417. [DOI] [Google Scholar]
- 29.Manivannan E., Karthikeyan C., Moorthy N.S.H.N., Chaturvedi S.C. The Rise and Fall of Chloroquine/Hydroxychloroquine as Compassionate Therapy of COVID-19. Front. Pharmacol. 2021;12:584940. doi: 10.3389/fphar.2021.584940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charan J., Kaur R.J., Bhardwaj P., Haque M., Sharma P., Misra S., Godman B. Rapid review of suspected adverse drug events due to remdesivir in the WHO database; findings and implications. Expert Rev. Clin. Pharmacol. 2020;14:95–103. doi: 10.1080/17512433.2021.1856655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abena P.M., Decloedt E.H., Bottieau E., Suleman F., Adejumo P., Sam-Agudu N.A., Muyembe TamFum J.-J., Seydi M., Eholie S.P., Mills E.J., et al. Chloroquine and Hydroxychloroquine for the Prevention or Treatment of novel coronavirus disease (COVID-19) in Africa: Caution for Inappropriate Off-label Use in Healthcare Settings. Am. J. Trop. Med. Hyg. 2020;102:1184–1188. doi: 10.4269/ajtmh.20-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.17863/cam.57006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.RECOVERY Collaborative Group Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO WHO Discontinues Hydroxychloroquine and Lopinavir/Ritonavir Treatment Arms for COVID-19. 2020. [(accessed on 1 August 2022)]. Available online: https://www.who.int/news/item/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19.
- 35.Dyer O. COVID-19: Remdesivir Has Little or No Impact on Survival, WHO Trial Shows. BMJ. 2020;371:m4057. doi: 10.1136/bmj.m4057. [DOI] [PubMed] [Google Scholar]
- 36.Deng J., Zhou F., Ali S., Heybati K., Hou W., Huang E., Wong C.Y. Correction to: Efficacy and safety of ivermectin for the treatment of COVID-19: A systematic review and meta-analysis. QJM Int. J. Med. 2022;114:721–734. doi: 10.1093/qjmed/hcab247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.RECOVERY Collaborative Group. Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R., Wiselka M., Ustianowski A., Elmahi E., et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CIOMS Medicines Assessment during Public Health Emergencies Needs Good Science, Best Practices and Proper Communication. 2020. [(accessed on 2 August 2022)]. Available online: https://cioms.ch/es-template/medicines-assessment-during-public-health-emergencies-needs-good-science-best-practices-and-proper-communication/
- 39.Grimes D.R. Medical disinformation and the unviable nature of COVID-19 conspiracy theories. PLoS ONE. 2021;16:e0245900. doi: 10.1371/journal.pone.0245900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neil S.J., Campbell E.M. Fake Science: XMRV, COVID-19, and the Toxic Legacy of Dr. Judy Mikovits. AIDS Res. Hum. Retrovir. 2020;36:545–549. doi: 10.1089/aid.2020.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carley S., Horner D., Body R., Mackway-Jones K. Evidence-based medicine and COVID-19: What to believe and when to change. Emerg. Med. J. 2020;37:572–575. doi: 10.1136/emermed-2020-210098. [DOI] [PubMed] [Google Scholar]
- 42.Kluge H.H.P., Wickramasinghe K., Rippin H.L., Mendes R., Peters D.H., Kontsevaya A., Breda J. Prevention and control of non-communicable diseases in the COVID-19 response. Lancet. 2020;395:1678–1680. doi: 10.1016/S0140-6736(20)31067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fatoye F., Gebrye T., Arije O., Fatoye C.T., Onigbinde O., Mbada C. Economic Impact of COVID-19 Lockdown on households. Pan Afr. Med. J. 2021;40:225. doi: 10.11604/pamj.2021.40.225.27446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glied S., Levy H. The Potential Effects of Coronavirus on National Health Expenditures. JAMA. 2020;323:2001. doi: 10.1001/jama.2020.6644. [DOI] [PubMed] [Google Scholar]
- 45.Richards F., Kodjamanova P., Chen X., Li N., Atanasov P., Bennetts L., Patterson B.J., Yektashenas B., Mesa-Frias M., Tronczynski K., et al. Economic Burden of COVID-19: A Systematic Review. Clin. Outcomes Res. 2022;14:293–307. doi: 10.2147/CEOR.S338225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buheji M., da Costa Cunha K., Beka G., Mavric B., De Souza Y.L., da Costa Silva S.S., Hanafi M., Yein T.C. The Extent of COVID-19 Pandemic Socio-Economic Impact on Global Poverty. A Global Integrative Multidisciplinary Review. Am. J. Econ. 2020;10:213–224. doi: 10.5923/j.economics.20201004.02. [DOI] [Google Scholar]
- 47.Martin A., Markhvida M., Hallegatte S., Walsh B. Socio-Economic Impacts of COVID-19 on Household Consumption and Poverty. Econ. Disasters Clim. Chang. 2020;4:453–479. doi: 10.1007/s41885-020-00070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The Economist What Is the Economic Cost of COVID-19? 2021. [(accessed on 1 August 2022)]. Available online: https://www.economist.com/finance-and-economics/2021/01/09/what-is-the-economic-cost-of-covid-19.
- 49.Suthar S., Das S., Nagpure A., Madhurantakam C., Tiwari S.B., Gahlot P., Tyagi V.K. Epidemiology and diagnosis, environmental resources quality and socio-economic perspectives for COVID-19 pandemic. J. Environ. Manag. 2020;280:111700. doi: 10.1016/j.jenvman.2020.111700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riera R., Bagattini M., Pacheco R.L., Pachito D.V., Roitberg F., Ilbawi A. Delays and Disruptions in Cancer Health Care Due to COVID-19 Pandemic: Systematic Review. JCO Glob. Oncol. 2021;7:311–323. doi: 10.1200/GO.20.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moraliyage H., De Silva D., Ranasinghe W., Adikari A., Alahakoon D., Prasad R., Lawrentschuk N., Bolton D. Cancer in Lockdown: Impact of the COVID -19 Pandemic on Patients with Cancer. Oncologist. 2020;26:e342–e344. doi: 10.1002/onco.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang H., Zhou Y., Tang W. Maintaining HIV care during the COVID-19 pandemic. Lancet HIV. 2020;7:e308–e309. doi: 10.1016/S2352-3018(20)30105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ataguba J.E. COVID-19 Pandemic, a War to be Won: Understanding its Economic Implications for Africa. Appl. Health Econ. Health Policy. 2020;18:325–328. doi: 10.1007/s40258-020-00580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abbas K., Procter S.R., van Zandvoort K., Clark A., Funk S., Mengistu T., Hogan D., Dansereau E., Jit M., Flasche S., et al. Routine childhood immunisation during the COVID-19 pandemic in Africa: A benefit-risk analysis of health benefits versus excess risk of SARS-CoV-2 infection. Lancet Glob. Health. 2020;8:e1264–e1272. doi: 10.1016/S2214-109X(20)30308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Causey K., Fullman N., Sorensen R.J.D., Galles N.C., Zheng P., Aravkin A., Danovaro-Holliday M.C., Martinez-Piedra R., Sodha S.V., Velandia-González M.P., et al. Estimating global and regional disruptions to routine childhood vaccine coverage during the COVID-19 pandemic in 2020: A modelling study. Lancet. 2021;398:522–534. doi: 10.1016/S0140-6736(21)01337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shet A., Carr K., Danovaro-Holliday M.C., Sodha S.V., Prosperi C., Wunderlich J., Wonodi C., Reynolds H.W., Mirza I., Gacic-Dobo M., et al. Impact of the SARS-CoV-2 pandemic on routine immunisation services: Evidence of disruption and recovery from 170 countries and territories. Lancet Glob. Health. 2022;10:e186–e194. doi: 10.1016/S2214-109X(21)00512-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Evans B., Jombart T. Worldwide routine immunisation coverage regressed during the first year of the COVID-19 pandemic. Vaccine. 2022;40:3531–3535. doi: 10.1016/j.vaccine.2022.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mohammed I., Nauman A., Paul P., Ganesan S., Chen K.-H., Jalil S.M.S., Jaouni S.H., Kawas H., Khan W.A., Vattoth A.L., et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum. Vaccines Immunother. 2022;18:2027160. doi: 10.1080/21645515.2022.2027160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang K., Wang L., Li M., Xie B., He L., Wang M., Zhang R., Hou N., Zhang Y., Jia F. Real-Word Effectiveness of Global COVID-19 Vaccines Against SARS-CoV-2 Variants: A Systematic Review and Meta-Analysis. Front. Med. 2022;9:820544. doi: 10.3389/fmed.2022.820544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feikin D.R., Higdon M.M., Abu-Raddad L.J., Andrews N., Araos R., Goldberg Y., Groome M.J., Huppert A., O’Brien K.L., Smith P.G. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Korang S.K., von Rohden E., Veroniki A.A., Ong G., Ngalamika O., Siddiqui F., Juul S., Nielsen E.E., Feinberg J.B., Petersen J.J., et al. Vaccines to prevent COVID-19: A living systematic review with Trial Sequential Analysis and network meta-analysis of randomized clinical trials. PLoS ONE. 2022;17:e0260733. doi: 10.1371/journal.pone.0260733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sallam M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines. 2021;9:160. doi: 10.3390/vaccines9020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeng B., Gao L., Zhou Q., Yu K., Sun F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: A systematic review and meta-analysis. BMC Med. 2022;20:200. doi: 10.1186/s12916-022-02397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chenchula S., Karunakaran P., Sharma S., Chavan M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review. J. Med. Virol. 2022;94:2969–2976. doi: 10.1002/jmv.27697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fajar J.K., Sallam M., Soegiarto G., Sugiri Y.J., Anshory M., Wulandari L., Kosasih S.A.P., Ilmawan M., Kusnaeni K., Fikri M., et al. Global Prevalence and Potential Influencing Factors of COVID-19 Vaccination Hesitancy: A Meta-Analysis. Vaccines. 2022;10:1356. doi: 10.3390/vaccines10081356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bono S.A., Faria de Moura Villela E., Siau C.S., Chen W.S., Pengpid S., Hasan M.T., Sessou P., Ditekemena J.D., Amodan B.O., Hosseinipour M.C., et al. Factors Affecting COVID-19 Vaccine Acceptance: An International Survey among Low- and Middle-Income Countries. Vaccines. 2021;9:515. doi: 10.3390/vaccines9050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Norhayati M.N., Yusof R.C., Azman Y.M. Systematic Review and Meta-Analysis of COVID-19 Vaccination Acceptance. Front. Med. 2022;8:783982. doi: 10.3389/fmed.2021.783982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kanyanda S., Markhof Y., Wollburg P., Zezza A. Acceptance of COVID-19 vaccines in sub-Saharan Africa: Evidence from six national phone surveys. BMJ Open. 2021;11:e055159. doi: 10.1136/bmjopen-2021-055159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adebisi Y.A., Alaran A.J., Bolarinwa O.A., Akande-Sholabi W., Lucero-Prisno D.E. When it is available, will we take it? Social media users’ perception of hypothetical COVID-19 vaccine in Nigeria. Pan Afr. Med. J. 2021;38:230. doi: 10.11604/pamj.2021.38.230.27325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adedeji-Adenola H., Olugbake O.A., Adeosun S.A. Factors influencing COVID-19 vaccine uptake among adults in Nigeria. PLoS ONE. 2022;17:e0264371. doi: 10.1371/journal.pone.0264371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oyekale A.S. Factors Influencing Willingness to Be Vaccinated against COVID-19 in Nigeria. Int. J. Environ. Res. Public Health. 2022;19:6816. doi: 10.3390/ijerph19116816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tobin E.A., Okonofua M., Adeke A., Obi A. Willingness to Accept a COVID-19 Vaccine in Nigeria: A Population-based Cross-sectional Study. Cent. Afr. J. Public Health. 2021;7:53. doi: 10.11648/j.cajph.20210702.12. [DOI] [Google Scholar]
- 73.Al-Ali D., Elshafeey A., Mushannen M., Kawas H., Shafiq A., Mhaimeed N., Mhaimeed O., Mhaimeed N., Zeghlache R., Salameh M., et al. Cardiovascular and haematological events post COVID-19 vaccination: A systematic review. J. Cell. Mol. Med. 2021;26:636–653. doi: 10.1111/jcmm.17137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hippisley-Cox J., Patone M., Mei X.W., Saatci D., Dixon S., Khunti K., Zaccardi F., Watkinson P., Shankar-Hari M., Doidge J., et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: Self-controlled case series study. BMJ. 2021;374:n1931. doi: 10.1136/bmj.n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kouhpayeh H., Ansari H. Adverse events following COVID-19 vaccination: A systematic review and meta-analysis. Int. Immunopharmacol. 2022;109:108906. doi: 10.1016/j.intimp.2022.108906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lau C.L., Galea I. Risk-benefit analysis of COVID-19 vaccines—A neurological perspective. Nat. Rev Neurol. 2022;18:69–70. doi: 10.1038/s41582-021-00606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tabong P.T., Opoku Mensah K., Asampong E. Preparation for COVID-19 vaccines rollout: Interventions to increase trust, acceptability, and uptake in West African countries. Int. J. Health Plan. Manag. 2022;37:1221–1228. doi: 10.1002/hpm.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.MacDonald N.E., Eskola J., Liang X., Chaudhuri M., Dube E., Gellin B., Goldstein S., Larson H., Manzo M.L., Reingold A., et al. Vaccine Hesitancy: Definition, Scope and Determinants. Vaccine. 2015;33:4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 79.Pires C. Global Predictors of COVID-19 Vaccine Hesitancy: A Systematic Review. Vaccines. 2022;10:1349. doi: 10.3390/vaccines10081349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sallam M., Al-Sanafi M., Sallam M. A Global Map of COVID-19 Vaccine Acceptance Rates per Country: An Updated Concise Narrative Review. J. Multidiscip. Healthc. 2022;15:21–45. doi: 10.2147/JMDH.S347669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leach M., MacGregor H., Akello G., Babawo L., Baluku M., Desclaux A., Grant C., Kamara F., Nyakoi M., Parker M., et al. Vaccine anxieties, vaccine preparedness: Perspectives from Africa in a COVID-19 era. Soc. Sci. Med. 2022;298:114826. doi: 10.1016/j.socscimed.2022.114826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dinga J.N., Sinda L.K., Titanji V.P.K. Assessment of Vaccine Hesitancy to a COVID-19 Vaccine in Cameroonian Adults and Its Global Implication. Vaccines. 2021;9:175. doi: 10.3390/vaccines9020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Faye S.L.B., Krumkamp R., Doumbia S., Tounkara M., Strauss R., Ouedraogo H.G., Sagna T., Barry A.M., Mbawah A.K., Doumbia C.O., et al. Factors influencing hesitancy towards adult and child COVID-19 vaccines in rural and urban West Africa: A cross-sectional study. BMJ Open. 2022;12:e059138. doi: 10.1136/bmjopen-2021-059138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ahiakpa J.K., Cosmas N.T., Anyiam F.E., Enalume K.O., Lawan I., Gabriel I.B., Oforka C.L., Dahir H.G., Fausat S.T., Nwobodo M.A., et al. COVID-19 vaccines uptake: Public knowledge, awareness, perception and acceptance among adult Africans. PLoS ONE. 2022;17:e0268230. doi: 10.1371/journal.pone.0268230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anjorin A.A., Odetokun I.A., Abioye A.I., Elnadi H., Umoren M.V., Damaris B.F., Eyedo J., Umar H.I., Nyandwi J.B., Abdalla M.M., et al. Will Africans take COVID-19 vaccination? PLoS ONE. 2021;16:e0260575. doi: 10.1371/journal.pone.0260575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Katoto P.D.M.C., Parker S., Coulson N., Pillay N., Cooper S., Jaca A., Mavundza E., Houston G., Groenewald C., Essack Z., et al. Predictors of COVID-19 Vaccine Hesitancy in South African Local Communities: The VaxScenes Study. Vaccines. 2022;10:353. doi: 10.3390/vaccines10030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nindrea R.D., Usman E., Katar Y., Sari N.P. Acceptance of COVID-19 vaccination and correlated variables among global populations: A systematic review and meta-analysis. Clin. Epidemiol. Glob. Health. 2021;12:100899. doi: 10.1016/j.cegh.2021.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shah J., Abeid A., Sharma K., Manji S., Nambafu J., Korom R., Patel K., Said M., Mohamed M.A., Sood M., et al. Perceptions and Knowledge towards COVID-19 Vaccine Hesitancy among a Subpopulation of Adults in Kenya: An English Survey at Six Healthcare Facilities. Vaccines. 2022;10:705. doi: 10.3390/vaccines10050705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mudenda S., Hikaambo C.N., Daka V., Chileshe M., Mfune R.L., Kampamba M., Kasanga M., Phiri M., Mufwambi W., Banda M., et al. Prevalence and factors associated with COVID-19 vaccine acceptance in Zambia: A web-based cross-sectional study. Pan Afr. Med. J. 2022;41:112. doi: 10.11604/pamj.2022.41.112.31219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cooper S., van Rooyen H., Wiysonge C.S. COVID-19 vaccine hesitancy in South Africa: How can we maximize uptake of COVID-19 vaccines? Expert Rev. Vaccines. 2021;20:921–933. doi: 10.1080/14760584.2021.1949291. [DOI] [PubMed] [Google Scholar]
- 91.Mundagowa P.T., Tozivepi S.N., Chiyaka E.T., Mukora-Mutseyekwa F., Makurumidze R. Assessment of COVID-19 vaccine hesitancy among Zimbabweans: A rapid national survey. PLoS ONE. 2022;17:e0266724. doi: 10.1371/journal.pone.0266724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Acheampong T., Akorsikumah E., Osae-Kwapong J., Khalid M., Appiah A., Amuasi J. Examining Vaccine Hesitancy in Sub-Saharan Africa: A Survey of the Knowledge and Attitudes among Adults to Receive COVID-19 Vaccines in Ghana. Vaccines. 2021;9:814. doi: 10.3390/vaccines9080814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murewanhema G., Musuka G., Mukwenha S., Chingombe I., Mapingure M.P., Dzinamarira T. Hesitancy, ignorance or uncertainty? The need for effective communication strategies as Zimbabwe’s uptake of COVID-19 vaccine booster doses remains poor. Public Health Pract. 2022;3:100244. doi: 10.1016/j.puhip.2022.100244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nyalile T., Loo L. Situating COVID-19 Vaccine Hesitancy in Tanzania. 2021. [(accessed on 2 August 2022)]. Available online: http://somatosphere.net/2021/covid-19-vaccine-hesitancy-tanzania.html/
- 95.Sippy P. Is It Too Late to Fight Covid Skepticism and Vaccine Hesitancy in Tanzania? 2021. [(accessed on 2 August 2022)]. Available online: https://qz.com/africa/2055780/covid-skepticism-and-vaccine-hesitancy-is-widespread-in-tanzania/
- 96.Ackah M., Ameyaw L., Salifu M.G., Asubonteng D.P.A., Yeboah C.O., Annor E.N., Ankapong E.A.K., Boakye H. COVID-19 vaccine acceptance among health care workers in Africa: A systematic review and meta-analysis. PLoS ONE. 2022;17:e0268711. doi: 10.1371/journal.pone.0268711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kanyike A.M., Olum R., Kajjimu J., Ojilong D., Akech G.M., Nassozi D.R., Agira D., Wamala N.K., Asiimwe A., Matovu D., et al. Acceptance of the coronavirus disease-2019 vaccine among medical students in Uganda. Trop. Med. Health. 2021;49:37. doi: 10.1186/s41182-021-00331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kabamba Nzaji M., Kabamba Ngombe L., Ngoie Mwamba G., Banza Ndala D.B., Mbidi Miema J., Lungoyo C.L., Mwimba B.L., Bene A.C.M., Musenga E.M. Acceptability of Vaccination Against COVID-19 Among Healthcare Workers in the Democratic Republic of the Congo. Pragmatic Obs. Res. 2020;11:103–109. doi: 10.2147/POR.S271096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.World Health Organization (WHO) Ten Threats to Global Health in 2019. 2019. [(accessed on 15 February 2022)]. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
- 100.Anakpo G., Mishi S. Hesitancy of COVID-19 vaccines: Rapid systematic review of the measurement, predictors, and preventive strategies. Hum. Vaccines Immunother. 2022;18:2074716. doi: 10.1080/21645515.2022.2074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Watson O.J., Barnsley G., Toor J., Hogan A.B., Winskill P., Ghani A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022;22:P1293–P1302. doi: 10.1016/S1473-3099(22)00320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kricorian K., Civen R., Equils O. COVID-19 vaccine hesitancy: Misinformation and perceptions of vaccine safety. Hum. Vaccines Immunother. 2021;18:1950504. doi: 10.1080/21645515.2021.1950504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Razai M.S., Chaudhry U.A.R., Doerholt K., Bauld L., Majeed A. Covid-19 vaccination hesitancy. BMJ. 2021;373:n1138. doi: 10.1136/bmj.n1138. [DOI] [PubMed] [Google Scholar]
- 104.Gerretsen P., Kim J., Caravaggio F., Quilty L., Sanches M., Wells S., Brown E.E., Agic B., Pollock B.G., Graff-Guerrero A. Individual determinants of COVID-19 vaccine hesitancy. PLoS ONE. 2021;16:e0258462. doi: 10.1371/journal.pone.0258462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Litaker J.R., Tamez N., Bray C.L., Durkalski W., Taylor R. Sociodemographic Factors Associated with Vaccine Hesitancy in Central Texas Immediately Prior to COVID-19 Vaccine Availability. Int. J. Environ. Res. Public Health. 2021;19:368. doi: 10.3390/ijerph19010368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kabakama S., Konje E.T., Dinga J.N., Kishamawe C., Morhason-Bello I., Hayombe P., Adeyemi O., Chimuka E., Lumu I., Amuasi J., et al. Commentary on COVID-19 Vaccine Hesitancy in sub-Saharan Africa. Trop. Med. Infect. Dis. 2022;7:130. doi: 10.3390/tropicalmed7070130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chan N.N., Ong K.W., Siau C.S., Lee K.W., Peh S.C., Yacob S., Chia Y.C., Seow V.K., Ooi P.B. The lived experiences of a COVID-19 immunization programme: Vaccine hesitancy and vaccine refusal. BMC Public Health. 2022;22:296. doi: 10.1186/s12889-022-12632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Afolabi A.A., Ilesanmi O.S. Dealing with vaccine hesitancy in Africa: The prospective COVID-19 vaccine context. Pan Afr. Med. J. 2021;38:3. doi: 10.11604/pamj.2021.38.3.27401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dzinamarira T., Nachipo B., Phiri B., Musuka G. COVID-19 Vaccine Roll-Out in South Africa and Zimbabwe: Urgent Need to Address Community Preparedness, Fears and Hesitancy. Vaccines. 2021;9:250. doi: 10.3390/vaccines9030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ekwebelem O.C., Yunusa I., Onyeaka H., Ekwebelem N.C., Nnorom-Dike O. COVID-19 vaccine rollout: Will it affect the rates of vaccine hesitancy in Africa? Public Health. 2021;197:e18–e19. doi: 10.1016/j.puhe.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Antimicrobial Resistance Collaborators Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alshaikh F.S., Godman B., Sindi O.N., Seaton R.A., Kurdi A. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: A systematic review and meta-analysis. PLoS ONE. 2022;17:e0272375. doi: 10.1371/journal.pone.0272375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Founou R.C., Blocker A.J., Noubom M., Tsayem C., Choukem S.P., Van Dongen M., Founou L.L. The COVID-19 pandemic: A threat to antimicrobial resistance containment. Futur. Sci. OA. 2021;7:FSO736. doi: 10.2144/fsoa-2021-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Razai M.S., Oakeshott P., Esmail A., Wiysonge C.S., Viswanath K., Mills M.C. COVID-19 vaccine hesitancy: The five Cs to tackle behavioural and sociodemographic factors. J. R. Soc. Med. 2021;114:295–298. doi: 10.1177/01410768211018951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roy D.N., Biswas M., Islam E., Azam S. Potential factors influencing COVID-19 vaccine acceptance and hesitancy: A systematic review. PLoS ONE. 2022;17:e0265496. doi: 10.1371/journal.pone.0265496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rutten L.J.F., Zhu X., Leppin A.L., Ridgeway J.L., Swift M.D., Griffin J.M., Sauver J.L.S., Virk A., Jacobson R.M. Evidence-Based Strategies for Clinical Organizations to Address COVID-19 Vaccine Hesitancy. Mayo Clin. Proc. 2021;96:699–707. doi: 10.1016/j.mayocp.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Trogen B., Pirofski L.-A. Understanding vaccine hesitancy in COVID-19. Nat. Med. 2021;2:498–501. doi: 10.1016/j.medj.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ekowo O.E., Manafa C., Isielu R.C., Okoli C.M., Chikodi I., Onwuasoanya A.F., Echendu S.T., Ihedoro I., Nwabueze U.D., Nwoke O.C. A cross sectional regional study looking at the factors responsible for the low COVID-19 vaccination rate in Nigeria. Pan Afr. Med. J. 2022;41:114. doi: 10.11604/pamj.2022.41.114.30767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wilson S.L., Wiysonge C. Social media and vaccine hesitancy. BMJ Glob. Health. 2020;5:e004206. doi: 10.1136/bmjgh-2020-004206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wawrzuta D., Klejdysz J., Jaworski M., Gotlib J., Panczyk M. Attitudes toward COVID-19 Vaccination on Social Media: A Cross-Platform Analysis. Vaccines. 2022;10:1190. doi: 10.3390/vaccines10081190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Iwu C.J., Jordan P., Jaja I.F., Iwu C.D., Wiysonge C.S. Treatment of COVID-19: Implications for antimicrobial resistance in Africa. Pan Afr. Med. J. 2020;35((Suppl. 2)):119. doi: 10.11604/pamj.supp.2020.35.2.23713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Troisi M., Andreano E., Sala C., Kabanova A., Rappuoli R. Vaccines as remedy for antimicrobial resistance and emerging infections. Curr. Opin. Immunol. 2020;65:102–106. doi: 10.1016/j.coi.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 123.Tagoe E.T., Sheikh N., Morton A., Nonvignon J., Sarker A.R., Williams L., Megiddo I. COVID-19 Vaccination in Lower-Middle Income Countries: National Stakeholder Views on Challenges, Barriers, and Potential Solutions. Front. Public Health. 2021;9:709127. doi: 10.3389/fpubh.2021.709127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Godman B., Egwuenu A., Haque M., Malande O., Schellack N., Kumar S., Saleem Z., Sneddon J., Hoxha I., Islam S., et al. Strategies to Improve Antimicrobial Utilization with a Special Focus on Developing Countries. Life. 2021;11:528. doi: 10.3390/life11060528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Godman B., Basu D., Pillay Y., Almeida P.H.R.F., Mwita J.C., Rwegerera G.M., Paramadhas B.D.A., Tiroyakgosi C., Patrick O., Niba L.L., et al. Ongoing and planned activities to improve the management of patients with Type 1 diabetes across Africa; implications for the future. Hosp. Prac. 2020;48:51–67. doi: 10.1080/21548331.2020.1745509. [DOI] [PubMed] [Google Scholar]
- 126.Godman B., Basu D., Pillay Y., Mwita J.C., Rwegerera G.M., Paramadhas B.D.A., Tiroyakgosi C., Okwen P.M., Niba L.L., Nonvignon J., et al. Review of Ongoing Activities and Challenges to Improve the Care of Patients with Type 2 Diabetes Across Africa and the Implications for the Future. Front. Pharmacol. 2020;11:108. doi: 10.3389/fphar.2020.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mwita J.C., Ogunleye O.O., Olalekan A., Kalungia A.C., Kurdi A., Saleem Z., Sneddon J., Godman B. Key Issues Surrounding Appropriate Antibiotic Use for Prevention of Surgical Site Infections in Low- and Middle-Income Countries: A Narrative Review and the Implications. Int. J. Gen. Med. 2021;14:515–530. doi: 10.2147/IJGM.S253216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Godman B., Leong T., Abubakar A.R., Kurdi A., Kalemeera F., Rwegerera G.M., Patrick O., Lum Niba L., Ibrahim K., Adefolarin A.A., et al. Availability and Use of Long-Acting Insulin Analogues Including Their Biosimilars across Africa: Findings and Implications. Intern. Med. 2021;11:343. [Google Scholar]
- 129.Godman B., Grobler C., Van-De-Lisle M., Wale J., Barbosa W.B., Massele A., Opondo P., Petrova G., Tachkov K., Sefah I., et al. Pharmacotherapeutic interventions for bipolar disorder type II: Addressing multiple symptoms and approaches with a particular emphasis on strategies in lower and middle-income countries. Expert Opin. Pharmacother. 2019;20:2237–2255. doi: 10.1080/14656566.2019.1684473. [DOI] [PubMed] [Google Scholar]
- 130.Godman B., Egwuenu A., Wesangula E., Schellack N., Kalungia A.C., Tiroyakgosi C., Kgatlwane J., Mwita J.C., Patrick O., Niba L.L., et al. Tackling antimicrobial resistance across sub-Saharan Africa; current challenges and implications for the future. Expert Opin. Drug Saf. 2022;21:1089–1111. doi: 10.1080/14740338.2022.2106368. [DOI] [PubMed] [Google Scholar]
- 131.Mudenda S., Mukosha M., Hikaambo C.N., Meyer J.C., Fadare J., Kampamba M., Kalungia A.C., Munsaka S., Okoro R., Daka V., et al. Awareness and acceptance of COVID-19 vaccines and associated factors among pharmacy students in Zambia. Malawi Med. J. 2022;34:236–243. doi: 10.4314/mmj.v34i4.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Godman B., Hill A., Simoens S., Selke G., Selke Krulichová I., Zampirolli Dias C., Martin A.P., Oortwijn W., Timoney A., Gustafsson L.L., et al. Potential approaches for the pricing of cancer medicines across Europe to enhance the sustainability of healthcare systems and the implications. Expert Rev. Pharmacoecon. Outcomes Res. 2021;21:527–540. doi: 10.1080/14737167.2021.1884546. [DOI] [PubMed] [Google Scholar]
- 133.Godman B., Haque M., McKimm J., Abu Bakar M., Sneddon J., Wale J., Campbell S., Martin A.P., Hoxha I., Abilova V., et al. Ongoing strategies to improve the management of upper respiratory tract infections and reduce inappropriate antibiotic use particularly among lower and middle-income countries: Findings and implications for the future. Curr. Med. Res. Opin. 2019;36:301–327. doi: 10.1080/03007995.2019.1700947. [DOI] [PubMed] [Google Scholar]
- 134.Gale N.K., Heath G., Cameron E., Rashid S., Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med. Res. Methodol. 2013;13:117. doi: 10.1186/1471-2288-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Allemang B., Sitter K., Dimitropoulos G. Pragmatism as a paradigm for patient-oriented research. Health Expect. 2021;25:38–47. doi: 10.1111/hex.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kaushik V., Walsh C.A. Pragmatism as a Research Paradigm and Its Implications for Social Work Research. Soc. Sci. 2019;8:255. doi: 10.3390/socsci8090255. [DOI] [Google Scholar]
- 137.Palinkas L.A., Horwitz S.M., Green C.A., Wisdom J.P., Duan N., Hoagwood K. Purposeful Sampling for Qualitative Data Collection and Analysis in Mixed Method Implementation Research. Adm. Policy Ment. Health Ment. Health Serv. Res. 2015;42:533–544. doi: 10.1007/s10488-013-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.World Bank World Bank National Accounts Data—GDP Per Capita (Current US$) [(accessed on 11 September 2020)]. Available online: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD.
- 139.Worldometer African Countries by Population. 2021. [(accessed on 7 September 2022)]. Available online: https://www.worldometers.info/population/countries-in-africa-by-population/
- 140.The Economist How We Estimated the True Death Toll of the Pandemic—Dealing with Potential Outcomes, Known Unknowns, and Uncertainty. 2021. [(accessed on 7 September 2022)]. Available online: https://www.economist.com/graphic-detail/2021/05/13/how-we-estimated-the-true-death-toll-of-the-pandemic.
- 141.Cox L., Yah C. Estimating actual COVID-19 case numbers using cumulative death count-A method of measuring effectiveness of lockdown of non-essential activities: A South African case study. Pan Afr. Med. J. 2020;35((Suppl. 2)):97. doi: 10.11604/pamj.supp.2020.35.2.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bradshaw D., Dorrington R.E., Laubscher R., Moultrie T.A., Groenewald P. Tracking mortality in near to real time provides essential information about the impact of the COVID-19 pandemic in South Africa in 2020. S. Afr. Med J. 2021;111:732. doi: 10.7196/SAMJ.2021.v111i8.15809. [DOI] [PubMed] [Google Scholar]
- 143.WHO COVID-19 Vaccination in the WHO African Region. Monthly Bulletin June 2022. [(accessed on 7 September 2022)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/359928/CV-20220714-eng.pdf.
- 144.Ritchie H.E., Mathieu L., Rodés-Guirao C., Appel C., Giattino E., Ortiz-Ospina J., Hasell B., Macdonald D., Roser B.A.M. Coronavirus (COVID-19) Vaccinations. 2020. [(accessed on 28 December 2021)]. Available online: https://ourworldindata.org/covid-vaccinations.
- 145.Mathieu E., Ritchie H., Ortiz-Ospina E., Roser M., Hasell J., Appel C., Giattino C., Rodés-Guirao L. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021;5:947–953. doi: 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
- 146.Rolfe R., Kwobah C., Muro F., Ruwanpathirana A., Lyamuya F., Bodinayake C., Nagahawatte A., Piyasiri B., Sheng T., Bollinger J., et al. Barriers to implementing antimicrobial stewardship programs in three low- and middle-income country tertiary care settings: Findings from a multi-site qualitative study. Antimicrob. Resist. Infect. Control. 2021;10:60. doi: 10.1186/s13756-021-00929-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wettermark B., Godman B., Jacobsson B., Haaijer-Ruskamp F.M. Soft regulations in pharmaceutical policy making: An overview of current approaches and their consequences. Appl. Health Econ. Health Policy. 2009;7:137–147. doi: 10.1007/BF03256147. [DOI] [PubMed] [Google Scholar]
- 148.Godman B. Health authority activities to enhance the quality and efficiency of medicine use and their impact. Adv. Hum. Biol. 2021;11:11. doi: 10.4103/2321-8568.308858. [DOI] [Google Scholar]
- 149.Moon J., Godman B., Petzold M., Alvarez-Madrazo S., Bennett K., Bishop I., Bucsics A., Hesse U., Martin A., Simoens S., et al. Different initiatives across Europe to enhance losartan utilization post generics: Impact and implications. Front. Pharmacol. 2014;5:219. doi: 10.3389/fphar.2014.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Godman B., Wettermark B., Van Woerkom M., Fraeyman J., Alvarez-Madrazo S., Berg C., Bishop I., Bucsics A., Campbell S., Finlayson A.E., et al. Multiple policies to enhance prescribing efficiency for established medicines in Europe with a particular focus on demand-side measures: Findings and future implications. Front. Pharmacol. 2014;5:106. doi: 10.3389/fphar.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Godman B., Malmström R.E., Diogene E., Gray A., Jayathissa S., Timoney A., Acurcio F., Alkan A., Brzezinska A., Bucsics A., et al. Are new models needed to optimize the utilization of new medicines to sustain healthcare systems? Expert Rev. Clin. Pharmacol. 2015;8:77–94. doi: 10.1586/17512433.2015.990380. [DOI] [PubMed] [Google Scholar]
- 152.Lima-Dellamora E.D.C., Caetano R., Gustafsson L.L., Godman B.B., Patterson K., Osorio-De-Castro C.G.S. An Analytical Framework for Assessing Drug and Therapeutics Committee Structure and Work Processes in Tertiary Brazilian Hospitals. Basic Clin. Pharmacol. Toxicol. 2014;115:268–276. doi: 10.1111/bcpt.12215. [DOI] [PubMed] [Google Scholar]
- 153.Matsitse T.B., Helberg E., Meyer J.C., Godman B., Massele A., Schellack N. Compliance with the primary health care treatment guidelines and the essential medicines list in the management of sexually transmitted infections in correctional centres in South Africa: Findings and implications. Expert Rev. Anti-Infect. Ther. 2017;15:963–972. doi: 10.1080/14787210.2017.1382354. [DOI] [PubMed] [Google Scholar]
- 154.Matlala M., Gous A.G., Godman B., Meyer J. Structure and activities of pharmacy and therapeutics committees among public hospitals in South Africa; findings and implications. Expert Rev. Clin. Pharmacol. 2017;10:1273–1280. doi: 10.1080/17512433.2017.1364625. [DOI] [PubMed] [Google Scholar]
- 155.Nguyen T.T.P., Do T.X., Nguyen H.A., Nguyen C.T.T., Meyer J.C., Godman B., Skosana P., Nguyen B.T. A National Survey of Dispensing Practice and Customer Knowledge on Antibiotic Use in Vietnam and the Implications. Antibiotics. 2022;11:1091. doi: 10.3390/antibiotics11081091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Alrasheedy A.A., Alsalloum M.A., Almuqbil F.A., Almuzaini M.A., Alkhayl B.S.A., Albishri A.S., Alharbi F.F., Alharbi S.R., Alodhayb A.K., Alfadl A.A., et al. The impact of law enforcement on dispensing antibiotics without prescription: A multi-methods study from Saudi Arabia. Expert Rev. Anti-Infect. Ther. 2019;18:87–97. doi: 10.1080/14787210.2020.1705156. [DOI] [PubMed] [Google Scholar]
- 157.Godman B., Haque M., Abubakar A.R., Ogunleye O.O., Sani I.H., Sefah I., Kurdi A., Islam S. Changes in availability, utilization, and prices of medicines and protection equipment for COVID-19 in an Urban population of Northern Nigeria. J. Res. Pharm. Prac. 2021;10:17–22. doi: 10.4103/jrpp.JRPP_20_92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gad M., Salem A., Oortwijn W., Hill R., Godman B. Mapping of Current Obstacles for Rationalizing Use of Medicines (CORUM) in Europe: Current Situation and Potential Solutions. Front. Pharmacol. 2020;11:144. doi: 10.3389/fphar.2020.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.World Health Organization COVAX. Working for Global Equitable Access to COVID-19 Vaccines. 2020. [(accessed on 19 February 2021)]. Available online: https://www.who.int/initiatives/act-accelerator/covax.
- 160.Ritchie H., Mathieu E., Rodés-Guirao L., Appel C., Giattino C., Ortiz-Ospina E., Hasell J., Macdonald B., Beltekian D., Roser M. Coronavirus Pandemic (COVID-19) 2020. [(accessed on 7 September 2022)]. Available online: https://ourworldindata.org/coronavirus.
- 161.Xinhua Botswana Launches Drive-through COVID-19 Caccination Campaign. 2021. [(accessed on 1 August 2022)]. Available online: http://www.news.cn/english/africa/2021-10/13/c_1310241172.htm.
- 162.Reliefweb Learning from Botswana’s COVID-19 Vaccine Rollout. 2021. [(accessed on 1 August 2022)]. Available online: https://reliefweb.int/report/botswana/learning-botswana-s-covid-19-vaccine-rollout.
- 163.Daily News Egypt Egypt Launches Campaign to Encourage Public to Register for COVID-19 Vaccination. 2021. [(accessed on 2 August 2022)]. Available online: https://www.zawya.com/en/life/egypt-launches-campaign-to-encourage-public-to-register-for-covid-19-vaccination-i6pousg2.
- 164.Saied S.M., Saied E.M., Kabbash I.A., Abdo S.A.E. Vaccine hesitancy: Beliefs and barriers associated with COVID-19 vaccination among Egyptian medical students. J. Med. Virol. 2021;93:4280–4291. doi: 10.1002/jmv.26910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Qunaibi E., Basheti I., Soudy M., Sultan I. Hesitancy of Arab Healthcare Workers towards COVID-19 Vaccination: A Large-Scale Multinational Study. Vaccines. 2021;9:446. doi: 10.3390/vaccines9050446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nabeth P., Hassan M., Adib K., Abubakar A., Brennan R. New COVID-19 resurgence in the WHO Eastern Mediterranean region. Lancet. 2021;397:1348–1349. doi: 10.1016/S0140-6736(21)00679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Reliefweb Eswatini: Vaccinating People against COVID-19 in Hard-to-Reach Communities. 2022. [(accessed on 2 August 2022)]. Available online: https://reliefweb.int/report/eswatini/eswatini-vaccinating-people-against-covid-19-hard-reach-communities.
- 168.Reliefweb In Eswatini, Pact Launches App to Fight COVID Misinformation, Vaccine Hesitancy. 2021. [(accessed on 2 August 2022)]. Available online: https://reliefweb.int/report/eswatini/eswatini-pact-launches-app-fight-covid-misinformation-vaccine-hesitancy.
- 169.Head M., Brackstone K., Boateng L. Vaccine Hesitancy Has Risen in Ghana: A Closer Look at WHO’S Worried. 2021. [(accessed on 2 August 2022)]. Available online: https://theconversation.com/vaccine-hesitancy-has-risen-in-ghana-a-closer-look-at-whos-worried-164733.
- 170.Brackstone K., Boateng L.A., Atengble K., Head M., Akinocho H., Osei K., Nuamah K. Examining Drivers of COVID-19 Vaccine Hesitancy in Ghana. University of Southampton; Southampton, UK: 2021. [DOI] [Google Scholar]
- 171.Alhassan R.K., Owusu-Agyei S., Ansah E.K., Gyapong M. COVID-19 vaccine uptake among health care workers in Ghana: A case for targeted vaccine deployment campaigns in the global south. Hum. Resour. Health. 2021;19:136. doi: 10.1186/s12960-021-00657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Agyekum M.W., Afrifa-Anane G.F., Kyei-Arthur F., Addo B. Acceptability of COVID-19 Vaccination among Health Care Workers in Ghana. Adv. Public Health. 2021;2021:9998176. doi: 10.1155/2021/9998176. [DOI] [Google Scholar]
- 173.Rufai N. After Botched Ebola Vaccine Trial, Ghana Struggles with Vaccine Hesitancy. 2021. [(accessed on 1 August 2022)]. Available online: https://www.pbs.org/newshour/show/after-botched-ebola-vaccine-trial-ghana-struggles-with-vaccine-hesitancy.
- 174.Afriyie D.K., Asare G.A., Amponsah S.K., Godman B. COVID-19 pandemic in resource-poor countries: Challenges, experiences and opportunities in Ghana. J. Infect. Dev. Ctries. 2020;14:838–843. doi: 10.3855/jidc.12909. [DOI] [PubMed] [Google Scholar]
- 175.Fan J., Wang X., Du S., Mao A., Du H., Qiu W. Discussion of the Trust in Vaccination against COVID-19. Vaccines. 2022;10:1214. doi: 10.3390/vaccines10081214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Osur J., Muinga E., Carter J., Kuria S., Hussein S., Ireri E.M. COVID-19 vaccine hesitancy: Vaccination intention and attitudes of community health volunteers in Kenya. PLoS Glob. Public Health. 2022;2:e0000233. doi: 10.1371/journal.pgph.0000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Orangi S., Pinchoff J., Mwanga D., Abuya T., Hamaluba M., Warimwe G., Austrian K., Barasa E. Assessing the Level and Determinants of COVID-19 Vaccine Confidence in Kenya. Vaccines. 2021;9:936. doi: 10.3390/vaccines9080936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Oyekale A.S. Compliance Indicators of COVID-19 Prevention and Vaccines Hesitancy in Kenya: A Random-Effects Endogenous Probit Model. Vaccines. 2021;9:1359. doi: 10.3390/vaccines9111359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Republic of Kenya Ministry of Health National COVID-19 Vaccine Deployment Plan, 2021. National Vaccine & Immunization Program—Acceleration of COVID-19 Vaccination Program in Kenya. [(accessed on 2 August 2022)]; Available online: https://www.health.go.ke/wp-content/uploads/2021/09/NATIONAL-COVID-19-VACCINE-DEPLOYMENT-PLAN-2021.pdf.
- 180.The World Bank Rolling Out COVID-19 Vaccines in Malawi Amid Hesitancy and Supply Challenges. 2021. [(accessed on 1 August 2022)]. Available online: https://www.worldbank.org/en/news/feature/2021/10/19/rolling-out-covid-19-vaccines-in-malawi-amid-hesitancy-and-supply-challenges.
- 181.WHO Malawi Malawi Marks One Year of COVID-19 Vaccination, 828, 080 People Receive Full Dose. 2022. [(accessed on 1 August 2022)]. Available online: https://www.afro.who.int/countries/malawi/news/malawi-marks-one-year-covid-19-vaccination-828-080-people-receive-full-dose.
- 182.Masina L. Malawi Fears Its COVID Vaccines Will Expire Due to Hesitancy. 2021. [(accessed on 1 August 2022)]. Available online: https://www.voanews.com/a/africa_malawi-fears-its-covid-vaccines-will-expire-due-hesitancy/6219387.html.
- 183.Tembo L. Tackling COVID-19 Vaccine Misinformation Through Faith Leaders—Faith Leaders Are Key in Behaviour Change. 2022. [(accessed on 1 August 2022)]. Available online: https://www.unicef.org/malawi/stories/tackling-covid-19-vaccine-misinformation-through-faith-leaders.
- 184.Ezigbo O. Nigeria: Tackling Vaccine Hesitancy Fuelled by Misinformation, Propaganda. 2021. [(accessed on 2 August 2022)]. Available online: https://allafrica.com/stories/202109230882.html.
- 185.Nomhwange T., Wariri O., Nkereuwem E., Olanrewaju S., Nwosu N., Adamu U., Danjuma E., Onuaguluchi N., Enegela J., Nomhwange E., et al. COVID-19 vaccine hesitancy amongst healthcare workers: An assessment of its magnitude and determinants during the initial phase of national vaccine deployment in Nigeria. eClinicalMedicine. 2022;50:101499. doi: 10.1016/j.eclinm.2022.101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Njoga E.O., Mshelbwala P.P., Abah K.O., Awoyomi O.J., Wangdi K., Pewan S.B., Oyeleye F.A., Galadima H.B., Alhassan S.A., Okoli C.E., et al. COVID-19 Vaccine Hesitancy and Determinants of Acceptance among Healthcare Workers, Academics and Tertiary Students in Nigeria. Vaccines. 2022;10:626. doi: 10.3390/vaccines10040626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Iliyasu Z., Umar A.A., Abdullahi H.M., Kwaku A.A., Amole T.G., Tsiga-Ahmed F.I., Garba R.M., Salihu H.M., Aliyu M.H. “They have produced a vaccine, but we doubt if COVID-19 exists”: Correlates of COVID-19 vaccine acceptability among adults in Kano, Nigeria. Hum. Vaccines Immunother. 2021;17:4057–4064. doi: 10.1080/21645515.2021.1974796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chukwu O.A., Nnogo C.C. Surmounting inherent challenges in healthcare service delivery for effective procurement and distribution of COVID-19 vaccines; A developing country context. Health Policy Technol. 2021;10:100518. doi: 10.1016/j.hlpt.2021.100518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nkanjeni U. Over 50? The Health Department Is Offering You Free Transport to Vaccination Sites. 2021. [(accessed on 2 August 2022)]. Available online: https://www.timeslive.co.za/news/south-africa/2021-08-26-over-50-the-health-department-is-offering-you-free-transport-to-vaccination-sites/
- 190.Department of Health Republic of South Africa Vooma Voucher Communication. 2021. [(accessed on 1 August 2022)]. Available online: https://sacoronavirus.co.za/vooma-voucher-communication.
- 191.Engelbrecht M., Heunis C., Kigozi G. COVID-19 Vaccine Hesitancy in South Africa: Lessons for Future Pandemics. Int. J. Environ. Res. Public Health. 2022;19:6694. doi: 10.3390/ijerph19116694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kollamparambil U., Oyenubi A., Nwosu C. COVID-19 vaccine intentions in South Africa: Health communication strategy to address vaccine hesitancy. BMC Public Health. 2021;21:2113. doi: 10.1186/s12889-021-12196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Swed S., Mohamed T., Sakkour R., Motawea K.R., Bohsas H. COVID-19 vaccine hesitancy among indigenous people in Sudan: An incipient crisis. Ann. Med. Surg. 2022;75:103379. doi: 10.1016/j.amsu.2022.103379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Udoh K. COVID-19 Vaccine Hesitancy in South Sudan; What Lessons Can be Learned From Angola’s Success Story? Am. J. Health Promot. 2022;36:579–581. doi: 10.1177/08901171211070955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Raja S.M., Osman M.E., Musa A.O., Hussien A.A., Yusuf K. COVID-19 vaccine acceptance, hesitancy, and associated factors among medical students in Sudan. PLoS ONE. 2022;17:e0266670. doi: 10.1371/journal.pone.0266670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bongomin F., Olum R., Andia-Biraro I., Nakwagala F.N., Hassan K.H., Nassozi D.R., Kaddumukasa M., Byakika-Kibwika P., Kiguli S., Kirenga B.J. COVID-19 vaccine acceptance among high-risk populations in Uganda. Ther. Adv. Infect. Dis. 2021;8:20499361211024376. doi: 10.1177/20499361211024376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Carcelen A.C., Prosperi C., Mutembo S., Chongwe G., Mwansa F.D., Ndubani P., Simulundu E., Chilumba I., Musukwa G., Thuma P., et al. COVID-19 vaccine hesitancy in Zambia: A glimpse at the possible challenges ahead for COVID-19 vaccination rollout in sub-Saharan Africa. Hum. Vaccines Immunother. 2021;18:1–6. doi: 10.1080/21645515.2021.1948784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.McAbee L., Tapera O., Kanyangarara M. Factors Associated with COVID-19 Vaccine Intentions in Eastern Zimbabwe: A Cross-Sectional Study. Vaccines. 2021;9:1109. doi: 10.3390/vaccines9101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dubé E. Addressing vaccine hesitancy: The crucial role of healthcare providers. Clin. Microbiol. Infect. 2017;23:279–280. doi: 10.1016/j.cmi.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 200.Dror A.A., Eisenbach N., Taiber S., Morozov N.G., Mizrachi M., Zigron A., Srouji S., Sela E. Vaccine hesitancy: The next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020;35:775–779. doi: 10.1007/s10654-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Freeman D., Waite F., Rosebrock L., Petit A., Causier C., East A., Jenner L., Teale A.-L., Carr L., Mulhall S., et al. Coronavirus conspiracy beliefs, mistrust, and compliance with government guidelines in England. Psychol. Med. 2020;52:251–263. doi: 10.1017/S0033291720001890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Huangfu L., Mo Y., Zhang P., Zeng D.D., He S. COVID-19 Vaccine Tweets After Vaccine Rollout: Sentiment–Based Topic Modeling. J. Med. Internet Res. 2022;24:e31726. doi: 10.2196/31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Robertson C.T., Bentele K., Meyerson B., Wood A.S.A., Salwa J. Effects of political versus expert messaging on vaccination intentions of Trump voters. PLoS ONE. 2021;16:e0257988. doi: 10.1371/journal.pone.0257988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ajayi O. COVID-19: Nigeria Develops Two Vaccines, Awaits Clinical Trials, Certification. 2021. [(accessed on 1 August 2022)]. Available online: https://www.vanguardngr.com/2021/03/covid-19-nigeria-develops-two-vaccines-awaits-clinical-trials-certification-2/
- 205.WHO Pan African Clinical Trials Registry (PACTR) 2022. [(accessed on 2 August 2022)]. Available online: https://www.who.int/clinical-trials-registry-platform/network/primary-registries/pan-african-clinical-trials-registry-pactr.
- 206.AHRI The PAVIA Project Aims to Strengthen Pharmacovigilance (PV) in Four African Countries. [(accessed on 2 August 2022)];2018 Available online: https://ahri.gov.et/2021/03/04/the-pavia-project-aims-to-strengthen-pharmacovigilance-pv-in-four-african-countries/
- 207.Kibuule D., Nambahu L., Sefah I.A., Kurdi A., Phuong T.N.T., Kwon H.-Y., Godman B. Activities in Namibia to Limit the Prevalence and Mortality from COVID-19 Including Community Pharmacy Activities and the Implications. Sch. Acad. J. Pharm. 2021;10:82–92. doi: 10.36347/sajp.2021.v10i05.001. [DOI] [Google Scholar]
- 208.Schellack N., Coetzee M., Schellack G., Gijzelaar M., Hassim Z., Milne M., Bronkhorst E., Padayachee N., Singh N., Kolman S., et al. COVID-19: Guidelines for pharmacists in South Africa. S. Afr. J. Infect. Dis. 2020;35:a206. doi: 10.4102/sajid.v35i1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cadogan C.A., Hughes C.M. On the frontline against COVID-19: Community pharmacists’ contribution during a public health crisis. Res. Soc. Adm. Pharm. 2020;17:2032–2035. doi: 10.1016/j.sapharm.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hedima E.W., Adeyemi M.S., Ikunaiye N.Y. WITHDRAWN: Community pharmacists: On the frontline of health service against COVID-19 in LMICs. Res. Soc. Adm. Pharm. 2020 doi: 10.1016/j.sapharm.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kharel R., Baird J., Vaishnav H., Chillara N., Lee J.A., Genisca A., Hayward A., Uzevski V., Elbenni A., Levine A.C., et al. Development and assessment of novel virtual COVID-19 trainer-of trainers course implemented by an academic–humanitarian partnership. Glob. Health Action. 2022;15:2010391. doi: 10.1080/16549716.2021.2010391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.WHO Second Training of Trainers on Infection Prevention and Control. 2021. [(accessed on 6 September 2022)]. Available online: https://www.afro.who.int/pt/node/14623.
- 213.Xinhua Skepticism Still Rife Despite Rollout of COVID-19 Vaccine in Zambia. 2021. [(accessed on 1 August 2022)]. Available online: https://www.macaubusiness.com/skepticism-still-rife-despite-rollout-of-covid-19-vaccine-in-zambia/
- 214.Shaaban R., Ghazy R.M., Elsherif F., Ali N., Yakoub Y., Aly M.O., ElMakhzangy R., Abdou M.S., McKinna B., Elzorkany A.M., et al. COVID-19 Vaccine Acceptance among Social Media Users: A Content Analysis, Multi-Continent Study. Int. J. Environ. Res. Public Health. 2022;19:5737. doi: 10.3390/ijerph19095737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Muric G., Wu Y., Ferrara E. COVID-19 Vaccine Hesitancy on Social Media: Building a Public Twitter Data Set of Antivaccine Content, Vaccine Misinformation, and Conspiracies. JMIR Public Health Surveill. 2021;7:e30642. doi: 10.2196/30642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.IMF Seven Finance & Trade Lessons from COVID-19 for Future Pandemics. 2022. [(accessed on 2 August 2022)]. Available online: https://www.imf.org/en/Publications/WP/Issues/2022/05/20/Seven-Finance-Trade-Lessons-from-COVID-19-for-Future-Pandemics-517755.
- 217.Chavula J. COVID-19 Vaccines on Wheels. 2022. [(accessed on 1 August 2022)]. Available online: https://www.unicef.org/malawi/stories/covid-19-vaccines-wheels.
- 218.Rahman W. Egypt Allocates Mobile COVID-19 Vaccination Clinics for Elderly, Pensioners. 2021. [(accessed on 1 August 2022)]. Available online: https://english.aawsat.com/home/article/3008776/egypt-allocates-mobile-covid-19-vaccination-clinics-elderly-pensioners.
- 219.WHO New Consortium Working to Boost Vaccine Production in South Africa. 2021. [(accessed on 2 August 2022)]. Available online: https://www.who.int/news/item/30-07-2021-new-consortium-working-to-boost-vaccine-production-in-south-africa.
- 220.DW COVID-19: South Africa Develops Own Coronavirus Vaccine. 2021. [(accessed on 2 August 2022)]. Available online: https://www.dw.com/en/covid-19-south-africa-develops-own-coronavirus-vaccine/a-60121009.
- 221.Sun T. Boosting COVID-19 Vaccine Production in Nigeria, Others. 2022. [(accessed on 1 August 2022)]. Available online: https://www.sunnewsonline.com/boosting-covid-19-vaccine-production-in-nigeria-others/
- 222.Al-Monitor Egypt Establishes Largest Coronavirus Vaccine Factory in Middle East. 2021. [(accessed on 2 August 2022)]. Available online: https://www.al-monitor.com/originals/2021/09/egypt-establishes-largest-coronavirus-vaccine-factory-middle-east.
- 223.Bloomberg Aspen’s Covid Flop Bodes Ill for Africa’s Vaccine Making Drive. 2022. [(accessed on 6 September 2022)]. Available online: https://www.engineeringnews.co.za/article/aspens-covid-flop-bodes-ill-for-africas-vaccine-making-drive-2022-05-18.
- 224.Van Nguyen D., Nguyen P.-H. Social media and COVID-19 vaccination hesitancy: The mediating role of the COVID-19 vaccine perception. Heliyon. 2022:e10575. doi: 10.1016/j.heliyon.2022.e10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.WHO COVID-19 and Mandatory Vaccination: Ethical Considerations. 2022. [(accessed on 2 August 2022)]. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Policy-brief-Mandatory-vaccination-2022.1.
- 226.Gibelli F., Ricci G., Sirignano A., De Leo D. COVID-19 Compulsory Vaccination: Legal and Bioethical Controversies. Front. Med. 2022;9:821522. doi: 10.3389/fmed.2022.821522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hsu J. How COVID-19 Is Accelerating the Threat of Antimicrobial Resistance. BMJ. 2020;369:m1983. doi: 10.1136/bmj.m1983. [DOI] [PubMed] [Google Scholar]
- 228.WHO Strategy to Achieve Global Covid-19 Vaccination by Mid-2022. [(accessed on 2 August 2022)]. Available online: https://cdn.who.int/media/docs/default-source/immunization/covid-19/strategy-to-achieve-global-covid-19-vaccination-by-mid-2022.pdf.
- 229.WHO COVID-19 Vaccination in Africa Increases by Almost Three-Quarters in June 2022. 2022. [(accessed on 6 September 2022)]. Available online: https://www.afro.who.int/news/covid-19-vaccination-africa-increases-almost-three-quarters-june-2022.
- 230.Lassi Z., Naseem R., Salam R., Siddiqui F., Das J. The Impact of the COVID-19 Pandemic on Immunization Campaigns and Programs: A Systematic Review. Int. J. Environ. Res. Public Health. 2021;18:988. doi: 10.3390/ijerph18030988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Godman B., Sefah I.A., Kordorwu H.E., Essah D.O., Kurdi A. Prevalence rate of spontaneously reported adverse events and determinants of serious adverse events amongst three outpatient care settings in Ghana: Findings and implications. Adv. Hum. Biol. 2021;11:97. doi: 10.4103/aihb.aihb_148_20. [DOI] [Google Scholar]
- 232.ECA African Vaccine Acquisition Trust Delivers 108,000 Doses of COVID-19 Vaccine to Botswana. 2021. [(accessed on 2 August 2022)]. Available online: https://www.uneca.org/?q=stories/african-vaccine-acquisition-trust-delivers-108%2C000-doses-of-covid-19-vaccine-to-botswana.
- 233.Ndi N. Cameroon Receives 200,000 Doses of COVID-19 Vaccines from China. 2021. [(accessed on 1 August 2022)]. Available online: https://www.theeastafrican.co.ke/tea/rest-of-africa/cameroon-receives-covid-19-vaccines-from-china-3362950.
- 234.Amani A., Djossaya D., Njoh A.A., Fouda A.A.B., Ndoula S., Abba-Kabir H.M., Mossus T., Nguefack-Tsague G., Kamgno J. The first 30 days of COVID-19 vaccination in Cameroon: Achievements, challenges, and lessons learned. Pan Afr. Med. J. 2022;41:201. doi: 10.11604/pamj.2022.41.201.30218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Unicef Egypt Egypt Receives 546,400 Doses of COVID-19 Vaccine Donated by France Through AVAT and COVAX Platforms. 2021. [(accessed on 2 August 2022)]. Available online: https://www.unicef.org/egypt/press-releases/egypt-receives-546400-doses-covid-19-vaccines-donated-france-through-avat-and-covax.
- 236.Al-Monitor Spanish Leader Promises COVID-19 Vaccines to Egypt. 2021. [(accessed on 2 August 2022)]. Available online: https://www.al-monitor.com/originals/2021/11/spanish-leader-promises-covid-19-vaccines-egypt.
- 237.Reliefweb Giving 110%: Eswatini’s Early Rollout of COVID-19 Vaccines. 2021. [(accessed on 1 August 2022)]. Available online: https://reliefweb.int/report/eswatini/giving-110-eswatini-s-early-rollout-covid-19-vaccines.
- 238.MyJoyOnline Akufo-Addo, Bawumia, Others Get Vaccinated against COVID-19. 2021. [(accessed on 2 August 2022)]. Available online: https://www.myjoyonline.com/livestream-akufo-addo-bawumia-others-get-vaccinated-against-covid-19/
- 239.United Nations News Ghana Receives First Historic Shipment of COVID-19 Vaccinations from International COVAX Facility. 2021. [(accessed on 2 August 2022)]. Available online: https://news.un.org/en/story/2021/02/1085572.
- 240.UNICEF Another 410,000 COVID-19 Vaccine Doses Arrive in Kenya, Donated by the, U.K. 2021. [(accessed on 1 August 2022)]. Available online: https://www.unicef.org/kenya/press-releases/another-410000-covid-19-vaccine-doses-arrive-kenya-donated-uk.
- 241.Njeru B. Kenya Receives Another Vaccine Donation. 2021. [(accessed on 2 August 2022)]. Available online: https://www.standardmedia.co.ke/health/health-science/article/2001417729/kenya-receives-another-vaccine-donation.
- 242.UNICEF UK Donates COVID-19 Vaccines to Malawi. 2021. [(accessed on 1 August 2022)]. Available online: https://www.unicef.org/malawi/press-releases/uk-donates-covid-19-vaccines-malawi.
- 243.UNICEF Malawi Receives First Shipment of COVID-19 Vaccines from COVAX. 2021. [(accessed on 2 August 2022)]. Available online: https://www.unicef.org/malawi/press-releases/malawi-receives-first-shipment-covid-19-vaccines-covax.
- 244.WHO Africa COVID-19 Vaccines Shipped by COVAX Arrive in Nigeria. 2021. [(accessed on 1 August 2022)]. Available online: https://www.afro.who.int/news/covid-19-vaccines-shipped-covax-arrive-nigeria.
- 245.Loembé M.M., Nkengasong J.N. COVID-19 Vaccine Access in Africa: Global Distribution, Vaccine Platforms, and Challenges Ahead. Immunity. 2021;54:1353–1362. doi: 10.1016/j.immuni.2021.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ezigbo O. Nigeria Receives 501, 600 Doses of COVID-19 Vaccine from France. 2022. [(accessed on 2 August 2022)]. Available online: https://www.thisdaylive.com/index.php/2021/10/09/nigeria-receives-501-600-doses-of-covid-19-vaccine-from-france/
- 247.Adebowale-Tambe N. COVID-19: Italian Govt Donates over Three Million Doses of Vaccine to Nigeria. 2022. [(accessed on 1 August 2022)]. Available online: https://www.premiumtimesng.com/news/top-news/523255-covid-19-italian-govt-donates-over-three-million-doses-of-vaccine-to-nigeria.html.
- 248.Premium Times COVID-19: Japanese Govt Donates over 800,000 Doses of AstraZeneca Vaccine to Nigeria. 2022. [(accessed on 1 August 2022)]. Available online: https://headtopics.com/ng/covid-19-japanese-govt-donates-over-800-000-doses-of-astrazeneca-vaccine-to-nigeria-24433360.
- 249.Chocomilo S. Spain Donates 4.4m COVID-19 Vaccines To Nigeria. 2022. [(accessed on 2 August 2022)]. Available online: https://www.withinnigeria.com/news/2022/05/26/spain-donates-4-4m-covid-19-vaccines-to-nigeria/
- 250.US Mission U.S. Donates an Additional 2.5 Million Doses of COVID-19 Vaccines to Nigeria This Week. [(accessed on 2 August 2022)];2021 Available online: https://ng.usembassy.gov/u-s-donates-an-additional-2-5-million-doses-of-covid-19-vaccines-to-nigeria-this-week/
- 251.Adepegba A. India Donates 100,000 Doses of COVID-19 Vaccines to Nigeria. 2021. [(accessed on 1 August 2022)]. Available online: https://punchng.com/india-donates-100000-doses-of-covid-19-vaccines-to-nigeria/
- 252.Unicef Sudan Sudan Joins the COVAX Facility to Ensure Equitable Access to COVID-19 Vaccines—Frequently Asked Questions about COVAX and Sudan’s Participation. 2021. [(accessed on 2 August 2022)]. Available online: https://www.unicef.org/sudan/stories/sudan-joins-covax-facility-ensure-equitable-access-covid-19-vaccines.
- 253.Ryeng H. The First COVID-19 Vaccines Have Arrived in Tanzania—An Important Milestone in the Fight against COVID-19. 2021. [(accessed on 1 August 2022)]. Available online: https://www.unicef.org/tanzania/stories/first-covid-19-vaccines-have-arrived-tanzania.
- 254.Africanews Tanzania Receives 500,000 Sinopharm Vaccines from China. 2021. [(accessed on 2 August 2022)]. Available online: https://www.africanews.com/2021/11/03/tanzania-receives-500-000-sinopharm-vaccines-from-china/
- 255.Kamoga J. Uganda to Roll Out First Round of COVID-19 Vaccine on March 10. 2021. [(accessed on 1 August 2022)]. Available online: https://www.theeastafrican.co.ke/tea/science-health/uganda-to-roll-out-first-round-jabs-3315226.
- 256.UNICEF Uganda to Benefit from UK COVID-19 Vaccines Donation. 2021. [(accessed on 2 August 2022)]. Available online: https://www.unicef.org/uganda/press-releases/uganda-benefit-uk-covid-19-vaccines-donation.
- 257.The Independent Uganda Receives COVID-19 Vaccine Donation from India. 2021. [(accessed on 1 August 2022)]. Available online: https://www.independent.co.ug/uganda-receives-covid-19-vaccine-donation-from-india/
- 258.James J. COVID-19 Vaccines Shipped by COVAX Arrive in Zambia—Donation by France; COVAX Local Press Statement on Arrival of 228,000 Vaccines in Zambia. 2021. [(accessed on 2 August 2022)]. Available online: https://www.unicef.org/zambia/press-releases/covid-19-vaccines-shipped-covax-arrive-zambia-donation-france.
- 259.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., Padayachee S.D., Dheda K., Barnabas S.L., Bhorat Q.E., et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021;384:1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sisonke Programme—Sisonke Study Protecting Healthcare Workers. 2021. [(accessed on 1 August 2022)]. Available online: https://sisonkestudy.samrc.ac.za/indexsisonke.html.
- 261.SAMRC Vast Majority of Breakthrough Infections in Vaccinated Health Workers Are Mild. 2021. [(accessed on 1 August 2022)]. Available online: https://www.samrc.ac.za/media-release/vast-majority-breakthrough-infections-vaccinated-health-workers-are-mild.
- 262.APA-Mbabane Eswatini Adds AstraZeneca to COVID-19 Booster Doses. 2022. [(accessed on 2 August 2022)]. Available online: http://apanews.net/en/news/eswatini-adds-astrazeneca-to-covid-19-booster-doses/
- 263.Wikipedia COVID-19 Vaccination in Ghana. 2021. [(accessed on 1 August 2022)]. Available online: https://en.wikipedia.org/wiki/COVID-19_vaccination_in_Ghana.
- 264.Larbie S. GHS Begins Administering COVID-19 Vaccine Booster Shots. 2022. [(accessed on 2 August 2022)]. Available online: https://www.gna.org.gh/1.21323473.
- 265.Saya M. Kenya Starts Giving COVID-19 Vaccine Booster Shots. 2021. [(accessed on 1 August 2022)]. Available online: https://www.the-star.co.ke/news/2021-12-25-kenya-starts-giving-covid-19-vaccine-booster-shots/
- 266.Kalekye M. Kenya Administers 114,007 Covid Booster Jabs. 2022. [(accessed on 2 August 2022)]. Available online: https://www.kbc.co.ke/kenya-administers-114007-covid-booster-jabs/
- 267.Silas D. FG Govt Approves COVID-19 Vaccines Booster for Nigerians. 2021. [(accessed on 1 August 2022)]. Available online: https://dailypost.ng/2021/12/04/fg-govt-approves-covid-19-vaccines-booster-for-nigerians/
- 268.The Independent Uganda Alters COVID-19 Vaccine Roll-Out Plan to Cover More People. 2021. [(accessed on 2 August 2022)]. Available online: https://www.independent.co.ug/uganda-alters-covid-19-vaccine-roll-out-plan-to-cover-more-people/
- 269.Nabatanzi V. COVID-19: Govt to Roll Out Vaccination to Children. 2022. [(accessed on 1 August 2022)]. Available online: https://www.bukedde.co.ug/health/130937/covid-19-govt-to-roll-out-vaccination-to-chil.
- 270.Unicef Uganda Country Office Annual Report 2021. [(accessed on 2 August 2022)]. Available online: https://www.unicef.org/media/116466/file/Uganda-2021-COAR.pdf.
- 271.AllAfrica Uganda: Health Ministry Approves COVID-19 Booster Shots for Ugandans. 2021. [(accessed on 1 August 2022)]. Available online: https://allafrica.com/stories/202112280071.html.
- 272.Jere J. Booster Jab Available in All Public Health Centers. 2021. [(accessed on 2 August 2022)]. Available online: https://www.znbc.co.zm/news/booster-jab-available-in-all-public-health-centers/
- 273.Cajnewsafrica Zimbabwe Takes Lead in COVID-19 Rollout. 2021. [(accessed on 1 August 2022)]. Available online: https://www.cajnewsafrica.com/2021/08/05/zimbabwe-takes-lead-in-covid-19-rollout/
- 274.Gonye V. Govt Gives Guidelines on Booster Shots. 2021. [(accessed on 2 August 2022)]. Available online: https://www.newsday.co.zw/2021/12/govt-gives-guidelines-on-booster-shots/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data can be obtained on reasonable request from the corresponding author.