Abstract
Objective
To summarise the clinical characteristics of patients with Stevens–Johnson syndrome/toxic epidermal necrolysis syndrome (SJS/TEN) and analyse the efficacy and safety of systemic glucocorticoid therapy.
Methods
This study was a retrospective study of 56 patients with SJS/TEN who had been systematically treated with glucocorticoids in the dermatology ward of Peking University Third Hospital from 2010 to 2020. The clinical characteristics, treatment regimen, effects on underlying diseases, incidence and outcome of hormone-related adverse reactions and skin lesion prognosis were summarised and analysed for each patient.
Results
① The allergenic drugs were found to be antibiotics (31.51%), antipyretic and analgesics (21.92%), traditional Chinese medicines and health products (15.07%) and neuropsychiatric drugs (13.70%). ② Based on the 56 patients’ scores of toxic epidermal necrosis at admission, the actual mortality rate was 1.8% (1/56), which was significantly lower than the average expected mortality rate of 15.0% (P = 0.032; standardised mortality ratio = 0.13; 95% confidence interval: 0.00–0.53). ③ A total of 33 patients (58.9%) had underlying diseases, of which 10 patients (30.3%) had underlying diseases that fluctuated during treatment but stabilised after symptomatic treatment. ④ During treatment, 73.2% (41/56) of patients had complications that may have been related to systemic glucocorticoids; 97.6% (40/41) had mild symptoms, and 92.7% (38/41) had improved/recovered complications at the time of discharge.
Conclusion
① Antibiotics are still the most common sensitising drugs, and traditional Chinese medicine and health products are also common sensitising drugs. ② Early systemic application of medium- to high-dose glucocorticoids is effective in the treatment of SJS/TEN, and it is beneficial in reducing mortality. ③ The short-term application of medium- to high-dose hormone therapy for SJS/TEN has little effect on underlying diseases. The related complications are mostly mild, and the treatment is safe.
Keywords: Stevens–Johnson syndrome, toxic epidermal necrolysis, SCORTEN, glucocorticoid-related complications, treatment
Introduction
Stevens–Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) is a rare, acute mucocutaneous syndrome characterised by peeling skin and necrosis. All instances of the disease are related to drugs. The clinical manifestations mainly include skin and mucous membrane pain, erythema, blisters, epidermal exfoliation and other characteristic skin lesions, which can involve the eye, oral cavity and/or urogenital mucosa. In severe cases, multi-system damage may occur. The reported case fatality rates of SJS and TEN can be as high as 5–10% and 20%–40%, respectively.1 Since patients with SJS and TEN share some common clinical features, it is currently believed that SJS and TEN represent a spectrum of diseases, and the diseases are classified according to epidermolysis area: SJS covers ≤ 10% of the body surface area (BSA), overlapping SJS/TEN covers 10–30% of the BSA, TEN covers ≥ 30% of the BSA. The disease incidence rate in this group is low. The annual incidence rate of SJS is (1~7)/million,2 and the annual incidence rate of TEN is (0.4~1.45)/million.3 The pathogenesis of SJS/TEN mainly relates to the drug and the patient’s genetic makeup. Histopathologically, the skin lesions are characterised by extensive apoptosis and necrosis of keratinocytes (KC), and this process is mainly mediated by drug-specific cytotoxic T cells.
At present, in addition to the immediate discontinuation of the suspected allergenic drugs and symptomatic and supportive treatment for SJS/TEN, a large number of studies have used systemic glucocorticoids, intravenous immunoglobulin and symptomatic and supportive treatment, and some studies have used cyclosporine, TNFα, plasma exchange and other treatments. Given the rarity of this type of disease, it is difficult to conduct randomised controlled trials, and there is no clear consensus on a standard treatment regimen. We retrospectively analysed the clinical characteristics, treatments and prognoses of all patients with SJS/TEN treated with systemic glucocorticoids in the dermatology ward of our hospital from 2010 to 2020, thereby hoping to provide a basis for the clinical diagnosis and treatment of patients suffering from such a severe disease.
Materials and Methods
Clinical information.
Research Objectives
Through the inpatient medical record system of Peking University Third Hospital, the clinical data of all 56 patients with SJS/TEN treated with systemic glucocorticoids in the dermatology ward from 2010 to 2020 were collected, including demographic, medical history and physical examination data, as well as auxiliary information on examinations, treatments and prognoses. This study protocol was approved by the Medical Research Ethics Committee of Peking University Third Hospital (IRB00006761-M2021261). There are currently no generally accepted diagnostic criteria for SJS/TEN.
The inclusion criteria for this study were as follows:4 ① a history of suggestive drug exposure or febrile illness; ② prodromal symptoms; ③ rapidly progressive painful rash, erythema, atypical target skin lesions or diffuse erythema determined to be progressing to vesicles and bullae; ④ positive Nikolsky sign; ⑤ oral, ocular and/or genital mucosal inflammation with painful mucosal erosion, epidermal necrosis and exfoliation to some degree; ⑥ systemic application of glucocorticoid therapy.
Exclusion criteria: ① Those who had not used glucocorticoids systematically. ② Those who could not cooperate with the relevant evaluation and follow-up of the study.
Treatment Plan and Evaluation Method
Score of Toxic Epidermal Necrosis (SCORTEN Score)
The following seven indicators were recorded within 24 hours of admission: ① age > 40 years; ② concurrent malignant tumor; ③ heart rate > 120 beats; ④ blood sugar > 14 mmol/L; ⑤ bicarbonate < 20 mmol/L; ⑥ epidermal exfoliation > 10% body surface area; ⑦ serum urea nitrogen > 10 mmol/L. Each indicator was counted as one point. If the criterium was not met, then a score of zero was given. The total score was 0~7 points. The corresponding predicted mortality rates were 1%, 4%, 12%, 32%, 62%, 85%, 95% and 99%, respectively.5 Standardised mortality ratio (SMR) = actual number of deaths/expected number of deaths.
Treatment Plan
The main systemic treatment drug was glucocorticoid (methylprednisolone or prednisone acetate/prednisolone or acetate/dexamethasone, etc.) The initial hormone dosage was selected according to symptom severity, and the patients with severe disease were treated with intravenous injection of human immunoglobulin. The hormone dosage was adjusted according to the patient’s vital signs and skin lesion changes.6 After the body temperature had dropped to normal and the rash and erosion surface had dried up and scabbed, the amount of hormone decreased depending on the patient’s condition, and the dose was reduced by 10% to 20% every 3–5 days until the drug treatment was discontinued. The treatment combined symptomatic treatment and supportive treatment (the management of wounds). The patients with symptoms involving the oral cavity, eyes and genital mucous membranes were treated accordingly after consultation and evaluation by specialists. According to the clinical symptoms and the results of laboratory tests, supportive therapy, such as fluid replacement, water and electrolyte balance and protein balance, was given.
Outcome
(1) In this study, ① the body temperature was reduced to normal, and the remaining vital signs were stable; ② the rash was completely dried up, and the mucosal involvement improved; ③ laboratory indicators returned to normal, etc., as clinical control.
(2) Monitoring complications, such as infection, visceral damage and ocular involvement. In this study, the correlation between comorbidities and the systemic application of glucocorticoids was evaluated for all patients and classified as related, possibly related or irrelevant. Potentially related adverse events were listed as systemic glucocorticoid-related side effects.
Statistical Analysis
Statistical analysis SPSS 20.0 statistical software was used for data analysis, and descriptive statistical analysis was used for the observation indexes and variables. The measurement data conforming to the normal distribution after the normality test were expressed as  . A t-test using two independent samples was used for univariate analysis. The comparison between the expected number of deaths and the actual number of deaths for each score was performed using Fisher’s exact probability method, with P < 0.05 as the criterion of significance.
. A t-test using two independent samples was used for univariate analysis. The comparison between the expected number of deaths and the actual number of deaths for each score was performed using Fisher’s exact probability method, with P < 0.05 as the criterion of significance.
Results
General Information
A total of 56 patients were diagnosed as having SJS/TEN. They were aged 18.0–76.0 (46.0±17.0) and included 31 males (55.4%) aged 18.0–75.0 (47.5±15.7) with body mass indexes (BMI) of 18.3–29.5 (23.5±3.0) and 26 females (44.6%) aged 18.0–76.0 (44.1±18.7) with BMI of 17.9–35.3 (22.9±3.5). A total of 13 cases (23.2%) had drug allergy histories, and 33 cases (58.9%) had other underlying diseases. The incubation period from medication to rash ranged from 1 to 120 days. Thirty-one cases (55.3%) had incubation periods of ≤ 7 days; ten cases (17.9%) had incubation periods of 7 to 14 days; nine cases (16.1%) had incubation periods of 14 to 60 days. A total of two cases (3.6%) had incubation periods longer than 60 days. The mean incubation period was 12.3 ± 21.3d, and the median incubation period (P25, P75) was 5.5d (1.0d, 12.75d). There were no significant differences in the male and female composition ratio, age, incubation period and underlying diseases among the subtypes (P > 0.05), as shown in Table 1.
Table 1.
Comparison of General Data of SJS/TEN
| Type Title |
SJS | SJS/TEN Overlapping Type | TEN |
|---|---|---|---|
| Case n(%) | 16 (28.6) | 8 (14.3) | 32 (57.1) |
| Sex | |||
| Male n(%) | 6 (37.5) | 6 (75.0) | 19 (59.4) |
| Female n(%) | 10 (62.5) | 2 (25.0) | 13 (40.6) |
| Age(years) | 44.3±17.1 | 43.6±16.2 | 47.4±17.6 |
| Incubation period(day) | 8.6±7.8 | 19.0±44.6 | 12.3±21.3 |
| Combined underlying diseasen(%) | 8 (50.0) | 5 (62.5) | 20 (62.5) |
Distribution of Sensitising Drugs
The suspected allergenic drugs are shown in Table 2. Fourteen patients had taken two or more suspected allergenic drugs. The allergenic drugs could not be determined for the other four patients.
Table 2.
Composition Ratio of Suspected Allergenic Drugs
| Suspected Allergens | Total (Case) | Composition Ratio (%) |
|---|---|---|
| Antibiotic | 23 | 31.51 |
| Antipyretic analgesics | 16 | 21.92 |
| Traditional Chinese Medicine and Health Products | 11 | 15.07 |
| Neuropsychiatric drugs | 10 | 13.70 |
| Anti-gout drugs | 3 | 4.11 |
| Antiviral | 1 | 1.37 |
| Ophthalmic medication | 1 | 1.37 |
| Lipid-lowering drugs | 1 | 1.37 |
| Antihypertensive drugs | 1 | 1.37 |
| Dehydration drugs | 1 | 1.37 |
| Antineoplastic drugs | 1 | 1.37 |
| Suspected sensitizing drug not available | 4 | 5.48 |
| Total | 73 | 100% |
The Appearance and Severity of Skin Lesions
The First Manifestation
Thirty-six patients (64.3%) had skin rashes as the first manifestation. Twenty patients (35.7%) suffered from mucosal erosion, including ten patients (17.9%) with oral mucosa, eight cases (14.3%) with eye mucosa and two cases (3.6%) with genital mucosa.
Body Temperature
The patients’ body temperatures upon admission were 36.2°C∼40.5°C (38.6 ± 1.2). Forty-six patients (82.1%) had temperatures of ≥ 37.3°C; five patients (8.9%) had temperatures of 37.3∼38°C, and twenty-one patients had temperatures of 38.1∼39°C (37.5%), with a total of twenty patients (35.7%) having temperatures of 39.1–41°C.
Severity
The exfoliation area percentage was 1–90; the median (P25, P75) was 46 (10, 60). Mucosal involvement:
Out of the 56 patients, 54 (96.4%) had symptoms that involved the oral mucosa; 45 (80.4%) patients had symptoms that involved the genital mucosa, and 33 (58.9%) patients had symptoms that involved the eye. Thirty-one patients (55.4%) had symptoms that involved the ocular, oral and genital mucosas. Fourteen patients (25.0%) had symptoms that involved two mucosal sites (twelve patients had symptoms that involved the oral and genital mucosas, and two patients had symptoms that involved the eye and the oral cavity). Eleven patients (19.6%) had symptoms that involved one mucosa (nine patients had symptoms that involved the oral cavity, and two patients had symptoms that involved the genital mucosa).
Complications
Forty-four (78.6%) patients suffered from other organ infections/complications, including twenty-six (46.2%) with comorbidities. Twelve (21.4%) patients suffered from secondary infections of skin lesions, and ten patients (17.9%) suffered from thrush; six patients (10.7%) suffered from respiratory tract infection; one patient (1.8%) suffered from each of bacteremia, peritonitis, urinary tract infection, herpes simplex infection and cholecystitis; five patients suffered from osteroid diabetes (8.9%); thirty-one patients suffered from electrolyte disturbance (55.4%); twenty-five patients (44.6%) suffered from elevated transaminase, and one patient (1.8%) suffered from bilateral calf intermuscular vein thrombosis.
Treatment Options
Glucocorticoids
All 56 patients were treated with systemic glucocorticoids. The initial dose was equivalent to 33.75 mg/d∼150 mg/d prednisone (0.39 mg/kg∙d∼2.59 mg/kg∙d, 1.02 ± 0.42 mg/kg.d). Two patients (3.6%) received low doses (<0.5 mg/kg.d); 33 patients received (58.9%) medium doses (0.5~1.0 mg/kg∙d); 33 patients (58.9%) received high doses (1.0~4.0 mg/kg∙d) in 21 cases (37.5%). The starting hormonal treatments for the patients with SJS, the overlapping type and TEN were 0.80 ± 0.26 mg/kg.d, 0.89 ± 0.21 mg/kg.d and 1.15 ± 0.47 mg/kg.d, respectively. The total hormone dose at the time of rash control was 8.44 ± 5.10 mg/kg. The total hormone doses for the patients with SJS, the overlapping type and TEN were 4.91 ± 2.98 mg/kg, 6.72 ± 1.71 mg/kg and 10.64 ± 5.39 mg/kg, respectively.
Human Immunoglobulin
Thirty-two patients (57.1%) received combined intravenous human immunoglobulin, including three patients (18.8%) suffering from SJS, four patients (50%) suffering from overlapping SJS/TEN, and twenty-five patients (78.1%) suffering from TEN. The single-day dose was 0.34 ± 0.13 g/kg.d; the application days totalled 3d∼12d (median 5d); the cumulative amount was 1.9 ± 0.7 g/kg.
Outcome
Mortality
The SCORTEN scores of the 56 patients upon admission were 0–5 points (mean: 1.70 ± 1.17). The median (P25, P75) was one point (1 point, 2.75 points); 7 patients (12.5%) had zero points; 22 patients (39.3%) had one point; 13 patients (23.2%) had two points; 10 patients (17.9%) had three points; 3 patients (5.4%) had four points, and 1 patient (1.8%) had five points. The actual number of deaths was one, and the actual mortality rate was 1.8% (1/56), which was significantly lower than the overall expected mortality rate of 15.0% (Fisher’s exact probability method, P = 0.032); SMR = 0.13; 95% confidence interval (CI): 0.00–0.53. The actual number of deaths was lower than the expected number of deaths, as shown in Table 3. The mortality rate of 32 TEN patients was 3.1% (1/32), which was lower than the expected mortality rate of 20.6%.5
Table 3.
Results of SCROTEN Score, Expected Number of Deaths and Actual Number of Deaths in 56 SJS/TEN Patients
| SCORTEN Score | Case(n) | Expected Mortality (%) | Expected Number of Deaths (n) | Actual Number of Deaths (n) | P |
|---|---|---|---|---|---|
| 0 | 7 | 1 | 0.07 | 0 | |
| 1 | 22 | 4 | 0.88 | 0 | |
| 2 | 13 | 12 | 1.56 | 0 | |
| 3 | 10 | 32 | 3.20 | 0 | |
| 4 | 3 | 62 | 1.86 | 1 | |
| 5 | 1 | 85 | 0.85 | 0 | |
| Total | 56 | 15 | 8.42 | 1 | 0.32 |
| SMR (95% CI) | 0.13 (0.00–0.53) |
The patient who died was male, 61 years old and suffered from TEN. This patient’s underlying diseases included diabetes, diabetic nephropathy, renal failure, peritoneal dialysis for three years, repeated pulmonary infection, immune thrombocytopenia three months before admission and acute cerebral infarction two months ago, moxifloxacin, cephalosporin and antipyretic analgesics. The patient had a history of sulfa allergy. The patient’s condition at admission was as follows. The epidermal exfoliation area was 80%; the SCORTEN score was four points, and creatinine was up to 1115 umol/L. After admission, 40 mg/day of methylprednisolone and 20 g/day of gamma globulin were given intravenously for one week to increase the frequency of peritoneal dialysis. After one week, the rash stably dried up, and the hormone was gradually reduced within 10 days. The rash did not recur after that. Dialysis-related peritonitis occurred during the period of hormone reduction, and Xanthomonas, Acinetobacter baumannii and Aspergillus were repeatedly cultured in sputum culture and stool culture. The patient died of septic shock, renal failure, uremia and multiple organ failure 25 days after admission.
Efficacy
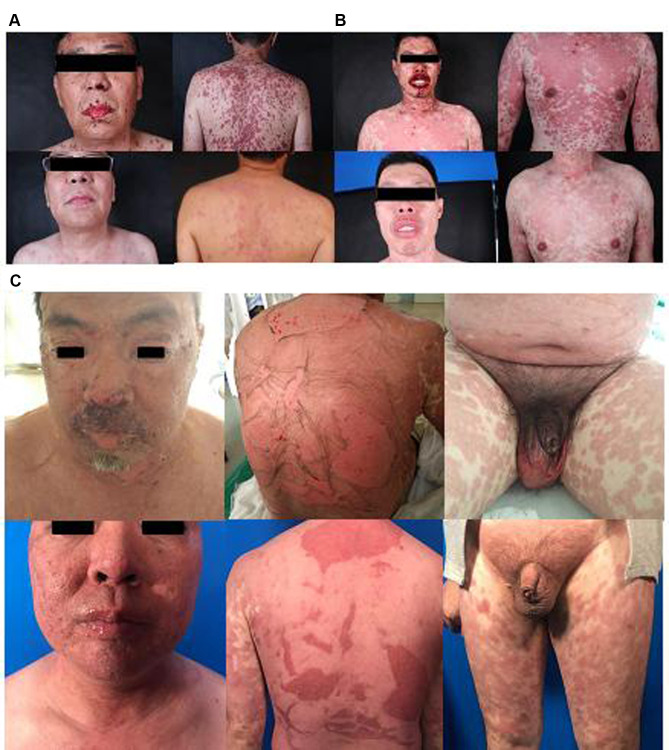
Excepting the one deceased patient, the remaining 55 patients had improved/recovered skin and mucous membranes, infections and visceral complications. Every patient was discharged when the steroid dose was tapered to oral administration. Every patient’s rash was cured from the time of hormone reduction to drug withdrawal, and the recovery rate from the rash was 98.2%. See Figure 1.
Figure 1.
Photos before and after treatment: (A) (SJS), (B) (SJS/TEN overlapping), (C) (TEN).
Hospitalization period:
The hospitalisation period was 3∼34d (15.4 ± 6.5d); the mean hospitalisation period for patients suffering from SJS, the overlapping type and TEN were 11.3 ± 5.0d, 13.9 ± 6.4 d and 17.9 ± 6.1d, respectively. The hospitalisation periods of patients suffering from SJS were significantly lower than those of patients suffering from TEN (t = −3.730; p = 0.001); the time it took to reach a clinical control state was 4–19 days (9.9 ± 3.8d). The days it took to reach a clinical control state for each patient suffering from SJS, the overlapping type and TEN were 6.8 ± 2.6d, 9.3 ± 1.3d and 11.7 ± 3.7d, respectively. The time to clinical control for patients suffering from SJS was significantly lower than the time to clinical control for patients suffering from the SJS/TEN overlap type (t = −2.576; p = 0.017), TEN (t = −4.747; p < 0.001), and the time to clinical control for patients suffering from the SJS/TEN overlap type was significantly lower than the time to clinical control for patients suffering from TEN (t = −3.022); p = 0.005).
Prognoses at the discharge of patients suffering from mucosal involvement:
Fifty-four (100%) patients suffering from oral mucosal and 45 (100%) patients suffering from genital mucosal involvement were discharged with mucosal recovery. Outcomes at the discharge of 33 patients suffering from ocular mucosal involvement: Nineteen (57.6%) patients recovered, six (18.2%) patients improved, and eight (24.2%) patients did not recover.
Prognoses of infections and visceral complications:
Thirty-eight patients (92.7%) had complication improvement/recovery at discharge, except the one patient who died. There was only one case of hypocalcemia and one case of liver damage, which did not improve. All complications were mild.
Safety Analysis of the Systemic Application of Glucocorticoids in the Treatment of SJS/TEN
Influence and Outcome of Underlying Diseases
A total of 33 patients (58.9%) out of 56 had underlying diseases, including 15 patients (15/32, 46.9%) suffering from SJS, 8 patients (8/10, 80.0%) suffering from SJS/TEN and 10 patients suffering from TEN (10/14, 80.0%). 71.4%). The underlying diseases included hypertension (twelve patients, 21.4%), cerebral infarction/intracerebral hemorrhage (eight patients, 14.3%), diabetes (seven patients, 12.5%), cardiovascular disease (seven patients, 12.5%), epilepsy (four patients, 7.1%), renal (two patients, 3.6%) of functional failure, tumors (four patients, 7.1%), lymphoma (two patients; one patient had liver cancer, and one patient had thyroid cancer), gastrointestinal disease (four patients, 7.1%), hepatitis B virus (two patients, 3.6%), one patient (1.8%) after aortic dissection, bullous pemphigoid (one patient, 1.8%) and fourteen other cases (25.0%). Eighteen patients (32.1%) had two or more underlying diseases at the same time.
① Two patients suffering from hypertension had increased blood pressure in the first week after admission, which stabilised after adjustments to the oral antihypertensive drug treatments. ② The blood sugar level was elevated in all patients with diabetes. Six patients with obvious increased blood sugar levels were treated with insulin, and one patient with a mild increase in the blood sugar level was stable after an adjustment to the oral hypoglycemic drug. ③ The medical histories of six patients with cerebral infarction included one patient each with a time span of one month, two months, one year and two years (with an aortic dissection one month after the operation) since the operation at the point of admission, and two patients had older cerebral infarctions. The medical histories of the two patients with cerebral hemorrhage showed that three months and two years had passed since the onset at the point of admission, and the medical history of one patient showed that one month had passed since the onset of cerebral infarction combined with aortic dissection. This patient developed transient coagulation abnormalities 10 days after admission with activated partial thromboplastin, 25.8 seconds; APTT ratio, 0.75; increased D-Dimer, 3.72 ug/mL; no thrombosis. The red blood cells were 2.97*1012/L; the hemoglobin was 82 g/L; the platelets were 70*109/L; conjunctival hemorrhage was also present. The symptoms improved after treatment, and the relevant laboratory tests processed for the other patients showed no abnormalities. ④ Cr increased in one patient with renal failure, who then underwent peritoneal dialysis treatment. After increasing the number of peritoneal dialyses, Cr was stable.
Hormone-Related Complications and Outcomes
Among the 56 patients treated with systemic glucocorticoids, 41 patients (73.2%) had complications that might have been related to systemic glucocorticoids, and 30 patients out of the 41 had two or more types of complications. Six patients suffered from the coexistence of both infections.
Among them, 40 cases (97.6%) were mild, and only one patient with renal failure complicated with pneumonia developed severe infection and electrolyte imbalance and eventually died.
After symptomatic treatments, 38 patients (92.7%) had improved/recovered complications at the time of discharge. One patient with hypocalcemia and one patient with liver function damage did not improve. Both cases were mild, and their calcium supplementation and liver protection treatments continued. The patient returned to normal after the one-month follow-up. The patient who died had peritonitis, eventual infectious shock and uncorrected hypokalemia, as shown in Table 4.
Table 4.
Complications and Prognosis Related to Systemic Glucocorticoids
| Types of Complications | SJS (n,%) | SJS/TEN (n,%) | TEN (n,%) | Case (n,%) | Healed at Discharge (%) | Better at Discharge (%) | Not Getting Better at Discharge (%) |
|---|---|---|---|---|---|---|---|
| Infection | 3 (18.8) | 3 (37.5) | 20 (62.5) | 26 (46.4) | 12 (46.2) | 13 (50.1) | 1 (3.8) |
| Secondary skin lesions | 1 (6.2) | 0 (0.0) | 11 (34.4) | 12 (21.4) | 8 (66.7) | 4 (33.3) | 0 |
| Thrush | 2 (12.5) | 1 (12.5) | 7 (21.9) | 10 (17.9) | 3 (30.0) | 7 (70.0) | 0 |
| Respiratory tract | 0 (0.0) | 1 (12.5) | 5 (15.6) | 6 (10.7) | 3 (50.0) | 3 (50.0) | 0 |
| Bacteremia | 0 | 0 | 1 (3.1) | 1 (1.8) | 1 (100.0) | 0 | 0 |
| Peritonitis | 0 | 0 | 1 (3.1) | 1 (1.8) | 0 | 0 | 1 (100.0) |
| Other infections | 1 (6.2) | 1 (12.5) | 0 | 0 | 0 | 2 (100.0) | 0 |
| Steroid diabetes | 1 (6.2) | 0 (0.0) | 4 (12.5) | 5 (8.9) | 1 (20.0) | 4 (80.0) | 0 |
| Electrolyte | 6 (37.5) | 2 (25.0) | 14 (43.8) | 22 (39.3) | 15 (68.2) | 5 (22.7) | 2 (9.1) |
| Low potassium | 4 (25.0) | 1 (12.5) | 6 (18.8) | 11 (19.6) | 9 (81.8) | 1 (9.1) | 1 (9.1) |
| Low calcium | 3 (18.8) | 1 (12.5) | 9 (28.1) | 13 (23.2) | 8 (61.5) | 4 (30.8) | 1 (7.7) |
| Liver damage | 2 (12.5) | 2 (25.0) | 9 (28.1) | 13 (23.2) | 4 (30.8) | 8 (61.5) | 1 (7.7) |
| Thrombosis | 0 | 0 | 1 (3.1) | 1 (1.8) | 0 | 1 (100.0) | 0 |
| Death | 0 | 0 | 1 (3.1) | 1 (1.8) | 0 | 0 | 1 |
| Total | 9 (56.2) | 4 (50.0) | 28 (87.5) | 41 (73.2) | 11 (26.8) | 27 (65.9) | 3 (7.3) |
Discussion
SJS/TEN is a serious skin and mucous membrane reactive disease. The pathogenesis is mainly related to drugs, the genetic background of the body and immunity. Histopathologically, the skin lesions are characterised by extensive apoptosis and necrosis of the epidermal KC. SJS/TEN is thought to be mainly caused by KC apoptosis and necrosis caused by a type IV hypersensitivity reaction mediated by drug-specific CD8+ T cells.7,8
In this study, it was found that 96.4% of the patients had an incubation period from medication to rash that lasted two months or less, and 73.2% of the patients had an incubation period of two weeks or less. Mockenhaupt et al9 analysed 379 SJS/TEN patients. The results showed that 85% to 100% of the patients were highly suspected to be sensitising drugs with an incubation period of eight weeks and an average of four weeks, which was consistent with the results of this study. Exogenous drugs that can induce SJS/TEN have been reported in the past, including anticonvulsants, antidepressants, sulfonamides, non-steroidal anti-inflammatory drugs, anti-infective drugs and targeted drugs that have been widely used in recent years.10 It is worth noting that the results of this study show that traditional Chinese medicine and health products are also common inducers of SJS/TEN. In recent years, reports of adverse reactions to traditional Chinese medicine have increased at home and abroad, with drug eruption the most common adverse reaction.11,12 More than 170 countries and regions around the world use traditional Chinese herbal medicines and medicines derived from plants and animals. These medicines were generally considered to be safer to use and had fewer adverse reactions. Our research shows that the application of Chinese herbal medicines and health care products may cause drug eruptions, even severe drug eruptions. With the expansion of the scope of application of such drugs, the proportion of allergenic drugs has increased accordingly, and sufficient attention must be paid to the screening of suspected allergenic traditional Chinese medicines, prescription components and monomer components.
There is currently no standard unified therapy for SJS/TEN, and whether glucocorticoids can reduce the mortality rate of SJS/TEN is being debated. Early studies suggest that the use of glucocorticoids in the treatment of SJS/TEN may increase the risk of sepsis without the benefit of significant mortality reduction. However, some recent case-cohort studies and systematic reviews and meta-analyses of systemic immunomodulatory therapy used in the treatment of SJS/TEN have demonstrated that13,14 compared with supportive care, systemic glucocorticoid therapy significantly improves outcomes in patients with SJS/TEN overlap and TEN. Micheletti et al15 retrospectively analysed a total of 377 SJS/TEN patients from 18 medical centres in the United States from 2000 to 2015. The mortality rate in the glucocorticoid treatment group was lower than the predicted mortality rate and the mortality rate in the non-glucocorticoid treatment group. The results of a systematic study of glucocorticoids used in the treatment of SJS/TEN in Thailand showed that early systemic glucocorticoid short-term pulse therapy was beneficial in reducing mortality.16
All patients in this study were systematically treated with glucocorticoids, and the actual mortality rate was 1.8%, which was significantly lower than the overall expected mortality rate of 15.0%. The SMR results also showed that the actual number of deaths was lower than the expected number of deaths. The average hospitalisation period was 15.4 days. The expected mortality rate of patients suffering from TEN (57.1%) was 20.6%; the actual mortality rate was 3.1%, and the average hospitalisation cycle was 17.9 days. The hospitalisation cycle and mortality were significantly lower than in previous studies in Europe and the United States. In a multicentre study of 377 patients suffering from SJS/TEN in the United States (39 Asians, TEN 22.8%),15 44.4% of the patients were treated with systemic hormones/hormones combined with gamma globulin; 25% with gamma globulin only; 29.3% with only symptomatic and supportive treatment. The expected mortality rate was 21.1%; the actual mortality rate was 14.7%, and the hospitalisation period was 21.9 days. A European multicentre study17 included a total of 212 patients (25 Asians) suffering from SJS/TEN, 35.4% of whom were treated with systemic glucocorticoids. 26.4% received gamma globulin/cyclosporine/TNFα; 38.2% received symptomatic and supportive treatment, and the six-week mortality rate was 20.8%. The mortality rate of this study is close to the results of a recent Asian study of partial systemic glucocorticoid therapy for SJS/TEN, which was a retrospective study of 213 patients with SJS/TEN in China18 in which all patients were treated with systemic glucocorticoids. The overall expected mortality rate was 8.6%, and the actual mortality rate was 3.8%. Among the patients, there were 53 patients suffering from TEN. The mortality rate was 5.7%, and the average hospital stay was 14.7 days. A recent study in Japan included 132 patients suffering from SJS/TEN (TEN 40.9%),19 of whom 97% were treated with systemic glucocorticoids, and the overall actual mortality rate was 5.3%, of which patients suffering from SJS had a mortality rate of 1.3% (1/78) and patients suffering from TEN had a mortality rate of 12.5% (6/54). We noticed that the mortality rates in the above studies of Asian patients with SJS/TEN were significantly lower than those in the European and American studies, and the reasons for the difference were analysed. In addition to the possible ethnic differences in different regions, there were differences in genetic susceptibility,20 differences in the distribution of disease types, comorbidities, complications and other clinical characteristics. We believe that the difference in treatment regimen is an important factor in the difference in mortality rates. The systemic glucocorticoid use rate (97–100%) of the studies in Asia was significantly higher than the rates used in the studies in Europe and the United States (35.4–44.4%), and the mortality rate was significantly lower than those in Europe and the United States. This also confirms that systemic glucocorticoid therapy may be beneficial in reducing the mortality of patients suffering from SJS/TEN.
In our study, excepting the one patient who died, the rash was cured from steroid dose reduction to discontinuation in the remaining patients, and the rash recovery rate was 98.2%. In one study,21 48 patients were included, all of whom were treated with glucocorticoids. One patient died after treatment, and the others all improved, which is consistent with our results. Taking into account the severe underlying diseases of our patient who died, the cause of death was less to do with hormones, and, if removed, the cure rate of the patient’s rash was almost 100%, which is very high, although there might have been errors. Most patients with mucosal involvement recovered before discharge, and only a small proportion of patients with ocular mucosal involvement did not recover. All of the above may indicate the effectiveness of early short-course, medium- to high-dose glucocorticoid treatment, which is very helpful for the improvement of patients’ symptoms and can be considered in clinical treatment. In addition, our study found that the long duration of hospitalisation was associated with the area of epidermolysis, and the larger the area, the longer the hospital stay and the longer the duration of hormone application.
Some patients with severe disease were also treated with a combination of human immunoglobulins, mainly patients with TEN, and the patients who were given immunoglobulins improved or were cured except for one death from TEN. For some patients with severe disease, human immunoglobulin in combination with treatment was required to improve the cure rate. Therefore, for severe cases, the effectiveness of systemic glucocorticoids has a certain relationship with the treatment regimen, and the combined use of some other drugs is required in order to achieve a better therapeutic effect.
There are potential benefits to the early use of glucocorticoids,22 but adverse reactions such as infection, hypertension, hyperglycemia, gastrointestinal bleeding, electrolyte disturbances, arrhythmias, osteoporosis, weight gain and the induction of grand mal seizures may occur. Especially for patients suffering from SJS/TEN who have underlying diseases, such as diabetes, hypertension, cataract and gastrointestinal bleeding, the risk of systemic application of high-dose hormones may increase the risk of related side effects.23 No study has evaluated the safety of using high-dose glucocorticoids in short-course treatments for patients with SJS/TEN. This study analysed the safety of this treatment from two aspects: the impact on underlying diseases and the incidence and outcome of systemic glucocorticoid-related adverse reactions.
At present, there are few reports on the specific risk of using systemic glucocorticoids to treat patients with SJS/TEN who have underlying diseases.18,24,25 There were five tumor patients in this study (three patients with TEN, one with the overlapping type and one with thyroid disease). After cancer surgery, the other two patients with lymphoma and one patient with liver cancer were under treatment. The rash was clinically cured, and there was no tumor aggravation/recurrence during the treatment. All patients with diabetes had elevated blood sugar, and 16.6% of patients with hypertension had elevated blood pressure. After adjusting blood sugar and blood pressure levels, both were stable Some patients in the study had cerebral infarction/cerebral hemorrhage histories at the same time (the patient histories were one month to two years from onset, including one month after fossa decompression after cerebral infarction), cardiovascular history, gastrointestinal history (including ten patients with Duodenal ulcers), history of epilepsy and history of carrying the hepatitis B virus. During the treatment process, the underlying diseases were stable, and there was no recurrence or aggravation of related diseases. Therefore, we believe that a short-term systemic application of moderate- to high-dose glucocorticoids in the treatment of SJS/TEN has little effect on underlying diseases. Routine monitoring of blood pressure, blood sugar and other indicators is required, and fluctuations can be controlled by symptomatic treatment.
In this study, all the comorbidities that cannot be ruled out (related/possibly related) as related to systemic glucocorticoids are listed as hormone-related complications, and disorders and elevated transaminases were the most common complications. 92.7% of patients had improved/recovered complications at the time of discharge. Except for the patients who died, the remaining patients with complications had recovered before the follow-up one month after discharge. Therefore, we believe that when it comes to a short course of medium- to high-dose hormone therapy for SJS/TEN, the early systemic application of high-dose hormones is prone to suspected hormone-related adverse reactions, but most are mild. With the reduction in steroid doses and related symptomatic treatment, these patients can rapidly improve/recover.
In this study, only one patient died. The patient had a history of chronic renal failure, and the creatinine level continued to rise after suffering from TEN. Studies have shown that dialysis is one of the high-risk factors for death in patients with SJS/TEN.26 Due to complications in the patient’s pulmonary infection, immune thrombocytopenia, cerebral infarction, diabetes and other underlying diseases, the cause of death in this patient was mainly related to the complex underlying diseases.
This study is a retrospective study, and its limitations include heterogeneity and potential bias in the assessment of patients’ skin lesion severity and supportive care and drug treatment decisions. In view of the rarity of the incidence of SJS/TEN, although this study spanned 10 years, the number of patients included is still relatively limited. In the future, it will be necessary to continue to collect cases for further stratification and correlation analysis of influencing factors. Furthermore, it will be necessary to conduct prospective multicentre studies and randomised controlled studies with standardised treatment regimens to further verify the conclusions of this study. Additionally, there is no relevant data available that can be used to analyse the effectiveness of hormones. More data will, therefore, be collected and analysed in the future so that the study can become complete.
Conclusion
This study analysed the efficacy and mortality rates of the short-course systemic application of a large number of glucoczborticoids in the treatment of SJS/TEN, and, for the first time, the impact of systemic glucocorticoids on underlying diseases, as well as the occurrence and outcome of glucocorticoid-related adverse reactions and other aspects, was analysed to determine the safety of the treatment regimen. The results of this study suggest that antibiotics are still the most common sensitising drugs, and traditional Chinese medicines and health products are also common sensitising drugs. Early systemic applications of medium and high doses of glucocorticoids can effectively treat SJS/TEN and reduce mortality with little effect on underlying diseases. The use of hormones is prone to related complications, which are mostly mild, and through the treatment of rapid recovery, the treatment is safe. Our results contain good clinical reference values for the treatment of patients with multiple underlying diseases.
Funding Statement
This study did not receive any funding in any form.
Data Sharing Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of Peking University Third Hospital. Written informed consent was obtained from all participants.
Consent for Publication
The authors affirm that human research participants provided informed consent for publication of the images in Figure(s) 1a, 1b and 1c.
Disclosure
All of the authors had no personal, financial, commercial, or academic conflicts of interest separately.
References
- 1.Estrella-Alonso A, Aramburu JA, González-Ruiz MY, Cachafeiro L, Sánchez MS, Lorente JA. Toxic epidermal necrolysis: a paradigm of critical illness. Rev Bras Ter Intensiva. 2017;29(4):499–508. doi: 10.5935/0103-507X.20170075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White KD, Abe R, Ardern-Jones M, et al. SJS/TEN 2017: building multidisciplinary networks to drive science and translation. J Allergy Clin Immunol Pract. 2018;6(1):38–69. doi: 10.1016/j.jaip.2017.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang MS, Lee JY, Kim J, et al. Incidence Of Stevens-Johnson syndrome and toxic epidermal necrolysis: a nationwide population-based study using national health insurance database in Korea. PLoS One. 2016;11(11):e0165933. doi: 10.1371/journal.pone.0165933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creamer D, Walsh SA, Dziewulski P, et al. U.K. guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults 2016. Br J Dermatol. 2016;174(6):1194–1227. doi: 10.1111/bjd.14530 [DOI] [PubMed] [Google Scholar]
- 5.Research Center for Adverse Drug Reactions, Dermatology and Venereology Branch of Chinese Medical Association. Expert consensus on diagnosis and treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis. Chin J Dermatol. 2021;54(05):376–381. [Google Scholar]
- 6.Nana C, Liming Z, Ting X. The treatment and prognosis of Stevens-Johnson syndrome: a retrospective analysis of 38 cases. Chin J Dermatol Venereol. 2016;30(08):796–798. [Google Scholar]
- 7.Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, Harr T. Current perspectives on Stevens-Johnson syndrome and toxic epidermal necrolysis. Clin Rev Allergy Immunol. 2018;54(1):147–176. doi: 10.1007/s12016-017-8654-z [DOI] [PubMed] [Google Scholar]
- 8.Chung WH, Hung SI, Yang JY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14(12):1343–1350. doi: 10.1038/nm.1884 [DOI] [PubMed] [Google Scholar]
- 9.Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008;128(1):35–44. doi: 10.1038/sj.jid.5701033 [DOI] [PubMed] [Google Scholar]
- 10.Sunaga Y, Kurosawa M, Ochiai H, et al. The nationwide epidemiological survey of Stevens-Johnson syndrome and toxic epidermal necrolysis in Japan, 2016–2018. J Dermatol Sci. 2020;100(3):175–182. doi: 10.1016/j.jdermsci.2020.09.009 [DOI] [PubMed] [Google Scholar]
- 11.Fanping Y, Shengan C, Qinyuan Z, et al. Analysis of sensitizing drugs in 1883 hospitalized patients with drug eruption. Chin J Clin Immunol Allergy. 2017;11(3):232–240. [Google Scholar]
- 12.Cheng L. Current pharmacogenetic perspective on Stevens-Johnson syndrome and toxic epidermal necrolysis. Front Pharmacol. 2021;12:588063. doi: 10.3389/fphar.2021.588063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai TY, Huang IH, Chao YC, et al. Treating toxic epidermal necrolysis with systemic immunomodulating therapies: a systematic review and network meta-analysis. J Am Acad Dermatol. 2021;84(2):390–397. doi: 10.1016/j.jaad.2020.08.122 [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann S, Sekula P, Venhoff M, et al. Systemic immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2017;153(6):514–522. doi: 10.1001/jamadermatol.2016.5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Micheletti RG, Chiesa-Fuxench Z, Noe MH, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis: a multicenter retrospective study of 377 adult patients from the United States. J Invest Dermatol. 2018;138(11):2315–2321. doi: 10.1016/j.jid.2018.04.027 [DOI] [PubMed] [Google Scholar]
- 16.Choonhakarn C, Limpawattana P, Chaowattanapanit S. Clinical profiles and treatment outcomes of systemic corticosteroids for toxic epidermal necrolysis: a retrospective study. J Dermatol. 2016;43(2):156–161. doi: 10.1111/1346-8138.13040 [DOI] [PubMed] [Google Scholar]
- 17.Kridin K, Brüggen MC, Chua SL, et al. Assessment of treatment approaches and outcomes in stevens-johnson syndrome and toxic epidermal necrolysis: insights from a Pan-European multicenter study. JAMA Dermatol. 2021;157(10):1182–1190. doi: 10.1001/jamadermatol.2021.3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L, Shou YH, Li F, Zhu XH, Yang YS, Xu JH. Retrospective study of 213 cases of Stevens-Johnson syndrome and toxic epidermal necrolysis from China. Burns. 2020;46(4):959–969. doi: 10.1016/j.burns.2019.10.008 [DOI] [PubMed] [Google Scholar]
- 19.Hsieh MH, Watanabe T, Aihara M. Recent dermatological treatments for Stevens-Johnson syndrome and toxic epidermal necrolysis in Japan. Front Med. 2021;8:636924. doi: 10.3389/fmed.2021.636924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neerukonda VK, Stagner AM. Stevens Johnson syndrome: a review of a vision and life-threatening mucocutaneous disease including histopathology with updates on pathogenesis and genetic risk factors. Semin Ophthalmol. 2021;36(4):270–281. doi: 10.1080/08820538.2021.1893764 [DOI] [PubMed] [Google Scholar]
- 21.Mockenhaupt M, Viboud C, Dunant A, et al.Stevens-Johnson syndrome and toxic epidermal necrolysis in 48 cases of clinical data analysis. J Clin Dermatol. 2019;48(11):665–667. [Google Scholar]
- 22.Houschyar KS, Tapking C, Borrelli MR, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. J Wound Care. 2021;30(12):1012–1019. doi: 10.12968/jowc.2021.30.12.1012 [DOI] [PubMed] [Google Scholar]
- 23.Suhler EB, Thorne JE, Mittal M, et al. Corticosteroid-related adverse events systematically increase with corticosteroid dose in noninfectious intermediate, posterior, or panuveitis: post hoc analyses from the VISUAL-1 and VISUAL-2 trials. Ophthalmology. 2017;124(12):1799–1807. doi: 10.1016/j.ophtha.2017.06.017 [DOI] [PubMed] [Google Scholar]
- 24.Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American association of clinical endocrinologists and American college of endocrinology-clinical practice guidelines for developing a diabetes mellitus comprehensive care plan-2015-executive summary. Endocr Pract. 2015;21(4):413–437. doi: 10.4158/EP15672.GL [DOI] [PubMed] [Google Scholar]
- 25.Wu J, Lee YY, Su SC, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis in patients with malignancies. Br J Dermatol. 2015;173(5):1224–1231. doi: 10.1111/bjd.14052 [DOI] [PubMed] [Google Scholar]
- 26.Weinand C, Xu W, Perbix W, et al. 27 years of a single burn centre experience with Stevens-Johnson syndrome and toxic epidermal necrolysis: analysis of mortality risk for causative agents. Burns. 2013;39(7):1449–1455. doi: 10.1016/j.burns.2013.03.011 [DOI] [PubMed] [Google Scholar]