Abstract
The objectives of this study were to investigate the effects of a novel method using flavonoids to inhibit Streptococcus mutans (S. mutans), Candida albicans (C. albicans) and dual-species biofilms and to protect enamel hardness in a biofilm-based caries model for the first time. Several flavonoids, including baicalein, naringenin and catechin, were tested. Gold-standard chlorhexidine (CHX) and untreated (UC) groups served as controls. Optimal concentrations were determined by cytotoxicity assay. Biofilm MTT, colony-forming-units (CFUs), biofilm biomass, lactic acid and polysaccharide production were evaluated. Real-time-polymerase-chain reaction (qRT-PCR) was used to determine gene expressions in biofilms. Demineralization of human enamel was induced via S. mutans-C. albicans biofilms, and enamel hardness was measured. Compared to CHX and UC groups, the baicalein group achieved the greatest reduction in S. mutans, C. albicans and S. mutans-C. albicans biofilms, yielding the least metabolic activity, polysaccharide synthesis and lactic acid production (p < 0.05). The biofilm CFU was decreased in baicalein group by 5 logs, 4 logs, 5 logs, for S. mutans, C. albicans and S. mutans-C. albicans biofilms, respectively, compared to UC group. When tested in a S. mutans-C. albicans in vitro caries model, the baicalein group substantially reduced enamel demineralization under biofilms, yielding an enamel hardness that was 2.75 times greater than that of UC group. Hence, the novel baicalein method is promising to inhibit dental caries by reducing biofilm formation and protecting enamel hardness.
Keywords: baicalein, Streptococcus mutans, Candida albicans, biofilm inhibition, dental caries
1. Introduction
Dental caries is one of the most widespread and costly biofilm-mediated oral infectious diseases, affecting people of all ages worldwide [1]. Among the several hundred bacterial species in the dental plaque, Streptococcus mutans (S. mutans) is a principal causative agent of caries [2]. Candida albicans (C. albicans) is one of the most common fungi in mouth [3,4], and oral colonization of C. albicans is increased in root caries (RC) and early childhood caries (ECC) lesions [5,6]. C. albicans has an extraordinary acidogenic capacity and acid tolerance, and its association with S. mutans results in increased exopolysaccharides (EPS) formation and acid production and yield-enhancing biofilm cariogenicity [7,8]. Indeed, the positive correlation between C. albicans and the risk of dental caries has been demonstrated [9], and individuals with higher yeast counts in saliva and dental biofilms have a higher incidence of RC and ECC [6,7,10].
Preventing biofilm formation is a key to avoid the occurrence of dental caries [11]. Chlorhexidine (CHX) is a potent dental agent against oral infections, due to its broad-spectrum antimicrobial efficacy [12,13]. CHX can increase the cellular membrane permeability of bacteria or fungi by interacting with the anionic receptors on the cell surface [14,15]. CHX is widely used in clinical dentistry, and its therapeutic benefit has been demonstrated in reducing dental plaque [16]. However, bacterial spores and mycobacteria are highly resistant to CHX [17]. Thus, novel and effective methods should be explored to fight and prevent cariogenic biofilms.
Flavonoids are a family of polyphenolic compounds possessing great antibacterial and antifungal action [18]. Studies have shown that flavonoids have potentially beneficial effects as antimicrobial agents in the therapy for human disease [19,20]. In view of the fact that flavonoids represent an emerging threat of dental microbe, the present study was designed to evaluate the effect of selected single flavonoids (baicalein, naringenin, and catechin) for their activities against dental biofilm bacteria and fungi. In addition, to date, there were few studies on the role of flavonoids in affecting S. mutans, C. albicans and the dual-species biofilm formation [21,22].
Therefore, the objectives of this study were to investigate, for the first time, that: (1) flavonoids could inhibit single (S. mutans or C. albicans) and dual-species (S. mutans-C. albicans) cariogenic biofilm formation, with CHX as control; (2) flavonoids would substantially reduce enamel demineralization and increase the enamel hardness under S. mutans-C. albicans biofilm.
2. Results
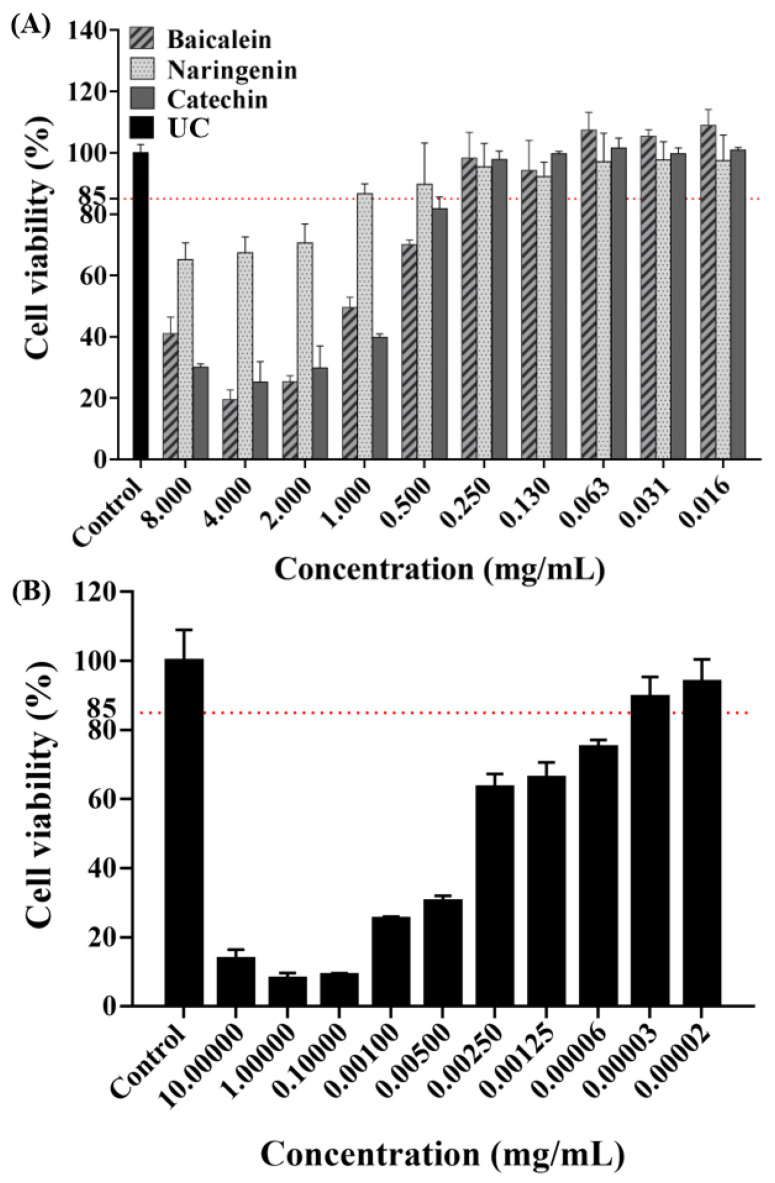
The concentration of antimicrobial agent in this experiment was screened using a cytotoxicity experiment. As shown by the results of CCK-8 assay (Figure 1), the absorbance rates decreased with increasing concentrations based on the negative values observed in the negative control group. As gold standard, CHX were chosen as our positive control to evaluate the cell toxicity. Based on the cell cytotoxicity test, the antibacterial concentrations were selected for our study as follows: 0.250 mg/mL of baicalein, 1.000 mg/mL of naringenin, 0.250 mg/mL of catechin, and 0.00031 mg/mL of CHX, respectively.
Figure 1.
Cell viability tests of HOK cells after being exposed to flavonoids (A) or CHX; (B) (mean ± sd; n = 3).
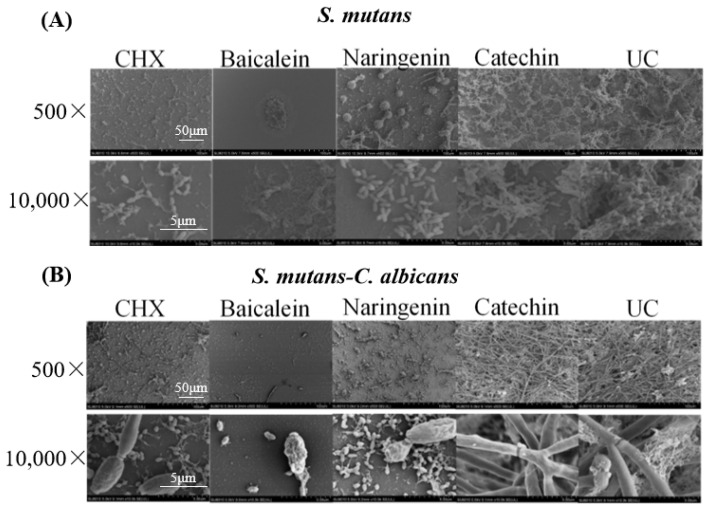
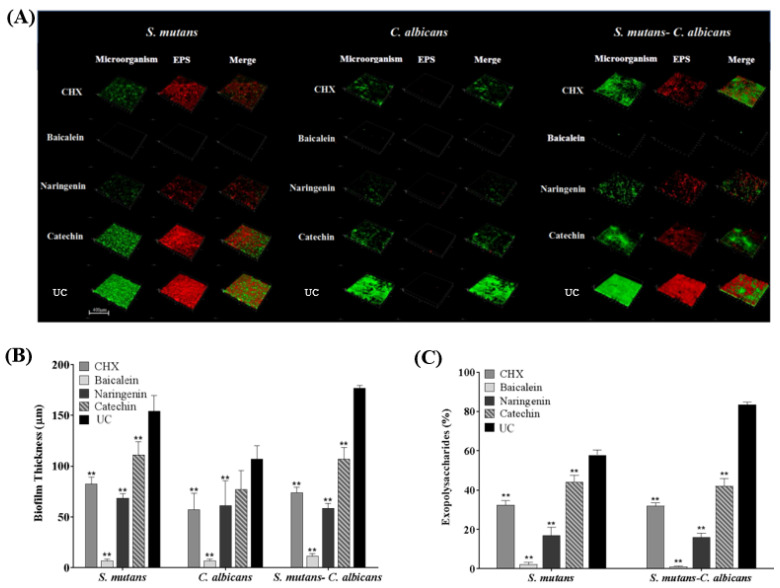
Representative SEM micrographs of typical biofilms on adhesive disks in different groups are shown in Figure 2. The five groups (CHX, baicalein, naringenin, catechin, and untreated control (UC)) were labeled in the images. The two types (S. mutans and dual-species) were labeled in these groups. Compared to the UC groups, biofilms of CHX, baicalein and naringenin groups on the adhesive disks did not fully develop even after 4 h. While the 0.250 mg/mL baicalein groups had the greatest antibacterial activity, only a few bacteria or fungi were observed on the surface of the disk.
Figure 2.
SEM observation of (A) S. mutans and (B) S. mutans-C. albicans biofilms after flavonoids or CHX treatment.
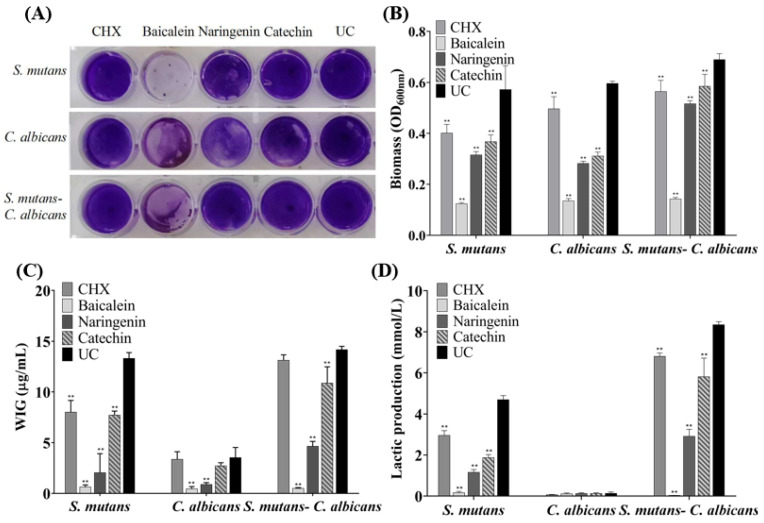
The CV staining image is presented in Figure 3A, and further biofilm biomass results are shown in Figure 3B (mean ± sd; n = 6). The drugs greatly reduced the biomass significantly containing flavonoid or CHX, compared to the UC group (p < 0.01), and the inhibition effect of baicalein is the greatest and much better than that of CHX (p < 0.01).
Figure 3.
Biofilm formation with or without drugs: (A) a CV staining photograph of biofilms grown in a 24-well polystyrene microtiter plate; (B) quantification of biofilm biomass; (C) polysaccharide synthesis and (D) lactic acid production of the single-species and dual-species biofilms after flavonoids or CHX treatment. The values represent the mean ± sd for six independent experiments (mean ± sd, n = 6; ** p < 0.01).
Six standard glucose concentrations of 0, 10, 20, 30, 40 and 50 μg/mL were used to plot the standard curve of OD620nm versus polysaccharide concentrations. A linear curve y = 0.0486x + 0.0614 was obtained, and the coefficient of determination (R2) was 0.9829. Figure 3C presents the polysaccharide synthesis with or without drugs (mean ± sd; n = 6). Two main results were obtained here (Figure 3C): (1) flavonoids and CHX remarkably decreased the polysaccharide production, compared to UC group (mean ± sd; n = 6; p < 0.01). (2) Using baicalein, the polysaccharide synthesis decreased more than 90%, which is much better than CHX control (p < 0.01).
Figure 3D plotted that S. mutans and dual-species biofilms had relatively higher levels of lactic acid production compared to C. albicans biofilms. The lactic acid production of single- and dual-species biofilms was greatly inhibited on the flavonoids and CHX groups (p < 0.01), and lactic acid production of baicalein group on biofilms was the lowest in all groups.
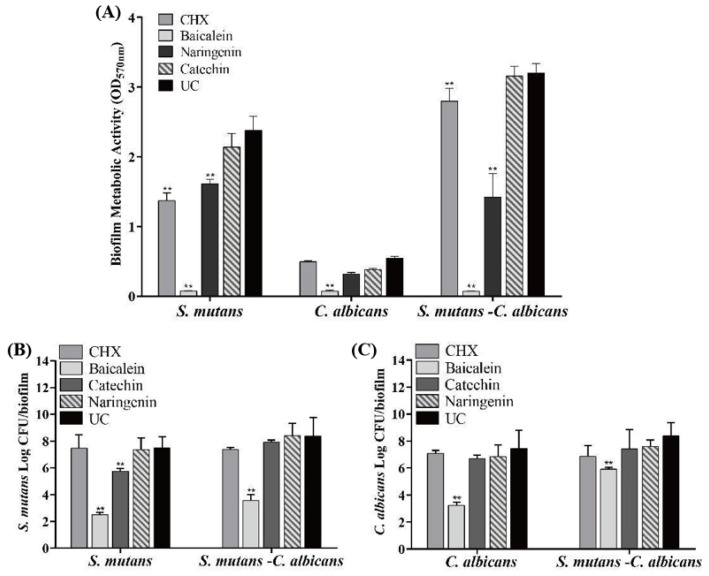
The antibacterial effects of flavonoids and CHX against the S. mutans, C. albicans and S. mutans-C. albicans biofilms are shown in Figure 4 (mean ± sd; n = 6). MTT metabolic activity and colony forming unit (CFU) were markedly decreased in the baicalein group (p < 0.01). Compared with the UC group, the CFU of S. mutans biofilm was decreased by nearly 5 logs and 4 logs in S. mutans and S. mutans-C. albicans biofilms, respectively, at 0.250 mg/mL baicalein (Figure 4B). While 4 logs and 1 log in C. albicans and S. mutans-C. albicans biofilms were reduced for C. albicans CFU at 0.250 mg/mL baicalein (Figure 4C). The use of baicalein caused the greatest reduction in biofilm activity, while the CHX group appeared to have no effect.
Figure 4.
Antimicrobial effects of flavonoids or CHX (mean ± sd; n = 6; ** p < 0.01): (A) MTT metabolic activity, (B) S. mutans colony-forming units (CFU) in S. mutans and S. mutans-C. albicans biofilms, and (C) C. albicans CFU in C. albicans and S. mutans-C. albicans biofilms.
CLSM showed that only small clusters of bacterial cells were observed in the baicalein and naringenin groups, while the CHX and catechin groups were similar with UC group (Figure 5A). It was concluded that baicalein and naringenin impaired EPS production and biofilm formation. Furthermore, we calculated the EPS/bacterial volume ratio, and baicalein exhibited the least value, which indicated the best anti-bacterial effect of baicalein in the EPS architecture development (Figure 5B,C). In addition, there was almost no EPS production of C. albicans biofilms in all groups, and therefore, we have deleted the results for C. albicans biofilms in Figure 5C.
Figure 5.
Representative CLSM staining images (A). Biofilm thickness (B) and EPS/bacterial or fungal ratio were calculated (C) (mean ± sd; n = 6; ** p < 0.01).
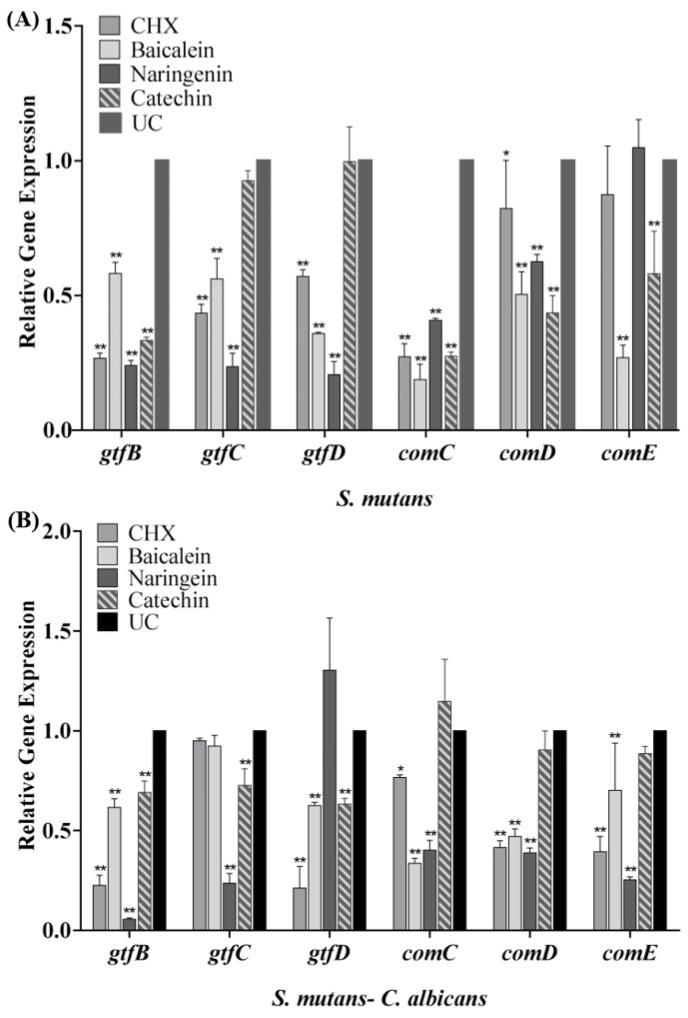
The expression profiles of gtfB/C/D and comC/D/E genes are shown in Figure 6. After the biofilm cultured with flavonoids or CHX, the transcription levels of gtfB/C/D and comC/D/E genes statistically declined (p < 0.05). Compared with the UC group in the S. mutans biofilm, the expression of gtfB/C/D in the baicalein group was down-regulated to 0.58, 0.56 and 0.35 folds, respectively (Figure 6A), while in the naringenin group, it was down-regulated to about 0.20 folds significantly. In dual-species biofilm, catechin down-regulate the expression of gtfB/C/D gene to 0.69–0.73 folds. In addition, the data revealed that the transcriptional levels of comC/D/E were significantly decreased in dual-species biofilm (Figure 6B). The expression of comC/D/E in the baicalein group was down-regulated to 0.34–0.70 folds, while in the naringenin group, it was down-regulated to 0.25–0.40 folds (Figure 6B).
Figure 6.
The S. mutans gtfB/C/D, comC/D/E gene expression in single-species (A) and dual-species biofilms (B) via qRT-PCR (mean ± sd, n = 3; * p < 0.05, ** p < 0.01).
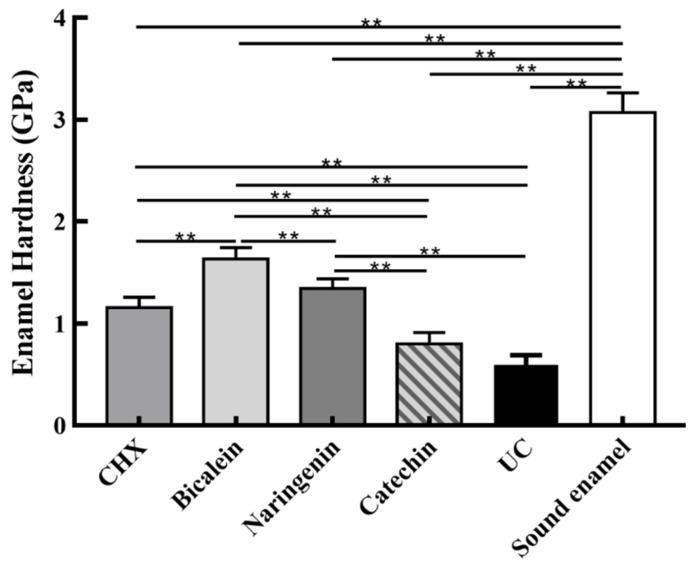
Enamel hardness at the enamel surface is plotted in Figure 7 (mean ± sd; n = 6). The sound enamel hardness was 3.08 ± 0.21 GPa. After 14 days under the S. mutans-C. albicans biofilm acid attack, enamel hardness decreased in every group, but the baicalein group had the highest enamel hardness (p < 0.01). These results showed that: (1) Flavonoids baicalein substantially increased the enamel hardness, by 2.75 folds, compared to the UC group. (2) The efficacy of baicalein and naringenin was much better than CHX in protecting the enamel hardness.
Figure 7.
Enamel hardness at the enamel surface after S. mutans-C. albicans biofilm acid attack for 14 days (mean ± sd, n = 6; ** p < 0.01).
3. Discussion
Flavonoids are a promising therapy against dental caries due to their antimicrobial activity and low toxicity. In the present study we investigated flavonoids with regard to their cytotoxicity on oral HOK cells and antimicrobial effects on cariogenic biofilm, using CHX as control group. There existed a certain usage concentration with regard to their cytotoxic activities in vitro. The antimicrobial ability observation and biofilm-based caries model proved that baicalein exerted instant plaque-inhibiting effects, helping control biofilm formation and protect tooth structure, which suggested that baicalein exerted great clinical application potentials.
Biocompatibility is a necessary indicators for evaluating all kinds of biomedical agents [23]. The toxicity to HOK cells was tested to explore the potential of flavonoids for dental clinical application [24]. An ideal antimicrobial agent should have effectiveness killing microbes while being safe to the host cell [23,25]. Therefore, in vitro and in vivo potential toxicity of antimicrobial agents should be evaluated before clinical use. However, sometimes in vitro studies used to evaluate drug toxicity tend to display cell toxicity, but they are still widely used in clinical treatment under certain conditions [23,26]. For example, 0.2% CHX is widely used in mouthwash, but 0.2% CHX in the present study revealed obvious toxic effects on human HOK cells viability. In the present study, we also evaluated the cytotoxic activities of flavonoids. Following current studies [23,27], we obtained the drug concentrations of more than 85% cell viability, and the results indicated that flavonoids concentrations of 0.250 mg/mL in baicalein, 1.000 mg/mL in naringenin and 0.250 mg/mL in catechin have good biocompatibility with HOK cells. Therefore, the concentrations above were used in the following experiments.
C. albicans is a Gram-positive fungal microorganism, which plays an important role in the development of dental caries because of its positive interactions with S. mutans [8]. The interaction between C. albicans and S. mutans can promote the occurrence and development of root caries and ECC [5,6]. C. albicans can enhance the sugar metabolism pathways of S. mutans [8]. S. mutans possesses an exceptional ability to produce EPS, and the EPS matrix is a key virulence factor for cariogenic biofilm [28,29]. As nutrients and the core of the matrix scaffold, EPS can enhance biofilm accumulation and stability [28,29]. In the present study, flavonoids disrupted the polysaccharide synthesis and biofilm formation in the S. mutans, C. albicans and the dual-species biofilms. Therefore, the use of drugs flavonoids might be a potential strategy for caries prevention since previous reports have indicated that the EPS initiates the adhesion for first colonizers and is critical for dental biofilms development.
In the present study, baicalein flavonoids substantially reduced the biofilm metabolic activity by more than 4 orders of magnitude. In sharp contrast, the biofilm CFU reduction in CHX had no significance compared to the UC group. The S. mutans and C. albicans in biofilms may be resistant to CHX [11]. Therefore, baicalein possesses anti-microbe effects of flavonoids that are much better than CHX.
Acidogenicity in S. mutans is a virulence factor associated with cariogenicity [1,30]. Biofilms can produce organic acids (mainly lactic acid) and induce teeth demineralization [28,30]. In the present study, the lactic acid secretion of biofilms decreased significantly in all drug groups, especially the baicalein group. The usage of baicalein achieved the greatest reduction in lactic acid production, more than 90% in S. mutans and dual-species biofilms. Such a major drop in biofilm acid production is expected to effectively inhibit dental caries, which warrants further study.
Sucrose-dependent adhesion is one of the major virulence factors [31,32]. The adhesion process is mainly mediated by glucans, which are synthesized from sucrose by glucosyltransferase (GTF) enzymes [33]. GTFB, GTFC and GTFD are the three important GTF enzymes [34]. The corresponding genes involved are gtfB, gtfC, and gtfD. The mRNA expression of gtfB, gtfC and gtfD of S. mutans and dual-species biofilm was examined using qRT-PCR in this study. The results showed that naringenin and baicalein could inhibit the mRNA expression for gtfB/C/D in S. mutans biofilm. The relative expressions of gtfB/D for S. mutans-C. albicans biofilms culturing in baicalein, of gtfB/C in naringenin and of gtfB/C/D in catechin also decreased. This demonstrated that flavonoids could reduce the sucrose-dependent adhesion of S. mutans and further decrease single- and dual-biofilm formation, which may neutralize the increase of sucrose independent adhesion, finally showing an inhibition effect.
The QS system controls biofilm formation and virulence factors release in cariogenic biofilms [35]. This system plays a key role in the competition and coexistence between microbe in biofilms and other significant processes [36]. In S. mutans, the most common intraspecific QS system is the competence-stimulating peptide (CSP)-QS system [37]. Hence, comC/D/E genes were tested, and the results proved that these genes were downregulated after incubation with naringenin and baicalein in the present study. In other words, naringenin and baicalein may inhibit the synthesis of QS system, which would reduce the internal signal transduction in S. mutans or S. mutans and C. albicans.
In the S. mutans-C. albicans in vitro caries model, the protection effects of flavonoids were verified. The hardness of the enamel in drug groups was all higher than that of UC groups. Compared to the CHX and UC groups, the enamel hardness in baicalein groups showed a significant increase at the enamel surface under 14 days of biofilm acid attack. These results demonstrate that baicalein could inhibit biofilm formation and hindered cariogenic activities, effectively inhibiting tooth demineralization and protecting tooth structure.
When the single- and dual-species biofilms were cultured in the flavonoids, the biofilm formation, metabolic activities and cariogenic activities were all significantly reduced. In addition, we proved the effect of flavonoids baicalein on inhibiting tooth demineralization under S. mutans-C. albicans biofilms for longer than 14 days. This study demonstrates the excellent antibacterial and antifungal effects of baicalein. Based on these results, the usage of baicalein could successfully inhibit dental caries pathogen biofilm formation. Further studies are needed to investigate the effect of baicalein in vivo, and more species biofilms also need to be studied regarding the complexity of oral microbe.
Regarding potential clinical applications, there is a major need in dentistry to decrease biofilm formation, as well as to inhibit caries and protect tooth structures. The present study demonstrated for the first time that the use of topical baicalein-containing solutions effectively suppressed caries associated S. mutans or C. albicans, yielding much greater enamel hardness under biofilm attacks. Dental applications of this novel method could include baicalein-based drugs for anti-caries clinical applications, such as dentifrices, mouthrinses, moisturizing gels, varnishes and chewing gums. Further studies are needed to investigate and realize these potentials.
4. Materials and Methods
4.1. Chemicals, Bacterial and Fungal Strains and Growth Conditions
Baicalein, naringenin, catechin and CHX were purchased commercially (Solarbio, Beijing, China). The use of all the bacterial species was approved by the Institutional Ethics Committee of Stomatological Hospital of Chongqing Medical University. S. mutans strain UA159 (ATCC 700610) and C. albicans SC5314 (ATCC MYA-2876) were provided by Sichuan University [3,15]. Precultures of S. mutans were grown in brain–heart infusion (BHI) medium at 37 °C under 5% CO2 [11]. Precultures of C. albicans were grown in YPD medium containing 1% yeast extract, 2% peptone, and 2% D-glucose at 37 °C under 5% CO2 as well [3]. The S. mutans biofilms were cultured in BHI supplemented with 1% sucrose (wt/vol) (BHIS). As for C. albicans and dual-species biofilms, YNBB (0.67% YNB, 75 mM Na2HPO4-NaH2PO4, 2.5 mM N-acetylglucosamine, 0.2% casamino acids, and 0.5% sucrose) was used [3].
4.2. Biofilm Formation
Precultures of S. mutans and C. albicans from single colonies were incubated overnight [3,11]. Then S. mutans were diluted to 2 × 106 CFU/mL into fresh BHIS, and C. albicans were diluted to 2 × 104 cell/mL into YNBB medium, with or without drugs. A volume of 2 mL bacteria dilution was cultured to form biofilms in 24-well plates. For dual-species biofilm, inoculum for the experiment was adjusted to 2 × 106 cell/mL of S. mutans, and 2 × 104 cell/mL of C. albicans [3]. Equal volumes of each strain (200 uL) and 1.6 mL YNBB medium with and without drugs were also incubated in 24-well plates for dual-species biofilm formation [38]. The plates were incubated at 37 °C under 5% CO2 for 4 h. The 4 h biofilms were then used for subsequent experiments.
4.3. Cytotoxicity Assays
Human oral keratinocyte cells (HOK cells) were used for cytotoxicity assays [39]. The HOK cells were cultured in a low-glucose Dulbecco’s modified eagle’s medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA, USA) (control media) [39,40]. The cells were incubated at 37 °C with 5% CO2, and the culture medium was changed every 2–3 days [40]. Each group, with or without drugs, and 8000 HOK cells were placed into a well of 96-well plates. Control medium was added accordingly. A cell counting kit (CCK-8, Dojindo, Tokyo, Japan) was used to evaluate cell proliferation [40]. Three replicates in each group were used for this assay (n = 3). After 24 h, the 96-well plate was washed twice with PBS. A volume of 100 uL of growth medium with 10% CCK-8 for 2 h was added into each well, and the cell proliferative rate was determined via measuring the absorbance at OD450nm using a microplate reader (SpectraMax M5, Molecular Devices, San Jose, CA, USA) [40]. Optimal tested concentrations were the drug concentrations with cell viability of above 85% [23]. Then the drugs (flavonoids and CHX) and UC groups were used in the following experiments.
4.4. Scanning Electron Microscopy (SEM)
The biofilm inhibition effects were observed using a scanning electron microscopy (SEM, FEI, Hillsboro, OR, USA). A volume of 2 mL 2 × 106 CFU/mL S. mutans dilution or 2 × 104 cell/mL C. albicans dilution was cultured to format single-species biofilm in a 24-well plate; and for dual-species biofilm, 2 × 106 CFU/mL of S. mutans and 2 × 104 cell/mL of C. albicans were used to form biofilms [3]. Each well of the 24-well plate contained a round glass slide (diameter = 14 mm) with 2 mL bacteria or fungal dilution for biofilm formation [41]. After 4 h anaerobic incubation, the biofilms were fixed with glutaraldehyde at room temperature for 12 h, then serially dehydrated in ethanol and tertiary butyl alcohol and sputter-coated with gold [41]. The specimens were examined at 500× and 10,000× magnification. Representative pictures are shown.
4.5. Crystal Violet (CV) Staining
The biomass of the biofilms with or without drugs was evaluated via CV staining, following previous studies [11,42]. Then S. mutans were diluted to 2 × 106 CFU/mL, and C. albicans were diluted to 2 × 104 cell/mL, and 2 mL dilution was cultured to format biofilms in 24-well plates. For dual-species biofilm, inoculum for the experiment was adjusted to 2 × 106 CFU/mL of S. mutans and 2 × 104 cell/mL of C. albicans [3]. After incubation for 4 h, the plates were washed twice with PBS [11]. The biofilms were air-dried and then stained with 0.1% (w/v) CV for 5 min at room temperature, washed three times with PBS to remove the unbound stain, dried and dissolved in 1 mL of 33% acetic acid [11]. The biofilm biomass was quantified by the optical density measured at a wavelength of 600 nm (OD600nm) [41]. The experiments were performed for six times in each group.
4.6. Polysaccharide Synthesis
The phenol-sulfuric acid method was used to measure the water-insoluble polysaccharides of biofilms [15]. Overnight cultures of S. mutans and C. albicans were respectively adjusted to 2 × 106 CFU/mL and 2 × 104 cell/mL for single- or dual-species biofilm formation. Biofilms with or without drugs were collected by scraping, and then, they were centrifuged (12,000 rpm) for 5 min at 4 °C and washed twice with PBS [15]. The precipitate was then resuspended in 1 M NaOH solution, placed in a constant temperature water bath at 37 °C and incubated for 3 h. Volumes of 100 μL of 6% phenol solution and 0.5 mL of 95–97% sulfuric acid were added, followed by incubation for 30 min [11]. Then, 200 μL of the solution was transferred into a 96-well plate, and OD620nm was determined with the microplate reader (SpectraMax M5, Molecular Devices, San Jose, CA, USA) [11,42]. Six glucose concentrations of 0, 10, 20, 30, 40 and 50 μg/mL were used to plot the standard curve of OD620nm readings to polysaccharide concentrations.
4.7. Lactic Acid Secretion
Overnight, bacterial cultures of S. mutans were diluted to 2 × 106 CFU/mL into fresh BHIS for biofilm formation with or without drugs. A volume of 2 mL 2 × 104 cell/mL C. albicans dilution was used for biofilm formation. For dual-species biofilm, inoculum for the experiment was adjusted to 2 × 106 CFU/mL of S. mutans and 2 × 104 cell/mL of C. albicans [3]. After incubation, the biofilms in 24-well plates were rinsed twice with PBS and then immersed in 1.5 mL buffered peptone water (BPW, Sigma-Aldrich, Saint Louis, MO, USA) with 0.2% sucrose and incubated at 37 °C for 3 h (n = 6) [15,42]. After removing planktonic cells by centrifugation, the supernatants were decanted to measure lactate concentrations according to the manuscript of the Lactate Assay Kit (MAK064, Sigma-Aldrich, Saint Louis, MO, USA) [43]. The absorbance at 530 nm was recorded using a microplate spectrophotometer, and lactate concentrations were calculated by the standard curves [15].
4.8. Biofilm Viability Using the MTT Assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (VWR Chemicals, OH, USA) assay was used to estimate the viability of bacteria and fungi in biofilms [15]. S. mutans were diluted to 2 × 106 CFU/mL in BHIS and C. albicans were diluted to 2 × 104 cell/mL in YNBB to form biofilm, with or without drugs. For dual-species biofilm, inoculum for the experiment was adjusted to 2 × 106 CFU/mL of S. mutans and 2 × 104 cell/mL of C. albicans [3]. Biofilms with or without drugs were washed twice with PBS (n = 6). A volume of 1mL MTT dye (0.5 mg/mL MTT in PBS) was added into each well and incubated at 37 °C under 5% CO2 for 1 h. A volume of 1 mL dimethyl sulfoxide (DMSO) was added in each well at room temperature for 20 min to dissolve the formazan crystals. After mixing via pipetting, 200 μL of the DMSO solution was collected and transferred into 96-well plate. OD570nm was determined using the microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA, USA).
4.9. Biofilm CFU Counts
Six wells of each group in 24-well plates were used for CFU counting [15]. The S. mutans were diluted to 2 × 106 CFU/mL into fresh BHIS, and 2 mL bacteria dilution was cultured to form biofilms in 24-well plates. A volume of 2 mL 2 × 106 CFU/mL C. albicans dilution was cultured in YNBB. For dual-species biofilm, inoculum for the experiment was adjusted to 2 × 106 CFU/mL of S. mutans, and 2 × 104 cell/mL of C. albicans [3]. Biofilms with or without drugs were transferred into tubes with 2 mL of PBS, and the biofilms were harvested by scraping and sonication/vortexing (Fisher, Pittsburg, PA, USA) [15]. The suspensions were serially diluted and spread onto BHI or YPD agar plates. For dual-species, we added 8 μg/mL amphotericin B in BHI agar plates and 8 μg/mL gentamicin in YPD agar plates to inhibit the growth of C. albicans and S. mutans, respectively [44]. After a 2-day incubation at 37 °C under 5% CO2, the colony number was counted and then CFU counts were determined.
4.10. Confocal Laser Scanning Microscopy (CLSM)
For analyzing EPS production and distribution within the biofilms with or without drugs, we used CLSM for EPS/bacterial staining. S. mutans were diluted to 2 × 106 CFU/mL into BHIS, and C. albicans were diluted to 2 × 104 cell/mL into YNBB for biofilm formation. For dual-species biofilm, inoculum for the experiment was adjusted to 2 × 106 CFU/mL of S. mutans, and 2 × 104 cell/mL of C. albicans [3]. As in previous studies, 1 μM Alexa Fluor 647 (Invitrogen, Eugene, OR, USA) and 2.5 μM SYTO 9 (Invitrogen, Carlsbad, CA, USA) were used to label EPS and bacterial cells, respectively [41,45]. Biofilms were grown on coverslips and observed by CLSM (Olympus FV1000, Tokyo, Japan) at a range of 495–515 nm for SYTO 9 and 655–690 nm for Alexa Fluor 647 [41,45]. Images of six random fields of each group were captured. A three-dimensional reconstruction of the biofilms was analyzed, and the EPS/bacteria ratio was calculated.
4.11. Quantitative Real-Time-Polymerase-Chain Reaction (qRT-PCR)
For qRT-PCR, UA159 biofilms were grown at 37 °C under anaerobic conditions (90% N2, 5% CO2, 5% H2) to 2 × 106 CFU/mL in fresh BHIS with or without drugs. Dual-species biofilms were cultured adjusted to 2 × 106 CFU/mL of S. mutans and 2 × 104 cell/mL of C. albicans [3]. Biofilms were grown in 24-well plates for 4 h. qRT-PCR was used to quantify expression of selected genes in biofilms, with gyrA of S. mutans as an internal control [41]. The cells were harvested from the biofilms and snap frozen in liquid nitrogen until they were needed [15]. Total bacterial RNA isolation, purification and reverse transcription of complementary DNA (cDNA) were performed as previously described [15]. All primers for qRT-PCR were obtained commercially (Sangon Biotech, Shanghai, China) and are listed in Table 1. Threshold cycle values (CT) were determined, and the data were analyzed by BIO-RAD CFX MANAGER software (version 2.0, Hercules, CA, USA) using the 2−ΔΔCT method [41].
Table 1.
Primers and probes used in qRT-PCR.
| Primer | Nucleotide Sequence | Reference |
|---|---|---|
| gyrA-F | 5′ ATTGTTGCTCGGGCTCTTCCAG 3′ | [46] |
| gyrA-R | 5′ ATGCGGCTTGTCAGGAGTAACC 3′ | |
| gtfB-F | 5′ ACACTTTCGGGTGGCTTG 3′ | [47] |
| gtfB-R | 5′ GCTTAGATGTCACTTCGGTTG 3′ | |
| gtfC-F | 5′ CCAAAATGGTATTATGGCTGTCG 3′ | [47] |
| gtfC-R | 5′ TGAGTCTCTATCAAAGTAACGCAG 3′ | |
| gtfD-F | 5′ AATGAAATTCGCAGCGGACTTGAG 3′ | [48] |
| gtfD-R | 5′ TTAGCCTGACGCATGTCTTCATTGTA 3′ | |
| comC-F | 5′ GACTTTAAAGAAATTAAGACTG 3′ | [47] |
| comC-R | 5′ AAGCTTGTGTAAAACTTCTGT 3′ | |
| comD-F | 5′ CTCTGATTGACCATTCTTCTGG 3′ | [47] |
| comD-R | 5′ CATTCTGAGTTTATGCCCCTC 3′ | |
| comE-F | 5′ CCTGAAAAGGGCAATCACCAG 3′ | [47] |
| comE-R | 5′ GGGGCATAAACTCAGAATGTGTCG 3′ |
4.12. Enamel Hardness Measurement
Extracted caries-free human teeth were obtained from the *** following a protocol approved by the *** Institutional Review Board. Teeth were cleaned and stored in 0.1% thymol solution at 4 °C before use. As previous study, enamel slabs were prepared to have a diameter of 6 mm and a thickness of 2 mm [42]. Except the enamel surfaces, the rest area was coated with two layers of acid-resistant nail varnish. The specimens were polished using sandpapers with grit of # 600, 1200, 2400 and 4000, consecutively, with copious water [42]. The 30 enamel slabs were randomly divided into five groups of 6 slabs each and incubated with or without drugs.
A hardness tester (HVS-10Z, Jingbo Company, Zhejiang, China) was used with a Vickers indenter, under a 50 g load with a dwell time of 20 s [42]. The area selected for indentation was the center of enamel surface. Every enamel specimen had six indentations. Measurements were conducted before and after biofilm acid attacks. The sterile specimens were placed in 24-well plates containing 2 mL 2 × 106 CFU/mL of S. mutans and 2 × 104 cell/mL C. albicans suspension, as a previous study described [42]. Each day, the slabs were placed into a well of 24-well plates and immersed in 2 mL of YNBB at pH 7.4 at 37 °C for 4 h. Then, they were removed and placed into a new plate with the YNBB-R medium which reduced the sucrose content at pH 7 for 20 h at 37 °C in 5% CO2. The daily medium change was done under aseptic conditions. Since this biofilm model took 14 days to finish, to avoid the biofilm becoming old and too thick which would deprive the interior bacteria from nutrients, a sterile paper was used to remove the biofilm from the slab every 24 h, which was then inoculated again to grow a new biofilm on the slab. This biofilm model and cyclic immersion treatment was repeated for 14 days.
4.13. Statistical Analysis
Data analyses were performed using Statistical Package for the Social Sciences (SPSS 22.0, Chicago, IL, USA). All data were expressed as the mean value ± standard deviation (mean ± sd). Statistical significance was analyzed by using the one-way analyses of variance (ANOVA) and Student–Newman–Keuls test. A confidence level of 95% (p < 0.05) was considered significant.
5. Conclusions
This study demonstrated for the first time that flavonoid baicalein had strong biofilm-suppression, caries-inhibition and enamel-protection capabilities. Baicalein showed the most potent and the greatest reduction in biofilm biomass, polysaccharide and lactic acid production among the test groups of baicalein, naringenin and catechin, and CHX. Baicalein reduced biofilm CFU by more than 4 orders of magnitude, and reduced biofilm acids by 99.99%, for S. mutans-C. albicans biofilm, compared to the UC group. In addition, the enamel hardness in the baicalein group showed only a minimal decrease under 14-day biofilm acid attacks, resulting in an enamel hardness that was 2.75 times greater than that of the untreated control group. Baicalein-based drugs are promising for a wide range of applications to suppress biofilms, prevent dental caries and protect tooth structures.
Author Contributions
Conceptualization, J.G., W.L., H.C., L.H. and H.H.K.X.; methodology, H.C., D.Y., S.X., L.H., H.H.K.X. and M.D.W.; investigation, S.X., H.C., W.L. and D.Y.; analysis and graphs—H.C., S.X., Y.T. and T.W.O.; writing—H.C., S.X. and D.Y.; writing—review and editing, H.H.K.X., H.C., D.Y. and J.G.; funding acquisition, D.Y., Y.T. and H.C.; resources, S.X., J.G., H.C., M.D.W. and T.W.O.; supervision, M.D.W., W.L., Y.T. and L.H.; project administration, H.H.K.X., D.Y. and H.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Stomatological Hospital of Chongqing Medical University.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by National Natural Science Foundation of China (No. 31970783 to D.Y., No. 82100991 to H.C.); Program for Top talent Distinguished Professor from Chongqing Medical University to D.Y., Program for Youth Innovation in Future Medicine from Chongqing Medical University (No. W0060 to D.Y.); the Science and Technology Research Program of Chongqing Municipal Education Commission (Grant No. KJQN201900401 to Y.T.) and Chongqing Special Postdoctoral Science Foundation (No. 2010010005994583 to H.C.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pitts N.B., Zero D.T., Marsh P.D., Ekstrand K., Weintraub J.A., Ramos-Gomez F., Tagami J., Twetman S., Tsakos G., Ismail A. Dental caries. Nat. Rev. Dis. Primers. 2017;3:17030. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 2.Ajdić D., McShan W.M., McLaughlin R.E., Savić G., Chang J., Carson M.B., Primeaux C., Tien R., Kenton F., Jia H., et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA. 2002;99:14434–14439. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du Q., Ren B., He J., Peng X., Guo Q., Zheng L., Li J., Dai H., Chen V., Zhang L., et al. Candida albicans promotes tooth decay by inducing oral microbial dysbiosis. ISME J. 2020;15:894–908. doi: 10.1038/s41396-020-00823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniluk T., Tokajuk G., Stokowska W., Fiedoruk K., Sciepuk M., Zaremba M.L., Rozkiewicz D., Cylwik-Rokicka D., A Kedra B., Anielska I., et al. Occurrence rate of oral Candida albicans in denture wearer patients. Adv. Med. Sci. 2006;51:77–80. [PubMed] [Google Scholar]
- 5.Takahashi N., Nyvad B. Ecological Hypothesis of Dentin and Root Caries. Caries Res. 2016;50:422–431. doi: 10.1159/000447309. [DOI] [PubMed] [Google Scholar]
- 6.Fakhruddin K.S., Samaranayake L.P., Egusa H., Ngo H.C., Panduwawala C., Venkatachalam T., Kumarappan A., Pesee S. Candida biome of severe early childhood caries (S-ECC) and its cariogenic virulence traits. J. Oral Microbiol. 2020;12:1724484. doi: 10.1080/20002297.2020.1724484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen S., Samaranayake L.P., Yip H.K., Dyson J.E. Bacterial and yeast flora of root surface caries in elderly, ethnic Chinese. Oral Dis. 2002;8:207–217. doi: 10.1034/j.1601-0825.2002.01796.x. [DOI] [PubMed] [Google Scholar]
- 8.He J., Kim D., Zhou X., Ahn S.-J., Burne R.A., Richards V., Koo H. RNA-Seq Reveals Enhanced Sugar Metabolism in Streptococcus mutans Co-cultured with Candida albicans within Mixed-Species Biofilms. Front. Microbiol. 2017;8:1036. doi: 10.3389/fmicb.2017.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira D., Seneviratne C., Ito C.K., Samaranayake L. Is the oral fungal pathogen Candida albicans a cariogen? Oral Dis. 2018;24:518–526. doi: 10.1111/odi.12691. [DOI] [PubMed] [Google Scholar]
- 10.Zaremba M.L., Stokowska W., Klimiuk A., Daniluk T., Rozkiewicz D., Cylwik-Rokicka D., Waszkiel D., Tokajuk G., Kierklo A., Abdelrazek S. Microorganisms in root carious lesions in adults. Adv. Med. Sci. 2006;51:237–240. [PubMed] [Google Scholar]
- 11.Chen H., Tang Y., Weir M.D., Gao J., Imazato S., Oates T.W., Lei L., Wang S., Hu T., Xu H.H. Effects of S. mutans gene-modification and antibacterial monomer dimethylaminohexadecyl methacrylate on biofilm growth and acid production. Dent. Mater. 2020;36:296–309. doi: 10.1016/j.dental.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Varoni E.M., Tarce M., Lodi G., Carrassi A. Chlorhexidine (CHX) in dentistry: State of the art. Minerva Stomatol. 2012;61:399–419. [PubMed] [Google Scholar]
- 13.Seneviratne C.J., Leung K.C.-F., Wong C.-H., Lee S.-F., Li X., Leung P.C., Lau C., Wat E., Jin L. Nanoparticle-Encapsulated Chlorhexidine against Oral Bacterial Biofilms. PLoS ONE. 2014;9:e103234. doi: 10.1371/journal.pone.0103234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar S.B. Chlorhexidine mouthwash-A review. J. Pharm. Pharm. Sci. 2017;9:1450. [Google Scholar]
- 15.Chen H., Zhang B., Weir M.D., Homayounfar N., Fay G.G., Martinho F., Lei L., Bai Y., Hu T., Xu H.H. S. mutans gene-modification and antibacterial resin composite as dual strategy to suppress biofilm acid production and inhibit caries. J. Dent. 2020;93:103278. doi: 10.1016/j.jdent.2020.103278. [DOI] [PubMed] [Google Scholar]
- 16.Sreenivasan P., Gaffar A. Antiplaque biocides and bacterial resistance: A review. J. Clin. Periodontol. 2002;29:965–974. doi: 10.1034/j.1600-051X.2002.291101.x. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadi Z., Abbott P.V. Antimicrobial substantivity of root canal irrigants and medicaments: A review. Aust. Endod. J. 2009;35:131–139. doi: 10.1111/j.1747-4477.2009.00164.x. [DOI] [PubMed] [Google Scholar]
- 18.Gutiérrez-Venegas G., González-Rosas Z. Apigenin reduce lipoteichoic acid-induced inflammatory response in rat cardiomyoblast cells. Arch. Pharmacal. Res. 2017;40:240–249. doi: 10.1007/s12272-016-0756-2. [DOI] [PubMed] [Google Scholar]
- 19.Serpa R., França E.J.G., Maia L., Andrade C.G.T.J., Diniz A., Furlaneto M.C. In vitro antifungal activity of the flavonoid baicalein against Candida species. J. Med. Microbiol. 2012;61:1704–1708. doi: 10.1099/jmm.0.047852-0. [DOI] [PubMed] [Google Scholar]
- 20.Gutiérrez-Venegas G., Gómez-Mora J.A., Meraz-Rodríguez M.A., Flores-Sánchez M.A., Ortiz-Miranda L.F. Effect of flavonoids on antimicrobial activity of microorganisms present in dental plaque. Heliyon. 2019;5:e03013. doi: 10.1016/j.heliyon.2019.e03013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lobo C.I.V., Lopes A.C.U.D.A., Klein M.I. Compounds with distinct targets present diverse antimicrobial and antibiofilm efficacy against Candida albicans and Streptococcus mutans, and combinations of compounds potentiate their effect. J. Fungi. 2021;7:340. doi: 10.3390/jof7050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi H.-A., Cheong D.-E., Lim H.-D., Kim W.-H., Ham M.-H., Oh M.-H., Wu Y., Shin H.-J., Kim G.-J. Antimicrobial and Anti-Biofilm Activities of the Methanol Extracts of Medicinal Plants against Dental Pathogens Streptococcus mutans and Candida albicans. J. Microbiol. Biotechnol. 2017;27:1242–1248. doi: 10.4014/jmb.1701.01026. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y., Wang S., Zhou X., Zou Y., Li M., Peng X., Ren B., Xu H.H.K., Weir M.D., Cheng L., et al. Short-Time Antibacterial Effects of Dimethylaminododecyl Methacrylate on Oral Multispecies Biofilm In Vitro. BioMed Res. Int. 2019;2019:6393470. doi: 10.1155/2019/6393470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weng L., Wu L., Guo R., Ye J., Liang W., Wu W., Chen L., Yang D. Lactobacillus cell envelope-coated nanoparticles for antibiotic delivery against cariogenic biofilm and dental caries. J. Nanobiotechnol. 2022;20:356. doi: 10.1186/s12951-022-01563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller H.-D., Eick S., Moritz A., Lussi A., Gruber R. Cytotoxicity and Antimicrobial Activity of Oral Rinses In Vitro. BioMed Res. Int. 2017;2017:4019723–4019729. doi: 10.1155/2017/4019723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyganowska-Swiatkowska M., Kotwicka M., Urbaniak P., Nowak A., Skrzypczak-Jankun E., Jankun J. Clinical implications of the growth-suppressive effects of chlorhexidine at low and high concentrations on human gingival fibroblasts and changes in morphology. Int. J. Mol. Med. 2016;37:1594–1600. doi: 10.3892/ijmm.2016.2550. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura M., Kawahara H., Kataoka Y., Maehara S., Izutani M., Taguchi H. Biocompatibility of dental amalgams in vitro during 52 week period. Shika Rikogaku Zasshi J. Jpn. Soc. Dent. Appar. Mater. 1980;21:228–244. [PubMed] [Google Scholar]
- 28.Koo H., Falsetta M., Klein M. The exopolysaccharide matrix: A virulence determinant of cariogenic biofilm. J. Dent. Res. 2013;92:1065–1073. doi: 10.1177/0022034513504218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koo H., Xiao J., I Klein M. Extracellular Polysaccharides Matrix—An Often Forgotten Virulence Factor in Oral Biofilm Research. Int. J. Oral Sci. 2009;1:229. doi: 10.4248/IJOS.09086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Law V., Seow W., Townsend G. Factors influencing oral colonization of mutans streptococci in young children. Aust. Dent. J. 2007;52:93–100. doi: 10.1111/j.1834-7819.2007.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg M. Microbial adhesion to hydrocarbons: Twenty-five years of doing MATH. FEMS Microbiol. Lett. 2006;262:129–134. doi: 10.1111/j.1574-6968.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- 32.Strużycka I. The Oral Microbiome in Dental Caries. Pol. J. Microbiol. 2014;63:127–135. doi: 10.33073/pjm-2014-018. [DOI] [PubMed] [Google Scholar]
- 33.Yue J., Yang H., Liu S., Song F., Guo J., Huang C. Influence of naringenin on the biofilm formation of Streptococcus mutans. J. Dent. 2018;76:24–31. doi: 10.1016/j.jdent.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Krzyściak W., Jurczak A., Kościelniak D., Bystrowska B., Skalniak A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. 2014;33:499–515. doi: 10.1007/s10096-013-1993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worthington R.J., Richards J.J., Melander C. Small molecule control of bacterial biofilms. Org. Biomol. Chem. 2012;10:7457–7474. doi: 10.1039/c2ob25835h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding Y., Wang W., Fan M., Tong Z., Kuang R., Jiang W., Ni L. Antimicrobial and anti-biofilm effect of Bac8c on major bacteria associated with dental caries and Streptococcus mutans biofilms. Peptides. 2014;52:61–67. doi: 10.1016/j.peptides.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 37.Suntharalingam P., Cvitkovitch D.G. Quorum sensing in streptococcal biofilm formation. Trends Microbiol. 2005;13:3–6. doi: 10.1016/j.tim.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Liu S., Qiu W., Zhang K., Zhou X., Ren B., He J., Xu X., Cheng L., Li M. Nicotine enhances interspecies relationship between Streptococcus mutans and Candida albicans. BioMed Res. Int. 2017;2017:7953920. doi: 10.1155/2017/5803246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagai J., Shi H., Kubota Y., Bundow K., Okudaira N., Uesawa Y., Sakagami H., Tomomura M., Tomomura A., Takao K., et al. Quantitative structure–cytotoxicity relationship of pyrano[4,3-b]chromones. Anticancer Res. 2018;38:4449–4457. doi: 10.21873/anticanres.12747. [DOI] [PubMed] [Google Scholar]
- 40.Chen H., Yang H., Weir M.D., Schneider A., Ren K., Homayounfar N., Oates T.W., Zhang K., Liu J., Hu T., et al. An antibacterial and injectable calcium phosphate scaffold delivering human periodontal ligament stem cells for bone tissue engineering. RSC Adv. 2020;10:40157–40170. doi: 10.1039/D0RA06873J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mao M.-Y., Yang Y.-M., Li K.-Z., Lei L., Li M., Yang Y., Tao X., Yin J.-X., Zhang R., Ma X.-R., et al. The rnc gene promotes exopolysaccharide synthesis and represses the vicRKX gene expressions via microRNA-size small RNAs in Streptococcus mutans. Front. Microbiol. 2016;7:687. doi: 10.3389/fmicb.2016.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen H., Tang Y., Weir M.D., Lei L., Masri R., Lynch C.D., Oates T.W., Zhang K., Hu T., Xu H.H.K. Effects of S. mutans gene-modification and antibacterial calcium phosphate nanocomposite on secondary caries and marginal enamel hardness. RSC Adv. 2019;9:41672–41683. doi: 10.1039/C9RA09220J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y., Wang X., Jiang W., Wang K., Luo J., Li W., Zhou X., Zhang L. Antimicrobial peptide GH12 suppresses cariogenic virulence factors of Streptococcus mutans. J. Oral Microbiol. 2018;10:1442089. doi: 10.1080/20002297.2018.1442089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Escobar I.E., Rossatto F.C.P., Kim S.M., Kang H.M., Kim W., Mylonakis E. Repurposing Kinase Inhibitor Bay 11–7085 to Combat Staphylococcus aureus and Candida albicans Biofilms. Front. Pharmacol. 2021;12:675300. doi: 10.3389/fphar.2021.675300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Z., Xiang Z., Zeng J., Li Y., Li J. A GntR family transcription factor in Streptococcus mutans regulates biofilm formation and expression of multiple sugar transporter genes. Front. Microbiol. 2019;9:3224. doi: 10.3389/fmicb.2018.03224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korithoski B., Levesque C.M., Cvitkovitch D.G. The involvement of the pyruvate dehydrogenase E1α subunit, in Streptococcus mutans acid tolerance. FEMS Microbiol. Lett. 2008;289:13–19. doi: 10.1111/j.1574-6968.2008.01351.x. [DOI] [PubMed] [Google Scholar]
- 47.Li B., Li X., Lin H., Zhou Y. Curcumin as a Promising Antibacterial Agent: Effects on Metabolism and Biofilm Formation in S. mutans. BioMed Res. Int. 2018;2018:4508709. doi: 10.1155/2018/4508709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mao M., Zhang W., Huang Z., Huang J., Wang J., Li W., Gu S. Graphene Oxide-Copper Nanocomposites Suppress Cariogenic Streptococcus mutans Biofilm Formation. Int. J. Nanomed. 2021;16:7727–7739. doi: 10.2147/IJN.S303521. [DOI] [PMC free article] [PubMed] [Google Scholar]