SUMMARY
The rapid rise in syphilis cases has prompted a number of public health campaigns to assist men who have sex with men (MSM) recognize and present early with symptoms. This study aimed to investigate the temporal trend of the duration of self-report symptoms and titre of rapid plasma reagin (RPR) in MSM with infectious syphilis. Seven hundred and sixty-one syphilis cases in MSM diagnosed at the Melbourne Sexual Health Centre (MSHC) from 2007–2013 were reviewed. Median duration of symptoms and RPR titres in each year were calculated. The median durations of symptoms with primary and secondary syphilis were 9 [interquartile range (IQR) 6–14] days and 14 (IQR 7–30) days, respectively. The overall median titre of RPR in secondary syphilis (median 128, IQR 64–256) was higher than in primary syphilis (median 4, IQR 1–32) and in early latent syphilis (median 32, IQR 4–64). The median duration of symptoms for primary syphilis, secondary syphilis and titre of RPR level did not change over time. Public health campaigns were not associated with a significant shorter time from onset of symptoms to treatment. Alternative strategies such as more frequent testing of MSM should be promoted to control the syphilis epidemic in Australia.
Key words: Men who have sex with men, public health, symptoms recognition, syphilis
INTRODUCTION
Up to a decade ago, syphilis was uncommon in gay men and other men who have sex with men (MSM) in Australia. In 2002, however, notifications began to increase nationally, and in Victoria, for example, have risen from nine cases in 2000 to 232 cases in 2006, and 660 cases in 2013 [1]. More than 90% of the syphilis cases in Victoria were males and the majority (>75%) of cases were attributed to male-to-male sexual contact [1]. Similar changes have occurred in both the UK [2] and United States [3]. Virtually no developed country has had any significant success in reducing syphilis rates in MSM in the last decade [4].
In an effort to reduce the risk of HIV and other sexually transmitted infections (STIs) in MSM in Victoria, a number of health promotion campaigns have been initiated (e.g. ‘Drama Down Under’, and ‘Staying Negative’), most since 2007 (Table 1) [5]. These campaigns aimed to increase health-seeking behaviours, awareness of typical STI symptoms, HIV/STI testing rates and condom use among MSM. A central principle that has underpinned strategies aimed at controlling syphilis is the detection and treatment of infectious stages of syphilis to interrupt further transmission. Early symptom recognition has the potential to lead to earlier diagnosis and treatment of infectious syphilis. One way to measure the effectiveness of these campaigns in the community is to determine whether the duration of symptoms reported by patients has shortened over time. Another approximate measure of early diagnosis of infectious syphilis could be obtained by tracking the rapid plasma reagin (RPR) titre; a rise in the median RPR titre over time could indicate an increasing delay in diagnosis.
Table 1.
Summary of HIV/STI health promotion campaigns and prevention initiatives in Victoria, Australia
| Campaign | Campaign period | Budgets (AUD) | Target group | Aims | Identification of syphilis symptoms | Information of STI testing |
|---|---|---|---|---|---|---|
| Drama Down Under (DDU) (www.dramadownunder.info) | February 2008 to June 2014 | 1 831 606 | All MSM |
|
Yes | Yes |
| Staying Negative (www.stayingnegative.net.au) | 2007 to present | 190 000 | All MSM |
|
Yes | Yes |
| Protection (www.protection.org.au) | February 2009 to October 2010 | 104 400 | All MSM |
|
No | Yes |
| Wherever Sex Happens (www.whereversexhappens.com) | February 2010 to July 2012 | 133 356 | All MSM |
|
No | Yes |
| Being Brendo (https://www.facebook.com/BeingBrendo) | April 2010 to April 2013 | 751 802 | All MSM |
|
No | Yes |
| Top2Bottom (http://www.top2bottom.org.au) | August to December 2012 | 170 000 | High-risk MSM – who have unprotected anal sex with regular or casual partners |
|
No | Yes |
| Ending HIV (http://endinghiv.org.au) | September 2013 to June 2014 | 426 533 | All MSM |
|
No | No |
STI, Sexually transmitted infection; MSM, men who have sex with men.
Mathematical models from over 10 years ago before the MSM epidemic began suggest that high level of symptom recognition could improve the control of syphilis [6]. Understanding the duration of syphilis symptoms would facilitate the development of mathematical models to inform syphilis control strategies in the current epidemic environment. To our knowledge there have been no published studies describing temporal trends of duration of symptoms in MSM infected with infectious syphilis since 2000. The aim of this study was to investigate if the duration of symptoms, or the median titre of the RPR test for infectious syphilis has reduced.
METHODS
Study population and data collection
We conducted a retrospective study examining clinical records of MSM diagnosed with infectious syphilis (primary, secondary, early latent) at Melbourne Sexual Health Centre (MSHC) in Australia, from 1 January 2007 to 31 December 2013. MSHC is the largest public sexual health clinic in the state of Victoria, and it is located in the city of Melbourne. It provides about 35 000 consultations per year and about 37% of the consultations are MSM [7]. The clinic provides a walk-in service, patients who are at higher risk of infections (e.g. a contact of an infection), or those who have notifiable STI-related symptoms are prioritized to the service. All services are free-of-charge and no referrals are required. Demographic characteristics (i.e. country of birth, age), sexual behaviours (i.e. sex overseas in the past 12 months), and ever injecting drug use were routinely collected from patients using computer-assisted self-interviewing on the day of consultation.
Clinical diagnosis of syphilis
Data on syphilis diagnoses in MSM were extracted from the electronic clinic database. MSM were defined as men who had had sex with other men in the 12 months before their first consultation to the clinic. Transgender MSM were excluded from this analysis. Clinicians routinely ask and record the symptoms (including the self-reported duration) in the clinical notes and we extracted these records for individuals diagnosed with symptomatic primary or secondary syphilis. A chart review on the self-reported symptoms and durations were conducted. Data were extracted and interpreted from the clinical notes by two junior clinicians (K.D. and J.P.). Discrepancies on symptom interpretation or classification were resolved by two senior sexual health clinicians (I.D., T.R.H.R.).
All syphilis cases were serologically confirmed by the RPR test, Treponema pallidum enzyme immunoassay (EIA) and T. pallidum particle agglutination assay (TPPA). Results of laboratory investigations were extracted and reviewed. Primary syphilis was defined by the presence of a genital ulcer with morphology typical of a chancre, together with a reactive syphilis serology, and/or positive dark-ground microscopy and/or T. pallidum PCR result. Secondary syphilis was defined by the presence of rash typical of secondary syphilis and/or mucosal lesions which were positive by dark-ground microscopy and/or T. pallidum PCR, and reactive syphilis serology with an RPR titre >4. Early latent syphilis was defined as reactive syphilis serology in the absence of symptoms or signs of syphilis, and documented to be of <2 years duration.
Statistical analysis
The median with interquartile range (IQR) and mean with standard derivation (s.d.) of duration of self-reported symptoms in MSM diagnosed with symptomatic (primary and secondary) syphilis were calculated in each calendar year. Duration of self-reported symptoms was defined as the number of days from the initial onset of self-identified symptoms to the day of clinical presentation. Cases with a mixture of primary and secondary symptoms were excluded from the analysis. The mean and median of duration of symptoms for primary and secondary syphilis were calculated by a range of risk factors (i.e. country of birth, sex overseas, injecting drug use, HIV serostatus). The median and IQR of RPR titres were calculated in each calendar year for all infectious stages (primary, secondary, early latent). The non-parametric Jonckheere–Terpstra test was performed to examine any temporal trends in the median duration of symptoms, and RPR titre over the study period. One-way analysis of variance was used to compare the mean duration of symptoms over the study period. The differences in mean duration of symptoms between risk factors were tested by independent two-sample t test; while the differences in median duration of symptoms between risk factors were tested by non-parametric Mann–Whitney U test. Spearman's rank correlation was performed to investigate the association between duration of symptoms and RPR titre in primary and secondary syphilis. Sensitivity analyses were conducted to examine the trend of mean/median duration of self-reported symptoms, and the median of RPR after the removal of repeat infections. Significance level of 0·05 was used for all statistical tests. All statistical analyses were performed using Stata software version 13.1 (StataCorp., USA).
Ethics consideration
Ethical approval was obtained from the Ethics Committee of Alfred Hospital, Melbourne, Australia (No.: 104/14).
RESULTS
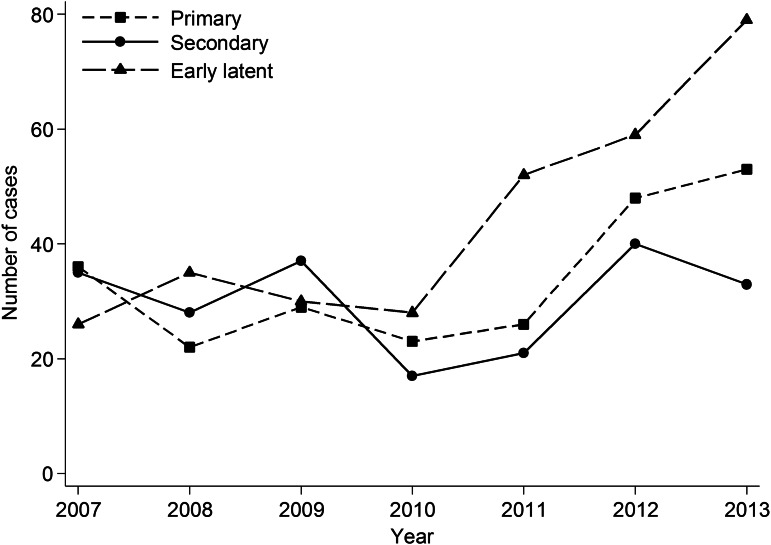
A total of 761 infectious syphilis cases were diagnosed in MSM attending MSHC from 2007 to 2013, and 228 (30·1%) syphilis cases were diagnosed in MSM with HIV. A total of 89 cases (11·7%) were repeat infections. The total number of infectious syphilis cases had nearly a twofold increase from 97 in 2007 to 165 in 2013. Of the 761 cases, 237 were primary, 211 were secondary, and 309 were early latent. Four cases were excluded from this analysis because we were unable to differentiate between primary and secondary syphilis in these cases. In addition, there was a rapid increase in the number of early latent syphilis cases from 26 in 2007 to 79 in 2013 (Fig. 1). The median age of MSM diagnosed with primary syphilis was 36 (IQR 28–47) years, secondary syphilis was 33 (IQR 26–44) years, and early latent syphilis was 35 (IQR 27–45) years; however, age was not significant between groups (P = 0·24).
Fig. 1.
Number of primary, secondary, and early latent syphilis cases in men who have sex with men attending Melbourne Sexual Health Centre, 2007–2013.
The proportion of cases diagnosed with symptomatic syphilis did not change significantly over time although HIV-positive MSM, who are screened for syphilis regularly as part of their HIV care were less likely to have symptoms (47·8%) compared to HIV-negative MSM (60·9%) (P < 0·001) (Table 2).
Table 2.
Proportion of men who have sex with men (MSM) with asymptomatic and symptomatic syphilis infections at Melbourne Sexual Health Centre, from 2007 to 2013, by HIV serostatus
| HIV-negative MSM, n (%) | HIV-positive MSM, n (%) | |||
|---|---|---|---|---|
| Year | Asymptomatic | Symptomatic | Asymptomatic | Symptomatic |
| 2007 | 19 (28·4) | 48 (71·6) | 12 (40·0) | 18 (60·0) |
| 2008 | 34 (49·3) | 35 (50·7) | 6 (37·5) | 10 (62·5) |
| 2009 | 22 (29·7) | 52 (70·3) | 10 (45·5) | 12 (54·5) |
| 2010 | 20 (37·7) | 33 (62·3) | 8 (53·3) | 7 (46·7) |
| 2011 | 34 (50·7) | 33 (49·3) | 20 (62·5) | 12 (37·5) |
| 2012 | 35 (33·7) | 69 (66·3) | 26 (59·1) | 18 (41·9) |
| 2013 | 43 (44·8) | 53 (55·2) | 37 (53·6) | 32 (46·4) |
| Total | 207 (39·1) | 323 (60·9) | 119 (52·2) | 109 (47·8) |
| P value* | 0·237 | 0·068 | ||
χ2 trend test.
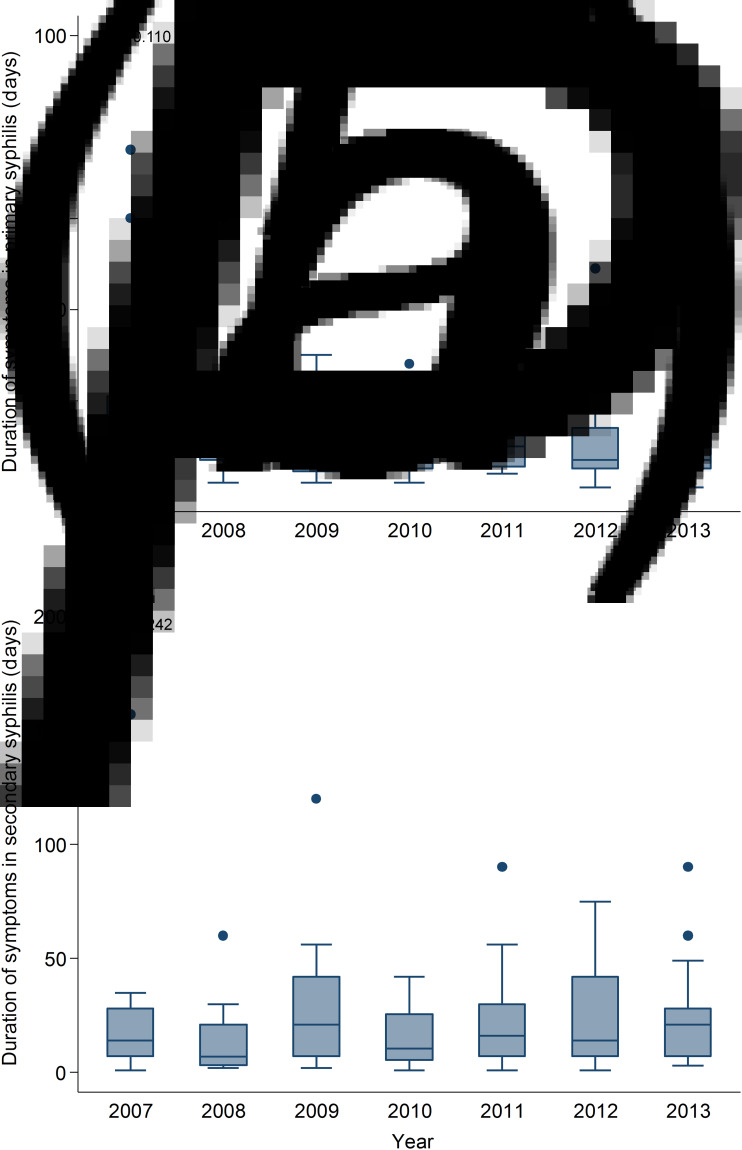
The median self-reported duration of symptoms for both primary and secondary syphilis remained stable (i.e. around 7–14 days) over the study period (Ptrend = 0·498).
Primary syphilis
Among 237 primary syphilis cases, 13 (5·5%) cases were repeat infections. Twelve out of 237 cases were serology positive but with a negative dark-ground microscopy or T. pallidum PCR, all 12 cases were included in this analysis because they were reviewed by two experienced sexual health clinicians (I.D., T.R.H.R.) who came to a consensus view on the stage of infection. A total of seven (3·0%) MSM did not have any symptoms at the time of the clinical consultation but perianal lesions or inguinal lymph nodes were found during clinical examination. Of the remaining 230 symptomatic patients, 194 (84·3%) MSM reported the duration of symptoms. The majority of symptomatic patients (57·0%) had a penile lesion (Table 3). Overall, the median and mean duration of self-reported symptoms was 9 (IQR 6–14) days and 13·6 (s.d. = 14·3) days, respectively. Although the median duration decreased slightly from 14 (IQR 7–21) days in 2007 to 7 (IQR 5–14) days in 2013, it was not significant over time (Ptrend = 0·11) (Fig. 2). Similarly, the mean duration of primary syphilis did not change over time (from 17·4 ± 16·3 days in 2007 to 13·7 ± 19·0 days in 2013; P = 0·65). The overall median duration of symptoms in HIV-positive and HIV-negative patients were 7 (IQR 5·5–14) days and 10 (IQR 6–14) days, respectively, and the median duration of symptoms did not differ between groups (P = 0·13) (Table 4) and the median duration in both groups did not change over time. There was no significant correlation between the duration of symptoms and age (r = 0·02, P = 0·08).
Table 3.
The five most common symptoms in men who have sex with men diagnosed with primary and secondary syphilis
| Symptoms | N | % |
|---|---|---|
| All primary syphilis | 237 | — |
| Symptomatic primary syphilis | 230 | 97·0 |
| Penile lesion | 131 | 57·0 |
| Penile rash | 21 | 9·1 |
| Pain | 18 | 7·8 |
| Anal lesion/sore/blister | 18 | 7·8 |
| Genital sores/ulcers | 17 | 7·4 |
| All secondary syphilis | 211 | — |
| Symptomatic secondary syphilis | 202 | 95·7 |
| Rash | 95 | 47·0 |
| Penile lesion | 31 | 15·3 |
| Penile rash | 24 | 11·9 |
| Anal lesion/sore/blister | 22 | 10·9 |
| Anal pain | 14 | 6·9 |
Fig. 2.
Box plots of duration of symptoms in (a) primary, and (b) secondary syphilis in men who have sex with men, 2007–2013.
Table 4.
Mean and median duration of symptoms in men who have sex with men diagnosed with primary and secondary syphilis at MSHC from 2007–2013, stratified by risk factors
| Number of symptomatic patients reporting duration of symptoms | Duration of symptoms (mean ± s.d.), days | Differences between group means (P value)* | Duration of symptoms (median, IQR), days | Differences between group medians (P value)† | |
|---|---|---|---|---|---|
| Primary syphilis | 194 | 13·6 ± 14·3 | — | 9 (6–14) | — |
| Country of birth | 0·867 | 0·494 | |||
| Australia | 115 | 12·9 ± 14·4 | 7 (6–14) | ||
| Overseas | 61 | 13·2 ± 11·9 | 10 (6–14) | ||
| Sex overseas | 0·490 | 0·843 | |||
| Yes | 23 | 15·4 ± 20·2 | 10 (5–14) | ||
| No | 88 | 13·2 ± 12·1 | 9 (7–14) | ||
| Injecting drug use | 0·161 | 0·673 | |||
| Yes | 9 | 19·9 ± 21·3 | 10 (7–28) | ||
| No | 104 | 13·1 ± 13·1 | 9 (6–14) | ||
| HIV serostatus | 0·066 | 0·131 | |||
| Positive | 40 | 9·9 ± 7·4 | 7 (5·5–14) | ||
| Negative | 154 | 14·6 ± 15·5 | 10 (6–14) | ||
| Secondary syphilis | 155 | 23·7 ± 26·8 | — | 14 (7–30) | — |
| Country of birth | 0·107 | 0·226 | |||
| Australia | 95 | 25·6 ± 29·3 | 14 (7–21) | ||
| Overseas | 49 | 18·1 ± 18·2 | 14 (7–30) | ||
| Sex overseas | 0·975 | 0·399 | |||
| Yes | 18 | 27·9 ± 25·9 | 21 (14–30) | ||
| No | 80 | 27·7 ± 32·1 | 16 (7–30) | ||
| Injecting drug use | 0·364 | 0·551 | |||
| Yes | 11 | 20·2 ± 18·0 | 19·5 (7–35) | ||
| No | 90 | 29·1 ± 31·9 | 18 (5–25·5) | ||
| HIV serostatus | 0·132 | 0·042 | |||
| Positive | 39 | 18·1 ± 22·4 | 8 (5–21) | ||
| Negative | 116 | 25·6 ± 27·9 | 15 (7–30) |
IQR, Interquartile range.
Independent two-sample t test.
Mann–Whitney U test.
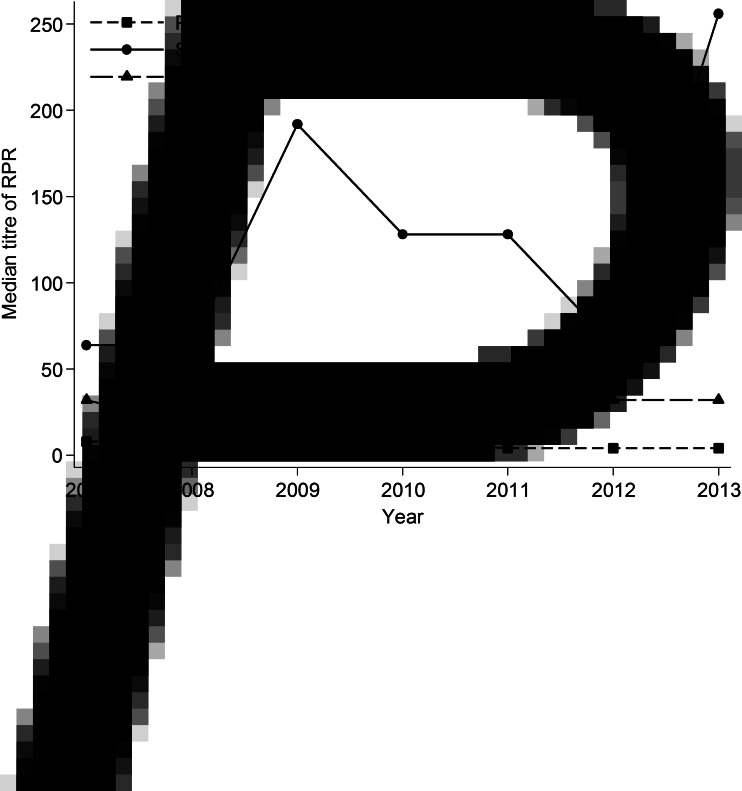
The overall median RPR titre was 4 (IQR 1–32): it decreased from 8 (IQR 1–32) in 2007 to 4 (IQR 1–16) in 2013 (Fig. 3); however, this temporal trend was not statistically significant (Ptrend = 0·35). There was a strong positive correlation between duration of symptoms and RPR titre (r = 0·36, P < 0·001).
Fig. 3.
The median rapid plasma reagin (RPR) titre with different stages of syphilis in men who have sex with men, 2007–2013.
No significant temporal trends on the mean, median duration of syphilis and median RPR titre level were observed, after removing repeat infections.
Secondary syphilis
A total of 211 secondary syphilis cases were diagnosed in MSM from 2007 to 2013 at MSHC, 19 (9·0%) cases were repeat infections. Nine (4·3%) out of 211 cases did not have any symptoms at the time of diagnosis. Of the remaining 202 symptomatic patients, 155 (76·7%) reported the duration of symptoms. The majority of symptomatic patients (47·0%) had a rash (Table 3). The overall median duration of self-reported symptoms was 14 (IQR 7–30) days, and it increased slightly from 14 (IQR 7–28) days in 2007 to 21 (IQR 7–28) days in 2013 but this was not significant (Ptrend = 0·24) (Fig. 2). Although the mean duration of secondary syphilis increased from 20·2 ± 29·6 days in 2007 to 26·6 ± 26·8 days in 2013, the trend was not statistically significant (P = 0·27). Overall, the median duration of symptoms in HIV-negative MSM (15, IQR 7–30 days) was significantly longer than in HIV-positive MSM (8, IQR 5–21 days) (P = 0·04) (Table 4); however, the median duration of symptoms did not differ over time in both groups. There was no significant relationship between the duration of symptoms and age (r = −0·132, P = 0·10).
The overall median RPR titre was 128 (IQR 64–256), it fluctuated across the study period, it remained stable at 64 in 2007 and 2008, but it increased to 128 during 2010–2011 and it reached the level of 256 in 2013 (Fig. 3); however, it did not significantly differ over time (Ptrend = 0·08). As observed with primary syphilis, there was a positive correlation between duration of symptoms and RPR titre (r = 0·16, P = 0·05) in secondary syphilis.
There were no significant temporal trends in the mean or median of the duration of symptoms, and the median of RPR titre level, after excluding the repeat infection.
Early latent syphilis
Overall, the median RPR titre level in early latent syphilis was 32 (IQR 4–64), and it remained stable at the level of 32 across the period (Ptrend = 0·85) (Fig. 3). Of the 309 early latent syphilis cases, 114 (36·9%) cases were also HIV positive. Fifty-seven (18·4%) out of 309 cases were repeat infections. The median RPR titre level did not change over time, after excluding the repeat infections.
DISCUSSION
In our study the duration of symptoms of primary or secondary syphilis cases prior to attending healthcare has not reduced significantly over the last 7 years (2007–2013) despite an increasing epidemic and focused health promotion campaigns. Some non-significant reduction in the duration of symptoms for primary syphilis did occur (median of 14 days to 7 days), but overall the differences were minimal. The duration of symptoms prior to presentation is now already relatively short (i.e. median of 9 days) for primary syphilis, and substantial effort would be needed to reduce this enough to realize a public health benefit. The somewhat longer duration of symptoms seen in cases with secondary syphilis offers some potential for a meaningful reduction but unfortunately no reduction in symptom duration was seen over the study period. However, the non-specific nature of the symptoms makes them difficult to recognize. Furthermore, the rapid increase in the number of early latent cases suggests individuals would have had the infection for a longer duration before seeking treatment. We saw no change in the median RPR titre over time in primary, secondary and early latent infections in keeping with the lack of change in the symptom duration data. The relative proportions of asymptomatic vs. symptomatic syphilis did not change, suggesting the lack of any impact of screening for syphilis. These data suggest that a very substantial public health effort would be needed to reduce the duration of symptoms further. It could be argued that if significant reductions of syphilis are to be achieved in MSM, marked increases in screening frequency of syphilis may be needed.
Our study has several limitations that are important to consider in interpreting the results. First, this is a retrospective analysis from a single urban sexual health centre and the findings may not be representative of other parts of Australia. However, MSHC does see about 30% of all cases of infectious syphilis in men in Victoria and this proportion has remained relatively stable over the 7 years (range 26–37%). Although two-thirds of MSM would have a HIV/STI test in general practice [5], it is possible that MSM attending a sexual health clinic are at higher risk than those in general practice [8], and therefore they are more aware of STIs, so interventions to shorten the duration of symptoms may only work in those at lower STI risk. Second, the duration of symptoms was self-reported by the patients and recall bias may have occurred in this analysis, although it is unlikely that this would have changed over time. Furthermore the absence of significant changes in the RPR titres supports the symptom duration findings. Third, a small number of cases (11·7%) are those who had previously had syphilis and this may have influenced the RPR result over time, and limited our ability to identify a fall. Fourth, the early stages of syphilis can be difficult to distinguish based on its clinical presentation and so some misclassification of primary and secondary syphilis cases may have occurred although all cases were carefully reviewed for their stage by a senior sexual health clinician (I.D.).
Victoria has spent approximately AUD 3·6 million (~USD 2·8 million) on several major sexual health education campaigns in the past few years; however, syphilis symptoms recognition and awareness have only been relatively small component parts of these campaigns and there has been no single health campaign specifically focused on syphilis. Our data suggest that these sexual health campaigns have not improved the recognition of syphilis symptoms in MSM, or if they have this has not shortened the period in which MSM seek clinical care after initial onset of symptoms. Previous studies have also shown that these campaigns did not increase the number of annual syphilis test (about 1·6 times) per individual [5].
Promoting frequent syphilis testing may be a more effective strategy in capturing syphilis in order to provide timely treatment and hence control the syphilis epidemic. However, the proportion of HIV-negative MSM who tested for syphilis in the past 12 months remains low and stable over time (from 62·4% in 2010 to 57·5% in 2013) in Melbourne [9].
Another effective strategy to control the syphilis epidemic is to increase the knowledge about syphilis in individuals and hence MSM would be more aware of syphilis (e.g. symptoms recognition, mode of transmission). Although there have been a few STI-related campaigns running, it is reported that knowledge about syphilis in MSM in the gay community has not changed over time [9].
Identifying the correct public health messages that will result in a significant reduction in syphilis cases is difficult. In the presence of an easy access to the health service, reducing the duration of symptoms may have limited impact because the duration of symptoms is already relatively low. By contrast, early latent syphilis is usually asymptomatic and therefore increasing the frequency of regular testing for syphilis in MSM in order to identify asymptomatic cases earlier can reduce further onward transmission. This has been very successfully achieved with MSM with HIV through opt-out programmes [10], but increasing testing of MSM without HIV is more difficult. Mathematical models suggest syphilis testing needs to become very frequent if it is to substantially reduce its incidence [11, 12]. Complicating this issue is the advance of rapid HIV testing, which could potentially reduce syphilis testing while increasing HIV testing and therefore could increase the delay of syphilis diagnoses. There is a need for campaigns to increase syphilis testing rates, particularly in MSM who are HIV-negative and not linked to care, to help to control the epidemic of syphilis in MSM. Furthermore, condoms are generally not used during oral sex by MSM and studies suggest a substantial proportion of syphilis is transmitted via oral sex [13, 14].
In conclusion, this is the first study to investigate the temporal trend of the duration of symptoms and the RPR titre in MSM diagnosed with infectious syphilis. It suggests that most MSM present soon after the initiation of symptoms and that reducing this further would need campaigns substantially more effective than are currently in place. Our findings, from a community with rapidly rising rates of syphilis, highlight the imperative of identifying other effective strategies for reducing the incidence of syphilis such as more frequent testing. It is important to scale up and promote more frequent testing in MSM and to integrate rapid HIV and syphilis testing.
ACKNOWLEDGEMENTS
This work was supported by the National Health and Medical Research Council (NHMRC) programme grant (grant number 568 971). EPFC is supported by the Early Career Fellowships from the Australian NHMRC (no. 1091226).
The authors thank A. Afrizal for his assistance with data extraction.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Victorian Department of Health. Victorian Prevention Epidemiology and Surveillance – sexually transmissible infections update. Melbourne, Australia, 2014.
- 2.Savage EJ, et al. Rapid increase in gonorrhoea and syphilis diagnoses in England in 2011. Eurosurveillance 2012; 17(29). [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2012. Atlanta, Georgia: Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC; 2014.
- 4.Read P, Fairley CK, Chow EP. Increasing trends of syphilis among men who have sex with men in high income countries. Sexual health 2015; 12: 155–163. [DOI] [PubMed] [Google Scholar]
- 5.Vella A, et al. Outcome evaluation of HIV prevention initiatives 2012–2013 in men who have sex with men in Victoria. Melbourne, Australia: Burnet Institute, 2014.
- 6.Garnett GP. An introduction to mathematical models in sexually transmitted disease epidemiology. Sexually Transmitted Infections 2002; 78: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow EP, et al. Testing commercial sex workers for sexually transmitted infections in Victoria, Australia: an evaluation of the impact of reducing the frequency of testing. PLoS ONE 2014; 9: e103081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poljski C, Atkin L, Williams H. Review of sexual health clinical services in Victoria. Melbourne, Victoria: Family Planning Victoria, 2003.
- 9.Lee E, et al. Gay Community Periodic Survey: Melbourne 2014. Sydney, Australia: Centre for Social Research in Health, Victorian AIDS Council/Gay Men's Health Centre, Department of Health Victoira, The Kirby Institute, 2014.
- 10.Bissessor M, et al. Frequent screening for syphilis as part of HIV monitoring increases the detection of early asymptomatic syphilis among HIV-positive homosexual men. Journal of Acquired Immune Deficiency Syndromes 2010; 55: 211–216. [DOI] [PubMed] [Google Scholar]
- 11.Gray RT, et al. Frequent testing of highly sexually active gay men is required to control syphilis. Sexually Transmitted Diseases 2010; 37: 298–305. [DOI] [PubMed] [Google Scholar]
- 12.Down I, et al. Increasing gay men's testing rates and enhancing partner notification can reduce the incidence of syphilis. Sexual Health 2012; 9: 472–480. [DOI] [PubMed] [Google Scholar]
- 13.Nash JL, et al. Contribution of sexual practices (other than anal sex) to bacterial sexually transmitted infection transmission in men who have sex with men: a cross-sectional analysis using electronic health records. Sexually Transmitted Infections 2014; 90: 55–57. [DOI] [PubMed] [Google Scholar]
- 14.Phang CW, et al. More than just anal sex: the potential for sexually transmitted infection transmission among men visiting sex-on-premises venues. Sexually Transmitted Infections 2008; 84: 217–219. [DOI] [PubMed] [Google Scholar]