Abstract
Results
2662 papers were identified with 37 selected for full-text review and one paper meeting criteria for inclusion. Ramadan fasting was the only time-restricted eating regimen trialled in this population with no strong evidence of a significant effect on insulin levels.
Conclusion
As the systematic review retrieved only one study investigating time-restricted eating to reduce insulin in patients with PCOS, there is no evidence to suggest that this intervention is effective. From the narrative review, based on studies in other patient groups, time-restricted eating could improve insulin resistance in those with PCOS; however, well-designed studies are required before this intervention can be recommended.
1. Introduction
Polycystic ovarian syndrome (PCOS) affects up to 10% of premenopausal women and is characterised by multiple ovarian cysts, hyperandrogenism causing hirsutism, hyperinsulinemia, insulin resistance, obesity, infertility, and mood disturbances [1]. Affected women have an increased risk of developing endometrial cancer, cardiovascular disease, hypercholesterolaemia, and type 2 diabetes mellitus meaning that early intervention and management are of crucial importance [2]. Both pharmacological [2–17] and nonpharmacological therapies (based around weight loss, diet, and exercise) are employed, the latter being the foundation of PCOS management.
The majority of women with PCOS have insulin resistance, independent of weight [18–20]. This is often subclinical and may not be reflected in routine biochemical tests [21]. Despite this, hyperinsulinemia and abnormalities in glucose haemostasis lead to cardiovascular and systemic inflammatory changes [22]. Women with PCOS are more likely to have postprandial hyperglycaemia owing to the peripheral insulin resistance rather than fasting hyperglycaemia due to intact endogenous glucose production mechanism [23]. Overall data suggests that 1 in 3 women with PCOS have impaired glucose tolerance and 1 in 10 have type 2 diabetes, with the majority (60%) being overweight or obese [1].
Hyperinsulinemia is linked with persistent hyperandrogenism, as well as its clinical manifestations, by directly driving excessive androgen production (Figure 1). Due to the relationship between increased insulin levels and androgens, the treatment of hyperinsulinemia with pharmacological and nonpharmacological approaches is essential, notwithstanding the preclinical effects of high insulin levels and predisposition to diabetes. Insulin resistance has also been implicated in cravings for carbohydrates and subsequently overeating, binge pattern eating, and weight gain [24, 25]. This has the potential to have confounding effects on the overall risk of hyperinsulinemia, subsequent diabetes, and cardiovascular disease. Obesity in PCOS is a key driver of deranged cardiometabolic parameters including insulin resistance, hyperandrogenism, and dyslipidaemia. Obesity exacerbates these conditions compared to women with PCOS who have a normal BMI. In particular, central obesity is associated with increased severity and worsening of insulin resistance [26].
Figure 1.
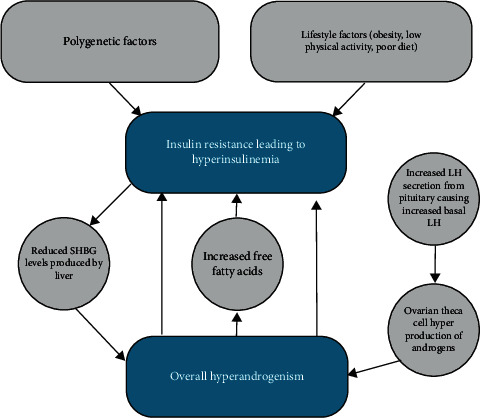
Hyperinsulinemia downstream effects on ovarian theca cell hyperproduction of androgens and subsequent pituitary feedback causing increased basal luteinizing hormone. Hyperandrogenism results in reduced SHBG (sex hormone binding globulin) (often used clinically for assessment of insulin resistance in PCOS).
Dietary interventions in PCOS are varied with many diets being unsustainable and most largely ineffective. Dietary regimens including energy (calorie) restriction [27, 28], Mediterranean diet [29], low carbohydrate diet [30], dietary approaches to stop hypertension (DASH) diet [31], pulse-based diet [32], low-starch diet [33], low-dairy diet [34], ketogenic diet [35], and low-glycaemic-index diet [36] have been trialled in patients with PCOS. Current dietary recommendations for patients with PCOS include regular eating, avoidance of refined sugars, low-glycaemic-index foods, and carbohydrate-rich diets, with calcium/vitamin D supplementation [37]. While the restriction of calories and an increase in exercise improve insulin sensitivity in overweight patients with PCOS, sustained calorie-restricted diets over a prolonged period of time are difficult to sustain [38]. More recently, the effect of time-restricted eating (also termed intermittent fasting) in PCOS has been examined [39]. In contrast to diets based specifically on food restriction, time-restricted eating, where patients are asked to consume all energy within a restricted daily time period, appears to offer more sustainable weight loss and cardiometabolic changes and may be more acceptable as a permanent lifestyle change [38].
Time-restricted eating involves a period of fasting, thereby allowing a decrease in insulin levels, an improvement in insulin sensitivity, and an improvement in glucose regulation. The short-term putative benefits of time-restricted eating include increased cell metabolic and oxidative stress resistance [40]. A period of daily fasting depletes liver glycogen stores and switches energy sources to fatty acid and ketones. This bioenergetic challenge activates signalling pathways that strengthen mitochondrial function and stress resistance and upregulate autophagy of damaged molecules adopting a stress resistance mode [40]. This reduces insulin signalling and overall protein synthesis. During refeeding following the daily “fast,” glucose levels rise and ketones are cleared with increased protein synthesis allowing growth and repair allowing more efficient cellular performance, leading to cellular resilience and disease resistance as a long-term adaptation [38]. A time-restricted eating regimen where evening food intake is restricted reportedly improves postprandial insulin and glucose handling due to alignment of circadian rhythm with diurnal food intake [41]. Several variations of time-restricted eating and terms are described in Table 1.
Table 1.
Summary of typical intermittent fasting/time-restricted eating regimens.
| Regimen | Description |
|---|---|
| Intermittent fasting/time-restricted eating | Most commonly involves fasting for 16–18 hours with an eating window of 6–8 hours, leveraging the natural circadian rhythm. Plain water and unsweetened fluids (black plain tea/coffee) are allowed |
| Time-restricted feeding | Restriction of caloric intake to specific time periods of the day, typically 8–12 hours during daytime hours. Term typically used in animal studies |
| Alternate day complete fasting | No calorie intake on fasting days, followed by a day of ad lib intake (eating to satiety) |
| Alternate day modified fasting | Restricted calorie intake on “fasting” days (<25% daily energy requirements), alternated with days of ad lib intake (eating to satiety) |
| Periodic fasting | 1-2 fasting days/week and 5-6 days of normal caloric intake |
| 5 : 2 | |
| 6 : 1 | |
| Ramadan fasting | Fasting from dawn until sunset followed by ad lib calorie intake after sunset to before dawn. Similar to time-restricted eating but conflicts with circadian rhythm |
| No water or fluids during fasting |
Fasting-induced fuel switching has the potential to overcome the issues seen in patients with PCOS, as seen in other populations with underlying insulin resistance, including metabolic syndrome, obesity, and type 2 diabetes [42–44]. Time-restricted eating represents a novel solution to aid in the control of insulin resistance in patients with PCOS in combination with already established nonpharmacological and pharmacological managements. This review aims to conduct a systematic review and meta-analysis of intervention studies on time-restricted eating in patients with PCOS and to conduct a further narrative review of the related literature to guide further research.
2. Methods
This systematic review followed PRISMA reporting guidelines [45]. This review was registered with the PROSPERO registry under registration number CRD42021267268.
2.1. Review Question
The PICO model was employed to expand the return from the review and applicability of data collected.
Population: Females above the age of eighteen years, diagnosed with polycystic ovarian syndrome using Rotterdam criteria.
Intervention: Any time-restricted feeding regimen, alone or in combination (pharmacological therapy, exercise, and weight loss). To include 16 : 8 method, 18 : 6 method, 5 : 2 diet, alternate day fasting, intermittent fasting, Ramadan or other religious fasting methods deemed suitable.
Comparator: Comparison to usual ad libitum diet or no dietary or fasting intervention, standard treatment-as-usual, and pharmacological therapy.
Outcomes: Metabolic parameters which represent effect on insulin levels, C peptide concentrations, glucagon, insulin-like growth factor 1 (IGF-1), glycated haemoglobin (HbA1c), homeostatic model assessment for insulin resistance (HOMA-IR), fasting blood glucose, sex hormone binding globulin (SHBG), or oral glucose tolerance test (OGTT).
2.2. Search Strategy and Included Study Selection
A preliminary scoping review was conducted to identify the nature and extent of the research available. A systematic search strategy was constructed with oversight of a medical librarian DM. Five search strategies were created for Medline, CINAHL (Cumulative Index to Nursing and Allied Health Literature), EMBASE, WOS (Web of Science), and Cochrane Library, the details of which are found in Appendix A. The five databases searched yielded 6340 potential papers: Medline (1737), CINAHL (339), EMBASE (1909), WOS (2086), and Cochrane Library (268). After removal of duplicates, 2739 papers remained. No further studies were identified by hand-reviewing citation lists of eligible studies, previous reviews, and field expert publications. 37 papers underwent full-text review and 1 met inclusion criteria (Figure 2). Keywords used for searches included “polycystic ovarian syndrome or PCOS,” “time-restricted feeding or TRF,” “insulin levels,” “metabolic changes,” “endocrine changes,” “diet,” and “Ramadan.” Full search details are available in Appendix A. Results were combined from the five databases and papers were selected for full-text review after dual-screening by two independent reviewers (R.F. and R.G.) who made independent decisions on inclusion. All conflicting decisions were resolved by a senior reviewer (S.D.). Further manual searches of the reference lists of all relevant papers were carried out for additional relevant papers. Studies were excluded based on incorrect study design, incorrect intervention, incorrect setting, incorrect population, failure to meet inclusion criteria, duplicate not detected, ongoing studies, still recruiting, noncontrolled trials, and different outcome measured with full details outlined in Table 2. The following data was extracted from each paper: study design, patients lost to follow-up, completion of intention-to-treat analysis, group allocation method, participant numbers, age range, study population, country, baseline preintervention weight and insulin levels, intervention type, comparison, and outcome assessment.
Figure 2.
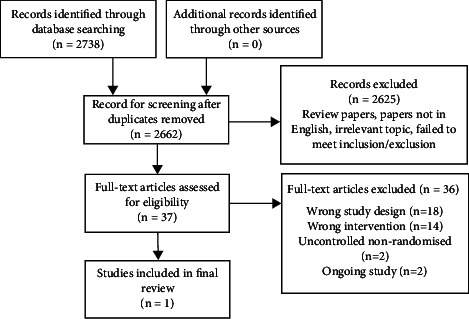
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram of systematic review search.
Table 2.
Excluded studies after full text review.
| Study ID number (Covidence) and author | Reason for exclusion |
|---|---|
| 1. #2560 Agowska 2021 | Wrong intervention |
| 2. #169 Altieri 2013 | Wrong study design |
| 3. #2263 Anderson 1995 | Wrong intervention |
| 4. #582 Armutcu 2019 | Wrong study design |
| 5. #2348 Asemi 2014 | Wrong intervention |
| 6. #419 Asemi 2015 | Nonrandomised and without control group |
| 7. #2213 Chiofalo 2017 | Wrong study design |
| 8. #1889 El-Bandrawy 2016 | Wrong intervention |
| 9. #2019 Farshchi 2007 | Wrong study design |
| 10. #2085 Fransk 1991 | Wrong study design |
| 11. #2652 Frary 2016 | Wrong study design |
| 12. #2097 Hartmann 2019 | Wrong study design |
| 13. #1369 Jyotsna 2018 | Wrong study design |
| 14. #651 Kiddy 1992 | Wrong intervention |
| 15. #2634 Kite 2019 | Wrong study design |
| 16. #2132 Lass 2011 | Wrong intervention |
| 17. #2181 Li 2021 | Nonrandomised and without control group |
| 18. #113 Marsh 2015 | Wrong study design |
| 19. #1920 Micić 2003 | Wrong intervention |
| 20. #2573 Moran 2006 | Wrong study design |
| 21. #1230 Moran 2017 | Wrong study design |
| 22. #432 Moran 2011 | Wrong study design |
| 23. #2345 Moran 2019 | Wrong study design |
| 24. #321 Moran 2006 | Wrong intervention |
| 25. #1066 NCT 2019 | Study ongoing |
| 26. #1065 NCT 2018 | Study ongoing |
| 27. #708 Papakonstantinou 2016 | Wrong intervention |
| 28. #742 Pasquali 2011 | Wrong study design |
| 29. #1284 Pundir 2019 | Wrong study design |
| 30. #1788 Shang 2020 | Wrong study design |
| 31. #1331 Song 2020 | Wrong intervention |
| 32. #943 Stamets 2004 | Wrong intervention |
| 33. #2543 vanDammen 2018 | Wrong intervention |
| 34. #462 Varady 2016 | Wrong study design |
| 35. #146 Wang 2016 | Wrong intervention |
| 36. #2489 Wong 2016 | Wrong intervention |
2.3. Inclusion/Exclusion Criteria
Timeline: Published before 10th of May 2021 when final searches were run.
Patients: Female patients with a diagnosis of polycystic ovarian syndrome by Rotterdam criteria.
Inclusion: All study designs considered which were published in peer-reviewed medical journals. Studies involving time-restricted eating, Ramadan fasting, and diets with any fasting regimen were considered, in addition to studies where time-restricted eating/intermittent fasting combined with concurrent medication use (combined oral contraceptive pill (COCP), metformin, spironolactone, etc.). Studies must have baseline and postintervention measures of outcomes. Pilot studies were assessed for inclusion if clear outcome effects were detailed. Only studies involving humans were included.
Exclusion: Conference papers, unpublished reports, letters to the editor, papers not in English were excluded. Studies considering administration of supplements, studies without a control group, studies without a baseline compared to postintervention measures, and nonhuman, preclinical, and animal studies were also excluded.
2.4. Assessment of Results
Results of included studies were extracted by two independent reviewers (R.F. and R.G.). Study characteristics were collected including details of participants, study methodology, intervention details, and effect on insulin and other metabolic parameters. Analysis to assess study quality and risk of bias was conducted in accordance with Cochrane Handbook for Systematic Reviews of Interventions.
2.5. Analysis/Data Synthesis
The studies included in the review were inherently heterogeneous due to varying interventions and outcomes. As a result, a descriptive presentation of the studies was used without pooling of outcomes for analysis.
3. Results
3.1. Populations and Interventions
The one study included was a nonrandomised controlled study with a Ramadan fasting regimen. This study recruited women with a PCOS diagnosis. The details of the study, population characteristics, and outcomes were extracted and reviewed.
3.2. Outcomes
Due to the lack of studies identified for inclusion and heterogeneity of study designs, it was not possible to conduct a meta-analysis.
3.2.1. Ramadan Fasting
One small study was identified which looked at 40 women with PCOS, 20 of whom completed Ramadan fasting for a mean of 26 days and were compared to a nonfasting control group. Details of the length of fasting times were not included but rather details of eating patterns before sunrise, after sunset, and between sunset and sunrise. There were no significant differences in levels of beta-endorphin (p=0.543), insulin (p=0.818), FSH, LH, testosterone, or adrenaline after undergoing Ramadan fasting. Significant effects of Ramadan fasting on reducing cortisol (p=0.049) and noradrenaline levels (p=0.047) were shown. Overall there was no benefit of Ramadan fasting shown in this study on insulin levels or glucose haemostasis [46].
3.3. Risk of Bias in Included Study
The risk of bias was categorised into low, high, or unclear risk for selection bias, reporting bias, performance bias, detection bias, and attrition bias. The risk of bias assessment was conducted by two independent reviewers (RF and RG). The overall risk of bias was deemed to be low.
4. Discussion
A systematic review of the literature found that the effects of time-restricted eating on insulin levels in patients with PCOS has not been investigated to date, and therefore there is no evidence to suggest that this intervention would be effective in reducing insulin in patients with PCOS. The systematic review retrieved just one study [46] which investigated on the effect of Ramadan fasting on insulin levels in patients with PCOS. This study showed no effect of Ramadan fasting on insulin and glucose homeostasis in patients with PCOS versus controls.
4.1. Narrative Review
The systematic review had excluded two studies investigating the effects of time-restricted eating as they were noncontrolled. The first of these [39] investigated the effect of a 16 : 8 time-restricted eating on anthropometric parameters, sex hormones, insulin resistance parameters, inflammatory markers, lipids, menstrual cycle, and eating behaviours in 18 women with PCOS. Participants completed a 16-hour fast daily for five weeks, preceded by a one-week baseline weight stabilisation period. Three of the 18 participants (16.7%) dropped out of the trial. Time-restricted eating (16 : 8) reduced fasting insulin levels (p=0.017), area under the curve for insulin (AUCIns) (p=0.007), ratio of AUCIns/AUCGlu (p=0.001), HOMA-IR (p=0.0025), SHBG (p < 0.001), body weight (p < 0.001), BMI (p < 0.001), body fat mass (p < 0.001), percentage of free androgen index (p=0.001), CRP (p=0.040), ALT (p=0.027), and IGF-1 (p=0.006). There were no significant differences in fasting glucose, area under the curve for glucose (AUCGlu), lipid profiles, and gonadal parameters including luteinizing hormone (LH), follicular stimulating hormone (FSH), and LH/FSH ratio or total testosterone. Overall, this study suggested that 16 : 8 time-restricted regimen improved parameters of glucose haemostasis and weight [39] in women with PCOS.
The second study [47] investigated the effects of Ramadan fasting in 27 women who fasted for a mean period of 16.5 hours a day for 29 days. There were no significant differences in weight (p=0.439), BMI (p=0.646), fasting blood glucose (p=0.183), insulin (p=0.474), HOMA-IR (p=0.364), HOMA-B (p=0.736), QUICKI (p=0.308), or lipid profiles. There were significant decreases in C-reactive protein levels (p=0.072), nitric oxide (NO) levels (p=0.003), and total glutathione levels (p=0.011). This study corroborated the systematic review finding that Ramadan fasting exerted no benefit on parameters of glucose haemostasis but had modest benefits on markers of inflammation and cardiovascular health [47]. Overall, considering both controlled and noncontrolled studies mentioned above, Ramadan fasting does not appear to have an effect on insulin levels or glucose haemostasis in individuals with PCOS.
There is some evidence from small studies in other patient groups that Ramadan fasting may have positive benefits on insulin levels and glucose haemostasis. Ramadan improved HbA1c levels in 29 patients with type 2 diabetes [48] and was associated with a decrease in glucose levels in 80 healthy subjects [49]. There appears to be modest benefits on hormonal markers of stress (cortisol and noradrenaline levels) [46], inflammation (C reactive protein or CRP levels), and cardiovascular health (NO and glutathione (GSH) levels) [47]. The underlying mechanism of these benefits remains to be defined and elucidated.
4.2. Weight Loss: A Potential Confounder?
In studies on time-restricted eating reporting weight loss [39], it is difficult to establish whether the improvements in glucose homeostasis are secondary to the concomitant weight loss rather than being a consequence of time-restricted eating. The majority of studies that cite therapeutic benefits of fasting regimens in various patient populations also report weight loss, with most beneficial cardiometabolic effects as a result of the latter [50–53]. Time-restricted eating may result in a reduction of energy intake from baseline levels. It is difficult to remove this confounding factor when designing studies on time-restricted eating, as asking patients to restrict their eating to a limited period might result in reduced calorie intake and subsequent weight loss. However, arguably this is an important outcome in itself. While “permanent” energy-restricted diets will result in weight loss, eating fewer calories appears to be virtually impossible in the long term. If time-restricted eating results in a sustainable, long-term reduction in energy intake due to its effect on appetite or satiety, then this will be of clear value for a myriad of patient types. Animal studies have shown that time-restricted feeding, without reduction of calories, shows protection against hyperinsulinemia and improves overall hepatic glucose metabolism [54–59]. Animal studies have also shown that time-restricted feeding of a high fat, diary, and sugary drinks containing western diet can have positive effect on metabolic effects without weight loss [58]. A small study in prediabetic men has shown benefits of a 6-hour time-restricted eating period on cardiometabolic profiles, including reduced blood pressure, reduced oxidative stress, and reduced appetite as well as increased insulin sensitivity and β cell responsiveness, independent of weight loss [60]. However, whether or not the benefits of time-restricted eating are independent of weight loss, particularly in patients with PCOS, is not known and has been poorly researched. Ideally, randomised human studies on patients with insulin resistance (including type 2 diabetes, metabolic syndrome, or PCOS) should be carried out. A study design comparing time-restricted eating with continuous eating, with both arms receiving isocaloric, meal frequency-matched diets, would be of value.
Li et al. observed significant results in a short, time-restricted eating regimen suggesting that improvements in hyperinsulinemia and insulin resistance can be seen without energy restriction [39]. This is contrary to a review reporting isocaloric time-restricted feeding which had a greater benefit in reducing fasting insulin and insulin resistance than ad libitum time-restricted feeding [61]. Li et al.'s study lacks power being a nonrandomised noncontrolled intervention study on a small number of participants. The short duration of the intervention was also a limitation of these studies, similar to the Ramadan studies. It is important to assess these over time to assess for compliance with fasting regimens.
4.3. Optimum Timeline
The optimum length of a time-restricted eating intervention to have a significant effect on insulin levels is undecided. Initially switching from standard dietary pattern to intermittent fasting, hunger, irritability, and reduced concentration ability while adapting to the new dietary regimen is expected [40]. These initial effects usually dissipate within one month potentially making this a reasonable minimum time for an intervention.
4.4. Low-Calorie Drinks
Li et al. [39] allowed the consumption of low-calorie sweetened drinks during the fasted period in their study on time-restricted eating. Although artificial sweeteners reportedly have negligible effects on insulin levels [62, 63], such ingredients (sucralose, aspartame, and saccharin) may adversely affect the gut microbiome which may indirectly impair glucose haemostasis, causing insulin resistance and contributing to metabolic disease [64]. While artificial sweetened and low- or zero-calorie diets facilitate a reduction in food energy content, promote satiety, and might ultimately reduce weight, their effects on insulin levels during a period of fasting should be considered. The effects of other ingredients present in artificially flavoured beverages have unknown effects on glucose and insulin levels during an otherwise fasted period. Arguably, a true fasted period should not include sweet-tasting beverages.
4.5. How the Intervention Might Work
Time-restricted eating reportedly causes a shift in fuel source from glucose to fatty acids during the fasting period [38]. Typically twelve hours into a fast, there is depletion of liver glycogen stores and fatty acids are mobilized and released as the main energy fuel [38]. By aligning meal times with the light-dark cycle, energy intake, weight control, and glucose and insulin levels may be optimised [65]. Shift workers who oppose the latter concept have higher rates of cardiometabolic dysfunction [65]. Time-restricted eating may also positively affect the gut microbiome [66]. The gut microbiome is heavily influenced by diet, as well as circadian rhythm, with time-restricted eating shown to improve intestinal bacteria microenvironment with increasingly favourable microbial profile. The downstream effects of this include favourable metabolic regulation and reduction in inflammation [67]. Time-restricted eating in non-PCOS patients resulted in weight loss, with meta-analysis showing a weighted mean difference of 2 kg in observational studies and 0.4 kg in randomised controlled trials analysed, but with limited improvements in insulin levels, glucose haemostasis, and lipid profiles [67]. Compliance is similar for patients following a time-restricted eating regimen compared to traditional calorie-restricted dietary regimens [50].
Typical ad libitum eating (with short fasting periods) might perturb normal glucose metabolism. Eating habits have largely changed with modern lifestyle. A longitudinal study of dietary habit changes over a 40-year timespan in USA showed higher daily energy intake, later breakfast and lunch times, and shorter time between dinner and a postdinner snack, with a significant increase in snacking among women in particular [68]. There are few, if any, human studies on continuous feeding and its effect on insulin levels. It could be hypothesised that long eating windows might increase the risk of insulin resistance and metabolic disorders. In animal studies comparing continuous ad libitum with time-restricted feeding, ad libitum feeding leads to increased rates of obesity, cancer, renal and cardiovascular disease, and decreased overall survival [69].
On a molecular level, the biochemical effects of time-restricted eating in PCOS are thought to include the following:
Increased recruitment of insulin receptors, internalised by the effects of persistent hyperinsulinemia as demonstrated in Figure 3.
Upregulation of insulin receptor expression which is downregulated by hyperinsulinemia.
Lowering insulin requirements with fasting. Fasting overcomes the issues seen in patients with PCOS including saturation of defected insulin receptors, beta cell dysfunction, and reduced hepatic insulin clearance [70–72].
Reduced leptin levels and increased adiponectin with an overall improved balance improving insulin resistance [42–44, 73, 74].
Figure 3.
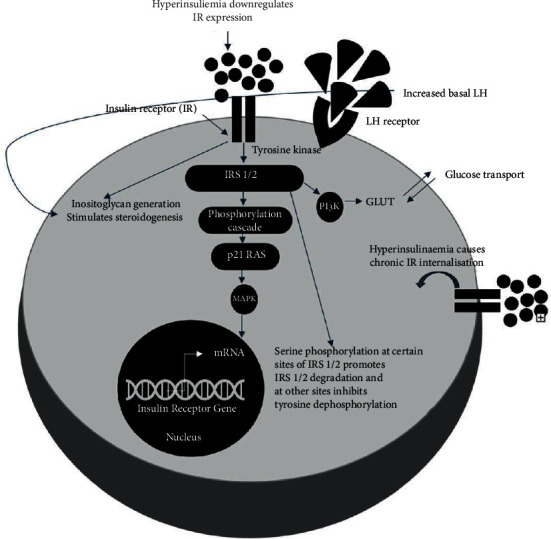
Illustration of the effect of hyperinsulinemia on insulin receptors and stimulation of steroidogenesis caused by hyperinsulinemia and increased LH (luteinizing hormone) levels. Insulin receptor defects are due to serine phosphorylation of the insulin receptor and IRS-1 (insulin receptor substrate 1) secondary to intracellular serine kinases. This results in reduced PI3K (phosphoinositide 3-kinase) downstream activity after insulin mediated activation and resistance to the metabolic actions of insulin. Activation of kinases in ERK/MAPK (extracellular signal-regulated kinases/mitogen-activated protein kinases) mitogenic pathway in PCOS (polycystic ovarian syndrome) causes inhibitory serine phosphorylation of IRS-1 in skeletal muscle in patients with PCOS as demonstrated here. These defects in the insulin receptor gene exist in patients with PCOS with extreme insulin resistance although insulin receptor numbers and affinity are similar to those in the patient without insulin receptor defects. Steroidogenesis is stimulated by both hyperinsulinemia causing inositolglycan generation and increased basal LH secreted from the anterior pituitary in response, also demonstrated below.
On a cellular level, time-restricted eating reportedly contributes to the fluctuating ratios of NAD+ to NADH, ATP to AMP, and acetyl CoA to CoA. These result in downstream activation of proteins including transcription factors (FOXOs, PGC-1α, and NRF2), AMP kinase, and deacetylases such as sirtuins (SIRTs) [38, 40], as illustrated in Figure 4. These might help to regulate cellular basic function and improve cellular stress resistance. Acetyl CoA and NAD+ serve as cofactors for these SIRTs epigenetic modifiers [38, 40]. SIRTs deacetylate transcription factors mentioned and promote gene expression, aiding with cellular stress resistance (Figure 4).
Figure 4.
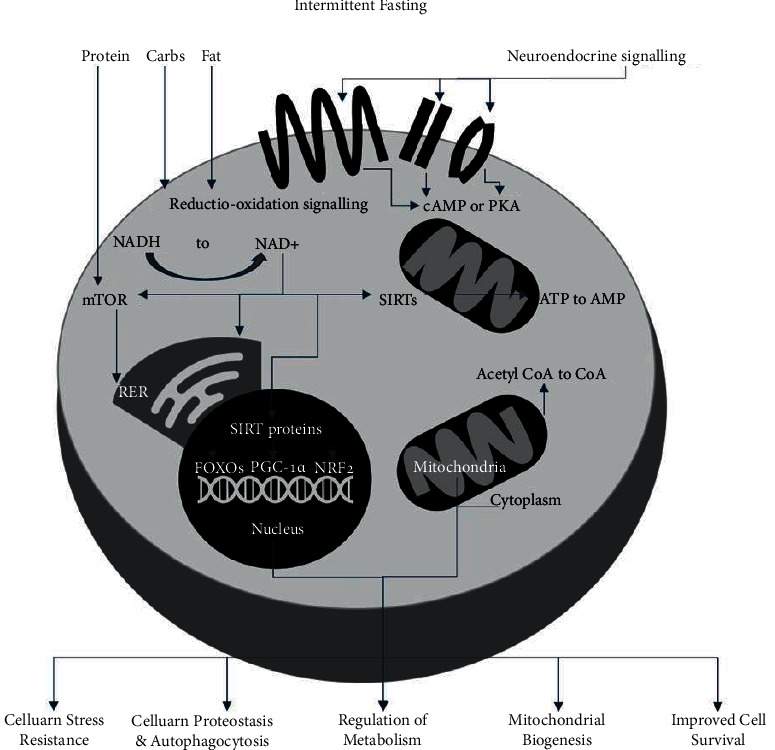
Cellular metabolic pathways and responses to intermittent fasting showing downstream cascade effects of fasting and resulting beneficial outcomes {NAD+ (nicotinamide adenine dinucleotide); transcription factors FOXOs (forkhead box Os), PGC-1α (proliferator-activated receptor γ coactivator 1α), and NRF2 (nuclear factor erythroid 2-related factor 2); kinases AMPK (AMP kinase) and cAMP cyclic AMP; and deacetylases sirtuins (SIRTs); mammalian target of rapamycin (mTOR); insulin-like growth factor 1 (IGF-1)}.
Fasting reportedly results in downregulation of the insulin-IGF-1 signalling pathway and reduced circulating amino acids causing reduced mTOR activity with subsequent inhibition of protein synthesis. All of the above processes allow activation of cellular repair and maintenance processes, stress resistance and mitochondrial biogenesis, cellular proteostasis, and autophagy with overall improved cell survival. The AMP to ATP ratio is increased by fasting, activating AMPK, which triggers repair and halts anabolic processes [40].
4.6. Suggested Future Research
A prospective randomised intervention study with crossover design would be best to determine the effect of a time-restricted eating pattern on insulin levels, androgens, fertility, and satiety. We suggest that a controlled feasibility study should be conducted to investigate the feasibility, safety, compliance, and acceptance of time-restricted eating in patients with PCOS, as well as its effects on insulin, androgens, and satiety, and have registered a study to investigate these outcomes (NCT05126199).
5. Conclusions
The systematic review found no studies investigating the effect of time-restricted eating on insulin levels in patients with PCOS, with the exception of one study on Ramadan fasting which showed no effect. Although a narrative review discussed an uncontrolled study which showed improvement in glucose homeostasis, weight, and androgens with 16 : 8 time-restricted eating in PCOS, overall we conclude that there is insufficient evidence that time-restricted eating works to reduce insulin levels in PCOS, and, pending further studies, the intervention should not be recommended in this group.
Acknowledgments
The authors received funding for this research by means of an unrestricted research grant from the Meath Foundation of Tallaght University Hospital.
Abbreviations
- PCOS:
Polycystic ovarian syndrome
- GLP-1:
Glucagon-like peptide 1
- DASH:
Dietary approach to stop hypertension
- TRF:
Time-restricted feeding
- PRISMA:
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PICO:
Population intervention comparison outcome
- IGF-1:
Insulin-like growth factor 1
- HbA1c:
Haemoglobin A1c
- HOMA-IR:
Homeostatic model assessment for insulin resistance
- HOMA-B:
Homeostatic model assessment beta cell
- QUICKI:
Quantitative insulin-sensitivity check index
- BMI:
Body mass index
- AUCIns:
Area under the curve insulin
- AUCGlu:
Area under the curve glucose
- CRP:
C-reactive protein
- ALT:
Alanine transaminase
- NO:
Nitric oxide
- GSH:
Glutathione
- SHBG:
Sex hormone binding globulin
- OGTT:
Oral glucose tolerance test
- HDLs:
High-density lipoproteins
- FSH:
Follicle stimulating hormone
- LH:
Luteinizing hormone
- E2:
Estradiol
- TFTs:
Thyroid function tests
- CINAHL:
Cumulative index to nursing and allied health literature
- EMBASE:
Excerpta Medica Database
- WOS:
Web of Science
- IF:
Intermittent fasting
- COCP:
Combine oral contraceptive pill
- PI3K:
Phosphoinositide 3-kinase
- mTOR:
Mammalian target of rapamycin
- AMPK:
AMP kinase
- (c)AMP:
(cyclic) Adenosine monophosphate
- ATP:
Adenosine triphosphate
- ERK:
Extracellular signal-regulated kinases
- MAPK:
Mitogen-activated protein kinases
- IRS-1:
Insulin receptor substrate 1
- mRNA:
Messenger ribonucleic acid
- SIRTs:
Sirtuins
- FOXOs:
Forkhead box Os
- PGC-1α:
Proliferator-activated receptor γ coactivator 1α
- NRF2:
Nuclear factor erythroid 2-related factor 2.
Appendix
A. Search Strategies
- EMBASE (1909 Results)
- “ovary polycystic disease”/exp
- (“stein leventhal syndrome” OR “cystic ovar∗” OR “micropolycystic ovar∗” OR “multiple follicle cyst∗” OR “ovar∗ polycystic disease” OR “ovar∗ polycystic syndrome” OR “polycystic ovarian disease” OR “polycystic ovar∗” OR “polycystic ovar∗ disease” OR “polycystic ovar∗ syndrome” OR “stein cohen leventhal syndrome” OR “stein leventhal disease” OR “syndrome stein leventhal”):ti,ab
- #1 OR #2
- “insulin”/exp OR “hyperinsulinism”/exp OR “insulin response”/exp
- insulin:ti,ab
- (“hyperinsulinism” OR “hyperinsulism” OR “hyperinsulinaemia” OR “hyperinsulinema” OR “hyperinsulinemia” OR “insulinaemia” OR “insulinemia” OR “insulin hypoglycaemia” OR “insulin hypoglycemia”):ti,ab
- #4 OR #5 OR #6
- “fasting”/exp
- (Time NEAR/2 restrict∗ NEAR/2 (Eating OR diet∗ OR feeding OR fast∗)):ti,ab
- ((“Feeding time∗” OR diet OR food OR fast∗) NEAR/3 restrict∗):ti,ab
- (Fasting OR “whole day fast∗” OR “food tim∗”):ti,ab
- (fast∗ NEAR/3 diet∗):ti,ab
- ((intermittent∗ OR alternat∗ OR modified) NEAR/3 fast∗):ti,ab
- (food NEAR/1 (abstinence or fast∗)):ti,ab
- (Ramadan or Ramadhan):ti,ab
- (“16 8 method” OR “5 2 diet”):ti,ab
- #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16
- #3 AND #7 AND #17
- “conference abstract”:it OR “conference report”:it OR letter:it OR editorial:it
- #18 NOT #19
- Medline (1737)
- exp Polycystic Ovary Syndrome/
- (stein leventhal syndrome OR cystic ovar∗ OR micropolycystic ovar∗ OR multiple follicle cyst∗ OR ovar∗ polycystic disease OR ovar∗ polycystic syndrome OR polycystic ovarian disease OR polycystic ovar∗ OR polycystic ovar∗ disease OR polycystic ovar∗ syndrome OR stein cohen leventhal syndrome OR stein leventhal disease OR syndrome stein leventhal).ti,ab.
- or/1-2
- exp Insulin/ OR exp Hyperinsulinism/
- (insulin OR hyperinsulinism OR hyperinsulism OR hyperinsulinaemia OR hyperinsulinema OR hyperinsulinemia OR insulinaemia OR insulinemia OR insulin hypoglycaemia OR insulin hypoglycemia).ti,ab.
- or/4-5
- fasting/
- (Time adj2 restrict∗ adj2 (Eating OR diet∗ OR feeding OR fast∗)).ti,ab.
- ((Feeding time∗ OR diet OR food OR fast∗) adj3 restrict∗).ti,ab.
- (Fasting OR whole day fast∗ OR food tim∗).ti,ab.
- (fast∗ adj3 diet∗).ti,ab.
- ((intermittent∗ OR alternat∗ OR modified) adj3 fast∗).ti,ab.
- (food adj1 (abstinence or fast∗)).ti,ab.
- (Ramadan or Ramadhan).ti,ab.
- (16 8 method OR 5 2 diet).ti,ab.
- or/7-15
- 3 AND 6 AND 16
- CINAHL (339)
- (MH “Polycystic Ovary Syndrome”)
- TI (“stein leventhal syndrome” OR “cystic ovar∗” OR “micropolycystic ovar∗” OR “multiple follicle cyst∗” OR “ovar∗ polycystic disease” OR “ovar∗ polycystic syndrome” OR “polycystic ovarian disease” OR “polycystic ovar∗” OR “polycystic ovar∗ disease” OR “polycystic ovar∗ syndrome” OR “stein cohen leventhal syndrome” OR “stein leventhal disease” OR “syndrome stein leventhal”) OR AB (“stein leventhal syndrome” OR “cystic ovar∗” OR “micropolycystic ovar∗” OR “multiple follicle cyst∗” OR “ovar∗ polycystic disease” OR “ovar∗ polycystic syndrome” OR “polycystic ovarian disease” OR “polycystic ovar∗” OR “polycystic ovar∗ disease” OR “polycystic ovar∗ syndrome” OR “stein cohen leventhal syndrome” OR “stein leventhal disease” OR “syndrome stein leventhal”)
- S1 OR S2
- (MH “Insulin+”) OR (MH “Insulin Sensitivity”) OR (MH “Hyperinsulinism+”)
- TI (insulin OR “hyperinsulinism” OR “hyperinsulism” OR “hyperinsulinaemia” OR “hyperinsulinema” OR “hyperinsulinemia” OR “insulinaemia” OR “insulinemia” OR “insulin hypoglycaemia” OR “insulin hypoglycemia”) OR AB (insulin OR “hyperinsulinism” OR “hyperinsulism” OR “hyperinsulinaemia” OR “hyperinsulinema” OR “hyperinsulinemia” OR “insulinaemia” OR “insulinemia” OR “insulin hypoglycaemia” OR “insulin hypoglycemia”)
- S4 OR S5
- (MH “Fasting”)
- TI (Time N2 restrict∗ N2 (Eating OR diet∗ OR feeding OR fast∗)) OR AB (Time N2 restrict∗ N2 (Eating OR diet∗ OR feeding OR fast∗))
- TI ((“Feeding time∗” OR diet OR food OR fast∗) N3 restrict∗) OR AB ((“Feeding time∗” OR diet OR food OR fast∗) N3 restrict∗)
- TI (Fasting OR “whole day fast∗” OR “food tim∗”) OR AB (Fasting OR “whole day fast∗” OR “food tim∗”)
- TI (fast∗ N3 diet∗) OR AB (fast∗ N3 diet∗)
- TI ((intermittent∗ OR alternat∗ OR modified) N3 fast∗) OR AB ((intermittent∗ OR alternat∗ OR modified) N3 fast∗)
- TI (food N1 (abstinence or fast∗)) OR AB (food N1 (abstinence or fast∗))
- TI (Ramadan or Ramadhan) OR AB (Ramadan or Ramadhan)
- TI (“16 8 method” OR “5 2 diet”) OR AB (“16 8 method” OR “5 2 diet”)
- S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15
- S3 AND S6 AND S16
- Cochrane Library (268)
- [mh “Polycystic Ovary Syndrome”]
- (“stein leventhal syndrome” OR “cystic ovar∗” OR “micropolycystic ovar∗” OR “multiple follicle cyst∗” OR “ovar∗ polycystic disease” OR “ovar∗ polycystic syndrome” OR “polycystic ovarian disease” OR “polycystic ovar∗” OR “polycystic ovar∗ disease” OR “polycystic ovar∗ syndrome” OR “stein cohen leventhal syndrome” OR “stein leventhal disease” OR “syndrome stein leventhal”):ti,ab,kw
- #1 OR #2
- [mh “Insulin”] OR [mh “Hyperinsulinism”]
- (Insulin OR hyperinsulinism OR hyperinsulism OR hyperinsulinaemia OR hyperinsulinema OR hyperinsulinemia OR insulinaemia OR insulinemia OR “insulin hypoglycaemia” OR “insulin hypoglycaemia”):ti,ab,kw
- #4 OR #5
- [mh “fasting”]
- (Time NEAR/2 restrict∗ NEAR/2 (Eating OR diet∗ OR feeding OR fast∗)):ti,ab,kw
- ((“Feeding time∗” OR diet OR food OR fast∗) NEAR/3 restrict∗):ti,ab,kw
- (Fasting OR “whole day fast∗” OR “food tim∗”):ti,ab,kw
- (fast∗ NEAR/3 diet∗):ti,ab,kw
- ((intermittent∗ OR alternat∗ OR modified) NEAR/3 fast∗):ti,ab,kw
- (food NEAR/1 (abstinence or fast∗)):ti,ab,kw
- (Ramadan or Ramadhan):ti,ab,kw
- (“16 8 method” OR “5 2 diet”):ti,ab,kw
- #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
- #3 AND #6 AND #16
- Web of Science (2086)
- TS =(“ovary polycystic disease” OR “stein leventhal syndrome” OR “cystic ovar∗” OR “micropolycystic ovar∗” OR “multiple follicle cyst∗” OR “ovar∗ polycystic disease” OR “ovar∗ polycystic syndrome” OR “polycystic ovar∗” OR “polycystic ovar∗ disease” OR “polycystic ovar∗ syndrome” OR “stein cohen leventhal syndrome” OR “stein leventhal disease” OR “syndrome stein leventhal”)
- TS =(Insulin OR “hyperinsulinism” OR “hyperinsulism” OR “hyperinsulinaemia” OR “hyperinsulinema” OR “hyperinsulinemia” OR “insulinaemia” OR “insulinemia” OR “insulin hypoglycaemia” OR “insulin hypoglycemia”)
- TS =((Time NEAR/1 restrict∗ NEAR/1 (Eating OR diet∗ OR feeding OR fast∗)) OR ((“Feeding time∗” OR diet OR food OR fast∗) NEAR/3 restrict∗) OR Fasting OR “whole day fast∗” OR “food tim∗” OR (fast∗ NEAR/3 diet∗) OR ((intermittent∗ OR alternat∗ OR modified) NEAR/3 fast∗) OR (food NEAR/1 (abstinence or fast∗)) OR Ramadan or Ramadhan OR “16 8 method” OR “5 2 diet”)
- #1 AND #2 AND #3
B. Excluded Trials
The study ID of the authors and their reason are given in the Table 2.
Data Availability
The data that support the findings of this study can be obtained from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
Ruairí Floyd (RF), Sinead Duggan (SD), and Lucy-Ann Behan (LAB) planned the study. RF and DM (David Mockler) defined the search strategies. RF conducted the review with RG (Róisín Gryson) as the second independent reviewer of papers when identifying papers for inclusion. Any conflicts were resolved by SD. RF wrote the review, and SD, LAB, and JG (James Gibney) reviewed and edited the paper. RF submitted the review. LAB is responsible for the overall content as guarantor.
References
- 1.Pirotta S., Joham A., Grieger J. A., et al. Obesity and the risk of infertility, gestational diabetes, and type 2 diabetes in polycystic ovary syndrome. Seminars in Reproductive Medicine . 2021;38(06):342–351. doi: 10.1055/s-0041-1726866. [DOI] [PubMed] [Google Scholar]
- 2.Ndefo U. A., Eaton A., Green M. R. Polycystic ovary syndrome: a review of treatment options with a focus on pharmacological approaches. P and T: A Peer-Reviewed Journal for Formulary Management . 2013;38(6):336–355. [PMC free article] [PubMed] [Google Scholar]
- 3.Costello M. F., Shrestha B., Eden J., Sjoblom P., Moran L. J. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database of Systematic Reviews . 2007;(1) doi: 10.1002/14651858.CD005552 [DOI] [PubMed] [Google Scholar]
- 4.Lord J. M., Flight I. H. K., Norman R. J., Lord J. M. Insulin-sensitising drugs (metformin, troglitazone, rosiglitazone, pioglitazone, D-chiro-inositol) for polycystic ovary syndrome. Cochrane Database of Systematic Reviews . 2003;(3) doi: 10.1002/14651858.CD003053.CD003053 [DOI] [PubMed] [Google Scholar]
- 5.Harmanci A., Cinar N., Bayraktar M., Yildiz B. O. Oral contraceptive plus antiandrogen therapy and cardiometabolic risk in polycystic ovary syndrome. Clinical Endocrinology . 2013;78(1):120–125. doi: 10.1111/j.1365-2265.2012.04466.x. [DOI] [PubMed] [Google Scholar]
- 6.Rabe T., Blume-Peytavi C., Egarter U., et al. Hirsutism—medicinal treatment. Joint statement of the German society of gynaecological endocrinology and reproductive medicine and the professional association of gynaecologists. Journal fur Reproduktionsmedizin und Endokrinologie . 2015;12(3):102–148. [Google Scholar]
- 7.Brown J., Farquhar C., Beck J., Boothroyd C., Hughes E. Clomiphene and anti‐oestrogens for ovulation induction in PCOS. Cochrane Database of Systematic Reviews . 2009;(4) doi: 10.1002/14651858.CD002249.CD002249 [DOI] [PubMed] [Google Scholar]
- 8.Niafar M., Pourafkari L., Porhomayon J., Nader N. A systematic review of GLP-1 agonists on the metabolic syndrome in women with polycystic ovaries. Archives of Gynecology and Obstetrics . 2016;293(3):509–515. doi: 10.1007/s00404-015-3976-7. [DOI] [PubMed] [Google Scholar]
- 9.Zhong W. Y., Peng H., Li H., et al. Effect of thiazolidinedione amide on insulin resistance, creactive protein and endothelial function in young women with polycystic ovary syndrome. Tropical Journal of Pharmaceutical Research . 2016;14(12):2287–2292. doi: 10.4314/tjpr.v14i12.19. [DOI] [Google Scholar]
- 10.Du Q., Yang S., Wang Y. J., Wu B., Zhao Y. Y., Fan B. Effects of thiazolidinediones on polycystic ovary syndrome: a meta-analysis of randomized placebo-controlled trials. Advances in Therapy . 2012;29(9):763–774. doi: 10.1007/s12325-012-0044-6. [DOI] [PubMed] [Google Scholar]
- 11.Fulghesu A. M., Ciampelli M., Muzj G., et al. N-acetyl-cysteine treatment improves insulin sensitivity in women with polycystic ovary syndrome. Fertility and Sterility . 2002;77(6):1128–1135. doi: 10.1016/s0015-0282(02)03133-3. [DOI] [PubMed] [Google Scholar]
- 12.Nehra J., Kaushal J., Rani Singhal S., Singh Ghalaut V. A comparative study of myo inositol versus metformin on biochemical profile in polycystic ovarian syndrome in women. International Journal of Pharmaceutical Sciences and Research . 2017;8(4):1664–1670. [Google Scholar]
- 13.Raval A. D., Hunter T., Stuckey B., Hart R. J. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database of Systematic Reviews . 2011;(10) doi: 10.1002/14651858.CD008565.CD008565 [DOI] [PubMed] [Google Scholar]
- 14.Tabrizi R., Ostadmohammadi V., Akbari M., et al. The effects of probiotic supplementation on clinical symptom, weight loss, glycemic control, lipid and hormonal profiles, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Probiotics and Antimicrobial Proteins . 2019;14(1):1–14. doi: 10.1007/s12602-019-09559-0. [DOI] [PubMed] [Google Scholar]
- 15.Heshmati J., Farsi F., Yosaee S., et al. The effects of probiotics or synbiotics supplementation in women with polycystic ovarian syndrome: a systematic review and meta-analysis of randomized clinical trials. Probiotics and Antimicrobial Proteins . 2019;11(4):1236–1247. doi: 10.1007/s12602-018-9493-9. [DOI] [PubMed] [Google Scholar]
- 16.Cozzolino M., Vitagliano A., Pellegrini L., et al. Therapy with probiotics and synbiotics for polycystic ovarian syndrome: a systematic review and meta-analysis. European Journal of Nutrition . 2020;59(7):2841–2856. doi: 10.1007/s00394-020-02233-0. [DOI] [PubMed] [Google Scholar]
- 17.de Medeiros S. F. Risks, benefits size and clinical implications of combined oral contraceptive use in women with polycystic ovary syndrome. Reproductive Biology and Endocrinology . 2017;15(1):p. 93. doi: 10.1186/s12958-017-0313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katsikis I., Karkanaki A., Misichronis G., Delkos D., Kandaraki E. A., Panidis D. Phenotypic expression, body mass index and insulin resistance in relation to LH levels in women with polycystic ovary syndrome. European Journal of Obstetrics & Gynecology and Reproductive Biology . 2011;156(2):181–185. doi: 10.1016/j.ejogrb.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Shamdeen M. Y., Saber M. A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome. Middle East Fertility Society Journal . 2005;10(3):223–230. [Google Scholar]
- 20.Marsden P. J., Murdoch A. P., Taylor R. Tissue insulin sensitivity and body weight in polycystic ovary syndrome. Clinical Endocrinology . 2001;55(2):191–199. doi: 10.1046/j.1365-2265.2001.01303.x. [DOI] [PubMed] [Google Scholar]
- 21.Bennett C. M., Guo M., Dharmage S. C. HbA(1c) as a screening tool for detection of Type 2 diabetes: a systematic review. Diabetic Medicine . 2007;24(4):333–343. doi: 10.1111/j.1464-5491.2007.02106.x. [DOI] [PubMed] [Google Scholar]
- 22.Tarkun I., Arslan B. C., Canturk Z., Turemen E., Sahi̇n T., Duman C. Endothelial dysfunction in young women with polycystic ovary syndrome: relationship with insulin resistance and low-grade chronic inflammation. Journal of Clinical Endocrinology and Metabolism . 2004;89(11):5592–5596. doi: 10.1210/jc.2004-0751. [DOI] [PubMed] [Google Scholar]
- 23.Amato M. C., Vesco R., Vigneri E., Ciresi A., Giordano C. Hyperinsulinism and polycystic ovary syndrome (PCOS): role of insulin clearance. Journal of Endocrinological Investigation . 2015;38(12):1319–1326. doi: 10.1007/s40618-015-0372-x. [DOI] [PubMed] [Google Scholar]
- 24.Farshchi H., Rane A., Love A., Kennedy R. L. Diet and nutrition in polycystic ovary syndrome (PCOS): pointers for nutritional management. Journal of Obstetrics and Gynaecology . 2007;27(8):762–773. doi: 10.1080/01443610701667338. [DOI] [PubMed] [Google Scholar]
- 25.Lindén Hirschberg A., Naessen S., Stridsberg M., Bystrom B., Holte J. Impaired cholecystokinin secretion and disturbed appetite regulation in women with polycystic ovary syndrome. Gynecological Endocrinology . 2004;19(2):79–87. doi: 10.1080/09513590400002300. [DOI] [PubMed] [Google Scholar]
- 26.Lim S. S., Norman R. J., Davies M. J., Moran L. J. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obesity Reviews . 2013;14(2):95–109. doi: 10.1111/j.1467-789x.2012.01053.x. [DOI] [PubMed] [Google Scholar]
- 27.Van Dam E. W. C. M., Roelfsema F., Veldhuis J. D., et al. Increase in daily LH secretion in response to short-term calorie restriction in obese women with PCOS. American Journal of Physiology. Endocrinology and Metabolism . 2002;282(4):865–872. doi: 10.1152/ajpendo.00458.2001. [DOI] [PubMed] [Google Scholar]
- 28.Legro R. S. Obesity and PCOS: implications for diagnosis and treatment. Seminars in Reproductive Medicine . 2012;30(06):496–506. doi: 10.1055/s-0032-1328878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrea L., Arnone A., Annunziata G., et al. Adherence to the mediterranean diet, dietary patterns and body composition in women with polycystic ovary syndrome (PCOS) Nutrients . 2019;11(10):p. 2278. doi: 10.3390/nu11102278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porchia L. M., Hernandez-Garcia S. C., Gonzalez-Mejia M. E., Lopez-Bayghen E. Diets with lower carbohydrate concentrations improve insulin sensitivity in women with polycystic ovary syndrome: a meta-analysis. European Journal of Obstetrics & Gynecology and Reproductive Biology . 2020;248:110–117. doi: 10.1016/j.ejogrb.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Shang Y., Zhou H., Hu M., Feng H. Effect of diet on insulin resistance in polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism . 2020;105(10):3346–3360. doi: 10.1210/clinem/dgaa425. [DOI] [PubMed] [Google Scholar]
- 32.Kazemi M., McBreairty L., Chizen D., Pierson R., Chilibeck P., Zello G. A comparison of a pulse-based diet and the therapeutic lifestyle changes diet in combination with exercise and health counselling on the cardio-metabolic risk profile in women with polycystic ovary syndrome: a randomized controlled trial. Nutrients . 2018;10:p. 1387. doi: 10.3390/nu10101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phy J. L., Pohlmeier A. M., Cooper J. A., et al. Low starch/low dairy diet results in successful treatment of obesity and Co-morbidities linked to polycystic ovary syndrome (PCOS) Journal of Obesity & Weight Loss Therapy . 2015;5(2):p. 259. doi: 10.4172/2165-7904.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pohlmeier A. M., Phy J. L., Watkins P., et al. Effect of a low-starch/low-dairy diet on fat oxidation in overweight and obese women with polycystic ovary syndrome. Applied Physiology Nutrition and Metabolism . 2014;39(11):1237–1244. doi: 10.1139/apnm-2014-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mavropoulos J. C., Yancy W. S., Hepburn J., Westman E. C. The effects of a low-carbohydrate, ketogenic diet on the polycystic ovary syndrome: a pilot study. Nutrition & Metabolism . 2005;2(1):p. 35. doi: 10.1186/1743-7075-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shishehgar F., Mirmiran P., Rahmati M., Tohidi M., Ramezani Tehrani F. Does a restricted energy low glycemic index diet have a different effect on overweight women with or without polycystic ovary syndrome? BMC Endocrine Disorders . 2019;19(1):p. 93. doi: 10.1186/s12902-019-0420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teede H. J., Misso M. L., Costello M. F., et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. International PCOS NetworkHuman reproduction (Oxford, England) . 2018;33(9):1602–1618. doi: 10.1093/humrep/dey256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anton S. D., Moehl K., Donahoo W. T., et al. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity . 2017;26(2):254–268. doi: 10.1002/oby.22065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C., Xing C., Zhang J., Zhao H., Shi W., He B. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. Journal of Translational Medicine . 2021;19(1):p. 148. doi: 10.1186/s12967-021-02817-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Cabo R., Mattson M. P. Effects of intermittent fasting on health, aging, and disease. New England Journal of Medicine . 2019;381(26):2541–2551. doi: 10.1056/nejmra1905136. [DOI] [PubMed] [Google Scholar]
- 41.Armutcu F. Fasting may be an alternative treatment method recommended by physicians. Electronic Journal of General Medicine . 2019;16(3) doi: 10.29333/ejgm/104620.em138 [DOI] [Google Scholar]
- 42.Brede S., Serfling G., Klement J., Schmid S. M., Lehnert H. Clinical scenario of the metabolic syndrome. Visceral Medicine . 2016;32(5):336–341. doi: 10.1159/000449028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.López-Jaramillo P., Gomez-Arbelaez D., Lopez-Lopez J., et al. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Hormone Molecular Biology and Clinical Investigation . 2014;18(1):37–45. doi: 10.1515/hmbci-2013-0053. [DOI] [PubMed] [Google Scholar]
- 44.Albosta M., Bakke J. Intermittent fasting: is there a role in the treatment of diabetes? A review of the literature and guide for primary care physicians. Clin Diabetes Endocrinol . 2021;7(1):p. 3. doi: 10.1186/s40842-020-00116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liberati A., Altman D. G., Tetzlaff J., et al. Research Methods & Reporting the PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ . 2009:p. 339. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zangeneh F., Salman Yazdi R., Naghizadeh M. M., Abedinia N. Effect of ramadan fasting on stress neurohormones in women with polycystic ovary syndrome. Journal of Family and Reproductive Health . 2015;9(2):51–57. [PMC free article] [PubMed] [Google Scholar]
- 47.Asemi Z., Samimi M., Taghizadeh M., Esmaillzadeh A. Effects of ramadan fasting on glucose homeostasis, lipid profiles, inflammation and oxidative stress in women with polycystic ovary syndrome in kashan, Iran. Archives of Iranian Medicine . 2015;18(12):806–810. [PubMed] [Google Scholar]
- 48.Yeoh E. C., Zainudin S. B., Loh W. N., et al. Fasting during ramadan and associated changes in glycaemia, caloric intake and body composition with gender differences in Singapore. Annals Academy of Medicine Singapore . 2015;44(6):202–206. doi: 10.47102/annals-acadmedsg.v44n6p202. [DOI] [PubMed] [Google Scholar]
- 49.Kiyani M. M., Memon A. R., Amjad M. I., Ameer M. R., Sadiq M., Mahmood T. Study of human biochemical parameters during and after ramadan. Journal of Religion and Health . 2017;56(1):55–62. doi: 10.1007/s10943-015-0084-8. [DOI] [PubMed] [Google Scholar]
- 50.Trepanowski J. F., Kroeger C. M., Barnosky A., et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Internal Medicine . 2017;177(7):930–938. doi: 10.1001/jamainternmed.2017.0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soeters M. R., Lammers N. M., Dubbelhuis P. F., et al. Intermittent fasting does not affect whole-body glucose, lipid, or protein metabolism. The American Journal of Clinical Nutrition . 2009;90(5):1244–1251. doi: 10.3945/ajcn.2008.27327. [DOI] [PubMed] [Google Scholar]
- 52.Harvie M. N., Pegington M., Mattson M. P., et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. International Journal of Obesity . 2011;35(5):714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halberg N., Henriksen M., Soderhamn N., et al. Effect of intermittent fasting and refeeding on insulin action in healthy men. Journal of Applied Physiology . 2005;99(6):2128–2136. doi: 10.1152/japplphysiol.00683.2005. [DOI] [PubMed] [Google Scholar]
- 54.Hatori M., Vollmers C., Zarrinpar A., et al. Time restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high fat diet. Cell Metabolism . 2012;15(6):848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belkacemi L., Selselet-Attou G., Hupkens E., et al. Intermittent fasting modulation of the diabetic syndrome in streptozotocin-injected rats. International Journal of Endocrinology . 2012;2012:12. doi: 10.1155/2012/962012.962012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olsen M. K., Choi M. H., Kulseng B., Zhao C. M., Chen D. Time-restricted feeding on weekdays restricts weight gain: a study using rat models of high-fat diet-induced obesity. Physiology & Behavior . 2017;173:298–304. doi: 10.1016/j.physbeh.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 57.Sherman H., Genzer Y., Cohen R., Chapnik N., Madar Z., Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. The FASEB Journal . 2012;26(8):3493–3502. doi: 10.1096/fj.12-208868. [DOI] [PubMed] [Google Scholar]
- 58.Woodie L. N., Luo Y., Wayne M. J., et al. Restricted feeding for 9h in the active period partially abrogates the detrimental metabolic effects of a Western diet with liquid sugar consumption in mice. Metabolism . 2018;82:1–13. doi: 10.1016/j.metabol.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 59.Wu T., Sun L., ZhuGe F., et al. Differential roles of breakfast and supper in rats of a daily three-meal schedule upon circadian regulation and physiology. Chronobiology International . 2011;28(10):890–903. doi: 10.3109/07420528.2011.622599. [DOI] [PubMed] [Google Scholar]
- 60.Sutton E. F., Beyl R., Early K. S., Cefalu W. T., Ravussin E., Peterson C. M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metabolism . 2018;27(6):1212–1221. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fauser B. C., Tarlatzis B. C., Rebar R. W., et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertility and Sterility . 2012;97(1):28–38. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 62.Anton S. D., Martin C. K., Han H., et al. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite . 2010;55(1):37–43. doi: 10.1016/j.appet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tey S. L., Salleh N. B., Henry J., Forde C. G. Effects of aspartame-monk fruit-stevia- and sucrose-sweetened beverages on postprandial glucose, insulin and energy intake. International Journal of Obesity . 2017;41(3):450–457. doi: 10.1038/ijo.2016.225. [DOI] [PubMed] [Google Scholar]
- 64.Nettleton J. E., Reimer R. A., Shearer J. Reshaping the gut microbiota: impact of low calorie sweeteners and the link to insulin resistance? Physiology & Behavior . 2016;164:488–493. doi: 10.1016/j.physbeh.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 65.Allaf M., Elghazaly H., Mohamed O. G., et al. Intermittent fasting for the prevention of cardiovascular disease. Cochrane Database of Systematic Reviews . 2019;1 doi: 10.1002/14651858.cd013496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zarrinpar A., Chaix A., Yooseph S., Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metabolism . 2014;20(6):1006–1017. doi: 10.1016/j.cmet.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pellegrini M., Cioffi I., Evangelista A., et al. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Reviews in Endocrine & Metabolic Disorders . 2020;21(1):17–33. doi: 10.1007/s11154-019-09524-w. [DOI] [PubMed] [Google Scholar]
- 68.Kant A. K., Graubard B. I. 40-year trends in meal and snack eating behaviors of American adults. Journal of the Academy of Nutrition and Dietetics . 2015;115(1):50–63. doi: 10.1016/j.jand.2014.06.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ritskes-Hoitinga M., Jensen T. L. The Laboratory Mouse . London, UK: Academic Press; 2012. Chapter 4.3—nutrition of the laboratory mouse. [Google Scholar]
- 70.Prelevic G. M., Wurzburger M. I., Peric L. A. Pancreatic beta cell function in polycystic ovary syndrome: its relationship to body weight, serum testosterone and serum prolactin levels. Experimental and Clinical Endocrinology & Diabetes . 2009;90(04):76–82. doi: 10.1055/s-0029-1210675. [DOI] [PubMed] [Google Scholar]
- 71.Malin S. K., Kirwan J. P., Sia C. L., Gonzalez F. Pancreatic β-cell dysfunction in polycystic ovary syndrome: role of hyperglycemia-induced nuclear factor-κB activation and systemic inflammation. American Journal of Physiology - Endocrinology and Metabolism . 2015;308(9):E770–E777. doi: 10.1152/ajpendo.00510.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dunaif A., Finegood D. T. Beta-cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism . 1996;81(3):942–947. doi: 10.1210/jc.81.3.942. [DOI] [PubMed] [Google Scholar]
- 73.Koleva D. I., Orbetzova M. M., Nikolova J. G., Tyutyundzhiev S. B. Adipokines and soluble cell adhesion molecules in insulin resistant and non-insulin resistant women with polycystic ovary syndrome. Archives of Physiology and Biochemistry . 2016;122(4):223–227. doi: 10.1080/13813455.2016.1190760. [DOI] [PubMed] [Google Scholar]
- 74.Gözüküçük M., Yarci Gursoy A., Destegul E., Taskin S., Satiroglu H. Adiponectin and leptin levels in normal weight women with polycystic ovary syndrome. Hormone Molecular Biology and Clinical Investigation . 2020;41(4) doi: 10.1515/hmbci-2020-0016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study can be obtained from the corresponding author upon reasonable request.


