Abstract
The aerobic metabolism of benzoate in the proteobacterium Azoarcus evansii was reinvestigated. The known pathways leading to catechol or protocatechuate do not operate in this bacterium. The presumed degradation via 3-hydroxybenzoyl-coenzyme A (CoA) and gentisate could not be confirmed. The first committed step is the activation of benzoate to benzoyl-CoA by a specifically induced benzoate-CoA ligase (AMP forming). This enzyme was purified and shown to differ from an isoenzyme catalyzing the same reaction under anaerobic conditions. The second step postulated involves the hydroxylation of benzoyl-CoA to a so far unknown product by a novel benzoyl-CoA oxygenase, presumably a multicomponent enzyme system. An iron-sulfur flavoprotein, which may be a component of this system, was purified and characterized. The homodimeric enzyme had a native molecular mass of 98 kDa as determined by gel filtration and contained 0.72 mol flavin adenine dinucleotide (FAD), 10.4 to 18.4 mol of Fe, and 13.3 to 17.9 mol of acid-labile sulfur per mol of native protein, depending on the method of protein determination. This benzoate-induced enzyme catalyzed a benzoyl-CoA-, FAD-, and O2-dependent NADPH oxidation surprisingly without hydroxylation of the aromatic ring; however, H2O2 was formed. The gene (boxA, for benzoate oxidation) coding for this protein was cloned and sequenced. It coded for a protein of 46 kDa with two amino acid consensus sequences for two [4Fe-4S] centers at the N terminus. The deduced amino acid sequence showed homology with subunits of ferredoxin-NADP reductase, nitric oxide synthase, NADPH-cytochrome P450 reductase, and phenol hydroxylase. Upstream of the boxA gene, another gene, boxB, encoding a protein of 55 kDa was found. The boxB gene exhibited homology to open reading frames in various other bacteria which code for components of a putative aerobic phenylacetyl-CoA oxidizing system. The boxB gene product was one of at least five proteins induced when A. evansii was grown on benzoate.
The aerobic metabolism of benzoate in the beta subclass proteobacterium Azoarcus evansii is an intriguing problem. All experimental data indicated that none of the known pathways are operable. Normally, benzoate is converted to catechol (1,2-dihydroxybenzene) or protocatechuate (3,4-dihydroxybenzoate), which are the substrates of ring fission dioxygenases. Catechol and protocatechuate dioxygenases could not be found in benzoate-grown cells; rather, gentisate (2,5-dihydroxybenzoate) 1,2-dioxygenase was found (38). Since 3-hydroxybenzoate 6-monooxygenase, which converts 3-hydroxybenzoate in an NADH-dependent reaction to gentisate (32), was also present, a new pathway of benzoate via 3-hydroxybenzoate and gentisate seemed likely.
The specific activities of the monooxygenase and dioxygenase in cell extracts met the requirements of growing cells, yet these activities were much higher in 3-hydroxybenzoate-grown cells (32). Furthermore, the postulated benzoate 3-monooxygenase could not be detected. An active benzoate-coenzyme A (CoA) ligase was unexpectedly present, suggesting that benzoate is first converted to benzoyl-CoA. This presumed first intermediate may subsequently become hydroxylated in the meta position, forming 3-hydroxybenzoyl-CoA (1). An NADH-oxidizing activity was found that was dependent on the addition of 3-hydroxybenzoyl-CoA, and a reaction product that cochromatographed with gentisate was formed (32). The sum of the new experimental evidence seemed to favor a modified pathway of benzoate via benzoyl-CoA and gentisate.
Cell extracts in fact contained an enzyme activity that oxidized NADPH in the presence of oxygen and flavin adenine dinucleotide (FAD) in a strictly benzoyl-CoA-dependent manner; the product formed seemed to comigrate with 3-hydroxybenzoyl-CoA on high-performance liquid chromatography (HPLC). The enzyme activity has been purified based on this spectrophotometric assay, but the product of oxidative transformation has not been studied. It was assumed to be 3-hydroxybenzoyl-CoA, and the enzyme was tentatively named benzoyl-CoA 3-monooxygenase (32).
There seem to be more unconventional catabolic pathways for monocyclic aromatic compounds in this bacterium; besides benzoate, also 2-aminobenzoate and phenylacetate are metabolized via their CoA thioesters. Whereas the dearomatizing reaction in case of 2-aminobenzoyl-CoA has been studied (2, 8, 9, 10, 16, 23, 24, 41), the fate of phenylacetyl-CoA is unknown (29). A similar aerobic phenylacetyl-CoA pathway seems to exist in Pseudomonas putida (28, 33), Escherichia coli (13, 38), and other bacteria (29, 38).
We have attempted to study in more detail the aerobic pathway of benzoyl-CoA. When the purified presumed benzoyl-CoA 3-monooxygenase was reinvestigated, to our surprise the purified protein did not hydroxylate benzoyl-CoA. Moreover, the presumed product 3-hydroxybenzoyl-CoA observed in cell extracts did not cochromatograph with the standard compound when a different and more sophisticated HPLC system for product separation was applied (A. Zaar, A. Bacher, W. Eisenreich, and G. Fuchs, submitted for publication). This paper presents extensive further studies on the new aerobic benzoyl-CoA pathway. The conclusions derived may well apply also to some other bacteria in which the aerobic benzoate pathway is at issue.
MATERIALS AND METHODS
Growth of bacteria.
A. evansii (3) was grown aerobically in chemically defined medium (8) with 5 mM benzoate, 3-hydroxybenzoate, phenylacetate, and acetate and was grown anaerobically with benzoate plus nitrate. A. evansii strain B5 (G. Fuchs, unpublished data) and Thauera strains LG356 (Fuchs, unpublished) and S2 (39) were also grown aerobically with 5 mM benzoate. Large-scale aerobic growth of A. evansii with benzoate was carried out in a 200-liter fermentor. A chemical mutant of A. evansii (Benz strain 38/7), which cannot grow on benzoate but still utilizes 3-hydroxybenzoate (1), was grown aerobically in the presence of 5 mM 3-hydroxybenzoate plus 5 mM benzoate. Growth was determined by following the optical density at 578 nm (diameter = 1 cm). An optical density of 1 corresponded to a cell concentration of approximately 0.3 g of cell dry mass per liter or 0.16 g of protein per liter.
Chemicals.
The chemicals and biochemicals used were obtained from Fluka (Neu Ulm, Germany), Sigma-Aldrich Chemie GmbH (Deisenhofen, Germany), and Amersham Pharmacia Biotech (Freiburg, Germany). [U-14C] benzoic acid was from American Radiolabelled Chemicals/Biotrend Chemikalien (Köln, Germany). CoA thioesters of benzoic acid and its hydroxyl derivatives were chemically synthesized as described elsewhere (15, 22).
Preparation of cell extracts.
The buffer used contained 10 mM Tris-HCl (pH 7.5), 2 mM MgCl2, 2 mM dithioerythritol, and 10% (wt/vol) glycerol. Frozen or fresh cells were suspended in the buffer (1 g of cells/2 ml of buffer), and DNase I (0.3 mg/g cells) was added. We used 15 g of frozen benzoate-grown cells for the purification of benzoyl-CoA ligase and 100 g of frozen benzoate-grown cells for the purification of benzoyl-CoA oxygenase. The cell suspension was passed twice through a French pressure cell at 137 MPa, and the crude cell extract was centrifuged at 100,000 or 150,000 × g (depending on the experiment) for 2 h. The resulting supernatant (cell extract) contained approximately 50 mg of protein/ml.
Enzymatic assays. (i) Benzoate-CoA ligase.
The coupled enzymatic assay of CoA ligase that monitors the oxidation of NADH was followed spectrophotometrically at 365 nm as previously described (46). A stoichiometry of 1 mol of NADH oxidized per mol of CoA thioester formed was taken as evidence for ADP formation, and a stoichiometry of 2 mol of NADH oxidized was taken as evidence for AMP formation. The protein fraction of the cell extract (100,000 × g supernatant) precipitated at 60% ammonium sulfate saturation was used, since the cell extract contained substances that interfered with this assay. Extracts of A. evansii cells aerobically grown on benzoate, 3-hydroxybenzoate, phenylacetate, and acetate and grown anaerobically with benzoate plus nitrate were screened for the presence of benzoate-CoA ligase activity.
(ii) Benzoyl-CoA oxygenase.
Enzyme activity was determined spectrophotometrically and polarographically. The spectrophotometric assay was followed at 365 nm as benzoyl-CoA-, oxygen-, and FAD-dependent oxidation of NADPH at 37°C. In the polarographic assay, benzoyl-CoA-, NADPH-, and FAD-dependent oxygen uptake was measured after correction for endogenous O2 uptake, using a Clark-type oxygen electrode (RE K1-1; Biolytik, Bochum, Germany). When O2 uptake was completed and remained at the original endogenous rate, 6,800 U of catalase was added and the regenerated O2 was measured. The standard spectrophotometric and polarographic assays (0.5 and 1 ml, respectively) contained 100 mM Tris-HCl (pH 8.0), 0.1 mM FAD, 0.3 mM NADPH, and cell extract (10 μl of 100,000 × g supernatant) or enzyme fraction. In both assays, adding 0.1 mM benzoyl-CoA started the reaction. Extracts of A. evansii cells grown on different aromatic substrates as well as extracts of other bacterial strains (A. evansii strain B5 and Thauera strains LG356 and S2) grown aerobically with benzoate were also tested.
(iii) Postulated 3-hydroxybenzoyl-CoA 6-monooxygenase and 3-hydroxybenzoate 6-monooxygenase.
The activities of the postulated monooxygenases (1, 32) were tested in extracts (100,000 × g supernatant) of A. evansii cells grown aerobically with benzoate and 3-hydroxybenzoate. These activities were tested at 37°C and monitored spectrophotometrically at 365 nm. The assay mixture (0.5 ml) contained 20 mM imidazole-HCl buffer (pH 7.4), 50 μM FAD, 0.4 mM NAD(P)H, and 10 μl of cell extract, and the addition of 0.2 mM 3-hydroxybenzoyl-CoA or 3-hydroxybenzoate started the reaction.
(iv) Gentisate 1,2-dioxygenase.
The oxygen-mediated ring cleavage of 2,5-dihydroxybenzoate (gentisate) was followed polarographically as described above. Oxygen consumption accompanying the addition of 0.5 mM gentisate or other dihydroxybenzoic acids was measured after correction for endogenous O2 uptake. The test was carried out in 100 mM Tris-HCl (pH 7.8) at 37°C. Activity was measured using 20 μl of cell extract (100,000 × g supernatant), and the addition of the respective dihydroxybenzoic acid started the test. This enzymatic activity was also assayed spectrophotometrically at 334 nm as the formation of maleylpyruvate, using the protein fraction of cell extract obtained by precipitation at 70% (NH4)2SO4 saturation (20).
Substrate-dependent oxygen consumption experiments.
Oxygen uptake by washed cell suspensions of A. evansii (1 ml of cell suspension with an optical density of 0.4 at 578 nm) or by cell extracts (100,00 × g supernatant, 3 mg of protein/1-ml assay) was measured polarographically by following oxygen consumption concomitant with oxidation of the aromatic substrate. These assays were performed at 37°C in growth medium or in 100 mM Tris-HCl (pH 7.5). Rates of oxidation of 0.5 mM benzoate, 3-hydroxybenzoate, gentisate, and other dihydroxybenzoic acids were determined after correction for endogenous O2 uptake.
Simultaneous adaptation experiments.
Cultures of A. evansii which were repeatedly grown on benzoate and 3-hydroxybenzoate were harvested during the exponential growth phase. After centrifugation, the cell pellet was washed once with the growth medium lacking the carbon source and resuspended in the same medium to give a final optical density at 578 nm of 25 to 30. The cell suspension was dispensed in three equal portions (3 ml each), and the experiment was started by the addition of 0.5 mM benzoate, 3-hydroxybenzoate, or gentisate. At different points in time, samples of 0.4 ml were withdrawn and immediately centrifuged in a precooled Eppendorf centrifuge at 10,000 rpm. The resulting supernatants were analyzed for the consumption of the different substrates by HPLC.
Purification of benzoate-CoA ligase.
Benzoate-CoA ligase was purified at 4°C using a simplified purification protocol based on a published method (1). The cell extract obtained after 150,000 × g centrifugation was used. The buffer used for preparation of the cell extract was used as equilibration buffer in the following chromatographic steps.
(i) Anion-exchange chromatography 1.
A DEAE-Sepharose Fast Flow (Pharmacia) column of 85-cm3 matrix was equilibrated at a flow rate of 4 ml min−1 with 500 ml of equilibration buffer supplemented with 70 mM KCl. After application of cell extract (38 ml), the column was washed with 250 ml of equilibration buffer (containing 70 mM KCl), and fractions of 12 ml were collected from the beginning of this washing step. The elution of the desired protein was achieved by a linear KCl gradient from 70 to 200 mM in 500 ml followed by 150 ml of 200 mM KCl in equilibration buffer. The benzoate-CoA ligase activity eluted between 150 and 200 mM KCl (fractions 49 to 62). Fractions 50 to 58 were pooled (108 ml).
(ii) Anion-exchange chromatography 2.
The pooled active protein fraction from the above step (104 ml) was applied to a 20-cm3 Q-Sepharose (Pharmacia) column which had been equilibrated with equilibration buffer containing 200 mM KCl. The flow rate was adjusted to 3 ml min−1. After sample application, fractions of 8 ml were collected, and the column was washed with 150 ml of equilibration buffer (containing 200 mM KCl) followed by 200 ml of a linear KCl gradient between 200 and 360 mM. Fractions 28 to 39 contained benzoate-CoA ligase activity; fractions 29 to 36 were pooled (64 ml), and the concentration of KCl was adjusted to 70 mM by dilution with 200 ml of equilibration buffer containing no KCl.
(iii) Affinity chromatography.
A part (20 ml) of the protein sample obtained from the preceding step after dilution was applied to a 15-cm3 reactive green-agarose (Sigma) column that had been equilibrated with equilibration buffer containing 70 mM KCl. The flow rate was adjusted to 1 ml min−1, and fractions of 3 ml were collected after sample application. The loaded column was washed with 60 ml of equilibration buffer followed by 75 ml of the same buffer supplemented with 5 mM potassium benzoate (pH 7.5) to specifically elute benzoate-CoA ligase in fractions 26 to 38. This chromatographic step yielded a homogeneous pure protein (26% yield after 180-fold enrichment) with a specific activity of 52 μmol min−1 mg of protein−1.
Purification of putative benzoyl-CoA oxygenase.
The buffer used throughout the following purification at 4°C was 10 mM Tris-HCl (pH 8.0) containing 2 mM dithioerythritol and various concentrations of KCl.
(i) Anion-exchange chromatography 1.
A DEAE-Sepharose (Pharmacia) column of 220-cm3 matrix was equilibrated with buffer at a flow rate of 4 ml min−1. After application of the cell extract (100,000 × g supernatant), fractions of 18 ml were collected and the column was washed with 220 ml of buffer. The column was washed with 440 ml of buffer containing 100 mM KCl and with 1 liter buffer containing 150 mM KCl. The desired protein was eluted with 900 ml of buffer containing 200 mM KCl. Elution started after 200 ml; 66 g of glycerol was added to the pooled fractions (670 ml), and the solution was kept frozen overnight.
(ii) Anion-exchange chromatography 2.
The pooled active protein fraction from the previous step was diluted by adding 245 ml of buffer and applied to a Q-Sepharose (Pharmacia) column of 58-cm3 matrix which had been equilibrated with buffer containing 150 mM KCl at a flow rate of 3 ml min−1. Fractions of 10 ml were collected after sample application. The loaded column was washed with 1.8 column volumes of buffer containing 150 mM KCl followed by 3.7 column volumes of buffer containing 200 mM KCl. A linear gradient from 200 to 300 mM KCl in buffer was then applied over 7.4 column volumes. The active enzyme eluted between 200 and 275 mM KCl.
(iii) Affinity chromatography.
A Cibacron blue 3GA column (Sigma) of 226 cm3 was equilibrated with 10 mM potassium phosphate buffer (pH 6.0) at a flow rate of 2 ml min−1. Then 16.5 ml of 200 mM potassium phosphate buffer (pH 6.0) was added to the pooled active protein fractions obtained from the preceding step (330 ml), and the pH was adjusted to pH 6.0 using HCl (1 M). The protein sample was loaded onto the column and then washed with 115 ml of equilibration buffer containing 150 mM KCl, 570 ml of the same buffer containing 400 mM KCl, and finally 420 ml of equilibration buffer without KCl. After sample application, fractions of 10 ml were collected in tubes containing 0.8 ml of 0.5 M Tris-HCl (pH 8). The desired protein was eluted by washing with 250 ml of equilibration buffer of pH 6 containing 50 μM benzoyl-CoA at a flow rate of 3 ml min−1. Fractions containing the active protein were combined and concentrated by dialysis. The purified protein was stored in the presence of 10% glycerol–2 mM dithioerythritol.
(iv) Gel permeation chromatography.
Part of the active protein fraction obtained from the above step (65 ml) was concentrated by ultrafiltration using a 30-kDa-cutoff membrane (Amicon, Beverly, Mass.). Part of the concentrated protein sample (1 ml) was applied to a calibrated Superdex 200 HR 10/30 (Pharmacia) column to determine the molecular mass of the enzyme, using 10 mM Tris-HCl (pH 8) containing 100 mM KCl. The flow rate was 0.2 ml min−1.
UV/visible spectroscopy of purified benzoyl-CoA oxygenase in the oxidized and reduced states.
Dithioerythritol was removed from the isolated enzyme by passing it over a PD-10 column (Pharmacia) using 10 mM Tris-HCl buffer (pH 8) at a flow rate of 2 ml min−1. The enzyme fraction obtained was considered to be in its oxidized form. The enzyme was reduced by adding a severalfold excess of dithionite. The spectra of the oxidized and reduced enzyme (0.76 mg/ml) were recorded against the buffer solution in which the enzyme was dissolved. The cuvette was evacuated and transferred to an anaerobic chamber (95% N2–5% H2 atmosphere) for displacement of oxygen. The dithionite stock solution (10 mM) was treated in the same way. The enzyme was reoxidized by stepwise addition of air to the stoppered sample using a gastight syringe, followed by stirring until the spectrum remained constant.
Electrophoresis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (11.5% polyacrylamide) was carried out with a discontinuous buffer system (12). Protein was visualized with Coomassie blue (41), and the molecular mass of the purified protein was compared with molecular mass protein standards phosphorylase, bovine serum albumin, ovalbumin, lactate dehydrogenase, carbonic anhydrase, and lysozyme (97, 67, 45, 34, 29, and 14 kDa, respectively).
Two-dimensional gel electrophoresis.
Cell extract proteins (100,000 × g supernatants) of A. evansii wild-type cells grown on benzoate or 3-hydroxybenzoate were separated by two-dimensional gel electrophoresis using the Immobiline Dry-Strip system (Amersham Pharmacia Biotech). The first dimension (isoelectric focusing [IEF]) was performed with IEF Dry-Strips and 0.2% ampholyte (pH 3 to 10; 40%, wt/vol; Bio-Rad, Hercules, Calif.). The second dimension was SDS-PAGE performed as described above. The proteins were transblotted onto a polyvinylidene difluoride membrane, and the N-terminal amino acid sequences of proteins induced by benzoate but not 3-hydroxybenzoate were determined as described below.
Electrophoretic transfer of protein, immunodetection, and determination of N-terminal amino acid sequences.
Extracts (100,000 × g supernatant) of different bacterial strains grown on benzoate (Azoarcus strains KB740 and B5 and Thauera strains LG356 and S2) as well as the purified enzymes (benzoate-CoA ligase and putative benzoyl-CoA oxygenase) and a cyanogen bromide cleavage fragment of putative benzoyl-CoA oxygenase were separated by SDS-PAGE and transferred to nitrocellulose filters (0.45-μm pore size) as described elsewhere (37). The transblotted proteins were detected by Ponceau S staining as described previously (12). The protein bands of the purified enzymes and the cyanogen bromide cleavage fragment were cut off, and the N-terminal amino acid sequences were determined by H. Schägger (Universität Frankfurt, Frankfurt, Germany) using gas-liquid-phase sequencing with an Applied Biosystem 473A sequencer. The polyclonal antibodies raised in rabbits against the putative benzoyl-CoA oxygenase of A. evansii were used to test the presence of similar proteins in different bacterial strains by Western blot analysis. Proteins were immunologically detected by using the Amersham ECL (enhanced chemiluminescence) system.
Protein determination.
The protein contents of the different samples were determined by the methods of Lowry and Bradford (12).
Extraction, identification, and quantification of the flavin cofactor.
All steps for extraction of the flavin cofactor from putative benzoyl-CoA oxygenase were carried out without light at 4°C. The purified enzyme obtained after the affinity chromatography step was used after concentration by ultrafiltration (0.43 mg/ml). The enzyme was denaturated by adding 3% (by volume) 70% perchloric acid. After incubation for 1 h at 4°C, precipitated protein was removed by centrifugation (10,000 × g, 10 min, 4°C). The flavin in the supernatant was identified and quantitated by HPLC, using an RP-C18 column (125 by 4 mm) and 9% acetonitrile in 20 mM ammonium acetate-HCl (pH 6.0) as a solvent. Detection was monitored at 375 nm at a flow rate of 1 ml min−1. Under these conditions, the retention times of FAD, flavin mononucleotide (FMN), and riboflavin were 6.3, 10.1, and 21.4 min, respectively. The amount of extracted flavin cofactor was determined by comparison of the peak area with that of standard solutions (5 to 25 μl of 0.12 mg/ml) of FAD, FMN, and riboflavin.
Determination of iron and acid-labile sulfur.
The iron content of the purified putative benzoyl-CoA oxygenase was determined photometrically at 546 nm, using FeCl3, as the standard (26). The acid-labile sulfur content of the dithioerythritol-free sample was determined photometrically at 670 nm, using Na2S · 9H2O as the standard (7).
Cloning, transformation, amplification, and purification of DNA and cloning of the genes for the putative benzoyl-CoA oxygenase.
Standard protocols were used for DNA cloning, transformation, amplification, and purification (5, 37). A λ-ZAP Express gene library containing chromosomal DNA of A. evansii after Sau3AI digestion was constructed as described in the ZAP Express cloning kit instruction manual (Stratagene). The 20-mer degenerate forward primer Mol (5′-ATG AAC GCS CCS GCS GAR CA-3′) and the 20-mer degenerate backward primer Morl (5′-CC GAA GTA SGG SAG YTC YTC-3′) were designed on the basis of the determined N-terminal amino acid sequences of purified benzoyl-CoA oxygenase and its cyanogen bromide cleavage fragment, using the codon usage of the organism. A 1-kbp PCR product was obtained and sequenced in PCRs containing these primers. This PCR fragment, which was identified as a part of the gene coding for the putative benzoyl-CoA oxygenase, was labeled with [γ32P]dCTP (Amersham) and used as a probe to screen the constructed gene library. Three positive clones were obtained, and the recombinant plasmids were maintained in E. coli XL1-Blue MRF′.
DNA sequencing and computer analysis.
Purification of plasmid DNA was carried out as described for the spin miniprep kit protocol (Qiagen, Hilden, Germany). Sequencing of the DNA insert was carried out by J. Alt-Mörbe (Labor für DNA-Analytik, Freiburg, Germany). DNA and amino acid sequences were analyzed with the BLAST network service at the National Center for Biotechnology Information (Bethesda, Md.).
HPLC.
Gentisate, 3-hydroxybenzoate, benzoate, 3-hydroxybenzoyl-CoA, benzoyl-CoA, and other intermediary products were separated, identified, and quantified using an HPLC equipped with a variable-wavelength UV or visible light monitor and a flowthrough radioactivity detector with a solid scintillator cell. The separation was carried out at room temperature at a flow rate of 1 ml min−1 and monitored at a wavelength of 260 nm. A reversed-phase C18 column (RP-C18, Grom-Sil octadecyl silane-4 hydrophilic end capped; particle size, 5 μm; 120 by 4 mm; Grom, Herrenberg, Germany) was used. First, 2% acetonitrile in 50 mM potassium phosphate buffer (pH 4.5) was applied for 15 min, followed by 11% acetonitrile in 50 mM potassium phosphate buffer, (pH 6.7) for 25 min. The retention times for the first five compounds mentioned above were 6, 9.5, 18, 25, and 32 min, respectively.
Thin-layer chromatography.
For separation and preliminary identification of benzoate, 3-hydroxybenzoate, and their CoA thioesters, and gentisate on silica gel aluminum thin-layer chromatography plates (thickness, 0.2 mm; 20 by 20 cm; Kieselgel type 60 F 254; Merck, Darmstadt, Germany), the following solvent systems were used: (i) n-butanol–acetic acid–water (12:3:5, vol/vol/vol) with Rf values of 0.9, 0.9, 0.75, 0.2, and 0.2 for benzoate, 3-hydroxybenzoate, gentisate, benzoyl-CoA, and 3-hydroxybenzoyl-CoA; and (ii) dichloroethane-acetic acid-water (2:1:1, vol/vol/vol; the lower aqueous phase of the mixture was used). Rf values of 0.9, 0.7, and 0.5 were observed for the first three compounds mentioned above. These compounds were visualized under UV light at 254 nm, and radioactive spots were localized either by autoradiography on X-ray films or with a phosphorimaging plate (Fuji Photo Film Co., Ltd., Kanagawa, Japan).
Radioactivity determination.
Radioactive peaks separated by HPLC were monitored by a flowthrough radioactivity detector with a solid scintillator cell. The amount of radioactivity of these fractions was quantified by determining the peak area in comparison to that of a standard labeled compound. Radioactivity in other samples was determined by liquid scintillation counting using the channel ratio method for quench correction.
Nucleotide sequence accession number.
The sequence data reported here were submitted to the EMBL database (accession no. AF220510).
RESULTS
Aerobic growth of A. evansii and simultaneous adaptation experiments.
A. evansii was grown aerobically with 5 mM benzoate or 3-hydroxybenzoate at 37°C. Benzoate-grown cultures were pale yellow, whereas 3-hydroxybenzoate-grown cultures had a brown color. The maximal growth rates were 0.23 and 0.28 h−1, and the molar growth yields were 58 and 62 g of dry cell mass formed per mol of benzoate and 3-hydroxybenzoate consumed, respectively. The estimated specific substrate consumption rates of these cultures were 110 and 126 nmol min−1 mg of protein−1, respectively. The consumption of benzoate and 3-hydroxybenzoate by suspensions of A. evansii cells adapted to either benzoate or 3-hydroxybenzoate was tested. Cells grown on benzoate consumed 3-hydroxybenzoate, though at one-half to one-third of the rate of benzoate consumption, whereas 3-hydroxybenzoate-grown cells started to consume benzoate only after a lag phase of 15 min. Benzoate-grown cells consumed 2,5-dihydroxybenzoate (gentisate) at one-third to one-fourth of the rate of gentisate consumption by 3-hydroxybenzoate-grown cells.
Substrate-dependent oxygen consumption by whole cells.
Washed cells grown on benzoate and 3-hydroxybenzoate were suspended in mineral growth medium to which different aromatic growth substrates were added. The ability of these cells to oxidize the aromatic substrate was monitored by measuring substrate-induced oxygen consumption. Benzoate-grown cells consumed O2 rapidly, with rates of 215 and 85 nmol of O2 min−1 mg of cell protein−1, when 0.5 mM benzoate and 3-hydroxybenzoate, respectively, were added. An oxygen consumption rate of 46 nmol O2 min−1 mg of cell protein−1 was observed when gentisate (2,5-dihydroxybenzoate) was added; other dihydroxybenzoate compounds (2,3-, 2,6-, 3,4-, and 3,5-dihydroxybenzoate) were inactive (<2 nmol min−1 mg of cell protein−1). Cells grown on 3-hydroxybenzoate consumed oxygen at rates of 245 and 155 nmol of O2 min−1 mg of cell protein−1 when 3-hydroxybenzoate and 2,5-dihydroxybenzoate, respectively, were added. When benzoate was added to these cells, no oxygen consumption was observed during 15 min of incubation.
Aerobic benzoate-CoA ligase and 3-hydroxybenzoate-CoA ligase.
Cells grown on benzoate contained benzoate-CoA ligase activity (Table 1). This activity could be measured only after precipitation of protein, e.g., with ammonium sulfate, and amounted to 196 nmol of benzoyl-CoA formed min−1 mg of protein−1 in the ammonium sulfate-precipitated protein fraction. The product of ATP cleavage was AMP, and the reaction catalyzed therefore was benzoate + MgATP + CoASH → benzoyl-SCoA + MgAMP + PPi. These cells also contained 3-hydroxybenzoate-CoA ligase activity (94 nmol min−1 mg of protein−1 in the ammonium sulfate-precipitated protein fraction). In contrast, in cells grown aerobically with 3-hydroxybenzoate, no benzoate-CoA ligase activity was detected, but these cells contained 3-hydroxybenzoate-CoA ligase activity (Table 1). In Benz mutant cells, which cannot grow aerobically with benzoate and were grown on a mixture of benzoate (as inducer) and 3-hydroxybenzoate (as growth-supporting substrate), benzoate-CoA ligase activity was detected but was less than 20% of wild-type activity. Cells grown anaerobically on benzoate and nitrate contained benzoate-CoA ligase activity as well. It is known that the anaerobically induced enzyme catalyzes the first step in the anaerobic metabolism of benzoate (17, 18). Low benzoate- and 3-hydroxybenzoate-CoA ligase activities were detected in acetate-grown cells (Table 1). Obviously, there are two distinct aerobically induced CoA ligases, one acting on benzoate and the other acting on 3-hydroxybenzoate.
TABLE 1.
CoA ligase activities in protein fraction (100,000 × g supernatant precipitated at 60% ammonium sulfate saturation) of A. evansii cells grown on different substrates
| Substrate with which cells were aerobically grown | CoA ligase activity (nmol min−1
mg of protein−1) with indicated substrate
|
||
|---|---|---|---|
| Benzoate | 3-Hydroxybenzoate | Benzoate + 3-hydroxybenzoate | |
| Benzoate | 196 | 94 | 238 |
| 3-Hydroxybenzoate | 8 | 144 | 132 |
| Benzoate + 3-hydroxybenzoate (Benz mutant) | 34 | 134 | 128 |
| Phenylacetate | 35 | 22 | NDa |
| Acetate | 56 | 11 | ND |
| Benzoate (anaerobic) | 105 | 3 | ND |
ND, not determined.
Substrate specificity and N-terminal amino acid sequence of the aerobically induced benzoate-CoA ligase.
The aerobically induced benzoate-CoA ligase was purified, and its substrate specificity was determined. The enzyme acted on benzoate (73 U/mg of protein) but was inactive with 3-hydroxybenzoate (<1%). The N-terminal amino acid sequence of the protein was determined as TTLSAADHSTSPPTItLPRQYNAad (lowercase letters represent uncertain amino acids). The N-terminal amino acid sequence differed from that of the anaerobically induced benzoate-CoA ligase: AELSVADhsVxPP (lowercase letters, uncertain amino acids; x, unknown) (2). This provided evidence that two isoenzymes are present in this organism that are induced by benzoate under aerobic and under denitrifying anaerobic conditions, respectively.
Purification of putative benzoyl-CoA oxygenase and molecular properties of the enzyme.
Extracts of benzoate-grown cells catalyzed the oxidation of NADPH with O2 when both FAD and benzoyl-CoA were present. The enzyme catalyzing the O2-, benzoyl-CoA-, and FAD-dependent oxidation of NADPH is tentatively termed benzoyl-CoA oxygenase. The specific activity of this putative benzoyl-CoA oxygenase in benzoate-grown cells was 57 nmol min−1 mg of cell protein−1, compared to 11 nmol min−1 mg of cell protein−1 in 3-hydroxybenzoate-grown cells (Table 2). The enzyme was purified from 100 g (fresh cell mass) of benzoate-grown cells with 30% yield (Table 2). A 300-fold enrichment yielded an almost homogeneous protein with a native molecular mass of approximately 98 kDa as determined by gel permeation chromatography. In SDS-PAGE, a strong protein band was seen at a molecular mass corresponding to 50 kDa (Fig. 1),and only traces of a 60-kDa contaminant protein were seen. Hence, the native protein appears to be a homodimer composed of 50-kDa subunits. The homodimeric brownish protein catalyzed NADPH oxidation at 17 to 24 μmol min−1 mg of protein−1, depending on the batch of enzyme.
TABLE 2.
Purification scheme for putative benzoyl-CoA oxygenase from A. evansii aerobically grown on benzoate
| Purification step | Total enzyme activity (μmol min−1) | Total protein (mg)a | Sp act (μmol min−1 mg of protein−1) | Recovery (%) | Level of purification (fold) |
|---|---|---|---|---|---|
| Cell extract (100,000 × g) | 512 | 8,990 | 0.057 | 100 | 1 |
| DEAE-Sepharose | 513 | 602 | 0.85 | 100 | 14.9 |
| Q-Sepharose | 393 | 250 | 1.57 | 76.7 | 27.5 |
| Cibacron blue | 157 | 9.18 | 17.0 | 30.6 | 299 |
Determined by the Bradford method.
FIG. 1.
SDS-PAGE of protein fractions collected during purification containing putative benzoyl-CoA oxygenase. Lane 1, molecular mass protein standards (see Materials and Methods); lane 2, cell extract (supernatant after centrifugation at 100,000 × g; 25 μg of protein); lane 3, DEAE pool (25 μg of protein); lanes 4 to 6, Q-Sepharose active fractions (20 μg of protein); lanes 7 and 8; Cibacron blue active fractions (9 μg of protein).
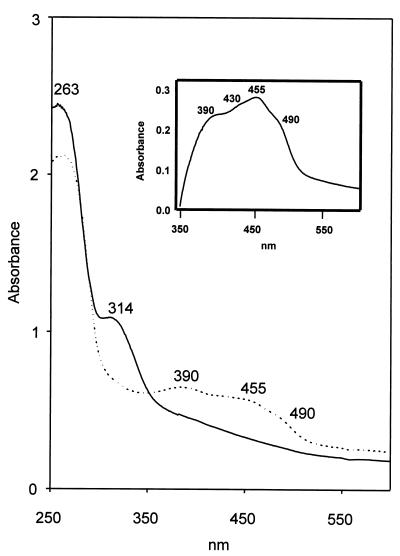
The visible and UV absorption spectra of the oxidized (as isolated) and dithionite-reduced enzyme preparations are shown in Fig. 2. Beside the absorption maximum of the protein, the oxidized enzyme exhibited absorption maxima at 390 and 455 nm and a shoulder at 490 nm. The molar absorption coefficients at 390 and 455 were estimated to be 80,000 and 70,000 M−1 cm−1, respectively, based on a molecular mass of 92 kDa per homodimer. This molecular mass was deduced from the gene sequence (see below) and will be used in the following. The reduced enzyme exhibited an absorption maximum in the protein absorption range; an additional maximum at 314 nm was due to an excess of dithionite. The inset in Fig. 2 shows the difference spectrum of the oxidized-minus-reduced enzyme with absorption maxima at 390 and 455 nm and shoulders at 430 and 490 nm. These spectra point to a flavin- and FeS-containing protein. The absorption maxima of the oxidized iron-sulfur clusters that absorb around 400 nm are superimposed over the absorption maximum of oxidized flavin around 375 and 450 nm. Determination of the iron content of the different protein batches varied, depending on the method used for protein determination, from 18.4 mol of iron per mol of native 92-kDa enzyme (Bradford) to 10.4 mol of iron per mol of 92-kDa enzyme (Lowry). The content of acid-labile sulfur varied from 17.9 (Bradford) to 13.3 (Lowry) mol per mol of 92-kDa protein. The flavin content was 0.72 mol per mol of 92-kDa enzyme (Lowry), FAD being the flavin nucleotide. The flavin nucleotide was identified and quantified as described in Materials and Methods.
FIG. 2.
UV/visible spectra of purified benzoyl-CoA oxygenase as isolated (oxidized; ····) and after reduction with dithionite (reduced; ——). The absorption spectra of the enzyme (0.76 mg of protein/ml, corresponding to a concentration of 8.3 μM dimeric protein of 92 kDa) were recorded under anaerobic conditions in 10 mM Tris-HCl (pH 8) against buffer blank. Addition of an at least fivefold excess of dithionite resulted in complete reduction of the enzyme. The insert shows the difference spectrum of oxidized benzoyl-CoA oxygenase minus the reduced enzyme.
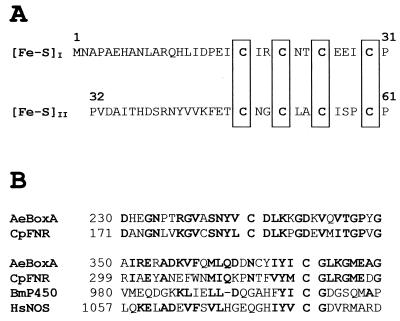
The N-terminal amino acid sequence was MNAPAEHANLARQHLIDPEsirsrnt (lowercase letters indicate uncertain amino acids; compare with Fig. 6A). The N-terminal amino acid sequence of a cyanogen bromide cleavage fragment was EGGELVLFFGARAPEELPYFgp (lowercase letters indicate uncertain amino acids).
FIG. 6.
Properties of BoxA. (A) N-terminal amino acid sequence of BoxA of A. evansii deduced from nucleotide sequence. Eight cysteine residues are clustered (in box), giving a typical consensus sequence for two [4Fe-4S] clusters, (Fe-S)I and (Fe-S)II. (B) Alignment of amino acid sequence regions of BoxA with corresponding regions of other proteins. The sequence abbreviations (and accession numbers) are as follows: AeBoxA, A. evansii benzoyl-CoA oxygenase (AF220510); CpFNR, Cyanophora paradoxa ferredoxin-NADP reductase (CAA47015); BmP450, Bacillus megaterium NADPH-cytochrome P450 reductase (P14799); HsNOS, Homo sapiens nitric oxide synthase (AF049656). Amino acids in boldface are conserved; i.e., they are identical or represent conservative exchanges.
Catalytic properties.
The purified enzyme catalyzed the benzoyl-CoA- and O2-dependent oxidation of NADPH (0.3 mM) with molecular oxygen when FAD or FMN (0.1 mM each) was present in the assay. The specific activity of 24 (in the presence of FAD) or 15 (in the presence of FMN) μmol min−1 mg of protein−1 was arbitrarily set 100%. NADH was not oxidized (<1%), and NADPH oxidation in the absence of FAD was 1%. When both FAD and benzoyl-CoA were lacking, no NADPH oxidation was detectable. Monohydroxybenzoyl-CoA analogues were active as well in stimulating NADPH oxidation, in the order 3-hydroxybenzoyl-CoA (70%), 2-hydroxybenzoyl-CoA (40%), and 4-hydroxybenzoyl-CoA (30%). The addition of the corresponding free aromatic acids, acetate, and CoA did not cause any oxidation of NADPH.
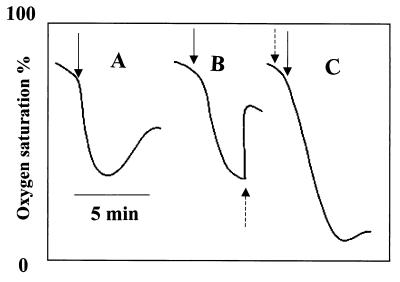
However, surprisingly no labeled product was formed enzymatically from [U-14C]benzoyl-CoA. There was an enzyme-independent decay of benzoyl-CoA to a polar product and benzoate in the presence of Tris-HCl (100 mM, pH 8) and the formation of another nonpolar product in the presence of dithioerythritol. The lack of product formation despite oxygen-dependent NADPH oxidation suggested that the enzyme catalyzed the FAD- or FMN-dependent oxidation of NADPH, with concomitant formation of H2O2, benzoyl-CoA, and analogues acting as nonproductive substrates. Addition of benzoyl-CoA to the enzyme assay caused immediate oxygen consumption. This oxygen consumption slowed down before the dissolved O2 was used up and was followed by a phase of slow oxygen generation (Fig. 3A). When the oxygen consumption phase was complete, addition of catalase resulted in rapid formation of oxygen, demonstrating that the product of the first reaction was H2O2 (Fig. 3B). The rate of oxygen consumption was equal to the rate of NADPH oxidation, and the amount of oxygen being formed after addition of catalase to the completed assay corresponded to half the amount of oxygen or NADPH initially consumed (Fig. 3B). Figure 3C shows complete oxygen consumption when catalase was added at the beginning. In the spectrophotometric assay, the rate of NADPH oxidation slowed before the dissolved O2 was consumed. This might have been due to the inactivation of the enzyme by accumulated H2O2. A chemical reaction between benzoyl-CoA and H2O2 resulting in the decay of benzoyl-CoA might also have occurred, although such a reaction seemed to require higher concentrations of H2O2.
FIG. 3.
Oxygen consumption by the putative benzoyl-CoA oxygenase in the presence of benzoyl-CoA, FAD, and NADPH (see Materials and Methods) with complete assay (A). As indicated by broken arrows, 6,800 U of catalase was added when the oxygen consumption phase was completed (B) and from the beginning (C). Solid arrows indicate times of benzoyl-CoA addition.
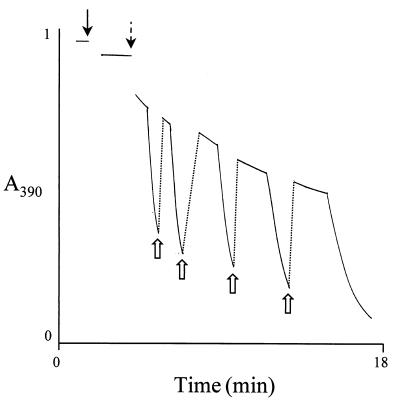
When benzoyl-CoA (0.1 mM) was added to an assay containing cell extract, FAD (0.1 mM), NADPH (2 mM), and oxygen (0.16 mM), and the reaction was followed spectrophotometrically at 390 nm, an absorption decrease was observed. The molecular absorption coefficients of NADPH (400 M−1 cm−1) and of FAD (6,800 M−1 cm−1) at this wavelength were obtained from the spectra of pure standard compounds using ɛ340 (NADPH) = 6,22 M−1 cm−1 and ɛ375 (FAD) = 9,300 M−1 cm−1 (6). Note that FADH2 hardly absorbs at 390 nm. The initial slow absorption decrease was obviously due to the oxidation of approximately 0.18 mM NADPH by oxygen (0.16 mM). When oxygen (initially present at approximately 0.16 mM) was completely consumed, a sudden absorption decrease followed due to reduction of FAD to FADH2 by NADPH. Shaking the cuvette, and thereby reintroducing molecular oxygen, resulted in a sudden absorption increase to nearly the initial value due to the reoxidation of FADH2. This cycle could be repeated many times (Fig. 4). These results suggested the following events catalyzed by the enzyme (reaction 1), occurring spontaneously (reaction 2), and catalyzed by catalase (reaction 3), respectively:
FIG. 4.
Oxidation of NADPH (2 mM) and reduction of FAD (0.1 mM) in the spectrophotometric assay of the putative benzoyl-CoA oxygenase as followed at 390 nm. The solid black arrow indicates the time of enzyme addition. After addition of benzoyl-CoA (0.4 mM; broken arrow), the decrease in absorption was monitored. The reaction showed two phases, a slow oxidation phase of NADPH in presence of O2 until O2 was consumed, followed by a rapid reduction phase of FAD in the absence of O2. Open arrows indicate the reintroduction of O2 by shaking the sample with air.
The formation of products from benzoyl-CoA under the experimental conditions was probably due to spontaneous chemical reactions in the presence of Tris, dithioerythritol, and H2O2. Addition of 3% H2O2 to assays containing [14C]benzoyl-CoA (0.2 mM) in the absence of enzyme resulted in complete decay of the CoA thioester within 3 min into labeled benzoate and traces of a second, very polar labeled product.
Whole-cell regulation and distribution of putative benzoyl-CoA oxygenase in different bacterial species.
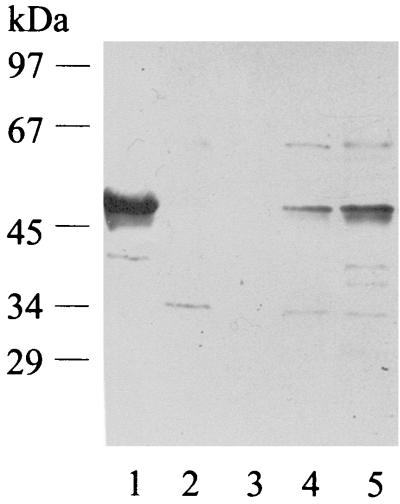
Benzoyl-CoA oxygenase activity was measured in extracts of cells grown on different substrates. The specific activity was approximately fivefold induced after growth with benzoate (57 nmol min−1 mg of protein−1) compared to cells grown on 3-hydroxybenzoate or phenylacetate (11 nmol min−1 mg of protein−1) and was even present in anaerobically grown cells (11 nmol min−1 mg of protein−1). The mutant, which was unable to utilize benzoate aerobically, still contained basic levels of this activity when grown on 3-hydroxybenzoate as growth-supporting substrate in the presence of benzoate (14 nmol min−1 mg of protein−1). The presence of a similar benzoyl-CoA oxygenase in A. evansii type strain KB740 and the newly isolated strain B5 (Fuchs, unpublished) and in two strains of Thauera, LG356 (Fuchs, unpublished) and S2 (39), was studied by Western blot analysis using polyclonal antibodies against the purified putative benzoyl-CoA oxygenase (Fig. 5) and by enzyme activity measurement. 16S rRNA sequencing of these strains indicated that they belong to either one of these two related genera of the beta group of proteobacteria (Fuchs, unpublished). Enzyme activity was measurable in strains KB740, B5, and LG356 (55, 27, and 17 nmol min−1 mg of protein−1, respectively) but was undetectable in strain S2. Polyclonal antibodies were raised against the enzyme of A. evansii and used for Western blot analysis of extracts of the different bacterial strains. A positive reaction with a band at approximately 50 kDa was observed only with the two Azoarcus strains (Fig. 5).
FIG. 5.
Western blot with polyclonal antibodies raised against purified benzoyl-CoA oxgenase. Lane 1, purified benzoyl-CoA oxygenase; lane 2, cell extract of Thauera strain LG356; lane 3, extract of Thauera strain S2; lane 4, extract of A. evansii B5; lane 5, extract of A. evansii KB740. Approximately 30 μg of protein was applied in the case of cell extracts, and 6 μg was used in the case of the purified enzyme.
3-Hydroxybenzoate 6-monooxygenase and gentisate 1,2-dioxygenase.
Extracts of benzoate-grown cells oxidized 3-hydroxybenzoate and 3-hydroxybenzoyl-CoA at rates of 80 and 50 nmol min−1 mg of protein−1, respectively. The oxidation of 3-hydroxybenzoate and 3-hydroxybenzoyl-CoA was NADH dependent. NADH oxidation after addition of 3-hydroxybenzoyl-CoA occurred only after a short lag phase that was not observed when the reaction was started by adding 3-hydroxybenzoate. Extracts rapidly catalyzed the hydrolysis of 3-hydroxybenzoyl-CoA into the free acid and CoA. In contrast, extracts of 3-hydroxybenzoate-grown cells oxidized 3-hydroxybenzoate at a much higher rate, accounting for more than 220 nmol min−1 mg of protein−1. The product of this monooxygenase reaction was identified as gentisate by HPLC. Cell extract (protein fraction precipitated at 70% ammonium sulfate saturation) of benzoate-grown cells oxidized gentisate at 70 nmol min−1 mg of protein−1, whereas the protein fraction of 3-hydroxybenzoate-grown cells contained at least threefold more activity.
Benzoate-induced proteins.
The results of the simultaneous adaptation and substrate-dependent oxygen consumption experiments suggested that benzoate-grown cells contained basic levels of the enzymes for 3-hydroxybenzoate metabolism, whereas 3-hydroxybenzoate-grown cells lacked all or some of the initial enzymes for benzoate metabolism. Therefore, the protein patterns of the two cell types were compared to identify benzoate-induced proteins. Benzoate-grown cells contained at least five and perhaps seven additional protein spots (data not shown). 3-Hydroxybenzoate-grown cells did not contain additional protein spots compared to benzoate-grown cells. Yet two spots, which also were present in benzoate-grown cells, were expressed to a greater extent. The N-terminal amino acid sequences of two benzoate-induced proteins were determined to be xINYSERIPNrxxL for a protein 1 with a molecular mass of about 50 kDa and qqAVANKPVAELvDYRtEPs for a protein 2 of about 60 kDa (x indicates unidentified amino acids; lowercase letters indicate uncertain amino acids).
Cloning and sequencing of the genes of major benzoate-induced proteins.
Primers derived from the determined N termini of the putative benzoyl-CoA oxygenase and its cyanogen bromide cleavage fragment were used in PCRs. A 1-kbp DNA fragment was obtained and used to screen a λ-ZAP Express gene library containing the chromosomal DNA of A. evansii. Three positive clones were obtained, and one of these clones, which contained an insert of 3 kbp, was sequenced. The nucleotide and deduced amino acid sequences of this DNA fragment showed the presence of two complete genes which were transcribed in the same direction. No potential open reading frames were found within 100 bp upstream or downstream of these genes.
The first gene (boxA, for benzoate oxidation) encoded a protein of 45,883 Da with a theoretical pI of 5.6 which had a deduced N-terminal amino acid sequence and internal sequence in very good agreement with that determined for the purified iron-sulfur flavoprotein component of the putative benzoyl-CoA oxygenase (Fig. 6A). A putative ribosome binding site (GGAG) was found 10 bases upstream of the potential ATG start codon of this gene. Near the N terminus of the boxA gene product were found two typical amino acid consensus sequences (CX2CX2CX3C) (30) (Fig. 6A), which most likely code for two [4Fe-4S] clusters. The second gene (boxB) was located at the 5′ end of and separated by 162 bp from boxA. It encoded a protein of 54,555 Da (473 amino acids) with a theoretical pI of 5.6. The boxB gene product had a deduced N-terminal amino acid sequence (MINYSERIPNNVNL), which agreed well with that determined for the benzoate-induced 50-kDa protein 1. Seven bases upstream of the ATG start codon of the boxB gene, a putative ribosome binding site (AGGAG) was found.
DISCUSSION
A. evansii metabolizes benzoate and 3-hydroxybenzoate both under aerobic and anaerobic conditions. Earlier results have suggested that the aerobic metabolism of benzoate involves the formation of benzoyl-CoA, 3-hydroxybenzoyl-CoA, gentisate, and maleylpyruvate mediated by benzoate-CoA ligase, two hypothetical monooxygenases termed benzoyl-CoA 3-monooxygenase and 3-hydroxybenzoyl-CoA 6-monooxygenase, and the ring-cleaving enzyme gentisate 1,2-dioxygenase. The postulated intermediary gentisyl-CoA was thought to be hydrolyzed to gentisate.
Role of benzoate-CoA ligase.
The role of an aerobically induced benzoate-CoA ligase isoenzyme in aerobic benzoate metabolism was corroborated. It was shown that whole cells rapidly formed benzoyl-CoA, which was consumed again (Zaar et al., submitted). The purified enzyme acted on benzoate and 2-aminobenzoate but not on 3-hydroxybenzoate (1). The role of the 3-hydroxybenzoate-CoA ligase activity observed in extracts of benzoate- and 3-hydroxybenzoate-grown cells remains enigmatic. Perhaps 3-hydroxybenzoate partly induces the enzymes required for anaerobic 3-hydroxybenzoate metabolism, including a 3-hydroxybenzoate-CoA ligase. We have recently studied the anaerobic 3-hydroxybenzoate metabolism in a related bacterium, T. aromatica (D. Laempe, M. Jahn, K. Breese, H. Schägger, and G. Fuchs, submitted for publication). This pathway involves a specific 3-hydroxybenzoate-CoA ligase.
Two pathways for benzoate and 3-hydroxybenzoate.
The following results are in support of the presence of two separate pathways for benzoate and 3-hydroxybenzoate. (i) Neither 3-hydroxybenzoyl-CoA nor gentisate could be detected in benzoate conversion by benzoate-adapted cells and in benzoyl-CoA conversion by cell extract (Zaar et al., submitted) (ii) 3-Hydroxybenzoate-grown cells started to consume benzoate only after a lag phase and contained higher activities of 3-hydroxybenzoate 6-monooxygenase and gentisate 1,2-dioxygenase than benzoate-grown cells. Vice versa, benzoate-grown cells metabolized 3-hydroxybenzoate and gentisate more slowly. (iii) Benzoate-grown cells exhibited at least five and perhaps seven additional protein spots in two-dimensional gel electrophoresis. In contrast, 3-hydroxybenzoate-grown cells did not exhibit major additional proteins. (iv) Benzoate- and 3-hydroxybenzoate-grown cultures had different colors; benzoate cultures were pale yellow, whereas 3-hydroxybenzoate cultures were brown.
3-Hydroxybenzoate indeed appears to be metabolized conventionally via gentisate, maleylpyruvate, and fumarylpyruvate involving 3-hydroxybenzoate 6-monooxygenase, gentisate 1,2-dioxygenase, and maleylpyruvate isomerase. The observed NADH oxidation upon addition of 3-hydroxybenzoyl-CoA is likely to be due to the combined action of two enzymes in cell extracts, an unspecific thioesterase hydrolyzing the CoA thioester followed by oxidation of the released 3-hydroxybenzoate by NADH-dependent 3-hydroxybenzoate 6-monooxygenase. This would explain the short lag phase in NADH oxidation observed after addition of 3-hydroxybenzoyl-CoA. It is likely that benzoate acted as a gratuitous inducer of the 3-hydroxybenzoate pathway.
Properties of putative benzoyl-CoA oxygenase.
The purified enzyme that catalyzes benzoyl-CoA-dependent NADPH oxidation was tentatively termed benzoyl-CoA oxygenase. The spectral properties of the purified enzyme pointed to a flavin- and FeS-containing protein. The flavin cofactor was FAD. The Fe-S content of the purified enzyme preparations varied depending on the method used for protein determination, with mean values of 14.4 mol of Fe and 15.6 mol of S per mol of homodimeric native protein. These values are close to the expected value of 16 mol per mol of native enzyme if four [4Fe-4S] centers were present. Also, the spectral properties concur this conclusion. With an average molar absorption coefficient per iron of 4,000 M−1 cm−1 at 400 nm (34), a molar absorption coefficient of the native enzyme of 64,000 M−1 cm−1 would be expected. Considering the presence of FAD, which also absorbs in this range, the observed value for the molar absorption coefficient of the native protein at 400 nm of about 75,000 M−1 cm−1 agrees well with the assumptions.
Nonproductive NAD(P)H oxidation.
The enzyme preparations catalyzed the benzoyl-CoA-dependent consumption of O2 and the stoichiometric oxidation of NADPH without hydroxylating benzoyl-CoA. In the presence of catalase, 50% of the consumed O2 was detected as hydrogen peroxide. It is well known that the catalysis by flavoprotein enzymes involves two half-reactions, (i) a reductive half-reaction in which the enzyme bound flavin is reduced and (ii) an oxidative half-reaction in which the reduced flavin is reoxidized to complete the catalytic cycle. The reduction of the flavin could be achieved by NADPH, as found in flavin-containing monooxygenases acting on anthranilate, phenol, and other aromatic substrates (14, 31, 35), or by reduced Fe-S centers in the protein, as in cytochrome P450 reductase, sulfite reductase, and nitrite reductase (35). Recently, a flavoprotein which hydroxylates 2-aminobenzoyl-CoA and reduces the enzyme-bound intermediate to a nonaromatic product has been studied in some detail (16). In the case of multicomponent dioxygenases, the reduced flavin donates electrons to a specific electron-accepting iron-sulfur protein. The latter in turn reduces the oxygenase component that catalyzes the dihydroxylation of the aromatic substrate (toluene, naphthalene, and benzoate dioxygenases [27]). In case of the absence of the oxygenase component, the reduced ferredoxin-like redox center reacts with O2, cytochrome c, or artificial electron acceptors as dichlorophenol indophenol, without hydroxylating the substrate. However, in contrast to the purified component of the benzoyl-CoA oxygenase, this unspecific reduction is not dependent on the presence of the substrate.
The presence of the benzoyl-CoA analogues 2-, 3-, and 4-hydroxybenzoyl-CoA also triggered H2O2 production from O2, albeit at a lower extent than benzoyl-CoA. Such pseudo-substrates (nonsubstrate effectors) that stimulate O2 consumption and nonproductive NAD(P)H oxidation have been found previously in flavin-containing monooxygenases (19, 25, 35). The effectors, like the native substrate, stimulate the rate of enzyme-flavin reduction by NAD(P)H by several orders of magnitude. The reduced enzyme-effector complex then reacts with O2 to form the flavin 4a-hydroperoxide, but since the effector is unsuitable for oxygenation, the 4a-hydroperoxyflavin decays to oxidized enzyme with the release of H2O2 (14).
The consumption of O2, the wasteful oxidation of NADH, and the production of H2O2 without hydroxylation of the substrate also have been reported for the flavoprotein component of some monooxygenases (14) such as 4-hydroxyphenylacetate 3-monooxygenase in P. putida and E. coli (4, 36). In this special case, even the substrate induced a nonproductive NADH oxidation when a subunit of the enzyme was lost. It is thought that the putative benzoyl-CoA oxygenase behaves in a similar manner. It is assumed that the Fe-S-flavoprotein is part of a more complex oxygenase. This Fe-S-flavoprotein, however, differs from the well-studied examples in several features, such as the unusual Fe-S clusters and the utilization of a CoA-activated aromatic substrate. Also, in contrast to the examples mentioned the nonproductive NADPH oxidation required the presence of FAD or FMN. The inability to hydroxylate benzoyl-CoA may be due to several reasons: the enzyme may become inactivated by H2O2, an essential subunit or cofactor is missing, or benzoyl-CoA is not the physiological substrate. However, the continuous removal of H2O2 by addition of catalase did not result in hydroxylation, suggesting that the enzyme was not inactivated by H2O2. Furthermore, benzoyl-CoA, but not 3-hydroxybenzoyl-CoA or benzoate, was indeed oxidized by cell extract (Zaar et al., submitted). The reduction of this activity in mutant cells that cannot utilize benzoate, and the presence of the boxA gene encoding this activity next to the boxB gene coding for benzoate-induced protein 1 are in favor of a role of these proteins in benzoyl-CoA metabolism.
Genes of benzoate-induced proteins.
Examination of the sequence of the 3-kbp DNA insert indicated that it contained two complete genes, designated boxA and boxB. The deduced N-terminal amino acid sequences of these two genes were in agreement with those determined for the Fe-S-flavoprotein and another benzoate-induced protein, confirming that we had isolated the corresponding genes. The gene product BoxA contained 13 cysteine residues; at the N terminus eight of these cysteine residues are clustered, giving a typical consensus sequence for two [4Fe-4S] clusters (Fig. 6A), confirming the experimental results (average of 7.2 mol of Fe and 7.8 mol of S/mol of 50-kDa subunit). Alignment searches (Fig. 6B) with the BLAST program for BoxA showed the following sequence identities with proteins which often contain conserved cysteine residues: 40% identity with many ferredoxin-NADP+ reductases (e.g., accession no. Q00598, Q55318, and CAA63961), 31% with NADPH-cytochrome P450 reductase (e.g., accession no. P14779), 29% with many inducible nitric oxide synthases (e.g., accession no. CAB46089), 23% with sulfite reductase (NADPH) flavoprotein (e.g., accession no. S56285), and 18% with phenol hydroxylase (accession no. AE001345). The experimental results indicated that BoxA contained 0.72 mol of FAD/mol of native enzyme. The loss of significant amounts of FAD during chromatography on ion-exchange resins is common with flavoproteins. It seems likely that the purified enzyme contains 1 or 2 mol of FAD/mol of native enzyme. In many—though not all—FAD-containing proteins, including nitric oxide synthase, cytochrome P450 reductase, and phenol hydroxylase, there are three conserved glycyl residues GXGXXG determining the FAD pyrophosphate binding segment (39). Despite the relatively high homology of BoxA with these proteins, no such FAD binding domain could be identified. Polyclonal antibodies against BoxA reacted only with Azoarcus strains, indicating that the system in other bacteria is significantly different.
BoxB showed weak sequence similarity to gene products (PaaA and PaaC or equivalents) of E. coli (13), P. putida (33), Bacillus halodurans (40), Deinococcus radiodurans (44), and A. evansii (M. Mohamed and G. Fuchs, unpublished data) which are involved in the aerobic metabolism of phenylacetate. This aerobic phenylacetate pathway also proceeds via the CoA thioester, and a multicomponent oxygenase acting on this intermediate has been postulated (13, 33). These similarities again suggest that the gene products BoxA and BoxB are functional components of a putative benzoyl-CoA oxygenase.
Distribution of the pathway.
None of the conventional aerobic pathways of benzoate metabolism could be detected for Bacillus stearothermophilus strains (11, 21). Unexpectedly, benzoate metabolism in the gram-negative A. evansii and in the gram-positive B. stearothermophilus seem to be similar. In both organisms, benzoyl-CoA is oxidized in an O2-and NADPH-dependent reaction in the absence of FAD or FMN to nonaromatic CoA thioesters. The earliest intermediate detected was 3,4-dehydroadipyl-CoA (Zaar et al., submitted). This points to an unprecedented aerobic benzoate pathway.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
Thanks are also due to Hans Heider, Universität Freiburg, for help and valuable discussions; to Juliane Alt-Mörbe, Laboratorium für DNA-Analytik, Freiburg, for DNA sequencing; and to Hermann Schägger, Universität Frankfurt, for determination of the N-terminal amino acid sequences.
REFERENCES
- 1.Altenschmidt U, Oswald B, Steiner E, Herrmann H, Fuchs G. New aerobic pathway via benzoyl-coenzyme A and 3-hydroxybenzoyl-coenzyme A in a denitrifying Pseudomonassp. J Bacteriol. 1993;175:4851–4858. doi: 10.1128/jb.175.15.4851-4858.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altenschmidt U, Oswald B, Fuchs G. Purification and characterization of benzoate-coenzyme A ligase and 2-aminobenzoate-coenzyme A ligases from a denitrifying Pseudomonassp. J Bacteriol. 1991;173:5494–5501. doi: 10.1128/jb.173.17.5494-5501.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anders J H, Kaetzke A, Kämpfer P, Ludwig W, Fuchs G. Taxonomic position of aromatic-degrading denitrifying Pseudomonas strains K172 and KB740 and their description as new members of genera Thauera, Thauera aromatica sp. nov., and Azoarcus, as Azoarcus evansiisp. nov., respectively, members of the beta subclass of Proteobacteria. Int J Syst Bacteriol. 1995;45:327–333. doi: 10.1099/00207713-45-2-327. [DOI] [PubMed] [Google Scholar]
- 4.Arunachalam U, Massey V, Vaidyanathan C S. p-Hydroxyphenylacetate-3-hydroxylase. J Biol Chem. 1992;267:25848–25855. [PubMed] [Google Scholar]
- 5.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 6.Beaucamp K, Kemp H U, Beuthler H-O. Coenzyme, Metabolite und sonstige biochemische Reagentien. In: Bermeyer H U, editor. Methoden der enzymatischen Katalyse. 3rd ed. Germany: Chemie Weinheim, Weinheim; 1974. pp. 558–590. [Google Scholar]
- 7.Beinert H, Thomson A J. Three-iron clusters in iron-sulfur proteins. Arch Biochem Biophys. 1983;222:333–361. doi: 10.1016/0003-9861(83)90531-3. [DOI] [PubMed] [Google Scholar]
- 8.Braun K, Gibson D T. Anaerobic degradation of 2-aminobenzoate (anthranilic acid) by denitrifying bacteria. Appl Environ Microbiol. 1998;48:102–107. doi: 10.1128/aem.48.1.102-107.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buder R, Fuchs G. 2-Aminobenzoyl-CoA monooxygenase/reductase, a novel type of flavoenzyme. Purification and some properties of the enzyme. Eur J Biochem. 1989;185:629–635. doi: 10.1111/j.1432-1033.1989.tb15159.x. [DOI] [PubMed] [Google Scholar]
- 10.Buder R, Ziegler K, Fuchs G, Lankau B, Ghisla S. 2-Aminobenzoyl-CoA monooxygenase/reductase, a novel type of flavoenzyme. Studies on the stoichiometry and the course of reaction. Eur J Biochem. 1989;185:637–643. doi: 10.1111/j.1432-1033.1989.tb15160.x. [DOI] [PubMed] [Google Scholar]
- 11.Clark J S, Buswell J A. Catabolism of gentisic acid by two strains of Bacillus stearothermophilus. J Gen Microbiol. 1979;112:191–195. [Google Scholar]
- 12.Coligan J E, Dunn B M, Ploegh H L, Speicher D W, Wingfield P T, editors. Current protocols in protein science. New York, N.Y: John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 13.Ferrandez A, Minambres B, Garcia B, Olivera E R, Luengo J M, Garcia J L, Diaz E. Catabolism of phenylacetic acid in Escherichia coli. Characterization of a new aerobic hybrid pathway. J Biol Chem. 1998;273:25974–86. doi: 10.1074/jbc.273.40.25974. [DOI] [PubMed] [Google Scholar]
- 14.Ghisla S, Massey V. Mechanism of flavoprotein-catalyzed reactions. Eur J Biochem. 1989;181:1–17. doi: 10.1111/j.1432-1033.1989.tb14688.x. [DOI] [PubMed] [Google Scholar]
- 15.Gross G G, Zenk M H. Darstellung und Eigenschaften von Coenzym A-Thioestern substituierter Zimtsäuren. Naturforschung. 1966;21b:683–690. [Google Scholar]
- 16.Hartmann S, Hultschig C, Eisenreich W, Bacher A, Ghisla S. NIH shift in flavin-dependent monooxygenation: mechanistic studies with 2-aminobenzoyl-CoA monooxygenase/reductase. Proc Natl Acad Sci USA. 1999;96:7831–7836. doi: 10.1073/pnas.96.14.7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heider J, Fuchs G. Anaerobic metabolism of aromatic compounds. Eur J Biochem. 1997;243:577–596. doi: 10.1111/j.1432-1033.1997.00577.x. [DOI] [PubMed] [Google Scholar]
- 18.Hutber G N, Ribbons D W. Involvement of coenzyme A esters in the metabolism of benzoate and cyclohexanecarboxylate by Rhodopseudomonas palustris. J Gen Microbiol. 1983;129:2413–2420. [Google Scholar]
- 19.Imbeault N Y, Powlowski J B, Colbert C L, Bolin J T, Eltis L D. Staedy-state kinetic characterization and crystallization of a polychlorinated biphenyl-transforming dioxygenase. J Biol Chem. 2000;275:12430–12437. doi: 10.1074/jbc.275.17.12430. [DOI] [PubMed] [Google Scholar]
- 20.Jones C N, Cooper R A. Catabolism of 3-hydroxybenzoate by the gentisate pathway in Klebsiella pneumoniaeM5a1. Arch Microbiol. 1990;154:489–495. doi: 10.1007/BF00245233. [DOI] [PubMed] [Google Scholar]
- 21.Kiemer P, Tshisuaka B, Fetzner S, Lingens F. Degradation of benzoate via benzoyl-coenzyme A and gentisate by Bacillus stearothermophilusPK1, and purification of gentisate 1,2-dioxygenase. Biol Fertil Soils. 1996;23:307–313. [Google Scholar]
- 22.Laempe D, Jahn M, Fuchs G. 6-Hydroxycyclohex-1-ene-1-carbonyl-CoA dehydrogenase and 6-oxocyclohex-1-ene-1-carbonyl-CoA hydroxylase, enzymes of the benzoyl-CoA pathway of anaerobic aromatic metabolism in the denitrifying bacterium Thauera aromatica. Eur J Biochem. 1999;263:420–429. doi: 10.1046/j.1432-1327.1999.00504.x. [DOI] [PubMed] [Google Scholar]
- 23.Lankau B, Ghisla S, Buder R, Ziegler K, Fuchs G. 2-Aminobenzoyl-CoA monoxygenase/reductase, a novel type of flavoenzyme. Identification of the products. Eur J Biochem. 1990;191:365–371. doi: 10.1111/j.1432-1033.1990.tb19131.x. [DOI] [PubMed] [Google Scholar]
- 24.Lankau B, Vock P, Massey V, Fuchs G, Ghisla S. 2-Aminobenzoyl-CoA monooxygenase/reductase. Evidence for two distinct loci catalyzing substrate monooxygenation and hydrogenation. Eur J Biochem. 1995;230:676–685. [PubMed] [Google Scholar]
- 25.Lee K. Benzene-induced uncoupling of naphthalene dioxygenase activity and enzyme inactivation by production of hydrogen peroxide. J Bacteriol. 1999;181:2719–2725. doi: 10.1128/jb.181.9.2719-2725.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovenberg W, Buchanan B B, Rabinowitz J L. Studies on the chemical nature of clostridial ferredoxin. J Biol Chem. 1963;238:3899–3913. [PubMed] [Google Scholar]
- 27.Manson J R, Cammack R. The electron-transport proteins of hydroxylating bacterial dioxygenases. Annu Rev Microbiol. 1992;46:277–305. doi: 10.1146/annurev.mi.46.100192.001425. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Blanco H, Reglero A, Rodriguez-Aparicio L B, Luengo J M. Purification and biochemical characterization of phenylacetyl-CoA ligase from Pseudomonas putida. A specific enzyme for the catabolism of phenylacetic acid. J Biol Chem. 1990;256:7084–7090. [PubMed] [Google Scholar]
- 29.Mohamed M E. Biochemical and molecular characterization of phenylacetate-coenzyme A ligase, an enzyme catalyzing the first step in aerobic metabolism of phenylacetic acid in Azoarcus evansii. J Bacteriol. 2000;182:286–294. doi: 10.1128/jb.182.2.286-294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mortenson L E, Nakos G. Bacterial ferredoxins an/or iron-sulfur proteins as electron carriers. In: Lovenberg W, editor. Iron-sulfur proteins as electron carriers. I. New York, N.Y: Academic Press; 1973. pp. 37–64. [Google Scholar]
- 31.Neujahr H Y, Kjellen K G. Phenol hydroxylase from yeast. J Biol Chem. 1978;253:8835–8841. [PubMed] [Google Scholar]
- 32.Niemetz R, Altenschmidt U, Brucker S, Fuchs G. Benzoyl-coenzyme A 3-monooxygenase, a flavin-dependent hydroxylase. Purification, some properties and its role in aerobic benzoate oxidation via gentisate in a denitrifying bacterium. Eur J Biochem. 1995;227:161–168. doi: 10.1111/j.1432-1033.1995.tb20372.x. [DOI] [PubMed] [Google Scholar]
- 33.Olivera E R, Minambres B, Garcia B, Muniz C, Moreno M A, Ferrandez A, Diaz E, Garcia J L, Luengo J M. Molecular characterization of the phenylacetic acid catabolic pathway in Pseudomonas putidaU: the phenylacetyl-CoA catabolon. Proc Natl Acad Sci USA. 1998;95:6419–6424. doi: 10.1073/pnas.95.11.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otaka E, Ooi T. Examination of protein sequence homologies. IV. Twenty-seven bacterial ferredoxins. J Mol Evol. 1987;26:257–267. doi: 10.1007/BF02099857. [DOI] [PubMed] [Google Scholar]
- 35.Powlowski J B, Dagley S, Massey V, Ballou D P. Properties of anthranilate hydroxylase (deaminating), a flavoprotein from Trichosporon cutaneum. J Biol Chem. 1987;262:69–74. [PubMed] [Google Scholar]
- 36.Prieto M A, Garcia J L. Molecular characterization of 4-hydroxyphenylacetate 3-hydroxylase of Escherichia coli. J Biol Chem. 1993;269:22823–22829. [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 38.Schennen U, Braun K, Knackmuss H J. Anaerobic degradation of 2-fluorobenzoate by benzoate-degrading, denitrifying bacteria. J Bacteriol. 1985;161:321–325. doi: 10.1128/jb.161.1.321-325.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seyfried B, Tschech A, Fuchs G. Anaerobic degradation of phenylacetate and 4-hydroxyphenyacetate by denitrifying bacteria. Arch Microbiol. 1991;155:249–255. doi: 10.1007/BF00252207. [DOI] [PubMed] [Google Scholar]
- 40.Takami H, Nakasone K, Ogasawara N, Hirama C, Nakamura Y, Masui N, Fuji F, Takaki Y, Inoue A, Horikoshi K. Sequencing of three lambda clones from the genome of alkaliphilic Bacillussp. strain C-125. Extremophiles. 1999;3:29–34. doi: 10.1007/s007920050096. [DOI] [PubMed] [Google Scholar]
- 41.Torres R A, Bruice T C. Theoretical investigation of the (1,2)-sigmatropic hydrogen migration in the mechanism of oxidation of 2-aminobenzoyl-CoA by 2-aminobenzoyl-CoA monooxygenase/reductase. Proc Natl Acad Sci USA. 1999;96:14748–14752. doi: 10.1073/pnas.96.26.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vitovski S. Phenylacetate-coenzyme A ligase is induced during growth on phenylacetic acid in different bacteria of several genera. FEMS Microbiol Lett. 1993;108:1–5. doi: 10.1016/0378-1097(93)90477-j. [DOI] [PubMed] [Google Scholar]
- 43.Wierenga R K, Terpstra P, Hol W G. Prediction of the occurrence of the ADP-binding beta alpha beta-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986;187:101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- 44.White O, Eisen J A, Heidelberg J F, Hickey E K, Peterson J D, Dodson R J, Haft D H, Gwinn M L, Nelson W C, Richardson D L, Moffat K S, Qin H, Jiang L, Pamphile W, Crosby M, Shen M, Vamathevan J J, Lam P, McDonald L, Utterback T, Zalewski C, Makarova K S, Aravind L, Daly M J, Fraser C M, et al. Genome sequence of the radioresistant bacterium Deinococcus radioduransR1. Science. 1999;286:1571–1577. doi: 10.1126/science.286.5444.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zehr B D, Savin T J, Hall R E. A one-step, low background Coomassie staining procedure for polyacrylamide gels. Anal Biochem. 1989;182:157–159. doi: 10.1016/0003-2697(89)90734-3. [DOI] [PubMed] [Google Scholar]
- 46.Ziegler K, Buder R, Winter J, Fuchs G. Activation of aromatic acids and aerobic 2-aminobenzoate metabolism in a denitrifying Pseudomonasstrain. Arch Microbiol. 1989;151:171–176. [Google Scholar]