Abstract
Due to their natural availability, biocompatibility, biodegradability, nontoxicity, flexibility, as well as improved structural and functional characteristics, pectin and pectin-based nanocomposites have become an interesting area of numerous researchers. Pectin is a polysaccharide that comes from plants and is used in a variety of products. The significance of pectin polysaccharide and its modified nanocomposites in a number of applications has been shown in numerous reviews. On their uses in pharmaceutical and medication delivery, there are, however, few review publications. The majority of papers on pectin polysaccharide do not structure their explanations of drug distribution and medicinal application. The biological application of pectin nanocomposite is also explained in this review, along with a recent publication. As a result, the goal of this review was in-depth analysis to summarize biological application of pectin and its modified nanocomposites. Due to their exceptional physicochemical and biological characteristics, pectin and its nanocomposites are remarkable materials for medicinal applications. In addition to enhancing the immune system, controlling blood cholesterol, and other things, they have been shown to have anticancer, antidiabetic, antioxidant, anti-inflammatory, immunomodulatory, and antibacterial properties. Because of their biocompatibility and properties that allow for regulated release, they have also received a lot of interest as drug carriers in targeted drug delivery systems. They have been used to administer medications to treat cancer, inflammation, pain, Alzheimer's, bacteria, and relax muscles. This review found that pectin and its derivatives have better drug delivery efficiency and are viable candidates for a wide range of medicinal applications. It has been advised to conduct further research on the subject of toxicity in order to produce commercial formulations that can serve as both therapeutic agents and drug carriers.
Keywords: Pectin, Polysaccharide, Nanocomposites, Pharmaceuticals, Drug delivery
Pectin; Polysaccharide; Nanocomposites; Pharmaceuticals; Drug delivery.
1. Introduction
Because of their abundance and natural availability, biopolymers are currently receiving a lot of interest in the food and pharmaceutical industries [1, 2]. Due to its intrinsic qualities like as biocompatibility, biodegradability, nontoxicity, flexibility, and improved structural and functional aspects, bio-nanocomposites have become the topic of substantial research [3, 4, 5, 6]. Pectin, chitosan, alginate, cellulose, agarose, guar gum, agar, carrageenan, and gelatin are examples of biopolymers and currently hot area research [4, 5]. Biopolymers are particularly intriguing because they are renewable, have a cheap production cost, and have a wide range of pharmaceutical applications [4, 6]. There are three main classes of biopolymers owing to their universal occurrence and abundance: (i) polynucleotides, (ii) polypeptides/poly amino acids, and (iii) polysaccharides [7, 8].
Pectin is a polysaccharide found in plants' cell walls that helps them to grow and extend their cells [5, 9]. Pectin is derived from plants and can be utilized as a bioplastic material for a range of applications [10, 11]. It is a carbohydrate polymer derived mostly from natural sources that serves as a structural component of plants' cell walls [12, 13]. Pectin is a biocompatible polysaccharide with biological activity that can take on many shapes depending on the source or extraction method [14, 15]. It is a poly α 1–4-galacturonic acid containing carboxylic acid residues that have been methylated to various degrees [8, 16]. The most critical parameter affecting pectin's solubility and gel forming characteristics is the degree of esterification of galacturonic acid residues [14, 17]. Since it has low cost, biodegradability, water solubility, and non-toxicity, pectin can be utilized for a variety of reasons [16, 17]. Due to outstanding thermal, mechanical, and biodegradable qualities, biopolymer-based nanocomposites including pectin nanocomposites have attracted a lot of attention in recent years [18, 19]. Pectin is employed in a number of pharmaceutical, cosmetic, food, and biological applications due to its biocompatibility, biodegradability, and non-toxicity [20]. In addition, pectin based bio nanocomposites have various applications in tissue engineering [21], gene transfer, wound healing, and dressings [22, 23], drug delivery [24], and cancer targeting [25]. In the cosmetics sector, it is utilized as an emulsifier. In oral formulations for drug administration to the colon, it is frequently employed in conjunction with kaolin [21]. Furthermore pectin are used to form edible films, and plasticizers [10, 11]. Pectin can have a range of structures depending on the source and extraction procedure [26, 27]. Numerous studies and review reports have demonstrated that pectin and its modified nanocomposites (NCs) are used for a variety of applications. However, there are a little number of reports on their pharmaceutical and drug delivery applications. Therefore, the aim of this review is to compile reports on the pharmaceutical applications of pectin and its modified NCs.
2. The review methodology
2.1. The study design
A comprehensive review study design were adopted to gather the general information regarding pectin polysaccharide and its modified NCs, including pharmacological and DDS applications, was assembled in this review.
2.2. The search strategy
The relevant sources were retrieved by using search engines such as Google scholar and PubMed. Particular keywords that helps to search related study phrases and synonyms such as biopolymer, pectin, polymer nanocomposite, pharmacological use/drug delivery application, biological properties and functionalization of polysaccharide, modification of biopolymer etc. were adopted.
2.3. Inclusion and exclusion criteria
Studies reporting pharmaceutical and/or drug delivery application of pectin and its modified NCs were as the other reports related biopolymer were excluded in this review. Studies published in languages other than English were omitted.
2.4. Study selection
A short study of the topics, abstracts, and conclusions of the sources was done to determine their eligibility after a relevant sources that helps to the review were gathering. The published paper/review article used as sources in these review were carefully examined in order to prepare this review paper.
2.5. Software used
Mendeley Desktop reference management software was utilized to generate references and citations for this review.
3. Pharmaceutical application of pectin and structurally modified pectin
Pectin and structurally modified pectin are the most promising pharmaceutical and medicinal applications as well as used for drug delivery system (DDS) [28, 29]. Increasing interest in pectin is due to its easy availability in nature and increasing availability in the pharmaceutical industry [30]. Due to their exceptional physical, chemical, and biological capabilities, biotechnologists and microbiologists have developed various types of biopolymers for specific applications in the biomedical and pharmaceutical industries [31, 32]. These functions make pectin biopolymer a noteworthy product for pharmaceutical and biological applications [26, 33]. The pharmacological properties of pectin and its modified NCs, which include anticancer, antidiabetic, antioxidant, anti-inflammatory, antibacterial, immune system strengthening, and blood cholesterol regulating properties, are discussed in the following section.
3.1. Pectin in the cancer treatment
The incidence of cancer is increasing owing to metastases and tumor cell medication tolerance, even though a wide range of scientific investigations have been stepped up to tackle this disease [25, 34]. Pectin has been shown in studies to play a role in the prevention of metastasis, which is especially true of pectin that has been broken down into smaller fragments with a lower molecular weight that the body can absorb [25, 34]. Pectin that has been altered by the use of chemicals, heat, radiation, and enzymes has stronger anticancer properties than unaltered pectin [26, 35]. The proliferation and migrating of colon carcinoma cells have been shown to be inhibited by functionally engineered pectin that contains neutral sugar sequences with a low degree of branching and is rich in galactose [36].
3.2. Pectin in the regulation of blood cholesterol level
Highly viscous pectin can have a greater impact on inhibiting the blood cholesterol level by disrupting the micelle formation, slowing down bile acid diffusion, blocking the absorption of micelles carrying cholesterol, and reducing bile acid diffusion rates [37, 38]. The cholesterol-lowering properties of citrus peels are likely due to pectin from the peels [39]. Without making any dietary changes, pectin consumption of at least 6 g/day can lower cholesterol levels in people with normal or elevated lipid levels, lowering the risk of coronary heart disease [37, 40]. The level of plasma triglycerides remained unaltered. Pectin from prickly pears has been found to affect guinea pigs' hepatic cholesterol homeostasis. Hepatic cholesterol homeostasis has been reported in guinea pigs to be altered by intake of pectin from prickly pear [37, 38]. The capacity of pectin varieties utilized to generate a viscous gastro-intestinal content, which is demonstrated to influence substantially the precise molecular composition, is proven to have a significant impact on the lowering of cholesterol and may also affect blood glucose levels [41].
3.3. Antioxidant activity
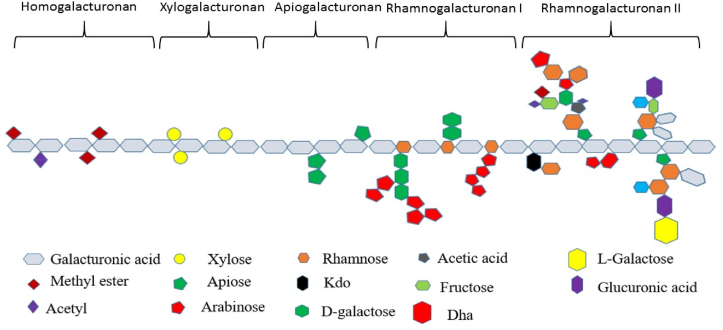
Pectin is a reliable antioxidant that possesses the ability to scavenge free radicals and surpass synthetic substances in the subject of health concerns. Pectin RG-I (rhamnogalacturonan I) exhibited good antioxidant activity as measured by the capture of DPPH- and ABTS- + radicals. Pectin having long HG-1 (homogalacturonan) segments alternated with RG-I segments, arabinogalactan type I and arabinanas side chains (Figure 1) has been found to provide effective protection against the oxidative action of intestinal stress [17]. The hydroxyl groups of polysaccharides in pectin can show good antioxidant activity when the viscosity is not too high [42]. The ferric reducing ability of plasma (FRAP) assay and DPPH scavenging activity [43]. The nano-scaled Fe3O4 with pectin nanoparticles had effective antioxidant activity based on the IC50 value. Recent nanoparticles appear to have an anti-human liver cancer effect due to their antioxidant properties [44].
Figure 1.
Structural characteristics of pectin molecules. There are different structural characteristics of pectin molecules including Homogalacturonan, Xylogalacturonan, Apiogalacturonan, Rhamnogalacturonan I and Rhamnogalacturonan.
3.4. Antidiabetic activity
Diabetes mellitus is a primary metabolic condition affecting 382 million people globally. The most common form of diabetes, type 2 diabetes mellitus, affects over 90% of people with the disease [45, 46]. Despite being often used to treat diabetes, artificial antidiabetic drugs have the potential to have negative side effects. As a result, natural compounds have gained a lot of attention in the fight against diabetes [45]. Citrus pectin has potential antidiabetic properties, which were studied in diabetic rats and effective in treating type 2 diabetes mellitus brought on by a modest dose of streptozotocin and a high-fat diet. It's also been demonstrated that citrus pectin improves hyperlipidemia, hepatic glycogen content, and glucose tolerance in diabetic rats [17]. According to earlier research [47], methoxylated apple pectin might be used as a functional ingredient to lower insulin resistance. Furthermore, soybean pectin improved glucose and insulin response in people of normal weight [46]. Based on earlier reported results, it is estimated that pectin and modified pectin have the potential to reduce blood sugar levels and insulin resistance.
3.5. Antimicrobial activity
S. aureus and E. coli are both significantly resistant to the antibacterial effects of pectin. The capacity of pectin-linoleate and pectin-oleate to 50–70% inhibit the growth of the selected bacteria was demonstrated. They have the greatest antibacterial power against S. aureus [42]. Additionally, pectin exhibits strong antibacterial activity against various strains of clinically isolated Helicobacter pylori [48]. Additionally, intriguing antibacterial activity has been seen in nanoparticles and nanocomposites that used pectin as the reducing and capping agent throughout production. The antibacterial activity of pectin-Ag NPs was also revealed against S. aureus and E. coli. Pectin-cadmium sulfide nanocomposite and pectin-based zirconium (IV) silicophosphate nanocomposite (Pc/ZrSPNC) were both found to exhibit strong antimicrobial activity against E. coli and S. aureus, respectively [17]. The antimicrobial and antiviral activities of pectin and structurally modified pectin have been the subject of numerous investigations, which are compiled in Table 1.
Table 1.
Antimicrobial activity of pectin based NCs.
| Pectin and its nanocomposites | Types of microbes | Microbial strains | Reference |
|---|---|---|---|
| Pectin-oleate, pectin-linoleate, and pectin palmitate | Bacteria | S. aureus and E. coli | [17] |
| Ag NPs using pectin as the reducing and capping | |||
| Pectin-based zirconium (IV) silicophosphate NCs | |||
| Pectin–CdS NCs | E. coli | ||
| pectin | Bacteria | H. pylori | [48] |
| Pectin-Amphotericin B imine and amine conjugates | Fungus | C. albicans and A. fumigatus | [49] |
| Pectin | Fungus | Colletotrichum gloeosporioides, Fusarium oxysporum, Sclerotinia sclerotiorum and Mucor sp. | [50] |
| Pectin | Fungus | C. albicans and S. cerevisiae | [51] |
| Pectin | Virus | Herpesvirus type 1 (HSV -1) poliovirus strain (PV). | [52] |
| Pectin methylesterase | Virus | Tobacco Mosaic Virus | [53] |
| Pectin | Virus | Hepatitis B Virus | |
| Pectin | Virus | Herpes Simplex Virus Type 2 | |
| Pectin | Virus | SARS-CoV-2 |
3.6. Anti-inflammatory and immunomodulatory activity
As models of inflammation, endotoxin shock, acetic acid-induced colitis, and blood leukocyte production of cytokines in response to lipopolysaccharide have all been used [54]. Intestinal inflammation can be reduced by low methyl-esterified pectin, but systemic and local inflammation can be diminished by high methyl esterified pectin [54, 55]. It has been shown that pectin from S. dendroideum leaves affects the release of pro- and anti-inflammatory cytokines by macrophages [17]. Citrus pectin inhibits chemotaxis and phagocytosis, the two main inflammatory processes of chicken monocytes, pointing to potential anti-inflammatory effects [56]. The immunologically stimulating effects of pectin may be due to these components [57]. Pectin were created from an aqueous extract of mulberry fruits that showed immune modulatory activity by improving macrophage function [58]. Furthermore, Rubus chingii Hu pectin can be utilized as a dietary supplement for the treatment of intestinal inflammation and also has a strong inhibitory action on the mRNA level [55]. Pectin polysaccharides exhibit good anti-inflammatory activity and can be improved for the production of anti-inflammatory agents [55, 58]. Further pharmaceutical applications of pectin and its modified NCs were summarized in Table 2.
Table 2.
Pharmaceutical applications of pectin and structurally modified pectin.
| Types of pectin | Sources of pectin | Pharmaceutical activity | Results | References |
|---|---|---|---|---|
| P/guar gum-ZnO | Cyamposis tetragonotobus (Seed) | Immuno-stimulator |
|
[59] |
| Pectin | Sambuci flos |
|
[60] | |
| Pectin | Citrus pectin (Commercial) | Treatment of neurological diseases |
|
[61] |
| Fe3O4@p- NPs | Orange peel | Antioxidant and anti-liver cancer |
|
[44] |
| Fe3O4/P NPs | Apple pomace |
|
[62] | |
| P/Ag and p/Au–Ag NCs | Citrus pectin (Commercial) |
|
[25] | |
| P/Tannic acid NCs | Apple pomace and citrus fruits | Anticancer activity |
|
[63] |
| P/Guar Gum/Zinc Oxide NCs | Commercial Citrus pectin |
|
[64] | |
| P/Guar Gum NCs | Citrus fruit peel | Control plasma hyperglycemia and hypercholesterolemia |
|
[65] |
| Pectin | Passiflora glandulosa cav | Reduce blood glucose |
|
[66] |
| Ginseng pectin (GP) | Citrus pectin (Commercial) | Neuroprotective effects |
|
[67] |
| Pectin | Citrus pectin (Commercial) |
|
[68] | |
| Pectin | Citrus sinensis (L.) Osbeck peel | Antidiabetic |
|
[69] |
| Pectin | Citrus peel |
|
[66] | |
| Pectin | Citrus fruit peel | Glycogen regulation |
|
[70] |
| P/galectin-3 carbohydrate | Citrus pectin (Commercial) | Nephroprotective activity |
|
[71] |
| Pectin | Citrus pectin (Commercial | Immuno modulatory activity |
|
[72] |
| P/Au NCs | Citrus pectin (Commercial |
|
[73] | |
| P/ZnO NPs | Pomelo and Citron peel | Antimicrobial activity |
|
[74] |
| P/Ag NPs | Citrus pectin (Commercial) |
|
[75] | |
| Pectin | Citrus pectin (Commercial | Hepatoprotective activity |
|
[76] |
| Pectin | Citrus | Antitumor activity |
|
[77] |
| Pectin nano-Se | Citrus |
|
[78] | |
| Pectin | Lonicera japonica |
|
[79] | |
| Rhamnogalacturonan-I (RG-I) | Potato pectin⁃ |
|
[80] | |
| Pectic acid | Apple⁃ |
|
[81] | |
| Rhamnogalacturonan-I (RG-I), Rhamnogalacturonan-I I (RG-II) | Persimmon leaves⁃ |
|
[82] | |
| High-methoxyl Homogalacturonan | Hippophae rhamnoide⁃ |
|
[83] | |
| Pectin | Portulaca oleracea L. C | Antiviral |
|
[51] |
| Pectin | Saussurea laniceps |
|
[84] | |
| Pectin | Inga spp⁃ |
|
[52] | |
| Pectin | Citreous peel⁃ |
|
[85] |
Key: P = pectin, NPs = nanoparticles, NCs = nanocomposites.
4. Pectin and its modified NCs for drug delivery applications
DDS is a formulation that allows a pharmaceutical agent to reach its target site of action while avoiding non-target cells [21]. In order for a pharmaceutical ingredient's therapeutic agent to be released in a controlled manner, DDS are devices that are meant to carry the substance throughout the body. The active ingredient is less likely to be disrupted physically, chemically, or enzymatically when the molecules are enclosed in a protective shell-like structure. As a result, not only is the active compound's bioavailability increased, but also the adverse effects linked to systemic, non-specific distribution are reduced. The number of dosages needed during therapy is decreased by nano-encapsulating bioactive chemicals, and it's possible that the drug will also be physically protected while being stored before being used for controlled drug release [86]. Natural polymers like pectin have gotten a lot of attention as drug carriers. Because of its biocompatibility, health advantages, nontoxicity, and biodegradability, pectin has been employed to make DDSs [86, 87]. It has a low production cost and is widely available [87]. Protective agents against enzymatic proteolysis have been found in a variety of polymers, including pectin. Because pectin stabilizes polypeptide drugs, they stay intact in the stomach and small intestine before being digested by colonic bacteria, resulting in drug molecule release [88]. Pectin has been employed in a variety of formulations, including hydrogels, films, microspheres, and nanoparticles, to target various medicines [87]. However, pectin's rich hydrophilic functional groups, including the hydroxyl, free carboxyl, and methyl ester groups, result in pectin's significant swelling qualities, limiting its potential use in DDS. Pectin formulations have the potential to expand under physiological conditions, resulting in premature drug release [88]. As a result, researchers have attempted to alter the structure of pectin in order to create pectin-based hybrid and composite materials [86, 88] using various chemical and physical approaches [87].
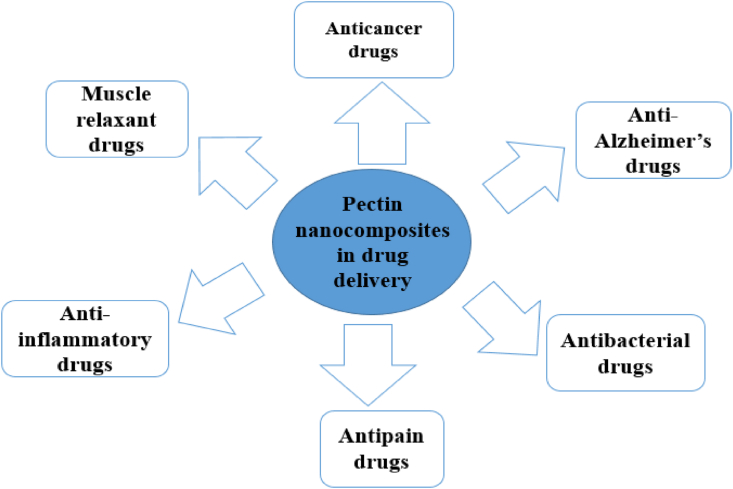
Polymer nanoparticles, such as pectin-based nanoparticles, have sparked attention in the biomedical area as gene/DDS due to their biocompatibility and controlled release [24]. Various interactions, such as hydrophobic interaction, electrostatic interaction, and covalent bonding, are commonly used to load medicines into nanoparticles [24, 89]. Drugs or biomolecules can be entrapped within the nanoparticles' internal structures, and/or they can be absorbed onto the nanoparticles' external surfaces [89]. Nanomaterials containing drugs or genes can enter cells by endocytosis rather than diffusion, and because of their small size, they can easily accumulate in target cells. As a result, nanoparticles as a delivery mechanism can reduce drug or gene loss while also increasing delivery efficiency [24]. Furthermore, nanoparticle-based DDS have a number of notable advantages, including the ability to easily pass through the smallest capillary vessels due to their ultra-small volume and avoid rapid clearance by phagocytes, extending their time in the blood stream; the ability to penetrate cells and tissue gaps to reach target organs; they have controlled release qualities [89]. Pectin-based nanomaterials are one strategy of delivering drugs to the colon known as colon targeted DDS. Pectin's swelling properties, as well as its capacity to withstand gastrointestinal degradation, have made it a popular carrier for colon-specific medication delivery. The biodegradability and gel-forming nature of this polysaccharide are the characteristics that drive its selection as a carrier for specific medication delivery [90]. Pectin-based NCs have also been studied for transdermal DDS, in addition to colon-targeted DDS. As shown in Table 3, numerous studies have been undertaken on the application of pectin-based nanocomposites for drug delivery, including anticancer, anti-inflammatory, antipain, anti-alzheimer, antibacterial, muscle relaxant, etc., as depicted in Figure 2.
Table 3.
Summary of applications of pectin based nanocomposites for delivery of different drugs.
| Delivery of anticancer drugs | ||||
|---|---|---|---|---|
| Pectin nanocomposites | Delivered drugs | Results | References | |
| Poly (acryl-amidoglycolic acid-covinyl caprolactam)/P/Ag NCs. | 5-Fluorouracil |
|
[22] | |
| P/magnetic graphene oxide nanohybrid. | Paclitaxel |
|
[91] | |
| P/tannic acid NCs. | 5-fluorouracil, gemcitabine, and irinotecan |
|
[92] | |
| P-nano-cell of core shell structure. | Doxorubicin |
|
[93] | |
| P/Poly (N,N-dimethylacrylamide-stat-4-formylphenyl acrylate) hydrogel | Doxorubicin |
|
[94] | |
| P-Graft-Copolymers with poly (vinyl alcohol) and their NCs. | 5-fluorouracil |
|
[95] | |
| P-/lactic acid-co- methacrylic acid hydrogels | Oxaliplatin |
|
[96] | |
| P-Based NPs | 5-fluorouracil |
|
[97] | |
| P-based hollow Nano capsules | Doxorubicin hydrochloride |
|
[98] | |
| P-based magnetic Nanocariers | Oxaliplatin |
|
[99] | |
| Delivery of anti-Alzheimer's drugs | ||||
| P/Ag NCs film | Donepezil |
|
[100] | |
| P/ZnO hybrid NCs | Donepezil |
|
[101] | |
| Delivery of antibacterial drugs | ||||
| P/polyvinylpyrrolidone,3-aminopropyl (diethoxy) methyl silane and sepiolite clay) hydrogel | Ceftriaxone sodium |
|
[102] | |
| P/Chitosan Polyelectrolyte NCs | Nisin |
|
[103] | |
| Delivery of antipain drugs | ||||
| P-based hydrogels | Ibuprofen |
|
[104] | |
| Methylcellulose/p/Montmorillonite NCs films | Ketorolac tromethamine |
|
[105] | |
| Delivery of anti-inflammatory drugs | ||||
| P-coated chitosan– layered double hydroxide bio- NCs beads | 5-aminosalicylic acid |
|
[106] | |
| P/Zn/Alginate Core-Shell Beads | Betamethasone |
|
[107] | |
| Delivery of muscle relaxant drugs | ||||
| P-coated baclofen-layered zinc hydroxide nanohybrid | Baclofen |
|
[107] | |
| P/chitosan/Eudragit®RS mixed-film coating | Theophylline |
|
[108] | |
| Delivery of other drugs/supplements | ||||
| P/Cu-based metal–organic framework nanofiber | Folic acid |
|
[109] | |
| P/hydroxyethyl methacrylate hydrogel NCs cross-linked with TiO2 | Vitamin B12 |
|
[110] | |
| P/chitosan hydrogels | Hesperidin |
|
[111] | |
| P/chitosan core–shell NPs | Resveratrol |
|
[112] | |
Key: P = pectin, NPs = nanoparticles, NCs = nanocomposites.
Figure 2.
Overview of applications of pectin nanocomposites in drug delivery systems.
5. Conclusion
Many in vitro and/or in vivo studies have revealed that pectin polysaccharide and pectin-based modified NCs have various pharmaceutical activities such as anticancer, antidiabetic, antioxidant, anti-inflammatory, antimicrobial, immune system boosting, blood cholesterol regulation, and so on. In addition to being utilized as active therapeutic agents, pectin-based nanocomposites have been employed in drug delivery systems, including the delivery of anticancer, anti-inflammatory, antipain, anti-alzheimer, antibacterial, muscle relaxant, and other drugs. According to the findings of this review, pectin and its modified NCs could be potential therapeutic agents and drug carriers in the near future if adequate toxicity studies are conducted to confirm their safety for human cells.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The data was collected from the internet, journals and books. All the statements taken from all the sources are cited in the proper manner.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Babu R.P., O’Connor K., Seeram R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013;2(1):8. doi: 10.1186/2194-0517-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes L.P., Paschoalin V.M.F., Del Aguila E.M. Chitosan nanoparticles: production, physicochemical characteristics and nutraceutical applications. Rev. Virtual Quim. 2017;9(1):387–409. [Google Scholar]
- 3.Gheorghita R., Anchidin-Norocel L., Filip R., Dimian M., Covasa M. Applications of biopolymers for drugs and probiotics delivery. Polymers. 2021;13(16):2723. doi: 10.3390/polym13162729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puri V., Sharma A., Kumar P. Thiolation of biopolymers for developing drug delivery systems with enhanced mechanical and mucoadhesive properties : a review. Polymers. 2020;12(8):1803. doi: 10.3390/polym12081803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor P., Ghai K., Gupta A.K., Gupta P.K. 2013, No. January 2015. Journal of Biologically Active Products from Nature Pectin : A Versatile Biopolymer with Numerous Health Benefits and Medical Uses; pp. 37–41. [Google Scholar]
- 6.Baranwal J., Barse B., Fais A., Delogu G.L., Kumar A. Biopolymer: a sustainable material for food and medical applications. Polymers. 2022;14:983. doi: 10.3390/polym14050983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Augustine R., Rajendran R., Cvelbar U., Mozetič M., George A. Biopolymers for health, food, and cosmetic applications. Handb. Biopolym. Mater. From Blends Compos. to Gels Complex Networks. 2013:801–849. [Google Scholar]
- 8.Wong T.W. 2011. Alginate Graft Copolymers and Alginate – Co-excipient Physical Physicochemical and Biological; pp. 1497–1512. [DOI] [PubMed] [Google Scholar]
- 9.Sharma K., Porat Z., Gedanken A. Designing natural polymer-based capsules and spheres for biomedical applications—a review. Polymers. 2021;13(24):1–41. doi: 10.3390/polym13244307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wankhade V. Elsevier; 2020. Animal-Derived Biopolymers in Food and Biomedical Technology; pp. 139–152. [Google Scholar]
- 11.De Alvarenga E. S. Characterization and properties of chitosan. Biotechnol. Biopolym. 2011:92–108. [Google Scholar]
- 12.Dassanayake R.S., Acharya S. Vol. 1. 2018. Biopolymer-Based Materials from Polysaccharides: Properties, Processing, Characterization and Sorption Applications; pp. 1–24. [Google Scholar]
- 13.Sahu M., Sahoo P.K. 2017. Bio Polymers: Sustainable Alternative for Food Packaging; pp. 28–32. [Google Scholar]
- 14.Pierce A., Zheng Y., Wagner W.L., Scheller H.V., Mohnen D., Tsuda A., Ackermann M., Mentzer S.J. Pectin biopolymer mechanics and microstructure associated with polysaccharide phase transitions. J. Biomed. Mater. Res., Part A. 2020;108(2):246–253. doi: 10.1002/jbm.a.36811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L., Fishman M.L., Hicks K.B. Pectin in controlled drug delivery - a review. Cellulose. 2007;14(1):15–24. [Google Scholar]
- 16.Mellinas C., Ramos M., Jiménez A., Garrigós M.C. Recent trends in the use of pectin from agro - waste residues as a natural - based biopolymer for food packaging applications. Materials. 2021;13(3):673. doi: 10.3390/ma13030673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sriamornsak P. Vol. 1. 2014. Chemistry of Pectin and its Pharmaceutical Uses : A Review; pp. 2–24. [Google Scholar]
- 18.Nemiwal M., Zhang T.C., Kumar D. Pectin modified metal nanoparticles and their application in property modification of biosensors. Carbohydr. Polym. Technol. Appl. 2021;2:100–164. [Google Scholar]
- 19.Ánchez D.S., Uguerza B.M., Oulay L.M., Ernández R.H., Iguel M.M., Leixandre A.A. 2008. Highly Methoxylated Pectin Improves Insulin Resistance and Other Cardiometabolic Risk Factors in Zucker Fatty Rats; pp. 3574–3581. [DOI] [PubMed] [Google Scholar]
- 20.Ranganathan P., Mutharani B., Chen S.M., Sireesha P. Biocompatible chitosan-pectin polyelectrolyte complex for simultaneous electrochemical determination of metronidazole and metribuzin. Carbohydr. Polym. 2019;214(March):317–327. doi: 10.1016/j.carbpol.2019.03.053. [DOI] [PubMed] [Google Scholar]
- 21.Rangelova N., Aleksandrov L., Nenkova S. Synthesis and characterization of pectin/SiO2 hybrid materials. J. Sol. Gel Sci. Technol. 2017;2(85):330–338. [Google Scholar]
- 22.Yadav P., Yadav H., Shah V.G., Shah G., Dhaka G. Biomedical biopolymers, their origin and evolution in biomedical sciences: a systematic review. J. Clin. Diagn. Res. 2015;9(9):21–25. doi: 10.7860/JCDR/2015/13907.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minzanova S.T., Mironov V.F., Arkhipova D.M., Khabibullina A.V., Mironova L.G., Zakirova Y.M., Milyukov V.A. 2018. Biological Activity and Pharmacological Application of Pectic Polysaccharides : A Review; pp. 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu B., Luo Y. A review on the preparation and characterization of chitosan-clay nanocomposite films and coatings for food packaging applications. Carbohydr. Polym. Technol. Appl. 2021;2:100–102. [Google Scholar]
- 25.Nagaraj B., Kwang-Hyun B. Advances in functional biopolymer-based nanocomposites for active food packaging applications. Polymers. 2021;13(23):4198. doi: 10.3390/polym13234198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prabu G., Muthusamy S., Selvaganesh B., Sivakumar N., Hosseini-bandegharaei A., Loke P. Journal of environmental chemical engineering biopolymers and composites : properties, characterization and their applications in food, medical and pharmaceutical industries. J. Environ. Chem. Eng. 2021;9(4):105–322. [Google Scholar]
- 27.Rojo J., Mascaraque A., Sevilla U. De. Perspectives of carbohydrates in drug discovery, third edit. Elsevier. 2017;1:577–610. [Google Scholar]
- 28.Reddy P.R.S., Eswaramma S., Rao K.S.V.K., Lee Y.I. Dual responsive pectin hydrogels and their silver nanocomposites : swelling studies. Contr. Drug Del. Antimicrob. Appl. 2014;35(8):2391–2399. [Google Scholar]
- 29.Lakouraj M.M., Alipour A., Najafiroudbari M., Ojani R. 2020. Fabrication of a Nanocomposite Based on Pectin/Polyaniline/Graphene Oxide as Novel Electrically Conductive Biomaterial for Biosensing Detection of Hemoglobin; pp. 295–303. [Google Scholar]
- 30.Zhao X.J., Zhou Z.Q. Synthesis and applications of pectin-based nanomaterials. Curr. Nanosci. 2016;12:103–109. [Google Scholar]
- 31.Kumari G.V., Mathavan T., Srinivasan R., Rajan M.A.J. Antioxidant properties of pectin/silver and pectin/gold-silver nanocomposite. AIP Conf. Proc. 2019;2115(7):1–5. [Google Scholar]
- 32.Maria C., Freitas P., Jane S., Gomes V., Souza L., Rita C. Structure and applications of pectin in food, biomedical, and pharmaceutical industry: a review, Coatings. 2021;11(8):922. [Google Scholar]
- 33.Ngenefeme J., Eko F.-T.J., Mbom N.D., Tantoh Y.D., Rui N.M., KW A one pot green synthesis and characterisation of iron oxide-pectin hybrid nanocomposite. Open J. Compos. Mater. 2013;3(2):30–37. [Google Scholar]
- 34.Kasirajan K., Balaji M., Nithya P., Sundrarajan M. International journal of biological macromolecules synthesis of biogenic chitosan-functionalized 2D layered MoS2 hybrid nanocomposite and its performance in pharmaceutical applications : in-vitro antibacterial and anticancer activity. Int. J. Biol. Macromol. 2020;149:1019–1033. doi: 10.1016/j.ijbiomac.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Hu Q., Luo Y. International journal of biological macromolecules chitosan-based nanocarriers for encapsulation and delivery of curcumin : a review. Int. J. Biol. Macromol. 2021;179:125–135. doi: 10.1016/j.ijbiomac.2021.02.216. [DOI] [PubMed] [Google Scholar]
- 36.Sriamornsak P. Vol. 1. 2003. Chemistry of Pectin and its Pharmaceutical Uses : A Review Pornsak Sriamornsak Pornsak Sriamornsak 207 207 207 207 207; pp. 206–228. 2003. [Google Scholar]
- 37.Orellano M.S., Longo G.S., Porporatto C., Correa N.M., Falcone R.D. Role of micellar interface in the synthesis of chitosan nanoparticles formulated by reverse micellar method. Colloids Surfaces A Physicochem. Eng. Asp. 2020;599(2):124876. [Google Scholar]
- 38.Saikia C., Gogoi P. Chitosan: a promising biopolymer in drug delivery applications. J. Mol. Genet. Med. 2015;7:4–6. [Google Scholar]
- 39.Sutapa B.M., Dhruti A.P.G., Gopa R.B. Therapeutic and pharmaceutical benefits of native and modified plant pectin. J. Med. Plants Res. 2018;12(1):1–6. [Google Scholar]
- 40.Deepika P., Ganguly M. Formulation and evaluation of a pectin based controlled. Res. J. Life Sci. Bioinformatics, Pharm. Chem. Sci. 2017;3(16):16–25. [Google Scholar]
- 41.Liu L.S., Fishman M.L., Hicks K.B. Pectin in controlled drug delivery - a review. Cellulose. 2007;14(1):15–24. [Google Scholar]
- 42.Sabra R., Billa N., Roberts C.J. An augmented delivery of the anticancer agent, curcumin, to the colon. React. Funct. Polym. 2018;123(12):54–60. [Google Scholar]
- 43.Burkatovskaya M., Castano A.P., Demidova-rice T.N., Tegos G.P., Hamblin M.R. Effect of chitosan acetate bandage on wound healing in infected and noninfected wounds in mice. Wound Repair Regen. 2008;16:425–431. doi: 10.1111/j.1524-475X.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiao Z., Wenyu C., Honglin G., Baoqing W., Wang He, Z Jimei, Li Wenlan. One-pot preparation of nano-scaled magnetic pectin particles (Fe3O4@pectin NPs): cytotoxicity, antioxidant, and anti-liver cancer properties. Eur. J. Nutr. 2002;17(1):326–333. [Google Scholar]
- 45.Schuchardt J.P., Heine S., Hahn A. A combination of palm oil tocotrienols and citrus peel polymethoxylated flavones does not influence elevated LDL cholesterol and high-sensitivity C-reactive protein levels. Eur. J. Clin. Nutr. 2015;69(11):1209–1214. doi: 10.1038/ejcn.2015.44. [DOI] [PubMed] [Google Scholar]
- 46.Brouns F., Theuwissen E., Adam A., Bell M., Berger A., Mensink R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012;66(5):591–599. doi: 10.1038/ejcn.2011.208. [DOI] [PubMed] [Google Scholar]
- 47.Wathoni N., Yuan Shan C., Yi Shan W., Rostinawati T., Indradi R.B., Pratiwi R., Muchtaridi M. Characterization and antioxidant activity of pectin from Indonesian mangosteen (Garcinia mangostana L.) rind. Heliyon. 2019;5(8) doi: 10.1016/j.heliyon.2019.e02299. 22–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Y., Liu H., Li Z., Huang D., Nong L., Ning Z., Hu Z., Xu C., Yan J.K. Pectin-decorated selenium nanoparticles as a nanocarrier of curcumin to achieve enhanced physicochemical and biological properties. IET Nanobiotechnol. 2019;13(8):880–886. doi: 10.1049/iet-nbt.2019.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q., Cui W., Guo H., Wang B., Wang H., Zhang J., Li W. One-Pot preparation of nano-scaled magnetic-pectin particles (Fe3O4 @pectin NPs): cytotoxicity, antioxidant, and anti-liver cancer properties. J. Exp. Nanosci. 2022;17(1):326–338. [Google Scholar]
- 50.Liu Y., Dong M., Yang Z., Pan S. Anti-diabetic effect of citrus pectin in diabetic rats and potential mechanism via PI3K/akt signaling pathway. Int. J. Biol. Macromol. 2016;89:484–488. doi: 10.1016/j.ijbiomac.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 51.Jones M., Gu X., Stebbins N., Crandall P., Ricke S., Lee S. Effects of soybean pectin on blood glucose and insulin responses in healthy men. Faseb. J. 2015;29(1):1–10. [Google Scholar]
- 52.Sánchez D., Muguerza B., Moulay L., Hernández R., Miguel M., Aleixandre A. Highly methoxylated pectin improves insulin resistance and other cardiometabolic risk factors in zucker fatty rats. J. Agric. Food Chem. 2008;56(10):3574–3581. doi: 10.1021/jf703598j. [DOI] [PubMed] [Google Scholar]
- 53.Daoud Z., Sura M., Abdel-Massih R.M. Pectin shows antibacterial activity against Helicobacter pylori. Adv. Biosci. Biotechnol. 2013;4(2):273–277. [Google Scholar]
- 54.Kothandaraman G.P., Ravichandran V., Bories C., Loiseau P.M., Jayakrishnan A. Anti-fungal and anti-leishmanial activities of pectin-amphotericin B conjugates. J. Drug Deliv. Sci. Technol. 2017;39:1–7. [Google Scholar]
- 55.Rojas R., Alvarez-Pérez O.B., Contreras-Esquivel J.C., Vicente A., Flores A., Sandoval J., Aguilar C.N. Valorisation of mango peels: extraction of pectin and antioxidant and antifungal polyphenols. Waste Biomass Valor. 2020;11(1):89–98. [Google Scholar]
- 56.Dong C.X., Hayashi K., Lee J.B., Hayashi T. Characterization of structures and antiviral effects of polysaccharides from portulaca oleracea L. Chem. Pharm. Bull. 2010;58(4):507–510. doi: 10.1248/cpb.58.507. [DOI] [PubMed] [Google Scholar]
- 57.De Godoi A.M., Faccin-Galhardi L.C., Rechenchoski D.Z., Arruda T.B.M.G., Cunha A.P., de Almeida R.R., Rodrigues F.E.A., Ricardo N.M.P.S., Nozawa C., Linhares R.E.C. Structural characterization and antiviral activity of pectin isolated from inga spp. Int. J. Biol. Macromol. 2019;139:925–931. doi: 10.1016/j.ijbiomac.2019.07.212. [DOI] [PubMed] [Google Scholar]
- 58.Odun-Ayo F., Reddy L. Vol. 4. 2021. Potential Roles of Modified Pectin Targeting Galectin-3 against Severe Acute Respiratory Syndrome Coronavirus-2. J; pp. 824–837. (4) [Google Scholar]
- 59.Hu S., Kuwabara R., Beukema M., Ferrari M., de Haan B.J., Walvoort M.T.C., de Vos P., Smink A.M. Low methyl-esterified pectin protects pancreatic β-cells against diabetes-induced oxidative and inflammatory stress via galectin-3. Carbohydr. Polym. 2020;249(8):116–863. doi: 10.1016/j.carbpol.2020.116863. [DOI] [PubMed] [Google Scholar]
- 60.Kong Y., Hu Y., Li J., Cai J., Qiu Y., Dong C. Anti-inflammatory effect of a novel pectin polysaccharide from Rubus chingii Hu on colitis mice. Front. Nutr. 2022;9(4):1–11. doi: 10.3389/fnut.2022.868657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ávila G., De Leonardis D., Grilli G., Lecchi C., Ceciliani F. Anti-inflammatory activity of citrus pectin on chicken monocytes’ immune response. Vet. Immunol. Immunopathol. 2021;237(1):110–269. doi: 10.1016/j.vetimm.2021.110269. [DOI] [PubMed] [Google Scholar]
- 62.Leclere L., Cutsem P. Van, Michiels C. Anti-cancer activities of PH- or heat-modified pectin. Front. Pharmacol. 2013;4(10):1–8. doi: 10.3389/fphar.2013.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang H., Wei G., Liu F., Banerjee G., Joshi M., Annie Bligh S.W., Shi S., Lian H., Fan H., Gu X., et al. Characterization of two homogalacturonan pectins with immunomodulatory activity from green tea. Int. J. Mol. Sci. 2014;15(6):9963–9978. doi: 10.3390/ijms15069963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hira I., Kumar A., Kumari R., Saini A.K., Saini R.V. Pectin-guar gum-zinc oxide nanocomposite enhances human lymphocytes cytotoxicity towards lung and breast carcinomas. Mater. Sci. Eng. C. 2018;90:494–503. doi: 10.1016/j.msec.2018.04.085. [DOI] [PubMed] [Google Scholar]
- 65.Ho G.T.T., Zou Y.F., Aslaksen T.H., Wangensteen H., Barsett H. Structural characterization of bioactive pectic polysaccharides from elderflowers (sambuci flos) Carbohydr. Polym. 2016;135(10):128–137. doi: 10.1016/j.carbpol.2015.08.056. [DOI] [PubMed] [Google Scholar]
- 66.Nuzzo D., Picone P., Giardina C., Scordino M., Mudò G., Pagliaro M., Scurria A., Meneguzzo F., Ilharco L.M., Fidalgo A., et al. New neuroprotective effect of lemon integro pectin on neuronal cellular model. Antioxidants. 2021;10:669. doi: 10.3390/antiox10050669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang C., Li G., Karmakar B., AlSalem H.S., Shati A.A., El-kott A.F., Elsaid F.G., Bani-Fwaz M.Z., Alsayegh A.A., Salem Alkhayyat S., et al. Pectin mediated green synthesis of Fe3O4/pectin nanoparticles under ultrasound condition as an anti-human colorectal carcinoma bionanocomposite. Arab. J. Chem. 2022;15(6):103–867. [Google Scholar]
- 68.Chauhan S.S., Shetty A.B., Hatami E., Chowdhury P., Yallapu M.M. Pectin-tannic acid nano-complexes promote the delivery and bioactivity of drugs in pancreatic cancer cells. Pharmaceutics. 2020;12:285. doi: 10.3390/pharmaceutics12030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hira I. Vol. 12. 2022. Apoptotic Cell Death Induction through Pectin, Guar Gum and Zinc Oxide Nanocomposite in A549 Lung Adenocarcinomas; pp. 1856–1869. (2) [Google Scholar]
- 70.Butt M.S., Ahmad A., Sharif M.K. Influence of pectin and guar gum composite flour on plasma biochemical profile of streptozotocin-induced diabetic male albino rats. Int. J. Food Prop. 2007;10(2):345–361. [Google Scholar]
- 71.Sousa R.V.R.B., Guedes M.I.F., Marques M.M.M., Viana D.A., Silva I.N.G. da; Rodrigues, P. A. S.; Vieira, Í. G. P. Hypoglycemic Effect of New Pectin Isolated From Passiflora Glandulosa Cav in Alloxan-Induced Diabetic Mice. World J. Pharm. Pharmaceut. Sci. 2015;4(1):1571–1586. [Google Scholar]
- 72.Fan Y., Sun C., Gao X., Wang F., Li X., Kassim R.M., Tai G., Zhou Y. Neuroprotective effects of ginseng pectin through the activation of ERK/MAPK and akt survival signaling pathways. Mol. Med. Rep. 2012;5(5):1185–1190. doi: 10.3892/mmr.2012.811. [DOI] [PubMed] [Google Scholar]
- 73.Nishikawa H., Liu L., Nakano F., Kawakita F., Kanamaru H., Nakatsuka Y., Okada T., Suzuki H. Modified citrus pectin prevents blood-brain barrier disruption in mouse subarachnoid hemorrhage by inhibiting galectin-3. Stroke. 2018;49(11):2743–2751. doi: 10.1161/STROKEAHA.118.021757. [DOI] [PubMed] [Google Scholar]
- 74.Srivastava R., Tripathi L., Swain S.R., Singh J. Neuroprotective validation of pectin in T2DM-induced allodynia and hyperalgesia in diabetic peripheral neuropathic pain. Arch. Physiol. Biochem. 2021:1–12. doi: 10.1080/13813455.2021.1884725. [DOI] [PubMed] [Google Scholar]
- 75.Kachare D.S., Ghadge P.K., Mali S.S., Student R. Role of citrus pectin in biological activity: a review. J Pharma Quality Assur. 2020;2(1):2–11. [Google Scholar]
- 76.Kolatsi-Joannou M., Price K.L., Winyard P.J., Long D.A. Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS One. 2011;6(4):18683. doi: 10.1371/journal.pone.0018683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Avila G., Leonardis D De, Grilli G., Lecchi C., Ceciliani F. Anti-inflammatory activity of citrus pectin on chicken monocytes. Imm. Resp. 2021;237(5) doi: 10.1016/j.vetimm.2021.110269. [DOI] [PubMed] [Google Scholar]
- 78.Viswanath K.B., Krithiga N., Vijayan M., Mideen A., Vasantha V.S. Vol. 10. 2018. Picomolar Level Detection of Insulin in Serum Using Pectin Gold Nanocomposite Platform Immunoassay; pp. 72–82. (11) [Google Scholar]
- 79.Hlaing T., Myint H.Z., Win N.N. Characterization and some bioactivities of the synthesized citrus pectin-ZnO nanocomposites from citron and pomelo fruits peels. Res. J. 2017:189–212. [Google Scholar]
- 80.Rao K.S.V.K., Reddy P.R., Rao K.M., Kumar S.P. Vol. 2016. 2015. Indian Journal of Advances in Chemical Science A Green Approach to Synthesize Silver Nanoparticles from Natural Polymer for Biomedical Application; pp. 340–344. [Google Scholar]
- 81.Nashwa M., Wagdi Fawzi E. Modified citrus pectin stops progression of liver fibrosis by inhibiting galactin-3 and inducing apoptosis of stellate cells. Can. J. Physiol. Pharmacol. 2016;94(5):554–562. doi: 10.1139/cjpp-2015-0284. [DOI] [PubMed] [Google Scholar]
- 82.Jackson C.L., Dreaden T.M., Theobald L.K., Tran N.M., Beal T.L., Eid M., Gao M.Y., Shirley R.B., Stoffel M.T., Kumar M.V., et al. Pectin induces apoptosis in human prostate cancer cells: correlation of apoptotic function with pectin structure. Glycobiology. 2007;17(8):805–819. doi: 10.1093/glycob/cwm054. [DOI] [PubMed] [Google Scholar]
- 83.Eliana M., Zapata V., Mina H., Blanca V., San J., Rojo L. Novel bioactive and antibacterial acrylic bone cement nanocomposites modified with graphene oxide and chitosan. Int. J. Mol. Sci. 2019;20:2938. doi: 10.3390/ijms20122938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin L., Wang P., Du Z., Wang W., Cong Q., Zheng C., Jin C., Ding K., Shao C. Structural elucidation of a pectin from flowers of Lonicera japonica and its antipancreatic cancer activity. Int. J. Biol. Macromol. 2016;88:130–137. doi: 10.1016/j.ijbiomac.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 85.Cheng H., Zhang Z., Leng J., Liu D., Hao M., Gao X., Tai G., Zhou Y. The inhibitory effects and mechanisms of rhamnogalacturonan i pectin from potato on HT-29 colon cancer cell proliferation and cell cycle progression. Int. J. Food Sci. Nutr. 2013;64(1):36–43. doi: 10.3109/09637486.2012.694853. [DOI] [PubMed] [Google Scholar]
- 86.Delphi L., Sepehri H. Apple pectin: a natural source for cancer suppression in 4T1 breast cancer cells in vitro and express P53 in mouse bearing 4T1 cancer tumors, in vivo. Biomed. Pharmacother. 2016;84:637–644. doi: 10.1016/j.biopha.2016.09.080. [DOI] [PubMed] [Google Scholar]
- 87.Park H.R., Hwang D., Hong H., Do, Shin K.S. Antitumor and antimetastatic activities of pectic polysaccharides isolated from persimmon leaves mediated by enhanced natural killer cell activity. J. Funct.Foods. 2017;37:460–466. [Google Scholar]
- 88.Wang H., Gao T., Du Y., Yang H., Wei L., Bi H., Ni W. Anticancer and immunostimulating activities of a novel homogalacturonan from hippophae rhamnoides L. Berry. Carbohydr. Polym. 2015;131:288–296. doi: 10.1016/j.carbpol.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 89.Chen W., Zhu X., Ma J., Zhang M., Wu H. Structural elucidation of a novel pectin-polysaccharide from the petal of saussurea laniceps and the mechanism of its anti-HBV activity. Carbohydr. Polym. 2019;223(3):115077. doi: 10.1016/j.carbpol.2019.115077. [DOI] [PubMed] [Google Scholar]
- 90.Utomo Yudi, Meiyanto R. Vol. 2. 2020. E. Revealing the Potency of Citrus and Galangal Constituents to Halt SARS-CoV-2 Infection; pp. 1–8. (March) [Google Scholar]
- 91.Vega-vásquez P., Mosier N.S., Irudayaraj J. Vol. 8. 2020. Nanoscale drug delivery systems : From Medicine to Agriculture; pp. 1–16. (February) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mishra R.K., Banthia A.K., Majeed A.B.A. Vol. 5. 2012. Pectin based formulations biomedical applications : A review; pp. 1–7. (4) [Google Scholar]
- 93.Morris G.A., Kök S.M., Harding S.E., Adams G.G., Morris G.A., Kök S.M., Harding S.E., Adams G.G., Kok S., Gordon A., et al. 2013. Polysaccharide Drug Delivery Systems Based on Pectin and Chitosan Polysaccharide Delivery Systems Systems Polysaccharide Drug Drug Delivery Based on Pectin and Chitosan Based on Pectin and Chitosan; p. 8725. [Google Scholar]
- 94.Chen L., Liu X., Wong K. Novel nanoparticle materials for drug/food delivery-polysaccharides. J. Phys. Sci. Rev. 2016:160–189. [Google Scholar]
- 95.Muvva A., Chacko I.A., Ghate V., Lewis S.A. Modified pectins for colon-specific drug delivery. Ind. J. Pharm. Edu. Res. 2020;54(2):12–18. [Google Scholar]
- 96.Journal A.I., Hussien N.A., Işıklan N., Türk M. Pectin-conjugated magnetic graphene oxide nanohybrid as a novel drug carrier for paclitaxel delivery. Artif. Cell Nanomed. Biotechnol. 2018;46(S1):264–273. doi: 10.1080/21691401.2017.1421211. [DOI] [PubMed] [Google Scholar]
- 97.Utrgv S., Chauhan S.S. Pectin-tannic acid nano-complexes promote the delivery and bioactivity of drugs in pancreatic cancer cells. Pharmaceutics. 2020;12:285. doi: 10.3390/pharmaceutics12030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ouyang J., Yang M., Gong T., Ou J., Tan Y., Zhang Z. 2020. Nanocell : A Novel Nanovehicle for Anticancer Agent Delivery with Multidrug Resistance Reversal; pp. 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen D., Chang L., Zhou Z., Bo Y., Wang Y., He Y., Qin J. Pectin - based self - healing hydrogel with - NaHCO 3 degradability for drug loading and release. J. Polym. Res. 2021:1–10. [Google Scholar]
- 100.Vijitha R., Reddy N.S., Nagaraja K., Vani T.J.S., Hanafiah M.M., Venkateswarlu K., Lakkaboyana S.K., Rao K.S.V.K., Rao K.M. Fabrication of polyelectrolyte membranes of pectin graft-copolymers with PVA and their composites with phosphomolybdic acid for drug delivery, toxic metal ion removal, and fuel cell applications. Membranes. 2021;11:792. doi: 10.3390/membranes11100792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ullah K., Sohail M., Buabeid M.A., Murtaza G., Ullah A., Rashid H., Khan M.A., Khan S.A. Pectin-based (LA-Co-maa) semi-IPNS as a potential biomaterial for colonic delivery of oxaliplatin. Int. J. Pharm. 2019:118–557. doi: 10.1016/j.ijpharm.2019.118557. [DOI] [PubMed] [Google Scholar]
- 102.Yu C., Wang Y., Li N., Liu G., Yang S., Tang G., He D., Tan X., Wei H. Vitro and in vivo evaluation of pectin-based nanoparticles for hepatocellular carcinoma drug chemotherapy. Mol. Pharmaceutics. 2014;11:638–644. doi: 10.1021/mp400412c. [DOI] [PubMed] [Google Scholar]
- 103.Ji F., Li J., Qin Z., Yang B., Zhang E., Dong D., Wang J., Wen Y., Tian L., Yao F. Engineering pectin-based hollow nanocapsules for delivery of anticancer drug. Carbohydr. Polym. 2017:2–10. doi: 10.1016/j.carbpol.2017.08.107. [DOI] [PubMed] [Google Scholar]
- 104.Kumar R., Sahu S. Results in pharma sciences development of oxaliplatin encapsulated in magnetic nanocarriers of pectin as a potential targeted drug delivery for cancer therapy. Results Pharma Sci. 2012;2:38–45. doi: 10.1016/j.rinphs.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kodoth A.K., Ghate V.M., Lewis S.A., Prakash B., Badalamoole V.P.T. Pectin-based silver nanocomposite film for transdermal delivery of Donepezil. Int. J. Biol. Macromol. 2019;134:269–279. doi: 10.1016/j.ijbiomac.2019.04.191. [DOI] [PubMed] [Google Scholar]
- 106.Kodoth A.K., Ghate V.M., Lewis S.A., Vishalakshi B. Vol. 1. 2018. Application of Pectin-Zinc Oxide Hybrid Nanocomposite in the Delivery of a Hydrophilic Drug and a Study of its Isotherm, Kinetics and Release Mechanism; pp. 1–36. [DOI] [PubMed] [Google Scholar]
- 107.Rehmat S., Rizvi N.B., Khan S.U., Ghaffar A., Islam A. Vol. 9. 2022. Novel Stimuli-Responsive Pectin-PVP-Functionalized Clay Based Smart Hydrogels for Drug Delivery and Controlled Release Application; pp. 1–15. (2) [Google Scholar]
- 108.Wang H., Yang B., Sun H. Vol. 3. 2017. Pectin-Chitosan Polyelectrolyte Complex Nanoparticles for Encapsulation and Controlled Release of Nisin Email Address; pp. 82–88. (5) [Google Scholar]
- 109.Sadeghi M. Vol. 2011. 2011. Pectin-Based Biodegradable Hydrogels with Potential Biomedical Applications as Drug Delivery Systems; pp. 36–40. (1) [Google Scholar]
- 110.Ranjan N., Sarkar G., Roy I., Rana D. Studies on methylcellulose/pectin/montmorillonite nanocomposite films and their application possibilities. Carbohydr. Polym. 2016;136:1218–1227. doi: 10.1016/j.carbpol.2015.10.046. [DOI] [PubMed] [Google Scholar]
- 111.Ribeiro L.N.M., Alcântara A.C.S., Darder M., Aranda P., Araújo-moreira F.M., Ruiz-hitzky E. Pectin-coated chitosan – LDH bionanocomposite beads as potential systems for colon-targeted drug delivery. Int. J. Pharm. 2014;463(1):1–9. doi: 10.1016/j.ijpharm.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 112.Auriemma G., Cerciello A., Aquino R.P., Gaudio P. Del, Fusco B.M., Russo P. Pectin and zinc alginate : the right inner/outer polymer combination for core-shell drug delivery systems. Pharmaceutics. 2020;12:87. doi: 10.3390/pharmaceutics12020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data was collected from the internet, journals and books. All the statements taken from all the sources are cited in the proper manner.