Background:
Cardiac sarcoidosis (CS) predisposes to sudden cardiac death (SCD). Guidelines for implantable cardioverter defibrillators (ICDs) in CS have been issued by the Heart Rhythm Society in 2014 and the American College of Cardiology/American Heart Association/Heart Rhythm Society consortium in 2017. How well they discriminate high from low risk remains unknown.
Methods:
We analyzed the data of 398 patients with CS detected in Finland from 1988 through 2017. All had clinical cardiac manifestations. Histological diagnosis was myocardial in 193 patients (definite CS) and extracardiac in 205 (probable CS). Patients with and without Class I or IIa ICD indications at presentation were identified, and subsequent occurrences of SCD (fatal or aborted) and sustained ventricular tachycardia were recorded, as were ICD indications emerging first on follow-up.
Results:
Over a median of 4.8 years, 41 patients (10.3%) had fatal (n=8) or aborted (n=33) SCD, and 98 (24.6%) experienced SCD or sustained ventricular tachycardia as the first event. By the Heart Rhythm Society guideline, Class I or IIa ICD indications were present in 339 patients (85%) and absent in 59 (15%), of whom 264 (78%) and 30 (51%), respectively, received an ICD. Cumulative 5-year incidence of SCD was 10.7% (95% CI, 7.4%–15.4%) in patients with ICD indications versus 4.8% (95% CI, 1.2%–19.1%) in those without (χ2=1.834, P=0.176). The corresponding rates of SCD were 13.8% (95% CI, 9.1%–21.0%) versus 6.3% (95% CI, 0.7%–54.0%; χ2=0.814, P=0.367) in definite CS and 7.6% (95% CI, 3.8%–15.1%) versus 3.3% (95% CI, 0.5%–22.9%; χ2=0.680, P=0.410) in probable CS. In multivariable regression analysis, SCD was predicted by definite histological diagnosis (P=0.033) but not by Class I or IIa ICD indications (P=0.210). In patients without ICD indications at presentation, 5-year incidence of SCD, sustained ventricular tachycardia, and emerging Class I or IIa indications was 53% (95% CI, 40%–71%). By the American College of Cardiology/American Heart Association/Heart Rhythm Society guideline, all patients with complete data (n=245) had Class I or IIa indications for ICD implantation.
Conclusions:
Current ICD guidelines fail to distinguish a truly low-risk group of patients with clinically manifest CS, the 5-year risk of SCD approaching 5% despite absent ICD indications. Further research is needed on prognostic factors, including the role of diagnostic histology. Meanwhile, all patients with CS presenting with clinical cardiac manifestations should be considered for an ICD implantation.
Keywords: death, sudden, cardiac; defibrillators, implantable; sarcoidosis
Clinical Perspective.
What Is New?
At least 85% of patients presenting with clinically manifest cardiac sarcoidosis had strong to modest indications for an implantable cardioverter defibrillator, and >50% of the rest either developed such indications or had an arrhythmic event within 5 years of follow-up.
Diagnosis of cardiac sarcoidosis based on myocardial histology predicted an increased risk of life-threatening arrhythmic events probably attributable to reflecting a more extensive myocardial involvement and scarring.
What Are the Clinical Implications?
Practically all patients with newly diagnosed cardiac sarcoidosis and clinical cardiac manifestations falling in the spectrum of our cohort can be expected to benefit from an implantable cardioverter defibrillator.
The pros and cons of an implantable cardioverter defibrillator should be discussed particularly thoroughly with patients having nondefinite cardiac sarcoidosis and no Class I or IIa indications.
If an implantable cardioverter defibrillator is not implanted, regularly repeated risk appraisal is needed during follow-up.
Editorial, see p 976
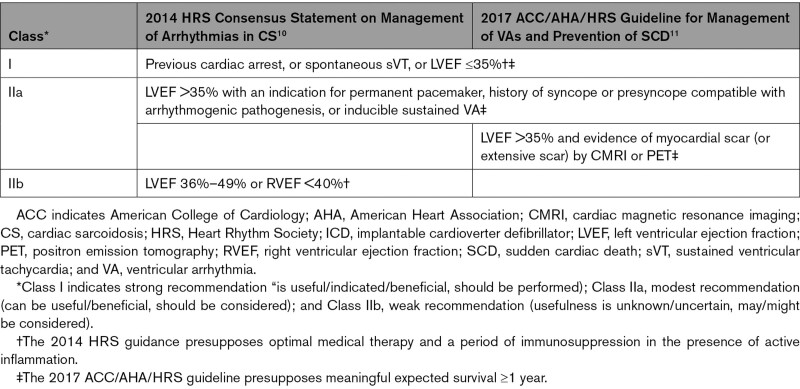
Cardiac sarcoidosis (CS) is currently included in the family of arrhythmogenic cardiomyopathies.1 It is caused by inflammatory granulomas injuring and scarring the myocardium and the conduction pathways. High-grade atrioventricular block (AVB) and life-threatening ventricular arrhythmias (VAs) constitute its most frequent clinical manifestations2 and explain the predominance of sudden cardiac death (SCD) among the fatalities from CS.3 The best treatment and prevention of sustained VAs in CS remain unknown. Both immunosuppression and antiarrhythmic drugs have unpredictable efficacy, and catheter ablation, although producing symptomatic benefit in most cases, cuts VA recurrences to a modest extent at best.4–6 An implantable cardioverter defibrillator (ICD) has lifesaving potential in CS,7–9 but its drawbacks appear increased in these patients in that up to 24% have been reported to receive inappropriate therapies7 and 15% may have complications such as infections and lead fracture or dislodgement.7,8 Current guidelines for ICD implantations in CS,10,11 detailed in Table 1, raise prior cardiac arrest, spontaneous sustained VAs, and left ventricular (LV) ejection fraction (LVEF) ≤35% as Class I indications (is useful), whereas a Class IIa recommendation (can be useful) is given in the presence of inducible sustained VA, need of permanent pacemaker, or history of arrhythmogenic syncope. In addition, the 2017 American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society (HRS) guideline11 recommends an ICD (Class IIa indication) if there is evidence of LV scar on cardiac imaging.
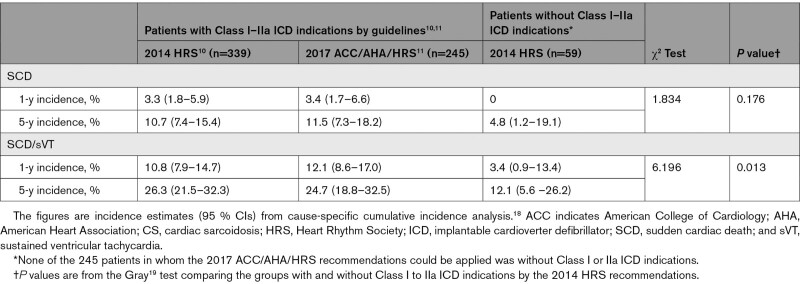
Table 1.
Current Indications by Societal Guidelines for an ICD in CS
We set out to assess how well the strong to modest ICD indications by current guidelines identify patients with CS at high versus low risk of fatal arrhythmias. To that aim, we analyzed the cumulative incidence of SCD and life-threatening VAs in nationwide cohorts of clinically manifest CS with and without Class I or IIa ICD indications at disease presentation. In particular, we wanted to explore the long-term safety of patients with clinically manifest CS who were considered not to benefit from an early ICD implantation. Here, we present findings suggesting that revision of the guidelines may be timely.
Methods
Individual-level data cannot be shared openly, and the data cannot be made available to other researchers for purposes of reproducing the results because of restrictions by the patient consent.
Study Population
At the end of 2017, the nationwide MIDFIN registry (Myocardial Inflammatory Diseases in Finland) included detailed data on 462 cases of definite (n=257) or probable (n=205) CS by the HRS diagnostic criteria (Table S1).10 Of them, 64 cases had been diagnosed at autopsy performed nearly always at coroner’s inquest for an unexpected SCD.3 Their particulars were reviewed, but all were ultimately excluded because diagnosis and inclusion through an event under investigation could have compromised survival analyses (possibility of reverse survivorship bias). The remaining 398 cases, the present study population, constitute a consecutive series of patients having CS by the HRS criteria10 and being diagnosed in the hospitals of the MIDFIN network between 1988 and the end of 2017.
MIDFIN Registry and Data Collection
The setup and methods of the MIDFIN registry have been detailed in our prior publications.2,3,12 The cases were identified and included in retrospect until the year 2010 but mainly prospectively thereafter. The registry includes information on patients’ demographics, clinical cardiac manifestations, associated diseases, results of diagnostic imaging and laboratory studies, details of treatment with drugs and devices, and occurrence of major adverse cardiac and noncardiac events throughout the disease course.2 The echocardiographic data on LVEF used in the present analyses were taken as originally done and reported by the attending cardiologists. In contrast, to assess the presence and extent of myocardial scar, the pertinent cardiac magnetic resonance imaging (CMRI) studies and myocardial perfusion scans were acquired for re-evaluation at the MIDFIN core center (Helsinki University Hospital).
CMRI Studies
Altogether, 274 patients had undergone CMRI examinations between 2003 and the end of 2017; the studies of 222 patients could be acquired for review. We accepted for the present analyses studies that either contributed to CS diagnosis or were done <3 months after the date of diagnosis. All examinations were conducted on 1.5-T or 3.-T cardiac magnetic resonance scanners using phased-array receiver coils and standard protocols.13 To assess LV and right ventricular volumes and EF, cine images were obtained in long-axis and short-axis planes covering both ventricles. Late gadolinium enhancement (LGE) imaging was performed 10 to 15 minutes after an intravenous injection of contrast agent (0.15 mmol/kg) using an inversion‐recovery gradient-echo sequence in views identical to cine imaging. Cine and LGE images were evaluated by a single cardiac magnetic resonance–trained cardiologist (P.P.) blinded to clinical data. LV and right ventricular volumes and LV mass were assessed using standard protocols14 with papillary muscles and outflow tract included in the LV volume. The presence of LGE was assessed visually, and the number of positive LV segments was counted according to the AHA 17-segment model.15 The extent of LGE as a percentage of LV mass was assessed with the full width at half-maximum method.16 Image analyses were performed with QMass MR software (version 8.1, Medis Medical Imaging Systems, Leiden, the Netherlands).
Myocardial Perfusion Studies
In total, 120 patients had undergone diagnostic myocardial perfusion scans with single-photon emission computed tomography (SPECT) from the beginning of 2007 to the end of 2017. Of them, 97 patients had scans that were both technically acceptable for analysis and dated at the time of presenting admission or <3 months after the diagnosis of CS. SPECT imaging was done at rest according to prevailing clinical practice with technetium-99m–labeled tetrofosmin (Myoview) as the radioactive tracer. The scans were obtained by standard dual-headed gamma cameras with low-energy, high-resolution collimators. For our work, the images were analyzed for the presence of LV scar by a nuclear medicine specialist (V.U.) blinded to clinical data. Perfusion defects not explained by common imaging artifacts were taken to represent scarred myocardium. The extent of LV scarring was estimated by calculating the summed rest score17 of perfusion defects according to the AHA 17-segment model.15
Definition of Outcome Events
The primary outcome event was SCD, fatal or aborted, the former defined as in our earlier report3 and the latter being a documented episode of ventricular fibrillation terminated successfully either by an ICD or by external defibrillation during resuscitation for sudden cardiac arrest. Our secondary end point was a composite of SCD or sustained ventricular tachycardia (sVT) needing for termination external synchronized cardioversion, ICD therapy, or rescue treatment with amiodarone infusion. The events occurring until the end of February 2018 were included in the present analyses. Their dates and characteristics were ascertained by reviews of medical records, 12-lead ECGs, and ICD reports read locally in the participating hospitals by the attending cardiologists and members of our research team. The causes of death were determined from medical records and findings at autopsy. The mortality data were double-checked from the Finnish Population Register.
Ethics Approvals
The MIDFIN registry study was approved by the national ethics review board in 2009 (STM/1219/2009), and all involved hospitals have granted approval to conduct the study. Written informed consent was obtained from each patient alive at the time of recruitment into the registry. The National Authority for Medicolegal Affairs (4615/06.01.03.01/2016) and the National Institute for Health and Welfare (THL/691/5.05.00/2016) approved the study of cases from the cause-of-death registry and the review of postmortem autopsy material.
Statistical Analyses
Patient characteristics are presented as means (±SDs) or medians (interquartile range) for continuous variables and as frequencies of categorical variables. Group comparisons were conducted with the Student t test, Kruskal-Wallis test, Fisher exact test, or χ2 test as appropriate. Follow-up times were calculated from disease presentation, defined as the date of the first medical contact for symptoms that led to the diagnosis of CS. Cause-specific cumulative incidence analysis18 was used to calculate the unadjusted incidence estimates with 95% CIs for the end point events and to construct the incidence-time curves; the Gray19 test was used for comparisons between groups. Cardiac transplantations and deaths attributable to either noncardiac causes or terminal heart failure were analyzed as competing events,18 and the Fine and Gray20 model was used to calculate subdistribution hazard ratios with 95% CIs. Values of P<0.05 were considered statistically significant. Analyses were performed with SPSS-26 for Macintosh (SPSS Inc, Chicago, IL), Xlstat Lifesciences (Addinsoft, Paris, France), and R (version 4.0.4, The R Foundation, Vienna, Austria).
Results
Characteristics of the Study Population
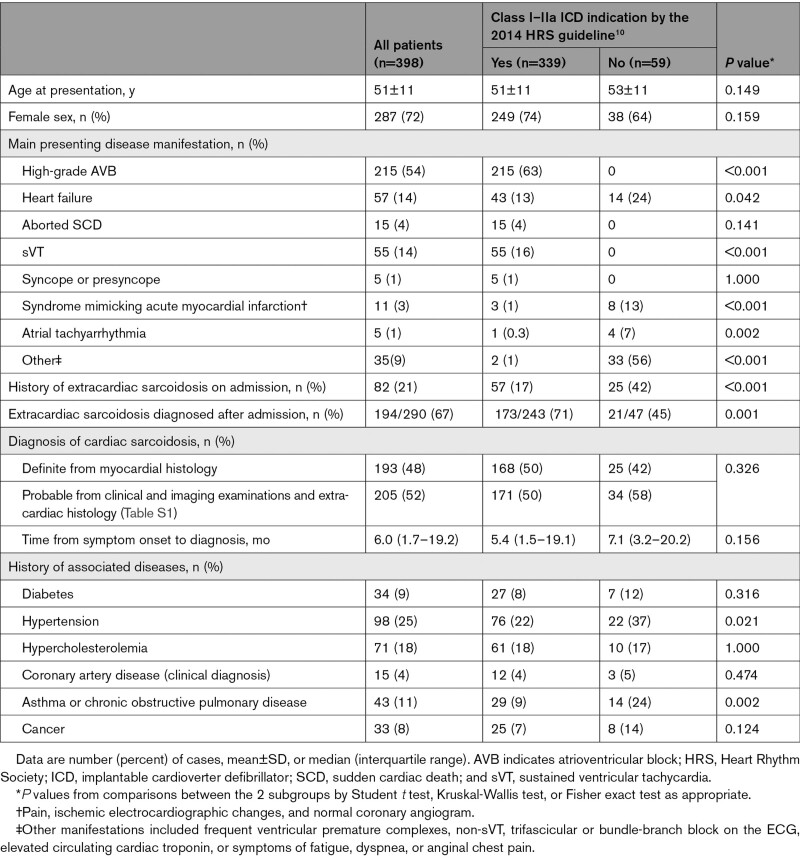
Table 2 summarizes the presenting clinical characteristics of the entire CS cohort and the subgroups with (n=339) and without (n=59) Class I or IIa indications for an early ICD implantation by the 2014 HRS guideline.10 Altogether, 135 patients had Class I indications, which included an aborted SCD, sVT, and LVEF ≤35% in 15, 70, and 59 patients, respectively. Class IIa indications included syncope or presyncope in 5 patients and need of a permanent pacemaker in 196 patients, of whom 192 had high-grade AVB (Mobitz II or complete AVB), 1 had trifascicular block, and 3 had sinus node dysfunction. No patient had a Class IIa indication based on inducible ventricular tachycardia. Among the 59 patients without Class I or IIa ICD indications, 4 had undergone programmed electric stimulation without showing inducible VAs. A Class IIb indication for an ICD (Table 1) was present in 23 of these 59 patients.
Table 2.
Clinical Characteristics of the Total Study Population and the Subgroups by ICD Indications
As Table 2 shows, the diagnosis of CS was definite, that is, based on myocardial histology, in 48% of patients, the rest having probable CS as detailed in the Table S1. Only a minority had known extracardiac sarcoidosis on admission. The presenting manifestations differed between the subgroups, with patients without ICD indications more often presenting with nonalarming cardiac signs or symptoms (Table 2).
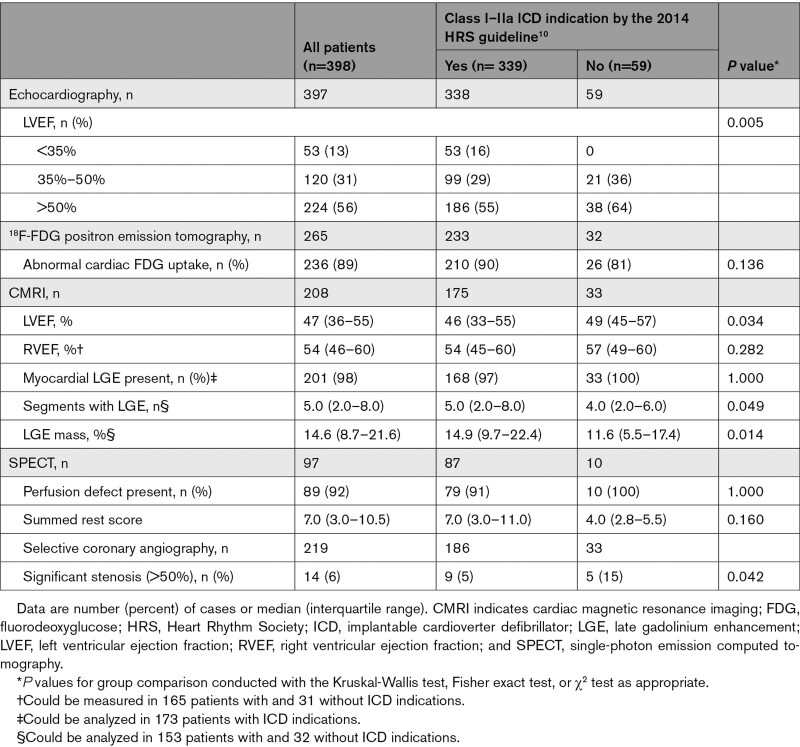
Table 3 summarizes the results of cardiac imaging studies done at or after presentation. As expected, impaired LVEF was more common in patients with Class I or IIa ICD indications. The prevalence of any myocardial LGE was nearly ubiquitous in either subgroup, but the number of LGE segments and the extent of LGE mass were higher in patients with ICD indications. Similar differences were not seen in the SPECT data. Angiographically significant coronary artery disease (presence of >50% stenosis) was more common in patients without ICD indications but rare overall. The detailed histories and CMRI studies of patients with coronary artery disease excluded myocardial ischemia or scarring from past infarction as the predominant cause of cardiac manifestations.
Table 3.
Results of Cardiac Imaging Studies Done at or After Presentation
Treatment in Brief
The decisions about treatment, including ICD implantations, were at the discretion of the individual hospitals participating in the study. Details of drug and device therapy are given in Table S2. At the outset of treatment, patients with Class I or IIa ICD indications by the HRS guideline10 received an ICD nearly twice as often as patients without; the ultimate implantation frequencies were 78% and 51% in the respective subgroups (P<0.001). Drug treatments were largely comparable in the 2 subgroups aside from a slight difference in the use of β-adrenergic blockers (Table S2).
Incidence of SCD and Life-Threatening VAs
Events in the Entire Cohort
The median follow-up time from presentation of CS to death, transplantation, or closure of the study on February 2018 was 4.8 years (2.5–8.4 years); there were no losses to follow-up. Of the 398-case cohort, 41patients (10.3 %) had either fatal (n=8) or aborted (n=33) SCD. The composite of SCD and sVT was recorded in 98 patients (24.6% of the cohort), among whom the first event was sVT in 63 patients and SCD in 35. Altogether, 23 experienced a competing event (14 heart transplantations, 6 noncardiac deaths, 3 deaths attributable to terminal heart failure). The cause-specific cumulative 5-year incidence was 9.8% (95% CI, 6.9%–13.9%) for SCD and 24.2% (95% CI, 19.8%–29.5%) for the composite of SCD and sVT. All except 1 of the 41 patients with a fatal or aborted SCD had received immunosuppressive therapy, and all were on β-adrenergic–blocking drugs. Among the 33 cases of aborted SCD, the causative ventricular fibrillation was defibrillated by ICD in 28 cases and externally during resuscitation for a sudden cardiac arrest in 5 cases.
Comparisons Between Groups With and Without Class I or IIa ICD Indications
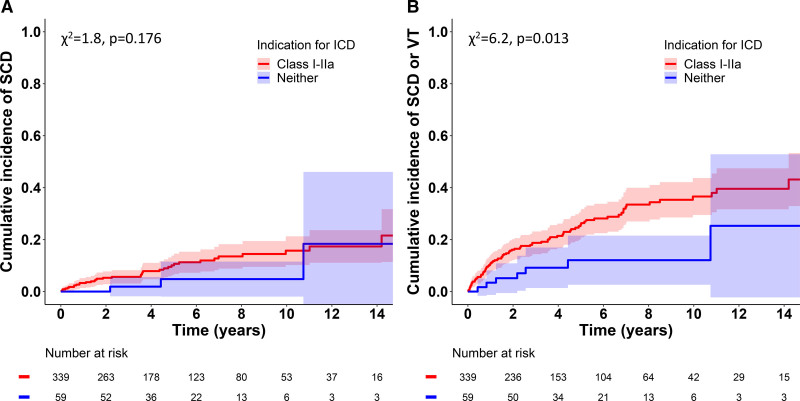
Figure 1 depicts the cumulative incidence graphs for SCD (Figure 1A) and the composite of SCD and sVT (Figure 1B) by the presence of Class I or IIa ICD indications, showing a statistically significant difference in the composite secondary end point but not in SCD alone. Table 4 summarizes the estimated 1- and 5-year cumulative rates of events in patients with and without combined Class I or IIa ICD indications. Pairwise comparisons between patients without ICD indications (n=59) and with Class IIa indications (n=205) showed no statistically significant difference in either SCD (χ2 test statistic=0.008, P=0.931) or SCD/sVT (χ2=1.318, P=0.251). The time-to-event graphs for the 3 individual groups are shown in Figure S1.
Figure 1.
Cumulative incidence of SCD and the composite of SCD and sustained VT. Incidence of sudden cardiac death (SCD; A) and the composite of SCD and ventricular tachycardia (VT; B) in 398 patients with cardiac sarcoidosis stratified by indications for an implantable cardioverter defibrillator (ICD) by the 2014 Heart Rhythm Society’s guideline.10 Shaded areas represent 95% CIs. Graphs were constructed by cause-specific cumulative incidence analysis18 with transplantations and deaths resulting from heart failure or noncardiac causes as competing events; comparisons were made with the Gray19 test.
Table 4.
Analyses Stratified by the Certainty of Diagnosis
In CS confirmed by myocardial histology (definite CS, n=193), cumulative 5-year incidence of SCD was 13.8% (9.1%–21.0%) in patients with Class I or IIa ICD indications (n=168) versus 6.3% (0.7%–54.0%) in those without (n=25; χ2=0.814, P=0.367). For the composite of SCD/sVT, the corresponding 5-year incidences were 35.4% (28.3%–44.3%) and 19.2% (7.7%–47.9%; χ2=2.541, P=0.111). In CS diagnosed by clinical examination, cardiac imaging, and extracardiac histology (probable CS, n=205), the 5-year rate of SCD was 7.6% (3.8%–15.1%) in the presence of Class I or IIa ICD indications (n=171) and 3.3% (0.5%–22.9%) in their absence (n=34; χ2=0.680, P=0.410); the corresponding rates for SCD/sVT were 16.8% (11.1%–25.5%) and 6.4% (1.7%–24.4%; χ2=2.981, P=0.084). The time-to-event graphs for these analyses are shown in Figures S2 and S3.
In multivariable competing-risk regression analysis, definite histological diagnosis of CS predicted the occurrence of SCD with a subdistribution hazard ratio of 2.11 (95% CI, 1.06–4.19; P=0.033), whereas Class I or IIa ICD indications, with a subdistribution hazard ratio of 2.10 (95% CI, 0.67–6.62), did not reach statistical significance (P=0.210). For the composite end point of SCD and sVT, the subdistribution hazard ratios were 2.25 (95% CI, 1.47–3.44; P=0.0002) for definite CS and 2.38 (95% CI, 1.12–5.06; P=0.024) for presence of Class I or IIa ICD indications. The characteristics of patients with definite versus probable CS revealed distinct differences in the severity of clinical manifestations and extent of LV involvement. Heart failure, aborted SCD, or sVT was the main presenting manifestation in 82 of 193 patients (42%) with definite CS versus 45 of 205 patients (22%) with probable CS (P<0.001), and LVEF was impaired (<50%) in 106 patients (55%) versus 67 patients (32%) of the definite and probable diagnosis groups, respectively (P<0.001). The median myocardial LGE mass on CMRI was 19.5% (13.0%–27.1%) in definite (n=86) and 11.4% (5.9%–17.7%) in probable (n=99; P<0.001) CS.
Emergence of ICD Indications During Follow-Up
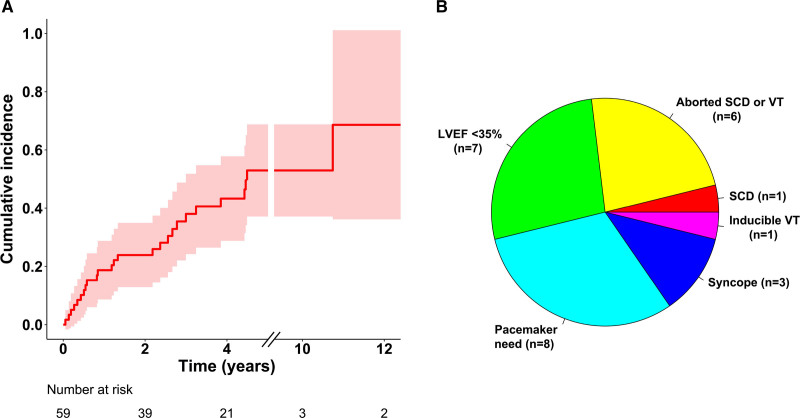
Of the 59 patients without an early ICD indication, 26 had a fatal or nonfatal event or developed on follow-up (median, 4.9 years) a cardiac condition meeting 1 of the Class I or IIa indications for an ICD. Figure 2 shows the timing (Figure 2A) and details (Figure 2B) of the events on follow-up. Their cumulative 5-year incidence was 53.0% (39.5%–70.9%). Figure S4 shows the incidence graphs individually for the subgroups of definite and probable CS. Arrhythmic events occurred in 2 of 21 patients (10%) with modest LV dysfunction (LVEF, 36%–50%) and 5 of 38 (13%) with normal LV function (EF >50%) at presentation.
Figure 2.
Emergence of ICD indications in follow-up. Incidence graph with 95% CIs for the composite of sudden cardiac death (SCD), sustained ventricular tachycardia (VT), and emergence of Class I or IIa indications for an implantable cardioverter defibrillator (ICD)10 in patients without such indications at presentation of cardiac sarcoidosis (A). Emerging ICD indications are specified in the pie chart (B). LVEF indicates left ventricular ejection fraction.
Application of the 2017 ACC/AHA/HRS Recommendations
As Table 1 shows, the 2017 ACC/AHA/HRS guideline shares Class I to IIa ICD indications with the 2014 HRS statement but recommends, in addition, implantation in the presence of LV scar on cardiac imaging (Class IIa indication). The phrasing of the recommendation alternates between “LV scar” and an undefined “extensive LV scar.”11 We applied the guideline to patients (n=245) in whom we had either 1 of or both high-quality CMRI or SPECT perfusion scan for reanalysis. Altogether, 240 of the 245 patients had myocardial LGE on CMRI (n=201 of 206) or a perfusion defect on SPECT (n=89 of 97) compatible with the presence of LV scar. Because the remaining 5 patients had other Class I or IIa indications, an ICD implantation should have been performed or considered by the guideline in 100% of cases. For a possible definition of extensive LV scar, we scrutinized the pertinent literature, identifying, as detailed in the literature review presented in the Supplemental Material, an extent of LGE mass >6% on CMRI as the risk discriminator best applicable to our cohort. Using this criterion as an index of extensive LV scar resulted in 95% of patients (176 of 185 with high-quality images) having Class I or IIa indications for ICD implantation. Of the remaining 9 patients, 1 individual developed a Class IIa indication on follow-up, but none had an arrhythmic event.
Discussion
The present study of a 30-year cohort of clinically manifest CS provides several noteworthy observations. The findings to take home first are the 5-year incidence of fatal or aborted SCD approaching 10% and the parallel rate of SCD or sVT amounting to 24% despite CS-targeted medical therapy. The other key observations relate to the retrospective application of the guidelines for ICD implantations in CS. By the 2014 HRS recommendations,10 85% of our patients had Class I or IIa indications for an early ICD implantation, and the remaining 15%, considered unlikely to benefit from an ICD, experienced fatal and nonfatal arrhythmic events at a rate comparable to the rate in patients with Class IIa indications. Furthermore, their combined incidence of arrhythmic events and emerging ICD indications exceeded 50% at 5 years of follow-up. By the 2017 ACC/AHA/HRS recommendations,11 in turn, every analyzable patient with CS had Class I or IIa indications for an early ICD implantation. Additional findings showed that CS diagnosis with histology of myocardial granulomas (definite CS) indicated an increased risk of fatal and nonfatal arrhythmic events.
Context of the Study
The clinical context of our study needs emphasis because it bears significantly on our observations and their implications. Most patients were admitted to cardiology services for new-onset and frequently serious cardiac manifestations, underwent diagnostic studies, and were found to have CS either during the first admission or in subsequent examinations. In this respect, our work differs substantially from the outcome studies done in patients with suspected CS only,21–23 in mixed groups with suspected and proven CS,24–26 or in patients with prevalent extracardiac sarcoidosis undergoing cardiac screening for research purposes regardless of symptoms.27–31 It is notable that only one-fifth of our patients had a history of preexisting systemic sarcoidosis on admission. Recognizing the fundamental differences in the patient phenotypes between such studies and ours helps put the present observations in perspective. Our findings reflect newly diagnosed and clinically manifest CS instead of the entire disease spectrum encompassing silent or even merely suspected cardiac involvement.
Guidelines for ICD Implantations in CS
Because of the frequency of life-threatening VAs and the risk of SCD, along with the high prevalence of AVB, clinicians often need to consider intracardiac device therapy in CS. The 2008 ACC/AHA/HRS general device guideline32 gave a Class IIa recommendation for ICD implantation in CS without further specifications. Because of the increased but difficult-to-stratify risk of SCD, acknowledging the lack of evidence, the contemporary experts thought it reasonable to consider ICD implantation in all patients with CS having a meaningful expected survival.32 More specific recommendations were issued by the HRS in 201410 and by the ACC/AHA/HRS consortium in 201811 (see Table 1). The Japanese Circulation Society has published national guidelines33 that, however, are more restrictive and were not applied here. Comparable guidance from the European Society of Cardiology does not exist, apart from the 2021 cardiac pacing guidelines34 stating briefly that patients with CS, high-grade AVB, and LVEF <50% should receive a resynchronization therapy defibrillator instead of a pacemaker.
Application to our CS cohort of the recommendations shown in Table 1 yielded surprising observations. By the 2014 HRS guideline,10 85% the cohort had Class I or IIa ICD indications; the remaining 15% of patients, considered not to need an ICD, had fatal and nonfatal arrhythmic events at a rate comparable to the rate in patients with Class IIa ICD indications. By the 2017 consortium guideline,11 in turn, every patient analyzable for the presence of LV scar met the criteria for ICD implantation. Of note, equal findings were recently found in a Japanese study applying the 2017 ACC/AHA/HRS recommendations to a cohort of 188 consecutive patients with CS.35 Even then, nearly all patients (95%) had Class I or IIa ICD indications, and the rest had serious events (SCD, ventricular fibrillation, or ventricular tachycardia) at a rate close to the annualized rate of 2.1% in patients with Class IIa indications.35 We believe that these observations reflect the high arrhythmogenicity of CS rather than just suggesting that the guidelines are poorly formulated. Still, their risk assessment tools need improvement. In the present cohort, definite CS diagnosis, reflecting the severity and extent of cardiac involvement, was an important indicator of the risk of arrhythmia, along with the presence of Class I or IIa ICD indications. This shows that prognostically important disease characteristics remain outside the criteria for increased SCD risk in the current ICD guidelines. Using more detailed information on the presenting manifestations, ventricular function, and findings on CMRI and positron emission tomography could be considered, perhaps in the form of a risk score. Positron emission tomography, for instance, can be expected to help because abnormal uptake of 18F-fluorodeoxyglucose associated with perfusion defects, suggesting coexistence of LV scar and active inflammation, predicts the outcome better than LV scar alone.36,37 New prognostic biomarkers have also been raised lately,38 and several works suggest that the status of the right ventricle deserves more prognostic emphasis.30,31,36 However, all of this requires additional research, preferably in even larger multicenter study populations than ours.
Dual Role of Myocardial LGE in CS
The inclusion of myocardial LGE among the Class IIa ICD indications in the consortium guideline11 is problematic because LGE also constitutes a major diagnostic criterion for CS.10,33 Taken to the letter, the recommendation would mean that whenever myocardial LGE is found in proven sarcoidosis, as is the case in ≈30% of patients,29 an ICD indication is born, symptoms notwithstanding. It is good to know, however, that the prognostic value of LGE is based on CMRI studies involving, partly or entirely, patients with sarcoidosis and suspected instead of proven cardiac involvement.21–31 It is self-evident that the presence of LGE comes out predictive from such analyses because it identifies individuals with the highest likelihood of true CS and thus an inherently high risk. Aside from our previous report,39 only 3 prognostic CMRI studies exist that involve exclusively patients with confirmed CS.40–42 In 2 of these studies,40,41 myocardial LGE was not a statistically significant predictor of arrhythmic events. Given the dual role of myocardial LGE, its extent appears a more reasonable prognostic factor. However, extensive LV scar in the prognostic sense is neither defined in the guideline11 nor agreed on in the CS literature. As our literature review in the Supplemental Material shows, the reported LGE mass thresholds for high risk differ widely and pertain to a variety of composite end points. In the present work, LGE mass >6% as an index of extensive LV scar resulted in 95% of patients still having an indication for ICD implantation. In comparison, in a large prospective study of nonischemic cardiomyopathy,43 the LGE mass threshold for optimal prediction of SCD was 2%, with values of 6% to 10% predicting a 5-year SCD risk of 6.4% even in the absence of severe LV dysfunction.
Clinical Implications
The present observations confirm the high arrhythmogenicity of clinically manifest CS. The 5-year risk of SCD or sVT was 24% in the entire cohort and 12% even in patients considered by the 2014 HRS guideline10 to have low risk and not to benefit from an ICD. The 5-year risk of SCD alone was close to 5% in the absence of ICD indications. In comparison, the European guideline for hypertrophic cardiomyopathy,44 another disease with a threat of arrhythmic SCD, states that ICD implantation should be considered if the estimated 5-year risk of SCD is ≥6% and may be considered if the risk is 4% to 6%. We think that discussion of the risk of fatal arrhythmias and the benefits and shortcomings of ICD implantation should be part of shared decision making with each newly diagnosed patient starting CS-targeted therapy. If an ICD is not implanted, repeat risk assessment is needed during surveillance.
Whether the prognostic role of myocardial versus extracardiac diagnostic histology should be factored into the risk assessment remains unanswered. The prevailing diagnostic strategies differ across countries and institutions, with many centers, unlike ours, preferring extracardiac over endomyocardial biopsies or diagnosing CS without any proof of histology.10,33,45,46 In our study, a more extensive cardiac involvement could explain both the positive endomyocardial biopsies and the poorer prognosis of definite CS. Yet, it cannot be excluded that part of the prognostic difference could be attributable to the probable CS category including less risky myocardial conditions being misdiagnosed as CS. A recent study of 422 patients from Japan also showed that myocardial histology–based CS diagnosis predicts poorer prognosis,45 but 43% of patients had no proof of sarcoidosis histology.
Strengths and Limitations
The strength of our work resides in the size and nationwide representativeness of our study population. The diagnosis of CS was based on widely used criteria,10 and nearly one-half of patients had CS confirmed by presence of myocardial granulomas. This reflects our practice of systematically preferring endomyocardial biopsies for proof of histology.47,48 In centers preferring extracardiac biopsies10 or emphasizing imaging-based CS diagnosis at the expense of histology,5,49 all our findings may not be applicable one to one. It is important to note that subclinical cases of CS, typically found on screening of patients with extracardiac sarcoidosis, were not studied here. We also excluded all 64 autopsy-diagnosed cases, among which 24 patients had presented with cardiac manifestations and undergone diagnostic examinations with CS remaining missed until autopsy. In retrospect, 21 of them met Class I or IIa criteria for ICD implantation by the 2014 HRS guideline. Had we included these cases in our analyses, the 5-year incidence of SCD would have been 14% in the total cohort and 8% in the absence of ICD indications. The availability of diagnostic examinations evolved over the 30-year coverage of our study, explaining the lack of modern cardiac imaging examinations in many patients. Therefore, the 2017 ACC/AHA/HRS recommendations11 could not be applied to the entire CS cohort. We used SPECT scans for LV scar quantification in addition to CMRI because positron emission tomography perfusion studies were rarely done over the decades of our work. Given the role assigned to programmed electric stimulation studies in the guidelines,10,11 their low number here is an important limitation. Although the size of our study was considerable given the rarity of CS, the number of patients and events remained small from the viewpoint of statistics. We acknowledge the limited power of our comparative analyses of time-to-event data. Last, because fewer patients without than with Class I or IIa ICD indications had an intracardiac device (54% versus 90%; Table S2), the sensitivity of our study to detect all episodes of ventricular tachycardia was inferior in the former group. The difference between the groups in the combined incidence of SCD and sVT may therefore have been even smaller than what our data show.
Conclusions
Our work shows that patients presenting with clinically manifest CS have a 10% cumulative risk of SCD at 5 years from disease presentation and a parallel 24% composite risk of SCD and sVT. By current guidelines, most patients (85%–100%) have at least 1 strong to modest indication for an early ICD implantation. Those without such an indication have a risk of SCD close to 5% and a combined incidence of life-threatening VAs and emerging ICD indications exceeding 50% at 5 years of follow-up. Diagnosis of CS based on myocardial histology (definite CS) predicts 2 times higher combined 5-year risk of SCD and serious VAs than diagnosis made by current consensus criteria without myocardial histology10 (probable CS). Patients with newly diagnosed CS should be informed of the risk of life-threatening VAs and the benefits and drawbacks of an early ICD implantation. Pending improved risk assessment, patients with CS with clinical manifestations falling inside the spectrum of our cohort should be recommended an ICD implantation.
Article Information
Acknowledgments
The authors are grateful to all the colleagues and research assistants who have helped them maintain the MIDFIN registry.
Sources of Funding
This work was supported by a Finnish government grant for medical research, Aarne Koskelo’s foundation, and the Finnish Foundation for Cardiovascular Research.
Disclosures
None.
Supplemental Material
Tables S1 and S2
Figures S1–S4
Literature Review with Table S3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ACC
- American College of Cardiology
- AHA
- American Heart Association
- AVB
- atrioventricular block
- CMRI
- cardiac magnetic resonance imaging
- CS
- cardiac sarcoidosis
- HRS
- Heart Rhythm Society
- ICD
- implantable cardioverter defibrillator
- LGE
- late gadolinium enhancement
- LV
- left ventricular
- LVEF
- left ventricular ejection fraction
- MIDFIN
- Myocardial Inflammatory Diseases in Finland
- SCD
- sudden cardiac death
- SPECT
- single-photon emission computed tomography
- sVT
- sustained ventricular tachycardia
- VA
- ventricular arrhythmia
Circulation is available at www.ahajournals.org/journal/circ
Supplemental Material, the podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.121.058120.
For Sources of Funding and Disclosures, see page 973.
Contributor Information
Pauli Pöyhönen, Email: pauli.poyhonen@hus.fi.
Jukka Lehtonen, Email: jukka.lehtonen@hus.fi.
Kaj Ekström, Email: kaj.ekstrom@helsinki.fi.
Valtteri Uusitalo, Email: valtteri.uusitalo@hus.fi.
Meri Niemelä, Email: meri.rainio@gmail.com.
Tapani Vihinen, Email: tapani.vihinen@tyks.fi.
Kari Kaikkonen, Email: kari.kaikkonen@ppshp.fi.
Petri Haataja, Email: petri.haataja@sydansairaala.fi.
Tuomas Kerola, Email: tuomas.kerola@phsotey.fi.
Tuomas T. Rissanen, Email: tuomas.rissanen@siunsote.fi.
Aleksi Alatalo, Email: aleksi.alatalo@epshp.fi.
Päivi Pietilä-Effati, Email: paivi.pietila-effati@ovph.fi.
Markku Kupari, Email: markku.kupari@hus.fi.
References
- 1.Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, Daubert JP, de Chillout C, DePasquale EC, Desai MY, et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16:e301–e372. doi: 10.1016/j.hrthm.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 2.Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, Kaikkonen K, Tuohinen S, Haataja P, Kerola T, et al. Cardiac sarcoidosis: epidemiology, characteristics and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–632. doi: 10.1161/CIRCULATIONAHA.114.011522 [DOI] [PubMed] [Google Scholar]
- 3.Ekström K, Lehtonen J, Nordenswan H-K, Mäyränpää MI, Räisänen-Sokolowski A, Kandolin R, Simonen P, Pietilä-Effati P, Alatalo A, Utriainen S, et al. Sudden death in cardiac sarcoidosis: an analysis of clinical and cause-of-death registries. Eur Heart J. 2019:3121–3128. doi: 10.1093/eurheartj/ehz428 [DOI] [PubMed] [Google Scholar]
- 4.Okada DR, Smith J, Derakhshan A, Gowani Z, Misra S, Berger RD, Calkins H, Tandri H, Chrispin J. Ventricular arrhythmias in cardiac sarcoidosis. Circulation. 2018;138:1253–1264. doi: 10.1161/CIRCULATIONAHA.118.034687 [DOI] [PubMed] [Google Scholar]
- 5.Gilotra N, Okada D, Sharma A, Crispin J. Management of cardiac sarcoidosis in 2020. Arrhythm Electrophysiol Rev. 2020;9:182–188. doi: 10.15420/aer.2020.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenfeld LE, Chung MK, Harding CV, Spagnolo P, Grunewald J, Appelbaum J, Sauer WH, Culver DA, Joglar JA, Lin BA, et al. Arrhythmias in cardiac sarcoidosis bench to bedside: a case-based review. Circ Arrhythm Electrophysiol. 2021;14:e009203. doi: 10.1161/CIRCEP.120.009203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kron J, Sauer W, Schuller J, Bogun F, Crawford T, Sarsam S, Rosenfeld L, Mitiku TY, Cooper JM, Mehta D, et al. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace. 2013;15:347–354. doi: 10.1093/europace/eus316 [DOI] [PubMed] [Google Scholar]
- 8.Halawa A, Jain R, Turagam MK, Kusumoto FM, Woldu HG, Gautam S. Outcome of implantable cardioverter-defibrillator in cardiac sarcoidosis: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2020;58:233–242. doi: 10.1007/s10840-020-00705-1 [DOI] [PubMed] [Google Scholar]
- 9.Takaya Y, Kusano K, Nishii N, Nakamura K, Ito H. Early and frequent defibrillator discharge in patients with cardiac sarcoidosis compared with patients with idiopathic dilated cardiomyopathy. Int J Cardiol. 2017;240:302–306. doi: 10.1016/j.ijcard.2017.04.044 [DOI] [PubMed] [Google Scholar]
- 10.Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043 [DOI] [PubMed] [Google Scholar]
- 11.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018;138:e272–e391. doi: 10.1161/CIR.0000000000000549 [DOI] [PubMed] [Google Scholar]
- 12.Ekström K, Räisänen-Sokolowski A, Lehtonen J, Nordenswan H-K, Mäyränpää MI, Kupari M. Idiopathic giant cell myocarditis or cardiac sarcoidosis? A retrospective audit of a nationwide case series. ESC Heart Fail. 2020;7:1362–1370. doi: 10.1002/ehf2.12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E; Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, Society for Cardiovascular Magnetic Resonance: Board of Trustees Task Force on Standardized Protocols. J Cardiovasc Magn Reson. 2008;10:35. doi: 10.1186/1532-429X-10-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post-Processing. J Cardiovasc Magn Reason. 2013;15:35. doi: 10.1186/1532-429X-15-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS; American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975 [DOI] [PubMed] [Google Scholar]
- 16.Flett AS, Hasleton J, Cook C, Hausenloy D, Quarta G, Ariti C, Muthurangu V, Moon JC. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging. 2011;4:150–156. doi: 10.1016/j.jcmg.2010.11.015 [DOI] [PubMed] [Google Scholar]
- 17.Hesse B, Tägil K, Cuocolo A, Anagnostopoulos C, Bardiés M, Bax J, Bengel F, Busemann Sokole E, Davies G, Dondi M, et al. ; EANM/ESC Group. EANM/ESC procedural guidelines for myocardial perfusion imaging in nuclear cardiology. Eur J Nucl Med Mol Imaging. 2005;32:855–897. doi: 10.1007/s00259-005-1779-y [DOI] [PubMed] [Google Scholar]
- 18.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 21.Agoston-Coldea L, Kouaho S, Sacre K, Dossier A, Escoubet B, Chillon S, Laissy J-P, Rouzet F, Kutty S, Extramiana F, et al. High mass (>18g) of late gadolinium enhancement on CMR imaging is associated with major cardiac events on long-term outcome in patients with biopsy-proven extracardiac sarcoidosis. Int J Cardiol. 2016;222:950–956. doi: 10.1016/j.ijcard.2016.07.233 [DOI] [PubMed] [Google Scholar]
- 22.Murtagh G, Laffin LJ, Beshai JF, Maffessanti F, Bonham CA, Patel AV, Yu Z, Addetia K, Mor-Avi V, Moss JD, et al. Prognosis of myocardial damage in sarcoidosis patients with preserved left ventricular ejection fraction: risk stratification using cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2016;9:e003738. doi: 10.1161/CIRCIMAGING.115.003738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flamée L, Symons R, Degtiarova G, Dresselaers T, Gheysens O, Wuyts W, Van Cleemput J, Bogaert J. Prognostic value of cardiovascular magnetic resonance in patients with biopsy-proven systemic sarcoidosis. Eur Radiol. 2020;30:3702–3710. doi: 10.1007/s00330-020-06765-1 [DOI] [PubMed] [Google Scholar]
- 24.Nadel J, Lancefield T, Voskoboinik A, Taylor AJ. Late gadolinium enhancement identified with cardiac magnetic resonance imaging in sarcoidosis patients is associated with long-term ventricular arrhythmia and sudden cardiac death. Eur Heart J Cardiovasc Imaging. 2015;16:634–641. doi: 10.1093/ehjci/jeu294 [DOI] [PubMed] [Google Scholar]
- 25.Yasuda M, Iwanaga Y, Kato T, Izumi T, Inuzuka Y, Nakamura T, Miyaji Y, Kawamura T, Ikeguchi S, Inoko M, et al. Risk stratification for major adverse cardiac events and ventricular tachyarrhythmias by cardiac MRI in patients with cardiac sarcoidosis. Open Heart. 2016;3:e000437. doi: 10.1136/openhrt-2016-000437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazmirczak F, Chen KA, Adabag S, von Wald L, Roukoz H, Benditt DG, Okasha O, Farzaneh-Far A, Markowitz J, Nijjar PS, et al. Assessment of the 2017 AHA/ACC/HRS guideline recommendations for implantable cardioverter-defibrillator implantation in cardiac sarcoidosis. Circ Arrhythm Electrophysiol. 2019;12:e007488. doi: 10.1161/CIRCEP.119.007488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, Meine TJ, White JB, Elliott MD, Kim HW, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–1977. doi: 10.1161/CIRCULATIONAHA.109.851352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greulich S, Deluigi CC, Gloekler S, Wahl A, Zürn C, Kramer U, Nothnagel D, Bültel H, Schumm J, Grün S, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2013;6:501–511. doi: 10.1016/j.jcmg.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 29.Kouranos V, Tzelepis GE, Rapti A, Mavrogeni S, Aggeli K, Douskou M, Prasad S, Koulouris N, Sfikakis P, Wells A, et al. Complementary role of CMR to conventional screening in the diagnosis and prognosis of cardiac sarcoidosis. JACC Cardiovasc Imaging. 2017;10:1437–1447. doi: 10.1016/j.jcmg.2016.11.019 [DOI] [PubMed] [Google Scholar]
- 30.Smedema J-P, van Geuns R-J, Ector J, Heidbuchel H, Ainslie G, Crijns HJGM. Right ventricular involvement and the extent of left ventricular enhancement with magnetic resonance predict adverse outcomes in pulmonary sarcoidosis. ESC Heart Fail. 2018;5:157–171. doi: 10.1002/ehf2.12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kagioka Y, Yasuda M, Okune M, Kakehi K, Kawamura T, Kobuke K, Miyazaki S, Iwanaga Y. Right ventricular involvement is an important prognostic factor and risk stratification tool in suspected cardiac sarcoidosis: analysis by cardiac magnetic resonance imaging. Clin Res Cardiol. 2020;109:988–998. doi: 10.1007/s00392-019-01591-y [DOI] [PubMed] [Google Scholar]
- 32.Epstein AE, DiMarco JP, Ellenbogen KA, Estes M, Friedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:e1–e62. doi: 10.1016/j.jacc.2008.02.032 [DOI] [PubMed] [Google Scholar]
- 33.Terasaki F, Azuma A, Anzai T, Ishizaka N, Ishida Y, Isobe M, Inomata T, Ishibashi-Ueda H, Eishi Y, Kitakaze M, et al. ; Japanese Circulation Society. Guidelines for the diagnosis and treatment of cardiac sarcoidosis. Circ J. 2019;83:2329–2388. doi: 10.1253/circj.CJ-19-0508 [DOI] [PubMed] [Google Scholar]
- 34.Glikson M, Nielsen JG, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, Barrabés JA, Boriani G, Braunschweig F, Brignole M, et al. ; ESC Scientific Document Group. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42:3427–3520. doi: 10.1093/eurheartj/ehab36434455430 [Google Scholar]
- 35.Takenaka S, Kobayashi Y, Nagai T, Kato Y, Komoriyama H, Nagano N, Kamiya K, Konishi T, Sato T, Omote K, et al. Applicability of the AHA/ACC/HRS guideline for implantable cardioverter-defibrillator implantation in Japanese patients with cardiac sarcoidosis. JACC Clin Electrophysiol. 2021;7:1410–1418. doi: 10.1016/j.jacep.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, Kazemian P, Kwong RY, Tokuda M, Skali H, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–336. doi: 10.1016/j.jacc.2013.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sperry BW, Tamarappoo BK, Oldan JD, Javed O, Culver DA, Brunken R, Cerqueira MD, Hachamovitch R. Prognostic impact of extent, severity, and heterogeneity of abnormalities on 18F-FDG PET scans for suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2018;11:336–345. doi: 10.1016/j.jcmg.2017.04.020 [DOI] [PubMed] [Google Scholar]
- 38.Yoshitomi R, Kobayashi S, Yano Y, Nakashima Y, Fujii S, Nanno T, Ishiguchi H, Fukuda M, Yoshiga Y, Okamura T, et al. Enhanced oxidative stress and presence of ventricular aneurysm for risk prediction in cardiac sarcoidosis. Heart. 2022;108:429–437. doi: 10.1136/heartjnl-2021-320244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ekström K, Lehtonen J, Hänninen H, Kandolin R, Kivistö S, Kupari M. Magnetic resonance imaging as a predictor of survival free of transplantation and life-threatening ventricular arrhythmias in cardiac sarcoidosis. J Am Heart Assoc. 2016;5:e003040. doi: 10.1161/JAHA.115.003040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shafee MA, Fukuda K, Wakayama Y, Nakano M, Kondo M, Hasebe Y, Kawana A, Shimokawa H. Delayed enhancement on cardiac magnetic resonance imaging is a poor prognostic factor in patients with cardiac sarcoidosis. J Cardiol. 2012;60:448–453. doi: 10.1016/j.jjcc.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 41.Ise T, Hasegawa T, Morita Y, Yamada N, Funada A, Takahama H, Amaki M, Kanzaki H, Okamura H, Kamakura S, et al. Extensive late gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in left ventricular function after steroid therapy in cardiac sarcoidosis. Heart. 2014;100:1165–1172. doi: 10.1136/heartjnl-2013-305187 [DOI] [PubMed] [Google Scholar]
- 42.Crawford T, Mueller G, Sarsam S, Prasitdumrong H, Chaiyen N, Gu X, Schuller J, Kron J, Nour KA, Cheng A, et al. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2014;7:1109–1115. doi: 10.1161/CIRCEP.113.000156 [DOI] [PubMed] [Google Scholar]
- 43.Klem I, Klein M, Khan M, Yang EY, Nabi F, Ivanov A, Bhatti L, Hayes B, Graviss EA, Nguyen DT, et al. Relationship of LVEF and myocardial scar to long-term mortality risk and mode of death in patients with nonischemic cardiomyopathy. Circulation. 2021;143:1343–1358. doi: 10.1161/CIRCULATIONAHA.120.048477 [DOI] [PubMed] [Google Scholar]
- 44.Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–2779. doi: 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
- 45.Kusano K, Ishibashi K, Noda T, Nakajima K, Nakasuka K, Terasaki S, Hattori Y, Nagayama T, Mori K, Takaya Y, et al. Prognosis and outcomes of clinically diagnosed cardiac sarcoidosis without positive endomyocardial biopsy findings. JACC Asia 2021;1:385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenbaum AN, Kolluri N, Elwazir MY, Kapa S, Abou Ezzeddine OF, Bois JP, Chareonthaitawee P, Schmidt TJ, Cooper LT. Identification of a novel presumed cardiac sarcoidosis category for patients at high risk of disease. Int J Cardiol. 2021;335:66–72. doi: 10.1016/j.ijcard.2021.04.022 [DOI] [PubMed] [Google Scholar]
- 47.Kandolin R, Lehtonen J, Graner M, Schildt J, Salmenkivi K, Kivistö SM, Kupari M. Diagnosing isolated cardiac sarcoidosis. J Intern Med. 2011;270:461–468. doi: 10.1111/j.1365-2796.2011.02396.x [DOI] [PubMed] [Google Scholar]
- 48.Simonen P, Lehtonen J, Kandolin R, Schildt J, Marjasuo S, Miettinen H, Airaksinen J, Vihinen T, Tuohinen S, Haataja P, et al. F-18-fluorodeoxyglucose positron emission tomography-guided sampling of mediastinal lymph nodes in the diagnosis of cardiac sarcoidosis. Am J Cardiol. 2015;116:1581–1585. doi: 10.1016/j.amjcard.2015.08.025 [DOI] [PubMed] [Google Scholar]
- 49.Miller EJ, Culver DA. Establishing an evidence-based method to diagnose cardiac sarcoidosis: the complementary use of cardiac magnetic resonance imaging and FDG-PET. Circ Cardiovasc Imaging. 2018;11:e007408. doi: 10.1161/CIRCIMAGING.117.007408 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.