Abstract
Purpose
To analyze reticular pseudodrusen (RPD) as an independent risk factor for progression to late age-related macular degeneration (AMD), alongside traditional macular risk factors (soft drusen and pigmentary abnormalities) considered simultaneously.
Design
Post hoc analysis of two clinical trial cohorts: Age-Related Eye Disease Study (AREDS) and AREDS2.
Participants
Eyes with no late AMD at baseline in AREDS (n=6959 eyes, 3780 participants; mean age 69.4y) and AREDS2 (n=3355 eyes, 2056 participants; mean age 72.3y).
Methods
Color fundus photographs (CFP) from annual study visits were graded for soft drusen, pigmentary abnormalities, and late AMD. RPD presence was determined by grading of fundus autofluorescence images (AREDS2) and deep learning grading of CFP (AREDS). Proportional hazards regression analyses were performed, considering AREDS AMD severity scales (modified simplified severity scale (person) and 9-step scale (eye)) and RPD presence simultaneously.
Main outcome measures
Progression to late AMD, geographic atrophy (GA), and neovascular AMD (NV).
Results
In AREDS, for late AMD analyses by person, in a model considering the modified simplified severity scale simultaneously, RPD presence was associated with higher risk of progression: hazard ratio (HR) 2.15 (95% CI 1.75–2.64). However, the risk associated with RPD presence differed significantly at different simplified severity scale levels: HR 3.23 (1.60–6.51), 3.81 (2.38–6.10), 2.28 (1.59–3.27), and 1.64 (1.20–2.24), at levels 0-1/2/3/4, respectively. Considering the 9-step scale (by eye), RPD presence was also associated with higher risk: HR 2.54 (2.07–3.13). The HRs were 5.11 (3.93–6.66) at levels 1–6 and 1.78 (1.43–2.22) at 7–8. In AREDS2, by person, RPD presence was not associated with higher risk: HR 1.18 (0.90–1.56); by eye, it was: HR 1.57 (1.31–1.89). No significant differences in risk were observed at different severity levels, for the limited spectrum in AREDS2. In both cohorts, RPD presence carried higher risk for GA than NV.
Conclusions
RPD represent an important anatomical risk factor for progression to late AMD, particularly GA. However, the added risk associated with RPD varies markedly by severity level. It carries highly increased risk at lower/moderate levels and less increased risk at higher levels. RPD status should be included in updated AMD classification systems, risk calculators, and clinical trials.
Précis
Reticular pseudodrusen presence is associated with increased risk of progression to late age-related macular degeneration, particularly geographic atrophy, above that captured by soft drusen and pigmentary abnormalities.
Introduction
Making accurate prognostic predictions of the risk of progression to late age-related macular degeneration (AMD), as a form of personalized medicine, is important for several reasons: to inform and counsel patients, to recommend evidence-based interventions where suitable, to make tailored plans for follow-up and reimaging at appropriate intervals, and/or for home monitoring, and for research and clinical trial recruitment.1–8 Making predictions of the differential risk of atrophic versus neovascular AMD would also be valuable, for similar reasons.
Two common methods for making predictions for progression to late AMD, using phenotypic information alone, have been described previously.9,10 The Age-Related Eye Disease Study (AREDS) 9-step Severity Scale is a detailed scale that can be applied to any eye without late AMD; a score from 1–9 is assigned (with 9 representing non-central geographic atrophy (GA)), based on quantitative assessment of soft drusen and AMD pigmentary abnormalities in the macula.9 Use of this scale is typically confined to the research and reading center settings, owing to its complexity. By contrast, the AREDS Simplified Severity Scale was designed for everyday use in the clinical setting.10 It is applied at the person level, as described below. In both cases, higher levels on the scale are associated with increased risk of progression to late AMD; the 5-year rates have been published previously.9,10 Hence, both of these common methods for making predictions of progression to late AMD rely on just two macular features: soft drusen and pigmentary abnormalities.
Since the publication of these scales, awareness has increased of a third common phenotypic feature in AMD: subretinal drusenoid deposits (SDD), also known as reticular pseudodrusen (RPD).11,12 RPD are often poorly visible on clinical examination or color fundus photography (CFP), but are well demonstrated on multimodal imaging, including fundus autofluorescence (FAF), near-infrared (NIR) reflectance, and optical coherence tomography (OCT).11–17 The availability of multimodal imaging and expert grading might represent a limitation to incorporating RPD into severity scales for routine clinical use. However, recent deep learning approaches have demonstrated that RPD presence can be detected automatically with high accuracy from FAF images or OCT images, and even with moderately high accuracy from CFP images alone.18–20 This has the potential to widen substantially the accessibility of RPD grading.
Importantly, longitudinal studies such as the AREDS2 have shown that RPD presence is associated with increased risk of progression to late AMD.15 It is therefore likely that incorporating this third feature into severity scales and prediction algorithms may improve their accuracy. However, in the previous AREDS2 analyses, the altered risk was reported in isolation, irrespective of AMD severity related to the two other macular features.15 It remains to be studied whether the increased risk of progression to late AMD is distributed evenly across the spectrum of non-advanced AMD severity.
The purpose of the current study was therefore to analyze rates of progression to late AMD outcomes, based on a combination of the new feature (RPD) and the two traditional features (soft drusen and pigmentary abnormalities), considered simultaneously. This represents an important step towards the development of an updated clinical classification system and improved risk prediction algorithms.
Methods
Study populations and procedures
The AREDS was a multicenter phase III randomized controlled clinical trial designed to assess the effects of nutritional supplements on AMD and cataract progression.21 The AREDS study design has been described previously.21 In brief, 4,757 participants aged 55 to 80 years were recruited between 1992 and 1998 at 11 retinal specialty clinics in the United States. The randomized clinical trial lasted five years and was followed by epidemiologic follow-up for another five years.
The AREDS2 was a multicenter phase III randomized controlled clinical trial designed to assess the effects of carotenoid and omega-3 fatty acid supplements on the course of AMD in people at moderate to high risk of progression to late AMD.22 The AREDS2 study design has been described previously.22 In brief, 4,203 participants aged 50 to 85 years were recruited between 2006 and 2008 at 82 retinal specialty clinics in the United States. Inclusion criteria at enrollment were the presence of either bilateral large drusen or advanced AMD in one eye and large drusen in the fellow eye. The randomized clinical trial lasted five years.
In both studies, at baseline and annual study visits, comprehensive eye examinations were performed, and CFP were taken by certified study personnel, according to standardized protocols. In both cases, the primary endpoint was progression to advanced AMD (defined as neovascular AMD or central GA), based on CFP grading and/or a history of treatment for neovascular AMD, as described previously.21,22 For both studies, institutional review board approval was obtained at each clinical site and written informed consent for the research was obtained from all study participants. The research was conducted according to the tenets of the Declaration of Helsinki. The AREDS2 complied with the Health Insurance Portability and Accountability Act.
AREDS2 fundus autofluorescence imaging and reading center grading for reticular pseudodrusen
The AREDS2 ancillary study of FAF imaging was conducted at 66 selected clinic sites, according to the availability of imaging equipment, as described previously.15 Sites were permitted to join the ancillary study at any time after FAF imaging equipment became available during the five-year study period. The FAF images were acquired from the Heidelberg Retinal Angiograph (Heidelberg Engineering, Heidelberg, Germany) and fundus cameras with autofluorescence capability by certified technicians using a standardized protocol. For the Heidelberg images, a single image was acquired at 30 degrees, centered on the fovea, captured in high-speed mode (768 ×768 pixels), with the automated real time mean function set at 14.
The FAF images were assessed for RPD presence by graders at the Wisconsin Reading Center. The grading protocol and definitions have been described previously.15 In brief, RPD were defined as clusters of discrete round or oval lesions of hypoautofluorescence, usually similar in size, or confluent ribbon-like patterns with intervening areas of normal or increased autofluorescence; a minimum of 0.5 disc areas (approximately five lesions) was required. Two primary graders at the reading center independently evaluated FAF images for the presence of RPD; in the case of disagreement, a senior grader would adjudicate the final grade. Inter-grader agreement for the presence/absence of RPD was 94%.15
The reading center graders were permitted to consider the whole time series of FAF images for each eye, particularly in the case of an individual image with poor quality for assessing RPD status.15 If the first FAF image available could not be graded for RPD status, but RPD were absent in subsequent FAF images, RPD status was considered absent in the first FAF image also. If RPD status could not be graded in the first FAF image, but RPD were present or could not be graded in subsequent FAF images, no such inference was made; RPD status remained missing for the first FAF image and that time point was excluded from the analyses (i.e., the baseline was shifted forwards in time, according to the approach described below).
AREDS deep learning algorithm grading of color fundus photographs for reticular pseudodrusen
Reading center grading for RPD presence was not available in the AREDS, owing to the absence of imaging other than CFP. Grades for RPD presence were therefore obtained by deep learning-based automated grading of the CFP. The algorithm and its performance metrics have been described previously.19 In brief, a deep learning algorithm was trained by exposing it to over 8000 AREDS2 CFP-FAF image pairs (from the AREDS2 ancillary FAF study described above). The ground truth labels for RPD presence/absence in each image pair came from grading of the FAF images, as described above (i.e., label transfer). Multimodal multitask training was used, whereby the algorithm first underwent joint training with two other deep learning algorithms (an FAF algorithm and a CFP-FAF algorithm), using a representation shared between the three algorithms. This was followed by additional training separately from the two other algorithms, i.e., fine-tuning training suitable for grading from CFP alone. The benefits of multimodal multitask training are that what is learned by each algorithm from each image modality can improve the training of the other algorithms (by sharing features that are complementary between the image modalities), i.e., the grading from CFP alone benefits from paired FAF images having been present during training. In previous evaluation of the deep learning algorithm on an AREDS2 test set of CFP images, it achieved an area under the receiver operating characteristic (AUROC) of 0.832; in external validation using an independent test set (Rotterdam Study, Netherlands), it achieved an AUROC of 0.965.19
Statistical methods
For the AREDS study population, eyes and participants were considered from the point of study baseline onwards, since RPD grades were available for all visits. For the AREDS2 study population, eyes and participants were considered from the earliest study visit possible, in the following way. In the case of AREDS2 eyes with RPD graded as absent at the first study visit with FAF available, RPD grades were defined as also absent in any previous study visits (i.e., those without FAF available); these eyes were considered from the time-point of study baseline onwards. In the case of AREDS2 eyes with RPD graded as present at the first study visit with FAF available, no assumptions were made about RPD presence/absence at previous time-points; these eyes were considered from the time-point of the first study visit with FAF available onwards (i.e., the baseline was shifted forwards in time).
Modified Simplified Severity Scale
The unit of analysis was at the person level, owing to the person-based nature of the severity scale. Analyses were conducted separately for the AREDS and AREDS2 datasets. In each case, eligible participants were those without late AMD (defined as neovascular AMD or any GA) in either eye at baseline and with at least two study visits. These participants were stratified on the basis of both (i) a modified simplified severity scale (0–4) at baseline and (ii) RPD presence/absence at baseline. The modified simplified severity scale was defined as follows, using information from both eyes: a score of 0–4 was recorded, based on the presence or absence of large drusen and AMD pigmentary abnormalities in the macula, with one point assigned for each feature in each eye (and, in the absence of large drusen in either eye, one point assigned if both eyes have medium-sized drusen). The modification from the original Simplified Severity Scale10 (in which non-central GA counted only as a pigmentary abnormality, rather than as part of the outcome) was made so that any GA (including non-central GA) could be considered as part of the outcome of late AMD, in line with modern practice. Multivariable proportional hazards regression was performed, with progression to (i) late AMD, (ii) neovascular AMD, and (iii) any GA as three separate outcomes, according to the modified severity scale and RPD presence. The models were adjusted for age, sex, smoking, and (for AREDS only) body mass index (BMI). The proportional hazards assumption was tested in all cases. Significance was set at p=0.05.
9-step Severity Scale
The unit of analysis was at the eye level, owing to the eye-based nature of this severity scale. Again, analyses were conducted separately for the AREDS and AREDS2 datasets. In each case, eligible eyes were those without late AMD (defined as neovascular AMD or any GA) at baseline and with at least two study visits. These eyes were stratified on the basis of both (i) 9-step Severity Scale at baseline and (ii) RPD presence/absence at baseline. Multivariable proportional hazards regression was performed, with progression to (i) late AMD, (ii) neovascular AMD, and (iii) GA as three separate outcomes, according to the 9-step Severity Scale and RPD presence. The models were adjusted for age, sex, smoking, BMI (for AREDS only), and correlation between eyes; adjustment for correlation between eyes was made in SAS by using the robust sandwich estimate for the covariance matrix in the Wald tests.23 The proportional hazards assumption was tested in all cases. Significance was set at p=0.05. All analyses were conducted using SAS version 9.4 (SAS Inc, Cary, NC).
Results
Risk of progression to late age-related macular degeneration by the modified simplified severity scale and reticular pseudodrusen status, at the person level, in the AREDS
The study population for these analyses comprised 3182 participants. Their demographic and clinical characteristics are shown in Table 1. The number of participants with RPD present at baseline (in either eye, by deep learning assessment of CFP) was 235 (7.4%). The number of participants that progressed to late AMD (defined as neovascular AMD or any GA) in either eye, during mean follow-up of 9.0 years, was 569 (17.9%).
Table 1.
Participant and eye characteristics of the study populations at baseline.
| AREDS, by person | AREDS, by eye | AREDS2, by person | AREDS2, by eye | |
|---|---|---|---|---|
| Participants/eyes | 3182 participants | 6959 eyes of 3780 participants | 1259 participants | 3355 eyes of 2056 participants |
| Mean age (years), mean (SD) | 69.0 (5.0) | 69.4 (5.1) | 71.0 (7.9) | 72.3 (7.9) |
| Female | 57.2% | 56.3% | 57.8% | 55.4% |
| Non-white | 5.1% | 4.5% | 4.8% | 4.7% |
| Current | 6.3% | 7.6% | 5.6% | 6.0% |
| >30 | 24.4% | 25.3% | ||
| RPD presence at baseline* | 7.4%† | 5.3% | 9.0%† | 11.4% |
| 4: 8.4% | 7–8: 16.1% | 4: 50.3% | 7–8: 51.9% | |
| Follow-up time (years), mean (SD) | 9.0 (2.9) | 8.8 (2.9) | 4.8 (0.8) | 4.8 (0.8) |
by deep learning-based automated grading of color fundus photographs (AREDS) or by reading center grading of fundus autofluorescence images (AREDS2)
for person-based analyses, defined as present if present in either eye
according to modified Simplified Severity Scale (levels 0–4), by person, or 9-step Severity Scale (levels 1–9), by eye
Abbreviations: AMD=age-related macular degeneration; AREDS=Age-Related Eye Disease Study; RPD=reticular pseudodrusen; SD=standard deviation
For the outcome of progression to late AMD, higher levels on the modified severity scale, considered in isolation from RPD status, were significantly associated with higher risks of progression. The hazard ratios were 7.0 (95% confidence interval 5.3–9.1, p<0.0001), 15.2 (11.7–19.9, p<0.0001), and 33.9 (26.1–44.0, p<0.0001) for severity levels 2, 3, and 4, respectively (compared with a reference level of 0–1). Similarly, RPD presence, considered in isolation from the modified severity scale, was associated with a significantly higher risk of progression: hazard ratio 4.7 (3.9–5.8, p<0.0001). However, with the severity scale and RPD presence considered simultaneously, a statistically significant interaction was observed between them: p=0.036. Stratified analyses were therefore performed. The hazard ratios associated with RPD presence, considered separately for severity levels 0–1, 2, 3, and 4, are shown in Table 2 and Figure 1. The hazard ratios associated with the severity scale, considered separately according to RPD presence and absence, are shown in Supplementary Table 1. The Kaplan-Meier curve of progression to late AMD, stratified according to the two variables considered simultaneously, is shown in Figure 2. In all analyses, similar results were obtained when considering RPD as a time-dependent variable.
Table 2.
Progression to late age-related macular degeneration and its subtypes in the Age-Related Eye Disease Study, by person: hazard ratios associated with reticular pseudodrusen presence, separately according to different levels on the modified Simplified Severity Scale.
| Late AMD | Geographic atrophy | Neovascular AMD | ||||
|---|---|---|---|---|---|---|
| Modified Simplified Severity Scale level | Hazard ratio associated with RPD presence (95% CI) | P value | Hazard ratio associated with RPD presence (95% CI) | P value | Hazard ratio associated with RPD presence (95% CI) | P value |
| 0–1 | 3.23 (1.60–6.51) | 0.001 | 3.47 (1.47–8.17) | 0.004 | 2.96 (1.03–8.46) | 0.043 |
| 2 | 3.81 (2.38–6.10) | <0.0001 | 4.97 (2.95–8.39) | <0.0001 | 3.03 (1.65–5.57) | 0.0004 |
| 3 | 2.28 (1.59–3.27) | <0.0001 | 3.08 (2.10–4.54) | <0.0001 | 1.13 (0.68–1.88) | 0.64 |
| 4 | 1.64 (1.20–2.24) | 0.002 | 1.91 (1.39–2.65) | <0.0001 | 0.96 (0.62–1.51) | 0.87 |
Abbreviations: AMD=age-related macular degeneration; CI=confidence interval; RPD=reticular pseudodrusen
Figure 1.
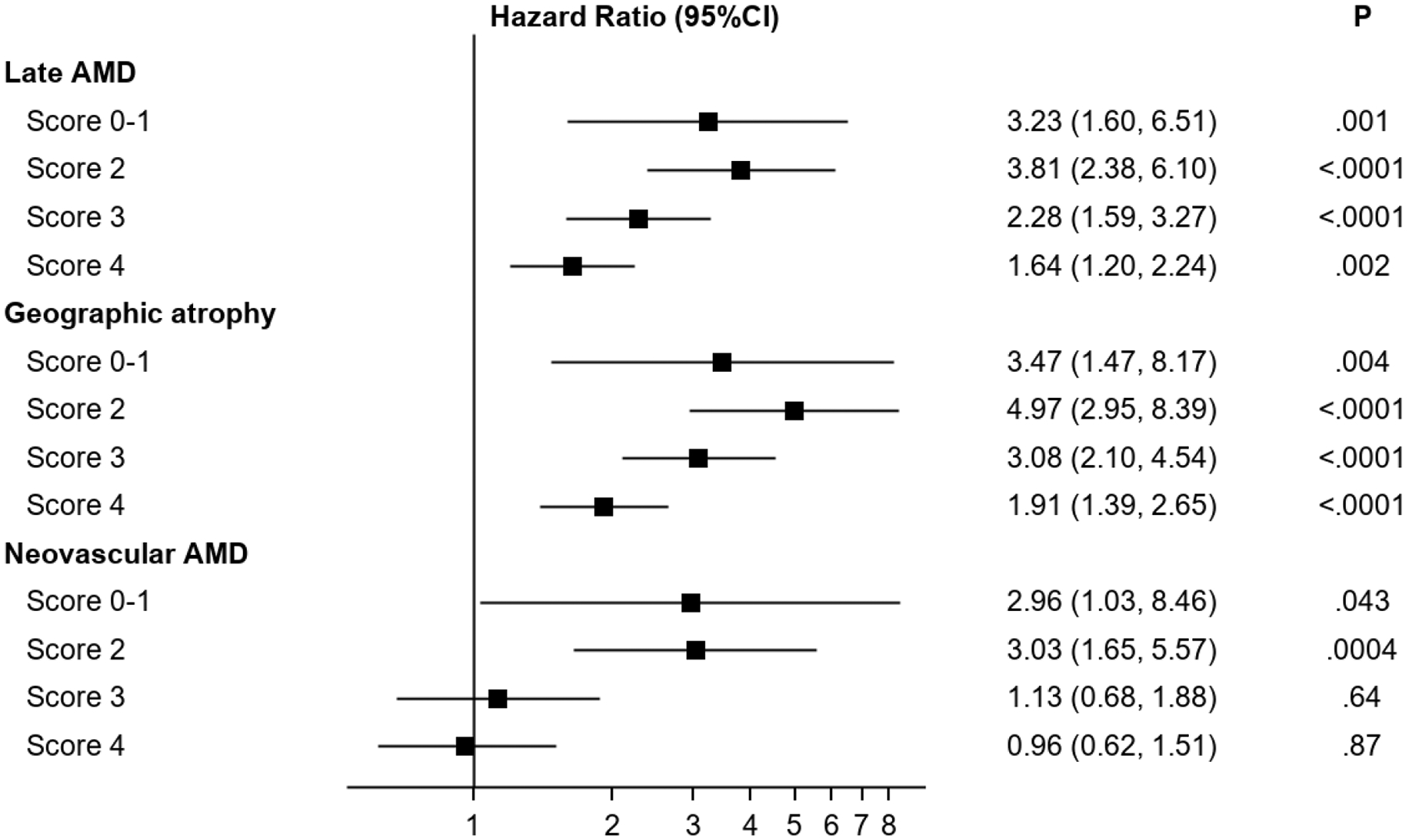
Forest plot of hazard ratios associated with reticular pseudodrusen presence for progression to late age-related macular degeneration (and its subtypes) in the Age-Related Eye Disease Study, at the person level, separately according to different levels on the modified Simplified Severity Scale. The 95% confidence intervals and associated p-values are also shown.
Figure 2.
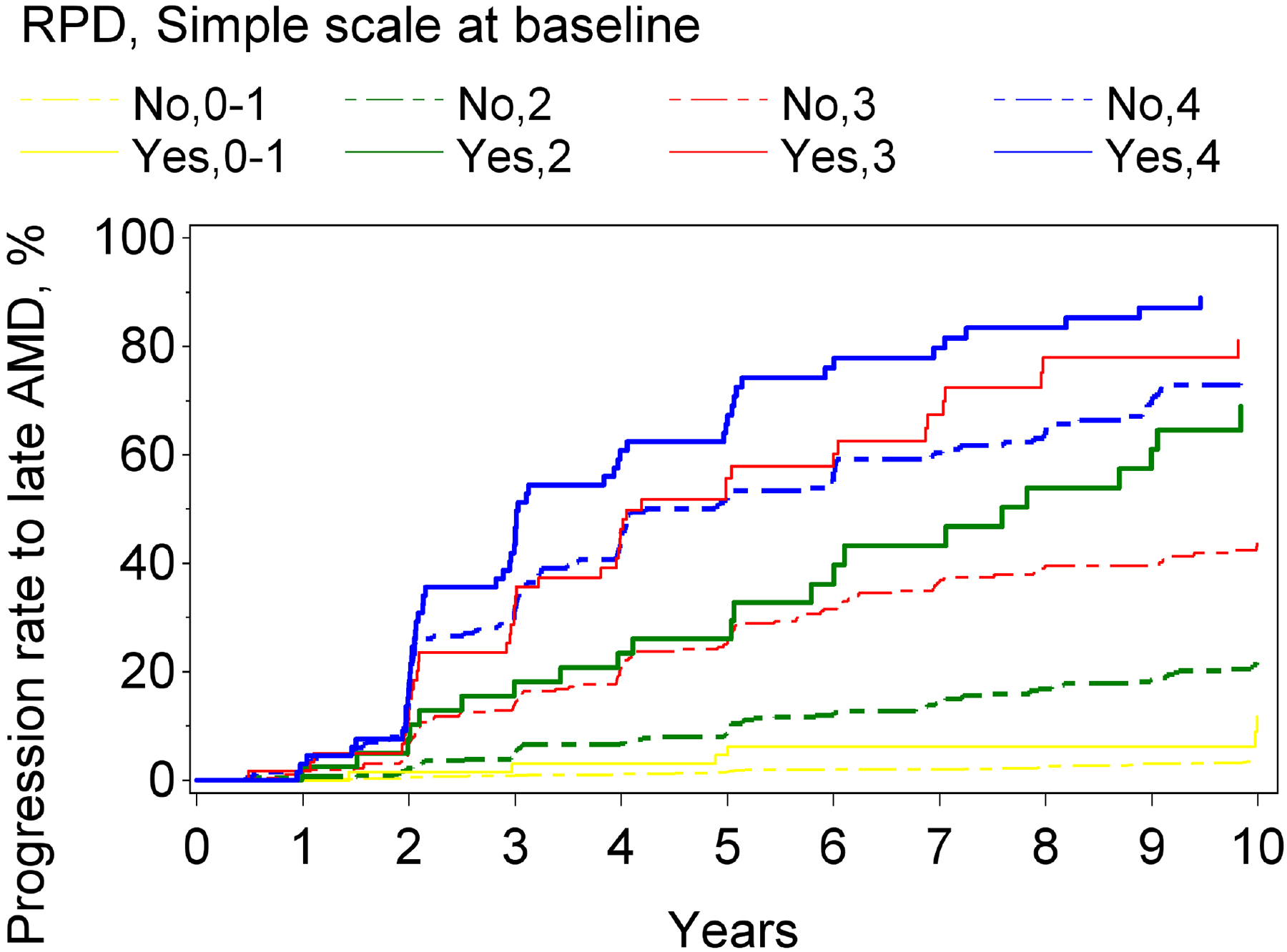
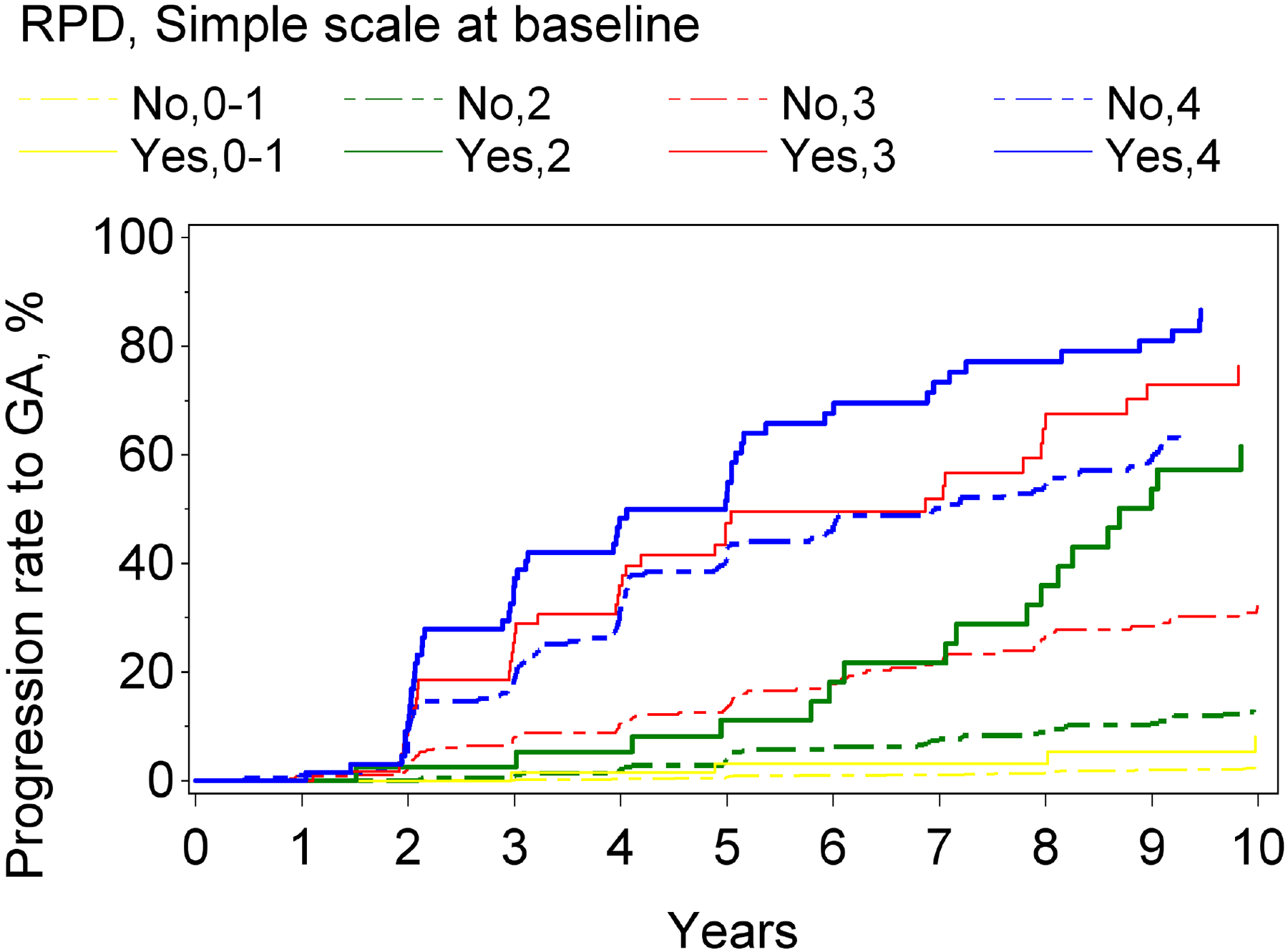
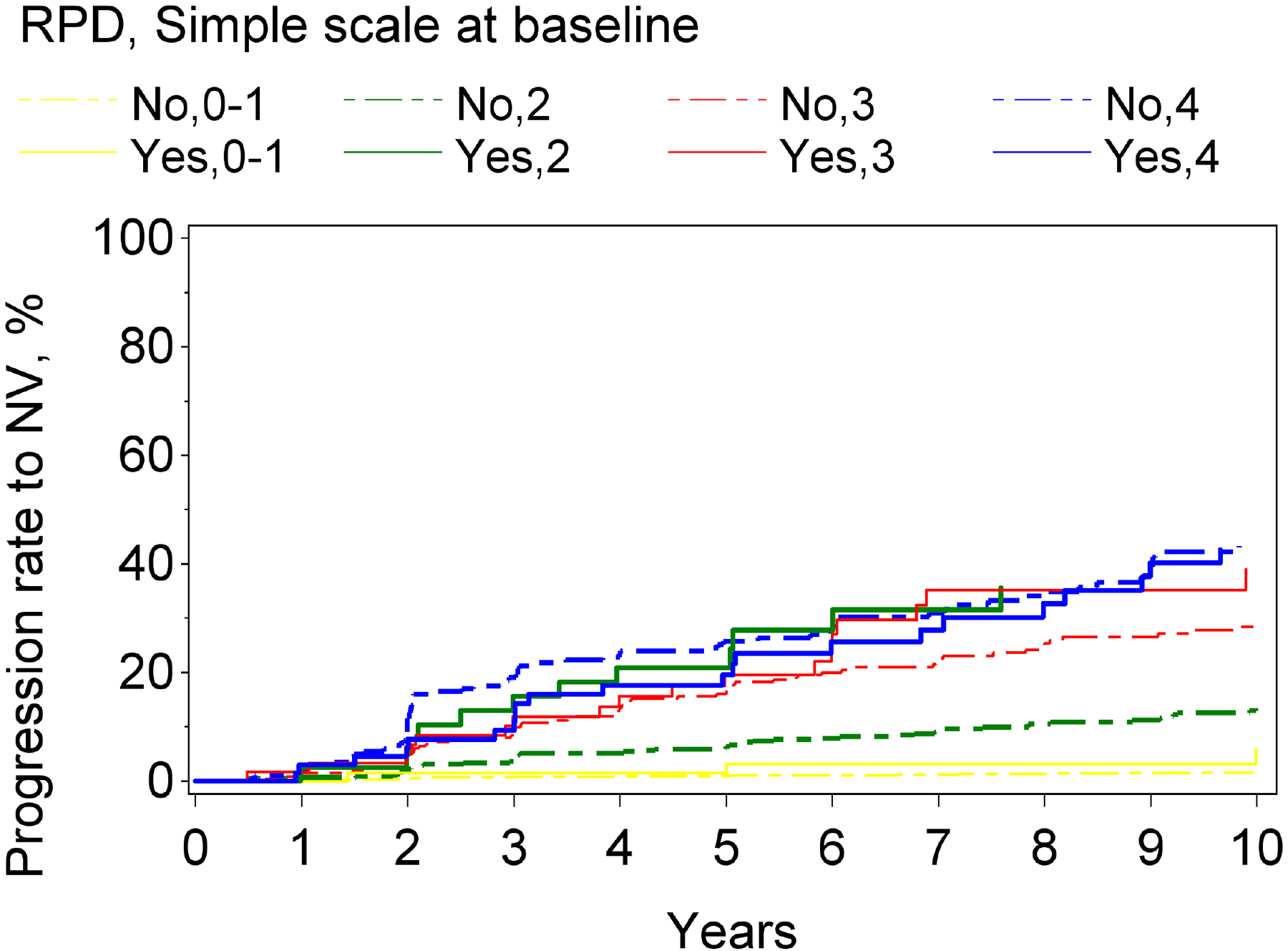
Kaplan-Meier curves of progression to late age-related macular degeneration and its subtypes, stratified according to reticular pseudodrusen presence and the modified Simplified Severity Scale, considered simultaneously, in the Age-Related Eye Disease Study, at the person level: (A) late age-related macular degeneration; (B) geographic atrophy; (C) neovascular age-related macular degeneration.
Geographic atrophy and neovascular age-related macular degeneration
For both progression to GA and to neovascular AMD, higher levels on the modified severity scale were significantly associated with higher risks of progression. Similarly, for both subtypes, RPD presence (considered in isolation from the modified severity scale) was associated with significantly higher risks: the hazard ratio was particularly high for GA (5.9, 4.8–7.3, p<0.0001), compared to neovascular AMD (3.1, 2.4–4.1, p<0.0001). With the severity scale and RPD presence considered simultaneously, a significant interaction was observed for neovascular AMD (p=0.01) and a borderline significant interaction for GA (p=0.059). In stratified analyses for progression to GA and to neovascular AMD, the hazard ratios associated with RPD presence, considered separately for severity levels 0–1, 2, 3, and 4, are shown in Table 2 and Figure 1. For each outcome, the increased risk associated with RPD presence was less prominent at higher severity levels. This was particularly true for neovascular AMD, for which no significantly increased risk was observed at levels 3 and 4. The Kaplan-Meier curves of progression to GA and neovascular AMD, stratified according to the two variables considered simultaneously, are shown in Figure 2.
Risk of progression to late age-related macular degeneration by the 9-step severity scale and reticular pseudodrusen status, at the eye level, in the AREDS
The study population for these analyses comprised 6959 eyes of 3780 participants. Their demographic and clinical characteristics are shown in Table 1. The number of eyes with RPD present at baseline (by deep learning assessment of CFP) was 368 (5.3%). The number of eyes that progressed to late AMD, during mean follow-up of 8.8 years, was 1183 (17.0%) eyes of 911 participants.
For the outcome of progression to late AMD, a higher level on the severity scale (1–6 vs 7–8), considered in isolation from RPD status, was significantly associated with higher risk of progression: hazard ratio 7.6 (6.6–8.6, p<0.0001). Similarly, RPD presence, considered in isolation from the severity scale, was associated with a significantly higher risk of progression: hazard ratio 4.3 (3.6–5.1, p<0.0001). However, with the severity scale and RPD presence considered simultaneously, a statistically significant interaction was observed between them: p<0.0001. Stratified analyses were therefore performed. For progression to late AMD, the hazard ratios associated with RPD presence, considered separately for severity scale levels 1–6 and 7–8, are shown in Table 3 and Figure 3. The hazard ratios associated with a higher level on the severity scale, considered separately according to RPD presence and absence, are shown in Supplementary Table 2. The Kaplan-Meier curve of progression to late AMD, stratified according to the two variables considered simultaneously, is shown in Figure 4. In all analyses, similar results were obtained when considering RPD as a time-dependent variable.
Table 3.
Progression to late age-related macular degeneration and its subtypes in the Age-Related Eye Disease Study, by eye: hazard ratios associated with reticular pseudodrusen presence, separately according to different levels on the 9-step Severity Scale.
| Late AMD | Geographic atrophy | Neovascular AMD | ||||
|---|---|---|---|---|---|---|
| 9-step Severity Scale levels | Hazard ratio associated with RPD presence (95% CI) | P value | Hazard ratio associated with RPD presence (95% CI) | P value | Hazard ratio associated with RPD presence (95% CI) | P value |
| 1–6 | 5.11 (3.93–6.66) | <0.0001 | 5.90 (4.19–8.32) | <0.0001 | 3.73 (2.64–5.27) | <0.0001 |
| 7–8 | 1.78 (1.43–2.22) | <0.0001 | 1.86 (1.45–2.40) | <0.0001 | 1.05 (0.77–1.43) | 0.76 |
Abbreviations: AMD=age-related macular degeneration; CI=confidence interval; RPD=reticular pseudodrusen
Figure 3.
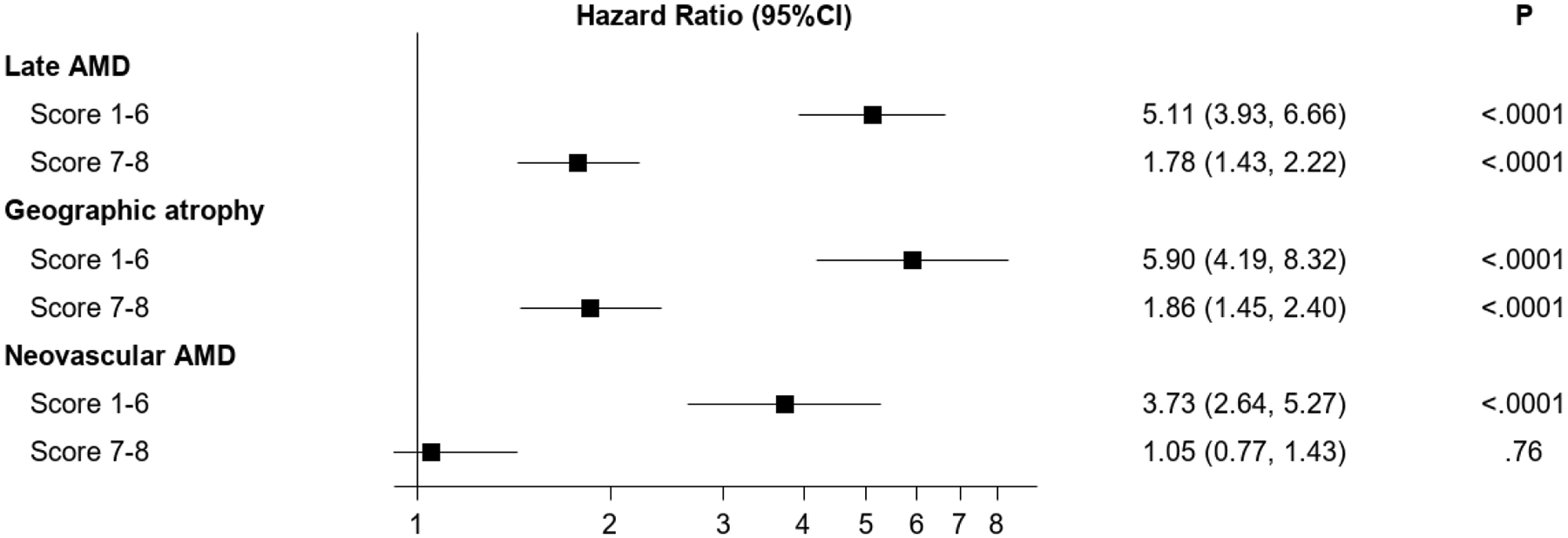
Forest plot of hazard ratios associated with reticular pseudodrusen presence for progression to late age-related macular degeneration (and its subtypes) in the Age-Related Eye Disease Study, at the eye level, separately according to different levels on the 9-step Severity Scale. The 95% confidence intervals and associated p-values are also shown.
Figure 4.
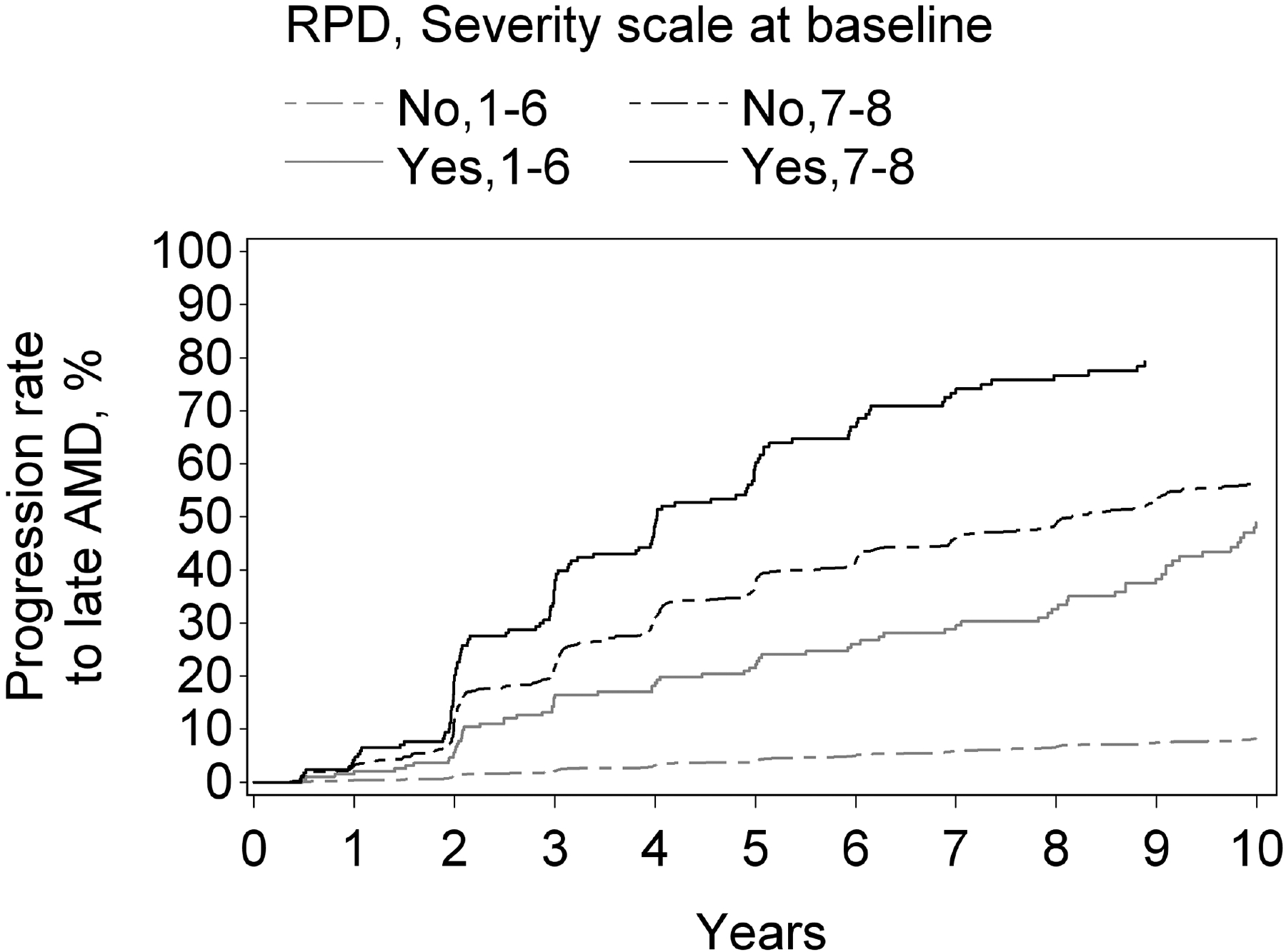
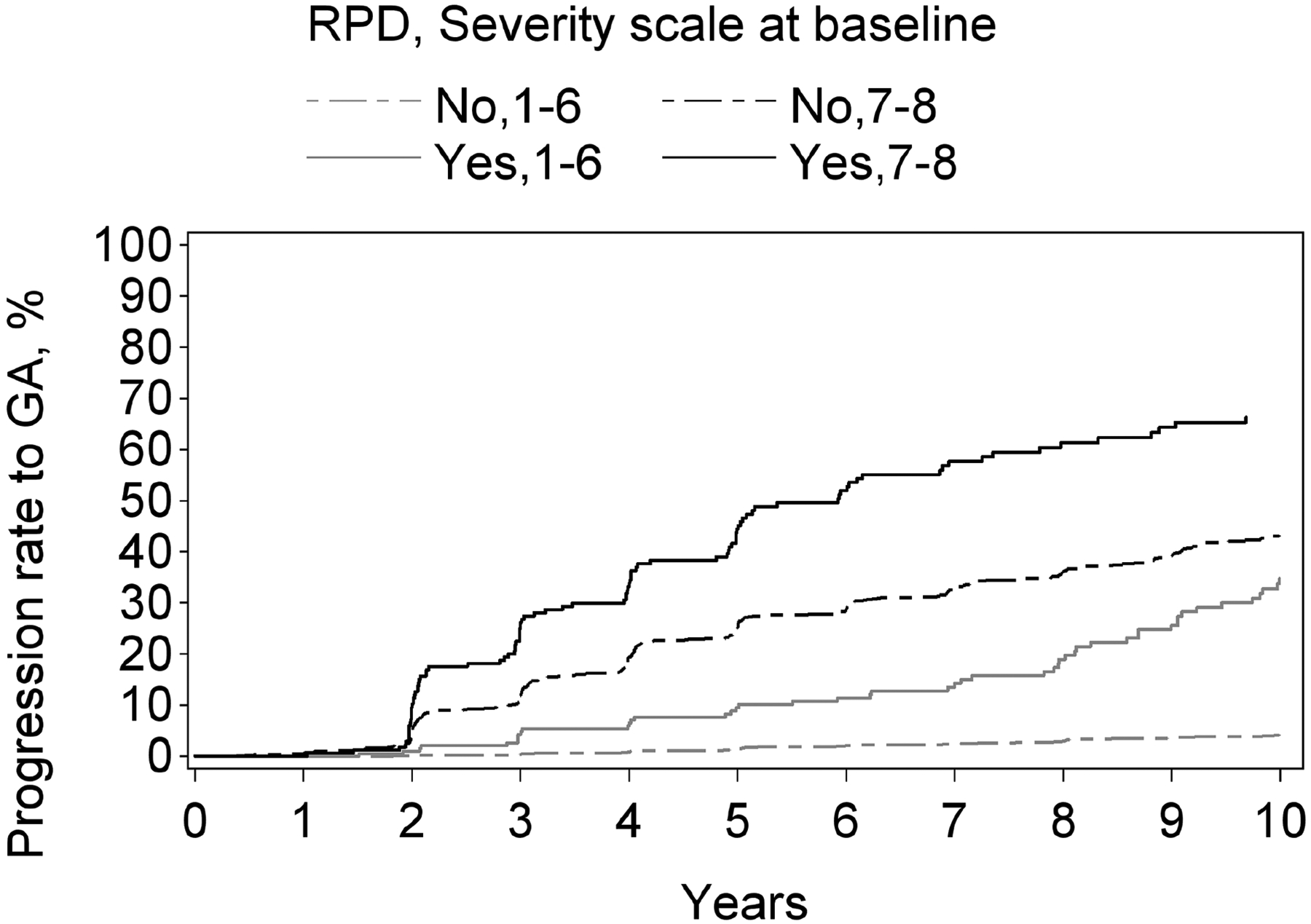
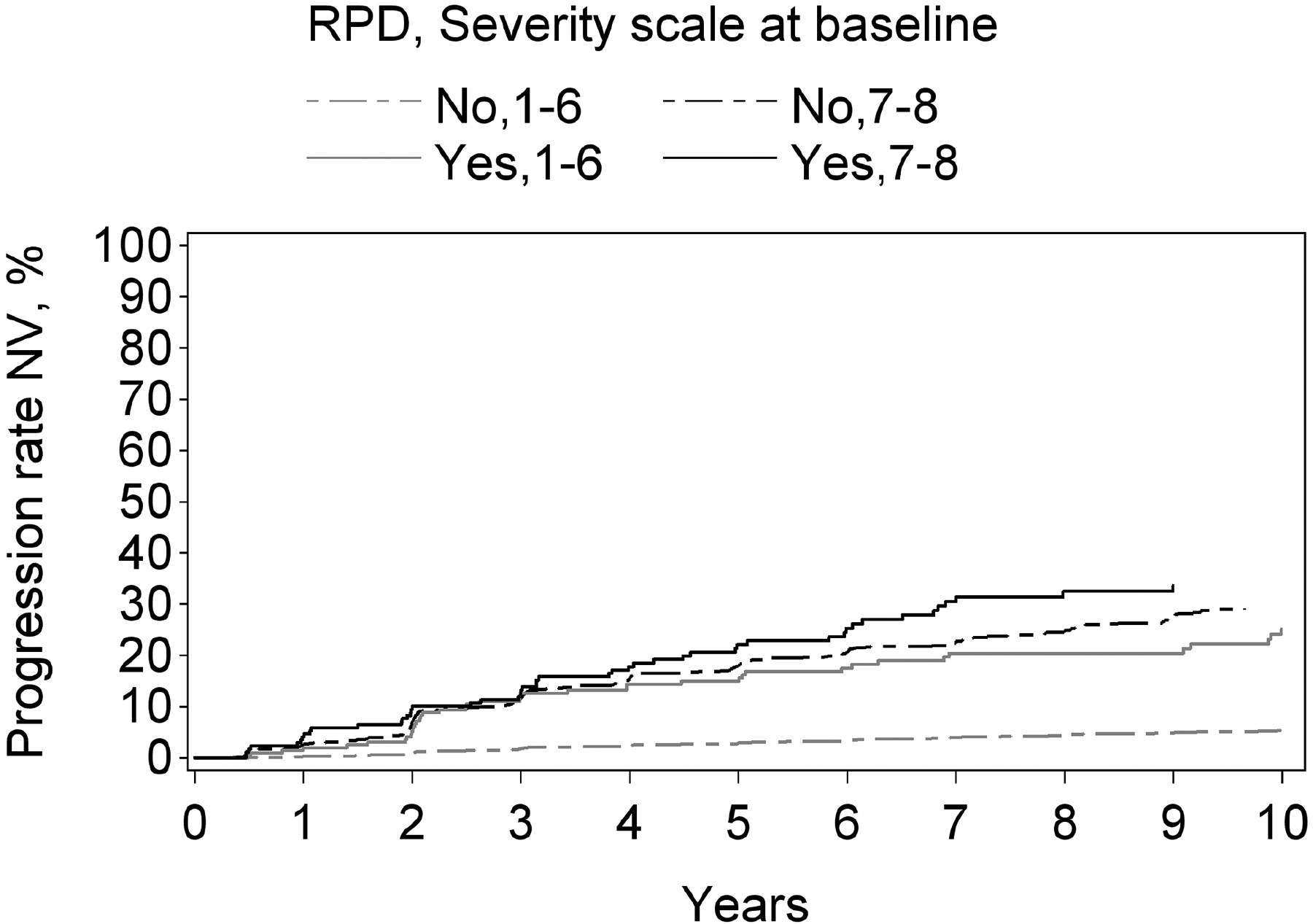
Kaplan-Meier curves of progression to late age-related macular degeneration and its subtypes, stratified according to reticular pseudodrusen presence and the 9-step Severity Scale, considered simultaneously, in the Age-Related Eye Disease Study, at the eye level: (A) late age-related macular degeneration; (B) geographic atrophy; (C) neovascular age-related macular degeneration.
Geographic atrophy and neovascular age-related macular degeneration
For both progression to GA and to neovascular AMD, a higher level on the 9-step severity scale (1–6 vs 7–8), considered in isolation from RPD status, was significantly associated with higher risk of progression: hazard ratio 9.6 (8.2–11.3, p<0.0001) for GA and 4.5 (3.8–5.4, p<0.0001) for neovascular AMD. Similarly, for both subtypes, RPD presence (considered in isolation from the severity scale) was associated with significantly higher risks. The hazard ratio was particularly high for GA (4.6, 3.7–5.7, p<0.0001), compared to neovascular AMD (2.8, 2.2–3.5, p<0.0001). With the severity scale and RPD presence considered simultaneously, a highly significant interaction was observed for both GA and neovascular AMD (each p<0.0001). In stratified analyses for progression to GA and to neovascular AMD, the hazard ratios associated with RPD presence, considered separately for severity scale levels 1–6 and 7–8, are shown in Table 3 and Figure 3. For each outcome, the increased risk associated with RPD presence was less prominent at higher severity levels. This was particularly true for neovascular AMD, for which no significantly increased risk was observed at levels 7–8. The Kaplan-Meier curves of progression to GA and neovascular AMD, stratified according to the two variables considered simultaneously, are shown in Figure 4.
Risk of progression to late age-related macular degeneration by the modified simplified severity scale and reticular pseudodrusen status, at the person level, in the AREDS2
The study population for these analyses comprised 1259 participants. Their demographic and clinical characteristics are shown in Table 1. Owing to the AREDS2 eligibility criteria, baseline AMD severity was generally much higher than for the AREDS, with a narrower spectrum (Table 1). The number of participants with RPD present at baseline (in either eye, by reading center grading of FAF images) was 113 (9.0%). The number of participants that progressed to late AMD in either eye, during mean follow-up of 4.8 years, was 371 (29.5%).
For the outcome of progression to late AMD, higher levels on the modified severity scale, considered in isolation from RPD status, were significantly associated with higher risks of progression. The hazard ratios were 2.2 (1.5–3.1, p<0.0001) and 4.1 (3.0–5.5, p<0.0001) for severity levels 3 and 4, respectively (compared with a reference level of 0–2). Similarly, RPD presence, considered in isolation from the modified severity scale, was associated with a significantly higher risk of progression: hazard ratio 1.54 (1.17–2.02, p=0.002). With the severity scale and RPD presence considered simultaneously, no statistically significant interaction was observed between them: p=0.19. In a model considering both simultaneously, the hazard ratios associated with the severity scale remained significant (2.16 (1.53–3.04, p<0.0001) and 4.00 (2.97–5.38, p<0.0001) for levels 3 and 4, respectively), while that associated with RPD presence was no longer significant (1.18 (0.90–1.56, p=0.24)). Similar results were obtained when considering RPD as a time-dependent variable.
Geographic atrophy and neovascular age-related macular degeneration
For both progression to GA and to neovascular AMD, higher levels on the modified severity scale, considered in isolation from RPD status, were significantly associated with higher risks of progression. RPD presence (considered in isolation from the modified severity scale) was associated with significantly higher risk of progression to GA (2.05, 1.47–2.86, p<0.0001) but not neovascular AMD (1.14, 0.76–1.71, p=0.53). With the severity scale and RPD presence considered simultaneously, no statistically significant interaction was observed between them for either GA (p=0.95) or neovascular AMD (p=0.37). In a model considering both simultaneously, for progression to GA, the hazard ratios associated with the severity scale remained significant, as did that associated with RPD presence (Supplementary Table 3). In a similar model for neovascular AMD, the hazard ratios associated with the severity scale remained significant, while that for RPD presence remained non-significant (Supplementary Table 3).
Risk of progression to late age-related macular degeneration by the 9-step severity scale and reticular pseudodrusen status, at the eye level, in the AREDS2
The study population for these analyses comprised 3355 eyes of 2056 participants. Their demographic and clinical characteristics are shown in Table 1. The number of eyes with RPD present at baseline (by reading center grading of FAF images) was 384 (11.4%). The number of eyes that progressed to late AMD, during mean follow-up of 4.8 years, was 1059 (31.6%) eyes of 876 participants.
For the outcome of progression to late AMD, higher levels on the severity scale (1–5/6/7/8), considered in isolation from RPD status, were significantly associated with higher risk of progression: hazard ratios were 1.76 (1.37–2.28, p<0.0001), 4.12 (3.29–5.16, p<0.0001), and 7.46 (5.86–9.50, p<0.0001) for levels 6, 7 and 8, respectively (compared with a reference level of 1–5). Similarly, RPD presence, considered in isolation from the severity scale, was associated with a significantly higher risk of progression: hazard ratio 2.21 (1.85–2.64, p<0.0001). With the 9-step severity scale (1–5/6/7/8) and RPD presence considered simultaneously, no statistically significant interaction was observed between them: p=0.22. In a model considering both simultaneously, each remained significantly associated with increased risk (Supplementary Table 4). In all analyses, similar results were obtained when considering RPD as a time-dependent variable.
Geographic atrophy and neovascular age-related macular degeneration
For both progression to GA and to neovascular AMD, higher levels on the modified severity scale, considered in isolation from RPD status, were significantly associated with higher risks of progression. RPD presence (considered in isolation from the modified severity scale) was associated with significantly higher risk of progression to GA (1.62 (1.29–2.04, p<0.0001)) but not neovascular AMD (0.80 (0.62–1.04, p=0.091)). With the severity scale and RPD presence considered simultaneously, a significant interaction was observed for GA (p=0.023) but not for neovascular AMD (p=0.22). In stratified analyses for progression to GA, the hazard ratios associated with RPD presence were 5.03 (1.43–17.73, p=0.012), 1.12 (0.43–2.95, p=0.82), 1.18 (0.87–1.59, p=0.29) and 0.73 (0.51–1.03, p=0.074) for severity levels 1–5, 6, 7 and 8, respectively.
All analyses were repeated with the omission of age from the statistical models. The pattern of results was very similar to those of the original analyses (data not shown), though the hazard ratios associated with RPD presence were generally slightly higher throughout.
Discussion
Main results and implications
In this study, RPD presence was associated with significantly increased risk of progression to late AMD, when considered in isolation from the other traditional macular risk features (i.e., soft drusen and pigmentary abnormalities). This was true in the AREDS2 study population, when RPD presence was defined directly by reading center grading of FAF images. It was also true in the AREDS study population, when RPD presence was assessed from CFP by a deep learning algorithm that had been developed using multimodal multitask training and label transfer (i.e., where the ground truth labels came from reading center grading of accompanying FAF images).19
The next important question is whether RPD presence confers additional risk of progression to late AMD, i.e., above that already captured by the other macular risk features. The results of this study demonstrate that RPD presence does indeed confer additional risk in most situations. In the AREDS population, this was true in separate analyses both by person and by eye. In the AREDS2 population, this was true in analyses by eye but not significantly in those by person (likely owing to lower power and the limited and higher spectrum of baseline severity); indeed, even in AREDS2 analyses by person, it was true for GA but not for neovascular AMD. This indicates that RPD represent a third macular risk factor for increased risk of progression to late AMD: one that is at least partially independent of soft drusen and pigmentary abnormality status and that captures additional risk above that already conferred by these two traditional macular risk features.
Next, it is important to know whether RPD presence is a completely independent risk factor, with the increased risk it confers distributed evenly across the spectrum of non-advanced AMD severity. This appears not to be the case. From analyses of rates of progression to late AMD outcomes, based on a combination of RPD presence and the two traditional features considered simultaneously, the increased risk was not distributed evenly. With milder AMD severity, RPD presence conferred highly increased risk of progression to late AMD. This was as high as a hazard ratio of 3.8, in the case of level 2 on the modified simplified scale in the AREDS. By contrast, with more severe AMD, RPD presence was more mildly associated with increased risk of progression. For example, for AREDS participants at level 4 on the modified simplified scale, the hazard ratio for RPD presence was more modest at 1.6. Indeed, the AREDS provided an ideal study population for this question, given the very wide spectrum of AMD severity at baseline. The AREDS2 contained a narrower and higher spectrum of AMD severity at baseline, with almost all participants at level 2 or greater on the modified simplified scale. However, the results from the two cohorts appeared consistent with each other, when comparing the AREDS2 results with those from the higher severity levels of AREDS (e.g., hazard ratio for RPD presence of 1.6 for AREDS eyes at 7–8 level only, compared to 1.8 for all AREDS2 eyes in the current study).
Hence, RPD status appears to be a particularly important and strong risk factor for eyes or individuals with low or moderate AMD severity, considered in the traditional way. However, for those with relatively high disease severity, it confers less additional risk than that already captured by soft drusen and pigmentary abnormality status; considered in this way, the risk of progression appears to be approaching saturation. Of course, RPD presence may confer important differences other than numerical risk of progression to late AMD. This appears to be true for the relative risk of GA versus neovascular AMD. In addition, these may include potential phenotypic differences in structure (e.g., choroidal thickness), function (e.g., dark adaptation), preferential progression by subtype (e.g., isolated outer retinal atrophy), and/or subsequent behavior (e.g., GA enlargement rate).24–26
An important distinction exists between the relative risk and absolute risk associated with RPD presence. The hazard ratios for RPD reported in this study relate to the relative risk, but this should be considered in the context of the risk already conferred by soft drusen and pigmentary abnormalities. The distinction is demonstrated well by the Kaplan-Meier curves. In general, for individuals or eyes with low severity levels, the relative risk of RPD presence is high. However, because the absolute risk at this severity level is low, this high relative risk equates to only a small increase in the absolute risk of progression to late AMD. For medium severity levels, the relative risk of RPD presence is also high. In this case, because the absolute risk at this severity level is moderate, this equates to a very large increase in the absolute risk. Finally, for high severity levels, the relative risk of RPD presence is lower. Because the absolute risk at this severity level is high, this equates to a moderate increase in absolute risk (that starts to approach 100% for 10-year progression rates in AREDS). Hence, in estimating the absolute risk of progression to late AMD, ascertaining RPD status is most important at medium severity levels and next most important at high severity levels. In addition, these data may shed light on the behavior of eyes with the specific combination of RPD presence but low burden of soft drusen or pigmentary abnormalities. Eyes with this phenotype are currently under longitudinal investigation in the AMD Ryan Initiative Study (NCT03092492). From the results of the current study, eyes like this appear to have low risk of progression to GA or neovascular AMD, despite RPD presence; considered another way, RPD presence may be able to explain progression to late AMD in some of the cases in which it did occur. Indeed, applying a deep learning algorithm to the historical AREDS dataset provided a rare opportunity to explore this question.
Regarding the secondary analyses that omitted age from the models, the higher hazard ratios associated with RPD presence likely arose because, when age is omitted from the models, some of the risk actually attributable to age is falsely attributed to RPD status (since both risk of progression to late AMD and RPD prevalence are higher with older age27,28).
The results of this study demonstrate that RPD status is important not only for assessing risk of progression to late AMD but also for considering the differential risk of progression to GA and neovascular AMD. In general, RPD presence was associated more strongly and with larger increased risks for progression to GA than to neovascular AMD. In the AREDS, the results were significant for both subtypes; in the AREDS2, which had lower power, they were significant for GA only. In the AREDS, a similar pattern of higher hazard ratios at lower severity levels and lower hazard ratios at higher severity levels was observed for both GA and neovascular AMD, considered separately; for GA, the hazard ratios remained significant at higher severity levels while, for neovascular AMD, they did not.
This situation differs from that for the other two macular risk features. The increased risk associated with soft drusen or pigmentary abnormalities is considered to occur in parallel for GA and neovascular AMD, such that neither is thought to increase the risk of one subtype disproportionately versus the other subtype. According to data from the AREDS, the risk of neovascular AMD always exceeds that of GA (as demonstrated in Figure 2).29 By contrast, the increased risk conferred by RPD presence is not distributed evenly between the two subtypes. Hence, the ascertainment of RPD status should improve not only overall estimates of progression risk but may greatly improve risk estimates for the two subtypes. In some circumstances with RPD present, the risk of GA will exceed that of neovascular AMD. These considerations may be particularly useful not only for risk calculators but also for the recruitment and stratification of clinical trials where progression to GA is the outcome of interest. In addition, the strong relationship between RPD and GA risk may provide biological insights into the nature of GA.
Indeed, these results suggest that incorporating RPD status into AMD scales and risk calculators is likely to lead to improved accuracy. However, it may be more appropriate for some risk calculators than for others, according to the desired use case and setting. For example, the AREDS Simple Scale10 has the advantage of simplicity, so may remain relevant for approximate risk calculation in routine clinical practice, without the incorporation of RPD status as a third feature. By contrast, more complex scales and calculators like the AREDS 9-Step Scale9 and Casey AMD calculator29 are typically used for more granular or continuous risk prediction, when higher accuracy and/or separate subtype predictions are required. Calculators like these may benefit more from incorporating RPD status. Future studies could repeat the original methods used to derive the scales (i.e., decision tree analysis and Cox proportional hazards analysis, respectively), with the addition of RPD status as another feature. Importantly, RPD presence can be assessed from OCT, which is considered highly sensitive and specific for detection27; this means that more accurate risk prediction would be possible even in routine clinical practice, where multimodal imaging is not always available. Indeed, AI algorithms are becoming available to detect RPD with high accuracy from OCT scans.30
Comparison with literature
We are not aware of any previous studies of large prospective datasets that have analyzed rates of progression to late AMD according to both RPD presence and traditional severity scales. Some large population-based AMD studies such as the Beaver Dam Eye Study and the Blue Mountains Eye Study were based on CFP, such that data on RPD status are not available.31,32 Others have reported data on RPD presence but not as potential risk factors for progression to late AMD.33–35
In previous analyses of the AREDS2, RPD presence was significantly associated with increased risk of progression to late AMD.15 This was also true for GA, considered separately, but not for neovascular AMD. The odds ratios over one year were 1.84 (1.47–2.31), 2.42 (1.80–3.24), and 1.21 (0.87–1.70), respectively. However, as described above, these analyses were performed irrespective of AMD severity related to other macular features. Instead, the odds ratios were adjusted for the 9-step AMD severity level. Additional differences include: (i) analysis of AREDS and AREDS2 in the current study, as opposed to AREDS2 only; (ii) use of time-to-event analysis with proportional hazards regression, as opposed to logistic regression for outcomes at each consecutive annual visit; and (iii) inclusion of a larger number of AREDS2 eyes/participants (specifically 3355 versus 1710 eyes), by considering all study visits for most participants, as opposed to only those from first FAF imaging.
Several cohort studies have analyzed potential associations between RPD and progression to late AMD in the specific setting of fellow eye of patients with unilateral neovascular AMD.36–43 In the Comparison of AMD Treatments Trials (CATT), RPD presence in the fellow eye at baseline was associated with increased risk of late AMD in those fellow eyes over two years, with a risk ratio of 2.09 (1.55–2.83).36 Following adjustment for a baseline severity score similar to the AREDS simplified scale (i.e., no large drusen or pigmentary abnormalities vs one vs both) and for dietary supplement use, RPD presence remained significantly associated with increased risk. For GA and neovascular AMD, the adjusted risk ratios were 2.05 (1.43–2.93) and 1.89 (1.13–2.17), respectively. In Kaplan-Meier analyses stratified by baseline severity in a binary way (i.e., large drusen or pigmentary abnormalities vs both), RPD presence was associated with higher risk of progression to late AMD in both cases. The authors did not present more detailed analyses, but the Kaplan-Meier plots are suggestive that RPD presence carried a higher level of risk at the lower level of severity, which could be consistent with the results from the current study. However, meaningful comparison with the results of the present study is very limited, since the previous study relates to the specific setting where one eye already had late AMD, and specifically neovascular AMD, such that the study population was not representative of the full spectrum of AMD.
We are not aware of any AMD risk prediction algorithms derived from prospectively obtained data that have included RPD presence alongside the other typical macular features of soft drusen and pigmentary abnormalities. In one case, data from 138 patients with AMD were reviewed and an OCT risk prediction algorithm proposed, which included RPD presence alongside three other binary variables: intraretinal hyperreflective foci, hyporeflective foci within a drusenoid lesion, and central drusen volume >0.03 mm3.44 However, the dataset was created by retrospective review of patients diagnosed with AMD as part of clinical care at one medical center and the potential risk factors were assessed against late AMD outcomes by Spearman’s rho correlation only. The retrospective ascertainment of eligible patients (together with the requirement for one year of follow-up) led to strong potential for bias and also meant that the large majority of patients had unilateral neovascular AMD. The algorithm was subsequently applied to a cohort of 501 fellow eyes with early or intermediate AMD from patients with neovascular AMD enrolled in the HARBOR study, which benefitted from prospective recruitment in a clinical trial setting.43 RPD presence had a hazard ratio of 1.95 (1.34–2.82) for progression to late AMD over two years, when considered in a multivariable Cox proportional hazards model alongside the three other binary OCT variables (together with age, sex, and smoking status). For GA and neovascular AMD, the hazard ratios were 2.05 (1.26–3.32) and 1.90 (1.05–3.44), respectively. Again, as for the CATT study, this represented the limited setting where one eye already had late AMD, specifically neovascular disease, such that the study population was not representative of the full spectrum of AMD.
Importantly, in both studies43,44, the authors did not analyze whether the increased risk associated with RPD presence was distributed evenly across the spectrum of non-advanced AMD severity. The estimate of increased risk is therefore an average across multiple AMD severity levels (weighted towards the particular severity levels in the population under study); such estimates are highly dependent on the characteristics of the particular study population and therefore likely to vary between studies and be less reflective of a true measure of the increased risk associated with RPD presence that would be meaningful and widely generalizable irrespective of the study population. Finally, in both the CATT and HARBOR studies36,43, RPD presence appeared to confer slightly higher risk of GA than of neovascular AMD, which would be consistent with the results from the current study. However, the differences appeared small and the confidence intervals wide; again, in both cases, the scenario of pre-existing neovascular AMD in all fellow eyes is not representative of the full spectrum of AMD, which makes these estimates less reliable.
Strengths and limitations
The strengths of this study include the use of two separate datasets, both with large size and long follow-up, which provided a high event rate for progression to late AMD. The datasets had the advantages of representing longitudinal multi-center studies with prospective recruitment and follow-up; regular imaging and centralized reading center grading according to standardized methods meant that all participants were well characterized for AMD severity. The two datasets were complementary, since AREDS contained the full spectrum of baseline AMD severity, while AREDS2 was enriched for participants with more severe disease at baseline. For each dataset, separate analyses were performed for both the 9-step and the simple scale, and for both late AMD and its subtypes as individual outcomes. RPD presence was considered at baseline and, in sensitivity analyses, as a time-dependent variable. The study was limited by the absence of reading center grading of RPD presence in AREDS, owing to the lack of FAF or other multimodal imaging at that time in history, though this information was available in AREDS2. In the AREDS dataset, RPD grading was therefore performed by deep learning-based grading of the fundus photographs. However, the ground truth of the RPD algorithm’s training was from reading center grading of FAF images (leading to high specificity of grading from CFP alone, in a previous study19). Since a study like AREDS (where several thousand participants with the full spectrum of baseline AMD severity followed for many years), is unlikely to be repeated with the addition of multimodal imaging, applying deep learning algorithms to the existing dataset may represent the optimum opportunity to explore the association between macular features and risk of disease progression. In the case of the AREDS analyses according to the modified simplified severity scale, if RPD were visible and large on CFP at the baseline visit, in some cases, these RPD may have contributed towards large drusen status being graded as positive instead of negative. Hence, in a small minority of participants with RPD, the severity score might be slightly inflated. For this reason, the hazard ratios associated with higher levels on the simplified scale might be slightly overestimated, while those associated with RPD presence might be slightly underestimated, though the differences are likely to be very modest. The assessments of RPD risk therefore err on the conservative side, and this consideration should not apply meaningfully to analyses by the 9-step scale or of the AREDS2.
The RPD prevalence rates reported in the current study may appear relatively low. For the AREDS2 analyses, this was expected through a combination of factors: cases with late AMD at baseline were excluded, prevalence rates were reported at baseline, a minimum area of 0.5 disc areas was required (in line with many other studies27), and FAF was used as the sole grading modality. OCT imaging is considered to have slightly higher sensitivity and specificity than FAF, but most grading definitions require presence on both OCT and FAF/NIR.27 Indeed, other studies have shown that very few eyes with intermediate AMD have RPD present on OCT but not on FAF/NIR.27 For the AREDS analyses, the relatively low RPD prevalence rate reported was also expected. This is partly for the same reasons, and because RPD prevalence is lower at the less severe spectrum of AMD severity captured in AREDS.27 However, it might also be partly because the AI algorithm used may miss some RPD cases, despite multimodal multitask deep learning training.19 With this technology, increasing sensitivity is not possible without losing specificity, and no ground truth for RPD status exists in AREDS, owing to the absence of multimodal imaging. The likely implication of some false negative RPD cases in the AREDS analyses is that the hazard ratios associated with RPD presence reported here may be underestimates.
This study considered RPD status at the image level in a binary way (i.e., present/absent), since that is how RPD grading was performed and how the AI algorithm was trained. Hence, we were unable to analyze potential associations according to RPD area or number of lesions. While binary grading and analyses may be sufficient to capture potential risk for most purposes, we aim to perform future studies that incorporate quantitative metrics of RPD status.
Conclusions
RPD represent a third anatomical risk factor, in addition to soft drusen and pigmentary abnormalities, for progression to late AMD. Importantly, RPD presence confers additional risk above that already captured by the two traditional macular features. However, it is not a totally independent risk factor, as its profile of additional risk is not distributed evenly across the spectrum of non-advanced AMD severity. It confers highly increased relative risk of late AMD at mild/moderate AMD severity levels and less highly increased risk at severe levels. However, in terms of absolute risk, ascertaining RPD status is most discriminative at medium severity levels, followed by high severity levels. Although RPD presence confers increased risk of both subtypes of late disease, the increased risk is weighted strongly towards GA; this differs markedly from the situation for the other two macular features. For these reasons, RPD status must be considered in combination with the traditional features for an accurate understanding of progression risk and subtype predictions. Ideally, RPD status should be included in updated AMD classification systems and risk calculators, as well as clinical trials.
Supplementary Material
Financial support
This research was supported by the Intramural Research Program of the National Eye Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland. It was also supported by contracts from the National Eye Institute (contract NOI-EY-0-2127 for AREDS and contracts HHS-N-260-2005-00007-C and ADB contract N01-EY-5-0007 for AREDS2). Funds were generously contributed to these contracts by the following NIH institutes: Office of Dietary Supplements; National Center for Complementary and Alternative Medicine; National Institute on Aging; National Heart, Lung, and Blood Institute; National Institute of Neurological Disorders and Stroke. The sponsor and funding organization participated in the design and conduct of the study, data collection, management, analysis, and interpretation, and preparation, review and approval of the manuscript. AD was supported in part by an unrestricted grant from Research to Prevent Blindness, Inc. to the University of Wisconsin Madison Department of Ophthalmology and Visual Sciences.
Abbreviations
- AMD
age-related macular degeneration
- AREDS
Age-Related Eye Disease Study
- AUROC
area under the receiver operating characteristic
- BMI
body mass index
- CATT
Comparison of AMD Treatments Trials
- CFP
color fundus photography
- FAF
fundus autofluorescence
- GA
geographic atrophy
- HR
hazard ratio
- NIR
near-infrared reflectance
- NV
neovascular AMD
- OCT
optical coherence tomography
- RPD
reticular pseudodrusen
- SDD
subretinal drusenoid deposit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
No conflicting relationship exists for any author.
References
- 1.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005–2015. [DOI] [PubMed] [Google Scholar]
- 3.Lawrenson JG, Evans JR. Advice about diet and smoking for people with or at risk of age-related macular degeneration: a cross-sectional survey of eye care professionals in the UK. BMC Public Health. 2013;13:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogg RE, Woodside JV. Mediterranean Diet and Age-Related Macular Degeneration: Is It Time to Attempt Dietary Modification? Ophthalmology. 2019;126(3):391–392. [DOI] [PubMed] [Google Scholar]
- 5.Domalpally A, Clemons TE, Bressler SB, et al. Imaging Characteristics of Choroidal Neovascular Lesions in the AREDS2-HOME Study: Report Number 4. Ophthalmol Retina. 2019;3(4):326–335. [DOI] [PubMed] [Google Scholar]
- 6.Chew EY, Clemons TE, Bressler SB, et al. Randomized trial of the ForeseeHome monitoring device for early detection of neovascular age-related macular degeneration. The HOme Monitoring of the Eye (HOME) study design - HOME Study report number 1. Contemp Clin Trials. 2014;37(2):294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wittenborn JS, Clemons T, Regillo C, Rayess N, Liffmann Kruger D, Rein D. Economic Evaluation of a Home-Based Age-Related Macular Degeneration Monitoring System. JAMA Ophthalmol. 2017;135(5):452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Academy of Ophthalmology. Age-related macular degeneration preferred practice patterns. Preferred practice patterns 2015; https://www.aao.org/preferred-practice-pattern/age-related-macular-degeneration-ppp-2015. Accessed 4/25/2019, 2019.
- 9.Davis MD, Gangnon RE, Lee LY, et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005;123(11):1484–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferris FL, Davis MD, Clemons TE, et al. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol. 2005;123(11):1570–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spaide RF, Ooto S, Curcio CA. Subretinal drusenoid deposits AKA pseudodrusen. Surv Ophthalmol. 2018;63(6):782–815. [DOI] [PubMed] [Google Scholar]
- 12.Wightman AJ, Guymer RH. Reticular pseudodrusen: current understanding. Clin Exp Optom. 2019;102(5):455–462. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz-Valckenberg S, Alten F, Steinberg JS, et al. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(9):5009–5015. [DOI] [PubMed] [Google Scholar]
- 14.Alten F, Clemens CR, Heiduschka P, Eter N. Characterisation of reticular pseudodrusen and their central target aspect in multi-spectral, confocal scanning laser ophthalmoscopy. Graefes Arch Clin Exp Ophthalmol. 2014;252(5):715–721. [DOI] [PubMed] [Google Scholar]
- 15.Domalpally A, Agron E, Pak JW, et al. Prevalence, Risk, and Genetic Association of Reticular Pseudodrusen in Age-related Macular Degeneration: Age-Related Eye Disease Study 2 Report 21. Ophthalmology. 2019;126(12):1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Grinsven MJ, Buitendijk GH, Brussee C, et al. Automatic identification of reticular pseudodrusen using multimodal retinal image analysis. Invest Ophthalmol Vis Sci. 2015;56(1):633–639. [DOI] [PubMed] [Google Scholar]
- 17.Ueda-Arakawa N, Ooto S, Tsujikawa A, Yamashiro K, Oishi A, Yoshimura N. Sensitivity and specificity of detecting reticular pseudodrusen in multimodal imaging in Japanese patients. Retina. 2013;33(3):490–497. [DOI] [PubMed] [Google Scholar]
- 18.Keenan TDL, Chen Q, Peng Y, et al. Deep Learning Automated Detection of Reticular Pseudodrusen from Fundus Autofluorescence Images or Color Fundus Photographs in AREDS2. Ophthalmology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Q, Keenan TDL, Allot A, et al. Multimodal, multitask, multiattention (M3) deep learning detection of reticular pseudodrusen: Toward automated and accessible classification of age-related macular degeneration. J Am Med Inform Assoc. 2021;28(6):1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha S, Nassisi M, Wang M, et al. Automated detection and classification of early AMD biomarkers using deep learning. Sci Rep. 2019;9(1):10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): design implications. AREDS report no. 1. Control Clin Trials. 1999;20(6):573–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AREDS2 Research Group, Chew EY, Clemons T, et al. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology. 2012;119(11):2282–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei LJ, Lin DY, Weissfeld L Regression analysis of multivariate incomplete failure time data by modeling marginal distribution. J Am Stat Assoc. 1989;84:1065–1073. [Google Scholar]
- 24.Keenan TD, Klein B, Agron E, Chew EY, Cukras CA, Wong WT. Choroidal Thickness and Vascularity Vary with Disease Severity and Subretinal Drusenoid Deposit Presence in Nonadvanced Age-Related Macular Degeneration. Retina. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flamendorf J, Agron E, Wong WT, et al. Impairments in Dark Adaptation Are Associated with Age-Related Macular Degeneration Severity and Reticular Pseudodrusen. Ophthalmology. 2015;122(10):2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spaide RF. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina. 2013;33(9):1800–1808. [DOI] [PubMed] [Google Scholar]
- 27.Wu Z, Fletcher EL, Kumar H, Greferath U, Guymer RH. Reticular pseudodrusen: A critical phenotype in age-related macular degeneration. Prog Retin Eye Res. 2021:101017. [DOI] [PubMed] [Google Scholar]
- 28.Ding Y, Liu Y, Yan Q, et al. Bivariate Analysis of Age-Related Macular Degeneration Progression Using Genetic Risk Scores. Genetics. 2017;206(1):119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein ML, Francis PJ, Ferris FL 3rd, Hamon SC, Clemons TE. Risk assessment model for development of advanced age-related macular degeneration. Arch Ophthalmol. 2011;129(12):1543–1550. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz R, Khalid H, Liakopoulos S, et al. A deep learning framework for the detection and quantification of drusen and reticular pseudodrusen on optical coherence tomography. 2022; https://arxiv.org/abs/2204.02406. Accessed 05/11/2022. [DOI] [PMC free article] [PubMed]
- 31.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99(6):933–943. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology. 1995;102(10):1450–1460. [DOI] [PubMed] [Google Scholar]
- 33.Buitendijk GH, Hooghart AJ, Brussee C, et al. Epidemiology of Reticular Pseudodrusen in Age-Related Macular Degeneration: The Rotterdam Study. Invest Ophthalmol Vis Sci. 2016;57(13):5593–5601. [DOI] [PubMed] [Google Scholar]
- 34.Dutheil C, Le Goff M, Cougnard-Gregoire A, et al. Incidence and Risk Factors of Reticular Pseudodrusen Using Multimodal Imaging. JAMA Ophthalmol. 2020;138(5):467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cleland SC, Domalpally A, Liu Z, et al. Reticular Pseudodrusen Characteristics and Associations in the Carotenoids in Age-Related Eye Disease Study 2 (CAREDS2), an Ancillary Study of the Women’s Health Initiative. Ophthalmol Retina. 2021;5(8):721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Q, Daniel E, Maguire MG, et al. Pseudodrusen and Incidence of Late Age-Related Macular Degeneration in Fellow Eyes in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2016;123(7):1530–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gil JQ, Marques JP, Hogg R, et al. Clinical features and long-term progression of reticular pseudodrusen in age-related macular degeneration: findings from a multicenter cohort. Eye (Lond). 2017;31(3):364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pumariega NM, Smith RT, Sohrab MA, Letien V, Souied EH. A prospective study of reticular macular disease. Ophthalmology. 2011;118(8):1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finger RP, Wu Z, Luu CD, et al. Reticular pseudodrusen: a risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularization. Ophthalmology. 2014;121(6):1252–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hogg RE, Silva R, Staurenghi G, et al. Clinical characteristics of reticular pseudodrusen in the fellow eye of patients with unilateral neovascular age-related macular degeneration. Ophthalmology. 2014;121(9):1748–1755. [DOI] [PubMed] [Google Scholar]
- 41.Sawa M, Ueno C, Gomi F, Nishida K. Incidence and characteristics of neovascularization in fellow eyes of Japanese patients with unilateral retinal angiomatous proliferation. Retina. 2014;34(4):761–767. [DOI] [PubMed] [Google Scholar]
- 42.Chang YS, Kim JH, Yoo SJ, Lew YJ, Kim J. Fellow-eye neovascularization in unilateral retinal angiomatous proliferation in a Korean population. Acta Ophthalmol. 2016;94(1):e49–53. [DOI] [PubMed] [Google Scholar]
- 43.Nassisi M, Lei J, Abdelfattah NS, et al. OCT Risk Factors for Development of Late Age-Related Macular Degeneration in the Fellow Eyes of Patients Enrolled in the HARBOR Study. Ophthalmology. 2019;126(12):1667–1674. [DOI] [PubMed] [Google Scholar]
- 44.Lei J, Balasubramanian S, Abdelfattah NS, Nittala MG, Sadda SR. Proposal of a simple optical coherence tomography-based scoring system for progression of age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2017;255(8):1551–1558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


