Abstract
Over the past decades, the incidence of early-onset cancers, often defined as cancers diagnosed in adults <50 years of age, in the breast, colorectum, endometrium, oesophagus, extrahepatic bile duct, gallbladder, head and neck, kidney, liver, bone marrow, pancreas, prostate, stomach, and thyroid has increased in multiple countries. Increased use of screening programmes has contributed to this phenomenon to a certain extent, although a genuine increase in the incidence of early-onset forms of several cancer types also seems to have emerged. Evidence suggests an aetiological role of risk factor exposures in early life and young adulthood. Since the mid-20th century, substantial multigenerational changes in the exposome have occurred (including in diet, lifestyle, obesity, environment and the microbiome), all of which might interact with genomic and/or genetic susceptibilities. However, the effects of individual exposures remain largely unknown. To study early-life exposures and their implications for multiple cancer types will require prospective cohort studies with dedicated biobanking and data collection technologies. Raising awareness among both the public and health-care professionals will also be critical. In this Review, we describe changes in the incidence of early-onset cancers globally and suggest measures that will likely reduce the burden of cancers and other chronic non-communicable diseases.
[H1] Introduction
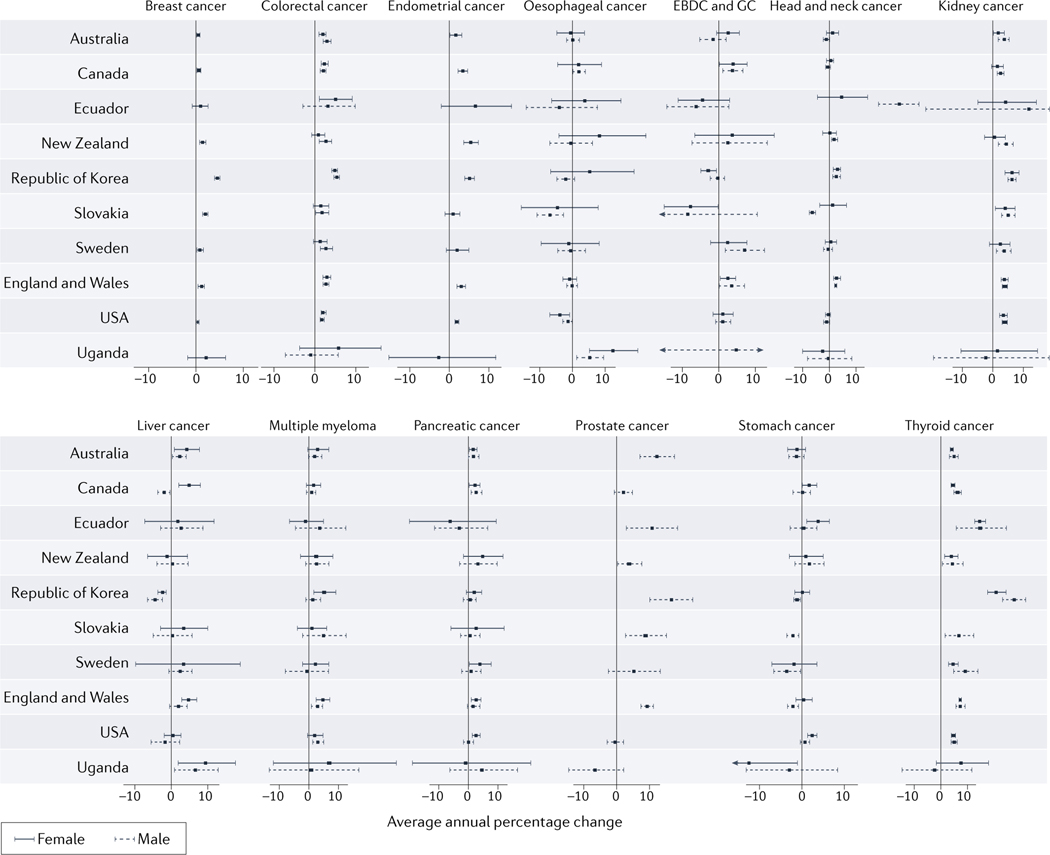
Cancer is a multifactorial disease that most commonly affects people ≥50 years of age. However, evidence indicates that the incidence of cancers of various organs (including those of the breast, colorectum, endometrium, oesophagus, extrahepatic bile duct, gallbladder, head and neck, kidney, liver, bone marrow (multiple myeloma), pancreas, prostate, stomach, and thyroid) has been rising in adults <50 years of age in many parts of the world.1–13 This trend is also observed in analyses using Global Cancer Observatory (GLOBOCAN) data (FIG 1 provides data on selected countries; more comprehensive data are provided in Supplementary Table 1). We herein use the term ‘early-onset’ to describe cancers diagnosed in adults <50 years of age, and a contrasting term ‘later-onset’ for those diagnosed at ≥50 years of age. Cancers diagnosed during childhood and adolescence (<20 years of age) are out of the scope of this Review.
Figure 1. Trends in incidence of selected early-onset cancers.
Trends in the incidence of 14 cancer types with increasing incidences among 20–49 year-old adults during the period of 2002–2012, by country and region. Age-standardized cancer incidence data were obtained from the Global Cancer Observatory (GLOBOCAN; https://gco.iarc.fr/). Horizontal bars indicate 95% confidence interval (CIs). Larger 95% CIs that do not fit onto the graph scale are indicated by arrows. Data were obtained from 44 countries that provided age-standardized data on cancer incidence during 2002–2012. Among these, we selected 10 countries that are indicative of trends in specific geographical regions. The full dataset, including data from all 44 countries is shown in Supplementary Table 1. Average annual percentage changes (AAPCs) with 95% confidence intervals (shown as horizontal bars) in incidence were calculated using the Joinpoint Regression Program (version 4.9.0.1) for data obtained for 2002–2012, except for Slovakia (2000–2010) owing to differences in data availability. A maximum of two joinpoints were permitted in this analysis. Although extrahepatic bile duct cancer and gallbladder cancer (EBDC & GC) are distinct cancer types, making precise classifications is often difficult, hence, these cancer types are often recorded and data calculated together. Data were not available on the incidence of thyroid cancer among women in Slovakia. AAPC, annual average percent increase; EBDC & GC, extrahepatic bile duct cancer and gallbladder cancer.
The rise of early-onset cancer has considerable personal, societal and economic implications. Survivors of early-onset cancers have a higher risk of long-term health problems such as infertility, cardiovascular disease and secondary cancers.14–16 Owing to this increasing cancer burden among young adults, which might be referred to as the ‘early-onset cancer epidemic’, the US National Cancer Institute listed this phenomenon as a research priority in one of its ‘Provocative Questions’ in 2020–2021.17
Differences in epidemiology and clinical, pathological, and molecular characteristics clearly exist between early-onset and later-onset cancers, although these features likely do not change dramatically at exactly 50 years of age.18 Furthermore, early-onset cancer in any given organ is not a homogeneous entity but rather encompasses a variable range of clinical and pathological features.18, 19 We acknowledge the limitations of applying a dichotomy at 50 years of age, although we selected this cutoff point to enable consistent collection and interpretation of current evidence on early-onset cancers. In reality, we also need to consider heterogeneity within this group. In addition, considering the variable distribution of age at cancer diagnosis by different organ sites, the optimal screening and treatment options for various age groups should be studied according to organ sites.
In this Review, we evaluate and summarize evidence on the pertinent features and possible risk-factor profiles of early-onset forms of cancer types with an increased incidence reported over the past decades. In-depth investigations of putative risk factors and tumour molecular characteristics across multiple early-onset cancer types could shed light on plausible common aetiologies. Improved knowledge of pathogenesis can also inform strategies for primary prevention, early detection and treatment. We also discuss strategies to address research gaps and promote prevention efforts, which have broader public health implications. We use the standardized nomenclature system for genes and gene products designated by the Human Genome Organisation (HUGO) Gene Nomenclature Committee (HGNC) along with colloquial names, to reduce ambiguity and increase clarity.20
[H1] Current evidence
[H3] Risk factors in early life and young adulthood.
The rising incidence of early-onset cancers is likely partially attributable to increasing uptake of screening and early detection before the age of 50, to variable degrees across certain cancer types, especially breast, prostate and thyroid cancers. However, an increasing incidence of early-onset cancers in several organs, such as colorectal and pancreatic cancers, that might not be fully explained by screening is also apparent. This trend could reflect increased risk factor exposures in early life and/or young adulthood.18 In this Review the term ‘early life’, is broadly defined as conception to the end of adolescence (19 years of age), and the contrasting term ‘adulthood’ is applied thereafter. Accumulating evidence suggests that the earliest phase of carcinogenesis might start in early life or young adulthood,18, 21 followed by intervals of up to several decades between initial cellular damage and clinical cancer detection.22, 23 Even in utero exposures can lead to cellular reprogramming, including epigenetic alterations, that might have long-lasting effects on susceptibility to chronic diseases.24–26 For example, data from the Dutch Famine Birth Cohort indicate that prenatal food restriction is associated with increased long-term risks of certain health conditions, including coronary heart disease and breast cancer, among adults.27, 28 Similarly, data from the Child Health and Development Studies suggest that maternal obesity increases the risk of colorectal cancer (CRC) in offspring.29 Data from another study indicate a positive association between birth weight and colon cancer risk in adulthood.30 Exposure to ionizing radiation and age at menarche are other factors occurring in childhood or young adulthood that can affect disease risk in later life.31, 32 These observations underscore the aetiological role of early-life exposures in cancer development.
Another aetiological insight into the rise of early-onset cancers can be derived from trends in CRC incidence in the USA; the incidence of later-onset CRC (in those born in the late 19th and early 20th centuries) started to increase in the 1950s whereas that of early-onset CRC (in those born in the mid-20th century) did not start to increase until the early 1990s.33, 34 Similar trends have been observed in other countries, including New Zealand, Australia and Canada.35 This time lag suggests that risk factor exposures, which started increasing similarly across broad age groups around the 1940–1950s and continued increasing for decades, might affect cancer risk in older individuals sooner than younger individuals, in whom the increase in cancer incidence appeared several decades later.18 Considering this time lag between increases in the incidence of later-onset and early-onset CRCs, dietary, lifestyle and environmental exposures (including less-studied exposures) that have increased since the 1940–1950s should merit further investigation. Many older individuals might have already accumulated cellular alterations (and/or developed premalignant lesions), which enabled an increased incidence of cancer within relatively short periods after factors such as the westernization of diet and lifestyle started spreading in the mid-20th century. By contrast, such (then early-life) exposures might have taken decades to increase the incidence of cancer in younger individuals who likely accumulated fewer potentially detrimental cellular alterations. Data from successive birth cohorts since the mid-20th century have demonstrated an increased incidence of cancers of the colorectum, endometrium, oesophagus, gallbladder, extrahepatic bile duct, kidney, thyroid and bone marrow (multiple myeloma).8, 36 This so-called birth cohort effect suggests an important role for early-life risk factor exposures for the observed increase in the incidence of early-onset cancers.
The early-onset cancer epidemic is likely attributable to changes in patterns of exposure in early life and/or young adulthood, although comprehensive analyses of individual risk factors in these early life stages remain limited. The possible long latency of exposure effects poses certain challenges. For example, unhealthy dietary components and other detrimental lifestyle behaviours are often correlated, making it difficult to disentangle confounding and estimate the true effect size of each individual risk factor. Confounding can also involve exposures at different stages of life. A childhood exposure (such as obesity37, 38) that is causally associated with an adulthood exposure (in this example obesity again) can become a confounder for the adulthood exposure. If such confounding exists, it becomes challenging to form accurate conclusions on the causality of the adulthood exposure unless the confounder can be accurately measured and analyzed.
[H3] Temporal trends in exposures.
In parallel with the global trends towards more-westernized diets, lifestyles and environments, the exposome (meaning the totality of exposures including, among others, diet, lifestyle, environment and the microbiota), during early life and young adulthood has changed substantially, albeit with large geographical variations, since the mid-20th century.8, 18, 21, 39 Temporal trends in putative risk factors since the mid-20th century (BOX 1) might have affected the incidence of early-onset cancer, starting from the 1990s. Briefly, trends have emerged towards increasing height,40 overweight and obesity,41, 42 type 2 diabetes,43–46 physical inactivity,47–49 Western-style diet (defined as a diet high in saturated fats, red meat, processed meat, sugar and ultra-processed foods, but low in fruits, vegetables, whole grains and fibre),50–56 and sugar-sweetened beverage intake55, 57, 58 in both children, adolescents, and adults worldwide (FIG 2). Per-capita alcohol consumption has also generally increased from 1960–2010, albeit with considerable variations between countries.59–63 Smoking habits have also changed in various ways during different time periods in different countries.64–69 In addition to personal smoking habits, effects of involuntary (secondhand or in utero) smoke exposures especially during but not limited to early life might not be trivial. Sleep patterns as well as the extent of exposure to bright lights at night have also changed in children, adolescents, and adults since the early 20th century.70–72 Reproductive factor exposures apply mostly to parents, especially mothers, although certain factors apply to both parents and offspring. Age at menarche and the overall number of childbirths have decreased,73–77 whereas age at both first and last birth, and oral contraceptive use have increased.78, 79 More-widespread use of infant formula led to a global decline in breastfeeding in the 20th century.80 Nonetheless, a trend towards increased breastfeeding has emerged in high-income countries since the 1990s.81
BOX 1. Temporal trends in exposuresa.
Alcohol
- Overall
- Per capita alcohol (ethanol) consumption among individuals of any age increased from the 1960s to early 2010s in many countries.59
- A decline in alcohol consumption was seen in Western Europe, whereas alcohol consumption increased in Eastern Europe, Asia and Middle Eastern countries during this period.59
- The ratio of male to female drinkers decreased in the Western Pacific region from 1990 to 2017, owing to an increase in the number of female drinkers.335
- Adolescents
- The prevalence of binge drinking (defined as having >4 drinks in a row at least once in past 2 weeks) among 12th-grade students in the USA peaked in 1979 and then declined from 41% in 1983 to 28% in 1992.60
- The National Survey on Drug Use and Health reported a consistent decline in the prevalence of alcohol consumption from 10.5% to 2.7% among adolescents 12–14 years of age and from 30% to 16% among those 15–17 years of age during 1991 to 2019.61
Antibiotics
- Overall
- Global per-capita consumption of antibiotics increased during 2000–2015.85
Height
- Overall
- Approximately 0.1 cm increase in average adult height (per 1 year increase in birth year) throughout the 20th century in the populations of several European countries.40
- Approximately 0.2 cm increase in average adult height (per 1 year increase in birth year) in the latter half of the 20th century in the population of South Korea.40
Obesity
- Adults
- Global age-standardized prevalence of obesity (BMI ≥30 kg/m2) increased from 3.2% to 10.8% in men, and from 6.4% to 14.9% in women during 1975–2014.41
- Children/adolescents (5–19 years of age)
- Global age-standardized prevalence of obesity in children and adolescents 5–19 years of age increased from 0.7% to 5.6% in girls and from 0.9% to 7.8% in boys during 1975–2016.41
Physical inactivity and sedentary lifestyle
Reproductive factors
- Overall
- Average age at menarche globally declined with greater declines in low/middle-income countries compared with high-income countries.73 Data from a UK-based study indicate that average age at menarche changed from 13.5 years of age in women born between 1908–1919 to 12.3 years of age in women born between 1990–1993.74
- Average age at first birth increased by 2–5 years during 1970–2018 in all OECD countries with available data.77
- Global fertility rate decreased from 5.0 births per women over a lifetime in 1950 to 2.5 in 2022.75
- The fertility rate decreased from 3.7 births per women over a lifetime in 1960 to 1.6 in 2020, and consequently the prevalence of women who never breastfed increased during the same period in the USA.73
- Global use of oral contraception increased from <40% to 60% of women 15–49 years of age during 1960s– 2009.78
Sleep duration and pattern changes (such as night shift work)
- Adults
- Reported sleep duration in adults has not changed significantly during 1960–2013 according to a systematic review of reports from Australia, Finland, Italy, Ireland, Japan, New Zealand, UK and the USA.71
- The prevalence of night shift workers has changed over the past few decades in Australia, Canada, China, Europe, Japan, South America and the USA.72
- Children/adolescents
- Reported sleep durations of children and adolescents have declined by >60 min per night during 1905–2008 according to a systematic review of reports from 20 countries in Asia, Europe, North America and Oceania.70
Smoking
- Adults
- The prevalence of smoking in men >20 years of age has decreased continuously in the USA, Canada, UK, Norway and Sweden during 1974–1987.64
- Smoking prevalence in women aged >20 years showed a slight fluctuation during the same period albeit with a general trend towards a decreasing prevalence (with the exception of Norway).64
- In the Netherlands, UK, Ireland and Denmark, smoking prevalence decreased continuously in men aged >15 years during 1950–1990, but increased in women aged >15 years, it increased during the mid-1960–1980s.65
- In China (urban areas), smoking prevalence increased in successive birth cohorts of men born in the 1920s to those born in the 1950s or later, whereas, in India, Japan, Singapore, South Korea and Taiwan, smoking prevalence plateaued in men born during the same period.68
- Smoking prevalence remained low in women born during the same period in these Asian countries.68
- The age-standardized prevalence of smoking has increased since 1990 in Africa and since 2010 in Latin America.69
- Adult smoking is directly linked with early-life involuntary smoke (secondhand or in utero) exposure in offspring.
- Adolescents (age 12–20 years)
- The global prevalence of smoking among adolescents 13–15 years of age decreased from 1999–2018.334
- The prevalence of cigarette smoking decreased among adolescents 12–16 years of age during 1974 to 1991 in the USA.66
- Smoking initiation rate during early (11–15 years of age) and late adolescence (16–20 years of age) declined for both men and women in most European countries during the 1970s–1980s.67
Type 2 diabetes mellitus
- Children/adolescents (<20 years of age)
Western-style diet and sweetened beverage consumption
- Overall
- Consumption of sugar-sweetened beverages has increased globally from 2000 to 2013.55
- Children/adolescents
- Increasing early-life calorie/food intake might have led to an increase in average height between 1896–1996 in 200 countries, although this trend varies by country.56
BMI, body mass index; CI, confidence interval; OECD, Organization for Economic Co-operation and Development.
Several decades will likely be required in order to observe possible effects of early-life exposures on the incidence of early-onset cancers. Hence, effects of a temporal trend associated with a specific early-life exposure in the past few decades are unlikely to have appeared in the literature on early-onset cancer incidence.
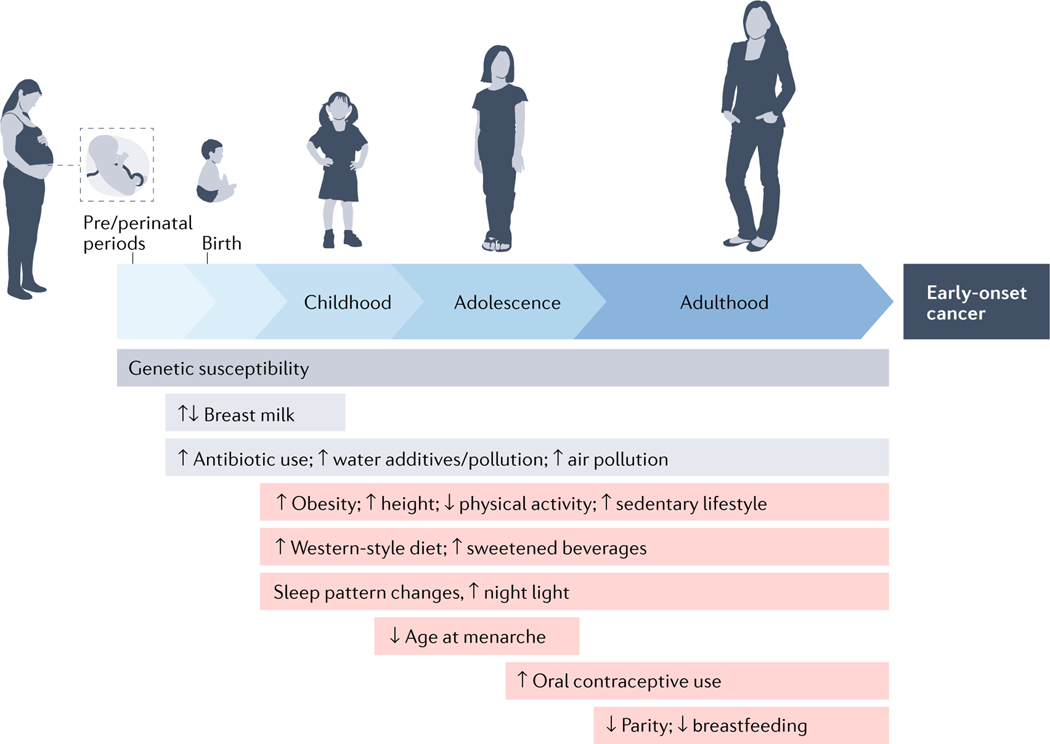
Figure 2. Individual life-course exposures and their relationship with the development of early-onset cancers.
An individual will encounter various exposures throughout life, from conception (or even the germ cell period before conception), some of which might also be cancer risk factors. Considering the long latency periods of neoplastic development, risk factor exposures in early life (from conception to adolescence) and during young adulthood are considered to have pathogenic roles in the development of early-onset cancer (defined here as cancer diagnosed in adults ≤50 years of age). Genetic susceptibility results from germline genetic variants with a spectrum from low to high penetrance. Gene–environment interactions can occur at any time throughout the lifetime of an individual. This figure also implies considerable challenges in studying the aetiology of early-onset cancers.
In addition to reproductive factors, smoking, diet, alcohol consumption, lifestyle and morbidities during pregnancy might all be relevant in utero exposures. These might affect the risk of cancers in variable target organs with different latency periods in mothers relative to offspring. Similarly, nonlinear time-varying changes in exposures involving both mother and offspring (such as breastfeeding and/or infant formula intake) in the past century might have influenced the incidence trends for various cancer types differentially in both mothers and offspring.
Notably, influences of any of these exposure trends in early life and young adulthood on cancer incidence are unlikely to appear until decades later. Therefore, a declining (or non-increasing) trend of a given risk factor in the past few decades (such as since the 1990s) does not imply that early-life exposure to that factor is not one of the causes of the early-onset cancer epidemic. This premise might hold true for early-life exposures to smoking, alcohol, or chronic hepatitis B virus (HBV), human papillomavirus (HPV), or H. pylori infections in certain countries. Data on exposure trends over the past several decades might be useful to predict future trends in cancer incidence across various age groups if we can elucidate their aetiological roles.
[H3] Other factors.
The microbiome is another notable contributor to tumour development.82 Among the 14 early-onset cancer types with a rising incidence, eight (those of the colorectum, oesophagus, extrahepatic bile duct, gallbladder, head and neck, liver, pancreas, and stomach) relate to the digestive system, indicating the potential pathogenic importance of both the oral and intestinal microbiome. Nutrition, lifestyle factors and antibiotic use can all influence the development of various chronic diseases through various physiological mechanisms, including microbial alterations.82–84 Antibiotic use, which has been associated with certain cancer types,85 has increased in both adults and children in many countries over the past half century.86–88 Inflammatory bowel disease (an established risk factor for CRC), in which the microbiome has a major pathogenic role, has increased in adolescents since the 1980s.89, 90 The early-life microbiome is known to influence the development of the immune system.91, 92 Therefore, tumour–microbial–immune interactions are an emerging research frontier,93 which will likely provide novel aetiological insights.
Interestingly, a polygenic risk score based on the presence of many low-penetrance variants is more strongly associated with early-onset CRC than later-onset CRC.94 Similar phenomena have been observed for breast and prostate cancers.95, 96 Certain environmental and/or lifestyle risk factors occurring in early life to young adulthood might make individuals carrying a higher number of low-penetrance risk variants more susceptible to cancer development.
Germline genetic variations in certain hereditary cancer-related genes are associated with early-onset cancers.97–99 Owing to advances in medicine over the past decades, individuals carrying certain high-penetrance variants (that would have tended to be removed from the population pool without advances in medicine) have increasingly had the chance to reproduce and therefore pass their variants to the next generation. This effect might be causing an increase in the population prevalence of high-penetrance variants of certain genes. However, multiple generations would likely be required to enable the detection of observable differences in the prevalence of such variants. Despite this speculation, thus far, no evidence for increased population prevalence of high-penetrance variants in any gene has emerged. Furthermore, at least another few decades would be required to observe an increased incidence of early-onset cancer in adults owing to an increase in the prevalence of high-penetrance variants (if it exists). The implications of an as yet unobserved but seemingly plausible increase in the prevalence of certain high-penetrance variants in the population will be an issue to be addressed in the future.
[H1] Organ-specific considerations
Evidence regarding risk factors has emerged for several early-onset cancers (TABLE 1; details in Supplementary Tables 2-12). Pertinent findings for each cancer type are summarized below. Many studies are reliant on small sample sizes and most of the results described require replication.
Table 1.
Possible risk factors for early-onset cancers.
| Cancer type | Factors with a generally increasing temporal trenda | Factors with a generally stable temporal trend | Factors with a generally decreasing or variable temporal trend* |
|---|---|---|---|
| Breast cancer | Younger age at menarche106–109, oral contraceptive use109, 110, nulliparity106, 109, older age at first birth106, 109, never breast feeding106, central obesity115, physical inactivity124, alcohol125–128, fat intake130, 131 | Family history of breast cancer97, 136 | Smoking132–134 |
| CRC | Obesity33, 146–156, sedentary behaviour160–162, metabolic syndrome151, 152, 163, type 2 diabetes148, 165–167, 336, hyperlipidaemia107,109,111,114,115,124,12, diet (such as western diet, sugar-sweetened beverages, low vitamin D intake, and red meat)168–170, 172, alcohol155, 165, 170, 174, 175, inflammatory bowel disease147 | Family history of CRC147–149, 155, 170, 177, 186–189 | Smoking149, 155, 164–166, 174, 175, 177–180 |
| Endometrial cancer | Obesity193–195, 337 | Family history of any cancer194, 196 | – |
| Oesophageal adenocarcinoma | Obesity199, recurrent gastroesophageal reflux199 | – | Smoking199 |
| Head and neck cancer | Alcohol consumption204, HPV infection in areas without comprehensive vaccination coverage205, 206 | – | Smoking (snuff use) 204, 338 |
| HPV infection in areas with comprehensive vaccination coverage205, 206 | |||
| Kidney cancer | Obesity207 | – | – |
| Liver cancer | – | Family history of liver cancer218 | Chronic HBV infection216 |
| Multiple myeloma | Obesity223, 224 | – | – |
| Pancreatic cancer | Obesity225, 226, alcohol intake227 | – | Smoking227 |
| Prostate cancer | – | Family history of prostate cancer238 | – |
| Stomach cancer | – | Family history of stomach or prostate cancer249, 251 | Helicobacter pylori infection242 |
CRC, colorectal cancer.
Several decades of follow-up monitoring will likely be required to confirm possible effects of early-life exposures on the development of early-onset cancer. Therefore, effects of temporal trends in early-life exposure seen over the past few decades are unlikely to have appeared in the available literature on the incidence of early-onset cancer.
[H3] Breast cancer.
Here we discuss premenopausal breast cancer, as studies have typically divided breast cancer cases based on menopausal status at diagnosis (instead of applying an exact age cutoff). The average age of menopause is 45–55 years globally.100
Enhanced screening and detection have likely contributed to an increased incidence of premenopausal breast cancer in countries with certain screening programmes, albeit to an unknown extent. Mammography screening has become increasingly prevalent worldwide during 2005–2015.101 In the USA, although the age at which screening is initiated has changed over time since the introduction of mammography screening in the 1980s,102, 103 the incidence of breast cancer has increased most prominently in women <40 years of age, who are below the routine screening age.104 The incidence of premenopausal breast cancer has also increased in countries that do not have routine screening programmes.105
Reproductive factors including younger age at menarche,106–109 oral contraceptive use,109, 110 nulliparity,106, 109 older age at first birth106, 109, 111 and never-breastfeeding106 are established risk factors for premenopausal breast cancer. Interestingly, trend analyses suggest that declining trends in fertility rates together with increasing trends in additional risk factor exposures since the 1930s might have contributed to the increased incidence of premenopausal breast cancer.105, 112, 113 Data also show that premenopausal breast cancer risk is reduced by a higher adulthood body mass index (BMI) and weight at age 18,114–120 but is increased by height, waist:hip ratio and weight gain in older adulthood.115, 121–123 The underlying mechanisms of these various relationships remain unclear.
A meta-analysis published in 2019 reported an inverse association between vigourous physical activity and premenopausal breast cancer risk.124 Alcohol consumption is also a risk factor for premenopausal breast cancer.125–128 Another meta-analysis did not find a statistically significant association between western diet and premenopausal breast cancer risk,129 although data from several studies suggest that greater fat intake during adolescence and animal fat intake during young adulthood are associated with increased premenopausal breast cancer risk.130, 131 Data from several studies suggest an increased risk of premenopausal breast cancer among smokers, with a prolonged latency period.132–135
Most premenopausal breast cancers arise sporadically, although a minority are hereditary and are caused by the presence of high-penetrance genetic variants (such as loss-of-function mutations in BRCA1 and BRCA2).97, 136 In one study, 12% of patients with breast cancer diagnosed at ≤40 years of age had germline mutations in BRCA1 or BRCA2.137 Data from other studies indicate that 5% of patients with breast cancer diagnosed at ≤35 years of age have germline TP53 mutations and 1% of those ≤40 years of age have germline PALB2 mutations.138, 139 In addition, many low-penetrance genetic variants are associated with breast cancer risk.140 However, none of these studies provided strong evidence for a role of gene–environment interactions in premenopausal breast cancer risk.141, 142
Based on current evidence, the trend of reproductive factors, central obesity, physical inactivity, alcohol consumption and dietary westernization since the mid-20th century might have contributed to the rising incidence of premenopausal breast cancer. Evidence also indicates differences in tumour molecular subtype distribution between premenopausal and postmenopausal breast cancers.143 Compared to women with postmenopausal breast cancer, those with premenopausal disease are more likely to have breast cancers of an ESR1 (also known as ER)-negative or triple-negative (ESR1-negative, PGR (also known as PR)-negative, ERBB2 (also known as HER2)-negative) subtype.144 In the USA, Black women tend to be diagnosed with breast cancer at an earlier age than women from other ethnic groups.145 Therefore, unidentified risk factors for certain breast cancer subtypes or specific populations might contribute to the observed increasing incidence of premenopausal breast cancer.
[H3] Colorectal cancer.
Data from previous studies have linked high adulthood BMI with early-onset CRC,146–155 and a few studies demonstrate an association between BMI during childhood or adolescence with early-onset CRC.146, 156 The association of adolescence or young adulthood obesity with early-onset CRC has been reported to be either stronger149–152 or weaker146–148 than that with later-onset CRC. BMI has also been associated more strongly with colon cancer than with rectal cancer;157 however, the rise of early-onset rectal cancer in the USA has outpaced that of early-onset colon cancer.158, 159 Therefore, further studies are needed to clarify the effects of BMI during childhood, adolescence and young adulthood on early-onset versus later-onset CRC risk by tumour location.
Sedentary lifestyle and physical inactivity likely both have a role in early-onset CRC.160–162 An analysis using the Nurses’ Health Study II found that prolonged sedentary television viewing time was associated with an increased incidence of early-onset CRC independent of exercise and BMI.160 Metabolic syndrome, which encompasses hypertension, hyperglycaemia, abdominal obesity and hyperlipidaemia,151, 152, 163 as well as individual metabolic comorbid conditions such as type 2 diabetes148, 152, 164–167 and hyperlipidaemia147–149, 151, 155, 165, 166 have also been associated with early-onset CRC risk.
Diet-related factors reported to be associated with early-onset CRC risk in certain studies include sweetened beverage intake during adolescence and young adulthood,168 western dietary patterns,169 processed meat intake,170 red meat intake,171 low vitamin D intake,172 limited intake of vegetables, fruits, and micronutrients,170 dietary and lifestyle index linked to hyperinsulinaemia173 and excessive alcohol consumption.155, 164, 165, 170, 171, 174, 175
Evidence supporting a role for other risk factors is limited. Aspirin and nonsteroidal anti-inflammatory drug use have been associated with a lower risk of early-onset CRC,171, 176 whereas inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease have been associated with an increased risk.147 Results from studies exploring associations between smoking and early-onset CRC have been inconsistent.149, 155, 164–166, 174, 175, 177–180 Long-term use of antibiotics has been associated with colorectal adenoma risk in a few studies,181, 182 although whether use of antibiotics is a risk factor for early-onset CRC remains to be determined.183–185
A family history of CRC has been associated with an increased risk of both early-onset CRC and advanced-stage adenoma,147, 148, 155, 170, 186 and the extent of increase in risk associated with a family history of CRC was higher for early-onset than for later-onset disease.147–149, 177, 186–189 Most early-onset CRCs are sporadic, although those with a family history of the disease constitute a heterogenous group with or without hereditary cancer syndromes, such as Lynch syndrome and familial adenomatous polyposis.190 Evidence suggests that 16–20% of patients with early-onset CRC have pathogenic germline variants, 5–8% with Lynch syndrome and 5% with polyposis syndromes.98, 99
Several studies have identified common genetic risk variants for early-onset CRC.94, 98, 99, 191 A large-scale consortium study demonstrated that a polygenic risk score based on 95 CRC-associated genetic variants was more strongly associated with early-onset than later-onset CRC, particularly in the absence of a family history of CRC and/or Lynch syndrome.94
[H3] Endometrial cancer.
Endometrial adenocarcinoma is the most common histological type of uterine corpus cancer.192 Obesity has been consistently associated with early-onset as well as overall endometrial cancer risk.193–195 Irregular menstruation and nulliparity are established risk factors for endometrial cancer; however, the role of reproductive factors such as decreasing fertility rates and increasing nulliparity in early-onset endometrial cancer remains to be determined. A family history of any cancer has been associated with early-onset endometrial cancer,194, 196 which might in part reflect the link between Lynch syndrome and early-onset cancer. In a cross-sectional analysis, 18% of individuals with early-onset endometrial cancers had presumptive Lynch syndrome.196
[H3] Oesophageal adenocarcinoma.
Squamous cell carcinoma and adenocarcinoma are the two main histological subtypes of oesophageal cancer, with differing risk factor profiles.197 Oesophageal adenocarcinomas tend to be most prevalent in western countries, whereas oesophageal squamous cell carcinomas are more common in certain parts of Asia and a few other geographical locations.198 According to GLOBOCAN data, the incidence of early-onset oesophageal cancer has increased in a few countries. Notably, an increase in the incidence of early-onset oesophageal adenocarcinoma has been observed in the USA.10
Possible risk factors for early-onset oesophageal adenocarcinoma include obesity, gastroesophageal reflux disease and smoking.199 Obesity is (likely causally) associated with gastroesophageal reflux disease200 and both obesity and gastroesophageal reflux disease have increased in most western countries over the past decades.201, 202 Helicobacter pylori infection has been inversely associated with gastroesophageal reflux disease203; therefore eradication of H. pylori infection might be associated with gastroesophageal reflux disease, which could partly account for the observed trends in the incidence of early-onset oesophageal adenocarcinoma.
[H3] Head and neck cancer.
Head and neck cancers include squamous cell carcinomas (most common), adenocarcinomas, lymphomas, sarcomas and other rarer subtypes. A pooled analysis of data from 25 case-control studies found associations of smoking, alcohol intake, a family history of cancer, and lower fruit and vegetable consumption with early-onset head and neck cancer (in the oral cavity, hypopharynx and larynx) defined as a diagnosis at <45 years of age.204 Behavioural factors relating to sexual activity, including oral sex (in adolescents), premarital sex, and the number of sexual partners have all changed since the mid-20th century in both North America and Europe.205, 206 These changes might have increased the prevalence of oral HPV infection, possibly leading to an increased incidence of early-onset head and neck cancer.205, 206 Whether and how increased uptake of HPV vaccination over the past two decades might reduce the incidence of early-onset cancer remains to be determined.
[H3] Kidney cancer.
Kidney cancer consists of renal cell carcinomas (RCCs) (most common), urothelial carcinoma of the kidney, and other rarer tumour subtypes. Most published data are based on either kidney cancer overall or renal cell carcinoma specifically.207, 208 Both smoking and physical inactivity have been associated with an increased overall risk of kidney cancer,209–211 although these risk factors have not been evaluated specifically in relation to early-onset cancers. In a registry study comprising 1.1 million adolescent males, BMI during adolescence ≥27.5 kg/m2 (relative to <22.5 kg/m2) was associated with a higher incidence of RCC (at a mean of 44 years of age at diagnosis).207 Having a first-degree relative (especially sibling proband) with RCC is also associated with a higher risk of early-onset RCC.208
[H3] Liver cancer.
Primary liver cancer mostly consists of hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma.212, 213 Most published data are based on either liver cancer overall or HCC. Established risk factors for HCC include chronic infection with HBV and hepatitis C virus (HCV), chronic hepatitis, cirrhosis, alcohol consumption, obesity, type 2 diabetes, nonalcoholic fatty liver disease and smoking.212 However, limited data exist on risk factors for early-onset HCC. Data from several studies indicate a lower prevalence of underlying cirrhosis in patients with early-onset HCCs compared with later-onset forms of this cancer,214 suggesting the existence of differences in aetiology.
Chronic HBV infection is associated with early-onset HCC.215, 216 Data from a case-control study involving HBV carriers indicate that smoking is associated with early-onset, but not later-onset HCC.217 A family history of HCC has been associated with an increased risk of an HCC diagnosis at <45 years of age, and the increase in risk was greater for HBV carriers than HBV noncarriers.218 Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis, which are associated with obesity, might also have contributed to the rise of early-onset HCC.219, 220 The implications of the declining global prevalence of chronic HBV infection, along with changes in smoking and alcohol intake over the past few decades,64, 219 for the incidence of HCC in different age groups remains to be determined.
[H3] Multiple myeloma.
Both the incidence and mean age at diagnosis of multiple myeloma (MM) varies greatly across ethnicities, regions and countries,221 although an increased incidence of early-onset MM has been reported in several countries.3, 8 This increase at least partly reflects increased uptake of screening for monoclonal gammopathy, although a genuine increase in incidence might also be emerging.
Established risk factors for MM include male sex, Black ethnicity, obesity and a family history of MM.222 However, data on risk factors for early-onset MM remain scarce. Compared with BMI later in adulthood, BMI at a younger age (25–30223; or 18–30224 years of age) is more strongly associated with MM risk. These studies223, 224 did not conduct a stratified analyses by age at diagnosis, but still suggest that the trend towards increasing obesity in younger people might contribute to the increasing incidence of early-onset MM.
[H3] Pancreatic cancer.
Obesity,225, 226 smoking227 and alcohol intake227 (all established risk factors for pancreatic cancer) have also been associated with early-onset pancreatic cancer risk. The trends of increasing obesity and alcohol intake since the early 20th century might have contributed to this rise. Obesity has been associated with a younger age at diagnosis among patients with pancreatic cancer.226 In a pooled analysis of data from case-control studies, alcohol intake was more strongly associated with a diagnosis of pancreatic cancer at <45 years of age than a diagnosis at ≥45 years.227 Studies have also shown that 0–8% and 3% of patients diagnosed with early-onset pancreatic cancers had a family history of the disease in any first-degree relative and Lynch syndrome, respectively.228, 229
[H3] Prostate cancer.
Determining the true trends in prostate cancer incidence independent of the effects of screening based on serum kallikrein related peptidase 3 (KLK3; also known as, prostate-specific antigen, (PSA)) is typically problematic. Routine serum KLK3 (PSA)-based screening has been less common in men <50 years of age,230 nonetheless, enhanced uptake of serum KLK3 (PSA) tests has likely contributed to an increase in the diagnosis of early-onset prostate cancer. However, a true increase in incidence also cannot be ruled out.
Established or possible risk factors for prostate cancer include Black ethnicity, tall height, obesity, early age at puberty, and high serum levels of testosterone and insulin-like growth factor 1 (IGF1).231–234 Evidence also suggests an aetiological role of genetic factors in early-onset prostate cancer.235 Data from the Nordic Twin Study suggest that the heritability (defined as the proportion of variance for a measure that is attributable to genetic differences in the study population) of prostate cancer risk is high (58%, 95% CI 52–63%).236 Data from another study suggest that 43% of prostate cancers diagnosed at <55 years of age arise from the presence of high-penetrance variants.237 The number of first-degree relatives with prostate cancer is also associated with early-onset prostate cancer risk.238
Data from a registry-based study from Sweden239 demonstrate an association between prostate cancer diagnosed <55 years of age with use of assisted reproduction techniques, which often reflect hampered spermatogenesis. How factors relating to the inability to reproduce naturally, undergoing enhanced medical work-up or both have contributed to this association remains unclear.
[H3] Stomach cancer.
Stomach (gastric) cancer, which mostly consists of adenocarcinomas and, less commonly, gastrointestinal stromal tumours, is anatomically classified into tumours of the cardia and non-cardia (fundus and antrum), with differing clinical and epidemiological features.240 Gastric non-cardia cancer is common in Eastern and Central Asia and Eastern Europe and is associated with Helicobacter pylori infection.13, 240, 241 Gastric cardia cancer is common in North America and Western Europe, and has been associated with obesity.241 Over the past several decades, the incidence of early-onset stomach (gastric) cancer has increased in some countries, although whether this reflects an increase in either early-onset cardia or non-cardia cancer, or both, remains unclear.
H. pylori infection has been associated with a diagnosis of stomach cancer at <40 years of age.242 The prevalence of H. pylori infection has declined in the USA, most European countries, China and Japan,243, 244 although it remains relatively high in developing and newly industrialized countries compared to economically developed countries.243, 245
Data from several studies from the USA indicate an association between heavy alcohol consumption and both early-onset and later-onset stomach cancer.246, 247 In a case-only study, BMI correlated inversely with age at stomach cancer diagnosis.226 Data from several studies demonstrate an association between a family history of stomach or prostate cancer and early-onset stomach cancer.248–251 Inherited cancer predisposition, such as hereditary diffuse gastric cancer (potentially owing to germline CDH1 mutations252) and Lynch syndrome, increase the risk of early-onset stomach cancer.253–255 Multiple lifestyle-related factors, including alcohol intake and obesity, and their interactions with genetic factors might also have a role in the rise of early-onset stomach cancer.
[H3] Thyroid cancer.
The increased incidence of thyroid cancer since the 1980s is likely attributable to increased use of diagnostic imaging techniques (such as ultrasonography, CT and MRI) and follow-up fine-needle aspiration biopsy sampling,256–260 which might have led to the detection of many clinically insignificant thyroid tumours. Notably, South Korea introduced a thyroid cancer screening programme in 1999, leading to substantial increases in the incidence of thyroid cancer shortly thereafter. Most papillary thyroid carcinomas (the most common thyroid cancer subtype) clinically behave as essentially benign tumours. However, because distinguishing between such benign tumours and truly malignant tumours is often impossible, all tumours with certain characteristic nuclear features are diagnosed as papillary thyroid carcinomas. Whether a true increase in the incidence of early-onset thyroid cancer incidence and related mortality exists remains to be determined.
[H1] Differences in aetiology and pathogenesis
Accumulating evidence indicates the existence of differences in both clinical and tumour characteristics between patients with certain forms of early-onset and later-onset cancer, including breast cancer,261–269 CRC,94, 98, 99, 187, 190, 270–282 endometrial cancer,196, 283–289 MM,290–294 pancreatic,228, 295–298 prostate299–305 and stomach cancers246, 253, 306–309 (TABLE 2). These findings suggest that early-onset and late-onset cancers might have somewhat different mechanisms of carcinogenesis. Tumour tissue analyses of molecular pathology, genomics, multi-omics, and/or immunological features can provide insights into pathology and explain the links between exposures and specific molecular signatures.310 Hence, the integration of tumour tissue analyses into epidemiological studies, so-called molecular pathological epidemiology (MPE) research,310 should be considered when feasible.
Table 2.
Clinical and tumour-specific characteristics of early-onset versus later-onset cancers
| Cancer type | Clinical characteristics | Tumour characteristics |
|---|---|---|
| Breast cancer | Advanced disease stages at diagnosis, inferior OS261, 263, 265, 339–342 | Adverse pathological features, including high tumour grade, triple-negative (ESR1 (also known as ER)-negative, PGR (also known as PR)-negative, ERBB2 (also known as HER2)-negative) subtype, ERBB2 (HER-2)-positive subtype, and MKI67 (Ki-67) overexpression.261–269 |
| CRC | Predilection to rectal and distal localization within the colon, advanced disease stages at diagnosis, inferior OS94, 187, 270, 271, 276, 280, 343–358 | Aggressive tumour phenotypes (excluding MSI-high status) such as poor differentiation, lymphovascular and perineural invasion, signet ring cell histology, LINE-1 hypomethylation, and lower lymphocytic immune reaction.190, 273–282 |
| Endometrial cancer | Inconsistent findings on OS and disease stages at diagnosis196, 283–289 | Certain studies suggest that early-onset endometrial cancer is associated with favourable features, such as well-differentiated carcinoma and adenoacanthoma283–287; whereas, others suggest that early-onset endometrial cancer is associated with unfavourable pathological features, including poor differentiation, high mitotic rates, and deep myometrial invasion.196, 288, 289 |
| Multiple myeloma | – | Certain studies suggest that younger patients have greater numbers of lytic lesions and high-risk cytogenetic abnormalities290, 291; whereas, other studies suggest that younger patients have similar or more-favourable tumour characteristics.291, 293, 294 |
| Pancreatic cancer | Advanced disease stages at diagnosis297, 298 | Poor differentiation, perineural invasion.228, 295–298 |
| Prostate cancer | Metastatic disease, resistance to androgen-deprivation therapy, and shorter OS299–302 | Genomic and epigenomic aberrations seen in patients with early-onset prostate cancer might be distinctly different to those seen in patients with later-onset disease303, 304; for example, clinically-advanced early-onset prostate cancers might be associated with TMPRSS2::ERG fusions and fewer AR, SPOP and ASXL1 alterations.305 |
| Stomach cancer | More common in women, advanced disease stages at diagnosis299–302 | Higher grades, advanced disease stages, signet ring cell or diffuse histology246, 253, 306; fewer somatic mutations in TP53 and more somatic mutations in MUC5B, BANP, CDH1, and TGFBR1.307, 308 |
CRC, colorectal cancer; LINE-1, long interspersed nucleotide element-1; MSI, microsatellite instability; OS, overall survival.
A major benefit of MPE resides in providing better definitions of tumour phenotype, thus improving our understanding of aetiologies related to host susceptibility and exposures. With the presence of a phenotype-specific association (of a given exposure), the MPE approach can reveal either a moderate or a strong effect size of a specific association, thereby helping to establish causality.311, 312 MPE can have a role in early-onset cancer research by linking unidentified or suspected risk factors (such as certain early-life exposures) to specific tumour phenotypes. Molecular pathological characteristics often by themselves imply specific aetiological underpinnings. For example, the detection of smoking, UV radiation and alkylating somatic mutational signatures suggests exposure to smoke, sunlight and red meat carcinogens, respectively.313, 314 Establishing new exposure-phenotype links will inform our effort to develop strategies for the prevention of early-onset cancer.
[H1] Public health and societal implications
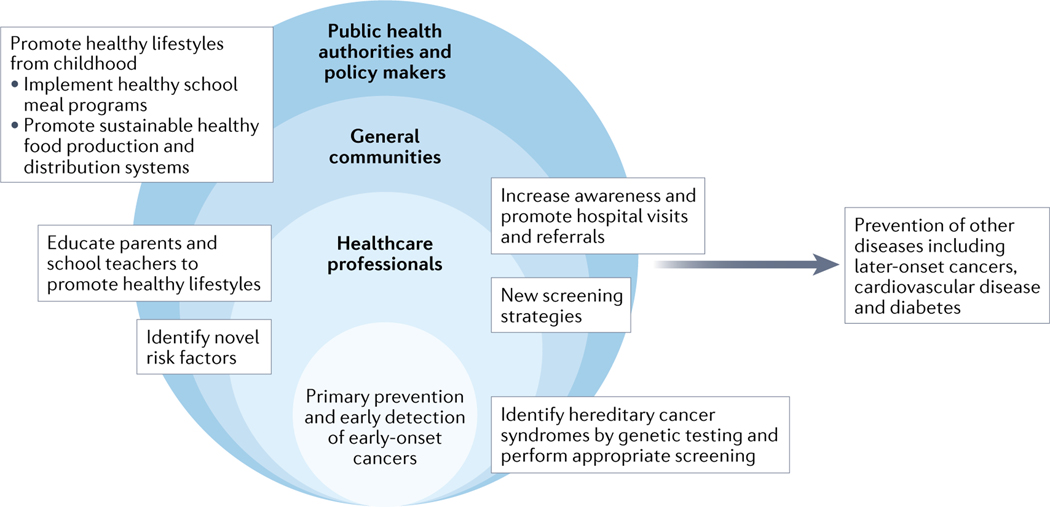
Several key steps need to be considered when attempting to address the current issues associated with early-onset cancer (FIG 3). Given the increasing incidence of several early-onset cancer types, we need to increase the awareness of this trend and potentially re-evaluate current screening guidelines and/or develop personalized screening approaches for early-onset cancer, although further research is needed in this area.
Figure 3. Broad implications and benefits of prevention efforts for early-onset cancers.
Collaborative interventions at multiple levels (such as those delivered by health-care professionals, general communities, public health authorities, and policy makers) promoting the early identification of hereditary cancer syndromes, healthy diet and lifestyle behaviours, behavioural education of children, and increasing cancer awareness will lead to not only prevention of early-onset cancers (defined here as cancer diagnosed in adults ≤50 years of age) and many other diseases including later-onset cancers (defined as cancers diagnosed at >50 years of age), but also ultimately more sustainable health-care practices.
Current evidence suggests that certain early-onset cancer types are more likely to be of an advanced-stage and have worse survival outcomes compared with their later-onset counterparts (TABLE 2). Therefore, research into therapeutics for early-onset cancers, in addition to primary prevention and early detection, is warranted. Researchers should aim to improve personalized therapeutic strategies. For example, given the established role of microsatellite instability (MSI) status in predicting a response to immune-checkpoint inhibitors, testing for MSI or mismatch repair (MMR) protein expression is indicated in patients with solid tumours, including those with early-onset solid tumours, for personalized treatment planning.315
Given the global changes in risk factor exposures over the past decades, a possibility emerges that, among all current birth cohorts, younger cohorts (current children, adolescents and young adults) might have higher age-specific risks of cancer throughout life compared to current older people (>50 years of age). The current generation of young adults already have a higher risk of early-onset cancer than that of older generations. Many cancer risk factors are also risk factors for other chronic diseases and the incidence and prevalence of other chronic diseases, such as diabetes and inflammatory bowel disease, among children, adolescents and young adults, have increased in multiple countries over the past several decades.45, 46, 316 Therefore, this ‘early-onset cancer epidemic’ could be just one manifestation (the tip of the iceberg) or example of an increasing trend towards greater incidences of many chronic diseases in young and/or future generations.
Cancer-related lifestyle factors in adulthood often originate from childhood and/or adolescence. Therefore, popularizing healthy diet and lifestyle (while avoiding unhealthy foods and/or beverages, a sedentary lifestyle and avoiding the development of an early interest in alcohol consumption and/or smoking) among children, possibly through school meal programmes and educational interventions, is an important method of primary cancer prevention.317 Avoidance of unhealthy behaviours can benefit both children and parents, enhance their wellbeing and reduce the societal burdens associated with many chronic diseases and/or conditions.
Reforming the food production and distribution system is also necessary to encourage people to eat healthier foods and fewer (ultra)processed foods and beverages. Policies implementing taxation on sugar-sweetened beverages could lead to reduced consumption and energy intake.318 Health-related taxes are recommended by the WHO319 and have been successfully implemented in several countries.320
In addition, considering the substantial environmental changes that have occurred since the mid-20th century, an improved understanding of the effects of exposures in the lived environment (such as air and water pollution) is also crucial. Other less-studied factors that affected several generations during the 20th century include changes in sleep patterns and night-time light exposure. Evidence indicates an epidemiological and pathobiological link between sleep patterns (such as night shift work) and systemic metabolic abnormalities such as obesity and type 2 diabetes,321, 322 both of which are cancer risk factors. The International Agency for Research on Cancer has classified night shift work as “probably carcinogenic to humans” (Group 2A).72 These exposures often relate not only to an individual’s habits but also the effects of certain societal, political, and environmental influences. For example, many individuals undertake regular night shifts and thus increase their exposure to light during night-time owing to societal demands for shift work. On the other hand, a policy that mandates increased payments for those doing night shifts will create financial incentives for employers to reduce the overall amount of night shift work to an essential minimum. Therefore, research on systematic and structural interventions at the societal level is warranted.
[H1] Research gaps and future directions
Substantial gaps exist in research on early-onset cancer. Data from many large-scale cross-sectional and case-control studies are available, although the design of such studies limits the ability to accurately assess early-life exposures, typically owing to reliance on personal recollection as the main source of data. Conducting prospective cohort studies involving early-life participants requires both the co-operation of parents and decades of follow-up monitoring. Currently, only very few cohort studies, in which most participants are of white ethnicity, have monitored individuals from early in life up to at least 40–50 years of age.29, 323–326
Certain technological advancements might help to address this research gap. First, data from sources such as electronic health records (EHRs) and computational analytical advances (such as those involving natural language processing and/or machine learning) provide many opportunities to evaluate the longitudinal relationships between early-life exposures and disease risk. The collection of EHR data will continue to expand worldwide, also integrating information from clinical omics testing.327 Second, geospatial data from early-life residence could be used to assess the effects of early-life environmental exposures.328, 329 Third, with rapid advances in biomedical sciences, including in omics analyses, we can assess the molecular profiles of early-onset cancers and compare them with later-onset cancers, which has the potential to inform on differences in aetiology and guide effective personalized treatment strategies. Furthermore, early-life biospecimens can provide reliable sources for objective biomarker measurements that reflect early-life biological information. Prospective cohort studies combined with early-life specimen collection (including blood, stool, saliva, urine, placenta, umbilical cord and others) would enable early-life factors to be studied in relation to various future health outcomes, including cancer.
Analyses of tumour tissue samples and other specimens can provide valuable pathological and biological insights, although the necessity to collect such specimens from sizable populations while minimizing selection bias remains a substantial issue. Close interdisciplinary collaborations involving investigators with divergent expertise in biomedical and population sciences are essential in addressing this challenge and opening up new opportunities.
Generally, research proposals that appear to promise more-foreseeable, shorter-term results have higher probabilities of receiving funding.330 To conduct prospective lifecourse cohort studies furnished with adequate specimen repositories would require a long-term commitment from one or more major funding bodies. In the meantime, existing datasets of populations that include patients with early-onset cancers could be utilized to provide research opportunities. If each dataset is small, pooling of datasets might be necessary to enable robust statistical analyses.
Research on disparities in cancer-related health between sexes and/or between ethnic, demographic, and socioeconomic groups currently lags behind the progress made in other areas of cancer research, such as genomics and therapeutics.331 For example, in the USA, trends in the incidence of early-onset CRC and pancreatic cancer differ substantially by race/ethnicity.332, 333 Many early-life exposures have a varying prevalence in these different societal groups. However, sufficient data on whether exposures differentially affect the risk of early-onset cancers according to sex, race/ethnicity or socioeconomic groups are currently unavailable. Therefore, addressing health disparities requires assessments of geospatial exposure and multilevel approaches encompassing social, epidemiological and pathobiological sciences.334 Furthermore, the integration of divergent research fields will contribute to a better understanding of early-onset cancers, ultimately leading to improved prevention and treatment strategies.
[H1] Conclusions
The incidence of many types of early-onset cancer (in those ≤50 years of age) has increased in many countries. The reasons for this phenomenon are not entirely clear but likely relate to changes in risk factor exposures in early-life and/or young adulthood from the mid-20th century onwards. The increased consumption of highly processed or westernized foods, changes in lifestyles, the environment, morbidities, and other factors might all have contributed to such changes in exposures. Therefore, although available data on the incidence of early-onset cancers in low and middle-income countries are currently limited, the rise of early-onset cancers will likely be increasingly prominent in those countries, potentially leading to a global early-onset cancer pandemic.
To study the aetiology of early-onset cancers, prospective life-course cohort studies that enable biomarker/omics analyses of specimens obtained during early life are needed. In addition, advances in information technologies combined with artificial intelligence should be leveraged to fill research gaps. We must raise awareness, among both the public and health-care professionals, of the rising incidence of early-onset cancer and aim to increase primary, secondary, and tertiary prevention efforts. A reasonable assumption exists that improving heath literacy and interventions that promote a healthy lifestyle, including a healthy diet, could reduce cancer risk. Beyond personal prevention efforts, systematic interventions that promote screening uptake and a healthier lifestyle at the societal level (such as, among others, regulation of industries that produce tobacco, ultra-processed foods and beverages) could potentially have an effect on cancer risk. We call for collaborations of researchers, health-care providers, public health practitioners, policymakers and the public to address the rising incidence of early-onset cancer.
Supplementary Material
Key points.
The incidence of cancers of various organs diagnosed in adults ≤50 years of age has been rising in many parts of the world since the 1990s.
Evidence suggests an aetiological role for risk factor exposures in early life and young adulthood, although specific effects of individual exposures remain largely unknown.
The early life exposome (including, among others, diet, lifestyle, obesity, environmental exposures and the microbiome) has changed substantially, with variable trends observed around the world since the mid-20th century.
The early-onset cancer epidemic might be one manifestation of increasing trends in the development of many chronic diseases in young and future generations.
Prospective cohort studies utilizing electronic health records and/or early-life biospecimen collection would enable the detailed investigation of early-life factors in relation to many future health outcomes, including cancer.
Raising awareness of the early-onset cancer epidemic and improving the early-life environment should be our immediate goals: this will likely reduce the burden of both early-onset and later-onset cancers.
Acknowledgements
The work of S.O. is supported in part by the U.S. National Institutes of Health grants (R35 CA197735 and R01 CA248857) and the Cancer Research UK Cancer Grand Challenge Award [6340201/A27140]. The work of T.U. is supported by grants from the Prevent Cancer Foundation, Japan Society for the Promotion of Science, and Mishima Kaiun Memorial Foundation.
Footnotes
Competing interests
E.W. is an employee of the IARC/WHO. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the IARC/WHO. The other authors declare no competing interests.
Reviewer information
Nature Reviews Clinical Oncology thanks C. Eng, L. Valle, and the other, anonymous, reviewer(s), for their contribution to the peer review of this work.
Related links
HGNC: the HUGO gene nomenclature committee database https://www.genenames.org/
References
- 1.Shah RR, et al. , Trends in the incidence of early-onset colorectal cancer in all 50 United States from 2001 through 2017. Cancer 2021. [DOI] [PubMed]
- 2.Islami F, et al. , Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. J Natl Cancer Inst 2021. [DOI] [PMC free article] [PubMed]
- 3.Gupta S, et al. , International Trends in the Incidence of Cancer Among Adolescents and Young Adults. J Natl Cancer Inst 2020, 112 (11), 1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heer E, et al. , Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health 2020, 8 (8), e1027–e1037. [DOI] [PubMed] [Google Scholar]
- 5.Lortet-Tieulent J; Ferlay J; Bray F; Jemal A, International Patterns and Trends in Endometrial Cancer Incidence, 1978–2013. J Natl Cancer Inst 2018, 110 (4), 354–361. [DOI] [PubMed] [Google Scholar]
- 6.Siegel RL; Miller KD; Fuchs HE; Jemal A, Cancer Statistics, 2021. CA Cancer J Clin 2021, 71 (1), 7–33. [DOI] [PubMed] [Google Scholar]
- 7.Ward EM, et al. , Annual Report to the Nation on the Status of Cancer, Featuring Cancer in Men and Women Age 20–49 Years. J Natl Cancer Inst 2019, 111 (12), 1279–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sung H; Siegel RL; Rosenberg PS; Jemal A, Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health 2019, 4 (3), e137–e147. [DOI] [PubMed] [Google Scholar]
- 9.Fidler MM, et al. , Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: a population-based study. Lancet Oncol 2017, 18 (12), 1579–1589. [DOI] [PubMed] [Google Scholar]
- 10.Codipilly DC, et al. , Epidemiology and Outcomes of Young-Onset Esophageal Adenocarcinoma: An Analysis from a Population-Based Database. Cancer Epidemiol Biomarkers Prev 2021, 30 (1), 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, et al. , Disease Burden, Risk Factors, and Recent Trends of Liver Cancer: A Global Country-Level Analysis. Liver Cancer 2021, 10 (4), 330–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J, et al. , Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology 2021, 160 (3), 744–754. [DOI] [PubMed] [Google Scholar]
- 13.Wong MCS, et al. , Global Incidence and Mortality of Gastric Cancer, 1980–2018. JAMA Netw Open 2021, 4 (7), e2118457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JS, et al. , Increased risk of second malignant neoplasms in adolescents and young adults with cancer. Cancer 2016, 122 (1), 116–23. [DOI] [PubMed] [Google Scholar]
- 15.Chao C, et al. , Cardiovascular Disease Risk Profiles in Survivors of Adolescent and Young Adult (AYA) Cancer: The Kaiser Permanente AYA Cancer Survivors Study. J Clin Oncol 2016, 34 (14), 1626–33. [DOI] [PubMed] [Google Scholar]
- 16.van Dorp W, et al. , Reproductive Function and Outcomes in Female Survivors of Childhood, Adolescent, and Young Adult Cancer: A Review. J Clin Oncol 2018, 36 (21), 2169–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.https://provocativequestions.cancer.gov/home (last accessed on May 26, 2022).
- 18.Akimoto N, et al. , Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol 2021, 18 (4), 230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chelmow D, et al. , Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet Gynecol 2020, 135 (6), 1457–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujiyoshi K, et al. , Opinion: Standardizing gene product nomenclature-a call to action. Proc Natl Acad Sci U S A 2021, 118 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofseth LJ, et al. , Early-onset colorectal cancer: initial clues and current views. Nat Rev Gastroenterol Hepatol 2020, 17 (6), 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song M; Vogelstein B; Giovannucci EL; Willett WC; Tomasetti C, Cancer prevention: Molecular and epidemiologic consensus. Science 2018, 361 (6409), 1317–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomasetti C; Vogelstein B, Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015, 347 (6217), 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker DJ, et al. , A possible link between the pubertal growth of girls and breast cancer in their daughters. Am J Hum Biol 2008, 20 (2), 127–31. [DOI] [PubMed] [Google Scholar]
- 25.Barker DJ, A new model for the origins of chronic disease. Med Health Care Philos 2001, 4 (1), 31–5. [DOI] [PubMed] [Google Scholar]
- 26.Barker DJ, In utero programming of chronic disease. Clin Sci (Lond) 1998, 95 (2), 115–28. [PubMed] [Google Scholar]
- 27.Bleker LS; de Rooij SR; Painter RC; Ravelli AC; Roseboom TJ, Cohort profile: the Dutch famine birth cohort (DFBC)- a prospective birth cohort study in the Netherlands. BMJ Open 2021, 11 (3), e042078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulz LC, The Dutch Hunger Winter and the developmental origins of health and disease. Proc Natl Acad Sci U S A 2010, 107 (39), 16757–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy CC, et al. , Maternal obesity, pregnancy weight gain, and birth weight and risk of colorectal cancer. Gut 2021. [DOI] [PMC free article] [PubMed]
- 30.Smith NR, et al. , Associations between birth weight and colon and rectal cancer risk in adulthood. Cancer Epidemiol 2016, 42, 181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakeford R, The risk of childhood leukaemia following exposure to ionising radiation--a review. J Radiol Prot 2013, 33 (1), 1–25. [DOI] [PubMed] [Google Scholar]
- 32.Charalampopoulos D; McLoughlin A; Elks CE; Ong KK, Age at menarche and risks of all-cause and cardiovascular death: a systematic review and meta-analysis. Am J Epidemiol 2014, 180 (1), 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegel RL, et al. , Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J Natl Cancer Inst 2017, 109 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoffel EM; Murphy CC, Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology 2020, 158 (2), 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel RL, et al. , Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68 (12), 2179–2185. [DOI] [PubMed] [Google Scholar]
- 36.Murphy CC; Yang YC, Use of age-period-cohort analysis in cancer epidemiology research. Curr Epidemiol Rep 2018, 5 (4), 418–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmonds M, et al. , The use of measures of obesity in childhood for predicting obesity and the development of obesity-related diseases in adulthood: a systematic review and meta-analysis. Health Technol Assess 2015, 19 (43), 1–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon-Larsen P; The NS; Adair LS, Longitudinal trends in obesity in the United States from adolescence to the third decade of life. Obesity (Silver Spring) 2010, 18 (9), 1801–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popkin BM; Adair LS; Ng SW, Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 2012, 70 (1), 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giovannucci E, A growing link-what is the role of height in cancer risk? Br J Cancer 2019, 120 (6), 575–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NCD Risk Factor Collaboration (NCD-RisC), Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390 (10113), 2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malik VS; Willet WC; Hu FB, Nearly a decade on - trends, risk factors and policy implications in global obesity. Nat Rev Endocrinol 2020, 16 (11), 615–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin X, et al. , Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep 2020, 10 (1), 14790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koopman RJ; Mainous AG 3rd; Diaz VA; Geesey ME, Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann Fam Med 2005, 3 (1), 60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayer-Davis EJ, et al. , Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N Engl J Med 2017, 376 (15), 1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fazeli Farsani S; van der Aa MP; van der Vorst MM; Knibbe CA; de Boer A, Global trends in the incidence and prevalence of type 2 diabetes in children and adolescents: a systematic review and evaluation of methodological approaches. Diabetologia 2013, 56 (7), 1471–88. [DOI] [PubMed] [Google Scholar]
- 47.Muthuri SK, et al. , Temporal trends and correlates of physical activity, sedentary behaviour, and physical fitness among school-aged children in Sub-Saharan Africa: a systematic review. Int J Environ Res Public Health 2014, 11 (3), 3327–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knuth AG; Hallal PC, Temporal trends in physical activity: a systematic review. J Phys Act Health 2009, 6 (5), 548–59. [DOI] [PubMed] [Google Scholar]
- 49.Yang L, et al. , Trends in Sedentary Behavior Among the US Population, 2001–2016. JAMA 2019, 321 (16), 1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.GBD 2019 Risk Factors Collaborators, Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396 (10258), 1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azzam A, Is the world converging to a ‘Western diet’? Public Health Nutr 2021, 24 (2), 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sans P; Combris P, World meat consumption patterns: An overview of the last fifty years (1961–2011). Meat Sci 2015, 109, 106–11. [DOI] [PubMed] [Google Scholar]
- 53.Clonan A; Roberts KE; Holdsworth M, Socioeconomic and demographic drivers of red and processed meat consumption: implications for health and environmental sustainability. Proc Nutr Soc 2016, 75 (3), 367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Micha R, et al. , Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys. BMJ 2014, 348, g2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Popkin BM; Hawkes C, Sweetening of the global diet, particularly beverages: patterns, trends, and policy responses. Lancet Diabetes Endocrinol 2016, 4 (2), 174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collaboration NCDRF, A century of trends in adult human height. Elife 2016, 5. [DOI] [PMC free article] [PubMed]
- 57.Wang YC; Bleich SN; Gortmaker SL, Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988–2004. Pediatrics 2008, 121 (6), e1604–14. [DOI] [PubMed] [Google Scholar]
- 58.Blecher E; Liber AC; Drope JM; Nguyen B; Stoklosa M, Global Trends in the Affordability of Sugar-Sweetened Beverages, 1990–2016. Prev Chronic Dis 2017, 14, E37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holmes AJ; Anderson K. J. J. o. W. E., Convergence in national alcohol consumption patterns: New global indicators. 2017, 12 (2), 117–148. [Google Scholar]
- 60.Johnston LD; O’Malley PM; Miech RA; Bachman JG; Schulenberg JE, Monitoring the Future national survey results on drug use, 1975–2015: Overview, key findings on adolescent drug use. 2016.
- 61.Chen CM; Yoon Y-H, TRENDS IN UNDERAGE DRINKING IN THE UNITED STATES, 1991–2019.
- 62.IARD, Trends Report: Underage Drinking. 2019.
- 63.Vashishtha R, et al. , Trends in adolescent drinking across 39 high-income countries: exploring the timing and magnitude of decline. Eur J Public Health 2021, 31 (2), 424–431. [DOI] [PubMed] [Google Scholar]
- 64.Pierce JP, International comparisons of trends in cigarette smoking prevalence. Am J Public Health 1989, 79 (2), 152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Graham H, Smoking prevalence among women in the European community 1950–1990. Soc Sci Med 1996, 43 (2), 243–54. [DOI] [PubMed] [Google Scholar]
- 66.Nelson DE, et al. , Trends in cigarette smoking among US adolescents, 1974 through 1991. Am J Public Health 1995, 85 (1), 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marcon A, et al. , Trends in smoking initiation in Europe over 40 years: A retrospective cohort study. PLoS One 2018, 13 (8), e0201881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang JJ, et al. , Tobacco Smoking and Mortality in Asia: A Pooled Meta-analysis. JAMA Netw Open 2019, 2 (3), e191474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.GBD 2019 Tobacco Collaborators, Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet 2021, 397 (10292), 2337–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matricciani L; Olds T; Petkov J, In search of lost sleep: secular trends in the sleep time of school-aged children and adolescents. Sleep Med Rev 2012, 16 (3), 203–11. [DOI] [PubMed] [Google Scholar]
- 71.Youngstedt SD, et al. , Has adult sleep duration declined over the last 50+ years? Sleep Med Rev 2016, 28, 69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.IARC Working Group on the Identification of Carcinogenic Hazards to Humans. Night Shift Work. Lyon (FR): International Agency for Research on Cancer; 2020. (IARC Monographs on the Identification of Carcinogenic Hazards to Humans, No. 124.) 1. Exposure Data. 2020. [PubMed] [Google Scholar]
- 73.https://data.worldbank.org/indicator/SP.DYN.TFRT.IN?locations=US (last accessed on July 5, 2022).
- 74.Leone T; Brown LJ, Timing and determinants of age at menarche in low-income and middle-income countries. BMJ Glob Health 2020, 5 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morris DH; Jones ME; Schoemaker MJ; Ashworth A; Swerdlow AJ, Secular trends in age at menarche in women in the UK born 1908–93: results from the Breakthrough Generations Study. Paediatr Perinat Epidemiol 2011, 25 (4), 394–400. [DOI] [PubMed] [Google Scholar]
- 76.Nations U, World population prospects 2019. 2019.
- 77.Liang M, et al. , The State of Adolescent Sexual and Reproductive Health. J Adolesc Health 2019, 65 (6S), S3–S15. [DOI] [PubMed] [Google Scholar]
- 78.OECD, Age of mothers at childbirth and age-specific fertility. 2018.
- 79.Darroch JE, Trends in contraceptive use. Contraception 2013, 87 (3), 259–63. [DOI] [PubMed] [Google Scholar]
- 80.Stevens EE; Patrick TE; Pickler R, A history of infant feeding. J Perinat Educ 2009, 18 (2), 32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Victora CG, et al. , Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387 (10017), 475–90. [DOI] [PubMed] [Google Scholar]
- 82.Rajpoot M; Sharma AK; Sharma A; Gupta GK, Understanding the microbiome: Emerging biomarkers for exploiting the microbiota for personalized medicine against cancer. Semin Cancer Biol 2018, 52 (Pt 1), 1–8. [DOI] [PubMed] [Google Scholar]
- 83.Rescigno T; Micolucci L; Tecce MF; Capasso A, Bioactive Nutrients and Nutrigenomics in Age-Related Diseases. Molecules 2017, 22 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamada T; Nowak JA; Milner DA Jr.; Song M; Ogino S, Integration of microbiology, molecular pathology, and epidemiology: a new paradigm to explore the pathogenesis of microbiome-driven neoplasms. J Pathol 2019, 247 (5), 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Petrelli F, et al. , Use of Antibiotics and Risk of Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Cancers (Basel) 2019, 11 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klein EY, et al. , Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–15: an analysis of pharmaceutical sales data. Lancet Infect Dis 2021, 21 (1), 107–115. [DOI] [PubMed] [Google Scholar]
- 87.Browne AJ, et al. , Global antibiotic consumption and usage in humans, 2000–18: a spatial modelling study. Lancet Planet Health 2021, 5 (12), e893–e904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McCaig LF; Hughes JM, Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA 1995, 273 (3), 214–9. [PubMed] [Google Scholar]
- 89.Zhou Q, et al. , Risk of Colorectal Cancer in Ulcerative Colitis Patients: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract 2019, 2019, 5363261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ghione S, et al. , Dramatic Increase in Incidence of Ulcerative Colitis and Crohn’s Disease (1988–2011): A Population-Based Study of French Adolescents. Am J Gastroenterol 2018, 113 (2), 265–272. [DOI] [PubMed] [Google Scholar]
- 91.O’Sullivan A; Farver M; Smilowitz JT, The Influence of Early Infant-Feeding Practices on the Intestinal Microbiome and Body Composition in Infants. Nutr Metab Insights 2015, 8 (Suppl 1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blaser MJ; Dominguez-Bello MG, The Human Microbiome before Birth. Cell Host Microbe 2016, 20 (5), 558–560. [DOI] [PubMed] [Google Scholar]
- 93.Mima K, et al. , The microbiome, genetics, and gastrointestinal neoplasms: the evolving field of molecular pathological epidemiology to analyze the tumor-immune-microbiome interaction. Hum Genet 2021, 140 (5), 725–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Archambault AN, et al. , Cumulative Burden of Colorectal Cancer-Associated Genetic Variants Is More Strongly Associated With Early-Onset vs Late-Onset Cancer. Gastroenterology 2020, 158 (5), 1274–1286.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yanes T; Young MA; Meiser B; James PA, Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res 2020, 22 (1), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Benafif S; Kote-Jarai Z; Eeles RA; Consortium P, A Review of Prostate Cancer Genome-Wide Association Studies (GWAS). Cancer Epidemiol Biomarkers Prev 2018, 27 (8), 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Daly AA; Rolph R; Cutress RI; Copson ER, A Review of Modifiable Risk Factors in Young Women for the Prevention of Breast Cancer. Breast Cancer (Dove Med Press) 2021, 13, 241–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stoffel EM, et al. , Germline Genetic Features of Young Individuals With Colorectal Cancer. Gastroenterology 2018, 154 (4), 897–905 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pearlman R, et al. , Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA oncology 2017, 3 (4), 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.WHO, Research on the menopause in the 1990s: report of a WHO scientific group. 1996. [PubMed]
- 101.OECD, “Screening, survival and mortality for breast cancer”, in Health at a Glance 2017. 2017.
- 102.Narayan AK; Lee CI; Lehman CD, Screening for Breast Cancer. Med Clin North Am 2020, 104 (6), 1007–1021. [DOI] [PubMed] [Google Scholar]
- 103.Smith RA; Cokkinides V; Brooks D; Saslow D; Brawley OW, Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010, 60 (2), 99–119. [DOI] [PubMed] [Google Scholar]
- 104.Kehm RD; Yang W; Tehranifar P; Terry MB, 40 Years of Change in Age- and Stage-Specific Cancer Incidence Rates in US Women and Men. JNCI Cancer Spectr 2019, 3 (3), pkz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lima SM; Kehm RD; Terry MB, Global breast cancer incidence and mortality trends by region, age-groups, and fertility patterns. EClinicalMedicine 2021, 38, 100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hankinson SE; Eliassen AH, Circulating sex steroids and breast cancer risk in premenopausal women. Horm Cancer 2010, 1 (1), 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Clavel-Chapelon F; Group ENE, Differential effects of reproductive factors on the risk of pre- and postmenopausal breast cancer. Results from a large cohort of French women. Br J Cancer 2002, 86 (5), 723–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Collaborative Group on Hormonal Factors in Breast, C., Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 2012, 13 (11), 1141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Al-Ajmi K; Lophatananon A; Ollier W; Muir KR, Risk of breast cancer in the UK biobank female cohort and its relationship to anthropometric and reproductive factors. PLoS One 2018, 13 (7), e0201097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Collaborative Group on Hormonal Factors in Breast, C., Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996, 347 (9017), 1713–27. [DOI] [PubMed] [Google Scholar]
- 111.Li CI, et al. , Timing of menarche and first full-term birth in relation to breast cancer risk. Am J Epidemiol 2008, 167 (2), 230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lima SM; Kehm RD; Swett K; Gonsalves L; Terry MB, Trends in Parity and Breast Cancer Incidence in US Women Younger Than 40 Years From 1935 to 2015. JAMA Netw Open 2020, 3 (3), e200929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bellanger M; Lima SM; Cowppli-Bony A; Molinie F; Terry MB, Effects of fertility on breast cancer incidence trends: comparing France and US. Cancer Causes Control 2021, 32 (8), 903–910. [DOI] [PubMed] [Google Scholar]
- 114.Premenopausal Breast Cancer Collaborative, G., et al. , Association of Body Mass Index and Age With Subsequent Breast Cancer Risk in Premenopausal Women. JAMA Oncol 2018, 4 (11), e181771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Amadou A, et al. , Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obes Rev 2013, 14 (8), 665–78. [DOI] [PubMed] [Google Scholar]
- 116.Renehan AG; Tyson M; Egger M; Heller RF; Zwahlen M, Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371 (9612), 569–78. [DOI] [PubMed] [Google Scholar]
- 117.Bergstrom A; Pisani P; Tenet V; Wolk A; Adami HO, Overweight as an avoidable cause of cancer in Europe. Int J Cancer 2001, 91 (3), 421–30. [DOI] [PubMed] [Google Scholar]
- 118.van den Brandt PA, et al. , Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol 2000, 152 (6), 514–27. [DOI] [PubMed] [Google Scholar]
- 119.Ursin G; Longnecker MP; Haile RW; Greenland S, A meta-analysis of body mass index and risk of premenopausal breast cancer. Epidemiology 1995, 6 (2), 137–41. [DOI] [PubMed] [Google Scholar]
- 120.Rosner B, et al. , Weight and weight changes in early adulthood and later breast cancer risk. Int J Cancer 2017, 140 (9), 2003–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van den Brandt PA, et al. , Body size and weight change over adulthood and risk of breast cancer by menopausal and hormone receptor status: a pooled analysis of 20 prospective cohort studies. Eur J Epidemiol 2021, 36 (1), 37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Houghton SC, et al. , Central Adiposity and Subsequent Risk of Breast Cancer by Menopause Status. J Natl Cancer Inst 2021, 113 (7), 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rosner B, et al. , Short-term weight gain and breast cancer risk by hormone receptor classification among pre- and postmenopausal women. Breast Cancer Res Treat 2015, 150 (3), 643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chan DSM, et al. , World Cancer Research Fund International: Continuous Update Project-systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control 2019, 30 (11), 1183–1200. [DOI] [PubMed] [Google Scholar]
- 125.Chen WY; Rosner B; Hankinson SE; Colditz GA; Willett WC, Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA 2011, 306 (17), 1884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kropp S; Becher H; Nieters A; Chang-Claude J, Low-to-moderate alcohol consumption and breast cancer risk by age 50 years among women in Germany. Am J Epidemiol 2001, 154 (7), 624–34. [DOI] [PubMed] [Google Scholar]
- 127.Godinho-Mota JCM, et al. , Sedentary Behavior and Alcohol Consumption Increase Breast Cancer Risk Regardless of Menopausal Status: A Case-Control Study. Nutrients 2019, 11 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Alcoholic drinks and the risk of cancer. Available at dietandcancerreport.org.
- 129.Xiao Y, et al. , Associations between dietary patterns and the risk of breast cancer: a systematic review and meta-analysis of observational studies. Breast Cancer Res 2019, 21 (1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Linos E; Willett WC; Cho E; Frazier L, Adolescent diet in relation to breast cancer risk among premenopausal women. Cancer Epidemiol Biomarkers Prev 2010, 19 (3), 689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Farvid MS; Cho E; Chen WY; Eliassen AH; Willett WC, Premenopausal dietary fat in relation to pre- and post-menopausal breast cancer. Breast Cancer Res Treat 2014, 145 (1), 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reynolds P, Smoking and breast cancer. J Mammary Gland Biol Neoplasia 2013, 18 (1), 15–23. [DOI] [PubMed] [Google Scholar]
- 133.Johnson KC, Accumulating evidence on passive and active smoking and breast cancer risk. Int J Cancer 2005, 117 (4), 619–28. [DOI] [PubMed] [Google Scholar]
- 134.Band PR; Le ND; Fang R; Deschamps M, Carcinogenic and endocrine disrupting effects of cigarette smoke and risk of breast cancer. Lancet 2002, 360 (9339), 1044–9. [DOI] [PubMed] [Google Scholar]
- 135.van den Brandt PA, A possible dual effect of cigarette smoking on the risk of postmenopausal breast cancer. Eur J Epidemiol 2017, 32 (8), 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lynch HT; Watson P; Conway T; Fitzsimmons ML; Lynch J, Breast cancer family history as a risk factor for early onset breast cancer. Breast Cancer Res Treat 1988, 11 (3), 263–7. [DOI] [PubMed] [Google Scholar]
- 137.Copson ER, et al. , Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol 2018, 19 (2), 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee DS, et al. , Comparable frequency of BRCA1, BRCA2 and TP53 germline mutations in a multi-ethnic Asian cohort suggests TP53 screening should be offered together with BRCA1/2 screening to early-onset breast cancer patients. Breast Cancer Res 2012, 14 (2), R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gomez-Flores-Ramos L; Alvarez-Gomez RM; Villarreal-Garza C; Wegman-Ostrosky T; Mohar A, Breast cancer genetics in young women: What do we know? Mutat Res Rev Mutat Res 2017, 774, 33–45. [DOI] [PubMed] [Google Scholar]
- 140.Mavaddat N, et al. , Prediction of breast cancer risk based on profiling with common genetic variants. J Natl Cancer Inst 2015, 107 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Barrdahl M, et al. , Gene-environment interactions involving functional variants: Results from the Breast Cancer Association Consortium. Int J Cancer 2017, 141 (9), 1830–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rudolph A; Chang-Claude J; Schmidt MK, Gene-environment interaction and risk of breast cancer. Br J Cancer 2016, 114 (2), 125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Carey LA, et al. , Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006, 295 (21), 2492–502. [DOI] [PubMed] [Google Scholar]
- 144.McCarthy AM, et al. , Relationship of established risk factors with breast cancer subtypes. Cancer Med 2021, 10 (18), 6456–6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ihemelandu CU, et al. , Molecular breast cancer subtypes in premenopausal African-American women, tumor biologic factors and clinical outcome. Ann Surg Oncol 2007, 14 (10), 2994–3003. [DOI] [PubMed] [Google Scholar]
- 146.Levi Z, et al. , Adolescent body mass index and risk of colon and rectal cancer in a cohort of 1.79 million Israeli men and women: A population-based study. Cancer 2017, 123 (20), 4022–4030. [DOI] [PubMed] [Google Scholar]
- 147.Gausman V, et al. , Risk Factors Associated With Early-Onset Colorectal Cancer. Clin Gastroenterol Hepatol 2020, 18 (12), 2752–2759 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim JY, et al. , Different risk factors for advanced colorectal neoplasm in young adults. World J Gastroenterol 2016, 22 (13), 3611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Syed AR, et al. , Old vs new: Risk factors predicting early onset colorectal cancer. World J Gastrointest Oncol 2019, 11 (11), 1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sanford NN; Giovannucci EL; Ahn C; Dee EC; Mahal BA, Obesity and younger versus older onset colorectal cancer in the United States, 1998–2017. J Journal of gastrointestinal oncology 2020, 11 (1), 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jung YS, et al. , Risk factors for colorectal neoplasia in persons aged 30 to 39 years and 40 to 49 years. Gastrointest Endosc 2015, 81 (3), 637–645 e7. [DOI] [PubMed] [Google Scholar]
- 152.Hong SN, et al. , Prevalence and risk of colorectal neoplasms in asymptomatic, average-risk screenees 40 to 49 years of age. Gastrointest Endosc 2010, 72 (3), 480–9. [DOI] [PubMed] [Google Scholar]
- 153.Hussan H, et al. , Rising incidence of colorectal cancer in young adults corresponds with increasing surgical resections in obese patients. Clinical translational gastroenterology 2020, 11 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu P-H, et al. , Association of obesity with risk of early-onset colorectal cancer among women. JAMA oncology 2019, 5 (1), 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.O’Sullivan DE, et al. , Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2021. [DOI] [PubMed]
- 156.Win AK, et al. , Body mass index in early adulthood and colorectal cancer risk for carriers and non-carriers of germline mutations in DNA mismatch repair genes. Br J Cancer 2011, 105 (1), 162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ma Y, et al. , Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One 2013, 8 (1), e53916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Siegel RL, et al. , Colorectal cancer statistics, 2020. CA Cancer J Clin 2020, 70 (3), 145–164. [DOI] [PubMed] [Google Scholar]
- 159.Cercek A, et al. , A Comprehensive Comparison of Early-Onset and Average-Onset Colorectal Cancers. J Natl Cancer Inst 2021. [DOI] [PMC free article] [PubMed]
- 160.Nguyen LH, et al. , Sedentary Behaviors, TV Viewing Time, and Risk of Young-Onset Colorectal Cancer. JNCI Cancer Spectr 2018, 2 (4), pky073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fraser G; Pearce N, Occupational physical activity and risk of cancer of the colon and rectum in New Zealand males. Cancer Causes Control 1993, 4 (1), 45–50. [DOI] [PubMed] [Google Scholar]
- 162.Peters RK; Garabrant DH; Yu MC; Mack TM, A case-control study of occupational and dietary factors in colorectal cancer in young men by subsite. Cancer Res 1989, 49 (19), 5459–68. [PubMed] [Google Scholar]
- 163.Chen H, et al. , Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut 2021, 70 (6), 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim JY, et al. , Development and validation of a scoring system for advanced colorectal neoplasm in young Korean subjects less than age 50 years. Intest Res 2019, 17 (2), 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Breau G; Ellis U, Risk Factors Associated With Young-Onset Colorectal Adenomas and Cancer: A Systematic Review and Meta-Analysis of Observational Research. Cancer Control 2020, 27 (1), 1073274820976670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Elangovan A, et al. , Colorectal Cancer, Age, and Obesity-Related Comorbidities: A Large Database Study. Dig Dis Sci 2020. [DOI] [PubMed]
- 167.Ali Khan U, et al. , Personal History of Diabetes as Important as Family History of Colorectal Cancer for Risk of Colorectal Cancer: A Nationwide Cohort Study. Am J Gastroenterol 2020, 115 (7), 1103–1109. [DOI] [PubMed] [Google Scholar]
- 168.Hur J, et al. , Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut 2021. [DOI] [PMC free article] [PubMed]
- 169.Zheng X, et al. , Comprehensive Assessment of Diet Quality and Risk of Precursors of Early-Onset Colorectal Cancer. J Natl Cancer Inst 2021, 113 (5), 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rosato V, et al. , Risk factors for young-onset colorectal cancer. Cancer Causes Control 2013, 24 (2), 335–341. [DOI] [PubMed] [Google Scholar]
- 171.Archambault AN, et al. , Nongenetic Determinants of Risk for Early-Onset Colorectal Cancer. JNCI Cancer Spectr 2021, 5 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim H, et al. , Total Vitamin D Intake and Risks of Early-Onset Colorectal Cancer and Precursors. Gastroenterology 2021, 161 (4), 1208–1217 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yue Y, et al. , Prospective evaluation of dietary and lifestyle pattern indices with risk of colorectal cancer in a cohort of younger women. Ann Oncol 2021, 32 (6), 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kwak JY, et al. , Prevalence of colorectal adenomas in asymptomatic young adults: a window to early intervention? Scand J Gastroenterol 2016, 51 (6), 731–8. [DOI] [PubMed] [Google Scholar]
- 175.Kim NH, et al. , Prevalence of and Risk Factors for Colorectal Neoplasia in Asymptomatic Young Adults (20–39 Years Old). Clin Gastroenterol Hepatol 2019, 17 (1), 115–122. [DOI] [PubMed] [Google Scholar]
- 176.Low EE, et al. , Risk Factors for Early-Onset Colorectal Cancer. Gastroenterology 2020, 159 (2), 492–501 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chung SJ, et al. , Prevalence and risk of colorectal adenoma in asymptomatic Koreans aged 40–49 years undergoing screening colonoscopy. J Gastroenterol Hepatol 2010, 25 (3), 519–25. [DOI] [PubMed] [Google Scholar]
- 178.Jung YS; Park CH; Kim NH; Lee MY; Park DI, Impact of Age on the Risk of Advanced Colorectal Neoplasia in a Young Population: An Analysis Using the Predicted Probability Model. Dig Dis Sci 2017, 62 (9), 2518–2525. [DOI] [PubMed] [Google Scholar]
- 179.Lee SE, et al. , Characteristics of and risk factors for colorectal neoplasms in young adults in a screening population. World J Gastroenterol 2016, 22 (10), 2981–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Buc E, et al. , Tobacco smoking: a factor of early onset of colorectal cancer. Diseases of the colon 2006, 49 (12), 1893–1896. [DOI] [PubMed] [Google Scholar]
- 181.Song M; Nguyen LH; Emilsson L; Chan AT; Ludvigsson JF, Antibiotic Use Associated With Risk of Colorectal Polyps in a Nationwide Study. Clin Gastroenterol Hepatol 2021, 19 (7), 1426–1435 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cao Y, et al. , Long-term use of antibiotics and risk of colorectal adenoma. Gut 2018, 67 (4), 672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McDowell R, et al. , Oral antibiotic use and early-onset colorectal cancer: findings from a case-control study using a national clinical database. Br J Cancer 2021. [DOI] [PMC free article] [PubMed]
- 184.Nguyen LH, et al. , Antibiotic Therapy and Risk of Early-Onset Colorectal Cancer: A National Case-Control Study. Clin Transl Gastroenterol 2022, 13 (1), e00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lu SSM, et al. , Antibiotics Use and Subsequent Risk of Colorectal Cancer: A Swedish Nationwide Population-Based Study. J Natl Cancer Inst 2022, 114 (1), 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fuchs CS, et al. , A prospective study of family history and the risk of colorectal cancer. New England Journal of Medicine 1994, 331 (25), 1669–1674. [DOI] [PubMed] [Google Scholar]
- 187.Chen FW; Sundaram V; Chew TA; Ladabaum U, Advanced-Stage Colorectal Cancer in Persons Younger Than 50 Years Not Associated With Longer Duration of Symptoms or Time to Diagnosis. Clin Gastroenterol Hepatol 2017, 15 (5), 728–737 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Heikkinen SM, et al. , Familial aggregation of early‐onset cancers. International journal of cancer 2020, 146 (7), 1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hall N; Bishop D; Stephenson B; Finan P; rectum, Hereditary susceptibility to colorectal cancer. Diseases of the colon rectum 1996, 39 (7), 739–743. [DOI] [PubMed] [Google Scholar]
- 190.Willauer AN, et al. , Clinical and molecular characterization of early-onset colorectal cancer. Cancer 2019, 125 (12), 2002–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Giráldez MD, et al. , Susceptibility genetic variants associated with early-onset colorectal cancer. Carcinogenesis 2012, 33 (3), 613–619. [DOI] [PubMed] [Google Scholar]
- 192.Lu KH; Broaddus RR, Endometrial Cancer. N Engl J Med 2020, 383 (21), 2053–2064. [DOI] [PubMed] [Google Scholar]
- 193.Raglan O, et al. , Risk factors for endometrial cancer: An umbrella review of the literature. Int J Cancer 2019, 145 (7), 1719–1730. [DOI] [PubMed] [Google Scholar]
- 194.Haidopoulos D, et al. , Risk factors in women 40 years of age and younger with endometrial carcinoma. Acta Obstet Gynecol Scand 2010, 89 (10), 1326–30. [DOI] [PubMed] [Google Scholar]
- 195.Soliman PT, et al. , Risk factors for young premenopausal women with endometrial cancer. Obstet Gynecol 2005, 105 (3), 575–80. [DOI] [PubMed] [Google Scholar]
- 196.Walsh MD, et al. , Molecular, pathologic, and clinical features of early-onset endometrial cancer: identifying presumptive Lynch syndrome patients. Clin Cancer Res 2008, 14 (6), 1692–700. [DOI] [PubMed] [Google Scholar]
- 197.Xie SH; Lagergren J, Risk factors for oesophageal cancer. Best Pract Res Clin Gastroenterol 2018, 36–37, 3–8. [DOI] [PubMed]
- 198.Arnold M; Ferlay J; van Berge Henegouwen MI; Soerjomataram I, Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut 2020, 69 (9), 1564–1571. [DOI] [PubMed] [Google Scholar]
- 199.Drahos J, et al. , Age-specific risk factor profiles of adenocarcinomas of the esophagus: A pooled analysis from the international BEACON consortium. Int J Cancer 2016, 138 (1), 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Anand G; Katz PO, Gastroesophageal reflux disease and obesity. Gastroenterol Clin North Am 2010, 39 (1), 39–46. [DOI] [PubMed] [Google Scholar]
- 201.El-Serag HB; Sweet S; Winchester CC; Dent J, Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014, 63 (6), 871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nelson SP, et al. , Pediatric gastroesophageal reflux disease and acid-related conditions: trends in incidence of diagnosis and acid suppression therapy. J Med Econ 2009, 12 (4), 348–55. [DOI] [PubMed] [Google Scholar]
- 203.Scida S, et al. , Relationship between Helicobacter pylori infection and GERD. Acta Biomed 2018, 89 (8-S), 40–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Toporcov TN, et al. , Risk factors for head and neck cancer in young adults: a pooled analysis in the INHANCE consortium. Int J Epidemiol 2015, 44 (1), 169–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shewale JB; Gillison ML, Dynamic factors affecting HPV-attributable fraction for head and neck cancers. Curr Opin Virol 2019, 39, 33–40. [DOI] [PubMed] [Google Scholar]
- 206.Marur S; D’Souza G; Westra WH; Forastiere AA, HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 2010, 11 (8), 781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Leiba A, et al. , Adolescent obesity and paternal country of origin predict renal cell carcinoma: a cohort study of 1.1 million 16 to 19-year-old males. J Urol 2013, 189 (1), 25–9. [DOI] [PubMed] [Google Scholar]
- 208.Hemminki K; Li X, Age-specific familial risks for renal cell carcinoma with evidence on recessive heritable effects. Kidney international 2004, 65 (6), 2298–2302. [DOI] [PubMed] [Google Scholar]
- 209.Behrens G; Leitzmann MF, The association between physical activity and renal cancer: systematic review and meta-analysis. Br J Cancer 2013, 108 (4), 798–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chow WH; Devesa SS, Contemporary epidemiology of renal cell cancer. Cancer J 2008, 14 (5), 288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Parker AS; Cerhan JR; Janney CA; Lynch CF; Cantor KP, Smoking cessation and renal cell carcinoma. Ann Epidemiol 2003, 13 (4), 245–51. [DOI] [PubMed] [Google Scholar]
- 212.McGlynn KA; Petrick JL; El-Serag HB, Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 Suppl 1, 4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Forner A; Reig M; Bruix J, Hepatocellular carcinoma. Lancet 2018, 391 (10127), 1301–1314. [DOI] [PubMed] [Google Scholar]
- 214.Lam CM, et al. , Different presentation of hepatitis B-related hepatocellular carcinoma in a cohort of 1863 young and old patients - implications for screening. Aliment Pharmacol Ther 2004, 19 (7), 771–7. [DOI] [PubMed] [Google Scholar]
- 215.Yang HI, et al. , Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med 2002, 347 (3), 168–74. [DOI] [PubMed] [Google Scholar]
- 216.Liu J, et al. , A viral exposure signature defines early onset of hepatocellular carcinoma. Cell 2020, 182 (2), 317–328. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wan DW, et al. , Risk factors for early-onset and late-onset hepatocellular carcinoma in Asian immigrants with hepatitis B in the United States. Am J Gastroenterol 2011, 106 (11), 1994–2000. [DOI] [PubMed] [Google Scholar]
- 218.Park C-H, et al. , Family history influences the early onset of hepatocellular carcinoma. World Journal of Gastroenterology 2012, 18 (21), 2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Liu Z, et al. , Global incidence trends in primary liver cancer by age at diagnosis, sex, region, and etiology, 1990–2017. Cancer 2020, 126 (10), 2267–2278. [DOI] [PubMed] [Google Scholar]
- 220.Doycheva I; Watt KD; Alkhouri N, Nonalcoholic fatty liver disease in adolescents and young adults: the next frontier in the epidemic. Hepatology 2017, 65 (6), 2100–2109. [DOI] [PubMed] [Google Scholar]
- 221.Cowan AJ, et al. , Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol 2018, 4 (9), 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.van de Donk N; Pawlyn C; Yong KL, Multiple myeloma. Lancet 2021, 397 (10272), 410–427. [DOI] [PubMed] [Google Scholar]
- 223.Birmann BM, et al. , Young Adult and Usual Adult Body Mass Index and Multiple Myeloma Risk: A Pooled Analysis in the International Multiple Myeloma Consortium (IMMC). Cancer Epidemiol Biomarkers Prev 2017, 26 (6), 876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Marinac CR, et al. , Body mass index throughout adulthood, physical activity, and risk of multiple myeloma: a prospective analysis in three large cohorts. Br J Cancer 2018, 118 (7), 1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Collaborators GBDPC, The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2019, 4 (12), 934–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Juo YY, et al. , Obesity Is Associated with Early Onset of Gastrointestinal Cancers in California. J Obes 2018, 2018, 7014073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.McWilliams RR, et al. , Risk Factors for Early-Onset and Very-Early-Onset Pancreatic Adenocarcinoma: A Pancreatic Cancer Case-Control Consortium (PanC4) Analysis. Pancreas 2016, 45 (2), 311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Piciucchi M, et al. , Early onset pancreatic cancer: risk factors, presentation and outcome. Pancreatology 2015, 15 (2), 151–5. [DOI] [PubMed] [Google Scholar]
- 229.Ntala C; Debernardi S; Feakins RM; Crnogorac-Jurcevic T, Demographic, clinical, and pathological features of early onset pancreatic cancer patients. BMC Gastroenterol 2018, 18 (1), 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Rebbeck TR; Haas GP, Temporal trends and racial disparities in global prostate cancer prevalence. Can J Urol 2014, 21 (5), 7496–506. [PMC free article] [PubMed] [Google Scholar]
- 231.Pernar CH; Ebot EM; Wilson KM; Mucci LA, The Epidemiology of Prostate Cancer. Cold Spring Harb Perspect Med 2018, 8 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hur J; Giovannucci E, Racial differences in prostate cancer: does timing of puberty play a role? Br J Cancer 2020, 123 (3), 349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Travis RC, et al. , A Meta-analysis of Individual Participant Data Reveals an Association between Circulating Levels of IGF-I and Prostate Cancer Risk. Cancer Res 2016, 76 (8), 2288–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Huggins C; Hodges CV, Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin 1972, 22 (4), 232–40. [DOI] [PubMed] [Google Scholar]
- 235.Lange EM, et al. , Early onset prostate cancer has a significant genetic component. Prostate 2012, 72 (2), 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hjelmborg JB, et al. , The heritability of prostate cancer in the Nordic Twin Study of Cancer. Cancer Epidemiol Biomarkers Prev 2014, 23 (11), 2303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Carter BS; Beaty TH; Steinberg GD; Childs B; Walsh PC, Mendelian inheritance of familial prostate cancer. Proc Natl Acad Sci U S A 1992, 89 (8), 3367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Brandt A; Bermejo JL; Sundquist J; Hemminki K, Age-specific risk of incident prostate cancer and risk of death from prostate cancer defined by the number of affected family members. European urology 2010, 58 (2), 275–280. [DOI] [PubMed] [Google Scholar]
- 239.Al-Jebari Y, et al. , Risk of prostate cancer for men fathering through assisted reproduction: nationwide population based register study. BMJ 2019, 366, l5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Arnold M, et al. , Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159 (1), 335–349 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Balakrishnan M; George R; Sharma A; Graham DY, Changing Trends in Stomach Cancer Throughout the World. Curr Gastroenterol Rep 2017, 19 (8), 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rugge M, et al. , Patients younger than 40 years with gastric carcinoma: Helicobacter pylori genotype and associated gastritis phenotype. Cancer 1999, 85 (12), 2506–11. [PubMed] [Google Scholar]
- 243.Sjomina O; Pavlova J; Niv Y; Leja M, Epidemiology of Helicobacter pylori infection. Helicobacter 2018, 23 Suppl 1, e12514. [DOI] [PubMed] [Google Scholar]
- 244.Peleteiro B; Bastos A; Ferro A; Lunet N, Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Dig Dis Sci 2014, 59 (8), 1698–709. [DOI] [PubMed] [Google Scholar]
- 245.Hooi JKY, et al. , Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153 (2), 420–429. [DOI] [PubMed] [Google Scholar]
- 246.Bergquist JR, et al. , Early-onset gastric cancer is a distinct disease with worrisome trends and oncogenic features. Surgery 2019, 166 (4), 547–555. [DOI] [PubMed] [Google Scholar]
- 247.Giryes A; Oweira H; Mannhart M; Decker M; Abdel-Rahman O, Exploring the differences between early-onset gastric cancer and traditional-onset gastric cancer. J Gastrointest Oncol 2018, 9 (6), 1157–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhou F; Shi J; Fang C; Zou X; Huang Q, Gastric Carcinomas in Young (Younger than 40 Years) Chinese Patients: Clinicopathology, Family History, and Postresection Survival. Medicine (Baltimore) 2016, 95 (9), e2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kwak HW, et al. , Individual having a parent with early-onset gastric cancer may need screening at younger age. World J Gastroenterol 2015, 21 (15), 4592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Chung HW; Noh SH; Lim JB, Analysis of demographic characteristics in 3242 young age gastric cancer patients in Korea. World J Gastroenterol 2010, 16 (2), 256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Grönberg H; Bergh A; Damber JE; Emanuelsson M, Cancer risk in families with hereditary prostate carcinoma: A possible link between prostate, breast, and gastric carcinoma. Cancer 2000, 89 (6), 1315–1321. [DOI] [PubMed] [Google Scholar]
- 252.Fitzgerald RC; Caldas C, Clinical implications of E-cadherin associated hereditary diffuse gastric cancer. Gut 2004, 53 (6), 775–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Li J, Gastric Cancer in Young Adults: A Different Clinical Entity from Carcinogenesis to Prognosis. Gastroenterol Res Pract 2020, 2020, 9512707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Bacani JT, et al. , CDH1/E-cadherin germline mutations in early-onset gastric cancer. J Med Genet 2006, 43 (11), 867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Huntsman DG, et al. , Early gastric cancer in young, asymptomatic carriers of germ-line E-cadherin mutations. N Engl J Med 2001, 344 (25), 1904–9. [DOI] [PubMed] [Google Scholar]
- 256.Vaccarella S, et al. , Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. N Engl J Med 2016, 375 (7), 614–7. [DOI] [PubMed] [Google Scholar]
- 257.Li M; Dal Maso L; Vaccarella S, Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol 2020, 8 (6), 468–470. [DOI] [PubMed] [Google Scholar]
- 258.Li M, et al. , Changing incidence and projections of thyroid cancer in mainland China, 1983–2032: evidence from Cancer Incidence in Five Continents. Cancer Causes Control 2021. [DOI] [PubMed]
- 259.Kitahara CM; Sosa JA, The changing incidence of thyroid cancer. Nat Rev Endocrinol 2016, 12 (11), 646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kim J; Gosnell JE; Roman SA, Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol 2020, 16 (1), 17–29. [DOI] [PubMed] [Google Scholar]
- 261.Lian W, et al. , The Impact of Young Age for Prognosis by Subtype in Women with Early Breast Cancer. Sci Rep 2017, 7 (1), 11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Colleoni M, et al. , Very young women (<35 years) with operable breast cancer: features of disease at presentation. Ann Oncol 2002, 13 (2), 273–9. [DOI] [PubMed] [Google Scholar]
- 263.Anders CK, et al. , Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 2008, 26 (20), 3324–30. [DOI] [PubMed] [Google Scholar]
- 264.Assi HA, et al. , Epidemiology and prognosis of breast cancer in young women. J Thorac Dis 2013, 5 Suppl 1, S2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Bharat A; Aft RL; Gao F; Margenthaler JA, Patient and tumor characteristics associated with increased mortality in young women (< or =40 years) with breast cancer. J Surg Oncol 2009, 100 (3), 248–51. [DOI] [PubMed] [Google Scholar]
- 266.Gnerlich JL, et al. , Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg 2009, 208 (3), 341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Keegan TH; DeRouen MC; Press DJ; Kurian AW; Clarke CA, Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res 2012, 14 (2), R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Murphy BL; Day CN; Hoskin TL; Habermann EB; Boughey JC, Adolescents and Young Adults with Breast Cancer have More Aggressive Disease and Treatment Than Patients in Their Forties. Ann Surg Oncol 2019, 26 (12), 3920–3930. [DOI] [PubMed] [Google Scholar]
- 269.Wang K, et al. , Comparison of Clinicopathological Features and Treatments between Young (</=40 Years) and Older (>40 Years) Female Breast Cancer Patients in West China: A Retrospective, Epidemiological, Multicenter, Case Only Study. PLoS One 2016, 11 (3), e0152312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kneuertz PJ, et al. , Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg 2015, 150 (5), 402–9. [DOI] [PubMed] [Google Scholar]
- 271.Myers EA, et al. , Colorectal cancer in patients under 50 years of age: a retrospective analysis of two institutions’ experience. World J Gastroenterol 2013, 19 (34), 5651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Liang JT, et al. , Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. Br J Surg 2003, 90 (2), 205–14. [DOI] [PubMed] [Google Scholar]
- 273.Ugai T, et al. , Immune cell profiles in the tumor microenvironment of early-onset, intermediate-onset, and later-onset colorectal cancer. Cancer Immunology, Immunotherapy 2021, (in press). [DOI] [PMC free article] [PubMed]
- 274.Akimoto N, et al. , Tumor Long Interspersed Nucleotide Element-1 (LINE-1) Hypomethylation in Relation to Age of Colorectal Cancer Diagnosis and Prognosis. Cancers (Basel) 2021, 13 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Holowatyj AN, et al. , Clinicopathologic and Racial/Ethnic Differences of Colorectal Cancer Among Adolescents and Young Adults. Clin Transl Gastroenterol 2019, 10 (7), e00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Rodriguez L, et al. , Disease Characteristics, Clinical Management, and Outcomes of Young Patients With Colon Cancer: A Population-based Study. Clin Colorectal Cancer 2018, 17 (4), e651–e661. [DOI] [PubMed] [Google Scholar]
- 277.Yeo H, et al. , Early-onset Colorectal Cancer is Distinct From Traditional Colorectal Cancer. Clin Colorectal Cancer 2017, 16 (4), 293–299 e6. [DOI] [PubMed] [Google Scholar]
- 278.Chang DT, et al. , Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol 2012, 25 (8), 1128–39. [DOI] [PubMed] [Google Scholar]
- 279.Antelo M, et al. , A high degree of LINE-1 hypomethylation is a unique feature of early-onset colorectal cancer. PLoS One 2012, 7 (9), e45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Sultan I, et al. , Distinct features of colorectal cancer in children and adolescents: a population-based study of 159 cases. Cancer 2010, 116 (3), 758–65. [DOI] [PubMed] [Google Scholar]
- 281.Baba Y, et al. , Epigenomic diversity of colorectal cancer indicated by LINE-1 methylation in a database of 869 tumors. Mol Cancer 2010, 9, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Yantiss RK, et al. , Clinical, pathologic, and molecular features of early-onset colorectal carcinoma. Am J Surg Pathol 2009, 33 (4), 572–82. [DOI] [PubMed] [Google Scholar]
- 283.Lachance JA, et al. , The effect of age on clinical/pathologic features, surgical morbidity, and outcome in patients with endometrial cancer. Gynecol Oncol 2006, 101 (3), 470–5. [DOI] [PubMed] [Google Scholar]
- 284.Tran BN; Connell PP; Waggoner S; Rotmensch J; Mundt AJ, Characteristics and outcome of endometrial carcinoma patients age 45 years and younger. Am J Clin Oncol 2000, 23 (5), 476–80. [DOI] [PubMed] [Google Scholar]
- 285.Pellerin GP; Finan MA, Endometrial cancer in women 45 years of age or younger: a clinicopathological analysis. Am J Obstet Gynecol 2005, 193 (5), 1640–4. [DOI] [PubMed] [Google Scholar]
- 286.Quinn MA; Kneale BJ; Fortune DW, Endometrial carcinoma in premenopausal women: a clinicopathological study. Gynecol Oncol 1985, 20 (3), 298–306. [DOI] [PubMed] [Google Scholar]
- 287.Silverberg SG; Makowski EL; Roche WD, Endometrial carcinoma in women under 40 years of age: comparison of cases in oral contraceptive users and non-users. Cancer 1977, 39 (2), 592–8. [DOI] [PubMed] [Google Scholar]
- 288.Evans-Metcalf ER; Brooks SE; Reale FR; Baker SP, Profile of women 45 years of age and younger with endometrial cancer. Obstet Gynecol 1998, 91 (3), 349–54. [DOI] [PubMed] [Google Scholar]
- 289.Gitsch G; Hanzal E; Jensen D; Hacker NF, Endometrial cancer in premenopausal women 45 years and younger. Obstet Gynecol 1995, 85 (4), 504–8. [DOI] [PubMed] [Google Scholar]
- 290.Cheema PK, et al. , Age 40 years and under does not confer superior prognosis in patients with multiple myeloma undergoing upfront autologous stem cell transmplant. Biol Blood Marrow Transplant 2009, 15 (6), 686–93. [DOI] [PubMed] [Google Scholar]
- 291.Jurczyszyn A, et al. , Characteristics and outcomes of patients with multiple myeloma aged 21–40 years versus 41–60 years: a multi-institutional case-control study. Br J Haematol 2016, 175 (5), 884–891. [DOI] [PubMed] [Google Scholar]
- 292.Blade J; Kyle RA; Greipp PR, Presenting features and prognosis in 72 patients with multiple myeloma who were younger than 40 years. Br J Haematol 1996, 93 (2), 345–51. [DOI] [PubMed] [Google Scholar]
- 293.Ludwig H, et al. , Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: an analysis of 10 549 patients from the International Myeloma Working Group. Blood 2008, 111 (8), 4039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Chretien ML, et al. , Age is a prognostic factor even among patients with multiple myeloma younger than 66 years treated with high-dose melphalan: the IFM experience on 2316 patients. Haematologica 2014, 99 (7), 1236–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kang JS; Jang JY; Kwon W; Han Y; Kim SW, Clinicopathologic and survival differences in younger patients with pancreatic ductal adenocarcinoma-A propensity score-matched comparative analysis. Pancreatology 2017, 17 (5), 827–832. [DOI] [PubMed] [Google Scholar]
- 296.Ramai D, et al. , Early- and late-onset pancreatic adenocarcinoma: A population-based comparative study. Pancreatology 2021, 21 (1), 124–129. [DOI] [PubMed] [Google Scholar]
- 297.Ansari D; Althini C; Ohlsson H; Andersson R, Early-onset pancreatic cancer: a population-based study using the SEER registry. Langenbecks Arch Surg 2019, 404 (5), 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Eguchi H, et al. , Clinicopathological Characteristics of Young Patients With Pancreatic Cancer: An Analysis of Data From Pancreatic Cancer Registry of Japan Pancreas Society. Pancreas 2016, 45 (10), 1411–1417. [DOI] [PubMed] [Google Scholar]
- 299.Shih HJ; Fang SC; An L; Shao YJ, Early-onset prostate cancer is associated with increased risks of disease progression and cancer-specific mortality. Prostate 2021, 81 (2), 118–126. [DOI] [PubMed] [Google Scholar]
- 300.Bleyer A; Spreafico F; Barr R, Prostate cancer in young men: An emerging young adult and older adolescent challenge. Cancer 2020, 126 (1), 46–57. [DOI] [PubMed] [Google Scholar]
- 301.Siegel DA; O’Neil ME; Richards TB; Dowling NF; Weir HK, Prostate Cancer Incidence and Survival, by Stage and Race/Ethnicity - United States, 2001–2017. MMWR Morb Mortal Wkly Rep 2020, 69 (41), 1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Sandhu DP; Munson KW; Benghiat A; Hopper IP, Natural history and prognosis of prostate carcinoma in adolescents and men under 35 years of age. Br J Urol 1992, 69 (5), 525–9. [DOI] [PubMed] [Google Scholar]
- 303.Gerhauser C, et al. , Molecular Evolution of Early-Onset Prostate Cancer Identifies Molecular Risk Markers and Clinical Trajectories. Cancer Cell 2018, 34 (6), 996–1011 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Weischenfeldt J; Korbel JO, Genomes of early onset prostate cancer. Curr Opin Urol 2017, 27 (5), 481–487. [DOI] [PubMed] [Google Scholar]
- 305.Chalmers ZR, et al. , Early-onset metastatic and clinically advanced prostate cancer is a distinct clinical and molecular entity characterized by increased TMPRSS2-ERG fusions. Prostate Cancer Prostatic Dis 2021, 24 (2), 558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zhou QP; Ge YH; Liu CY, Comparison of metastasis between early-onset and late-onset gastric signet ring cell carcinoma. BMC Gastroenterol 2020, 20 (1), 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Cho SY, et al. , Sporadic Early-Onset Diffuse Gastric Cancers Have High Frequency of Somatic CDH1 Alterations, but Low Frequency of Somatic RHOA Mutations Compared With Late-Onset Cancers. Gastroenterology 2017, 153 (2), 536–549 e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Mun DG, et al. , Proteogenomic Characterization of Human Early-Onset Gastric Cancer. Cancer Cell 2019, 35 (1), 111–124 e10. [DOI] [PubMed] [Google Scholar]
- 309.De B, et al. , Gastric adenocarcinoma in young adult patients: patterns of care and survival in the United States. Gastric Cancer 2018, 21 (6), 889–899. [DOI] [PubMed] [Google Scholar]
- 310.Ogino S; Nowak JA; Hamada T; Milner DA Jr.; Nishihara R, Insights into Pathogenic Interactions Among Environment, Host, and Tumor at the Crossroads of Molecular Pathology and Epidemiology. Annu Rev Pathol 2019, 14, 83–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Ogino S; Chan AT; Fuchs CS; Giovannucci E, Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut 2011, 60 (3), 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Ogino S, et al. , Review Article: The Role of Molecular Pathological Epidemiology in the Study of Neoplastic and Non-neoplastic Diseases in the Era of Precision Medicine. Epidemiology 2016, 27 (4), 602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Kucab JE, et al. , A Compendium of Mutational Signatures of Environmental Agents. Cell 2019, 177 (4), 821–836 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Gurjao C, et al. , Discovery and Features of an Alkylating Signature in Colorectal Cancer. Cancer Discov 2021, 11 (10), 2446–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Bai J; Chen H; Bai X, Relationship between microsatellite status and immune microenvironment of colorectal cancer and its application to diagnosis and treatment. J Clin Lab Anal 2021, 35 (6), e23810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Kuenzig ME, et al. , Twenty-first Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology 2022, 162 (4), 1147–1159 e4. [DOI] [PubMed] [Google Scholar]
- 317.Asakura K; Sasaki S, School lunches in Japan: their contribution to healthier nutrient intake among elementary-school and junior high-school children. Public Health Nutr 2017, 20 (9), 1523–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Yoshida Y; Simoes EJ, Sugar-Sweetened Beverage, Obesity, and Type 2 Diabetes in Children and Adolescents: Policies, Taxation, and Programs. Curr Diab Rep 2018, 18 (6), 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Cathaoir KO, Childhood Obesity and the Right to Health. Health Hum Rights 2016, 18 (1), 249–262. [PMC free article] [PubMed] [Google Scholar]
- 320.Wright A; Smith KE; Hellowell M, Policy lessons from health taxes: a systematic review of empirical studies. BMC Public Health 2017, 17 (1), 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Beccuti G; Pannain S, Sleep and obesity. Curr Opin Clin Nutr Metab Care 2011, 14 (4), 402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Ogilvie RP; Patel SR, The Epidemiology of Sleep and Diabetes. Curr Diab Rep 2018, 18 (10), 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Chavarro JE, et al. , Contributions of the Nurses’ Health Studies to Reproductive Health Research. Am J Public Health 2016, 106 (9), 1669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Cirillo PM; Cohn BA, Pregnancy complications and cardiovascular disease death: 50-year follow-up of the Child Health and Development Studies pregnancy cohort. Circulation 2015, 132 (13), 1234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Morton SM, et al. , Cohort profile: growing up in New Zealand. Int J Epidemiol 2013, 42 (1), 65–75. [DOI] [PubMed] [Google Scholar]
- 326.Connelly R; Platt L, Cohort profile: UK Millennium Cohort Study (MCS). Int J Epidemiol 2014, 43 (6), 1719–25. [DOI] [PubMed] [Google Scholar]
- 327.Carter AB, et al. , Electronic Health Records and Genomics: Perspectives from the Association for Molecular Pathology Electronic Health Record (EHR) Interoperability for Clinical Genomics Data Working Group. J Mol Diagn 2022, 24 (1), 1–17. [DOI] [PubMed] [Google Scholar]
- 328.Maitre L, et al. , Human Early Life Exposome (HELIX) study: a European population-based exposome cohort. BMJ Open 2018, 8 (9), e021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.van den Bosch M, et al. , Green CURIOCITY: a study protocol for a European birth cohort study analysing childhood heat-related health impacts and protective effects of urban natural environments. BMJ Open 2022, 12 (1), e052537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Alberts B; Kirschner MW; Tilghman S; Varmus H, Rescuing US biomedical research from its systemic flaws. Proc Natl Acad Sci U S A 2014, 111 (16), 5773–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Zavala VA, et al. , Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer 2021, 124 (2), 315–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Huang BZ, et al. , Rising incidence and racial disparities of early-onset pancreatic cancer in the United States, 1995–2018. Gastroenterology 2022. [DOI] [PMC free article] [PubMed]
- 333.Murphy CC; Wallace K; Sandler RS; Baron JA, Racial Disparities in Incidence of Young-Onset Colorectal Cancer and Patient Survival. Gastroenterology 2019, 156 (4), 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Dai J, et al. , Revisiting social MPE: an integration of molecular pathological epidemiology and social science in the new era of precision medicine. Expert Rev Mol Diagn 2021, 1–18. [DOI] [PMC free article] [PubMed]
- 335.Manthey J, et al. , Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet 2019, 393 (10190), 2493–2502. [DOI] [PubMed] [Google Scholar]
- 336.Mikaeel RR, et al. , Young-onset colorectal cancer is associated with a personal history of type 2 diabetes. Asia Pac J Clin Oncol 2021, 17 (1), 131–138. [DOI] [PubMed] [Google Scholar]
- 337.Rosen MW, et al. , Risk Factors for Endometrial Cancer or Hyperplasia in Adolescents and Women 25 Years Old or Younger. J Pediatr Adolesc Gynecol 2019, 32 (5), 546–549. [DOI] [PubMed] [Google Scholar]
- 338.Campbell BR, et al. , Early onset oral tongue squamous cell carcinoma: Associated factors and patient outcomes. Head Neck 2019, 41 (6), 1952–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Arvold ND, et al. , Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol 2011, 29 (29), 3885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.El Saghir NS, et al. , Effects of young age at presentation on survival in breast cancer. BMC Cancer 2006, 6, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Chung M; Chang HR; Bland KI; Wanebo HJ, Younger women with breast carcinoma have a poorer prognosis than older women. Cancer 1996, 77 (1), 97–103. [DOI] [PubMed] [Google Scholar]
- 342.de la Rochefordiere A, et al. , Age as prognostic factor in premenopausal breast carcinoma. Lancet 1993, 341 (8852), 1039–43. [DOI] [PubMed] [Google Scholar]
- 343.Jin Z, et al. , Clinicopathological and Molecular Characteristics of Early-Onset Stage III Colon Adenocarcinoma: An Analysis of the ACCENT Database. J Natl Cancer Inst 2021. [DOI] [PMC free article] [PubMed]
- 344.Lieu CH, et al. , Association of age with survival in patients with metastatic colorectal cancer: analysis from the ARCAD Clinical Trials Program. J Clin Oncol 2014, 32 (27), 2975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Kim TJ; Kim ER; Hong SN; Chang DK; Kim YH, Long-Term Outcome and Prognostic Factors of Sporadic Colorectal Cancer in Young Patients: A Large Institutional-Based Retrospective Study. Medicine (Baltimore) 2016, 95 (19), e3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Chou CL; Tseng CJ; Shiue YL, The impact of young age on the prognosis for colorectal cancer: a population-based study in Taiwan. Jpn J Clin Oncol 2017, 47 (11), 1010–1018. [DOI] [PubMed] [Google Scholar]
- 347.Blanke CD, et al. , Impact of young age on treatment efficacy and safety in advanced colorectal cancer: a pooled analysis of patients from nine first-line phase III chemotherapy trials. J Clin Oncol 2011, 29 (20), 2781–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Pokharkar AB, et al. , Young Vs Old Colorectal Cancer in Indian Subcontinent: a Tertiary Care Center Experience. Indian J Surg Oncol 2017, 8 (4), 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Rho YS, et al. , Comparing Clinical Characteristics and Outcomes of Young-onset and Late-onset Colorectal Cancer: An International Collaborative Study. Clin Colorectal Cancer 2017, 16 (4), 334–342. [DOI] [PubMed] [Google Scholar]
- 350.Yang Z, et al. , Characteristics and long-term survival of colorectal cancer patients aged 44 years and younger. Clin Transl Oncol 2012, 14 (12), 896–904. [DOI] [PubMed] [Google Scholar]
- 351.McMillan DC; McArdle CS, The impact of young age on cancer-specific and non-cancer-related survival after surgery for colorectal cancer: 10-year follow-up. Br J Cancer 2009, 101 (4), 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Manjelievskaia J, et al. , Chemotherapy Use and Survival Among Young and Middle-Aged Patients With Colon Cancer. JAMA Surg 2017, 152 (5), 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Schellerer VS, et al. , Despite aggressive histopathology survival is not impaired in young patients with colorectal cancer : CRC in patients under 50 years of age. Int J Colorectal Dis 2012, 27 (1), 71–9. [DOI] [PubMed] [Google Scholar]
- 354.Wang MJ, et al. , The prognostic factors and multiple biomarkers in young patients with colorectal cancer. Sci Rep 2015, 5, 10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Quah HM, et al. , Young age influences treatment but not outcome of colon cancer. Ann Surg Oncol 2007, 14 (10), 2759–65. [DOI] [PubMed] [Google Scholar]
- 356.Boyce S, et al. , Young-onset colorectal cancer in New South Wales: a population-based study. Med J Aust 2016, 205 (10), 465–470. [DOI] [PubMed] [Google Scholar]
- 357.O’Connell JB, et al. , Do young colon cancer patients have worse outcomes? World J Surg 2004, 28 (6), 558–62. [DOI] [PubMed] [Google Scholar]
- 358.Hubbard J, et al. , Benefits and adverse events in younger versus older patients receiving adjuvant chemotherapy for colon cancer: findings from the Adjuvant Colon Cancer Endpoints data set. J Clin Oncol 2012, 30 (19), 2334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.