Abstract
Background
Delirium is a common neurocognitive disorder in hospitalised older adults with vast negative consequences. The predominant method of subtyping delirium is by motor activity profile into hypoactive, hyperactive and mixed groups.
Objective
This systematic review and meta-analysis investigated how predisposing factors differ between delirium motor subtypes.
Methods
Databases (Medline, PsycINFO, Embase) were systematically searched for studies reporting predisposing factors (prior to delirium) for delirium motor subtypes. A total of 61 studies met inclusion criteria (N = 14,407, mean age 73.63 years). Random-effects meta-analyses synthesised differences between delirium motor subtypes relative to 22 factors.
Results
Hypoactive cases were older, had poorer cognition and higher physical risk scores than hyperactive cases and were more likely to be women, living in care homes, taking more medications, with worse functional performance and history of cerebrovascular disease than all remaining subtypes. Hyperactive cases were younger than hypoactive and mixed subtypes and were more likely to be men, with better cognition and lower physical risk scores than all other subtypes. Those with no motor subtype (unable to be classified) were more likely to be women and have better functional performance. Effect sizes were small.
Conclusions
Important differences in those who develop motor subtypes of delirium were shown prior to delirium occurrence. We provide robust quantitative evidence for a common clinical assumption that indices of frailty (institutional living, cognitive and functional impairment) are seen more in hypoactive patients. Motor subtypes should be measured across delirium research. Motor subtyping has great potential to improve the clinical risk assessment and management of delirium.
Keywords: systematic review, risk factor, older people, mixed, hypoactive, hyperactive
Key Points
Individuals who develop hypoactive delirium show more indices of frailty than other subtypes.
Men are more likely to develop hyperactive delirium and women are more likely to develop hypoactive delirium.
Differences between subtypes were more pronounced in analyses in older adults.
Incorporating risk scores for individual motor subtypes could improve delirium prediction tools and the planning of care.
Background
Delirium is an acute and fluctuating disorder characterised by deficits in attention commonly seen in older adults across settings (e.g. surgical, medical, community, palliative care, aged care), with highest rates in intensive care (19–82%) [1–3]. Several negative consequences are associated with delirium, including functional and cognitive decline, increased institutionalisation, dementia and death [4–7].
Delirium subtypes can be derived from its motor activity profile: hyperactive (increased quantity of motor activity, loss of control of activity, agitation), hypoactive (decreased activity and speech, reduced speed and alertness) and mixed (fluctuations between hyperactive and hypoactive) [8]. There has been recent work to refine the systematic classification of motor features [9, 10]. Notwithstanding their defining motor differences, motor subtypes of delirium also differ in detection rates, treatment and outcomes [10, 11]. Recent delirium research emphasises the importance of considering subtypes [2].
A considerable proportion (30–40%) of delirium is preventable, yet current risk prediction tools lack sensitivity [1, 12, 13]. Risk factors for delirium can be categorised as precipitating (e.g. surgery type or infection which represents the acute insult that drives delirium) or predisposing (individual factors which represent vulnerability to delirium). A focus on predisposing factors represents a broad approach encompassing general vulnerability to delirium across various precipitants. Understanding predisposing risk factors for delirium subtypes is of emerging importance as the incorporation of these nuanced factors could improve prediction tool accuracy. The literature has yet to be systematically and quantitatively synthesised. Many studies appear to have assessed only a small number of predisposing factors for delirium motor subtypes, or only assessed one setting (e.g. intensive care) [14–16]. Studies report associations between poorer functional status, increased comorbidity, intravenous access and hypoactive delirium, while antipsychotic prescriptions have been associated with the hyperactive subtype [15, 16], though both these observations may be subject to reverse causation. Through a semi-quantitative analysis, a recent review in critical care [14] reported inconsistent findings or no association for differences between the motor subtypes in relation to age, sex and mortality risk score.
Quantifying how predisposing factors differ between delirium motor subtypes could inform our understanding of the neurobiological bases of delirium [2, 17, 18]. Despite different symptomatology, no theories of delirium neurobiology make differential predictions relative to subtype [19, 20]. This contrasts with other disorders such as attention deficit hyperactivity disorder [21], where subtypes are theoretically and empirically considered, leading to improved assessment and treatment, along with neurobiological understanding.
This systematic review and meta-analysis will investigate how predisposing factors (e.g. demographic and medical factors) differ between delirium motor subtypes. Findings will (i) enable clinical delirium risk prediction tools to be improved, and (ii) indicate whether neurobiological mechanisms underlying subtypes diverge.
Methods
This work was conducted according to the PRISMA 2020 statement [22] (see supplementary data for Supplementary Table S1 for PRISMA checklist) and was registered prior to data extraction (osf.io/j69g2).
Search strategy and selection criteria
Databases (Medline, PsycINFO, Embase) were searched without database limits through Ovid from inception to 7 June 2021. The complete search strategy was (delirium/OR deliri*) AND (hypoactive OR hypo-active OR hyperactive OR hyper-active OR mixed OR subtype OR sub-type OR motor*). We did not include search terms for confusion or encephalopathy as, while they share similar features, delirium is a distinct concept [23] and the focus of the current review. All identified records were screened by two reviewers, first by title and abstract, and then by full text, with disagreements resolved through reviewer discussion and consensus.
Prospective and retrospective original empirical research papers published in English language after 1990 (first formal definition of motor subtypes [8]) including adult participants (>18 years) were eligible for inclusion. Studies could measure delirium and motor subtype (hypoactive, hyperactive, mixed or no motor subtype for those unable to be classified) using any method. Data to calculate an effect size for an appropriate factor must have been reported for two motor subtypes. Predisposing factors must have been measured prior to (not during or after) delirium for those which change over time (e.g. cognition), but may have been measured during or after delirium if stable over time (e.g. sex). Precipitating factors (e.g. surgery type, aetiology) were not included. To be included, studies must report sufficient data (N > 1) for at least two delirium motor subtype groups.
Data collection and coding
Data were extracted independently by two reviewers with discrepancies resolved through discussion and consensus. We extracted study and sample information (country, design, setting, demographics), delirium and subtype assessment details, and statistics for an appropriate factor and motor subtypes of delirium. Factors were grouped (see supplementary data for Supplementary Table S2) by author discussion and consensus.
Risk of bias in individual studies
Risk of bias was assessed with the Risk of Bias for Non-randomised studies (RoBANS) [24] tool for observational studies (including randomised controlled trials where only observational data, pre-intervention, was used). Two independent reviewers assessed risk of bias with disagreements resolved through discussion and consensus.
Data analysis
All data analyses were conducted in R using the metafor package [25], with data and code publicly available (github.com/ericaghezzi/delirium_subtypes_metaanalysis). All medians were converted to means and standard deviations using the quantile estimation method within the estmeansd package [26].
Ten delirium motor subtype comparisons were considered in analyses (Table 1). Random-effects models were used to calculate effect sizes for differences between Group A and Group B for each factor/subtype comparison reported by >2 studies. Statistical dependency in analyses was accounted for by averaging effect sizes and variances within studies to produce a single study-level estimate for each analysis. We used the Paule and Mandel estimator [27] of between-study variance and the Knapp and Hartung method [28]. Between-study variance was quantified using tau2 and the proportion of between-study heterogeneity out of total variance was assessed using the I2 statistic (classified as low (25%), moderate (50%) or high (75%) [29]).
Table 1.
Motor subtype comparison groupings
| Motor subtype comparison | Group A | Group B |
|---|---|---|
| 1 | Hypoactive | Hyperactive |
| 2 | Hypoactive | Mixed |
| 3 | Hypoactive | No motor subtype |
| 4 | Hyperactive | Mixed |
| 5 | Hyperactive | No motor subtype |
| 6 | Mixed | No motor subtype |
| 7 | Hypoactive | Remaining |
| 8 | Hyperactive | Remaining |
| 9 | Mixed | Remaining |
| 10 | No motor subtype | Remaining |
Note: ‘Remaining’ in comparisons 7–10 refers to the average of all other reported motor subtypes (not including Group A).
Categorical and continuous factors were summarised separately (Hedges’ g for continuous, odds ratio for categorical). Positive Hedges’ g represents higher scores in Group A compared with group B. An odds ratio greater than 1 represents greater likelihood of the factor being present in Group A compared with Group B.
Effects are unadjusted for important variables (e.g. age) as too few studies reported multivariate analyses for these to be analysed. To provide an indication of the effect of age, we ran a subgroup analysis stratified by two groups: (i) studies which included all adults and (ii) those which only included participants >60 years (by some inclusion/exclusion criteria, or by reported age range where minimum was ≥60). We also ran a subgroup analysis stratified by delirium type (incident or prevalent) to investigate the predictive ability of factors (for incident delirium). With generally low heterogeneity across analyses, further subgroup analyses were deemed unnecessary.
Funnel plots of effect size versus standard error for all significant analyses were visually examined for symmetry to assess for bias across studies due to the small-study effect [30]. In analyses with at least 10 studies, the small-study effect was formally tested using Egger’s intercept test [31]. If evidence of asymmetry (one-tailed P < 0.1 on the Egger’s test) was found, Duval and Tweedie’s [32] trim and fill method was used to quantify the magnitude of potential bias.
Certainty in the body of evidence was assessed using the GRADE approach (Grading of Recommendations, Assessment, Development and Evaluation) [33]. Overall certainty was categorised as high, moderate, low or very low according to assessments of the eight GRADE criteria: risk of bias, inconsistency of results, indirectness of evidence, imprecision, publication bias, magnitude of effect, dose–response gradient and influence of residual plausible confounding.
Results
Database searching identified 3,432 unique articles, and 863 were screened by full-text following initial title/abstract screening (Supplementary Figure S1). A total of 62 studies satisfied the inclusion criteria; however, one study [34] did not include any factors reported in at least two other studies. Therefore, 61 studies were included in the quantitative synthesis (Table 2).
Table 2.
Overview of included studies
| Lead author, yeara |
Study design | Countryh | Age, mean (SD) | Male/female, N | Sample size | Delirium type | When/where delirium diagnosed |
Delirium diagnosis tool/s |
Subtype classification method/s |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All delirium | Hyper active | Hypo active | Mixed | No motor subtype | Combined | |||||||||
| Avelino-Silva, 2018 [44] | Prospective cohort | BR | 83.00 (8.00) | 250/407 | 657 | 112 | 348 | 197 | - | - | Prevalent | Geriatric ward admission/stay | Short CAM | Observation of clinical features |
| Balan, 2003 [56] | Prospective cohort | IL | 83.50 (7.10) | 13/18 | 31 | 7 | 10 | 14 | - | - | Incident | During medical ward stay | ICD-10 | Symptoms |
| Boettger, 2011ab [57] | Retrospective cohort | US | 69.60 (11.90) | 10/11 | 21 | 12 | 9 | - | - | - | Prevalent | Hospitalised cancer patients | DSM-IV, MDAS | NR |
| Boettger, 2011bb [58] | Retrospective cohort | US | 65.60 (13.60) | 65/46 | 111 | 61 | 50 | - | - | - | Prevalent | Cancer patients referred for delirium management |
DSM-IV-TR, MDAS | MDAS |
| Boettger, 2011cc [59] | Retrospective cohort | US | 58.36 (16.65) | 52/48 | 100 | - | 53 | - | - | 47 (hyper/mix) | Prevalent | Inpatients receiving cancer care | DSM-IV, MDAS | MDAS |
| Boettger, 2014b, c [60] | Retrospective cohort | US | 65.57 (13.71) | 65/46 | 111 | 62 | 49 | - | - | - | Prevalent | Patients undergoing cancer treatment referred for delirium management |
DSM-IV-TR, MDAS | NR |
| Boettger, 2017 [61] | Retrospective cohort | CH | 74.44 (10.74) | 63/28 | 91 | 11 | 38 | 42 | - | - | Prevalent | Medical and surgical referral patients |
DOS, S-CAM | DOS |
| Bui, 2017 [62] | Retrospective cohort | US | 65.00 (16.00) | 214/209 | 423 | - | 170 | - | - | 253 (hyper/mix) | Prevalent | During surgical ICU stay | CAM-ICU, ICD-9-CM | RASS |
| Camus, 2000 [63] | Prospective cohort | FR, CH | 84.10 (5.90) | 63/120 | 183 | 85 | 48 | 50 | - | - | Prevalent | Admission to geriatric ward | DSM-III-R | Symptom checklist |
| Chong, 2013d [64] | Prospective cohort | SG | 84.20 (7.40) | 99/129 | 228 | 117 | 42 | 69 | - | - | Prevalent | Geriatric Admission Unit (admission or during hospital stay) |
CAM | Activity pattern |
| Chong, 2015d [65] | Prospective cohort | SG | 84.13 (7.36) | 102/132 | 234 | 121 | 46 | 67 | - | - | Prevalent | Admission to Geriatric Monitoring Unit |
CAM | Observation of psychomotor activity |
| Daly, 2018 [66] | Cross-sectional | IE | 79.10 (8.20) | 100/99 | 199 | 41 | 38 | 16 | 104 | - | Prevalent | Medical inpatients referred to consultation liaison psychiatry service |
DRS-R98, DSM-IV | NR |
| DeCrane, 2012 [67] | Prospective cohort | US | 89.95 (NR) | 71/249j | 70 | 14 | 33 | 23 | - | - | Prevalent | Long-term care residents during 28-day surveillance period |
CAM, NEECHAM, MMSE, Vigilance A test, CAC-A | CAC-A |
| Eriksson, 2002 [68] | Prospective cohort | SE | 72.50 (4.06) | 8/4 | 12 | 5 | 6 | 1 | - | - | Prevalent | During hospital stay for cardiac surgery (pre- or post-surgery) |
DSM-IV, CAM | Symptoms |
| Evensen, 2019ae [47] | Cross-sectional | NO | 86.70 (5.20) | 29/31 | 60 | 15 | 20 | 17 | 8 | - | Prevalent | NA | DSM-5 | DMSS |
| Evensen, 2019bh [69] | Prospective cohort | NO | 86.75 (5.19) | 43/50 | 93 | 27 | 30 | 24 | 12 | - | Prevalent | Geriatric ward (during hospital stay) |
DSM-5, chart-review, interviews with nurses | DMSS |
| Fialho Silva, 2021 [70] | Prospective cohort | BR | 67.99 (12.92) | 38/33 | 71 | 9 | 41 | 21 | - | - | Prevalent | Patients in acute phase of stroke admitted to Stroke Unit |
CAM | RASS |
| Franco, 2014 [71] | Case–control | CO | 78.30 (8.96) | 13/21 | 34 | 10 | 13 | 11 | - | - | Incident | Internal medicine ward stay | CAM-S, DRS-R98 | DRS-R98 |
| Glynn, 2021 [72] | Cross-sectional | IE, IN | 56.60 (20.30) | 1125/632 | 1757 | 844 | 298 | 426 | 189 | - | Prevalent | Patients in palliative care, old age liaison psychiatry and general adult liaison psychiatry settings | DSM-IV | DMSS-4 |
| Godfrey, 2009 [73] | Prospective cohort | IE | 70.70 (11.60) | 16/9 | 25 | 12 | 4 | 9 | - | - | Prevalent | Patients in palliative care | DSM-IV | DMC |
| Grover, 2014 [74] | Prospective cohort | IN | 49.00 (17.62) | 228/93 | 321 | 161 | 64 | 79 | 17 | - | Prevalent | Patients referred to psychiatry consultation liaison services from any medical or surgical ward |
DSM-IV-TR | DMSS |
| Gual, 2018 [16] | Prospective cohort | ES | 87.41 (6.00) | 140/203 | 343 | 143 | 91 | 109 | - | - | Prevalent | Admission to subacute care unit | CAM | DMSS |
| Hayhurst, 2020f [75] | Prospective cohort | US | 61.00 (13.34) | 343/239j | - | 100 | 411 | - | - | - | Prevalent | Adult medical and surgical ICU patients with respiratory failure or shock (during ICU stay) | CAM-ICU, RASS | RASS |
| Heymann, 2007 [76] | Retrospective cohort | DE | 64.00 (22.00) | 114/82 | 196 | 55 | - | - | - | - | Prevalent | Admission to anaesthesiology ICU or intermediate care unit |
DDS | DDS |
| Horacek, 2016 [77] | Prospective cohort | CZ | 68.21 (12.07) | 100/40 | 140 | 54 | 27 | 59 | - | - | Prevalent | Intensive care unit (during stay) | Validated chart review, Riker SAS, RASS | NR |
| Hughes, 2021f [78] | Prospective cohort | US | 63.00 (13.34) | 435/305 | 740 | 185 | 733 | - | - | - | Prevalent | Adult medical and surgical ICU patients with respiratory failure or shock (during ICU stay) | CAM-ICU, RASS | RASS |
| Jackson, 2017 [79] | Prospective cohort | GB | 85.50 (6.15) | 21/34 | 55 | 12 | 34 | 9 | - | - | Prevalent | Newly admitted medical patients | DSM-IV-TR, CAM, AMTS, digit span test, medical notes | DRS-R98 |
| Khurana, 2011 [80] | Prospective cohort | IN | 70.86 (8.86) | 224/176 | 400 | 102 | 259 | 39 | - | - | Prevalent | Medical ward (admission or during hospitalisation) |
DSM-IV, CAM | Symptoms |
| Kiely, 2007 [81] | RCT | US | 84.00 (7.30) | 162/295 | 457 | 47 | 212 | 55 | 143 | - | Prevalent | Admission to post-acute care | CAM | MDAS |
| Kim, 2018 [82] | Prospective cohort | KR | 69.30 (10.60) | 156/68 | 224 | 144 | 25 | 33 | 22 | - | Prevalent | Nonpsychiatric inpatients referred to the consultation liaison psychiatric service |
DSM-IV-TR, CAM | DMSS |
| Kobayashi, 1992 [83] | Prospective cohort | JP | 74.58 (9.47) | 63/43 | 106 | 83 | 7 | 16 | - | - | Prevalent | Referral to Division of Neuropsychiatry | NR | Clinical characteristics (Lipowski) |
| Kumar, 2015 [84] | Prospective cohort | IN | 33.78 (7.18) | 57/23 | 80 | 56 | 24 | - | - | - | Prevalent | Patients in psychiatry ward, general medical and surgical/postoperative wards and intensive care unit | DSM-IV-TR | MDAS |
| Lee, 2018 [85] | Prospective cohort | HK | 64.81 (NR) | 55/28 | 83 | 24 | 26 | 33 | - | - | Incident | Following urgent and elective cardiac surgery |
CAM-ICU | RASS |
| Leonard, 2011g [86] | Prospective cohort | IE | 70.30 (10.50) | 51/49 | 100 | 18 | 33 | 26 | 23 | - | Prevalent | Palliative care patients | CAM, DSM-IV | DMC |
| Liptzin, 1992 [87] | Prospective cohort | US | 86.10 (NR) | 47/78 | 125 | 19 | 24 | 65 | 17 | - | Prevalent | Admission for medical or surgical care (non-ICU), throughout stay |
DSM-III, DSI | DSI |
| Lixouriotis, 2011 [88] | Retrospective cohort | GR | 76.30 (8.00) | 5/4 | 9 | 6 | 1 | 2 | - | - | Prevalent | Patients examined at regional medical office |
ICD-10 | Clinical description (Liptzin and Levkoff) |
| Lundström, 2012 [89] | RCT | SE | 82.93 (6.15) | 36/93 | 129 | 56 | 43 | 28 | 2 | - | Incident | Following femoral neck fracture surgery | Modified OBS | NR |
| Marcantonio, 2002 [90] | Prospective cohort | US | 79.00 (8.00) | 9/39 | 49 | - | 34 | - | 1 | 14 (hyper/mix) | Incident | Following acute hip fracture surgery |
CAM | MDAS |
| Margiotta, 2006 [91] | Prospective cohort | IT | 81.60 (7.20) | 26/37 | 63 | 26 | 7 | 30 | - | - | Prevalent | Admission to acute medical care unit |
CAM | DRS, ODFS |
| Meagher, 2000 [92] | Prospective cohort | IE | 60.10 (19.50) | 20/26 | 46 | 14 | 11 | 21 | - | - | Prevalent | Referral from general medical wards to psychiatric consultation service |
ICD-10, DRS | Case records and information from the consultation |
| Meagher, 2012 [93] | Prospective cohort | IE | 70.20 (10.50) | 51/49 | 100 | 10 | 28 | 18 | 6 | - | Prevalent | Palliative care inpatients with cancer diagnoses |
DSM-IV | DMSS |
| Morandi, 2017 [94] | Cross-sectional | IT | 85.00 (6.70) | 113/162 | 275 | 59 | 106 | 75 | 35 | - | Prevalent | Patients in acute and rehabilitation hospital wards | 4AT | DMSS |
| Morandi, 2020 [95] | Cross-sectional | IT | 85.98 (NR) | 186/185 | 371 | 95 | 123 | 128 | 25 | - | Prevalent | Patients in acute and rehabilitation hospital wards | 4AT | DMSS |
| O’Keeffe, 1999 [96] | Prospective cohort | IE | 82.44 (4.63) | NR | 94 | 20 | 27 | 40 | 7 | - | Prevalent | Admission to acute care geriatric unit |
DSM-III, DAS | DAS |
| Özkul, 2019 [97] | Case–control | TR | 70.10 (13.60) | 29/24 | 53 | 34 | 14 | 5 | - | - | Prevalent | Admission to ICU | DSM-IV, CAM-ICU | NR |
| Park, 2016 [98] | Retrospective cohort | KR | 71.21 (13.16) | 132/78 | 210 | - | 67 | - | - | 143 (hyper/mix) | Prevalent | Referral from ward physicians to consultation liaison psychiatry service |
DRS-R98, CAM | RASS |
| Pasinska, 2019 [99] | Prospective cohort | PL | 77.47 (10.54) | 84/119 | 203 | 31 | 85 | 77 | 10 | - | Prevalent | Patients with stroke/TIA admitted to Stroke Unit |
bCAM, CAM-ICU, DSM-5 | DMSS-4 |
| Price, 2017 [100] | Prospective cohort | US | 70.50 (9.70) | 75/62 | 137 | 10 | 108 | 19 | - | - | Incident | Following elective cardiac surgery | CAM-ICU, RASS | RASS |
| Radinovic, 2019 [101] | Prospective cohort | RS | 80.95 (7.12) | NR | 148 | 37 | 111 | - | - | - | Incident | Following bipolar hemiarthroplasty or compression hip screw procedure |
CAM | Symptoms |
| Rawle, 2020 [102] | Retrospective cohort | GB | 86.00 (7.60) | 73/61j | 56 | 43 | 13 | - | - | - | Prevalent | Admission to hospital with COVID-19 diagnosis | Review medical records | Symptoms |
| Robinson, 2011 [44] | Prospective cohort | US | 69.11 (9.42) | 166/6j | 74 | 1 | 50 | 23 | - | - | Incident | Following elective operation with planned postoperative ICU admission |
CAM-ICU, validated medical record review | RASS |
| Rood, 2019 [103] | Retrospective cohort | NL | 64.64 (13.90) | 994/606 | 1,600 | 111 | 433 | 571 | - | - | Prevalent | Admission to ICU (during stay) | CAM-ICU, RASS | RASS |
| Santana Santos, 2005 [104] | Prospective cohort | SE | 82.90 (6.30) | 6/13 | 19 | 9 | 5 | 5 | - | - | Incident | Following operation for hip fracture |
CAM, DSM-IV | Classified according to Lipowski |
| Slor, 2013 [105] | Prospective cohort | NL | 85.75 (5.09) | 7/23 | 30 | 7 | 5 | 6 | 1 | - | Incident | Following surgery for hip fracture | CAM | DRS-R98 |
| Trzepacz, 2018g [106] | Cross-sectional | BR, CO, IE, JP, KR, TW, US | 68.00 (14.96) | 258/148 | 406 | 172 | 64 | 146 | 24 | - | Prevalent | During hospitalisation in general or rehabilitation hospital settings or referral to psychiatric services |
DSM-IV | DRS-R98 |
| van den Boogaard, 2012 [107] | Prospective cohort | NL | 64.00 (15.00) | 235/176 | 411 | 44 | 148 | 219 | - | - | Incident | During ICU stay | CAM-ICU, DOS | NR |
| van der Kooi, 2013 [108] | Retrospective cohort | NL | 66.71 (13.97) | 15/9 | 24 | 0 | 6 | 18 | - | - | Prevalent | During ICU stay | CAM-ICU, review of medical records | RASS |
| van Keulen, 2018 [109] | Prospective cohort | NL | 62.76 (14.24) | 257/153 | 410 | 0 | 124 | 286 | - | - | Prevalent | During ICU stay | CAM-ICU | RASS |
| van Velthuijsen, 2018 [110] | Retrospective cohort | NL | 81.00 (7.00) | 234/167 | 401 | - | 94 | - | - | 307 (hyper/mix) | Prevalent | During hospital admission | File review | Clinical judgement/DOS |
| Yang, 2019 [111] | Prospective cohort | KR | 77.58 (7.26) | 55/40 | 95 | 34 | 25 | 30 | 6 | - | Prevalent | Patients with cerebral infarction admitted to stroke unit (during stay) |
CAM | K-DMSS |
| Zipser, 2020 [112] | Prospective cohort | CH | 71.56 (13.88) | 379/223 | 602 | 72 | 229 | 301 | - | - | Prevalent | During hospital stay | DOS, S-CAM | DOS |
Note:-= Not applicable;
aReferences 68 and beyond are provided in Supplementary Materials.
b,c,d,e,f,g,Overlapping samples;
h2-digit ISO country code.
iSex reported for entire sample, not delirium only;
AMTS: Abbreviated Mental Test Score; bCAM: Abbreviated Confusion Assessment Method; CAC-A: Vermeersch Clinical Assessment of Confusion Form A; CAM: Confusion Assessment Method; CAM-ICU: Confusion Assessment Method for the Intensive Care Unit; CAM-S: Spanish Confusion Assessment Method; DAS: Delirium Assessment Scale; DMC: Delirium Motoric Checklist; DMSS: Delirium Motor Subtype Scale; DMSS-4: Abbreviated version of the Delirium Motor Subtype Scale; DOS: Delirium Observation Screening scale; DRS: Delirium Rating Scale; DRS-R98: Delirium Rating Scale – Revised-98; DSI: Delirium Symptom Interview; DSM-5: The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; DSM-III-R: The Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised; DSM-IV: The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; DSM-IV-TR: The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision; ICD-10: International Classification of Diseases, Tenth Revision; ICD-9-CM: International Classification of Diseases, Ninth Revision, Clinical Modification; ICU: intensive care unit; K-DMSS: Delirium Motor Subtype Scale, Korean Version; MDAS: Memorial Delirium Assessment Scale; MMSE: Mini-Mental State Examination; NEECHAM: The Neelon and Champagne Confusion Scale; NR: not reported; OBS: Organic Brain Syndrome Scale; ODFS: One Day Fluctuation Scale; RASS: Richmond Agitation Sedation Scale; Riker SAS: Riker Sedation-Agitation Scale; S-CAM: Short Confusion Assessment Method; TIA: transient ischemic attack.
The included studies comprised 14,407 cases of delirium (55% male, mean age 73.63 years). Most studies (50/61) reported prevalence of delirium across various settings (mostly acute) and the remaining reported incidence (mostly post-surgery). Subtype proportions differed greatly across studies, and not all reported every subtype. On average when measured across studies, hyperactive delirium was seen in 33% of cases, hypoactive in 34%, mixed in 31% and no motor subtype in 12%. Subtype categorisation methods varied, but most involved clinical observation of symptoms (13/61), a Richmond Agitation Sedation Scale cut-off (11/61) or the Delirium Motor Subtype Scale (11/61) (Table 2).
A robust analysis of more factors than anticipated was conducted, so we did not qualitatively synthesise remaining factors or pool descriptive data for categorical factors. Supplementary data item Supplementary Table S3 lists factors with insufficient data for meta-analysis (e.g. biomarkers, smoker status, depression, frailty).
The following sections summarise significant results from 122 individual analyses. The analyses each study contributed to are shown in Supplementary Table S4 in the supplementary data. Detailed model results are shown in Supplementary Table S5 in the supplementary data, and all factor/subtype comparisons are shown in Figures 1 and 2.
Figure 1.
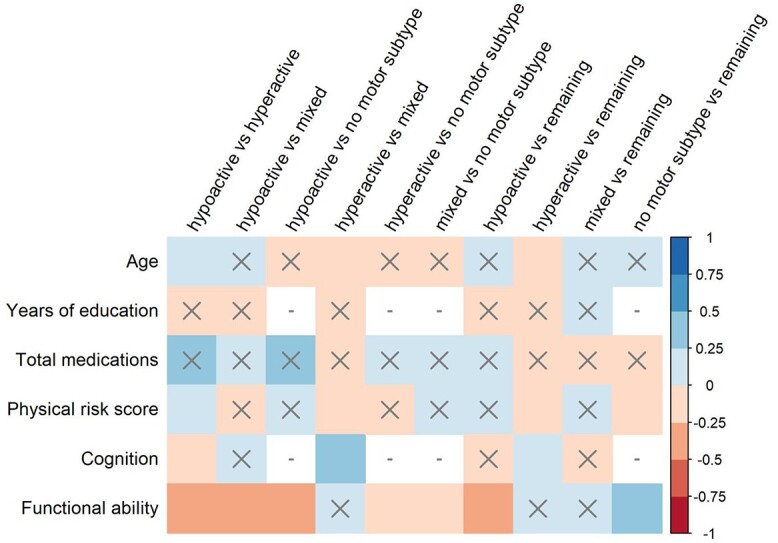
Effect sizes (Hedges’ g) for random-effects meta-analyses conducted on differences between motor subtypes of delirium on continuous predisposing factors. Positive Hedges’ g indicates higher scores on factor in Group A compared with Group B. X = non-significant result (P > 0.05), − = analysis unable to be conducted (insufficient data).
Figure 2.
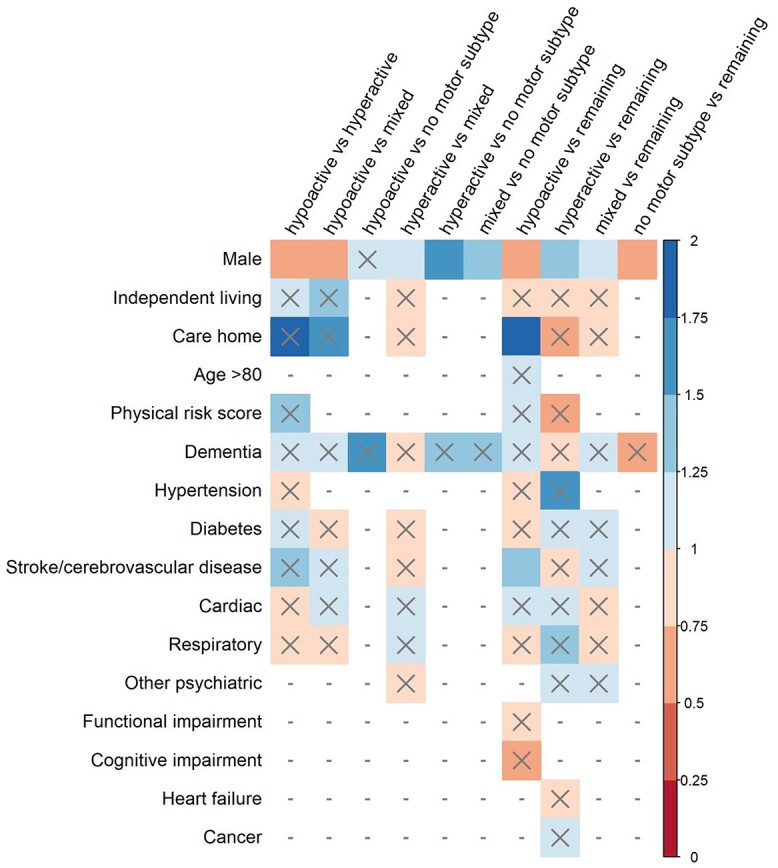
Effect sizes (odds ratio) for random-effects meta-analyses conducted on differences between motor subtypes of delirium on categorical predisposing factors. OR > 1 indicates greater likelihood of the factor being present in Group A compared with Group B. X = non-significant result (P > 0.05), − = analysis unable to be conducted (insufficient data).
Demographic factors
At least one subtype comparison was assessed for eight demographic factors (Figures 1 and 2). The hypoactive group was significantly older than hyperactive, and hyperactive was significantly younger than the mixed group and all non-hyperactive subtypes pooled. The hypoactive group was taking significantly more daily medications than all non-hypoactive subtypes pooled and was significantly more likely to live in a care home compared with all non-hypoactive subtypes pooled. There were significant sex differences in all comparisons except hypoactive versus no motor subtype. Hypoactive and no motor subtype groups were less likely to be men, and hyperactive and mixed groups were more likely. Null to moderate heterogeneity was seen across these analyses (I2 median [range] = 19.19% [0–72.14%]).
Comorbidities and risk scores
Thirteen comorbidities and one risk score (combined physical score, see Supplementary Table S2 in the supplementary data) were investigated for at least one subtype comparison (Figures 1 and 2). Significantly lower cognitive scores were seen for hypoactive compared with hyperactive, and higher cognitive scores for hyperactive compared with mixed and all non-hyperactive groups pooled. Significantly worse functional performance was seen for hypoactive compared with mixed and all non-hypoactive subtypes pooled. Better functional performance was also seen for no motor subtype compared with hyperactive, mixed, and all non-no motor subtype groups pooled. The hypoactive group was also significantly more likely to have history of stroke/cerebrovascular disease than all remaining subtypes. The hypoactive group had significantly higher physical risk scores than hyperactive, and hyperactive had lower scores than mixed and all non-hyperactive groups pooled. Null to high heterogeneity was seen across these analyses (I2 median [range] = 0% [0–81.23%]).
Subgroup analyses
Differing from the main analyses, in the older group of the age stratification analysis the hypoactive group were significantly more likely to reside in a care home and less likely to live independently than all non-hypoactive pooled together. Dementia was also less likely for the no motor subtype compared with all remaining subtypes. Differences were no longer found between subtypes on functional performance in the group with all ages. The hypoactive group was also more likely to have cardiac comorbidities compared with mixed group, and the mixed group had significantly more years of education than all other subtypes pooled. No other notable differences were seen in the age stratification analyses (Supplementary Tables S6 and S7 in the supplementary data).
No differences between the main analyses (mainly prevalent delirium studies) and the prevalent subgroup analyses were found. The incident delirium analyses comprised fewer studies, and the only significant differences were seen for higher physical risk scores for the mixed subtype compared with hypoactive and all remaining subtypes pooled (small effect sizes). Importantly, while non-significant, most analyses demonstrated similar effect sizes to the prevalence analyses. There was one case where the effect size was in the opposite direction: the hyperactive group was more likely to be women than the mixed group (not statistically significant). In some cases, smaller, non-significant effect sizes were seen. This was true for the sex comparison between hyperactive and remaining subtypes, as well as the cognition comparisons for hypoactive versus hyperactive and hyperactive versus remaining subtypes (Supplementary Tables S8 and S9 in the supplementary data).
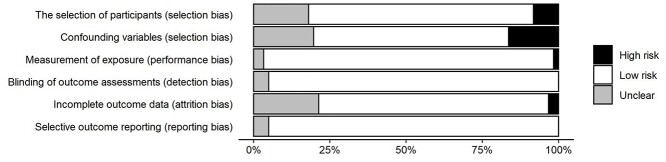
Risk of bias
Overall risk of bias across assessed domains (RoBANS [24]) was low (see Figure 3 for summary). Some studies did not clearly report the study location or time period or failed to adjust for major confounding variables (age, sex) through their design or analysis. As such, some selection bias was shown across studies. Attrition bias was deemed unclear for studies where reasons for participant drop-out or exclusion were not clearly reported and, as such, potential risk of bias was unable to be ascertained. Risk of bias assessments for individual studies are presented in Supplementary Table S10 in the supplementary data.
Figure 3.
Percentage of studies with high risk (black), low risk (white) or unclear (grey) risk of bias ratings for each Risk of Bias for Non-randomised Studies (RoBANS) item assessed by authors.
Reporting biases
Potential small study effect was found in two analyses. Trim and fill estimation led to an increase in the size of the effect for the difference in the likelihood of being male between the hyperactive group and both the mixed and all non-hyperactive subtypes (Supplementary Table S11 in the supplementary data).
Certainty of evidence
Using the GRADE approach, the overall certainty in the body of evidence presented here was deemed to be moderate: we are moderately confident in reported effect estimates. The true effect is likely to be close to reported estimates but may be considerably different. We identified some imprecision, with large confidence intervals and smaller sample sizes in some analyses.
Discussion
Our quantitative synthesis of the literature identified important differences in predisposing factors between delirium motor subtypes. Those who developed hypoactive delirium were more likely to be older, have poorer cognition and higher physical risk scores than those with hyperactive delirium (small effect sizes), and were more likely to be women, living in care homes, take more daily medications, have worse functional performance and history of cerebrovascular disease compared with all remaining subtypes (all with negligible to small effect sizes). Those who developed hyperactive delirium were more likely to be men and younger, with better cognition and lower physical risk scores than all other subtypes (all with negligible to small effect sizes). Individuals with mixed delirium were more likely to be men than remaining subtypes (negligible effect size). Those who developed no motor subtype were more likely to be women and have better functional performance than all remaining subtypes (albeit predominantly with small effect sizes). Taken together, we describe how different delirium motor subtypes arise from heterogeneity of baseline factors.
Identification of these differences extends recent discussions about delirium as a unitary condition, and whether further classification, as has been done for other brain disorders (e.g. stroke, dementia), would be useful for delirium research [2]. We now extend the argument for motor subtype classification, bolstering previous research demonstrating differences in their aetiology, treatment and outcomes [10, 11], now showing key individual differences (small effect sizes) between motor subtypes even prior to delirium occurring.
Key differences in predisposing factors between delirium subtypes
Although we could not directly investigate standardised frailty measures, indications of frailty (lack of independent living, increased medication use, cognitive and functional impairment) were more common in those who develop hypoactive delirium. We provide quantitative evidence for a commonly held clinical assumption that more frail, older individuals develop hypoactive delirium [10]. Results demonstrating better functional performance prior to delirium occurrence in those with no motor subtype compared with all other subtypes support literature, indicating that those with no motor subtype have lower risk or less severe delirium [11].
The most consistent difference across subtypes were sex differences: men were more likely to have hyperactive and mixed delirium, while women more likely to have hypoactive and no motor subtype. It should be noted sex differences were most consistent in the all-age stratified analysis (less in the older age analysis). Sex differences are also apparent in the behavioural symptoms of late-life dementia, with men displaying more aggressive symptoms and women more mood symptoms [35]. Given that dementia is a significant risk factor for incident delirium [2, 36], and delirium a risk factor for incident dementia [37], it is perhaps unsurprising to find sex differences here. The underlying biology likely relates to hormonal and metabolic differences [38], as well as neuroplastic changes across the lifespan related to stress, along with social, educational and occupational opportunities [39, 40].
It is likely that factors associated with age (residual confounding), rather than age specifically, are driving the development of delirium subtypes. While patients with hypoactive delirium were found to be older than those with hyperactive delirium, age differences for most subtype comparisons were not statistically significant. Clinically, this calls for comprehensive geriatric assessments for delirium patients, for causes to be investigated beyond age [41, 42]. Differences between subtypes in terms of functional status, physical risk scores and presence of dementia were more pronounced (and statistically significant) in the older age group. We could not test whether these functional factors were driving associations between age and delirium.
Future directions
Several central risk factors for delirium were lacking sufficient data for meta-analysis. These include direct frailty measures, depression, alcohol use and poor nutritional status [2]. Future research should further investigate differences in these factors between subtypes. Individual studies also demonstrated differences between motor subtypes on factors which had insufficient data for quantitative investigation here. These factors include biomarkers (e.g. haematocrit levels) [43], depression [44], frailty [45], physical function [46] and substance abuse [47].
Classification methods for subtypes differed across studies, with clinical observation most common (22% of studies). To ensure consistency in research on motor subtypes of delirium, future research must employ standardised tools for classification (e.g. Delirium Motor Subtype Scale [9]). Considering its relatively high rate across included studies (12%), delirium with no motor subtype has received little attention in the broader literature. With important differences in vulnerability for no motor subtype compared with other motor subtypes shown here, future research should consider those who do not experience a motor subtype as an important group in delirium research.
Differences in the vulnerability profile between motor subtypes have significance for clinical practice. Up to 30–40% of delirium is preventable, and effective risk prediction is essential to target preventative interventions towards at-risk patients [1, 12]. However, existing risk prediction tools for delirium are limited and lack sensitivity [13], possibly due to heterogeneity in predisposing factors for delirium in general, as we have demonstrated here. The addition of risk scores for motor subtypes within risk prediction modelling has potential to improve the sensitivity of these tools. Stratifying risk according to motor subtype would allow for tailored patient and family education on delirium risk prior to elective procedures, as well as increasing awareness of the subtypes and improving detection, especially for hypoactive delirium [48, 49]. Future research would need to directly assess the utility of risk stratification by subtype in delirium prediction tools. The incident subgroup analyses provide an idea of prediction to subtype. Despite reduced power and non-significant effects, effect sizes and direction were relatively stable, providing evidence that identified factors appear to differentiate between subtypes prospectively. Further prospective research is needed to examine the specific predictive ability of risk factors for the individual delirium subtypes. Regarding delirium prevention, our results indicate that current preventative interventions [12, 50], which focus on cognitive activation and mobilisation, mostly target those at risk for hypoactive delirium. This focus is warranted considering the poorer outcomes for hypoactive delirium, including higher mortality [11, 14, 15, 51].
Leading neurobiological theories state that delirium vulnerability is characterised by functional brain disintegration [19, 20]. It is thought that vulnerability for delirium is determined by two important factors, and their interaction: baseline brain network connectivity and level of inhibitory tone [19]. Non-modifiable factors such as cognitive impairment, age, dementia and depression are thought to impact baseline level of brain connectivity. We show that hypoactive cases are older (than hyperactive) with lower cognition, which potentially indicates greater network connectivity breakdown than other subtypes. In contrast, level of inhibitory tone is thought to be influenced by modifiable factors such as infection, inflammation and medication (e.g. benzodiazepines) [20]. There is some indication of hyperactive delirium being more common in medication or GABA-withdrawal states (e.g. benzodiazepine, alcohol) [10, 52, 53]. These differences in the predisposing risk factors (modifiable and non-modifiable) between subtypes may lead to differing degrees of brain network connectivity and level of inhibitory tone. Future empirical and theoretical work needs to address neurobiological differences in delirium subtypes, including the roles of predisposing and precipitating factors.
Limitations and conclusion
Only English-language studies were included. Due to minimal reporting of multivariate comparisons between subtypes, these were not used, and effects were unadjusted for important covariates (e.g. age). However, we did investigate this with a stratified age cut-off analysis. Patient populations and delirium assessment methods were heterogeneous across studies. This was not reflected statistically, with low heterogeneity found across most analyses. Additionally, pooling of different delirium groups strengthens our findings with large samples and effects shown across contexts.
Limitations also remain in subtype diagnostics, and current delirium motor subtypes may require refinement. The fact that 12% of cases across included studies could not be subtyped is perhaps cause for concern and provides an impetus for subtype clinical categorisation refinement. It is important to note that while delirium categorisations may be valuable for research, the unitary umbrella definition may be more suited to some training and education contexts. Delirium is still widely underdiagnosed [2, 54, 55], with fewer than half of cases detected in hospital settings, and effective means of raising awareness are vital. Further, research has shown there is a bias for the overrepresentation of hyperactive delirium diagnoses, especially in chart documentation [49]. The inclusion of retrospective study designs (21% of included studies), which often rely on chart review to identify delirium, may have led to an underrepresentation of hypoactive delirium in our included sample.
Through a robust quantitative synthesis of available literature, we found significant differences between delirium motor subtypes, albeit with small effect sizes, in their characteristics prior to delirium occurrence. Importantly, indices of frailty (institutional living, increased medication use, cognitive and functional impairment) are seen more in hypoactive patients, who are also more likely to be women. In conjunction with research demonstrating differences by subtype for pathophysiology, treatment experience and outcomes [10], these results highlight the importance of considering motor subtypes in all delirium research. There is great potential to improve delirium theory, prediction and clinical management by considering subtypes.
Supplementary Material
Contributor Information
Erica S Ghezzi, Cognitive Ageing and Impairment Neurosciences Laboratory, Justice and Society, University of South Australia, Adelaide, Australia.
Danielle Greaves, Cognitive Ageing and Impairment Neurosciences Laboratory, Justice and Society, University of South Australia, Adelaide, Australia.
Monique S Boord, Cognitive Ageing and Impairment Neurosciences Laboratory, Justice and Society, University of South Australia, Adelaide, Australia.
Daniel Davis, MRC Unit for Lifelong Health and Ageing Unit at UCL, London, UK.
Sara Knayfati, Cognitive Ageing and Impairment Neurosciences Laboratory, Justice and Society, University of South Australia, Adelaide, Australia.
Jack M Astley, Cognitive Ageing and Impairment Neurosciences Laboratory, Justice and Society, University of South Australia, Adelaide, Australia.
Rhianna L S Sharman, Cognitive Ageing and Impairment Neurosciences Laboratory, Justice and Society, University of South Australia, Adelaide, Australia.
Stephanie I Goodwin, Cognitive Ageing and Impairment Neurosciences Laboratory, Justice and Society, University of South Australia, Adelaide, Australia.
Hannah A D Keage, Cognitive Ageing and Impairment Neurosciences Laboratory, Justice and Society, University of South Australia, Adelaide, Australia.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
H.A.D.K. is supported by a NHMRC Dementia Research Leadership Fellowship (GNT1135676). D.D. is supported by a Wellcome Trust Intermediate Clinical Fellowship (WT107467). M.S.B. is supported by the Australian Government Research Training Program Scholarship. We acknowledge the Brain Foundation for their Project Grant funding.
References
- 1. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. The Lancet 2014; 383: 911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilson JE, Mart MF, Cunningham Cet al. . Delirium. Nat Rev Dis Primers 2020; 6: 90. 10.1038/s41572-020-00223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Inouye SK, Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. Ann Intern Med 1990; 113: 941–8. [DOI] [PubMed] [Google Scholar]
- 4. Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg 2009; 249: 173–8. [DOI] [PubMed] [Google Scholar]
- 5. Crocker E, Beggs T, Hassan Aet al. . Long-term effects of postoperative delirium in patients undergoing cardiac operation: a systematic review. Ann Thorac Surg 2016; 102: 1391–9. [DOI] [PubMed] [Google Scholar]
- 6. Witlox J, Eurelings LSM, Jonghe JFM, Kalisvaart KJ, Eikelenboom P, Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 2010; 304: 443–51. [DOI] [PubMed] [Google Scholar]
- 7. Davis DHJ, Muniz Terrera G, Keage Het al. . Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain 2012; 135: 2809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lipowski ZJ. Delirium: Acute Confusional States. New York, NY: Oxford University Press, 1990. [Google Scholar]
- 9. Meagher D, Moran M, Raju Bet al. . A new data-based motor subtype schema for delirium. J Neuropsychiatry Clin Neurosci 2008; 20: 185–93. [DOI] [PubMed] [Google Scholar]
- 10. Meagher D. Motor subtypes of delirium: past, present and future. Int Rev Psychiatry 2009; 21: 59–73. [DOI] [PubMed] [Google Scholar]
- 11. Meagher DJ, Leonard M, Donnelly S, Conroy M, Adamis D, Trzepacz PT. A longitudinal study of motor subtypes in delirium: relationship with other phenomenology, etiology, medication exposure and prognosis. J Psychosom Res 2011; 71: 395–403. [DOI] [PubMed] [Google Scholar]
- 12. Siddiqi N, Harrison JK, Clegg Aet al. . Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev 2016; 3: CD005563. 10.1002/14651858.CD005563.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lindroth H, Bratzke L, Purvis Set al. . Systematic review of prediction models for delirium in the older adult inpatient. BMJ Open 2018; 8: e019223. 10.1136/bmjopen-2017-019223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krewulak KD, Stelfox HT, Ely EW, Fiest KM. Risk factors and outcomes among delirium subtypes in adult ICUs: a systematic review. J Crit Care 2020; 56: 257–64. [DOI] [PubMed] [Google Scholar]
- 15. Gual N, Inzitari M, Carrizo Get al. . Delirium subtypes and associated characteristics in older patients with exacerbation of chronic conditions. Am J Geriatr Psychiatry 2018; 26: 1204–12. [DOI] [PubMed] [Google Scholar]
- 16. Morandi A, Zambon A, Di Santo SGet al. . Understanding factors associated with psychomotor subtypes of delirium in older inpatients with dementia. J Am Med Dir Assoc 2020; 21: 486–92.e7. [DOI] [PubMed] [Google Scholar]
- 17. Ghezzi ES, Ross TJ, Sharman Ret al. . The neuropsychological profile of delirium vulnerability: a systematic review and meta-analysis. Neurosci Biobehav Rev 2022; 132: 248–59. [DOI] [PubMed] [Google Scholar]
- 18. Boord MS, Moezzi B, Davis Det al. . Investigating how electroencephalogram measures associate with delirium: a systematic review. Clin Neurophysiol 2021; 132: 246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanders RD. Hypothesis for the pathophysiology of delirium: role of baseline brain network connectivity and changes in inhibitory tone. Med Hypotheses 2011; 77: 140–3. [DOI] [PubMed] [Google Scholar]
- 20. Maldonado JR. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry 2018; 33: 1428–57. [DOI] [PubMed] [Google Scholar]
- 21. Hodgson K, Hutchinson AD, Denson L. Nonpharmacological treatments for ADHD: a meta-analytic review. J Atten Disord 2012; 18: 275–82. [DOI] [PubMed] [Google Scholar]
- 22. Page MJ, McKenzie JE, Bossuyt PMet al. . The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Slooter AJC, Otte WM, Devlin JWet al. . Updated nomenclature of delirium and acute encephalopathy: statement of ten Societies. Intensive Care Med 2020; 46: 1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim SY, Park JE, Lee YJet al. . Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 2013; 66: 408–14. [DOI] [PubMed] [Google Scholar]
- 25. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36: 1–48. 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 26. McGrath S, Zhao X, Steele R, Benedetti A. Estimating the Sample Mean and Standard Deviation from Commonly Reported Quantiles in Meta-Analysis. 2020; Available from:; 29: 2520–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paule RC, Mandel J. Consensus values and weighting factors. J Res Natl Bur Stand 1982; 87: 377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med 2003; 22: 2693–710. [DOI] [PubMed] [Google Scholar]
- 29. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sterne JAC, Sutton AJ, Ioannidis JPAet al. . Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343: d4002. 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 31. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56: 455–63. [DOI] [PubMed] [Google Scholar]
- 33. Guyatt GH, Oxman AD, Vist GEet al. . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Simons KS, Boogaard M, Hendriksen Eet al. . Temporal biomarker profiles and their association with ICU acquired delirium: a cohort study. Crit Care 2018; 22: 137. 10.1186/s13054-018-2054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lövheim H, Sandman P-O, Karlsson S, Gustafson Y. Sex differences in the prevalence of behavioral and psychological symptoms of dementia. Int Psychogeriatr 2009; 21: 469–75. [DOI] [PubMed] [Google Scholar]
- 36. Gross AL, Jones RN, Habtemariam DAet al. . Delirium and long-term cognitive trajectory among persons with dementia. Arch Intern Med 2012; 172: 1324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Richardson SJ, Davis DHJ, Stephan BCMet al. . Recurrent delirium over 12 months predicts dementia: results of the Delirium and Cognitive Impact in Dementia (DECIDE) study. Age Ageing 2021; 50: 914–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cavedo E, Chiesa PA, Houot Met al. . Sex differences in functional and molecular neuroimaging biomarkers of Alzheimer's disease in cognitively normal older adults with subjective memory complaints. Alzheimers Dement 2018; 14: 1204–15. [DOI] [PubMed] [Google Scholar]
- 39. Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol 2012; 11: 1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002; 8: 448–60. [PubMed] [Google Scholar]
- 41. Australian Commission on Safety and Quality in Health Care. Delirium Clinical Care Standard 2021; 2021. [Google Scholar]
- 42. Scottish Intercollegiate Guidelines Network . Risk reduction and management of delirium: a national clinical guideline, 2019. [DOI] [PubMed]
- 43. Robinson TN, Raeburn CD, Tran ZV, Brenner LA, Moss M. Motor subtypes of postoperative delirium in older adults. Arch Surg 2011; 146: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Avelino-Silva TJ, Campora F, Curiati JAE, Jacob-Filho W. Prognostic effects of delirium motor subtypes in hospitalized older adults: a prospective cohort study. PLoS One 2018; 13: e0191092. 10.1371/journal.pone.0191092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hayhurst CJ, Marra A, Han JHet al. . Association of hypoactive and hyperactive delirium with cognitive function after critical illness. Crit Care Med 2020; 48: e480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Evensen S, Bourke AK, Lydersen Set al. . Motor activity across delirium motor subtypes in geriatric patients assessed using body-worn sensors: a Norwegian cross-sectional study. BMJ Open 2019a; 9: e026401. 10.1136/bmjopen-2018-026401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heymann A, Sander M, Krahne Det al. . Hyperactive delirium and blood glucose control in critically ill patients. J Int Med Res 2007; 35: 666–77. [DOI] [PubMed] [Google Scholar]
- 48. Rice KL, Bennett M, Gomez M, Theall KP, Knight M, Foreman MD. Nurses' recognition of delirium in the hospitalized older adult. Clin Nurse Spec 2011; 25: 299–311. [DOI] [PubMed] [Google Scholar]
- 49. Albrecht JS, Marcantonio ER, Roffey DMet al. . Stability of postoperative delirium psychomotor subtypes in individuals with hip fracture. J Am Geriatr Soc 2015; 63: 970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hshieh TT, Yue J, Oh Eet al. . Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med 2015; 175: 512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jackson TA, Wilson D, Richardson S, Lord JM. Predicting outcome in older hospital patients with delirium: a systematic literature review. Int J Geriatr Psychiatry 2016; 31: 392–9. [DOI] [PubMed] [Google Scholar]
- 52. Morita T, Tei Y, Tsunoda J, Inoue S, Chihara S. Underlying pathologies and their associations with clinical features in terminal delirium of cancer patients. J Pain Symptom Manage 2001; 22: 997–1006. [DOI] [PubMed] [Google Scholar]
- 53. Steiner LA. Postoperative delirium. Part 1: Pathophysiology and risk factors. Eur J Anaesthesiol 2011; 28: 628–36. [DOI] [PubMed] [Google Scholar]
- 54. Meagher D, Leonard M. The active management of delirium: improving detection and treatment. Adv Psychiatr Treat 2008; 14: 292–301. [Google Scholar]
- 55. Geriatric Medicine Research Collaborative . Delirium is prevalent in older hospital inpatients and associated with adverse outcomes: results of a prospective multi-centre study on World Delirium Awareness Day. BMC Med 2019; 17: 229. 10.1186/s12916-019-1458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.