Abstract
Background
our aim was to assess the effectiveness of medication review and deprescribing interventions as a single intervention in falls prevention.
Methods
Design
systematic review and meta-analysis.
Data sources
Medline, Embase, Cochrane CENTRAL, PsycINFO until 28 March 2022.
Eligibility criteria
randomised controlled trials of older participants comparing any medication review or deprescribing intervention with usual care and reporting falls as an outcome.
Study records
title/abstract and full-text screening by two reviewers.
Risk of bias
Cochrane Collaboration revised tool.
Data synthesis
results reported separately for different settings and sufficiently comparable studies meta-analysed.
Results
forty-nine heterogeneous studies were included.
Community
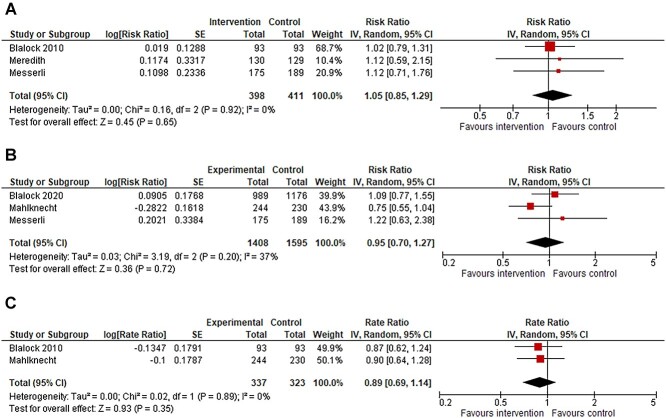
meta-analyses of medication reviews resulted in a risk ratio (RR) of 1.05 (95% confidence interval, 0.85–1.29, I2 = 0%, 3 studies(s)) for number of fallers, in an RR = 0.95 (0.70–1.27, I2 = 37%, 3 s) for number of injurious fallers and in a rate ratio (RaR) of 0.89 (0.69–1.14, I2 = 0%, 2 s) for injurious falls.
Hospital
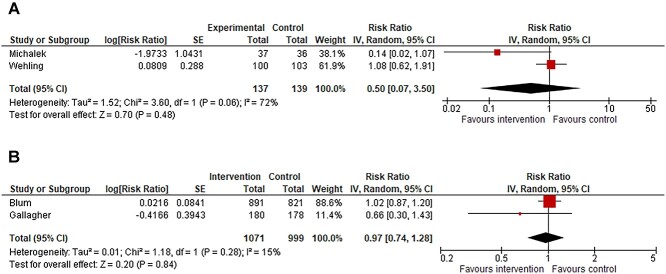
meta-analyses assessing medication reviews resulted in an RR = 0.97 (0.74–1.28, I2 = 15%, 2 s) and in an RR = 0.50 (0.07–3.50, I2 = 72% %, 2 s) for number of fallers after and during admission, respectively.
Long-term care
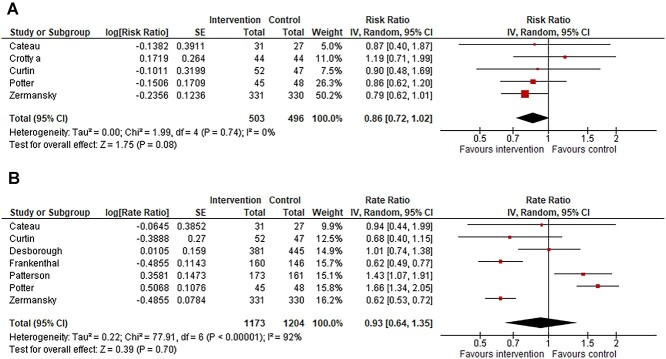
meta-analyses investigating medication reviews or deprescribing plans resulted in an RR = 0.86 (0.72–1.02, I2 = 0%, 5 s) for number of fallers and in an RaR = 0.93 (0.64–1.35, I2 = 92%, 7 s) for number of falls.
Conclusions
the heterogeneity of the interventions precluded us to estimate the exact effect of medication review and deprescribing as a single intervention. For future studies, more comparability is warranted. These interventions should not be implemented as a stand-alone strategy in falls prevention but included in multimodal strategies due to the multifactorial nature of falls.
PROSPERO registration number: CRD42020218231
Keywords: accidental falls, medication review, deprescribing, fall-risk-increasing drugs, older people, systematic review
Key Points
A medication review with the aim of deprescribing is an important component of a multifactorial falls prevention strategy.
However, there is uncertainty related to the effectiveness of these interventions as a single intervention for falls prevention.
In meta-analyses, no significant associations between medication reviews and fall outcomes were found in any of the settings.
However, there was a trend for a lower number of fallers in the meta-analysis assessing medication reviews in long-term care.
In a frail subgroup of older persons, medication review might be effective even as a single intervention.
Introduction
Falls incidents are a common and growing threat for both immediate- and long-term health and functional independence of older adults [1]. Approximately a third of community dwellers aged 65 years and older will sustain at least one fall each year, and in long-term care, fall injuries are even more common as more than half of the residents will fall at least annually [2–4]. Furthermore, falls are the most frequently reported safety incident in adult inpatients [5]. In general, falls are multifactorial and often result from several interacting risks [3]. Due to the burden related to fall injuries from both an individual and a societal perspective, identification of effective falls prevention approaches, such as appropriate multifactorial interventions, is of utmost importance.
An important component of a multifactorial falls prevention strategy is a medication review with the aim of judicious deprescribing of certain medications [6]. The rationale behind this intervention is that the potential adverse effects of fall-risk-increasing drugs (FRIDs) that may contribute to falls such as orthostatic hypotension and sedation are reversible after deprescribing [7–9]. However, there is uncertainty related to the effectiveness of medication review and deprescribing interventions as a single intervention for falls prevention.
To date, few systematic reviews and meta-analyses have focused on the topic [10–13]. However, comparing these systematic reviews has proved challenging due to the variation of included trials in the reviews. The most recent systematic review found no effect of FRIDs deprescribing on any falls outcomes [13]. However, the authors excluded studies assessing medication review and management with a broader focus on reducing polypharmacy and potentially inappropriate prescribing. Likewise, Cameron et al. [11] stated that a general medication review may make little or no difference to the rate of falls or risk of falling in long-term care facilities. However, they only identified one medication review intervention study that was performed in a hospital [11].
Therefore, there is a need for a broad and detailed update of the available falls prevention literature, focusing on all settings in which older people receive healthcare and all medication review and deprescribing interventions, as several important medication review and deprescribing trials have been published in recent years. The aim of this systematic review and meta-analysis was to assess the effectiveness of any medication review or deprescribing intervention as a single intervention in falls prevention performed among older people in any care setting.
Methods
This systematic review was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14] and conducted following the guidance of Cochrane Handbook [15]. A protocol was registered to PROSPERO (registration number: CRD42020218231) and was recently published [16].
Eligibility criteria
Type of studies
Randomised controlled trials (RCTs), including quasi-randomised trials, cluster-randomised trials and trials in which treatment allocations were inadequately concealed were considered for inclusion. Language restriction was not applied.
Types of participants
Studies conducted in any setting were considered for inclusion if they included participants aged ≥60 years or if the majority of participants were aged >65 years or the mean age was >65 years.
Type of interventions
The intervention could be any type of deprescribing or medication review intervention. The interventions could be, for example, medication reviews led by pharmacist or physician, education programmes for prescribers or clinical decision support systems (CDSS). The intervention could target specific drug classes (e.g. psychotropics) or general medication regimens (i.e. comprehensive medication review). The intervention might have targeted multiple medication issues in case of comprehensive medication review in addition to deprescribing such as non-adherence and starting medications. If the intervention was part of a multi-modal intervention (e.g. intervention including also physical exercise), the study was excluded.
Type of control
The comparison intervention was usual care (i.e. no deprescribing/medication review conducted or no change in usual activities of care).
Type of outcomes
Studies that reported raw data or statistics related to any type of falls outcomes (e.g. number of falls, number of fallers or frequent fallers and time to first fall) were considered for inclusion. Our secondary outcome was injurious falls (e.g. fall-related fractures).
Information sources
A search was performed in the Cochrane Central Register of Controlled Trials (CENTRAL), Medline, Embase and PsycINFO to search for literature published from inception until 28 March 2022. A customised search strategy was built by a librarian for each database. When a relevant conference abstract or protocol was identified, we emailed the authors to obtain the full text article. Reference lists of included studies, reviews (e.g. Cochrane reviews) and falls prevention guidelines were assessed to identify additional studies. Finally, additional studies were aimed to identify through trial registers (clinicaltrials.gov, EU clinical trials register, International Clinical Trials Registry Platform).
Search strategy
The search included the following search concepts: (i) ‘deprescribing’ AND ‘falls/healthcare assessment’ AND ‘geriatric’ OR (ii) ‘specific prescription tools’ [17]. Eventually, these were combined with ‘RCTs’ as follows: (([deprescribing] AND [falls/healthcare assessment] AND [geriatric]) OR [specific prescription tools]) AND [RCT’s]. The full search strategy is provided in Supplementary Text 1.
Data records and management
First, title and abstract screening was performed independently by two reviewers using Rayyan [18]. Then, a full-text screening was done by two independent reviewers. In both phases, a third reviewer was consulted in case of disagreement.
Finally, data from the articles were extracted independently by two authors using a structured data collection form. In case of disagreement, the disagreements were discussed or a third reviewer was consulted. The following data items were collected: study design, country, setting, inclusion criteria, total number of participants and age of the participants (preferably mean and standard deviation), intervention type, control type, all fall-related outcomes and how data on these outcomes was collected, adjustment of outcomes if applicable and follow-up duration.
Risk of bias
The Cochrane Collaboration revised tool of Risk of Bias was applied by two reviewers independently to assess the risk of bias [19]. In case of disagreement, the disagreements were discussed or a third reviewer was consulted.
Data synthesis
We present the results separately for every setting: community, hospital or long-term care facilities.
When a group of studies with a sufficiently comparable intervention and outcome and performed in a same setting was identified, a meta-analysis using random-effects model was conducted applying the intention-to-treat principle. We reported the treatment effects between the intervention and control group as a Rate Ratio (RaR) or as Risk Ratio (RR) together with the 95% confidence intervals (95% CI). We used the unadjusted effect sizes, unless the adjustment was performed due to clustering. We adjusted for clustering, if not already done in the published report using average cluster size and following intra-cluster coefficient estimates: 0.01 in case of community setting, 0.01 in case of hospital wards, 0.07 in case of long-term care facilities and number of fallers, and 0.1 in case of long-term care facilities and fall rates [20–22]. We tried to minimise the expected heterogeneity by pooling the studies from a similar setting and with a comparable intervention. The remaining heterogeneity was assessed within a pooled group of studies with help of visual inspection of the forest plots and Chi2 test (with statistical significance set at P < 0.10), and the I2 statistics [15]. We explored the heterogeneity by conducting pre-specified subgroup analysis described in our protocol [16]. Furthermore, we conducted pre-specified sensitivity analyses based on overall study quality and by comparing random and fixed effect models. Funnel plots and Eggers tests were not conducted as none of the analyses included >10 studies. For all statistical tests, the software Review Manager Version 5.4.1 was used.
Confidence in cumulative evidence
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) was applied by two reviewers independently to assess the confidence of the effect estimates of the meta-analyses [23]. In case of disagreement, the disagreements were discussed or a third reviewer was consulted.
Results
Study inclusion
The initial search yielded 5,887 records after the removal of duplicates. Of these, 437 full texts were assessed for eligibility, 49 articles were included in the qualitative synthesis of this article and 17 articles in the separate quantitative analyses. Supplementary Figure 1 shows the flow chart of study screening and inclusion.
Studies conducted in the community setting
Nineteen studies were conducted in the community. Eleven of the included studies were individually randomised [24–34], and eight studies were cluster-randomised [35–42]. Eight of the studies were conducted in the United States [24, 28–30, 33, 35, 38, 40], eight in Europe [25–27, 31, 37, 41, 42] and two in Australia [36, 39] and one in New Zealand [32]. The sample size ranged from 81 to 3,904 participants, and the mean age between 67 and 85 years. The follow-up period for falls ranged from 45 days to 24 months. The study characteristics and results are summarised in Supplementary Table 1 and the risk of bias assessment is provided in Supplementary Figures 2 and 3.
In 10 studies, the intervention was a medication review by a healthcare professional. Three of these studies were pooled, which resulted in an RR of 1.05 (95% CI 0.85–1.29) for the number of fallers, with a heterogeneity of I2 = 0% (Figure 1a). The GRADE assessment was low for this effect estimate (Supplementary Table 2). Sensitivity and subgroup analyses are shown in Supplementary Figure 4. In addition, three of the studies were pooled for the meta-analysis of number of injurious fallers which resulted in an RR of 0.95 (95% CI 0.70–1.27) with a heterogeneity of I2 = 37% (Figure 1b). The GRADE assessment was very low for this effect estimate (Supplementary Table 2). Sensitivity and subgroup analyses are shown in Supplementary Figure 5. Finally, two of the studies were pooled for the meta-analysis of number of injurious falls that resulted in RaR of 0.89 (95% CI 0.69–1.14) with a heterogeneity of I2 = 0% (Figure 1c). The GRADE assessment was low for this effect estimate (Supplementary Table 2). Sensitivity analysis is shown in Supplementary Figure 6.
Figure 1.
(a) Forest plot of meta-analysis assessing medication review versus usual care, outcome number of fallers during follow-up. (b) Forest plot of meta-analysis assessing medication review versus usual care, outcome number of injurious fallers during follow-up. *Blalock 2020 and Mahlknecht adjusted for clustering by review authors and the totals are design effect corrected totals. (c) Forest plot of meta-analysis assessing medication review versus usual care, outcome number of injurious falls during follow-up. *Mahlknecht adjusted for clustering by review authors and the totals are design effect corrected totals.
The interventions of the trials that were included in the meta-analyses are shown in Table 1. Four studies that were not included in the meta-analyses are described in the Supplementary Text 2.
Table 1.
Medication review interventions in the meta-analysed studies
| Study | Intervention |
|---|---|
| Community | |
| Blalock 2010 | Medication review by community pharmacist with special attention to FRIDs. When a drug therapy problem was identified, the pharmacist discussed it with the patient. If patient was interested, pharmacist contacted their physician. |
| Blalock 2020 | Community pharmacy staff screened patients for fall risk using STEADI algorithm. If patient was screened positive, patient was eligible to receive a pharmacist-conducted medication review. Recommendations were sent to patients’ healthcare providers following the review. |
| Meredith | Medication use improvement programme addressing for home healthcare patients: (i) unnecessary therapeutic duplication, (ii) cardiovascular medication problems, (iii) use of psychotropic drugs in patients with possible adverse psychomotor or adrenergic effects and (iv) use of non-steroidal anti-inflammatory drugs in patients at high risk of peptic ulcer complications. Development of plan by pharmacist to address the identified problem and the plan presented for the physician. The nurse assisted the patient with medication changes and monitoring the effect. |
| Messerli | Polymedication Check, a community pharmacist-led medication review including a structured face-to-face counselling with the patient and screening all medicines currently used. Possible resulting interventions were for example consultation with the general practitioner (GP), referral of the patient, potential suggestion and implementation of a weekly dose reminder system, an individual patient education and a medication plan. |
| Mahlknecht | A review of patient’s medication regimens by three experts who gave specific recommendations for drug discontinuation. If at least two experts concorded regarding a specific recommendation, the respective recommendation and a brief explanation was forwarded to the respective GP. The GPs were invited to reflect on the recommendations in a shared decision-making process with the patient. |
| Hospital | |
| Blum | A structured pharmacotherapy optimisation intervention jointly by a physician and a pharmacist at the individual level with the support of CDSS deploying the STOPP/START criteria. |
| Gallagher | Physician applied STOPP/START criteria. These were immediately discussed with the attending medical team and followed up with a written communication within 24 hours. Medication changes were included in the discharge summary to the patient’s general practitioner. |
| Wehling | A FORTA team instructed ward physicians on FORTA. The physicians convened with the FORTA-intervention team weekly, to discuss medication plans. Physician’s own judgement was leading over FORTA-based suggestions. |
| Michalek | The drugs were evaluated according to the FORTA list and changed as guided by FORTA within the 1st week in the hospital if possible. |
| Long-term care facilities | |
| Zermansky | Clinical medication review by a pharmacist including a review of the GP clinical records and a consultation with the patient and carer. The pharmacist passed the formulated recommendations on a written proforma to the GP for acceptance and implementation. |
| Patterson | Specially trained pharmacists visited intervention homes monthly for 12 months and reviewed residents’ clinical and prescribing information, applied an algorithm that guided them in assessing the appropriateness of psychoactive medication and worked with GPs to improve the prescribing of these drugs. |
| Frankenthal | Screening medications with STOPP/START criteria by study pharmacist followed up with recommendations to the chief physician. Review at baseline, 6 and 12 months later. |
| Desborough | Multi-professional medication review meetings involving a clinical pharmacist and pharmacy technician, care home staff and GP(s) responsible for the medical care of residents. Review at baseline and 6 months. The outcome of the meeting was an agreed medicine-related action plan |
| Crotty 2004a | Receiving the services of the pharmacist transition coordinator for the patients transferring 1st time from hospital to long-term care facility including medication management transfer summaries from hospitals, timely coordinated medication reviews by accredited community pharmacists and case conferences with physicians and pharmacists. |
| Curtin | STOPPFrail-guided deprescribing plan for the patients discharged from acute hospital to nursing home devised by the research physician. The plan was communicated directly to one of the participant’s attending physicians and also documented in the patient’s medical record. |
| Potter | A medication review followed by the planned deprescribing of non-beneficial medicines. The aim was to reduce the total number of medications. GP and a geriatrician who was also a clinical pharmacologist of older people led the review. The plan was implemented over several months. Participants were reviewed weekly during deprescribing. |
| Cateau 2020a | The intervention consisted of a deprescribing-focused medication review, performed by the pharmacists, followed by the creation of a treatment-modification plan in collaboration with nurses and physicians. Once agreed upon by the professionals, the plan was submitted to the participating resident, or her/his representative, before implementation. |
Furthermore, nine other studies with various interventions are described in the Supplementary Text 2.
Studies conducted in the hospital setting
Seven studies were conducted in hospital setting. Two of the included studies were individually randomised using a randomisation algorithm [43, 44], two of the studies were quasi randomised trials [45, 46], one of the studies was stepped-wedge cluster RCT [47], in one of the studies the randomisation had to be partly guided by random availability of beds [48] and in the OPERAM trial, clusters were defined at the level of attending physicians [49]. Six studies were conducted in Europe [43–46, 48, 49] and one in Canada [47]. The sample size varied from 114 to 5,698 participants. In four of the studies, the mean or median age was above 80 years [43, 45, 46, 48]. The study characteristics and results are summarised in Supplementary Table 3 and the risk of bias assessment in Supplementary Figures 7 and 8.
Three studies evaluated falls during hospital stay and investigated optimisation of medication regimen. In two of these studies, the medications were evaluated according to the Fit fOR The Aged (FORTA) criteria and these were pooled for meta-analysis of the number of fallers, which resulted in an RR 0.50 (0.07–3.50) with a heterogeneity of I2 = 72% (Figure 2a). The GRADE assessment was very low for this effect estimate (Supplementary Table 4). Sensitivity analysis is shown in Supplementary Figure 9. The interventions of the trials that were included in the meta-analysis are shown in Table 1. The study that was not included in the meta-analysis is described in the Supplementary Text 2.
Figure 2.
(a) Forest plot of meta-analysis assessing evaluation of medications according to the FORTA criteria versus usual care, outcome number of fallers during hospital admission. *Both trials adjusted for clustering by review authors and the totals are design effect corrected totals. (b) Forest plot of meta-analysis assessing medication review versus usual care, outcome number of fallers after hospital admission. *Blum adjusted for clustering by review authors and the totals of Blum are design effect corrected totals.
Five of the seven studies evaluated falls after discharge. In four of these studies pharmacotherapy optimisation interventions were performed. Two of these studies were pooled for meta-analysis of the number of fallers, which resulted in an RR of 0.97 (95% CI 0.74–1.28), with a heterogeneity of I2 = 15% (Figure 2b). The GRADE assessment was very low for this effect estimate (Supplementary Table 4). Sensitivity analysis is shown in Supplementary Figure 10. The interventions of the trials that were included in the meta-analysis are shown in Table 1. Two studies that were not included in the meta-analysis and additional study investigating implementation of electronic deprescribing decision support tool providing personalised deprescribing are described in the Supplementary Text 2.
Studies conducted in the long-term care setting
Twenty-three studies conducted in long-term care facilities were identified. Nine of the included studies were individually randomised [50–58], eleven studies were randomised by cluster [59–69] and three were stepped wedge cluster RCTs [70–72]. Fifteen of the studies were conducted in Europe [50, 52–54, 56, 58, 60–63, 65, 67–70], four in North America [57, 64, 66, 72], three in Australia [51, 55, 59] and one in Singapore [71]. The sample size varied from 19 to 5,363 participants and the mean ages varied between 79 and 90 years. The study characteristics and results are summarised in Supplementary Table 5 and the risk of bias assessment is provided in Supplementary Figures 11 and 12.
Eleven of the 22 studies investigated medication reviews or medication withdrawal plans. Five of these 11 studies were pooled for meta-analysis of the number of fallers, which resulted in an RR of 0.86 (95% CI 0.72–1.02), with a heterogeneity of I2 = 0% (Figure 3a). The GRADE assessment was moderate for this effect estimate (Supplementary Table 6). Sensitivity and subgroup analyses are shown in Supplementary Figure 13. Seven of these 11 studies were pooled for meta-analysis of the number of falls, which resulted in an RaR of 0.93 (95% CI 0.64–1.35), with a heterogeneity of I2 = 92% (Figure 3b). The GRADE assessment was very low for this effect estimate (Supplementary Table 6). Sensitivity and subgroup analyses are shown in Supplementary Figure 14. The interventions of the trials that were included in the meta-analyses are shown in Table 1. Three studies that were not included in the meta-analysis are described in the Supplementary Text 2.
Figure 3.
(a) Forest plot of meta-analysis assessing medication review versus usual care among long-term care facility residents, outcome number of fallers in follow up. (b) Forest plot of meta-analysis assessing medication review versus usual care among long-term care facility residents, outcome number of falls in follow up. *Rate ratio of Patterson et al., adjusted for clustering by review authors. All of the totals are complete totals of the trials independent of trial design.
Furthermore, 12 other studies with various interventions are described in the Supplementary Text 2.
Discussion
We conducted a comprehensive systematic review and meta-analysis assessing the effectiveness of medication review and deprescribing interventions as a single falls prevention intervention by focusing on all care settings in which older people receive healthcare. In total, 49 studies for the qualitative synthesis were identified, with very heterogeneous results as well as interventions including medication review by a healthcare professional, targeted FRIDs withdrawal by a research team, educational interventions and implementation of CDSS. The heterogeneity precludes us estimating the effect of medication review and/or deprescribing as a single intervention. However, we assessed the effect of medication reviews on different fall outcomes by pooling the studies with a sufficiently comparable intervention (17 studies in four different quantitative analyses). No significant effects on fall outcomes of a medication review as a single intervention were found for any of the settings. Thus, although multifactorial falls preventive interventions, including a medication review, have been proven effective [3, 73], this cannot be concluded for a medication review as a single intervention in falls prevention. However, there was a trend for a lower number of fallers in the meta-analysis assessing medication reviews in long-term care possibly indicating that in a frail subgroup of older persons, medication review intervention might be effective also as a single intervention.
To the best of our knowledge, there have not been previous meta-analyses pooling the studies regarding medication reviews as a single intervention that reported falls during or after admission to hospital as an outcome. Since the number of identified trials was low, further studies in this setting with sufficient power are warranted to detect potential differences in fall-related outcomes. Our findings in community and long-term care settings are, overall, aligned with previous systematic reviews regarding the effectiveness of medication reviews as a single intervention in falls prevention. Cameron et al. [11] found comparable effect estimates for the studies conducted in long-term care facilities. Our analysis for number of fallers resulted in a slightly lower effect size due to the inclusion of two newer studies and, on the other hand, exclusion of two studies concerning educational approach/outreach intervention and one concerning implementation of a CDSS from the meta-analysis. Furthermore, our meta-analysis regarding the studies conducted in community, which included a newer study compared to the meta-analysis by Gillespie et al. still resulted in a similar non-significant effect estimate compared with their analysis [10]. In addition, we were able to pool RCTs investigating injurious falls but these meta-analyses led to non-significant findings. Thus, given the current evidence, there is no strong support to perform a medication review as a single intervention to prevent falls in any settings in which older people receive healthcare. Nevertheless, this does not preclude performing medication reviews as a general measure in geriatric patients in different settings, aiming among others to minimise the burden of adverse drug reactions, improve quality of life and functioning [74]. Furthermore, a trend was found in the meta-analysis of long-term care setting, and the trial by Crotty et al. included in the meta-analysis showing the highest effect size was conducted probably in the least frail population in comparison with the other studies [51]. Therefore, future studies in frail populations in all settings are warranted.
On the other hand, some studies did show a significant effect. First, in the trial by Campbell et al. [32], only patients who were thought to benefit from the psychotropic withdrawal were included, and the uptake of the deprescribing intervention in this placebo-controlled trial was far beyond the uptake of the interventions in other trials. Also, in the trial by Frankenthal et al. [53], there was a high acceptance rate of STOPP (82.4%) and START (92.6%) recommendations by the chief physician. Interestingly, three further studies showing an effect were educational interventions or included an educational component [36, 62, 63]. This is in line with evidence on effective implementation strategies in general falls prevention as active training and support of healthcare professionals is identified as an essential component in these strategies [75], and education is identified as one of the main facilitators of implementation efforts of deprescribing [76]. This highlights the importance of conducting future studies regarding educational interventions on medication review and deprescribing interventions in falls prevention and the importance of training and engaging the providers.
There are several potential explanations for the lack of significant findings in this systematic review. First, it should be noted that the majority of the individual studies included were not adequately powered to detect differences in fall-related outcomes. Second, it might be that a medication review as a single intervention is simply not sufficient to induce a relevant decrease in fall risk. Falls are usually of a multifactorial nature, warranting a multifactorial approach. Thus, medication review and deprescribing interventions should not be implemented as a stand-alone strategy in falls prevention but should rather be a part of multimodal strategy due to this multifactorial nature. Nevertheless, considering the trend found in frail older persons, long-term care residents might already benefit even when FRID withdrawal is introduced as a single intervention.
Third, the effect of FRIDs on fall risk appears to be dependent on patient characteristics such as history of previous fall injuries [77]. These characteristics including co-morbidities, frailty, other geriatric syndromes and patient’s preferences should be taken into account when performing a medication review. Therefore, a holistic assessment to apply a personalised medication strategy instead of a stand-alone one-time medication review is needed. The need for such an assessment may explain the unsuccessful outcomes of trials that took a simpler approach, and it may also explain the low uptake that was occasionally present concerning advice of the medication review. In particular, the acceptance of recommendations provided by community pharmacists to providers seems to be low. In a recent trial by Blalock et al. [40], ~15% of the providers planned to change the medication based on the recommendations. There can be healthcare professionals, individuals and the public, healthcare organisation and environment- (e.g. regulatory, policy, financial) related reluctance for deprescribing [78]. To successfully implement deprescribing interventions, unique barriers arising from all different levels of the healthcare system should be considered [78].
Fourth, the interventions varied greatly in terms of which medication classes were targeted, from focusing on a single medication class of FRIDs to a complete medication regimen. Moreover, even regarding the definition of FRIDs, there were great differences between the trials, varying between few psychotropic medication classes and a comprehensive list of cardiovascular, psychotropic and other medication classes making comparison difficult [25, 65]. For future studies, more comparability is warranted. Recently, a European Delphi consensus effort has been performed to develop a consensus FRIDs list and accompanying STOPPFall deprescribing tool [9]. Finally, the compliance to deprescribing FRIDs is often poor. For example, Campbell et al. demonstrated that deprescribing can be difficult as 47% of the participants failed permanent withdrawal of psychotropics, and in an RCT by Boye et al., 35% of attempted withdrawal was unsuccessful [25, 32]. Provision of monitoring, support and documentation of deprescribing decisions and the process undertaken that led to these decisions are crucial for the long-term success of deprescribing and are essential in both future trials and clinical practice [79].
Limitations
There are several limitations to be mentioned. First, we were only able to identify few studies conducted in hospital settings, limiting the quality of evidence related to this setting. Second, most of the studies conducted among community-dwelling older adults were not targeted to only fallers even though history of falls is the strongest fall risk factor. Thus, future studies focusing on high-risk populations e.g. fall clinic visitors are warranted. Third, majority of the identified studies were medication review interventions and not targeted FRIDs deprescribing interventions. Fourth, the meta-analysis of number of fallers regarding medication reviews in long-term care facilities led to very high heterogeneity, and the source of the heterogeneity was not revealed in our subgroup analyses. Fall rates are also vulnerable to non-normal distribution as seen in trial by Potter et al., in which three participants in the intervention group had >30 falls [11]. Fifth, the quality of the included studies was low or moderate according to the risk of bias assessment, and definition for falls was often lacking. Sixth, the studies were often small and not powered to detect differences in falls outcomes, since falls were often measured as secondary outcomes. Sixth, outcomes number of falls and number of fallers were measured most often, and only a few studies evaluated fall-related injuries. Finally, almost all of the studies were conducted in Europe, North America or Oceania, limiting the worldwide generalisability of the results.
Conclusion
In our systematic review and meta-analysis on the effectiveness of medication review and deprescribing interventions as a single intervention for falls prevention in older people, no significant associations between medication reviews and fall outcomes were found in any of the settings in which older people receive healthcare. However, there was a trend for a lower number of fallers in the meta-analysis assessing medication reviews in long-term care, possibly indicating that in a frail subgroup of older persons, medication review might be effective even as a single intervention. Furthermore, several other studies with heterogeneous interventions and results not included in the meta-analyses were identified. Since the conducted studies are very heterogeneous, it is difficult to estimate the effect of medication review and/or deprescribing as a single intervention.
Based on the existing evidence, medication review and deprescribing interventions should not be implemented as a stand-alone strategy in falls prevention but as a part of multimodal strategy due to the multifactorial nature of falls [73]. The interventions should involve older individuals, their representatives and healthcare professionals to focus on the multidisciplinary team-centred approach to facilitate the implementation. For future, more studies actively targeting FRIDs withdrawal are warranted, and also, more comparability is needed in terms of targeted medication classes.
Supplementary Material
Contributor Information
Lotta J Seppala, Amsterdam UMC location University of Amsterdam, Internal Medicine, Section of Geriatric Medicine, Meibergdreef 9, Amsterdam, The Netherlands; Amsterdam Public Health Research Institute, Amsterdam, The Netherlands.
Nellie Kamkar, Gait and Brain Laboratory, Lawson Research Health Institute, Parkwood Hospital, London Ontario, Canada; Department of Epidemiology and Biostatistics, University of Western Ontario, London Ontario, Canada.
Eveline P van Poelgeest, Amsterdam UMC location University of Amsterdam, Internal Medicine, Section of Geriatric Medicine, Meibergdreef 9, Amsterdam, The Netherlands; Amsterdam Public Health Research Institute, Amsterdam, The Netherlands.
Katja Thomsen, Department of Geriatric Medicine, Odense University Hospital, Odense, Denmark; Geriatric Research Unit, Department of Clinical Research, University of Southern Denmark, Odense, Denmark.
Joost G Daams, Research Support, Medical Library, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105AZ Amsterdam, The Netherlands.
Jesper Ryg, Department of Geriatric Medicine, Odense University Hospital, Odense, Denmark; Geriatric Research Unit, Department of Clinical Research, University of Southern Denmark, Odense, Denmark; ODIN (Odense Deprescribing INitiative), Denmark.
Tahir Masud, Nottingham University Hospitals NHS Trust, Nottingham, UK.
Manuel Montero-Odasso, Gait and Brain Laboratory, Lawson Research Health Institute, Parkwood Hospital, London Ontario, Canada; Schulich School of Medicine and Dentistry, London Ontario, Canada; Departments of Medicine (Geriatrics) and of Epidemiology and Biostatistics, University of Western Ontario, London Ontario, Canada.
Sirpa Hartikainen, School of Pharmacy, University of Eastern Finland, Kuopio, Finland.
Mirko Petrovic, Department of Internal Medicine and Paediatrics (Section of Geriatrics), Ghent University, Ghent, Belgium.
Nathalie van der Velde, Amsterdam UMC location University of Amsterdam, Internal Medicine, Section of Geriatric Medicine, Meibergdreef 9, Amsterdam, The Netherlands; Amsterdam Public Health Research Institute, Amsterdam, The Netherlands.
the Task Force on Global Guidelines for Falls in Older Adults:
Mirko Petrovic, Alice Nieuwboer, Ellen Vlaeyen, Koen Milisen, Jesper Ryg, Rose Anne Kenny, Robert Bourke, Sirpa Hartikainen, Tischa Van der Cammen, Nathalie van der Velde, Eveline Poelgeest, Anton Jellema, Lotta J Seppala, Tahir Masud, Chris Todd, Finbarr C Martin, David R Marsh, Sallie Lamb, James Frith, Pip Logan, Dawn Skelton, Hubert Blain, Cedric Anweiller, Ellen Freiberger, Clemens Becker, Lorenzo Chiari, Matteo Cesari, Alvaro Casas-Herrero, Javier Perez Jara, Christina Alonzo Bouzòn, Ana-Karim Welmer, Stephanie Birnghebuam, Reto Kressig, Manuel Montero-Odasso, Mark Speechley, Bill McIlroy, Frederico Faria, Munira Sultana, Susan Muir-Hunter, Richard Camicioli, Kenneth Madden, Mireille Norris, Jennifer Watt, Louise Mallet, David Hogan, Joe Verghese, Ervin Sejdic, Luigi Ferrucci, Lewis Lipsitz, David A Ganz, Neil B Alexander, Nancy Kathryn Latham, Fabiana Giber, Marcelo Schapira, Ricardo Jauregui, Felipe Melgar-Cuellar, Roberto Alves Lourenço, Daniela Cristina Carvalho de Abreu, Monica Perracini, Alejandro Ceriani, Pedro Marín-Larraín, Homero Gac Espinola, José Fernando Gómez-Montes, Carlos Alberto Cano-Gutierrez, Xinia Ramirez Ulate, José Ernesto Picado Ovares, Patricio Gabriel Buendia, Susana Lucia Tito, Diego Martínez Padilla, Sara G Aguilar-Navarro, Alberto Mimenza, Rogelio Moctezum, Alberto Avila-Funes, Luis Miguel Gutiérrez-Robledo, Luis Manuel Cornejo Alemán, Edgar Aguilera Caona, Juan Carlos Carbajal, José F Parodi, Aldo Sgaravatti, Stephen Lord, Cathie Sherrington, Cathy Said, Ian Cameron, Meg Morris, Gustavo Duque, Jacqueline Close, Ngaire Kerse, Maw Pin Tan, Leilei Duan, Ryota Sakurai, Chek Hooi Wong, Irfan Muneeb, Hossein Negahban, Canan Birimoglu, Chang Won Won, Jeffrey Huasdorff, Sebastiana Kalula, and Olive Kobusingye
Declarations of Conflicts of Interest
None.
Declaration of Sources of Funding
Canadian Institute of Health Research (CIHR; MOP 211220, PTJ 153100) and Clementine Brigitta Maria Dalderup Fund (grant number 7303), which is an Amsterdam University fund. The sponsors played no part in the design, execution, analysis and interpretation of data or writing of the study.
References
- 1. Montero-Odasso M, van der Velde N, Alexander NB et al. New horizons in falls prevention and management for older adults: a global initiative. Age Ageing 2021; 50: 1499–507. [DOI] [PubMed] [Google Scholar]
- 2. Berry SD, Miller RR. Falls: epidemiology, pathophysiology, and relationship to fracture. Curr Osteoporos Rep 2008; 6: 149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ganz DA, Latham NK. Prevention of falls in community-dwelling older adults. N Engl J Med 2020; 382: 734–43. [DOI] [PubMed] [Google Scholar]
- 4. Kennedy CC, Ioannidis G, Thabane L et al. Successful knowledge translation intervention in long-term care: final results from the vitamin D and osteoporosis study (ViDOS) pilot cluster randomized controlled trial. Trials 2015; 16: 214. 10.1186/s13063-015-0720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morris R, O'Riordan S. Prevention of falls in hospital. Clin Med (Lond) 2017; 17: 360–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hopewell S, Adedire O, Copsey BJ et al. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2018; 2018: CD012221. 10.1002/14651858.CD012221.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Velde N, Stricker BH, Pols HA, van der Cammen TJM. Withdrawal of fall-risk-increasing drugs in older persons: effect on mobility test outcomes. Drugs Aging 2007; 24: 691–9. [DOI] [PubMed] [Google Scholar]
- 8. van der Velde N, van den Meiracker AH, Pols HA, Stricker BHC, van der Cammen TJM. Withdrawal of fall-risk-increasing drugs in older persons: effect on tilt-table test outcomes. J Am Geriatr Soc 2007; 55: 734–9. [DOI] [PubMed] [Google Scholar]
- 9. Seppala LJ, Petrovic M, Ryg J et al. STOPPFall (Screening Tool of Older Persons Prescriptions in older adults with high fall risk): a Delphi study by the EuGMS Task and Finish Group on fall-risk-increasing drugs. Age Ageing 2020; 50: 1189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gillespie LD, Robertson MC, Gillespie WJ et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012; Cd007146. 10.1002/14651858.ED000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cameron ID, Dyer SM, Panagoda CE et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev 2018; 9: CD005465. 10.1002/14651858.CD005465.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hart LA, Phelan EA, Yi JY, Marcum ZA, Gray SL. Use of fall risk–increasing drugs around a fall-related injury in older adults: a systematic review. J Am Geriatr Soc 2020; 68: 1334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee J, Negm A, Peters R, Wong EKC, Holbrook A. Deprescribing fall-risk increasing drugs (FRIDs) for the prevention of falls and fall-related complications: a systematic review and meta-analysis. BMJ Open 2021; 11: e035978. 10.1136/bmjopen-2019-035978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. www.training.cochrane.org/handbook. Accessed, 10.1002/9781119536604. [DOI] [Google Scholar]
- 16. Seppala LJ, Kamkar N, Ryg J et al. Protocol for a systematic review and meta-analysis assessing the effectiveness of deprescribing in falls prevention in older people. BMJ Open 2021; 11: e047190. 10.1136/bmjopen-2020-047190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Motter FR, Fritzen JS, Hilmer SN, Paniz ÉV, Paniz VMV. Potentially inappropriate medication in the elderly: a systematic review of validated explicit criteria. Eur J Clin Pharmacol 2018; 74: 679–700. [DOI] [PubMed] [Google Scholar]
- 18. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sterne JAC, Savović J, Page MJ et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 20. Smeeth L, Ng ES-W. Intraclass correlation coefficients for cluster randomized trials in primary care: data from the MRC trial of the assessment and management of older people in the community. Control Clin Trials 2002; 23: 409–21. [DOI] [PubMed] [Google Scholar]
- 21. Cumming RG, Sherrington C, Lord SR et al. Cluster randomised trial of a targeted multifactorial intervention to prevent falls among older people in hospital. BMJ 2008; 336: 758–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dyer CA, Taylor GJ, Reed M et al. Falls prevention in residential care homes: a randomised controlled trial. Age Ageing 2004; 33: 596–602. [DOI] [PubMed] [Google Scholar]
- 23. Guyatt G, Oxman AD, Akl EA et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64: 383–94. [DOI] [PubMed] [Google Scholar]
- 24. Blalock SJ, Casteel C, Roth MT, Ferreri S, Demby KB, Shankar V. Impact of enhanced pharmacologic care on the prevention of falls: a randomized controlled trial. Am J Geriatr Pharmacother 2010; 8: 428–40. [DOI] [PubMed] [Google Scholar]
- 25. Boyé ND, van der Velde N, de Vries OJ et al. Effectiveness of medication withdrawal in older fallers: results from the Improving Medication Prescribing to reduce Risk Of FALLs (IMPROveFALL) trial. Age Ageing 2017; 46: 142–6. [DOI] [PubMed] [Google Scholar]
- 26. Sheppard JP, Burt J, Lown M et al. Effect of antihypertensive medication reduction vs usual care on short-term blood pressure control in patients with hypertension aged 80 years and older: the OPTIMISE Randomized Clinical Trial. JAMA 2020; 323: 2039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Meer HG, Wouters H, Pont LG, Taxis K. Reducing the anticholinergic and sedative load in older patients on polypharmacy by pharmacist-led medication review: a randomised controlled trial. BMJ Open 2018; 8: e019042. 10.1136/bmjopen-2017-019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Devanand DP, Mintzer J, Schultz SK et al. Relapse risk after discontinuation of risperidone in Alzheimer's disease. N Engl J Med 2012; 367: 1497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elliott LS, Henderson JC, Neradilek MB, Moyer NA, Ashcraft KC, Thirumaran RK. Clinical impact of pharmacogenetic profiling with a clinical decision support tool in polypharmacy home health patients: a prospective pilot randomized controlled trial. PLoS One 2017; 12: e0170905. 10.1371/journal.pone.0170905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meredith S, Feldman P, Frey D et al. Improving medication use in newly admitted home healthcare patients: a randomized controlled trial. J Am Geriatr Soc 2002; 50: 1484–91. [DOI] [PubMed] [Google Scholar]
- 31. Messerli M, Blozik E, Vriends N, Hersberger KE. Impact of a community pharmacist-led medication review on medicines use in patients on polypharmacy--a prospective randomised controlled trial. BMC Health Serv Res 2016; 16: 145. 10.1186/s12913-016-1384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Campbell A, Robertson M, Gardner MM, Norton RN, Buchner DM. Psychotropic medication withdrawal and a home-based exercise program to prevent falls: a randomized, controlled trial. J Am Geriatr Soc 1999; 47: 850–3. [DOI] [PubMed] [Google Scholar]
- 33. Gurwitz JH, Kapoor A, Garber L et al. Effect of a multifaceted clinical pharmacist intervention on medication safety after hospitalization in persons prescribed high-risk medications: a randomized clinical trial. JAMA Intern Med 2021; 181: 610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kornholt J, Feizi ST, Hansen AS et al. Effects of a comprehensive medication review intervention on health-related quality of life and other clinical outcomes in geriatric outpatients with polypharmacy: a pragmatic randomized clinical trial. Br J Clin Pharmacol 2022; 88: 3360–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mott DA, Martin B, Breslow R et al. Impact of a medication therapy management intervention targeting medications associated with falling: results of a pilot study. J Am Pharm Assoc 2003; 56: 22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pit SW, Byles JE, Henry DA, Holt L, Hansen V, Bowman DA. A quality use of medicines program for general practitioners and older people: a cluster randomised controlled trial. Med J Aust 2007; 187: 23–30. [DOI] [PubMed] [Google Scholar]
- 37. Romskaug R, Skovlund E, Straand J et al. Effect of clinical geriatric assessments and collaborative medication reviews by geriatrician and family physician for improving health-related quality of life in home-dwelling older patients receiving polypharmacy: a cluster randomized clinical trial. JAMA Intern Med 2020; 180: 181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weber V, White A, McIlvried R. An electronic medical record (EMR)-based intervention to reduce polypharmacy and falls in an ambulatory rural elderly population. J Gen Intern Med 2008; 23: 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kouladjian O'Donnell L, Gnjidic D, Sawan M et al. Impact of the goal-directed medication review electronic decision support system on drug burden index: a cluster-randomised clinical trial in primary care. Br J Clin Pharmacol 2020; 87: 1499–511. [DOI] [PubMed] [Google Scholar]
- 40. Blalock SJ, Ferreri SP, Renfro CP et al. Impact of STEADI-Rx: a community pharmacy-based fall prevention intervention. J Am Geriatr Soc 2020; 68: 1778–86. [DOI] [PubMed] [Google Scholar]
- 41. Rieckert A, Reeves D, Altiner A et al. Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomised controlled trial. BMJ 2020; 369: m1822. 10.1136/bmj.m1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mahlknecht A, Wiedermann CJ, Sandri M et al. Expert-based medication reviews to reduce polypharmacy in older patients in primary care: a northern-Italian cluster-randomised controlled trial. BMC Geriatr 2021; 21: 659. 10.1186/s12877-021-02612-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sjoberg C, Wallerstedt SM. Effects of medication reviews performed by a physician on treatment with fracture-preventing and fall-risk-increasing drugs in older adults with hip fracture-a randomized controlled study. J Am Geriatr Soc 2013; 61: 1464–72. [DOI] [PubMed] [Google Scholar]
- 44. Gallagher PF, O'Connor MN, O'Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther 2011; 89: 845–54. [DOI] [PubMed] [Google Scholar]
- 45. Michalek C, Wehling M, Schlitzer J, Frohnhofen H. Effects of “Fit fOR The Aged” (FORTA) on pharmacotherapy and clinical endpoints--a pilot randomized controlled study. Eur J Clin Pharmacol 2014; 70: 1261–7. [DOI] [PubMed] [Google Scholar]
- 46. Van der Linden L, Decoutere L, Walgraeve K et al. Combined use of the rationalization of home medication by an adjusted STOPP in older patients (RASP) list and a pharmacist-led medication review in very old inpatients: impact on quality of prescribing and clinical outcome. Drugs Aging 2017; 34: 123–33. [DOI] [PubMed] [Google Scholar]
- 47. McDonald EG, Wu PE, Rashidi B et al. The MedSafer study-electronic decision support for deprescribing in hospitalized older adults: a cluster randomized clinical trial. JAMA Intern Med 2022; 182: 265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wehling M, Burkhardt H, Kuhn-Thiel A et al. VALFORTA: a randomised trial to validate the FORTA (Fit fOR The Aged) classification. Age Ageing 2016; 45: 262–7. [DOI] [PubMed] [Google Scholar]
- 49. Blum MR, Sallevelt BTGM, Spinewine A et al. Optimizing therapy to prevent avoidable hospital admissions in multimorbid older adults (OPERAM): cluster randomised controlled trial. BMJ 2021; 374: n1585-n. 10.1136/bmj.n1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bergh S, Selbaek G, Engedal K. Discontinuation of antidepressants in people with dementia and neuropsychiatric symptoms (DESEP study): double blind, randomised, parallel group, placebo controlled trial. BMJ 2012; 344: e1566. 10.1136/bmj.e1566. [DOI] [PubMed] [Google Scholar]
- 51. Crotty M, Rowett D, Spurling L, Giles LC, Phillips PA. Does the addition of a pharmacist transition coordinator improve evidence-based medication management and health outcomes in older adults moving from the hospital to a long-term care facility? Results of a randomized, controlled trial. Am J Geriatr Pharmacother 2004; 2: 257–64. [DOI] [PubMed] [Google Scholar]
- 52. Curtin D, Jennings E, Daunt R et al. Deprescribing in older people approaching end of life: a randomized controlled trial using STOPPFrail criteria. J Am Geriatr Soc 2019; 68: 762–9. [DOI] [PubMed] [Google Scholar]
- 53. Frankenthal D, Kalendaryev E, Lerman Y. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Am Geriatr Soc 2014; 62: 1658–65. [DOI] [PubMed] [Google Scholar]
- 54. Peyro Saint Paul L, Martin J, Gaillard C et al. Moderate, potentially drug-induced hyponatremia in older adults: is there a benefit in drug reduction? J Am Geriatr Soc 2012; 60: 1991–3. [DOI] [PubMed] [Google Scholar]
- 55. Potter K, Flicker L, Page A, Etherton-Beer C. Deprescribing in frail older people: a randomised controlled trial. PLoS One 2016; 11: e0149984. 10.1371/journal.pone.0149984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zermansky AG, Alldred DP, Petty DR et al. Clinical medication review by a pharmacist of elderly people living in care homes--randomised controlled trial. Age Ageing 2006; 35: 586–91. [DOI] [PubMed] [Google Scholar]
- 57. Medication Treatment for Depression in Nursing Home Residents https://clinicaltrials.gov/ct2/show/record/NCT00076622?view=record (accessed 4 August 2021).
- 58. Cateau D, Ballabeni P, Niquille A. Effects of an interprofessional deprescribing intervention in Swiss nursing homes: the Individual Deprescribing Intervention (IDeI) randomised controlled trial. BMC Geriatr 2021; 21: 655. 10.1186/s12877-021-02465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Crotty M, Whitehead C, Rowett D et al. An outreach intervention to implement evidence based practice in residential care: a randomized controlled trial [ISRCTN67855475]. BMC Health Serv Res 2004; 4: 6. 10.1186/1472-6963-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Desborough JA, Clark A, Houghton J et al. Clinical and cost effectiveness of a multi-professional medication reviews in care homes (CAREMED). Int J Pharm Pract 2020; 28: 626–34. [DOI] [PubMed] [Google Scholar]
- 61. Furniss L, Burns A, Craig SK, Scobie S, Cooke J, Faragher B. Effects of a pharmacist's medication review in nursing homes. Randomised controlled trial. Br J Psychiatry 2000; 176: 563–7. [DOI] [PubMed] [Google Scholar]
- 62. Garcia-Gollarte F, Baleriola-Julvez J, Ferrero-Lopez I et al. An educational intervention on drug use in nursing homes improves health outcomes resource utilization and reduces inappropriate drug prescription. J Am Med Dir Assoc 2014; 15: 885–91. [DOI] [PubMed] [Google Scholar]
- 63. Juola A-L, Bjorkman MP, Pylkkanen S et al. Nurse education to reduce harmful medication use in assisted living facilities: effects of a randomized controlled trial on falls and cognition. Drugs Aging 2015; 32: 947–55. [DOI] [PubMed] [Google Scholar]
- 64. Lapane KL, Hughes CM, Daiello LA, Cameron KA, Feinberg J. Effect of a pharmacist-led multicomponent intervention focusing on the medication monitoring phase to prevent potential adverse drug events in nursing homes. J Am Geriatr Soc 2011; 59: 1238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Patterson SM, Hughes CM, Crealey G, Cardwell C, Lapane KL. An evaluation of an adapted U.S. model of pharmaceutical care to improve psychoactive prescribing for nursing home residents in northern ireland (fleetwood northern ireland study). J Am Geriatr Soc 2010; 58: 44–53. [DOI] [PubMed] [Google Scholar]
- 66. Tadrous M, Fung K, Desveaux L et al. Effect of academic detailing on promoting appropriate prescribing of antipsychotic medication in nursing homes: a cluster randomized clinical trial. JAMA Netw Open 2020; 3: e205724. 10.1001/jamanetworkopen.2020.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wouters H, Scheper J, Koning H et al. Discontinuing inappropriate medication use in nursing home residents: a cluster randomized controlled trial. Ann Intern Med 2017; 167: 609–17. [DOI] [PubMed] [Google Scholar]
- 68. Cateau D, Ballabeni P, Niquille A. Effects of an interprofessional Quality Circle-Deprescribing Module (QC-DeMo) in Swiss nursing homes: a randomised controlled trial. BMC Geriatr 2021; 21: 289. 10.1186/s12877-021-02220-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Junius-Walker U, Krause O, Thürmann P et al. Drug safety for nursing-home residents-findings of a pragmatic, cluster-randomized, controlled intervention trialin 44 nursing homes. Dtsch Arztebl Int 2021; 118: 705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jordan S, Gabe-Walters ME, Watkins A et al. Nurse-led medicines' monitoring for patients with dementia in care homes: a pragmatic cohort stepped wedge cluster randomised trial. PLoS One 2015; 10: e0140203. 10.1371/journal.pone.0140203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kua CH, Yeo CYY, Tan PC et al. Association of deprescribing with reduction in mortality and hospitalization: a pragmatic stepped-wedge cluster-randomized controlled trial. J Am Med Dir Assoc 2020; 15:82–89.e3. [DOI] [PubMed] [Google Scholar]
- 72. Kirkham J, Maxwell C, Velkers C, Leung R, Moffat K, Seitz D. Optimizing prescribing of antipsychotics in long-term care (OPAL): a stepped-wedge trial. J Am Med Dir Assoc 2020; 21: 381–7.e3. [DOI] [PubMed] [Google Scholar]
- 73. Dautzenberg L, Beglinger S, Tsokani S et al. Interventions for preventing falls and fall-related fractures in community-dwelling older adults: a systematic review and network meta-analysis. J Am Geriatr Soc 2021; 69: 2973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Beuscart JB, Pelayo S, Robert L, Thevelin S, Marien S, Dalleur O. Medication review and reconciliation in older adults. Eur Geriatr Med 2021; 12: 499–507. [DOI] [PubMed] [Google Scholar]
- 75. Goodwin V, Jones-Hughes T, Thompson-Coon J, Boddy K, Stein K. Implementing the evidence for preventing falls among community-dwelling older people: a systematic review. J Safety Res 2011; 42: 443–51. [DOI] [PubMed] [Google Scholar]
- 76. Conklin J, Farrell B, Suleman S. Implementing deprescribing guidelines into frontline practice: barriers and facilitators. Res Social Adm Pharm 2019; 15: 796–800. [DOI] [PubMed] [Google Scholar]
- 77. Tinetti ME, Han L, Lee DS et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med 2014; 174: 588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sawan M, Reeve E, Turner J et al. A systems approach to identifying the challenges of implementing deprescribing in older adults across different health-care settings and countries: a narrative review. Expert Rev Clin Pharmacol 2020; 13: 233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmacol 2014; 78: 738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.