Abstract
Ciliogenesis is a complex multistep process used to describe assembly of cilia and flagella. These organelles play essential roles in motility and signaling on the surface of cells. Cilia are built at the distal ends of centrioles through the formation of an axoneme that is surrounded by the ciliary membrane. As is the case in the biogenesis of other cellular organelles, regulators of membrane trafficking play essential roles in ciliogenesis, albeit with a unique feature that membranes are organized around microtubule-based structures. Membrane association with the distal end of the centriole is a critical initiating step for ciliogenesis. Studies of this process in different cell types suggests that a singular mechanism may not be utilized to initiate cilium assembly. In this review, we focus on recent insights into cilium biogenesis and the roles membrane trafficking regulators play in described ciliogenesis mechanisms with relevance to human disease.
Introduction
Most human cells have a single immotile primary cilium or one or more motile cilia. Defects in the functions of these organelles cause genetic diseases, referred to as ciliopathy, with more than 30 human disorders described thus far. Motile cilia are associated with movement related functions, while primary cilia are important in signaling needed for normal development and tissue homeostasis in humans (1). Depending on the tissue, these functions include mechanosensation, light sensation, chemosensation, and morphogen and growth factors signaling.
The structure of the cilium is highly conserved in prokaryotes and eukaryotes, with a core nine-microtubule-doublet-based axoneme surrounded by a ciliary membrane. Motile cilia have an extra pair of microtubules in the middle of the axoneme needed for movement associated functioning. The 9-fold axoneme symmetry is carried over from the centrioles, which have a 9-triplet microtubule architecture structure, and form the base of the cilium, referred to as the basal body. Cilia are assembled through a complex process referred to as ciliogenesis (Figure 1). Centrioles competent to assemble cilia contain proteinaceous subdistal appendage (SDA) and distal appendage (DA) structures (2,3). Between the basal body and the axoneme is a transition zone (TZ), which is critical for regulating trafficking in and out of the cilium mediated by intraflagellar transport proteins (IFT) complexes, IFT-A and IFT-B. IFT complexes display anterograde and retrograde movement via association with kinesin and dynein motors, respectively. Like other membrane-bound organelles, the cilium lacks protein synthesis machinery (4). Consequently, it is not surprising that membrane trafficking regulators have been shown to be important for ciliogenesis including members of the RAB and ARF GTPases (5,6). This review will focus on how the ciliary membrane is formed and the role membrane trafficking regulators play in ciliogenesis.
Figure 1. Ciliogenesis mechanisms.
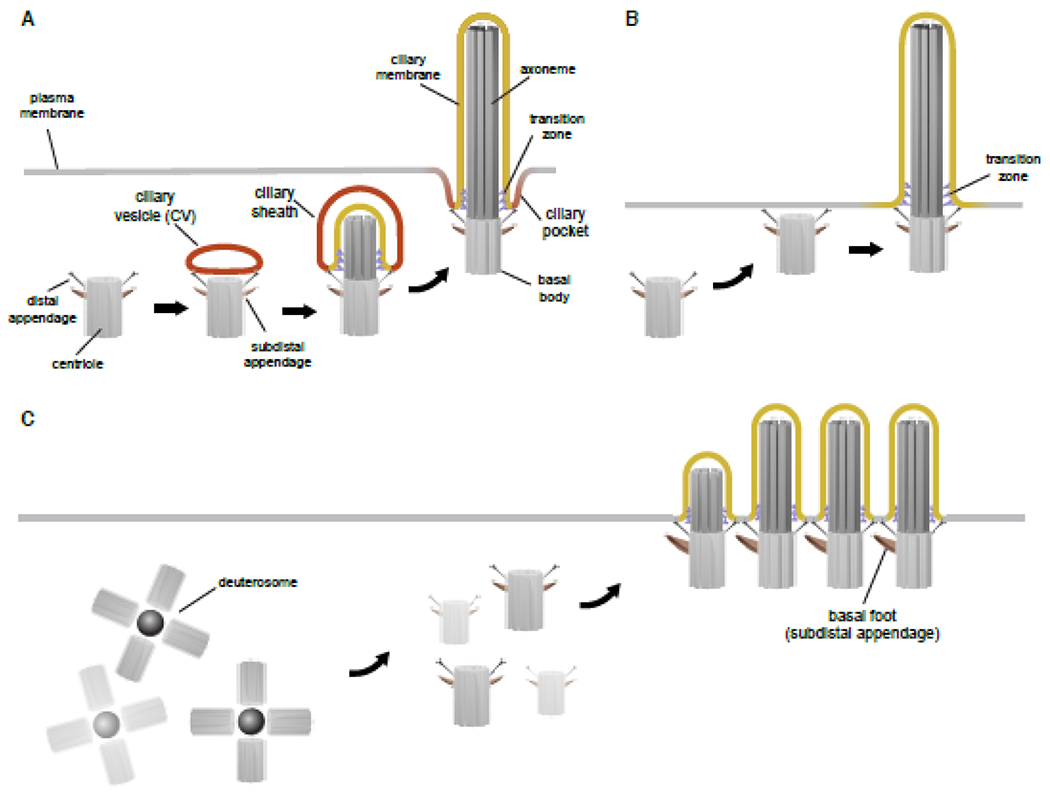
A)Intracellular ciliogenesis. In the cytoplasm a ciliary vesicle (CV) organizes at the distal appendages of centrioles prior to axoneme and ciliary membrane formation at the sheath stage. In the mature cilium a ciliary pocket membrane partially embeds the organelle in the cell. B) Extracellular ciliogenesis. Mature centrioles migrate and dock to the plasma membrane via distal appendages followed by axoneme formation. C) Multiciliogenesis. Centriole duplication occurs through the deuterosome. Centrioles develop DAs and SDAs while migrating towards the cell surface to form the axoneme. The transition zone forms prior to axoneme growth at the base of the cilium (A,B,C). Subdistal appendages are organized into the basal foot in multiciliated cells.
Cilium formation occurs in the G1/G0 phase of the cell cycle, and in dividing cells, the cilium is disassembled prior to mitosis as the centrioles participate in spindle formation. An essential early step of ciliogenesis involves the trafficking and docking of centrioles to cellular membranes via the DA of the mother centriole (MC) (7) (Figures 1A, B and 2). Distal appendage proteins (DAPs) serve to tether the basal body to the membrane, and in mature cilia are referred to as transition fibers. Centriole-membrane docking is required for the removal of CP110-CEP97 proteins from the MC distal end (2), a process needed to transform the MC into the basal body (BB) in order for the axoneme to grow (Figure 2). Prior to axoneme growth the Y-shaped TZ structure is built to bridge the ciliary membrane to the microtubule doublets (8). The IFT-B complex regulates the transport of factors at the cilium base needed for growth of cilium.
Figure 2. Intracellular ciliogenesis and membrane trafficking regulation.
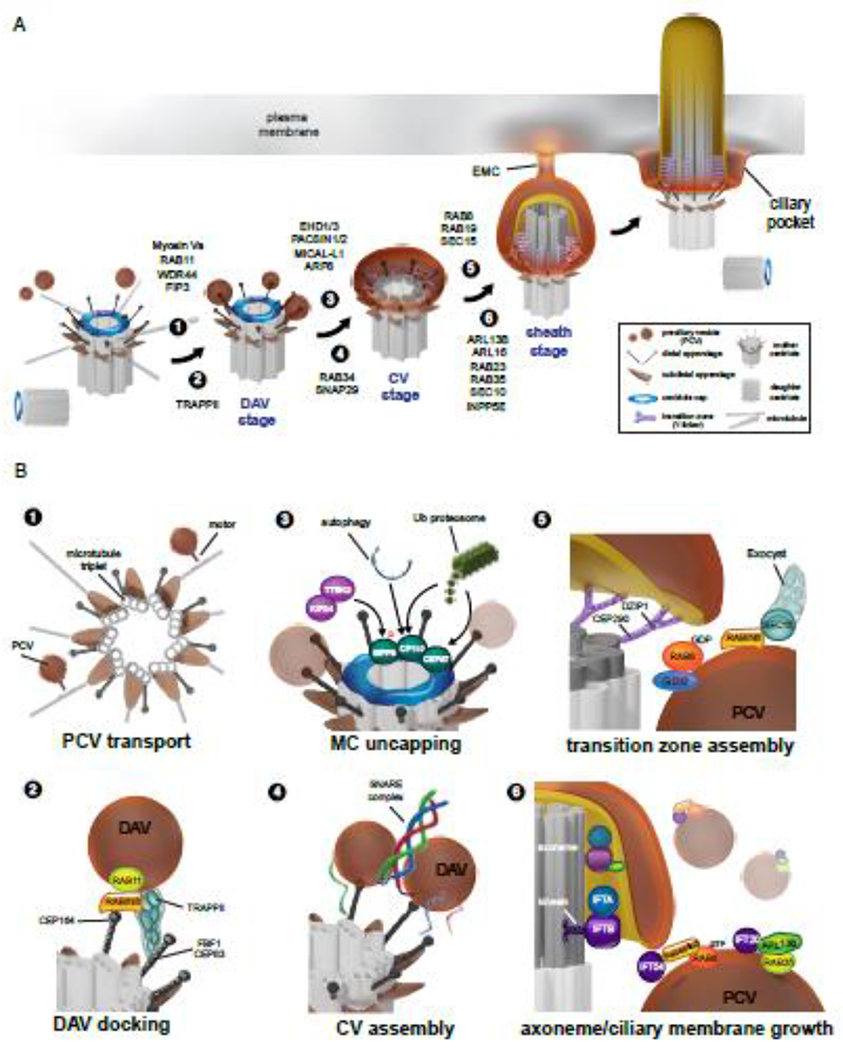
(A) Model showing the stages of intracellular ciliogenesis and requirements of membrane trafficking regulators in associated ciliogenesis processes (1-6) shown in (B). (B) Expanded view of ciliogenesis processes (1-6) shown in (A). (B) Expanded view of ciliogenesis processes (1-6) from (A). (1) Preciliary vesicle (PCV) trafficking to the mother centriole (MC). (2) Docking of PCVs to the MC mediated by distal appendage proteins (DA) proteins. Distal appendage vesicles – DAVs. (3) Uncapping of the mother centriole. TTBK2-mediated phosphorylation of MMP9 promotes ubiquitin proteasomal cascade to remove CP110 and CEP97. Autophagy has also been shown to regulate CP110 MC uncapping. (4) SNARE mediated fusion of DAVs to assemble the ciliary vesicle (CV). (5) Transition zone (TZ) protein association with RAB8 activation and the CV stage. (6) Axoneme and ciliary membrane growth regulation by the IFT-B complex and associated trafficking regulators including ARL13B and RAB GTPases. Extracellular membrane channel (EMC) connects the developing intracellular cilium membrane with the plasma membrane. The ciliary membrane is marked in yellow.
A. Intracellular and Extracellular Ciliogenesis:
It is generally thought that there are two mechanisms by which centriole-membrane docking occurs in ciliogenesis: an extracellular- and intracellular pathway. In the extracellular pathway, cilium assembly begins following migration and docking of the centriole to the plasma membrane (PM) (9) (Figure 1B); while in the intracellular pathway, ciliogenesis is initiated by the trafficking and docking of membranes to the centriole in the cytoplasm (Figures 1A and 2). Cells with primary cilia such as mesenchymal cells, fibroblasts, retinal pigmented epithelial (RPE1) and photoreceptors, which likewise have 9 + 0 axonemes, are thought to use the intracellular pathway, while polarized epithelial cells with primary cilia or motile monocilia, and multiciliated cells (Figure 1C) may use the extracellular pathway. Membrane trafficking regulators have been linked to both ciliogenesis schemes.
i. Intracellular ciliogenesis assembly mechanisms
In the 1960’s, work by Sorokin using transmission electron microscopy (TEM) provided the first evidence that the primary cilium begins to assemble in the cytoplasm (10). Mother centrioles with a ~300nm vesicle were observed attached to the DAs that was referred to as the ciliary vesicle (CV) (Figures 1A and 2). Axonemal structures surrounded by a double membrane sheath were also observed, suggesting that the CV reorganizes to encase the axoneme. Renewed interest in this pathway began following the discovery that the membrane trafficking regulator RAB8 was associated with ciliogenesis in RPE1 cells (11,12). Clues for how the CV assembles once again came from electron microscopy observations in RPE1 cells and mouse rod photoreceptors that smaller ~50 nm diameter preciliary vesicles (PCVs) dock to DAs, referred to as distal appendage vesicles (DAVs), and likely fuse together to generate the CV (13,14). Factors important for membrane vesicle shaping and fusion, including EHD1/EHD3, were detected on DAVs suggesting a mechanism for how the CV is assembled (15). This work also demonstrated that the CP110 is removed in association with CV assembly stage (Figure 2).
Cells utilizing the intracellular pathway have been linked to the presence of a ciliary pocket (16–18) (Figure 1A). This membrane connects the base of the cilium to the plasma membrane. The ciliary pocket membrane is likely formed from the cytoplasmic face of the ciliary sheath membrane following fusion with the PM, which allows the cilium to emerge from the cell. However, little is known about how this fusion process occurs. We recently showed that membrane tubules form from the CV and sheath, and fuse to the PM (15). These tubular structures establish a membrane channel that exposes the ciliary membrane to the extracellular environment, called extracellular membrane channels (EMCs) (Figure 2), providing a possible molecular explanation for how the cilium emerges from the cytoplasm. EMC formation requires microtubules suggesting that these membranes are guided to the cell surface by the cytoskeleton. The observation that membrane trafficking regulators localizes to EMCs suggests that factors needed for ciliogenesis could be delivered to the developing cilium through these membranes, possibly originating from the PM. Based on these recent findings, it seems likely that further high-resolution structural studies of the intracellular ciliogenesis pathway will reveal new understanding about ciliary membrane assembly.
ii: Extracellular ciliogenesis pathways:
There is considerably less known about the processes regulating centriole docking to membranes in the extracellular pathway. As mentioned above, the absence of ciliary pockets is a hallmark of cells using the extracellular pathway, including some polarized epithelial cells, sperm and multiciliated cells (MCCs) (Figure 1B, C). As in the intracellular pathway, the mother centriole docks to the cell surface membrane via DAPs, triggering MC uncapping (19). However, unlike the EMC strategy used in intracellular ciliogenesis, centrioles are thought to be transported to cell surface membrane via a poorly understood mechanism, which appears to be cell/tissue specific.
Madin-Dardy canine kidney (MDCK) cells are polarized epithelial cells that may use the extracellular ciliogenesis pathway since the intracellular intermediates are not readily observed (20,21). In these cells cortical actin clearing and apical membrane partitioning proceds centriole docking to the plasma membrane (20,22). Membrane trafficking regulators important for the intracellular pathway are dispensable for extracellular ciliogenesis or to have different ciliogenesis associated function in MDCK cells (20,23). These observations indicate that membrane trafficking regulators could play distinct roles in the polarized epithelial cell ciliogenesis. An alternative model for the centrosome docking mechanism to the cell surface membrane that has been put forth suggests that the cytokinesis midbody remnant (MBR), a membrane inherited by one of the daughter cells, directs the site of ciliogenesis at the apical surface of MDCK cells (21). During cytokinesis, ingression of the cleavage furrow leads to the formation of a narrow membrane bridge connecting the two nascent daughter cells called the midbody (24), and following division the MBR, can remain attached to the PM in one of the daughter cells. Physical removal of the MBR reduces ciliogenesis in MDCK cells and the MBR contains IFT-B components and RAB8 (21), although the latter is consistent with non-ciliary functions of these proteins in cytokinesis (25). Upon cilium formation, the MBR is shed and therefore does not become part of the ciliary membrane. It is not clear how ciliogenesis initiation is controlled in this mechanism, in particular how MC uncapping is regulated if the centrioles are already docked to the MBR membrane.
In the case of MCCs, these cells can generate hundreds of centrioles via a deuterosome-mediated mechanism (Figure 1C). Following dissociation from deuterosomes, centrioles migrate and dock to the apical surface to nucleate motile cilia (19). Centriole migration and docking involves actin filaments but not microtubules. The molecular mechanisms of actin-dependent centriole migration have only recently begun to be illuminated. Multiple components of the planar cell polarity (PCP) signaling pathway govern the apical centriole migration, including core PCP proteins, such as Dishevelled (DVL) and Vangle2 (26,27), along with the PCP effector proteins Inturned (INTU), Fuzzy (FUZ), DRG1 and the small GTPase RhoA (28–30). Focal adhesion complex proteins, nucleotide binding protein 1 (NUBP1) and WDR5 were also shown to regulate the actin network for apical centriole migration and docking in Xenopus multiciliated cells (31,32). Notably, there have been limited investigations into membrane trafficking regulator roles in multiciliogenesis.
The sperm flagellum is a specialized ‘cilium’, which possesses additional structures including outer dense fibers and the fibrous sheath (33). Despite the importance of sperm flagella for fertilization, how the sperm flagellar structure is built is not well understood. During spermiogenesis, the cell surface membrane docked to the basal body-flagella complex invaginates and attaches to the nuclear envelope through a tail-head connecting piece at the concave implantation fossa (33). The manchette is another microtubule based structure that surrounds the elongating spermatid head and is important for nuclear shaping and sperm flagella formation (34). It is hypothesized that structural cargo proteins are transported through the manchette to the construction site in the flagellum by either the IFT-B complex along the microtubule tracks or via F-actin related transport. Membrane trafficking regulators have also been linked to the mammalian sperm tail assembly suggestive of an involvement of vesicle trafficking (34).
Interestingly, kidney epithelial mouse inner medullary collecting duct cells (mIMCD3) can use either the intracellular or extracellular pathway depending on cell densities (35,36). Small vesicles were also observed near the distal appendages of centrioles in MCCs by TEM (18). Moreover, multiciliogenesis is blocked in mouse airway MCCs when Chibby (CBY), a reported distal appendage protein, is removed and membrane vesicles consistent with DAV and CVs were detected near the centrioles (37). Consequently, these findings raise the question of whether cells can use both ciliogenesis mechanisms.
B. Organizing the basal body and cilium:
i. Centriole sub-distal and distal appendages
DAs are critical for membrane docking to the MC to initiate ciliogenesis, whereas SDAs appear to be dispensable (2,3). As with the 9-fold microtubule architecture observed in the centrioles and axonemes, there are 9 DAs and possibly 9 SDAs (Figure 2), although SDAs do not have a symmetrical arrangement (Figure 2) (38). In somatic cells, the older mother centriole has DAs and SDAs, while the younger daughter centriole does not, which determines the status of centriole maturation for the subsequent ciliation (Figure 2). These modifications are not added until the next cell cycle. In MCCs, DA and SDA assemblies proceed after centriole duplication (39,40) (Figure 1C).
To date, the only SDA protein essential for ciliogenesis is ODF2, with the caveat that it is also required for the assembly of DAs (41,42). ODF2 specifically interacts with RAB8A (12). While it has been proposed that microtubule anchoring to SDAs could serve as a route for vesicular membrane transport to the centrioles (10,12,41,43,44), other microtubules near the distal end of the MC could more directly deliver vesicles to the DAs (38). Cells using an extracellular pathway would likely not require SDAs for ciliogenesis due to direct docking of the centriole to the plasma membrane. In the case of motile cilia, the SDAs form the basal foot important for the direction of effective stroke of ciliary beating during movement (45,46).
DAPs identified to date include CEP164, CEP123/CEP89, CCDC41/CEP83, SCLT1, FBF1, C2CD3, ANKRD26 and CEP90, most of which are required for primary ciliogenesis (2,3,47,48). EM studies have shown that depletion of the CEP164, CEP89/CEP123, C2CD3, or CCDC41/CEP83 prevents vesicle docking to the MC and the removal of the MC cap. Phosphorylation of CEP83 by the Tau tubulin kinase 2 (TTBK2) kinase is required to initiate ciliogenesis (49). TTBK2 also phosphorylates CEP164, which inhibits the DAPs interaction with Dishevelled-3 (50,51), a protein important for apical docking and planar polarization of basal bodies in ciliogenesis of epithelial cells (52). Investigation of the structural organization of the DAs indicates that CCDC41/CEP83 and SCLT1 are located to the inner DA structure with FBF1, CEP164 and ANKRD26 decorating the outer surface, where vesicles were observed to dock to the MC (Figure 2) (38,48,53).
ii. Mother centriole uncapping mechanisms
Proteins localized to the distal end of centrioles including CP110 and CEP97 control centriole length and prevent axoneme formation in mammalian studies (3). CEP97 ciliogenesis function has also been linked to centriole stability (54). Removal of these proteins along with MPP9 specifically from the MC is associated with membrane vesicle docking to DAPs (Figure 2 ) (7), although it is not clear how membrane docking affects MC uncapping. This MC uncapping process requires TTBK2 and the proteasomal pathway (Figure 2) (55–57). While some of these factors localize to the MC, others are recruited upon initiation of ciliogenesis, suggestive of a directed transport mechanism. Under non-ciliation conditions, the motor protein KIF24 maintains MPP9 at the MC cap, and MPP9 affects CP110-CEP97 MC localization through direct binding to CEP97 (58). During ciliogenesis KIFC1 recruits TTBK2 to the MC (59) where it phosphorylates MPP9 on Ser629 resulting in its removal from the mother centriole via the ubiquitin-proteasome degradation (58). TTBK2 MC recruitment is also regulated by the phosphoinositide PI(4)P which associates with CEP164 and prevents TTBK2 binding to this DAP. MPP9 phosphorylation by TTBK2 triggers a ubiquitin proteasomal cascade whereby CUL3-RBX1-KCTD10 mediates CEP97 degradation and the E3 ligases HERC2, SCFcyclinF, the linear ubiquitin chain assembly complex (LUBAC), and EDD1-DYRK2-DDB1VPRBP have been linked to CP110 degradation during ciliogenesis (56,57,60–63). Recently, CP110 removal was shown to be associated with the autophagy-dependent degradation pathway mediated by LC3 and NUDCL2 in murine embryonic fibroblasts (MEF) (64). It is not clear if these proteolytic factors are already present at the MC prior to ciliogenesis initiation, although the HERC2 cofactor NEURL4 translocates from the daughter centriole to the MC during ciliogenesis (65). Given that blockage of ciliogenesis at the DAV stage can prevent MC uncapping, vesicular trafficking could direct factors to the MC needed to remove MC cap proteins. In addition to functioning as a suppressor of cilia formation, CP110 and CEP97 are also needed for ciliogenesis. Studies in CP110−/− mice MEFs display aberrant ciliary vesicle docking (66). Furthermore, CP110 is also required for primary and motile cilia formation in Xenopus (67). These results suggest that CP110 and CEP97 may function in the initial recruitment of PCVs to the MC.
iii. Transition zone assembly:
The TZ is critical for cilium formation and function and it helps to establish a unique protein and lipid composition through regulating the entry and exit of proteins and lipids at the cilium base (8). Predicted transmembrane domain containing TZ proteins such as TMEM67, TMEM216, TMEM231 and TMEM237 presumably link the ciliary membrane to the soluble TZ elements in the Y-shaped structures that are connected to axonemal microtubule doublets (8,68). TZ protein recruitment to the MC coincides with the appearance of the CV (Figure 2) (14,15), consistent with a mechanism whereby the TZ assembles prior to axoneme growth (Figure 2). The TZ proteins CBY and CEP290 have been linked to the docking of membranes to the DAPs and interact with membrane trafficking regulators associated with RAB8 (Figure 2)(37,69–71). Functional disruption of CEP290, CBY and other TZ proteins including TMEM67, AHI1, and DZIP1/DZIPL1 affects ciliogenesis at the CV stage (37,69,70,72–76). While it remains to be shown, it is anticipated that membrane transport of TZ factors represents an essential step in the progression of cilium assembly.
iv. Axoneme and ciliary membrane growth
Evidence of tight coupling between the formation of the ciliary membrane and the axoneme comes from the observation that ciliogenesis is stalled at the CV stage if regulators of either of these processes are disrupted (14,77–79). Upon the removal of the cap proteins from the MC, the centriolar A- and B-tubules extend to form the ciliary axoneme, whereas the C-tubule terminates within the distal centriole region (Figure 2)(80). During ciliogenesis axonemal tubulin is transported into the cilium via the IFT-B complex (81–83). IFT complexes are assembled at the ciliary base and trafficked toward the cilium tip with ciliary cargo proteins as well as the Bardet–Biedl syndrome (BBS) complex by the anterograde motor kinesin in association with the IFT-B subcomplex. At the ciliary tip, cargo proteins are unloaded and the IFT motor switches to the retrograde motor dynein associated with the of the IFT-A subcomplex, which mediates the return of the IFT complex and associated proteins to the base of the cilium (84). Exceptions to this rule of directional IFT transport have been reported (85). Mutations in the IFT-B complex components cause shortened or missing cilia, while mutations in the IFT-A complex components typically result in stumpy cilia that accumulate IFT-B proteins (86). IFT complexes are observed at the base of the cilium on “train’-like structures associated with adjoining cell surface membrane. Moreover, IFT-B components are known to associate with membrane vesicles and the Golgi (87,88), and have been shown to localize at the developing primary cilium with the appearance of ciliary membrane components (14). IFT-B complex proteins play a critical role in coordinating axoneme growth via interactions with molecular motors, ciliary cargos and the ciliary membrane (84,89). IFT-B proteins have also been shown to cooperate in cilia growth with the membrane trafficking associated ARF GTPase ARL13B (Figure 2)(83). Notably, ARL13B localizes to the ciliary membrane after the CV stage (90). IFT-B components and ARL13B also associate with Rab GTPases that have been linked to cilia membrane growth (Figure 2), which will be described in detail later in this review. Interestingly, phylogenetic analysis indicates that IFT complexes share homology with the coat protein I (COPI), important in regulating endosomal vesicular transport (25). IFT-coated vesicles carrying axonemal proteins have been observed at the base of the Chlamydomonas flagellum (91). Moreover, a recent study demonstrated that IFT-A components may function as a vesicle coat (92). IFT121/WDR35 was found to be important for coat formation on Golgi-associated vesicles associated with cilium elongation during ciliogenesis. Together these observations support a direct role of the IFT-B complex is modifying and regulating the delivery of PCVs to the developing cilium.
C. Ciliary membrane assembly mechanisms
Because the majority of what is understood about ciliary membrane assembly comes from studies of the intracellular pathway, findings discussed in this section will be assumed to be related to this mechanism unless otherwise stated.
i). Rabs in ciliogenesis:
Rabs are members of the Ras super family of small GTPases that function in membrane trafficking important for the biogenesis, transport, tethering, and fusion of membranous structures in the cell (5). Rabs associate with cellular membranes via COOH-terminal lipid modifications. In the active GTP bound state, Rabs bind to effector molecules that participate in membrane trafficking functions. Rab active states are controlled by guanine nucleotide exchange factors (GEFs) that promote GTP binding, and GTPase activating proteins (GAPs) which stimulate GTP hydrolysis to convert the Rab to the inactive GDP bound state. Several Rabs, Rab effectors, GEFs and GAPs have been linked to ciliogenesis (7, 101). In addition, RAB8A, RAB10, RAB27 and RABL2 have been linked to the mammalian sperm tail assembly (93–101).
In this section, we review what is known about Rab membrane trafficking regulation of ciliogenesis.
RAB11-RAB8 cascade:
RAB8 was the first membrane trafficking regulator shown to localize to the primary cilium and function in ciliogenesis (11,12,14). Its GEF, RABIN8, and GAP, TBC1D30, are also important for ciliogenesis. RAB11, a regulator of the endocytic recycling compartment (ERC), binds RABIN8 in a GTP-dependent manner and transports the GEF on PCVs to the mother centriole to activate RAB8, which is known as the RAB11-RAB8 cascade (Figure 2)(102,103). The RAB11 effector FIP3 forms a complex with RAB11-RABIN8 to regulate PCV transport to the MC (104,105). This RAB11-RABIN8-RAB8 cascade is also involved in regulating the Golgi-to-cilia rhodopsin transport carriers (RTCs) in photoreceptors (106,107).
Serum starvation induces PCV trafficking and ciliogenesis initiation (102). In cultured cells this process is regulated by the serum mitogen lysophosphatidic acid (LPA) signaling through LPA receptors and the downstream activation of the PI3K-Akt signaling (104). Akt directly phosphorylates the RAB11 effector WDR44 which stabilizes its binding to RAB11, preventing the formation of a PCV complex with FIP3 and RABIN8. Preciliary trafficking of RABIN8 to the MC also depends on microtubules and the TRAPPII complex protein TRAPPC14, which interacts with RABIN8 (Figure 2) (102,108). It is not clear if this MC directed transport occurs directly to the DAs, or to the more proximal MC SDA (Figure 2)(38,109). Although the SDA protein CEP128 mediates RAB11 association with the MC (110), CEP128 is dispensable for ciliogenesis (110) suggesting direct transport of PCVs may occur to the vicinity of the DAs. PCV docking to the MC is mediated by interactions between PCV proteins and DAPs. Biochemical studies have shown that RABIN8 interacts with CEP164 (51) and TRAPPC14 interacts with FBF1 and CEP83 (Figure 2)(108).
EM studies of RAB8A and RAB8B depleted cells indicate that this Rab is associated with the CV stage (Figure 2)(14), consistent with a model whereby RAB11 traffics RABIN8 on PCV to the MC to activate RAB8. This is supported by live cell imaging studies showing that RAB8 accumulates after preciliary membranes are detected at the MC (14). RAB8 activation by RABIN8 may also be controlled by Akt as it phosphorylates RABIN8 in the GEF domain, which was shown to affect RAB8 activation in vitro (104). The recruitment and activation of RAB8 at the developing cilia also requires TZ proteins (Figure 2), consistent with timing of TZ formation. DZIP1 regulates the dissociation of RAB8-GDP from GDI2, which is needed for RAB8 activation (111). CEP290 is also required for RAB8 targeting to the MC (69). A post CV role for RAB8 in cilia growth is supported by the observation that RAB8 overexpression promotes cilium elongation (102). Moreover, following ciliogenesis, RAB8 is lost from cilia (102), which is likely related to the cessation of RABIN8 PCV trafficking resulting from phosphorylation by the NDR2 kinase (102,112). Presumably this mechanism is important for establishing and maintaining proper cilium length, however it is not clear how RAB8 functions in these processes. Rabaptin5, a RAB8 effector, binds to the IFT-B protein IFT54 (Figure 2)(113), suggesting a possible mechanism whereby the IFT-B complex can associate with RAB8-associated vesicles.
Interestingly, MEFs from RAB8A and RAB8B knockout (KO) mice displayed normal ciliogenesis (114). However, depletion of the closely related RAB10 blocked ciliation in RAB8A+8B KO MEFs suggesting RAB8 ciliogenesis function can be compensated by another Rab (114). Notably, in another study, a triple KO of RAB8A/8B/10 in RPE1 cells only had a modest effect on ciliation (115). Together these results suggest that other Rabs that have been linked to ciliogenesis function after the CV stage could cooperate to grow the cilium.
RAB34:
RAB34 was functionally linked to cilia function by observations that RAB34 KO mice displayed characteristic defects associated with the cilium and Hedgehog signaling (116). Several groups have recently shown that RAB34 localizes to MC associated vesicles in the intracellular ciliogenesis pathway (Figure 2)(23,36,115,116), but was dispensable for ciliogenesis in MDCK cells. Disruption of DAs or TTBK2 reduces RAB34 accumulation at the MC (36). Interestingly, three-dimensional electron microscopy studies of RAB34-KO RPE1 cells detected PCV vesicles associated with the DAs, suggesting RAB34 functions to assemble the CV (23). RAB34 was not observed in the cilium or ciliary pocket, but rather is only observed on the ciliary sheath, which supports a specific function in the intracellular pathway needed to assemble the CV (23). Interestingly, RAB34 is dispensable for RABIN8 preciliary trafficking suggesting that RAB11 and RAB34 may transport discrete PCV cargo to the MC. Consistent with this idea, RAB34 localizes to the Golgi apparatus, and therefore it may be involved in trafficking ciliogenic cargo from this organelle (117). RAB34 was not required for MC uncapping and IFT recruitment (23,115), however, there is some discrepancy in the literature about its requirements in regulating EHD1 and ARL13B localization during ciliogenesis. Further evidence for discrete vesicle compartments for EHD proteins and RAB34 comes from observations that these proteins do not colocalize on membrane tubules associated with the developing cilia (36). While known RAB34 effectors RILPL1 and RILPL2 are important for ciliogenesis (118), their functions were not associated with RAB34 (36). Thus, future investigations into RAB34 ciliogenesis effectors will be important for understanding how this Rab specifically functions in ciliogenesis.
Other Rabs involved in ciliogenesis:
RAB10 is present in the primary cilia of epithelial MDCK and pig kidney LLC-PK1 cells and in rodent renal tubules (119). As mentioned, RAB10 depletion reduces ciliation in RAB8A+8B KO MEFs, however, in RPE1 and human lung adenocarcinoma A549 cells RAB10 depletion promotes ciliation (120). The LRRK2 kinase, which is associated with Parkinson’s disease, phosphorylates RAB10 promoting its association with the effector RILPL1 on pericentriolar membranes, which inhibits ciliogenesis by preventing MC uncapping (Figure 2)(121). While it is not clear how RAB10 and RILPL1 prevents ciliogenesis, they could be influencing RAB8 or RAB34 function in ciliogenesis as RILPL1 can also bind RAB8 and RAB34 as an effector (120,122). Opposing ciliogenesis functions of RAB10 and RAB8 could also be mediated by RABIN8 which can activate both Rabs (123,124).
RAB 19 is required for ciliogenesis in the intracellular and the extracellular pathway (20). In MDCK cells, RAB19 ciliogenesis function is associated with the RAB-GAP TBC1D4 and the homotypic fusion and protein sorting (HOPS) complex, which coordinate cortical actin clearing. In RPE1 cells, RAB 19 depleted cells were able to establish the CV (Figure 2) suggesting this Rab functions in cilia growth associated with actin-regulation of ciliogenesis.
RAB23 was originally linked to the cilium based on observations it regulates Hedgehog signaling (125). RAB23 also localizes to the cilium, however the evidence that this protein regulates ciliation via the intracellular pathway is less clear (Figure 2). RAB23 dominant-negative expression reduces cilium length in mouse fibroblast NIH 3T3 cells (126) and Evi5L, a putative RAB23 GAP important for ciliogenesis in RPE1 cells (12). RAB23 KO in granule precursor cells in mice brains showed reduced ciliation but paradoxically caused upregulated Hedgehog signaling (127). Interestingly, ciliation in IMCD3 cells requires RAB23 and its GEF complex INTU and FUZ, which are components of the ciliogenesis and planar cell polarity effector (CPLANE) complex (128). INTU and FUZ are required to activate RAB23 at post-CV ciliogenic stages, following RAB8 accumulation. Interestingly, INTU also interacts with another GTPase, RSG1, which is needed for assembly of the axoneme (79), suggesting another possible link between ciliary membrane and axoneme growth.
RAB29 localizes to a vesicular compartment at the base of the cilium in NIH-3T3 and IMCD cells and is required for proper cilium length in these cells (129). The mechanism for how RAB29 functions in cilia growth is not known although it interacts with RAB8, RAB11 and IFT20.
RAB35 localizes to the cilium and functions in regulating cilium length (130). Moreover, the RAB35 GEF, DENND1B, and GAP, TBC1D10A regulate RAB35 ciliary localization and function (130). RAB35 affects ciliary ARL13B and the phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2] lipid levels suggesting it could function with axonemal IFT-B complex through ARL13B in coordinating growth of the axoneme and the ciliary membrane.
Finally, the Rab-like small GTPases RABL5/IFT22 and RABL4/IFT27 are components of the IFT-A and IFT-B complexes, respectively, while RABL2 regulates ciliary transport (101). Functional studies revealed that RABL4 and RABL2 are required for sperm flagella formation but MEFs derived from RABL4 or RABL2 KO mice exhibit normal ciliogenesis. Direct roles for these proteins in the regulation of vesicle trafficking in ciliogenesis remain to be determined.
ii). ARF GTPases and ciliogenesis
ARFs family members also regulate membrane trafficking in ciliogenesis and ciliary membrane transport (6). Several ARFs have been linked to the regulation of ciliary membrane cargo, and ARL13B, ARL16, and ARF6 function in ciliogenesis (131–134). Studies in multiple organisms have shown that ablation of ARL13B function leads to short cilia and defects in the ciliary axoneme structures (6). As mentioned previously, ARL13B ciliogenesis function may be attributed to its association with the IFT-B complex and RAB proteins (Figure 2). ARL13B can also function as a GEF for ARL3 (135,136), and disruptions of ARL13B or ARL3 lead to defective outer segment formation in retinal photoreceptors (137). Finally, ARL13B associates with the ciliary axoneme through direct interaction with tubulin, which may explain how ciliary membrane proteins are anchored to the axoneme (138). Studies in ARL16 KO MEFs indicate that this protein functions downstream of MC uncapping and it has been linked to Golgi transport of IFT-B component (131). Similar to findings with ARL13B and ARL16, it has recently been shown that the ARF GAPs ELMOD1 and ELMOD3 function in ciliation downstream of MC uncapping (139). Likewise, ARF6 and its GEF EFA6A are required for MC uncapping and may also be associated with downstream ARL13B and RAB8 ciliogenesis function (Figure 2)(134). Together, these observations support functions for ARFs in ciliary extension in concert with the IFT-B complex.
iii). Shaping the developing ciliary membrane:
The endosomal trafficking and membrane shaping factors EHD1 and EHD3, along with their binding partners MICAL-L1 and the F-BAR containing PACSIN1 and −2 proteins function in the assembly of the CV from DAVs (Figure 2) (14,15,140). Depletion of these proteins prevents MC uncapping in RPE1 cells (Figure 2), raising the possibility that docking of DAVs and MC capping processes are linked. It remains to be seen if DAVs could help recruit factors needed for the proteolytical degradation of CP110-CEP97-MPP9. These membrane shaping factors also localize to the CV and the sheath membranes and are associated with the tubulation of these membranes that generate EMCs. As these proteins are not observed in the ciliary membrane, which has a negative curvature, these different ciliary localizations indicate a preference for positive curvature membranes, as would be expected for F-BAR associated proteins. The F-BAR domain in PACSIN proteins can sense and/or establish positive membrane curvature (141) and Eps15 homology domain proteins EHD1 and EHD3 recruit PACSINs to membranes, but may also influence membrane shaping directly through partial insertion into bilayers (142). Membrane shaping is likely to be important for reorganizing the CV from a spherical-like structure into the double membrane ciliary sheath, with both positive and negative membrane curvatures, that become the ciliary pocket and ciliary membrane. One possibility is that the establishment of the TZ at the CV stage prevents EHD, PACSIN and MICAL-1 from interfering with the formation of the negative membrane curvature of the ciliary membrane that opposes the axoneme (Figure 2). The TZ protein FAM92 also has a BAR domain but its role in ciliary membrane shaping remains to be established (143). IFT172 remodels membranes in vitro, suggesting that IFT-B components could be involved in shaping the developing ciliary membrane (144). Establishment of ciliary membrane curvature could also involve an unknown I-Bar protein to generate its negative curvature. However, ciliary membrane association with the axoneme could be sufficient to generate the membrane curvature observed inside the cilium.
iv). Transport, targeting, tethering, and fusion in ciliogenesis:
PCV transport to the MC during ciliogenesis is regulated by the motor protein Myosin Va, an actin-based motor protein (Figure 2)(35,145). However, the actin cytoskeleton prevents cilium growth (77,146–148). Although, inhibition of actin polymerization with cytochalasin D is able to rescue ciliogenesis defects induced by depletion of TZ and IFT-B proteins (149). Interestingly, RILPL2 and LLRK2-phosporylated RAB10 interacts with Myosin Va, which may block the motor’s ciliogenesis function (150). Myosin Va also localizes to the manchette and manchette-associated vesicles during spermiogenesis (94,95). Interestingly, Myosin Va is not associated with RABIN8, EHD1 and RAB34 preciliary trafficking further suggesting that more than one type of PCVs may be involved in cilium assembly (35). The Golgi-associated motors dynein-2 and kinesin-14 (KIFC1) are also important for ciliogenesis (59,151–153), but connections to ciliogenesis PCV trafficking has not been investigated.
The assembly of the CV and downstream ciliogenesis membrane structures requires fusion of PCVs (Figure 2). Membrane vesicle fusion requires four SNAREs that form a transcomplex between two membrane compartments in order to overcome the electrostatic repulsive forces of the lipid head groups on these membranes. This SNARE complex is comprised of one helix from an R-SNARE, one from a Syntaxin (Qa-SNARE), and two from the Qb, Qc and/or Qbc SNAREs (154). Presently, SNAP29, a Qbc SNARE, is the only SNARE that has been identified to function at the CV stage and is recruited to DAVs by EHD1 (14). The R-SNARE VAMP7 is required for ciliogenesis in MDCK cells, but it is not known what ciliogenesis process is affected (155). Clues for membrane fusion mechanisms during ciliogenesis could come from studies in photoreceptors, where it has been shown that VAMP7, which interacts with RAB11, RAB8, and RABIN8, cooperates with Syntaxin 3 and SNAP25, to regulate fusion of RTCs (156).
Larger molecular weight complexes are important in targeting membrane vesicles to specific membranes for fusion, and can serve as tethers prior to SNAREs engagement. Evidence for involvement of multimeric complexes in ciliogenesis include the exocyst, TRAPPII, and HOPS complexes (20,102,108,112,157,158). The TRAPPII complex functions in recruiting RABIN8-PCVs to the mother centriole (Westlake et al., 2011; Cuenca et al., 2019). Interestingly, the TRAPPII component TRAPPC14 interacts with the DAPs CEP83 and FBF1 suggesting tethering complexes could participate in docking of PCVs to the MC structures (Figure 2)(108). The targeting/tethering function of large molecule weight complexes appears to also be important in the coordination of ciliary membrane and axoneme growth. The exocyst component Sec15 is an effector of RAB8 that is needed for ciliogenesis, and is coupled to the regulation of RABIN8 function (Figure 2)(159). The exocyst regulator SEC10 accumulates in the cilium and associates with ciliogenic proteins IFT20 and IFT88 (158,160). Moreover, the exocyst ciliogenic function is associated with ARL13B (90,161,162).
v). Populating ciliary lipids
The ciliary membrane is continuous with the plasma membrane. However, compared with the adjacent plasma membrane, the ciliary membrane contains high levels of PI(4)P while PI(4,5)P2 is largely excluded. The latter is required for ciliary protein localization and Hedgehog signaling (163,164). PI(4)P is also enriched in the ERC and in Golgi-derived vesicles which supports the involvement of these compartments in delivering membranes to the developing cilium (165,166). As mentioned, the TZ is important for the lipid composition of the ciliary membrane. The unique ciliary distribution of lipids is also established by a phosphoinositide 5-phosphatase, INPP5E, which is recruited to the cilium and hydrolyzes PI(4,5)P2 and PI(3,4,5)P3 to generate PI(4)P (167), The ciliary targeting of INPP5E is facilitated by its interactions with prenyl-binding protein phosphodiesterase 6D (PDE6D), ARL13B and CEP164 (168). One prediction is that the population of lipids in the ciliary membrane begins early in ciliogenesis coinciding with recruitment of TZ proteins associated with CV establishment. However, PI(4)P and INPP5E prevent ciliogenesis initiation suggesting that membrane vesicles associated with early ciliogenesis stages would have a different phosphatidylinositol composition (169). Careful analysis of MC PIs in conjunction with membrane trafficking regulators targeting to the MC could shed light on the mechanism associated with building the unique ciliary membrane.
D. Autophagy links to ciliogenesis:
Autophagy is an intracellular degradation process mediated by lysosomes and has recently been linked to ciliogenesis (170). Notably, both ciliogenesis and autophagy are triggered by serum starvation in cultured cells. Membrane trafficking regulators important for ciliogenesis have also been associated with autophagy, including RAB8, RAB11, RAB23 and RAB34 (171,172). Furthermore, SNAP29 is essential for SNARE mediated fusion of the autophagosome with lysosomes (173,174). Interestingly, IFT20-deficient cells displayed defects in autophagy that was ascribed to dysfunction of the lysosome (175,176). Consistent with this role, IFT20 is associated with the basal body localization of pallidin (177), a subunit of biogenesis of lysosome-related organelles complex-1 (BLOC-1), which regulates lysosome maturation (178,179). These findings suggest the IFT-B complex could help target BLOC-1 endosomes to the basal body for an as yet to be determined function in ciliogenesis membrane assembly.
Autophagy has both been proposed to be a both a positive and negative regulator for ciliogenesis. Blockage of autophagy enhances primary cilia growth through preventing the degradation of proteins required for intraflagellar transport (180,181). In contrast, the ciliogenesis regulator OFD1 is degraded by autophagy following serum starvation to promote ciliogenesis (182). Moreover, removal of CP110 from the MC has been recently linked to autophagic degradation pathway (Figure 2)(64). Selective autophagy-dependent degradation of CP110 at the MC is regulated by LC3 via the autophagy receptor NUDCL2. In addition, autophagic degradation of NEK9 and MYH9 is required for ciliogenesis via affecting actin dynamics (183).
While more direct links to lysosomal trafficking regulators are not known, they should be considered in future investigations of ciliogenesis. Notably, in Niemann–Pick type C1 (NPC1) disease, characterized by lysosomal cholesterol accumulation, alteration of the primary cilium has been reported suggesting cholesterol transport or distribution could be tightly coupled with ciliogenesis (184). TMEM135, a peroxisome localized protein, affects lysosome–peroxisome contact, causing lysosomal cholesterol accumulation, which affects primary ciliogenesis. This led to defects in RAB8 trafficking and activation needed for CV extension, which could be partially rescued by treating TMEM135 depleted cells with the cholesterol–cyclodextrin complex (185). Together, these observations suggest that membrane trafficking process in autophagy may help coordinate early ciliogenesis initiation by remodeling the MC to enable cilium growth.
Conclusion
Cilia/flagella are an evolutionarily conserved organelle implicated in multiple cellular pathways, which makes the process of cilium biogenesis complex with a high probability of protein functional redundancies. In this review, we summarize current knowledge of membrane trafficking regulators in different ciliogenesis pathways adapted in cell types. The critical nature of membrane trafficking in cilium functioning is evident by findings that mutations in ARL13B, RAB23, and the exocyst subunits EXOC8 and SEC8 cause or have been linked to ciliopathy (186–189). Furthermore, mutations in ciliogenic factors associated with membrane trafficking including INPP5E (190), IFT172 (191,192), and transition zone proteins (1) and DAPs (193) also cause ciliopathy. Notably, ciliopathy causing mutations in TZ protein CEP290 have been shown to affect ciliogenesis at the CV stage (76). Although numerous membrane trafficking regulators have been characterized in ciliogenesis over the past decade, our understanding of this process is still limited. Most of what is known about ciliogenesis has come from in vitro studies in cells that utilize the intracellular assembly scheme. Consequently, broader investigations are needed that examine the organization and dynamics of membrane compartments and their trafficking regulators that coordinate with centrioles to assemble the cilium in different cell types and tissues. Key areas for future investigation include characterizing the ciliogenesis pathways adapted by epithelial cells, multiciliated cells and sperm cells and determining the molecular mechanism of ciliogenesis in in vivo physiological process such as embryogenesis and organogenesis.
Acknowledgement
We thank Joseph Meyer for assistance in preparing figures and Drs. Christine Kettenhofen, Cherry Liu, and Ipsita Saha for critical reading of the manuscript. The authors are funded by the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary
- BB
basal body
- BBS
Bardet–Biedl syndrome
- BLOC-1
biogenesis of lysosome-related organelles complex-1
- CBY
Chibby
- COPI
coat protein I
- CPLANE
ciliogenesis and planar cell polarity effector
- CV
ciliary vesicle
- DA
distal appendage
- DAP
distal appendage protein
- DAV
distal appendage vesicle
- DVL
Dishevelled
- EMC
extracellular membrane channel
- ERC
endocytic recycling compartment
- FUZ
Fuzzy
- GAP
GTPase activating protein
- GEF
guanine nucleotide exchange factor
- HOPS
homotypic fusion and protein sorting
- IFT
intraflagellar transport
- INPP5E
inositol polyphosphate-5-phosphotase E
- INTU
Inturned
- KO
knockout
- LPA
lysophosphatidic acid
- LUBAC
linear ubiquitin chain assembly complex
- MBR
midbody remnant
- MC
mother centriole
- MCC
multiciliated cell
- MDCK
Madin-Dardy canine kidney
- MEF
murine embryonic fibroblast
- mIMCD3
mouse inner medullary collecting duct cells
- NPC1
Niemann–Pick type C1
- NUBP1
nucleotide binding protein 1
- PCP
planar cell polarity
- PCV
preciliary vesicle
- PDE6D
prenyl-binding protein phosphodiesterase 6D
- PIPKIγ
type Iγ phosphatidylinositol 4-phosphate [PI(4)P] 5-kinase
- PM
plasma membrane
- RPE1
retinal pigment epithelial 1 cell
- RTC
rhodopsin transport carrier
- SDA
subdistal appendage
- TEM
transmission electron microscopy
- TTBK2
Tau tubulin kinase 2
- TZ
transition zone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Reiter JF, and Leroux MR (2017) Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol 18, 533–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar D, and Reiter J (2021) How the centriole builds its cilium: of mothers, daughters, and the acquisition of appendages. Curr Opin Struct Biol 66, 41–48 [DOI] [PubMed] [Google Scholar]
- 3.Breslow DK, and Holland AJ (2019) Mechanism and Regulation of Centriole and Cilium Biogenesis. Annu Rev Biochem 88, 691–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson KA, and Rosenbaum JL (1992) Replication of basal bodies and centrioles. Curr Opin Cell Biol 4, 80–85 [DOI] [PubMed] [Google Scholar]
- 5.Homma Y, Hiragi S, and Fukuda M (2021) Rab family of small GTPases: an updated view on their regulation and functions. FEBS J 288, 36–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher S, Kuna D, Caspary T, Kahn RA, and Sztul E (2020) ARF family GTPases with links to cilia. Am J Physiol Cell Physiol 319, C404–C418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shakya S, and Westlake CJ (2021) Recent advances in understanding assembly of the primary cilium membrane. Fac Rev 10, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goncalves J, and Pelletier L (2017) The Ciliary Transition Zone: Finding the Pieces and Assembling the Gate. Mol Cells 40, 243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, and Dynlacht BD (2018) The regulation of cilium assembly and disassembly in development and disease. Development 145, dev151407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorokin S (1962) Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol 15, 363–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, and Jackson PK (2007) A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129, 1201–1213 [DOI] [PubMed] [Google Scholar]
- 12.Yoshimura S, Egerer J, Fuchs E, Haas AK, and Barr FA (2007) Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol 178, 363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedmak T, and Wolfrum U (2011) Intraflagellar transport proteins in ciliogenesis of photoreceptor cells. Biol Cell 103, 449–466 [DOI] [PubMed] [Google Scholar]
- 14.Lu Q, Insinna C, Ott C, Stauffer J, Pintado PA, Rahajeng J, Baxa U, Walia V, Cuenca A, Hwang YS, Daar IO, Lopes S, Lippincott-Schwartz J, Jackson PK, Caplan S, and Westlake CJ (2015) Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat Cell Biol 17, 228–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Insinna C, Lu Q, Teixeira I, Harned A, Semler EM, Stauffer J, Magidson V, Tiwari A, Kenworthy AK, Narayan K, and Westlake CJ (2019) Investigation of F-BAR domain PACSIN proteins uncovers membrane tubulation function in cilia assembly and transport. Nat Commun 10, 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chemes HE, Puigdomenech ET, Carizza C, Olmedo SB, Zanchetti F, and Hermes R (1999) Acephalic spermatozoa and abnormal development of the head-neck attachment: a human syndrome of genetic origin. Hum Reprod 14, 1811–1818 [DOI] [PubMed] [Google Scholar]
- 17.Rangel L, Bernabe-Rubio M, Fernandez-Barrera J, Casares-Arias J, Millan J, Alonso MA, and Correas I (2019) Caveolin-1alpha regulates primary cilium length by controlling RhoA GTPase activity. Sci Rep 9, 1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorokin SP (1968) Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci 3, 207–230 [DOI] [PubMed] [Google Scholar]
- 19.Spassky N, and Meunier A (2017) The development and functions of multiciliated epithelia. Nat Rev Mol Cell Biol 18, 423–436 [DOI] [PubMed] [Google Scholar]
- 20.Jewett CE, Soh AWJ, Lin CH, Lu Q, Lencer E, Westlake CJ, Pearson CG, and Prekeris R (2021) RAB19 Directs Cortical Remodeling and Membrane Growth for Primary Ciliogenesis. Dev Cell 56, 325–340 e328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernabe-Rubio M, Andres G, Casares-Arias J, Fernandez-Barrera J, Rangel L, Reglero-Real N, Gershlick DC, Fernandez JJ, Millan J, Correas I, Miguez DG, and Alonso MA (2016) Novel role for the midbody in primary ciliogenesis by polarized epithelial cells. J Cell Biol 214, 259–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis SS, Sfakianos J, Lo B, and Mellman I (2011) A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. J Cell Biol 193, 219–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganga AK, Kennedy MC, Oguchi ME, Gray S, Oliver KE, Knight TA, De La Cruz EM, Homma Y, Fukuda M, and Breslow DK (2021) Rab34 GTPase mediates ciliary membrane formation in the intracellular ciliogenesis pathway. Curr Biol 31, 2895–2905 e2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labat-de-Hoz L, Rubio-Ramos A, Casares-Arias J, Bernabe-Rubio M, Correas L, and Alonso MA (2020) A Model for Primary Cilium Biogenesis by Polarized Epithelial Cells: Role of the Midbody Remnant and Associated Specialized Membranes. Front Cell Dev Biol 8, 622918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, and Huang K (2019) Dissecting the Vesicular Trafficking Function of IFT Subunits. Front Cell Dev Biol 7, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park TJ, Mitchell BJ, Abitua PB, Kintner C, and Wallingford JB (2008) Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet 40, 871–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vladar EK, Bayly RD, Sangoram AM, Scott MP, and Axelrod JD (2012) Microtubules enable the planar cell polarity of airway cilia. Curr Biol 22, 2203–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park TJ, Haigo SL, and Wallingford JB (2006) Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet 38, 303–311 [DOI] [PubMed] [Google Scholar]
- 29.Pan J, You Y, Huang T, and Brody SL (2007) RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. J Cell Sci 120, 1868–1876 [DOI] [PubMed] [Google Scholar]
- 30.Lee M, Hwang YS, Yoon J, Sun J, Harned A, Nagashima K, and Daar IO (2019) Developmentally regulated GTP-binding protein 1 modulates ciliogenesis via an interaction with Dishevelled. J Cell Biol 218, 2659–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith CEL, Lake AVR, and Johnson CA (2020) Primary Cilia, Ciliogenesis and the Actin Cytoskeleton: A Little Less Resorption, A Little More Actin Please. Front Cell Dev Biol 8, 622822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulkarni SS, Griffin JN, Date PP, Liem KF Jr., and Khokha MK (2018) WDR5 Stabilizes Actin Architecture to Promote Multiciliated Cell Formation. Dev Cell 46, 595–610 e593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu B, Gao H, Liu C, and Li W (2020) The coupling apparatus of the sperm head and tail†. Biology of Reproduction 102, 988–998 [DOI] [PubMed] [Google Scholar]
- 34.Lehti MS, and Sironen A (2016) Formation and function of the manchette and flagellum during spermatogenesis. Reproduction 151, R43–54 [DOI] [PubMed] [Google Scholar]
- 35.Wu CT, Chen HY, and Tang TK (2018) Myosin-Va is required for preciliary vesicle transportation to the mother centriole during ciliogenesis. Nat Cell Biol 20, 175–185 [DOI] [PubMed] [Google Scholar]
- 36.Stuck MW, Chong WM, Liao JC, and Pazour GJ (2021) Rab34 is necessary for early stages of intracellular ciliogenesis. Curr Biol 31, 2887–2894 e2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burke MC, Li FQ, Cyge B, Arashiro T, Brechbuhl HM, Chen X, Siller SS, Weiss MA, O’Connell CB, Love D, Westlake CJ, Reynolds SD, Kuriyama R, and Takemaru K (2014) Chibby promotes ciliary vesicle formation and basal body docking during airway cell differentiation. J Cell Biol 207, 123–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chong WM, Wang WJ, Lo CH, Chiu TY, Chang TJ, Liu YP, Tanos B, Mazo G, Tsou MB, Jane WN, Yang TT, and Liao JC (2020) Super-resolution microscopy reveals coupling between mammalian centriole subdistal appendages and distal appendages. Elife 9, e53580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao H, Chen Q, Fang C, Huang Q, Zhou J, Yan X, and Zhu X (2019) Parental centrioles are dispensable for deuterosome formation and function during basal body amplification. EMBO Rep 20, e46735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao H, Chen Q, Li F, Cui L, Xie L, Huang Q, Liang X, Zhou J, Yan X, and Zhu X (2021) Fibrogranular materials function as organizers to ensure the fidelity of multiciliary assembly. Nat Commun 12, 1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishikawa H, Kubo A, Tsukita S, and Tsukita S (2005) Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat Cell Biol 7, 517–524 [DOI] [PubMed] [Google Scholar]
- 42.Hall NA, and Hehnly H (2021) A centriole’s subdistal appendages: contributions to cell division, ciliogenesis and differentiation. Open Biol 11, 200399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hehnly H, Chen CT, Powers CM, Liu HL, and Doxsey S (2012) The centrosome regulates the Rab11- dependent recycling endosome pathway at appendages of the mother centriole. Curr Biol 22, 1944–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang J, Seo SG, Lee KH, Nagashima K, Bang JK, Kim BY, Erikson RL, Lee KW, Lee HJ, Park JE, and Lee KS (2013) Essential role of Cenexin1, but not Odf2, in ciliogenesis. Cell Cycle 12, 655–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen QPH, Liu Z, Albulescu A, Ouyang H, Zlock L, Coyaud E, Laurent E, Finkbeiner W, Moraes TJ, Raught B, and Mennella V (2020) Comparative Super-Resolution Mapping of Basal Feet Reveals a Modular but Distinct Architecture in Primary and Motile Cilia. Dev Cell 55, 209–223 e207 [DOI] [PubMed] [Google Scholar]
- 46.Ryu H, Lee H, Lee J, Noh H, Shin M, Kumar V, Hong S, Kim J, and Park S (2021) The molecular dynamics of subdistal appendages in multi-ciliated cells. Nat Commun 12, 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar D, Rains A, Herranz-Perez V, Lu Q, Shi X, Swaney DL, Stevenson E, Krogan NJ, Huang B, Westlake C, Garcia-Verdugo JM, Yoder BK, and Reiter JF (2021) A ciliopathy complex builds distal appendages to initiate ciliogenesis. J Cell Biol 220, e202011133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang TT, Chong WM, Wang WJ, Mazo G, Tanos B, Chen Z, Tran TMN, Chen YD, Weng RR, Huang CE, Jane WN, Tsou MB, and Liao JC (2018) Super-resolution architecture of mammalian centriole distal appendages reveals distinct blade and matrix functional components. Nat Commun 9, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lo CH, Lin IH, Yang TT, Huang YC, Tanos BE, Chou PC, Chang CW, Tsay YG, Liao JC, and Wang WJ (2019) Phosphorylation of CEP83 by TTBK2 is necessary for cilia initiation. J Cell Biol 218, 3489–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cajanek L, and Nigg EA (2014) Cep164 triggers ciliogenesis by recruiting Tau tubulin kinase 2 to the mother centriole. Proc Natl Acad Sci U S A 111, E2841–2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt KN, Kuhns S, Neuner A, Hub B, Zentgraf H, and Pereira G (2012) Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J Cell Biol 199, 1083–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oda T, Chiba S, Nagai T, and Mizuno K (2014) Binding to Cep164, but not EB1, is essential for centriolar localization of TTBK2 and its function in ciliogenesis. Genes Cells 19, 927–940 [DOI] [PubMed] [Google Scholar]
- 53.Bowler M, Kong D, Sun S, Nanjundappa R, Evans L, Farmer V, Holland A, Mahjoub MR, Sui H, and Loncarek J (2019) High-resolution characterization of centriole distal appendage morphology and dynamics by correlative STORM and electron microscopy. Nat Commun 10, 993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dobbelaere J, Schmidt Cernohorska M, Huranova M, Slade D, and Dammermann A (2020) Cep97 Is Required for Centriole Structural Integrity and Cilia Formation in Drosophila. Curr Biol 30, 3045–3056 e3047 [DOI] [PubMed] [Google Scholar]
- 55.Bernatik O, Pejskova P, Vyslouzil D, Hanakova K, Zdrahal Z, and Cajanek L (2020) Phosphorylation of multiple proteins involved in ciliogenesis by Tau Tubulin kinase 2. Mol Biol Cell 31, 1032–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagai T, Mukoyama S, Kagiwada H, Goshima N, and Mizuno K (2018) Cullin-3-KCTD10-mediated CEP97 degradation promotes primary cilium formation. J Cell Sci 131, jcs219527. [DOI] [PubMed] [Google Scholar]
- 57.Spektor A, Tsang WY, Khoo D, and Dynlacht BD (2007) Cep97 and CP110 suppress a cilia assembly program. Cell 130, 678–690 [DOI] [PubMed] [Google Scholar]
- 58.Huang N, Zhang D, Li F, Chai P, Wang S, Teng J, and Chen J (2018) M-Phase Phosphoprotein 9 regulates ciliogenesis by modulating CP110-CEP97 complex localization at the mother centriole. Nat Commun 9, 4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee SH, Joo K, Jung EJ, Hong H, Seo J, and Kim J (2018) Export of membrane proteins from the Golgi complex to the primary cilium requires the kinesin motor, KIFC1. FASEB J 32, 957–968 [DOI] [PubMed] [Google Scholar]
- 60.D’Angiolella V, Donato V, Vijayakumar S, Saraf A, Florens L, Washburn MP, Dynlacht B, and Pagano M (2010) SCF(Cyclin F) controls centrosome homeostasis and mitotic fidelity through CP110 degradation. Nature 466, 138–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al-Hakim AK, Bashkurov M, Gingras AC, Durocher D, and Pelletier L (2012) Interaction proteomics identify NEURL4 and the HECT E3 ligase HERC2 as novel modulators of centrosome architecture. Mol Cell Proteomics 11, M111 014233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen XL, Yuan JF, Qin XH, Song GP, Hu HB, Tu HQ, Song ZQ, Li PY, Xu YL, Li S, Jian XX, Li JN, He CY, Yu XP, Liang LY, Wu M, Han QY, Wang K, Li AL, Zhou T, Zhang YC, Wang N, and Li HY (2022) LUBAC regulates ciliogenesis by promoting CP110 removal from the mother centriole. J Cell Biol 221, e202105092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hossain D, Javadi Esfehani Y, Das A, and Tsang WY (2017) Cep78 controls centrosome homeostasis by inhibiting EDD-DYRK2-DDB1(Vpr)(BP). EMBO Rep 18, 632–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu M, Zhang W, Li M, Feng J, Kuang W, Chen X, Yang F, Sun Q, Xu Z, Hua J, Yang C, Liu W, Shu Q, Yang Y, Zhou T, and Xie S (2021) NudCL2 is an autophagy receptor that mediates selective autophagic degradation of CP110 at mother centrioles to promote ciliogenesis. Cell Res, 31, 1199–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loukil A, Tormanen K, and Sutterlin C (2017) The daughter centriole controls ciliogenesis by regulating Neurl-4 localization at the centrosome. J Cell Biol 216, 1287–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yadav SP, Sharma NK, Liu C, Dong L, Li T, and Swaroop A (2016) Centrosomal protein CP110 controls maturation of the mother centriole during cilia biogenesis. Development 143, 1491–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walentek P, Quigley IK, Sun DI, Sajjan UK, Kintner C, and Harland RM (2016) Ciliary transcription factors and miRNAs precisely regulate Cp110 levels required for ciliary adhesions and ciliogenesis. Elife 5, e17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gogendeau D, Lemullois M, Le Borgne P, Castelli M, Aubusson-Fleury A, Arnaiz O, Cohen J, Vesque C, Schneider-Maunoury S, Bouhouche K, Koll F, and Tassin AM (2020) MKS-NPHP module proteins control ciliary shedding at the transition zone. PLoS Biol 18, e3000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim J, Krishnaswami SR, and Gleeson JG (2008) CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet 17, 3796–3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kobayashi T, Kim S, Lin YC, Inoue T, and Dynlacht BD (2014) The CP110-interacting proteins Talpid3 and Cep290 play overlapping and distinct roles in cilia assembly. J Cell Biol 204, 215–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu Z, Pang N, Zhang Y, Chen H, Peng Y, Fu J, and Wei Q (2020) CEP290 is essential for the initiation of ciliary transition zone assembly. PLoS Biol 18, e3001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Munoz-Estrada J, and Ferland RJ (2019) Ahil promotes Arl13b ciliary recruitment, regulates Arl13b stability and is required for normal cell migration. J Cell Sci 132, jcs230680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lapart JA, Gottardo M, Cortier E, Duteyrat JL, Augiere C, Mange A, Jerber J, Solassol J, Gopalakrishnan J, Thomas J, and Durand B (2019) Dzip1 and Fam92 form a ciliary transition zone complex with cell type specific roles in Drosophila. Elife 8, e49307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu H, Galeano MCR, Ott E, Kaeslin G, Kausalya PJ, Kramer C, Ortiz-Bruchle N, Hilger N, Metzis V, Hiersche M, Tay SY, Tunningley R, Vij S, Courtney AD, Whittle B, Wuhl E, Vester U, Hartleben B, Neuber S, Frank V, Little MH, Epting D, Papathanasiou P, Perkins AC, Wright GD, Hunziker W, Gee HY, Otto EA, Zerres K, Hildebrandt F, Roy S, Wicking C, and Bergmann C (2017) Mutations in DZIP1L, which encodes a ciliary-transition-zone protein, cause autosomal recessive polycystic kidney disease. Nat Genet 49, 1025–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang C, Li J, Takemaru KI, Jiang X, Xu G, and Wang B (2018) Centrosomal protein Dzip1l binds Cby, promotes ciliary bud formation, and acts redundantly with Bromi to regulate ciliogenesis in the mouse. Development 145, dev164236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shimada H, Lu Q, Insinna-Kettenhofen C, Nagashima K, English MA, Semler EM, Mahgerefteh J, Cideciyan AV, Li T, Brooks BP, Gunay-Aygun M, Jacobson SG, Cogliati T, Westlake CJ, and Swaroop A (2017) In Vitro Modeling Using Ciliopathy-Patient-Derived Cells Reveals Distinct Cilia Dysfunctions Caused by CEP290 Mutations. Cell Rep 20, 384–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, and Gleeson JG (2010) Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 464, 1048–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Malicki JJ, and Johnson CA (2017) The Cilium: Cellular Antenna and Central Processing Unit. Trends Cell Biol 27, 126–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Agbu SO, Liang Y, Liu A, and Anderson KV (2018) The small GTPase RSG1 controls a final step in primary cilia initiation. J Cell Biol 217, 413–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garcia G 3rd, and Reiter JF (2016) A primer on the mouse basal body. Cilia 5, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Craft JM, Harris JA, Hyman S, Kner P, and Lechtreck KF (2015) Tubulin transport by IFT is upregulated during ciliary growth by a cilium-autonomous mechanism. J Cell Biol 208, 223–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhogaraju S, Cajanek L, Fort C, Blisnick T, Weber K, Taschner M, Mizuno N, Lamla S, Bastin P, Nigg EA, and Lorentzen E (2013) Molecular basis of tubulin transport within the cilium by IFT74 and IFT81. Science 341, 1009–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nozaki S, Katoh Y, Terada M, Michisaka S, Funabashi T, Takahashi S, Kontani K, and Nakayama K (2017) Regulation of ciliary retrograde protein trafficking by the Joubert syndrome proteins ARL13B and INPP5E. J Cell Sci 130, 563–576 [DOI] [PubMed] [Google Scholar]
- 84.Taschner M, and Lorentzen E (2016) The Intraflagellar Transport Machinery. Cold Spring Harb Perspect Biol 8, a028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mukhopadhyay S, Wen X, Chih B, Nelson CD, Lane WS, Scales SJ, and Jackson PK (2010) TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev 24, 2180–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang W, Jack BM, Wang HH, Kavanaugh MA, Maser RL, and Tran PV (2021) Intraflagellar Transport Proteins as Regulators of Primary Cilia Length. Front Cell Dev Biol 9, 661350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Follit JA, Tuft RA, Fogarty KE, and Pazour GJ (2006) The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell 17, 3781–3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sedmak T, and Wolfrum U (2010) Intraflagellar transport molecules in ciliary and nonciliary cells of the retina. J Cell Biol 189, 171–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ishikawa H, and Marshall WF (2017) Intraflagellar Transport and Ciliary Dynamics. Cold Spring Harb Perspect Biol 9, a021998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seixas C, Choi SY, Polgar N, Umberger NL, East MP, Zuo X, Moreiras H, Ghossoub R, Benmerah A, Kahn RA, Fogelgren B, Caspary T, Lipschutz JH, and Barral DC (2016) Arl13b and the exocyst interact synergistically in ciliogenesis. Mol Biol Cell 27, 308–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wood CR, and Rosenbaum JL (2015) Ciliary ectosomes: transmissions from the cell’s antenna. Trends Cell Biol 25, 276–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quidwai T, Wang J, Hall EA, Petriman NA, Leng W, Kiesel P, Wells JN, Murphy LC, Keighren MA, J AM, Lorentzen E, Pigino G, and Mill P (2021) A WDR35-dependent coat protein complex transports ciliary membrane cargo vesicles to cilia. Elife 10, e69786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brohmann H, Pinnecke S, and Hoyer-Fender S (1997) Identification and characterization of new cDNAs encoding outer dense fiber proteins of rat sperm. J Biol Chem 272, 10327–10332 [DOI] [PubMed] [Google Scholar]
- 94.Hayasaka S, Terada Y, Suzuki K, Murakawa H, Tachibana I, Sankai T, Murakami T, Yaegashi N, and Okamura K (2008) Intramanchette transport during primate spermiogenesis: expression of dynein, myosin Va, motor recruiter myosin Va, VIIa-Rab27a/b interacting protein, and Rab27b in the manchette during human and monkey spermiogenesis. Asian J Androl 10, 561–568 [DOI] [PubMed] [Google Scholar]
- 95.Kierszenbaum AL, Rivkin E, and Tres LL (2003) The actin-based motor myosin Va is a component of the acroplaxome, an acrosome-nuclear envelope junctional plate, and of manchette-associated vesicles. Cytogenet Genome Res 103, 337–344 [DOI] [PubMed] [Google Scholar]
- 96.Lin YH, Ke CC, Wang YY, Chen MF, Chen TM, Ku WC, Chiang HS, and Yeh CH (2017) RAB10 Interacts with the Male Germ Cell-Specific GTPase-Activating Protein during Mammalian Spermiogenesis. Int J Mol Sci 18, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lo JC, Jamsai D, O’Connor AE, Borg C, Clark BJ, Whisstock JC, Field MC, Adams V, Ishikawa T, Aitken RJ, Whittle B, Goodnow CC, Ormandy CJ, and O’Bryan MK (2012) RAB-like 2 has an essential role in male fertility, sperm intraflagellar transport, and tail assembly. PLoS Genet 8, e1002969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nishijima Y, Hagiya Y, Kubo T, Takei R, Katoh Y, and Nakayama K (2017) RABL2 interacts with the intraflagellar transport-B complex and CEP19 and participates in ciliary assembly. Mol Biol Cell 28, 1652–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kanie T, Abbott KL, Mooney NA, Plowey ED, Demeter J, and Jackson PK (2017) The CEP19-RABL2 GTPase Complex Binds IFT-B to Initiate Intraflagellar Transport at the Ciliary Base. Dev Cell 42, 22–36 e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Duan S, Li H, Zhang Y, Yang S, Chen Y, Qiu B, Huang C, Wang J, Li J, Zhu X, and Yan X (2021) Rabl2 GTP hydrolysis licenses BBSome-mediated export to fine-tune ciliary signaling. EMBO J 40, e105499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yan X, and Shen Y (2021) Rab-like small GTPases in the regulation of ciliary Bardet-Biedl syndrome (BBS) complex transport. FEBS J (2021) doi: 10.1111/febs.16232 [DOI] [PubMed] [Google Scholar]
- 102.Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, Sheffield VC, Scheller RH, and Jackson PK (2011) Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A 108, 2759–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Knodler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, and Guo W (2010) Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A 107, 6346–6351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walia V, Cuenca A, Vetter M, Insinna C, Perera S, Lu Q, Ritt DA, Semler E, Specht S, Stauffer J, Morrison DK, Lorentzen E, and Westlake CJ (2019) Akt Regulates a Rab11-Effector Switch Required for Ciliogenesis. Dev Cell 50, 229–246 e227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vetter M, Stehle R, Basquin C, and Lorentzen E (2015) Structure of Rab11-FIP3-Rabin8 reveals simultaneous binding of FIP3 and Rabin8 effectors to Rab11. Nat Struct Mol Biol 22, 695–702 [DOI] [PubMed] [Google Scholar]
- 106.Deretic D, Huber LA, Ransom N, Mancini M, Simons K, and Papermaster DS (1995) rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J Cell Sci 108 ( Pt 1), 215–224 [DOI] [PubMed] [Google Scholar]
- 107.Moritz OL, Tam BM, Hurd LL, Peranen J, Deretic D, and Papermaster DS (2001) Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell 12, 2341–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cuenca A, Insinna C, Zhao H, John P, Weiss MA, Lu Q, Walia V, Specht S, Manivannan S, Stauffer J, Peden AA, and Westlake CJ (2019) The C7orf43/TRAPPC14 component links the TRAPPII complex to Rabin8 for preciliary vesicle tethering at the mother centriole during ciliogenesis. J Biol Chem 294, 15418–15434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schroder JM, Larsen J, Komarova Y, Akhmanova A, Thorsteinsson RI, Grigoriev I, Manguso R, Christensen ST, Pedersen SF, Geimer S, and Pedersen LB (2011) EB1 and EB3 promote cilia biogenesis by several centrosome-related mechanisms. J Cell Sci 124, 2539–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Monnich M, Borgeskov L, Breslin L, Jakobsen L, Rogowski M, Doganli C, Schroder JM, Mogensen JB, Blinkenkjaer L, Harder LM, Lundberg E, Geimer S, Christensen ST, Andersen JS, Larsen LA, and Pedersen LB (2018) CEP128 Localizes to the Subdistal Appendages of the Mother Centriole and Regulates TGF-beta/BMP Signaling at the Primary Cilium. Cell Rep 22, 2584–2592 [DOI] [PubMed] [Google Scholar]
- 111.Zhang B, Zhang T, Wang G, Wang G, Chi W, Jiang Q, and Zhang C (2015) GSK3beta-Dzip1-Rab8 cascade regulates ciliogenesis after mitosis. PLoS Biol 13, e1002129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chiba S, Amagai Y, Homma Y, Fukuda M, and Mizuno K (2013) NDR2-mediated Rabin8 phosphorylation is crucial for ciliogenesis by switching binding specificity from phosphatidylserine to Sec15. EMBO J 32, 874–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Omori Y, Zhao C, Saras A, Mukhopadhyay S, Kim W, Furukawa T, Sengupta P, Veraksa A, and Malicki J (2008) Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat Cell Biol 10, 437–444 [DOI] [PubMed] [Google Scholar]
- 114.Sato T, Iwano T, Kunii M, Matsuda S, Mizuguchi R, Jung Y, Hagiwara H, Yoshihara Y, Yuzaki M, Harada R, and Harada A (2014) Rab8a and Rab8b are essential for several apical transport pathways but insufficient for ciliogenesis. J Cell Sci 127, 422–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oguchi ME, Okuyama K, Homma Y, and Fukuda M (2020) A comprehensive analysis of Rab GTPases reveals a role for Rab34 in serum starvation-induced primary ciliogenesis. J Biol Chem 295, 12674–12685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu S, Liu Y, Meng Q, and Wang B (2018) Rab34 small GTPase is required for Hedgehog signaling and an early step of ciliary vesicle formation in mouse. J Cell Sci 131, jcs213710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang T, and Hong W (2002) Interorganellar regulation of lysosome positioning by the Golgi apparatus through Rab34 interaction with Rab-interacting lysosomal protein. Mol Biol Cell 13, 4317–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schaub JR, and Stearns T (2013) The Rilp-like proteins Rilpl1 and Rilpl2 regulate ciliary membrane content. Mol Biol Cell 24, 453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Babbey CM, Bacallao RL, and Dunn KW (2010) Rab10 associates with primary cilia and the exocyst complex in renal epithelial cells. Am J Physiol Renal Physiol 299, F495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dhekne HS, Yanatori I, Gomez RC, Tonelli F, Diez F, Schule B, Steger M, Alessi DR, and Pfeffer SR (2018) A pathway for Parkinson’s Disease LRRK2 kinase to block primary cilia and Sonic hedgehog signaling in the brain. Elife 7, e40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sobu Y, Wawro PS, Dhekne HS, Yeshaw WM, and Pfeffer SR (2021) Pathogenic LRRK2 regulates ciliation probability upstream of tau tubulin kinase 2 via Rab10 and RILPL1 proteins. Proc Natl Acad Sci U S A 118, e2005894118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, Olkkonen VM, and Neefjes J (2007) Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol 176, 459–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hattula K, Furuhjelm J, Arffman A, and Peranen J (2002) A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell 13, 3268–3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Homma Y, and Fukuda M (2016) Rabin8 regulates neurite outgrowth in both GEF activity-dependent and -independent manners. Mol Biol Cell 27, 2107–2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Eggenschwiler JT, Espinoza E, and Anderson KV (2001) Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature 412, 194–198 [DOI] [PubMed] [Google Scholar]
- 126.Lim YS, and Tang BL (2015) A role for Rab23 in the trafficking of Kif17 to the primary cilium. J Cell Sci 128, 2996–3008 [DOI] [PubMed] [Google Scholar]
- 127.Hor CHH, Lo JCW, Cham ALS, Leong WY, and Goh ELK (2021) Multifaceted Functions of Rab23 on Primary Cilium-Mediated and Hedgehog Signaling-Mediated Cerebellar Granule Cell Proliferation. J Neurosci 41, 6850–6863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gerondopoulos A, Strutt H, Stevenson NL, Sobajima T, Levine TP, Stephens DJ, Strutt D, and Barr FA (2019) Planar Cell Polarity Effector Proteins Inturned and Fuzzy Form a Rab23 GEF Complex. Curr Biol 29, 3323–3330 e3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Onnis A, Finetti F, Patrussi L, Gottardo M, Cassioli C, Spano S, and Baldari CT(2015) The small GTPase Rab29 is a common regulator of immune synapse assembly and ciliogenesis. Cell Death Differ 22, 1687–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kuhns S, Seixas C, Pestana S, Tavares B, Nogueira R, Jacinto R, Ramalho JS, Simpson JC, Andersen JS, Echard A, Lopes SS, Barral DC, and Blacque OE(2019) Rab35 controls cilium length, function and membrane composition. EMBO Rep 20, e47625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dewees SL, Vargová R, Hardin KR, Turn RE, Devi S, Linnert J, Wolfrum U, Caspary T, Eliáš M, and Kahn RA (2021) Phylogenetic profiling and cellular analyses of ARL16 reveal roles in traffic of IFT140 and INPP5E. bioRxiv, 2021.2010.2014.464442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Y, Wei Q, Zhang Y, Ling K, and Hu J (2010) The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J Cell Biol 189, 1039–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gigante ED, Taylor MR, Ivanova AA, Kahn RA, and Caspary T (2020) ARL13B regulates Sonic hedgehog signaling from outside primary cilia. Elife 9, e50434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Partisani M, Baron CL, Ghossoub R, Fayad R, Pagnotta S, Abelanet S, Macia E, Brau F, Lacas-Gervais S, Benmerah A, Luton F, and Franco M (2021) EFA6A, an exchange factor for Arf6, regulates early steps in ciliogenesis. J Cell Sci 134, jcs249565. [DOI] [PubMed] [Google Scholar]
- 135.Gotthardt K, Lokaj M, Koerner C, Falk N, Giessl A, and Wittinghofer A (2015) A G-protein activation cascade from Arl13B to Arl3 and implications for ciliary targeting of lipidated proteins. Elife 4, e11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ivanova AA, Caspary T, Seyfried NT, Duong DM, West AB, Liu Z, and Kahn RA (2017) Biochemical characterization of purified mammalian ARL13B protein indicates that it is an atypical GTPase and ARL3 guanine nucleotide exchange factor (GEF). J Biol Chem 292, 11091–11108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hanke-Gogokhia C, Wu Z, Gerstner CD, Frederick JM, Zhang H, and Baehr W (2016) Arf-like Protein 3 (ARL3) Regulates Protein Trafficking and Ciliogenesis in Mouse Photoreceptors. J Biol Chem 291, 7142–7155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Revenkova E, Liu Q, Gusella GL, and Iomini C (2018) The Joubert syndrome protein ARL13B binds tubulin to maintain uniform distribution of proteins along the ciliary membrane. J Cell Sci 131, jcs212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Turn RE, Hu Y, Dewees SI, Devi N, East MP, Hardin KR, Khatib T, Linnert J, Wolfrum U, Lim MJ, Casanova JE, Caspary T, and Kahn RA (2021) The ARF GAPs ELMOD1 and ELMOD3 act at the Golgi and Cilia to Regulate Ciliogenesis and Ciliary Protein Traffic. Mol Biol Cell, mbcE21090443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xie S, Farmer T, Naslavsky N, and Caplan S (2019) MICAL-L1 coordinates ciliogenesis by recruiting EHD1 to the primary cilium. J Cell Sci 132, jcs233973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McDonald NA, and Gould KL (2016) Linking up at the BAR: Oligomerization and F-BAR protein function. Cell Cycle 15, 1977–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Naslavsky N, and Caplan S (2020) Endocytic membrane trafficking in the control of centrosome function. Curr Opin Cell Biol 65, 150–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li FQ, Chen X, Fisher C, Siller SS, Zelikman K, Kuriyama R, and Takemaru KI (2016) BAR Domain-Containing FAM92 Proteins Interact with Chibby1 To Facilitate Ciliogenesis. Mol Cell Biol 36, 2668–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang Q, Taschner M, Ganzinger KA, Kelley C, Villasenor A, Heymann M, Schwille P, Lorentzen E, and Mizuno N (2018) Membrane association and remodeling by intraflagellar transport protein IFT172. Nat Commun 9, 4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Assis LH, Silva-Junior RM, Dolce LG, Alborghetti MR, Honorato RV, Nascimento AF, Melo-Hanchuk TD, Trindade DM, Tonoli CC, Santos CT, Oliveira PS, Larson RE, Kobarg J, Espreafico EM, Giuseppe PO, and Murakami MT (2017) The molecular motor Myosin Va interacts with the cilia-centrosomal protein RPGRIP1L. Sci Rep 7, 43692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bershteyn M, Atwood SX, Woo WM, Li M, and Oro AE (2010) MIM and cortactin antagonism regulates ciliogenesis and hedgehog signaling. Dev Cell 19, 270–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cao J, Shen Y, Zhu L, Xu Y, Zhou Y, Wu Z, Li Y, Yan X, and Zhu X (2012) miR-129-3p controls cilia assembly by regulating CP110 and actin dynamics. Nat Cell Biol 14, 697–706 [DOI] [PubMed] [Google Scholar]
- 148.Kohli P, Hohne M, Jungst C, Bertsch S, Ebert LK, Schauss AC, Benzing T, Rinschen MM, and Schermer B (2017) The ciliary membrane-associated proteome reveals actin-binding proteins as key components of cilia. EMBO Rep 18, 1521–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Barbelanne M, Song J, Ahmadzai M, and Tsang WY (2013) Pathogenic NPHP5 mutations impair protein interaction with Cep290, a prerequisite for ciliogenesis. Hum Mol Genet 22, 2482–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dhekne HS, Yanatori I, Vides EG, Sobu Y, Diez F, Tonelli F, and Pfeffer SR (2021) LRRK2-phosphorylated Rab10 sequesters Myosin Va with RILPL2 during ciliogenesis blockade. Life Sci Alliance 4, e202101050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Asante D, Maccarthy-Morrogh L, Townley AK, Weiss MA, Katayama K, Palmer KJ, Suzuki H, Westlake CJ, and Stephens DJ (2013) A role for the Golgi matrix protein giantin in ciliogenesis through control of the localization of dynein-2. J Cell Sci 126, 5189–5197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Morthorst SK, Christensen ST, and Pedersen LB (2018) Regulation of ciliary membrane protein trafficking and signalling by kinesin motor proteins. FEBS J 285, 4535–4564 [DOI] [PubMed] [Google Scholar]
- 153.Vuolo L, Stevenson NL, Heesom KJ, and Stephens DJ (2018) Dynein-2 intermediate chains play crucial but distinct roles in primary cilia formation and function. Elife 7, e39655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dingjan I, Linders PTA, Verboogen DRJ, Revelo NH, Ter Beest M, and van den Bogaart G (2018) Endosomal and Phagosomal SNAREs. Physiol Rev 98, 1465–1492 [DOI] [PubMed] [Google Scholar]
- 155.Szalinski CM, Labilloy A, Bruns JR, and Weisz OA (2014) VAMP7 modulates ciliary biogenesis in kidney cells. PLoS One 9, e86425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tian X, Teng J, and Chen J (2021) New insights regarding SNARE proteins in autophagosome-lysosome fusion. Autophagy 17, 2680–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zuo X, Lobo G, Fulmer D, Guo L, Dang Y, Su Y, Ilatovskaya DV, Nihalani D, Rohrer B, Body SC, Norris RA, and Lipschutz JH (2019) The exocyst acting through the primary cilium is necessary for renal ciliogenesis, cystogenesis, and tubulogenesis. J Biol Chem 294, 6710–6718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zuo X, Guo W, and Lipschutz JH (2009) The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol Biol Cell 20, 2522–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Feng S, Knodler A, Ren J, Zhang J, Zhang X, Hong Y, Huang S, Peranen J, and Guo W (2012) A Rab8 guanine nucleotide exchange factor-effector interaction network regulates primary ciliogenesis. J Biol Chem 287, 15602–15609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fogelgren B, Lin SY, Zuo X, Jaffe KM, Park KM, Reichert RJ, Bell PD, Burdine RD, and Lipschutz JH (2011) The exocyst protein Sec10 interacts with Polycystin-2 and knockdown causes PKD-phenotypes. PLoS Genet 7, e1001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Caspary T, Larkins CE, and Anderson KV (2007) The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell 12, 767–778 [DOI] [PubMed] [Google Scholar]
- 162.Lipschutz JH, and Mostov KE (2002) Exocytosis: the many masters of the exocyst. Curr Biol 12, R212–214 [DOI] [PubMed] [Google Scholar]
- 163.Chavez M, Ena S, Van Sande J, de Kerchove d’Exaerde A, Schurmans S, and Schiffmann SN (2015) Modulation of Ciliary Phosphoinositide Content Regulates Trafficking and Sonic Hedgehog Signaling Output. Dev Cell 34, 338–350 [DOI] [PubMed] [Google Scholar]
- 164.Garcia-Gonzalo FR, Phua SC, Roberson EC, Garcia G 3rd, Abedin M, Schurmans S, Inoue T, and Reiter JF (2015) Phosphoinositides Regulate Ciliary Protein Trafficking to Modulate Hedgehog Signaling. Dev Cell 34, 400–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.D’Angelo G, Vicinanza M, Di Campli A, and De Matteis MA (2008) The multiple roles of PtdIns(4)P -- not just the precursor of PtdIns(4,5)P2. J Cell Sci 121, 1955–1963 [DOI] [PubMed] [Google Scholar]
- 166.Campa CC, and Hirsch E (2017) Rab11 and phosphoinositides: A synergy of signal transducers in the control of vesicular trafficking. Adv Biol Regul 63, 132–139 [DOI] [PubMed] [Google Scholar]
- 167.Park J, Lee N, Kavoussi A, Seo JT, Kim CH, and Moon SJ (2015) Ciliary Phosphoinositide Regulates Ciliary Protein Trafficking in Drosophila. Cell Rep 13, 2808–2816 [DOI] [PubMed] [Google Scholar]
- 168.Humbert MC, Weihbrecht K, Searby CC, Li Y, Pope RM, Sheffield VC, and Seo S (2012) ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc Natl Acad Sci U S A 109, 19691–19696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xu Q, Zhang Y, Wei Q, Huang Y, Hu J, and Ling K (2016) Phosphatidylinositol phosphate kinase PIPKIgamma and phosphatase INPP5E coordinate initiation of ciliogenesis. Nat Commun 7, 10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yamamoto Y, and Mizushima N (2021) Autophagy and Ciliogenesis. JMA J 4, 207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ao X, Zou L, and Wu Y (2014) Regulation of autophagy by the Rab GTPase network. Cell Death Differ 21, 348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang J, Zhang X, Liu G, Chang D, Liang X, Zhu X, Tao W, and Mei L (2016) Intracellular Trafficking Network of Protein Nanocapsules: Endocytosis, Exocytosis and Autophagy. Theranostics 6, 2099–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Huang L, Yuan P, Yu P, Kong Q, Xu Z, Yan X, Shen Y, Yang J, Wan R, Hong K, Tang Y, and Hu J (2018) O-GlcNAc-modified SNAP29 inhibits autophagy- mediated degradation via the disturbed SNAP29-STX17-VAMP8 complex and exacerbates myocardial injury in type I diabetic rats. Int J Mol Med 42, 3278–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Morelli E, Ginefra P, Mastrodonato V, Beznoussenko GV, Rusten TE, Bilder D, Stenmark H, Mironov AA, and Vaccari T (2014) Multiple functions of the SNARE protein Snap29 in autophagy, endocytic, and exocytic trafficking during epithelial formation in Drosophila. Autophagy 10, 2251–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang Z, Li W, Zhang Y, Zhang L, Teves ME, Liu H, Strauss JF 3rd, Pazour GJ, Foster JA, Hess RA, and Zhang Z (2016) Intraflagellar transport protein IFT20 is essential for male fertility and spermiogenesis in mice. Mol Biol Cell, 27, 3705–3716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Finetti F, Cassioli C, Cianfanelli V, Zevolini F, Onnis A, Gesualdo M, Brunetti J, Cecconi F, and Baldari CT (2021) The Intraflagellar Transport Protein IFT20 Recruits ATG16L1 to Early Endosomes to Promote Autophagosome Formation in T Cells. Front Cell Dev Biol 9, 634003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Monis WJ, Faundez V, and Pazour GJ (2017) BLOC-1 is required for selective membrane protein trafficking from endosomes to primary cilia. J Cell Biol 216, 2131–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cai Q, Lu L, Tian JH, Zhu YB, Qiao H, and Sheng ZH (2010) Snapin-regulated late endosomal transport is critical for efficient autophagy-lysosomal function in neurons. Neuron 68, 73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yuzaki M (2010) Snapin snaps into the dynein complex for late endosome-lysosome trafficking and autophagy. Neuron 68, 4–6 [DOI] [PubMed] [Google Scholar]
- 180.Pampliega O, Orhon I, Patel B, Sridhar S, Diaz-Carretero A, Beau I, Codogno P, Satir BH, Satir P, and Cuervo AM (2013) Functional interaction between autophagy and ciliogenesis. Nature 502, 194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Struchtrup A, Wiegering A, Stork B, Ruther U, and Gerhardt C (2018) The ciliary protein RPGRIP1L governs autophagy independently of its proteasome-regulating function at the ciliary base in mouse embryonic fibroblasts. Autophagy 14, 567–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tang Z, Lin MG, Stowe TR, Chen S, Zhu M, Stearns T, Franco B, and Zhong Q (2013) Autophagy promotes primary ciliogenesis by removing OFD1 from centriolar satellites. Nature 502, 254–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yamamoto Y, Chino H, Tsukamoto S, Ode KL, Ueda HR, and Mizushima N (2021) NEK9 regulates primary cilia formation by acting as a selective autophagy adaptor for MYH9/myosin IIA. Nat Commun 12, 3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Canterini S, Dragotto J, Dardis A, Zampieri S, De Stefano ME, Mangia F, Erickson RP, and Fiorenza MT (2017) Shortened primary cilium length and dysregulated Sonic hedgehog signaling in Niemann-Pick C1 disease. Hum Mol Genet 26, 2277–2289 [DOI] [PubMed] [Google Scholar]
- 185.Maharjan Y, Lee JN, Kwak SA, Dutta RK, Park C, Choe SK, and Park R (2020) TMEM135 regulates primary ciliogenesis through modulation of intracellular cholesterol distribution. EMBO Rep 21, e48901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cantagrel V, Silhavy JL, Bielas SL, Swistun D, Marsh SE, Bertrand JY, Audollent S, Attie-Bitach T, Holden KR, Dobyns WB, Traver D, Al-Gazali L, Ali BR, Lindner TH, Caspary T, Otto EA, Hildebrandt F, Glass IA, Logan CV, Johnson CA, Bennett C, Brancati F, International Joubert Syndrome Related Disorders Study, G., Valente EM, Woods CG, and Gleeson JG (2008) Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet 83, 170–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jenkins D, Seelow D, Jehee FS, Perlyn CA, Alonso LG, Bueno DF, Donnai D, Josifova D, Mathijssen IM, Morton JE, Orstavik KH, Sweeney E,Wall SA, Marsh JL, Nurnberg P, Passos-Bueno MR, and Wilkie AO (2007) RAB23 mutations in Carpenter syndrome imply an unexpected role for hedgehog signaling in cranial-suture development and obesity. Am J Hum Genet 80, 1162–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shaheen R, Faqeih E, Alshammari MJ, Swaid A,Al-Gazali L, Mardawi E, Ansari S, Sogaty S, Seidahmed MZ, AlMotairi MI, Farra C, Kurdi W, Al-Rasheed S, and Alkuraya FS (2013) Genomic analysis of Meckel-Gruber syndrome in Arabs reveals marked genetic heterogeneity and novel candidate genes. Eur J Hum Genet 21, 762–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dixon-Salazar TJ, Silhavy JL, Udpa N, Schroth J, Bielas S, Schaffer AE, Olvera J, Bafna V, Zaki MS, Abdel-Salam GH, Mansour LA, Selim L, Abdel-Hadi S, Marzouki N, Ben-Omran T, Al-Saana NA, Sonmez FM, Celep F, Azam M, Hill KJ, Collazo A, Fenstermaker AG, Novarino G, Akizu N, Garimella KV, Sougnez C, Russ C, Gabriel SB, and Gleeson JG (2012) Exome sequencing can improve diagnosis and alter patient management. Sci Transl Med 4, 138ra178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, Pernot E, Kisseleva MV, Compere P, Schiffmann SN, Gergely F, Riley JH, Perez-Morga D, Woods CG, and Schurmans S (2009) INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet 41, 1027–1031 [DOI] [PubMed] [Google Scholar]
- 191.Halbritter J, Bizet AA, Schmidts M, Porath JD, Braun DA, Gee HY, McInerney-Leo AM, Krug P, Filhol E, Davis EE, Airik R, Czarnecki PG, Lehman AM, Trnka P, Nitschke P, Bole-Feysot C, Schueler M, Knebelmann B, Burtey S, Szabo AJ, Tory K, Leo PJ, Gardiner B, McKenzie FA, Zankl A, Brown MA, Hartley JL, Maher ER, Li C, Leroux MR, Scambler PJ, Zhan SH, Jones SJ, Kayserili H, Tuysuz B, Moorani KN, Constantinescu A, Krantz ID, Kaplan BS, Shah JV, Consortium UK, Hurd TW, Doherty D, Katsanis N, Duncan EL, Otto EA, Beales PL, Mitchison HM, Saunier S, and Hildebrandt F (2013) Defects in the IFT-B component IFT172 cause Jeune and Mainzer-Saldino syndromes in humans. Am J Hum Genet 93, 915–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bujakowska KM, Zhang Q, Siemiatkowska AM, Liu Q, Place E, Falk MJ, Consugar M, Lancelot ME, Antonio A, Lonjou C, Carpentier W, Mohand-Said S, den Hollander AI, Cremers FP, Leroy BP, Gai X, Sahel JA, van den Born LI, Collin RW, Zeitz C, Audo I, and Pierce EA (2015) Mutations in IFT172 cause isolated retinal degeneration and Bardet-Biedl syndrome. Hum Mol Genet 24, 230–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mansour F, Boivin FJ, Shaheed IB, Schueler M, and Schmidt-Ott KM (2021) The Role of Centrosome Distal Appendage Proteins (DAPs) in Nephronophthisis and Ciliogenesis. Int J Mol Sci 22 [DOI] [PMC free article] [PubMed] [Google Scholar]


