Abstract
Pancreatic ductal adenocarcinoma (PDAC) tumor microenvironment (TME) consists of multiple cell types interspersed by dense fibrous stroma. These cells communicate through low molecular weight signaling molecules called cytokines. The cytokines, through their receptors, facilitate PDAC initiation, progression, metastasis, and distant colonization of malignant cells. These signaling mediators secreted from tumor-associated macrophages, and cancer-associated fibroblasts in conjunction with oncogenic Kras mutation initiate acinar to ductal metaplasia (ADM), resulting in the appearance of early preneoplastic lesions. Further, M1- and M2-polarized macrophages provide proinflammatory conditions and promote deposition of extracellular matrix, whereas myofibroblasts and T-lymphocytes, such as Th17 and T-regulatory cells, create a fibroinflammatory and immunosuppressive environment with a significantly reduced cytotoxic T-cell population. During PDAC progression, cytokines regulate the expression of various oncogenic regulators such as NFκB, c-myc, growth factor receptors, and mucins resulting in the formation of high-grade PanIN lesions, epithelial to mesenchymal transition, invasion, and extravasation of malignant cells, and metastasis. During metastasis, PDAC cells colonize to the premetastatic niche created in the liver, and lung, an organotropic function primarily executed by cytokines in circulation or loaded in the exosomes from the primary tumor cells. The indispensable contribution of these cytokines at every stage of PDAC tumorigenesis makes them exciting candidates in combination with immune-, chemo- and targeted radiation therapy.
1. Introduction
The cytokines are low molecular weight signal transducers responsible for autocrine and paracrine communications during tissue injury, inflammation, and malignancies. The pancreas, a retroperitoneal organ located behind the stomach, is a unique organ due to the presence of exocrine and endocrine functions. During pancreatic tissue injury and inflammation, cytokines released at the injury site generate inflammatory response resulting in the accumulation of effector cells for wound healing-like response. The secretion of chemokines is switched off as soon as the injury and inflammation are resolved [1]. In addition to benign inflammation, the pancreatic malignancies, mainly pancreatic ductal adenocarcinoma (PDAC), intraductal papillary mucinous neoplasm (IPMN), and mucinous cystic neoplasm, are also associated with chronic inflammation. The PDAC constitutes 95% of all pancreatic malignancies that develop sporadically due to the presence of constitutively active Kras mutations. The initiating events set off by Kras lead to the development of a complex tumor microenvironment (TME) consisting of multiple cell types, including fibroblast, immune cells, endothelial cells, and the extensive deposition of extracellular matrix (ECM) proteins. The dense fibrotic stroma, a characteristic hallmark of PDAC, also modulates the primary function of these cells to support tumor growth and metastasis [2–4]. Though Kras mutation is the major driver of PDAC tumorigenesis; however, activating oncogenic Kras mutations alone is not sufficient for tumor onset and essentially requires various cytokines produced by different cell types from TME to cooperate with Ras signaling [5–7].
The first histologically unique event during PDAC pathogenesis is acinar cell trans differentiation into duct-like cells called acinar to ductal metaplasia (ADM) [8–10]. ADM is required by the acinar cells for pancreatic regeneration and is accompanied by loss of cell-cell or cell-ECM contact or loss of polarity. However, inflammatory cytokines in the presence of oncogenic Kras halt the acinar reversibility and progress ADM to PanIN lesions [8]. Depletion of macrophages, the warehouse of cytokines and inflammatory mediators, has been shown to reduce ADM formation and tumor onset. Pancreas infiltrating macrophages secrete inflammatory cytokines such as RANTES (Regulated on Activation Normal T Cell Expressed and Secreted) and tumor necrosis factor-alpha (TNF-α) that induce ADM by activating nuclear factor-κB (NF-κB) signaling and expression of matrix metalloproteinases (MMPs) [11, 12]. Mice treated with cholecystokinin analog, cerulein, accelerates ADM formation associated with the increased presence of macrophages in the TME and activation of NF-κB signaling [12, 13]. Similarly, transforming growth factor-alpha (TGF-α) activates growth receptor signaling potentiating mutant Kras activation and PDAC initiation [7, 14, 15]. The interleukin (IL)-6 secreted by tumor resident macrophages promote STAT signaling resulting in tumor growth and progression [16, 17]. The initial secretion of cytokines further recruits various lymphoid and myeloid subsets into the TME, secreting more cytokines, setting up a feed-forward loop leading to chronically inflamed tissues. The major stimulus for the infiltration of these immune cells into the PDAC tissue is mediated by the signaling through a family of G-protein coupled receptors [18]. Depending upon the conserved cysteine residues, chemokines and their receptors are classified as C, CXC, CX3C, and CC. The C-C chemokine receptor type 2 (CCR2), C-X-C receptor type 2 (CXCR2), C-X-C receptor type 3 (CXCR3), and C-X-C receptor type 4 (CXCR4) are some known chemokine receptors expressed on the cells infiltrating PDAC TME. The tumor-infiltrating lymphocytes (TILs) create an inflammatory niche conducive for tumor growth and immune evasion [19, 20]. Immune profiling on 57 PDAC patient samples revealed that accumulation of CD3+ TILs in the tumor correlates with better survival [21]. Further, the large-scale meta-analysis of TILs as a prognostic marker on four different datasets established their contribution to disease prognosis, survival outcomes, and chemotherapy response [22]. Although cytokine and chemokine therapy has not gained much success in PDAC so far, the advancement in the knowledge and utility of cytokines receptors such as IL-2, interferon-gamma (IFN-γ), IL-6, CCR2, and CXCR4, as new therapeutic targets provide a ray of hope for their use as promising candidates [23–27]. The major hurdle in the success of cytokine-mediated therapy is an absurdly immunosuppressive and fibroinflammatory TME created by the immune cells in the surrounding cytokine milieu. This review highlights the distinctive role of cytokines in PDAC tumorigenesis, major advancements in their role in PDAC pathology, and their success in PDAC therapy so far.
2. Source of Cytokines in PDAC Tumor Microenvironment
The cytokines communicate through their canonical receptors present on the cell surface. The extent of cytokine binding to their canonical or non-canonical receptor and the intensity of generated stimulus depends upon specificity and binding affinity [28–30]. This well-orchestrated network is hijacked by the cancer cells during malignant transformation for their survival benefits and growth.
Pancreatic stellate cells (PSCs) secrete fibrogenic cytokines such as platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), and connective tissue growth factor (CTGF), which are potent proliferative and connective tissue secreting factors. The TGF-β promotes the production of MMPs such as MMP2 involved in modulating collagen dynamics in TME. Other cytokines secreted by PSCs include MCP-1, IL-1, IL-8, IL-15, and RANTES. These cytokines also stimulate PSCs in an autocrine manner. Treatment of PSCs with TNF-α increases collagen synthesis, whereas no effect of IL-1, IL-6, and IL-10 is observed on stellate cell proliferation [31, 32]. The PSCs during PDAC initiation and progression are themselves activated by numerous inflammatory factors such as TNFα, ROS, IL-1, IL-6, FGF, and activin A, facilitating processes involved in progression to metastatic colonization [33, 34]. In addition to PDGF and TGF-β, PDAC associated fibroblasts (CAFs) secrete CXCL12, IL-8, IL-6, IL-33, thymic stromal lymphopoietin (TSLP), which play an important role in PDAC cell invasion and metastasis. Based on inflammatory and fibrogenic characteristics, CAFs are further classified as inflammatory CAFs (iCAFs), predominantly secreting IL-6 and IL-1 promoting inflammatory state, and myofibroblastic CAFs (myCAFs), secreting fibrotic factors such as PDGF and TGF-β constructing complex ECM composition [35, 36]. Apart from PDAC, iCAFs are present in abundance in both low- and high-grade IPMNs and drive the pathology of IPMN development [37]. MyCAFs have heterogenous distribution in PDAC. These FAP+ positive fibroblasts express low (FAP+ αSMAlow) or high (FAP+ αSMAhigh) α smooth muscle actin levels and are distributed heterogeneously in the tumor with (FAP+ αSMAlow) myCAFs occupying the periglandular region. Fibrogenic cytokines secreted by these myCAFs contribute exclusively to driving low-grade ducts to high-grade lesions [36].
In addition to CAFs, immune cells are other major sources of cytokines. The tumor-associated macrophages (TAMs) in PDAC secrete cytokines such as TGF-β1 that upregulates CTGF, high mobility group box protein 1 (HMGB1), IL-10, IL-1α, IL-1β, IL-8, TNF-α, and CCL18 depending upon their activation status as M1 or M2 phenotype [38–40]. Single-cell RNA sequencing (scRNA-seq) analysis from PDAC samples and peripheral blood mononuclear cells showed that TAMs are predominantly present in the PDAC tissues, indicating the significant function of macrophages in PDAC tumorigenesis and establishment of suppressive TME [41]. High TAMs tumor infiltration correlates with lymph node metastasis and poor prognosis. IL-8 secreted by PDAC cells activates the STAT3 pathway resulting in TWIST expression and increased cell motility and invasiveness. High CD68 TAMs and stromal IL-8 expression correlate with lymph node metastasis and poor patient outcomes [39]. The M2 TAMs drive inflammation in PDAC by releasing IL-1β due to stimulation by cancer cell-derived debris and IgG antibodies. IL-1β secretion from M2-TAMs promotes EMT phenotype and increases metastasis [40]. The T-cells, B-cells, and natural killer (NK) cells constitute most of the PDAC lymphocyte population. The CD4+ and CD8+ T-cells population is relatively low in PDAC. The T-regulatory cells (Tregs) create an immunosuppressive environment in PDAC and precancerous lesions by secreting IL-10 and TGFβ, resulting in lower CD8+ and CD4+ T-cell subsets. The T-helper 17 (Th17) cells produce proinflammatory cytokine 1L-17 involved in tumor development by mediating the interaction between immune cells and PanIN lesions expressing IL-17 receptor A (IL-17RA) on their surface. The Kras mutation results in the expression of IL-17RA on PanIN lesions, and IL-17 from lymphocytes promote tumor progression and induce cancer stemness feature through IL-6/pStat3 and NF-κB signaling [42–44]. The Tregs and exhausted T-cells number increase with the PDAC progression resulting in immunosuppression of cytotoxic, effector, memory T-cell, infiltrating the PDAC TME, resulting in antitumor effects and upregulation of immune checkpoint inhibitors [41, 45, 46]. Increased IL-35 levels from pro-tumorigenic B-cells are responsible for PDAC growth [47]. Apart from IL-35, pro-tumorigenic B-cells also secrete immunosuppressive IL-10 and exhibit tumor infiltration by sensing CXCL3 secreted by primary cancer cells [48, 49]. The cancer cells also secrete cytokines that act in an autocrine and paracrine manner. The PDAC cells attract neutrophils by secreting CXCL8 and CXCL18 [50]. Furthermore, cancer cells secrete granulocyte macrophage-colony stimulating factor (GM-CSF) and granulocyte-colony stimulating factor (G-CSF), resulting in leukocyte recruitment in the TME [51, 52]. PDAC cells also secrete IL-6, IL-10, IL-13, TGF-β, and vascular endothelial growth factor (VEGF). These pro-and anti-inflammatory cytokines create inflammatory milieu, immunosuppressive TME, and desmoplastic reaction during PDAC initiation and progression [54]. These cytokines also serve as biomarkers for PDAC diagnosis and improve the sensitivity and specificity of the widely used marker, CA19.9. The change in the levels of these cytokines in the patients after surgery or chemotherapy further explains their role in early diagnosis. IL-6 secreted by pancreatic cancer cells stimulates the secretion of Th2 type cytokines in cancer cells, which regulates the expression of VEGF and neuropilin-1 (NRP-1) responsible for angiogenesis and cell proliferation by activating the ERK2 signaling pathway [53]. PDAC patients with the advanced and metastatic disease show significantly higher IL-6, IL-10, and TGFβ2 serum cytokine levels than healthy controls. This is in line with the significantly increased secretion of these cytokines, as shown in PT-5, Capan-2, and BxPC-3 pancreatic cancer cell lines [55].
3. Contribution of Cytokines to PDAC Biology:
Cytokines play a diverse role from inception to distant metastasis (Figure 1). The following is their contribution at each step from malignant transformation to distant colonization of the PDAC cells.
Figure 1: Role of cytokine in PDAC pathology.
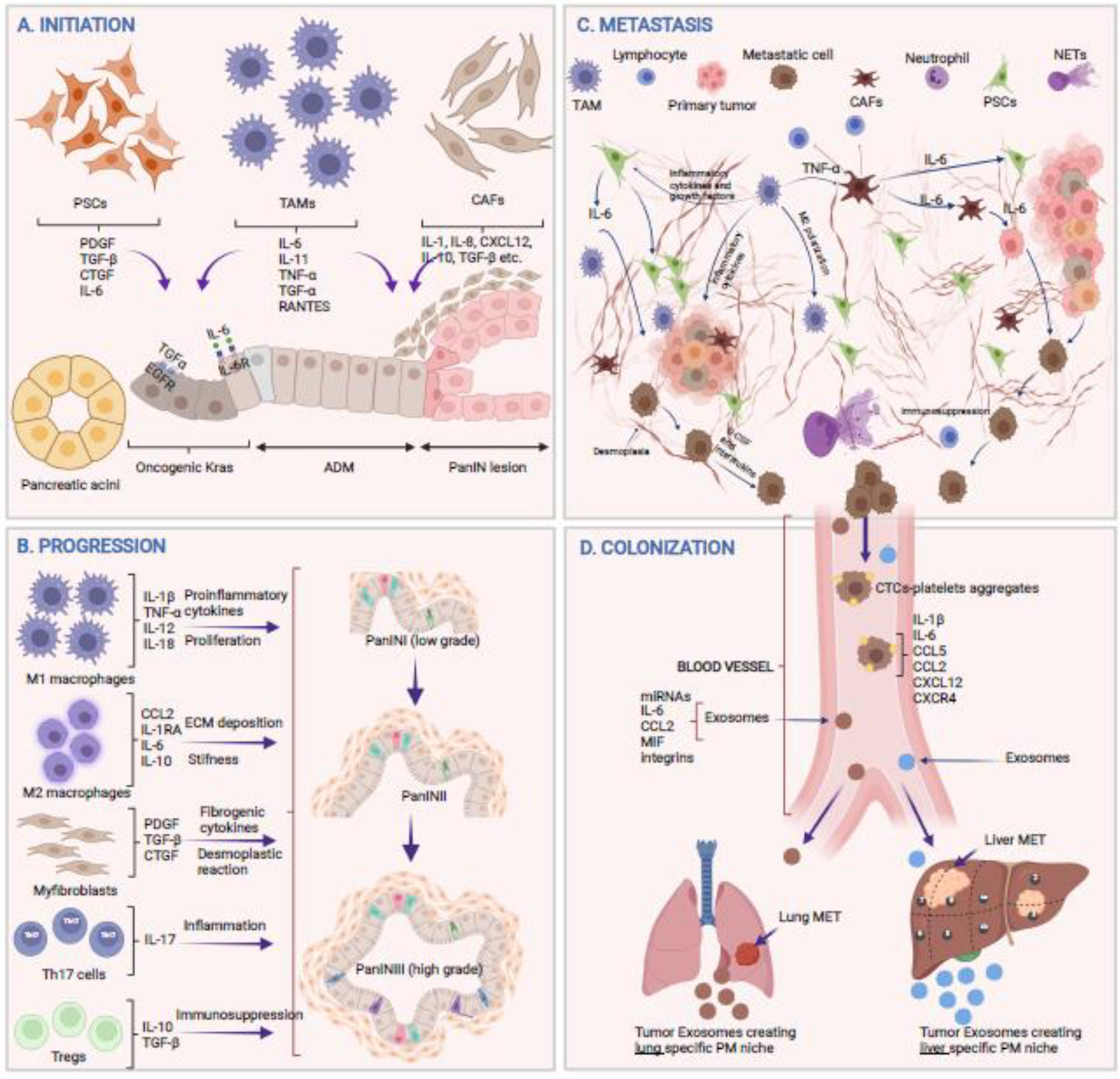
A) Involvement of cytokines secreted from various cell types during different steps of tumor initiation. B) Cytokine milieu secreted by immune cells present in TME driving tumor progression from low-grade to high-grade lesions. C) The crosstalk between various cell types within the TME orchestrating complex metastatic events resulting in EMT, invasion, extravasation, and dislodging of tumor cells into the vasculature D) Cytokines, either free or loaded in exosomes, create lung or liver tissue-specific premetastatic (PM) niche while circulating tumor cells (CTCs) interact with platelets or endothelial cells and colonize to a favorable niche at the metastatic sites (Mets). Figure created with BioRender.com
3.1. PDAC initiation and progression
Although mutations play an important role in cancer initiation and progression, cytokines (either pro- or anti-inflammatory) and their receptors, expressed by the resident or infiltrating immune cells, provide a necessary signal and create a favorable malignant transformation environment. As described earlier, the tumorigenesis is dependent on chronic inflammation, demonstrated by a 13.3-fold higher risk of developing PDAC in patients with chronic pancreatitis [56–58]. However, it is not necessary that chronic inflammation precedes tumorigenesis as some solid tumors show the presence of lymphoid or myeloid cells irrespective of chronic inflammation [22, 59]. Generally, high serum IL-6, IL-8, and IL-10 levels are associated with PDAC [60–62]. During acinar cell transformation to ductal lesions, secretion of IL-6 and IL-11 upon cerulein-induced inflammation potentiate STAT3 signaling, which promotes Kras-driven PDAC development. More precisely, IL-6 secreted from myeloid cells binds to the IL-6 receptor (IL-6R) and gp130 on epithelial cells promoting STAT3 signaling and PanIN lesion development [62–64]. Further, oncogenic Kras activation results in the production of IL-1α, a proinflammatory cytokine, from PDAC cells resulting in downstream NF-κB-mediated activation and enhanced tumorigenesis. Similarly, IL-6 cytokine gene polymorphism has been shown to increase the risk of PDAC, which is attributed to the activation of IL-6 induced oncogenic signaling in the presence of Kras mutation [65–67]. During pancreatitis in the presence of IL-13, macrophages produce TNFα and RANTES, which activate NF-κB signaling in acinar cells leading to MMP-9 expression and ADM [12, 38, 68]. Chemokines also promote immunosuppression and PDAC cell migration leading to early dissemination and invasiveness of PDAC cells even before the formation of high-grade PanIN lesions. Chemokine receptor CXCR2 is upregulated in PDAC and is predominantly expressed by neutrophil/myeloid-derived suppressor cells. PDAC cells also express CXCR2 and its ligands such as CXCL1, CXCL2, CXCL5 that are involved in PDAC initiation and stroma development [69, 70]. PDAC cells promote chemokine pathways to recruit bone marrow myeloid cells through CCL2 secretion, resulting in immunosuppressive TME. Combined targeting of CCR2 expressing Tumor-associated neutrophils and CCR2 expressing TAMs reduces tumor infiltration and promotes robust antitumor response [71]. IL-17 also plays an important role in PDAC initiation. Activating Kras mutation induces the expression of IL-17 receptor on transformed preneoplastic cells, and IL-17 secreted from infiltrating γδT cells dramatically accelerates PDAC initiation. This was further explained by delayed PanIN initiation upon genetic ablation and pharmacological neutralization of IL-17A [43].
In addition to the initiation, cytokines play an important role in the progression of low-grade lesions to high-grade lesions and a clinically detectable tumor. The classically activated macrophages, M1, generate a strong inflammatory reaction resulting in aggressive phenotype and invasive tumor and angiogenesis [72]. On the other hand, alternatively activated (IL-4 mediated) macrophages, M2, promote ECM deposition and stiffness, resulting in faster tumor progression and EMT [73, 74]. Progression of low-grade PanINs to high-grade PanINs requires high levels of ERK1/2 signaling, which is mainly influenced by CCL2 and IL-1RA secreted by M2-polarized macrophages [68]. In addition, expression of EGFR-ligands like EGF, HBEGF, TGFα secreted by macrophages promote ERK1/2 signaling and expression of Sox9 in driving tumor progression [9, 75–77]. IL-6 secreted by the infiltrating macrophages activates STAT3 signaling in the PDAC cells, whereas IL-10, IL-13, and IL-4 produced by M2 macrophages secrete extracellular matrix components by CAFs, resulting in remodeling of TME and helping in tumor progression [16]. Tumor-associated neutrophils (TANs) express lipocalin 2 in PanIN lesions that promotes PDAC progression and is a suggested biomarker for early diagnosis of PDAC and severe acute pancreatitis [78–80]. TANs are an important source of cytokines such as CXCL5, TNF, and TGFβ and are detected in very high levels in the KPC murine model of PDAC.
The contribution of CAFs in PDAC progression is dichotomous, as highlighted by multiple studies. During PDAC, stellate cells are transformed into myofibroblasts-like cells characterized by the expression of α-SMA, which are responsible for increased secretion of fibrogenic cytokines and ECM deposition, supporting cancer cell growth [81]. Targeting M2 macrophages by clodronate liposomes, TLR4 depletion, and neutralizing antibodies resulted in a significant reduction in tumor stroma and increased proliferation and severity, suggesting that stroma plays both tumor regressing and promoting role in a context-dependent manner [38, 82]. The M2 macrophages also express the inflammasome protein NLRP3, which actively participates in the proliferation of PDAC cells [83]. These macrophages also increase the proliferation of various T-cell subsets like Th17 or Tregs, which promote cancer cell growth by limiting the activated CD8+ T-cell population. Analysis of PDAC patient samples shows high CD4+CD25+Foxp3+ Tregs in PDAC TME. Blockade of CD25 by monoclonal antibody daclizumab decreases CD4+CD25+Foxp3+ Tregs and reprogrammed Tregs population to CD45RA− Tregs resulting in decreased suppressive function and increased IFN-γ secretion with improved immunotherapy in PDAC patients [84]. On the contrary, the depletion of Tregs in the pancreatic cancer mouse model decreases TGFβ expression and accelerates tumor progression due to the loss of constraining CAFs. This leads to the increased secretion of Ccl3, Ccl6, and Ccl8 in the TME, promoting immune infiltration and restoration of immunosuppressive environment for tumor progression [85]. The interferon-gamma (IFN-γ) and neutrophil elastase also regulate the expression of mucins such as MUC4 and MUC5AC, which are involved in tumor progression from early to later stages by maintaining stemness [86–89]. In addition, the expression of these mucins is also induced by CXCL2 and IL-17 in PDAC. Mucins are also regarded as the hallmark of PDAC pathology, and their regulation by cytokines further establishes the role of cytokines during PDAC development. IL-6 and IL-24 regulate MUC4 via STAT3 signaling, an important axis in PDAC progression. MUC1 is also regulated by various proinflammatory cytokines such as IL-1β, IL-6, TNFα, and IFN-γ [90]. A recent study from our lab shows the importance of four mucin-based subtypes of PDAC and determines their contribution to patient survival [91]. PDAC also expresses CXCR3 and its cognate ligands, CXCL9 and CXCL10. The CXCR3-ligands are thought to induce immunosuppression, while CXCR3 expression correlates with increased CD8+ T-cell signature and is associated with poor survival of PDAC patients, suggesting the differential contribution of chemokine receptors based on their expression either on cancer cells or immune cells [92–94].
3.2. Pancreatic Cancer Metastasis
The metastatic process is a continuum that involves a series of well-coordinated processes to carve a niche favorable for invasion into the surrounding tissue, extravasation into the circulation, and colonization at distant sites. The initial microenvironment during preneoplastic lesion may be tumor restraining; however, cancer cell-mediated reprogramming educates cells in the TME to favor tumor growth and metastasis [95]. Over disease progression, the cytokines and other inflammatory mediators create an environment conducive for tumor metastasis and actively participate in cancer cell dissemination (Figure 2). At a cellular level, Ras and MYC oncogenes help remodel the TME by recruiting proinflammatory cytokines facilitating cancer metastasis. At the molecular level, cytokines increased the expression of genes required for epithelial to mesenchymal transition (EMT) to enhance the motility of the tumor cells to facilitate metastasis.
Figure 2: Cytokine crosstalk in PDAC tumor microenvironment.
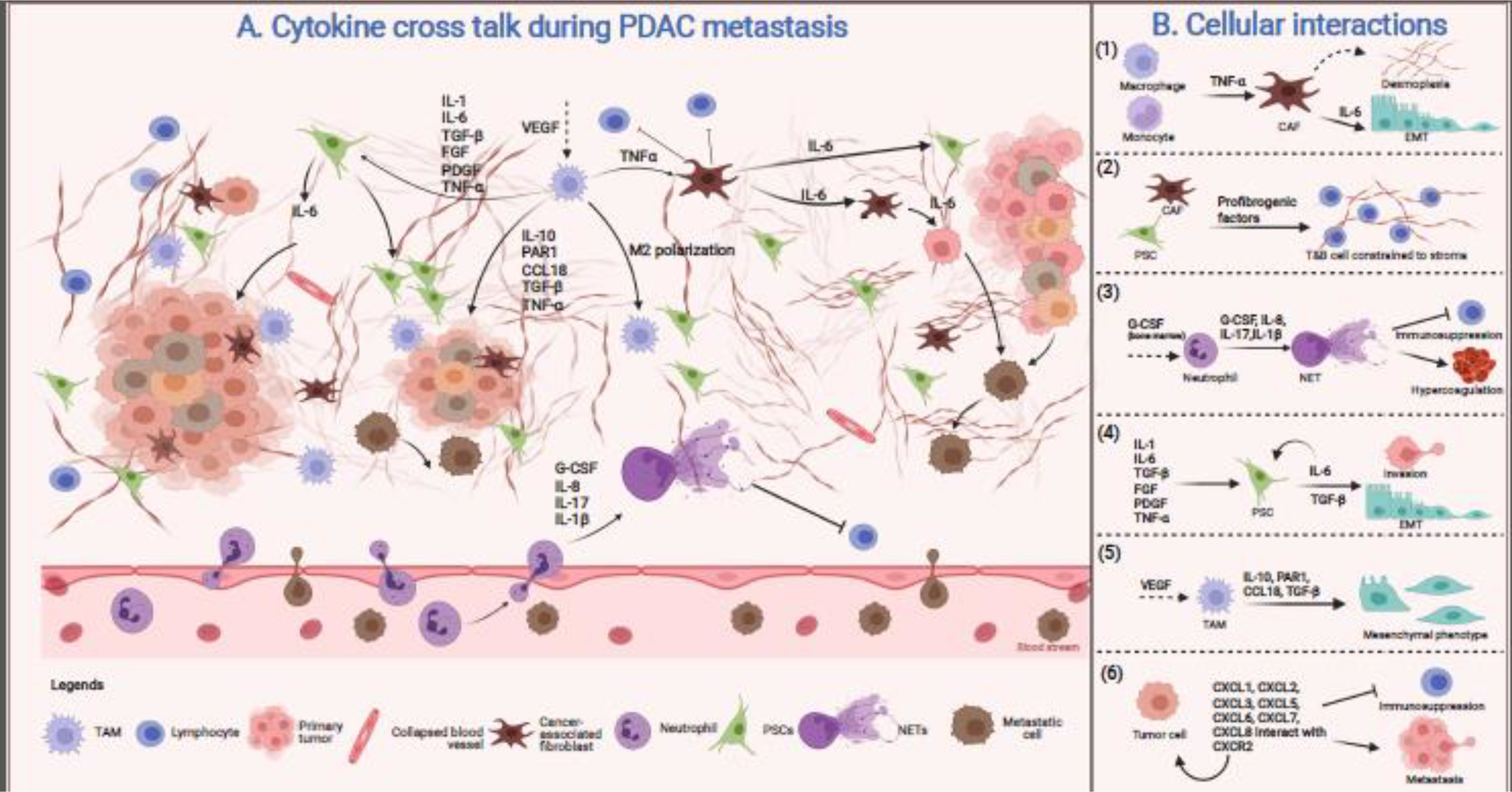
A) The metastasis-promoting microenvironment is created by crosstalk between a labyrinth of versatile cells present in the TME. This complex network secretes various cytokines resulting in immune infiltration to facilitate migration and invasion. B) TNFα is an inflammatory cytokine secreted by macrophages and monocytes, which stimulates CAFs to secrete more fibrous stroma and participate in various signaling pathways promoting EMT (1). Cells such as CAFs and PSCs secrete profibrogenic factors that increase ECM deposition constraining lymphoid (B- and T-) cells to the tumor periphery, physically preventing their access to cancer cells (2). Secretion of G-CSF and other factors from the TME mobilize neutrophils from the bone marrow and triggers NET formation in the primary tumor and circulation. The NETs further promote immunosuppression as well as hypercoagulation (3). Cytokine milieu triggers PSCs to aid in invasion and EMT through an IL-6/STAT3 mediated pathway (4). The M2 polarized macrophages secrete inflammatory cytokines altering cancer cell morphology to a mesenchymal phenotype (5). Tumor cells secrete various chemokine, CXC-ligands, and express CXC-receptors, facilitating immunosuppression and dislodging of the cancer cells into the circulation for distant colonization (6). Figure created with BioRender.com.
3.2.1. Epithelial to mesenchymal transition
The EMT of the cancer cells is facilitated predominantly by CAFs and immune cells. One of the important cytokines involved in EMT is TGF-β, which has immunosuppressive and anti-inflammatory actions at early stages of tumorigenesis; however, it is shown to induce EMT in cancer cells [96]. The pancreatic stroma plays a critical role as it secretes TGF-β, which in turn regulates PSCs biology and promotes myofibroblast phenotype [97, 98]. So, the stromal cell behaviors can significantly contribute to the establishment of a pro-metastatic microenvironment. TNF-α is another inflammatory cytokine produced by macrophages and monocytes, with a well-documented role in a range of signaling cascades involved in EMT. In fact, TNFα regulates different cellular interactions, and each of these facilitates metastasis through different mechanisms. One study in PDAC shows that TNF-α induces endothelial-mesenchymal transition (EndMT), a form of EMT, giving rise to tumor cells with multipotent potential [99]. This process occurs partially through Tyrosine Kinase with Immunoglobulin Like and EGF-like Domains 1 downregulation, and removal of TNF-α can revert the process to MEndT, which is a critical step in re-establishing the tumor cells at distant metastatic sites [99]. Simultaneously, in vivo administration of TNFα increases the desmoplastic stroma surrounding the pancreatic tumors through the formation of CAFs, which secrete chemokines and further facilitate the establishment of metastatic foci [99]. This is a classic example of how a single inflammatory mediator can potentiate numerous inter-related pathways, culminating in a common end goal, the metastasis of the tumor cells.
Cytokines derived from tumor-associated macrophages (TAMs) are also known to promote EMT in PDAC through TLR4/IL10 activation [100, 101] and PAR1 [100, 102] signaling pathways. TAMs secrete CCL18, which induces VCAM-1 expression in PDAC, increasing lactate production in PDAC cells and polarizing macrophages towards the M2 phenotype. When co-cultured with alternatively activated M2 macrophages, PDAC cells demonstrate an increase in fibroblastic morphology as well as upregulation of mesenchymal markers and concomitant downregulation of epithelial markers [100, 101]. Similarly, serum IL-6 levels are significantly increased in PDAC patients relative to healthy patients [103–106], with the highest levels found in patients with metastatic disease [103]. Treating PDAC cells with IL-6 modulated the expression of EMT-related molecules like upregulation of Snail, Slug, N-cadherin, vimentin, fibronectin, collagen, and TWIST2, and downregulation of E-cadherin [107]. The in vitro and in vivo studies point to the contribution of the IL-6/STAT3 signaling axis in regulating these proteins, promoting metastasis. Wartenberg et al. and others explain that a metastatically-favorable environment contains very few T and B-cells, probably because they are constrained to the stromal compartment, and this correlated with the high-grade of tumor budding, which is known to be associated with EMT, metastasis, and poor patient outcomes [108, 109].
3.2.2. Invasion:
The TAMs are thought to enhance metastasis by promoting invasive behavior and angiogenesis. They are one of the earliest infiltrating cells and increase as the tumor progresses. A study assessing their role in PDAC tumors found high numbers of M2-polarized TAMs, which correlate with an increased incidence of lymph node metastasis [110]. Cytokines and VEGF attract TAMs to the TME and support the proliferation of PDAC cells by secreting growth factors [111]. Of these secreted factors, IL-10 and TGF-β contribute to the immunosuppressive phenotypes of the TME by restricting dendritic cell-mediated antitumor immune response. The CAFs also promote invasion, thus contributing to metastasis of tumor cells, especially in solid cancers. A study by Liu et al. explored the relationship between CAFs and the malignant phenotype and observed significant overexpression of CAF markers in PDAC tissues [112]. The in vivo studies also demonstrate that a gain-of-function mutation of p53 in PDAC induces a degree of CAF heterogeneity that allows the tumor cells to adopt invasive phenotype and establishment of pro-metastatic niche [109, 113]. These CAFs secrete proteoglycan called perlecan, which promotes the formation of the CAF-induced pro-metastatic niche [109, 113].
3.2.3. Migration and Intravasation:
Chemokines control the directional movement of migrating cells under normal physiological conditions; however, its dysregulation contributes to numerous pathologies. The shifts in bioenergetic metabolism during PDAC migration, the receptor: ligand interactions are serving as a protective mechanism for migrating cells, and overexpression of certain cytokine receptors directs those tumor cells towards ligands, which then serves as metastatic sites [114]. Traditionally, chemotactic factors recruit immune cells to inflammatory sites [109]. However, PDAC models have shown their role in restricting immune cells in the stromal compartment, preventing their ability to invade the tumor nest. There are numerous studies documenting chemokines and their corresponding receptors, which contribute to immune infiltration. The CXCR2 is the receptor for the chemokine ligands CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8. Studies show that CXCR2 recruits MDSCs, neutrophils, and other inflammatory cell types to pancreatic tumors, promoting tumor progression [109, 115, 116]. CXCR2/CXCL is a powerful axis in tumor cell migration, most likely due to their involvement with Rho, Rac, and MAPK signaling pathways involved with cell migration [117]. A recent report identified that CCL21 and CXCL10 promote PDAC cell migration towards intrapancreatic sensory neurons [118]. Another study identified the biochemical mechanism by which chemokines such as CXCL12 stimulate PDAC cell migration [114]. Singh et al. demonstrated that the CCR5/CCL5 axis promoted tumor cell invasion and proliferation [119]. Other studies showed that radiation exposure increases CXCL12 secretion from CAFs, which promotes migratory and invasive capabilities of cancer cells in vivo [120, 121].
The neutrophil extracellular traps (NETs) from activated neutrophils are well-documented for their role in capturing and killing pathogens. Over time, studies have implicated NETs in conjunction with secreted chemokines in cancer metastasis, primarily by capturing circulating cancer cells and aiding in their migration capabilities. In fact, G-CSF and IL-8 are responsible for triggering NETosis by cancer cells [122]. An in vitro study conducted by Jung et al. showed a PDAC cell line, AsPC-1 induced NET formation, likely in response to the components of the AsPC-1 conditioned medium. The same study also linked the NET formation to enhanced PDAC cell migration, invasion, and angiogenesis [123]. A more in-depth analysis of chemokines linked to NETs was done using the KPC model where authors found the upregulation of genes such as Lcn2, Ltf, CXCL5, CXCL3, Csf3, Ltbp2, Duoxa1, Rab15, Noxa1, Tnf, Cbr2, and IL-6 in response to IL-17; all of which are associated with the induction of NETosis [124]. A study conducted by Zhang et al. in PDAC patient serum samples demonstrated an imbalance between NET formation and degradation such that there is excess NETs production. This study concluded that IL17 promotes immunosuppression by inducing neutrophil infiltration and NETs in pancreatic tumors. The evidence supporting the role of NETs in PDAC patients is so compelling a study called for the integration of tumor-infiltrating NETs with the standard TNM staging system for the prediction of postsurgical patient survival. A follow-up study done by the same team demonstrated that NETs in PDAC patients could promote migration and invasion through the EGFR/ERK pathway and addition of neutralizing antibodies for IL-1β could block the pro-metastatic effects, suggesting the contribution of IL-1β/EGFR/ERK axis in PDAC cell migration and metastasis.
3.2.4. Distant colonization and organotropism
Cytokines, through multiple mechanisms, create a suitable niche for colonizing circulating tumor cells (CTCs) at distant organs such as the lung and liver. Primary tumor cells secrete cytokines and exosomes that target the specific organ creating a conducive environment for incoming CTCs to attach and proliferate [125]. The activation of the STAT3-MAPK-NFκB signaling axis in lung stroma leads to establishing a premetastatic niche (PMN), resulting in stromal modifications and activation of pro-metastatic pathways [126]. Colonization of CTCs to the lung tissues is significantly reduced upon IL-1β knockdown. IL-6 and Timp-1 are trophic factors secreted by thymus cells upon therapy exposure, creating a suitable niche for cancer cells promoting disease relapse [127]. The microRNAs from the primary tumor are taken up by the cells of distant organs; for instance, miR-21 having an affinity for TLR7 present on macrophages leads to the secretion of IL-6 and S100A family members, thus supporting liver metastasis and proliferation [128]. Exosomes from the primary pancreatic tumor containing macrophage inhibitory factor (MIF) stimulate hepatic stellate cells through TGF-β secretion resulting in fibronectin deposition and recruitment of bone marrow-derived cells helping liver colonization and metastasis [129]. The chemokines such as CCL2 and CCL5 recruit myeloid cells to promote distant colonization of CTCs to the lung and liver. The CTCs aggregate platelets in the vasculature and lead to CCL5 expression from endothelial cells and mesenchymal stem cells, resulting in increased metastasis to the lungs [130].
When conducting an in-depth exploration of mechanistic orchestrators of site-specific metastasis, in the context of chemokines, it is important to refer to Steve Paget’s “seed and soil” theory from 1889, which emphasizes the importance of the communication channels between the disseminating cancer cells (“seed”) and the surrounding metastatic TME (“soil”). Under the context of this theory, chemokines appear to act as an intentional guiding compass that directs the tumor cells to specific organs such as the liver, lungs, peritoneum, and spleen. To a degree, the localization of PDAC mets can be attributed to proximity with respect to blood vessels, kidneys, and the spleen. However, key studies have demonstrated the role of chemokines in the deliberate patterns of metastasis. For example, the CXCL12, through its receptor CXCR4, is shown to play an important role in colonizing cancer cells to their metastatic target organs. Similarly, their expression in PDAC TME might also play an important role in CTCs colonization to common metastatic sites like the lung and liver [131]. This is further supported by the increase in immune response in metastatic lesions in PDAC patients treated with continuous administration of CXCR4 inhibitor AMD3100 (plerixafor, Mozobil) [23]. MIF+ exosomes have been shown to target Kupffer cells and recruit BMDCs, facilitating liver metastases [132]. Similarly, lymphatic metastasis appears to be guided by SDF-1 and CCL19/CCL21 [133]. PDAC cells also secrete CXCL1 and CCL2, which are myeloid chemoattractants, and this may support the “seeding” of the circulating tumor cells [134]. Additionally, inflammation appears to enhance the receptivity of an organ to deposition of CTCs, and the development of metastatic lesions, so inflammatory chemokines might also have a hand in establishing a metastatic niche.
4. Cytokine therapy in pancreatic cancer
Significant amounts of literature have accumulated over the years suggesting therapeutic potential in modulating the cytokine profile of the TME. Multiple strategies for cytokine-mediated therapies have been tested over the last couple of decades as both monotherapy and adjuvant therapy. These include direct delivery of recombinant proinflammatory cytokines, antagonists of inflammatory signaling pathways, reprogramming and reintroduction of immune cells to shift the TME away from an immunosuppressive environment, and other alternative therapies that may impact the cytokine profile. However, the clinical efficacy of this type of immunotherapy remains inconclusive, underscored by the complex interplay of pleiotropic cytokines as well as diverse cell types present in the TME deriving PDAC initiation, progression, and metastasis. Although the determination of clinical efficacy remains challenging, the prognostic value of cytokine profiles in PDAC tissues has shown promise [135–137]. This indicates that effective therapeutic strategies may be reached while underscoring the complexity of the cytokine crosstalk present in PDAC TME. In this regard, recently published clinical data within the last five years involving cytokine modulation as therapies in PDAC will be discussed in the following section.
4.1. Cytokines in combination with immunotherapy
Few studies have investigated the effects of direct administration of cytokines on PDAC. These therapeutic targeted cytokines include interferons [138], IL-2 [139], IL-7 and CCL19 [140], IL-10 [141], and GM-CSF [142, 143]. Albeit the study regarding recombinant IL-2 involves treatment of metastases from non-small cell lung cancer, colorectal cancer, renal cell cancer, and melanoma rather than primary pancreatic tumors [139]. As a follow-up from their initial report in 2008 [144], one study investigated the effects of concurrent IFN-α administration and chemoradiation on the survival of PDAC patients [138]. Although the overall median survival of patients was significantly improved to 25 months, this treatment regimen also incurred significant early and late-stage toxicities. During treatment, 68% of patients experienced a grade three or greater toxicity [138]. However, in the recent long-term follow-up [138], patterns of recurrence and tumor characteristics, as well as complications, were identified. For example, 73% of patients had a recurrence of PDAC with a relationship between positive microscopic margins and overall distal recurrence, likely due to lymph node invasion of tumor cells [138]. Additionally, 17% of patients experienced infectious complications, while 15% of patients experienced gastrointestinal complications at 10-year follow-up. Overall, while there was a small survival benefit with concurrent IFN-α treatment and chemoradiation, the severe toxicities led to discontinuing this regimen [138]. The delivery of IL-7 and CCL19 via CAR-T-cells engineered against GPC3, MSN, and CD20 has been proposed as another therapeutic strategy targeting PDAC [140]. Treatment of murine CAR-T-cells with IL-7 and CCL19 has been demonstrated to promote the invasion and survival of these cells in solid tumors [145]. The intravenous injection of these engineered CAR-T-cells induced a complete response and abrogation of any further spread from the initial lymph node metastasis 240 days after final treatment [140]. However, important to note is that this was not a comprehensive clinical trial (i.e., a single pancreatic cancer patient), but planning to initiate phase I trial with a much larger patient cohort [140].
A couple of recent studies have also investigated the use of GM-CSF administration to strengthen the immune reaction against pancreatic tumor cells [142, 143]. One recent phase I/II clinical trial involved the concurrent administration of the Kras-specific antigen immunotherapy, TG01, with GM-CSF (TG01/GM-CSF) in generating and enhancing Kras-specific cytotoxic T-cell responses, respectively, combined with adjuvant gemcitabine treatment [142]. Previous studies utilizing TG01 in clinical trials have been documented, wherein mutant Kras-specific immune responses were reliably induced, and the median survival of patients was around 28 months [146]. In this most recent clinical trial, a total of 32 patients were enrolled with a confirmed diagnosis of stage I or II PDAC, undergone successful (R0 or R1) surgical resection, had an ECOG status of 0 or 1, and a predicted life expectancy of at least 6 months [142]. Although there were over 60 treatment-emergent adverse events, most of these were reported to be related to chemotherapy (gemcitabine) rather than TG01/GM-CSF, indicating this regimen to be relatively well tolerated. Additionally, 94% of patients reported positive immune response as indicated by DHT and T-cell proliferation assays, suggesting neither GM-CSF nor concurrent gemcitabine administration interferes with the immune response [142]. Moreover, this modality has some clinical efficacy, as median overall survival improved to 34.3 months and median disease-free survival was 19.5 months. However, the authors did note adverse events in four patients, and several patients in the cohort were reported to have incidences of recurrence [142].
A separate clinical study has investigated the efficacy of GM-CSF-allogeneic pancreatic tumor cells (GVAX) and ipilimumab as maintenance immunotherapy for patients with metastatic PDAC [143]. Many patients were unable to tolerate multiagent chemotherapies such as FOLFIRINOX for longer than 4–6 months, leading to a need to develop alternative approaches for metastatic PDAC. The patients with metastatic PDAC and treated with FOLFIRINOX for 8–12 doses were split into two arms, either consisting of the GVAX + ipilimumab or continuation of FOLFIRINOX [143]. The allogeneic PDAC cells lines in GVAX serve as a polyvalent source of antigens shared between the vaccine tumor cell lines and the patient’s tumor. These cell lines are also engineered to secrete GM-CSF, which allows for the maturation of dendritic cells and enables robust tumor-specific antigen presentation for immune-mediated cytotoxicity of tumor cells. The final piece of this regimen included ipilimumab, a CTLA-4 inhibitor to promote a more immunogenic PDAC TME. Although well tolerable, with treatment-related adverse events of grade 2 or less (mild) attributed with GVAX, the GVAX + ipilimumab treatment strategy had significantly reduced efficacy in improving overall patient survival compared to FOLFIRINOX continuation [143]. This was demonstrated by median overall survival of 9.38 months for GVAX-treated patients and a median overall survival of 14.7 months for FOLFIRINOX-treated patients. Interestingly, of the two immune-related responses reported from GVAX + ipilimumab treated patients, metastatic pancreatic tumors were characterized by increases in late effector T-cells, memory CD8+ T-cells, and a shift toward M1 macrophages in the tumor microenvironment [143]. Albeit these observations were made from a very small sample size of four patients. Thus, although GVAX + ipilimumab treatment induces changes in immune cell subsets within the TME, other barriers from the stromal compartment, such as immunosuppressive cytokines, may be preventing an effective antitumor response.
4.2. Cytokines in combination with chemotherapy
Another recent study has reported a phase III clinical trial targeting the IL-10 axis in patients with gemcitabine-resistant metastatic PDAC [141]. The patients were separated into two main treatment arms, either pegylated IL-10 + FOLFOX or FOLFOX alone, with almost all patients previously treated with gemcitabine or nab-paclitaxel and tumors graded ECOG of 0 or 1 [141]. The median PEG treatment duration was 10 weeks, with patients in this treatment group experiencing treatment-emergent adverse events such as thrombocytopenia, anemia, fatigue, and neutropenia. However, most of the patients were discontinued due to progressive disease instead of severe adverse events [141]. The exploratory analysis of tumors from both treatment arms revealed significant changes in Th1-specific cytokines such as increases in IL-18 and IFN-γ, as well as a reduction in immunosuppressive TGF-β in PEG-IL-10-treated patients, and a correlation between IL-18 levels and better clinical outcomes in patients. [141]. The granzyme B was also increased, suggesting activation of PD-1+CD8+ T-cells. Unfortunately, there was a lack of survival benefit in gemcitabine refractory metastatic PDAC patients through stimulating the IL-10 pathway, as both treatment arms had a median overall survival of around six months and a median progression-free survival around 2 months [141].
Other therapeutic strategies have directed focus toward antagonizing critical cytokine receptors [147–149]. One recent strategy has been to inhibit TGF-β signaling. The members of the TGF-β signaling pathway are frequently mutated in PDAC, and this pathway has been associated with promoting tumor growth, EMT, ECM remodeling, and stemness of cancer cells [150]. Since TGF-β is viewed as an immunosuppressive cytokine, a recent clinical trial evaluated the efficacy and safety of the first orally available type I TGF-β receptor antagonist, galunisertib, in combination with gemcitabine as a first-line treatment option for PDAC [147]. Almost all patients were diagnosed with stage III/IV disease, and patients received a median of two cycles (28-day cycles of 14 days on and 14 days off) of 300mg/day dose of galunisertib. The study reported a modest extension in median overall survival with galunisertib combination treatment compared with gemcitabine alone, extending survival from 7.1 months to 8.9 months [147]. Evaluation of plasma proteins identified two immunosuppressive cytokines (interferon-γ-induced protein 10 (IP-10) and macrophage inflammatory protein-1-a (MIP-1a)) as predictive biomarkers for overall survival, with galunisertib combination treatment providing the greatest survival benefit [147].
BL-8040 (motixafortide), a small synthetic peptide antagonist of CXCR4, has been tested in a recent clinical trial, COMBAT, to determine its efficacy in treating chemotherapy-refractory metastatic PDAC [148]. Since CXCR4-CCL12 signaling has been documented to modulate the immune microenvironment of solid tumors, the study hypothesized that inhibition of CXCR4 signaling would promote tumor invasion of T-cells, B-cells, and NK cells while limiting the numbers of MDSCs and tumor-associated macrophages. By combining this with pembrolizumab, a PD-1 inhibitor, tumor cells would be more sensitive to infiltrating effector CD8+ T-cells [148]. While median overall survival for patients undergoing BL-8040 combination as third-line treatment was 3.3 months, utilization of this therapy as second-line treatment increased median overall survival to 7.5 months [148], an improvement from the 6.1 months historically reported with the currently approved second-line chemotherapy, NAPOLI-1 [148, 151]. Although the authors report a relatively modest improvement in median overall survival, several immunogenic responses from BL-8040 combination treatment were also reported. For example, the study notes an increase in circulating white blood cells and lymphocytes with a concurrent decrease in circulating Treg cells [148]. Additionally, both BL-8040 monotherapy and BL-8040 combination with pembrolizumab increased cell densities of CD3+, CD4+, CD8+, and activated CD3+CD8+granzyme B+ T-cells while decreasing MDSCs in the TME. Furthermore, the combination treatment incurred minimal additional toxicity, with <5% of patients experiencing treatment-related adverse events severe enough to warrant discontinuation. Still, due to the low sample size of this study, conclusions drawn from this combination therapy need to be exercised cautiously [148].
A separate chemokine receptor antagonist, PF-04136309, specific for CCR2, has also been evaluated in combination with chemotherapy for treatment of metastatic PDAC; although, this was to determine its efficacy as first-line treatment [149]. The study hypothesized that targeted inhibition of the CCR2 axis might relieve a mechanism involved in creating an immunosuppressive TME, thus facilitating enhanced tumor immunogenicity [149]. Based on pharmacodynamic data of the 21-patient cohort, the authors proposed PF-04136309 acts by reducing the trafficking of CD14+CCR2+ inflammatory monocytes from the bone marrow to the solid tumor. Unfortunately, the study also reported a questionable safety profile for the combination of PF-04136309 and gemcitabine/nab-paclitaxel, as a significant portion of the patients (25%) presented with pulmonary toxicity. Furthermore, there was no additional benefit to clinical efficacy with PF-04136309 combination compared to gemcitabine + nab-paclitaxel [149].
Several other recent clinical trials have utilized indirect methods to modulate cytokine signaling in PDAC treatments [152–156]. For example, a recent phase Ib/II clinical trial has demonstrated some clinical activity using itacitanib, a JAK1 specific inhibitor, in combination with nab-paclitaxel and gemcitabine in patients with advanced-stage PDAC [152]. Since JAK/STAT is a critical pathway in cytokine-mediated signaling, inhibiting this pathway could limit the inflammatory response, which is well documented to promote the progression of PDAC and sensitize tumors to chemotherapy. This therapeutic strategy incurred mild toxicity (grade 3 neutropenia in 12% of patients) with an overall response rate of 24%, where all patients in this subset had partial response [152]. However, this trial was terminated early due to a separate phase III trial using a JAK1/2 inhibitor, indicating an absence of improved clinical efficacy [152]. The STARPAC phase I clinical trial has investigated the use of all-trans retinoic acid (ATRA) in combination with nab-paclitaxel and gemcitabine to treat PDAC [153]. The authors hypothesized that administration of ATRA would reprogram pancreatic stroma (via replenishing retinoic acid stores), reversing the inhibitory effects of activated pancreatic stellate cells (PSCs) on cytotoxic immune cells infiltration into the TME and modulate the response of PSCs to cytokines. Patients treated with 45mg/m2 of ATRA combined with chemotherapy had improved median overall survival to 11.7 months with an acceptable cytotoxicity profile [153]. The authors also note that ATRA may dampen the neurotoxicity from nab-paclitaxel, although this is yet to be tested on a more comprehensive scale [153]. The heparin sulfate mimetic, necuparanib, has been suggested as a possible first-line treatment in metastatic PDAC [154]. Glycosaminoglycans, such as heparin sulfate, can modulate the stromal environment by binding to cytokines, localizing concentrations of certain immune factors, and even facilitating interactions between cytokines and their receptors. Treatment of patients with 5mg/kg one daily per week for three weeks in a four-week cycle led to a response rate of 35% (out of 62 patients) with a median overall survival of 13.1 months [154]. Of note, this trial reported increased hematological and gastrointestinal toxicity but ultimately evaluated this treatment to have an acceptable safety profile. However, there was no improvement in survival outcome from necuparanib combination therapy compared to standard therapy [154]. Another clinical trial has tested the effects of eicosapentaenoic acid (EPA)-enriched nutrition for use as a pre-operative agent in conjunction with chemoradiation for treatment of PDAC [155]. While the focus of this study was to determine the efficacy of EPA to abrogate cancer cachexia syndrome, EPA can also reduce the secretion of proinflammatory cytokines [155]. Albeit, the effect of EPA supplementation on tumor progression and its influence on the efficacy of chemoradiation remains in question. Lastly, a phase I trial has investigated the effect of Thermatron RF-8-mediated tumor hyperthermia in combination with immunotherapies (ACT and/or anti-PD-1) to treat advanced solid tumors, including PDAC [156]. The authors proposed that exposing tumors to high temperatures (40–43°C) would increase antigen presentation and exposure to antigen-presenting cells, enhancing the efficacy of immunotherapies. Although the study recorded an objective response rate of 30% with an acceptable safety profile and changes in circulating cytokine profiles [156], conclusions regarding the clinical benefits of hyperthermia as a cancer treatment remain constrained by the nature of phase I clinical trial. Furthermore, only two patients with PDAC were part of the patient cohort [156], putting into question the effectiveness of this treatment modality in the context of PDAC.
Mixed results have come from recent clinical trials directly or indirectly targeting the cytokine signaling pathways within the TME. Although some offer promise, most recent trials have reported modest clinical efficacy by modulating cytokine profiles in PDAC TME. This underscores the complexity of the TME and highlights the multiple dynamic interactions that serve as mechanisms in the initiation, progression, and metastasis of PDAC. Still, several clinical trials are ongoing that are exploring other combinations of immunotherapies, chemotherapies, radiation, and cytokine therapies to improve upon the limited therapeutic modalities available to PDAC patients.
5. Conclusion and future directions:
Pancreatic cancer is a lethal malignancy that is notorious for its penchant to metastasize to distant organs and establish tumor colonies. It is characteristically immunosuppressive, and communication between constituents of the complex PDAC TME are critical for cancer cell sustenance. This communication is a bilateral process engaging a methodical and interdependent series of events culminating in metastatic spread and chemoresistance. Cytokines serve as a liaison between the tumor cells and other cells present in the TME, thus facilitating this critical communication. From a birds-eye-view, the TME constitutes a plethora of cell types such as pancreatic stellate cells, cancer-associated fibroblasts, tumor-associated macrophages, and neutrophils, which are stimulated by the cancer cells to secrete various chemokines that either drive inflammation, immunosuppression, cell migration, and/or colonization while simultaneously forming a positive feedback loop to potentiate the tumorigenic environment. Upregulated chemokine receptors like CXCR2, in conjunction with the expression of cytokines and ligands like IL-6, IL-17, EGF, and TGF-α, further promote signaling pathways that drive tumor initiation and progression. On top of cancer progression, it appears that these chemokines also contribute to the characteristic chemoresistance that is a major obstacle in PDAC-therapy development. MUC4 and MUC5AC contribute to chemoresistance, and chemokines have been demonstrated to enhance their expression. Numerous studies have unveiled the importance of mucin biology in the PC TME through chemokine signaling [157] and CAF-mediated enrichment of the cancer stem cell population [158]. Further exploration of this avenue of research could prove beneficial to the overall PDAC field, especially with respect to reducing metastasis and chemoresistance. Due to the importance of host-derived chemokines in PDAC, numerous drugs are also in clinical trials to target them directly or indirectly. However, further understanding of the complex communication networks needs to be better understood to establish functional therapies with minimal off-target effects. Overall, the present review provides valuable information regarding the function of cytokines and their receptors at every stage of PDAC development, simultaneously providing their current status in therapy. Further understanding their role as drivers of the early onset and their significant systemic elevation in PDAC during tumorigenesis makes them important candidates for early detection and diagnosis, an area of focus in PDAC research and therapy.
Acknowledgments:
This author’s/work was partly supported by funding from the National Institutes of Health (P01 CA217798, U01 CA210240, U01 CA200466, R01 CA206444, R01 CA228524, and R44 CA235991).
Footnotes
Conflicts of Interest: SKB is one of the founders of Sanguine Diagnostics and Therapeutics, Inc. Other authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kozłowska A, Wojtacha P, Majewski M, Równiak M, The cytokine alterations/abnormalities and oxidative damage in the pancreas during hypertension development, Pflugers Arch 471(10) (2019) 1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Xie D, Xie K, Pancreatic cancer stromal biology and therapy, Genes Dis 2(2) (2015) 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tian C, Clauser KR, Öhlund D, Rickelt S, Huang Y, Gupta M, Mani DR, Carr SA, Tuveson DA, Hynes RO, Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells, Proc Natl Acad Sci U S A 116(39) (2019) 19609–19618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tian C, Huang Y, Clauser KR, Rickelt S, Lau AN, Carr SA, Vander Heiden MG, Hynes RO, Suppression of pancreatic ductal adenocarcinoma growth and metastasis by fibrillar collagens produced selectively by tumor cells, Nat Commun 12(1) (2021) 2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Waters AM, Der CJ, KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer, Cold Spring Harb Perspect Med 8(9) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lennerz JK, Stenzinger A, Allelic ratio of KRAS mutations in pancreatic cancer, Oncologist 20(4) (2015) e8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ardito CM, Grüner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK, Delgiorno KE, Carpenter ES, Halbrook CJ, Hall JC, Pal D, Briel T, Herner A, Trajkovic-Arsic M, Sipos B, Liou GY, Storz P, Murray NR, Threadgill DW, Sibilia M, Washington MK, Wilson CL, Schmid RM, Raines EW, Crawford HC, Siveke JT, EGF receptor is required for KRAS-induced pancreatic tumorigenesis, Cancer Cell 22(3) (2012) 304–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Storz P, Acinar cell plasticity and development of pancreatic ductal adenocarcinoma, Nat Rev Gastroenterol Hepatol 14(5) (2017) 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris J.P.t., Pan FC, Akiyama H, Wright CV, Jensen K, Hebrok M, Sander M, Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma, Cancer Cell 22(6) (2012) 737–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Parte S, Nimmakayala RK, Batra SK, Ponnusamy MP, Acinar to ductal cell transdifferentiation: A prelude to dysplasia and pancreatic ductal adenocarcinoma, Biochim Biophys Acta Rev Cancer 1877(1) (2021) 188669. [DOI] [PubMed] [Google Scholar]
- [11].Poh AR, Ernst M, Tumor-Associated Macrophages in Pancreatic Ductal Adenocarcinoma: Therapeutic Opportunities and Clinical Challenges, Cancers (Basel) 13(12) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liou GY, Döppler H, Necela B, Krishna M, Crawford HC, Raimondo M, Storz P, Macrophage-secreted cytokines drive pancreatic acinar-to-ductal metaplasia through NF-κB and MMPs, J Cell Biol 202(3) (2013) 563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Deschênes-Simard X, Mizukami Y, Bardeesy N, Macrophages in pancreatic cancer: starting things off on the wrong track, J Cell Biol 202(3) (2013) 403–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Perera RM, Bardeesy N, Ready, set, go: the EGF receptor at the pancreatic cancer starting line, Cancer Cell 22(3) (2012) 281–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu J, Akanuma N, Liu C, Naji A, Halff GA, Washburn WK, Sun L, Wang P, TGF-β1 promotes acinar to ductal metaplasia of human pancreatic acinar cells, Sci Rep 6 (2016) 30904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Klöppel G, Yoshimura A, Reindl W, Sipos B, Akira S, Schmid RM, Algül H, Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer, Cancer Cell 19(4) (2011) 456–69. [DOI] [PubMed] [Google Scholar]
- [17].Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, Rossaint R, Heinrich PC, Müller-Newen G, Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3, J Immunol 170(6) (2003) 3263–72. [DOI] [PubMed] [Google Scholar]
- [18].Sriram K, Salmerón C, Wiley SZ, Insel PA, GPCRs in pancreatic adenocarcinoma: Contributors to tumour biology and novel therapeutic targets, Br J Pharmacol 177(11) (2020) 2434–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gong R, Ren H, Targeting chemokines/chemokine receptors: a promising strategy for enhancing the immunotherapy of pancreatic ductal adenocarcinoma, Signal Transduct Target Ther 5(1) (2020) 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Raman D, Baugher PJ, Thu YM, Richmond A, Role of chemokines in tumor growth, Cancer Lett 256(2) (2007) 137–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Miksch RC, Schoenberg MB, Weniger M, Bösch F, Ormanns S, Mayer B, Werner J, Bazhin AV, D’Haese JG, Prognostic Impact of Tumor-Infiltrating Lymphocytes and Neutrophils on Survival of Patients with Upfront Resection of Pancreatic Cancer, Cancers (Basel) 11(1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gao Y, Chen S, Vafaei S, Zhong X, Tumor-Infiltrating Immune Cell Signature Predicts the Prognosis and Chemosensitivity of Patients With Pancreatic Ductal Adenocarcinoma, Front Oncol 10 (2020) 557638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Biasci D, Smoragiewicz M, Connell CM, Wang Z, Gao Y, Thaventhiran JED, Basu B, Magiera L, Johnson TI, Bax L, Gopinathan A, Isherwood C, Gallagher FA, Pawula M, Hudecova I, Gale D, Rosenfeld N, Barmpounakis P, Popa EC, Brais R, Godfrey E, Mir F, Richards FM, Fearon DT, Janowitz T, Jodrell DI, CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response, Proc Natl Acad Sci U S A 117(46) (2020) 28960–28970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mace TA, Shakya R, Pitarresi JR, Swanson B, McQuinn CW, Loftus S, Nordquist E, Cruz-Monserrate Z, Yu L, Young G, Zhong X, Zimmers TA, Ostrowski MC, Ludwig T, Bloomston M, Bekaii-Saab T, Lesinski GB, IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer, Gut 67(2) (2018) 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vitale G, van Eijck CH, van Koetsveld Ing PM, Erdmann JI, Speel EJ, van der Wansem Ing K, Mooij DM, Colao A, Lombardi G, Croze E, Lamberts SW, Hofland LJ, Type I interferons in the treatment of pancreatic cancer: mechanisms of action and role of related receptors, Ann Surg 246(2) (2007) 259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Angelini C, Bovo G, Muselli P, Mussi C, Crippa S, Caprotti R, Uggeri F, Preoperative interleukin-2 immunotherapy in pancreatic cancer: preliminary results, Hepatogastroenterology 53(67) (2006) 141–4. [PubMed] [Google Scholar]
- [27].Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, Toriola AT, Nieman RK, Worley LA, Yano M, Fowler KJ, Lockhart AC, Suresh R, Tan BR, Lim KH, Fields RC, Strasberg SM, Hawkins WG, DeNardo DG, Goedegebuure SP, Linehan DC, Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial, Lancet Oncol 17(5) (2016) 651–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Spangler JB, Moraga I, Mendoza JL, Garcia KC, Insights into cytokine-receptor interactions from cytokine engineering, Annu Rev Immunol 33 (2015) 139–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Moraga I, Spangler J, Mendoza JL, Garcia KC, Multifarious determinants of cytokine receptor signaling specificity, Adv Immunol 121 (2014) 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kufareva I, Salanga CL, Handel TM, Chemokine and chemokine receptor structure and interactions: implications for therapeutic strategies, Immunol Cell Biol 93(4) (2015) 372–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Apte MV, Pirola RC, Wilson JS, Pancreatic stellate cells: a starring role in normal and diseased pancreas, Front Physiol 3 (2012) 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mews P, Phillips P, Fahmy R, Korsten M, Pirola R, Wilson J, Apte M, Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis, Gut 50(4) (2002) 535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Algül H, Treiber M, Lesina M, Schmid RM, Mechanisms of disease: chronic inflammation and cancer in the pancreas—a potential role for pancreatic stellate cells?, Nature Clinical Practice Gastroenterology & Hepatology 4(8) (2007) 454–462. [DOI] [PubMed] [Google Scholar]
- [34].Xiao Z, Luo G, Liu C, Wu C, Liu L, Liu Z, Ni Q, Long J, Yu X, Molecular mechanism underlying lymphatic metastasis in pancreatic cancer, BioMed research international 2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Biffi G, Oni TE, Spielman B, Hao Y, Elyada E, Park Y, Preall J, Tuveson DA, IL1-Induced JAK/STAT Signaling Is Antagonized by TGFβ to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma, Cancer Discov 9(2) (2019) 282–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio II, Hwang CI, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, Tuveson DA, Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer, J Exp Med 214(3) (2017) 579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hernandez-Barco YG, Bardeesy N, Ting DT, No Cell Left Unturned: Intraductal Papillary Mucinous Neoplasm Heterogeneity, Clin Cancer Res 25(7) (2019) 2027–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yang S, Liu Q, Liao Q, Tumor-Associated Macrophages in Pancreatic Ductal Adenocarcinoma: Origin, Polarization, Function, and Reprogramming, Front Cell Dev Biol 8 (2020) 607209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chen SJ, Lian GD, Li JJ, Zhang QB, Zeng LJ, Yang KG, Huang CM, Li YQ, Chen YT, Huang KH, Tumor-driven like macrophages induced by conditioned media from pancreatic ductal adenocarcinoma promote tumor metastasis via secreting IL-8, Cancer Med 7(11) (2018) 5679–5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen Q, Wang J, Zhang Q, Zhang J, Lou Y, Yang J, Chen Y, Wei T, Zhang J, Fu Q, Ye M, Zhang X, Dang X, Liang T, Bai X, Tumour cell-derived debris and IgG synergistically promote metastasis of pancreatic cancer by inducing inflammation via tumour-associated macrophages, Br J Cancer 121(9) (2019) 786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lee JJ, Bernard V, Semaan A, Monberg ME, Huang J, Stephens BM, Lin D, Rajapakshe KI, Weston BR, Bhutani MS, Haymaker CL, Bernatchez C, Taniguchi CM, Maitra A, Guerrero PA, Elucidation of Tumor-Stromal Heterogeneity and the Ligand-Receptor Interactome by Single-Cell Transcriptomics in Real-world Pancreatic Cancer Biopsies, Clin Cancer Res 27(21) (2021) 5912–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gnerlich JL, Mitchem JB, Weir JS, Sankpal NV, Kashiwagi H, Belt BA, Porembka MR, Herndon JM, Eberlein TJ, Goedegebuure P, Linehan DC, Induction of Th17 cells in the tumor microenvironment improves survival in a murine model of pancreatic cancer, J Immunol 185(7) (2010) 4063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, Rattigan Y, Roeser JC, Lankapalli RH, Zhang H, Jaffee EM, Drake CG, Housseau F, Maitra A, Kolls JK, Sears CL, Pardoll DM, Leach SD, Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia, Cancer Cell 25(5) (2014) 621–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang Y, Zoltan M, Riquelme E, Xu H, Sahin I, Castro-Pando S, Montiel MF, Chang K, Jiang Z, Ling J, Gupta S, Horne W, Pruski M, Wang H, Sun SC, Lozano G, Chiao P, Maitra A, Leach SD, Kolls JK, Vilar E, Wang TC, Bailey JM, McAllister F, Immune Cell Production of Interleukin 17 Induces Stem Cell Features of Pancreatic Intraepithelial Neoplasia Cells, Gastroenterology 155(1) (2018) 210–223.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chen K, Wang Q, Li M, Guo H, Liu W, Wang F, Tian X, Yang Y, Single-cell RNA-seq reveals dynamic change in tumor microenvironment during pancreatic ductal adenocarcinoma malignant progression, EBioMedicine 66 (2021) 103315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Steele NG, Carpenter ES, Kemp SB, Sirihorachai V, The S, Delrosario L, Lazarus J, Amir ED, Gunchick V, Espinoza C, Bell S, Harris L, Lima F, Irizarry-Negron V, Paglia D, Macchia J, Chu AKY, Schofield H, Wamsteker EJ, Kwon R, Schulman A, Prabhu A, Law R, Sondhi A, Yu J, Patel A, Donahue K, Nathan H, Cho C, Anderson MA, Sahai V, Lyssiotis CA, Zou W, Allen BL, Rao A, Crawford HC, Bednar F, Frankel TL, Pasca di Magliano M, Multimodal Mapping of the Tumor and Peripheral Blood Immune Landscape in Human Pancreatic Cancer, Nat Cancer 1(11) (2020) 1097–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pylayeva-Gupta Y, Das S, Handler JS, Hajdu CH, Coffre M, Koralov SB, Bar-Sagi D, IL35-Producing B Cells Promote the Development of Pancreatic Neoplasia, Cancer Discov 6(3) (2016) 247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M, B-cell-derived lymphotoxin promotes castration-resistant prostate cancer, Nature 464(7286) (2010) 302–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Liou GY, Inflammatory Cytokine Signaling during Development of Pancreatic and Prostate Cancers, J Immunol Res 2017 (2017) 7979637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Foucher ED, Ghigo C, Chouaib S, Galon J, Iovanna J, Olive D, Pancreatic Ductal Adenocarcinoma: A Strong Imbalance of Good and Bad Immunological Cops in the Tumor Microenvironment, Front Immunol 9 (2018) 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D, Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia, Cancer Cell 21(6) (2012) 836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH, Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer, Cancer Cell 21(6) (2012) 822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Feurino LW, Zhang Y, Bharadwaj U, Zhang R, Li F, Fisher WE, Brunicardi FC, Chen C, Yao Q, Min L, IL-6 stimulates Th2 type cytokine secretion and upregulates VEGF and NRP-1 expression in pancreatic cancer cells, Cancer Biol Ther 6(7) (2007) 1096–100. [DOI] [PubMed] [Google Scholar]
- [54].Yako YY, Kruger D, Smith M, Brand M, Cytokines as Biomarkers of Pancreatic Ductal Adenocarcinoma: A Systematic Review, PLoS One 11(5) (2016) e0154016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bellone G, Carbone A, Smirne C, Scirelli T, Buffolino A, Novarino A, Stacchini A, Bertetto O, Palestro G, Sorio C, Scarpa A, Emanuelli G, Rodeck U, Cooperative induction of a tolerogenic dendritic cell phenotype by cytokines secreted by pancreatic carcinoma cells, J Immunol 177(5) (2006) 3448–60. [DOI] [PubMed] [Google Scholar]
- [56].Kirkegård J, Mortensen FV, Cronin-Fenton D, Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis, Am J Gastroenterol 112(9) (2017) 1366–1372. [DOI] [PubMed] [Google Scholar]
- [57].Malka D, Hammel P, Maire F, Rufat P, Madeira I, Pessione F, Lévy P, Ruszniewski P, Risk of pancreatic adenocarcinoma in chronic pancreatitis, Gut 51(6) (2002) 849–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bhatia R, Thompson C, Ganguly K, Singh S, Batra SK, Kumar S, Alcohol and Smoking Mediated Modulations in Adaptive Immunity in Pancreatitis, Cells 9(8) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N, Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer, Br J Cancer 108(4) (2013) 914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Feng L, Qi Q, Wang P, Chen H, Chen Z, Meng Z, Liu L, Serum levels of IL-6, IL-8, and IL-10 are indicators of prognosis in pancreatic cancer, J Int Med Res 46(12) (2018) 5228–5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kim HW, Lee JC, Paik KH, Kang J, Kim J, Hwang JH, Serum interleukin-6 is associated with pancreatic ductal adenocarcinoma progression pattern, Medicine (Baltimore) 96(5) (2017) e5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].van Duijneveldt G, Griffin MDW, Putoczki TL, Emerging roles for the IL-6 family of cytokines in pancreatic cancer, Clin Sci (Lond) 134(16) (2020) 2091–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Scholz A, Heinze S, Detjen KM, Peters M, Welzel M, Hauff P, Schirner M, Wiedenmann B, Rosewicz S, Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer, Gastroenterology 125(3) (2003) 891–905. [DOI] [PubMed] [Google Scholar]
- [64].Corcoran RB, Contino G, Deshpande V, Tzatsos A, Conrad C, Benes CH, Levy DE, Settleman J, Engelman JA, Bardeesy N, STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis, Cancer Res 71(14) (2011) 5020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zdanov S, Mandapathil M, Abu Eid R, Adamson-Fadeyi S, Wilson W, Qian J, Carnie A, Tarasova N, Mkrtichyan M, Berzofsky JA, Whiteside TL, Khleif SN, Mutant KRAS Conversion of Conventional T Cells into Regulatory T Cells, Cancer Immunol Res 4(4) (2016) 354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhang D, Zhou Y, Wu L, Wang S, Zheng H, Yu B, Li J, Association of IL-6 gene polymorphisms with cachexia susceptibility and survival time of patients with pancreatic cancer, Ann Clin Lab Sci 38(2) (2008) 113–9. [PubMed] [Google Scholar]
- [67].Talar-Wojnarowska R, Gasiorowska A, Smolarz B, Romanowicz-Makowska H, Kulig A, Malecka-Panas E, Clinical significance of interleukin-6 (IL-6) gene polymorphism and IL-6 serum level in pancreatic adenocarcinoma and chronic pancreatitis, Dig Dis Sci 54(3) (2009) 683–9. [DOI] [PubMed] [Google Scholar]
- [68].Liou GY, Bastea L, Fleming A, Döppler H, Edenfield BH, Dawson DW, Zhang L, Bardeesy N, Storz P, The Presence of Interleukin-13 at Pancreatic ADM/PanIN Lesions Alters Macrophage Populations and Mediates Pancreatic Tumorigenesis, Cell Rep 19(7) (2017) 1322–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Purohit A, Varney M, Rachagani S, Ouellette MM, Batra SK, Singh RK, CXCR2 signaling regulates KRAS(G12D)-induced autocrine growth of pancreatic cancer, Oncotarget 7(6) (2016) 7280–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Steele CW, Karim SA, Leach JDG, Bailey P, Upstill-Goddard R, Rishi L, Foth M, Bryson S, McDaid K, Wilson Z, Eberlein C, Candido JB, Clarke M, Nixon C, Connelly J, Jamieson N, Carter CR, Balkwill F, Chang DK, Evans TRJ, Strathdee D, Biankin AV, Nibbs RJB, Barry ST, Sansom OJ, Morton JP, CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma, Cancer Cell 29(6) (2016) 832–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Nywening TM, Belt BA, Cullinan DR, Panni RZ, Han BJ, Sanford DE, Jacobs RC, Ye J, Patel AA, Gillanders WE, Fields RC, DeNardo DG, Hawkins WG, Goedegebuure P, Linehan DC, Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma, Gut 67(6) (2018) 1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mielgo A, Schmid MC, Impact of tumour associated macrophages in pancreatic cancer, BMB Rep 46(3) (2013) 131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Huang J, Zhang L, Wan D, Zhou L, Zheng S, Lin S, Qiao Y, Extracellular matrix and its therapeutic potential for cancer treatment, Signal Transduct Target Ther 6(1) (2021) 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ahmad RS, Eubank TD, Lukomski S, Boone BA, Immune Cell Modulation of the Extracellular Matrix Contributes to the Pathogenesis of Pancreatic Cancer, Biomolecules 11(6) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Grimont A, Pinho AV, Cowley MJ, Augereau C, Mawson A, Giry-Laterrière M, Van den Steen G, Waddell N, Pajic M, Sempoux C, Wu J, Grimmond SM, Biankin AV, Lemaigre FP, Rooman I, Jacquemin P, SOX9 regulates ERBB signalling in pancreatic cancer development, Gut 64(11) (2015) 1790–9. [DOI] [PubMed] [Google Scholar]
- [76].Lankadasari MB, Mukhopadhyay P, Mohammed S, Harikumar KB, TAMing pancreatic cancer: combat with a double edged sword, Mol Cancer 18(1) (2019) 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ray KC, Moss ME, Franklin JL, Weaver CJ, Higginbotham J, Song Y, Revetta FL, Blaine SA, Bridges LR, Guess KE, Coffey RJ, Crawford HC, Washington MK, Means AL, Heparin-binding epidermal growth factor-like growth factor eliminates constraints on activated Kras to promote rapid onset of pancreatic neoplasia, Oncogene 33(7) (2014) 823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Moniaux N, Chakraborty S, Yalniz M, Gonzalez J, Shostrom VK, Standop J, Lele SM, Ouellette M, Pour PM, Sasson AR, Brand RE, Hollingsworth MA, Jain M, Batra SK, Early diagnosis of pancreatic cancer: neutrophil gelatinase-associated lipocalin as a marker of pancreatic intraepithelial neoplasia, Br J Cancer 98(9) (2008) 1540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bhatia R, Muniyan S, Thompson CM, Kaur S, Jain M, Singh RK, Dhaliwal A, Cox JL, Akira S, Singh S, Batra SK, Kumar S, Neutrophil Gelatinase-Associated Lipocalin Protects Acinar Cells From Cerulein-Induced Damage During Acute Pancreatitis, Pancreas 49(10) (2020) 1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chakraborty S, Kaur S, Muddana V, Sharma N, Wittel UA, Papachristou GI, Whitcomb D, Brand RE, Batra SK, Elevated serum neutrophil gelatinase-associated lipocalin is an early predictor of severity and outcome in acute pancreatitis, Am J Gastroenterol 105(9) (2010) 2050–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Cannon A, Thompson CM, Bhatia R, Armstrong KA, Solheim JC, Kumar S, Batra SK, Molecular mechanisms of pancreatic myofibroblast activation in chronic pancreatitis and pancreatic ductal adenocarcinoma, J Gastroenterol 56(8) (2021) 689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Partecke LI, Günther C, Hagemann S, Jacobi C, Merkel M, Sendler M, van Rooijen N, Käding A, Nguyen Trung D, Lorenz E, Diedrich S, Weiss FU, Heidecke CD, von Bernstorff W, Induction of M2-macrophages by tumour cells and tumour growth promotion by M2-macrophages: a quid pro quo in pancreatic cancer, Pancreatology 13(5) (2013) 508–16. [DOI] [PubMed] [Google Scholar]
- [83].Ju M, Bi J, Wei Q, Jiang L, Guan Q, Zhang M, Song X, Chen T, Fan J, Li X, Wei M, Zhao L, Pan-cancer analysis of NLRP3 inflammasome with potential implications in prognosis and immunotherapy in human cancer, Brief Bioinform 22(4) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rech AJ, Mick R, Martin S, Recio A, Aqui NA, Powell DJ Jr., Colligon TA, Trosko JA, Leinbach LI, Pletcher CH, Tweed CK, DeMichele A, Fox KR, Domchek SM, Riley JL, Vonderheide RH, CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients, Sci Transl Med 4(134) (2012) 134ra62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zhang Y, Lazarus J, Steele NG, Yan W, Lee HJ, Nwosu ZC, Halbrook CJ, Menjivar RE, Kemp SB, Sirihorachai VR, Velez-Delgado A, Donahue K, Carpenter ES, Brown KL, Irizarry-Negron V, Nevison AC, Vinta A, Anderson MA, Crawford HC, Lyssiotis CA, Frankel TL, Bednar F, Pasca di Magliano M, Regulatory T-cell Depletion Alters the Tumor Microenvironment and Accelerates Pancreatic Carcinogenesis, Cancer Discov 10(3) (2020) 422–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Bhatia R, Gautam SK, Cannon A, Thompson C, Hall BR, Aithal A, Banerjee K, Jain M, Solheim JC, Kumar S, Batra SK, Cancer-associated mucins: role in immune modulation and metastasis, Cancer Metastasis Rev 38(1–2) (2019) 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Gautam SK, Kumar S, Cannon A, Hall B, Bhatia R, Nasser MW, Mahapatra S, Batra SK, Jain M, MUC4 mucin- a therapeutic target for pancreatic ductal adenocarcinoma, Expert Opin Ther Targets 21(7) (2017) 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rachagani S, Torres MP, Kumar S, Haridas D, Baine M, Macha MA, Kaur S, Ponnusamy MP, Dey P, Seshacharyulu P, Johansson SL, Jain M, Wagner KU, Batra SK, Mucin (Muc) expression during pancreatic cancer progression in spontaneous mouse model: potential implications for diagnosis and therapy, J Hematol Oncol 5 (2012) 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ganguly K, Bhatia R, Rauth S, Kisling A, Atri P, Thompson C, Vengoji R, Ram Krishn S, Shinde D, Thomas V, Kaur S, Mallya K, Cox JL, Kumar S, Batra SK, Mucin 5AC Serves as the Nexus for β-Catenin/c-Myc Interplay to Promote Glutamine Dependency During Pancreatic Cancer Chemoresistance, Gastroenterology 162(1) (2022) 253–268.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Li X, Wang L, Nunes DP, Troxler RF, Offner GD, Proinflammatory cytokines upregulate MUC1 gene expression in oral epithelial cells, J Dent Res 82(11) (2003) 883–7. [DOI] [PubMed] [Google Scholar]
- [91].Thompson CM, Cannon A, West S, Ghersi D, Atri P, Bhatia R, Smith L, Rachagani S, Wichman C, Kumar S, Batra SK, Mucin Expression and Splicing Determine Novel Subtypes and Patient Mortality in Pancreatic Ductal Adenocarcinoma, Clin Cancer Res 27(24) (2021) 6787–6799. [DOI] [PubMed] [Google Scholar]
- [92].Cannon A, Thompson CM, Maurer HC, Atri P, Bhatia R, West S, Ghersi D, Olive KP, Kumar S, Batra SK, CXCR3 and Cognate Ligands are Associated with Immune Cell Alteration and Aggressiveness of Pancreatic Ductal Adenocarcinoma, Clin Cancer Res 26(22) (2020) 6051–6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Cannon A, Thompson CM, Bhatia R, Kandy RRK, Solheim JC, Batra SK, Kumar S, Contribution of CXCR3-mediated signaling in the metastatic cascade of solid malignancies, Biochim Biophys Acta Rev Cancer 1876(2) (2021) 188628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Pandey V, Fleming-Martinez A, Bastea L, Doeppler HR, Eisenhauer J, Le T, Edenfield B, Storz P, CXCL10/CXCR3 signaling contributes to an inflammatory microenvironment and its blockade enhances progression of murine pancreatic precancerous lesions, Elife 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Maman S, Witz IP, A history of exploring cancer in context, Nature Reviews Cancer 18(6) (2018) 359–376. [DOI] [PubMed] [Google Scholar]
- [96].Greer JB, Whitcomb DC, Inflammation and pancreatic cancer: an evidence-based review, Current opinion in pharmacology 9(4) (2009) 411–418. [DOI] [PubMed] [Google Scholar]
- [97].Fan Y, Lesina M, Algül H, Subtypes of pancreatic stellate cells and distant metastasis of pancreatic ductal adenocarcinoma, Annals of Translational Medicine 8(11) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Cukierman E, Bassi DE, Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors, Seminars in cancer biology, Elsevier, 2010, pp. 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Adjuto-Saccone M, Soubeyran P, Garcia J, Audebert S, Camoin L, Rubis M, Roques J, Binétruy B, Iovanna JL, Tournaire R, TNF-α induces endothelial–mesenchymal transition promoting stromal development of pancreatic adenocarcinoma, Cell Death & Disease 12(7) (2021) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Poh AR, Ernst M, Tumor-Associated Macrophages in Pancreatic Ductal Adenocarcinoma: Therapeutic Opportunities and Clinical Challenges, Cancers 13(12) (2021) 2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Liu C-Y, Xu J-Y, Shi X-Y, Huang W, Ruan T-Y, Xie P, Ding J-L, M2-polarized tumor-associated macrophages promoted epithelial–mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway, Laboratory investigation 93(7) (2013) 844–854. [DOI] [PubMed] [Google Scholar]
- [102].Tekin C, Aberson HL, Waasdorp C, Hooijer GK, de Boer OJ, Dijk F, Bijlsma MF, Spek CA, Macrophage-secreted MMP9 induces mesenchymal transition in pancreatic cancer cells via PAR1 activation, Cellular Oncology 43(6) (2020) 1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].van Duijneveldt G, Griffin MD, Putoczki TL, Emerging roles for the IL-6 family of cytokines in pancreatic cancer, Clinical Science 134(16) (2020) 2091–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ebrahimi B, Tucker SL, Li D, Abbruzzese JL, Kurzrock R, Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis, Cancer: Interdisciplinary International Journal of the American Cancer Society 101(12) (2004) 2727–2736. [DOI] [PubMed] [Google Scholar]
- [105].Miron N, Miron M-M, Milea V, Cristea V, Proinflammatory cytokines: an insight into pancreatic oncogenesis, Roum Arch Microbiol Immunol 69(4) (2010) 183–189. [PubMed] [Google Scholar]
- [106].Okada S, Okusaka T, Ishii H, Kyogoku A, Yoshimori M, Kajimura N, Yamaguchi K, Kakizoe T, Elevated serum interleukin-6 levels in patients with pancreatic cancer, Japanese journal of clinical oncology 28(1) (1998) 12–15. [DOI] [PubMed] [Google Scholar]
- [107].Abaurrea A, Araujo AM, Caffarel MM, The Role of the IL-6 Cytokine Family in Epithelial–Mesenchymal Plasticity in Cancer Progression, International Journal of Molecular Sciences 22(15) (2021) 8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wartenberg M, Cibin S, Zlobec I, Vassella E, Eppenberger-Castori S, Terracciano L, Eichmann MD, Worni M, Gloor B, Perren A, Integrated genomic and immunophenotypic classification of pancreatic cancer reveals three distinct subtypes with prognostic/predictive significance, Clinical cancer research 24(18) (2018) 4444–4454. [DOI] [PubMed] [Google Scholar]
- [109].Gorchs L, Kaipe H, Interactions between Cancer-Associated Fibroblasts and T Cells in the Pancreatic Tumor Microenvironment and the Role of Chemokines, Cancers 13(12) (2021) 2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Evans A, Costello E, The role of inflammatory cells in fostering pancreatic cancer cell growth and invasion, Frontiers in physiology 3 (2012) 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Noy R, Pollard JW, Tumor-associated macrophages: from mechanisms to therapy, Immunity 41(1) (2014) 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Wang Z, Liu J, Huang H, Ye M, Li X, Wu R, Liu H, Song Y, Metastasis-associated fibroblasts: an emerging target for metastatic cancer, Biomarker Research 9(1) (2021) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Vennin C, Mélénec P, Rouet R, Nobis M, Cazet AS, Murphy KJ, Herrmann D, Reed DA, Lucas MC, Warren SC, CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan, Nature communications 10(1) (2019) 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Roy I, McAllister DM, Gorse E, Dixon K, Piper CT, Zimmerman NP, Getschman AE, Tsai S, Engle DD, Evans DB, Pancreatic cancer cell migration and metastasis is regulated by chemokine-biased agonism and bioenergetic signaling, Cancer research 75(17) (2015) 3529–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Chao T, Furth EE, Vonderheide RH, CXCR2-dependent accumulation of tumor-associated neutrophils regulates T-cell immunity in pancreatic ductal adenocarcinoma, Cancer Immunology Research 4(11) (2016) 968–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Steele CW, Karim SA, Leach JD, Bailey P, Upstill-Goddard R, Rishi L, Foth M, Bryson S, McDaid K, Wilson Z, CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma, Cancer cell 29(6) (2016) 832–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Hertzer KM, Donald GW, Hines OJ, CXCR2: a target for pancreatic cancer treatment?, Expert opinion on therapeutic targets 17(6) (2013) 667–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Hirth M, Gandla J, Höper C, Gaida MM, Agarwal N, Simonetti M, Demir A, Xie Y, Weiss C, Michalski CW, CXCL10 and CCL21 promote migration of pancreatic cancer cells toward sensory neurons and neural remodeling in tumors in mice, associated with pain in patients, Gastroenterology 159(2) (2020) 665–681. e13. [DOI] [PubMed] [Google Scholar]
- [119].Singh SK, Mishra MK, Eltoum I-EA, Bae S, Lillard JW, Singh R, CCR5/CCL5 axis interaction promotes migratory and invasiveness of pancreatic cancer cells, Scientific reports 8(1) (2018) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Li D, Qu C, Ning Z, Wang H, Zang K, Zhuang L, Chen L, Wang P, Meng Z, Radiation promotes epithelial-to-mesenchymal transition and invasion of pancreatic cancer cell by activating carcinoma-associated fibroblasts, American journal of cancer research 6(10) (2016) 2192. [PMC free article] [PubMed] [Google Scholar]
- [121].Wang Z, Tang Y, Tan Y, Wei Q, Yu W, Cancer-associated fibroblasts in radiotherapy: challenges and new opportunities, Cell Communication and Signaling 17(1) (2019) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Masucci MT, Minopoli M, Del Vecchio S, Carriero MV, The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis, Frontiers in Immunology 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Jung HS, Gu J, Kim J-E, Nam Y, Song JW, Kim HK, Cancer cell–induced neutrophil extracellular traps promote both hypercoagulability and cancer progression, PLoS One 14(4) (2019) e0216055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Zhang Y, Chandra V, Riquelme Sanchez E, Dutta P, Quesada PR, Rakoski A, Zoltan M, Arora N, Baydogan S, Horne W, Interleukin-17–induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer, Journal of Experimental Medicine 217(12) (2020) e20190354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Wortzel I, Dror S, Kenific CM, Lyden D, Exosome-Mediated Metastasis: Communication from a Distance, Dev Cell 49(3) (2019) 347–360. [DOI] [PubMed] [Google Scholar]
- [126].Maji S, Chaudhary P, Akopova I, Nguyen PM, Hare RJ, Gryczynski I, Vishwanatha JK, Exosomal Annexin II Promotes Angiogenesis and Breast Cancer Metastasis, Mol Cancer Res 15(1) (2017) 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Gilbert LA, Hemann MT, DNA damage-mediated induction of a chemoresistant niche, Cell 143(3) (2010) 355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Shao Y, Chen T, Zheng X, Yang S, Xu K, Chen X, Xu F, Wang L, Shen Y, Wang T, Zhang M, Hu W, Ye C, Yu X, Shao J, Zheng S, Colorectal cancer-derived small extracellular vesicles establish an inflammatory premetastatic niche in liver metastasis, Carcinogenesis 39(11) (2018) 1368–1379. [DOI] [PubMed] [Google Scholar]
- [129].Massagué J, Obenauf AC, Metastatic colonization by circulating tumour cells, Nature 529(7586) (2016) 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Borsig L, Wolf MJ, Roblek M, Lorentzen A, Heikenwalder M, Inflammatory chemokines and metastasis--tracing the accessory, Oncogene 33(25) (2014) 3217–24. [DOI] [PubMed] [Google Scholar]
- [131].Wendel C, Hemping-Bovenkerk A, Krasnyanska J, Mees ST, Kochetkova M, Stoeppeler S, Haier J, CXCR4/CXCL12 participate in extravasation of metastasizing breast cancer cells within the liver in a rat model, PLoS One 7(1) (2012) e30046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, García-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D, Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver, Nat Cell Biol 17(6) (2015) 816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Xiao Z, Luo G, Liu C, Wu C, Liu L, Liu Z, Ni Q, Long J, Yu X, Molecular mechanism underlying lymphatic metastasis in pancreatic cancer, Biomed Res Int 2014 (2014) 925845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Thomas SK, Lee J, Beatty GL, Paracrine and cell autonomous signalling in pancreatic cancer progression and metastasis, EBioMedicine 53 (2020) 102662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Merz V, Zecchetto C, Santoro R, Simionato F, Sabbadini F, Mangiameli D, Piro G, Cavaliere A, Deiana M, Valenti MT, Bazan D, Fedele V, Lonardi S, Melisi D, Plasma IL8 Is a Biomarker for TAK1 Activation and Predicts Resistance to Nanoliposomal Irinotecan in Patients with Gemcitabine-Refractory Pancreatic Cancer, Clin Cancer Res 26(17) (2020) 4661–4669. [DOI] [PubMed] [Google Scholar]
- [136].Kruger D, Yako YY, Devar J, Lahoud N, Smith M, Inflammatory cytokines and combined biomarker panels in pancreatic ductal adenocarcinoma: Enhancing diagnostic accuracy, PLoS One 14(8) (2019) e0221169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Vicente D, Lee AJ, Hall CS, Lucci A, Lee JE, Kim MP, Katz MHG, Hurd MW, Maitra A, Rhim Md AD, Tzeng CD, Circulating Tumor Cells and Transforming Growth Factor Beta in Resected Pancreatic Adenocarcinoma, J Surg Res 243 (2019) 90–99. [DOI] [PubMed] [Google Scholar]
- [138].Ohman KA, Liu J, Linehan DC, Tan MC, Tan BR, Fields RC, Strasberg SM, Hawkins WG, Interferon-based chemoradiation followed by gemcitabine for resected pancreatic adenocarcinoma: long-term follow-up, HPB (Oxford) 19(5) (2017) 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Van Limbergen EJ, Hoeben A, Lieverse RIY, Houben R, Overhof C, Postma A, Zindler J, Verhelst F, Dubois LJ, De Ruysscher D, Troost EGC, Lambin P, Toxicity of L19-Interleukin 2 Combined with Stereotactic Body Radiation Therapy: A Phase 1 Study, Int J Radiat Oncol Biol Phys 109(5) (2021) 1421–1430. [DOI] [PubMed] [Google Scholar]
- [140].Pang N, Shi J, Qin L, Chen A, Tang Y, Yang H, Huang Y, Wu Q, Li X, He B, Li T, Liang B, Zhang J, Cao B, Liu M, Feng Y, Ye X, Chen X, Wang L, Tian Y, Li H, Li J, Hu H, He J, Hu Y, Zhi C, Tang Z, Gong Y, Xu F, Xu L, Fan W, Zhao M, Chen D, Lian H, Yang L, Li P, Zhang Z, IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin, J Hematol Oncol 14(1) (2021) 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Hecht JR, Lonardi S, Bendell J, Sim HW, Macarulla T, Lopez CD, Van Cutsem E, Munoz Martin AJ, Park JO, Greil R, Wang H, Hozak RR, Gueorguieva I, Lin Y, Rao S, Ryoo BY, Randomized Phase III Study of FOLFOX Alone or With Pegilodecakin as Second-Line Therapy in Patients With Metastatic Pancreatic Cancer That Progressed After Gemcitabine (SEQUOIA), J Clin Oncol 39(10) (2021) 1108–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Palmer DH, Valle JW, Ma YT, Faluyi O, Neoptolemos JP, Jensen Gjertsen T, Iversen B, Amund Eriksen J, Moller AS, Aksnes AK, Miller R, Dueland S, TG01/GM-CSF and adjuvant gemcitabine in patients with resected RAS-mutant adenocarcinoma of the pancreas (CT TG01–01): a single-arm, phase 1/2 trial, Br J Cancer 122(7) (2020) 971–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Wu AA, Bever KM, Ho WJ, Fertig EJ, Niu N, Zheng L, Parkinson RM, Durham JN, Onners B, Ferguson AK, Wilt C, Ko AH, Wang-Gillam A, Laheru DA, Anders RA, Thompson ED, Sugar EA, Jaffee EM, Le DT, A Phase II Study of Allogeneic GM-CSF-Transfected Pancreatic Tumor Vaccine (GVAX) with Ipilimumab as Maintenance Treatment for Metastatic Pancreatic Cancer, Clin Cancer Res 26(19) (2020) 5129–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Linehan DC, Tan MC, Strasberg SM, Drebin JA, Hawkins WG, Picus J, Myerson RJ, Malyapa RS, Hull M, Trinkaus K, Tan BR Jr., Adjuvant interferon-based chemoradiation followed by gemcitabine for resected pancreatic adenocarcinoma: a single-institution phase II study, Ann Surg 248(2) (2008) 145–51. [DOI] [PubMed] [Google Scholar]
- [145].Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, Tamada K, IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor, Nat Biotechnol 36(4) (2018) 346–351. [DOI] [PubMed] [Google Scholar]
- [146].Weden S, Klemp M, Gladhaug IP, Moller M, Eriksen JA, Gaudernack G, Buanes T, Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras, Int J Cancer 128(5) (2011) 1120–8. [DOI] [PubMed] [Google Scholar]
- [147].Melisi D, Garcia-Carbonero R, Macarulla T, Pezet D, Deplanque G, Fuchs M, Trojan J, Oettle H, Kozloff M, Cleverly A, Smith C, Estrem ST, Gueorguieva I, Lahn MMF, Blunt A, Benhadji KA, Tabernero J, Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer, Br J Cancer 119(10) (2018) 1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Bockorny B, Semenisty V, Macarulla T, Borazanci E, Wolpin BM, Stemmer SM, Golan T, Geva R, Borad MJ, Pedersen KS, Park JO, Ramirez RA, Abad DG, Feliu J, Munoz A, Ponz-Sarvise M, Peled A, Lustig TM, Bohana-Kashtan O, Shaw SM, Sorani E, Chaney M, Kadosh S, Vainstein Haras A, Von Hoff DD, Hidalgo M, BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial, Nat Med 26(6) (2020) 878–885. [DOI] [PubMed] [Google Scholar]
- [149].Noel M, O’Reilly EM, Wolpin BM, Ryan DP, Bullock AJ, Britten CD, Linehan DC, Belt BA, Gamelin EC, Ganguly B, Yin D, Joh T, Jacobs IA, Taylor CT, Lowery MA, Phase 1b study of a small molecule antagonist of human chemokine (C-C motif) receptor 2 (PF-04136309) in combination with nab-paclitaxel/gemcitabine in first-line treatment of metastatic pancreatic ductal adenocarcinoma, Invest New Drugs 38(3) (2020) 800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Greer JB, Whitcomb DC, Inflammation and pancreatic cancer: an evidence-based review, Curr Opin Pharmacol 9(4) (2009) 411–8. [DOI] [PubMed] [Google Scholar]
- [151].Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, Hubner RA, Chiu CF, Schwartsmann G, Siveke JT, Braiteh F, Moyo V, Belanger B, Dhindsa N, Bayever E, Von Hoff DD, Chen LT, Group N-S, Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial, Lancet 387(10018) (2016) 545–557. [DOI] [PubMed] [Google Scholar]
- [152].Beatty GL, Shahda S, Beck T, Uppal N, Cohen SJ, Donehower R, Gabayan AE, Assad A, Switzky J, Zhen H, Von Hoff DD, A Phase Ib/II Study of the JAK1 Inhibitor, Itacitinib, plus nab-Paclitaxel and Gemcitabine in Advanced Solid Tumors, Oncologist 24(1) (2019) 14–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Kocher HM, Basu B, Froeling FEM, Sarker D, Slater S, Carlin D, deSouza NM, De Paepe KN, Goulart MR, Hughes C, Imrali A, Roberts R, Pawula M, Houghton R, Lawrence C, Yogeswaran Y, Mousa K, Coetzee C, Sasieni P, Prendergast A, Propper DJ, Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer, Nat Commun 11(1) (2020) 4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].O’Reilly EM, Barone D, Mahalingam D, Bekaii-Saab T, Shao SH, Wolf J, Rosano M, Krause S, Richards DA, Yu KH, Roach JM, Flaherty KT, Ryan DP, Randomised phase II trial of gemcitabine and nab-paclitaxel with necuparanib or placebo in untreated metastatic pancreas ductal adenocarcinoma, Eur J Cancer 132 (2020) 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Akita H, Takahashi H, Asukai K, Tomokuni A, Wada H, Marukawa S, Yamasaki T, Yanagimoto Y, Takahashi Y, Sugimura K, Yamamoto K, Nishimura J, Yasui M, Omori T, Miyata H, Ochi A, Kagawa A, Soh Y, Taniguchi Y, Ohue M, Yano M, Sakon M, The utility of nutritional supportive care with an eicosapentaenoic acid (EPA)-enriched nutrition agent during pre-operative chemoradiotherapy for pancreatic cancer: Prospective randomized control study, Clin Nutr ESPEN 33 (2019) 148–153. [DOI] [PubMed] [Google Scholar]
- [156].Qiao G, Wang X, Zhou X, Morse MA, Wu J, Wang S, Song Y, Jiang N, Zhao Y, Zhou L, Zhao J, Di Y, Zhu L, Hobeika A, Ren J, Lyerly HK, Immune correlates of clinical benefit in a phase I study of hyperthermia with adoptive T cell immunotherapy in patients with solid tumors, Int J Hyperthermia 36(sup1) (2019) 74–82. [DOI] [PubMed] [Google Scholar]
- [157].Ganguly K, Cox JL, Ghersi D, Grandgenett PM, Hollingsworth MA, Jain M, Kumar S, Batra SK, Mucin 5AC-mediated CD44/ITGB1 clustering mobilizes adipose-derived mesenchymal stem cells to modulate pancreatic cancer stromal heterogeneity, Gastroenterology (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Nallasamy P, Nimmakayala RK, Karmakar S, Leon F, Seshacharyulu P, Lakshmanan I, Rachagani S, Mallya K, Zhang C, Ly QP, Myers MS, Josh L, Grabow CE, Gautam SK, Kumar S, Lele SM, Jain M, Batra SK, Ponnusamy MP, Pancreatic Tumor Microenvironment Factor Promotes Cancer Stemness via SPP1-CD44 Axis, Gastroenterology 161(6) (2021) 1998–2013.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]


