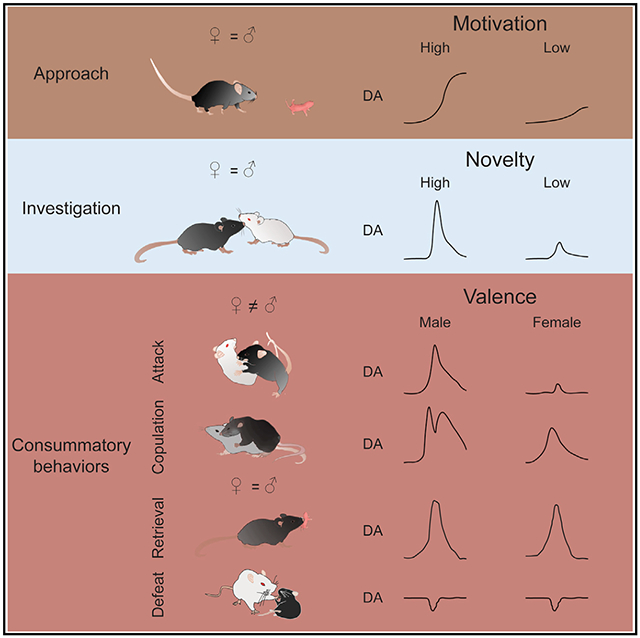
SUMMARY
Social behaviors are among the most important motivated behaviors. How dopamine (DA), a “reward” signal, releases during social behaviors has been a topic of interest for decades. Here, we use a genetically encoded DA sensor, GRABDA2m, to record DA activity in the nucleus accumbens (NAc) core during various social behaviors in male and female mice. We find that DA releases during approach, investigation and consummation phases of social behaviors signal animals’ motivation, familiarity of the social target, and valence of the experience, respectively. Positive and negative social experiences evoke opposite DA patterns. Furthermore, DA releases during mating and fighting are sexually dimorphic with a higher level in males than in females. At the functional level, increasing DA in NAc enhances social interest toward a familiar conspecific and alleviates defeat-induced social avoidance. Altogether, our results reveal complex information encoded by NAc DA activity during social behaviors and their multistage functional roles.
Graphical Abstract
In brief
Dai et al. report complex and sexually dimorphic DA release in nucleus accumbens (NAc) core during various social behaviors in mice. DA release during approach, investigation, and action phases of social behaviors carries distinct information. When DA release is artificially boosted, it enhances social interest and reduces defeat-induced social avoidance.
INTRODUCTION
Social behaviors, such as sexual and parental behaviors, are among the most important motivated behaviors, that is, behaviors driven by rewarding goals (Trezza et al., 2011). The end goals of these behaviors, e.g., reproduction and fostering youngsters, are essential for the survival of a species. Thus, animals are innately motivated to engage in social behaviors and in some cases, willing to work hard for such opportunities. Social behaviors can serve as unconditioned stimulus for both Pavlovian and instrumental conditioning, suggesting the intrinsic hedonic value of these behaviors. For example, male and female rodents show a preference for the context associated with copulation, and postpartum female rats can learn to lever press to gain access to pups (Hauser and Gandelman, 1985; Tzschentke, 2007; Wilsoncroft, 1968).
Given the important role of dopamine (DA) in reward, motivation, and behavior activation, many studies have been carried out since the early 1990s to ask how DA level changes in NAc during social behaviors (Berridge and Robinson, 1998; Bromberg-Martin et al., 2010; Wise, 2004). Early microdialysis studies with a temporal resolution of minutes revealed a gradual increase of DA in NAc in male rats that copulated to ejaculation with receptive females (Damsma et al., 1992; Fumero et al., 1994; Pfaus et al., 1990; Pleim et al., 1990; Wang et al., 1995; Wenkstern et al., 1993). Later fast-scan cyclic voltammetry (FSCV) recording with a higher temporal resolution (subsecond) found that DA transients increased mainly during initial female encounter but not during consummatory phase of sexual behaviors, such as deep thrust (Robinson et al., 2001, 2002). This is surprising given that consummatory sexual actions are required for establishing conditioned place preference—typically a DA-dependent learning process (Kippin and Pfaus, 2001; Tenk et al., 2009). Recently, we used a genetically encoded DA sensor, namely GRABDA, to optically record the DA signal in NAc in male mice with millisecond resolution and found time-locked DA increase during each episode of thrust and ejaculation (Sun et al., 2018, 2020). Thus, the full details of DA responses during social behaviors remain incompletely understood.
Similar to male sexual behaviors, DA increases in NAc during female sexual behaviors and pup interactions have been reported using microdialysis and to a lesser extent voltammetry (Afonso et al., 2008, 2009, 2013; Becker et al., 2001; Champagne et al., 2004; Hansen et al., 1993; Jenkins and Becker, 2003; Kohlert and Meisel, 1999; Lavi-Avnon et al., 2008; Meisel et al., 1993; Mermelstein and Becker, 1995; Pfaus et al., 1995; Shnitko et al., 2017). These studies unequivocally suggested that the DA release in NAc is correlated with the animal’s sexual or maternal motivation (Afonso et al., 2009; Champagne et al., 2004; Kohlert and Meisel, 1999; Mermelstein and Becker, 1995). However, considering the poor temporal resolution of microdialysis, DA responses during individual behavioral events, especially those lasting for just a second or two, remain unclear. It also remains to be determined whether the slow changes in DA levels truly reflect slow dynamics of DA or whether they simply reflect methodological limitations. Furthermore, nearly all studies on parental behaviors have focused on mothers, probably because the mother is the main caregiver. One study showed that DA release to pups in NAc in naive and pair-bonded male prairie voles were quantitatively similar, although pair-bonded males show enhanced paternal behavior (Lei et al., 2017). These results raise the question of whether pup-triggered DA release in males varies with the paternal state as is the case in females (Afonso et al., 2008, 2009; Champagne et al., 2004).
Aggression is another important type of motivated behavior toward a social target. Animals are willing to work for the opportunity to attack a conspecific, especially when the outcome of attack is likely winning (Falkner et al., 2016; Fish et al., 2002, 2005, 2008; Golden et al., 2017, 2019b; May and Kennedy, 2009). Winning also supports associative learning: animals demonstrate a preference for the context where winning occurs (Aleyasin et al., 2018; Golden et al., 2016, 2019b; Martinez et al., 1995; Stagkourakis et al., 2018). Several studies investigated changes in DA levels related to aggressive behaviors and found a gradual and sustained increase in NAc (Beiderbeck et al., 2012; van Erp and Miczek, 2000); however, unlike sexual behaviors, DA was found to rise slowly and remain elevated for more than an hour (Beiderbeck et al., 2012; van Erp and Miczek, 2000). Whether this particularly slow and long-lasting DA rise is a unique feature of aggression or whether it is caused by low sampling rate in those studies remains to be investigated. Further complicating the findings is the observation that NAc DA also increased after defeat (Anstrom et al., 2009; Tidey and Miczek, 1996). Since defeat is clearly a negative experience, it suggests that DA increase during social behaviors may signal salience instead of valence. Alternatively, these results may reflect different subregions targeted in different studies. Recent studies found heterogeneity in DA release pattern in NAc subdivisions (de Jong et al., 2019; Yuan et al., 2019).
Taken together, while DA release in NAc during social behaviors has been a topic of interest for the past 3 decades, many questions remain unaddressed due to technical limitations, biased choices of subjects’ sex, and differences in methodological details and recording sites across studies. Thus, the goal of our current study was to comprehensively investigate the DA release in NAc during various social behaviors in both males and females using an optical recording method with fast temporal resolution and cell type specificity. Furthermore, guided by the recording results, we investigated the functional role of NAc DA release during different stages of social behaviors. Here, we specifically focused on NAc core given that this region is known to signal both motivation and reward, two important variables relevant for social behaviors (Aitken et al., 2016; Hamid et al., 2016; Mohebi et al., 2019).
RESULTS
Synchronized DA transients in left and right NAc core
We performed optical recording of DA signal by virally expressing Cre-dependent (Cre-on) GRABDA2m, a genetically encoded fluorescent DA sensor, bilaterally in the NAc core of Drd1-Cre mice, and implanting 400-μm optic fiber(s) immediately above the virus injection site(s) (Sun et al., 2018, 2020). In a subset of animals, we also injected Cre-on or Cre-off GRABDA2m virus into contralateral sides of NAc core to compare DA release onto D1R and non-D1R cells (Figures S1A–S1C). Control animals were injected with Cre-on GFP virus into both sides. Only animals with correct fiber targeting were included in the analysis (Figure S2).
In animals with bilateral GRABDA expression in NAc D1R cells, we observed highly correlated activity regardless of the presence of an intruder, suggesting synchronized DA release in the two hemispheres (Figures S1 D, S1G, and S1J). Furthermore, in animals with GRABDA expression in D1R and non-D1R cells in the contralateral sides of NAc core, we observed similarly highly correlated DA signals, suggesting that D1R and non-D1R cells likely sense similar levels of DA fluctuation (Figures S1E, S1H, and S1J). The similar DA signal sensed by D1R and non-D1R cells perhaps is not surprising given that D1R and non-D1R cells are intermingled and DA mainly uses volume transmission (Liu et al., 2021; Ren et al., 2017). The highly synchronized activity could not be fully accounted for by signal fluctuation related to locomotion, as we observed significantly lower correlation in fluorescence signals between the two hemispheres in GFP animals (Figures S1F, S1I, and S1J). Furthermore, we observed no significant change in fluorescence in GFP-expressing animals during any tested behaviors (Figure S3). Given the highly correlated DA signal sensed by D1R and non-D1R cells, our subsequent recordings were only obtained from D1R cells in unilateral NAc core (Figures 1A–1C).
Figure 1. DA responses during approaching social and non-social targets.
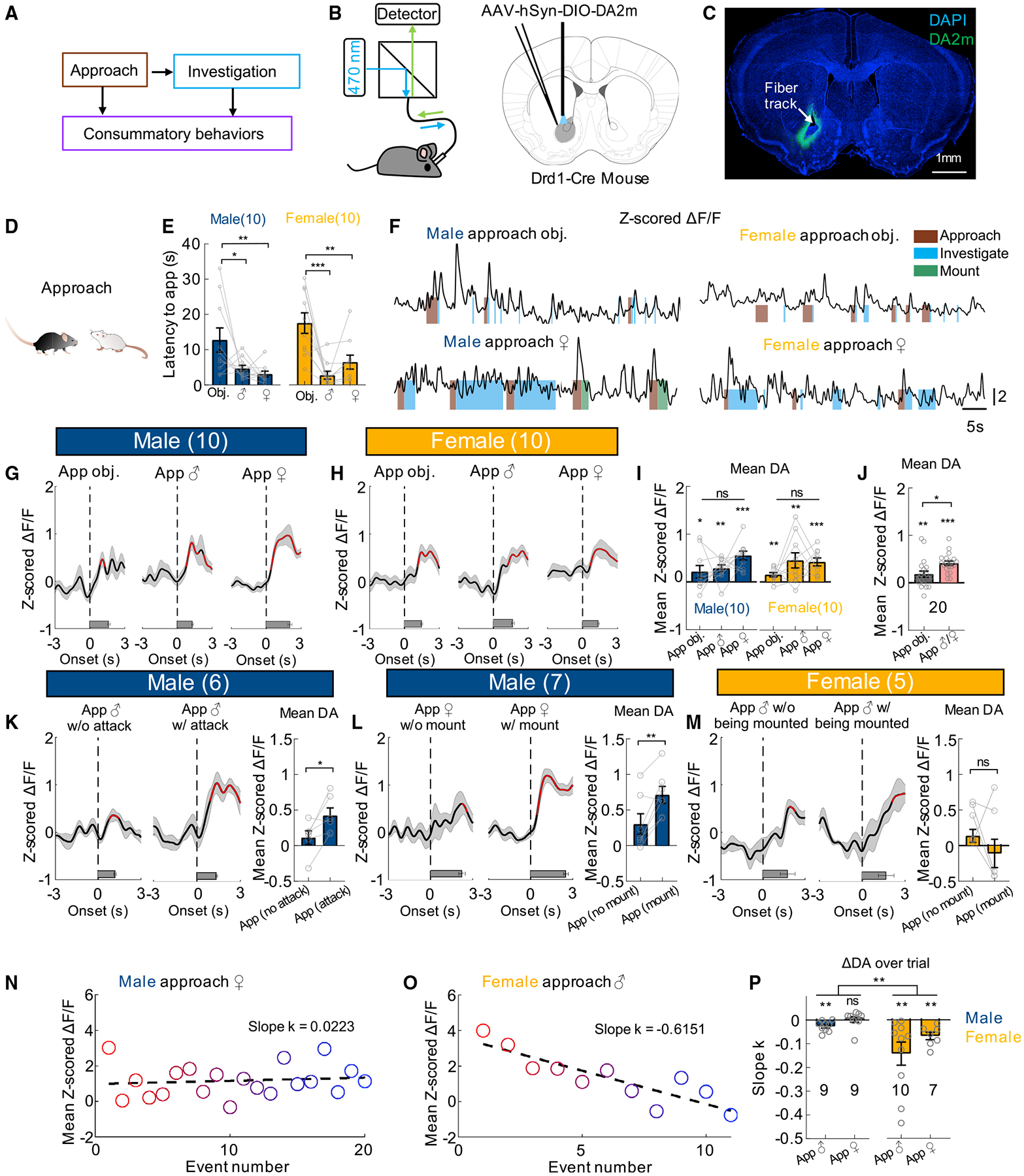
(A) Different stages of social behaviors.
(B) The fiber photometry setup and virus injection. Atlas image adopted from Franklin and Paxinos (2007).
(C) Representative image showing the expression of GRABDA2m and optic track. Scale bar, 1 mm.
(D) Cartoon illustration of social approach.
(E) Latency to approach different targets in male and female mice.
(F) Representative traces of Z scored ΔF/F of GRABDA2m during social and non-social interaction from example male and female mice. Color shades indicate annotated behaviors.
(G–H) Average post-event histograms (PETHs) aligned to the onset of approach for male (G) and female mice (H).
(I) Averaged Z scored GRABDA2m responses during approaching various targets.
(J) Averaged Z scored GRABDA2m responses during approaching non-social and social targets (average responses of male approach and female approach).
(K–M) Left: Average PETHs aligned to the onset of approach followed/not followed by attacking male (K)/mounting female (L) in male mice and being mounted by the male in female mice (M). Right: Mean GRABDA2m responses during indicated approach events.
(N and O) A scatterplot showing mean Z scored GRABDA2m responses during repeated male-approach-female events (N) or female-approach-male events (O), overlaid with a linear regression line.
(P) Average slope of the linear regression lines of GRABDA2m responses over trials.
Numbers on the graphs represent number of test animals. Error bars and shaded areas in (E, G–M, and P) represent ±SEM. In (G, H, and K–M), red line indicates periods with significantly increased DA level, and horizontal bars indicate average durations of the behaviors. (E, I) two-way ANOVA followed by multiple comparison test with Tukey’s correction; (J) Wilcoxon test; (K, L, M) paired t test; (P) two-way mixed model ANOVA. *p < 0.05, **p < 0.01, and ***p < 0.001. See also Figures S1–S6 and Table S1 for detailed statistics.
DA release during social approach
To understand DA responses during social behaviors, we sequentially introduced a conspecific male, a female, and a novel object into the recording mouse’s home cage in a randomized order, each for 10 min or until ejaculation in the case of opposite sex interaction. After stimulus introduction, the recording animal quickly approached the target (Figure 1D). For both male and female mice, the latency to approach an adult social target for the first time was shorter than that to an object, suggesting higher interest toward the social targets (Figure 1E). The approach onset, defined as the first step toward the target, was manually annotated frame-by-frame and machine learning algorithm-based tracking showed that the distance between the two animals decreased at, or slightly before, the approach onset, supporting the high accuracy of human annotation (Figures S4 and S5). At the approach onset, the DA level was not significantly elevated in any group and then during approach, DA level gradually increased (Figures 1F–1H). For both males and females, the average DA increase during approach did not significantly vary with the target (Figure 1I), although when we compared all approach trials toward a social target versus an object, DA elevated slightly more during social approach (Figure 1J). Importantly, the magnitude of DA increase during social approach varied with immediate subsequent behaviors (Figures 1K–1L). In male mice, when approach was followed by attack or mount, DA increase was significantly higher than approach that was not (Figures 1K–1L). Females did not typically initiate specific behaviors toward other adult males or females after approach. We noticed no difference in DA activity in female approach trials that were followed by male mounting and those not, suggesting that DA increase during approach did not simply vary with subsequent experience (Figure 1M). Interestingly, DA response during approach showed sexually dimorphic change over trials. In males that consistently attacked and mounted, we observed little adaptation in DA signal during approach over repeated trials (Figures 1N and 1P). In contrast, DA signal in females quickly decreased over repeated approach (Figures 1O and 1P).
To address whether the response during approach was due to movement per se, we tracked the test animal in the absence of an intruder and identified time points when the animal initiated locomotion (Figures S6A and S6B). No increase in DA activity was observed at the onset of locomotion (Figures S6C and S6D). In fact, DA slightly but significantly decreased when the animal initiated locomotion and the movement velocity was significantly negatively correlated with the DA signal, suggesting that the DA increase during approach cannot be accounted for by increased locomotion (Figures S6C–S6F).
DA release during investigatory phase signals novelty versus familiarity
Upon reaching the target, test animals closely investigated it (Figure 2A). For both females and males, the average duration of investigation bout was longer toward adult conspecifics than that toward novel objects (Figure 2B). DA level at the onset of investigation was already significantly elevated, likely due to DA increase during approach (Figures 2C–2E). During investigation, the DA further rose transiently before it dropped (Figures 2D and 2E). The mean DA increases during initial social investigation were significantly higher than those during initial object investigation, but did not differ among social targets themselves in either sex (Figure 2F). Furthermore, unlike DA response during approach, DA increase during social investigation trials followed by attack/mount did not differ from those not (Figure S7).
Figure 2. DA responses during male and female investigatory behaviors.
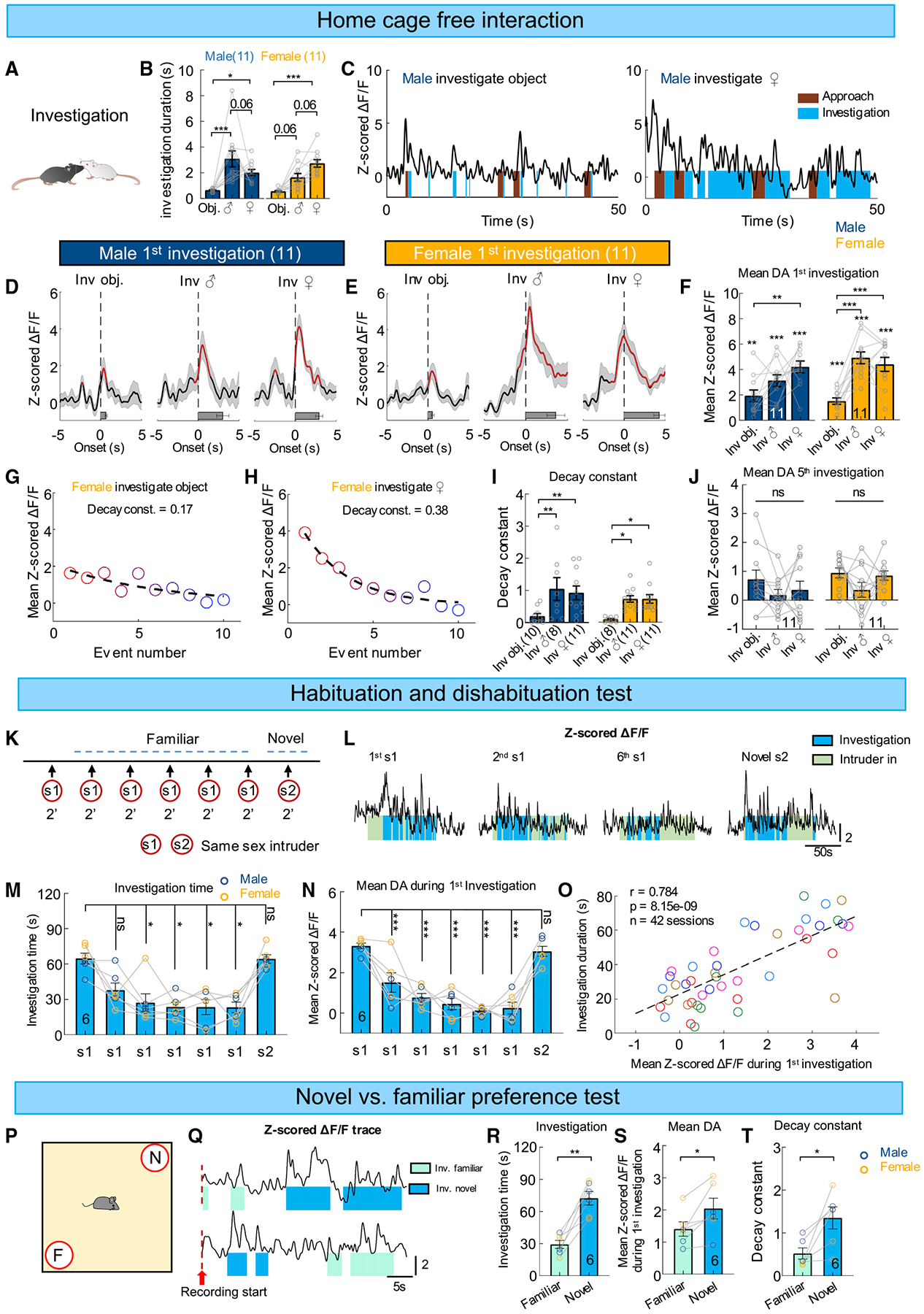
(A) A cartoon illustration of social investigation.
(B) Averaged investigation duration toward various targets.
(C) Representative traces of Z scored ΔF/F of GRABDA2m during object and female interaction from an example male mouse.
(D and E) Average PETHs aligned to the onset of the first investigation bout toward a novel object, a male intruder, and a female intruder for male (D) and female mice (E).
(F) Averaged Z scored GRABDA2m responses during the first investigation bout toward various targets. Stimulus.
(G and H) Scatterplots showing mean Z scored GRABDA2m responses over the first 10 investigation bouts from a female-object (G) and a female-female
(H) encounter session, overlaid with fitted one exponential curves.
(I) Decay constants of the one exponential curve fitted with the first 10 investigation bouts toward a novel object, a male, and a female conspecific in male and female mice.
(J) Averaged Z scored GRABDA2m responses during the fifth investigation bout toward various targets.
(K) The experimental design of the habituation and dishabituation test.
(L) Representative traces of Z scored ΔF/F of GRABDA2m during the first, second, and sixth 2-min social interactions with the s1 intruder, and with the novel s2 intruder.
(M and N) Total investigation duration (M) and mean Z scored GRABDA2m responses during the first investigation (N) in each session of the habituation and dishabituation test.
(O) A scatterplot showing Pearson correlation between total investigation duration and mean Z scored GRABDA2m responses during the first investigation in each session. Each color represents one individual animal.
(P) The experimental design of novel versus familiar preference test.
(Q) Representative traces of Z scored ΔF/F of GRABDA2m during the novel versus familiar preference test from two different example animals. Red dashed lines indicate the start of video recording that occurred immediately after placing the test mouse in the arena center.
(R) The total investigation duration toward familiar and novel mice.
(S) Mean Z scored GRABDA2m responses during the first investigation toward familiar and novel mice.
(T) Decay constant of the one exponential function fitted to the first 10 investigation trials toward familiar and novel mice.
Numbers on the graphs represent number of test animals. Error bars and shaded areas in (B, D–F, I, J, M, N, and R–T) represent ±SEM. In (D and E), red line indicates periods with significantly increased DA level, and horizontal bars indicate average durations of the behaviors. (B, F, I, J) Two-way ANOVA followed by multiple comparison test with Tukey’s correction; (F) one-sample t test followed by two-stage step-up method of Benjamini, Krieger, and Yekutieli with 0.05 false discovery rate. (M) Friedman test with Dunn’s multiple comparison test; (N) one-way ANOVA with Tukey’s multiple comparisons test; (R, S) paired t test; (T) Wilcoxon test. *p < 0.05, **p < 0.01, and ***p < 0.001. See also Figures S3 and S7, and Table S1 for detailed statistics.
Notably, DA response during investigation adapted quickly over repeated bouts and can be best fit with exponential decay functions (Figures 2G–2I). While initial DA response during social investigation was higher than that during object investigation, this difference no longer existed by the fifth investigation due to the faster decay of DA signal during social investigation (Figure 2J). These results suggest that DA increase during social investigation likely signals animals’ familiarity with the target.
To further understand the relationship between DA response and familiarity of a social target, we used a habituation-dishabituation task (Gabor et al., 2012). In the task, we presented the same same-sex intruder briefly (2 min) for six times before introducing a new same-sex intruder (Figure 2K). As expected, we found that both male and female test mice gradually decreased investigation time toward the repeatedly presented intruder while the investigation time recovered completely when a novel mouse was introduced (Figures 2L and 2M). When the test mouse decreased its interest toward the repeatedly presented intruder, we observed a rapid decline in initial DA increase during intruder investigation in each 2-min session and a robust increase in DA response when a novel intruder was introduced (Figures 2L and 2N). The initial DA response during intruder investigation was highly correlated with the accumulated investigation duration in a 2-min session, consistent with a role of NAc DA in signaling novelty versus familiarity and potentially guiding social investigation accordingly (Figure 2O).
In a separate social preference test, we recorded NAc DA signal when the test mice freely explored a familiar conspecific (cagemate) and a novel same-sex mouse that were presented simultaneously (Figure 2P) (Gabor et al., 2012). Behaviorally, as expected, the test animal spent significantly more time investigating the novel mouse than the familiar mouse (Figure 2R). DA response during the first investigation of the novel mouse was significantly higher than that of the familiar mouse regardless of which mouse was encountered first (Figures 2Q and 2S). Similar to the DA response during free social interaction, DA response to both novel and familiar conspecific gradually decreased with repeated investigation and the response to the novel mouse decayed faster than that to the familiar mouse likely due to the higher initial value (Figure 2T).
We next asked whether the decreased NAc DA release to a familiar conspecific is functionally relevant for decreased social interest. We virally expressed ChR2-EYFP in DA neurons in the ventral tegmental area (VTA), the main source of DA to NAc and implanted optic fibers above NAc core bilaterally to activate VTA dopaminergic terminals in NAc (VTADA-NAc) (Figures 3A and 3B). Control animals expressed GFP in VTADA cells (Figure 3A). Sham (0 mW) or blue light (20 ms, 20 Hz, 2 min) was delivered to the NAc in both groups of animals when the test mice encountered the same stimulus animal repeatedly in the habituation-dishabituation test (Figure 3C). We found that while both groups of animals reduced social investigation to the intruder after its repeated presentation under sham light condition, with light stimulation, ChR2 mice, but not GFP control mice, maintained high interest toward the stimulus animal even during the sixth presentation of the same intruder (Figure 3D).
Figure 3. Increasing NAc DA promotes social investigation toward a familiar conspecific.
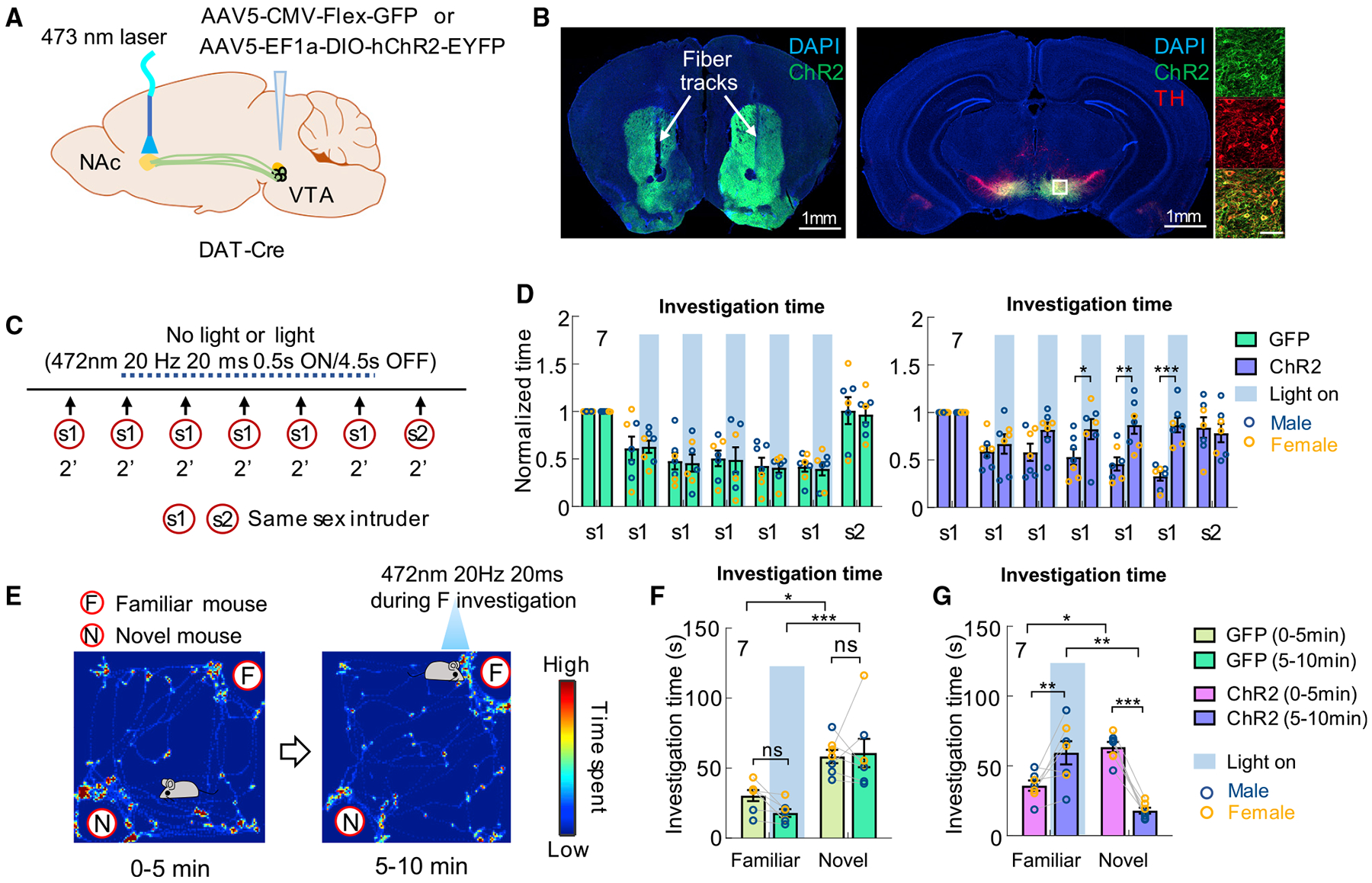
(A) Experimental strategy.
(B) Representative images showing ChR2-EYFP in the VTADA cells and NAc, amplified by immunostaining, and optic fiber tracks. Right shows the enlarged view of the boxed area. Scale bars, 1 mm (overview) and 100 μm (zoom-in view).
(C) The light stimulation protocol during habituation and dishabituation test.
(D) Normalized accumulated investigation duration in each 2-min session of the habituation and dishabituation test in GFP (left) and ChR2 animals (right).
(E) The light stimulation protocol during novel versus familiar preference test and heatmap showing the body center distribution of an example mouse during the test.
(F and G) Total investigation time of familiar and novel mice during different periods in GFP control animals (F) and ChR2 test animals (G).
Numbers in bar graphs represent number of test animals. Error bars in (D, F, and G) represent ±SEM. (D, F, G) Two-way ANOVA with Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01, and ***p < 0.001. See also Figure S2 and Table S1 for detailed statistics.
In addition, we tested the effect of VTADA-NAc activation on social interest when a cagemate and a novel same-sex mouse were simultaneously presented (Figure 3E). During the test, the test animal first freely interacted with the caged stimulus animals for 5 min without any light delivery. Then, the light was delivered to activate VTADA-NAc terminals for 5 min whenever the test mouse investigated the cagemate (Figure 3E). While both GFP and ChR2 animals showed a clear preference toward the novel animal in the first 5 min, ChR2 animals, but not GFP animals, strongly preferred the cagemate during the second 5 min (Figures 3F and 3G). The light-induced higher interest toward a familiar animal than a novel animal likely reflects the fact that DA release to the novel animal gradually decreases as the animal gains familiarity while it stays at a supernatural high level during investigation of the familiar animal due to optogenetic activation. Altogether, these results support that NAc DA signals novelty versus familiarity of a conspecific and such information could play an important role in guiding moment-to-moment social interaction.
Sexually dimorphic DA release during sexual behaviors
During male and female encounters, after a period of investigation, male mice initiate consummatory sexual actions toward the females (Figure 4A). Male sexual behaviors can be separated into three stages: mounting, intromission, and ejaculation. During mounting, the male clasps the flank of the female and establishes an on-top position. If the female is receptive, the male advances mounting to intromission—a rhythmic pelvic movement presumably resulting in penile insertion. After repeated intromission, males achieve ejaculation, which is characterized as a sudden cessation of all movements (Liu et al., 2020). The DA level changed in a stage-locked manner: it increased transiently from the onset of each stage of male sexual behaviors, including mounting, intromission, and ejaculation, and dropped before the offset (Figures 4B and 4D). The peak DA release escalated as the sexual behavior advanced and reached the maximum during ejaculation (Figures 4D and 4G). Interestingly, we noticed that after the males terminated mounting (not followed by intromission) and intromission (not followed by ejaculation), the DA level transiently decreased below the baseline (Figure 4D).
Figure 4. DA responses during male and female sexual behaviors.
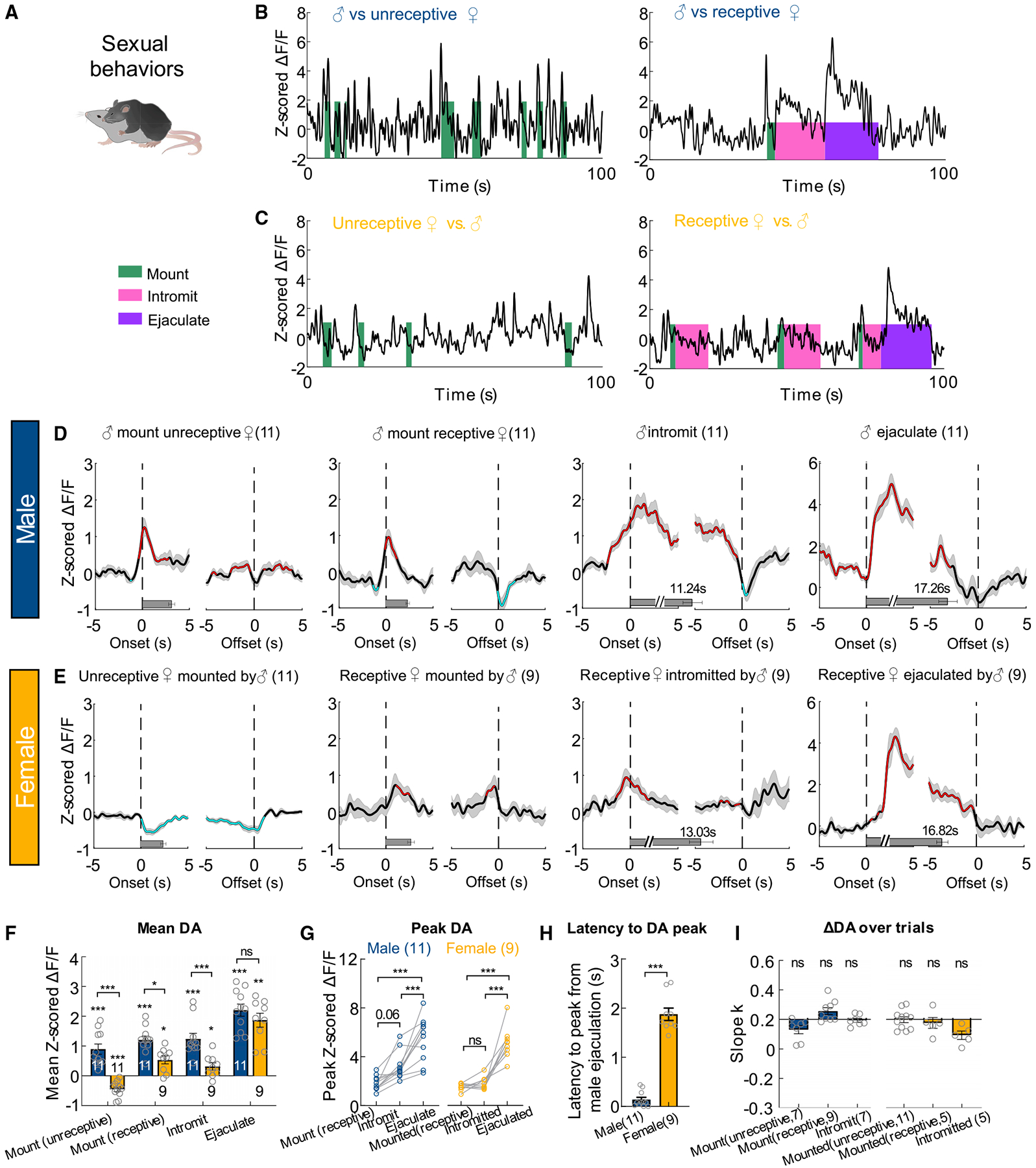
(A) A cartoon illustration of mouse mating.
(B and C) Representative traces of Z scored ΔF/F of GRABDA2m during various stages of mating in male (B) and female (C) mice.
(D and E) Average PETHs aligned to the onset (left) and offset (right) of various mating events, when the female mice were unreceptive or receptive.
(F) Averaged Z scored GRABDA2m responses during various mating events, when the female mice were unreceptive or receptive.
(G) Comparison of GRABDA2m peak responses during different stages of sexual behaviors in males and females.
(H) The latency from the onset of ejaculation to the moment of DA increase.
(I) Slope of the best fitted line of the GRABDA2m responses over repeated mating trials.
Numbers in bar graphs represent number of test animals. Error bars and shaded areas in (D–I) represent ±SEM. (D and E) Red and cyan respectively indicate periods with significantly increased and decreased DA level; horizontal bars indicate average durations of the behaviors. (F) Two-way mixed model ANOVA with Bonferroni’s multiple comparison test; (G) two-way ANOVA followed by multiple comparison test with Tukey’s correction; (H) Mann-Whitney test; one-sample Wilcoxon test (in F) or one-sample t test (in I) followed by false discovery rate correction. *p < 0.05, **p < 0.01, and ***p < 0.001. See Table S1 for detailed statistics.
NAc DA release during female sexual behaviors differ from that in males in several ways (Figures 4B–4H). First, while males showed DA increase during forceful mounting of a non-receptive female, NAc DA decreased in non-receptive females that were being forcefully mounted (Figures 4B–4F). Second, the mean DA increase during mounting and intromission was significantly higher in males than in females (Figure 4F). Third, while NAc DA level increased in males during intromission, it monotonically decreased in females (Figures 4B–4E). Thus, the peak DA was higher during intromission than mounting in males but not in females (Figure 4G). Fourth, the DA increase in females occurred approximately 2 s after male ejaculation while DA increased immediately at the onset of ejaculation in males (Figures 4D, 4E and 4H). Last, NAc DA showed a transient suppression after the termination of sexual behaviors in males but not in females (Figures 4D–4E). However, common to both males and females, the mean DA response during sexual actions did not decrease over repeated trials (Figure 4I).
Opposite DA responses during attack and defeat
When test males encountered male intruders, they initiated attack after a period of investigation (Figure 5A). At the onset of each attack, DA level was already elevated, likely due to increase during approach or investigation (Figures 5B and 5C). During attack, DA rose transiently, reached peak, and then dropped (Figures 5B and 5C). DA response did not adapt significantly over repeated attacks (Figure 5F). The average DA increase during male aggression was quantitatively comparable to that during sexual intercourse, supporting the notion that aggression could be rewarding (Figure 4F).
Figure 5. DA responses during aggressive behaviors and social defeat.
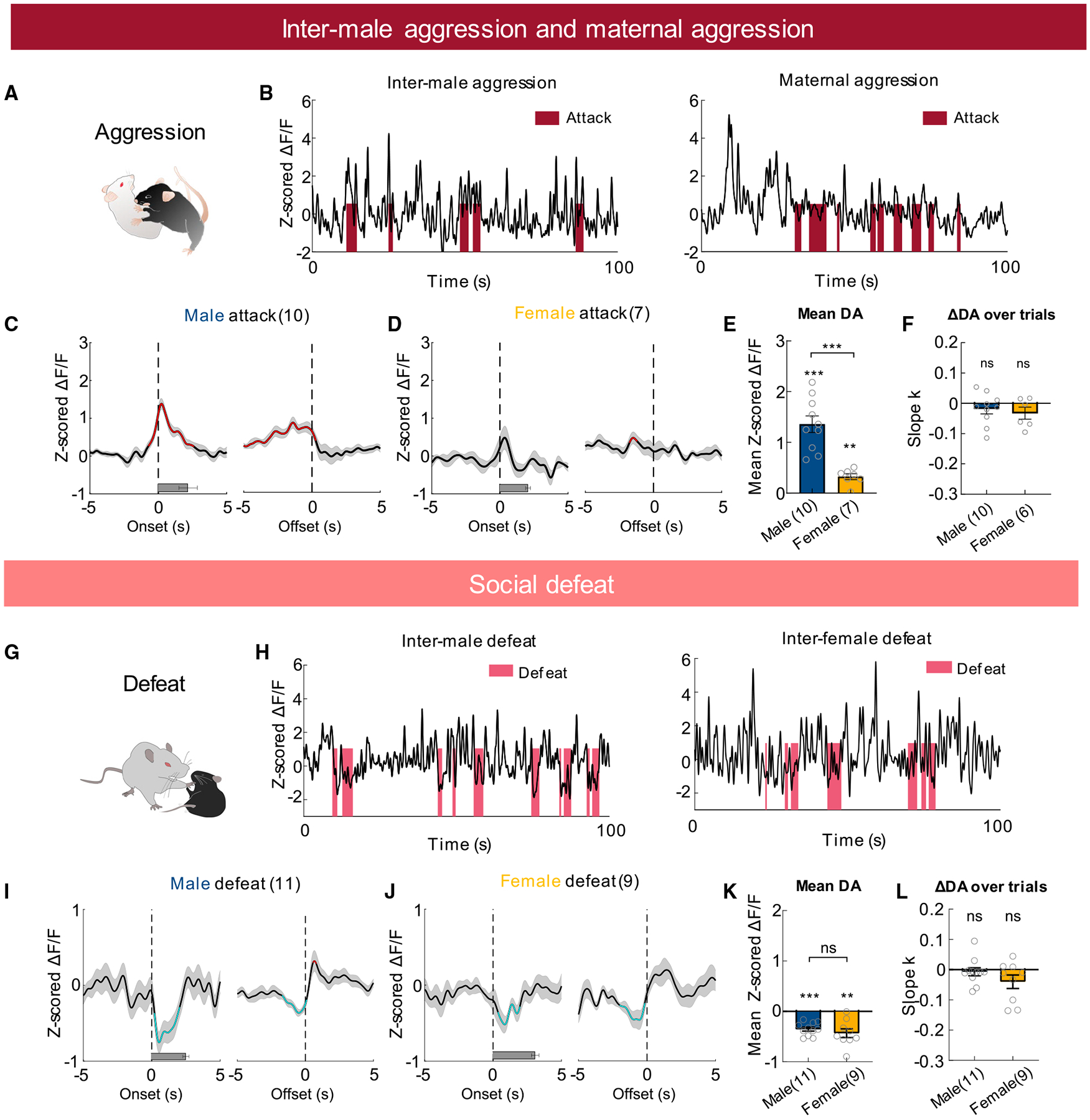
(A) A cartoon illustration of mouse attack.
(B) Representative traces of Z scored ΔF/F of GRABDA2m during inter-male and maternal aggression.
(C and D) Average PETHs aligned to the onset (left) and offset (right) of attack during male-male (C) and female-female agonistic encounters (D).
(E) Mean Z scored GRABDA2m responses during attack.
(F) Slope of the best fitted line of GRABDA2m responses over repeated attack events.
(G) A cartoon illustration of social defeat.
(H) Representative traces of Z scored ΔF/F of GRABDA2m during inter-male defeat (left) and inter-female defeat (right).
(I and J) Average PETHs aligned to the onset (left) and offset (right) of being attacked during inter-male defeat (I) and inter-female defeat (J).
(K) Mean Z scored GRABDA2m responses during social defeat.
(L) Slope of the best fitted line of GRABDA2m responses over repeated defeat events.
Numbers in bar graphs represent number of test animals. In (C, D, I, and J), red and cyan, respectively, indicate periods with significantly increased and decreased DA level; horizontal bars indicate average durations of the behaviors. (E, K) Unpaired t tests and one-sample t test; (F) one-sample t test; (L) one-sample Wilcoxon test. Error bars and shaded areas in (C–F and I–L) represent ±SEM. **p < 0.01 and ***p < 0.001. See also Figures S3 and S8, and Table S1 for detailed statistics.
Non-lactating female mice typically show little aggression toward conspecific intruders. During lactation, however, females show a marked increase in aggression toward all intruders except pups, a phenomenon known as maternal aggression (St John and Corning, 1973). Maternal aggression contains both offensive and defensive attacks and its main purpose is to protect the young (Ferrari et al., 2000; Flannelly and Flannelly, 1985). During female attack, DA increase barely reached significance at any given time (Figures 5B and 5D). In comparison with male aggression, average DA increase during female attack was significantly lower (Figure 5E). The sex difference in DA release during attack was not due to difference in attack duration: in both males and females, each attack bout lasted approximately 2 s (Figures 5C and 5D).
Previous microdialysis studies suggest a sustained elevation of DA level after male-male confrontation (Beiderbeck et al., 2012; van Erp and Miczek, 2000). We thus analyzed the accumulated DA signal before, during, and after inter-male aggression and maternal aggression (Figures S8A–S8D). While DA level was significantly elevated during initial encounter with male intruders in males, we found no difference in DA level among pre-intruder period, later period of intruder encounter, and post-intruder period (Figure S8B). In females, no difference in overall DA level was observed between pre-intruder period and any other periods (Figure S8D). To ensure that our recording method can detect a sustained increase in DA, we intraperitoneally injected DA transporter (DAT) inhibitor (GBR12909 20 mg/kg) in a subset of animals. DAT mediates DA reuptake and its blockage is known to cause an elevation of extracellular DA concentration (Westerink et al., 1987). Ten minutes after injecting DAT inhibitor but not saline, GRABDA2m signal showed a consistent and sustained upward shift, supporting the capability of our method to detect a tonic increase in DA level (Figures S8E–S8H).
Previous microdialysis and FSCV found that NAc DA increased after both attack and defeat, suggesting that DA signals salience instead of valence during consummatory social behaviors (Anstrom et al., 2009; Tidey and Miczek, 1996). We revisited this question by recording NAc GRABDA2m signal during defeat (Figure 5G). We introduced the recording male mouse into the home cage of an aggressive Swiss Webster (SW) male mouse for 10 min and all SW aggressors quickly attacked the recording mice. Upon being attacked, the recording male mouse attempted to fight back initially but quickly stopped and tried to escape by flight and pushing. After several bouts of agonistic interactions, the recording male was clearly defeated: the SW intruder initiated all attacks and the recording mice stayed in corners and showed submissive postures. In contrast to DA increase during attack, DA consistently and transiently decreased during defeat (Figures 5H, 5I, and 5K). To induce defeat in females, we introduced each recording female mouse to the home cage of a lactating SW female and also observed decreased DA signal during defeat (Figures 5H and 5J–5K). In both males and females, DA decrease during defeat did not adapt significantly over repeated trials (Figure 5L).
DA decrease during defeat is functionally important for defeat-induced social avoidance
The opposite response patterns during attack and defeat suggest that DA potentially signals the valence of social experience. To test this hypothesis functionally, we artificially elevated NAc DA level during defeat by optogenetically activating VTADA-NAc projection whenever the ChR2 expressing test mice were defeated by the aggressor (Figures 6A–6C). Control animals were injected with GFP and went through the same testing protocol (Figure 6A). During the 5-min aggressor encounter, the total defeat duration and the average inter-male distance did not differ between ChR2 and GFP groups, suggesting that the stimulation did not change the defeat experience per se (Figures 6D and 6E). Strikingly, while GFP mice strongly avoided the aggressor in a social interaction test 30 min after defeat, ChR2 test animals remained interactive with the aggressor (Figures 6F and 6G). When we calculated the social preference index, defined as the percentage of time spent in aggressor containing half of the cage divide the percentage of time in the other half of the cage, the GFP and ChR2 animals showed similar preference toward the cupped aggressor before defeat, whereas control animals, but not ChR2 animals, showed strong aversion toward the aggressor side after defeat (Figure 6H). These results support that DA response during the consummatory phase of social behaviors signals the valence of the experience and is functionally important for altering subsequent social tendency toward the conspecific linked to the experience.
Figure 6. DA decrease during social defeat is important for defeat-induced social avoidance.
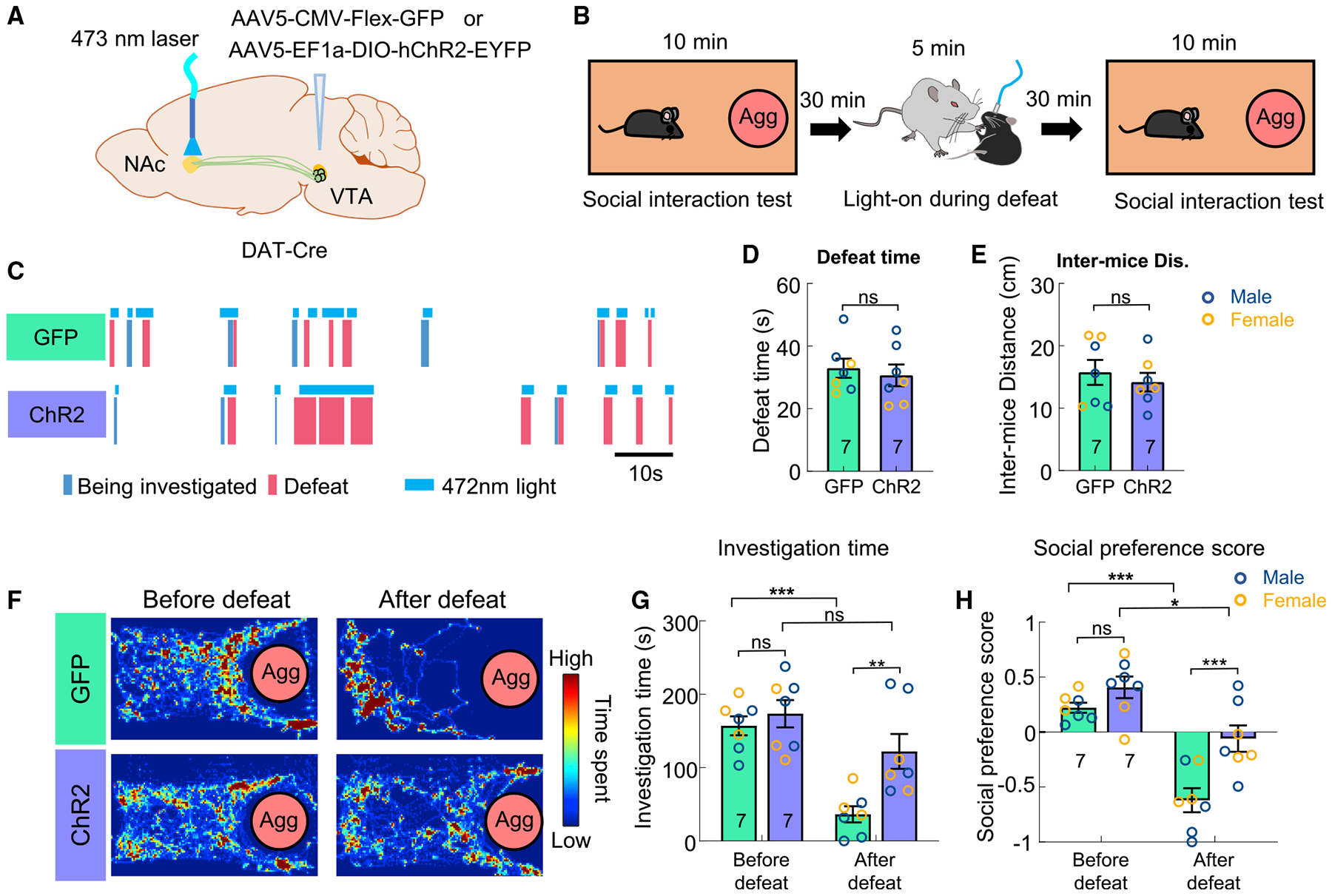
(A) Experimental strategy.
(B) Behavioral test protocol.
(C) Raster plots showing light delivery during defeat (legend below) in a GFP (top) and a ChR2 (bottom) animal.
(D) The total defeat time during aggressor encounter in GFP and ChR2 animals.
(E) Inter-mice distance during aggressor encounter in GFP and ChR2 animals.
(F) Heatmaps showing the distribution of body center location of example GFP (top) and ChR2 (bottom) animals during the social interaction test before and after defeat.
(G) The total investigation duration toward aggressors before and after defeat in GFP and ChR2 animals.
(H) Social preference score before and after defeat in GFP and ChR2 animals.
Numbers in bar graphs represent number of test animals. Error bars in (D, E, G, and H) represent ±SEM. (D, E) Unpaired t tests. (G, H) Two-way ANOVA with Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01, and ***p < 0.001. See also Figure S2 and Table S1 for detailed statistics.
DA release during pup-directed social behaviors
Lastly, we examined NAc DA release during pup-directed behaviors. Pup is a unique social stimulus, as the animal’s behavior toward pups and the rewarding value of pups vary dramatically with reproductive state (Figure 7A). In our recording mice, half of naive males (5 of 10) were hostile to pups, while the remaining half ignored pups after brief investigation. Ten of 16 naive female mice ignored pups while 6 of 16 spontaneously retrieved pups, a characteristic parental behavior. All mothers and fathers quickly retrieved pups upon their introduction. During the test, we first introduced one pup into the home cage of the test animal and if the pup was retrieved to the nest, we then introduced a new pup. Up to seven pups were introduced in each 10-min recording session.
Figure 7. DA responses during pup-directed behaviors in naive and parental mice.
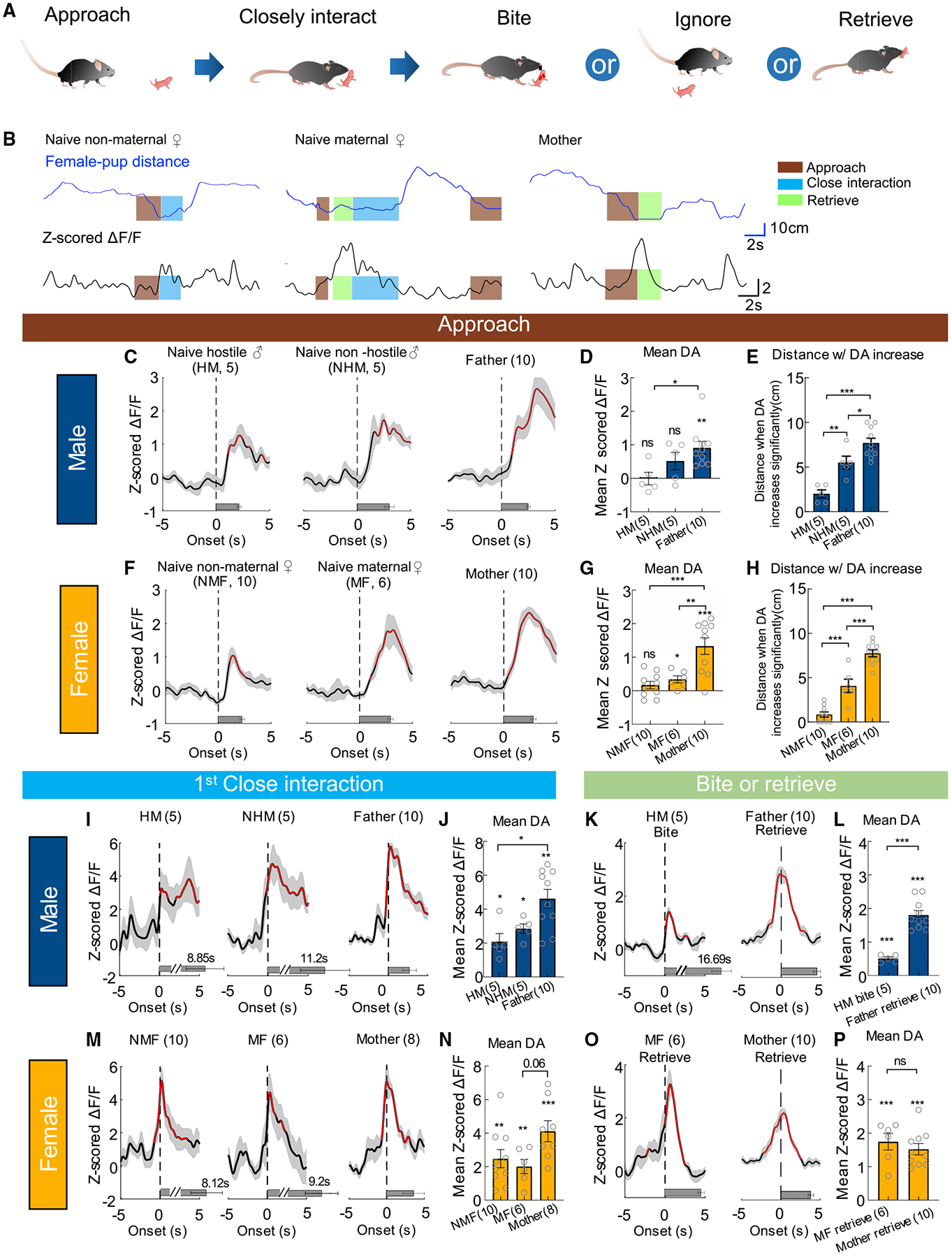
(A) A cartoon showing different stages of pup-directed behaviors.
(B) Representative traces showing distance from a pup (top) and Z scored ΔF/F of GRABDA2m (bottom) during pup interaction of an example naive non-maternal female mouse (NMF), a naive maternal female (MF), and a mother. Color shades indicate annotated behaviors.
(C) Average PETHs aligned to the onset of pup approach for male mice.
(D) Mean Z scored GRABDA2m responses during approaching pups for male mice.
(E) Distance from pups when the GRABDA2m responses become significantly increased during pup approach in male mice.
(F–H) The responses during pup approach in females, following the conventions as in (C–E).
(I) Average PETHs aligned to the onset of the first close interaction toward pups for male mice.
(J) Mean Z scored GRABDA2m responses during the first close interaction with a pup for male mice.
(K) Average PETHs aligned to the onset of biting pups in HMs and retrieving pups in fathers.
(L) Averaged Z scored GRABDA2m responses during biting pups and retrieving pups.
(M and N) The responses during the first close interaction with a pup in NMFs, MFs, and mothers, following the conventions as in (I and J). Two mothers were excluded as they retrieved pup without investigation.
(O and P) The responses during retrieving pups in MFs and mothers, following the conventions as in (K and L).
Numbers in bar graphs represent number of test animals. Error bars and shaded areas in (C–P) represent ±SEM. In (C, F, I, K, M, and O), red indicates periods with significantly increased DA level; horizontal bars indicate average durations of the behaviors. (D, E, G, H, N) One-way ANOVA with Tukey’s multiple comparisons test; (J) Kruskal-Wallis test with Dunn’s multiple comparisons test; one-sample t test (in D, G, and N) and one-sample Wilcoxon test (in J) followed by false discovery rate correction; (L, P) unpaired t test and one-sample t test. *p < 0.05, **p < 0.01, and ***p < 0.001. See also Figures S5, S6 and S9, and Table S1 for detailed statistics.
NAc DA increase during pup approach varied significantly among animals at different reproductive states: it was significantly higher in parents than naive animals in both males and females (Figures 7B–7D, 7F, and 7G). As an alternative way to quantify DA response, we examined the distance at which DA became significantly elevated (Z > 2) during approach and found that DA increased at a farther distance from pups in parents than naive animals while naive hostile males and non-maternal females only showed DA increase when they nearly reached the pup (Figures 7E and 7H). Also, similar to adult approach, DA increased after, instead of before, the onset of approach in all groups (Figures 7C and 7F).
Both virgin and parental animals closely interacted with pups, including investigating, licking, and grooming the pups. As pups were often occluded by the body of recording mice, we did not attempt to distinguish these behaviors. DA increase during initial pup interaction did not differ significantly between naive females or males regardless of whether the animals later attacked, retrieved, or ignored pups (Figures 7I, 7J, 7M, and 7N). However, fathers and mothers generally showed higher DA responses during first close pup interaction than naive animals (Figures 7J and 7N). Consistent with fast adaptation in activity during adult investigation, DA response in both males and females quickly decreased as the mice repeatedly interacted with the same pup (Figures S9A–S9E and S9G). In comparison, DA response during initial close interaction with different pups stayed relatively high (Figures S9F and S9H). These results further support that NAc DA response during investigation/close interaction mainly signals familiarity versus novelty of a social target.
After interacting with a pup for some time, hostile naive males attacked the pup, while fathers, mothers, and naive maternal females retrieved the pup back to the nest. The DA response magnitude during pup retrieval was high and comparable to that during ejaculation (Figures 7L, 7P, and 4F). It was time-locked (Figures 7K, 7L, 7O, and 7P) and stable across trials (Figure S9I–S9K). In comparison, DA response during pup biting was significantly lower although clearly positive (Figures 7K and 7L). These results suggest that reunion with a lost young and bringing it back home is a highly positive experience for parental animals.
DISCUSSION
Using a recently developed genetically encoded DA sensor, our study revealed detailed DA response patterns in NAc core during approach, investigatory, and consummatory phases of social behaviors. We found that DA signals distinct information during each phase of social behaviors and identified several sex differences in response patterns. Optogenetic activation of NAc DA release further revealed functional roles of NAc DA in modulating moment-to-moment social interest and valence of social experience.
DA release during approach signals motivational state
The magnitude of DA increase during approach is found to be related to the motivational level of an animal. Here, we define motivation as an internal state that determines the likelihood of an animal to express certain behaviors. By this definition, immediately before the onset of attack and mount, the aggressive motivation and sexual motivation is high. This high motivational state is associated with large DA increase during approach. Furthermore, DA increase in NAc core during pup approach is higher in parents than naive animals and parents are known to be highly motivated for pups as shown by their readiness to care for pups and willingness to work hard or give up other high-value rewards to gain access to pups (Hauser and Gandelman, 1985; Lee et al., 2000; Mattson et al., 2001; Wang et al., 2012; Wilsoncroft, 1968).
What is the functional relevance of the graded DA increase during approach? As DA rise is found to always follow, instead of precede, approach onset, it is unlikely to play a role in initiating approach per se. This temporal pattern is in line with the functional experiments showing that initiation of a response habit (e.g., approach) toward a reward-associated stimulus (e.g., smell of food) is not dependent on DA release (Halbout et al., 2019; Wise, 2004; Wise et al., 1978). After blocking DA receptors, animals continue to show conditioned instrumental responses for rewards, at least initially (Fouriezos and Wise, 1976; Gerber et al., 1981; Wise, 2004; Wise et al., 1978). However, if the response repeats without activation of DA receptors, it progressively weakens, suggesting that DA release during or right after approach could be essential for maintaining approach (Fouriezos and Wise, 1976; Gerber et al., 1981; Wise et al., 1978). DA elevation during approach may also invigorate the ongoing process, which could be particularly important when the target is hard to reach (Kurniawan et al., 2011; Salamone et al., 2003). In support of this idea, increased DA release in NAc by activating inputs from the medial preoptic nucleus to the VTA promotes pup and male approach in female mice (Fang et al., 2018; McHenry et al., 2017).
DA release during social investigation signals novelty versus familiarity
Surprisingly, DA increase during investigation is largely invariant with social target or motivational level of the animal. For example, DA increase during investigation is similar regardless of whether it is followed by mount/attack or not. Instead, DA response during social investigation appears to signal novelty versus familiarity of the target. One prominent feature of investigation-associated DA response is its fast adaptation. While DA increases strongly during initial investigation, it diminishes exponentially. When the recording animal was presented with a novel and a familiar mouse at the same time, DA response to the novel mouse was significantly higher than that to the familiar mouse. These results are consistent with previous FSCV recording showing robust increase of DA transients to unexpected salient stimuli while repeated presentation of the same stimuli reduced the responses (Rebec et al., 1997; Robinson et al., 2002). Similarly, responses of VTA dopaminergic cells have been found to adapt rapidly over repeated social investigation (Gunaydin et al., 2014). Importantly, the differential DA responses to familiar and novel targets appear to be functionally relevant for guiding social investigation, a commonly used behavior readout for social interest. When NAc DA release was artificially boosted optogenetically, animals failed to reduce interest to a social target after its familiarity increased with repeated presentation. These recording and activation results suggest that NAc DA release during social investigation quickly adjusts to perhaps guide the animal to direct its attention to the least known target and maximize its information gain through investigation. These findings complement a recent study showing that chemogenetic inhibition of VTADA cells attenuated interaction with a novel conspecific (Bariselli et al., 2018).
Sexually dimorphic DA responses during consummatory social behaviors
DA consistently and transiently increases during the consummatory phase of social behaviors with little adaptation over repeated trials. During each episode of consummatory social behaviors, DA rises, reaches its peak level soon after behavior onset, and gradually drops afterward. At the offset of the behavior episode, DA shows a transient dip in some cases. These response patterns suggest that DA signals may be most important for marking transitions in behaviors instead of their maintenance. Our results differ from a previous FSCV study that found few DA transients during male copulation (Robinson et al., 2001). This discrepancy may be due to the low sensitivity of FSCV in the previous study as reflected by the overall low number of detected DA transients (Robinson et al., 2001).
The sexual dimorphism of DA release is most noticeable during the consummatory phase. During attack, NAc DA increases more in males than postpartum females. During mounting, DA always increases in males, whereas only receptive females, not unreceptive females, show DA increase when being mounted. When a male ejaculates, NAc DA increases in both males and females although the increase in female occurs approximately 2 s after that in males. While it is hard to know exactly what female mice experience during male ejaculation, the large increase of DA in female NAc could suggest a high positive valence of this experience. In humans, imaging study supports activation of ventral midbrain during female orgasm (Georgiadis et al., 2006). The sensory trigger of the DA surge in females is unknown, but could be related to the mechanic and chemosensory stimulation caused by penis cup and the forceful expulsion of ejaculatory fluid. The sex difference in DA release during aggression and sexual behaviors could be due to the sex-specific sensory inputs associated with those behaviors or sexually dimorphic inputs to VTA DA cells. Regardless of the cause, this difference in release pattern could reinforce the behavior in a sex-specific way. Indeed, while in male mice repeated attacks lead to an increase in aggression (winner effect) and preference to the winning-associated context, such behavioral changes are not observed in female mice (Aubry et al., 2022; Hashikawa et al., 2018).
In contrast to aggression and sexual behaviors, DA release during pup retrieval is similar in fathers and mothers and higher than the release during hostile behaviors expressed by naive animals. This result is in contrast to a previous report showing that DA response to pups does not differ in naive male prairie voles and pair-bonded males that show a high level of parental behaviors (Lei et al., 2017) and suggests that parental behaviors are likely of high positive valence to both fathers and mothers.
The functional role of NAc DA during consummatory social behaviors
Consummatory social behaviors are generally accompanied by an increase in NAc DA with two exceptions. First, when an unreceptive female is forcefully mounted by a male, NAc DA decreases. Second, when males and females are defeated by stronger opponents, NAc DA decreases. These response patterns are consistent with a role of NAc DA in valence coding. What is the functional importance of NAc DA change during consummatory social behaviors? Under non-social contexts, NAc DA has been shown to be essential for reinforcement and avoidance learning—that is, to reinforce actions (including pursuing predictive cues) that lead to positive experience while reducing actions (including avoiding predictive cues) that lead to negative experience (Glimcher, 2011; Ilango et al., 2012). When DA signaling is blocked in NAc, normally rewarding stimuli can no longer reinforce actions or cues associated with the stimuli (Wise, 2004).
Here, we found that NAc DA release during social experience could be similarly important for social avoidance learning. Specifically, animals quickly learn to avoid the aggressor after defeat, and this behavior change is dependent on DA decrease during defeat. When we artificially increased DA release during defeat and thus altered the valence associated with defeat, animals showed reduced social avoidance to the aggressor after defeat. This result is consistent with a recent study showing that increasing DA level in NAc but not in tail striatum during 10-day chronic defeat led to increased resilience of defeated animals, measured as decreased social avoidance (Willmore et al., 2022). In a separate study, Xie et al. found that optogenetic inhibition of VTA reduced “rewarding history” of pup interaction and slowed the emergence of parental behaviors (Xie et al., 2022).
Given that DA release generally precedes the onset of consummatory behaviors, could DA release also play a role in generating the behavior? The answer to this question could be behavior-specific. Pup retrieval, for example, has been shown to be critically dependent on DA receptor activation in the NAc shell (Keer and Stern, 1999; Numan et al., 2005). Blocking D1 receptor signaling in NAc disrupts pup retrieval, whereas D1 receptor agonist in the NAc facilitates the onset of maternal behavior (Numan et al., 2005; Stolzenberg et al., 2007). Inhibiting D1R expressing cells in NAc reduced attack duration in male mice (Golden et al., 2019a). In contrast, male sexual behaviors are not affected by DA depletion in the NAc, although manipulated males showed a decrease in noncontact erection, suggesting a decrease in sexual motivation (Liu et al., 1998; Moses et al., 1995). The differential importance of DA signaling in the expression of various social behaviors suggests distinct roles of NAc cells in driving these behaviors. Indeed, NAc has been suggested as a key part of the maternal circuit but is largely left out of consummatory sexual behavior circuits (Jennings and de Lecea, 2020; Kohl et al., 2017; Numan, 2007).
In summary, our study demonstrated dynamic DA release in NAc core during approach, investigatory, and consummatory phases of social behaviors, which could signal motivation, familiarity, and valence of the experience, respectively. DA release during consummatory phase shows the largest sex differences and may underlie certain aspects of behavioral differences (e.g., winner effect) between sexes. Guided by the recording results, behavior-locked optogenetic activation further demonstrated the functional relevance of NAc DA signal in determining moment-to-moment social interest and mediating social learning. These results help to achieve a better understanding of the complex, multistage and important functions of NAc DA signals in social behaviors.
Limitations of the study
Fiber photometry records DA signals at the population level. Although our results suggest similar DA inputs to D1R and non-D1R neurons, this pattern could be violated at the microscopic level (Liu et al., 2018). Furthermore, GRABDA reports changes instead of absolute values of DA level and hence does not allow us to reveal differences in baseline DA across animals, e.g., mothers versus naive females. Our attempt to optogenetically suppress VTA DA release in the NAc through VTADA terminal inhibition was unsuccessful, as determined by a lack of real-time place aversion with light delivery. Thus, it remains unclear whether VTA DA release at NAc is necessary for the increased investigation to a novel conspecific. Future studies with more efficient terminal inhibition tools will help answer this question.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dr. Dayu Lin (dayu.lin@nyulangone.org).
Materials availability
The plasmid for expressing Cre-out GRABDA2m used in this study has been deposited to Addgene (Addgene Plasmid ID 189751).
Data and code availability
All data reported in this paper will be shared by the lead contact upon request.
All original code has been deposited at Zenodo and is publicly available as of the date of publication. DOIs are listed in the key resources table.
Data and any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Chicken anti-GFP | Abcam | Cat#ab13970; RRID: AB_300798 |
| Sheep anti-TH | Pel Freeze | Cat#P60101-0; RRID: AB_461070 |
| Donkey anti-chicken Alexa Fluor 488 | Jackson ImmunoResearch | Cat#703-545-155; RRID: AB_2340375 |
| Donkey anti-sheep Cyanine Cy™5 | Jackson ImmunoResearch | Cat#713-175-147; RRID: AB_2340730 |
| Bacterial and Virus Strains | ||
| AAV9-hSyn-DIO-GRAB.DA2m | (Sun et al., 2020); Vigene Biosciences | NA |
| AAV9-hSyn-loxP-GRAB.DA2m-loxP | Current study; Vigene Biosciences | Addgene# 189751 |
| AAV2-CAG-Flex-GFP | (Oh et al., 2014); UNC Vector Core; | Cat#AV-2-ALL854 |
| AAV5-CAG-Flex-GFP | (Oh et al., 2014); UNC Vector Core; | Cat#AV4531b |
| AAV5-Ef1a-DIO-hChR2(H134R)-EYFP-WPRE-pA | UNC Vector Core; Karl Deisseroth lab (unpublished) | Cat#AV-4313H1 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Normal donkey serum | Jackson ImmunoResearch | Cat#017-000-121; RRID:AB_2337258 |
| GBR 12909 dihydrochloride | Tocris | Cat#0421 |
| 0.9% NaCl | Hanna Pharmaceutical Supply | Cat#NC9054335 |
| DAPI | Thermo Fisher Scientific | Cat#62248 |
| Fluoromount-G™ Mounting Medium | Thermo Fisher Scientific | Cat#00-4958-02 |
| Experimental Models: Organisms/Strains | ||
| Drd1-cre mice | MMRRC | RRID: MMRRC_030989-UCD |
| DAT-ires-cre mice | Jackson Laboratory | RRID: IMSR_JAX:006660 |
| Ai9 mice | Jackson Laboratory | RRID: IMSR_JAX:007909 |
| C57BL/6N mice | Charles River | Strain #027 |
| Swiss Webster mice | Charles River | Strain #024 |
| Software and Algorithms | ||
| MATLAB R2019b | MathWorks |
https://www.mathworks.com RRID:SCR_001622 |
| Prism 9 | GraphPad Software |
https://www.graphpad.com RRID:SCR_002798 |
| StreamPix 8 | NorPix |
https://www.norpix.com/products/streampix/streampix.php RRID:SCR_015773 |
| Deeplabcut | (Mathis et al., 2018) | https://github.com/DeepLabCut |
| SLEAP | (Pereira et al., 2022) | https://sleap.ai/ |
| SLEAP Extension | Current study | https://zenodo.org/badge/latestdoi/400214215 |
| Adobe Photoshop 2020 | Adobe |
https://www.adobe.com/; RRID:SCR 014199 |
| Adobe Illustrator 2020 | Adobe |
https://www.adobe.com/; RRID:SCR_010279 |
| other | ||
| Optic fibers (200 um) | Thorlabs | Cat#FT200EMT |
| Optic fibers (400 um) | Thorlabs | Cat#CF440-10 |
| Ceramic Ferrule for MM Fiber (230 um) | Thorlabs | Cat#CFLC230-10 |
| Stainless Steel Ferrule for MM Fiber (440 um) | Thorlabs | Cat#SFLC440-10 |
| Ceramic split matching sleeves | Thorlabs | Cat#ADAL1 |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
All procedures were approved by the IACUC of NYULMC in compliance with the NIH guidelines for the care and use of laboratory animals. Mice were housed at 18–23°C with 40–60% humidity under a 12 h light–dark cycle (dark cycle, 10 p.m. to 10 a.m.), with food and water available ad libitum. Test animals were adult Drd1-cre (>8 weeks, RRID: MMRRC_030989-UCD) and DAT-ires-cre (>8 weeks, Jackson, RRID: IMSR_JAX:006660) mice. Ai9 mice (Jackson, RRID: IMSR_JAX:007909) were crossed with Drd1-cre mice for revealing D1R expression. Stimulus animals were adult C57BL/6N male and female mice, adult BALB/c male and female mice, or sexually experienced C57BL/6N and Swiss Webster males (aggressor) purchased from Charles River, and 3–7 days old pups were from test mice or wildtype C57BL/6N breeders. After surgery, all the animals are single housed. All experiments were performed during the dark cycle of the animals.
Viruses
Viruses AAV9-hSyn-DIO-GRABDA2m (3.60 × 1013 vg/mL) and AAV9-hSyn-loxP-GRABDA2m-loxP (1.43 × 1013 vg/mL) were purchased from Vigene Biosciences. Viruses AAV2-CAG-Flex-GFP (4.00 × 1012 vg/mL), AAV5-CAG-Flex-GFP (8.70 × 1012 vg/mL), and AAV5-Ef1a-DIO-hChR2(H134R)-EYFP-WPRE-pA (9.80 × 1012 vg/mL) were purchased from University of North Carolina vector core facility. All viruses were stored in aliquots at 80°C until use.
METHOD DETAILS
Immunofluorescence
For histological analysis, animals were deeply anesthetized and transcardially perfused with 20 mL of PBS, followed by 20 mL of 4% paraformaldehyde (Electron Microscopy Sciences, cat. no. 15714) in PBS. After perfusion, brains were harvested, post-fixed in 4% paraformaldehyde for 4h at 4°C and cryoprotected in 20% (w/v) sucrose for 24h. The brains were then embedded in an O.C.T compound (Fisher Healthcare, cat. no. 23730571) and sectioned into 60-μm-thick slices using a CM1900 cryostat (Leica). GRABDA2m, GFP and ChR2-EYFP were immunostained using a chicken anti-GFP antibody (1:1,000, Abcam, Cat#ab13970, RRID: AB_300798) followed by an Alexa 488-conjugated donkey anti-chicken secondary antibody (1:1,000, Jackson ImmunoResearch, Cat#703-545-155, RRID: AB_2340375). Tyrosine Hydroxylase was immunostained using a sheep anti-TH antibody (1:750, Pel Freeze, Cat#P60101–0, RRID: AB_461070) followed by a Cy5-conjugated donkey anti-sheep secondary antibody(1:1,000, Jackson ImmunoResearch, Cat#713-175-147, RRID: AB_2340730). DAPI (1:20,000; Thermo Fisher, cat. no. D1306) was used with the secondary antibody to visualize the nucleus. The GRABDA2m fluorescence images were acquired with a virtual slide microscope (Olympus, VS120) in 10x mode or a confocal microscope (Zeiss LSM 510 or 700 microscope) for the high-resolution image in 40x mode.
Fiber photometry
The male mice (8–15 weeks old) were screened for aggression before the surgery, and the female mice (8–12 weeks old) were randomly selected. For the test mouse with a single fiber implanted, 80 nL AAV9.hSyn.DIO.GRABDA2m (4.97 × 1013 gc/mL) was injected into one side of NAc core (anterior-posterior (AP): +0.98 mm relative to bregma; medial-lateral (ML): ±1.2mm relative to bregma; dorsal-ventral (DV): 4.2 mm from brain surface) using a nanoinjector (World Precision Instruments, Nanoliter, 2000). For bilateral recording, AAV9.hSyn.DIO.GRABDA2m was injected into one side and AAV9.hSyn.DIO.GRABDA2m or AAV9.hSyn.loxP. GRABDA2m.loxP (1.43 × 1013 gc/mL) was injected into the contralateral side of NAc core for the D1-D1 mice or D1-nonD1 mice respectively. Control mice were injected with AAV2.CAG.Flex.GFP (4.00 × 1012 gc/mL) bilaterally. After virus injection, a custom-made optic fiber assembly (Thorlabs, FT400EMT and SFLC440-10) was implanted approximately 300 μm above each injection site. All the mice were single housed after surgery. Fiber photometry recording was performed two weeks after AAV injection.
The setup used for recording was constructed as described previously (Falkner et al., 2016). In brief, a 400-Hz 472-nm bandpass (passing band: 472 ± 15 nm, FF02-472/30-25, Semrock) filtered light-emitting diode (Thorlabs, LED light: M470F1; LED driver: LEDD1B) was used to excite GRABDA2m or GFP. The emission light collected from the recording site was bandpass filtered (passing bands: 535 ± 25 nm, FF01-535/505, Semrock), detected by a Femtowatt Silicon Photoreceiver (Newport, 2151), and recorded using a real-time processor (RZ5, TDT). The 400-Hz signals carrying fluorescence intensity of GRABDA2m or GFP were extracted in real time using a custom TDT program.
To analyze the recording data, the MATLAB function “msbackadj” with a moving window of 25% of the total recording duration was first applied to obtain the instantaneous baseline signal. The instantaneous ΔF/F was calculated as (Fraw −Fbaseline)/Fbaseline. The Z scored ΔF/F of the entire recording session was calculated as (ΔF/F – mean(ΔF/F))/std(ΔF/F). The peri-event histogram (PETH) of a given behavior was plotted by aligning the Z scored ΔF/F signal to the onset or offset of the behavior. In the PETHs, the periods that showed significant difference from zero were obtained by running a column t test followed by a false discovery rate correction (FDR = 0.05). The response during each behavior episode was defined as the average Z score during the behavior. For Figure 2F, the response at the first investigation toward male and female intruders was defined as the average Z score during the first 0.63s (the average investigation duration toward object) after the onset of the behavior. For Figure 4G, the peak Z scored ΔF/F was defined as the maximum Z score during the behavior. For the Figures 1P, 4I, 5F, and 5L, the slope k of ΔDA over trials was calculated based on the following steps: (1) select the test session that had 10 or more than 10 trials; (2) calculate the average Z score of each trial; (3) fit the obtained values using a linear regression function “regression” in MATLAB, and report the slope of fitted curve. For Figures 2I and 2T, the decay constants were calculated by fitting the average Z score of the first 10 investigation trials using one exponential model y = a*exp(b*x) in MATLAB Curve Fitting Toolbox with constraints a>0 and b < 0.
Optogenetic activation
Test DAT-Cre animals were injected with 90 nL AAV5-Ef1a-DIO-hChR2(H134R)-EYFP-WPRE-pA bilaterally into the VTA (AP: −3.16mm, ML: 0.5mm, DV: 4.3mm) at 30 nL/min. Control animals were injected with AAV5-CAG-Flex-GFP using the same condition. After injection, a custom-made optic fiber assembly (Thorlabs, FT400EMT and SFLC440-10) was implanted approximately 400 μm above NAc Core (AP: +0.98 mm, ML: ±1.2mm, DV: 4.2mm) unilaterally or bilaterally and was secured using dental cement (C&B Metabond, S380). The test animals were single housed for at least 5 weeks before testing.
Before each testing session, a 400-μm patch cord was coupled to each implanted fiber with a ceramic split sleeve (Thorlabs, ADAL1-5). The fiber patch cord was connected to a 473nm blue laser (Shanghai Dream Lasers Technology, SDL-473-1015 100MFL). The laser output was regulated by a custom written TDT circuit by generating TTL pulses at desired frequency and duration.
In habituation and dishabituation test, light stimulation was delivered during the 2nd, 3rd, 4th, 5th, and 6th test session, and each light stimulation lasted for 2 mins (3 mW, 20 Hz, 20 ms, 0.5 s ON and 4.5 s OFF) to cover the entire social interaction. The sham (0mW) control was performed similarly on a different day.
In the social preference test, we attached the patch cord to the test animal and then introduced the animal to the center of test arena. The test animals first freely interacted with the novel and familiar mice for 5 min without light delivery. Then, light pulses (3 mW, 20 Hz, 20 ms) were manually triggered whenever the test animals investigated the familiar mouse for 5 min.
During the social defeat session preceding social interaction test, the test mice were introduced to the home cage of an SW aggressor. The light pulses (3 mW, 20 Hz, 20 ms) were manually triggered whenever the aggressor investigated or attacked the test mice.
Habituation and dishabituation test
We attached a path cord to the implanted fiber assembly of test mice and waited for at least 5 min before starting the recording. A 3-min baseline period was first acquired, then a same-sex BALB/c intruder was introduced into the home cage of test mice for 2 min. One minute after removing the intruder, the same intruder was introduced again and the procedure was repeated for 5 times, meaning that we introduced the same BALB/c intruder for 6 times in total. During the 7th trial, a different same-sex BALB/c intruder was introduced into the home cage of the test mice for 2 min.
Social preference test
A same-sex BALB/c mouse was introduced into the cage of test mice and housed with it for at least two days before the test. The test arena (L × W × H: 18 inches × 18 inches × 15 inches) was custom made with acrylic sheets (Canal Plastics Center). Its bottom was covered with fresh bedding, and two metal pencil cups (SPECTRUM, 31570 GLXY PNCL CP) were placed at two randomly selected corners diagonally. Test animals were habituated in the test arena containing two empty cups for 30 min the day before the test. On the test day, the cage mate and a novel same-sex BALB/c mouse were each placed under a cup in the arena for 30 min. Then, the test mouse was introduced into the arena and freely interacted with the cupped mice for 10 min.
Social interaction test
During habituation period, the aggressor was placed under in a metal pencil cup that sit at one end of a clean cage for 30 min. During the test, the test animal was introduced into the cage and allowed to freely interact with the cupped aggressor for 10 min. This social interaction test was performed twice, once at 30 min before social defeat and once at 30 min after defeat. During defeat, the test mouse was introduced into the home cage of the same aggressor for 5–6 min. To calculate social preference score, the cage was divided evenly from the midline on the recorded video and the side with aggressor was named as social side, the other side was named as non-social side. The social preference score can be expressed as:
Tracking
Body parts of black and white mice were tracked using Deeplabcut (Mathis et al., 2018). Skeletons of two black mice in each frame were identified using SLEAP (Pereira et al., 2022) and the identity of each mouse was then determined based on its unique feature (implantation or not) using a custom written python program (https://zenodo.org/badge/latestdoi/400214215). The distance between the resident and intruder mice was calculated based on animals’ head center positions. The velocity of the animal was calculated as the displacement of the body center location between neighboring frames. The onset of the movement was defined as the movement following at least 2-s immobility (velocity <2 cm/s) and reached a velocity of minimally 15 cm/s for at least 1.5 s. The correlation coefficient was calculated using MATLAB function ‘corrcoef’.
Behavioral paradigm and analysis
Animal behaviors in all experiments were video recorded from both the side and top of the cage using two synchronized cameras (Basler, acA640-100 gm) and a commercial video acquisition software (StreamPix 8, Norpix) in a semi-dark room with infrared illumination at a frame rate of 25 frames/s. Manual behavioral annotation was performed on a frame-by-frame basis using custom software written in MATLAB (https://pdollar.github.io/toolbox/).
Social interaction between animals of the same sex
An adult BALB/c male was introduced to the home cage of the test male mouse, or an adult BALB/c female was introduced to the home cage of the test female mouse. If the test female was lactating, pups were removed 10 min prior to the intruder introduction. During social encounters, we identified three behaviors of the test mice – approach, investigation and attack. “Approach” was defined as continuous movement toward a stationary intruder mouse until the center mass of the two animals are below 100 pixels. “Investigation” was defined as close contact to any part of the intruder’s body. “Attack” was defined as a suite of intense actions aiming at biting the intruders, including push, lunge, bite, tumbling, and fast locomotion episodes between these movements.
Social interaction between animals of different sexes
Female receptivity was first assessed visually based on female’s vaginal opening (Ajayi and Akhigbe, 2020). For females that appear to be in vaginal estrus, we further examined its behavioral receptivity by introducing it into the cage of a highly sexually experienced male briefly. In all cases, the male quickly attempted to mount the female. If the female showed no resistance and male was able to achieve intromission within the first 5 mounting attempts, we considered the female being receptive. During the test, an adult receptive or unreceptive female intruder mouse was introduced into the home cage of the test male mouse. For female test mouse, an adult sexually experienced male mouse was introduced into the female’s home cage. During social encounters, we annotated “approach” and “investigation” of the test mouse, and “mount”, “intromit” and “ejaculate” of the male mouse. “Mount” is when the male grasped and mounted the female’s flanks. “Intromit” includes both rapid thrust against the female’s rear and deep rhythmic thrust. “Ejaculate” starts when the male suddenly ceases all thrusting movements but still holding onto the female’s flank and then after a few seconds slumps to the side of the female. “Ejaculate” ends when the male resumes movements.
Pup-related behaviors.
At the beginning of a test session, we introduced a pup into the home cage of the recording mouse. If the pup was retrieved to the nest during the recording session, we introduced another pup. Up to 7 pups were introduced during a 10-min recording session. The pups are either from C57BL/6N breeders or the test mice themsleves. Approach, close interaction, biting, and retrieval are annotated. “Approach” is defined as continuous movement toward a pup until the head of the test mouse is above the pup. “Close interaction” is defined as close contact with pups including sniffing, licking, and grooming. “Biting” is when the test mouse holds and bites the pup and causes harm. “Retrieval” starts when the mouse picks up a pup with its mouth and ends when it drops the pup into or around the nest.
Social defeat
To induce defeat, the male test mouse was introduced into a Swiss Webster male’s (aggressor) cage for 5–15 min (5 min for ChR2 experiment and 10–15 for fiber photometry recording). The female test mouse was introduced into a lactating Swiss Webster female’s cage. During interaction with the aggressor, we annotated “being attacked” of test mice, which is defined as when the aggressor attacks the test mouse.
The social stimulus and object was introduced in a randomized order. However, aggressor encounter was always the last test in a recording day. No other behavior tests were performed after social defeat for at least 24 h.
Drug injection
The test mouse was habituated for head fixation for 3 consecutive days. On the test day, the mouse was head-fixed while DA signal was continuously monitored. After 10 min baseline, we intraperitoneally injected 0.9% NaCl (10mL/kg, Hanna Pharmaceutical Supply, Cat. # NC9054335) and 40 min later, 20 mg/kg GBR 12909 (Tocris, Cat. # 0421). DA signal was recorded for at least 30 min after last injection.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data were tested for normality first using Kolmogorov-Smirnov test. If all data points were normally distributed, paired t tests for within animal comparison between two groups, unpaired t tests for between animal comparison between two groups and one-way ANOVA with Turkey’s post hoc test for comparison across multiple groups were performed. In addition, one sample t test was performed to determine whether the Z score was significantly different from 0, followed by false discovery rate correction with a false discovery rate of 0.05. If data points in one or more groups were not normally distributed, Mann–Whitney test, Wilcoxon matched-pairs signed-rank test and the Friedman test with Dunn’s multiple-comparison post hoc test were performed. Wilcoxon rank test was used to compare the means of two groups or the mean of a single group with 0, followed by false discovery rate correction with a false discovery rate of 0.05. For comparison of values across two categorical variables, we used ordinary (or mixed effect) two-way ANOVA with Bonferroni’s multiple-comparison post hoc test for between group comparisons and Turkey’s multiple-comparison post hoc test for within group comparisons, and repeated-measures two-way ANOVA with Turkey’s or Bonferroni’s multiple-comparison test for matched data. In these cases, normality was not formally tested. Details of each statistical test can be found in the Table S1. All error bars or error shades represent ±SEM. * <0.05; ** <0.01; *** <0.001. All p or q values equal or smaller than 0.06 were indicated on the figures.
Supplementary Material
Highlights.
DA in NAc core during social approach signals motivation
DA in NAc core during social investigation signals novelty of the social target
DA in NAc core during social actions signals valence and is sexually dimorphic
Increase NAc DA promotes social interest and alleviates defeat-induced avoidance
ACKNOWLEDGMENTS
We thank Y. Jiang for help with genotyping; J. Fan for assistance with video annotation; and N. Tritsch, A. Carter, and U. Kang for helpful discussions. This research was supported by NIH grants R01MH101377, 1R01HD092596, U19NS107616 (D.L.), and U01NS113358 (D.L. and Y.L.); the Mathers Foundation (D.L.); the Vulnerable Brain Project (D.L.); NIH grant U01NS120824 (Y.L.); the General Program of the National Natural Science Foundation of China project nos. 31871087 and 31925017 (Y.L.); the Uehara Memorial Foundation, JSPS Overseas Research Fellowship, and Osamu Hayaishi Memorial Scholarship (T.O.).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2022.111246.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Afonso VM, Grella SL, Chatterjee D, and Fleming AS (2008). Previous maternal experience affects accumbal dopaminergic responses to pup-stimuli. Brain Res. 1198, 115–123. [DOI] [PubMed] [Google Scholar]
- Afonso VM, King S, Chatterjee D, and Fleming AS (2009). Hormones that increase maternal responsiveness affect accumbal dopaminergic responses to pup-and food-stimuli in the female rat. Horm. Behav 56, 11–23. [DOI] [PubMed] [Google Scholar]
- Afonso VM, Shams WM, Jin D, and Fleming AS (2013). Distal pup cues evoke dopamine responses in hormonally primed rats in the absence of pup experience or ongoing maternal behavior. J. Neurosci 33, 2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken TJ, Greenfield VY, and Wassum KM (2016). Nucleus accumbens core dopamine signaling tracks the need-based motivational value of food-paired cues. J. Neurochem 136, 1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajayi AF, and Akhigbe RE (2020). Staging of the estrous cycle and induction of estrus in experimental rodents: an update. Fertil. Res. Pract 6, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleyasin H, Flanigan ME, Golden SA, Takahashi A, Menard C, Pfau ML, Multer J, Pina J, McCabe KA, Bhatti N, et al. (2018). Cell-type-specific role of DeltaFosB in nucleus accumbens in modulating intermale aggression. J. Neurosci 38, 5913–5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstrom KK, Miczek KA, and Budygin EA (2009). Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience 161, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry A, Burnett CJ, Goodwin NL, Li L, Navarrete J, Zhang Y, Tsai V, Durand-de Cuttoli R, Golden SA, and Russo SJ (2022). Dynamic sex differences in appetitive and reactive aggression. Preprint at bioRxiv. 10.1101/2022.02.22.481480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariselli S, Hörnberg H, Prévost-Solié C, Musardo S, Hatstatt-Burklé L, Scheiffele P, and Bellone C (2018). Role of VTA dopamine neurons and neuroligin 3 in sociability traits related to nonfamiliar conspecific interaction. Nat. Commun 9, 3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Rudick CN, and Jenkins WJ (2001). The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J. Neurosci 21, 3236–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiderbeck DI, Reber SO, Havasi A, Bredewold R, Veenema AH, and Neumann ID (2012). High and abnormal forms of aggression in rats with extremes in trait anxiety–involvement of the dopamine system in the nucleus accumbens. Psychoneuroendocrinology 37, 1969–1980. [DOI] [PubMed] [Google Scholar]
- Berridge KC, and Robinson TE (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev 28, 309–369. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, and Hikosaka O (2010). Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68, 815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, and Meaney MJ (2004). Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J. Neurosci 24, 4113–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsma G, Pfaus JG, Wenkstern D, Phillips AG, and Fibiger HC (1992). Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav. Neurosci 106, 181–191. [DOI] [PubMed] [Google Scholar]
- de Jong JW, Afjei SA, Pollak Dorocic I, Peck JR, Liu C, Kim CK, Tian L, Deisseroth K, and Lammel S (2019). A neural circuit mechanism for encoding aversive stimuli in the mesolimbic dopamine system. Neuron 101, 133–151.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner AL, Grosenick L, Davidson TJ, Deisseroth K, and Lin D (2016). Hypothalamic control of male aggression-seeking behavior. Nat. Neurosci 19, 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang YY, Yamaguchi T, Song SC, Tritsch NX, and Lin D (2018). A hypothalamic midbrain pathway essential for driving maternal behaviors. Neuron 98, 192–207.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari P, Palanza P, and Parmigiani S (2000). Does fear modulate defensive and offensive types of maternal attack in mice? Aggress. Behav 26, 193–203. [Google Scholar]
- Fish EW, DeBold JF, and Miczek KA (2005). Escalated aggression as a reward: corticosterone and GABA A receptor positive modulators in mice. Psychopharmacology 182, 116–127. [DOI] [PubMed] [Google Scholar]
- Fish EW, De Bold JF, and Miczek KA (2002). Aggressive behavior as a reinforcer in mice: activation by allopregnanolone. Psychopharmacology 163, 459–466. [DOI] [PubMed] [Google Scholar]
- Fish EW, McKenzie-Quirk SD, Bannai M, and Miczek KA (2008). 5-HT(1B) receptor inhibition of alcohol-heightened aggression in mice: comparison to drinking and running. Psychopharmacology (Berl) 197, 145–156. [DOI] [PubMed] [Google Scholar]
- Flannelly KJ, and Flannelly L (1985). Opponents’ size influences maternal aggression. Psychol. Rep 57, 883–886. [DOI] [PubMed] [Google Scholar]
- Fouriezos G, and Wise RA (1976). Pimozide-induced extinction of intracranial self-stimulation: response patterns rule out motor or performance deficits. Brain Res. 103, 377–380. [DOI] [PubMed] [Google Scholar]
- Franklin Keith, and Paxinos George (2007). The Mouse Brain in Stereotaxic Coordinates, 3rd Edn (San Diego, CA: Academic Press; ). [Google Scholar]
- Fumero B, Fernandez-Vera JR, Gonzalez-Mora JL, and Mas M (1994). Changes in monoamine turnover in forebrain areas associated with masculine sexual behavior: a microdialysis study. Brain Res. 662, 233–239. [DOI] [PubMed] [Google Scholar]
- Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, and Choleris E (2012). Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav. Neurosci 126, 97–109. [DOI] [PubMed] [Google Scholar]
- Georgiadis JR, Kortekaas R, Kuipers R, Nieuwenburg A, Pruim J, Reinders AATS, and Holstege G (2006). Regional cerebral blood flow changes associated with clitorally induced orgasm in healthy women. Eur. J. Neurosci 24, 3305–3316. [DOI] [PubMed] [Google Scholar]
- Gerber GJ, Sing J, and Wise RA (1981). Pimozide attenuates lever pressing for water reinforcement in rats. Pharmacol. Biochem. Behav 14, 201–205. [DOI] [PubMed] [Google Scholar]
- Glimcher PW (2011). Understanding dopamine and reinforcement learning: the dopamine reward prediction error hypothesis. Proc. Natl. Acad. Sci. USA 108, 15647–15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Heins C, Venniro M, Caprioli D, Zhang M, Epstein DH, and Shaham Y (2017). Compulsive addiction-like aggressive behavior in mice. Biol. Psychiatr 82, 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, Aleyasin H, Menard C, Zhang H, Hodes GE, et al. (2016). Basal forebrain projections to the lateral habenula modulate aggression reward. Nature 534, 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Jin M, Heins C, Venniro M, Michaelides M, and Shaham Y (2019a). Nucleus accumbens Drd1-expressing neurons control aggression self-administration and aggression seeking in mice. J. Neurosci 39, 2482–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Jin M, and Shaham Y (2019b). Animal models of (or for) aggression reward, addiction, and relapse: behavior and circuits. J. Neurosci 39, 3996–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, et al. (2014). Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbout B, Marshall AT, Azimi A, Liljeholm M, Mahler SV, Wassum KM, and Ostlund SB (2019). Mesolimbic dopamine projections mediate cue-motivated reward seeking but not reward retrieval in rats. Elife 8, e43551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, Kennedy RT, Aragona BJ, and Berke JD (2016). Mesolimbic dopamine signals the value of work. Nat. Neurosci 19, 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, and Nyiredi S (1993). Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol. Biochem. Behav 45, 673–676. [DOI] [PubMed] [Google Scholar]
- Hashikawa K, Hashikawa Y, Lischinsky J, and Lin D (2018). The neural mechanisms of sexually dimorphic aggressive behaviors. Trends Genet. 34, 755–776. [DOI] [PubMed] [Google Scholar]
- Hauser H, and Gandelman R (1985). Lever pressing for pups: evidence for hormonal influence upon maternal behavior of mice. Horm. Behav 19, 454–468. [DOI] [PubMed] [Google Scholar]
- Ilango A, Shumake J, Wetzel W, Scheich H, and Ohl FW (2012). The role of dopamine in the context of aversive stimuli with particular reference to acoustically signaled avoidance learning. Front. Neurosci 6, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins WJ, and Becker JB (2003). Dynamic increases in dopamine during paced copulation in the female rat. Eur. J. Neurosci 18, 1997–2001. [DOI] [PubMed] [Google Scholar]
- Jennings KJ, and de Lecea L (2020). Neural and hormonal control of sexual behavior. Endocrinology 161, bqaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keer SE, and Stern JM (1999). Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol. Behav 67, 659–669. [DOI] [PubMed] [Google Scholar]
- Kippin TE, and Pfaus JG (2001). The development of olfactory conditioned ejaculatory preferences in the male rat. I. Nature of the unconditioned stimulus. Physiol. Behav 73, 457–469. [DOI] [PubMed] [Google Scholar]
- Kohl J, Autry AE, and Dulac C (2017). The neurobiology of parenting: a neural circuit perspective. Bioessays 39, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlert JG, and Meisel RL (1999). Sexual experience sensitizes mating-related nucleus accumbens dopamine responses of female Syrian hamsters. Behav. Brain Res 99, 45–52. [DOI] [PubMed] [Google Scholar]
- Kurniawan IT, Guitart-Masip M, and Dolan RJ (2011). Dopamine and effort-based decision making. Front. Neurosci 5, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi-Avnon Y, Weller A, Finberg JPM, Gispan-Herman I, Kinor N, Stern Y, Schroeder M, Gelber V, Bergman SY, Overstreet DH, and Yadid G (2008). The reward system and maternal behavior in an animal model of depression: a microdialysis study. Psychopharmacology (Berl) 196, 281–291. [DOI] [PubMed] [Google Scholar]
- Lee A, Clancy S, and Fleming AS (2000). Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav. Brain Res 108, 215–231. [DOI] [PubMed] [Google Scholar]
- Lei K, Liu Y, Smith AS, Lonstein JS, and Wang Z (2017). Effects of pair bonding on parental behavior and dopamine activity in the nucleus accumbens in male prairie voles. Eur. J. Neurosci 46, 2276–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Goel P, and Kaeser PS (2021). Spatial and temporal scales of dopamine transmission. Nat. Rev. Neurosci 22, 345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Kershberg L, Wang J, Schneeberger S, and Kaeser PS (2018). Dopamine secretion is mediated by sparse active zone-like release sites. Cell 172, 706–718.e15. e715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC, Sachs BD, and Salamone JD (1998). Sexual behavior in male rats after radiofrequency or dopamine-depleting lesions in nucleus accumbens. Pharmacol. Biochem. Behav 60, 585–592. [DOI] [PubMed] [Google Scholar]
- Liu ZW, Jiang N, Tao X, Wang XP, Liu XM, and Xiao SY (2020). Assessment of sexual behavior of male mice. JoVE. 10.3791/60154. [DOI] [PubMed] [Google Scholar]
- Martínez M, Guillén-Salazar F, Salvador A, and Simón VM (1995). Successful intermale aggression and conditioned place preference in mice. Physiol. Behav 58, 323–328. [DOI] [PubMed] [Google Scholar]
- Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, and Bethge M (2018). DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci 21, 1281–1289. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, and Morrell JI (2001). Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav. Neurosci 115, 683–694. [DOI] [PubMed] [Google Scholar]
- May ME, and Kennedy CH (2009). Aggression as positive reinforcement in mice under various ratio-and time-based reinforcement schedules. J. Exp. Anal. Behav 91, 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry JA, Otis JM, Rossi MA, Robinson JE, Kosyk O, Miller NW, McElligott ZA, Budygin EA, Rubinow DR, and Stuber GD (2017). Hormonal gain control of a medial preoptic area social reward circuit. Nat. Neurosci 20, 1427–1430. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Camp DM, and Robinson TE (1993). A microdialysis study of ventral striatal dopamine during sexual behavior in female Syrian hamsters. Behav. Brain Res 55, 151–157. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, and Becker JB (1995). Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behav. Neurosci 109, 354–365. [DOI] [PubMed] [Google Scholar]
- Mohebi A, Pettibone JR, Hamid AA, Wong JMT, Vinson LT, Patriarchi T, Tian L, Kennedy RT, and Berke JD (2019). Dissociable dopamine dynamics for learning and motivation. Nature 570, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses J, Loucks JA, Watson HL, Matuszewich L, and Hull EM (1995). Dopaminergic drugs in the medial preoptic area and nucleus accumbens: effects on motor activity, sexual motivation, and sexual performance. Pharmacol. Biochem. Behav 51, 681–686. [DOI] [PubMed] [Google Scholar]
- Numan M (2007). Motivational systems and the neural circuitry of maternal behavior in the rat. Dev. Psychobiol 49, 12–21. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, and Smith CD (2005). The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav. Neurosci 119, 1588–1604. [DOI] [PubMed] [Google Scholar]
- Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, et al. (2014). A mesoscale connectome of the mouse brain. Nature 508, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira TD, Tabris N, Matsliah A, Turner DM, Li J, Ravindranath S, Papadoyannis ES, Normand E, Deutsch DS, Wang ZY, et al. (2022). SLEAP: a deep learning system for multi-animal pose tracking. Nat. Methods 19, 486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaus JG, Damsma G, Nomikos GG, Wenkstern DG, Blaha CD, Phillips AG, and Fibiger HC (1990). Sexual behavior enhances central dopamine transmission in the male rat. Brain Res. 530, 345–348. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Damsma G, Wenkstern D, and Fibiger HC (1995). Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res. 693, 21–30. [DOI] [PubMed] [Google Scholar]
- Pleim ET, Matochik JA, Barfield RJ, and Auerbach SB (1990). Correlation of dopamine release in the nucleus accumbens with masculine sexual behavior in rats. Brain Res. 524, 160–163. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Christensen JR, Guerra C, and Bardo MT (1997). Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 776, 61–67. [DOI] [PubMed] [Google Scholar]
- Ren K, Guo B, Dai C, Yao H, Sun T, Liu X, Bai Z, Wang W, and Wu S (2017). Striatal distribution and cytoarchitecture of dopamine receptor subtype 1 and 2: evidence from double-labeling transgenic mice. Front. Neural Circuits 11, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Heien MLAV, and Wightman RM (2002). Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J. Neurosci 22, 10477–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Phillips PE, Budygin EA, Trafton BJ, Garris PA, and Wightman RM (2001). Sub-second changes in accumbal dopamine during sexual behavior in male rats. Neuroreport 12, 2549–2552. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, and Weber SM (2003). Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J. Pharmacol. Exp. Therapeut 305, 1–8. [DOI] [PubMed] [Google Scholar]
- Shnitko TA, Mace KD, Sullivan KM, Martin WK, Andersen EH, Williams Avram SK, Johns JM, and Robinson DL (2017). Use of fast scan cyclic voltammetry to assess phasic dopamine release in rat models of early postpartum maternal behavior and neglect. Behav. Pharmacol 28, 648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John RD, and Corning PA (1973). Maternal aggression in mice. Behav. Biol 9, 635–639. [DOI] [PubMed] [Google Scholar]
- Stagkourakis S, Spigolon G, Williams P, Protzmann J, Fisone G, and Broberger C (2018). A neural network for intermale aggression to establish social hierarchy. Nat. Neurosci 21, 834–842. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, McKenna JB, Keough S, Hancock R, Numan MJ, and Numan M (2007). Dopamine D₁ receptor stimulation of the nucleus accumbens or the medial preoptic area promotes the onset of maternal behavior in pregnancy-terminated rats. Behav. Neurosci 121, 907–919. [DOI] [PubMed] [Google Scholar]
- Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, Luo Y, Li F, Wang H, Yamaguchi T, et al. (2018). A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell 174, 481–496.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Zhou J, Dai B, Qian T, Zeng J, Li X, Zhuo Y, Zhang Y, Wang Y, Qian C, et al. (2020). Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat. Methods 17, 1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenk CM, Wilson H, Zhang Q, Pitchers KK, and Coolen LM (2009). Sexual reward in male rats: effects of sexual experience on conditioned place preferences associated with ejaculation and intromissions. Horm. Behav 55, 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, and Miczek KA (1996). Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 721, 140–149. [DOI] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, and Vanderschuren LJMJ (2011). Evaluating the rewarding nature of social interactions in laboratory animals. Dev. Cogn. Neurosci 1, 444–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM (2007). Review on CPP: measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addiction Biol. 12, 227–462. [DOI] [PubMed] [Google Scholar]
- van Erp AM, and Miczek KA (2000). Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J. Neurosci 20, 9320–9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CT, Huang RL, Tai MY, Tsai YF, and Peng MT (1995). Dopamine release in the nucleus accumbens during sexual behavior in prenatally stressed adult male rats. Neurosci. Lett 200, 29–32. [DOI] [PubMed] [Google Scholar]
- Wang J, Tai F, Yu P, and Wu R (2012). Reinforcing properties of pups versus cocaine for fathers and associated central expression of Fos and tyrosine hydroxylase in Mandarin voles (Microtus mandarinus). Behav. Brain Res 230, 149–157. [DOI] [PubMed] [Google Scholar]
- Wenkstern D, Pfaus JG, and Fibiger HC (1993). Dopamine transmission increases in the nucleus accumbens of male rats during their first exposure to sexually receptive female rats. Brain Res. 618, 41–46. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Damsma G, De Vries JB, and Koning H (1987). Dopamine re-uptake inhibitors show inconsistent effects on the in vivo release of dopamine as measured by intracerebral dialysis in the rat. Eur. J. Pharmacol 135, 123–128. [DOI] [PubMed] [Google Scholar]
- Willmore L, Cameron C, Yang J, Witten I, and Falkner A (2022). Behavioral and dopaminergic signatures of resilience. Preprint at bioRxiv. 10.1101/2022.03.18.484885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsoncroft W (1968). Babies by bar-press: maternal behavior in the rat. Behav. Res. Methods Instrum 1, 229–230. [Google Scholar]
- Wise RA (2004). Dopamine, learning and motivation. Nat. Rev. Neurosci 5, 483–494. [DOI] [PubMed] [Google Scholar]
- Wise RA, Spindler J, deWit H, and Gerberg GJ (1978). Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food. Science 201, 262–264. [DOI] [PubMed] [Google Scholar]
- Xie Y, Huang L, Corona A, Pagliaro AH, and Shea SD (2022). A reinforcement learning algorithm shapes maternal care in mice. Preprint at bio-Rxiv. 10.1101/2022.03.21.485130. [DOI] [Google Scholar]
- Yuan L, Dou YN, and Sun YG (2019). Topography of reward and aversion encoding in the mesolimbic dopaminergic system. J. Neurosci 39, 6472–6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
All original code has been deposited at Zenodo and is publicly available as of the date of publication. DOIs are listed in the key resources table.
Data and any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Chicken anti-GFP | Abcam | Cat#ab13970; RRID: AB_300798 |
| Sheep anti-TH | Pel Freeze | Cat#P60101-0; RRID: AB_461070 |
| Donkey anti-chicken Alexa Fluor 488 | Jackson ImmunoResearch | Cat#703-545-155; RRID: AB_2340375 |
| Donkey anti-sheep Cyanine Cy™5 | Jackson ImmunoResearch | Cat#713-175-147; RRID: AB_2340730 |
| Bacterial and Virus Strains | ||
| AAV9-hSyn-DIO-GRAB.DA2m | (Sun et al., 2020); Vigene Biosciences | NA |
| AAV9-hSyn-loxP-GRAB.DA2m-loxP | Current study; Vigene Biosciences | Addgene# 189751 |
| AAV2-CAG-Flex-GFP | (Oh et al., 2014); UNC Vector Core; | Cat#AV-2-ALL854 |
| AAV5-CAG-Flex-GFP | (Oh et al., 2014); UNC Vector Core; | Cat#AV4531b |
| AAV5-Ef1a-DIO-hChR2(H134R)-EYFP-WPRE-pA | UNC Vector Core; Karl Deisseroth lab (unpublished) | Cat#AV-4313H1 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Normal donkey serum | Jackson ImmunoResearch | Cat#017-000-121; RRID:AB_2337258 |
| GBR 12909 dihydrochloride | Tocris | Cat#0421 |
| 0.9% NaCl | Hanna Pharmaceutical Supply | Cat#NC9054335 |
| DAPI | Thermo Fisher Scientific | Cat#62248 |
| Fluoromount-G™ Mounting Medium | Thermo Fisher Scientific | Cat#00-4958-02 |
| Experimental Models: Organisms/Strains | ||
| Drd1-cre mice | MMRRC | RRID: MMRRC_030989-UCD |
| DAT-ires-cre mice | Jackson Laboratory | RRID: IMSR_JAX:006660 |
| Ai9 mice | Jackson Laboratory | RRID: IMSR_JAX:007909 |
| C57BL/6N mice | Charles River | Strain #027 |
| Swiss Webster mice | Charles River | Strain #024 |
| Software and Algorithms | ||
| MATLAB R2019b | MathWorks |
https://www.mathworks.com RRID:SCR_001622 |
| Prism 9 | GraphPad Software |
https://www.graphpad.com RRID:SCR_002798 |
| StreamPix 8 | NorPix |
https://www.norpix.com/products/streampix/streampix.php RRID:SCR_015773 |
| Deeplabcut | (Mathis et al., 2018) | https://github.com/DeepLabCut |
| SLEAP | (Pereira et al., 2022) | https://sleap.ai/ |
| SLEAP Extension | Current study | https://zenodo.org/badge/latestdoi/400214215 |
| Adobe Photoshop 2020 | Adobe |
https://www.adobe.com/; RRID:SCR 014199 |
| Adobe Illustrator 2020 | Adobe |
https://www.adobe.com/; RRID:SCR_010279 |
| other | ||
| Optic fibers (200 um) | Thorlabs | Cat#FT200EMT |
| Optic fibers (400 um) | Thorlabs | Cat#CF440-10 |
| Ceramic Ferrule for MM Fiber (230 um) | Thorlabs | Cat#CFLC230-10 |
| Stainless Steel Ferrule for MM Fiber (440 um) | Thorlabs | Cat#SFLC440-10 |
| Ceramic split matching sleeves | Thorlabs | Cat#ADAL1 |


