Summary:
The understanding of the molecular and cellular basis of aging has grown exponentially over recent years, and it is now accepted within the scientific community that aging is a malleable process; just as it can be accelerated, it can also be slowed and even reversed. This has far-reaching implications for our attitude and approach toward aging, presenting the opportunity to enter a new era of cellular regenerative medicine to not only manage the external signs of aging but also to develop therapies that support the body to repair and restore itself back to a state of internal well-being. A wealth of evidence now demonstrates that a decline in cellular nicotinamide adenine dinucleotide (NAD+) is a feature of aging and may play a role in the process. NAD+ plays a pivotal role in cellular metabolism and is a co-substrate for enzymes that play key roles in pathways that modify aging. Thus, interventions that increase NAD+ may slow aspects of the aging trajectory, and there is great interest in methods for cellular NAD+ restoration. Given these recent advancements in understanding the cellular aging process, it is important that there is an integration between the basic scientists who are investigating the underlying mechanisms of cellular aging and the surgeons and aesthetic practitioners who are providing antiaging therapies. This will allow the effective translation of this vastly complex area of biology into clinical practice so that people can continue to not only stay looking younger for longer but also experience improved health and wellness.
In general terms, aging is considered the organism-wide loss of homeostasis, innate repair, and regenerative capacity, resulting in an accumulation of damage and the development of multiple copathologies. Aging is a complex and multifactorial phenomenon that includes many effects at the systemic level which are ultimately driven by critical changes at the cellular level. It is recognized that there are nine key cellular changes that underpin the cascade of events that lead to systemic age-related decline. These cellular causes of aging have been well characterized and are collectively referred to within the aging research community as the “hallmarks of aging.”1
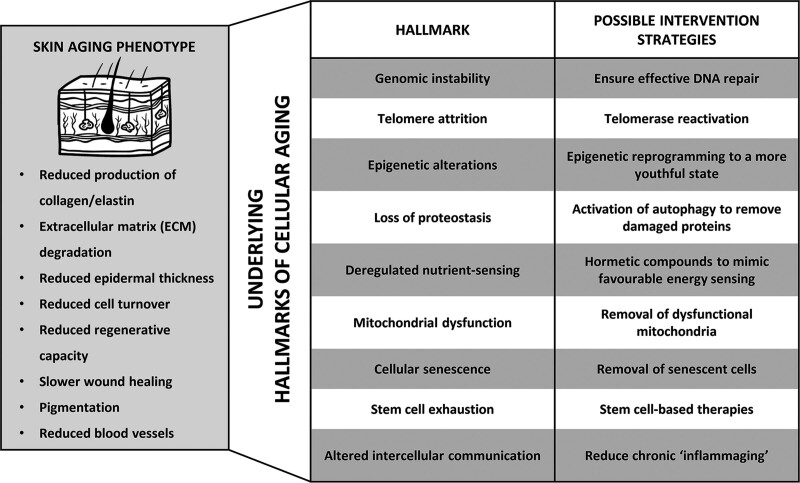
The identification of the hallmarks of aging has marked a shift toward understanding aging not as a single process, but instead as a combination of multiple cellular changes. This has allowed the molecular and cellular root causes of many common aging phenotypes to be identified. For example, skin aging—arguably the most recognizable sign of aging—has traditionally been described at the histological level, but it is now understood that these changes result from more specific failures at a cellular level, revealing new therapeutic targets with the potential to address aging at its root cause (Fig. 1).
Fig. 1.
Traditionally, age-related dysfunction has been described at the histological level, but it is now known that these changes result from more specific failures at the cellular level. These key cellular changes are collectively known as the “hallmarks of aging” and designing interventions that target these hallmarks is currently an area of intense research.
NAD+ AS A TARGET FOR CELLULAR AGING
One area of intense research within the field of cellular aging surrounds the molecule nicotinamide adenine dinucleotide (NAD+). Metabolomics-based studies of aging have identified NAD+ as a central metabolic intermediate linked to many of the hallmarks of aging.2 NAD+ is a cellular coenzyme that plays an essential role in both metabolic and signaling reactions. During its role in metabolism, NAD+ participates in redox reactions leading to the formation of ATP. Aside from this key role, NAD+ is also a critical regulator of a wide array of enzymes involved in making posttranslational modifications to proteins that change their activity.3 This combination of metabolic and cell-signaling functions means that NAD+ acts as a metabolic messenger providing an important link between the energy status of the cell and downstream signaling for appropriate cellular adaptation to bioenergetic stress. Therefore, a proper maintenance of NAD+ levels is required to maintain tissue homeostasis and stress response.4
Despite its critical role, an age-dependent decrease of cellular NAD+ is observed across species. In humans, age-related NAD+ decline has been observed in the liver,5 skin,6 brain,7,8 plasma,9 skeletal muscle,10 and monocyte-derived macrophages.11 Chronically low NAD+ has been observed in accelerated aging disorders12 and age-related disease states,13–15 and has been linked to multiple hallmarks of aging.
Low NAD+ contributes to aging because NAD+ serves as an exclusive co-substrate for two key families of enzymes that affect cellular repair and longevity—the sirtuins (SIRTs) and the poly(ADP-ribose) polymerases (PARPs). These enzyme families regulate many signaling processes associated with cellular health and longevity and are directly dependent on NAD+ availability to perform their functions.
PRECLINICAL BENEFITS OF NAD+ RESTORATION
Restoration of NAD+ in vivo has been investigated extensively and has demonstrated whole-body benefits. This has been reviewed in detail recently.16 Briefly, in mice, NAD+ levels have been found to decrease twofold by mid-age, correlating with the onset of multiple age-related issues.5 Successful restoration of NAD+ to youthful levels resulted in cardiovascular improvements17 and the reversal of multiple metabolic conditions.18–21 Improvements to muscle function and endurance20 were also observed together with increased mitochondrial function, ATP production14 and an increased number and quality of muscle stem cells.22 An increased capacity for organ protection and regeneration after injury was found in the liver, heart, and kidneys,23–25 and NAD+ restoration was also found to rescue vision by reversing retinal degeneration.26 Significant neurological benefits have also been demonstrated in Alzheimer disease animal models on NAD+ restoration, including improved cognition and nerve regeneration. NAD+ availability also appears to impact fertility, as strategies to boost NAD+ levels were found to improve oocyte quality and restore fertility in aged mice.27
CLINICAL BENEFITS OF NAD+ RESTORATION
These impressive preclinical results have now shifted the focus to human clinical trials with the hope of translating the benefits of NAD+ restoration to humans (see Table, Supplemental Digital Content 1, which shows human clinical trials that have measured NAD+ levels and clinical outcomes after NAD+ restoration, http://links.lww.com/PRS/F374). Notable observations so far include a trend towards an improvement in indicators of cardiovascular function including lower systolic blood pressure and aortic stiffness,28 a promising reduction in the levels of circulating inflammatory cytokines in older males after only 3 weeks of NAD+ restoration29 and an NAD+-associated increase in mitochondrial function and decreased proinflammatory factors in heart failure patients.30 The diverse protective and regenerative capacity of NAD+ has been attributed to its involvement in the prevention of multiple hallmarks of cellular aging (Table 1).
Table 1.
Hallmarks of Aging Are Key Cellular Changes That Underpin the Cascade of Events that Lead to Systemic Age-Related Decline*
| Hallmark of Aging | Role of NAD+ | References |
|---|---|---|
| Genomic instability | Adequate NAD+ availability is critical to drive DNA repair enzymes and pathways such as PARP1, SIRT1, and SIRT6 | 31–33 |
| Cellular senescence | Low NAD+ promotes senescence in skin whilst restoration of NAD+ reduces the burden of senescent cells | 40,41 |
| Epigenetic alterations | NAD+-dependent sirtuins are critical for youthful epigenetic regulation. Reduced NAD+ means sirtuins cannot perform this critical role | 43 |
| Mitochondrial dysfunction | Adequate NAD+ is critical to healthy mitochondrial function and for the removal of damaged mitochondria | 79 |
| Telomere attrition | NAD+ restoration is found to alleviate telomere dysfunction | 80 |
| Altered intracellular communication | Low NAD+ promotes age-related inflammation | 81 |
| Loss of proteostasis | NAD+ is required for SIRT1-mediated activation of autophagy to clear damaged cellular proteins | 60,61 |
| Deregulated nutrient sensing | NAD+ levels are critical to sense the energetic status of the cell for adaptation to energy stress | 4 |
| Stem cell exhaustion | NAD+ restoration leads to stem cell rejuvenation | 22 |
NAD+ has been identified as a key metabolic intermediate linked to many of the hallmarks of aging.
THE ROLE OF NAD+ IN SKIN AGING
There is also growing evidence that NAD+ decline plays a critical role in the biology of skin aging. DNA damage and genomic instability are key features of skin aging due to the continued exposure of the skin to UV radiation and sophisticated DNA repair mechanisms exist to quickly repair damage before it becomes harmful to the cell. It has emerged that several of these repair mechanisms are directly dependent on NAD+ to perform their function, so its decline with age is problematic. For example, the DNA repair enzyme PARP1, and SIRTs 1 and 6, which are integral elements of the DNA repair response, are all critically dependent on NAD+ to function.31–33 Decreasing NAD+ levels therefore contribute to reduced DNA repair and an accumulation of DNA damage. Accordingly, an age-associated decrease in both NAD+ and SIRT1 is observed in skin, whilst DNA damage is found to accumulate,6 ultimately triggering other hallmarks of aging such as cellular senescence.
Cellular senescence is characterized by the cell entering a state of irreversible cell cycle arrest.34 Both fibroblasts and keratinocytes have been found to become senescent with age35 and, while they persist in the skin and remain metabolically active, they do not perform their normal function in contributing to skin health. For example, senescent fibroblasts no longer produce collagen and elastin resulting in a dysfunctional support matrix and skin that is not capable of efficient damage repair.36 Senescent cells also have a distinct inflammatory secretory profile termed the “senescence associated secretory phenotype” (SASP), which has a profound detrimental effect on surrounding cells, leading to altered intercellular communication.37 Senescent fibroblasts promote degradation of the extracellular matrix (ECM) by secreting matrix metalloproteinase-1 (MMP1) and other proinflammatory factors,38 ultimately leading to thinning of the epidermis and decreased barrier function,39 while the selective removal of senescent cells leads to normalization of the ECM and a reduction in inflammation.37 Low NAD+ has also been found to promote senescence by reducing SIRT1 activity, which in turn reduces p63 expression, leading to a reduction in cell proliferation.40 NAD+, SIRT1, and p63 are all found to decline in aged keratinocytes leading to senescence,35 while the restoration of NAD+ reduces the senescent cell burden in dermal fibroblasts.41
As well as genomic instability, the aging process is characterized by changes to DNA methylation patterns known as “epigenetic drift,” which ultimately alters gene expression.42 This has led to the development of “DNA methylation clocks” that predict the biological age of cells based on these measurable epigenetic changes. The NAD+-dependent SIRTs play a key role in epigenetic regulation, highlighting a major role for NAD+ in the cross talk between the metabolic state of the cell and epigenetic regulation of gene expression.43 Indeed, many of the beneficial antiaging effects of healthy lifestyle practices such as fasting and exercise are coordinated by increasing NAD+ levels, which in turn activates SIRT1 to change the expression of beneficial genes. In skin, the SIRTs are linked to the preservation of collagen in the dermis and their activation is important in wound healing and regeneration of skin by promoting keratinocyte proliferation.44 SIRT6 promotes genes associated with collagen production,45 and both SIRT1 and SIRT6 mediate the inhibition of MMP-1 gene transcription, which degrades collagen.46 Both SIRT1 and SIRT6 are found to be downregulated in older skin, and this correlates with a reduction in available NAD+.47,48
Aged skin also demonstrates mitochondrial dysfunction,49 an aging hallmark that is directly linked to oxidative stress, increased MMP-1 expression, dermal atrophy, and epidermal hyperplasia.50 Adequate cellular NAD+ is critical for normal mitochondrial function both directly through its role in oxidative phosphorylation and indirectly through activation of SIRT1 and SIRT3, which are involved in the biogenesis and degradation of damaged mitochondria.51,52 Increasing cellular NAD+ has been found to improve mitochondrial function, activate mitophagy (the recycling of dysfunctional mitochondria), and improve keratinocyte regenerative capacity.53,54
An age-dependent dermal accumulation of oxidatively modified and damaged proteins has also been found to cause skin dysfunction.55 ECM proteins such as collagen become glycated to form Advanced Glycation End products (AGES) leading to dermal stiffness and decreased flexibility.56 Autophagy mediates the recycling of AGES and other defective proteins and an age-related reduction in autophagic activity in dermal fibroblasts is found to reduce collagen, hyaluronan, and elastin, collectively leading to deterioration of dermal integrity and skin fragility.57–59 Increasing data indicates that the maintenance of high NAD+ is critical to SIRT1-mediated activation of autophagy pathways and the clearance of damaged cellular proteins.60,61
CAUSES OF NAD+ DECLINE
The above discussion demonstrates clear evidence for the role of NAD+ decline in the development of the hallmarks of aging in the skin, and there has been great interest in understanding the root causes of NAD+ decline to determine strategies to successfully restore cellular NAD+ levels.
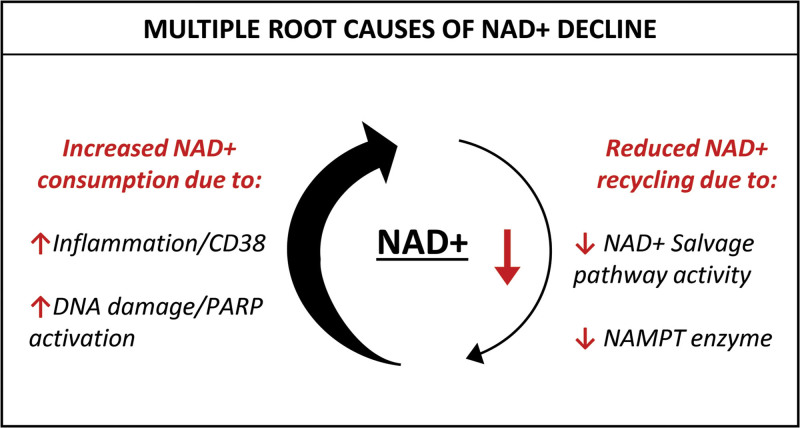
It is known that NAD+ metabolism comprises multiple precursors, production routes, recycling pathways, and a myriad of consuming enzymes. Evidence now suggests that a major cause of NAD+ decline is a disruption of this finely controlled network. Specifically, it has been found that NAD+ consumption starts to outpace NAD+ production and recycling with age62 (Fig. 2).
Fig. 2.
There are multiple root causes of NAD+ decline. Older cells exhibit excessive NAD+ consumption due to chronic inflammation and DNA damage which increases the activity of the NAD+ consumers CD38 and the PARPs. At the same time, reduced expression of the NAMPT enzyme means the salvage pathway is less efficient at recycling NAD+, resulting in cells that struggle to meet the demand for NAD+.
In its role as a coenzyme, NAD+ acts as a substrate that is irreversibly degraded by NAD+-consuming enzymes, including the SIRTs, PARPs, and CD38. The expression and activity of these NAD+-consuming enzymes have been found to increase with age meaning that the demand for NAD+ also increases. For example, age-associated increases in DNA damage activates NAD+-dependent PARP1,63,64 and although PARP1 is a critical DNA repair enzyme, its persistent activation is harmful due to this contribution to NAD+ depletion. Indeed, overactivation of PARPs with resulting severe depletion of NAD+ has been highlighted recently by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), which is found to severely deplete cellular NAD+ due to overactivation of PARPs.65
The NAD+ glycohydrolase CD38 has also been recognized to consume large quantities of NAD+.66 CD38 is found throughout the body and plays an important role in multiple aspects of the inflammatory response.67 It is now clear that CD38 becomes overexpressed during aging due to chronic activation from persistent low-level “inflammaging,” which in turn results in NAD+ depletion.68,69
This overactivation of NAD+-consuming pathways with age and disease can severely compromise NAD+ availability in cells, subsequently limiting utilization of NAD+ by other critical NAD+-dependent enzymes that promote good health, such as the SIRTs. Despite this increased demand for NAD+ throughout life, NAD+ levels should, in theory, be self-sustaining as cells have the ability to rapidly recycle the breakdown products of NAD+ consumption to replenish NAD+. This occurs via the salvage pathway, which plays a major role in restoring NAD+. Nicotinamide phosphoribosyltransferase (NAMPT) is the rate-limiting enzyme in this recycling process,70 and it is now known that NAMPT levels decline with age in parallel with the decline of NAD+ in aged tissues.5,71–77 This reduction in NAD+ biosynthesis via the salvage pathway is a significant factor in older cells because, as NAD+ consumption increases concurrently with age and demands for NAD+ replenishment and recycling increase, the resulting degraded NAD+ is no longer efficiently recycled, exacerbating a situation of declining NAD+ levels.5
METHODS TO RESTORE NAD+
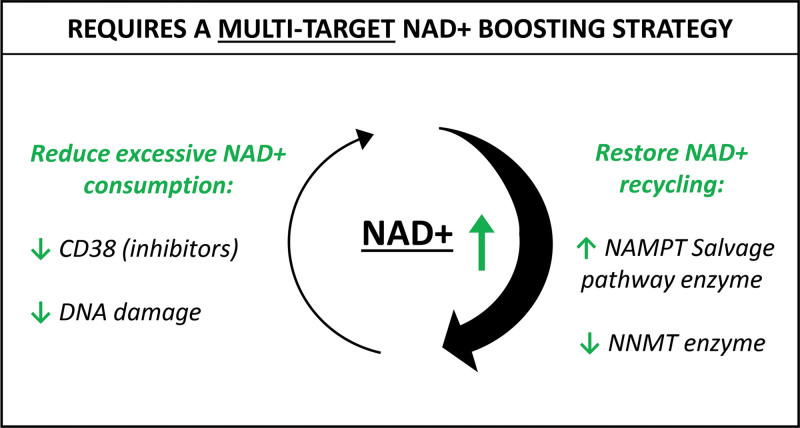
The evidence demonstrates that the biology behind NAD+ decline is complex. NAD+ restoration using pure exogenous NAD+ is often not practical due to its unstable nature and poor bioavailability to most cell types, so efforts have focused on oral supplementation with the NAD+ precursor compounds nicotinamide (NAM), nicotinamide riboside (NR), and nicotinamide mononucleotide (NMN). These precursors are utilized by NAD+ biosynthesis pathways and converted to NAD+ within the cell. However, it is now clear that this popular approach ignores the root causes of NAD+ decline meaning supplementation with precursors alone such as NR or NMN do not offer a long-term efficacious solution for NAD+ restoration. Instead, strategies that simultaneously address the multiple root causes of NAD+ decline such as the combined administration of a NAD+ precursor, a CD38 inhibitor, and an NAMPT activator, hold potential for NAD+ restoration with greater measurable benefits (Fig. 3). Furthermore, there is already a wealth of evidence to support these targets as interventions to successfully restore cellular NAD+ levels and multiple safe and well-tolerated active ingredients against these targets already exist.62 This presents the opportunity for the development of oral, topical, and injectable formulations, allowing for both whole-body NAD+-restoring benefits alongside more targeted administration, resulting in the ultimate inside-outside approach to aging (Table 2).
Fig. 3.
Successful NAD+ restoration requires a multitargeted strategy that simultaneously addresses the root causes of NAD+ decline. Therapies must reduce the excessive consumption of NAD+ with approaches such as CD38 inhibition and reduction of DNA damage, while improving the efficiency of NAD+ recycling by promoting upregulation of the rate-limiting salvage pathway enzyme NAMPT and inhibition of NNMT, an enzyme that promotes the removal of NAD+ breakdown products from the cell rather than recycling.
Table 2.
Potential Clinical Applications, Routes of Administration and Benefits of NAD+-Restoration Therapies
| Method of Administration | Potential Benefits | |
|---|---|---|
| Systemic applications for NAD+-restoration | Oral supplementation | Whole-body improvements in cellular health contributing to improved healthspan |
| Improved energy, cognitive function, and sense of well-being | ||
| Improved sleep quality | ||
| Intravenous | Pre-procedure administration to prime cells for optimal response to aesthetic treatments | |
| Improve healing/regenerative capacity pre/postsurgery | ||
| Localized applications for NAD+-restoration | Topical | Concentrated treatment for problematic areas |
| Concentrated application to improve healing/regeneration postsurgery | ||
| Injectable | Use in combination with aesthetic procedures such as microneedling |
THE FUTURE OF NAD+ IN CLINICAL PRACTICE
Until recently, antiaging therapies were limited to repairing the consequences of aging, but now there is clear evidence that aging can be targeted from its root cellular cause giving the opportunity to slow and even reverse aspects of aging.78
NAD+ restoration has been identified as a key therapeutic target that can positively impact many of the hallmarks of cellular aging. Not only does it play a key role in skin aging but also demonstrates a great potential to improve multiple aspects of age-related decline across the whole body. This offers an unprecedented opportunity for practitioners to introduce NAD+-restoring therapies that not only impact the appearance of their patients but also their health and well-being (Table 2).
Given the rapidly aging population, addressing aging at the cellular level is now critically important. Many surgical procedures rely on the healing and regenerative capacity of the skin which is known to decline with age, leading to disappointing results or negative postsurgery outcomes. Improving health and resilience at the cellular level with NAD+ restoration could be harnessed as a way to ensure consistent results and recovery irrespective of patient age. It should also be noted that the efficacy of many nonsurgical aesthetic procedures such as microneedling and laser technologies ultimately rely on the activation of cellular stress pathways to trigger the clearance of damaged cells and stimulate the production of new collagen. Many of these pathways require adequate NAD+ levels for optimal function, meaning NAD+ restoration before treatment could be a strategy to ensure the underlying cells are in an optimal condition to respond to the treatment.
With this greater understanding of the benefits of NAD+ and how to design targeted strategies to maintain its availability, collaboration with clinical practitioners, who have firsthand experience of the clinical manifestations of aging and access to patient groups for clinical trials, is now crucial to translate this exciting science into the clinic.
Supplementary Material
Footnotes
Presented at the Science of Aging Symposium 2021, held virtually, September 15, 2021.
Disclosure: Dr. Conlon is chief executive officer and shareholder of Nuchido Ltd. and has filed patents on NAD+ boosting therapies.
Related digital media are available in the full-text version of the article on www.PRSJournal.com.
REFERENCES
- 1.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma R, Ramanathan A. The aging metabolome-biomarkers to hub metabolites. Proteomics. 2020;20:e1800407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ansari HR, Raghava GP. Identification of NAD interacting residues in proteins. BMC Bioinformatics. 2010;11:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantó C, Auwerx J. NAD+ as a signaling molecule modulating metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou CC, Yang X, Hua X, et al. Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br J Pharmacol. 2016;173:2352–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012;7:e42357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagga P, Hariharan H, Wilson NE, et al. Single-voxel 1 H MR spectroscopy of cerebral nicotinamide adenine dinucleotide (NAD+) in humans at 7T using a 32-channel volume coil. Magn Reson Med. 2020;83:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci USA. 2015;112:2876–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clement J, Wong M, Poljak A, Sachdev P, Braidy N. The plasma NAD+ metabolome is dysregulated in “normal” aging. Rejuvenation Res. 2019;22:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamb DA, Moore JH, Mesquita PHC, et al. Resistance training increases muscle NAD+ and NADH concentrations as well as NAMPT protein levels and global sirtuin activity in middle-aged, overweight, untrained individuals. Aging (Albany NY). 2020;12:9447–9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minhas PS, Liu L, Moon PK, et al. Macrophage de novo NAD+ synthesis specifies immune function in aging and inflammation. Nat Immunol. 2019;20:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheibye-Knudsen M, Mitchell SJ, Fang EF, et al. A high-fat diet and NAD(+) activate Sirt1 to rescue premature aging in cockayne syndrome. Cell Metab. 2014;20:840–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang EF, Lautrup S, Hou Y, et al. NAD+ in aging: Molecular mechanisms and translational implications. Trends Mol Med. 2017;23:899–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes AP, Price NL, Ling AJ, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdin E. NAD+ in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–1213. [DOI] [PubMed] [Google Scholar]
- 16.Reiten OK, Wilvang MA, Mitchell SJ, Hu Z, Fang EF. Preclinical and clinical evidence of NAD+ precursors in health, disease, and ageing. Mech Ageing Dev. 2021;199:111567. [DOI] [PubMed] [Google Scholar]
- 17.de Picciotto NE, Gano LB, Johnson LC, et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15:522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbosa MT, Soares SM, Novak CM, et al. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J. 2007;21:3629–3639. [DOI] [PubMed] [Google Scholar]
- 20.Cantó C, Houtkooper RH, Pirinen E, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gariani K, Ryu D, Menzies KJ, et al. Inhibiting poly ADP-ribosylation increases fatty acid oxidation and protects against fatty liver disease. J Hepatol. 2017;66:132–141. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Ryu D, Wu Y, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–1443. [DOI] [PubMed] [Google Scholar]
- 23.Horton JL, Martin OJ, Lai L, et al. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight. 2016;2:e84897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran MT, Zsengeller ZK, Berg AH, et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherjee S, Chellappa K, Moffitt A, et al. Nicotinamide adenine dinucleotide biosynthesis promotes liver regeneration. Hepatology. 2017;65:616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin JB, Kubota S, Ban N, et al. NAMPT-mediated NAD(+) biosynthesis is essential for vision in mice. Cell Rep. 2016;17:69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertoldo MJ, Listijono DR, Ho WJ, et al. NAD+ repletion rescues female fertility during reproductive aging. Cell Rep. 2020;30:1670–1681.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martens CR, Denman BA, Mazzo MR, et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun. 2018;9:1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elhassan YS, Kluckova K, Fletcher RS, et al. Nicotinamide riboside augments the aged human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 2019;28:1717–1728.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou B, Wang DD, Qiu Y, et al. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J Clin Invest. 2020;130:6054–6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saville KM, Clark J, Wilk A, et al. NAD+-mediated regulation of mammalian base excision repair. DNA Repair (Amst). 2020;93:102930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurtado-Bagès S, Knobloch G, Ladurner AG, Buschbeck M. The taming of PARP1 and its impact on NAD+ metabolism. Mol Metab. 2020;38:100950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imai SI, Guarente L. It takes two to tango: NAD+ and sirtuins in aging/longevity control. NPJ Aging Mech Dis. 2016;2:16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28:436–453. [DOI] [PubMed] [Google Scholar]
- 35.Cordisco S, Maurelli R, Bondanza S, et al. Bmi-1 reduction plays a key role in physiological and premature aging of primary human keratinocytes. J Invest Dermatol. 2010;130:1048–1062. [DOI] [PubMed] [Google Scholar]
- 36.Quan T, Qin Z, Voorhees JJ, Fisher GJ. Cysteine-rich protein 61 (CCN1) mediates replicative senescence-associated aberrant collagen homeostasis in human skin fibroblasts. J Cell Biochem. 2012;113:3011–3018. [DOI] [PubMed] [Google Scholar]
- 37.Victorelli S, Lagnado A, Halim J, et al. Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. EMBO J. 2019;38:e101982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malaquin N, Vercamer C, Bouali F, et al. Senescent fibroblasts enhance early skin carcinogenic events via a paracrine MMP-PAR-1 axis. PLoS One. 2013;8:e63607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinmüllner R, Zbiral B, Becirovic A, et al. Organotypic human skin culture models constructed with senescent fibroblasts show hallmarks of skin aging. NPJ Aging Mech Dis. 2020;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rivetti di Val Cervo P, Lena AM, Nicoloso M, et al. p63-microRNA feedback in keratinocyte senescence. Proc Natl Acad Sci U S A. 2012;109:1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bierman JC, Laughlin T, Tamura M, et al. Niacinamide mitigates SASP-related inflammation induced by environmental stressors in human epidermal keratinocytes and skin. Int J Cosmet Sci. 2020;42:501–511. [DOI] [PubMed] [Google Scholar]
- 42.Raj K, Horvath S. Current perspectives on the cellular and molecular features of epigenetic ageing. Exp Biol Med (Maywood). 2020;245:1532–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Etchegaray JP, Mostoslavsky R. Interplay between metabolism and epigenetics: A nuclear adaptation to environmental changes. Mol Cell. 2016;62:695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spallotta F, Cencioni C, Straino S, et al. A nitric oxide-dependent cross-talk between class I and III histone deacetylases accelerates skin repair. J Biol Chem. 2013;288:11004–11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baohua Y, Li L. Effects of SIRT6 silencing on collagen metabolism in human dermal fibroblasts. Cell Biol Int. 2012;36:105–108. [DOI] [PubMed] [Google Scholar]
- 46.Ohguchi K, Itoh T, Akao Y, Inoue H, Nozawa Y, Ito M. SIRT1 modulates expression of matrix metalloproteinases in human dermal fibroblasts. Br J Dermatol. 2010;163:689–694. [DOI] [PubMed] [Google Scholar]
- 47.Bielach-Bazyluk A, Zbroch E, Mysliwiec H, et al. Sirtuin 1 and skin: Implications in intrinsic and extrinsic aging-a systematic review. Cells. 2021;10:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim KS, Park HK, Lee JW, Kim YI, Shin MK. Investigate correlation between mechanical property and aging biomarker in passaged human dermal fibroblasts. Microsc Res Tech. 2015;78:277–282. [DOI] [PubMed] [Google Scholar]
- 49.Mellem D, Sattler M, Pagel-Wolff S, et al. Fragmentation of the mitochondrial network in skin in vivo. PLoS One. 2017;12:e0174469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh B, Schoeb TR, Bajpai P, Slominski A, Singh KK. Reversing wrinkled skin and hair loss in mice by restoring mitochondrial function. Cell Death Dis. 2018;9:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalfalah F, Sobek S, Bornholz B, et al. Inadequate mito-biogenesis in primary dermal fibroblasts from old humans is associated with impairment of PGC1A-independent stimulation. Exp Gerontol. 2014;56:59–68. [DOI] [PubMed] [Google Scholar]
- 52.Tang BL. Sirt1 and the mitochondria. Mol Cells. 2016;39:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan CL, Chin T, Tan CYR, et al. Nicotinamide metabolism modulates the proliferation/differentiation balance and senescence of human primary keratinocytes. J Invest Dermatol. 2019;139:1638–1647.e3. [DOI] [PubMed] [Google Scholar]
- 54.Kang HT, Hwang ES. Nicotinamide enhances mitochondria quality through autophagy activation in human cells. Aging Cell. 2009;8:426–438. [DOI] [PubMed] [Google Scholar]
- 55.Sander CS, Chang H, Salzmann S, et al. Photoaging is associated with protein oxidation in human skin in vivo. J Invest Dermatol. 2002;118:618–625. [DOI] [PubMed] [Google Scholar]
- 56.Laughlin T, Tan Y, Jarrold B, et al. Autophagy activators stimulate the removal of advanced glycation end products in human keratinocytes. J Eur Acad Dermatol Venereol. 2020;34 Suppl 3:12–18. [DOI] [PubMed] [Google Scholar]
- 57.Tashiro K, Shishido M, Fujimoto K, et al. Age-related disruption of autophagy in dermal fibroblasts modulates extracellular matrix components. Biochem Biophys Res Commun. 2014;443:167–172. [DOI] [PubMed] [Google Scholar]
- 58.Rajawat YS, Hilioti Z, Bossis I. Aging: central role for autophagy and the lysosomal degradative system. Ageing Res Rev. 2009;8:199–213. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Wen X, Hao D, et al. Insights into autophagy machinery in cells related to skin diseases and strategies for therapeutic modulation. Biomed Pharmacother. 2019;113:108775. [DOI] [PubMed] [Google Scholar]
- 60.Fang EF, Kassahun H, Croteau DL, et al. NAD+ replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair. Cell Metab. 2016;24:566–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang DX, Zhang JP, Hu JY, Huang YS. The potential regulatory roles of NAD(+) and its metabolism in autophagy. Metabolism. 2016;65:454–462. [DOI] [PubMed] [Google Scholar]
- 62.Conlon N, Ford D. A systems-approach to NAD+ restoration. Biochem Pharmacol. 2022;198:114946. [DOI] [PubMed] [Google Scholar]
- 63.Dollé ME, Giese H, Hopkins CL, Martus HJ, Hausdorff JM, Vijg J. Rapid accumulation of genome rearrangements in liver but not in brain of old mice. Nat Genet. 1997;17:431–434. [DOI] [PubMed] [Google Scholar]
- 64.Krichevsky S, Pawelec G, Gural A, et al. Age related microsatellite instability in T cells from healthy individuals. Exp Gerontol. 2004;39:507–515. [DOI] [PubMed] [Google Scholar]
- 65.Heer CD, Sanderson DJ, Voth LS, et al. Coronavirus infection and PARP expression dysregulate the NAD metabolome: an actionable component of innate immunity. J Biol Chem. 2020;295:17986–17996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chini CCS, Tarragó MG, Chini EN. NAD and the aging process: role in life, death and everything in between. Mol Cell Endocrinol. 2017;455:62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piedra-Quintero ZL, Wilson Z, Nava P, Guerau-de-Arellano M. CD38: an immunomodulatory molecule in inflammation and autoimmunity. Front Immunol. 2020;11:597959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Polzonetti V, Carpi FM, Micozzi D, Pucciarelli S, Vincenzetti S, Napolioni V. Population variability in CD38 activity: correlation with age and significant effect of TNF-α -308G>A and CD38 184C>G SNPs. Mol Genet Metab. 2012;105:502–507. [DOI] [PubMed] [Google Scholar]
- 69.Amici SA, Young NA, Narvaez-Miranda J, et al. CD38 is robustly induced in human macrophages and monocytes in inflammatory conditions. Front Immunol. 2018;9:1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang H, Yang T, Baur JA, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koltai E, Szabo Z, Atalay M, et al. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev. 2010;131:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma C, Pi C, Yang Y, et al. Nampt expression decreases age-related senescence in rat bone marrow mesenchymal stem cells by targeting Sirt1. PLoS One. 2017;12:e0170930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu LY, Wang F, Zhang XY, et al. Nicotinamide phosphoribosyltransferase may be involved in age-related brain diseases. PLoS One. 2012;7:e44933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jadeja RN, Powell FL, Jones MA, et al. Loss of NAMPT in aging retinal pigment epithelium reduces NAD+ availability and promotes cellular senescence. Aging (Albany NY). 2018;10:1306–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xing S, Hu Y, Huang X, Shen D, Chen C. Nicotinamide phosphoribosyltransferase-related signaling pathway in early Alzheimer’s disease mouse models. Mol Med Rep. 2019;20:5163–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stein LR, Imai S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014;33:1321–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Guia RM, Agerholm M, Nielsen TS, et al. Aerobic and resistance exercise training reverses age-dependent decline in NAD+ salvage capacity in human skeletal muscle. Physiol Rep. 2019;7:e14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fahy GM, Brooke RT, Watson JP, et al. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell. 2019;18:e13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cantó C, Menzies KJ, Auwerx J. NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 2015;22:31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun C, Wang K, Stock AJ, et al. Re-equilibration of imbalanced NAD metabolism ameliorates the impact of telomere dysfunction. EMBO J. 2020;39:e103420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schultz MB, Bochaton T, Bonkowski M, et al. NAD+ depletion as a cause ofinflammaging. Innov Aging. 2018;2(S1):746–747. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.