Abstract
It is interesting that high iron is an independent inducer or cofactor of hepatocellular carcinoma (HCC) while the amount of iron is decreased in the liver tumor tissues. Due to the previous findings that iron deficiency promoted HCC metastasis, it is of significance to identify the underlying mechanism of iron deficiency in HCC. The tumor iron content and expressions of iron‐metabolic molecules were observed in the primary liver cancers of rats and mice. The molecules that changed independently of iron were identified by comparing the expression profiles in the human HCC tissues and iron‐deprived HCC cells. The downstream effects of these molecules on regulating intracellular iron content were investigated in vitro and further validated in vivo. Both in primary liver cancers of rats and mice, we confirmed the decreased iron content in tumor tissues and the altered expressions of iron‐metabolic molecules, including transferrin receptor 1 (TfR1), six‐transmembrane epithelial antigen of prostate 3 (STEAP3), divalent metal transporter 1 (DMT1), SLC46A1, ferroportin, hepcidin, and ferritin. Among these, STEAP3, DMT1, and SLC46A1 were altered free of iron deficiency. However, only silence or overexpression of SLC46A1 controlled the intracellular iron content of HCC cells. The interventions of STEAP3 or DMT1 could not change the intracellular iron content. Lentivirus‐mediated regain of SLC46A1 expression restored the iron content in orthotopically implanted tumors, with correspondingly changes in the iron‐metabolic molecules as iron increasing. Conclusion: Taken together, these results suggest that the loss of SLC46A1 expression leads to iron deficiency in liver tumor tissues, which would be an effective target to manage iron homeostasis in HCC.
Iron metabolism is dysrupted in hepatocellular cacinoma with decreased tumor iron content and abnormal expressions of iron‐metabolic molecules. Among these, the decrease of SLC46A1 is responsible for the dyshomeostasis of iron; the regain of it increases the tumor iron content and restores the expressions of iron‐metabolic molecules.
INTRODUCTION
The trace element iron impacts various aspects of the tumor, from the tumor cell proliferation, in which iron is indispensable for cell replication, to the tumor cell death, in which iron is necessary for ferroptosis.[ 1 ] Thus, iron excess or iron deficiency may be a double‐edged sword for tumor initiation and progression.[ 2 ] In past decades, iron overload was deemed as a trigger of gene mutation for its production of overburdened free radicals. The liver is the most vulnerable organ due to the storage of excess iron. The connection between iron overload and hepatocarcinoma is undoubted basing on observations on patients with hereditary hemochromatosis and many kinds of iron overload animal models.[ 3 ] Interestingly, unignorable findings indicated that iron is deficient and iron metabolism is dysregulated in liver tumor tissues of both patients and mice.[ 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 ] In our last study, we found that iron deficiency promoted pulmonary metastasis of liver cancer through immunosuppression and immune‐independent mechanisms.[ 13 ] However, the reason for iron deficiency in the liver tumor has not been clearly understood so far.
Iron homeostasis is primarily regulated by liver–specifically secreted hepcidin (HAMP), which binds with ferroportin (FPN), leading to its degradation and thus decreasing the export of iron from enterocytes, hepatocytes, and macrophages to the blood.[ 14 ] On the contrary, there are three known ways for iron to enter hepatocytes: the principal transferrin (Tf)–transferrin receptor (TfR) pathway for transferrin binding iron (Holo‐Tf) and H‐ferritin‐iron[ 15 ]; the divalent metal transporter 1 (DMT1) for ferrous iron, which is reduced from Tf‐ferric iron by the metalloreductase six‐transmembrane epithelial antigen of prostate 3 (STEAP3) or rarely existed in blood; and the SLC46A1, also named as proton‐coupled folate transporter or heme carrier protein 1, which is the main importer of heme‐iron and folate. SLC46A1 transcripts are abundant in the duodenum and the liver.[ 16 , 17 ] The transport of folic acid is deemed to be the main function of SLC46A1 due to the affinity of SLC46A1 with folate (1.67 μm), which is far beyond with heme (85 μm).[ 18 ] However, the affinity of folate with SLC46A1 is dramatically declined in the liver because SLC46A1 transports folate in a proton‐coupled manner, which is optimum in the small intestine with pH in the range of 5.8–6.0.[ 19 ] Although SLC46A1 transports heme with low affinity, it is independent of pH.[ 20 ] Our previous results showed that the SLC46A1 contributed to hepatic iron metabolism by importing heme in hepatocytes, which was unaffected by folate.[ 21 ]
It is difficult to determine the causality between iron content and iron‐metabolic molecules. Because changes in iron transporters will lead to changes in intracellular iron content, changes in intracellular iron content also alter the expressions of these iron‐responsive transporters through diversified feedback manners. In the present study, we first investigated the iron contents and iron‐metabolic molecules in the liver tumor tissues of rats and mice. Then we identified the molecules possibly leading to iron deficiency in the liver tumor by comparing the expression profiles of iron transporters in the HCC tumor tissues with their expressions in manipulated iron‐deficient HCC cells. The molecules with different alterations in HCC tissues and iron‐deprived HCC cells, which meant they were not regulated by iron deficiency, were possible causes of iron deficiency. Finally, we further verified the functions of these molecules in regulating iron content and iron homeostasis both in vitro and in vivo to elucidate the mechanism of iron deficiency in HCC tissues.
METHODS
Animals
Animal studies were performed under the approval of the Institutional Animal Care and Use Committee of Second Military Medical University, following the guidance of “Guide for the Care and Use of Laboratory Animals.” Rats and mice were purchased and reared in the Animal Center of Second Military Medical University, with normal circadian rhythm and food or water ad libitum in the SPF grade environment. The number of animals used in each experiment is shown in the figures.
Induction of primary liver cancer
Male Wistar rats, 7 weeks old, were intraperitoneally injected with diethylnitrosamine (DEN, Cat. 73861; Sigma‐Aldrich) at 50 mg/kg once a week for 16 weeks. Male C57/BL6 mice, 2 weeks old, were intraperitoneally injected with DEN at 25 mg/kg for one time. Rats or mice were injected with an equal volume of phosphate buffered saline for negative controls. Mice were sacrificed at 1 month, 3 months, 5 months, 7 months, and 9 months to observe tumorigenesis and iron metabolism. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) contents were determined by Hitachi 7600 Automatic Biochemical Analyzer (Hitachi). Serum alpha‐fetoprotein (AFP) content was measured by an enzyme‐linked immunosorbent assay kit (Cat. ab210969; Abcam).
Clinic liver tumor tissues
The liver tumor tissues and nontumor counterparts used in this study were identical to our previous report.[ 13 ] Written informed consent was received from participants before inclusion in the study. Research Ethics Committees of Changhai Hospital, Second Military Medical University, granted ethical approval for the use of human liver tumor samples. Sample sizes are reported in the figure legend.
Cells and chemicals
Hepatoma cell lines Huh7 cells, HepG2 cells, and Hepa1‐6 cells were cultured in Dulbecco's modified Eagle's medium (Hyclone; GE Lifesciences) containing 10% fetal bovine serum (Cat. 10099141; Gibco). Cells were obtained from Stem Cell Bank, Chinese Academy of Sciences, with STR authentication and detected without mycoplasma by the Myco‐Lumi Luminescent Mycoplasma Detection Kit (Cat. C0298 M; Beyotime Biotech). Holo‐Tf (Low Endotoxin, Cat. 616424) was purchased from Merck Millipore. Hemin (Cat. 51280) and deferoxamine (DFO; Cat. D9533) were purchased from Sigma‐Aldrich.
Cell transfection
Small interfering RNAs (siRNAs) and plasmids were transfected by Lipofectamine 3000 reagent (Cat. L3000015; Thermo Fisher) according to its protocols. Nonsense oligos or empty plasmids were used as negative control (NC). Forty‐eight hours following transfection, cells were used for downstream applications. The synthesis of siRNAs and plasmids was commissioned to GenePharma. The sequences of siRNAs were given in Table S1.
SLC46A1 overexpression
Hepa1‐6 cells were cocultured with lentivirus‐NC or lentivirus‐SLC46A1 for 48 h at the approximate ratio of 1:1000. After expansion to sufficient amount, the cells were treated with puromycin for 48 h to clear uninfected cells. Then the infected cells were detected for expression of SLC46A1. The lentivirus vectors, with EF‐1a promoter and resistance to puromycin, were packaged with nonsense oligos (lentivirus‐NC) or SLC46A1 messenger RNA (mRNA) (NM_026740.2, lentivirus‐SLC46A1) by GenePharma Biotech and validated by sequencing and titer determination.
Orthotopic implantation of liver tumor
Hepa1‐6 cells were mixed with Matrigel (Cat. 356237; Corning) to 5 × 107 cells/ml and kept at 4°C. Mice were anesthetized and an aseptic micro‐incision was cut along the lower edge of the left rib to show out the liver. Then 20‐μl cell suspension was slowly injected into the largest liver lobe and held for 10 s to concrete the Matrigel. If bleeding, we pressed with a gelatin sponge for 30 s and then sutured the abdominal muscles and the skin layer by layer.
Iron content determination
The intracellular iron content and tumor iron content were determined by the Iron Colorimetric Assay Kit (Cat. K390‐100; BioVision). Briefly, cells or tissues were homogenized in the Iron Assay Buffer. Then ferric iron was reduced to ferrous iron by Iron Reducer and the total ferrous iron was measured by Iron Probe. The iron content was calculated according to the standard curve.
Real‐time polymerase chain reaction
RNA was extracted from cells or tissues using Trizol reagent (Cat. 15596–026; Invitrogen) following its protocol. A total of 1 μg RNA proceeded to be reverse‐transcribed using PrimeScript RT Master Mix (Cat. RR037A; Takara). Real‐time quantitative polymerase chain reaction was performed by the SYBR Green Kit (Cat. QPK201; Toyobo Bio Inc.) using the QuantStudio 1 (Applied Biosystems). The mRNA expressions of target genes were normalized by internal reference 18 s. All primers were synthesized and high‐performance liquid chromatography–purified in Sangon Biotech. The sequences of primers are provided in Table S2.
Western blot
Total proteins were extracted by the total protein extraction kit (Keygene Biotech). Western blot assays were performed and analyzed as before.[ 22 ] Antibodies used in this study were β‐actin (Cat. ab8227; Abcam), SLC46A1 (Cat. ab25134; Abcam), STEAP3 (Cat. ab180770; Abcam), DMT1 (Cat. ab55735; Abcam), TfR1 (Cat. ab84036; Abcam), FPN (Cat. ab85370; Abcam), HAMP (Cat. ab30760; Abcam), and ferritin (Cat. ab75973; Abcam). The IRDye secondary antibody (LI‐COR) was used, and immunoblots were scanned by Odyssey dual‐color infrared fluorescence imaging system. The grayscale of each band was obtained from Odyssey software.
Staining of tissue slices
Hematoxylin and eosin (H&E) staining and immunohistochemical staining were described previously.[ 23 ] Briefly, tissues were perfused with 0.9% NaCl, fixed in 4% paraformaldehyde overnight, and embedded in paraffin. The slices were dewaxed for H&E staining. For immunohistochemical staining, slices were repaired antigen in antigen repair buffer (Cat. C1032; Solarbio Science & Technology), blocked by Normal Donkey Serum (Cat. ab7475; Abcam), and incubated by primary and secondary antibodies. The images of all slices were taken by an inverted fluorescence microscope (Leica).
Statistics
The t test was used for two‐group comparisons, and one‐way or two‐way analysis of variance was used for multigroup comparison if the data obeyed the normal distribution. The Mann–Whitney test was used for two‐group comparisons, and the Kruskal‐Wallis test was used for multigroup comparisons if the data did not obey the normal distribution. Tukey's multiple comparisons test was used for post hoc tests. The difference was significant when p < 0.05.
RESULTS
Decreased iron content and dysregulated iron‐metabolic molecules in primary liver tumor tissues of rats and mice
We used DEN to induce primary hepatocarcinoma in rats and mice to investigate changes in the contents of iron and expressions of iron‐metabolic molecules. In rats, DEN was used at 50 mg/kg intraperitoneally (i.p.) for 16 weeks, once a week. At the end of treatment, the liver presented obvious oncogenesis (Figure 1A). The serum contents of ALT, AST, and liver/body weight ratio were significantly increased (Figure 1B–D). The iron content in liver tumor tissues was significantly reduced (Figure 1E). The mRNA and protein expressions of iron‐metabolic molecules, including TFRC (TfR1), STEAP3, DMT1, SLC46A1, HAMP, FPN, and ferritin, were changed in iron‐deficient tumor tissues (Figure 1F,G). The TFRC (TfR1) was increased, and the STEAP3, DMT1, SLC46A1, HAMP, and FPN were decreased. However, the protein expression of FPN was decreased while its mRNA level was increased, in line with previous findings,[ 24 ] probably due to the post‐transcription inhibition by iron deficiency through iron‐responsive elements.[ 25 ]
FIGURE 1.
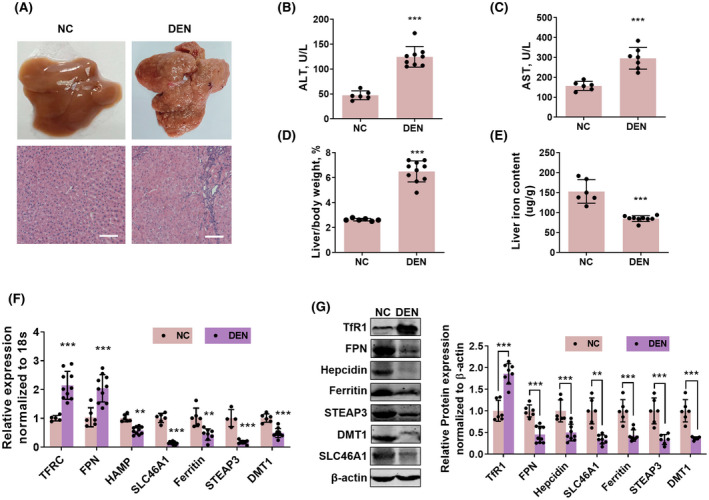
Iron content and expressions of iron‐metabolic molecules in liver tumor of rats. (A) The liver and hematoxylin and eosin (H&E) staining of liver slices of rats received intraperitoneal (i.p.) injection of 50 mg/kg DEN for 16 weeks, once a week. Scale bar: 100 μm. Serum alanine aminotransferase (ALT) content (B) and serum aspartate aminotransferase (AST) content (C), as well as liver/body weight ratio (D), were increased in rats with diethylnitrosamine (DEN)–induced liver cancer. (E) Liver iron content was decreased in rats with DEN‐induced liver cancer. Messenger RNA (mRNA) expression (F) and protein expressions of TFRC (transferrin receptor 1 [TfR1]), ferroportin (FPN), hepcidin (HAMP), ferritin, six‐transmembrane epithelial antigen of prostate 3 (STEAP3), divalent metal transporter 1 (DMT1), and SLC46A1 (G) were altered in rats with DEN‐induced liver cancer. Compared with the negative control (NC) group: **p < 0.01; ***p < 0.001; tested by unpaired t test.
In mice, we set five timepoints to analyze changes in iron‐metabolic molecules during the oncogenesis of hepatocarcinoma. DEN was given at 25 mg/kg i.p. at the age of 2 weeks of mice for one time. Not until 7 months in our experiment did the mice show tumor tissues in the liver (Figure 2A). The serum AFP level was increased from 5 months (Figure 2B), earlier than the macroscopic tumor tissues (Figure 2A) and the increases in serum ALT (Figure 2C), AST (Figure 2D), and liver/body weight ratio (Figure 2E). The liver iron contents were not significantly changed in 1 month, 3 months, and 5 months (Figure S1) but decreased at 7 months and 9 months (Figure 2F). The iron‐metabolic molecules, TFRC (TfR1), HAMP, FPN, SLC46A1, STEAP3, DMT1, and ferritin, were also changed from 7 months, both at mRNA levels (Figure 2G,H) and protein levels (Figure 2I,J). Same as in the tumor tissues of rats, the expression of TFRC (TfR1) was increased in tumor tissues compared with normal liver tissues or surrounding nontumor tissues, while the expressions of HAMP, FPN, SLC46A1, STEAP3, DMT1, and ferritin were decreased. These molecules were not significantly altered at 1 month, 3 months, and 5 months (Figure S2). Noteworthy, the earlier change in AFP level than iron content and iron‐metabolic molecules hinted that the transformation of hepatocytes to tumor cells was earlier than its dyshomeostasis of iron metabolism.
FIGURE 2.
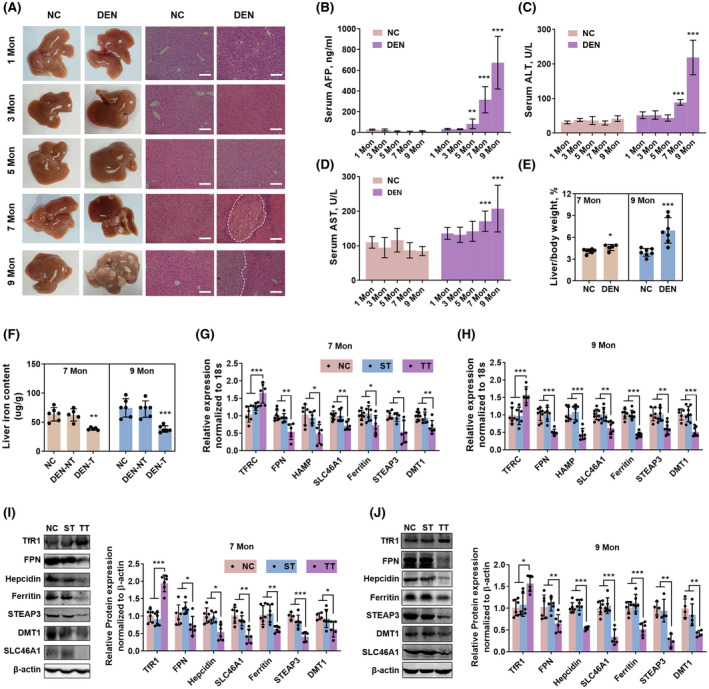
Iron content and expressions of iron‐metabolic molecules during hepatic tumorigenesis in mice. (A) The liver and H&E staining of liver slices of mice received i.p. injection of 25 mg/kg DEN at 2 weeks of age for one time. From 7 months, the liver exhibited obvious tumor tissue. Scale bar: 100 μm. Serum alpha‐fetoprotein (AFP) content (B), serum ALT content (C), and serum AST content (D) were escalated during the tumorigenesis of mice. (E) The liver/body weight ratio was increased in DEN‐injected mice at 7 months and 9 months. (F) Iron content was decreased in DEN‐induced tumor tissue (DEN‐T) compared with non‐tumor liver tissue (DEN‐NT) and liver tissue in the NC group. (G,H) mRNA expressions of TFRC, FPN, HAMP, SLC46A1, FTL, STEAP3, and DMT1 were disrupted in tumor tissues (TT) of DEN‐injected mice at 7 months and 9 months, compared with tumor surrounding tissues (ST) and normal tissues in the NC group. (I,J) Protein expressions of TfR1, FPN, HAMP, ferritin, STEAP3, DMT1, and SLC46A1 were similarly changed in TT of DEN‐injected mice at 7 months and 9 months. Compared with the NC group: *p < 0.05; **p < 0.01; ***p < 0.001; tested by one‐way analysis of variance (ANOVA) or unpaired t test.
SLC46A1, STEAP3, and DMT1 were altered iron‐independently in liver tumor tissues
In line with others' reports, our previous study confirmed that iron content was decreased in human liver tumor tissues compared with adjacent nontumor tissues (n = 29).[ 13 ] To further investigate the alterations of iron‐metabolic molecules in HCC, we first retrieved the RNA‐sequencing date of liver tumor in The Cancer Genome Atlas (TCGA) database. Iron‐related genes were classified as iron ion homeostasis, iron ion transport, regulation of iron ion transport, and response to iron ion, according to Gene Ontology functions (Figure 3A). The expression profiles of these iron‐related genes were significantly changed in tumor tissues (n = 371) compared with control tissues (n = 50), suggesting that iron metabolism was dysregulated in the liver tumor. Then we validated the protein levels of TfR1, STEAP3, DMT1, SLC46A1, HAMP, and FPN in the same batch samples of our previous study. The protein expressions of TfR1 and FPN were increased in tumor tissues (T) compared with non‐tumor tissues (NT), while the protein expressions of STEAP3, DMT1, SLC46A1, and HAMP were decreased (Figure 3B).
FIGURE 3.
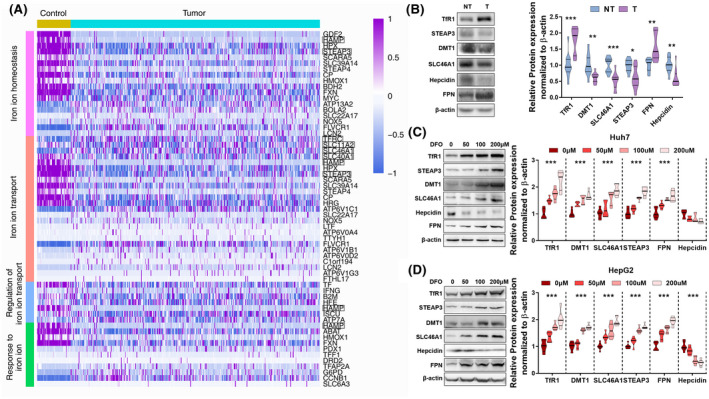
Expressions of iron‐metabolic molecules in liver cancer and iron‐deprived HCC cells. (A) Disruption of iron‐metabolic molecules expressions in liver tumor tissues (n = 371) compared with control tissues (n = 50). Data were retrieved from The Cancer Genome Atlas (TCGA). The iron‐metabolic genes with significant differences (tested by Welch's T‐test) were plotted by the R package. The genes that were validated by protein expressions were marked by rectangles. Solute carrier family 11 member 2 (SLC11A2): DMT1; solute carrier family 40 member 1 (SLC40A1): FPN. (B) Protein expressions of iron‐metabolic molecules TfR1, STEAP3, DMT1, SLC46A1, FPN, and Hepcidin in liver tumor tissues (T) compared with surrounding nontumor tissues (NT) (n = 29). (C,D) Protein expressions of TfR1, STEAP3, DMT1, SLC46A1, FPN, and Hepcidin in Huh7 (C) and HepG2 (D) cells treated by 0, 50, 100, and 200 μM iron chelator DFO for 24 h (n = 6). The changes in expressions of STEAP3, DMT1, and SLC46A1 in iron‐deprived HCC cells were opposite to those in iron‐deficient human liver tumor tissues. Compared with control group: *p < 0.05; **p < 0.01; ***p < 0.001; tested by one‐way ANOVA or unpaired t test.
To determine whether these alterations of iron‐related molecules in human liver tumor tissues were induced by iron deficiency, we tested their expressions in iron chelator–treated human liver cancer Huh7 and HepG2 cells (Figure 3C,D). Interestingly, we found that three transporters, STEAP3, DMT1, and SLC46A1, changed oppositely in iron‐deficient liver tumor tissues and iron chelator–treated hepatocarcinoma cells, as they were decreased in the former but up‐regulated in the latter. The rest of the iron‐related molecules showed the same direction of change in tumor tissues and iron‐deprived cells. Thus, these three proteins were probably the cause, rather than the result, of iron deficiency in tumor tissues.
SLC46A1, not STEAP3 or DMT1, regulated the intracellular iron content of HCC cells in vitro
Next, we observed the effects of silencing these three proteins on the intracellular iron content in Huh7 and HepG2 cells. Validation of the silencing efficacy is shown in Figure S3. The silence of STEAP3 or DMT1 did not influence the entrance of transferrin‐binding iron (Holo‐Tf), nor the combination silence of STEAP3 and DMT1 (Figure 4A). Surely, the silence of SLC46A1 did not inhibit the transport of Holo‐Tf (Figure 4B), as it is the transporter of heme.[ 21 ] The heme treatment could significantly increase intracellular iron content in human liver cancer cell lines Huh7 and HepG2, and mouse liver cancer cell line Hepa1‐6 (Figure 4C), showing that heme could be transported into the cytoplasm in hepatocarcinoma cells like hepatocytes. In this circumstance, both silence and overexpression of SLC46A1 could manipulate the intracellular iron content in Huh7, HepG2, and Hepa1‐6 cells (Figure 4D,E; validation of SLC46A1 silence or overexpression is shown in Figure S3). These results showed that the low expression of SLC46A1, rather than STEAP3 or DMT1, might lead to iron deficiency in liver tumor tissues.
FIGURE 4.
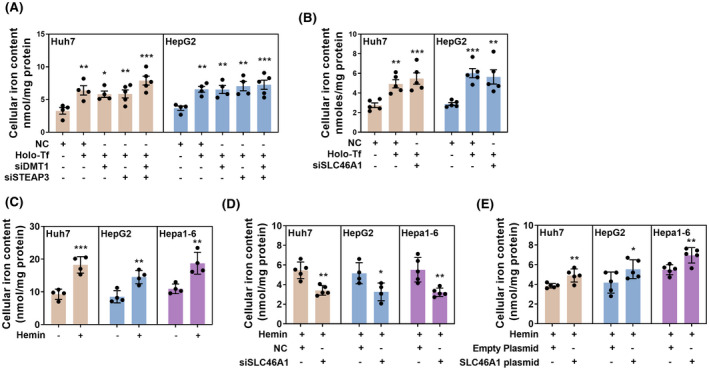
Validation of SLC46A1, STEAP3, and DMT1 in regulating intracellular iron content of HCC cells. The silencing or overexpression efficacy is shown in Figure S3. Nonsense oligos or empty plasmids were used as NC. (A) Silence of STEAP3 or DMT1 or co‐silence of them did not influence intracellular iron content in Huh7 and HepG2 cells treated with 30 μM Holo‐Tf. (B) Silence of SLC46A1 did not influence intracellular iron content in Huh7 and HepG2 cells treated with 30 μM Holo‐Tf. (C) 30 μM hemin treatment increased intracellular iron content in Huh7 and HepG2 cells and mouse liver cancer cell line Hepa1‐6 cells. (D) Silence of SLC46A1 decreased intracellular iron content in Huh7, HepG2, and Hepa1‐6 cells treated with 30 μM hemin. (I) Overexpression of SLC46A1 increased intracellular iron content in Huh7, HepG2, and Hepa1‐6 cells treated with 30 μM hemin. Compared with the NC group: *p < 0.05; **p < 0.01; ***p < 0.001; tested by one‐way ANOVA or unpaired t test.
Overexpression of SLC46A1 restored tumor iron homeostasis
To validate the role of SLC46A1 in regulating tumor iron metabolism in vivo, mouse liver cancer Hepa1‐6 cells were transfected with lentivirus‐carrying SLC46A1 protein‐coding sequence (SLC46A1). The lentivirus‐carrying nonsense sequence was used as negative control (NC). After transfection and killing untransfected cells, the cells stably overexpressing SLC46A1 were used for the next experiments (Figure 5A,B). In vitro, the SLC46A1‐overexpressed cells had more iron content in the hemin‐added medium (Figure 5C). The iron‐metabolic molecules also responded to the increase of intracellular iron content, especially the HAMP mRNA expression, in Hepa1‐6‐SLC46A1 cells compared with Hepa1‐6‐NC cells (Figure S4). In vivo, the immunohistochemistry staining of SLC46A1 exhibited weak staining in mice primary liver tumor tissues and orthotopic implantation of Hepa1‐6‐NC cells, while it was regained in tumor tissues of Hepa1‐6‐SLC46A1 cells (Figure 5D). The implanted tumor iron content (Figure 5E) was increased by the recovery of SLC46A1 expression (Figure 5F,G), followed by the changes in mRNA (Figure 5F) and protein (Figure 5G) expressions of iron‐metabolic molecules TfR1, FPN, HAMP, ferritin, STEAP3, and DMT1. These results further confirmed that SLC46A1 regulated tumor iron homeostasis, and the loss of SLC46A1 decreased tumor iron content.
FIGURE 5.
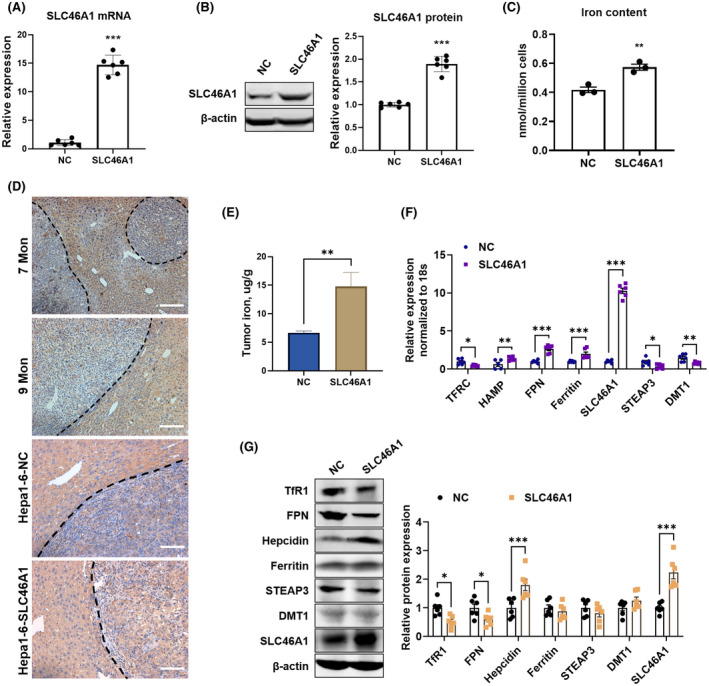
Overexpression of SLC46A1 increased tumor iron content and restored tumor iron homeostasis. mRNA expression (A) and protein expression (B) of SLC46A1 in Hepa1‐6 cells after being transfected with lentivirus‐carrying mouse SLC46A1 protein code sequence and (SLC46A1) or nonsense sequence (NC). (C) Intracellular iron content in Hepa1‐6‐SLC46A1 cells with the addition of 30 μm hemin for 2 h. (D) Immunohistochemistry staining of SLC46A1 in liver slices of mice injected with DEN at 7 months and 9 months (up) and in orthotopic implantation tumor tissues from Hepa1‐6‐NC cells and Hepa1‐6‐SLC46A1 cells (down). Scale bar: 100 μm. (E) Iron content was increased in Hepa1‐6‐SLC46A1 cell–implanted tumor tissues compared with Hepa1‐6‐NC cell–implanted tumor tissues. (F) mRNA expressions of TFRC, HAMP, FPN, ferritin, SLC46A1, STEAP3, and DMT1 in implanted tumor tissues. (G) Protein expressions of TfR1, FPN, HAMP, ferritin, STEAP3, DMT1, and SLC46A1 in implanted tumor tissues. Compared with the NC group: *p < 0.05; **p < 0.01; ***p < 0.001; tested by unpaired t test.
DISCUSSION
Iron deficiency in liver tumor tissues was noticed but not well understood in past decades. It was, to some extent, paradoxical to another reality that iron excess acted as an independent initiator or cofactor to induce and promote liver cancer. An interesting thing was that, even in iron overload–induced primary hepatocarcinoma of patients with hemochromatosis and high iron feeding rats, the iron content in tumor tissues was lower than surrounding tissues, exhibiting iron‐free neoplastic nodules.[ 10 , 11 , 12 ] Evidence in non–iron overload–induced hepatocarcinoma was more. A systematic review had indicated that iron content was lower in HCC than in surrounding tissues or normal controls.[ 26 ] Our last and present studies also showed that iron content was lower in tumor tissues than adjacent normal tissues in liver cancer[ 13 ] and in DEN‐induced hepatocarcinoma of rats and mice. Noteworthily, during the oncogenesis of our mice model, we found that the increase of AFP (at 5 months) was earlier than the changes in iron regulatory genes (at 7 months). These findings declared that iron metabolism was disturbed by the oncogenic mutation of hepatocytes.
Previous studies had observed the changes in expressions of iron regulatory genes in liver tumor tissues[ 7 , 24 ]; however, it was hard to dictate the reason for iron deficiency due to the complicated mutual regulatory pathways. For example, the TfR is the main route of iron entering cells; hence, its increase will definitely lead to more intracellular iron content. However, iron deficiency also counter‐leads to its overexpression; moreover, the increase of soluble TfR in serum is the most reliable indicator for iron deficiency anemia.[ 27 ] Thus, the up‐regulation of TfR1 and down‐regulation of HAMP in liver tumor tissues were more likely the results of iron deficiency. In 2009, two independent groups reported that expressions of STEAP3 and DMT1 were altered independently of iron status in liver tumor tissues.[ 7 , 9 ] Unfortunately, subsequent studies on DMT1 knockout[ 28 ] and STEAP3 knockout[ 29 ] mice indicated that their absences did not affect iron content in the liver. Our in vitro results also supported these findings. Recently, we reported that SLC46A1 was iron‐responsive in the liver, shown to be increased by iron deficiency and decreased by iron overload.[ 21 ] However, its expression in liver tumor tissues was also decreased independently by iron deficiency. The recombinant adeno‐associated virus–mediated silence decreased the liver iron content in vivo.[ 21 ] In liver cancer cells, both silence and overexpression of SLC46A1 could result in corresponding changes in intracellular iron content. Moreover, its overexpression effectively increased the iron content of implanted tumors. Considering the loss of its expression in liver tumor tissues of patients, rats and mice, it was reasonable to propose that its down‐regulation led to iron deficiency in liver tumor tissues.
HAMP, together with FPN, control iron homeostasis in the body. In the normal physiological condition, it is predominantly produced in hepatocytes. However, its expression was down‐regulated in liver tumor tissues, while increased in tumor tissues of almost all other cancer types (Figure S5, retrieved from TCGA database by GEPIA2[ 30 ]). This difference was in line with the decreased tumor iron content in liver tumor tissues but increased in tumor tissues of breast cancer,[ 31 , 32 , 33 , 34 ] lung cancer,[ 35 , 36 ] prostate cancer,[ 37 , 38 ] colorectal cancer,[ 39 , 40 , 41 ] and so on. Iron deficiency inhibits the production and secretion of HAMP to disable the degradation of FPN, thus increasing the absorption of exogenous iron into the body.[ 14 ] Also, iron deficiency decreases the translation of FPN mRNA through its 5′ UTR iron‐response element to achieve a dynamic balance of FPN expression.[ 42 ] Therefore, the down‐regulation of HAMP in liver tumor tissues and up‐regulation of HAMP in other types of tumor tissues were more likely the responses to iron conditions. The expression profiles of FPN, both at mRNA level and protein level, were not totally coincident in liver tumor tissues of patients,[ 7 , 43 ] rats,[ 24 ] and mice,[ 44 ] suggesting different regulatory mechanisms of FPN in the oncogenesis of different species. Importantly, as recorded by TCGA, the down‐regulation of HAMP was correlated with poor prognosis, implying potential influences of iron deficiency on liver cancer progressions.[ 13 ] In addition, the HAMP‐FPN axis, the main regulators of iron homeostasis in vivo, was identified to influence tumor malignancy, progress and prognosis, which highlighted the importance of iron metabolism in tumor biology.
In our last study, we found that iron deficiency promoted pulmonary metastasis of liver cancer through immunosuppression and immune‐independent mechanisms.[ 13 , 45 ] Indeed, iron deficiency led to inhibition of the immune system, especially the T cells' immune responses.[ 46 ] The observations on patients with iron deficiency anemia and iron‐deficient mice demonstrated the decreased amounts and the inhibited maturation of immune cells, as well as the reduced synthesis of cytokines.[ 47 , 48 , 49 ] The immune‐independent mechanism of metastasis probably involved the decreased degradation of hypoxia inducible factor 1 alpha subunit protein by iron deficiency, which is important for epithelial–mesenchymal transition, migration, and invasion of cancer cells.[ 50 ] In the present study, we showed that the loss of SLC46A1 in HCC tissues led to the decreased tumor iron content, which was restored by SLC46A1 overexpression. Therefore, it is worthwhile to further investigate the roles and mechanisms of rebalance of iron homeostasis in HCC metastasis and the effective measures targeting tumor iron metabolism, including SLC46A1 manipulation.
CONCLUSIONS
The results of the present study revealed the dysregulated iron metabolism in HCC tissues and the key role of SLC46A1 in the tumor iron metabolism of HCC. Loss of SLC46A1 expression decreased the tumor iron content, while its overexpression effectively restored the iron homeostasis of liver cancer. The role of targeting iron homeostasis by SLC46A1 in HCC metastasis and the functional mechanisms need to be further explored.
ACKNOWLEDGMENT
This work was supported by the National Natural Science Foundation of China (81903306, to Yuxiao Tang; 82003711, to Dongyao Wang), the Shanghai Sailing Program (19YF1459400, to Dongyao Wang), and the Shanghai Natural Science Foundation (19ZR1469700, to Yuxiao Tang).
AUTHOR CONTRIBUTIONS
Experiments and manuscript draft: Dr. Dongyao Wang, Ms. Huiwen Wu, and Mr. Jianxin Yang. Experiment assistance: Mr. Zelong Gao. Technical and material support: Dr. Min Li and Dr. Hongtao Lu. Clinical samples and manuscript revisions: Dr. Changquan Ling. Data interpretation and critical revision of the manuscript: Dr. Hui Shen. Study proposal and design: Dr. Yuxiao Tang.
FUNDING INFORMATION
Supported by the National Natural Science Foundation of China (81903306 and 82003711), the Shanghai Sailing Program (19YF1459400), and the Shanghai Natural Science Foundation (19ZR1469700).
CONFLICT OF INTEREST
Nothing to report.
Supporting information
Appendix S1 Supporting Information
Wang D, Wu H, Yang J, Li M, Ling C, Gao Z, et al. Loss of SLC46A1 decreases tumor iron content in hepatocellular carcinoma. Hepatol Commun. 2022;6:2914–2924. 10.1002/hep4.2031
Dongyao Wang, Huiwen Wu, and Jianxin Yang contributed equally to this work.
Contributor Information
Hui Shen, Email: shenhui@smmu.edu.cn.
Yuxiao Tang, Email: tangyuxiao@smmu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen X, Kang R, Kroemer G, Tang D. Targeting ferroptosis in pancreatic cancer: a double‐edged sword. Trends Cancer. 2021;7:891–901. [DOI] [PubMed] [Google Scholar]
- 3. Torti SV, Torti FM. Iron: the cancer connection. Mol Aspects Med. 2020;75:100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jungst C, Cheng B, Gehrke R, Schmitz V, Nischalke HD, Ramakers J, et al. Oxidative damage is increased in human liver tissue adjacent to hepatocellular carcinoma. Hepatology. 2004;39:1663–72. [DOI] [PubMed] [Google Scholar]
- 5. Park KS, Kim H, Kim NG, Cho SY, Choi KH, Seong JK, et al. Proteomic analysis and molecular characterization of tissue ferritin light chain in hepatocellular carcinoma. Hepatology. 2002;35:1459–66. [DOI] [PubMed] [Google Scholar]
- 6. Sakurai K, Sohda T, Ueda S, Tanaka T, Hirano G, Yokoyama K, et al. Immunohistochemical demonstration of transferrin receptor 1 and 2 in human hepatocellular carcinoma tissue. Hepatogastroenterology. 2014;61:426–30. [PubMed] [Google Scholar]
- 7. Tan MG, Kumarasinghe MP, Wang SM, Ooi LL, Aw SE, Hui KM. Modulation of iron‐regulatory genes in human hepatocellular carcinoma and its physiological consequences. Exp Biol Med (Maywood). 2009;234:693–702. [DOI] [PubMed] [Google Scholar]
- 8. Zhou XD, DeTolla L, Custer RP, London WT. Iron, ferritin, hepatitis B surface and core antigens in the livers of Chinese patients with hepatocellular carcinoma. Cancer. 1987;59:1430–7. [DOI] [PubMed] [Google Scholar]
- 9. Youn P, Kim S, Ahn JH, Kim Y, Park JD, Ryu DY. Regulation of iron metabolism‐related genes in diethylnitrosamine‐induced mouse liver tumors. Toxicol Lett. 2009;184:151–8. [DOI] [PubMed] [Google Scholar]
- 10. Asare GA, Paterson AC, Kew MC, Khan S, Mossanda KS. Iron‐free neoplastic nodules and hepatocellular carcinoma without cirrhosis in Wistar rats fed a diet high in iron. J Pathol. 2006;208:82–90. [DOI] [PubMed] [Google Scholar]
- 11. Deugnier YM, Guyader D, Crantock L, Lopez JM, Turlin B, Yaouanq J, et al. Primary liver cancer in genetic hemochromatosis: a clinical, pathological, and pathogenetic study of 54 cases. Gastroenterology. 1993;104:228–34. [DOI] [PubMed] [Google Scholar]
- 12. Deugnier YM, Charalambous P, Le Quilleuc D, Turlin B, Searle J, Brissot P, et al. Preneoplastic significance of hepatic iron‐free foci in genetic hemochromatosis: a study of 185 patients. Hepatology. 1993;18:1363–9. [PubMed] [Google Scholar]
- 13. Li M, Tang Y, Wang D, Zhai X, Shen H, Zhong C, et al. Sphingosine‐1‐phosphate transporter spinster homolog 2 is essential for iron‐regulated metastasis of hepatocellular carcinoma. Mol Ther. 2022;30:703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Billesbolle CB, Azumaya CM, Kretsch RC, Powers AS, Gonen S, Schneider S, et al. Structure of hepcidin‐bound ferroportin reveals iron homeostatic mechanisms. Nature. 2020;586:807–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawabata H. Transferrin and transferrin receptors update. Free Radic Biol Med. 2019;133:46–54. [DOI] [PubMed] [Google Scholar]
- 16. Shayeghi M, Latunde‐Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, et al. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. [DOI] [PubMed] [Google Scholar]
- 17. Sid V, Siow YL, Shang Y, Woo CW, O K. High‐fat diet consumption reduces hepatic folate transporter expression via nuclear respiratory factor‐1. J Mol Med (Berl). 2018;96:1203–13. [DOI] [PubMed] [Google Scholar]
- 18. Le Blanc S, Garrick MD, Arredondo M. Heme carrier protein 1 transports heme and is involved in heme‐Fe metabolism. Am J Physiol Cell Physiol. 2012;302:C1780–E1785. [DOI] [PubMed] [Google Scholar]
- 19. Radziejewska A, Suliburska J, Kolodziejski P, Chmurzynska A. Simultaneous supplementation with iron and folic acid can affect Slc11a2 and Slc46a1 transcription and metabolite concentrations in rats. Br J Nutr. 2020;123:264–72. [DOI] [PubMed] [Google Scholar]
- 20. Zhao R, Aluri S, Goldman ID. The proton‐coupled folate transporter (PCFT‐SLC46A1) and the syndrome of systemic and cerebral folate deficiency of infancy: hereditary folate malabsorption. Mol Aspects Med. 2017;53:57–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li H, Wang D, Wu H, Shen H, Lv D, Zhang Y, et al. SLC46A1 contributes to hepatic iron metabolism by importing heme in hepatocytes. Metabolism. 2020;110:154306. [DOI] [PubMed] [Google Scholar]
- 22. Tang Y, Wang D, Zhang H, Zhang Y, Wang J, Qi R, et al. Rapid responses of adipocytes to iron overload increase serum TG level by decreasing adiponectin. J Cell Physiol. 2021;236:7544–53. [DOI] [PubMed] [Google Scholar]
- 23. Tang Y, Wang D, Niu X, Wu H, Yang J, Zhang Y, et al. Mild iron overload induces TRIP12‐mediated degradation of YY1 to trigger hepatic inflammation. Free Radic Biol Med. 2020;161:187–97. [DOI] [PubMed] [Google Scholar]
- 24. Holmstrom P, Gafvels M, Eriksson LC, Dzikaite V, Hultcrantz R, Eggertsen G, et al. Expression of iron regulatory genes in a rat model of hepatocellular carcinoma. Liver Int. 2006;26:976–85. [DOI] [PubMed] [Google Scholar]
- 25. Wang L, Liu X, You LH, Ci YZ, Chang S, Yu P, et al. Hepcidin and iron regulatory proteins coordinately regulate ferroportin 1 expression in the brain of mice. J Cell Physiol. 2019;234:7600–7. [DOI] [PubMed] [Google Scholar]
- 26. Gurusamy K. Trace element concentration in primary liver cancers—a systematic review. Biol Trace Elem Res. 2007;118:191–206. [DOI] [PubMed] [Google Scholar]
- 27. Naess‐Andresen ML, Eggemoen AR, Berg JP, Falk RS, Jenum AK. Serum ferritin, soluble transferrin receptor, and total body iron for the detection of iron deficiency in early pregnancy: a multiethnic population‐based study with low use of iron supplements. Am J Clin Nutr. 2019;109:566–75. [DOI] [PubMed] [Google Scholar]
- 28. Wang CY, Knutson MD. Hepatocyte divalent metal‐ion transporter‐1 is dispensable for hepatic iron accumulation and non‐transferrin‐bound iron uptake in mice. Hepatology. 2013;58:788–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang F, Tao Y, Zhang Z, Guo X, An P, Shen Y, et al. Metalloreductase Steap3 coordinates the regulation of iron homeostasis and inflammatory responses. Haematologica. 2012;97:1826–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large‐scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47:W556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang S, Chen Y, Guo W, Yuan L, Zhang D, Xu Y, et al. Disordered hepcidin‐ferroportin signaling promotes breast cancer growth. Cell Signal. 2014;26:2539–50. [DOI] [PubMed] [Google Scholar]
- 32. Higgy NA, Salicioni AM, Russo IH, Zhang PL, Russo J. Differential expression of human ferritin H chain gene in immortal human breast epithelial MCF‐10F cells. Mol Carcinog. 1997;20:332–9. [DOI] [PubMed] [Google Scholar]
- 33. Rehman S, Husnain SM. A probable risk factor of female breast cancer: study on benign and malignant breast tissue samples. Biol Trace Elem Res. 2014;157:24–9. [DOI] [PubMed] [Google Scholar]
- 34. Mannello F, Tonti GA, Medda V, Simone P, Darbre PD. Analysis of aluminium content and iron homeostasis in nipple aspirate fluids from healthy women and breast cancer‐affected patients. J Appl Toxicol. 2011;31:262–9. [DOI] [PubMed] [Google Scholar]
- 35. Chen Q, Wang L, Ma Y, Wu X, Jin L, Yu F. Increased hepcidin expression in non‐small cell lung cancer tissue and serum is associated with clinical stage. Thorac Cancer. 2014;5:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muller CI, Miller CW, Kawabata H, McKenna RJ Jr, Marchevsky AM, Koeffler HP. Do cancer cells selectively mutate HFE to increase their intracellular iron? Oncol Rep. 2005;14:299–303. [PubMed] [Google Scholar]
- 37. Chen Y, Zhang Z, Yang K, Du J, Xu Y, Liu S. Myeloid zinc‐finger 1 (MZF‐1) suppresses prostate tumor growth through enforcing ferroportin‐conducted iron egress. Oncogene. 2015;34:3839–47. [DOI] [PubMed] [Google Scholar]
- 38. Sarafanov AG, Todorov TI, Centeno JA, Macias V, Gao W, Liang WM, et al. Prostate cancer outcome and tissue levels of metal ions. Prostate. 2011;71:1231–8. [DOI] [PubMed] [Google Scholar]
- 39. Brookes MJ, Hughes S, Turner FE, Reynolds G, Sharma N, Ismail T, et al. Modulation of iron transport proteins in human colorectal carcinogenesis. Gut. 2006;55:1449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Han HS, Lee SY, Seong MK, Kim JH, Sung IK, Park HS, et al. Presence of iron in colorectal adenomas and adenocarcinomas. Gut Liver. 2008;2:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rinaldi L, Barabino G, Klein JP, Bitounis D, Pourchez J, Forest V, et al. Metals distribution in colorectal biopsies: new insight on the elemental fingerprint of tumour tissue. Dig Liver Dis. 2015;47:602–7. [DOI] [PubMed] [Google Scholar]
- 42. Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. [DOI] [PubMed] [Google Scholar]
- 43. Kijima H, Sawada T, Tomosugi N, Kubota K. Expression of hepcidin mRNA is uniformly suppressed in hepatocellular carcinoma. BMC Cancer. 2008;8:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shen Y, Li X, Zhao B, Xue Y, Wang S, Chen X, et al. Iron metabolism gene expression and prognostic features of hepatocellular carcinoma. J Cell Biochem. 2018;119:9178–204. [DOI] [PubMed] [Google Scholar]
- 45. Wang D, Li M, Shen H, Yang J, Gao Z, Tang Y. Iron deficiency increases phosphorylation of SP1 to upregulate SPNS2 expression in hepatocellular carcinoma. Biol Trace Elem Res. 2022. May 26. 10.1007/s12011-022-03296-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46. Pfeifhofer‐Obermair C, Tymoszuk P, Petzer V, Weiss G, Nairz M. Iron in the tumor microenvironment—connecting the dots. Front Oncol. 2018;8:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bonaccorsi‐Riani E, Danger R, Lozano JJ, Martinez‐Picola M, Kodela E, Mas‐Malavila R, et al. Iron deficiency impairs intra‐hepatic lymphocyte mediated immune response. PLoS One. 2015;10:e0136106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jabara HH, Boyden SE, Chou J, Ramesh N, Massaad MJ, Benson H, et al. A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency. Nat Genet. 2016;48:74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kuvibidila SR, Velez M, Gardner R, Penugonda K, Chandra LC, Yu L. Iron deficiency reduces serum and in vitro secretion of interleukin‐4 in mice independent of altered spleen cell proliferation. Nutr Res. 2012;32:107–15. [DOI] [PubMed] [Google Scholar]
- 50. Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352:175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


