Abstract
All‐trans retinoic acid (AtRA) is an active metabolite of vitamin A that influences many biological processes in development, differentiation, and metabolism. AtRA functions through activation of retinoid acid receptors (RARs). AtRA is shown to ameliorate hepatic steatosis, but the underlying mechanism is not well understood. In this study, we investigated the role of hepatocyte RAR alpha (RARα) in mediating the effect of AtRA on hepatosteatosis in mice. Hepatocyte‐specific Rarα −/− (L‐Rarα −/− ) mice and their control mice were fed a chow diet, high‐fat diet (HFD), or a high‐fat/cholesterol/fructose (HFCF) diet. Some of the mice were also treated with AtRA. Loss of hepatocyte RARα‐induced hepatosteatosis in chow‐fed aged mice and HFD‐fed mice. AtRA prevented and reversed HFCF diet–induced obesity and hepatosteatosis in the control mice but not in L‐Rarα −/− mice. Furthermore, AtRA reduced hepatocyte fatty acid uptake and lipid droplet formation, dependent on hepatocyte RARα. Our data suggest that hepatocyte RARα plays an important role in preventing hepatosteatosis and mediates AtRA's effects on diet‐induced hepatosteatosis.
Loss of hepatocyte retinoic acid receptor alpha (RARa) induces liver steatosis in aged mice or high fat diet‐fed mice. All‐trans retinoic acid attenuates diet‐induced hepatosteaosis largely dependent on activation of hepatocyte RARa.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the world. NAFLD constitutes a spectrum of conditions ranging from nonalcoholic fatty liver or simple steatosis to nonalcoholic steatohepatitis, which may further progress to cirrhosis and hepatocellular carcinoma. So far, the underlying mechanism for the pathogenesis of NAFLD is not fully understood. NAFLD is often associated with insulin resistance and is the hepatic manifestation of metabolic syndrome. During insulin resistance, hepatic de novo lipogenesis and fatty acid uptake are increased to induce hepatosteatosis and lipotoxic lipid accumulation, which may further cause liver inflammation and fibrogenesis.[ 1 , 2 , 3 ]
Vitamin A is important for many biological processes, such as embryogenesis, cell proliferation and differentiation, vision, immune regulation, and metabolism.[ 4 ] Patients with NAFLD have often shown disrupted vitamin A homeostasis.[ 4 , 5 , 6 ] The liver harbors the most vitamin A in hepatic stellate cells (HSCs). When HSCs are activated, ‐they lose the retinyl ester stores, leading to fibrosis. Vitamin A exerts its functions through active metabolites—all trans retinoic acid (AtRA) and 9‐cis retinoic acid (9‐cis‐RA), which activate the nuclear hormone receptors retinoic acid receptors (RARα, RARβ, and RARγ) and retinoid X receptors (RXRα, RXRβ, and RXRγ), respectively. RARs heterodimerize with RXR and bind to the DNA motifs known as retinoic acid response elements (RAREs) to regulate the expression of genes involved in cell growth or differentiation.
AtRA has been shown to reduce liver steatosis via a pathway involving peroxisome proliferator‐activated receptor gamma (PPARγ) repression in mice,[ 7 ] and improves liver steatosis in rabbits.[ 8 ] Treatment of KK‐A y mice or high‐fat/high fructose diet–fed mice with AtRA has been shown to ameliorate insulin resistance.[ 9 ] Despite these beneficial effects of AtRA, it remains unclear how AtRA improves hepatosteatosis. In addition, the role of genetic loss of hepatocyte RARα in fatty liver disease has not been investigated. In this study, we investigated the role of loss of hepatocyte RARα in high‐fat/cholesterol/fructose (HFCF) diet–induced hepatosteatosis and in mediating AtRA‐induced amelioration of fatty liver disease. Our data show that AtRA treatment prevents and reverses HFCF diet–induced hepatosteatosis via hepatocyte RARα‐dependent regulation of lipid droplet formation and fatty acid uptake.
MATERIALS AND METHODS
Mice
The floxed Rarα (Rarαfl/fl) mice (on a C57BL/6 background) were generously gifted by Dr. Yasmine Balkaid (National Institutes of Health/National Institute of Allergy and Infectious Diseases) and have been previously described by Dr. Chambon and colleagues.[ 10 ] Albumin cre (Alb‐Cre) mice were purchased from the Jackson Laboratory. Rarαfl/fl mice were crossed with Alb‐cre mice to generate germline hepatocyte‐specific Rarα−/− (gL‐Rarα−/−) and control littermates (Rarαfl/fl mice). AAV8‐TBG‐Null or AAV8‐TBG‐Cre was intravenously injected to 8‐week‐old male Rarαfl/fl mice to generate control mice (Rarαfl/fl) or adult‐onset hepatocyte‐specific Rarα−/− (L‐Rarα−/−) mice, respectively. Some of the mice were also gavaged with either vehicle (0.5% carboxymethyl cellulose) or AtRA (15 mg/kg/day).[ 7 ] All mice were housed in a temperature and humidity‐controlled room with a 12‐h light/12‐h dark cycle under pathogen‐free conditions. Mice are fasted for 5–6 h during the light cycle before euthanasia. All animal experiments were approved by the Institutional Animal Care and Use Committee at Northeast Ohio Medical University.
Diets
The HFCF diet contained 40% fat, 0.2% cholesterol (AIN‐76A Western diet from TestDiet), and 4.2% fructose (in drinking water). Eight‐week‐old male mice were used and fed an HFCF diet for 16 or 20 weeks. The high‐fat diet (HFD) containing 60% kcal from fat was purchased from Research Diets (Cat. #D12492). Eight‐week‐old mice were used and fed an HFD for 20 weeks.
Adeno‐associated viruses
Adeno‐associated virus 8 (AAV8)–TBG‐null (control) and AAV8‐TBG‐Cre were produced and titrated by Vector BioLabs. Each mouse was intravenously injected with 3 × 1011 genome copies of AAVs.
Primary hepatocyte isolation and culture
Mouse primary hepatocytes were isolated as described.[ 11 ] Mice were anesthetized by intraperitoneal injection of Xylazine/Ketamine. The portal vein was cannulated with a 23‐gauge plastic cannula. Mouse livers were perfused with Hank's Balanced Salt Solution (HBSS; 14170112; Thermo Fisher Scientific). Subsequently, livers were perfused with HBSS with calcium and magnesium (14,025,092; Thermo Fisher Scientific) containing 0.8 mg/ml collagenase from Clostridium histolyticum type IV (Sigma). Primary hepatocytes were released and collected in a 50‐ml centrifuge tube. After centrifugation at 50 g for 3 min and washing with Dulbecco's modified Eagle's medium (DMEM), cells were cultured in DMEM plus 10% fetal bovine serum (FBS) in plates or 6‐well dishes precoated with 0.1% gelatin. Primary hepatocytes were cultured in DMEM containing vehicle or lipid mixture (100 μm palmitate, 100 μm oleic acid, 100 μm linoleic acid, and 1 μg/ml cholesterol). After 24 or 48 h, messenger RNA (mRNA) levels and intracellular lipid levels were determined.
Real‐time polymerase chain reaction
Total RNA was isolated using Trizol Reagent (Invitrogen), and mRNA levels were quantified by quantitative real‐time PCR (PCR) using PowerUP SYBR Green Master Mix (Thermo Fisher) on a 7500 Real‐Time PCR machine (Applied Biosystems). Results were calculated using cycle threshold values and normalized to 36B4 mRNA level.
Analysis of intracellular and hepatic lipids
About 100‐mg livers were homogenized in methanol, and lipids in the livers or hepatocytes were extracted in chloroform/methanol (2:1 vol/vol) as described previously.[ 12 ] Hepatic and intracellular triglyceride and total cholesterol levels were quantified using Infinity reagents from Thermo Fisher Scientific. Free cholesterol and nonesterified free fatty acids (NEFAs) were determined using a kit from Wako Chemicals USA.
Analysis of plasma aspartate aminotransferase, alanine aminotransferase, and lipids and hepatic hydroxyproline
Plasma triglycerides, cholesterol, glucose, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were determined using Infinity reagents from Thermo Scientific. NEFAs were determined using a kit from Wako Chemicals USA. About 40–50 mg of the liver was homogenized in distilled water and hydroxyproline levels in the liver were quantified using a kit from Cell BioLabs (Cat. #STA675).
Oil Red O, hematoxylin and eosin, or picrosirius red staining
Liver tissues were fixed in 10% formalin and then embedded in optimal cutting temperature or paraffin. Liver sections were stained with Oil Red O (ORO), hematoxylin and eosin (H&E), or picrosirius red. Images were acquired using an Olympus microscope.
Body composition analysis and energy expenditure
Mouse body fat mass was measured by Echo‐MRI‐700 (Echo Medical Systems). Oxygen consumption, carbon dioxide production, heat production, and physical activity were determined in a Comprehensive Lab Animal Monitoring System using Columbus Instruments hardware and Oxymax software (Columbus Instruments) as detailed previously.[ 13 ] In brief, mice underwent an acclimation period, and a 24‐h measurement of energy expenditure was determined using an eight‐chamber system. Each run included two genotypes and two treatments with two mice per group.
Fatty acid oxidation
Primary hepatocytes were isolated, and then cultured in DMEM containing 10% FBS in 12‐well dishes in the presence of vehicle or AtRA (2 μm). After 48 h, the media were removed and washed with 1× phosphate‐buffered saline. The cells were then cultured in DMEM containing 0.5% fatty acid–free BSA, 0.5 μCi [3H]palmitate, and 500 μm cold palmitate. Radioactivity was measured and fatty acid oxidation (FAO) was determined as described previously.[ 14 , 15 ]
Fatty acid uptake
Primary hepatocytes were isolated and plated at 5 × 104 cells per well in 96‐well plates. Cells were incubated in DMEM plus 10% FBS in the presence of vehicle or AtRA (4 μm) overnight. Before the assay, hepatocytes were fasted 2 h by changing to a serum‐free media. Fatty acid uptake was measured using a QBT fatty acid uptake assay kit (Molecular Devices) by reading the plate at 485 nm excitation/535 nm emission. The end‐point fatty acid uptake after 1 h was determined as described.[ 16 ]
Hepatic lipogenesis
Mice were fasted for 2 h and then injected intraperitoneally with 2H2O (20–30 μl/g). After 4 h, liver and plasma were snap‐frozen. The newly synthesized triglycerides were measured by gas chromatography–mass spectrometry as described.[ 17 ]
Intestinal fat absorption and hepatic very‐low density lipoprotein secretion
To determine intestinal fat absorption, mice were fasted for 6 h, and then intravenously injected with 100 μl Tyloxapol (500 mg/kg). Mice were then gavaged with olive oil (15 μl/g body weight) and plasma triglyceride (TG) levels were determined at defined time points as described previously.[ 18 , 19 ] For the VLDL secretion assay, mice were fasted for 6 h and then intravenously injected with 100–200 μl of Tyloxapol (500 mg/kg). Blood was collected at indicated time points and plasma TG levels were determined.
Statistical analysis
All data were expressed as mean ± SEM. Statistical significance was analyzed using an unpaired Student t test or analysis of variance (ANOVA; for more than two groups) by using GraphPad Prism (GraphPad Software). Differences were considered statistically significant at p < 0.05.
RESULTS
RARα is required to protect against intracellular lipid accumulation in hepatocytes
To determine the role of hepatocyte RARα in lipid metabolism, we isolated primary hepatocytes from Rarα fl/fl mice and mice with germline deficiency of Rarα in hepatocytes (gL‐Rarα −/− ), and treated them with a lipid cocktail of free fatty acids and cholesterol because they are known to be elevated in NAFLD. Loss of hepatocyte Rarα significantly increased cellular levels of TG (Figure 1A), NEFAs (Figure 1B), total cholesterol (TC), and free cholesterol (FC) (Figure 1C). Consistent with these findings, quantitative real‐time PCR data show that some genes were induced by Rarα deficiency, including genes involved in lipogenesis (peroxisome proliferator‐activated receptor γ1 (Pparγ1), Pparγ2, acetyl‐CoA carboxylase 1 (Acc1), Acc2) or lipid droplet formation (cell death–inducing DNA fragmentation factor‐like effector A [Cidea], Cidec, perilipin 4 [Plin4]), or inflammation (tumor necrotic factor α (Tnfα)) (Figure 1D). Rarα deficiency inhibited the expression of peroxisome proliferator‐activated receptor β/δ (Pparβ/δ), but had no impact on the expression of genes involved in lipolysis, such as adipose triglyceride lipase (Atgl) (Figure 1D).
FIGURE 1.
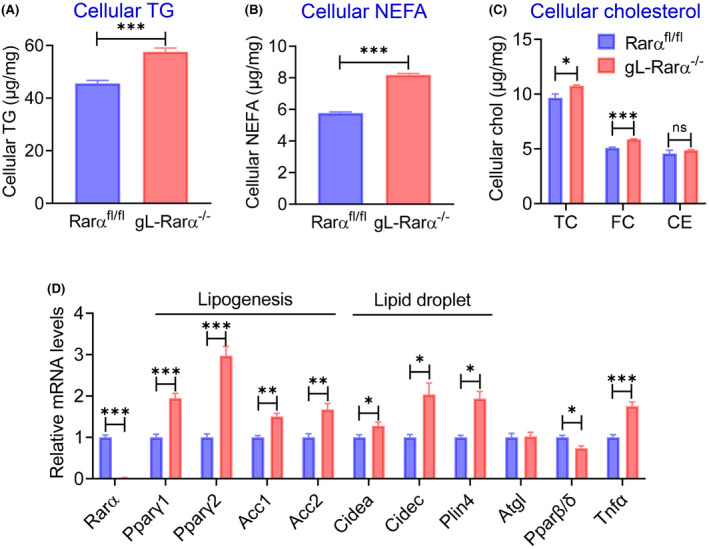
Retinoid acid receptor alpha (RARα) is required to protect against intracellular lipid accumulation in hepatocytes. Primary hepatocytes were isolated from Rarα fl/fl mice and mice with germline deficiency in Rarα (gL‐Rarα −/− ) and cultured in the presence of lipids (100 μm oleic acid, linoleic acid, palmitic acid, and 1 μg/ml cholesterol) for 48 h (intracellular lipids) and 24 h (messenger RNA [mRNA]). Intracellular levels of triglycerides (TG) (A), nonesterified fatty acids (NEFAs) (B), total cholesterol (TC), free cholesterol (FC), and cholesterol esters (CE) (C) were measured. (D) mRNA levels were determined. All values are expressed as mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001. Acc1, acetyl‐CoA carboxylase 1; Atgl, adipose triglyceride lipase; Chol, cholesterol; ns, not significant; Cidea, cell death–inducing DNA fragmentation factor‐like effector A; Plin4, perilipin 4; PPARγ, peroxisome proliferator‐activated receptor gamma; Tnfα, tumor necrosis factor alpha.
Germline loss of hepatocyte RARα induces hepatosteatosis in chow‐fed aged mice
To determine whether hepatocyte RARα affected the development of NAFLD on a chow diet, we studied 1‐year‐old male Rarα fl/fl mice versus gL‐Rarα −/− mice. Compared with Rarα fl/fl mice, gL‐Rarα −/− mice had significantly higher body weight, body fat content (%), and plasma NEFA levels, whereas the ratio of the liver to body weight and plasma levels of glucose, TG, or cholesterol were unchanged (Figure S1A–G).
Surprisingly, gL‐Rarα −/− mice had higher plasma AST and ALT levels (Figure 2A), indicative of liver injury in these mice (Figure 2A). gL‐Rarα −/− mice also had higher levels of TG (Figure 2B) and NEFA (Figure 2C) in the liver, whereas hepatic TC or FC was unchanged despite a subtle increase in cholesterol ester (CE) levels (Figure 2D). Histological staining with Oil Red O or H&E showed increased hepatosteatosis in gL‐Rarα −/− mice (Figure 2E). Consistent with the increased hepatic lipid accumulation, hepatic mRNA levels involved in lipogenesis (sterol regulatory element binding protein 1c [Srebp1c], Acc1, Acc2, ATP‐citrate lyase (Acly)) and lipid droplet formation (Cidea, Plin4) were significantly increased (Figure 2F). Hepatic Cd36 and diacylglycerol acyltransferase 1 (Dgat1) also tended to increase (Figure 2F). Thus, germline loss of hepatocyte RARα is sufficient to induce hepatosteatosis on a chow diet.
FIGURE 2.
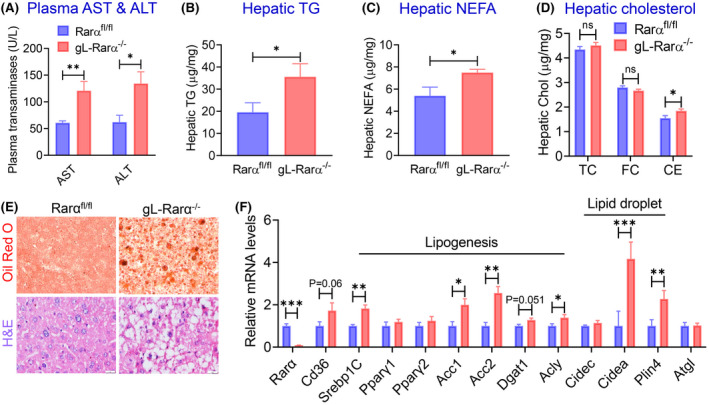
Germline loss of hepatocyte RARα induces hepatosteatosis in chow‐fed aged mice. One‐year‐old male Rarα fl/fl mice and gL‐Rarα −/− male mice were fed a chow diet (n = 7–8). Plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels (A) as well as hepatic levels of TG (B), NEFA (C), TC, FC, and CE were determined. (E) Representative liver images of Oil Red O staining (upper panels) and hematoxylin and eosin (H&E) staining (lower panels) (scale bars = 20 μm). (F) Hepatic mRNA levels were quantified. All values are expressed as mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001. ns, not significant. Acly, ATP citrate lyase; Cd36, cluster of differentiation 36; Dgat1, diacylglycerol o‐acyltransferase 1; Srebp1C, sterol regulatory element‐binding protein 1C.
Germline loss of hepatocyte RARα induces hepatosteatosis in HFD‐fed mice
Next, we fed 8‐week‐old male Rarα fl/fl mice and gL‐Rarα −/− mice a HFD (60% kcal from fat) or a chow diet for 20 weeks. In HFD‐fed mice we observed a modest increase in the plasma NEFA levels in gL‐Rarα −/− mice, but there was no change in body weight, body fat content, the ratio of liver to body weight, hepatic cholesterol (TC, FC, and CE), or plasma levels of glucose, TG, or TC between Rarα fl/fl mice and gL‐Rarα −/− mice (Figure S2A–H). However, there was a > 50% increase in plasma AST and ALT levels (Figure 3A,B), hepatic TG (Figure 3C), and hepatic NEFA (Figure 3D) levels in HFD‐fed gL‐Rarα −/− mice compared with HFD‐fed Rarα fl/fl mice with no changes observed in the age‐matched chow‐fed mice (Figure 3A–D). The increase in hepatic TG accumulation was also confirmed by ORO staining and H&E staining in HFD‐fed mice with no significant changes in ORO or H&E staining in chow‐fed mice (Figure 3E,F).
FIGURE 3.
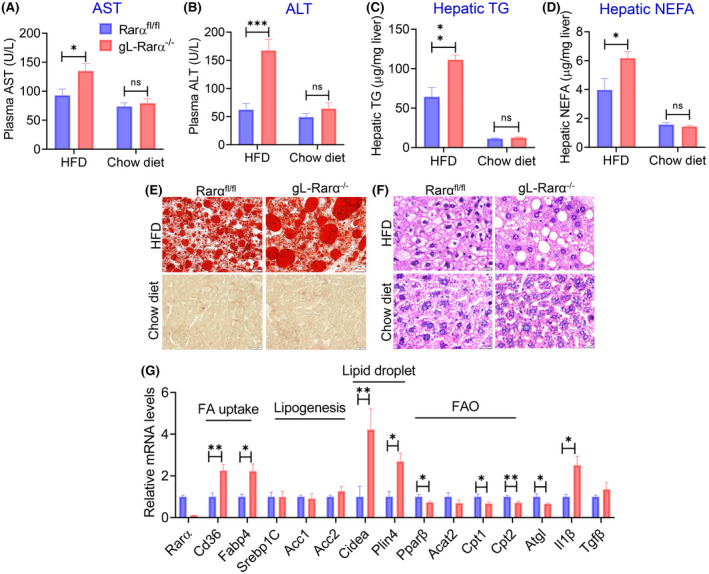
Germline loss of hepatocyte RARα induces hepatosteatosis in high‐fat diet–fed mice. Eight‐week‐old male Rarα fl/fl and gL‐Rarα −/− mice were fed a high‐fat diet (HFD) (n = 7) or a chow diet (n = 8) for 20 weeks. Plasma AST (A) and ALT (B) levels, and hepatic TG (C) and NEFA (D) levels were determined. (E,F) Representative liver images of Oil Red O staining (E) and H&E staining (F) (scale bars = 20 μm). (G) Hepatic mRNA levels of HFD‐fed mice were measured. All values are expressed as mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001. Cpt1, carnitine palmitoyltransferase 1; FA, fatty acid; FAO, fatty acid oxidation; Fabp4, fatty acid binding protein 4; Il1β, interleukin 1β; Tgfβ, tumor growth factorβ.
In the liver, genes involved in fatty acid uptake (Cd36, fatty acid binding protein 4 [Fabp4]), lipid droplet formation (Cidea, Plin4), and inflammation (interleukin‐1β) were significantly induced, whereas genes involved in FAO (Pparβ, carnitine palmitoyl transferase 1 [Cpt1], Cpt2) or lipolysis (Atgl) were significantly reduced (Figure 3G). Interestingly, genes involved in lipogenesis (Srebp1c, Acc1, Acc2) or fibrogenesis (transforming growth factor β) were unchanged (Figure 3G). Thus, the data in Figure 3 indicate that germline loss of hepatocyte RARα aggravates HFD‐induced hepatosteatosis.
AtRA protects from HFCF diet–induced obesity in a hepatocyte RARα‐dependent manner
To determine whether hepatocyte RARα played a role in AtRA‐mediated regulation of lipid homeostasis, we intravenously injected AAV8‐TBG‐null or AAV8‐TBG‐iCRE to Rarα fl/fl mice to generate adult‐onset hepatocyte‐specific Rarα knockout (L‐Rarα −/− ) mice and their control mice. These mice were then fed a HFCF diet with a daily gavage with either vehicle or AtRA (15 mg/kg) for 16 weeks. Interestingly, AtRA reduced body weight (Figure 4A) and body fat content (Figure 4B) in Rarα fl/fl mice but not in the L‐Rarα −/− mice. Consistent with these observations, AtRA increased oxygen consumption (Figure 4C), CO2 production (Figure 4D), and energy expenditure (Figure 4E) during the day and dark time in Rarα fl/fl mice but not in the L‐Rarα −/− mice. There was no change in locomotor activity (Figure 4F). In brown adipose tissue, there was a modest increase in uncoupled protein 3 (Ucp3) and a tendency in induction of peroxisome proliferator‐activated receptor gamma coactivator 1α (Pgc1α) in the L‐Rarα −/− mice (Figure 4G). These results indicate that retinoic acid prevents HFCF diet–induced obesity via hepatocyte RARα‐dependent induction of energy expenditure.
FIGURE 4.
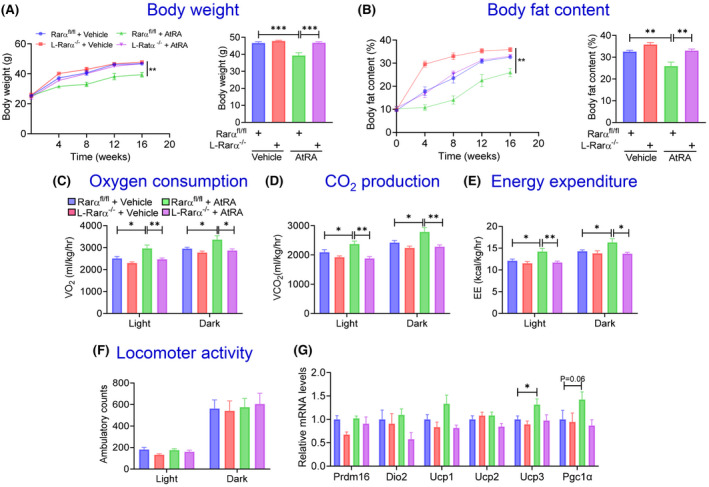
All‐trans retinoic acid (AtRA) protects from high fat/cholesterol/fructose (HFCF) diet–induced obesity in a hepatocyte RARα‐dependent manner. Rarα fl/fl mice were intravenously injected with adeno‐associated virus 8 (AAV8)–TBG‐null or AAV8‐TBG‐iCRE and then gavaged with vehicle or AtRA (15 mg/kg) for 16 weeks (n = 8 per group). These mice were also fed a HFCF diet. Body weight over the 16 weeks (A, left panel) or for the final week (A, right panel) was measured. Body fat content over the 16 weeks (B, left panel) or the for the final week (B, right panel) was determined. Oxygen consumption (C), CO2 production (D), energy expenditure (E), and ambulatory locomotor activity (F) during the 24‐h light or dark time were analyzed. mRNA levels in brown adipose tissue were determined (G). All values are expressed as mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001. Dio2, iodothyroine deiodinase 2; Pgc1α, peroxisome proliferator‐activated recetpor gamma coactivator 1‐α; Prdm16, PR domain containing 16; UCP1, uncoupling protein 1.
AtRA protects from HFCF diet–induced hepatosteatosis in a RARα‐dependent manner
Previous studies have shown that AtRA treatment ameliorates diet‐induced hepatosteatosis in mice.[ 8 ] However, it is unclear whether hepatocyte RARα plays a role in this process. The data in Figure 5 show that AtRA modestly reduced plasma ALT and AST levels (Figure 5A) as well as the ratio of the liver to body weight (Figure 5B), and decreased hepatic TG levels by 67% (Figure 5C) in Rarα fl/fl mice. However, these reductions were largely abolished in L‐Rarα −/− mice (Figure 5A–C). The data from ORO staining (Figure 5D) or H&E staining (Figure 5E) further confirmed these findings. Interestingly, AtRA treatment had not much effect on hepatic NEFA, cholesterol, or hydroxyproline levels in Rarα fl/fl mice but reduced hepatic NEFA, TC, and FC levels in L‐Rarα −/− mice (Figure S3A–D), suggesting that AtRA may regulate hepatic NEFA, TC, or FC levels in the absence of hepatocyte RARα. These results indicate that hepatocyte RARα is important for AtRA to attenuate diet‐induced hepatosteatosis.
FIGURE 5.
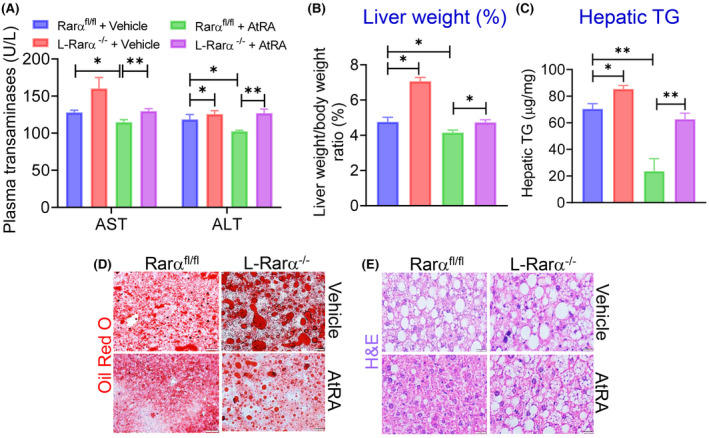
AtRA protects from HFCF diet–induced hepatosteatosis in a RARα‐dependent manner. Rarα fl/fl mice were intravenously injected with AAV8‐TBG‐null or AAV8‐TBG‐iCRE and then gavaged with vehicle or AtRA (15 mg/kg) for 16 weeks (n = 8 per group). These mice were also fed a HFCF diet. (A) Plasma AST and ALT levels were analyzed. (B) The liver weight–to–body weight ratio (percentage) was measured. (C) Hepatic TG levels were quantified. (D,E) Representative liver images of Oil Red O staining (D) or hematoxylin and eosin (H&E) staining (E) are presented (scale bars = 20 μm). All values are expressed as mean ± SEM; *p < 0.05, **p < 0.01.
AtRA reduces hepatosteatosis by inhibiting hepatocyte fatty acid uptake and lipid droplet formation via hepatocyte RARα
To understand how AtRA attenuates HFCF diet–induced hepatosteatosis via hepatocyte RARα, we measured hepatic gene expression and performed some functional assays. AtRA treatment inhibited the expression of genes involved in lipid droplet formation (Cidea, Cidec, Plin2, Plin3, Plin4, Plin5) or fatty acid uptake (Cd36, Fabp4) in the Rarα fl/fl mice, and these reductions were largely abolished in L‐Rarα −/− mice (Figure 6A,B). Although AtRA treatment reduced some of the genes involved in lipogenesis (Srebp1c, Pparg1, Pparg2, Scd1, Scd2), other lipogenic genes (Acc1, Acc2, Fasn) were unchanged (Figure 6D). Importantly, AtRA did not regulate hepatic TG synthesis although loss of hepatocyte RARα significantly induced hepatic TG synthesis (Figure 6D). AtRA reduced hepatic apolipoprotein b and microsomal triglyceride transfer protein expression, but did not have much impact on hepatic genes involved in FAO (Cpt1, Cpt2, Pparα) except for a significant induction of Cpt1c (Figure 4A,B). Further studies showed that loss of hepatocyte RARα did not affect VLDL secretion or intestinal fat absorption (Figure 4C,D).
FIGURE 6.
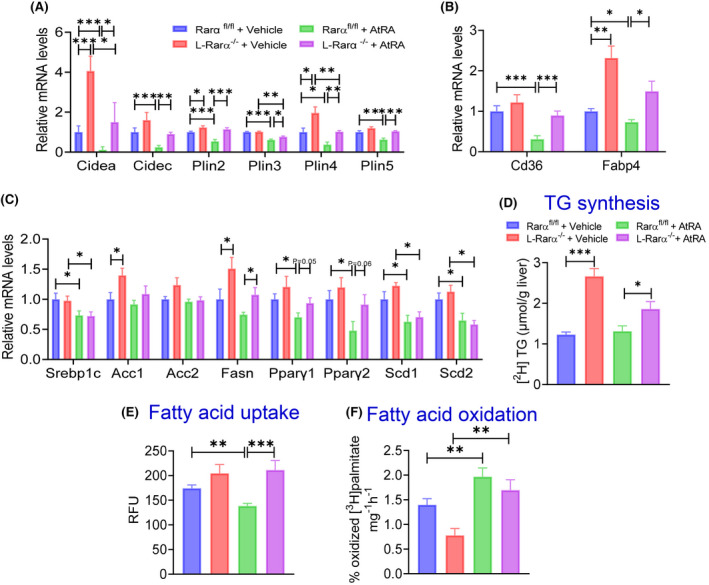
AtRA reduces hepatosteatosis by inhibiting hepatocyte fatty acid uptake and lipid droplet formation. Rara fl/fl mice were intravenously injected with AAV8‐TBG‐null or AAV8‐TBG‐iCRE and then gavaged with vehicle or AtRA (15 mg/kg) for 16 weeks (n = 8 per group). These mice were also fed a HFCF diet. Hepatic mRNA levels of genes involved in lipid droplet formation (A), fatty acid uptake (B), and lipogenesis (C) were determined. (D) Hepatic newly synthesized TG levels were analyzed. (E) Fatty acid uptake was determined in primary hepatocytes treated with either vehicle or AtRA (4 μm) (n = 10 per group). (F) FAO was performed in isolated primary hepatocytes treated with either vehicle or AtRA (2 μm), with each well seeded with the same amounts of cells. All values are expressed as mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001. RFU, relative fluorescence unit.
We then determined the role of AtRA in fatty acid uptake and FAO by hepatocytes. AtRA treatment reduced fatty acid uptake in Rarα fl/fl mice but not in L‐Rarα −/− mice (Figure 6E). In contrast, AtRA induced FAO in both Rarα fl/fl mice and L‐Rarα −/− mice (Figure 6F). Taken together, the data in Figures 5 and 6 indicate that AtRA prevents diet‐induced hepatosteatosis through hepatocyte RARα‐dependent inhibition of fatty acid uptake and lipid droplet formation.
AtRA reverses HFCF diet–induced obesity and hepatosteatosis dependent on hepatocyte RARα
To investigate whether AtRA could reverse HFCF diet–induced hepatosteatosis, we fed Rarα fl/fl mice and gL‐Rarα −/− mice an HFCF diet for 20 weeks. During the last 10 weeks, these mice were also gavaged with either vehicle or AtRA. Treatment with AtRA significantly reduced body weight (Figure 7A,B), body fat content (Figure 7C), and hepatic TG levels (Figure 7D) in the Rarα fl/fl mice, and these reductions were largely abolished in the L‐Rarα −/− mice (Figure 7A–D). The changes in hepatic TG levels were also corroborated by hepatic ORO staining (Figure 7E) and H&E staining (Figure 7F). Thus, the data in Figure 7 show that hepatocyte RARα is essential for AtRA to reverse diet‐induced obesity and hepatosteatosis.
FIGURE 7.
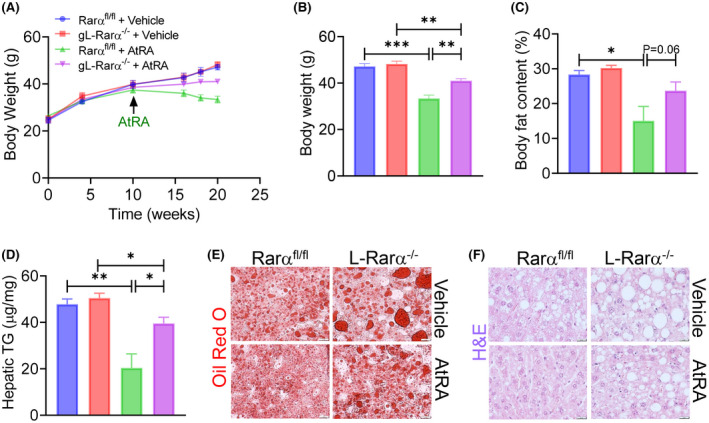
AtRA reverses HFCF diet–induced obesity and hepatosteatosis dependent on hepatocyte RARα. Eight‐week‐old male Rara fl/fl mice and gL‐Rara −/− mice were fed an HFCF diet for a total of 20 weeks. After they were fed an HFCF diet for 10 weeks, they were also gavaged with vehicle or AtRA (15 mg/kg, once a day) for another 10 weeks. (A) Body weight over the 20 weeks was measured. (B,C) At the conclusion of the study, body weight (B) and body fat content (C) were also measured. (D) Hepatic TG levels were determined. (E,F) Representative liver images of Oil Red O staining (E) and H&E staining (F) are shown (scale bars = 20 μm). All values are expressed as mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001.
DISCUSSION
Clinical studies have shown that retinoic acid signaling is impaired in NAFLD.[ 4 , 5 , 6 ] Treatment of mice[ 7 ] or rabbits[ 8 ] with AtRA attenuates diet‐induced hepatosteatosis, suggesting that impaired retinoic acid signaling may promote or aggravate the development of NAFLD. Indeed, our current studies show that loss of RARα in hepatocytes is sufficient to cause hepatosteatosis in aged mice or HFD‐fed mice. In line with our studies, expression of dominant negative RARα in the liver is also shown to promote liver steatosis.[ 20 ]
Aging is known to promote hepatic steatosis, and the progression of NAFLD via cellular senescence and impaired mitochondrial activity.[ 21 , 22 ] Retinoic acid signaling is known to enhance mitochondrial activity.[ 23 ] However, the role of RARα in senescence is not well understood. Our studies show that loss of hepatic RARα aggravates steatosis in 1‐year‐old mice but not in age‐matched wild‐type mice. It will be interesting to investigate whether hepatocyte senescence plays a role in hepatic steatosis induced by RARα deficiency.
So far, the exact mechanism by which AtRA inhibits liver steatosis remains to be fully understood. A previous study show that AtRA may prevent hepatosteatosis via inhibition of PPARγ signaling.[ 7 ] AtRA is known to function via activation of RARα, RARβ or RARγ, which are expressed in many tissues and cell types. In the current study, we demonstrate that AtRA attenuates diet‐induced liver steatosis via activation of hepatocyte RARα and subsequent inhibition of fatty acid uptake by hepatocytes and lipid droplet formation. AtRA promotes FAO in both Rarα fl/fl mice and L‐Rarα −/− mice, suggesting that AtRA‐regulated FAO does not play a role in RARα‐mediated reduction of hepatosteatosis. Also, we show that AtRA does not regulate hepatic TG synthesis or hepatic expression of genes involved in lipolysis or VLDL secretion. Collectively, our data indicate that AtRA ameliorates diet‐induced hepatosteatosis via hepatocyte RARα‐mediated inhibition of hepatic fatty acid uptake and lipid droplet formation.
In addition to attenuating diet–induced hepatosteatosis, AtRA treatment also prevents diet‐induced obesity in a hepatocyte RARα‐dependent manner. A previous study shows that activation of RARβ induces hepatic fibroblast growth factor 21 to stimulate FAO and promote whole body energy metabolism.[ 24 ] Nonetheless, we still do not fully understand how activation of hepatic RARα is responsible for AtRA to induce energy expenditure and inhibit diet‐induced obesity.
Because activation of hepatic RARα prevents and reverses hepatosteatosis, and retinoic acid signaling is impaired in NAFLD, targeting hepatic RARα or retinoic acid signaling may be a promising strategy for treatment of hepatosteatosis. Whether and how retinoic acid signaling regulates steatohepatitis remains to explored.
AUTHOR CONTRIBUTIONS
Study concept and design, data interpretation, and manuscript preparation: Fathima N. Cassim Bawa and Yanqiao Zhang. Experiments: Fathima N. Cassim Bawa, Yanyong Xu, Raja Gopoju, Amy Shiyab, Shuwei Hu, Shaoru Chen, Yingdong Zhu, and Kavita Jadhav. Data analysis: Noel‐Marie Plonski. Gas chromatography–mass spectrometry studies: Takhar Kasumov.
FUNDING INFORMATION
Supported by the National Institutes of Health (R01DK102619 and R01DK118941).
CONFLICT OF INTEREST
Nothing to report.
Supporting information
Figure S1 Effect of germline loss of hepatocyte retinoid acid receptor alpha (RARα) on body weight, body fat, liver weight, and plasma lipids. One‐year‐old Rarα fl/fl and gL‐Rarα −/− male mice were fed a chow diet (n = 7–8). Body weight (A), body fat content (B), and ratio of liver to body weight (C) were measured. Plasma glucose (D), triglycerides (TG) (E), nonesterified free fatty acids (NEFAs) (F), and total cholesterol (TC) (G) were analyzed. All values are expressed as mean ± SEM; *p < 0.05. Abbreviation: ns, not significant
Figure S2 Effect of germline loss of hepatocyte Rarα on body weight, body fat, liver weight, and plasma lipids in high‐fat diet (HFD)–fed mice. Eight‐week‐old male Rarα fl/fl mice and gL‐Rarα −/− mice were fed a HFD for 20 weeks (n = 7). Body weight (A), body fat content (B), and liver weight (C) were measured. Plasma levels of glucose (D), TG (E), NEFAs (F), and TC were analyzed. All values are expressed as mean ± SEM; *p < 0.05, **p < 0.01
Figure S3 Effects of hepatocyte RARα deficiency and all‐trans retinoic acid (AtRA) on hepatic lipid and hydroxyproline levels. Rarα fl/fl mice were intravenously injected with adeno‐associated virus 8 (AAV8)–TBG‐null or AAV8‐TBG‐iCRE and then gavaged with vehicle or AtRA (15 mg/kg) for 16 weeks (n = 8 per group). These mice were also fed a high‐fat/cholesterol/fructose (HFCF) diet. Hepatic levels of NEFAs (A), cholesterol (B), and hydroxyproline (C) were determined. (D) Representative liver images of picosirius red staining (scare bars = 20 μm). All values are expressed as mean ± SEM; *p < 0.05, **p < 0.01
Figure S4 AtRA or RARα deficiency has little impact on very‐low density lipoprotein (VLDL) secretion or intestinal fat absorption. Rarα fl/fl mice were intravenously injected with AAV8‐TBG‐null or AAV8‐TBG‐iCRE and then gavaged with vehicle or AtRA (15 mg/kg) for 16 weeks (n = 8 per group). These mice were also fed a HFCF diet. (A,B) Hepatic messenger RNA (mRNA) levels were determined. VLDL secretion (C) or intestinal fat absorption (D) was performed in HFCF diet–fed Rarα fl/fl mice or gL‐Rarα −/− mice (n = 8 per group). All values are expressed as mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001
Figure S5 Additional Oil Red O or hematoxylin and eosin (H&E) staining images for Figures 2E, 3E, 5D,E, and 7E,F
Cassim Bawa FN, Xu Y, Gopoju R, Plonski N‐M, Shiyab A, Hu S, et al. Hepatic retinoic acid receptor alpha mediates all‐trans retinoic acid's effect on diet‐induced hepatosteatosis. Hepatol Commun. 2022;6:2665–2675. 10.1002/hep4.2049
REFERENCES
- 1. Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–64. [DOI] [PubMed] [Google Scholar]
- 2. Friedman SL, Neuschwander‐Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pan X, Zhang Y. Hepatocyte nuclear factor 4alpha in the pathogenesis of non‐alcoholic fatty liver disease. Chin Med J (Engl). 2022. Feb 21. 10.1097/CM9.0000000000002092. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saeed A, Dullaart RPF, Schreuder T, Blokzijl H, Faber K. Disturbed vitamin A metabolism in non‐alcoholic fatty liver disease (NAFLD). Nutrients. 2017;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhong G, Kirkwood J, Won KJ, Tjota N, Jeong H, Isoherranen N. Characterization of vitamin a metabolome in human livers with and without nonalcoholic fatty liver disease. J Pharmacol Exp Ther. 2019;370:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaves GV, Pereira SE, Saboya CJ, Spitz D, Rodrigues CS, Ramalho A. Association between liver vitamin A reserves and severity of nonalcoholic fatty liver disease in the class III obese following bariatric surgery. Obes Surg. 2014;24:219–24. [DOI] [PubMed] [Google Scholar]
- 7. Kim SC, Kim CK, Axe D, Cook A, Lee M, Li T, et al. All‐trans‐retinoic acid ameliorates hepatic steatosis in mice by a novel transcriptional cascade. Hepatology. 2014;59:1750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zarei L, Farhad N, Abbasi A. All‐trans retinoic acid (atRA) effectively improves liver steatosis in a rabbit model of high fat induced liver steatosis. Arch Physiol Biochem. 2020;23:1–6. [DOI] [PubMed] [Google Scholar]
- 9. Tsuchiya H, Ikeda Y, Ebata Y, Kojima C, Katsuma R, Tsuruyama T, et al. Retinoids ameliorate insulin resistance in a leptin‐dependent manner in mice. Hepatology. 2012;56:1319–30. [DOI] [PubMed] [Google Scholar]
- 10. Chapellier B, Mark M, Messaddeq N, Calléja C, Warot X, Brocard J, et al. Physiological and retinoid‐induced proliferations of epidermis basal keratinocytes are differently controlled. EMBO J. 2002;21:3402–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edwards M, Houseman L, Phillips IR, Shephard EA. Isolation of mouse hepatocytes. Methods Mol Biol. 2013;987:283–93. [DOI] [PubMed] [Google Scholar]
- 12. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y, Ge X, Heemstra LA, Chen WD, Xu J, Smith JL, et al. Loss of FXR protects against diet‐induced obesity and accelerates liver carcinogenesis in ob/ob mice. Mol Endocrinol. 2012;26:272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rune A, Osler ME, Fritz T, Zierath JR. Regulation of skeletal muscle sucrose, non‐fermenting 1/AMP‐activated protein kinase‐related kinase (SNARK) by metabolic stress and diabetes. Diabetologia. 2009;52:2182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Y, Zalzala M, Jadhav K, Xu Y, Kasumov T, Yin L, et al. Carboxylesterase 2 prevents liver steatosis by modulating lipolysis, endoplasmic reticulum stress, and lipogenesis and is regulated by hepatocyte nuclear factor 4 alpha in mice. Hepatology. 2016;63:1860–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liao J, Sportsman R, Harris J, Stahl A. Real‐time quantification of fatty acid uptake using a novel fluorescence assay. J Lipid Res. 2005;46:597–602. [DOI] [PubMed] [Google Scholar]
- 17. Xu J, Li Y, Chen WD, Xu Y, Yin L, Ge X, et al. Hepatic carboxylesterase 1 is essential for both normal and farnesoid X receptor‐controlled lipid homeostasis. Hepatology. 2014;59:1761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khalifeh‐Soltani A, McKleroy W, Sakuma S, Cheung YY, Tharp K, Qiu Y, et al. Mfge8 promotes obesity by mediating the uptake of dietary fats and serum fatty acids. Nat Med. 2014;20:175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu Y, Li Y, Jadhav K, Pan X, Zhu Y, Hu S, et al. Hepatocyte ATF3 protects against atherosclerosis by regulating HDL and bile acid metabolism. Nat Metab. 2021;3:59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yanagitani A, Yamada S, Yasui S, Shimomura T, Murai R, Murawaki Y, et al. Retinoic acid receptor alpha dominant negative form causes steatohepatitis and liver tumors in transgenic mice. Hepatology. 2004;40:366–75. [DOI] [PubMed] [Google Scholar]
- 21. Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, et al. Cellular senescence drives age‐dependent hepatic steatosis. Nat Commun. 2017;8:15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aravinthan A, Scarpini C, Tachtatzis P, Verma S, Penrhyn‐Lowe S, Harvey R, et al. Hepatocyte senescence predicts progression in non‐alcohol‐related fatty liver disease. J Hepatol. 2013;58:549–56. [DOI] [PubMed] [Google Scholar]
- 23. Tripathy S, Chapman JD, Han CY, Hogarth CA, Arnold SLM, Onken J, et al. All‐trans‐retinoic acid enhances mitochondrial function in models of human liver. Mol Pharmacol. 2016;89:560–74. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Li Y, Wong K, Walsh K, Gao B, Zang M. Retinoic acid receptor beta stimulates hepatic induction of fibroblast growth factor 21 to promote fatty acid oxidation and control whole‐body energy homeostasis in mice. J Biol Chem. 2013;288:10490–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Effect of germline loss of hepatocyte retinoid acid receptor alpha (RARα) on body weight, body fat, liver weight, and plasma lipids. One‐year‐old Rarα fl/fl and gL‐Rarα −/− male mice were fed a chow diet (n = 7–8). Body weight (A), body fat content (B), and ratio of liver to body weight (C) were measured. Plasma glucose (D), triglycerides (TG) (E), nonesterified free fatty acids (NEFAs) (F), and total cholesterol (TC) (G) were analyzed. All values are expressed as mean ± SEM; *p < 0.05. Abbreviation: ns, not significant
Figure S2 Effect of germline loss of hepatocyte Rarα on body weight, body fat, liver weight, and plasma lipids in high‐fat diet (HFD)–fed mice. Eight‐week‐old male Rarα fl/fl mice and gL‐Rarα −/− mice were fed a HFD for 20 weeks (n = 7). Body weight (A), body fat content (B), and liver weight (C) were measured. Plasma levels of glucose (D), TG (E), NEFAs (F), and TC were analyzed. All values are expressed as mean ± SEM; *p < 0.05, **p < 0.01
Figure S3 Effects of hepatocyte RARα deficiency and all‐trans retinoic acid (AtRA) on hepatic lipid and hydroxyproline levels. Rarα fl/fl mice were intravenously injected with adeno‐associated virus 8 (AAV8)–TBG‐null or AAV8‐TBG‐iCRE and then gavaged with vehicle or AtRA (15 mg/kg) for 16 weeks (n = 8 per group). These mice were also fed a high‐fat/cholesterol/fructose (HFCF) diet. Hepatic levels of NEFAs (A), cholesterol (B), and hydroxyproline (C) were determined. (D) Representative liver images of picosirius red staining (scare bars = 20 μm). All values are expressed as mean ± SEM; *p < 0.05, **p < 0.01
Figure S4 AtRA or RARα deficiency has little impact on very‐low density lipoprotein (VLDL) secretion or intestinal fat absorption. Rarα fl/fl mice were intravenously injected with AAV8‐TBG‐null or AAV8‐TBG‐iCRE and then gavaged with vehicle or AtRA (15 mg/kg) for 16 weeks (n = 8 per group). These mice were also fed a HFCF diet. (A,B) Hepatic messenger RNA (mRNA) levels were determined. VLDL secretion (C) or intestinal fat absorption (D) was performed in HFCF diet–fed Rarα fl/fl mice or gL‐Rarα −/− mice (n = 8 per group). All values are expressed as mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001
Figure S5 Additional Oil Red O or hematoxylin and eosin (H&E) staining images for Figures 2E, 3E, 5D,E, and 7E,F


