Abstract
Purpose
The purpose of this study was to determine the most optimal central venous catheter (CVC) for pediatric patients with Hodgkin lymphoma (HL) in terms of complications.
Methods
A retrospective study including patients diagnosed with HL from 2015 to 2021 at the Princess Máxima Center was performed. Patients were followed from CVC insertion until removal or 06–2021, whichever came first. The primary outcome was the CVC-related complication incidence rate (IR) per 1000 CVC-days. Furthermore, the incidence rate ratio (IRR) was calculated by comparing complication IRs between peripherally inserted central catheters (PICC) and totally implantable venous access ports (TIVAP). Additionally, risk factors for central venous thrombosis (CVT) were identified.
Results
A total of 98 patients were included. The most frequently observed complications were local irritation/infections (18%; IR 0.93), malfunctions (15%; IR 0.88), and CVC-related CVTs (10%; IR 0.52). Single lumen PICCs were associated with a higher risk of complications (49% vs. 26%; IRR 5.12, CI95% 2.76–9.50), severe complications (19% vs. 7%; IRR 11.96, CI95% 2.68–53.42), and early removal (18% vs. 7%; IRR 9.96, CI95% 2.18–45.47). A single lumen PICC was identified as a risk factor for CVC-related CVT when compared to TIVAPs (12% vs. 7%, IRR 6.98, CI95% 1.45–33.57).
Conclusion
The insertion of a TIVAP rather than a PICC should be recommended for pediatric patients with HL, especially in the presence of CVT-related risk factors. Future trials should evaluate the efficacy and safety of direct oral anticoagulants for the primary prevention of CVT in pediatric patients with a PICC and other CVT-related risk factors.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00520-022-07256-3.
Keywords: Hodgkin lymphoma, Central venous catheter, Pediatric, Complications, Thrombosis
Introduction
The vast majority of pediatric patients diagnosed with Hodgkin lymphoma (HL) will receive a central venous catheter (CVC) at the start of their treatment. Multiple CVC types are available, but single lumen totally implantable central venous access ports (TIVAP) and peripherally inserted central venous catheters (PICC) are the most frequently used CVCs in this patient group. Insertion of a PICC is still considered favorable in patients with HL since insertion is possible without the need for general anesthesia (which is especially favorable in children with mediastinal masses causing airway problems) and is considered safe because of the relatively short treatment period and larger peripheral vessels of this, usually older, pediatric patient group [1].
In contrast to PICCs, TIVAPs can stay in situ for a longer period and give patients more freedom of movement. However, general anesthesia is needed for insertion, sedation for removal, percutaneous punctures to access the port, and a larger scar will remain visible after removal, whereas these disadvantages do not apply to PICCs. On the other hand, higher incidence rates of mechanical failure, CVC-related infections, and CVC-related central venous thrombosis (CVT) have been associated with PICCs when compared to other CVC-types in a variety of adult and pediatric patients (i.e., oncology, intensive care unit, total parenteral nutrition) [1–10]. However, the incidence of all CVC-related complications for patients with HL specifically has not been described previously.
Based on studies in adults and children, the risk of CVC-related CVTs has been described to be higher in patients with HL, compared to other oncology patients [3, 9]. Suggested risk factors that may contribute to this difference are tumor-associated inflammation and compression of veins typically occurring in the upper body, the older age of patients, and frequent high-dose corticosteroid treatment, which are all known risk factors for thrombosis [1, 3, 11–15]. Since pediatric patients with HL might be at a higher risk of CVC-related CVTs and since the risk of other CVC-related complications per CVC type for this patient group is currently unknown, it is of importance that the most optimal CVC for these children is identified. In this study, we have analyzed all CVC complications and their outcomes.
Methods
Study design and participants
A retrospective study including all consecutive patients diagnosed with HL, who received a CVC and who were treated in the Princess Máxima Center for pediatric oncology (Utrecht, The Netherlands) from January 2015 until March 2021, was performed. Patients were excluded if their CVC was inserted in any other hospital than the Princess Máxima Center, if they were older than 18 years at CVC insertion, or if they did not give their consent to use their data for scientific research (n = 24). Each patient was followed up from first CVC insertion until first CVC removal or June 2021, whichever came first. Patients were treated following the guidelines of the European Network-Pediatric Hodgkin’s Lymphoma Study Group (EuroNet-PHL: C1, C2, or LP1) in an outpatient setting. The medical ethics committee of the University Medical Center Utrecht (Utrecht, The Netherlands) waived the need for official approval by the medical ethics committee (File number: 21/723).
Data collection and definitions
The primary outcome was the incidence rate (IR) per 1000 CVC-days for all observed CVC-related complications in total and per CVC-type. This aggregated outcome was chosen to give an overview of the overall risk of CVC-related complications for pediatric HL patients. Secondary outcomes were the IRs of each complication type as described below per CVC-type, of early removal due to complications per CVC-type and of CVC-related CVTs per risk factor.
The patient files were assessed retrospectively for the occurrence of the following CVC-related complications: intra-operative complications, central line–associated bloodstream infections (CLABSI), local irritation/infection, CVC-related CVT, malfunctions, and mechanical complications (dislocations, ruptures, and dislodgement). Intra-operative complications were defined as any abnormalities during or directly after CVC insertion (e.g., pneumothorax, arterial puncture, dislocation, bleeding, malfunction). CLABSIs were defined following the Centers for Disease Control and Prevention criteria [16]. Local irritation/infections were defined as a positive exit-site culture, erythema, purulent drainage, or tenderness within 2 cm of the CVC track. CVTs were scored if their presence was confirmed by the radiology department in the imaging report. Imaging was performed due to the presence of CVT-related symptoms or for routine tumor response evaluation. Malfunctions were defined as the inability to flush and/or aspirate requiring the need of thrombolysis or CVC removal. Mechanical complications were defined as the detachment of CVC components, dislocation of the CVC diagnosed by a chest radiograph or a visible cuff, and rupture of the CVC components causing leakage. All complications were thereafter scored following the Clavien-Dindo classification [17]. Severe complications were defined as a Clavien-Dindo classification of III or higher. The Clavien-Dindo definitions are described in Online Resource 1 (Online Resource 1).
Furthermore, to evaluate the presence of CVT-related risk factors, the following data were collected: age, sex, obesity [18], HL type and stage following the Ann-Arbor classification [19, 20], presence of a mediastinal mass (i.e., confirmed by radiologist), smoking, thrombosis or laboratory-confirmed thrombophilia in medical (family) history, use of hormonal contraceptives, compression of the veins in the trajectory of the CVC as confirmed by a radiologist at insertion (for the vena cava superior specifically < 50% or > 50% compression), preference to not insert the CVC under general anesthesia as evaluated retrospectively by two pediatric lymphoma specialists, pediatric intensive care unit admission from diagnosis until end of study period, prophylactic anticoagulant use, signs of infection during CVT diagnosis, > one insertion attempt, CVC type, CVC side and lumen size, CVC use for total parenteral nutrition, and CVC to vein ratio for PICCs specifically. Additionally, the following information was extracted from the patient files: diagnosis date, CVC insertion date, end of treatment date, complication date, CVC insertion method, CVC insertion vein, reason for CVC removal, complication treatment, and hospital/intensive care unit admission due to complications. For CVT events specifically, the severity, symptoms, and complications (e.g., pulmonary embolism, CLABSI, vena cava superior syndrome, and post-thrombotic syndrome scored following the modified Villalta score [21]) were collected. If data was not explicitly reported in the patient files, this was reported as missing data.
CVC insertion and maintenance
The vast majority of patients diagnosed with Hodgkin will receive either a PICC or TIVAP at the start of their treatment. A non-tunneled CVC is only inserted for a short period in case of an emergency setting, positive blood cultures, or if the insertion of a tunneled CVC is not possible. If a PICC or TIVAP is to be inserted, it is determined by the expected treatment duration, lumen needed, presence of a tumor causing airway problems, and the wishes of the patient. In case of an expected treatment duration of more than 6 months, the insertion of a TIVAP is recommended. In patients with a tumor causing airway problems, the insertion of a PICC is preferred to avoid the need for general anesthesia or a TIVAP is inserted after steroid treatment. In all other cases, the (dis)advantages of both CVC types are discussed and the patient is thereafter free of choice. All CVCs were inserted by a specialized vascular access team or a pediatric oncology surgeon. All CVCs were inserted ultrasound-guided, only by exception CVCs were inserted percutaneously based on anatomical land marks. The insertion vein was chosen based on the availability and quality of the veins (i.e., adequate blood-flow through vein and CVC-to-vein ratio of < 45% [22] for PICCs assessed by ultrasound), with a preference for the right jugular vein for the non-PICC CVCs. CVC care was performed by or under supervision of specialized pediatric oncology nurses following international guidelines [23, 24]. The CVCs were flushed with NaCl 0.9% before use and locked with heparin 100 international units per milliliter after every use. The locks were replaced once every 8 weeks for the TIVAP and once every week for the other CVC types if the CVC was not used.
Statistical analysis
Differences between patients with a SL PICC and TIVAP with respect to baseline characteristics were analyzed using a Fisher Exact or Wilcoxon rank sum test, depending on the variable. The IRs per 1000 CVC-days were calculated with the number of all CVC-related events observed and total CVC-days (i.e., sum of the days from insertion until the end of follow-up, during in- and outpatient settings). Additionally, the IRs per 1000 CVC treatment days were calculated with the number of CVC-related events during the treatment period and total CVC-treatment days (i.e., sum of days during in- and outpatient settings from insertion until the end of follow-up or the last day of treatment, whichever came first; in case of a recurrence during insertion of the primary CVC, the days from recurrence diagnosis until the end of follow-up or last day of treatment, whichever came first, were added up to the total sum). This last calculation was performed since the frequency of complications might be higher during the treatment period and some CVCs remained in situ without treatment due to clinical follow-up and delays in the surgical lists.
Incidence rate ratios (IRR) along with their 95% confidence intervals (CI) were computed (1) to compare the IRs per 1000 CVC-days and CVC-treatment days for all complications between single lumen PICCs and TIVAPs and (2) to compare the IRs per 1000 CVC-days for CVC-related CVT between patients with and without CVT-related risk factors. The second analysis was performed for two different settings: (1) including only single lumen PICCs and TIVAPs (most commonly inserted CVCs) and (2) excluding patients where CVC insertion under general anesthesia was not preferred due to disease severity resulting in the insertion of a PICC instead of a TIVAP (since these patients possibly have a high risk of CVT and TIVAP insertion is not possible [1]). The exact confidence limits for the IRRs were computed based on the polynomial algorithm for person time data [25, 26]. The mean CVC to vein ratio for patients with a CVT compared to patients without a CVT was compared using an independent t-test. IBM SPSS Statistics for Windows version 26.0 (IBM Corp, USA) was used to perform all statistical analyses.
Results
Patient and CVC characteristics
In total 98 patients were included with a median age at diagnosis of 15 years (6–17). Most patients (96%) were diagnosed with classic HL. Compression of the veins due to lymphoma in the CVC tract was observed in 18 (18%) patients. Two patients (2%) received anticoagulants before CVC insertion and kept using it during CVC insertion due to a non-CVC-related thrombosis and venous compression (prophylactic dose n = 1, therapeutic dose n = 1). Additionally, one more patient (1%) received anticoagulants during CVC insertion due to venous compression (prophylactic dose n = 1). General anesthesia at diagnosis was not preferred in 14 (14%) patients, resulting in prephase therapy with steroids. In only five (36%) of these patients a PICC instead of a TIVAP was eventually inserted since general anesthesia was still not preferred (Table 1). Baseline characteristics for patients receiving a TIVAP or SL PICC are described separately in Online Resource 2. Patients with a SL PICC differed from patients in the TIVAP group in terms of age at diagnosis, Ann-Arbor stage, and CVC-(treatment) days (Online Resource 2).
Table 1.
Baseline characteristics
| Patient characteristics (N=98) | CVC characteristics (N=98) | ||||
|---|---|---|---|---|---|
| Sex, N (%) | Male | 50 (51.0) | Days from diagnosis until insertion, median (range) | 11 (0-41) | |
| Female | 48 (49.0) | CVC-days, median; sum (range) | 143; 19 341 (0-717) | ||
| Age at diagnosis, median (range) | 15 (6-17) | CVC-treatment days, median; sum (range) | 118; 11 158 (0-308) | ||
| Hodgkin type, N (%) | Classic | 94 (95.9) | CVC type, N (%) | TIVAP | 31 (31.6) |
| NLPHL | 4 (4.1) | SL PICC | 57 (58.2) | ||
| Ann-Arbor staging, N (%) | I | 2 (2.0) | DL PICC | 9 (9.2) | |
| II | 43 (43.9) | Non-tunneled | 1 (1.0)d | ||
| III | 27 (27.6) | Introduction method, N (%) | Ultrasound | 96 (98.0) | |
| IV | 26 (26.5) | Anatomic landmarks | 1 (1.0) | ||
| EuroNet-PHL protocol, N (%) | C1 | 2 (2.0) | Missing | 1 (1.0) | |
| C2 | 92 (93.9) | Lumen number, N (%) | Single | 88 (89.8) | |
| LP1 | 4 (4.1) | Double | 9 (9.2) | ||
| Mediastinal mass, N (%) | No | 8 (8.2) | Triple | 1 (1.0) | |
| Yes | 90 (91.8) | Lumen diameter, N (%) | <6.5 Fr | 65 (66.3) | |
| Obesity at diagnosisa, N (%) | No | 80 (81.6) | ≥6.5 Fr | 31 (31.6) | |
| Yes | 18 (18.4) | Missing | 2 (2.0) | ||
| Smoking, N (%) | No | 60 (61.2) | Insertion vein, N (%) | Jugular | 29 (29.6) |
| Yes | 3 (3.1) | Subclavian | 2 (2.0) | ||
| Passive | 6 (6.1) | Brachial | 39 (39.8) | ||
| Missing | 29 (29.6) | Cephalic | 1 (1.0) | ||
| Oral anti-conceptive use, N (%) | No | 82 (83.7) | Basilica | 26 (26.5) | |
| Progesterone | 4 (4.1) | Femoral | 1 (1.0) | ||
| Progesterone and estrogen | 12 (12.3) | Insertion side, N (%) | Right | 86 (87.8) | |
| Thrombophilia, N (%) | No | 2 (20) | Left | 12 (12.2) | |
| Yes | 3 (3.1) | Long-term anticoagulant use during CVC-insertione, N (%) | No | 95 (96.9) | |
| Not tested | 93 (94.9) | Prophylactic | 1 (1.0) | ||
| Thrombotic family history, N (%) | Negative | 61 (62.2) | Therapeutic | 2 (2.0) | |
| Positive | 5 (5.1) | >1 Insertion attempt, N (%) | No | 92 (93.9) | |
| Missing | 32 (32.7) | Yes | 2 (2.0) | ||
| Compression veins, N (%) | No | 80 (81.6) | Missing | 4 (4.1) | |
| Yes | 18 (18.4) | TPN over CVC, N (%) | TPNf | 4 (4.1) | |
| VCS compression, N (%) | No | 84 (85.7) | No TPN | 94 (95.9) | |
| <50% | 10 (10.2) | CVC to vein ratio for PICCs, median (range) | 0.27 (0.15-0.33) | ||
| >50% | 4 (4.1) | ||||
| Thrombosis before insertion, N (%) | No | 97 (99.0) | |||
| Yes | 1 (1.0) | ||||
| Anticoagulant use in period before and at insertion, N (%) | No | 96 (98.0) | |||
| Prophylactic | 1 (1.0) | ||||
| Therapeutic | 1 (1.0) | ||||
| CVC insertion under general anesthesia not preferredb, N (%) | No | 93 (94.9) | |||
| Yes | 5 (5.1) | ||||
| PICU admission, N (%) | No | 95 (96.9) | |||
| Yes | 3c (3.1) | ||||
CVCs central venous catheter, NLPHL nodular lymphocyte-predominant Hodgkin lymphoma, PHL pediatric Hodgkin lymphoma, TIVAP totally implantable venous access port, PICC peripherally inserted central catheter, Fr French, VCS vena cava superior, SL single lumen, DL double lumen, TPN total parenteral nutrition, PICU pediatric intensive care unit, N number.
aObesity was scored following: Cole 200018.
bBased on clinical evaluation by two lymphoma specialists.
cPICU admissions due to respiratory or circulatory insufficiency, CVT-related PICU admission registered as “no.”
dInserted during an emergency setting due to an anaphylactic reaction to contrast.
eCVCs where thrombolytics were given for only a short period of time due to for example hospitalization or where thrombolytics were given after a CVT was observed are registered as “no.” Reasons for anticoagulant use were the following: not CVC-related thrombosis (n = 2) and venous compression (n = 1).
fMedian (range) days of TPN: 4.5 (1–13)
Mainly single lumen CVCs (90%) were inserted; single lumen PICCs (65%) and TIVAPs (35%). The CVCs were in situ for a total of 19,341 CVC-days and 11,158 CVC-treatment days. TIVAPs were in situ for a median of 377 (33–717) days and single lumen PICCs for 105 (0–208) days. Of all CVCs, 12 (12%) were removed due to complications, 75 (77%) due to end of treatment, three (3%) due to the need for another CVC type, one (1%) since the patient was not content with the location, and seven (7%) were still in situ at the end of this study (Table 1).
Complications
A total of 58 complications were observed with an IR of 3.00 per 1000 CVC-days. In 42% of all CVCs, at least one complication was observed. The most frequently observed complications per 1000 CVC-days were local irritation/infections (18%; IR 0.93), malfunctions (14%; IR 0.88), and CVC-related CVT (10%; IR 0.52). The IRs per 1000 CVC-treatment days were comparable or higher for all complication types. All complications were observed after a median of 63 (3–378) days. CVC-related CVT was the most frequently observed reason for early CVC removal (50% of CVCs removed early due to complications, 6% of all inserted CVCs). Hospital admission was mainly observed in patients experiencing a CLABSI. One patient was admitted to the intensive care unit due to a combined CVT and CLABSI episode (Table 2).
Table 2.
Incidence of CVC-related complications
| Complications | Events, n (%) | CVCse, n (% all CVCs) | IR per 1000 CVC-days | IR per 1000 CVC-treatment days | Days until complication, median (range) | CVC removal, n (% all CVCs) | Hospital admission days, median (range) |
|---|---|---|---|---|---|---|---|
| Intra-operative | 6 (10.3) | 6 (6.1) | NA | NA | NA | 1 (1.0) | 0 (0–1) |
| CLABSI | 3 (5.2) | 3 (3.1) | 0.16 | 0.27 | 19 (13–99) | 1a (1.0) | 6 (5–10) |
| Local irritation/infection | 18 (31.0) | 18 (18.4) | 0.93 | 1.43 | 66 (30–139) | 2 (2.0) | 0 (0–5) |
| CVT | 10 (17.2) | 10 (10.2) | 0.52 | 0.81 | 19 (3–374) | 6a (6.1) | 0 (0–28) |
| Dislocation | 2 (3.4) | 2 (2.0) | 0.10 | 0.09 | 4 (3–5) | 0 (0.0) | 0 (0–0) |
| Malfunction | 17b (29.3) | 14 (14.3) | 0.88 | 1.43 | 67 (10–378) | 2 (2.0) | 0 (0–0) |
| Rupture | 1 (1.4) | 1 (1.0) | 0.05 | 0.09 | 16 (16–16) | 1 (1.0) | 0 (0–0) |
| Dislodgement | 1 (1.4) | 1 (1.0) | 0.05 | 0.09 | 39 (39–39) | 0 (0.0) | 0 (0–0) |
| Total | 58 (100.0) | 41 (41.8)c | 3.00 | 4.75 | 63 (3–378) | 12d (12.2) | 0 (0–28) |
CVCs central venous catheter, CLABSI central line associated bloodstream infection, CVT central venous thrombosis, IR incidence rate. In total, five (8.6%) CVC-related complications were observed after the end of treatment. aOne CVC was removed due to a combined CVT and CLABSI episode. bMalfunctions successfully treated with thrombolysis (n = 12), unsuccessful thrombolysis resulting in removal (n = 2), unsuccessful thrombolysis due to the presence of a CVT for which the CVC was removed (n = 1), unsuccessful thrombolysis after which malfunction was treated with pulsatile flushes of regular saline (n = 1). cIf multiple complications were identified in one CVC, this was counted as one to calculate this percentage. This means that 42.3% of all patients experienced one or more complications during CVC insertion. dCombined CVT and CLABSI episode counted as one in total. eFor each complication type, only the first was counted per CVC
In total, ten CVC-related CVT events were observed, among which eight CVTs were identified due to symptoms and two due to a routine ultrasound and magnetic-resonance imaging for tumor response evaluation. In four patients, the CVT resulted in complications; three short-term complications (i.e., vena cava superior syndrome causing chylothorax, pulmonary embolisms, and septic thrombophlebitis); and one long-term complication (i.e., post-thrombotic syndrome; modified Villalta score 4 indicating a moderate post-thrombotic syndrome). The CVTs were diagnosed after a median of 19 (3–374) days after insertion. All CVT events were treated with anticoagulants; in six cases, the CVC was removed; one patient required a thrombectomy and another required a percutaneous transluminal angioplasty. None of the patients with a CVT received thrombosis prophylaxis before the CVT occurred. In four CVT cases, simultaneous clinical signs of infection were present (Online Resource 3).
Complications TIVAP versus single lumen PICC
During CVC-insertion, single lumen PICCs were associated with a significantly higher risk of complications (49% vs. 26%; IRR 5.12, CI95%2.76–9.50) and removal due to complications (18% vs. 7%; IRR 9.96, CI95%2.18–45.47), when compared to TIVAPs during complete CVC insertion. Specifically, a higher risk of local irritation/infections (26% vs. 7%; IRR 14.95, CI95%3.42–65.35) and CVC-related CVTs (12% vs. 7%; IRR 6.98, CI95%1.45–33.57) was associated with the insertion of single lumen PICCs compared to TIVAPs. A Clavien-Dindo grade of I or II was scored for the vast majority of complications. Of all complications, a grade of III or higher was scored in 27.6%. Single lumen PICCs were associated with a significantly higher risk of severe complications, i.e., Clavien-Dindo grade of III or higher, when compared to TIVAPs (19% vs. 7%; IRR 11.96, CI95%2.68–53.42) (Table 3 and Online Resource 1).
Table 3.
Comparison of CVC-related complication incidence rates between TIVAPs and single lumen PICCs
| TIVAP n = 31 12,258 CVC days and 4193 CVC-treatment days |
Single lumen PICC n = 57 6151 CVC days and 6033 CVC-treatment days |
IRR (CI95%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Complications | Events, n (%) | CVCs, n (% all CVCs) | IR per 1000 CVC days | IR per 1000 CVC treatment days | Events, n (%) | CVCs, n (% all CVCs) | IR per 1000 CVC days | IR per 1000 CVC treatment days | During CVC insertion | During treatment |
| Intra-operative | 2 (14.3) | 2 (6.5) | NA | NA | 2 (5.6) | 2 (3.5) | NA | NA | NA | NA |
| CLABSI | 1 (7.1) | 1 (3.2) | 0.08 | 0.24 | 2 (5.6) | 2 (3.5) | 0.33 | 0.33 | 3.99 (0.36–43.95) | 1.39 (0.13–15.33) |
| Local infection | 2 (14.3) | 2 (6.5) | 0.16 | 0.48 | 15 (41.7) | 15 (26.3) | 2.44 | 2.15 | 14.95 (3.42–65.35) | 4.52 (1.02–20.02) |
| CVT | 2 (14.3) | 2 (6.5) | 0.16 | 0.24 | 7 (19.24) | 7 (12.3) | 1.14 | 1.16 | 6.98 (1.45–33.57) | 4.87 (0.60–39.54) |
| Malfunction | 6 (46.9) | 4 (12.9) | 0.49 | 1.19 | 8 (22.2) | 6 (10.5) | 1.30 | 1.33 | 2.66 (0.92–7.66) | 1.11 (0.36–3.40) |
| Dislocation | 0 (0.0) | 0 (0.0) | 0.00 | 0.00 | 1 (2.8) | 1 (1.8) | 0.16 | 0.17 | Undefined | Undefined |
| Rupture | 0 (0.0) | 0 (0.0) | 0.00 | 0.00 | 1 (2.8) | 1 (1.8) | 0.16 | 0.17 | Undefined | Undefined |
| Dislodgement | 1 (7.1) | 1 (32) | 0.08 | 0.24 | 0 (0.0) | 0 (0.0) | 0.00 | 0.00 | Undefined | Undefined |
| Total | 14 (100.0) | 8 (25.8) | 1.14 | 2.86 | 36 (100.0) | 28 (49.1) | 5.85 | 5.64 | 5.12 (2.76–9.50) | 1.97 (1.02–3.80) |
| Clavien-Dindo grade of ≥ 3 | 2 (14.3) | 2a (6.5) | 0.16 | 0.24 | 12 (33.3) | 11a (19.3) | 1.95 | 1.99 | 11.96 (2.68–53.42) | 8.34 (1.09–64.13) |
| Removal due to complications | 2 (NA) | 2 (6.5) | 0.16 | 0.48 | 10 (NA) | 10 (17.5) | 1.63 | 1.66 | 9.96 (2.18–45.47) | 3.48 (0.76–15.86) |
CVCs central venous catheter, CLABSI central line associated bloodstream infection, CVT central venous thrombosis, TIVAP totally implantable venous access port, PICC peripherally inserted central catheter, IR incidence rate, IRR incidence rate ratio, CI confidence interval, N number, NA not applicable. The events, CVCs, and IR per 1000 CVC days columns include all CVC-related complications observed. The IR per 1000 CVC treatment days includes all CVC-related complications observed from CVC insertion until the end of treatment or removal of the first CVC, whichever comes first. aHighest Clavien-Dindo grade per CVC counted
During treatment, single lumen PICCs were also associated with a significantly higher risk of complications (IRR 1.97, CI95% 1.02–3.80), local irritation/infections in particular (IRR 4.52, CI95% 1.02–20.02), and complications with a Clavien-Dindo grade of III or higher (IRR 8.34, CI95% 1.09–64.13) (Table 3 and Online Resource 1).
Risk factors for CVC-related CVT
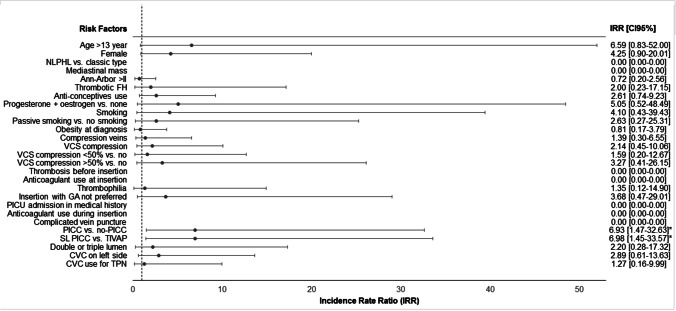
A (single lumen) PICC compared to a TIVAP or other non-PICC CVC was identified as the only significant risk factors for a CVC-related CVT. All other IRRs per CVT-related risk factor and their associated 95%CI are described in Fig. 1 and in more detail in the supporting information (Fig. 1 and Online Resource 4).
Fig. 1.
Risk factors for CVC-related CVT in pediatric Hodgkin lymphoma patients. CVC: central venous catheters, CVT: central venous thrombosis, TIVAP: totally implantable venous access port, PICC: peripherally inserted central catheter, FH: family history, NLPHL: nodular lymphocyte-predominant Hodgkin lymphoma, TPN: total parenteral nutrition, PICU: pediatric intensive care unit, SL: single lumen, GA: general anesthesia, VCS: vena cava superior, IRR: incidence rate ratio, CI: confidence interval. Mean CVC to vein ratio (calculated only for patients with a PICC) did not differ between patients with and without a CVC-related CVT (0.25 versus 0.27; CI95% − 0.02–0.06)
When the analysis was repeated twice including only single lumen PICCs and TIVAPs (n = 88) and excluding patients where general anesthesia was not preferred resulting in the insertion of a PICC instead of a TIVAP (n = 5), the insertion of a PICC was still identified as a risk factor. For patients with a single lumen PICC or TIVAP, only the female sex was additionally identified as a risk factor (Online Resource 5 and 6).
Discussion
In this study, we investigated the incidence of CVC-related complications in patients treated for HL. We found at least one complication in 42% of all patients. Complications were more often observed, more severe, and resulted in more frequent early CVC removal in patients receiving a PICC compared to patients with a TIVAP. One of the most frequent and severe complications was a CVC-related CVT, which occurred in one out of ten patients with HL. The incidence rate of CVC-related CVT in this study was seven times higher for patients with a single lumen PICC compared to patients with a TIVAP.
CVT is a severe complication, as most patients will receive anticoagulant therapy for months and CVC replacement is often necessary. In severe cases, CVT-infections, vena cava superior syndrome, embolisms, and long-term complications like post-thrombotic syndrome can occur. In this study, a CVC-related CVT incidence of 10% was observed. This incidence falls within the wide range reported for children with cancer in general of 2–50% [27–29]. The risk of CVC-related CVTs has been described to be higher in patients with HL compared to other oncology patients, presumably caused by the frequent presence of risk factors for CVT (e.g., vein compression in the upper body, high-dose corticosteroid treatment), as also described in the “Introduction” section [1, 3, 9, 11–14, 28]. However, the difference between HL and non-HL patients might also be explained by the CVC types included in the previously performed studies, i.e., only 0–3% PICCs [28, 29]. Previous studies including lymphoma patients only reported CVC-related CVT incidences of 7–9% for adults [30] and 3–7% for pediatric patients [1, 14]. The low incidence of 3% (definite CVC-related CVT) as reported by Schonning et al. [14] might be explained by the inclusion of mainly patients with a TIVAP and that the other not-definite CVTs reported by the authors might also have been related to the CVC.
The results of this study suggest that single lumen PICCs are associated with much higher complication rates (49% vs. 26%; IRR 5.12), early removal (18% vs. 7%; IRR 9.96), and more severe complications (19% vs. 7%; IRR 11.96) when compared to TIVAPs. The high rate of complications associated with PICCs has also been previously reported in a variety of patient populations [1–9]. In this study, the incidence rate of local irritation/infections and CVC-related CVTs specifically was higher in patients receiving single lumen PICCs compared to TIVAPs (26% vs. 7%; IRR 14.95 and 12% vs. 7%; IRR 6.98, respectively), suggesting that TIVAPs are more suitable for this patient group compared to PICCs in terms of CVC-related complications and the risk of early removal. However, due to the non-randomized retrospective nature of this study, differences in baseline characteristics between patients with a SL PICC and TIVAP were observed. Patients with a SL PICC were slightly older, were more often diagnosed with Ann-Arbor stage I or II, and had their CVC in situ for a shorter (treatment) period compared to patients with a TIVAP. The older age might be explained by the fact that PICC insertion without anesthesia is less preferable in younger patients. This might result in some bias since older age has been described to be associated with a higher incidence of CVT [1]. Furthermore, patients with a higher Ann-Arbor stage are more frequently expected to have a longer treatment duration, resulting in the more frequent insertion of a TIVAP in this group. Higher rates of CVT in children and adults with higher Ann-arbor stage have been previously described, but these results were not significant [11, 15]. In this study however, patients with a TIVAP developed less CVTs compared to patients with a PICC. The lower number of CVC-(treatment) days might be explained by the fact that PICCs are removed much sooner after the end of treatment. This can be explained by two reasons: (1) TIVAPs are more often left in situ for the clinical follow-up period compared to PICCs and (2) the surgical waiting lists for TIVAP removal. Since the risk of some complications might be higher during treatment (i.e., intensive use of CVC), the IRs per 1000 CVC-treatment days were also calculated, which still showed that the insertion of a SL PICC is associated with a significantly higher risk of (severe) complications and local infections in particular compared to a TIVAP.
The high rate of complications associated with PICCs was also reported previously in adult patients with HL and pediatric oncology patients in general [1, 9–13]. Three studies did investigate risk factors for CVT (CVC and non-CVC-related) specifically in pediatric patients with lymphoma. Gartrell et al. [1] identified the insertion of a PICC as a risk factor, but noted that this result might be biased since patients unstable for sedation with large mediastinal masses initially received a PICC. Insertion of a PICC as an independent risk factor for CVC-related CVT was not observed by Athale et al. [15] and Schonning et al. [14]; however, almost all patients included in these studies received a TIVAP. Furthermore, Athale et al. [15] identified the presence of a mediastinal mass as a risk factor. Schonning et al. [14] did not identify any risk factors [1, 14, 15]. Previous studies suggested that the device-specific quality of life was lower and costs were higher for oncology patients in general with a PICC compared to a TIVAP [4, 31]. Taxbro et al. [31] pointed out that this increase in costs was mainly caused by the costs related to complications.
Based on the complications found in this study together with the current literature, the insertion of a TIVAP rather than a PICC should be advised by physicians in pediatric patients diagnosed with HL, especially in case of CVT-related risk factors. This is in line with the conclusions drawn by Taxbro et al. [6] for oncology patients in general.
If a PICC is preferred, for example since general anesthesia is preferably avoided, the use of prophylactic anticoagulants could be considered, particularly when other risk factors for thrombosis in pediatric oncology patients like age, sex, thrombophilia, and vein compression are present [1]. In adult oncology patients with a PICC, the use of direct oral anticoagulants (DOAC) and low-molecular weight heparin for primary CVT prevention resulted in a significant decrease in the incidence of CVT with comparable safety outcomes [32, 33], but the evidence to use primary prophylaxis for patients with cancer and a CVC is still scarce and guidelines therefore do not recommend primary prophylaxis [34]. The first results of phase III trials investigating DOACs in children showed that DOACs are at least as efficient and safe as low-molecular weight heparin and vitamin K antagonists for the treatment and secondary prophylaxis of thrombotic events in children with different clinical conditions [35–38]. Future trials should be focusing on the use of DOACs as primary prevention for pediatric patients.
This study shows the high risk of CVC-related complications associated with pediatric patients diagnosed with HL receiving a PICC. Strengths are that this study describes a large pediatric HL cohort, that this study investigates all CVC-related complications, that CVC-related complication severity and outcomes were investigated, that multiple CVT-related risk factors were evaluated, and that separate analyses were performed excluding patients requiring a PICC instead of a TIVAP since CVC insertion under general anesthesia was not preferred. Limitations of this study are the retrospective study design, differences in baseline characteristics between the SL PICC and TIVAP group, and the impossibility to perform a multivariate analysis due to the small patient group.
In conclusion, PICCs were associated with a higher risk of (severe) complications, CVTs specifically, and subsequent CVC removal when compared to TIVAPs. The insertion of a TIVAP rather than a PICC should therefore be advised by physicians in pediatric patients diagnosed with HL, especially in case of CVT-related risk factors. Future trials should evaluate the efficacy and safety of direct oral anticoagulants for the primary prevention of CVT in pediatric patients with a PICC and other CVT-related risk factors.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge all patients and parents who gave permission to the use of their clinical data for research. Furthermore, we would like to acknowledge the Princess Máxima Center for Pediatric Oncology for the support of this research.
Author contribution
Methodology: C.B., J.S., A.B., M.F., I.K., L.S., M.W., and M.vdW. Formal analysis and investigation: C.B., J.S., M.F., and A.B. Writing–original draft: C.B. and J.S. Writing and editing: C.B., I.K., A.B., J.S., I.M., M.F., L.S., M.vdW, and F.M.-W. Supervision: J.S., A.B., M.F., L.S., M.W., and M.vdW.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Code availability
The custom code that supports the findings of this study is available on request from the corresponding author.
Declarations
Ethics approval
The medical ethics committee of the University Medical Center Utrecht (Utrecht, The Netherlands) waived the need for official approval by the medical ethics committee (File number: 21/723) in view of the retrospective nature of the study and all the procedures being performed were part of the routine care. The study was performed in accordance with the Declaration of Helsinki and its later amendments.
Consent to participate
Written informed consent was obtained from all individual participants and/or their parents depending on the age included in the study.
Consent for publication
Not applicable since the manuscript does not contain any individual person’s data (individual details, images, or videos).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gartrell J, Kaste SC, Sandlund JT, Flerlage J, Zhou Y, Cheng C, Estepp J, Metzger ML. The association of mediastinal mass in the formation of thrombi in pediatric patients with non-lymphoblastic lymphomas. Pediatr Blood Cancer. 2020;67:e28057. doi: 10.1002/pbc.28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chopra V, Anand S, Hickner A, Buist M, Rogers MA, Saint S, Flanders SA. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382:311–325. doi: 10.1016/S0140-6736(13)60592-9. [DOI] [PubMed] [Google Scholar]
- 3.Ellis ML, Okano S, McCann A, McDowall A, Van Kuilenburg R, McCarthy AL, Joubert W, Harper J, Jones M, Mollee P. Catheter-related thrombosis incidence and risk factors in adult cancer patients with central venous access devices. Intern Med J. 2020;50:1475–1482. doi: 10.1111/imj.14780. [DOI] [PubMed] [Google Scholar]
- 4.Moss JG, Wu O, Bodenham AR, Agarwal R, Menne TF, Jones BL, Heggie R, Hill S, Dixon-Hughes J, Soulis E, Germeni E, Dillon S, McCartney E, group Ct, Central venous access devices for the delivery of systemic anticancer therapy (CAVA): a randomised controlled trial. Lancet. 2021;398:403–415. doi: 10.1016/S0140-6736(21)00766-2. [DOI] [PubMed] [Google Scholar]
- 5.Pu YL, Li ZS, Zhi XX, Shi YA, Meng AF, Cheng F, Ali A, Li C, Fang H, Wang C. Complications and costs of peripherally inserted central venous catheters compared with implantable port catheters for cancer patients: a meta-analysis. Cancer Nurs. 2020;43:455–467. doi: 10.1097/NCC.0000000000000742. [DOI] [PubMed] [Google Scholar]
- 6.Taxbro K, Chopra V. Appropriate vascular access for patients with cancer. Lancet. 2021;398:367–368. doi: 10.1016/S0140-6736(21)00920-X. [DOI] [PubMed] [Google Scholar]
- 7.Taxbro K, Hammarskjold F, Thelin B, Lewin F, Hagman H, Hanberger H, Berg S. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: an open-label, randomised, two-centre trial. Br J Anaesth. 2019;122:734–741. doi: 10.1016/j.bja.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 8.Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136:1331–1344. doi: 10.1542/peds.2015-1507. [DOI] [PubMed] [Google Scholar]
- 9.Hsiao W, Krava E, Wee CP, Chau E, Jaffray J. The incidence and risk factors for venous thromboembolism in adolescent and young adult oncology patients. Pediatr Blood Cancer. 2021;68:e28957. doi: 10.1002/pbc.28957. [DOI] [PubMed] [Google Scholar]
- 10.Jaffray J, Witmer C, O'Brien SH, Diaz R, Ji L, Krava E, Young G. Peripherally inserted central catheters lead to a high risk of venous thromboembolism in children. Blood. 2020;135:220–226. doi: 10.1182/blood.2019002260. [DOI] [PubMed] [Google Scholar]
- 11.Borchmann S, Muller H, Hude I, Fuchs M, Borchmann P, Engert A. Thrombosis as a treatment complication in Hodgkin lymphoma patients: a comprehensive analysis of three prospective randomized German Hodgkin Study Group (GHSG) trials. Ann Oncol. 2019;30:1329–1334. doi: 10.1093/annonc/mdz168. [DOI] [PubMed] [Google Scholar]
- 12.Connors JM. Hodgkin lymphoma, treatment and thrombosis: a dangerous mix. Ann Oncol. 2019;30:1192–1193. doi: 10.1093/annonc/mdz174. [DOI] [PubMed] [Google Scholar]
- 13.Newman GM. Thrombosis: risk factors among pediatric patients with cancer. Clin J Oncol Nurs. 2020;24:58–64. doi: 10.1188/20.CJON.58-64. [DOI] [PubMed] [Google Scholar]
- 14.Schonning A, Karlen J, Frisk T, Heyman M, Svahn JE, Ora I, Kawan L, Holmqvist BM, Bjorklund C, Harila-Saari A, Ranta S. Venous thrombosis in children and adolescents with Hodgkin lymphoma in Sweden. Thromb Res. 2017;152:64–68. doi: 10.1016/j.thromres.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Athale UH, Nagel K, Khan AA, Chan AK. Thromboembolism in children with lymphoma. Thromb Res. 2008;122:459–465. doi: 10.1016/j.thromres.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC) (2022) Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-central Line Associted Bloodstream Infection). CDC National Healthcare Safety Network (NHSN). https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf. Accessed 01 Jan 2022
- 17.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg SA. Validity of the Ann Arbor staging classification for the non-Hodgkin’s lymphomas. Cancer Treat Rep. 1977;61:1023–1027. [PubMed] [Google Scholar]
- 20.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, Alliance AL. Lymphoma G. Eastern Cooperative Oncology G. European Mantle Cell Lymphoma C. Italian Lymphoma F. European Organisation for R. Treatment of Cancer/Dutch Hemato-Oncology G. GrupoEspanol de Medula O. German High-Grade Lymphoma Study G. German Hodgkin's Study G. Japanese Lymphorra Study G. Lymphoma Study A. Group NCT. Nordic Lymphoma Study G. Southwest Oncology G. United Kingdom National Cancer Research I Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Betensky M, Goldenberg NA. Post-thrombotic syndrome in children. Thromb Res. 2018;164:129–135. doi: 10.1016/j.thromres.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Sharp R, Carr P, Childs J, Scullion A, Young M, Flynn T, Kirker C, Jackson G, Esterman A. Catheter to vein ratio and risk of peripherally inserted central catheter (PICC)-associated thrombosis according to diagnostic group: a retrospective cohort study. BMJ Open. 2021;11:e045895. doi: 10.1136/bmjopen-2020-045895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52:162–193. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin DAH. An efficient program for computing conditional maximum likelihood estimates and exact confidence limits for a common odds ratio. Epidemiology. 1991;2:359–362. doi: 10.1097/00001648-199109000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Martin DAH. Exact estimates for a rate ratio. Epidemiology. 1996;7:29–33. doi: 10.1097/00001648-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Ko RH, Thornburg CD. Venous thromboembolism in children with cancer and blood disorders. Front Pediatr. 2017;5:12. doi: 10.3389/fped.2017.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoot RA, van de Wetering MD, Stijnen T, Tissing WJ, Michiels E, Abbink FC, Raphael MF, Heij HA, Zwaan M, Lieverst JA, Caron HN, van Ommen H, Group DC-ASCW Prevalence of symptomatic and asymptomatic thrombosis in pediatric oncology patients with tunneled central venous catheters. Pediatr Blood Cancer. 2016;63:1438–1444. doi: 10.1002/pbc.26036. [DOI] [PubMed] [Google Scholar]
- 29.van den Bosch CH, van der Bruggen JT, Frakking FNJ, Terwisscha van Scheltinga CEJ, van de Ven CP, van Grotel M, Wellens LM, Loeffen YGT, Fiocco M, Wijnen M. Incidence, severity and outcome of central line related complications in pediatric oncology patients; a single center study. J Pediatr Surg. 2019;54:1894–1900. doi: 10.1016/j.jpedsurg.2018.10.054. [DOI] [PubMed] [Google Scholar]
- 30.Hohaus S, Bartolomei F, Cuccaro A, Maiolo E, Alma E, D'Alo F, Bellesi S, Rossi E, De Stefano V. Venous thromboembolism in lymphoma: risk stratification and antithrombotic prophylaxis. Cancers. 2020;12:1259. doi: 10.3390/cancers12051291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taxbro K, Hammarskjold F, Juhlin D, Hagman H, Bernfort L, Berg S. Cost analysis comparison between peripherally inserted central catheters and implanted chest ports in patients with cancer-a health economic evaluation of the PICCPORT trial. Acta Anaesthesiol Scand. 2020;64:385–393. doi: 10.1111/aas.13505. [DOI] [PubMed] [Google Scholar]
- 32.Lv S, Liu Y, Wei G, Shi X, Chen S, Zhang X. The anticoagulants rivaroxaban and low molecular weight heparin prevent PICC-related upper extremity venous thrombosis in cancer patients. Medicine. 2019;98:e17894. doi: 10.1097/MD.0000000000017894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khachatryan T, Hauschild C, Hoff J, Contractor T, Khachatryan A, Tran H, Matsuo B, Jacobson A, Hilliard A. Review of direct oral anticoagulants and guide for effective drug utilization. Am J Cardiovasc Drugs. 2019;19:525–539. doi: 10.1007/s40256-019-00344-6. [DOI] [PubMed] [Google Scholar]
- 34.Lyman GH, Carrier M, Ay C, Di Nisio M, Hicks LK, Khorana AA, Leavitt AD, Lee AYY, Macbeth F, Morgan RL, Noble S, Sexton EA, Stenehjem D, Wiercioch W, Kahale LA, Alonso-Coello P. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5:927–974. doi: 10.1182/bloodadvances.2020003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albisetti M. Use of direct oral anticoagulants in children and adolescents. Hamostaseologie. 2020;40:64–73. doi: 10.1055/s-0039-3400491. [DOI] [PubMed] [Google Scholar]
- 36.Male C, Lensing AWA, Palumbo JS, Kumar R, Nurmeev I, Hege K, Bonnet D, Connor P, Hooimeijer HL, Torres M, Chan AKC, Kenet G, Holzhauer S, Santamaria A, Amedro P, Chalmers E, Simioni P, Bhat RV, Yee DL, Lvova O, Beyer-Westendorf J, Biss TT, Martinelli I, Saracco P, Peters M, Kallay K, Gauger CA, Massicotte MP, Young G, Pap AF, Majumder M, Smith WT, Heubach JF, Berkowitz SD, Thelen K, Kubitza D, Crowther M, Prins MH, Monagle P, Investigators EI-JP. Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematol. 2020;7:18–27. doi: 10.1016/S2352-3026(19)30219-4. [DOI] [PubMed] [Google Scholar]
- 37.Halton J, Brandao LR, Luciani M, Bomgaars L, Chalmers E, Mitchell LG, Nurmeev I, Sharathkumar A, Svirin P, Gorbatikov K, Tartakovsky I, Simetzberger M, Huang F, Sun Z, Kreuzer J, Gropper S, Reilly P, Brueckmann M, Albisetti M, Investigators DT. Dabigatran etexilate for the treatment of acute venous thromboembolism in children (DIVERSITY): a randomised, controlled, open-label, phase 2b/3, non-inferiority trial. Lancet Haematol. 2021;8:22–33. doi: 10.1016/S2352-3026(20)30368-9. [DOI] [PubMed] [Google Scholar]
- 38.Brandao LR, Albisetti M, Halton J, Bomgaars L, Chalmers E, Mitchell LG, Nurmeev I, Svirin P, Kuhn T, Zapletal O, Tartakovsky I, Simetzberger M, Huang F, Sun Z, Kreuzer J, Gropper S, Brueckmann M, Luciani M, Investigators DS. Safety of dabigatran etexilate for the secondary prevention of venous thromboembolism in children. Blood. 2020;135:491–504. doi: 10.1182/blood.2019000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
The custom code that supports the findings of this study is available on request from the corresponding author.