Abstract
Background
First-line standard-of-care therapy for advanced cholangiocarcinoma is gemcitabine plus cisplatin; there is no established second-line systemic therapy. Fibroblast growth factor receptor (FGFR)-2 fusions/rearrangements can be oncogenic drivers, occurring almost exclusively in intrahepatic cholangiocarcinoma, but little is known about whether FGFR2 status affects the response to systemic chemotherapy.
Objective
We aimed to evaluate the effects of FGFR2 status on survival outcomes in patients receiving systemic therapy for intrahepatic cholangiocarcinoma.
Methods
In this retrospective analysis, patients treated with systemic therapy at Memorial Sloan Kettering Cancer Center for intrahepatic cholangiocarcinoma were categorized into three cohorts: FGFR2 fusions; other FGFR2 alterations; no FGFR2 alterations. Endpoints were overall survival and progression-free survival per therapy line.
Results
In total, 132 patients with intrahepatic cholangiocarcinoma were included (FGFR2 fusions, n = 15; other FGFR2 alterations, n = 2 [data not reported]; no FGFR2 alterations, n = 115). First-line therapy was platinum based in 93% of patients; 80% received platinum/pyrimidine-based second-line therapy. For patients with FGFR2 fusions and no FGFR2 alterations, respectively, median overall survival from diagnosis was 31.3 months (95% confidence interval [CI] 5.8–not estimable months) [n = 9] and 21.7 months (95% CI 16.1–26.6) [n = 109]; median progression-free survival in first-line therapy was 6.2 months (95% CI 2.0–16.8) [n = 15] and 7.2 months (95% CI 5.0–8.3) [n = 107], and median progression-free survival in second-line therapy was 5.6 months (95% CI 2.8–10.3) [n = 8] and 3.7 months (95% CI 2.6–5.6) [n = 81].
Conclusions
Patients with intrahepatic cholangiocarcinoma and FGFR2 fusions may have a better prognosis than those without FGFR2 alterations in terms of overall survival, and progression-free survival on second-line, but not first-line systemic therapy. Progression-free survival improvement on second-line chemotherapy may imply an important impact of prior chemotherapy as first line.
Plain Language Summary
Intrahepatic cholangiocarcinoma (iCCA) can be caused by changes in many different genes. One type of change in iCCA is a fibroblast growth factor receptor 2 gene (FGFR2) fusion. In fusions, the FGFR2 gene has fused to another gene. Our study examined people with iCCA to compare the overall survival following diagnosis for people with FGFR2 changes and people without. We also measured progression-free survival, which is the time from their first chemotherapy dose until their cancer got worse. All participants had iCCA and their first or second treatment was chemotherapy. Fifteen participants had FGFR2 fusions and 115 had no FGFR2 changes. We found that participants with FGFR2 fusions lived longer (median 31 months) than those without these fusions (median 22 months). During their first treatment, median progression-free survival was similar for participants with and without FGFR2 fusions. After the second chemotherapy, median progression-free survival was about 2 months longer for participants with FGFR2 fusions than those without. Results will vary from person to person and will depend on other factors. However, people with iCCA with FGFR2 fusions may stay slightly longer on their second treatment without their cancer getting worse. With chemotherapy, they may also live somewhat longer than those without FGFR2 fusions.
KeyPoints
| Patients with intrahepatic cholangiocarcinoma and fibroblast growth factor receptor (FGFR)-2 alterations appear to have longer overall survival and longer progression-free survival on second-line systemic therapy compared with patients without FGFR alterations. |
| The apparent advantage in progression-free survival during second-line systemic therapy in patients with intrahepatic cholangiocarcinoma and FGFR2 alterations may have an implication to the value of first-line chemotherapy. |
Introduction
Cholangiocarcinoma (CCA), involving the intrahepatic, perihilar, or distal biliary tree, is the second most common hepatic malignancy [1]. Symptoms of CCA are often nonspecific, including abdominal pain, malaise, night sweats, cachexia, fatigue, and jaundice [2, 3]. Most patients with CCA are diagnosed at an advanced disease stage and are not qualified for potentially curative surgery; for patients who do undergo surgery, the relapse rate is 49–64% [2]. First-line standard-of-care therapy for advanced/metastatic biliary tract cancer, including CCA, is gemcitabine plus cisplatin (GemCis) [4], with reported median overall survival (OS) of 11.2–11.7 months and progression-free survival (PFS) of 5.8–8.0 months [5, 6]. For molecularly unselected patients who have progressed on first-line therapy, there is no established systemic therapy; current second-line chemotherapy regimens are associated with limited survival outcomes (OS 6.2–11.0 months; PFS 3.2–4.0 months) [7–11].
Fibroblast growth factor receptor (FGFR)-2 fusions or rearrangements can be oncogenic drivers, occurring almost exclusively in patients with intrahepatic CCA (iCCA) [12, 13]. In patients with CCA, FGFR alterations (predominantly FGFR2 fusions or rearrangements) occur more frequently in younger patients and women [14, 15]. Supported by data from the phase II FIGHT-202 study (NCT02924376) [16], pemigatinib, a selective, potent, oral FGFR 1–3 inhibitor [17], was the first to receive approval for the treatment of patients with previously treated, locally advanced or metastatic CCA harboring an FGFR2 fusion or other rearrangement in Canada, Europe, Japan, and the USA [18–21]. The selective, potent, oral FGFR1–4 inhibitor, infigratinib, is also approved for previously treated, unresectable locally advanced or metastatic CCA with an FGFR2 fusion or other rearrangement [22, 23]; the selective irreversible FGFR1–4 inhibitor, futibatinib, was granted a priority review by the US Food and Drug Administration for the treatment of patients with locally advanced or metastatic cholangiocarcinoma with FGFR2 rearrangements [24].
The question of whether FGFR2 status affects the response to systemic chemotherapy remains to be resolved. Evidence in favor of this was provided by a retrospective study of 377 patients with biliary tract cancer (72% with iCCA) predominantly receiving one line or more of standard chemotherapy (91%), which demonstrated significantly longer OS in patients with (n = 95) versus patients without (n = 282) FGFR alterations (37 vs 20 months; p < 0.001) [15]. In the same study, PFS in patients who had received first-line chemotherapy was not significantly different between those with versus those without FGFR alterations (33 vs 25 months; p = 0.074) [15]. A more recent post hoc data analysis from the phase II FIGHT-202 study assessed response to systemic therapy in patients with CCA harboring FGFR2 fusions or rearrangements before enrollment. It demonstrated a median PFS of 5.6 and 4.4 months on prior first-line systemic therapy and second-line systemic therapy, respectively [25]. Of note, median PFS on first-line systemic therapy or second-line systemic therapy received before FIGHT-202 enrollment were both shorter than the observed median PFS on second-line pemigatinib received during FIGHT-202 (7.0 months) [16].
The Memorial Sloan Kettering Cancer Center (MSK) [New York, NY, USA] obtains genomic sequencing data for patients with iCCA treated at the institution, allowing genomic profiling data to be overlaid with clinical data to facilitate a meaningful understanding of patient outcomes, and to suggest potential therapeutic options. This retrospective analysis evaluated OS and PFS of patients with iCCA harboring FGFR2 fusions who received systemic therapy at MSK.
Methods
Patients
Patients with iCCA treated at MSK were included in this study; clinical and genomic data (based on tissue biopsy) were obtained from the MSK database. Patients who received FGFR inhibitors or isocitrate dehydrogenase inhibitors were excluded from the analysis. Patients with iCCA were categorized into three groups according to FGFR2 status obtained from next-generation sequencing (MSK-IMPACT [26]) data: patients with FGFR2 fusions; patients with other FGFR2 alterations; and patients with no FGFR2 alterations. Clinical data included disease history and exposure to prior lines of systemic therapy in the advanced setting; only patients with complete data for initiation and completion of prior lines of therapy were analyzed.
Endpoints
Overall survival was defined as the duration from iCCA diagnosis (of any stage) until death; PFS was defined as the duration from the first dose of first-line or second-line systemic therapy until progression, death, last visit, or end-of-line treatment/cycle. Patients who were lost to follow-up were censored at the last known follow-up date.
Statistical Analysis
Continuous variables were summarized using descriptive statistics, including median and range. Binary variables were described using number and percentage, with 95% confidence intervals (CIs) calculated where appropriate. Overall survival and PFS distributions were calculated using the Kaplan–Meier method. Statistical analysis was conducted using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
Patients
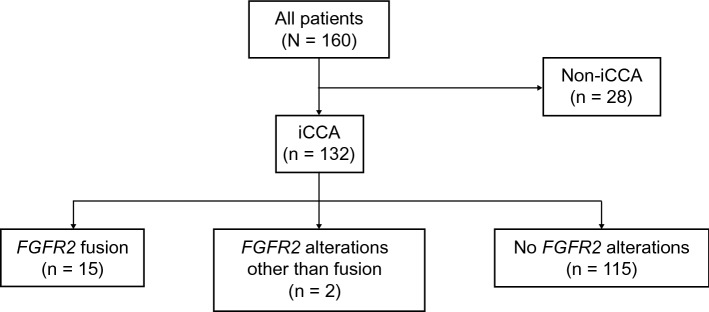
Of 160 patients with CCA treated at MSK from 2013 to 2019, 132 de-identified patients with iCCA were included in this analysis: 15 patients (11%) with FGFR2 fusions; two patients (2%) with other FGFR2 alterations; and 115 patients (87%) with no FGFR2 alterations (Fig. 1). Because there were only two patients with other FGFR2 alterations, data from this group are not reported in this article. Three patients received FGFR inhibitors in second-line therapy, and three patients received FGFR inhibitors in third-line therapy; these patients were therefore excluded from PFS and OS analyses in accordance with the eligibility criteria. In addition, six patients received isocitrate dehydrogenase inhibitors and were ineligible for OS calculations.
Fig. 1.
Distribution of patients. FGFR2 fibroblast growth factor receptor 2, iCCA intrahepatic cholangiocarcinoma
In all 132 patients, median age at diagnosis was 62.0 years, 54.5% were women, and 21.1% had received more than three lines of therapy (Table 1). Patients with FGFR2 fusions were younger than those with no FGFR2 alterations (median, 58.0 vs 64.0 years), and other patient characteristics were similar across cohorts (Table 1). The median follow-up was 21.2 months (range, 2–161 months); median durations of first-line and second-line treatments were 4.6 months (< 0.1–36.6 months) and 3.1 months (< 0.1–85.0 months), respectively. First-line therapy was platinum based in 93% (107/115) of patients; 80% (71/89) of patients received platinum-based or pyrimidine-based second-line therapy. The major reason for discontinuation of first-line or second-line treatment was disease progression (62% [77/125]; 73/93 [78%]).
Table 1.
Demographics and clinical characteristics of patients with iCCA
| Characteristics | FGFR2 fusions (n = 15) | No FGFR2 alterations (n = 115) | All patients with iCCA (n = 132)a |
|---|---|---|---|
| Age at diagnosis, median (range), years | 58.0 (36–73) | 64.0 (28–86) | 62.0 (28–86) |
| < 40, n (%) | 2 (13.3) | 5 (4.3) | 7 (5.3) |
| 40 to <65, n (%) | 10 (66.7) | 55 (47.8) | 66 (50.0) |
| ≥ 65, n (%) | 3 (20.0) | 55 (47.8) | 59 (44.7) |
| Sex, n (%) | |||
| Male | 6 (40.0) | 53 (46.1) | 60 (45.5) |
| Female | 9 (60.0) | 62 (53.9) | 72 (54.5) |
| Race, n (%) | |||
| Evaluable, n | 14 | 106 | 121 |
| White | 12 (85.7) | 96 (90.6) | 109 (90.1) |
| Black | 1 (7.1) | 5 (4.7) | 6 (5.0) |
| Asian | 1 (7.1) | 5 (4.7) | 6 (5.0) |
| Prior resection, n (%) | |||
| Yes | 5 (33.3) | 36 (31.3) | 42 (31.8) |
| No | 10 (66.7) | 79 (68.7) | 90 (68.2) |
| Disease stage at diagnosis, n (%) | |||
| 1 | 3 (20.0) | 9 (7.8) | 13 (9.8) |
| 2 | 0 | 18 (15.7) | 18 (13.6) |
| 3 | 3 (20.0) | 26 (22.6) | 30 (22.7) |
| 4 | 9 (60.0) | 62 (53.9) | 71 (53.8) |
| Number of treatment lines following initial diagnosis, n (%) | |||
| Evaluable, n | 15 | 111 | 128 |
| 0 | 0 | 4 (3.6) | 4 (3.1) |
| 1 | 4 (26.7) | 26 (23.4) | 31 (24.2) |
| 2 | 1 (6.7) | 29 (26.1) | 31 (24.2) |
| 3 | 3 (20.0) | 32 (28.8) | 35 (27.3) |
| >3 | 7 (46.7) | 20 (18.0) | 27 (21.1) |
| 1L treatment, n (%) | |||
| Evaluable, n | 15 | 99 | 115 |
| Platinum basedb | 14 (93.3) | 92 (92.9) | 107 (93.0) |
| Non-platinum basedc | 1 (6.7) | 7 (7.1) | 8 (7.0) |
| 2L treatment, n (%) | |||
| Evaluable, n | 11 | 77 | 89 |
| Pyrimidine based | 7 (63.6) | 45 (58.4) | 53 (59.6) |
| Pyrimidine/platinum | 1 (9.1) | 17 (22.1) | 18 (20.2) |
| Other | 3 (27.3) | 15 (19.5) | 18 (20.2) |
| Duration of 1L therapy | |||
| Evaluable, n | 13 | 89 | 103 |
| Overall, median (range), days | 105.0 (43–770) | 140.0 (1–1114) | 139.0 (1–1114) |
| Evaluable, n | 10 | 70 | 81 |
| Platinum-based, median (range), days | 98.0 (43–770) | 175.0 (1–1114) | 141.0 (1–1114) |
| Evaluable, n | 3 | 19 | 22 |
| Non-platinum-based, median (range), days | 169.0 (97–505) | 113.0 (36–751) | 117.0 (36–751) |
| Duration of 2L therapy | |||
| Evaluable, n | 10 | 71 | 82 |
| Overall, median (range), days | 185.5 (80–1149) | 76.0 (1–2584) | 93.5 (1–2584) |
1L first-line, 2L second-line, FGFR2 fibroblast growth factor receptor 2, iCCA intrahepatic cholangiocarcinoma
aTwo patients with other FGFR2 alterations are not presented
bGemcitabine + cisplatin; gemcitabine, cisplatin + other therapy; gemcitabine, platinum therapy (not cisplatin) + other therapy; or platinum therapy + other therapy
cGemcitabine monotherapy; gemcitabine, nonplatinum therapy + other therapy; or other therapy
Overall Survival and Progression-Free Survival
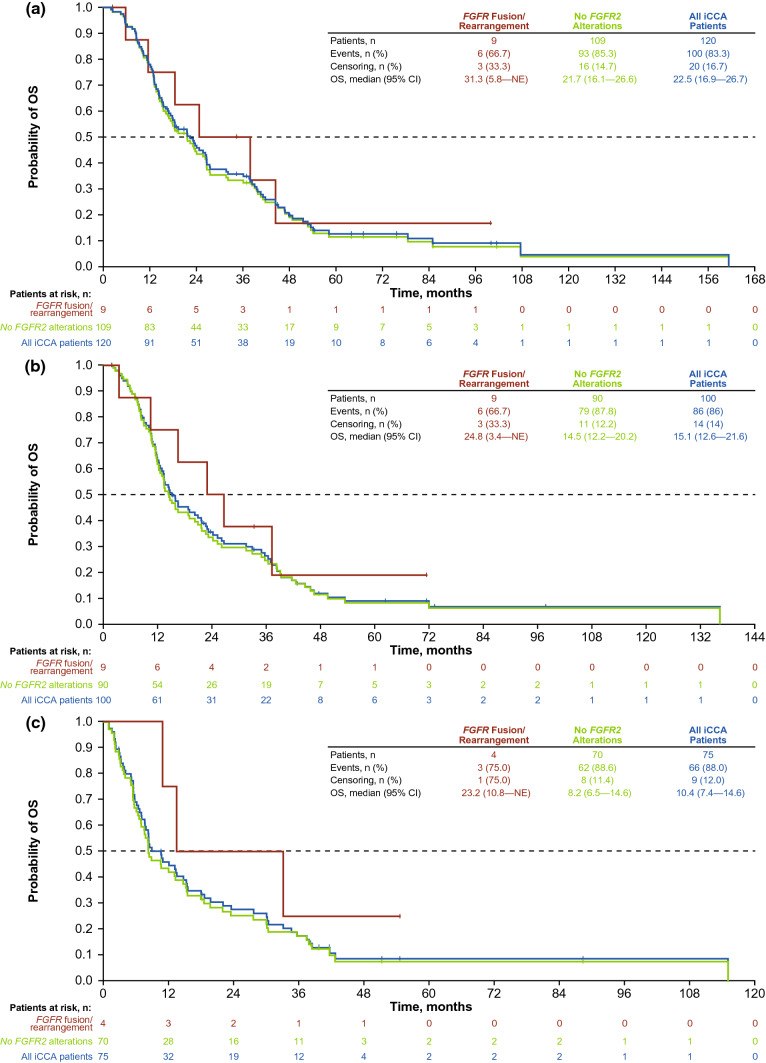
Median OS for all evaluable patients included in the analysis was 22.5 months (95% CI 16.9–26.7 months [n = 120]) (Table 2; Fig. 2a). Twelve patients were not evaluable for OS calculations: six patients who received isocitrate dehydrogenase inhibitors and six patients who received FGFR inhibitors. Median OS was 31.3 months (95% CI 5.8–not estimable) in patients with FGFR2 fusions (n = 9) and 21.7 months (95% CI 16.1–26.6) for patients with no FGFR2 alterations (n = 109). From the start of first-line therapy, patients with FGFR2 fusions had a median OS of 24.8 months (95% CI 3.4–not estimable [n = 9]), and patients with no FGFR2 alterations had a median OS of 14.5 months (95% CI 12.2–20.2 [n = 90]) (Table 2; Fig. 2b). From the start of second-line therapy, patients with FGFR2 fusions had a median OS of 23.2 months (95% CI 10.8–not estimable [n = 4]), and patients with no FGFR2 alterations had a median OS of 8.2 months (95% CI 6.5–14.6 [n = 70]) (Table 2; Fig. 2c). Small sample sizes preclude interpretation of median OS following second-line therapy.
Table 2.
OS in patients with iCCA
| Variable | FGFR2 fusions (n = 15) | No FGFR2 alterations (n = 109) | All patients with iCCA (n = 126)a |
|---|---|---|---|
| OS since diagnosis | |||
| Evaluable, n | 9 | 109 | 120 |
| Median (95% CI), months | 31.3 (5.8–NE) | 21.7 (16.1–26.6) | 22.5 (16.9–26.7) |
| OS since start of 1L therapy | |||
| Evaluable, n | 9 | 90 | 100 |
| Median (95% CI), months | 24.8 (3.4–NE) | 14.5 (12.2–20.2) | 15.1 (12.6–21.6) |
| OS since start of 2L therapy | |||
| Evaluable, n | 4 | 70 | 75 |
| Median (95% CI), months | 23.2 (10.8–NE) | 8.2 (6.5–14.6) | 10.4 (7.4–14.6) |
1L first-line, 2L second-line, CI confidence interval, FGFR2 fibroblast growth factor receptor 2, iCCA intrahepatic cholangiocarcinoma, NE not estimable, OS overall survival
aTwo patients with other FGFR2 alterations are not presented
Fig. 2.
Overall survival (OS) a since diagnosis, b since the start of first-line therapy, and c since the start of second-line therapy. CI confidence interval, FGFR fibroblast growth factor receptor, FGFR2 fibroblast growth factor receptor 2, iCCA intrahepatic cholangiocarcinoma, NE not estimable
Median PFS with first-line therapy was 7.1 months (95% CI 5.0–8.3) for all patients (n = 124), 6.2 months (95% CI 2.0–16.8) for patients with FGFR2 fusions (n = 15), and 7.2 months (95% CI 5.0–8.3) for patients with no FGFR2 alterations (n = 107) (Table 3). Median PFS with second-line therapy was 5.6 months (95% CI 2.8–10.3) for patients with FGFR2 fusions (n = 8) and 3.7 months (95% CI 2.6–5.6) for patients with no FGFR2 alterations (n = 81) (Table 3). Median PFS following second-line therapy should be interpreted with caution owing to the small sample sizes.
Table 3.
PFS in patients with iCCA
| Variable | FGFR2 fusions (n = 15) | No FGFR2 alterations (n = 115) | All patients with iCCA (n = 132)a |
|---|---|---|---|
| PFS since start of 1L therapy | |||
| Evaluable, n | 15 | 107 | 124 |
| Overall, median (95% CI), months | 6.2 (2.0–16.8) | 7.2 (5.0–8.3) | 7.1 (5.0–8.3) |
| Evaluable, n | 12 | 78 | 91 |
| Platinum-based, median (95% CI), months | 4.0 (1.9–NE) | 7.1 (4.9–8.2) | 6.7 (4.6–8.2) |
| Evaluable, n | 3 | 21 | 24 |
| Non-platinum-based, median (95% CI), months | 6.2 (3.2–NE) | 5.3 (3.0–11.2) | 5.3 (3.2–11.2) |
| PFS since start of 2L therapy | |||
| Evaluable | 8 | 81 | 90 |
| Median (95% CI), months | 5.6 (2.8–10.3) | 3.7 (2.6–5.6) | 3.7 (2.8–5.6) |
1L first-line, 2L second-line, CI confidence interval, FGFR2 fibroblast growth factor receptor 2, iCCA intrahepatic cholangiocarcinoma, NE not estimable, PFS progression-free survival
aTwo patients with other FGFR2 alterations are not presented
Discussion and Conclusions
Comprehensive molecular profiling studies have demonstrated that actionable genetic alterations are present in approximately 45% of patients with CCA [13, 27], prompting a wealth of research into personalized treatment regimens targeting specific oncogenic drivers. However, few studies have looked at how these genetic alterations may affect outcomes following standard systemic therapy. This study provides real-world evidence on the characteristics and treatment outcomes of patients with and without FGFR2 fusion-driven iCCA using next-generation sequencing data obtained by MSK-IMPACT.
In general, the baseline demographics and clinical characteristics of patients with FGFR2 fusions included in this retrospective study are similar to those of patients enrolled in phase II studies of pemigatinib (FIGHT-202) [16], and infigratinib [28]. In addition, the percentage of FGFR2 fusions detected in this cohort (15/132; 11%) is consistent with the published values (9–14%) [13, 14, 16, 29]. Although comparisons across groups were rendered difficult by the small numbers of patients and the fact that they were not randomly assigned to each cohort, the observation that patients with iCCA harboring FGFR2 fusions were younger compared with those with no FGFR2 alterations is consistent with the published literature [14, 16].
Median OS in all patients with iCCA, regardless of genomic status, was 22.5 months; this is longer than the median OS of 12.6 months reported in a recent pooled post hoc analysis of 109 molecularly unselected patients with iCCA receiving first-line chemotherapy in the ABC-01, ABC-02, and ABC-03 trials [30]. A previous retrospective study assessed the natural history of CCA harboring FGFR alterations in 377 patients with biliary tract cancer (72% iCCA; 12% extrahepatic CCA; 16% gallbladder) [15]. Among 341 patients in this analysis who had not received FGFR-directed therapy, those harboring FGFR genomic alterations (n = 59) were found to have significantly longer OS compared with those who did not have FGFR alterations (n = 282) [30 vs 20 months; p = 0.027] [15]. Another observational study of 571 patients with advanced CCA also demonstrated that median OS was prolonged for those with FGFR2 fusions or rearrangements compared with those without FGFR2 alterations (12.1 vs 7.1 months), although the difference was not statistically significant (p > 0.05) and FGFR2 status was not found to be a significant covariate of OS [31]. Consistent with these previous results, our findings suggest that median OS was numerically longer in patients with iCCA harboring FGFR2 fusions compared with those with no FGFR2 alterations (31.3 vs 21.7 months). Overall survival from the start of first-line or second-line therapy also appeared more favorable in patients with FGFR2 fusions (median 24.8 and 23.2 months, respectively) compared with patients with no FGFR2 alterations (median 14.5 and 8.2 months, respectively), although only four patients with FGFR2 fusions were evaluable for OS from the start of second-line therapy.
A previously published retrospective study demonstrated that the median PFS of patients receiving first-line chemotherapy predominantly for CCA was not statistically significantly different for cohorts with FGFR alterations versus those without FGFR alterations (33.9 vs 25.4 weeks [1.1 vs 0.8 months]; p = 0.07) [15]. Consistent with this finding, it was observed here that the median PFS associated with first-line systemic therapy in patients with FGFR2 fusions was shorter than that observed in patients with no FGFR2 alterations (6.2 vs 7.2 months). Of note, the observed median PFS associated with platinum-based therapy in first-line therapy was also shorter in patients with FGFR2 fusions versus PFS in patients with no FGFR2 alterations (4.0 vs 7.1 months), supporting the use of targeted first-line therapy in patients with FGFR2 fusions. Furthermore, there is the observation that median PFS following second-line systemic therapy was longer in patients with versus patients without FGFR2 alterations (5.6 vs 3.7 months). These results support the investigation of novel treatment approaches for CCA in second-line therapy, particularly in patients without FGFR2 alterations given the shorter PFS following second-line systemic therapy in these patients.
The efficacy and safety of several systemic chemotherapeutic regimens have been assessed previously for the treatment of patients with molecularly unselected advanced biliary tract cancer in second-line therapy [7, 8, 10, 11, 32, 33]. Second-line chemotherapies are associated with limited survival outcomes [9, 34]. In the ABC-06 trial, molecularly unselected patients with locally advanced/metastatic biliary tract cancer who had progressed on first-line GemCis were randomized to FOLFOX plus active symptom control or to active symptom control alone [10]. For these cohorts, median OS was 6.2 months and 5.3 months for all patients with biliary tract cancer; and was 5.7 months and 5.2 months for a subgroup of patients with iCCA. In the FOLFOX plus active symptom control arm, median PFS was 4.0 months for all patients with biliary tract cancer and 3.3 months for those with iCCA [10]. The phase II NIFTY trial randomized 178 molecularly unselected patients with metastatic biliary tract cancer who had progressed on GemCis to either liposomal irinotecan plus fluorouracil and leucovorin or to fluorouracil and leucovorin alone [35]. Among 174 patients analyzed for efficacy, the median PFS in the liposomal irinotecan plus fluorouracil and leucovorin cohort was observed to be significantly longer than in the fluorouracil and leucovorin alone cohort (7.1 vs 1.4 months; p = 0.0019) [35]. Support for these findings was provided by a small retrospective study of 14 patients receiving nanoliposomal irinotecan in combination with leucovorin plus fluorouracil for advanced biliary tract cancer, who had initially received platinum-based chemotherapy [36]. Among 11 patients analyzed, the results demonstrated median PFS and OS on second-line chemotherapy of 6.1 months and 12.1 months, respectively [36].
The median PFS observed here is slightly longer than that observed in a post hoc analysis of response to systemic therapy in patients with FGFR2 fusions or rearrangements enrolled in FIGHT-202 (first-line: PFS 6.2 vs 5.6 months; second-line PFS 5.6 vs 4.4 months) [37]. However, these comparisons should be interpreted with caution because of the small numbers of patients and differences in the study design (e.g., prospective vs retrospective). In a recent retrospective analysis in patients with advanced CCA harboring FGFR2 fusions who received second-line chemotherapy, median PFS was 4.6 months [38]. Despite the observation that patients with FGFR2 fusions receiving second-line systemic therapy have longer PFS compared with patients with no FGFR2 alterations, PFS appears to be further improved by treatment with FGFR inhibitors. Recently updated data from FIGHT-202 in patients with CCA who received pemigatinib in second-line therapy showed a median PFS of 7.0 months for patients with FGFR2 fusions or rearrangements [39]. In a pivotal phase II study, infigratinib was associated with a median PFS of 7.3 months in 108 patients with FGFR2 fusions or rearrangements who had previously received one or more lines of therapy [28]. The final analysis of the FOENIX-CCA2 trial of futibatinib in patients with iCCA and FGFR2 fusion or rearrangements who had received one or more prior treatments including gemcitabine plus platinum reported a median PFS of 8.9 months [40]. Data for PFS among patients receiving infigratinib or futibatinib in second-line therapy only were not reported.
Taken together, median PFS with first-line systemic therapy does not appear to be substantially affected by FGFR2 fusion status; patients harboring FGFR2 fusions or rearrangements may experience longer PFS with second-line systemic therapy compared with patients who do not harbor FGFR2 fusions or rearrangements—a PFS advantage that may be further enhanced with targeted therapy. However, these observations require confirmation in future prospective randomized controlled studies.
In this study, data interpretation was limited by the small population of patients with FGFR2 fusions; therefore, numerical differences between patient populations for some analyses should be interpreted with caution. Nevertheless, the results of this retrospective analysis suggest that patients with iCCA harboring FGFR2 fusions may have a better prognosis compared with patients without FGFR2 alterations in terms of overall OS and PFS on second-line systemic therapy, but not first-line systemic therapy. Further research is warranted on the prognostic value of FGFR2 fusion-driven iCCA in response to systemic therapy through prospectively designed studies. The FGFR-targeted inhibitors, pemigatinib and infigratinib, are approved for the treatment of patients with unresectable locally advanced or metastatic CCA with an FGFR fusion or other rearrangement in the second-line setting [18–21, 41]. However, the present observation that for patients with FGFR2 fusions receiving second-line systemic therapy PFS may be further improved by FGFR inhibitor treatment raises the question of whether FGFR inhibitors (or other targeted therapies) could provide additional survival benefit in first-line compared with standard-of-care GemCis. Evidence that this may be the case for the programmed death-ligand 1-targeted monoclonal antibody durvalumab was provided by an interim analysis of data from the phase III randomized TOPAZ-1 trial (NCT03875235), which is evaluating durvalumab plus GemCis versus placebo plus GemCis in 685 patients with locally advanced or metastatic biliary tract cancer in the first-line setting [42]. Importantly, durvalumab plus GemCis was associated with significant improvements in OS (hazard ratio, 0.80; 95% CI 0.66–0.97; p = 0.021; median, 12.8 vs 11.5 months) and PFS (hazard ratio, 0.75; 95% CI 0.64–0.89; p = 0.001; median, 7.2 vs 5.7 months) compared with placebo plus GemCis. It might therefore be conjectured, and further research would thus be warranted to evaluate, that inclusion of an FGFR inhibitor in first-line treatment could also provide a survival advantage compared with GemCis alone. Phase III studies are currently ongoing comparing FGFR inhibitors with GemCis for the first-line treatment of patients with unresectable, locally advanced, or metastatic CCA and FGFR2 fusions or rearrangements, including FIGHT-302 (NCT03656536) with pemigatinib [43], the PROOF trial (NCT03773302) with infigratinib [44], and the FOENIX-CCA3 (NCT04093362) with futibatinib [45].
Acknowledgments
The study was funded by Incyte Corporation. The authors wish to thank the participants, investigators, and site personnel who participated in this study. Medical writing assistance was provided by Abigail Marmont, PhD, CMPP, of Envision Pharma Group (Philadelphia, PA, USA), and funded by Incyte Corporation.
Declarations
Funding
This study was sponsored by Incyte Corporation (Wilmington, DE, USA).
Conflicts of interest/Competing interests
Ghassan K. Abou-Alfa has received research grants from Agios, Arcus, AstraZeneca, Bayer, BioNTech, BMS, Celgene, Flatiron, Genentech/Roche, GeoScience, Incyte Corporation, Polaris, Puma, QED, SillaJen, and Yiviva; and acted as a consultant for Agios, Alnylam, AstraZeneca, Autem, Bayer, Beigene, Berry Genomics, Celgene, CytomX, Eisai, Eli Lilly and Company, Exelixis, Flatiron, Genentech/Roche, Genoscience, Helio, Incyte Corporation, Ipsen, Legend Biotech, Loxo, Merck, MINA, QED, Rafael, Redhill, Silenseed, Sillajen, Sobi, Surface Oncology, Therabionics, twoXAR, Vector, and Yiviva. Nikolaus Schultz, Amin Yaqubie, and Brittanie Millang report no conflicts of interest. Kristen Bibeau, Haobo Ren, and Luis Féliz report employment by and stock ownership in Incyte Corporation.
Ethics approval
Ethics approval was not required for this non-interventional retrospective study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data generated or analyzed during this study are included in this article and/or its supplementary material files. Further inquiries can be directed to the corresponding author.
Code availability
Not applicable.
Authors’ contributions
All authors contributed to the conceptualization and study design, data collection, analysis and interpretation, and drafting/editing of the manuscript.
Footnotes
Prior Presentation: American Society of Clinical Oncology—Gastrointestinal Cancers Symposium (ASCO-GI), Virtual Meeting, 15–17 January 2021. J Clin Oncol 2021;39(Suppl.):Abstract 303. Available from: https://ascopubs.org/doi/10.1200/JCO.2021.39.3_suppl.303.
References
- 1.Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019;39(Suppl. 1):19–31. doi: 10.1111/liv.14095. [DOI] [PubMed] [Google Scholar]
- 2.Blechacz B. Cholangiocarcinoma: current knowledge and new developments. Gut Liver. 2017;11:13–26. doi: 10.5009/gnl15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huguet JM, Lobo M, Labrador JM, Boix C, Albert C, Ferrer-Barceló L, et al. Diagnostic-therapeutic management of bile duct cancer. World J Clin Cases. 2019;7:1732–1752. doi: 10.12998/wjcc.v7.i14.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tefferi A, Cervantes F, Mesa R, Passamonti F, Verstovsek S, Vannucchi AM, et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood. 2013;122:1395–1398. doi: 10.1182/blood-2013-03-488098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103:469–474. doi: 10.1038/sj.bjc.6605779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 7.Brieau B, Dahan L, De Rycke Y, Boussaha T, Vasseur P, Tougeron D, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: a large multicenter study by the Association des Gastro-Entérologues Oncologues. Cancer. 2015;121:3290–3297. doi: 10.1002/cncr.29471. [DOI] [PubMed] [Google Scholar]
- 8.Kim ST, Oh SY, Lee J, Kang JH, Lee HW, Lee MA, et al. Capecitabine plus oxaliplatin as a second-line therapy for advanced biliary tract cancers: a multicenter, open-label, phase II trial. J Cancer. 2019;10:6185–6190. doi: 10.7150/jca.37610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014;25:2328–2338. doi: 10.1093/annonc/mdu162. [DOI] [PubMed] [Google Scholar]
- 10.Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22:690–701. doi: 10.1016/s1470-2045(21)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowery MA, Goff LW, Keenan BP, Jordan E, Wang R, Bocobo AG, et al. Second-line chemotherapy in advanced biliary cancers: a retrospective, multicenter analysis of outcomes. Cancer. 2019;125:4426–4434. doi: 10.1002/cncr.32463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arai Y, Totoki Y, Hosoda F, Shirota T, Hama N, Nakamura H, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology. 2014;59:1427–1434. doi: 10.1002/hep.26890. [DOI] [PubMed] [Google Scholar]
- 13.Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin Cancer Res. 2018;24:4154–4161. doi: 10.1158/1078-0432.Ccr-18-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham RP, Barr Fritcher EG, Pestova E, Schulz J, Sitailo LA, Vasmatzis G, et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol. 2014;45:1630–1638. doi: 10.1016/j.humpath.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Jain A, Borad MJ, Kelley RK, Wang Y, Abdel-Wahab R, Meric-Bernstam F, et al. Cholangiocarcinoma with FGFR genetic aberrations: a unique clinical phenotype. JCO Precis Oncol. 2018;2:1–12. doi: 10.1200/PO.17.00080. [DOI] [PubMed] [Google Scholar]
- 16.Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu PCC, Koblish H, Wu L, Bowman K, Diamond S, DiMatteo D, et al. INCB054828 (pemigatinib), a potent and selective inhibitor of fibroblast growth factor receptors 1, 2, and 3, displays activity against genetically defined tumor models. PLoS ONE. 2020;15:e0231877. doi: 10.1371/journal.pone.0231877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Incyte Corporation. Pemazyre™ (pemigatinib) prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213736s000lbl.pdf. Accessed 20 May 2021.
- 19.Medicines.org. Pemazyre (pemigatinib) SmPC. https://www.medicines.org.uk/emc/product/12485/smpc. Accessed 20 May 2021.
- 20.CheckOrphan. Press release: Incyte announces Health Canada conditional approval of Pemazyre® (pemigatinib) as first targeted treatment for adults with previously treated, unresectable locally advanced or metastatic cholangiocarcinoma. https://checkorphan.org/news/incyte-announces-health-canada-conditional-approval-of-pemazyre-pemigatinib-as-first-targeted-treatment-for-adults-with-previously-treated-unresectable-locally-advanced-or-metastatic-cholangi/#:~:text=MONTREAL%20%E2%80%93%20Incyte%20%28NASDAQ%3A%20INCY%29%20today%20announced%20that,factor%20receptor%202%20%28FGFR2%29%20fusion%20or%20other%20rearrangement. Accessed 18 Mar 2022.
- 21.Incyte Corporation. Press release: Incyte announces approval of Pemazyre® (pemigatinib) in Japan for the treatment of patients with unresectable biliary tract cancer (BTC) with a fibroblast growth factor receptor 2 (FGFR2) fusion gene, worsening after cancer chemotherapy. https://investor.incyte.com/press-releases/press-releases/2021/Incyte-Announces-Approval-of-Pemazyre-pemigatinib-in-Japan-for-the-Treatment-of-Patients-with-Unresectable-Biliary-Tract-Cancer-BTC-with-a-Fibroblast-Growth-Factor-Receptor-2-FGFR2-Fusion-Gene-Worsening-After-Cancer-Chemotherapy/default.aspx. Accessed 18 Mar 2022.
- 22.Kang C. Infigratinib: first approval. Drugs. 2021;81:1355–1360. doi: 10.1007/s40265-021-01567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang C. Correction to: infigratinib: first approval. Drugs. 2022;82:93. doi: 10.1007/s40265-021-01652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taiho Oncology Inc. U.S. FDA accepts for priority review new drug application of futibatinib for advanced cholangiocarcinoma. https://www.taiho.co.jp/en/release/files/pdf/20220330.pdf. Accessed 3 Aug 2022.
- 25.Abou-Alfa G, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. SO-4 Progression-free survival in patients with cholangiocarcinoma with FGFR2 fusions or rearrangements: a FIGHT-202 post-hoc analysis of prior systemic therapy response [abstract] Ann Oncol. 2021;32(Suppl 3):S203–S204. doi: 10.1016/j.annonc/2021.05.028. [DOI] [Google Scholar]
- 26.Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- 27.Silverman IM, Hollebecque A, Friboulet L, Owens S, Newton RC, Zhen H, et al. Clinicogenomic analysis of FGFR2-rearranged cholangiocarcinoma identifies correlates of response and mechanisms of resistance to pemigatinib. Cancer Discov. 2021;11:326–339. doi: 10.1158/2159-8290.Cd-20-0766. [DOI] [PubMed] [Google Scholar]
- 28.Javle M, Roychowdhury S, Kelley RK, Sadeghi S, Macarulla T, Weiss KH, et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol Hepatol. 2021;6:803–815. doi: 10.1016/S2468-1253(21)00196-5. [DOI] [PubMed] [Google Scholar]
- 29.Farshidfar F, Zheng S, Gingras MC, Newton Y, Shih J, Robertson AG, et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep. 2017;19:2878–2880. doi: 10.1016/j.celrep.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamarca A, Ross P, Wasan HS, Hubner RA, McNamara MG, Lopes A, et al. Advanced intrahepatic cholangiocarcinoma: post hoc analysis of the ABC-01, -02, and -03 clinical trials. J Natl Cancer Inst. 2020;112:200–210. doi: 10.1093/jnci/djz071. [DOI] [PubMed] [Google Scholar]
- 31.Shroff RT, Rearden J, Li A, Moran S, Peacock Shepherd S, Lamarca A. Natural history of patients (pts) with advanced cholangiocarcinoma (CCA) with FGFR2 gene fusion/rearrangement or wild-type (WT) FGFR2. J Clin Oncol. 2021;39(15 Suppl.):Abstract4089. doi: 10.1200/JCO.2021.39.15_suppl.4089. [DOI] [Google Scholar]
- 32.Rogers JE, Law L, Nguyen VD, Qiao W, Javle MM, Kaseb A, et al. Second-line systemic treatment for advanced cholangiocarcinoma. J Gastrointest Oncol. 2014;5:408–413. doi: 10.3978/j.issn.2078-6891.2014.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schweitzer N, Kirstein MM, Kratzel AM, Mederacke YS, Fischer M, Manns MP, et al. Second-line chemotherapy in biliary tract cancer: outcome and prognostic factors. Liver Int. 2019;39:914–923. doi: 10.1111/liv.14063. [DOI] [PubMed] [Google Scholar]
- 34.Ying J, Chen J. Combination versus mono-therapy as salvage treatment for advanced biliary tract cancer: a comprehensive meta-analysis of published data. Crit Rev Oncol Hematol. 2019;139:134–142. doi: 10.1016/j.critrevonc.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Yoo C, Kim KP, Jeong JH, Kim I, Kang MJ, Cheon J, et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): a multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021;22:1560–1572. doi: 10.1016/S1470-2045(21)00486-1. [DOI] [PubMed] [Google Scholar]
- 36.Allo G, Can AD, Wahba R, Vogel N, Goeser T, Kutting F, et al. Nanoliposomal irinotecan in combination with leucovorin and 5-fluorouracil in advanced biliary tract cancers. Mol Clin Oncol. 2022;16:52. doi: 10.3892/mco.2021.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro GM, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated locally advanced/metastatic cholangiocarcinoma (CCA): update of FIGHT-202. J Clin Oncol. 2021;39(15 Suppl.):Abstract4086. doi: 10.1200/JCO.2021.39.15_suppl.4086. [DOI] [Google Scholar]
- 38.Javle MM, Sadeghi S, El-Khoueiry AB, Goyal L, Philip PA, Kelley RK, et al. A retrospective analysis of post second-line chemotherapy treatment outcomes for patients with advanced or metastatic cholangiocarcinoma and FGFR2 fusions. J Clin Oncol. 2020;38(15 Suppl.):Abstract4591. doi: 10.1200/JCO.2020.38.15_suppl.4591. [DOI] [Google Scholar]
- 39.Bibeau K, Féliz L, Lihou CF, Ren H, Abou-Alfa GK. Progression-free survival in patients with cholangiocarcinoma with or without FGF/FGFR alterations: a FIGHT-202 post hoc analysis of prior systemic therapy response. JCO Precis Oncol. 2022 doi: 10.1200/po.21.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goyal L, Meric-Bernstam F, Hollebecque A, Morizane C, Valle JW, Karasic TB, et al. Updated results of the FOENIX-CCA2 trial: efficacy and safety of futibatinib in intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 fusions/rearrangements. J Clin Oncol. 2022;40:4009. doi: 10.1200/JCO.2022.40.16_suppl.4009. [DOI] [Google Scholar]
- 41.QED Therapeutics Inc. Truseltiq (infigratinib) capsules, for oral use. Highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214622s000lbl.pdf. Accessed 3 Aug 2022.
- 42.Oh DY, He AR, Qin S, Chen LT, Okusaka T, Vogel A, et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J Clin Oncol. 2022;40(4 Suppl.):Abstract378. doi: 10.1200/JCO.2022.40.4_suppl.378. [DOI] [Google Scholar]
- 43.Bekaii-Saab TS, Valle JW, Van Cutsem E, Rimassa L, Furuse J, Ioka T, et al. FIGHT-302: first-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncol. 2020;16:2385–2399. doi: 10.2217/fon-2020-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makawita SGKA-A, Roychowdhury S, Sadeghi S, Borbath I, Goyal L, et al. Infigratinib in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: the PROOF 301 trial. Future Oncol. 2020;16:2375–2384. doi: 10.2217/fon-2020-0299. [DOI] [PubMed] [Google Scholar]
- 45.Borad MJ, Bridgewater JA, Morizane C, Shroff RT, Oh D-Y, Moehler MH, et al. A phase III study of futibatinib (TAS-120) versus gemcitabine-cisplatin (gem-cis) chemotherapy as first-line (1L) treatment for patients (pts) with advanced (adv) cholangiocarcinoma (CCA) harboring fibroblast growth factor receptor 2 (FGFR2) gene rearrangements (FOENIX-CCA3) J Clin Oncol. 2020;38:TPS600-TPS. doi: 10.1200/JCO.2020.38.4_suppl.TPS600. [DOI] [Google Scholar]