Abstract
Coronavirus disease 2019 (COVID-19) poses a serious threat to human health and life. The effective prevention and treatment of COVID-19 complications have become crucial to saving patients’ lives. During the phase of mass spread of the epidemic, a large number of patients with pulmonary fibrosis and lung cancers were inevitably infected with the SARS-CoV-2 virus. Lung cancers have the highest tumor morbidity and mortality rates worldwide, and pulmonary fibrosis itself is one of the complications of COVID-19. Idiopathic lung fibrosis (IPF) and various lung cancers (primary and metastatic) become risk factors for complications of COVID-19 and significantly increase mortality in patients. Therefore, we applied bioinformatics and systems biology approaches to identify molecular biomarkers and common pathways in COVID-19, IPF, colorectal cancer (CRC) lung metastasis, SCLC and NSCLC. We identified 79 DEGs between COVID-19, IPF, CRC lung metastasis, SCLC and NSCLC. Meanwhile, based on the transcriptome features of DSigDB and common DEGs, we identified 10 drug candidates. In this study, 79 DEGs are the common core genes of the 5 diseases. The 10 drugs were found to have positive effects in treating COVID-19 and lung cancer, potentially reducing the risk of pulmonary fibrosis.
Subject terms: Computational biology and bioinformatics, Cancer
Introduction
SARS-CoV-2 is a novel coronavirus. It has directly contributed to the worldwide COVID-19 pandemic1. SARS-CoV-2 infection can have serious pulmonary fibrosis consequences2. IPF is a chronic progressive lung disease, nintedanib and pirfenidone are FDA-approved for the treatment of IPF. After a COVID-19 infection, survivors are likely to develop bilateral interstitial pneumonia, which often leads to acute respiratory distress syndrome and pulmonary fibrosis. A number of risk factors are shared by both IPF and lung cancer, and patients with both conditions have a worse prognosis than patients with either. In relation to the severity of IPF, the lung cancer stage matters more than the interval between diagnosis and lung cancer3. Patients with cancer appear to be particularly vulnerable to COVID-19, as tumors can severely affect the immunological response to viral infection4. Delays in screening, diagnosis and treatment due to the COVID-19 pandemic could lead to excess cancer deaths and delay or even reverse projected mortality declines for some cancers5; i.e., cancer patients with COVID-19 have a significantly higher mortality rate than infected patients without cancer6. As a result, patients with underlying diseases such as pulmonary fibrosis and lung cancer are more likely to suffer serious complications or even death after SARS-CoV-2 infection.
Patients with lung cancer, whose underlying lung function and endurance are poor, are more likely to experience more severe hypoxia and to progress more rapidly with COVID-19 infection, so treatment is urgently needed for lung cancer patients infected with COVID-197. Of all lung cancers, small cell lung cancer (SCLC), although less common, is a rapidly fatal disease for which there is little effective clinical treatment8. Most patients will die within one year. Non-small cell lung cancer (NSCLC) is the most common type of lung cancer and although the death rate is lower than that of SCLC, it kills more people than SCLC because of the disproportionate number of cases9. In addition to primary lung cancer, secondary lung cancer caused by metastases from other types of cancer also constitutes an important risk factor after SARS-CoV-2 infection.
The lungs are the second most common site of metastasis from CRC after the liver. 11% of CRC patients present with isolated lung metastases and surgical removal of lung metastases is effective in some cases, but the prognosis is poor10. Surfactant protein D (SP-D) downregulates EGF signaling and inhibits lung cancer cell growth11. As an in vitro and in vivo test, SP-D was shown to suppress pulmonary metastases from CRC12.
In our study, five datasets were used to identify the biological relationships between COVID-19 and IPF, lung metastases from CRC, SCLC and NSCLC. First, differentially expressed genes were identified from the dataset, and then common differential genes were found for the five diseases. Using these common differential genes, further analyses, including enrichment analysis and pathway analysis, are performed to understand the biological processes involved in genome-based expression studies. PPI networks were made using common differential genes to collect the full range of hub genes. Common DEGs were also tracked according to GSE186460, GSE17978, GSE41258, GSE40275 and GSE33532 for transcriptional regulators. Finding the top 10 genes from hub genes is a key step in predicting potential drugs. Finally, an outlook on potential drugs for the hub gene is provided.
Materials and methods
Datasets used in this study
In order to obtain common genes between COVID-19, IPF, CRC lung metastasis, SCLC and NSCLC, we searched the GEO database from the NCBI (https://www.ncbi.nlm.nih.gov/geo/)13 to find datasets with both onset lung tissue and normal lung tissue controls and to download the full data. The GEO accession ID for the COVID-19 dataset is GSE18646014, which consists of 4 COVID-19 samples and 11 normal lung tissue samples sequenced by a high-throughput sequencing system called Illumina HiSeq 2500 (Homo sapiens) provided by Dobosh B et al. The GEO accession ID for the IPF dataset is GSE1797815, which includes 38 IPF samples and 20 normal lung tissue samples sequenced by the high-throughput sequencing system called Duke Human Operon 36 k v4.0 spotted microarray provided by Emblom-Callahan MC et al.; the GEO accession ID for the CRC lung metastasis dataset is GSE4125816, which includes 20 lung metastasis samples from CRC and 7 normal lung tissue samples sequenced by the high-throughput sequencing system called [HG-U133A] Affymetrix Human Genome U133A Array provided by Sheffer M et al. The GEO accession ID for the SCLC datasets GSE4027517, comprising 15 SCLC samples and 43 normal lung tissue samples, was sequenced by the high-throughput sequencing system called Human Exon 1.0 ST Array [CDF: Brainarray Version 9.0.1, HsEx10stv2_Hs_REFSEQ] provided by Kastner et al. The GEO accession ID of the NSCLC dataset is GSE3353218, including 80 NSCLC samples and 20 normal lung tissue samples, provided by Meister M et al. called [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array. See Table 1.
Table 1.
A description of the datasets in this analysis together with their geo-features and quantitative measurements.
| Disease name | GEO accession | GEO platform | Total DEGs count | Up regulated DEGs count | Down regulated DEGs count |
|---|---|---|---|---|---|
| COVID-19 | GSE186460 | GPL16791 | 7640 | 7211 | 429 |
| IPF | GSE17978 | GPL8903 | 2272 | 1420 | 852 |
| NSCLC | GSE33532 | GPL570 | 4407 | 2103 | 2304 |
| SCLC | GSE40275 | GPL15974 | 7164 | 4088 | 3076 |
| CRC lung metastases | GSE41258 | GPL96 | 2875 | 1387 | 1488 |
Identification of differentially expressed genes in COVID-19, IPF, CRC lung metastasis, SCLC and NSCLC
A gene is described as differentially expressed when there are statistically significant differences between experimental conditions at the transcription level19. The key role of this analysis was to obtain DEGs for datasets GSE186460, GSE17978, GSE40275, GSE41258 and GSE33532. Using the R LIMMA package together with Benjamini-Hochberg procedure we have tackled the problem of multiple comparisons due to the DESEq2 analysis. The final list of DEGs were determined by applying the following thresholds: adj. P-value < 0.05 and |logFC| ≥ 1.0. Jvenn, an online VENN analysis tool, was used to gather mutual DEGs for GSE186460, GSE17978, GSE40275, GSE41258 and GSE3353220.
Gene ontology (GO) and pathway enrichment analyses
Analyzing gene set enrichment is an important analytical exercise for classifying common biological insights, such as biological processes and chromosomal locations associated with different interlinked diseases21. Through EnrichR (https://maayanlab.cloud/Enrichr/)—a comprehensive gene set enrichment web tool, GO enrichment and functional enrichment (biological processes, cellular composition, and molecular functions) studies were conducted22 to characterize biological mechanisms and signaling pathways for shared DEGs. At the time, we used four databases, including KEGG (Kyoto Encyclopedia of Genes and Genomes)23, WikiPathways, Reactome, and BioCarta, as sources of pathway classification to identify shared pathways between COVID-19, IPF, CRC lung metastasis, SCLC, and NSCLC. Typically, the KEGG pathway controls metabolic processes and makes genome analysis quite useful. The P-value < 0.05 was considered a standard indicator for quantifying the highest pathways listed.
PPI construction
Proteins conclude their journey into a cell with a similar protein affiliation formed by a Protein–Protein Interaction network, which indicates the protein mechanisms. In cell and systems biology, assessment and analysis of PPI networks and their functionalities are fundamental and key objectives for interpreting and gaining insight into cellular machinery operations. Using the STRING (https://string-db.org/) (Version 11.5)24 repository, we construct a protein PPI network from shared DEGs to describe functional and physical interactions between COVID-19, IPF, CRC lung metastasis, SCLC, and NSCLC. STRING envisions expanding PPI awareness through active interactive channels, including text mining, experimental databases, coexpression, culture, gene fusion, and coexistence under different taxonomic confidence levels (low, medium, and high). Moderate confidence was set at 0.5 to generate a common DEG PPI network. We then consumed our PPI network into Cytoscape (v.3.8.2) for visualization and further experimental PPI network studies. Cytoscape (v.3.8.2)-an open-source web visualization platform-serves as a flexible tool for combining multiple datasets to improve the performance of different interactions such as PPI, genetic interactions, and protein-DNA interactions25.
Hub gene extraction and submodule analysis
A PPI network consists of nodes, edges, and their connections, where hub genes are the most entangled nodes. Cytohubba (http://apps.cytoscape.org/apps/Cytohubba)-a novel Cytoscape-plugin for ranking and extracting central or potential or target elements of biological networks based on various network characteristics. Cytohubba has 11 methods of investigating networks from different angles, with Maximal Clique Centrality (MCC) being the best of them26. Using the MCC method of Cytohubba, we identified the top 10 hub genes in the PPI network. Based on the proximity ranking characteristics of Cytohubba, we also categorized the shortest available pathways across hub genes.
Evaluation of the applied medicine
In this study, one of the most important aspects is the prediction of protein-drug interactions (PDI) or the identification of drug molecules. Drug molecules were identified by Enrichr using the Drug Signatures database (DSigDB) based on COVID-19, IPF, CRC lung metastasis, SCLC, and NSCLC. The Enrichr web portal is one of the most popular online resources to explore the enrichment of gene sets across a genome-wide scale. DSigDB is the global archive for the reidentification of targeted drugs associated with DEGs27. The database has 22,527 gene sets, and access to the DSigDB database is via Enrichr in disease/ drug function.
Ethics approval
Our study did not require ethical board approval because it did not contain human or animal trials. GEO belongs to public databases. The patients involved in the database have obtained ethical approval. Users can download relevant data for free for research and publish relevant articles. Our study is based on open source data, so there are no ethical issues and other conflicts of interest.
Results
Identification of common DEGs between COVID-19, IPF, CRC lung metastasis, SCLC and NSCLC
To explore the interrelationships and significance of COVID-19, IPF, CRC lung metastasis, SCLC, and NSCLC, based on the human RNA-seq and microarray datasets from the NCBI, we identified dysregulated genes that stimulate COVID-19, IPF, CRC lung metastasis, SCLC, and NSCLC. The RNA-seq and microarray dataset experiments were performed in an R-language environment characterized by DESeq2 and limit packs with Benjamin-Hochberg's false discovery rate.
COVID-19 has 7640 differential genes, of which 7211 are up-regulated and 429 are down-regulated; similarly, IPF has 2272 differential genes, of which 1420 are up-regulated and 852 are down-regulated; lung metastases from CRC have 2875 differential genes, of which 1387 are up-regulated and 1488 are down-regulated. SCLC has a total of 7164 differential genes, of which a total of 4088 are up-regulated and 3076 are down-regulated; NSCLC has a total of 4407 differential genes, of which a total of 2103 are up-regulated and 2304 are down-regulated.
All significant DEGs were extracted at P-value < 0.05 and |logFC|≥ 1. After cross-referencing Jvenn (a reliable Venn analytics web portal), we identified 79 common DEGs from COVID-19, IPF, CRC lung metastasis, SCLC, and NSCLC datasets. This common set of genes was used to complete further experiments. The five diseases are linked because they share one or more genes. Figure 1 shows cumulative comparative evaluation and mutual DEGs retrieval of the five datasets.
Figure 1.
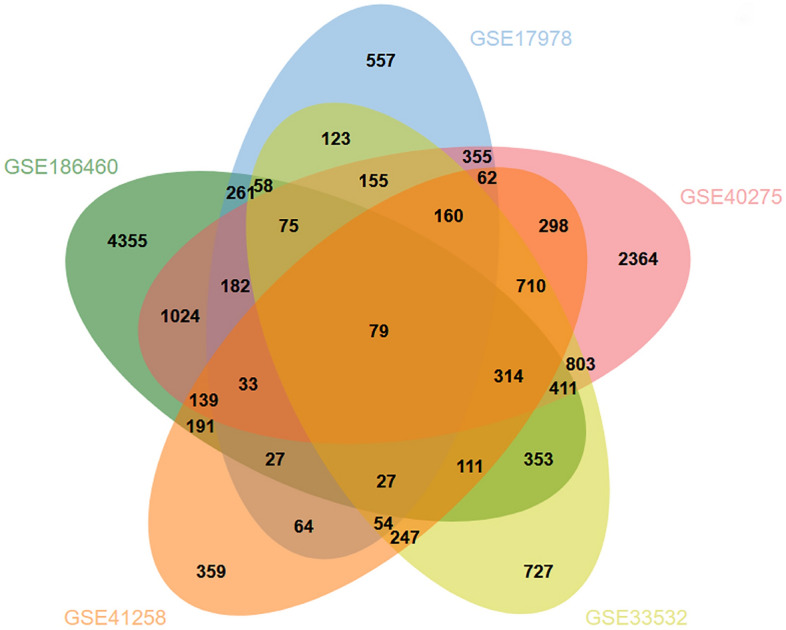
Among the datasets included in this study, IPF (GSE17978), COVID-19 (GSE186460), SCLC (GSE40275), NSCLC (GSE33532), and CRC lung metastasis (GSE41258) are analyzed using microarrays and RNA-seq. Based on this integrated analysis, we discovered 79 DEGs that are common among IPF, COVID-19, SCLC, NSCLC, and CRC lung metastases.
GO and pathway enrichment analyses
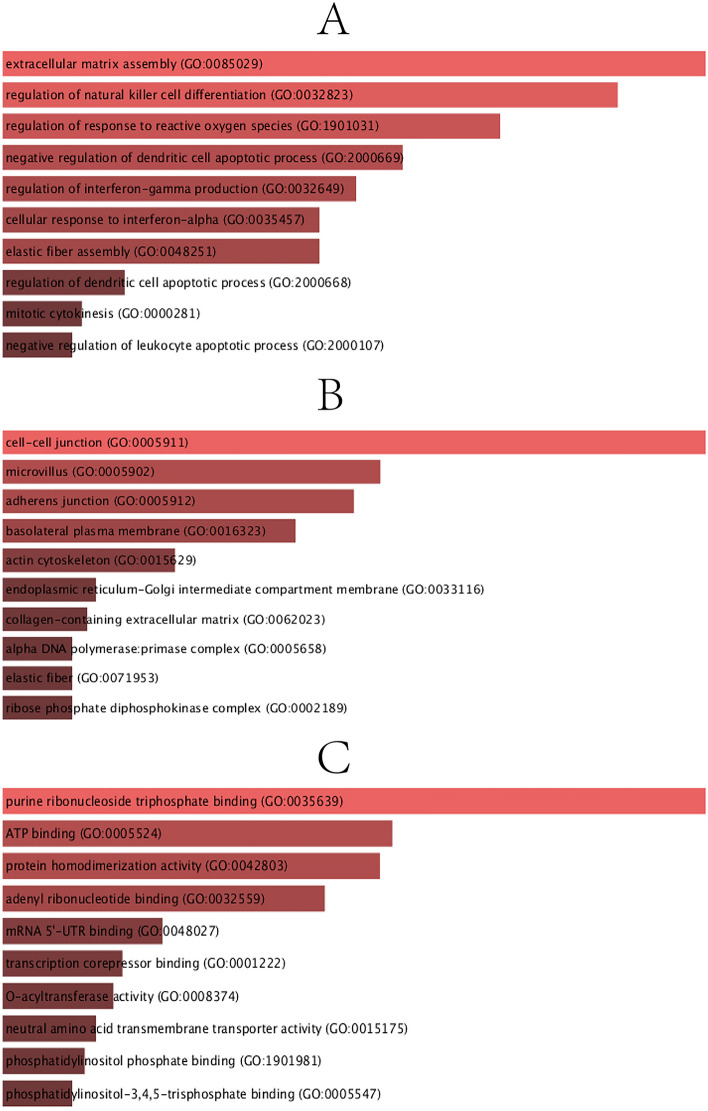
To determine the biological importance, pathway enrichment, and sharing of the DEGs highlighted in this study. GO and pathway enrichment analysis was carried out using Enrichr. Enrichr has a potentially improved method to compute enrichment, and the researchers demonstrated that this method might be better than the currently widely used Fisher exact test22. We have corrected the results for GO and Pathways using the improved method (Enrichr) to take the multiple comparison problem. GO, taking into account gene function and its composition provides a wide range of extensive computable knowledge resources for humans. Ontologies define theoretically defined bodies of information. An ontology and annotation serve to perform a detailed biological structural model, primarily for biological applications. The GO analysis was performed in terms of biological processes, cellular composition, and molecular function, and the GO database was selected as an annotation source. The top 10 terms for biological processes, molecular functions and cell composition categories are summarized in Table 2. Figure 2 also describes the linear features of the overall ontological analysis of each category in a bar graph.
Table 2.
A descriptive analysis of the DEGs that are common to IPF, COVID-19, SCLC, NSCLC, and Colon cancer lung metastases.
| Category | GO ID | Term | P-values | Genes |
|---|---|---|---|---|
| GO Biological Process | GO:0,085,029 | Extracellular matrix assembly | 1.13E-04 | MFAP4;LTBP3;GAS6 |
| GO:0,032,823 | Regulation of natural killer cell differentiation | 1.53E-04 | AXL;GAS6 | |
| GO:1,901,031 | Regulation of response to reactive oxygen species | 2.29E-04 | DHFR;STK26 | |
| GO:2,000,669 | Negative regulation of dendritic cell apoptotic process | 3.19E-04 | AXL;GAS6 | |
| GO:0,032,649 | Regulation of interferon-gamma production | 3.74E-04 | SLC7A5;AXL;GAS6;IL18R1 | |
| GO:0,035,457 | Cellular response to interferon-alpha | 4.25E-04 | AXL;GAS6 | |
| GO:0,048,251 | Elastic fiber assembly | 4.25E-04 | MFAP4;LTBP3 | |
| GO:2,000,668 | Regulation of dendritic cell apoptotic process | 8.28E-04 | AXL;GAS6 | |
| GO:0,000,281 | Mitotic cytokinesis | 9.59E-04 | KIF4A;MYH10;CEP55 | |
| GO:2,000,107 | Negative regulation of leukocyte apoptotic process | 9.91E-04 | AXL;GAS6 | |
| GO Cellular Component | GO:0,005,911 | Cell–cell junction | 9.79E-05 | TMEM47;CEACAM1;CADM1;PTPRM;FLNA;PLPP3;RND1 |
| GO:0,005,902 | Microvillus | 0.001488612 | SLC7A5;MYO1B;MSN | |
| GO:0,005,912 | Adherens junction | 0.001858139 | CEACAM1;PTPRM;PLPP3;RND1 | |
| GO:0,016,323 | Basolateral plasma membrane | 0.003025032 | SLC7A5;CADM1;EPCAM;PLPP3 | |
| GO:0,015,629 | Actin cytoskeleton | 0.00829552 | MYO1B;AXL;FLNA;LPXN;RND1 | |
| GO:0,033,116 | Endoplasmic reticulum-Golgi intermediate compartment membrane | 0.016068342 | SERPINA1;PLPP3 | |
| GO:0,062,023 | Collagen-containing extracellular matrix | 0.017279767 | MFAP4;SERPINA1;ADAMTS1;HDGF;LTBP3 | |
| GO:0,005,658 | Alpha DNA polymerase:primase complex | 0.019596385 | PRIM1 | |
| GO:0,071,953 | Elastic fiber | 0.019596385 | MFAP4 | |
| GO:0,002,189 | Ribose phosphate diphosphokinase complex | 0.019596385 | PRPS2 | |
| GO Molecular Function | GO:0,035,639 | Purine ribonucleoside triphosphate binding | 8.24E-05 | PRPS2;RASL12;RRM1;MYO1B;SNRK;STK26;SCG5;MYH10;RND1 |
| GO:0,005,524 | ATP binding | 8.13E-04 | PRPS2;RRM1;MYO1B;SNRK;STK26;MYH10 | |
| GO:0,042,803 | Protein homodimerization activity | 8.90E-04 | GGCT;PRPS2;CEACAM1;PLN;CADM1;ZBTB16;STK26;RPE;FLNA | |
| GO:0,032,559 | Adenyl ribonucleotide binding | 0.001331367 | PRPS2;RRM1;MYO1B;SNRK;STK26;MYH10 | |
| GO:0,048,027 | mRNA 5'-UTR binding | 0.004357181 | MYH10;CCT5 | |
| GO:0,001,222 | Transcription corepressor binding | 0.005836836 | ZBTB16;HDGF | |
| GO:0,008,374 | O-acyltransferase activity | 0.006237826 | LPCAT1;PLA2G4A | |
| GO:0,015,175 | Neutral amino acid transmembrane transporter activity | 0.00707639 | SLC7A5;SLC1A4 | |
| GO:1,901,981 | Phosphatidylinositol phosphate binding | 0.00769414 | MYO1B;ARAP3;PLA2G4A | |
| GO:0,005,547 | Phosphatidylinositol-3,4,5-trisphosphate binding | 0.008424198 | MYO1B;ARAP3 |
Figure 2.
The bar graphs of ontological analysis of shared DEGs among IPF, COVID-19, SCLC, NSCLC, and CRC lung metastases performed by the Enrichr online tool: here, (A) Biological Processes, (B) Cellular Component, and (C) Molecular Function.
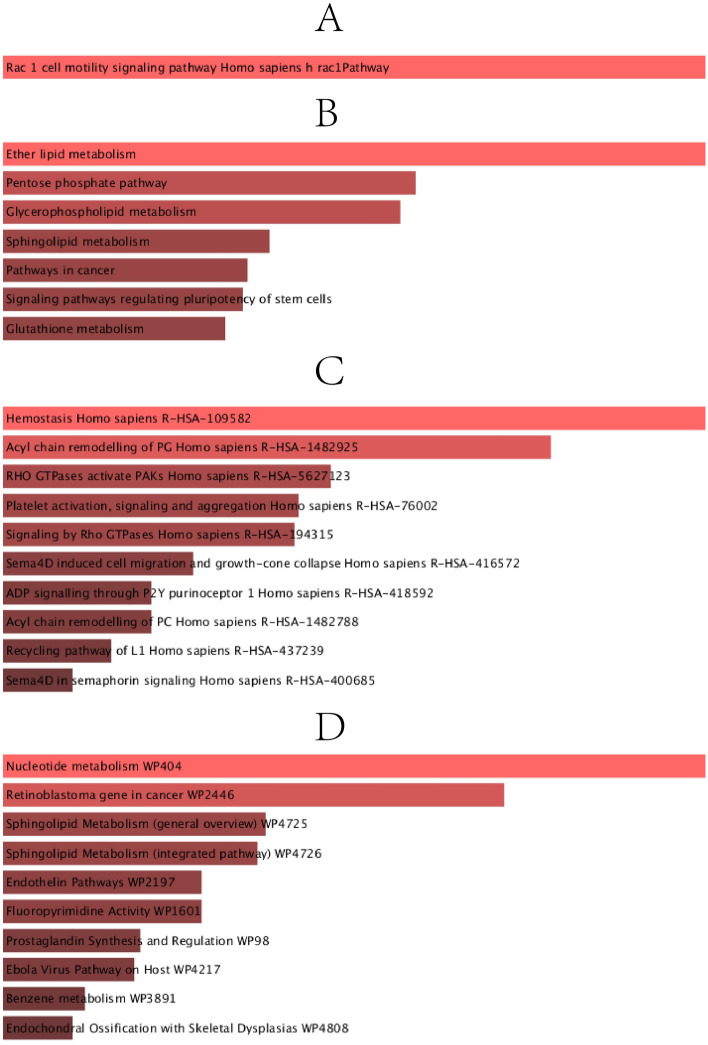
Pathways analysis reveals how organisms respond to their inherent modifications. It is a model technique that demonstrates the interaction between diseases through fundamental molecular or biological processes. The most affected paths of the COVID-19, IPF, CRC lung metastasis, SCLC, and NSCLC joint DEGs were collected from four global databases, including KEGG, WikiPathways, Reactome and BioCarta. Table 3 shows the main paths from the selected datasets. In Fig. 3, the pathway enrichment analysis is also shown as bar graphs to illustrate this better.
Table 3.
Analysis of pathway enrichment among IPF, COVID-19, SCLC, NSCLC, and CRC lung metastases.
| Category | Pathways | P-values | Genes |
|---|---|---|---|
| BioCarta | Rac 1 cell motility signaling pathway Homo sapiens h rac1Pathway | 0.008897062 | CADM1;CHN1 |
| KEGG | Ether lipid metabolism | 9.59E-04 | LPCAT1;PLA2G4A;PLPP3 |
| Pentose phosphate pathway | 0.006237826 | PRPS2;RPE | |
| Glycerophospholipid metabolism | 0.006892716 | LPCAT1;PLA2G4A;PLPP3 | |
| Sphingolipid metabolism | 0.016068342 | CERS6;PLPP3 | |
| Pathways in cancer | 0.018517416 | EDNRB;GNG4;ZBTB16;IL7R;CKS1B;RASGRP3 | |
| Signaling pathways regulating pluripotency of stem cells | 0.019064495 | ID4;ID3;TBX3 | |
| Glutathione metabolism | 0.021369678 | GGCT;RRM1 | |
| Reactome | Hemostasis Homo sapiens R-HSA-109582 | 0.001496763 | SLC7A5;CEACAM1;SERPINA1;GNG4;KIF4A;PLA2G4A;FLNA;GAS6 |
| Acyl chain remodelling of PG Homo sapiens R-HSA-1482925 | 0.002016063 | LPCAT1;PLA2G4A | |
| RHO GTPases activate PAKs Homo sapiens R-HSA-5627123 | 0.003081354 | FLNA;MYH10 | |
| Platelet activation, signaling and aggregation Homo sapiens R-HSA-76002 | 0.003278966 | SERPINA1;GNG4;PLA2G4A;FLNA;GAS6 | |
| Signaling by Rho GTPases Homo sapiens R-HSA-194315 | 0.003306344 | CHN1;ARAP3;FLNA;MYH10;NDC80;NUP37 | |
| Sema4D induced cell migration and growth-cone collapse Homo sapiens R-HSA-416572 | 0.004018856 | MYH10;RND1 | |
| ADP signalling through P2Y purinoceptor 1 Homo sapiens R-HSA-418592 | 0.004357181 | GNG4;PLA2G4A | |
| Acyl chain remodelling of PC Homo sapiens R-HSA-1482788 | 0.004357181 | LPCAT1;PLA2G4A | |
| Recycling pathway of L1 Homo sapiens R-HSA-437239 | 0.004708244 | KIF4A;MSN | |
| Sema4D in semaphorin signaling Homo sapiens R-HSA-400685 | 0.005071943 | MYH10;RND1 | |
| Wiki | Nucleotide metabolism WP404 | 5.49E-05 | PRPS2;DHFR;RRM1 |
| Retinoblastoma gene in cancer WP2446 | 3.91E-04 | DHFR;RRM1;PRIM1;KIF4A | |
| Sphingolipid Metabolism (general overview) WP4725 | 0.004018856 | CERS6;PLPP3 | |
| Sphingolipid Metabolism (integrated pathway) WP4726 | 0.004357181 | CERS6;PLPP3 | |
| Endothelin Pathways WP2197 | 0.007513763 | CNN1;EDNRB | |
| Fluoropyrimidine Activity WP1601 | 0.007513763 | DHFR;RRM1 | |
| Prostaglandin Synthesis and Regulation WP98 | 0.013664889 | EDNRB;PLA2G4A | |
| Ebola Virus Pathway on Host WP4217 | 0.014520104 | AXL;FLNA;GAS6 | |
| Benzene metabolism WP3891 | 0.023469932 | EPHX1 | |
| Endochondral Ossification with Skeletal Dysplasias WP4808 | 0.026520035 | ADAMTS1;CTSV |
Figure 3.
The bar graphs of pathway enrichment analysis of shared DEGs among IPF, COVID-19, SCLC, NSCLC, and CRC lung metastases performed by the Enrichr online tool: here, (A) BioCarta pathway, (B) KEGG pathway, (C) Reactome pathway, and (D) Wiki pathway.
Classification of hub genes and submodule construction
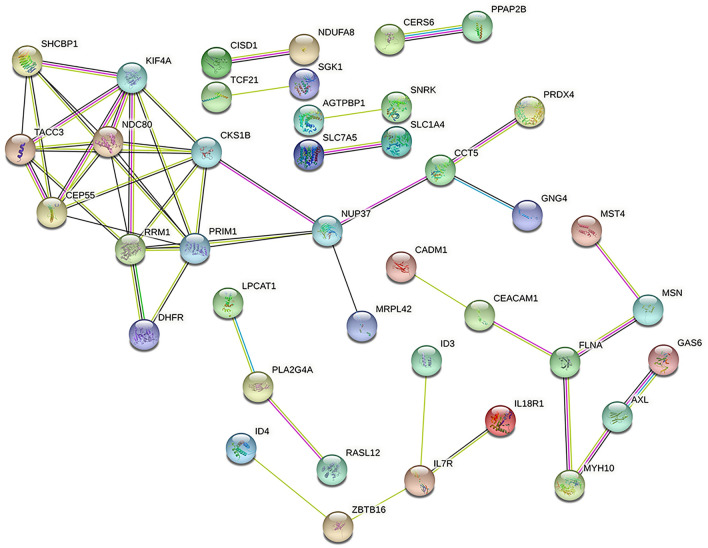
We used the online tool STRING to construct the protein network, the PPI network contains 79 nodes and 51 edges, see Fig. 4, and using the Cytohubba plugin in Cytoscape, we listed the 10 (10.87%) most influential genes as NDC80, KIF4A, CKS1B, CEP55, PRIM1, TACC3, RRM1, SHCBP1, NUP37, and CCT5. These hub genes could be potential biomarkers, which could also lead to new therapeutic strategies to treat the studied diseases. Since the central gene is latent, with the help of the Cytohubba plugin, we also constructed a submodule network (Fig. 5). By of Hub-gene interactions derived from the PPI network.
Figure 4.
PPI network of common DEGs among IPF, COVID-19, SCLC, NSCLC, and CRC lung metastases.
Figure 5.
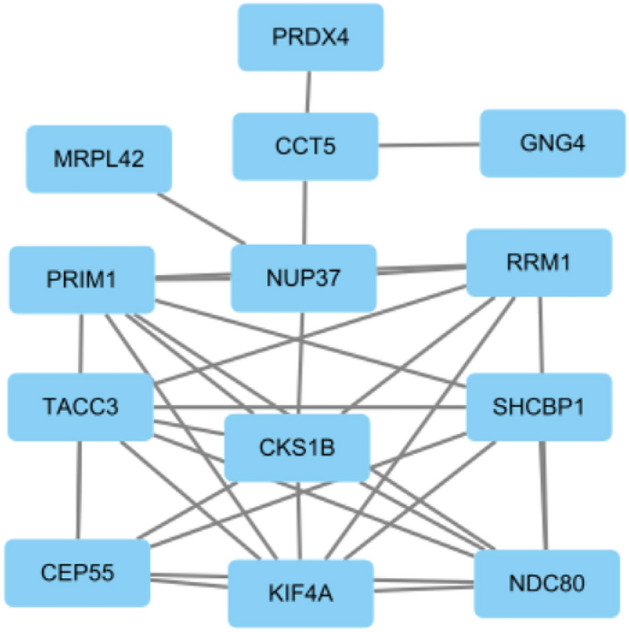
Determination of hub genes from the PPI network by using the Cytohubba plugin in Cytoscape. The latest MCC procedure of the Cytohubba plugin was pursued to obtain hub genes.
Identification of candidate drugs
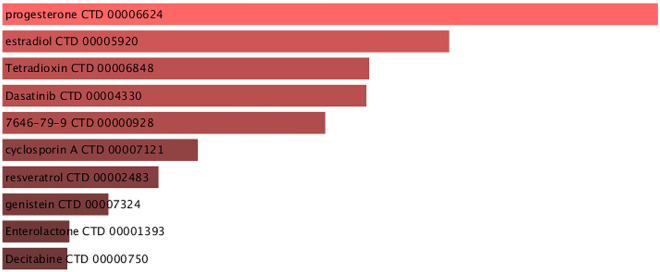
Assessing protein-drug interactions is important for understanding the structural characteristics of receptor sensitivity. In conjoint DEGs for COVID-19, IPF, CRC lung metastasis, SCLC, and NSCLC, we identified 10 potential drug molecules based on transcriptome signatures of DSigDB; these potential drugs are recommended for corporate diseases; and they can be collective compounds for treating five diseases. Figure 6 shows the active agents of commonly used drugs in the DSigDB database. The criteria for candidate drugs screening are to select the top 10 in ascending order of Adjusted P-value (Table 4). Adjusted P-value < 0.05 was considered statistically significant.
Figure 6.
List of the suggested drugs among COVID-19, IPF, NSCLC, SCLC and CRC lung metastases.
Table 4.
A list of candidate drugs that are common to IPF, COVID-19, SCLC, NSCLC, and CRC lung metastases.
| Drugs | Adjusted P-value | Genes |
|---|---|---|
| progesterone CTD 00,006,624 | 1.22E-13 | RASL12;PAK1IP1;SLC1A4 |
| estradiol CTD 00,005,920 | 2.17E-10 | COLEC12;RASL12;SERPINA1 |
| Tetradioxin CTD 00,006,848 | 2.77E-09 | COLEC12;RASL12; CCDC69 |
| Dasatinib CTD 00,004,330 | 2.77E-09 | RRM1;PRIM1;EPHX1 |
| 7646–79-9 CTD 00,000,928 | 1.11E-08 | PRPS2;SERPINA1;WBP2 |
| cyclosporin A CTD 00,007,121 | 1.36E-06 | PRPS2;WBP2;PAK1IP1 |
| resveratrol CTD 00,002,483 | 5.44E-06 | PRPS2;RRM1;CERS6 |
| genistein CTD 00,007,324 | 3.38E-05 | SERPINA1;PRIM1;CCDC69 |
| Enterolactone CTD 00,001,393 | 1.35E-04 | RRM1;CADM1;PRIM1 |
| Decitabine CTD 00,000,750 | 1.35E-04 | RASL12;SERPINA1;CADM1 |
Discussion
As the COVID-19 pandemic continues, the number of new COVID-19 cases continues to rise globally as the SARS-CoV-2 Omicron variant spreads28. Some experts predict that humans and COVID-19 may coexist in the long term29. Patients with cancer, including those with lung cancer, are more likely to contract SARS-CoV-2 regardless of vaccination status due to reduced immunity and to develop complications after infection. Patients with lung cancer are at high risk of hospitalization and death due to COVID-1930. Several non-coding RNAs that inhibit SARS-CoV-2 gene expression have been proposed to prevent multiple viral infections, pulmonary hypertension, and related diseases31.
In this paper, 10 hub genes related to five diseases of COVID-19, IPF, CRC lung metastasis, SCLC, and NSCLC were identified by means of bioinformatics, namely NDC80, KIF4A, CKS1B, CEP55, PRIM1, TACC3, RRM1, SHCBP1, NUP37 and CCT5. By analyzing these genes, the following related conclusions were obtained. TACC3 is involved in regulating normal cell growth and differentiation. Overexpression of TACC3 was previously associated with poor prognosis in lung cancer, and its expression levels were associated with clinical outcomes of lung cancer patients32. SHCBP1 plays an important role in the development of NSCLC33. The protein levels of NDC80, which is required for chromosome segregation, are kept low early in the cell cycle to allow the normal assembly of meiotic I kinetochores34. CEP55 protein levels are significantly reduced in extracellular vesicles from SDCBP-knockout lines35. RRM1 overexpression has been associated with gemcitabine resistance36. RRM1 can be used as a biomarker of gemcitabine resistance, and its increased expression has been shown to lead to gemcitabine resistance37. Furthermore, EGFR and KRAS are the most common mutated oncogenic drivers in lung adenocarcinoma38. While KRAS, HRAS and NRAS are all known oncogenes, the KRAS isoform is most commonly mutated in cancer, particularly in lung adenocarcinoma39. Studies on lung cancer have shown that deregulated expression or genomic alterations of KRAS oncogenes contribute to tumor progression and metastasis40. The absence of KEAP1 promoted metastasis in their lung cancer model expressing KRAS41.
The best way to understand how an organism responds is through its pathway analysis. A KEGG pathway containing 79 common DEGs has been identified as a similar pathway in five diseases. The top ten KEGG human pathways include ether lipid metabolism, pentose phosphate pathway, glycerophospholipid metabolism, sphingolipid metabolism, pathways in cancer, signaling pathways regulating pluripotency of stem cell, glutathione metabolism, TGF-beta signaling pathway, Fc gamma R-mediated phagocytosis and choline metabolism in cancer. Etheric lipid metabolism is strongly associated with lung cancer42.
We identified 10 drug candidates: progesterone, estradiol, tetradioxin, dasatinib, decitabine, 7646-79-9 (cobalt chloride), cyclosporine A, resveratrol alcohol, genistein, and enterolactone. These drugs may have potential activity in 5 diseases.
The anticancer agent dasatinib induces the apoptosis of cancer cells43. 5-Fu-induced apoptosis in CRC is significantly reduced by dasatinib by inhibiting Src activation44. TRAF6 depletion induces decitabine resistance in triple-negative breast cancer by blocking decitabine-induced DNA methyltransferase DEGs radiation45. Cyclosporin A is a powerful immunosuppressive agent that acts on T-lymphocytes and blocks effective T-cell receptor signaling46,47. By blocking MEK/ERK/c-Fos pathways, immunosuppressants like cyclosporine A, which is used to treat non-small lung cancer, reduce TRPM6 expression48. Resveratrol alcohol has shown promising activity in preventing and treating cancer49. Resveratrol inhibits cancer progression by inducing p53-dependent cell death50. Pancreatic cancer cells can be inhibited in their growth and apoptosis can be induced by Genistein by inhibiting oncogenic miR-223 expression51. There is strong evidence that higher serum enterolactone concentrations and increased lignan intake improve patients' prognoses after menopause52.
About 15 million people died in the first two years of the COVID-19 pandemic, according to new data from the World Health Organization (WHO). WHO estimates the global excess death toll in 2020 and 2021 at 14.9 million. Most of these deaths (84%) were concentrated in Southeast Asia, Europe and the Americas, and more than two-thirds (68%) occurred in just 10 countries53. Children infected with Omicron have a lower risk of serious illness than children infected with Delta54.
The current global COVID-19 pandemic is caused by the rapid international spread of SARS-CoV-2 infection55–57. In essence, COVID-19 can range from mild, self-limiting respiratory dis- ease to severe progressive pneumonia, multiorgan failure, and even death. SARS-CoV-2 infection, especially severe infection, is associated with an increased risk of longitudinal cognitive decline58. At the same time, SARS-CoV-2 has a high transmission rate, and there is a lack of adequate and effective treatments. Consequently, the increase in the number of respiratory distress cases has the potential to overwhelm global health care capacity59.
A recent study shows that tissue damage related to COVID-19 is mainly mediated by host innate immunity60. In critically ill COVID-19 patients, the host immune response is thought to play a key role in driving acute pneumonia with diffuse alveolar injury, inflammatory infiltration and microvascular thrombosis61. Expression of cell receptors (such as IFNGR1 and CXCR4) was reduced by a viral infection and is associated with suppression of associated signaling pathways and immune function62. A systematic evaluation in 2020 estimated that 28% of patients with severe COVID-19 had venous thromboembolism. Complications of COVID-19 thrombosis include arterial and venous events, and microvascular thrombosis may lead to diffuse alveolar damage, the main source of lung injury in COVID-19 patients63,64. Among COVID-19 patients, acute respiratory failure is the main reason for admission to the intensive care unit, and hypoxic respiratory failure is the most common life-threatening complication of COVID-1965,66.
In addition, in patients with COVID-19, ACE2 may play a causative role in cardiovascular complications such as thrombosis, heart damage and heart failure. ACE2 may be the link between SARS-CoV-2 and the cardiac manifestations identified in global data on the COVID-19 pandemic67. Recent studies have proven that COVID-19 is an independent risk factor for ischaemic stroke and acute myocardial infarction68. Additionally, older age is a significant independent predictor of mortality from SARS and Middle East respiratory syndrome (MERS). Similarly, increased age is associated with death among patients with COVID-1969. Many patients who die from COVID-19 are older and weaker with significantly compromised lung and/or immune function70.
Thus, to combat the COVID-19 pandemic, there is an urgent need to identify effective SARS-CoV-2 therapeutics that improve prognosis, particularly those with utility in the outpatient setting71. While new treatments are being developed, there is growing interest in repurposing existing drugs for COVID-1972,73. Aside from the recently licensed LY-CoV1404 (bebtelovimab), there are no licensed monoclonal antibody therapies that adequately target all Omicron variants74. Itaconic acid supplementation and CLYBL inhibition are possible therapeutic options for the treatment of COVID-19 that aim to modulate host defense to combat SARS-CoV-2 infection75. Hydroxychloroquine has been widely promoted as a potential treatment for COVID-19 due to its anti-inflammatory effects and antiviral activity in vitro studies76. Azithromycin is a widely used drug that may reduce viral load when combined with hydroxychloroquine in patients with non-severe COVID-1977. Trials of the JAK inhibitors baricitinib and ruxolitinib have shown promise in controlling excessive inflammation in COVID-19 patients78. Bamlanivimab monotherapy was reported to reduce the incidence of SARS-CoV-2 infection. Tocilizumab is an effective treatment for COVID-19 in patients with evidence of hypoxia and inflammation79.
Another strategy for combatting the global COVID-19 pandemic is to develop and produce COVID-19 vaccines80. Vaccination will be a key strategy for limiting the spread of SARS-CoV-2, reducing mortality, and controlling the COVID-19 pandemic81,82. The Pfizer-BioNTech vaccine is 95% effective against laboratory-confirmed COVID-19, and the Oxford–AstraZeneca vaccine was found to be 70% effective against COVID-19 in seronegative participants83.
Several guidelines issued at the start of the COVID-19 pandemic recommended delaying systemic anticancer treatment until COVID-19 symptoms had completely subsided84. However, failure to provide effective cancer treatment to many cancer patients during a pandemic increases cancer morbidity and mortality, which may be more serious than COVID-19 itself85. Patients with cancer are ostensibly more likely to develop and succumb to COVID-19 due to immunosuppression, increased comorbidity, and in the case of those with lung malignancies, potential pre-existing lung damage86.
Results from a recent study suggest that severe COVID-19 can lead to bilateral interstitial pneumonia, which usually results in acute respiratory distress syndrome and pulmonary fibrosis in survivors87. In essence, IPF is a chronic, progressive lung disease with a median survival time of 5.7 years88. Pulmonary fibrosis, similar to other types of fibrosis, is characterized by excessive accumulation of collagen and other matrix proteins, leading to distorting of pulmonary tissue structure and ultimately pulmonary failure89. Moreover, epithelial cell apoptosis and impaired autophagy are increasingly recognized as hallmarks of pulmonary fibrosis90. The causal role of endoplasmic reticulum stress in the pathogenesis of pulmonary fibrosis has been investigated91. The importance of CD4 + T cells producing interleukin-17A has been demonstrated in pulmonary fibrosis92.
Previously, IPF has been treated with a variety of chemical agents and drugs. For example, nintedanib and pirfenidone are FDA-approved for the treatment of IPF because they slow disease progression and improve lung function, exercise tolerance, and progression-free survival93. Compared to these two FDA-approved therapeutics for IPF, nebulized triiodothyronine has shown comparable or better effects on pulmonary fibrosis or survival rates94.
According to one study, those with IPF had a significantly higher chance of developing lung cancer than those of the same sex and age. Research supports that lung cancer is the leading cause of cancer-related deaths worldwide, with NSCLC accounting for 85% of cases95. The median overall survival (OS) and 5-year survival rates have historically been poor for patients with NSCLC96. The historical 5-year OS rate for locally advanced NSCLC patients treated with radical concurrent radiotherapy ranges from 25 to 30%97. As the leading cause of cancer-related deaths worldwide, adenocarcinoma of the lung accounts for approximately 50% of lung cancers, making it the most common subtype of NSCLC98–100. The highly aggressive NSCLC subtype squamous cell carcinoma of the lung accounts for one-third of all lung cancer cases101,102. The Keap1–Nrf2 pathway was associated with lung squamous cell carcinogenesis and chemoresistance103,104.
Besides, there is growing interest in the potential benefits of antimetastatic treatment for cancers such as lung adenocarcinoma, as a large number of patients are initially diagnosed with localized disease105. Maintenance therapy has become the standard of care for patients with advanced non-squamous NSCLC106. Of this total, approximately 50% of lung adenocarcinomas are molecularly subdivided, and their treatment depends on the presence of different molecular alterations, including EGFR mutations and ALK or ROS1 fusions, that confer sensitivity to selective kinase inhibitors107. For advanced EGFR-mutated lung adenocarcinoma, first-line therapy involves treatment with EGFR tyrosine kinase inhibitors108. There has been a study indicating that gene-based targeted therapy is the standard of care for patients with advanced NSCLC109. Atezolizumab monotherapy is effective in patients with PD-L1-selected advanced NSCLC110. In parallel, advances in immunotherapy have improved outcomes for a variety of cancers, including NSCLC111. For example, cancer immunotherapy with checkpoint inhibitors improves the survival rate of patients with NSCLC112. Based on the ability of immune checkpoint inhibitors to improve survival rates, these therapeutics have been approved for the treatment of a wide range of cancers, including NSCLC, melanoma, and uroepithelial cancer113. However, STK11 (LKB1) mutations are a major cause of primary resistance to immunotherapy in NSCLC114.
Surgical resection is currently the treatment option that provides the greatest long-term survival benefit for patients with early-stage NSCLC115. All studies suggest that pneumonectomy/metastasectomy is safe and can be performed in the absence of significant morbidity116. For patients with non-metastatic lung cancer, a proportion of patients can be cured after initial surgical resection, radiotherapy and/or a combination of therapeutic approaches117.
Oxaliplatin is a first-line treatment for breast cancer and NSCLC118. Triple-negative breast cancer is an aggressive form of invasive breast cancer defined by the lack of significant expression of the therapeutic target estrogen receptor, progesterone receptor and HER2119. circKIF4A is a prognostic biomarker and therapeutic target for triple-negative breast cancer120.
Several other studies have reported that loss-of-function mutations in the RB1 gene are common in several refractory cancers, such as SCLC and triple-negative breast cancer121. Among lung cancer cases, 15–30% are SCLC and one-third are diagnosed at the limited stage122. SCLC is an aggressive high-grade neuroendocrine malignancy and one of the deadliest solid tumors123. Among cancer patients, those with SCLC have one of the worst survival rates, with an overall 5-year survival rate of approximately 5%124,125. SCLC is characterized by a rapid doubling time and high growth fraction, and approximately two-thirds of patients present with metastases at the time of diagnosis126. SCLC is thought to acquire metastatic ability early during tumor progression. A previous study found that NFIB was highly expressed in more than 50% of human SCLC metastases, suggesting that upregulation of this transcription factor may be a driver of SCLC metastasis127. CREBBP plays a key role as a tumor suppressor in SCLC128.
SCLC is a rapidly fatal disease with few treatment options 129. The combination of a platinum drug and etoposide remains the mainstay treatment for SCLC130. However, cisplatin and etoposide improved progression-free survival (PFS) but failed to improve OS in patients with an extensive stage (ES)-SCLC131. The only FDA-approved drug for recurrent or progressive SCLC is topotecan, which has a response rate of 24% in patients with the platinum-sensitive disease and 2–6% in patients with platinum-refractory SCLC132. Although patients with SCLC usually respond initially to chemotherapy, the tumors almost always recur within 6–12 months, resulting in a 5-year survival rate of less than 7%133. Both DDR inhibition and immune checkpoint blockade are therapeutic strategies in preclinical and clinical development for patients with SCLC134. Patients with stage I to II SCLC have achieved long-term survival after radiotherapy with acceptable toxicity135. Suppression of DNA damage repair by poly [ADP-ribose] polymerase inhibitors have emerged as a potential therapeutic strategy for SCLC136. SCLC is sensitive to THZ1, a covalent CDK7 inhibitor with single-agent activity in T-cell acute lymphoblastic leukemia, MYCN- dependent neuroblastoma, and triple-negative breast cancer137,138. Pembrolizumab monotherapy is approved as a third-line or later therapy for metastatic SCLC139.
CRC is the second leading killer behind lung cancer, but must not be underestimated. CRC is one of the most common malignancies of the digestive system and the second most common cancer in the United States; in this country, CRC causes more than 50,000 deaths each year140–142. Dietary and lifestyle factors may significantly affect the risk of recurrence and death from CRC143. For example, obesity is associated with reduced survival among patients with metastatic CRC, particularly those receiving antiangiogenic therapy144.
COX-2 overexpression has been observed in a variety of malignancies, including lung and CRCs145. Aspirin may be more effective in preventing sporadic CRC in which COX-2 is over-expressed. In CRC, delayed initiation of chemotherapy is associated with a reduction in overall survival146. In patients with stage III CRC, adjuvant chemotherapy may improve OS147. Activation of p38-MAPK signaling due to KRAS mutation in CRC enables secondary colonization of the lungs by established liver metastases148.
The specific EGFR inhibitor cetuximab has been used to treat metastatic CRC, metastatic NSCLC and head and neck cancer149. Human metastatic lung cancer has high levels of HO1 and Bach1; thus, HO1 inhibitors represent an effective therapeutic strategy to prevent lung cancer metastasis150.
Since the World Health Organization declared a global pandemic on 11 March 2020, SARS- CoV-2 has caused more than 6.2 million deaths worldwide151. Compared to infection with the Delta variant, with Omicron carries a significantly lower risk of serious outcomes, with a greater reduction in the risk of more serious endpoints but the significant variation with age152. Since the beginning of the pandemic, more than 400 million cases of SARS-CoV-2 infection have been confirmed, including in people with chronic diseases such as cancer, diabetes and heart disease. These people are at high risk of serious illness and death associated with SARS-CoV-2 infection due to poor immune function as a result of pre-existing disease. The ultimate aim of this study was to improve the survival rate of patients with chronic diseases who are infected with SARS-CoV-2.
The COVID-19 pandemic poses an unprecedented challenge to global healthcare resources153. The pandemic has severely crowded out medical resources, and deaths from other diseases, such as CRC, have risen sharply. There are two main targets of CRC metastasis, the liver and lungs, but liver metastases are the most common. The liver has the considerable regenerative capacity and can regrow after partial surgical resection, whereas the lungs have no regenerative capacity, and lung function is irreversibly impaired after lung lobe removal due to CRC metastasis. The respiratory system is overwhelmed when patients with CRC lung metastasis become infected with SARS- CoV-2.
Recent studies have shown that a network where nodes are people and edges represent their social connections can effectively mimic the spreading of the virus154. Some graphics-based epidemiological models to simulate disease production and transmission pathways can significantly improve disease transmission control155.
There are many limitations to the treatment of cancer. One flaw of chemotherapy drugs is the inability to distinguish between malignant and normal cells; although chemotherapeutics kills cancer cells, they also non-selectively kill normal stem cells that must divide to maintain tissue homeostasis. In contrast, targeted drugs affect specific lesions, accumulating at the target site or releasing an active ingredient at the target site. However, mutation of the target renders the targeted drug ineffective, and drug resistance can occur with the long-term application. Immunotherapy aims to activate the host immune system, relying on its function to kill cancer cells and debulk tumors; this treatment approach is currently only effective in some cancers, and long-term treatment carries the risk of the cytokine storm.
Chemotherapy, targeted therapy and immunotherapy essentially kill cancer cells but have no effect on SARS-CoV-2. Only symptomatic treatment for COVID-19 is available, and eliminating the infection is dependent on the host immune system. Chemotherapy, targeted therapy and immunotherapy suppress immune function in cancer patients to varying degrees. If these patients are infected with SARS-CoV-2 while on treatment, their immune function will decrease drastically or even collapse, leading to a variety of serious complications and eventually systemic organ failure and death.
As mentioned above, although some specific drugs against SARS-COV-2 have been successfully developed, it is unclear whether they can have a positive effect in the treatment of lung cancer. People are more willing to discover and adopt existing drugs that are now well established to treat covid-19 and its complications. This article uses bioinformatics methods to screen out the 10 most meaningful drugs that act together on these five diseases. We found that these drugs have a positive effect in treating COVID-19 and lung cancer, potentially reducing the risk of pulmonary fibrosis caused by COVID-19. During the COVID-19 pandemic, in-depth research on these drugs may have certain reference significance for the prevention and treatment of complications in lung cancer patients infected with SARS-COV-2.
Conclusion
In this study, we identified 79 DEGs between COVID-19, IPF, CRC lung metastasis, SCLC and NSCLC. In our opinion, these DEGs are the common core genes of the 5 diseases. Meanwhile, based on the transcriptome features of DSigDB and common DEGs, we identified 10 drug candidates. The treatment of COVID-19 and lung cancer with these drugs may show positive results, potentially reducing the risk of COVID-19-induced pulmonary fibrosis. This research provides new ideas that have not yet been experimentally validated, and additional scientific research is needed to validate our findings and hypotheses.
Acknowledgements
We acknowledge the GEO database for providing their platforms and contributors for uploading their meaningful datasets.
Abbreviations
- COVID-19
Coronavirus disease 2019
- SARS-CoV-2
Syndrome coronavirus 2
- SCLC
Small cell lung cancer
- NSCLC
Non-small cell lung cancer
- IPF
Idiopathic lung fibrosis
- CRC
Colorectal cancer
- BP
Biological process
- CC
Cellular component
- DEGs
Differentially expressed gene
- KEGG
Kyoto encyclopedia of genes and genomes
- MF
Molecular function
- PPI
Protein- Protein Interaction
- EGFR
Epidermal growth factor receptor
- SP-D
Surfactant protein D
Author contributions
Y.L. collected and analysed the data. L.P. N. designed and supervised the study. Y.L. drafted the first version of the manuscript. L.P. N. reviewed and revised the manuscript. Y.L. constructed the diagrams and tables of the article. All authors read and approved the final manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. The datasets (GSE186460, GSE17978, GSE41258, GSE40275 and GSE33532) analyzed during the current study are available in the Gene Expression Omnibus (GEO) repository (http://www.ncbi.nlm.nih.gov/geo/).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singh S, et al. How an outbreak became a pandemic: A chronological analysis of crucial junctures and international obligations in the early months of the COVID-19 pandemic. Lancet. 2021;398:2109–2124. doi: 10.1016/s0140-6736(21)01897-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020;8:807–815. doi: 10.1016/s2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang HJ, et al. The relationship between the severity of pulmonary fibrosis and the lung cancer stage. J. Cancer. 2021;12:2807–2814. doi: 10.7150/jca.51445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sica A, et al. Immunometabolic status of COVID-19 cancer patients. Physiol. Rev. 2020;100:1839–1850. doi: 10.1152/physrev.00018.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells CR, Galvani AP. Impact of the COVID-19 pandemic on cancer incidence and mortality. Lancet Public Health. 2022;7:e490–e491. doi: 10.1016/s2468-2667(22)00111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogado J, et al. 1685P is cancer what determines COVID-19 oncological patient’s outcome or are other external factors involved? Experience in a hospital in Madrid, Spain. Ann. Oncol. 2020;31:S997. doi: 10.1016/j.annonc.2020.08.1749. [DOI] [Google Scholar]
- 7.Zhang L, et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang F, et al. Inosine monophosphate dehydrogenase dependence in a subset of small cell lung cancers. Cell Metab. 2018;28:369–382.e365. doi: 10.1016/j.cmet.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, et al. Characterization of a novel HDAC/RXR/HtrA1 signaling axis as a novel target to overcome cisplatin resistance in human non-small cell lung cancer. Mol. Cancer. 2020;19:134. doi: 10.1186/s12943-020-01256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lote H, et al. Carbon dating cancer: Defining the chronology of metastatic progression in colorectal cancer. Ann. Oncol. 2017;28:1243–1249. doi: 10.1093/annonc/mdx074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasegawa Y, et al. Surfactant protein D suppresses lung cancer progression by downregulation of epidermal growth factor signaling. Oncogene. 2015;34:4285–4286. doi: 10.1038/onc.2015.266. [DOI] [PubMed] [Google Scholar]
- 12.Tajima Y, et al. Association of surfactant protein D with pulmonary metastases from colon cancer. Oncol. Lett. 2020;20:322. doi: 10.3892/ol.2020.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrett T, et al. NCBI GEO: Archive for functional genomics data sets–10 years on. Nucleic Acids Res. 2011;39:D1005–1010. doi: 10.1093/nar/gkq1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobosh B, et al. Baricitinib attenuates the proinflammatory phase of COVID-19 driven by lung-infiltrating monocytes. Cell Rep. 2022;39:110945. doi: 10.1016/j.celrep.2022.110945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emblom-Callahan MC, et al. Genomic phenotype of non-cultured pulmonary fibroblasts in idiopathic pulmonary fibrosis. Genomics. 2010;96:134–145. doi: 10.1016/j.ygeno.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Sheffer M, et al. Association of survival and disease progression with chromosomal instability: A genomic exploration of colorectal cancer. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7131–7136. doi: 10.1073/pnas.0902232106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kastner S, et al. Expression of G protein-coupled receptor 19 in human lung cancer cells is triggered by entry into S-phase and supports G(2)-M cell-cycle progression. Mol. Cancer Res. 2012;10:1343–1358. doi: 10.1158/1541-7786.Mcr-12-0139. [DOI] [PubMed] [Google Scholar]
- 18.Meister M, et al. Intra-tumor heterogeneity of gene expression profiles in early stage non-small cell lung cancer. J. Bioinf. Res. Stud. 2014;1:1. [Google Scholar]
- 19.Anjum A, et al. Identification of differentially expressed genes in RNA-seq data of Arabidopsis thaliana: A compound distribution approach. J. Comput. Biol. 2016;23:239–247. doi: 10.1089/cmb.2015.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bardou P, Mariette J, Escudié F, Djemiel C, Klopp C. jvenn: An interactive Venn diagram viewer. BMC Bioinf. 2014;15:293. doi: 10.1186/1471-2105-15-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joly JH, Lowry WE, Graham NA. Differential gene set enrichment analysis: A statistical approach to quantify the relative enrichment of two gene sets. Bioinformatics. 2021;36:5247–5254. doi: 10.1093/bioinformatics/btaa658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen EY, et al. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49:D545–D551. doi: 10.1093/nar/gkaa970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szklarczyk D, et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shannon P, et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chin CH, et al. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014;8(Suppl 4):S11. doi: 10.1186/1752-0509-8-s4-s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoo M, et al. DSigDB: Drug signatures database for gene set analysis. Bioinformatics. 2015;31:3069–3071. doi: 10.1093/bioinformatics/btv313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon S, et al. Association of social distancing and face mask use with risk of COVID-19. Nat. Commun. 2021;12:3737. doi: 10.1038/s41467-021-24115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray CJL. COVID-19 will continue but the end of the pandemic is near. Lancet. 2022;399:417–419. doi: 10.1016/s0140-6736(22)00100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agbarya A, et al. 1683P SARS-CoV-2 infection and lung cancer management in Europe. Ann. Oncol. 2020;31:S996–S997. doi: 10.1016/j.annonc.2020.08.1747. [DOI] [Google Scholar]
- 31.Natarelli, L. et al. MicroRNAs and Long Non-Coding RNAs as Potential Candidates to Target Specific Motifs of SARS-CoV-2. Noncoding RNA7. 10.3390/ncrna7010014 (2021). [DOI] [PMC free article] [PubMed]
- 32.Uhlen, M. et al. A pathology atlas of the human cancer transcriptome. Science357. 10.1126/science.aan2507 (2017). [DOI] [PubMed]
- 33.Shi W, et al. Hyperactivation of HER2-SHCBP1-PLK1 axis promotes tumor cell mitosis and impairs trastuzumab sensitivity to gastric cancer. Nat. Commun. 2021;12:2812. doi: 10.1038/s41467-021-23053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng Z, et al. Pervasive, Coordinated Protein-Level Changes Driven by Transcript Isoform Switching during Meiosis. Cell. 2018;172:910–923.e916. doi: 10.1016/j.cell.2018.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luck K, et al. A reference map of the human binary protein interactome. Nature. 2020;580:402–408. doi: 10.1038/s41586-020-2188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin-Broto J, et al. Gemcitabine plus sirolimus for relapsed and progressing osteosarcoma patients after standard chemotherapy: a multicenter, single-arm phase II trial of Spanish Group for Research on Sarcoma (GEIS) Ann. Oncol. 2017;28:2994–2999. doi: 10.1093/annonc/mdx536. [DOI] [PubMed] [Google Scholar]
- 37.Lankadasari MB, et al. Targeting S1PR1/STAT3 loop abrogates desmoplasia and chemosensitizes pancreatic cancer to gemcitabine. Theranostics. 2018;8:3824–3840. doi: 10.7150/thno.25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maity TK, et al. Loss of MIG6 Accelerates Initiation and Progression of Mutant Epidermal Growth Factor Receptor-Driven Lung Adenocarcinoma. Cancer Discov. 2015;5:534–549. doi: 10.1158/2159-8290.Cd-14-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly MR, et al. Combined Proteomic and Genetic Interaction Mapping Reveals New RAS Effector Pathways and Susceptibilities. Cancer Discov. 2020;10:1950–1967. doi: 10.1158/2159-8290.Cd-19-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weng CW, et al. Pharmacophore-based virtual screening for the identification of the novel Src inhibitor SJG-136 against lung cancer cell growth and motility. Am. J. Cancer Res. 2020;10:1668–1690. [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson NM, Simon MC. BACH1 Orchestrates Lung Cancer Metastasis. Cell. 2019;178:265–267. doi: 10.1016/j.cell.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 42.El-Aarag SA, et al. In silico identification of potential key regulatory factors in smoking-induced lung cancer. BMC Med. Genomics. 2017;10:40. doi: 10.1186/s12920-017-0284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sikora E, Bielak-Zmijewska A, Mosieniak G. Targeting normal and cancer senescent cells as a strategy of senotherapy. Ageing Res. Rev. 2019;55:100941. doi: 10.1016/j.arr.2019.100941. [DOI] [PubMed] [Google Scholar]
- 44.Fu Y, et al. Dasatinib reduces 5-Fu-triggered apoptosis in colon carcinoma by directly modulating Src-dependent caspase-9 phosphorylation. Cell Death Discov. 2018;4:61. doi: 10.1038/s41420-018-0062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu YT, et al. The KBTBD6/7-DRD2 axis regulates pituitary adenoma sensitivity to dopamine agonist treatment. Acta Neuropathol. 2020;140:377–396. doi: 10.1007/s00401-020-02180-4. [DOI] [PubMed] [Google Scholar]
- 46.Slütter B, Kuiper J. Immune Responses in Context. Circulation. 2019;139:2567–2569. doi: 10.1161/CIRCULATIONAHA.119.040651. [DOI] [PubMed] [Google Scholar]
- 47.Kitanosono T, Masuda K, Xu P, Kobayashi S. Catalytic Organic Reactions in Water toward Sustainable Society. Chem. Rev. 2018;118:679–746. doi: 10.1021/acs.chemrev.7b00417. [DOI] [PubMed] [Google Scholar]
- 48.Takashina, Y. et al. Sodium Citrate Increases Expression and Flux of Mg(2+) Transport Carriers Mediated by Activation of MEK/ERK/c-Fos Pathway in Renal Tubular Epithelial Cells. Nutrients10. 10.3390/nu10101345 (2018). [DOI] [PMC free article] [PubMed]
- 49.Venturelli S, et al. Resveratrol as a pan-HDAC inhibitor alters the acetylation status of histone [corrected] proteins in human-derived hepatoblastoma cells. PLoS ONE. 2013;8:e73097. doi: 10.1371/journal.pone.0073097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva, J. L., Cino, E. A., Soares, I. N., Ferreira, V. F. & G, A. P. d. O. Targeting the Prion-like Aggregation of Mutant p53 to Combat Cancer. Acc. Chem. Res.51, 181–190. 10.1021/acs.accounts.7b00473 (2018). [DOI] [PubMed]
- 51.Javed Z, et al. Correction: Genistein as a regulator of signaling pathways and microRNAs in different types of cancers. Cancer Cell. Int. 2022;22:256. doi: 10.1186/s12935-022-02667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Z, et al. Lignans intake and enterolactone concentration and prognosis of breast cancer: a systematic review and meta-analysis. J. Cancer. 2021;12:2787–2796. doi: 10.7150/jca.55477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adam D. 15 million people have died in the pandemic, WHO says. Nature. 2022;605:206. doi: 10.1038/d41586-022-01245-6. [DOI] [PubMed] [Google Scholar]
- 54.Mallapaty S. Most US kids have caught the coronavirus, antibody survey finds. Nature. 2022;605:207. doi: 10.1038/d41586-022-01231-y. [DOI] [PubMed] [Google Scholar]
- 55.Caricchio R, et al. Effect of Canakinumab vs Placebo on Survival Without Invasive Mechanical Ventilation in Patients Hospitalized With Severe COVID-19: A Randomized Clinical Trial. JAMA. 2021;326:230–239. doi: 10.1001/jama.2021.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dhaka S, Beniwal SK, Arora V. CN45 Effect of SARS-CoV-2 on management of paediatric blood malignancy: A regional cancer centre study. Ann. Oncol. 2021;32:S1272. doi: 10.1016/j.annonc.2021.08.674. [DOI] [Google Scholar]
- 57.Thoms M, et al. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science. 2020;369:1249–1255. doi: 10.1126/science.abc8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Douaud G, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richmond P, et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:682–694. doi: 10.1016/s0140-6736(21)00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verdoni L, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/s0140-6736(20)31103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abaleke E, et al. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet. 2021;397:605–612. doi: 10.1016/s0140-6736(21)00149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sansico F, et al. COVID-19 specific immune markers revealed by single cell phenotypic profiling. Biomedicines. 2021 doi: 10.3390/biomedicines9121794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lopes RD, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): An open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/s0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bradley BT, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: A case series. Lancet. 2020;396:320–332. doi: 10.1016/s0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grieco DL, et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: The HENIVOT randomized clinical trial. JAMA. 2021;325:1731–1743. doi: 10.1001/jama.2021.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dequin PF, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically Ill patients with COVID-19: A randomized clinical trial. JAMA. 2020;324:1298–1306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Narula S, et al. Plasma ACE2 and risk of death or cardiometabolic diseases: A case-cohort analysis. Lancet. 2020;396:968–976. doi: 10.1016/s0140-6736(20)31964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Fors Connolly AM. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: A self-controlled case series and matched cohort study. Lancet. 2021;398:599–607. doi: 10.1016/s0140-6736(21)00896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mehta V, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10:935–941. doi: 10.1158/2159-8290.Cd-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bojkova D, et al. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583:469–472. doi: 10.1038/s41586-020-2332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iwanami S, et al. Detection of significant antiviral drug effects on COVID-19 with reasonable sample sizes in randomized controlled trials: A modeling study. PLoS Med. 2021;18:e1003660. doi: 10.1371/journal.pmed.1003660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bouhaddou M, et al. The global phosphorylation landscape of SARS-CoV-2 infection. Cell. 2020;182:685–712.e619. doi: 10.1016/j.cell.2020.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hong Q, et al. Molecular basis of receptor binding and antibody neutralization of Omicron. Nature. 2022;604:546–552. doi: 10.1038/s41586-022-04581-9. [DOI] [PubMed] [Google Scholar]
- 75.Liu Y, et al. A urinary proteomic landscape of COVID-19 progression identifies signaling pathways and therapeutic options. Sci. China Life Sci. 2022 doi: 10.1007/s11427-021-2070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Self WH, et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: A randomized clinical trial. JAMA. 2020;324:2165–2176. doi: 10.1001/jama.2020.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Furtado RHM, et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): A randomised clinical trial. Lancet. 2020;396:959–967. doi: 10.1016/s0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Desai N, et al. Temporal and spatial heterogeneity of host response to SARS-CoV-2 pulmonary infection. Nat. Commun. 2020;11:6319. doi: 10.1038/s41467-020-20139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/s0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazereel V, Van Assche K, Detraux J, De Hert M. COVID-19 vaccination for people with severe mental illness: Why, what, and how? Lancet Psychiatry. 2021;8:444–450. doi: 10.1016/s2215-0366(20)30564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Logunov DY, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/s0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stephenson KE, et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. Jama. 2021;325:1535–1544. doi: 10.1001/jama.2021.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vasileiou E, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: A national prospective cohort study. Lancet. 2021;397:1646–1657. doi: 10.1016/s0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aguinaga L, et al. 1721P Prolonged positive SARS-CoV-2 RT-PCR in cancer outpatients requires specific reorganization of cancer centres. Ann. Oncol. 2020;31:S1010. doi: 10.1016/j.annonc.2020.08.1785. [DOI] [Google Scholar]
- 85.Lee LY, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/s0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nichetti F, Bini M, Dotti KF, Ottini A, Braud F. 1702P COVID-19 risk for patients undergoing anticancer treatment at the outpatient clinic of the National Cancer Institute of Milan: The COVINT study. Ann. Oncol. 2020;31:S1003. doi: 10.1016/j.annonc.2020.08.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khalaj K, Figueira RL, Antounians L, Lauriti G, Zani A. Systematic review of extracellular vesicle-based treatments for lung injury: Are EVs a potential therapy for COVID-19? J. Extracell. Vesicles. 2020;9:1795365. doi: 10.1080/20013078.2020.1795365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilson AM, et al. Effect of co-trimoxazole (Trimethoprim-Sulfamethoxazole) vs placebo on death, lung transplant, or hospital admission in patients with moderate and severe idiopathic pulmonary fibrosis: The EME-TIPAC randomized clinical trial. JAMA. 2020;324:2282–2291. doi: 10.1001/jama.2020.22960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lagares D, et al. ADAM10-mediated ephrin-B2 shedding promotes myofibroblast activation and organ fibrosis. Nat. Med. 2017;23:1405–1415. doi: 10.1038/nm.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Araya J, et al. Insufficient autophagy in idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013;304:L56–69. doi: 10.1152/ajplung.00213.2012. [DOI] [PubMed] [Google Scholar]
- 91.Borok Z, et al. Grp78 loss in epithelial progenitors reveals an age-linked role for endoplasmic reticulum stress in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2020;201:198–211. doi: 10.1164/rccm.201902-0451OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Celada LJ, et al. PD-1 up-regulation on CD4(+) T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci. Transl. Med. 2018 doi: 10.1126/scitranslmed.aar8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Du J, et al. Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF-β production. Blood. 2017;129:2570–2580. doi: 10.1182/blood-2017-01-758854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu G, et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat. Med. 2018;24:39–49. doi: 10.1038/nm.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wei F, et al. Exosomes derived from gemcitabine-resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Mol. Cancer. 2017;16:132. doi: 10.1186/s12943-017-0694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garon EB, et al. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE-001 study. J. Clin. Oncol. 2019;37:2518–2527. doi: 10.1200/jco.19.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jabbour SK, et al. Phase 1 trial of pembrolizumab administered concurrently with chemoradiotherapy for locally advanced non-small cell lung cancer: A nonrandomized controlled trial. JAMA Oncol. 2020;6:848–855. doi: 10.1001/jamaoncol.2019.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jordan EJ, et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov. 2017;7:596–609. doi: 10.1158/2159-8290.Cd-16-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li F, et al. In vivo epigenetic CRISPR screen identifies Asf1a as an immunotherapeutic target in kras-mutant lung adenocarcinoma. Cancer Discov. 2020;10:270–287. doi: 10.1158/2159-8290.Cd-19-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim JW, et al. Antitumor activity of an engineered decoy receptor targeting CLCF1-CNTFR signaling in lung adenocarcinoma. Nat. Med. 2019;25:1783–1795. doi: 10.1038/s41591-019-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Torres-Ayuso P, et al. TNIK is a therapeutic target in lung squamous cell carcinoma and regulates FAK activation through merlin. Cancer Discov. 2021;11:1411–1423. doi: 10.1158/2159-8290.Cd-20-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Momcilovic M, et al. The GSK3 signaling axis regulates adaptive glutamine metabolism in lung squamous cell carcinoma. Cancer Cell. 2018;33:905–921.e905. doi: 10.1016/j.ccell.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang JJ, et al. Dietary fat intake and lung cancer risk: A pooled analysis. J. Clin. Oncol. 2017;35:3055–3064. doi: 10.1200/jco.2017.73.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eckhardt M, et al. Multiple routes to oncogenesis are promoted by the human papillomavirus-host protein network. Cancer Discov. 2018;8:1474–1489. doi: 10.1158/2159-8290.Cd-17-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chuang CH, et al. Molecular definition of a metastatic lung cancer state reveals a targetable CD109-Janus kinase-Stat axis. Nat. Med. 2017;23:291–300. doi: 10.1038/nm.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ramalingam SS, et al. Pemetrexed, bevacizumab, or the combination as maintenance therapy for advanced nonsquamous non-small-cell lung cancer: ECOG-ACRIN 5508. J. Clin. Oncol. 2019;37:2360–2367. doi: 10.1200/jco.19.01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tsukumo Y, Naito M, Suzuki T. Influence of EGFR-activating mutations on sensitivity to tyrosine kinase inhibitors in a KRAS mutant non-small cell lung cancer cell line. PLoS ONE. 2020;15:e0229712. doi: 10.1371/journal.pone.0229712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bria E, et al. Outcome of advanced NSCLC patients harboring sensitizing EGFR mutations randomized to EGFR tyrosine kinase inhibitors or chemotherapy as first-line treatment: A meta-analysis. Ann. Oncol. 2011;22:2277–2285. doi: 10.1093/annonc/mdq742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jänne PA, et al. Selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRAS-mutant advanced non-small cell lung cancer: The SELECT-1 randomized clinical trial. JAMA. 2017;317:1844–1853. doi: 10.1001/jama.2017.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peters S, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH) J. Clin. Oncol. 2017;35:2781–2789. doi: 10.1200/jco.2016.71.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haratani K, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4:374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Berner F, et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 2019;5:1043–1047. doi: 10.1001/jamaoncol.2019.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Azad NS, et al. Nivolumab is effective in mismatch repair-deficient noncolorectal cancers: Results from Arm Z1D-A subprotocol of the NCI-MATCH (EAY131) study. J. Clin. Oncol. 2020;38:214–222. doi: 10.1200/jco.19.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Della Corte CM, Byers LA. Evading the STING: LKB1 loss leads to STING silencing and immune escape in KRAS-mutant lung cancers. Cancer Discov. 2019;9:16–18. doi: 10.1158/2159-8290.Cd-18-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Quan YH, et al. Evaluation of intraoperative near-infrared fluorescence visualization of the lung tumor margin with indocyanine green inhalation. JAMA Surg. 2020;155:732–740. doi: 10.1001/jamasurg.2020.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.O'Neill AF, et al. Characterization of pulmonary metastases in children with hepatoblastoma treated on children's oncology group protocol AHEP0731 (The treatment of children with all stages of hepatoblastoma): A report from the children's oncology group. J. Clin. Oncol. 2017;35:3465–3473. doi: 10.1200/jco.2017.73.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chaudhuri AA, et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov. 2017;7:1394–1403. doi: 10.1158/2159-8290.Cd-17-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bruno PM, et al. A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat. Med. 2017;23:461–471. doi: 10.1038/nm.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Keren L, et al. A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell. 2018;174:1373–1387.e1319. doi: 10.1016/j.cell.2018.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tang H, et al. circKIF4A acts as a prognostic factor and mediator to regulate the progression of triple-negative breast cancer. Mol. Cancer. 2019;18:23. doi: 10.1186/s12943-019-0946-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gong X, et al. Aurora a kinase inhibition is synthetic lethal with loss of the RB1 tumor suppressor gene. Cancer Discov. 2019;9:248–263. doi: 10.1158/2159-8290.Cd-18-0469. [DOI] [PubMed] [Google Scholar]
- 122.Pezzi TA, et al. Barriers to combined-modality therapy for limited-stage small cell lung cancer. JAMA Oncol. 2018;4:e174504. doi: 10.1001/jamaoncol.2017.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li L, et al. Identification of DHODH as a therapeutic target in small cell lung cancer. Sci Transl Med. 2019 doi: 10.1126/scitranslmed.aaw7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schenk MW, et al. Soluble guanylate cyclase signalling mediates etoposide resistance in progressing small cell lung cancer. Nat. Commun. 2021;12:6652. doi: 10.1038/s41467-021-26823-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Drapkin BJ, et al. Genomic and functional fidelity of small cell lung cancer patient-derived xenografts. Cancer Discov. 2018;8:600–615. doi: 10.1158/2159-8290.Cd-17-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rudin CM, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: Randomized, double-blind, phase III KEYNOTE-604 study. J. Clin. Oncol. 2020;38:2369–2379. doi: 10.1200/jco.20.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang D, et al. Intertumoral heterogeneity in SCLC is influenced by the cell type of origin. Cancer Discov. 2018;8:1316–1331. doi: 10.1158/2159-8290.Cd-17-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Horton SJ, et al. Early loss of Crebbp confers malignant stem cell properties on lymphoid progenitors. Nat. Cell Biol. 2017;19:1093–1104. doi: 10.1038/ncb3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li H, et al. Targeting brain lesions of non-small cell lung cancer by enhancing CCL2-mediated CAR-T cell migration. Nat. Commun. 2022;13:2154. doi: 10.1038/s41467-022-29647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marcoux N, et al. EGFR-mutant adenocarcinomas that transform to small-cell lung cancer and other neuroendocrine carcinomas: Clinical outcomes. J. Clin. Oncol. 2019;37:278–285. doi: 10.1200/jco.18.01585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thomas M, et al. Immunotherapeutic maintenance treatment with toll-like receptor 9 agonist lefitolimod in patients with extensive-stage small-cell lung cancer: Results from the exploratory, controlled, randomized, international phase II IMPULSE study. Ann. Oncol. 2018;29:2076–2084. doi: 10.1093/annonc/mdy326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pietanza MC, et al. Randomized, double-blind, phase II study of temozolomide in combination with either veliparib or placebo in patients with relapsed-sensitive or refractory small-cell lung cancer. J. Clin. Oncol. 2018;36:2386–2394. doi: 10.1200/jco.2018.77.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang H, et al. CDK7 inhibition potentiates genome instability triggering anti-tumor immunity in small cell lung cancer. Cancer Cell. 2020;37:37–54.e39. doi: 10.1016/j.ccell.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sen T, et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 2019;9:646–661. doi: 10.1158/2159-8290.Cd-18-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Salem A, et al. Association of chemoradiotherapy with outcomes among patients with stage I to II vs stage III small cell lung cancer: Secondary analysis of a randomized clinical trial. JAMA Oncol. 2019;5:e185335. doi: 10.1001/jamaoncol.2018.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Farago AF, et al. Combination olaparib and temozolomide in relapsed small-cell lung cancer. Cancer Discov. 2019;9:1372–1387. doi: 10.1158/2159-8290.Cd-19-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang C, et al. A CRISPR screen identifies CDK7 as a therapeutic target in hepatocellular carcinoma. Cell Res. 2018;28:690–692. doi: 10.1038/s41422-018-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rusan M, et al. Suppression of adaptive responses to targeted cancer therapy by transcriptional repression. Cancer Discov. 2018;8:59–73. doi: 10.1158/2159-8290.Cd-17-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pacheco JM, Byers LA. Temozolomide plus PARP inhibition in small-cell lung cancer: Could patient-derived xenografts accelerate discovery of biomarker candidates? Cancer Discov. 2019;9:1340–1342. doi: 10.1158/2159-8290.Cd-19-0850. [DOI] [PubMed] [Google Scholar]
- 140.Ding D, et al. Multifunctional nanodrug mediates synergistic photodynamic therapy and MDSCs-targeting immunotherapy of colon cancer. Adv. Sci. (Weinh) 2021;8:e2100712. doi: 10.1002/advs.202100712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Smith TG, et al. Perceptions of patients with breast and colon cancer of the management of cancer-related pain, fatigue, and emotional distress in community oncology. J. Clin. Oncol. 2019;37:1666–1676. doi: 10.1200/jco.18.01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ganesh K, et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat. Med. 2019;25:1607–1614. doi: 10.1038/s41591-019-0584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fadelu T, et al. Nut consumption and survival in patients with stage III colon cancer: Results from CALGB 89803 (Alliance) J. Clin. Oncol. 2018;36:1112–1120. doi: 10.1200/jco.2017.75.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Incio J, et al. Obesity promotes resistance to anti-VEGF therapy in breast cancer by up-regulating IL-6 and potentially FGF-2. Sci. Transl. Med. 2018 doi: 10.1126/scitranslmed.aag0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hamy AS, et al. Celecoxib with neoadjuvant chemotherapy for breast cancer might worsen outcomes differentially by COX-2 expression and ER status: Exploratory analysis of the REMAGUS02 trial. J. Clin. Oncol. 2019;37:624–635. doi: 10.1200/jco.18.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Salazar MC, et al. Association of delayed adjuvant chemotherapy with survival after lung cancer surgery. JAMA Oncol. 2017;3:610–619. doi: 10.1001/jamaoncol.2016.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tie J, et al. Circulating tumor DNA analyses as markers of recurrence risk and benefit of adjuvant therapy for stage III colon cancer. JAMA Oncol. 2019;5:1710–1717. doi: 10.1001/jamaoncol.2019.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jiang R, et al. The long noncoding RNA lnc-EGFR stimulates T-regulatory cells differentiation thus promoting hepatocellular carcinoma immune evasion. Nat. Commun. 2017;8:15129. doi: 10.1038/ncomms15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lignitto L, et al. Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of bach1. Cell. 2019;178:316–329.e318. doi: 10.1016/j.cell.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Johns Hopkins University & Medicine. COVID-19 Dashboard, https://coronavirus.jhu.edu/map.html (2022).
- 152.Nyberg T, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/s0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Oliveira JF, et al. Mathematical modeling of COVID-19 in 14.8 million individuals in Bahia, Brazil. Nat. Commun. 2021;12:333. doi: 10.1038/s41467-020-19798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Petrizzelli F, Guzzi PH, Mazza T. Beyond COVID-19 pandemic: Topology-aware optimization of vaccination strategy for minimizing virus spreading. Comput. Struct. Biotechnol. J. 2022;20:2664–2671. doi: 10.1016/j.csbj.2022.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hiram Guzzi P, Petrizzelli F, Mazza T. Disease spreading modeling and analysis: A survey. Brief. Bioinform. 2022 doi: 10.1093/bib/bbac230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. The datasets (GSE186460, GSE17978, GSE41258, GSE40275 and GSE33532) analyzed during the current study are available in the Gene Expression Omnibus (GEO) repository (http://www.ncbi.nlm.nih.gov/geo/).