Abstract
Experimental evidence has implicated multiple neurotransmitter systems in either the direct or indirect modulation of cortical arousal and attention circuitry. In this review, we selectively focus on three such systems: 1) norepinephrine (NE)-containing neurons of the locus coeruleus (LC), 2) acetylcholine (ACh)-containing neurons of the basal forebrain (BF), and 3) parvalbumin (PV)-containing gamma-aminobutyric acid neurons of the BF. Whereas BF-PV neurons serve as a rapid and transient arousal system, LC-NE and BF-ACh neuromodulation are typically activated on slower but longer-lasting timescales. Recent findings suggest that the BF-PV system serves to rapidly respond to even subtle sensory stimuli with a microarousal. We posit that salient sensory stimuli, such as those that are threatening or predict the need for a response, will quickly activate the BF-PV system and subsequently activate both the BF-ACh and LC-NE systems if the circumstances require longer periods of arousal and vigilance. We suggest that NE and ACh have overlapping psychological functions with the main difference being the precise internal/environmental sensory situations/contexts that recruit each neurotransmitter system – a goal for future research to determine. Implications of dysfunction of each of these three attentional systems for our understanding of neuropsychiatric conditions are considered. Finally, the contemporary availability of research tools to selectively manipulate and measure the activity of these distinctive neuronal populations promises to answer longstanding questions, such as how various arousal systems influence downstream decision-making and motor responding.
Keywords: Vigilance, Wakefulness, Norepinephrine, Acetylcholine, Parvalbumin, GABA
1. Multiple systems for arousal and attention
Arousal and attention are two closely interacting and mutually beneficial psychological processes which have been the focus of intense scrutiny for decades. General arousal can be thought of as “a continuum of sensitivity to environmental stimuli,” a definition emphasizing its dynamic and changing nature (Berridge, 2008). Additionally, arousal can be increased by elevated levels of motor activity and/or sympathetic activation independent of sensitivity to the environment. The relationship between arousal and cognitive performance follows an inverted-U function whereby optimal attention, learning, and working memory are associated with intermediate levels of arousal (Alhola and Polo-Kantola, 2007; McCoy and Strecker, 2011; Van Dongen et al., 2003; Yerkes and Dodson, 1908). Thus, a range of perceptual, affective, motoric, and cognitive processes including normal attentional function depend on sufficient and balanced arousal. Attention can be broadly described as the active cortical response to specific sensory stimuli in a way that informs action and enhances learning. Understanding the neural regulation of arousal as well as how it contributes to and is differentiated from attention remains an ongoing challenge for neuroscientists. Considering that virtually all higher cognitive and affective processes are dependent on the integrity of multiple subcortical arousal and attention systems, research aimed at parsing the unique contributions of distinct populations of neurons in these overlapping mental states is highly justified.
Experimental evidence has implicated multiple brainstem neurotransmitter systems in either the direct control or indirect modulation of cortical arousal and attention circuitry, including glutamatergic neurons of the parabrachial nucleus, serotonin neurons of the raphe nuclei, dopaminergic neurons in the periaqueductal grey (PAG), acetylcholine neurons of the dorsal pons, and norepinephrinergic neurons of the locus coeruleus (LC) (Brown et al., 2012; Kaur et al., 2017; Magoun, 1952; Scammell et al., 2017; Steriade, 1996). These brainstem sites contribute efferent fibers to the ventral branch of the ascending reticular activating system (ARAS) which innervates arousal promoting neurons in the lateral hypothalamus (LH), tuberomammillary nucleus (TMN) of the hypothalamus as well as basal forebrain (BF). For example, orex-in/hypocretin neurons of the LH and histamine neurons of the TMN then become additional players in the ARAS; with the former promoting and extending bouts of wakefulness through the widespread release of orexin neuropeptides (Scammell et al., 2017; Yu et al., 2021) and the latter exhibiting a wake-on firing pattern (Scammell et al., 2019; Strecker et al., 2002; Thakkar, 2011), though a recent study suggests TMN histamine neurons increase arousal during behavioral challenges but not during baseline conditions (Venner et al., 2019).
The simultaneous activity of these ARAS-associated neurotransmitter systems begets wakefulness and alertness by directly or indirectly stimulating cortical networks associated with sensory, motor, and associational processing, including the prefrontal cortex (PFC), which receives highly-processed information and exerts top-down control and allocation of attentional resources towards stimuli and cues of interest such as reward or threat (Brown et al., 2012; Hanson et al., 2021; Magoun, 1952; Miller et al., 2002). Ultimately, by facilitating wakefulness and tonic alertness, the activity of multiple neurotransmitter populations in the ARAS yields a generalized readiness to quickly process and respond to events in the environment. It is both empirically and intuitively understood that adequate arousal is a prerequisite for normal attentional functioning (Lim and Dinges, 2008; Oken et al., 2006); as such, neuromodulatory corticopetal brainstem and forebrain projections which promote arousal also influence vigilance and attentional performance. In addition to our understanding of the bottom-up processes governing attentional allocation towards pertinent stimuli, the volitional deployment of attention is also mediated by top-down cortico--cortical pathways that prioritize attentional resources based on internal behavioral goals (Noudoost et al., 2010).
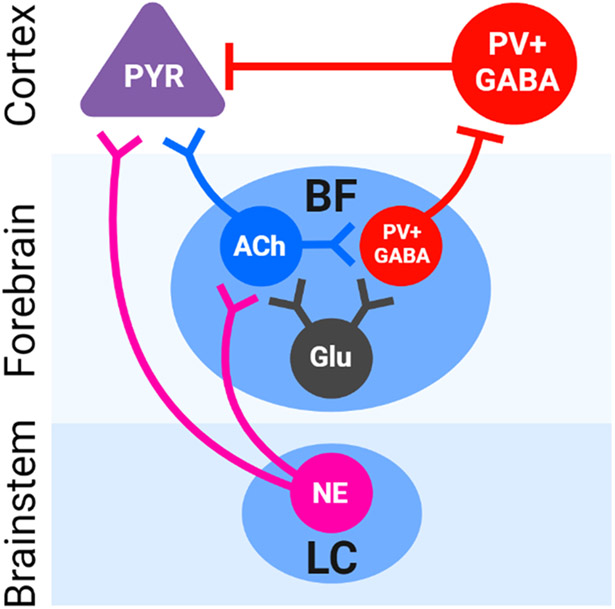
Despite progress, the precise functional characterization of these multiple arousal systems such as how they contribute to specific types of attentional processing and performance remain unanswered (Waterhouse and Navarra, 2019). In this review, we selectively focus on and compare the LC and the BF, two arousal promoting structures whose ascending projections promote wakefulness, arousal, alertness, and attentiveness. The LC contains norepinephrine (NE) arousal-producing neurons that project diffusely throughout the forebrain including the BF. The BF, constituted of the substantia innominata, horizontal limb of the diagonal band, magnocellular preoptic nucleus and ventral pallidum, has been considered a final common pathway of the ventral branch of the ARAS, and largely contains glutamatergic, cholinergic and parvalbumin (PV)-containing gamma-aminobutyric acid (GABA) neurons which together optimize sensory and information processing in the cortex through differing mechanisms (Fig. 1; Anaclet et al., 2015; Do et al., 2016; Henny and Jones, 2008; McKenna et al., 2021; Xu et al., 2015; Yang et al., 2017; Zant et al., 2016).
Fig. 1.
Simplified circuit diagram showing interactions between ascending norepinephrinergic, cholinergic, and gamma-Aminobutyric (GABA)-ergic pathways and their effects on cortical arousal- and attention-relevant neurons. Both norepinephrine (NE) from the locus coeruleus (LC) and acetylcholine (ACh) from the basal forebrain (BF) enhance sensitivity of pyramidal (PYR) excitatory neurons to incoming salient sensory inputs, exerting a slower but longer-lasting effect through volume transmission and actions at G protein-coupled NE and ACh receptors. LC norepinephrinergic neurons also project to and increase the output of BF nuclei. BF corticopetal parvalbumin (PV)-positive GABAergic neurons evoke a rapid yet excitatory effect through the suppression of PYR-innervating cortical GABAergic interneurons. Though not discussed in depth in this review, BF glutamate (Glu) neurons promote cortical activity by exciting BF-ACh and BF-PV neurons (Yang et al., 2017).
We firstly summarize literature which supports the idea that, while arousal effects of the LC-NE and BF-acetylcholine (ACh) systems are typically slower, longer-lasting, and associated with amplified excitability of principal cortical neurons, the influence of the corticopetal BF-PV system is more immediate and shorter in latency, instigating rapid transitions from low to high states of alertness. Differences in duration of activation, patterns of neural discharge, and other properties may provide clues into understanding the distinct roles served by each system in enhancing arousal and attentional processing as well as their involvement in psychiatric conditions for which these psychological states are compromised. In the final section, we discuss how recently developed selective research tools will allow for a more granular differentiation of the circuitry, neurochemistry, and function of different arousal systems and enable researchers to explore the possible framework that LC-NE, BF-ACh, and BF-PV projections contribute to differing, yet overlapping, elements of wakefulness, alertness, and vigilant attention.
2. The LC norepinephrinergic system in arousal and attention
The LC, which is a small, NE-synthesizing nucleus located at the rostral pons, is part of a larger brainstem network consisting of multiple populations of neurons which operate within the ARAS. NE is stored in not only small synaptic vesicles, which is typical of fast-acting transmitters, but also in large, dense core vesicles known as varicosities, with its release acting in a paracrine fashion via this mechanism (Jones, 2020; Poe et al., 2020). As such, NE can be considered a broad-reaching, rather than a targeted and specific, stimulatory regulator of neuronal activity. In support of this idea, the relatively large NE-containing neurons originating in the LC have diffuse collateral branches and send projections to the spinal cord, brainstem, cerebellum, BF, thalamus, hypothalamus, hippocampus, amygdala, basal ganglia, and cerebral cortex (Jones, 2020), suggesting that the direct and indirect influence of NE is nearly ubiquitous in the CNS.
The historical assumption has been that NE serves as a general neuromodulator, having a fairly homogenous effect on neuronal function and behavioral output. Indeed, retrograde tracing showed that norepinephrinergic neurons emanating from the LC project diffusely to multiple sensory cortices, in contrast to BF-ACh projections, which were modality specific (Kim et al., 2016). Furthermore, optogenetic activation of LC-NE neurons induced broad desynchronization across multiple sensory cortices (Kim et al., 2016). However, the historical viewpoint of NE as a uniform neuromodulator seems overly simplified. Recent studies on the LC (reviewed in Poe et al., 2020) employing a wide range of techniques (i.e., optogenetics, chemogenetics, viral tract tracing, RNA sequence and molecule-specific labeling combined with behavioral assays) provide a more nuanced picture. The LC may be more heterogenous than previously appreciated, with clusters of some NE-containing neurons organized into modules and distributed in coordinated fashion to targeted circuits serving particular functions (Waterhouse and Chandler, 2016). A few examples of specific subgroups of NE-containing neurons and proposed roles include analgesia in the spinal cord, aversion and/or anxiety in the anterior cingulate, amygdala and prefrontal cortex (Hirschberg et al., 2017; McCall et al., 2017), and motor function in the striatum (Zerbi et al., 2019), the latter of which was not previously recognized. Thus, the notion of NE as a homogenous neuromodulator of function is inconsistent with more recent data (Poe et al., 2020).
As opposed to fast-acting synaptic transmission, non-synaptic volume transmission of NE has temporally slow effects which occur on a longer timescale and produce a prolonged state of neuronal excitation in LC-NE-recipient regions (Taber and Hurley, 2014). The concept of volume transmission has been challenged with regard to NE released by LC neurons (Poe et al., 2020) as well as ACh released by BF neurons (Sarter et al., 2009). The limited temporal resolution associated with in vivo microdialysis studies, for example, calls into question how rate of firing corresponds to transmitter release and the resultant physiological effects (Poe et al., 2020). Recent advances in fast scan voltammetry that have a range of seconds may be able to address these issues. Notwithstanding this contention, evidence is consistent with the notion that tonic release of NE (Taber and Hurley, 2014) is important in the maintenance of vigilance states, while phasic or transient discharge of NE is proposed to mediate other important functions.
2.1. LC-NE neurons in wakefulness and arousal
The widespread and prolific influence of LC projections establishes the norepinephrinergic system as a key regulator of wakefulness and arousal. A substantial focus of research exploring the various roles of LC-NE neurons has centered around their role in stimulating wakefulness and facilitating awareness of the external world by increasing cortical excitability and instigating transitions to states of heightened wakefulness and alertness. In general, basal discharge rates of LC-NE neurons are highest during periods of wakefulness, significantly lower during slow wave sleep, and absent during REM sleep (Swift et al., 2018). The classic work of Moruzzi and Magoun (1949) demonstrated that electrical stimulation of the ARAS in experimental animals induced a change in the predominant electroencephalographic (EEG) pattern from synchronous, high-voltage slow wave activity to desynchronous, low-voltage, fast wave activity. In their research, it was noted that this alteration in EEG pattern resembled the change that occurred in their human patients when they directed their focus towards a visual stimulus. Aston-Jones and Bloom (1981) published a seminal paper which characterized the role of the LC-NE neurons within the ARAS by demonstrating electrical discharge from single and multiple unit electrodes implanted in the pontine LC neurons of unanesthetized rats. From these and subsequent studies, it has become clear that, by enhancing cortical activity and modulating the responsiveness of cortical neurons to important sensory inputs, NE-producing LC neurons play an important regulatory role in facilitating not only global consciousness, but awareness of and attention towards important stimuli and cues.
The LC is the sole source of NE to the cortex, with its corticopetal projections providing diffuse norepinephrinergic inputs via primarily non-junctional varicosities and allowing for coordinated activation of groups of neurons within large diffusion zones (Agnati et al., 1995; O’Donnell et al., 2012). In addition to these direct corticopetal projections, LC-NE activity indirectly promotes cortical excitation and wakefulness through its innervations of numerous non-cortical targets, including the thalamus, which is an important brain state-regulating hub. Thalamic control of sleep and arousal involves complex interactions of thalamocortical neurons, corticothalamic neurons, and thalamic reticular neurons. Li et al. (2017) posit a correlation between the degree of norepinephrinergic modulation of cortically-projecting thalamic neurons and various states of consciousness. During slow-wave sleep (SWS) – which is characterized by low-frequency burst spiking of thalamocortical neurons – a near-silence of afferent inputs from norepinephrinergic neurons to the thalamus hyperpolarizes the membrane potential of cortex-innervating thalamic cells, resulting in reduced sensation-induced outputs to thalamo-recipient cortical neurons. Upon waking, NE released from terminals of LC neurons binds with alpha-1 and beta adrenoceptors expressed on thalamocortical neurons, thereby increasing the frequency of intrinsic oscillations and, in doing so, promoting the transition from unconsciousness to consciousness (McCormick and Bal, 1997; Steriade and McCarley, 1990, Brown et al., 2012). In this regard, alongside inputs from other ascending neurotransmitter systems, norepinephrinergic depolarization of corticopetal thalamic projections greatly influences the extent of cortical activation and the degree of arousal, with NE-mediated thalamic activation being low during light sleep, moderate during relaxed wakefulness, and high during periods of focused attention (Li et al., 2017).
Though lesions of LC-NE efferents and the ensuing depletion of NE in the cortex do not necessarily impair wakefulness (Berridge et al., 2012), targeted optogenetic stimulation of LC-NE neurons activates the cortex and promotes indicators of behavioral arousal, such as increased locomotion (Carter et al., 2010). Thus, while the LC-NE system is not required to maintain waking consciousness, it exercises a key role in the activation of other arousal-inducing populations of neurons. Super-imposed on these state-dependent changes in basal firing, recordings from LC-NE neurons in vivo were also shown to be altered in response to sensory stimulation in rats and monkeys (Foote et al., 1980). Noteworthy was the observation that impulse activity in the LC selectively increased in response to both physiologically relevant stimuli (e.g., food) as well as to noxious stimuli (e.g., pain). Waterhouse et al. (1988) suggest that NE facilitates the postsynaptic response of neurons which would not ordinarily respond to a given stimulus in the absence of NE, highlighting the LC as a principal gatekeeper of sensory and information processing. Additionally, electrical activation of the LC increased neuronal responding to strong, but not weak, inputs, suggesting that NE normally functions to bias processing towards important stimuli (Dahl et al., 2022; Ross and Van Bockstaele, 2021). These findings establish that the LC-NE system contributes to target impulse activity. Whether this can be interpreted as an increase in signal-to-noise ratio is debatable. On one hand, both NE and ACh can suppress synaptic responding to one input while having a less suppressive effect or even increasing responsivity to another input (Hasselmo et al., 1997; Hayat et al., 2020; Hsieh et al., 2000; Manella et al., 2017). This has been interpreted by some as an increase in signal-to-noise ratio, but it could also simply reflect increases in tonic or baseline firing rate, which is known to occur for both transmitters under many circumstances (for review see McBurney-Lin et al., 2019).
Other evidence that the effects of NE can be long lasting comes from the interaction of NE with the neuroendocrine system. NE is highly involved in the enduring neuronal and behavioral responses to stress, with both central and peripheral norepinephrinergic signals increasing in response to stressors and during behaviors which coincide with heightened arousal, such as that which occurs during the fight-or-flight response (Esler et al., 1988; Morilak et al., 2005). In general, increased NE efflux temporarily makes afferent inputs, rather than intrinsic excitation, the primary facilitator of neuronal activity, promoting a shift in focus from internal representations towards external events (Hasselmo et al., 1997). The LC-NE system acts in coordinated fashion to the hypothalamic-pituitary-adrenal (HPA) axis during acute and chronic stress. The first limb of the HPA axis that is engaged following a stressful experience consists of corticotropic releasing factor (CRF)-producing neurons of the hypothalamic paraventricular nucleus (PVN), the activation of which then promotes the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary gland into the blood. By binding to glucocorticoid and mineralocorticoid receptors on the adrenal gland, circulating ACTH levels activate a release of the stress hormone cortisol (corticosterone in rodents), inducing the release of glucose from the liver to aid in response to the threat (Thau et al., 2022). CRF released from the hypothalamic PVN depolarizes LC-NE neurons inducing widespread release of NE from terminals throughout the neuroaxis (Snyder et al., 2012). Tract tracing studies have demonstrated that CRF from an additional site, the central nucleus of the amygdala, also primes the LC-NE system and is thought to be especially important in cognitive processes such as decision-making under duress (Ross and Van Bockstaele, 2021). This parallel activation of the HPA and LC ultimately produces a cascade of events in response to stress that are long-acting, ensuring that an organism is awake and alert over an extended period of time.
2.2. LC-NE neurons in attention and cognition
Arousal exists on a continuum, with sleepiness and fatigue at the lower end, hypervigilance and fear at the higher end, and normal waking alertness - which is most conducive for attentional processing and cognitive performance - located between the two extremes. The extent of arousal can be conceptualized as a function of NE-mediated modulation of the signal-to-noise ratio in the cortex, with two modes of norepinephrinergic release dictating the neuronal response to a perceived stimulus: a phasic, burst-like firing pattern and a longer-lasting tonic firing pattern (Calderon et al., 2016). Phasic, or transient, discharge of NE-generating LC neurons incites high-frequency rhythmic activity throughout the cortex, acting as a “neuronal interrupter” by driving rapid and flexible reorientation in behaviorally-relevant contexts and promoting maximal engagement with an important stimulus, such as during periods of selective and sustained attention (Dayan and Yu, 2006). Additionally, a phasic or transient release of NE from LC neurons was shown to be necessary for attention-relevant gamma oscillations in the PFC induced by a brief train of foot shocks (Neves et al., 2018), suggesting that transient increases in cortical NE efflux play a critical role in the neurological and behavioral response to alarming or noxious stimuli.
Conversely, a transition from phasic-to-tonic discharge pattern of LC-NE neurons is associated with stress-induced disengagement from salient stimuli and increased environmental exploration (Aston-Jones and Cohen, 2005). This idea is supported by recent research from Dahl et al. (2022) which demonstrated that tonic optogenetic activation of LC-NE neurons in mice results in a prolonged heightened state of arousal and behavioral activation accompanied by enhanced and suppressed highl- and low-frequency cortical oscillations, respectively (see also Marzo et al., 2014). Optimal performance in an auditory signal detection task was associated with intermediate norepinephrinergic activity, as both diminished and excessive NE-mediated cortical activation coincided with worsened task performance (Dahl et al., 2022). It can therefore be surmised that, while brief, phasic NE release enhances informational processing by biasing attentional resources towards meaningful stimuli, increased tonic firing of LC-NE neurons - which occurs when stressors induce higher arousal - can result in suboptimal goal-directed cognitive performance.
Thus, a critical function of the LC norepinephrinergic system is instigating wakefulness and alertness in response to stressors in the immediate environment - particularly in the context of an alarming stimulus or event - and enabling an organism to adequately respond in demanding or potentially dangerous situations. In doing so, LC-NE neurons have demonstrated attention-enhancing capabilities. Attentional processes can be divided into three stages - alerting (heightened vigilance or sustained attention), orienting (the initial selection and turning towards a salient environmental stimulus), and executive attention (the ability to flexibly regulate the direction of one’s focused attention; reviewed in Posner and Boies, 1971) - and research has shown that separable neural networks govern each stage (Raz and Buhle, 2006). In this context, the LC norepinephrinergic system primarily modulates the initial alerting function by enhancing the responsiveness of key neurons to incoming information pertinent to noteworthy stimuli, especially to stressors that necessitate immediate and longer-lasting arousal (Beane and Marrocco, 2004).
Consistent with the longer-lasting nature of NE-facilitated activation, LC neurons also play an important role in attentional shifting and the reorientation of attention towards behaviorally-relevant cues in the environment (Sara, 2009). If a noxious or threatening stimulus is detected, the stressor would activate the HPA axis in parallel with the LC-NE system, facilitating an extended state of alertness and heightened sensory processing. The recognition of the threat would induce a highly aroused and attentive state of readiness for action that befits the nature of the threat. The sustained, or tonic, activity of the LC-NE, along with effects associated with activation of the sympathetic nervous system, will ensure that a return to a state of low arousal in this case will not be forthcoming for some time, an outcome that is advantageous in potentially dangerous situations. This example highlights the overarching notion that tonic LC-NE activity may be beneficial and even necessary to support the threat response of an organism emerging from a low arousal state.
3. The BF cholinergic system in arousal and attention
Norepinephrinergic neurons of the LC, as well as other brainstem (i. e., raphe nucleus, parabrachial nucleus, and PAG) and hypothalamic nuclei (i.e., LH and TMN) contribute efferents to the ventral pathway of the ARAS which converge directly onto caudal aspects of the BF, a region of magnocellular neurons that receives and orchestrates inputs from these lower arousal-related nuclei and generates stimulatory outputs to consciousness-relevant brain regions (Sarter et al., 2009). The BF is much larger and contains significantly more neurons that the LC. In contrast to norepinephrinergic neurons, cholinergic neurons of the BF have projections to the cortex that are predominantly sensory modality specific (Kim et al., 2016; McBurney-Lin et al., 2019).
Pathways originating in the BF are heterogenous, with cholinergic and non-cholinergic neurons projecting to multiple cortical and subcortical regions, including primary sensory cortices, the medial PFC (mPFC), and the hippocampus. Neuroanatomical tract tracing and optogenetic studies have revealed that projections to the neocortex from the BF exhibit a degree of topographical specificity not previously appreciated (Chaves-Coira et al., 2018; Zaborszky et al., 2015). For example, neurons of the vertical and horizontal diagonal band of Broca (VDB/HDB) in particular, located in the rostral BF, send specific projections to primary sensory cortices, and also maintain reciprocal connections with mPFC (Chaves-Coira et al., 2018). Neurons of the basal magnocellular nucleus, in the caudal BF, target sensory and motor cortices with less specificity than VDB/HDB neurons. Overall, cholinergic and non-cholinergic neurons of the BF are organized into segregated or overlapping groups of neurons, with the extent of overlap correlating with the degree of connectivity between specific cortical target areas (Chaves-Coira et al., 2018; Zaborszky et al., 2015). These overlapping populations of BF projections to specific cortical areas may constitute an elementary circuit by which state-related activity may selectively modulate cognitive processes. Neurons in the rostral aspect of BF, including the medial septum and vertical limb of the diagonal band, also project to the hippocampus, and play an essential role in driving theta oscillations implicated in mnemonic function and evident in REM sleep (Buzsáki, 2002; Freund and Antal, 1988; Kang et al., 2017; Vertes and Kocsis, 1997).
Specific cell markers combined with anterograde and retrograde tract tracing techniques have established that there are three main types of cortically-projecting neurons from the BF based on neurotransmitter phenotype: cholinergic, GABAergic and glutamatergic neurons (Henny and Jones, 2008). While somatostatin-expressing GABAergic cells of the BF promote sleep, its populations of cholinergic, PV + GABAergic, and glutamatergic neurons instigate wakefulness and arousal (see Yang et al., 2017 for a review). Here we focus only on the BF-ACh and BF-PV cell types and their role in arousal and attention.
3.1. BF-ACh neurons in wakefulness and arousal
In relation to the control of the sleep-wake cycle, attention, and motivated behaviors by the BF, focus has been primarily on its cholinergic efferents, with ACh neurons localized to the caudal BF innervating the entirety of the cortical mantle and activating low-voltage fast EEG activity (LVFA) characteristic of wakefulness (Duque et al., 2000). ACh exerts its excitatory influence through two types of cholinergic receptors: ionotropic nicotinic receptors, which facilitate presynaptic glutamate release and depolarize cortical interneurons, and G protein-coupled muscarinic receptors, the stimulation of which enhances pyramidal neuron activity through the blockade of leak potassium conductance, generation of 20–40 Hz oscillations (i.e., beta and gamma range), and inhibition of slow afterhyperpolarizations (reviewed in Brown et al., 2012). ACh additionally serves a similar role as NE in stimulating wakefulness through its actions in the thalamus during wakefulness and vigilance (Brown et al., 2012; Sarter and Bruno, 1999).
Also, like NE, cholinergic modulation of cholinoceptive cortical and thalamocortical neurons is largely absent in SWS, and the lack of thalamus-mediated sensory transmission during deep sleep is due, in part, to reductions in brainstem and forebrain cholinergic thalamic outputs (Brown et al., 2012; Hayat et al., 2020; Li et al., 2017). However, unlike LC-NE neurons, which are REM silent, cholinergic tone increases from NREM to REM sleep (Thakkar et al., 1998), and reticular thalamic GABAergic neurons revert to tonic firing mode. As a result, LVFA returns during REM sleep, producing a similar EEG pattern to what is observed during wakefulness (McCormick and Bal, 1997; Zagha and McCormick, 2014). Ultimately, the sleep-to-wake transition has been primarily attributed to parallel activation of cholinergic and norepinephrinergic circuitry (Li et al., 2017; McCormick, 1992, 1989), and it is through these mechanisms that BF corticopetal cholinergic projections subserve not only arousal, but normal and sustained attentional functioning as well (Sarter et al., 1999).
3.2. BF-ACh neurons in attention and cognition
In general, ACh is posited to subserve attention by strengthening the response of sensory-recipient neurons to incoming signals while suppressing those which come from unimportant sources. In doing so, there is extensive overlap in the influence of NE and ACh on circuit dynamics in brain regions associated with arousal and attentional processing. Like NE, ACh has the capability of exerting cellular effects via brief phasic signaling (Sarter et al., 2009), but its volume transmission establishes it as a predominantly slower-acting neuromodulator that can induce a prolonged amplification of cortical and hippocampal pyramidal neuron responsivity (Gulledge et al., 2007; Hasselmo et al., 1997; Hasselmo and Giocomo, 2006; Hopkins and Johnston, 1988). Behaviorally relevant cues evoke cholinergic transients just as they do with norepinephrinergic transients, and the temporary activity of both systems increase the signal-to-noise ratio in arousal- and attention-relevant cortical regions. Also, like NE, the propensity for ACh to have enduring effects on network excitability results in a robust enhancement of attentional processing and performance by magnifying the response of pyramidal neurons to incoming sensory inputs (Sarter et al., 2009).
While NE release may be highest during alarming circumstances, cortical ACh efflux may bias attentional resources towards relevant stimuli and cues during active attentional effort, such as when deliberately focusing on the novelty or familiarity of various elements in the external world (Hasselmo et al., 1997). Additionally, a pathway from the LC norepinephrinergic system to the BF represents an important junction of multiple components of vigilance, with the magnitude of the LC-NE-mediated alerting response informing BF-ACh cells of attention-worthy stimuli and events and promoting ACh efflux in the cortex (Breton-Provencher et al., 2021). Thus, in situations where heightened alertness may be required to inform immediate decision-making, simultaneous activation of otherwise redundant arousal regulators which have sustained effects is likely advantageous.
4. The BF-GABA/parvalbumin system in arousal and attention
Tract tracing studies, three-dimensional reconstruction and computational analysis has revealed that BF cholinergic and non-cholinergic projection neurons are segregated into overlapping pools of neurons. The extent of overlap between BF populations is associated with degree of connectivity of cortical target cells, forming multiple functional “triangles” in which BF cell groups may provide parallel modulation of multiple groups of interconnected cortical regions (Zaborszky et al., 2015). GABAergic neurons from the BF, which comprise the majority of all BF neurons, mostly innervate cortical GABAergic interneurons (Henny and Jones, 2008) which, in turn, form synapses on pyramidal neurons in the cortex. Rather than simple inhibition or disinhibition, regular tonic or phasic activity of ascending GABA neurons is associated with the timing or gating of large, principle cortical neurons via the action of cortical GABA neurons (i.e., fast inhibitory postsynaptic potentials) (Hassani et al., 2009). One subtype of ascending BF arousal projection is comprised of large (on average > 20 μm somatic large axis diameter), cortically-projecting GABAergic neurons which express the calcium binding protein PV (McKenna et al., 2013). Activity of these corticopetal GABAergic BF-PV efferents has been shown to promote cortex-wide stimulation through the perisomatic inhibition of cortical GABAergic interneurons which ordinarily suppress the activity of pyramidal neurons (Alitto and Dan, 2013).
4.1. BF-PV neurons in wakefulness and arousal
Gain of function experiments have shown that activation of BF-ACh and BF-PV neurons separately facilitate arousal from sleep, with optogenetic stimulation of BF-PV neurons in mice inducing NREM-to-wake, but not REM-to-wake, arousal latencies that were significantly shorter than the arousal latencies produced by stimulation of cholinergic BF neurons (McKenna et al., 2020; Zant et al., 2016). While cholinergic stimulation produced bouts of wakefulness which lasted ~ 13.5 s in our laboratory (Zant et al., 2016) and 25–45 s in another laboratory (Ozen Irmak & de Lecea, 2014), the vast majority of arousal latencies from NREM sleep in mice following optogenetic stimulation targeting BF-PV neurons occurred within a five-second window (McKenna et al., 2020). The transient nature of the waking effect of this stimulation may be suggestive of the specific role of PV in rapid arousal. These investigations, though, employed differing optogenetic stimulation protocols (for example, wattage of light stimulation, stimulation frequency), warranting a more systematic investigation to comprehensively compare these different BF neuronal subtypes and their role in NREM-to-wake transitions. In our laboratory, optogenetic inhibition of these BF-PV neurons attenuated arousals induced by hypercapnia as well as auditory stimulation, findings which are consistent with a specific role for the BF-PV system in quick and transient arousals from sleep (McKenna et al., 2020). Brief periods of wakefulness of this nature occur through the sleep period in healthy individuals, though a much higher frequency of such arousals is observed in sleep apnea (Guilleminault et al., 1976), insomnia (Levenson et al., 2015), and aging (Li et al., 2018). Overall, these findings suggest that fast-acting BF-PV neurons operate alongside slower but longer-lasting BF-ACh neurons to provide excitatory inputs to cortical neurons.
4.2. BF-PV neurons in attention and cognition
Optogenetic stimulation of BF-PV neurons in vivo has been shown to promote wakefulness (Xu et al., 2015; McKenna et al., 2020) and produce cortical gamma oscillations (Espinosa et al., 2019b; Kim et al., 2015; McNally et al., 2021). As shown in Fig. 1, GABAergic BF-PV neurons largely innervate GABAergic PV+ interneurons in the PFC, and BF-PV stimulation induces wakefulness through the disinhibition of pyramidal neurons. This circuit has also been shown to be critical for performance on learned tasks that make demands on working memory. Normal cognition depends on the ability of neural networks to maintain stable, but not static, levels of activity. The ratio of activity of excitatory and inhibitory components of the cortical circuitry, referred to as the excitatory/inhibitory (E/I) balance, provides a measure of stable global activity within a circuit, though groups of neurons are dynamic and can exhibit transient imbalances. A signal-to-noise ratio in the cortex that is conducive for normal cognitive functioning reflects an optimal E/I balance, and excessive excitation of key neurons yields a reduced signal-to-noise ratio by amplifying irrelevant noise and, as a result, de-emphasizing important, information-bearing signals (Sohal and Rubenstein, 2019). By generating cortical gamma oscillations, BF-PV neurons appear to be crucial for maintaining balanced cortical activation, and EEG power in the gamma range is a common metric used to assess this E/I balance. In support of this, increasing inhibitory tone by optogenetically stimulating PV+ interneurons has been shown to impair delayed alternation performance in mice without affecting general activity or motivation (Rossi et al., 2012). Other studies manipulating PV+ interneurons demonstrate that cortical over-excitation can result in severe social deficits that could be reversed by increasing inhibitory tone (Yizhar et al., 2011). Taken together, these findings suggest that the maintenance of the E/I balance in the cortex may represent a unifying concept for the understanding of a range of psychiatric disorders.
Until recently, the importance of the BF in attentional processing was predominantly attributed to the activity of corticopetal ACh-generating neurons, with the role of the BF cholinergic system in attention having been extensively studied and reviewed (Klinkenberg et al., 2011; Villano et al., 2017). Overall, elevated cholinergic tone in the cortex, particularly in the PFC, is conducive for normal attentional functioning and is especially important for attentional performance in challenging, distracting, and otherwise demanding circumstances (Himmelheber et al., 2000; Kozak et al., 2006). In a similar vein, the lesioning of corticopetal cholinergic projections impairs accuracy in tasks of sustained attention (Hasselmo and McGaughy, 2004; McGaughy et al., 2002; Voytko et al., 1994). However, lesions of cholinergic BF neurons do not impair theta and gamma activity to the same degree as diffuse cortical lesions or in activation of the total BF cell populations (Gerashchenko et al., 2001; McGaughy et al., 1996), suggesting that non-cholinergic cell groups make an important contribution to arousal-dependent cognitive processes as well. As more targeted tools became available to exclusively manipulate other populations of BF neurons, it became clear that BF-PV neurons also play an important role in attentional processing (Burk and Sarter, 2001; Kim et al., 2015, McNally et al., 2021). Co-release of more than one chemical messenger enhances neural network flexibility, for example, by expanding the range of temporal and spatial representation (Nusbaum, 2001). When comparing the influence of BF-PV neurons to that of LC-NE and BF-ACh-mediated neuromodulation, the former is capable of far more rapid, but transient, effects that occur on the scale of milliseconds rather than seconds (Shine, 2019). Thus, being comprised of multiple arousal systems establishes the BF as a critical anatomical substrate of consciousness and vigilant attention, with the role of BF-PV neurons in these psychological states currently under investigation.
Perhaps the most important function of BF-PV neurons is associated with gamma band oscillations (GBOs) which serve important roles in attention, consciousness, and working memory. While BF-ACh neurons have a low tonic firing rate of ~ 14 Hz and long afterhyperpolarizations, BF-PV neurons exhibit narrow action potentials and high maximal discharge rates (McKenna et al., 2013; Yang et al., 2014, 2017). In terms of conscious perception, research implicates an important function of cortical oscillations in the gamma range (30–80 Hz, centered at ~ 40 Hz) in the binding of sensory information (Gray and Singer, 1989). This process, referred to as feature detection or feature binding, involves the integration of different stimulus properties (e.g., shape, form, color, depth) into one unified object. Optogenetic stimulation of GABAergic BF-PV neurons, which comprise ~ 25 % of large (> 20 μm long-axis diameter) GABAergic neurons in the BF (McKenna et al., 2013), have been shown to preferentially enhance cortical GBOs (Kim et al., 2015), an effect which occurs independently of but is enhanced by BF cholinergic neuron activity. Conversely, the 40 Hz auditory steady-state response (ASSR) in the cortex was inhibited by optogenetic suppression of corticopetal BF-PV projections, further establishing the distinct role of this population of neurons in generating cortical GBOs and enabling the psychological processes that depend on or are enhanced by neuronal activity in the gamma range.
Hwang et al. (2019) used optogenetic stimulation of BF-PV neurons in combination with high-density EEG recordings, which allows for the determination of spatio-temporal patterns of cortical activity, in a series of experiments in mice. Selective targeting of BF-PV neurons in mice facilitated time-locked GBOs preferentially in frontal cortices. Furthermore, the effect of BF-PV activation on an ASSR was used since a similar paradigm has been employed in human patients with certain neuropsychiatric conditions in which cortical abnormalities in stimulus-driven neuronal responding have been detected (see McNally and McCarley, 2016, for a review). It also established that those cortical projections from PV-containing GABAergic neurons of the BF may be central to cortical reorganization upon which important cognitive processes, including attention, depend. Additionally, manipulation of attentional variables has been shown to enhance sensory stimulus evoked GBOs through cortico-cortical interactions of the frontal lobe with primary sensory cortices in a way that is not recapitulated with the occurrence of non-stimulus-linked spontaneous GBOs (Welle and Contreras, 2016). Overall, recent research converges on the idea that optogenetic stimulation of BF-PV neurons strongly enhanced auditory stimuli driven GBOs in a manner similar to the effects of attention, strongly implicating BF-PV neurons in mediation of attentional processes (Hwang et al., 2019), at least in part through the targeting of cortical PV interneurons (Kim et al., 2015).
In addition to diffuse cortical projections, GABAergic BF-PV neurons have also been shown to innervate the anterior cingulate cortex (ACC), a central hub of the default mode network (DMN) (Do et al., 2016). Furthermore, gamma band activity in the BF is functionally coupled with GBOs in the ACC during the performance of tasks requiring response to external stimuli. Contrary to its role in the facilitation of attentional and sensory processes described above, BF gamma band activity is generated in rats during quiet wakefulness when DMN activity is elevated and suppressed during performance of tasks that require a response to external stimuli (Nair et al., 2018). A paper by Espinosa et al. (2019) suggests a role for BF somatostatin neurons in the control of the DMN as well, highlighting an important function of the BF as a “subcortical switch” which redirects attentional resources towards either internal or external states. One future direction for research will address the seeming paradoxical roles for BF-PV neurons in attention on the one hand and in the DMN on the other.
Taken together, LC-NE, BF-ACh, and BF-PV neurons - all of which are considered critical components of the ARAS - support and enhance attentional processing through their wakefulness-promoting qualities. The existence of parallel arousal pathways, especially ones which operate on dissociable timescales, is evolutionarily advantageous as it relates to rapidly identifying potential threats in the immediate environment and producing a state of prolonged vigilance to activate appropriate behavioral responses when quick responding may be necessary. Fast but transiently-active arousal systems like the corticopetal BF-PV innervation would be beneficial for instigating transitions from sleep or low arousal to high arousal, and those that are slower but have longer-lasting effects, such as LC norepinephrinergic and BF cholinergic systems, would be recruited to maintain a state of cortical arousal that primes the response of sensory-recipient neurons to salient inputs over an extended period of time. In orchestration with the sympathetic nervous system and the HPA axis, NE is preferentially released in times of alarm and heightened stress, resulting in enhanced attentional processing at moderate levels and worsened attention when norepinephrinergic efflux is in excess, such as in frightening situations. Cortical ACh provided by BF cholinergic neurons has a similar faciliatory effect on alertness and vigilance as NE, with the primary reported difference between NE and ACh being the contexts which preferentially evoke their activation. In summary, we propose that the BF-PV serves non-discriminatively as a quick, initial response system to sensory stimulation, while the specific function of the LC-NE and BF-ACh systems may come into play when sensory stimulation is alarming and recognized as a genuine threat or during periods of increased attentional effort.
5. Clinical relevance, future directions, and contemporary tools
5.1. Clinical relevance
Arousal is the foundation by which all volitional behaviors arise, and attention is the lens through which the world is perceived and processed; as such, the dysfunction of neural systems supporting these psychological states negatively impacts daily functioning and is implicated in numerous psychiatric and neurological disorders. Because of their overlapping contributions to arousal and vigilance, alterations in NE and ACh activity are implicated in similar psychiatric conditions for which consciousness and attention are disrupted. For example, abnormal regulation of both norepinephrinergic and cholinergic systems are associated with sleep disorders such as narcolepsy and insomnia (Adamantidis et al., 2020; Reid et al., 1994; Roth and Ancoli-Israel, 1999; Shiromani et al., 1987; Szabo et al., 2019), attention deficit/-hyperactivity disorder (Borodovitsyna et al., 2017; Todd et al., 2003), and dementing conditions such as Alzheimer’s disease and Parkinson’s disease (Francis et al., 1999; Gesi et al., 2000; Kelly et al., 2017; Perez-Lloret and Barrantes, 2016; Perry et al., 1978; Tejani-Butt et al., 1993). Dysfunctional LC-NE and BF-ACh activity also contributes to the hyperarousal-linked attentional impairments observed in anxiety, post-traumatic stress disorder, and schizophrenia (Berntson et al., 1998; Gong et al., 2021; Lake et al., 1980; Lustig and Sarter, 2015; Redmond and Huang, 1979; Swift et al., 2018). Additionally, while the clinical involvement of BF-PV neurons is an emergent line of research, anomalous spontaneous and evoked cortical gamma oscillatory activity – which together are putative indicators of compromised BF-PV neuron functioning – have been noted in individuals with schizophrenia (Hirano et al., 2015; McNally and McCarley, 2016; Spencer et al., 2008), suggesting that corticopetal parvalbuminergic BF projections may underlie the treatment-resistant attentional dysfunction of this disorder as well as other disorders for which cortical E/I balance is compromised.
Despite the evidence cited above that LC and BF arousal networks play a role in normal attention and contribute to the attentional dysfunction in a variety of psychiatric conditions, it is still unclear exactly how LC-NE, BF-ACh, and BF-PV systems uniquely contribute to cortical activity depending on the demands of the external world (Dahl et al., 2022). In their recent review of the LC-NE system, Waterhouse and Navarra (2019) illustrate a need for experiments to determine how discharge rates of norepinephrinergic neurons vary and change in response to various stimuli, during decision-making, and in tasks requiring motoric responding. The same might be said about other arousal circuits, such as the cholinergic and non-cholinergic systems of the BF. Much like the LC, the BF is a phylogenetically-conserved region of the vertebrate brain that has been intractable to anatomical characterization. Indeed, the diverse cell types of the BF, as well as their properties, interactions, and projections have been described as a “menagerie” by Yang et al. (2017), suggesting that more research is needed to parse the contributions of distinct populations of BF neurons in distinct aspects of attentional processing, particularly as it relates to cortical activation. This is particularly true of BF-PV neurons, the attention-promoting qualities of which have only recently begun to be elucidated through selective targeting techniques (Schiffino et al., 2021). Ultimately, better understanding of the brain circuitry controlling attention through the employment of novel and emergent technologies and methodologies can solidity our understanding of the contributions of individual brain systems to consciousness and vigilance as well as guide the development of treatments to ameliorate the attention and cognitive impairments in a variety of conditions.
5.2. Contemporary tools and future directions
Recent innovations in neuronal manipulation techniques can help researchers parse the neuropsychology of arousal, alerting, and attention to an extent that was not previously possible. For example, optogenetics, which uses light to control the activity of neurons in the living brain that have been genetically altered to express light-sensitive ion channels, has revolutionized neuroscience by providing a way to alter a neuron of a specific type in each brain region in vivo while leaving other neuron types unaffected (Deisseroth, 2011). This enables selective targeting of distinct neuronal populations for stimulation or inhibition, including the previously elusive BF-PV neurons, without affecting other adjacent systems, such as BF cholinergic and non-cholinergic neurons. Temporal precision is a second major advantage; while traditional genetic manipulations that allow for the study of gain- or loss-of-function occur over the course of hours, days, or longer, optogenetics operates on the order of milliseconds, allowing for the addition or deletion of precise neural activity patterns in selective cells within the brains of intact animals (Chen et al., 2018).
Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) are artificially engineered protein receptors (usually G protein-coupled) that are exclusively activated by selective synthetic ligands which can be used to manipulate neurons of a specific type in vitro or in vivo (Roth, 2016; Urban and Roth, 2015). This chemogenetic approach is beneficial not only because of its selectivity, but designer drugs can be delivered in ways which are non-invasive and clinically-relevant, including via peripheral injections and oral administration. Promising optical methods for recording the activity of distinct nuclei have also emerged in the last decade. Fiber photometry, one such technique, permits simultaneous photometric calcium recording of the activity of multiple genetically-defined downstream brain regions and neuronal populations, changes which can be analyzed alongside changes in behavior and outcomes in various cognitive tasks (Sych et al., 2019). Another recent contender, G protein-coupled receptor activation-based (GRAB) sensors, like other genetically-encoded fluorescent neurotransmitter sensors, enable the imaging of subtle alterations in extracellular dynamics in ex vivo brain tissue and behaving animals (Sun et al., 2020).
These, and other, novel methodologies will allow investigators to address formerly intractable problems such as differentiation of the role of NE in attention from that of ACh as well as other neuromodulators implicated in the arousing influence of the ARAS (Burk et al., 2018). Indeed, previously-cited optogenetic experiments (Carter et al., 2010; Hwang et al., 2019; Dahl et al., 2022) have begun to elucidate NE- and ACh-specific effects of cortical activation and ensuing arousal and vigilance, and DREADDs have already been employed in probe circuitry in both the BF (Kucinski et al., 2019) as well as the LC (Ao et al., 2021). Numerous GRAB sensors have also been created to measure monoaminergic, cholinergic, and orexinergic receptor activity in target downstream regions, and these G protein-coupled receptor-based encoded sensors can allow for precise measurements of NE, ACh, and other arousal- and attention-relevant systems based on the activity of their constituent metabotropic receptors (Chen et al., 2021; Duffet et al., 2022). Moreover, these same techniques can allow investigators to differentiate the role of different cell types in the BF as well as more effectively parse the arousal- and attention-promoting qualities of BF-PV neurons from those of NE and ACh. The invention of imaging techniques such as the optical fiberscope or miniscope allows for calcium imaging at a single cell resolution in freely moving experimental animals. Hence, the relationship between neural circuits and behavior can finally be elucidated at a single-cell resolution in animals while they perform an attentional task.
Advances in selective manipulation and imaging of the neural circuitry will not yield meaningful results unless equal attention is paid to the behavioral side of the equation. The National Institute of Mental Health (NIMH) has recently brought to light limitations associated with the study of disorders or disease states in isolation. The Research Domain Criteria (RDoC) issued by the NIMH recommends that constructs should generate testable predictions concerning neurobiological mechanisms underlying the behavioral elements that comprise that construct. Therefore, careful consideration should be given to the operational definition of constructs so that results can be compared across studies and that the full range of behavior be represented by the construct. For example, an individual construct such as attention can be further divided into sub-constructs, including sustained attention (i.e., vigilance), divided attention, selective attention, and the higher order attentional set-shifting (see Zomeren and Brouwer, 1994). Further, we contend that it may be implausible to expect widespread agreement among scientists in terms of operational definitions and consistent use of terms to describe a construct. Alternatively, we propose that the way forward would be facilitated if the same behavioral assays were employed for different transmitter systems involved in arousal and attention. Regardless of the term used by an investigator, one could then make quantitative comparisons among different neural circuits or different neurotransmitters which will help discern how each differs.
In our laboratory, we employ an integrative approach to study attention by combining optogenetic or chemogenetic manipulations of BF-PV neurons while measuring neural activity via established electrophysiological approaches, including electrode recordings of local field potentials, as well as more recently the use of fiber photometry. The effect of optogenetic (or chemogenetic) excitation or inhibition of this population of BF neurons can then be assessed on attention-related performance. A rodent psychomotor vigilance task (rPVT) has been developed to assess sustained attention by measuring reaction time in a manner analogous to the human PVT task (see Christie et al., 2008; Davis et al., 2016). For example, we have found that BF-PV optogenetic inhibition and sleep deprivation both produce similar impairments in the rPVT, revealing an important role of these neurons in wakefulness-dependent attentional performance (Schiffino et al., 2021). Optogenetic excitation of BF-PV neurons can then be evaluated for its ability to ameliorate the impairments in rPVT performance associated with sleep loss. The addition of fiber photometry will allow us to determine if BF-PV population activity correlates with sustained attention, such as reaction time in the rPVT as well as response inhibition in tasks of executive functioning, such as the Go/No-Go odor discrimination task.
The role of attention in associative learning can be assessed using Pavlovian conditioning paradigms, since investigators are able to condition an orienting response. In associative learning, the discrepancy between expected events and actual events is represented by prediction error (Iordanova et al., 2021), and is known as “surprise”. In Pavlovian tasks, cues are more rapidly associated with surprising events (Holland and Schiffino, 2016). A distinction has been made between reinforcement models and attention models. Traditional associative learning theory has emphasized the role of the reinforcement. Referred to as “signed” prediction error (PE), the extent of PE determines the reinforcing power of a surprising event (Iordanova et al., 2021). By contrast, attention models stipulate that the “absolute value” of PE governs the eligibility of cues for associative learning and these “unsigned” PE signals should be encoded similarly across bidirectional types of errors (reviewed in Holland and Schiffino, 2016). That is, in attention models, identical neurophysiological signals represent the surprising occurrence of unexpected events as well as the surprising omission of expected events. Surprising events alert the animal of its ignorance about predictors for a given event, and that its current model of the world needs to be updated (Rescorla, 1988). There is reason to believe that surprise (unsigned PE signals) may be encoded by GBOs. Since BF-PV neurons have been shown to modulate GBOs (Kim et al., 2015), we have proposed that these neurons may be critical to the generation of the cortical signal representing surprise. These hypotheses can be evaluated by combining optogenetic manipulation of BF-PV neurons with performance on the 2-choice visual discrimination task. Moreover, electrophysiological recordings and fiber photometry can be used to determine whether BF-PV activity correlates with salience enhancement in separate experiments that use Pavlovian conditioning to enhance stimulus salience.
Taken together, the BF is a region comprised of multiple types of neurons and projection pathways, including cholinergic and PV-expressing GABAergic neurons which innervate areas of the cortex responsible for sensory and information processing. Aberrations in cholinergic signaling have been well-documented in several psychiatric conditions, many of which are also characterized by some degree of norepinephrinergic abnormalities, potentially a testament to their similarities in transmission and psychological outcomes. The influence of BF-PV neurons in these same states and disorders, on the other hand, has only recently begun to be elucidated, in large part due to the recent advent of technologies which allow for targeted manipulations of these neurons.
6. Conclusion
To summarize, we have examined and compared three (among multiple) arousal systems in the brain: the LC-NE system originating in the brainstem as well as cholinergic and PV-containing GABAergic systems located in the BF. Whereas the BF-PV system serves as a rapid and transient arousal system, LC-NE and BF-ACh neuromodulation are typically activated on slower but longer-lasting timescales (Shine, 2019). NE and ACh serve similar functions, have largely overlapping psychological influences, and are implicated in many of the same disorders. Their main difference is in what situations and contexts they are recruited. The LC-NE system provides a general external awareness and is likely to be most active in times of stress. The BF-ACh system is most active during periods of attentional effort and active focusing on details about a stimulus/the environment. Heightened levels of activity during wakefulness characterize the three systems, and all are activated following an awakening from slumber. Optogenetic stimulation of BF-PV neurons during sleep evokes a rapid but short-lived awakening from sleep, akin to a microarousal. By contrast, activation of the LC-NE system elicits a longer lasting period of wakefulness that occurs alongside activation of the HPA axis.
We suggest that it would be adaptive for animals to have evolved a rapid response system alongside purportedly redundant neuromodulatory pathways in order to respond to even subtle sensory stimuli (e.g., a sound) with at least a microarousal. If the stimulus is benign, it would make sense for sleep to be disturbed only minimally. On the other hand, a loud or alarming sound may be predictive of a genuine threat to survival; in such cases, the BF-PV system would be initially activated and soon followed by activation of the LC-NE system and HPA axis to mount a maximal response to the threat and BF-ACh neurons enhancing the processing of and biasing attentional resources towards potential dangers in the external world. While the systems may have evolved to serve separate functions, research on the LC and on the BF has been beset by similar problems that have prevented full characterization. In both regions, cell bodies clustered in relatively small areas send projections diffusely throughout the brain and appear to modulate specific, yet overlapping, cognitive and affective processes; however, it has remained difficult to disentangle distinct subpopulations and to determine specificity of function. Investigations into the distinct roles of these arousal-implicated nuclei would benefit from clear identification of domains that depend on adequate activity of the ARAS - including tonic alertness and attention - and the direct comparison of these networks using the same physiological and behavioral tests and measurements for each respective arousal system. Additionally, a new array of powerful research tools now allows investigators to address questions formerly beyond our grasp, such as how and when various arousal systems influence downstream decision-making and motor responding.
Acnowledgements
This work was supported by VA Biomedical Laboratory Research and Development Service Merit Awards I01 BX002774 (RES), I01 BX004500 (JMM), I01 BX001356, VA CDA IK2 BX002130 (JMM) and VA RCS Award IK6 BX005714 (RES); and NIH support from P01 HL095491 (RES), R21 MH125242 (JTM), T32 HL07901 (FLS), F32 MH119838 (FLS), and K99 AG066819 (FLS). We thank our colleagues Drs. Brown, Basheer, and McNally (JMM) for helpful discussions and ideas. EBM, JTM, FLS, & RES are scientists at VA Boston Healthcare System, West Roxbury, MA. JTM received partial salary compensation and funding from Merck MISP (Merck Investigator Sponsored Programs) but has no conflict of interest with this work. The contents of this work do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
References
- Adamantidis AR, Schmidt MH, Carter ME, Burdakov D, Peyron C, Scammell TE, 2020. A circuit perspective on narcolepsy. Sleep 43 (5), zsz296. 10.1093/sleep/zsz296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnati LF, Bjelke B, Fuxe K, 1995. Volume versus wiring transmission in the brain: a new theoretical frame for neuropsychopharmacology. Med. Res. Rev 15 (1), 33–45. 10.1002/med.2610150104. [DOI] [PubMed] [Google Scholar]
- Alhola P, Polo-Kantola P, 2007. Sleep deprivation: impact on cognitive performance. Neuropsychiatr. Dis. Treat 3 (5), 553–567. [PMC free article] [PubMed] [Google Scholar]
- Alitto HJ, Dan Y, 2013. Cell-type-specific modulation of neocortical activity by basal forebrain input. Front. Syst. Neurosci 6. 10.3389/fnsys.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaclet C, Pedersen NP, Ferrari LL, Venner A, Bass CE, Arrigoni E, Fuller PM, 2015. Basal forebrain control of wakefulness and cortical rhythms. Nat. Commun 6 (1), 8744. 10.1038/ncomms9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao Y, Yang B, Zhang C, Wu B, Zhang X, Xing D, Xu H, 2021. Locus coeruleus to paraventricular thalamus projections facilitate emergence from isoflurane anesthesia in mice. Front. Pharm 12, 643172 10.3389/fphar.2021.643172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom F, 1981. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci 1 (8), 876–886. 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD, 2005. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J. Comp. Neurol 493 (1), 99–110. 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Beane M, Marrocco RT, 2004. Norepinephrine and acetylcholine mediation of the components of reflexive attention: implications for attention deficit disorders. Prog. Neurobiol 74 (3), 167–181. 10.1016/j.pneurobio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT, 1998. Anxiety and cardiovascular reactivity: the basal forebrain cholinergic link. Behav. Brain Res 94 (2), 225–248. 10.1016/S0166-4328(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Berridge CW, 2008. Noradrenergic modulation of arousal. Brain Res. Rev 58 (1), 1–17. 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Schmeichel BE, España RA, 2012. Noradrenergic modulation of wakefulness/arousal. Sleep Med. Rev 16 (2), 187–197. 10.1016/j.smrv.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodovitsyna O, Flamini M, Chandler D, 2017. Noradrenergic modulation of cognition in health and disease. Neural Plast. 2017, 1–14. 10.1155/2017/6031478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton-Provencher V, Drummond GT, Sur M, 2021. Locus coeruleus norepinephrine in learned behavior: anatomical modularity and spatiotemporal integration in targets. Front. Neural Circuits 15, 638007. 10.3389/fncir.2021.638007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW, 2012. Control of sleep and wakefulness. Physiol. Rev 92 (3), 1087–1187. 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk JA, Sarter M, 2001. Dissociation between the attentional functions mediated via basal forebrain cholinergic and GABAergic neurons. Neuroscience 105 (4), 899–909. 10.1016/S0306-4522(01)00233-0. [DOI] [PubMed] [Google Scholar]
- Burk JA, Blumenthal SA, Maness EB, 2018. Neuropharmacology of attention. Eur. J. Pharm 835, 162–168. 10.1016/j.ejphar.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, 2002. Theta oscillations in the hippocampus. Neuron 33 (3), 325–340. 10.1016/S0896-6273(02)00586-X. [DOI] [PubMed] [Google Scholar]
- Calderon DP, Kilinc M, Maritan A, Banavar JR, Pfaff D, 2016. Generalized CNS arousal: an elementary force within the vertebrate nervous system. Neurosci. Biobehav. Rev 68, 167–176. 10.1016/j.neubiorev.2016.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L, 2010. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci 13 (12), 1526–1533. 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves-Coira I, Martín-Cortecero J, Nuñez A, Rodrigo-Angulo ML, 2018. Basal forebrain nuclei display distinct projecting pathways and functional circuits to sensory primary and prefrontal cortices in the rat. Front. Neuroanat 12, 69. 10.3389/fnana.2018.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I-W, Papagiakoumou E, Emiliani V, 2018. Towards circuit optogenetics. Curr. Opin. Neurobiol 50, 179–189. 10.1016/j.conb.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cho KE, Skwarzynska D, Clancy S, Conley NJ, Clinton SM, Li X, Lin L, Zhu JJ, 2021. The property-based practical applications and solutions of genetically encoded acetylcholine and monoamine sensors. J. Neurosci 41 (11), 2318–2328. 10.1523/JNEUROSCI.1062-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie MA, Mckenna JT, Connolly NP, Mccarley RW, Strecker RE, 2008. 24 h of sleep deprivation in the rat increases sleepiness and decreases vigilance: introduction of the rat-psychomotor vigilance task. J. Sleep Res 17 (4), 376–384. 10.1111/j.1365-2869.2008.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl MJ, Mather M, Werkle-Bergner M, 2022. Noradrenergic modulation of rhythmic neural activity shapes selective attention. Trends Cogn. Sci 26 (1), 38–52. 10.1016/j.tics.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CM, Roma PG, Hienz RD, 2016. The rodent psychomotor vigilance test (rPVT): a method for assessing neurobehavioral performance in rats and mice. J. Vis. Exp 118, 54629. 10.3791/54629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Yu AJ, 2006. Phasic norepinephrine: a neural interrupt signal for unexpected events. Netw.: Comput. Neural Syst 17 (4), 335–350. 10.1080/09548980601004024. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, 2011. Optogenetics. Nat. Methods 8 (1), 26–29. 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do JP, Xu M, Lee S-H, Chang W-C, Zhang S, Chung S, Yung TJ, Fan JL, Miyamichi K, Luo L, Dan Y, 2016. Cell type-specific long-range connections of basal forebrain circuit. ELife 5, e13214. 10.7554/eLife.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffet L, Kosar S, Panniello M, Viberti B, Bracey E, Zych AD, Radoux-Mergault A, Zhou X, Dernic J, Ravotto L, Tsai Y-C, Figueiredo M, Tyagarajan SK, Weber B, Stoeber M, Gogolla N, Schmidt MH, Adamantidis AR, Fellin T, Patriarchi T, 2022. A genetically encoded sensor for in vivo imaging of orexin neuropeptides. Nat. Methods 19 (2), 231–241. 10.1038/s41592-021-01390-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque A, Balatoni B, Detari L, Zaborszky L, 2000. EEG correlation of the discharge properties of identified neurons in the basal forebrain. J. Neurophysiol 84 (3), 1627–1635. 10.1152/jn.2000.84.3.1627. [DOI] [PubMed] [Google Scholar]
- Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G, 1988. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension 11 (1), 3–20. 10.1161/01.HYP.11.1.3. [DOI] [PubMed] [Google Scholar]
- Espinosa N, Alonso A, Lara-Vasquez A, Fuentealba P, 2019. Basal forebrain somatostatin cells differentially regulate local gamma oscillations and functionally segregate motor and cognitive circuits. Sci. Rep 9 (1), 2570. 10.1038/S41598-019-39203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa N, Alonso A, Morales C, Espinosa P, Chávez AE, Fuentealba P, 2019. Basal forebrain gating by somatostatin neurons drives prefrontal cortical activity. Cereb. Cortex 29 (1), 42–53. 10.1093/cercor/bhx302. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE, 1980. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc. Natl. Acad. Sci. USA 77 (5), 3033–3037. 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis PT, Palmer AM, Snape M, Wilcock GK, 1999. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J. Neurol. Neurosurg. Psychiatry 66 (2), 137–147. 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Antal M, 1988. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature 336 (6195), 170–173. 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Salin-Pascual R, Shiromani PJ, 2001. Effects of hypocretin–saporin injections into the medial septum on sleep and hippocampal theta. Brain Res. 913 (1), 106–115. 10.1016/S0006-8993(01)02792-5. [DOI] [PubMed] [Google Scholar]
- Gesi M, Soldani P, Giorgi FS, Santinami A, Bonaccorsi I, Fornai F, 2000. The role of the locus coeruleus in the development of Parkinson’s disease. Neurosci. Biobehav. Rev 24 (6), 655–668. 10.1016/S0149-7634(00)00028-2. [DOI] [PubMed] [Google Scholar]
- Gong L, Shi M, Wang J, Xu R, Yu S, Liu D, Ding X, Zhang B, Zhang X, Xi C, 2021. The abnormal functional connectivity in the locus coeruleus-norepinephrine system associated with anxiety symptom in chronic insomnia disorder. Front. Neurosci 15, 678465 10.3389/fnins.2021.678465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, Singer W, 1989. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc. Natl. Acad. Sci. USA 86 (5), 1698–1702. 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilleminault C, Tilkian A, Dement WC, 1976. The sleep apnea syndromes. Annu. Rev. Med 27 (1), 465–484. 10.1146/annurev.me.27.020176.002341. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Park SB, Kawaguchi Y, Stuart GJ, 2007. Heterogeneity of phasic cholinergic signaling in neocortical neurons. J. Neurophysiol 97 (3), 2215–2229. 10.1152/jn.00493.2006. [DOI] [PubMed] [Google Scholar]
- Hanson E, Brandel-Ankrapp KL, Arenkiel BR, 2021. Dynamic cholinergic tone in the basal forebrain reflects reward-seeking and reinforcement during olfactory behavior. Front. Cell. Neurosci 15, 635837 10.3389/fncel.2021.635837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Lee MG, Henny P, Jones BE, 2009. Discharge profiles of identified GABAergic in comparison to cholinergic and putative glutamatergic basal forebrain neurons across the sleep-wake cycle. J. Neurosci 29 (38), 11828–11840. 10.1523/JNEUROSCI.1259-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, McGaughy J, 2004. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. In: Progress in Brain Research, 145. Elsevier, pp. 207–231. 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Giocomo LM, 2006. Cholinergic modulation of cortical function. J. Mol. Neurosci 30 (1–2), 133–136. 10.1385/JMN:30:1:133. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Linster C, Patil M, Ma D, Cekic M, 1997. Noradrenergic suppression of synaptic transmission may influence cortical signal-to-noise ratio. J. Neurophysiol 77 (6), 3326–3339. 10.1152/jn.1997.77.6.3326. [DOI] [PubMed] [Google Scholar]
- Hayat H, Regev N, Matosevich N, Sales A, Paredes-Rodriguez E, Krom AJ, Bergman L, Li Y, Lavigne M, Kremer EJ, Yizhar O, Pickering AE, Nir Y, 2020. Locus coeruleus norepinephrine activity mediates sensory-evoked awakenings from sleep. Sci. Adv 6 (15), eaaz4232. 10.1126/sciadv.aaz4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny P, Jones BE, 2008. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur. J. Neurosci 27 (3), 654–670. 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP, 2000. Increases in cortical acetylcholine release during sustained attention performance in rats. Cogn. Brain Res 9 (3), 313–325. 10.1016/S0926-6410(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Oribe N, Kanba S, Onitsuka T, Nestor PG, Spencer KM, 2015. Spontaneous gamma activity in schizophrenia. JAMA Psychiatry 72 (8), 813. 10.1001/jamapsychiatry.2014.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg S, Li Y, Randall A, Kremer EJ, Pickering AE, 2017. Functional dichotomy in spinal- vs prefrontal-projecting locus coeruleus modules splits descending noradrenergic analgesia from ascending aversion and anxiety in rats. ELife 6, e29808. 10.7554/eLife.29808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Schiffino FL, 2016. Mini-review: prediction errors, attention and associative learning. Neurobiol. Learn. Mem 131, 207–215. 10.1016/j.nlm.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WF, Johnston D, 1988. Noradrenergic enhancement of long-term potentiation at mossy fiber synapses in the hippocampus. J. Neurophysiol 59 (2), 667–687. 10.1152/jn.1988.59.2.667. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Cruikshank SJ, Metherate R, 2000. Differential modulation of auditory thalamocortical and intracortical synaptic transmission by cholinergic agonist. Brain Res. 880 (1–2), 51–64. 10.1016/S0006-8993(00)02766-9. [DOI] [PubMed] [Google Scholar]
- Hwang E, Brown RE, Kocsis B, Kim T, McKenna JT, McNally JM, Han H-B, Choi JH, 2019. Optogenetic stimulation of basal forebrain parvalbumin neurons modulates the cortical topography of auditory steady-state responses. Brain Struct. Funct 224 (4), 1505–1518. 10.1007/s00429-019-01845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanova MD, Yau JO-Y, McDannald MA, Corbit LH, 2021. Neural substrates of appetitive and aversive prediction error. Neurosci. Biobehav. Rev 123, 337–351. 10.1016/j.neubiorev.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE, 2020. Arousal and sleep circuits. Neuropsychopharmacology 45 (1), 6–20. 10.1038/s41386-019-0444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Ding M, Topchiy I, Kocsis B, 2017. Reciprocal interactions between medial septum and hippocampus in theta generation: Granger causality decomposition of mixed spike-field recordings. Front. Neuroanat 11, 120. 10.3389/fnana.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Wang JL, Ferrari L, Thankachan S, Kroeger D, Venner A, Lazarus M, Wellman A, Arrigoni E, Fuller PM, Saper CB, 2017. A genetically defined circuit for arousal from sleep during hypercapnia. Neuron 96 (5), 1153–1167. 10.1016/j.neuron.2017.10.009 (.e5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SC, He B, Perez SE, Ginsberg SD, Mufson EJ, Counts SE, 2017. Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta Neuropathol. Commun 5 (1), 8. 10.1186/s40478-017-0411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Ährlund-Richter S, Wang X, Deisseroth K, Carlén M, 2016. Prefrontal parvalbumin neurons in control of attention. Cell 164 (1–2), 208–218. 10.1016/j.cell.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Thankachan S, McKenna JT, McNally JM, Yang C, Choi JH, Chen L, Kocsis B, Deisseroth K, Strecker RE, Basheer R, Brown RE, McCarley RW, 2015. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc. Natl. Acad. Sci. USA 112 (11), 3535–3540. 10.1073/pnas.1413625112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg I, Sambeth A, Blokland A, 2011. Acetylcholine and attention. Behav. Brain Res 221 (2), 430–442. 10.1016/j.bbr.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Kozak R, Bruno JP, Sarter M, 2006. Augmented prefrontal acetylcholine release during challenged attentional performance. Cereb. Cortex 16 (1), 9–17. 10.1093/cercor/bhi079. [DOI] [PubMed] [Google Scholar]
- Kucinski A, Kim Y, Sarter M, 2019. Basal forebrain chemogenetic inhibition disrupts the superior complex movement control of goal-tracking rats. Behav. Neurosci 133 (1), 121–134. 10.1037/bne0000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake CR, Sternberg DE, van Kammen DP, Ballenger JC, Ziegler MG, Post RM, Kopin IJ, Bunney WE, 1980. Schizophrenia: elevated cerebrospinal fluid norepinephrine. Science 207 (4428), 331–333. 10.1126/science.7350667. [DOI] [PubMed] [Google Scholar]
- Levenson JC, Kay DB, Buysse DJ, 2015. The pathophysiology of insomnia. Chest 147 (4), 1179–1192. 10.1378/chest.14-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Henriquez CS, Fröhlich F, 2017. Unified thalamic model generates multiple distinct oscillations with state-dependent entrainment by stimulation. PLoS Comput. Biol 13 (10), e1005797 10.1371/journal.pcbi.1005797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KZH, Bherer L, Mirelman A, Maidan I, Hausdorff JM, 2018. Cognitive involvement in balance, gait and dual-tasking in aging: a focused review from a neuroscience of aging perspective. Front. Neurol 9, 913. 10.3389/fneur.2018.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Dinges DF, 2008. Sleep deprivation and vigilant attention. Ann. N. Y. Acad. Sci 1129 (1), 305–322. 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- Lustig C, Sarter M, 2015. Attention and the cholinergic system: relevance to schizophrenia. In: Robbins TW, Sahakian BJ (Eds.), Translational Neuropsychopharmacology, 28. Springer International Publishing, pp. 327–362. 10.1007/7854_2015_5009. [DOI] [PubMed] [Google Scholar]
- Magoun HW, 1952. An ascending reticular activating system in the brain stem. Arch. Neurol. Psychiatry 67 (2), 145. 10.1001/archneurpsyc.1952.02320140013002. [DOI] [PubMed] [Google Scholar]
- Manella LC, Petersen N, Linster C, 2017. Stimulation of the locus ceruleus modulates signal-to-noise ratio in the olfactory bulb. J. Neurosci 37 (48), 11605–11615. 10.1523/JNEUROSCI.2026-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo A, Totah NK, Neves RM, Logothetis NK, Eschenko O, 2014. Unilateral electrical stimulation of rat locus coeruleus elicits bilateral response of norepinephrine neurons and sustained activation of medial prefrontal cortex. J. Neurophysiol 111 (12), 2570–2588. 10.1152/jn.00920.2013. [DOI] [PubMed] [Google Scholar]
- McBurney-Lin J, Lu J, Zuo Y, Yang H, 2019. Locus coeruleus-norepinephrine modulation of sensory processing and perception: a focused review. Neurosci. Biobehav. Rev 105, 190–199. 10.1016/j.neubiorev.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall JG, Siuda ER, Bhatti DL, Lawson LA, McElligott ZA, Stuber GD, Bruchas MR, 2017. Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. ELife 6, e18247. 10.7554/eLife.18247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D, 1992. Cellular mechanisms underlying cholinergic and noradrenergic modulation of neuronal firing mode in the cat and guinea pig dorsal lateral geniculate nucleus. J. Neurosci 12 (1), 278–289. 10.1523/JNEUROSCI.12-01-00278.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, 1989. Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 12 (6), 215–221. 10.1016/0166-2236(89)90125-2. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Bal T, 1997. Sleep and arousal: thalamocortical mechanisms. Annu. Rev. Neurosci 20 (1), 185–215. 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- McCoy JG, Strecker RE, 2011. The cognitive cost of sleep lost. Neurobiol. Learn. Mem 96 (4), 564–582. 10.1016/j.nlm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M, 1996. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav. Neurosci 110 (2), 247–265. 10.1037/0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW, 2002. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a five-choice serial reaction time task. J. Neurosci 22 (5), 1905–1913. 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JT, Yang C, Franciosi S, Winston S, Abarr KK, Rigby MS, Yanagawa Y, McCarley RW, Brown RE, 2013. Distribution and intrinsic membrane properties of basal forebrain GABAergic and parvalbumin neurons in the mouse. J. Comp. Neurol 521 (6), 1225–1250. 10.1002/cne.23290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JT, Yang C, Bellio T, Anderson-Chernishof MB, Gamble MC, Hulverson A, McCoy JG, Winston S, Hodges E, Katsuki F, McNally JM, Basheer R, Brown RE, 2021. Characterization of basal forebrain glutamate neurons suggests a role in control of arousal and avoidance behavior. Brain Struct. Funct 226 (6), 1755–1778. 10.1007/s00429-021-02288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JT, Thankachan S, Uygun DS, Shukla C, McNally JM, Schiffino FL, Cordeira J, Katsuki F, Zant JC, Gamble MC, Deisseroth K, McCarley RW, Brown RE, Strecker RE, Basheer R, 2020. Basal forebrain parvalbumin neurons mediate arousals from sleep induced by hypercarbia or auditory stimuli. Curr. Biol 30 (12), 2379–2385. 10.1016/j.cub.2020.04.029 (.e4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally JM, McCarley RW, 2016. Gamma band oscillations: a key to understanding schizophrenia symptoms and neural circuit abnormalities. Curr. Opin. Psychiatry 29 (3), 202–210. 10.1097/YCO.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally JM, Aguilar DD, Katsuki F, Radzik LK, Schiffino FL, Uygun DS, McKenna JT, Strecker RE, Deisseroth K, Spencer KM, Brown RE, 2021. Optogenetic manipulation of an ascending arousal system tunes cortical broadband gamma power and reveals functional deficits relevant to schizophrenia. Mol. Psychiatry 26 (7), 3461–3475. 10.1038/s41380-020-0840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Freedman DJ, Wallis JD, 2002. The prefrontal cortex: categories, concepts and cognition. Philos. Trans. R. Soc. Lond. Ser. B: Biol. Sci 357 (1424), 1123–1136. 10.1098/rstb.2002.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO, 2005. Role of brain norepinephrine in the behavioral response to stress. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 29 (8), 1214–1224. 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Moruzzi G, Magoun HW, 1949. Brain stem reticular formation and activation of the EEG. Electroencephalogr. Clin. Neurophysiol 1 (1–4), 455–473. 10.1016/0013-4694(49)90219-9. [DOI] [PubMed] [Google Scholar]
- Nair J, Klaassen A-L, Arato J, Vyssotski AL, Harvey M, Rainer G, 2018. Basal forebrain contributes to default mode network regulation. Proc. Natl. Acad. Sci. USA 115 (6), 1352–1357. 10.1073/pnas.1712431115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves RM, van Keulen S, Yang M, Logothetis NK, Eschenko O, 2018. Locus coeruleus phasic discharge is essential for stimulus-induced gamma oscillations in the prefrontal cortex. J. Neurophysiol 119 (3), 904–920. 10.1152/jn.00552.2017. [DOI] [PubMed] [Google Scholar]
- Noudoost B, Chang MH, Steinmetz NA, Moore T, 2010. Top-down control of visual attention. Curr. Opin. Neurobiol 20 (2), 183–190. 10.1016/j.conb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum M, 2001. The roles of co-transmission in neural network modulation. Trends Neurosci. 24 (3), 146–154. 10.1016/S0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- O’Donnell J, Zeppenfeld D, McConnell E, Pena S, Nedergaard M, 2012. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem. Res 37 (11), 2496–2512. 10.1007/s11064-012-0818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken BS, Salinsky MC, Elsas SM, 2006. Vigilance, alertness, or sustained attention: physiological basis and measurement. Clin. Neurophysiol 117 (9), 1885–1901. 10.1016/j.clinph.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozen Irmak S, de Lecea L, 2014. Basal forebrain cholinergic modulation of sleep transitions. Sleep 37 (12), 1941–1951. 10.5665/sleep.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Lloret S, Barrantes FJ, 2016. Deficits in cholinergic neurotransmission and their clinical correlates in Parkinson’s disease. Npj Parkinson’s Dis. 2 (1), 16001. 10.1038/npjparkd.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry RH, 1978. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. BMJ 2 (6150), 1457–1459. 10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe GR, Foote S, Eschenko O, Johansen JP, Bouret S, Aston-Jones G, Harley CW, Manahan-Vaughan D, Weinshenker D, Valentino R, Berridge C, Chandler DJ, Waterhouse B, Sara SJ, 2020. Locus coeruleus: a new look at the blue spot. Nat. Rev. Neurosci 21 (11), 644–659. 10.1038/s41583-020-0360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Boies SJ, 1971. Components of attention. Psychol. Rev 78 (5), 391–408. 10.1037/h0031333. [DOI] [Google Scholar]
- Raz A, Buhle J, 2006. Typologies of attentional networks. Nat. Rev. Neurosci 7 (5), 367–379. 10.1038/nrn1903. [DOI] [PubMed] [Google Scholar]
- Redmond DE, Huang YH, 1979. New evidence for a locus coeruleus-norepinephrine connection with anxiety. Life Sci. 25 (26), 2149–2162. 10.1016/0024-3205(79)90087-0. [DOI] [PubMed] [Google Scholar]
- Reid MS, Siegel JM, Dement WC, Mignot E, 1994. Cholinergic mechanisms in canine narcolepsy—II. Acetylcholine release in the pontine reticular formation is enhanced during cataplexy. Neuroscience 59 (3), 523–530. 10.1016/0306-4522(94)90174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, 1988. Pavlovian conditioning: it’s not what you think it is. Am. Psychol 43 (3), 151–160. 10.1037/0003-066X.43.3.151. [DOI] [PubMed] [Google Scholar]
- Ross JA, Van Bockstaele EJ, 2021. The locus coeruleus-norepinephrine system in stress and arousal: unraveling historical, current, and future perspectives. Front. Psychiatry 11, 601519. 10.3389/fpsyt.2020.601519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi MA, Hayrapetyan VY, Maimon B, Mak K, Je HS, Yin HH, 2012. Prefrontal cortical mechanisms underlying delayed alternation in mice. J. Neurophysiol 108 (4), 1211–1222. 10.1152/jn.01060.2011. [DOI] [PubMed] [Google Scholar]
- Roth BL, 2016. DREADDs for neuroscientists. Neuron 89 (4), 683–694. 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T, Ancoli-Israel S, 1999. Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. II. Sleep 22 (Suppl. 2), S354–S358. [PubMed] [Google Scholar]
- Sara SJ, 2009. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 10 (3), 211–223. 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, 1999. Cortical cholinergic inputs mediating arousal, attentional processing and dreaming: differential afferent regulation of the basal forebrain by telencephalic and brainstem afferents. Neuroscience 95 (4), 933–952. 10.1016/S0306-4522(99)00487-X. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Turchi J, 1999. Basal forebrain afferent projections modulating cortical acetylcholine, attention, and implications for neuropsychiatric disorders. Ann. N. Y. Acad. Sci 877 (1 ADVANCING FRO), 368–382. 10.1111/j.1749-6632.1999.tb09277.x. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM, 2009. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat. Rev. Neurosci 10 (5), 383–390. 10.1038/nrn2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Arrigoni E, Lipton JO, 2017. Neural circuitry of wakefulness and sleep. Neuron 93 (4), 747–765. 10.1016/j.neuron.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Jackson AC, Franks NP, Wisden W, Dauvilliers Y, 2019. Histamine: neural circuits and new medications. Sleep 42 (1). 10.1093/sleep/zsy183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffino FL, McNally JM, Brown RE, Strecker RE, 2021. Basal forebrain parvalbumin neurons modulate vigilant attention [Preprint]. Neuroscience. 10.1101/2021.04.19.440515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, 2019. Neuromodulatory influences on integration and segregation in the brain. Trends Cogn. Sci 23 (7), 572–583. 10.1016/j.tics.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, Gillin JC, Henriksen SJ, 1987. Acetylcholine and the regulation of REM sleep: basic mechanisms and clinical implications for affective illness and narcolepsy. Annu. Rev. Pharm. Toxicol 27 (1), 137–156. 10.1146/annurev.pa.27.040187.001033. [DOI] [PubMed] [Google Scholar]
- Snyder K, Wang WW, Han R, McFadden K, Valentino RJ, 2012. Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacol. 37 (2), 520–530. 10.1038/npp.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Rubenstein JLR, 2019. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol. Psychiatry 24 (9), 1248–1257. 10.1038/s41380-019-0426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM., Niznikiewicz MA, Shenton ME, McCarley RW, 2008. Sensory-evoked gamma oscillations in chronic schizophrenia. Biol. Psychiatry 63 (8), 744–747. 10.1016/j.biopsych.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, 1996. Arousal—revisiting the reticular activating system. Science 272 (5259). 10.1126/science.272.5259.225 (225–225). [DOI] [PubMed] [Google Scholar]
- Steriade M, McCarley RW, 1990. Brainstem Control of Wakefulness and Sleep. Springer, US. 10.1007/978-1-4757-4669-3. [DOI] [Google Scholar]
- Strecker RE, Nalwalk J, Dauphin LJ, Thakkar MM, Chen Y, Ramesh V, Hough LB, McCarley RW, 2002. Extracellular histamine levels in the feline preoptic/anterior hypothalamic area during natural sleep–wakefulness and prolonged wakefulness: an in vivo microdialysis study. Neuroscience 113 (3), 663–670. 10.1016/S0306-4522(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Sun F, Zhou J, Dai B, Qian T, Zeng J, Li X, Zhuo Y, Zhang Y, Wang Y, Qian C, Tan K, Feng J, Dong H, Lin D, Cui G, Li Y, 2020. Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat. Methods 17 (11), 1156–1166. 10.1038/s41592-020-00981-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift KM, Gross BA, Frazer MA, Bauer DS, Clark KJD, Vazey EM, Aston-Jones G, Li Y, Pickering AE, Sara SJ, Poe GR, 2018. Abnormal locus coeruleus sleep activity alters sleep signatures of memory consolidation and impairs place cell stability and spatial memory. Curr. Biol 28 (22), 3599–3609. 10.1016/j.cub.2018.09.054 (.e4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sych Y, Chernysheva M, Sumanovski LT, Helmchen F, 2019. High-density multi-fiber photometry for studying large-scale brain circuit dynamics. Nat. Methods 16 (6), 553–560. 10.1038/s41592-019-0400-4. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Thorpy MJ, Mayer G, Peever JH, Kilduff TS, 2019. Neurobiological and immunogenetic aspects of narcolepsy: implications for pharmacotherapy. Sleep Med. Rev 43, 23–36. 10.1016/j.smrv.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber KH, Hurley RA, 2014. Volume transmission in the brain: beyond the synapse. J. Neuropsychiatry Clin. Neurosci 26 (1) 10.1176/appi.neuropsych.13110351 (iv-4). [DOI] [PubMed] [Google Scholar]
- Tejani-Butt SM, Yang J, Zaffar H, 1993. Norepinephrine transporter sites are decreased in the locus coeruleus in Alzheimer’s disease. Brain Res. 631 (1), 147–150. 10.1016/0006-8993(93)91201-3. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, 2011. Histamine in the regulation of wakefulness. Sleep Med. Rev 15 (1), 65–74. 10.1016/j.smrv.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Strecker RE, McCarley RW, 1998. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: a simultaneous unit recording and microdialysis study. J. Neurosci 18 (14), 5490–5497. 10.1523/JNEUROSCI.18-14-05490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thau L, Gandhi J, Sharma S, 2022. Physiology, Cortisol. In: StatPearls. StatPearls Publishing. ⟨http://www.ncbi.nlm.nih.gov/books/NBK538239/⟩. [PubMed] [Google Scholar]
- Todd RD, Lobos EA, Sun L-W, Neuman RJ, 2003. Mutational analysis of the nicotinic acetylcholine receptor alpha 4 subunit gene in attention deficit/-hyperactivity disorder: evidence for association of an intronic polymorphism with attention problems. Mol. Psychiatry 8 (1), 103–108. 10.1038/sj.mp.4001257. [DOI] [PubMed] [Google Scholar]
- Urban DJ, Roth BL, 2015. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu. Rev. Pharm. Toxicol 55 (1), 399–417. 10.1146/annurev-pharmtox-010814-124803. [DOI] [PubMed] [Google Scholar]
- Van Dongen HPA, Maislin G, Mullington JM, Dinges DF, 2003. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 26 (2), 117–126. 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Venner A, Mochizuki T, De Luca R, Anaclet C, Scammell TE, Saper CB, Arrigoni E, Fuller PM, 2019. Reassessing the role of histaminergic tuberomammillary neurons in arousal control. J. Neurosci 39 (45), 8929–8939. 10.1523/JNEUROSCI.1032-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B, 1997. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience 81 (4), 893–926. 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Villano I, Messina A, Valenzano A, Moscatelli F, Esposito T, Monda V, Esposito M, Precenzano F, Carotenuto M, Viggiano A, Chieffi S, Cibelli G, Monda M, Messina G, 2017. Basal forebrain cholinergic system and orexin neurons: effects on attention. Front. Behav. Neurosci 11. 10.3389/fnbeh.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko M, Olton D, Richardson R, Gorman L, Tobin J, Price D, 1994. Basal forebrain lesions in monkeys disrupt attention but not learning and memory [published erratum appears in J Neurosci 1995 Mar;15(3): following table of contents]. J. Neurosci 14 (1), 167–186. 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse BD, Chandler DJ, 2016. Heterogeneous organization and function of the central noradrenergic system. Brain Res. 1641, v–x. 10.1016/j.brainres.2015.12.050. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Navarra RL, 2019. The locus coeruleus-norepinephrine system and sensory signal processing: a historical review and current perspectives. Brain Res. 1709, 1–15. 10.1016/j.brainres.2018.08.032. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Sessler FM, Jung-Tung C, Woodward DJ, Azizi SA, Moises HC, 1988. New evidence for a gating action of norepinephrine in central neuronal circuits of mammalian brain. Brain Res. Bull 21 (3), 425–432. 10.1016/0361-9230(88)90154-2. [DOI] [PubMed] [Google Scholar]
- Welle CG, Contreras D, 2016. Sensory-driven and spontaneous gamma oscillations engage distinct cortical circuitry. J. Neurophysiol 115 (4), 1821–1835. 10.1152/jn.00137.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Chung S, Zhang S, Zhong P, Ma C, Chang W-C, Weissbourd B, Sakai N, Luo L, Nishino S, Dan Y, 2015. Basal forebrain circuit for sleep-wake control. Nat. Neurosci 18 (11), 1641–1647. 10.1038/nn.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Thankachan S, McCarley RW, Brown RE, 2017. The menagerie of the basal forebrain: how many (neural) species are there, what do they look like, how do they behave and who talks to whom. Curr. Opin. Neurobiol 44, 159–166. 10.1016/j.conb.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, McKenna JT, Zant JC, Winston S, Basheer R, Brown RE, 2014. Cholinergic neurons excite cortically projecting basal forebrain GABAergic neurons. J. Neurosci 34 (8), 2832–2844. 10.1523/JNEUROSCI.3235-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD, 1908. The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. Psychol 18 (5), 459–482. 10.1002/cne.920180503. [DOI] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K, 2011. Optogenetics in neural systems. Neuron 71 (1), 9–34. 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Yu X, Franks NP, Wisden W, 2021. Brain clocks, sleep, and mood. In: Engmann O, Brancaccio M (Eds.), Circadian Clock in Brain Health and Disease, 1344. Springer International Publishing, pp. 71–86. 10.1007/978-3-030-81147-1_5. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, Vadasz C, Nadasdy Z, 2015. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cereb. Cortex 25 (1), 118–137. 10.1093/cercor/bht210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagha E, McCormick DA, 2014. Neural control of brain state. Curr. Opin. Neurobiol 29, 178–186. 10.1016/j.conb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zant JC, Kim T, Prokai L, Szarka S, McNally J, McKenna JT, Shukla C, Yang C, Kalinchuk AV, McCarley RW, Brown RE, Basheer R, 2016. Cholinergic neurons in the basal forebrain promote wakefulness by actions on neighboring non-cholinergic neurons: an opto-dialysis study. J. Neurosci 36 (6), 2057–2067. 10.1523/JNEUROSCI.3318-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbi V, Floriou-Servou A, Markicevic M, Vermeiren Y, Sturman O, Privitera M, von Ziegler L, Ferrari KD, Weber B, De Deyn PP, Wenderoth N, Bohacek J, 2019. Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron 103 (4), 702–718. 10.1016/j.neuron.2019.05.034 (.e5). [DOI] [PubMed] [Google Scholar]
- Zomeren AH van, Brouwer WH, 1994. Clinical Neuropsychology of Attention. Oxford University Press. [Google Scholar]