Most theories of meal-induced thermogenesis involve a gut-brain-brown adipose tissue axis driving sympathetic nervous system-mediated thermogenesis. Li et al. demonstrate that secretin released by the gut after a meal binds to abundant receptors in brown adipose tissue to stimulate thermogenesis, inhibiting food intake and thereby suggesting a novel role for secretin regulating satiety.
Secretin is a 27-amino-acid polypeptide secreted by the duodenum that inhibits gastric acid secretion and stimulates pancreatic bicarbonate production. In this issue of Cell, Li et al. (Li et al., 2018) now propose a new function for this digestive hormone, the first hormone ever discovered in 1902 (Bayliss and Starling, 1902). The authors performed an impressive series of experiments in cells, mice, and humans and propose secretin as a key player in a gut-brown adipose tissue-brain axis. Indeed, in response to a meal, serum secretin increases and binds to its abundant receptors in brown adipose tissue (BAT), which subsequently produces heat (and probably BATokines) that the brain senses. Consequently, the brain hypothalamus generates a satiation response with inhibition of orexigenic (neuropeptide Y [NPY] and agouti-related peptide [AgRP]) neurons and stimulation of anorexic signals via pro-opiomelanocortin (POMC) neurons (Figure 1). In short, the secretin released after a meal causes an increase in energy expenditure (meal-induced thermogenesis) with a subsequent suppression of hunger and enhancement of fullness, leading to meal termination. It almost sounds too good to be true since such mechanisms of action for secretin would most likely lead to negative energy balance and weight loss not only in rodents but probably also in humans.
Figure 1.
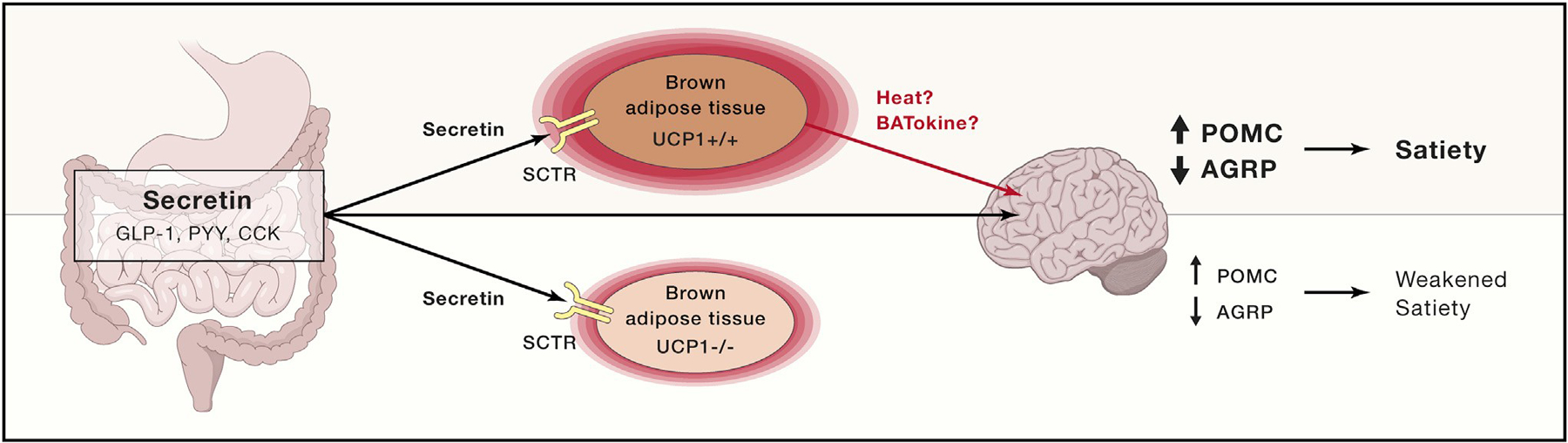
Proposed Mechanism of Action for Secretin
Following a meal, secretin and other peptides are released from the gastrointestinal tract. Secretin receptors (SCTR) are expressed at high level in interscapular brown adipose tissue (iBAT). SCTR is a Gs-protein-coupled receptor that activates lipolysis, uncoupling protein 1 (UCP1), and heat production. The hypothalamus seems to sense the increased heat production from brown adipose tissue after a meal (or secreted proteins called BATokines), causing an inhibition of agouti- related peptide (AgRP) neurons and a stimulation of pro-opiomelanocortin (POMC) neurons, resulting in improved satiety and decreased food intake. GLP-1, glucagon-like peptide-1; PYY, peptide YY; CCK, cholecystokinin.
Meal-induced thermogenesis is the increase in energy expenditure caused by the obligatory cost of digesting, absorbing, and storing the ingested food and a facultative component in response to sympathetic nervous system stimulation (Acheson et al., 1984). On a standard eucaloric and equilibrated diet, meal-induced thermogenesis accounts for approximately 10% of daily energy expenditure, half of which is facultative. Notably, secretin seems to increase facultative thermogenesis by stimulating brown adipose tissue metabolism, thus producing heat with an elevation of temperature not only in this tissue but in the whole body. The brain senses these rises in temperature and/or the proteins secreted by brown adipose tissue (BATokines) and triggers a response leading to meal termination.
In a series of well-designed experiments, Li et al. (Li et al., 2018) first show that the secretin receptor (a Gs-protein-coupled receptor that activates lipolysis) is highly expressed in interscapular brown adipose tissue, and its expression increases if differentiating brown adipocytes are incubated in the presence of secretin. Furthermore, the addition of secretin to primary brown adipocytes increases oxygen consumption with a potency 50-fold higher than isoproterenol by a mechanism independent of sympathetic stimulation. In addition to the robust in vitro data, the authors report that secretin injection increases in vivo heat production in control mice but not in mice deficient in uncoupling protein 1 (Ucp1), i.e., without active BAT. In addition, humans exhibit increased serum secretin concentrations following a single meal, which is associated with increased energy expenditure. The authors provide more evidence for the thermogenic action of secretin in brown adipose tissue by FDG-PET-CT, showing that secretin administration significantly increases glucose uptake in human brown adipose tissue.
Surprisingly, and in parallel to the induction of heat production in brown adipose tissue, secretin injection reduces short-term food intake in mice. This effect is attenuated in mice deficient in Ucp1 but not diminished by beta receptor-blockade (propranolol), clearly indicating an effect independent of sympathetic nervous system activation. The authors note that the decreased food intake occurs in parallel with a decrease in hypothalamic AgRP (potent stimulant of food intake) expression and an increase in POMC (potent effector of satiety) expression. The expression of the transient receptor potential cation channel subfamily V member 1 (TRPV1, also known as the capsaicin receptor) is upregulated, supporting the effect of local warming of these temperature-sensitive ion channels expressed in POMC neurons. Unfortunately, the authors did not test whether the satiety effect disappeared in mice with TRPV1 deletion. The effects of secretin are observed in the hypothalamic tissues of wild-type mice but not in mice deficient in Ucp1. Because of the temporal effect of body temperature on meal pattern (initiation and termination), the authors propose a novel gut-secretin-brown adipose tissue-brain (hypothalamus) axis, which mediates facultative meal-induced thermogenesis and satiation leading to meal termination.
Blocking secretin action with a polyclonal secretin antibody decreases meal-induced thermogenesis and increases meal size and duration yet has little effect on total food intake. Similarly, chronic infusion of secretin in diet-induced obese mice has only a transient short-term effect on reducing food intake with no effect on weight and a marginal effect on fat mass loss. These results are consistent with the lack of effect of genetic deletion of secretin or its receptor on body weight or food intake (Cheng et al., 2011, Sekar and Chow, 2014). Such observations are reminiscent of the short-term effect of cholecystokinin on satiety with no overall effect on food intake since animals compensate by increasing meal frequency.
The data generated by Li et al. leave us with an unfinished story on the potential of targeting secretin for weight control in humans. Indeed, if secretin is part of facultative meal-induced thermogenesis and induces satiety, it seems a logical target for weight management since it acts on both arms of the energy balance equation, i.e., increase in energy expenditure and decrease in energy intake. Unfortunately, humans have much less brown adipose tissue than rodents (Marlatt et al., 2018), and stimulation of this tissue by secretin is unlikely to sufficiently boost energy expenditure and decrease energy intake via increased hypothalamic temperature. Studies of the acute and prolonged effects of secretin analogues in humans are now warranted. Only such studies will tell us whether this old hormone holds a true burning secret.
REFERENCES
- Acheson KJ, Ravussin E, Wahren J, and Jequier E (1984). Thermic effect of glucose in man. Obligatory and facultative thermogenesis. J. Clin. Invest 74, 1572–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss WM, and Starling EH (1902). The mechanism of pancreatic secretion. J. Physiol 28, 325–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Chu JY, and Chow BK (2011). Central and peripheral administration of secretin inhibits food intake in mice through the activation of the melanocortin system. Neuropsychopharmacology 36, 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Schnabl K, Gabler S-M, Willershauser M, Reber J, Karlas A, Laurila S, Lahesmaa M, Din u, Bast-Habersbrunner M, et al. (2018). Secretin-activated brown fat mediates prandial thermogenesis to induce satiation. Cell 175, this issue, 1561–1574. [DOI] [PubMed] [Google Scholar]
- Marlatt KL, Chen KY, and Ravussin E (2018). Is activation of human brown adipose tissue a viable target for weight management? Am. J. Physiol. Regul. Integr. Comp. Physiol 315, R479–R483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar R, and Chow BK (2014). Lipolytic actions of secretin in mouse adipocytes. J. Lipid Res 55, 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]


