Abstract
Background
Carmustine (Cr) is an important chemotherapeutic drug, widely used in the treatment of brain tumors. Herein, the protective role of Codiaeum variegatum leaves ethyl acetate fraction was determined against genotoxicity of Cr. The technique HPLC-qTOF-MS/MS was used to identify the constituents in C. variegatum.
Materials
90 male mice were used to evaluate micronuclei (MPCEs) in bone marrow, chromosomal aberration (CAs) in bone marrow and mouse spermatocytes, sperm abnormalities, and gene expression (qRT-PCR). The following groups were included, I: Negative control (ethanol 30%), II: Positive control (i.p injected once with 30 mg/kg Cr), III: Control orally treated with C. variegatum at 500 mg/kg, four days. IV-VI: treated with 100, 300, and 500 mg/kg of the plant (4 days) plus a single dose of Cr.
Results
In bone marrow, Cr induced significant increase in MPCEs and CAs by 3 and 7-folds respectively over the control. Cr also induced a significant percentage of CAs in spermatocytes in meiosis in the form of univalent (X–Y and autosomal univalent) and also a significant percentage of morphological sperm abnormalities was recorded. A large number of coiled tail abnormalities were detected indicating the effect of Cr in sperm motility. Cr induced an overexpression of p53 gene. C. variegatum mitigated all deleterious genotoxic effects of Cr. Chemical analysis showed that flavones (35.21%) and phenolic acids (17.62%) constitute the main components.
Conclusions
The results indicated that Cr is genotoxic in both somatic and germ cells. The active components in C. variegatum together participate in the obtained protective role.
Keywords: Carmustine, Genotoxicity, Gene expression, Codiaeum variegatum, Protective role, HPLC-qTOF-MS/MS, C-glycosyl flavones
Introduction
Codiaeum is an important member of the family Euphorbiaceae, which includes about 300 genera and 8900 species, several of which are widely used in traditional medicine [1, 2]. Among them, Codiaeum is a valuable genus in the family and it includes approximately 200 species. Different Codiaeum species are used in folklore medicine. This genus was demonstrated to be rich in various bioactive components including mainly polyphenols [2, 3]. Phytochemicals, especially polyphenols are known to be responsible for antioxidant and free radical scavenging activities which are involved in many diseases such as cancer, diabetes, aging and cardiovascular problems [4]. Codiaeum variegatum was known as "garden croton῍ and has the common name Croton. Its leaves are considered with sedative, purgative, anti-amoebic, antioxidant activities, anti-inflammatory, and anticancerous properties [2]. Root decoction is taken to treat gastric ulcers. The fresh latex and leaves extract of C. variegatum are active against influenza A virus and herpes simplex virus, respectively [2]. Antifungal, antibacterial, insecticidal, antimalarial activity and treatment of kidney stones, and immunostimulant properties were also reported [2].
Carmustine (1,3-bis(2-chloroethyl)-1-nitrosourea) is an alkylating agent in the haloethylnitrosourea family and is also known by its brand name, BCNU. It is a common synthetic antineoplastic agent frequently employed, as a systemic monotherapy or in established combination therapy with other approved chemotherapeutic drugs or with other therapeutic measures (surgery, radiotherapy). It is used in the managements of various types of malignancies, e.g. brain tumors, multiple myeloma, Hodgkin’s and non-Hodgkin’s lymphomas [5]. It is highly lipophilic, capable of readily crossing the blood–brain barrier and thus it is useful and important as a chemotherapeutic agent against brain tumors [6, 7]. As with other nitrosoureas and under physiological conditions, it undergoes spontaneous nonenzymatic decomposition to release 2 reactive metabolites with alkylating and carbamoylating activities. Such metabolites are thought to be responsible for the antineoplastic activities of Cr [8]. It has been established as genotoxic both in vivo and in vitro and is classified as an animal carcinogen [9]. The most common adverse effects of Cr are bone marrow suppression, pulmonary toxicity, genotoxicity and fetal harm when administered to a pregnant woman [10, 11, 12]. It alkylates DNA and RNA and induces DNA cross-linking [7, 13]. In addition, secondary tumors were reported to be associated with the long-term use of nitrosoureas [7]. Recently, genotoxicity has fully developed into a serious question for the cause of several cancers. Due to the low toxicity, effective pharmacological activities and economic viability, new therapeutic applications of plants have been examined in the light of recent systematic developments throughout the world [14]. Accordingly, we decided to determine the genotoxic effect of carmustine at different cytogenetic endpoints and the possible ameliorative effect of different doses of the C. variegatum ethyl acetate fraction from leaves was evaluated. The main groups of metabolites and their active components in C. variegatum L. was demonstrated by high-performance liquid chromatography-quadrupole, time-of-flight, and tandem mass spectrometry (the data is under publications).
Materials and methods
Plant material
Codiaeum variegatum L. cv. Gold Dust leaves were collected from Al- Zohriya Garden, Giza, Egypt in 2019. The plant was kindly identified by Therese Labib, Herbarium Section, El-Orman Botanical Garden, Giza (Egypt). A voucher specimen (02Cva/2019) was prepared and deposited at the Chemistry of Natural Compounds Department, National Research Centre (NRC), Dokki-Giza (Egypt).
Preparation of extract
The air-dried leaves of C. variegatum (1.8 kg) were crushed and extracted with 80℅ ethanol by maceration at room temperature. A rotary evaporator was used to evaporate the solvent under vacuum at 50 °C. The resulting ethanol extract was sequentially defatted and desalted by CHCl3 and C2H5OH, respectively, by warming under reflux conditions. Thereafter, the residue was taken in ethyl acetate affording an ethyl acetate-soluble fraction that yielded 14.96 g.
Cytogenetic studies
Chemicals
Carmustine (Cr) was purchased from Sigma–Aldrich Corp. (St. Louis, MO,
USA), dissolved in ethanol 30% and injected i.p. in a single dose of 30 mg/kg body weight. This dose was comparable to the human therapeutic dose for the treatment of brain tumors [15] based on body surface area conversion ratios [16].
Experimental animals
Mature Swiss male mice of 8–12 weeks and weighing approximately 25 g were obtained from the animal house colony of the NRC, Dokki-Giza (Egypt). Mice were housed in plastic boxes in an air-conditioned room of temperature (23 ± 1 °C) and relative humidity (50 ± 20%) with a 12-h light/dark cycle. Mice were provided with a standard balanced pelleted chow diet and chlorinated tap water ad libitum.
Experimental design
In these experiments, a total of 90 mice were divided as follows: 30 mice for micronucleus test and the real-time polymerase chain reaction (real-time PCR), 30 mice for chromosomal aberrations in the bone marrow and mouse spermatocytes, and 30 mice for sperm abnormalities. In each of these tests, mice were subdivided into groups (5 /group).
The main groups in all tests examined were, Group I: Negative control (30% ethanol), Group II: Mice i.p injected with a single dose of Cr (positive control) at the dose level of 30 mg/kg, Group III: Plant control orally administrated the extract of C. variegatum at the highest tested dose (500 mg/kg) for 4 successive days, Groups IV-VI: Represent the combined groups in which mice received repeated oral treatment with C. variegatum fraction (100, 300 and 500 mg/kg) for four days and at the last day received i.p injection with Cr (30 mg/kg). In all experiments, except sperm analysis, samples were taken 24 h after the last treatment. In the test of sperm abnormalities, samples were taken 35 days after the 1st injection.
Cytogenetic analysis
Micronucleus test
The micronucleus preparation from bone marrow was performed following the standard test protocol of Schmid [17] and according to the Guideline OECD 474 for the Testing of Chemicals [18]. Briefly, the bone marrow cells were collected from bilateral femurs after separation in 3 mL of fetal bovine serum, centrifuged and smeared on slides. The air-dried slides were fixed by submerging in absolute methanol (for 10–20 min). Fixed slides were stained with May Grünwald—Giemsa protocol. Micronuclei were identified as dark blue staining bodies in the cytoplasm of polychromatic erythrocytes (MPCEs). The ratio of erythrocytes to nucleated cells was expressed as the percentage of PCE’s/100 nucleated cells (PCE᾽s + NCE᾽s). Total of 2000 cells (PCEs + NCEs) were scored/animal (5 animals/group). Scoring was performed under 1000 × magnification with a light microscope.
Chromosomal aberration assay in mouse bone marrow and spermatocytes
Mitotic and meiotic chromosomes were prepared from bone marrow and testis of the same animal, respectively. Bone marrow chromosomes were prepared according to the technique described by Fahmy et al. [19]. In brief, mouse bone-marrow cells were collected from both femurs, cells were incubated in hypotonic solution (KCL 0.075 M) for 20 min at 37 °C, and then centrifuged. The cell pellets were suspended in a fixative (methanol/glacial acetic acid 3:1). This step was repeated at least twice, then the cells were suspended in a few drops of fixative and spread onto frozen slides, air-dried, stained with 10% Giemsa for 30 min, washed, and air dried again.
Spermatocyte chromosomes were prepared from the testes according to the protocol described by Evans et al. [20] with some modifications [21]. Briefly, the testis was removed and squashed into a petri dish containing an isotonic solution of 2.2% trisodium citrate. Then the cell suspension was centrifuged for 5 min at 1500 rpm. The cell pellet was incubated in a hypotonic solution of 1.1% trisodium citrate for 20 min at 37 °C followed by centrifugation. The cell pellet was washed twice by a freshly prepared fixative. A few drops of the fixative cell suspension were dropped in clean microscopic slides, air-dried and stained with 10% Giemsa stain. In each, one hundred well-spread metaphases were analyzed per mouse describing different kinds of chromosome abnormalities (CAs). Scoring for CAs was performed under ×2000 magnification with a light microscope.
Morphological sperm abnormalities
Sperm were prepared according to the recommended method of Wyrobek and Bruce [22] with some modifications recorded by Fahmy et al. [19]. Smears were stained with 1% Eosin Y. A total of 1000 sperm was counted per animal (5000/each treatment), and different types of sperm abnormalities were scored (Head and Tail abnormalities). Sperm preparations were examined by light microscopy at ×1000 magnification.
Expression of p53
Isolation of total RNA
Total RNA was isolated from liver tissues of male mice by the standard TRIzol® Reagent extraction method (cat#15596-026, Invitrogen, Germany). Briefly, tissue samples were homogenized in mortar with liquid nitrogen and 1 mL of TRIzol® Reagent. RNA was dissolved in diethylpyrocarbonate (DEPC)-treated water up to use.
Reverse transcription (RT) reaction
The complete Poly(A)+ RNA isolated from liver tissue samples was reverse transcribed into cDNA in a total volume of 20 µl using RevertAid™ First Strand cDNA Synthesis Kit (MBI Fermentas, Germany).
Real time-polymerase chain reaction (qRT-PCR)
Step One™ Real-Time PCR System from Applied Biosystems (Thermo Fisher Scientific, Waltham, MA USA) was used to determine the tissues samples of mice copy number. The sequences of specific primer of the genes used are listed in Table 1. At the end of each qPCR a melting curve analysis was performed at 95.0 °C to check the quality of the used primers. The relative quantification of the target to the reference was determined by using the 2−ΔΔCT method.
Table 1.
Primers sequence used for qRT-PCR
| Gene | Primers sequence | NCBI Reference |
|---|---|---|
| p53 | F: ACA GTC GGA TAT CAG CCT CG | AB021961.1 |
| R: GCT TCA CTT GGG CCT TCA AA | ||
| GAPDH | F:GGA TGC AGG GAT GAT GTT CT | NM_017008.3 |
| R:GAA GGG CTC ATT GAC CAC AGTT |
GAPDH: glyceraldehyde-3-phosphate dehydrogenase,
P53: tumor protein p53 gene
Data analysis
Data were analyzed using computerized software SPSS (Statistical Package of Social Science, version 20, Armonk, New York: IBM Corp). The data were checked for normality and the homogeneity of the variance using the Kolmogorov–Smirnov's test and Levene's test, respectively. The differences among groups with normal distribution were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey HSD test. The results were regarded as significant when the P-value was less than or equal to 0.05.
Results
Chemical composition of C. variegatum
Depending on the structural information obtained from HPLC/QTOF–MS/MS data, in [M + H]− ion, total ion current chromatogram (TIC), a total of 115 components were unambiguously identified. The flavonoids were the main component identified (including flavones (35.21%) of C- and/or O-hexosides nature, flavonols (3.40%), isoflavones (0.53%). Also, the study can identify other important classes of chemicals e.g. phenolic acids (17.62%), coumarins (8.29%), indoles (4.10%), fatty acids (0.932%), amino acid derivatives (0.53%), and polyols (0.49%) based on their fragments (data of chemical analysis is under publication).
Cytogenetic analysis
Micronucleus analysis
Table 2 presents the frequency of MPCEs induced after treatment of Cr with and without C. variegatum fraction at different doses (100,300 and 500 mg/kg). Also, the percentage of PCEs to the total counted cells was recorded. The results demonstrated a highly significant percentage of MPCEs after Cr treatment reached 9.88 ± 0.58 compared to 2.63 ± 0.63 and 2.97 ± 0.27 for negative and plant control respectively. The extract of C. variegatum at the dose levels of 300 and 500 mg/kg was significantly reduced such effect to be reached to 6.67 ± 0.49 and 5.75 ± 0.26 respectively. The results also indicated that Cr induced an increase in the number of PCEs/total counted cells, indicating bone marrow toxicity. The extract of C. variegatum at different doses ameliorates such percentage in a dose-dependent manner.
Table 2.
Percentage of polychromatic erythrocytes (PCEs) and PCEs with micronuclei (MPCEs); chromosomal aberrations induced in mouse bone marrow cells after treatment with carmustine and Codiaeum variegatum
| Treatment and doses | No. and percentage of PCEs/(PCEs + NCPs) | No. and percentage of MPCEs | Total abnormal metaphases | No and (%) of metaphases with different types of chromosome aberrations | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NO | Mean ± S.E | NO | Mean ± S.E | No | Mean (%) ± SE | Gap | Fragment and/or break | Multiple aberrations | R.T | |
| I. Control (30% ethanol) | 419 | 4.19 ± 0.37a | 11 | 2.63 ± 0.63a | 23 | 4.60 ± 0.4 a |
7 (1.40) |
16 (3.20) |
– | – |
| II. Carmustine (30 mg / kg) | 1448 | 14.48 ± 0.49d | 143 | 9.88 ± 0.58c | 163 | 32.60 ±0.0.75 e |
12 (2.40) |
42 (8.40) |
102 (20.40) |
7 (1.40) |
| III. Codiaeum variegatum (500 mg/kg) | 438 | 4.38 ± 0.54a | 13 | 2.97 ± 0.27a | 16 | 3.20 ± 0.58a |
7 (1.40) |
9 (1.80) |
– | – |
| IV-VI- Carmustine + Codiaeum variegatum | ||||||||||
| + 100 mg/kg | 1132 | 11.32 ± 0.53c | 88 | 7.77 ± 0.61 c | 130 | 26.0 ± 0.89d |
14 (2.80) |
37 (7.40) |
72 (14.40) |
7 (1.40) |
| + 300 mg/kg | 1005 | 10.05 ± 0.49b | 67 | 6.67 ± 0.49 b | 107 | 21.40 ± 0.98 c |
13 (2.60) |
41 (8.20) |
49 (9.80) |
4 (0.80) |
| + 500 mg/kg | 887 | 8.87 ± 0.32b | 51 | 5.75 ± 0.26 b | 86 | 17.20 ± 0.86b |
11 (2.20) |
35 (7.0) |
36 (7.20) |
4 (0.80) |
A total of 500 cells were analyzed (5 mice per group; 100 cells/mouse) for chromosomal aberrations. R.T.: Robertsonian translocation. No. of examined nucleated cells = 2000/mouse (5 mice/group) for micronuclei test.One way ANOVA–Tukey’s multiple comparisons test was used. The values having different superscript letters in each column are significantly different from one another at p < 0.05
Chromosomal aberrations (CAs) in bone marrow cells
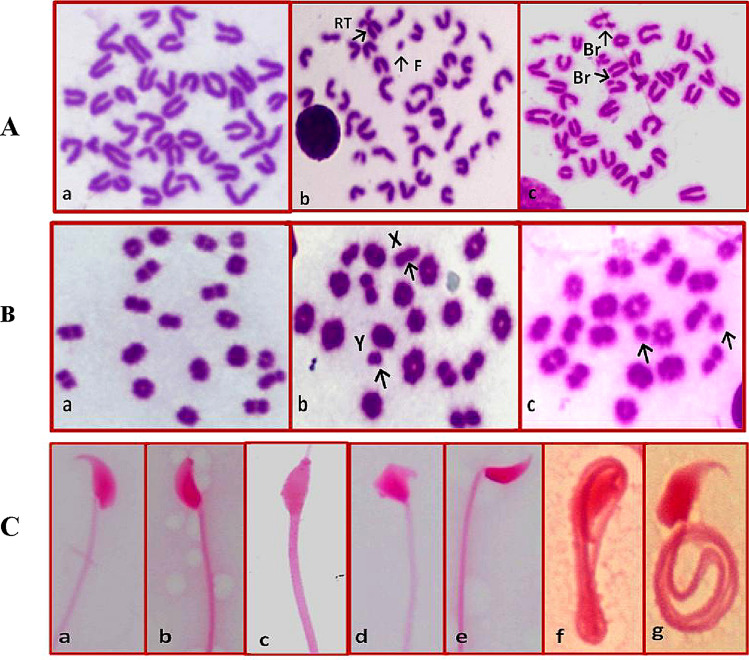
Table 2 also revealed that Cr (30 mg/kg) induced a significant percentage of CAs in bone marrow cells reaching 32.60 ± 0.75 vs 4.60 ± 0.40 for control. Metaphases contain multiple aberrations and metaphases with breaks/fragments are the most pronounced after Cr treatment. Robertsonian translocations were also recorded (Fig. 1A). The results also demonstrated that C. variegatum at the highest tested dose (500 mg/kg) has approximately the same effect as the control negative. Moreover, the tested concentrations of C. variegatum significantly decreased Cr-induced aberrations recording a dose-dependent relationship.
Fig. 1.
A Chromosomal abnormalities in bone marrow cells in mice showing (a) normal (b) fragment and Robertsonian translocation and (c) breaks. B Chromosomal abnormalities in diakinase- metephase 1 cells in mice showing (a) normal, (b) XY univalent and (c) autosomal univalent. C Sperm abnormalities in mice showing (a) normal, (b) without hook, (c) amorphous, (d) triangular, (e) banana and (f, g) coiled tail
Chromosomal aberrations in spermatocyte cells
Table 3 recorded that Cr induced a significant percentage of chromosomal aberrations in mouse spermatocytes reached 16.80 ± 0.97 vs 3.80 ± 0.37 and 3.20 ± 0.49 for control negative and control plant respectively. The three tested concentrations of C. variegatum are significantly mitigated the frequency of aberrations in a dose-dependent manner. The majority of aberrations induced after Cr treatment are univalent (X–Y and autosomal univalent (Fig. 1B).
Table 3.
Percentage of chromosomal aberrations in mouse spermatocytes and sperm abnormalities induced after treatment with carmustine and Codiaeum variegatum
| Treatment and doses | Total abnormal metaphases | No. of different types of sperm abnormalities | Total abnormal sperm | |||
|---|---|---|---|---|---|---|
| No | Mean(%) ± SE | Head abnormalities | Tail abnormalities | No | Mean (%) ± SE | |
| I. Control (30% ethanol) | 19 | 3.80 ± 0.37 a | 126 | 86 | 212 | 4.24 ± 0.19a |
| II.Carmustine (30 mg / kg) | 84 | 16.80 ± 0.97c | 274 | 252 | 526 | 10.52 ± 0.21e |
| III. Codiaeum variegatum (500 mg/kg) | 16 | 3.20 ± 0.49 a | 122 | 104 | 226 | 4.52 ± 0.38a |
| IV-VI- Carmustine + Codiaeum variegatum | ||||||
| + 100 mg/kg | 54 | 10.80 ± 0.97 b | 276 | 201 | 477 | 9.54 ± 0.46d |
| + 300 mg/kg | 46 | 9.20 ± 0.58b | 191 | 191 | 382 | 7.64 ± 0.31c |
| + 500 mg/kg | 43 | 8.60 ± 0.75 b | 156 | 164 | 320 | 6.40 ± 0.24 b |
A total of 500 cells were analyzed for chromosomal aberrations (5 mice per group; 100 cells/mouse). Total number of examined sperms 5000 per each treatment (1000 /mouse, 5 mice/group). One way ANOVA–Tukey’s multiple comparisons test was used. The values having different superscript letters in each column are significantly different from one another at p < 0.05
Morphological sperm abnormalities
Sperm abnormalities induced after treatment with Cr and C. variegatum was presented in Table 3. The results indicated that Cr induced a significant percentage of sperm defects that reached 10.52 ± 0.21 vs 4.24 ± 0.19 for the negative control. The results also indicated that C. variegatum has approximately the same effect as control. Cr induced both head and tail abnormalities but the coiled tail abnormality was the most pronounced type of sperm defects induced after Cr treatment. Different doses of C. variegatum ameliorated such effect in a dose dependent manner. The maximum protective effect was recorded with the dose 500 mg/kg of plant extract. Figure 1C represents different types of the induced sperm abnormalities.
Gene expression of p53 gene as indicated by qRT-PCR
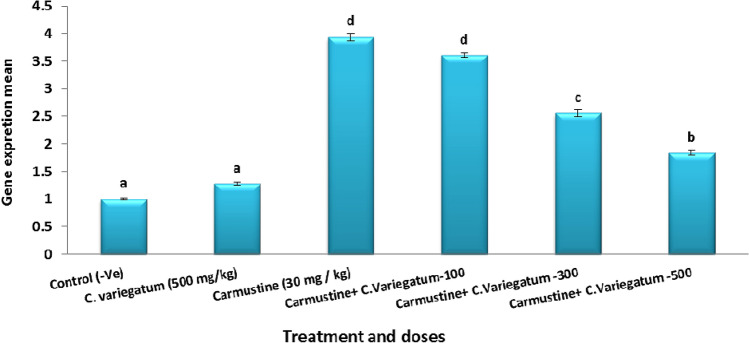
The results in Fig. 2 and Table 4 indicated an over expression of the gene p53 after treatment with Cr 3.94 ± 0.07 vs 1.0 ± 0.02 and 1.28 ± 0.03 for the negative control and Codiaeum variegatum (500 mg/kg, control plant) respectively. The results also showed that the combined groups (Cr plus C. variegatum) have lower values of p53 expression than Cr group. The maximum modulating effect was demonstrated with the highest tested dose of plant where the value of p53 expression reached 1.84 ± 0.04.
Fig. 2.
P53 gene expression as indicated by q RT-PCR in mice liver cells
Table 4.
The gene expression of P53 gene as indicated by RT-PCR
| Treatment | Mean | SEM |
|---|---|---|
| I. Control (30% ethanol) | 1 | 0.02 |
| II.Carmustine (30 mg / kg) | 3.94 | 0.07 |
| III. Codiaeum variegatum (500 mg/kg) | 1.28 | 0.03 |
| IV-VI- Carmustine + Codiaeum variegatum | ||
| + 100 mg/kg | 3.61 | 0.04 |
| + 300 mg/kg | 2.56 | 0.07 |
| + 500 mg/kg | 1.84 | 0.04 |
Discussion
Carmustine (Cr) is an important chemotherapeutic drug for treating brain tumors, lymphoma, and multiple myeloma [23]. Genotoxicity is a common side effect of all chemotherapeutic agents which may lead to secondary malignancies. Herein the genotoxicity of Cr was evaluated using various cytogenetic parameters as the first goal of this work. To the best of our knowledge the present study is the first one referring to the previous works on Cr genotoxicity. The protective role of different doses of ethyl acetate fraction of C. variegatum leaves was also evaluated which represents the second goal of this study. Cr was found to induce a genotoxic effect in somatic cells evidenced by an increase in the frequency of MPCEs and CAs in bone marrow cells by about 3- and 7- fold increases over that of the negative control respectively. The percentage of the induced CAs in bone marrow reached 32.60 ± 0.75 compared with 4.60 ± 0.40 for the control. Cells with multiple aberrations were the most pronounced. This result is in the same line as that obtained in the study of Tates et al. [24]. The results of genotoxicity in bone marrow cells emphasize the positive correlation between micronucleus and chromosomal aberration induction. This indicates that one parameter has predictive value for the other. Fahmy et al. [25] suggested that CAs induced by chemical agents is essential events for the induction of MPCEs of bone marrow. The genotoxicity of Cr in bone marrow cells that is detected in the present work may be an indication for inability of this tissue to repair DNA damage induced by Cr efficiently [25, 26]. The results coincide with Tofilon et al. [27] who demonstrated that Cr induced sister chromatid exchanges in 9L rat brain tumor cells. The present results also demonstrated that Cr is toxic to bone marrow cells evidenced by increase in the percentage of PCEs/ (NCEs + PCEs). Previous studies recorded myelotoxicity and myelosuppression of bone marrow cells after Cr treatment [28]. El-Sayed et al. [29] reported that i.p injection of rats with Cr (30 mg/kg) significantly increased bone marrow apoptosis and bone marrow content of malondialdehyde which is a lipid peroxidation marker. Also, Lytle et al. [30] demonstrated that Cr chemotherapy down-regulates Bcl-xL and Bcl-2 in the bone marrow resulting in enhancement of apoptosis. Cr, like certain other nitrosoureas, decomposes spontaneously into 2 active intermediates: chloroethyl diazohydroxide and an isocyanate group. The DNA alkylation leads to the formation of irreversible DNA-DNA and DNA–protein crosslinks which are mediated by the chloroethyl diazohydroxide intermediate. The isocyanate intermediate produces carbamoylation of amino groups, which inhibits DNA repair, depletes glutathione and interferes with DNA/RNA synthesis [29].
Cr was reported to inhibit the antioxidant enzyme glutathione reductase (GR) massively [31]. As one of the most effective and abundant ROS-scavenging systems, glutathione plays a critical role in the maintenance of the redox balance in virtually all cells including normal and neoplastic cells. Therefore, inhibition of GR by Cr leads to the accumulation of reactive oxygen species which has been considered as one of the mechanisms responsible for Cr-genotoxicity and cytotoxicity. Recently, it was demonstrated that depletion of reduced GR enhanced the production ROS after opening the mitochondrial permeability transition pore [32]. ROS, such as the superoxide anion (O2−), hydrogen peroxide H2O2 and the hydroxyl radical, are thought to be involved in mediating the cytotoxic pathways induced by many anti-cancer agents [14, 33]. Cr also reduced the activity of other antioxidant enzymes SOD, CAT, and GPx [29]. It has been reported that GPx is capable of reducing free hydrogen peroxide, while CAT and SOD provide the first line defense against oxygen radical toxicity [34].
Concerning gonadal cells, the present results illustrated that Cr induced a significant percentage of CAs in mouse spermatocytes. The majority is in the form of univalent (X–Y and autosomal univalent). It also induced morphological sperm defects. These results are in the same line with the reports of Meistrich et al. [35].The univalent chromosome formation in spermatocytes is thought to be developed due to meiotic division that cannot be successfully completed. The study by Inanc et al. [23] finds a high level of caspase 3 in the cells undergoing meiosis after treatment with Cr. This could be explained the induction of univalent chromosomes and genotoxicity of Cr that ultimately reached to apoptosis in these cells as one of the defense mechanisms. Generally, it was reported that alkylating agents induce toxic effects on gonads [36]. Also some nitrosourea chemotherapeutic agents like 5-FU induced the same effect in mouse spermatocytes [14]. Cr in particular has been demonstrated to induce oxidative stress in the form of reactive oxygen species and lipid peroxidation in testicular tissues [37]. Lipid peroxidation has been reported to induce inflammation in gonads which may lead to toxic alterations in the cells associated with spermatogenesis and reduce spermatozoa motility. Cr generates ROS in testicular cells in the form of hydroxide radicals (OH), superoxide anions (O2−) and hydrogen peroxide H2O2 which can damage cellular macromolecules, DNA, RNA, protein and lipids in the testes [23].
It was recorded that the measurements of morphological sperm abnormalities could be used as an indication of damage that spermatogenic cells have suffered from chemical or physical agents [14]. The present results also recorded a significant percentage of morphological sperm abnormalities after Cr treatment with malformation of sperm head and tail. Coiled tail sperm was the most pronounced type of abnormalities induced after Cr treatment. The coiling of the sperm tail greatly affects its motility and function, accordingly this decrease its ability to fertilize an egg. These results are coincide well with the results of Inanc et al. [23] who reported that Cr negatively affected spermatozoa motility and induced sperm toxic effect as a result of oxidative stress and DNA damage. Defective sperm function has been identified as the most common cause of infertility [14].
The results of the present work also revealed an over expression of the gene p53 after Cr treatment which may be an indication of cellular DNA damage. Most chemotherapeutic drugs cause DNA damage, which is sensed by p53; the cell can then try to repair the damage or induce cell suicide [38].
Nowadays, the search for new and effective antioxidants is of a great importance especially, that extracted from plant origin. Herein the ethyl acetate fraction of C. variegatum leaves was evaluated as a positive chemo-preventive agent. Its genotoxicity/anti-genotoxicity was discussed and the main effective constituents were evaluated. The results showed that C. variegatum has no genotoxic effect in all tests examined. Moreover, a significant decrease in MPECs, CAs, and the toxicity of bone marrow in the combined groups treated with the plant and Cr was recorded in comparison with Cr group. The results also showed that C. variegatum could potentially protect against genotoxicity in germ cells. The results demonstrated about 50% decrease in the percentage of Cr- induced MPCEs, CAs in bone marrow and mouse spermatocytes with the highest tested dose of the extract. Also, a dose-dependent decrease was reported in sperm abnormalities in the combined groups (Cr and plant). C. variegatum is known to have medicinal properties like antioxidant, anti-inflammatory and anticancerious properties [2]. In a previous study, the phenolic compounds extracted from C. variegatum leaves showed an ameliorative effect against CAs induced by mitomycin C (MMC) in bone marrow and mouse spermatocytes. Likewise, the extract declined cytotoxicity and DNA damage (as indicated by comet assay) induced by MMC in bone marrow cells [39]. The same authors demonstrated that the highest concentration (500 mg/kg) of this extract did not induce cytotoxicity, CAs, or DNA damage. Moreover, it was reported that the aqueous extract from C. variegatum did not induce cytotoxic or genotoxic effects on mouse lymphoma cells [2, 39]. The goal of HPLC-qTOF-MS/MS analysis was performed to identify and quantify all secondary metabolites belonging to different chemical groups. The flavonoids (including flavones (35.21%) of C- and/or O-hexosides nature, flavonols (3.99%) and phenolic acids (17.62%), were the main identified polyphenols. Polyphenols have antioxidant activities and could improve sperm parameters. Plants extremely rich in natural antioxidants, such as flavonoids and phenolic acids have a protective role against genotoxic hazards both in vitro and in vivo [14]. Our study is characterized by a large range of C-glycosylflavonoids and vitexin was the main C-flavone glycoside (28.97%). Vitexin is a powerful antioxidant against ROS and lipid peroxidation. Vitexin activates the signaling pathways that depend on its antioxidant activity and has anti-inflammatory and antiangiogenic properties [40]. Isovitexin, orientin and their multiglycosides such as vicenin-2 were detected in the fraction. These compounds were also previously isolated from C. variegatum leaves [41]. Different biological properties including antioxidant, anti-cancerous, anti-inflammatory and chemo-preventive have been attributed to the C- and O-glycosyl flavones content of flavonoids [42]. Flavones are the most likely candidates among the compounds known for preventing oxidative lesions and providing antigenotoxic effects. Many other flavones were also identified such as rhoifolin, acacetin, apigenin, and diosmin with the amount range of 0.17–2.08%. The flavone; rhoifolin was reported to possesses a variety of significant biological activities including anti-inflammatory, antioxidant, and anticancer effects [43]. Acacetin is known to possess numerous pharmacological properties, including cardioprotective, neuroprotective, anti-inflammatory, antidiabetic and anticancer activities [44, 45]. Apigenin is known for its significant antioxidant and anti-proliferative activity and scavenges reactive oxygen species. Some derivatives of kaempferol were also identified (0.32–1.62%). This class of polyphenol secondary metabolites was demonstrated to have anti-proliferative activities on a variety of human cancer cell lines [40]. Phenolic acids (17.61%) were the second main group of compounds. Some phenolic acids showed an ameliorative effect on sperm DNA damage caused by oxidative stress and on testicular function and semen quality in human and animal models exposed to toxins. One of identified phenolic acids was rosmarinic acid (1.58%). Recently, the compound was reviewed in many in vitro and in vivo studies for its potent anti-inflammatory effects against inflammatory diseases [46]. In the current study different mono- and di-glycoside of quercetin were also identified (0.92%). The plant extract that contains quercetin and/or its derivatives has been reported to have antioxidant and antimutagenic properties [47]. In human lymphocytes, quercetin and some of its derivatives have shown a protective effect against oxidative DNA damage in vitro. Also, the anti-mutagenic activity against some oxidative mutagens in the Ames assay was reported [47]. Of the important compounds detected coumarins which represent 8.29% of the extract. Coumarin and its derivatives represent one of the most active classes of compounds that display a wide range of biological activities e.g. anti-inflammatory, antioxidants, anticancerious, radical scavenging activities as well as antibacterial and anti-HIV [48]. Indolic compounds (4.10%) are proven to be very efficient antioxidants, protecting cell proteins and lipids from peroxidation. The indole structure influences the antioxidant efficacy in biological systems [49].
In conclusion, the bioactivity of C. variegatum which was detected in the present work by inducing anti-genotoxicity in somatic and germ cells of male mice could be attributed to the major compounds or synergy between the major and minor constituents which can act by different mechanisms e.g. free radical scavengers, immune-stimulants, DNA repairs, termination of oxidative stress and activation of antioxidant enzymes.
Acknowledgements
The authors would like to declare that the funder had no role in the idea, practical work, discussion, and publication of the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This is a collaborative study between the Department of Genetics and Cytology and Chemistry of Natural Compounds Department, National Research Centre (NRC) Dokki-Giza (Egypt). This work is a part of the in-house project of the NRC, Dokki-Giza (Egypt) which is under the NO: 12060167. NRC provided all necessary facilities to complete this work.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All experimental procedures were carried out following the guiding principles for the care and use of laboratory animals approved by the NRC, Dokki-Giza (Egypt). The Approval Certificate is under the number: 19-163.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abd-Alla HI, Moharram FA, Gaara AH, El-Safty MM. Phytoconstituents of Jatropha curcas L. leaves and their immunomodulatory activity on humoral and cell-mediated immune response in chicks. Z Naturforsch C. 2009;64:495–501. doi: 10.1515/znc-2009-7-805. [DOI] [PubMed] [Google Scholar]
- 2.Njoya EM, Moundipa PF, Niedermeyer TH. Codiaeum variegatum (L.) Rumph. ex A. Juss. (Euphorbiaceae): an overview of its botanical diversity, traditional uses, phytochemistry, pharmacological effects and perspectives towards developing its plant-based products. J Ethnopharmacol. 2021 doi: 10.1016/j.jep.2021.114244. [DOI] [PubMed] [Google Scholar]
- 3.Salatino A, Salatino ML, Negri G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae) J Braz Chem Soc. 2007;18:11–33. doi: 10.1590/S0103-50532007000100002. [DOI] [Google Scholar]
- 4.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panigrahi M, Das PK, Parikh PM. Brain tumor and Gliadel wafer treatment. Indian J Cancer. 2011;48:11–17. doi: 10.4103/0019-509X.76623. [DOI] [PubMed] [Google Scholar]
- 6.Nagaimo S, Yamamoto YL, Diksic M, et al. Neurotoxicity after intracarotid 1,3-bis(2-chloroethyl)-1-nitrosourea administration in the rat: hemody-namic changes studied by double-tracer autoradiography. Neurosurgery. 1991;29:19–25. doi: 10.1097/00006123-199107000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Das D, Preet R, Mohapatra P, et al. 1,3-Bis(2-chloroethyl)-1- nitrosourea enhances the inhibitory effect of Resveratrol on 5-fluorouracil sensitive/resistant colon cancer cells. World J Gastroenterol. 2013;19:7374–7388. doi: 10.3748/wjg.v19.i42.7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wait SD, Prabhu RS, Burri SH, et al. Polymeric drug delivery for the treatment of glioblastoma. Neuro Oncol. 2015;17:9–23. doi: 10.1093/neuonc/nou360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hang B, Chenna A, Guliaev AB, et al. Miscoding prop-erties of 1, N6-ethanoadenine, a DNA adduct derived from reaction with the antitumor agent 1,3-bis(2-chloroethyl)-1-nitrosourea. Mutat Res. 2003;531:191–203. doi: 10.1016/j.mrfmmm.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Volkin RL, Shadduck RK, Winkelstein A, et al. Potentiation of carmustine-cranial irradiation-induced myelosuppression by cimetidine. Arch Intern Med. 1982;142:243–245. doi: 10.1001/archinte.1982.00340150043011. [DOI] [PubMed] [Google Scholar]
- 11.O'Driscoll BR, Hasleton PS, Path FRC, et al. Active lung fibrosis up to 17 years after chemotherapy with Carmustine (BCNU) in childhood. N Engl J Med. 1990;323:378–382. doi: 10.1056/NEJM199008093230604. [DOI] [PubMed] [Google Scholar]
- 12.Autio K, Rassnick KM, Bedford-Guaus SJ. Chemotherapy during pregnancy: a review of the literature. Vet Comp Oncol. 2007;5:61–75. doi: 10.1111/j.1476-5829.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- 13.Ludlum DB. DNA alkylation by the haloethylnitrosoureas: nature of modifications produced and their enzymatic repair or removal. Mutat Res. 1990;233:117–126. doi: 10.1016/0027-5107(90)90156-X. [DOI] [PubMed] [Google Scholar]
- 14.Fahmy MA, Abd-Alla HI, Entesar E, et al. Genotoxicity and sperm defects induced by 5-FU in male mice and the possible protective role of Pentas lanceolata-iridoids. Mutat Res Genet Toxicol Environ Mutagen. 2020;850–851:503145. doi: 10.1016/j.mrgentox.2020.503145. [DOI] [PubMed] [Google Scholar]
- 15.Chu E, Sartorelli AC. Cancer chemotherapy. In: Katzung BG, editor. Basic and clinical pharmacology, Chapter 55. New York: McGraw Hill Medical; 2007. pp. 878–907. [Google Scholar]
- 16.Paget GE, Barnes JM. Interspecies dosage conversion scheme in evaluation of results and quantitative application in different species. In: Laurence DR, Bacharach AL, editors. Evaluation of drug activities: pharmacometrics. New York: Academic Press; 1964. pp. 160–162. [Google Scholar]
- 17.Schmid W. The micronucleus test for cytogenetic analysis. In: Hollaender A, editor. Chemical mutagenesis, principals and methods for their detection. New York: Plenum Press; 1976. [Google Scholar]
- 18.OECD (2016) Guidelines for the testing of chemicals, genetic toxicology: mammalian erythrocyte micronucleus test, Organisation for Economic Co-operation and Development, Paris, TG474
- 19.Fahmy MA, Hassan NHA, El-Fiky SA, et al. A mixture of honey bee products ameliorates the genotoxic side effects of cyclophosphamide. Asian Pac J Trop Dis. 2015;5:638–644. doi: 10.1016/S2222-1808(15)60904-5. [DOI] [Google Scholar]
- 20.Evans EP, Breckon G, Ford CE. An air drying method for meiotic preparation from mammalian testes. Cytogenetics. 1964;3:289–329. doi: 10.1159/000129818. [DOI] [PubMed] [Google Scholar]
- 21.Hassan NHA, Fahmy MA, Farghaly AA, et al. Antimutagenic effects of selenium and vitamins against the genotoxicity induced by cobalt chloride in mice. Cytologia. 2006;71:201–222. doi: 10.1508/cytologia.71.213. [DOI] [Google Scholar]
- 22.Wyrobek AJ, Bruce WR. Chemical induction of sperm-shape abnormalities in mice. New York: Plenum Press; 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inanc ME, Gungor S, Yeni D, et al. Protective role of the dried white mulberry extract on the reproductive damage and fertility in rats treated with carmustine. Food Chem Toxicol. 2022;163:12979. doi: 10.1016/j.fct.2022.112979. [DOI] [PubMed] [Google Scholar]
- 24.Tates AD, Natarajan AT, De Vogel N, et al. A correlative study on the genetic damage induced by chemical mutagens in bone marrow and spermatogonia of mice : III. 1,3-Bis (2-chloroethyl)-3-nitrosourea (BCNU) Mut Res. 1977;44:87–95. doi: 10.1016/0027-5107(76)90041-5. [DOI] [PubMed] [Google Scholar]
- 25.Gerson SL, Trey JE, Miller K, et al. Comparison of O6-alkylguanine- DNA alkyltransferase activity based on cellular DNA content in human, rat and mouse tissues. Carcinogenesis. 1986;7:745–749. doi: 10.1093/carcin/7.5.745. [DOI] [PubMed] [Google Scholar]
- 26.Maze R, Carney JP, Kelley MR, et al. Increasing DNA repair methyltransferase levels via bone marrow stem cell transduction rescues mice from the toxic effects of 1,3-bis(2-chloroethyl)-1-nitrosourea, a chemotherapeutic alkylating agent. Proc Natl Acad Sci. 1996;93:206–210. doi: 10.1073/pnas.93.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tofilon PJ, Williams ME, Deen DF. Nitrosourea-induced sister chromatid exchanges and correlation to cell survival in 9L rat brain tumor cells. Cancer Res. 1983;43:473–475. [PubMed] [Google Scholar]
- 28.Maxwell SR. Prospects for the use of antioxidant therapies. Drugs. 1995;49:345–361. doi: 10.2165/00003495-199549030-00003. [DOI] [PubMed] [Google Scholar]
- 29.El-Sayed SM, Abdel-Aziz AH, Helal GK, et al. Protective effect of N-acetylcysteine against carmustine-induced myelotoxicity in rats. Food Chem Toxicol. 2010;48:1576–1580. doi: 10.1016/j.fct.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Lytle RA, Jiang Z, Zheng X, et al. BCNU down-regulates anti-apoptotic proteins Bcl-xL and Bcl-2 in association with cell death in oligodendroglioma-derived cells. J Neuro-Oncol. 2004;68:233–241. doi: 10.1023/b:neon.0000033382.40601.5a. [DOI] [PubMed] [Google Scholar]
- 31.Helal GK, Helal OK. Metallothionein attenuates carmustine-induced oxidative stress and protects against pulmonary fibrosis in rats. Arch Toxicol. 2009;83:87–94. doi: 10.1007/s00204-008-0325-7. [DOI] [PubMed] [Google Scholar]
- 32.Bizzozero OA, Ziegler JL, De Jesus G, et al. Acute depletion of reduced glutathione causes extensive carbonylation of rat brain proteins. Neurosci Res. 2006;83:656–667. doi: 10.1002/jnr.20771. [DOI] [PubMed] [Google Scholar]
- 33.Berthiaume JM, Wallace KB. Persistent alterations to the gene expression profile of the heart subsequent to chronic doxorubicin treatment. Cardiovasc Toxicol. 2007;7:178–191. doi: 10.1007/s12012-007-0026-0. [DOI] [PubMed] [Google Scholar]
- 34.Sundaresan S, Subramanian P. S-allylcysteine inhibits circulatory lipid peroxidation and promotes antioxidants in N-nitrosodiethylamine-induced carcinogenesis. Pol J Pharmacol. 2003;55:37–42. [PubMed] [Google Scholar]
- 35.Meistrich ML, Finch M, Da Cunha MF, et al. Damaging effects of fourteen chemotherapeutic drugs on mouse testis cells. Cancer Res. 1982;42:122–131. [PubMed] [Google Scholar]
- 36.Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. JNCI Monogr. 2005;34:12–17. doi: 10.1093/jncimonographs/lgi003. [DOI] [PubMed] [Google Scholar]
- 37.Kaneko T, Iuchi Y, Kobayashi T, et al. The expression of glutathione reductase in the male reproductive system of rats supports the enzymatic basis of glutathione function in spermatogenesis. Eur J Biochem. 2002;269:1570–1578. doi: 10.1046/j.1432-1033.2002.02809.x. [DOI] [PubMed] [Google Scholar]
- 38.Ye S, Shen J, Choy E, et al. p53 overexpression increases chemosensitivity in multidrug resistant osteosarcoma cell lines. Cancer Chemother Pharmacol. 2016;77:349–356. doi: 10.1007/s00280-015-2944-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abo-Zeid MAM, Farghaly AA, Hassan EM, et al. Phenolic compounds of Codiaeum variegatum spirale lessened cytotoxic and genotoxic effects of mitomycin C in mice somatic and germ cells. Cytol Genet. 2019;53:494–501. doi: 10.3103/S0095452719060057. [DOI] [Google Scholar]
- 40.Babaei F, Moafizad A, Darvishv Z, et al. Review of the effects of vitexin in oxidative stress-related diseases. Food Sci Nutr. 2020;8:2569–2580. doi: 10.1002/fsn3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hassan EM, Hassan RA, Salib JY, et al. Chemical constituents and cytotoxic activity of Codiaeum variegatum cv. petra. J Appl Sci Res. 2013;9:4884–4888. [Google Scholar]
- 42.Awad HM, Abd-Alla HI, Mahmoud KH, et al. In vitro anti-nitrosative, antioxidant, and cytotoxicity activities of plant flavonoids: a comparative study. Med Chem Res. 2014;23:3298–3307. doi: 10.1007/s00044-014-0915-2. [DOI] [Google Scholar]
- 43.Refaat J, Desoukey SY, Ramadan MA, et al. Rhoifolin: a review of sources and biological activities. Int J Pharmacogn. 2015;2:102–109. doi: 10.13040/IJPSR.0975-8232.IJP.2(3).102-09. [DOI] [Google Scholar]
- 44.Shen KH, Hung SH, Yin LT, et al. Acacetin, a flavonoid, inhibits the invasion and migration of human prostate cancer DU145 cells via inactivation of the p38 MAPK signaling pathway. Mol Cell Biochem. 2010;333:279–291. doi: 10.1007/s11010-009-0229-8. [DOI] [PubMed] [Google Scholar]
- 45.Sarker SD, Nahar L. Medicinal natural products: a disease-focused approach. Cambridge: Elsevier - Academic Press; 2020. [Google Scholar]
- 46.Luo C, Zou L, Sun H, et al. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front Pharmacol. 2020;11:153. doi: 10.3389/fphar.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D'Andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256–271. doi: 10.1016/j.fitote.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 48.Ma QG, Wei RR, Yang M, et al. Molecular characterization and bioactivity of Coumarin derivatives from the fruits of Cucumis bisexualis. J Agric Food Chem. 2018;66:22. doi: 10.1515/znc-2019-0202. [DOI] [PubMed] [Google Scholar]
- 49.Estevão MS, Carvalho LC, Ribeiro D, et al. Antioxidant activity of unexplored indole derivatives: synthesis and screening. Eur J Med Chem. 2010;45:4869–4878. doi: 10.1016/j.ejmech.2010.07.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.