Abstract
Zinc deficiency impairs the antibody-mediated immune response and is common in children from lower-income countries. This study aimed to investigate the impact of different zinc supplementation regimens (7, 10 or 20 mg/day elemental zinc)—therapeutic dispersible zinc tablets (TZ), daily multiple micronutrient powder (MNP), daily preventive zinc tablets (PZ) and placebo powder (control)—and compare between baseline and endline antibody production against pathogenic Escherichia coli in Laotian children (aged 6–23 months). Fifty representative plasma samples of each treatment group were randomly selected from 512 children to determine anti-E. coli IgG antibody levels and avidity. Of the 200 children, 78.5% had zinc deficiency (plasma zinc concentration < 65 µg/dL) and 40% had anaemia before receiving zinc supplementation. aAfter receiving the TZ, MNP or PZ regimen, the plasma anti-E. coli IgG levels were significantly increased compared with baseline; the effect on the antibody level was more pronounced in children with zinc deficiency. Interestingly, there was increased anti-E. coli IgG avidity in the control and PZ groups. This study suggests that PZ might be the optimal zinc supplementation regimen to increase both the quantity and quality of antibody responses in children with zinc deficiency. Clinical trial registration: https://clinicaltrials.gov/ct2/show/NCT02428647 (NCT02428647, 29/04/2015).
Subject terms: Immunology, Medical research
Introduction
Zinc is an essential micronutrient to maintain regular biological maturation, neurocognitive development as well as immune function1. Immune response modulation by zinc has been reported via release of glucocorticoids, decreased thymulin and antioxidant activity2. Dysregulation of zinc homeostasis affects adaptive immune responses and causes immunodeficiency3. A major component of the adaptive immune system is the humoral immune response, also called the antibody-mediated immune response4. Binding of zinc to SLC39A10/ZIP10 or zinc transporter modulates the B-cell receptor (BCR) signal strength, resulting in the induction of an antibody-mediated immune response5.
The prevalence of zinc deficiency is estimated to range from 7.5% in high-income regions to 30% in South Asia6. Multiple systematic reviews have reported that preventive zinc supplementation is associated with a decrease in diarrhoea- and pneumonia-related morbidity and mortality in children in lower-income countries7,8. Diarrhoeal disease is the second leading cause of death in children under 5 years old9. In those areas, pathogenic Escherichia coli strains and Salmonella are commonly found in children with persistent diarrhoea10. Moreover, sepsis is present in about 22.5% of children with diarrhoea; it is cause by the translocation of gram-negative bacteria through the diseased and inflamed gut11. Antibodies, especially immunoglobulin G (IgG), encounter and neutralise the bacteria and their toxins12,13. Antibody avidity has been used as a measure of functional maturation of the humoral immune response, and increases in antibody avidity over time have been shown after both infection and vaccination14,15.
A previous randomised controlled trial investigated zinc supplementation in 512 rural Laotian children. There were four intervention groups: (1) daily placebo (Control), (2) therapeutic dispersible zinc tablets (TZ) as part of 10-day treatment of diarrhoea, (3) daily multiple micronutrient powder including zinc (MNP) and (4) daily preventive zinc tablets (PZ). Children aged 6–23 months were randomly assigned to one of these interventions in a community-based intervention trial for approximately 9 months. The parent studies examined child growth, diarrhoeal morbidity and the haematologic and micronutrient statuses16–23. PZ and MNP supplementation significantly increased the plasma zinc concentration compared with the control and TZ groups, but there was no impact on growth or overall diarrhoea burden19. Interestingly, the previous sub-study on the immune response found that zinc supplementation, especially PZ supplementation, decreased lymphocyte and eosinophil concentrations, although there was no effect on cytokine concentrations or T cell levels17. However, the humoral antibody response of this trial has not yet been reported.
This report represents a sub-study to investigate the impact of zinc supplementation on antibody production against pathogenic E. coli assayed in surplus aliquots of plasma samples from the parent trial in Laotian children. Plasma IgG levels and the avidity against pathogenic E. coli were quantified by enzyme-linked immunosorbent assay (ELISA) and analysed with respect to the zinc status at baseline. TZ, MNP and PZ could increase the plasma IgG level, but only PZ could improve the avidity of anti-E. coli antibodies.
Results
Demographic characteristics, zinc status and complete blood count (CBC) data
At baseline, the mean ± standard deviation (SD) age of children was 15.1 ± 5.4 months and 56.5% of them were male (Table 1). The mean ± SD plasma zinc concentration was 56.1 ± 12.7 μg/dL, with 78.5% zinc deficient based on the cut-off of 65 μg/dL24. The mean ± SD haemoglobin (Hb) concentration was 11.1 ± 0.9 g/dL with 40.0% of children at < 11 g/dL (lower limit of normal Hb in children aged 0.5–4 years25), suggesting that they had anaemia prior to the intervention. According to the Nakhonphanom Hospital’s reference ranges, 2.5% of children had increased white blood cells (range 4–11 × 103 cells/µL) with increased percentages of neutrophils (range 50–70%), lymphocytes (range 20–40%), monocytes (range 0–7%), basophils (range 0–1%) and eosinophils (range 0–1%), indicating inflammation or infection.
Table 1.
Baseline characteristics of the total study population (All) and each intervention group.
| Characteristic | All (n = 200) | Control (n = 50) | TZ (n = 50) | MNP (n = 50) | PZ (n = 50) |
|---|---|---|---|---|---|
| Age, month(s) | 15.1 ± 5.4 | 14.4 ± 5.7 | 15.2 ± 5.2 | 15.5 ± 5.3 | 15.3 ± 5.4 |
| Males | 56.5 (113) | 62.0 (31) | 46.0 (23) | 58.0 (29) | 61.2 (30) |
| Plasma zinc, µg/dL | 56.1 ± 12.7 | 56.1 ± 12.3 | 57.6 ± 12.4 | 54.3 ± 10.0 | 56.5 ± 15.7 |
| Hb, g/dL | 11.1 ± 0.9 | 11.1 ± 0.8 | 11.1 ± 0.9 | 11.1 ± 0.8 | 11.1 ± 0.9 |
| White blood cells, × 103/µL | 11.4 ± 3.2 | 11.5 ± 3.2 | 11.2 ± 3.2 | 11.4 ± 2.7 | 11.4 ± 3.6 |
| Neutrophils, % | 27.8 ± 11.8 | 28.0 ± 12.1 | 28.3 ± 11.9 | 27.6 ± 11.5 | 27.1 ± 11.8 |
| Lymphocytes, % | 55.9 ± 13.2 | 57.6 ± 13.2 | 55.2 ± 13.1 | 55.0 ± 14.0 | 55.9 ± 12.8 |
| Monocytes, % | 5.8 ± 3.8 | 5.8 ± 3.2 | 5.9 ± 3.7 | 5.4 ± 3.1 | 6.3 ± 5.1 |
| Eosinophils, % | 4.0 ± 4.2 | 3.5 ± 3.6 | 4.0 ± 3.4 | 4.0 ± 3.7 | 4.6 ± 5.7 |
| Basophils, % | 0.4 ± 0.6 | 0.3 ± 0.4 | 0.5 ± 0.7 | 0.3 ± 0.4 | 0.5 ± 0.7 |
The values are the mean ± standard deviation or % (n).
Control, placebo; TZ therapeutic dispersible zinc tablets, MNP daily multiple micronutrient powder, PZ daily preventive zinc tablets, Hb haemoglobin.
Plasma IgG levels in response to pathogenic E. coli
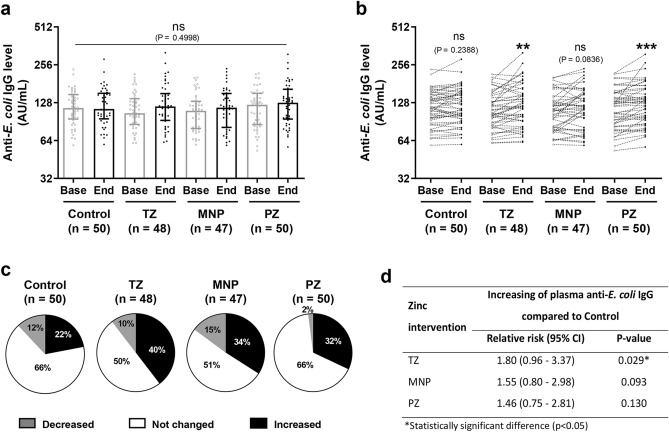
This sub-study was part of a randomised controlled trial and the acute diarrhoea incidence was low (~ 0.6 episodes per 100 days at risk) and similar among the four intervention groups during the study period20. Thus, the chance of infection was assumed to be equal across the intervention groups. To examine the effect of zinc intervention on antibody response, the plasma IgG level was determined against pathogenic E. coli. Data from all samples were analysed for outliners in the anti-E. coli IgG level at baseline. Two samples from the TZ group and three samples from the MNP group were excluded (Supplementary Figure S1). Comparison of plasma anti-E. coli IgG levels from all samples showed no significant differences among the groups (P = 0.4998; Fig. 1a and Supplementary Table S1). However, paired-sample analysis revealed significantly increased anti-E. coli IgG levels from baseline to endline in the TZ and PZ groups (Fig. 1b). While supplementation with MNP showed a trend for an increase, it did not reach statistical significance (P = 0.0863).
Figure 1.
Plasma IgG levels in response to pathogenic Escherichia coli of the four intervention groups. (a) Plasma samples from baseline and endline of each intervention group (Control, n = 50; TZ, therapeutic zinc with diarrhoea, n = 48; MNP, zinc-containing micronutrient powder, n = 47; PZ, preventive zinc, n = 50) were used to determine the anti-E. coli IgG levels. The bars represent the median with the interquartile range of each group and each dot represents an individual sample. The P-value was determined with the Kruskal–Wallis test. (b) Changes in the antibody level in each individual. The P-values indicate significant differences between baseline and endline of each intervention group determined by the Wilcoxon matched-pairs signed rank test (**P < 0.01; ***P < 0.001; ns, non-significant). (c) The pie charts show the percentages of children who showed no change or an increase or decrease of at least 10% in antibody levels at endline compared with baseline for each group. (d) Chi-square analysis of the relative risk and P-values of increasing antibody levels for each intervention group compared with the Control group.
For each group, the percentages of children who showed changes in their anti-E. coli IgG levels (an increase or decrease of at least 10% when comparing between baseline and endline) were calculated and are presented in pie charts (Fig. 1c). After the 9-month intervention, more than 30% of children who had received zinc supplementation had increased IgG levels—40%, 34% and 32% of children in TZ, MNP, and PZ groups, respectively – while 22% of children in the Control group had increased IgG levels (Fig. 1c and Supplementary Table S2). The TZ group showed the highest percentage of increased IgG levels while the PZ group showed the lowest percentage of children with decreased IgG levels (2%). However, the Control group showed the lowest percentage of increased IgG. These findings are consistent with the fact that TZ, MNP and PZ were the only groups with significantly increased plasma IgG levels at endline compared with baseline. Statistical analysis of the proportion of samples with increased antibody levels revealed a significant different between the TZ and Control groups (chi-square test, P = 0.029) and a relative risk of 1.80 (Fig. 1d). Moreover, the relative risk for increased antibody levels was 1.55 for MNP and 1.46 for PZ.
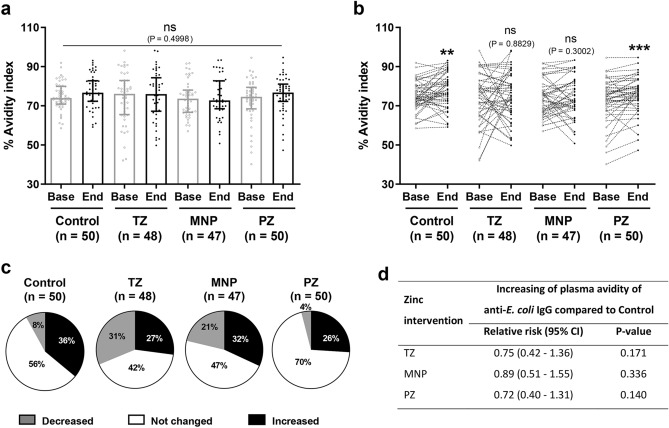
Changes in the avidity index in response to pathogenic E. coli
Changes in the avidity index were determined as maturation predictors of the antibody response to pathogenic E. coli. Similarly to the antibody levels, the overall avidity index was not different among the groups (P = 0.4998; Fig. 2a). Analysis of paired samples within each group revealed that the avidity index had increased significantly in the Control and PZ groups at endline compared with baseline (Fig. 2b). However, only the PZ group showed a significant increase in both the IgG level and avidity index at endline compared with baseline (Figs. 1b and 2b). For each group, the percentages of children who showed a change in the avidity index (increase or decrease of at least 5% when comparing between endline and baseline) were calculated and are presented in pie chart (Fig. 2c and Supplementary Table S3). The Control group showed 36% of children had an increase in the avidity index and 8% had a decrease in the avidity index. In the PZ group, there were fewer children who show a decrease in the avidity index (4%). There were high percentages of children in the TZ and MNP groups with an increased avidity index: 31% and 21%, respectively. Overall, most children had no changes in their IgG levels and avidity index after the 9-month intervention (Fig. 2b,c). Statistical analysis of the proportion of samples with increased avidity showed no significant difference in any of the treatment groups compared with the Control group, with a relative risk at 0.75 for the TZ group, 0.89 for the MNP group and 0.72 for the PZ group (Fig. 2d).
Figure 2.
Changes in the avidity index of children among the four intervention groups. (a) Plasma samples from baseline and endline of each intervention group (Control, n = 50; TZ, therapeutic zinc with diarrhoea, n = 50; MNP, zinc-containing micronutrient powder, n = 50; PZ, preventive zinc, n = 50) were used to determine the avidity index. Bars represent the median with the interquartile range of each group; each dot represents an individual sample. The P-value was determined with the Kruskal–Wallis test. (b) Changes in the avidity index of each individual. The P-values indicate significant differences between baseline and endline of each intervention group determined by the Wilcoxon matched-pairs signed rank test (**P < 0.01; ***P < 0.001; ns non-significant). (c) The pie charts show the percentages of children who showed no change or an increase or decrease of at least 5% in the avidity index at endline compared with baseline for each group. (d) Chi-square analysis of relative risk and P-values of increasing avidity from each intervention group compared with the Control group.
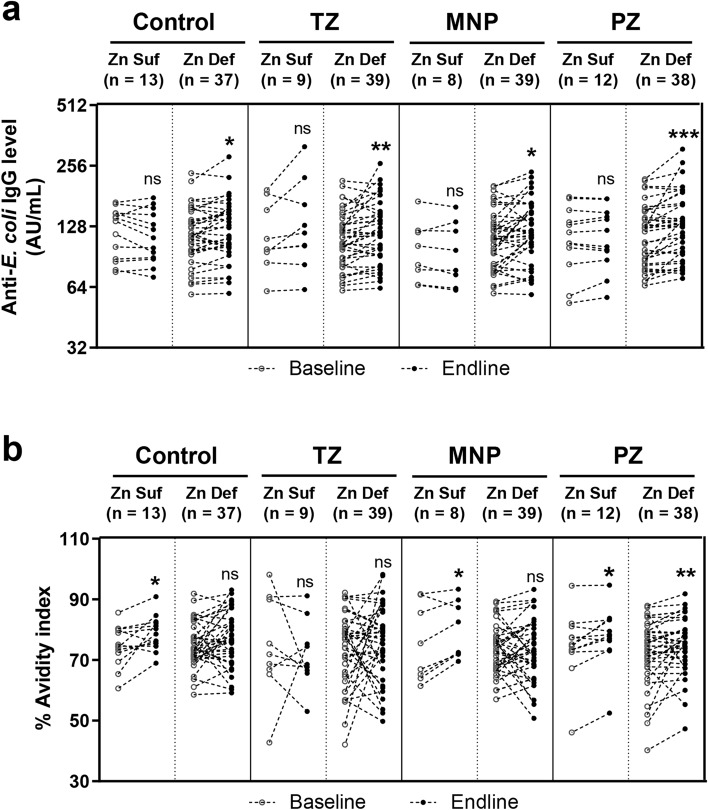
Plasma IgG levels and changes in the avidity index in zinc-sufficient or zinc-deficient children
More than 79% of children in this study were zinc deficient and the effect of zinc supplementation might be more pronounced in zinc-deficient children. Children in each intervention group were divided into zinc-sufficient and zinc-deficient sub-groups based on the plasma zinc cut-off of 65 μg/dL24. At endline, in all intervention groups there were significantly increased IgG levels in children who were zinc deficient. Only zinc-sufficient children in the TZ group showed significantly increased IgG levels (Fig. 3a). Zinc-sufficient children in the Control, MNP and PZ groups showed a significant increase in the avidity index. Only zinc-deficient children in the PZ group showed a significant increase in the avidity index (Fig. 3b). Interestingly, only zinc-deficient children in the PZ group showed significant increases in IgG levels and the avidity index.
Figure 3.
The plasma IgG level and avidity index of the four intervention groups divided by the baseline zinc status. For each group, the (a) plasma IgG level and (b) avidity index were divided into zinc-sufficient and zinc-deficient sub-groups (C control; TZ therapeutic zinc with diarrhoea; MNP zinc-containing micronutrient powder; PZ preventive zinc). Children who had baseline plasma zinc lower than 65 μg/dL were categorised in the zinc-deficient sub-group. The sample sizes are indicated above each graph. Each dot represents an individual sample. Zn Suf zinc sufficient group; Zn Def zinc deficient group. The P-values indicate significant differences between baseline and endline of each intervention group determined by the Wilcoxon matched-pairs signed rank test. *P < 0.05; **P < 0.01; ***P < 0.001; ns non-significant.
Discussion
The present study determined the IgG levels and avidity index (as a measure of the responsiveness of antibody maturation) of children who received different interventions of zinc supplementation. The different zinc supplementation regimens affected these endpoints. It was especially notable among zinc-deficient children given PZ supplementation, who showed increases in their IgG levels and avidity index. This sub-study of antibody response focussed on the two daily zinc supplementation regimens, PZ and MNP, which both increased plasma zinc and decreased the prevalence of zinc deficiency over the course of the study, as was also reported by the parent study using a slightly larger sample size19.
Although zinc supplementation had no effect on cytokine concentrations or T cell counts, interestingly our previous sub-study of immune functions showed that the PZ regimen, given daily as a 7-mg supplement, increased the number of total lymphocytes (perhaps B cells), suggesting further examination of the effect of zinc intervention on these cell subsets or the humoral response to zinc supplementation17. Zinc deficiency reduces the B cell response and T cell–dependent antibody responses of B cells3. Hence, it makes sense that the children who were zinc deficient at baseline in the PZ group had increases in both IgG levels and the avidity index after the 9-month intervention. From our published data of the parent study at endline, there was a significantly lower prevalence of zinc deficiency in the PZ (60%) and the MNP (67%) groups compared with the TZ (85%) and Control (85%) groups19. Moreover, the acute diarrhoea incidence was low and similar among the four intervention groups. A diarrhoea episode lasted for about 2 days, with no group-wise differences in the diarrhoeal duration20. These data imply that the results of this study are due to the treatments rather than the exposure to the pathogen. Taken together, these results suggest that intervention by daily preventive zinc tablets is able to increase plasma zinc concentrations and enhance antibody maturation in response to pathogenic bacteria that cause diarrhoea.
In previous studies of the cholera vaccine, children with malnutrition received zinc supplementation for 1 month after vaccination with oral cholera vaccine; they presented enhanced seroconversion of vibriocidal antibodies26,27 and cell-mediated immunity in response to cholera27. Moreover, a 14-day zinc supplementation during acute shigellosis in children with moderate malnutrition significantly increased antigen-specific antibody levels and lymphocyte proliferation28. Furthermore, zinc supplementation as an adjunctive therapy during acute shigellosis significantly improved seroconversion of shigellacidal antibodies and enhanced the number of circulating B lymphocyte subpopulations and plasma cells29. These observations are consistent with our results, indicating that daily zinc tablet supplementation improves antibody response and maturation against pathogenic E. coli, particularly in zinc-deficient children.
Both the PZ and Control groups showed an increase in the avidity index. Given that avidity maturation is based on hyper-mutation and clonal selection of B cells, maintained and optimal availability of antigen seems to be necessary for complete affinity maturation30–32. Lowering the antigenic load through treatment of HIV or tuberculosis infections leads to incomplete avidity maturation33,34. However, there were no differences in diarrhoea duration among the four supplementation groups20. There are two factors that could affect avidity besides zinc supplementation. (1) Previous infections or exposure (including the number of infections and diarrhoeal duration) of each child, especially to E. coli, before being enrolled to the parent study could have affected avidity. (2) Confirmatory bacterial culture from children who had diarrhoea was not performed in the study. The children might be infected with other enteropathogens including Shigella spp., Salmonella spp., Vibrio cholerae and Campylobacter spp.10. These factors should be considered for future studies. Moreover, the possible reason that some children showed a decrease in the avidity index could be related to E. coli exposure during the 9-month intervention. These children might have developed different or new B cell clones35, leading to a reduction in avidity in response to intact E. coli at endline. However, all supplementation groups had a similar incidence of diarrhoea and the chance of bacterial exposure was assumed to be similar among the groups.
The present study includes samples from an immunology sub-study and the main study, which enrolled a large number of children in a population with a high prevalence of zinc deficiency and close proximity to laboratory facilities suitable for analysis of immune function. Although the outcome of the trial is that a majority of the participants remained zinc deficient at the endline, this present study suggests that taking zinc tablets daily might be the optimal regimen for zinc supplementation based on the increased plasma zinc concentration and enhanced antibody response.
Methods
Study design and recruitment
This study used surplus aliquots of plasma samples from a previous sub-study of immune function17 nested within a community-based randomised controlled trial examining the optimal zinc supplementation strategy for improving growth, diarrhoeal morbidity and the haematologic and micronutrient statuses in rural Laotian children at risk of zinc deficiency among 3407 children aged 6–23 months16. The full study design, method and rationale for the main randomised controlled trial are described by Wessells et al.16. Briefly, the previous sub-study aimed to determine the effect of zinc supplementation on immune function in young Laotian children in Khammouane Province17. At baseline, non-fasting blood samples of children aged 6–23 months were received at Nakonphanom Hospital, and CBC analysis was performed. Approximately 82% of participants were re-examined at endline after 9 months. The previous analysis of the sub-study included a total of 512 children for whom we had both baseline and endline data. This present study included 200 samples, with 50 samples randomly selected from each of the four intervention groups. These samples had an adequate amount of plasma remaining for the assays performed in the present study.
Children in the parent study were randomised to four intervention groups: (1) daily placebo (Control) powders as the negative control group; (2) 10 days of therapeutic dispersible zinc (TZ) tablets (containing 20 mg zinc) for episodes of diarrhoea; (3) daily preventive multiple micronutrient powders (MNP; containing 10 mg zinc, 6 mg iron, and 13 other vitamin and minerals/day); or (4) daily preventive zinc (PZ) supplements (7 mg zinc/day). The supplementation regimen for the preventive supplements (PZ and MNP) was given daily for 9 months, and caregivers were encouraged to provide therapeutic supplements (TZ) along with ORS starting on the first day of diarrhoea for 10 day for episodes of diarrhoea. According to the intervention protocol16, the supplements were given either with or without food.
The trial was approved by the National Ethics Committee for Health Research, Ministry of Health, Lao PDR (NECHR; 040/2014, 069/2015, 039/2016); the University of California, Davis Institutional Review Board (IRB; 626187); and the Khon Kaen University (Thailand) Ethics Committee in Health Research (HE642232). All experiments were performed in accordance with the relevant named guidelines and regulations. Informed consent was obtained from their legal guardians.
Blood collection and transport
At baseline and endline, venous blood was collected into 7.5-mL lithium heparin tubes (Sarstedt AG & Co; ref. 01.1604.400) for immune assays and plasma zinc measurement, and into a 1.2-mL EDTA tubes (Sarstedt; ref.06.1666.100) for CBC analysis. Plasma separated from 2-mL aliquots of heparinised whole blood was stored at −20 °C prior to laboratory analyses.
CBC with a five-part differential
This analysis was performed following Nakhonphanom Hospital laboratory protocols as stated previously7.
Plasma zinc analysis
Plasma zinc was analysed by inductively coupled plasma optical emission spectrophotometry (5100 ICP-OES SVDV, Agilent, Santa Clara, CA, USA) at the Children’s Hospital of Oakland Research Institute as described previously36.
IgG antibody measurement
Plasma IgG was detected by ELISA as described by Capelli et al.37 Paraformaldehyde-fixed intact pathogenic E. coli clinical strain, isolated from clinical samples of 10-month-old girl who had been admitted to Nakhonphanom Hospital, Thailand, was used to coat 96-well polystyrene plate (Nunc, Denmark) at 106 colony-forming units (CFU) with carbonate bicarbonate buffer (pH 9.6) overnight at 4 °C. The plates were washed with washing buffer, 0.1% Tween-20 in phosphate-buffered saline (PBS, pH 7.4), before blocking with 10% foetal bovine serum (FBS) in PBS at room temperature for 2 h. Heparinised plasma samples were diluted at 1:100 with assay diluent (10% FBS in PBS with 0.05% Tween-20). The solution was removed from the pre-coated plates before adding diluted plasma samples and incubated at room temperature for 2 h. After washing, a detection antibody mixture [0.1 μg/mL biotinylated anti-human IgG (BD Biosciences, Franklin Lakes, NJ, USA)], 1:1000 sAv-HRP (BD Biosciences) diluted in assay diluent) was added and incubated for 2 h at room temperature. After washing seven times, 3,3',5,5'-tetramethylbenzidine (TMB) substrate (BD Biosciences) was added and incubated at room temperature for 10 min, before stopping the reaction with 2 N H2SO4. The absorbance of each well was read at 490 nm by an ELISA reader. The results are shown as AU/mL calculated based on a standard curve of known concentrations of purified human IgG standard38,39. The standard curve and quality control chart of each experiment are shown in Supplementary Fig. S2.
The modified protocol to measure the avidity index of plasma IgG was done by duplicate measurements. The protocol involved adding 7 M urea to the samples and incubating them for 1 h prior to the measurement40,41. The results are presented as the avidity index (a percentage) calculated with the following formula:
Statistical analysis
The normality of the data in each intervention group was tested by the Anderson–Darling test and re-checked for outliners by using a normal Q-Q plot. Outliers at baseline were identified and excluded from the data analysis by using the ROUT method with Q = 10%, the most aggressive setting to remove likely outliners (Supplementary Fig. S1). Differences between the interventions groups were tested with the non-parametric Kruskal–Wallis test. Differences between baseline and endline of each intervention group were tested with the Wilcoxon matched-pairs signed rank test. All analysis was performed using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). P ≤ 0.05 was considered to be significant. The power of each test was calculated by post-hoc power analysis with 95% confidence interval and > 80% was acceptable for all experiments.
Supplementary Information
Acknowledgements
We wish to acknowledge the help of Chidchanok Promkong, Head of the Department of Medical Technology and Pathology; Inthira Tussakhon; and all members at Nakhonphanom Hospital, Thailand, for facilitating our project. We also thank all members of the Cellular and Molecular Immunology Unit, CMDL, Faculty of AMS, Khon Kaen University. We thank Charles Arnold for his suggestions on statistical analysis. The Lao ZincStudy was funded by a grant from Nutrition International co-funded with the Mathile Institute for the Advancement of Human Nutrition (grant number 10-1347-UCALIF-07; S. Hess, PI) and the Bill & Melinda Gates Foundation (grant number OPP1134272; S. Hess, PI). The immune study was supported by USDA-ARS Project Number 2032-53000-001-00-D (C. Stephensen, PI).
Abbreviations
- CBC
Complete blood count
- Hb
Haemoglobin
- MNP
Daily multiple micronutrient powder
- PZ
Daily preventive zinc tablets
- TZ
Therapeutic dispersible zinc tablets
Author contributions
C.K. and G.L. performed conceptualisation; C.K., M.M.S. and A.N. curated the data; A.N., C.K. and M.M.S. performed formal analysis; G.H., M.A.B., C.K., M.M.S., A.N. and A.K. performed the investigation; G.L., A.N. and C.K. provided methodology; C.B.S., G.L., S.K. and S.Y.H. were project administrators; C.B.S., G.L., C.K. and S.Y.H. supervised the project; C.K. and M.M.S wrote the first draft; C.B.S., G.L., K.R.W. and S.Y.H reviewed and edited the manuscript. All authors reviewed the manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The spouse of SYH worked for the Bill & Melinda Gates Foundation, which provided part of the financial support. The other authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chidchamai Kewcharoenwong and Myint Myint Sein.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-20445-8.
References
- 1.Wu D, Lewis ED, Pae M, Meydani SN. Nutritional modulation of immune function: Analysis of evidence, mechanisms, and clinical relevance. Front. Immunol. 2018;9:3160. doi: 10.3389/fimmu.2018.03160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maywald M, Wessels I, Rink L. Zinc signals and immunity. Int. J. Mol. Sci. 2017 doi: 10.3390/ijms18102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hojyo S, Fukada T. Roles of zinc signaling in the immune system. J. Immunol. Res. 2016;2016:6762343. doi: 10.1155/2016/6762343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allman D, Pillai S. Peripheral B cell subsets. Curr. Opin. Immunol. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hojyo S, et al. Zinc transporter SLC39A10/ZIP10 controls humoral immunity by modulating B-cell receptor signal strength. Proc. Natl. Acad. Sci. USA. 2014;111:11786–11791. doi: 10.1073/pnas.1323557111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE. 2012;7:e50568. doi: 10.1371/journal.pone.0050568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown KH, Peerson JM, Baker SK, Hess SY. Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food Nutr. Bull. 2009;30:S12–40. doi: 10.1177/15648265090301S103. [DOI] [PubMed] [Google Scholar]
- 8.Mayo-Wilson E, Imdad A, Junior J, Dean S, Bhutta ZA. Preventive zinc supplementation for children, and the effect of additional iron: A systematic review and meta-analysis. BMJ Open. 2014;4:e004647. doi: 10.1136/bmjopen-2013-004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. Diarrhoeal Disease. https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (2017).
- 10.Abba K, Sinfield R, Hart CA, Garner P. Pathogens associated with persistent diarrhoea in children in low and middle income countries: Systematic review. BMC Infect. Dis. 2009;9:88. doi: 10.1186/1471-2334-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarmin M, et al. Predictor of death in diarrheal children under 5 years of age having severe sepsis in an urban critical care ward in Bangladesh. Glob. Pediatr. Health. 2019;6:2333794X19862716. doi: 10.1177/2333794X19862716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Frey E, Mackenzie AM, Finlay BB. Human response to Escherichia coli O157:H7 infection: Antibodies to secreted virulence factors. Infect. Immun. 2000;68:5090–5095. doi: 10.1128/IAI.68.9.5090-5095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giuntini S, et al. Identification and characterization of human monoclonal antibodies for immunoprophylaxis against enterotoxigenic Escherichia coli infection. Infect. Immun. 2018 doi: 10.1128/IAI.00355-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alam MM, et al. Antigen-specific memory B-cell responses to enterotoxigenic Escherichia coli infection in Bangladeshi adults. PLoS Negl. Trop. Dis. 2014;8:e2822. doi: 10.1371/journal.pntd.0002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alam MM, et al. Antibody avidity in humoral immune responses in Bangladeshi children and adults following administration of an oral killed cholera vaccine. Clin. Vaccine Immunol. 2013;20:1541–1548. doi: 10.1128/CVI.00341-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wessells KR, et al. Comparison of two forms of daily preventive zinc supplementation versus therapeutic zinc supplementation for diarrhea on young children's physical growth and risk of infection: Study design and rationale for a randomized controlled trial. BMC Nutr. 2018;4:39. doi: 10.1186/s40795-018-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kewcharoenwong C, et al. Daily preventive zinc supplementation decreases lymphocyte and eosinophil concentrations in rural Laotian children from communities with a high prevalence of zinc deficiency: Results of a randomized controlled trial. J. Nutr. 2020;150:2204–2213. doi: 10.1093/jn/nxaa037. [DOI] [PubMed] [Google Scholar]
- 18.Wessells KR, et al. Impact of daily preventive zinc or therapeutic zinc supplementation for diarrhea on plasma biomarkers of environmental enteric dysfunction among rural laotian children: A randomized controlled trial. Am. J. Trop. Med. Hyg. 2020;102:415–426. doi: 10.4269/ajtmh.19-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barffour MA, et al. Effects of daily zinc, daily multiple micronutrient powder, or therapeutic zinc supplementation for diarrhea prevention on physical growth, anemia, and micronutrient status in rural Laotian children: A randomized controlled trial. J. Pediatr. 2019;207:80–89e82. doi: 10.1016/j.jpeds.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barffour MA, et al. Effects of therapeutic zinc supplementation for diarrhea and two preventive zinc supplementation regimens on the incidence and duration of diarrhea and acute respiratory tract infections in rural Laotian children: A randomized controlled trial. J. Glob. Health. 2020;10:010424. doi: 10.7189/jogh.10.010424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hess SY, et al. Iron status and inherited haemoglobin disorders modify the effects of micronutrient powders on linear growth and morbidity among young Lao children in a double-blind randomised trial. Br. J. Nutr. 2019;122:895–909. doi: 10.1017/S0007114519001715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinnouho GM, et al. Impact of Two forms of daily preventive zinc or therapeutic zinc supplementation for diarrhea on hair cortisol concentrations among rural Laotian children: A randomized controlled trial. Nutrients. 2018 doi: 10.3390/nu11010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinnouho GM, et al. Impact of different strategies for delivering supplemental zinc on selected fecal markers of environmental enteric dysfunction among young Laotian children: A randomized controlled trial. Am. J. Trop. Med. Hyg. 2020;103:1416–1426. doi: 10.4269/ajtmh.20-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Zinc Nutrition Consultative, G. et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull.25, S99–S203 (2004). [PubMed]
- 25.Garvin, J. H. Principles of Gender-Specific Medicine. 2nd edn. (ed. Legato, M. J.). 51–61 (Academic Press, 2010).
- 26.Karlsen TH, et al. Intestinal and systemic immune responses to an oral cholera toxoid B subunit whole-cell vaccine administered during zinc supplementation. Infect. Immun. 2003;71:3909–3913. doi: 10.1128/IAI.71.7.3909-3913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albert MJ, et al. Supplementation with zinc, but not vitamin A, improves seroconversion to vibriocidal antibody in children given an oral cholera vaccine. J. Infect. Dis. 2003;187:909–913. doi: 10.1086/368132. [DOI] [PubMed] [Google Scholar]
- 28.Raqib R, et al. Effect of zinc supplementation on immune and inflammatory responses in pediatric patients with shigellosis. Am. J. Clin. Nutr. 2004;79:444–450. doi: 10.1093/ajcn/79.3.444. [DOI] [PubMed] [Google Scholar]
- 29.Rahman MJ, et al. Effects of zinc supplementation as adjunct therapy on the systemic immune responses in shigellosis. Am. J. Clin. Nutr. 2005;81:495–502. doi: 10.1093/ajcn.81.2.495. [DOI] [PubMed] [Google Scholar]
- 30.Kontio M, Jokinen S, Paunio M, Peltola H, Davidkin I. Waning antibody levels and avidity: implications for MMR vaccine-induced protection. J. Infect. Dis. 2012;206:1542–1548. doi: 10.1093/infdis/jis568. [DOI] [PubMed] [Google Scholar]
- 31.Brokstad KA, Cox RJ, Major D, Wood JM, Haaheim LR. Cross-reaction but no avidity change of the serum antibody response after influenza vaccination. Vaccine. 1995;13:1522–1528. doi: 10.1016/0264-410x(95)00095-i. [DOI] [PubMed] [Google Scholar]
- 32.Struck F, et al. Incomplete IgG avidity maturation after seasonal coronavirus infections. J. Med. Virol. 2022;94:186–196. doi: 10.1002/jmv.27291. [DOI] [PubMed] [Google Scholar]
- 33.Re MC, et al. Incomplete IgG response to HIV-1 proteins and low avidity levels in recently converted HIV patients treated with early antiretroviral therapy. Int. J. Infect. Dis. 2010;14:e1008–1012. doi: 10.1016/j.ijid.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Arias-Bouda LM, et al. Changes in avidity and level of immunoglobulin G antibodies to Mycobacterium tuberculosis in sera of patients undergoing treatment for pulmonary tuberculosis. Clin. Diagn. Lab. Immunol. 2003;10:702–709. doi: 10.1128/cdli.10.4.702-709.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viant C, et al. Antibody affinity shapes the choice between memory and germinal center B cell fates. Cell. 2020;183:1298–1311.e11. doi: 10.1016/j.cell.2020.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Killilea DW, Ames BN. Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts. Proc. Natl. Acad. Sci. USA. 2008;105:5768–5773. doi: 10.1073/pnas.0712401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capelli R, et al. BPSL1626: Reverse and structural vaccinology reveal a novel candidate for vaccine design against Burkholderia pseudomallei. Antibodies (Basel) 2018 doi: 10.3390/antib7030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gourlay LJ, et al. Exploiting the Burkholderia pseudomallei acute phase antigen BPSL2765 for structure-based epitope discovery/design in structural vaccinology. Chem. Biol. 2013;20:1147–1156. doi: 10.1016/j.chembiol.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Nithichanon A, et al. Sequence- and structure-based immunoreactive epitope discovery for Burkholderia pseudomallei flagellin. PLoS Negl. Trop. Dis. 2015;9:e0003917. doi: 10.1371/journal.pntd.0003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saenwongsa W, et al. Metformin-induced suppression of IFN-alpha via mTORC1 signalling following seasonal vaccination is associated with impaired antibody responses in type 2 diabetes. Sci. Rep. 2020;10:3229. doi: 10.1038/s41598-020-60213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nithichanon A, et al. Boosting of post-exposure human T-cell and B-cell recall responses in vivo by Burkholderia pseudomallei-related proteins. Immunology. 2017;151:98–109. doi: 10.1111/imm.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.