Summary
Trogocytosis is a cellular process whereby a cell acquires a membrane fragment from a donor cell in a contact-dependent manner allowing for the transfer of surface proteins with functional integrity. It is involved in various biological processes, including cell-cell communication, immune regulation, and response to pathogens and cancer cells, with poorly defined molecular mechanisms. With the exception of eosinophils, trogocytosis has been reported in most immune cells and plays diverse roles in the modulation of anti-tumor immune responses. Here, we report that eosinophils acquire membrane fragments from tumor cells early after contact through the CD11b/CD18 integrin complex. We discuss the impact of trogocytosis in innate immune cells on cancer progression in the context of the evidence that eosinophils can engage in trogocytosis with tumor cells. We also discuss shared and cell-specific mechanisms underlying this process based on in silico modeling and provide a hypothetical molecular model for the stabilization of the immunological synapse operating in granulocytes and possibly other innate immune cells that enables trogocytosis.
Subject areas: Biophysics, cancer, cell biology, immunology
Graphical abstract
Highlights
-
•
Trogocytosis in innate immune cells can regulate immune responses to cancer
-
•
Eosinophils engage in trogocytosis with tumor cells via CD11b/CD18 integrin complex
-
•
CD11b/CD18 integrin, focal adhesion molecules and actin network enable trogocytosis
Biophysics; Cancer; Cell biology; Immunology
Introduction
Trogocytosis is a biological process whereby a cell (receiving cell) nibbles membrane fragments from another cell (donor cell) and subsequently to cell-cell contact. This bite-and-detach event has been first reported in the Naegleria fowleri ameba, where this eukaryotic microorganism exploits trogocytosis to bite rat neuroblastoma cells, resulting in a markedly compromised nervous system in hosts (Marciano-Cabral et al., 1990). Here, transmission electron microphotography showed that N. fowleri amebae can engage nerve cells via strict membrane-to-membrane contact, and radiolabeling experiments demonstrated the transfer of little membrane pieces from B013 rat neuroblastoma cell to the receiving microorganism causing nerve cell injury. After about a decade, this phenomenon was coined as trans-endocytosis to correctly define the passage of material from a donor cell to a receiving one (Klueg and Muskavitch, 1999). However, the definition trogocytosis (from Greek trogo = nibble) gradually became the most common term to describe these sequential biological events. It is now well established that trogocytosis is a process largely diffused in and between species, although the biological impact, as well as the molecular basis behind this complex event, are still under investigation.
In the cells of the immune system, the first hint of trogocytosis dates back to 1972, when Cone et al. noted the presence of allogeneic MHC class II molecules on adoptively transferred T cells (Cone et al., 1972). This observation was confirmed by subsequent studies but the mechanism of molecule transfer was only described in the early 2000s (Hudrisier and Bongrand, 2002; Joly and Hudrisier, 2003). To date, trogocytosis has been reported in many immune cell types, including T and B lymphocytes, natural killer (NK) cells, macrophages and monocytes, dendritic cells (DCs), neutrophils and basophils (Miyake and Karasuyama, 2021), whereas it has never been described in eosinophils. In general, trogocytosis is an active process that requires actin cytoskeleton remodeling and, in the majority of immune cells, is triggered by receptor-ligand interaction. For example, T cells are able to engage in trogocytosis with antigen-presenting cells (APCs) during the interaction of the T cell receptor (TCR) with MHC molecules restricted by antigen-derived peptides. This event requires the formation of an immunological synapse (IS) that allows the internalization into T cells of peptide-MHC (pMHC) complexes and adjacent membrane patches that include co-stimulatory (i.e., CD80, CD86, OX40L) inhibitory (PD-L1) and adhesion (ICAM-1) molecules (Reed et al., 2021). The molecules transferred via trogocytosis are functional and influence the response of the receiving cell, thus implying consequences for the modulation of immune responses. Therefore, trogocytosis represents an important process for cell-cell communication, immune regulation, and response to pathogens and cancerous cells (Li et al., 2021; Miyake and Karasuyama, 2021).
In the tumor microenvironment (TME), malignant cells may exploit trogocytosis as a mechanism to escape immunity. This may occur by the removal of tumor antigens from cancer cells that reduce “immunovisibility” or by the replacement of stimulatory with inhibitory molecules on immune effector cells that limit immune surveillance (Zhao et al., 2022). On the other hand, trogocytosis can be a strategy that immune cells adopt to limit cancer expansion. By hijacking protein antigens from tumor cell membrane immune cells may acquire enhanced anti-tumor function (Lu et al., 2021; Machlenkin et al., 2008; Miyake and Karasuyama, 2021). In addition, as we will discuss, innate immune cells may employ prolonged trogocytosis to induce tumor cell death (Matlung et al., 2018; Velmurugan et al., 2016). The contact between different types of immune cells via trogocytosis is a phenomenon also occurring in the TME as a strategy to modulate the function of immune cells, thus indirectly affecting cancer progression (Li et al., 2021). In this respect, trogocytosis can be regarded as a double-edge sword in cancer immunity involved in both activation and repression pathways of immune responses to cancer. In this study, we report that eosinophils can engage in trogocytosis with several tumor cell types, providing evidence of trogocytosis in this granulocyte subset. We discuss our data in the context of trogocytosis in innate immune cells and the role of this biological process in the regulation of immune responses to cancer. Finally, we discuss the mechanisms underlying trogocytosis in innate immune cells, highlighting similarities and distinct traits among cell lineages, and propose a model for the stabilization of the IS.
Results and discussion
Eosinophils engage trogocytosis with cancer cells via CD18/CD11b-guided immunological synapse
Eosinophils are major actors in allergic and parasitic diseases. Moreover, eosinophils infiltrate a variety of solid cancers and play diverse roles in tumor progression (Varricchi et al., 2018). Human and mouse eosinophils express various integrins and adhesion molecules that allow direct interaction with their targets, including tumor cells (Barthel et al., 2008; Gatault et al., 2015; Mattei et al., 2020). Whether eosinophils can carry out trogocytosis following cell-cell contact is currently unknown. Our previous work showed that eosinophils activated with the cytokine IL-33 up-regulate adhesion (CD11b/CD18, ICAM-1) and activation (CD63, CD69) molecules resulting in tight binding to target tumor cells, formation of an IS, and tumor cell killing via degranulation (Andreone et al., 2019). We here report that, following direct contact with a variety of tumor cell lines, activation of eosinophils with IL-33 induces trogocytosis of membrane fragments from the target cells.
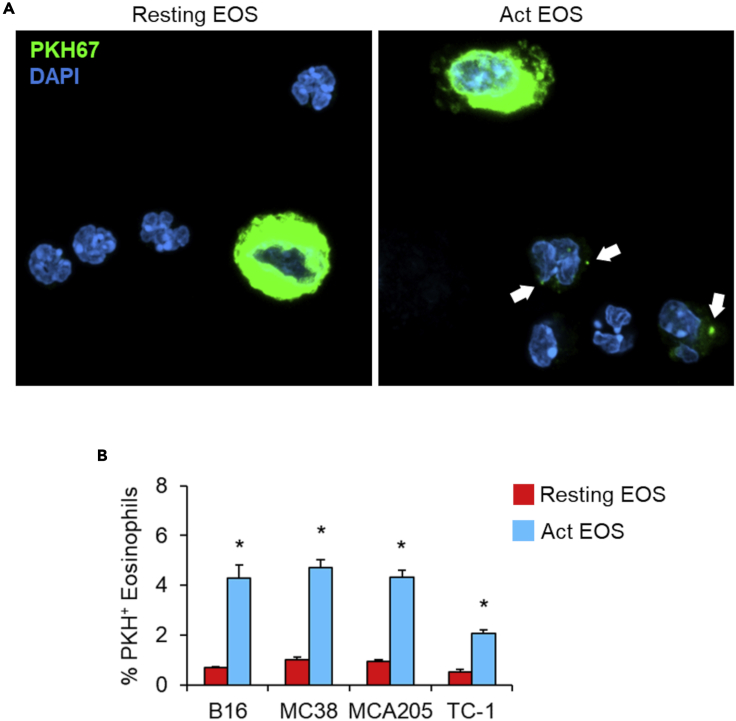
After 1-h co-culture with tumor cells labeled with the PKH membrane dye, activated, but not resting, eosinophils acquired the PKH fluorescent dye, as visualized by confocal microscopy (Figure 1A) and flow cytometry (Figure 1B). Time-lapse fluorescence microscopy revealed that trogocytosis by activated eosinophils occurs rapidly (within 2 min; Figure 2), as described for other innate immune cells. Of note, transmission electron microscopy of eosinophil and tumor cell conjugates showed the formation of typical protrusions (Figure 3), which resemble the tubular structures found in neutrophils during their trogocytic engagement with cancer cells (Matlung et al., 2018).
Figure 1.
Eosinophils engage in trogocytosis with cancer cells
(A) Resting and IL-33 activated bone marrow-derived murine eosinophils were co-cultured with PKH67-labeled EG7.OVA lymphoma cells (4:1 ratio) for 1 h at 37°C. DAPI stain allowed distinguishing multilobate eosinophil nuclei. Fluorescence images show the capture of PKH67 membrane dye by activated but not resting eosinophils. Bar = 10 μm. One representative experiment out of three is shown.
(B) Trogocytosis in resting vs activated eosinophils co-cultured (2:1 ratio) with the indicated tumor cell lines labeled with PKH26 dye was evaluated by flow cytometry. Data are expressed as mean values of culture triplicates +/− SD ∗p < 0.05. One-way ANOVA test. One representative experiment out of three is shown.
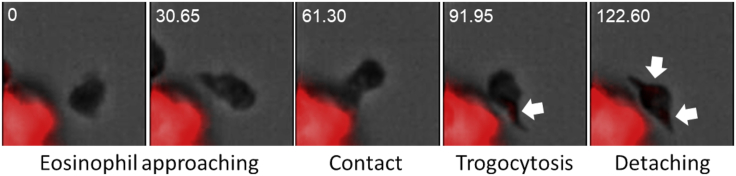
Figure 2.
Trogocytosis in eosinophils occurs early after contact with cancer cells
Time-lapse fluorescence microscopy images showing an in vivo trogocytic event between an eosinophil and an MC38 tumor cell (red). Arrows indicate the acquisition of red fluorescence in the eosinophil following cell contact and within 2 min. Numbers indicate seconds elapsed from the PKH26 labeling.
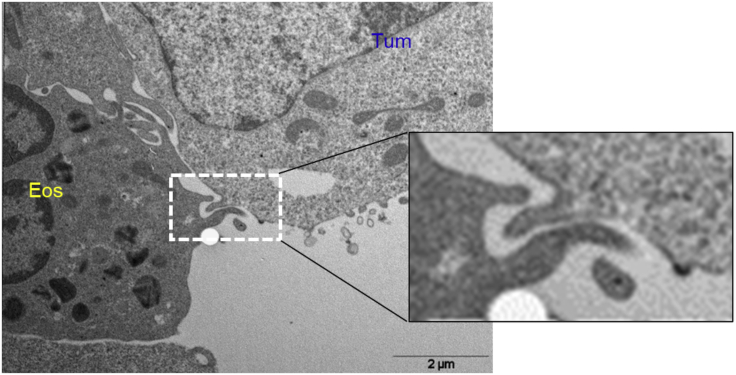
Figure 3.
Evidence of trogocytosis by transmission electronic microscopy
Ultrastructural analysis showing a trogocytic invagination at the activated eosinophil interface with a tumor cell (B16.F10 melanoma cell) after 1-h co-culture at 10:1 ratio. The right rectangle delineates a magnified image of the white dashed contour.
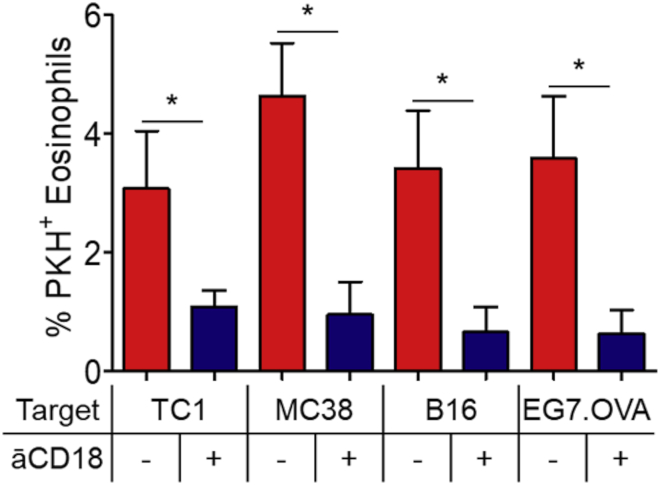
As we will discuss later in discussion, this is an early event preceding trogocytosis that, in granulocytes, implies the formation of a stable complex between membrane integrins and F-actin in the cytoplasmic region of the granulocyte via the intermediate actin linkers talin-2 and kindlin-3. Moreover, it has been demonstrated that kindlin-3, but not kindlin-1, is selectively expressed in hematopoietic cells and is central for the CD11b/CD18 integrin complex stability (Fagerholm et al., 2014). As the formation of conjugates between eosinophils and cancer cells is strictly dependent on CD11b/CD18-mediated adhesion (Andreone et al., 2019) and eosinophil trogocytosis is inhibited by CD11b/CD18 blockade (Figure 4), it is likely that eosinophils carry out adhesion molecule-dependent trogocytosis with a similar mechanism as neutrophils (Matlung et al., 2018). It remains to be established whether trogocytosis in eosinophils results in trogoptosis and, if this is the case, what is the contribution of this process to tumor killing.
Figure 4.
Trogocytosis in eosinophils is CD11b/CD18-dependent
IL-33 activated eosinophils were incubated with 10 μg/mL anti-CD18/CD11b blocking antibody for 25 min at 4°C prior to the co-culture with indicated PKH26-labelled tumor cells (4:1 ratio). Trogocytosis was evaluated by fluorescence microscopy. Data represent the mean percentages of trogocytic eosinophils of multiple fields (at least 400 cells/condition) +/− SD ∗p < 0.05. One-way ANOVA test. One representative experiment out of two is shown.
Lastly, the observation that trogocytosis is increased in activated eosinophils is in line with what was observed with activated NK cells (Caumartin et al., 2007; Hasim et al., 2022) and suggests a correlation between cell activation, adhesion, and trogocytosis.
Trogocytosis in innate immune cells is a double-edged sword in cancer immunity
Innate immune cells spatially and temporally interact with tumor cells, both systemically and locally in the TME (Costa et al., 2021). As we will discuss, trogocytosis between immune cells does not cause cell death and is generally employed as a means of immune regulation whereby cells acquire or down-regulate a certain function. Instead, trogocytosis between innate immune and cancer cells may ensue in cancer cell death, in a process termed trogoptosis. Furthermore, although trogocytosis is performed by most, if not all, cells of hemopoietic origin, the molecular pathways and mechanisms involved can differ depending on the lineage of the immune cell (see Table 1).
Table 1.
Trogocytosis in innate immune cells: role in the regulation of anti-tumor immune responses
| Cell type | Target cell | Acquired molecule | Donated molecule | Molecular mechanism | Outcome | References |
|---|---|---|---|---|---|---|
| NK | Various target cells | MHC-I | Ly49A; KIR | Ly49 A/H-2D; KIR2DL1/HLA-C; ILT2/HLA-G; ATP-dependent | Reduction in NK cell cytotoxic activity | (Carlin et al., 2001; Sjöström et al., 2001; Vanherberghen et al., 2004; Zimmer et al., 2001) |
| DCs; tumor cells | CCR7 | LFA-1 and CD2-mediated adhesion; prevented by KIR/HLA-class I interaction | Acquired NK cell migration to lymph nodes | (Marcenaro et al., 2009) | ||
| Tumor cells | Rae-1 | NKG2D | NK fratricide killing | (Nakamura et al., 2013) | ||
| Tumor cells | CD9 | NE | Impaired NK cell function | (Gonzalez et al., 2021) | ||
| Tumor cells | HER2; TYRO | NE | NK activation and degranulation; unaffected anti-tumor immunity | (Lu et al., 2021; Suzuki et al., 2015) | ||
| Tumor cells | PD-1 | SLAM receptors | NK activation and degranulation; reduced anti-tumor immunity | (Hasim et al., 2022) | ||
| DCs | APCs; tumor cells | pMHC-I | NE | Enhanced Ag presentation by cross-dressing | (Das Mohapatra et al., 2020; Zhang et al., 2008) | |
| Treg | CD70, CD80, CD86 | CTLA-4 mediated IS formation | Decreased APC function; increased expression of free PD-L1 | (Dhainaut et al., 2015; Gu et al., 2012; Tekguc et al., 2021) | ||
| CD8 T cells | PD-L1 | pMHC-I/TCR recognition | Fratricide CD8 T cell killing | (Gary et al., 2012) | ||
| CD8 T cells | pMHC-I | Phosphatidylserine/Tim-3 | Fratricide CD8 T cell killing | (Pagliano et al., 2022) | ||
| pDC | Tumor cells | pMHC-I | NE | CD8 T cell priming by cross-dressing | (Bonaccorsi et al., 2014) | |
| Macrophages | mAb-opsonized tumor cells | TAA | FcγR-mediated | TAA shaving; impaired mAb-based immunotherapy efficiency | (Beum et al., 2006, 2008; Taylor and Lindorfer, 2015) | |
| mAb-opsonized tumor cells | TAA | FcγR-mediated with increased affinity | Persistent trogocytosis leads to tumor cell death | (Velmurugan et al., 2016; Vijayaraghavan et al., 2020) | ||
| Tumor cells | PD-L1/PD-L2 | NE | Inhibition of T cell activation | (Kawashima et al., 2020) | ||
| Neutrophils | mAb-opsonized tumor cells | NE | NE | FcγR-mediated; CD11b/CD18-mediated adhesion; enhanced by CD47/SIRPα blockade | Tumor cell death by prolonged trogocytosis (trogoptosis) | (Bouti et al., 2021; Horner et al., 2007; Martinez Sanz et al., 2021; Matlung et al., 2018; van Rees et al., 2022) |
| Basophils | DCs | pMHC-II | LFA-1 and ICAM-1-mediated adhesion; CD11b/CD18 independent | Acquisition of APC function by cross-dressing | (Miyake et al., 2017) | |
| Eosinophils | Tumor cells | NE | NE | CD11b/CD18-mediated adhesion | NE | Figure 4 |
TAA, Tumor-associated antigens; mAb, monoclonal antibody; Ag, antigen; IS, immunological synapse; NE, not explored.
NK cells are able to form tight IS with the target cell which enable the exchange of membrane patches early after conjugation. Trogocytosis in NK cells is an active process that is controlled by ATP, tyrosine kinase activation, and intracellular Ca2+ and involves rearrangements of the actin cytoskeleton, as it is inhibited at low temperature (4°C), resistant to tubulin inhibitors but sensitive to inhibitors of actin polymerization (i.e., latrunculin B and Cytochalasin D) (Alari-Pahissa et al., 2021; Tabiasco et al., 2002). These findings have suggested a role for ATP/ADP balance in the actin polymerization/depolymerization process of cytoskeleton architecture during the trogocytic events.
Early studies have shown that mouse and human NK cells acquire from their targets class I p-MHC (pMHC-I) complexes through NK inhibitory receptors, respectively, mouse Ly49 (Sjöström et al., 2001; Zimmer et al., 2001) and human killer cell Ig-like receptors (KIR) (Carlin et al., 2001) with the bidirectional transfer of inhibitory receptors onto target cells (Vanherberghen et al., 2004). In mice, trogocytosis of MHC-I (H-2Dd) through Ly49A is accompanied by partial inactivation of cytotoxic activity of NK cells (Sjöström et al., 2001; Zimmer et al., 2001). Similarly, trogocytosis-mediated acquisition of tumor-derived NKG2D ligands (i.e., Rae-1, the NKG2D ligand retinoic acid early inducible protein 1) by NK cells leads to fratricide killing (Nakamura et al., 2013). Activated, but not resting, human NK cells that capture the immunosuppressive molecule human leukocyte antigen (HLA)-G1 from melanoma cells through the NK inhibitory receptor ILT2 have impaired effector function and acquire suppressive function toward neighboring NK cells (Caumartin et al., 2007). Thus, trogocytosis through these inhibitory receptors negatively regulate NK responses and can be a major mechanism of immune escape.
Trogocytosis of tumor molecules can also result in reduced NK effector function. NK cells that have captured the CD9 molecule from ovarian cancer cells exhibit impaired anti-tumor cytokine production and cytotoxicity that can be restored with a CD9-blocking antibody in the co-culture (Gonzalez et al., 2021). Hasim et al. reported that NK cells acquire the immune checkpoint molecule programmed death-1 (PD-1) from leukemia cells in vitro and in vivo through trogocytosis via the signaling lymphocytic activation molecule (SLAM) family of receptors (Hasim et al., 2022). PD-1 expressing NK cells have impaired anti-tumor activities and respond to PD-1 blockade in tumor-bearing mice, indicating full functionality of trogocytosed PD-1. Of interest, NK cells acquiring PD-1 expressed higher levels of activating receptors and maturation markers (i.e., CD11b), produced more IFNγ, and exhibited higher degranulation (Hasim et al., 2022). This observation suggests that activated NK cells are more likely to directly interact with tumor cells but are also more susceptible to taking up PD-1 and, consequently, to its inhibitory effects.
In some circumstances, trogocytosis may promote NK cell activation. NK cells that capture the CCR7 chemokine receptor by trogocytosis upon interaction with CCR7+ cells acquire migrating properties to lymph nodes (Marcenaro et al., 2009). This mechanism is dependent on adhesion molecules, including leukocyte function-associated antigen 1 (LFA-1) and CD2, while it is prevented by KIR-mediated recognition of HLA class I. Human NK cells that have acquired HER2 from trastuzumab-opsonized HER2-expressing breast cancer cells via trogocytosis exhibited greater expression of the degranulation marker CD107a than non-HER2-trogocytosed cells (Suzuki et al., 2015). Moreover, human NK cells were shown to take up the tyrosine kinase receptor TYRO from tumor cells in vitro and in vivo via rapid (within 5 min) and cell-cell contact-dependent trogocytosis (Lu et al., 2021). Trogocytosis of TYRO by NK cells positively correlated with the tumor expression level of TYRO and with the activation status of NK cells. TYRO-expressing NK cells acquired enhanced effector functions and proliferation in vitro, but had similar anti-tumor functions in vivo with respect to TYRO− NK cells, probably owing to the limited half-life (8 h) of the acquired TYRO. Thus, although the current literature suggests that trogocytosis in NK cells may be exploited for immune regulation or activation, in the latter case it is unclear whether NK activation translates into the gain of anti-tumor effector function.
Dendritic cells (DC) are professional APCs that prime naive CD4+ and CD8+ T cell responses. Priming of CD8+ T cells occurs via three mechanisms (Miyake and Karasuyama, 2021). The first is the direct presentation of endogenous antigens that are processed and presented by the pMHC-I complex, typically when the DC is directly infected by a virus. The second is the cross-presentation of exogenous antigens, such as those derived from engulfed dying cells, via re-routing of proteins into specific endosomal compartments where antigens are processed and loaded onto MHC I molecules. A third mechanism described for MHC-I restricted presentation is cross-dressing, whereby pMHC-I complexes are borrowed from neighboring APCs and thus antigen presentation occurs without antigen processing. It is now established that this latter mechanism can occur through trogocytosis of pMHC-I complexes from neighboring APC or non-APC cells (Nakayama, 2014). Trogocytosis of pMHC-I complexes derived from tumors and infected cells results in enhanced DC cross-priming and promotes adaptive CD8+ T cell responses (Dolan et al., 2006; MacNabb et al., 2022; Wakim and Bevan, 2011; Zhang et al., 2008). Of note, trogocytosis of tumor-associated antigens was shown to be the primary mechanism for cross-priming mediated by CD8α+ DCs in mice immunized with small numbers of live tumor cells (Das Mohapatra et al., 2020). Moreover, the acquisition of tumor-derived antigens via trogocytosis was shown as an important mechanism for antigen presentation by plasmacytoid DC (pDC). Although human pDCs are inefficient in the internalization of tumor cells by phagocytosis, they can rapidly acquire membrane antigens and HLA molecules from melanoma cells in a cell-cell contact-dependent manner, and subsequently stimulate melanoma antigen-specific CD8+ T cell responses (Bonaccorsi et al., 2014). Overall, cross-dressing via trogocytosis of pMHC-I complexes represents an important mechanism of antigen presentation by DC in cancer.
Through trogocytic capture by neighboring immune cells, DC can be deprived of surface molecules that impair their function. For example, regulatory T cells (Tregs) can strip co-stimulatory molecules, such as CD70 (Dhainaut et al., 2015), CD80, and CD86 (Gu et al., 2012) from the membrane of DCs, gaining the enhanced suppressive ability and resulting in defective APC function by the DCs. This process occurs via Treg-expressed CTLA-4, which facilitates the IS formation with DCs and concomitant transfer of CD80/CD86 molecules from DCs to Tregs by trogocytosis (Qureshi et al., 2011; Tekguc et al., 2021). Of note, Treg-dependent depletion of CD80 disrupted cis-CD80/programmed death ligand-1 (PD-L1) heterodimers and increased free PD-L1 on DCs, further inhibiting DC activity. T cell ability to engage trogocytosis with DCs represents a key example of how immune cells can interchange membrane molecules that may affect anti-tumor immune responses. In a recent report, expression of Tim-3 by DCs engaged its receptor phosphatidylserine on tumor-infiltrating CD8+ T cells (TILs) to promote the trogocytosis of myeloid molecules by tumor antigen-specific TILs, including pMHC-I complexes, which converted these TILs into a target for fratricide CD8+ T cell killing (Pagliano et al., 2022). Moreover, trogocytic transfer of PD-L1 from human DCs to CD8+ T cells upon antigen-specific recognition endowed PD-L1+ CD8+ T cells with fratricide-killing properties (Gary et al., 2012). Therefore, in DCs active trogocytosis is a mechanism of cross-dressing that strengthens DC presentation of tumor antigens, whereas the transfer of co-stimulatory and immune checkpoints from DCs to neighboring immune cells may hamper anti-tumor immunity.
Monocytes and macrophages express Fcγ receptors through which they interact with antibody-opsonized cancer cells resulting in either phagocytosis of whole cells or trogocytosis of membrane patches (Miyake and Karasuyama, 2021). Trogocytosis can result in the removal of relevant antigens from target tumor cells that can compromise the efficiency of anticancer immunotherapeutic monoclonal antibodies. This is the case of the anti-CD20 antibody Rituximab for the treatment of patients with chronic lymphocytic leukemia, whereby antibody opsonization of leukemia cells produced the stripping (shaving) of CD20 molecule from target tumor cells by monocytes or macrophages via Fcγ receptors that limited tumor cell clearance (Beum et al., 2006). This mechanism was later reported for other immunotherapeutic antibodies targeting malignant cells (Beum et al., 2008; Taylor and Lindorfer, 2015).
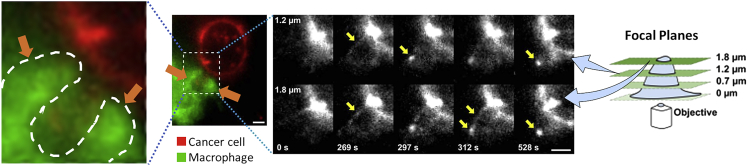
However, persistent trogocytosis of antibody-opsonized cancer cells can result in tumor killing. Trogocytosis of antibody-opsonized HER2+ human breast carcinoma cells mediated by macrophages was shown to lead to tumor killing in vitro (Velmurugan et al., 2016). Trogocytosis involved the extension of tubular structures in the macrophage that result in the formation of an invagination embracing a piece of the tumor cell membrane. Membrane patches of the cancer cell are then enclosed by the macrophage membrane surface and eventually bit off by the latter. This membrane tubulation event can be considered an early biomechanical force initiating the trogocytic process (Figure 5).
Figure 5.
Role of tubular structures in macrophage trogocytosis
Fluorescence image acquired at 1020 s inside the 1.8 and 1.2 μm focal planes showing the Trastuzumab signal in cancer cells (red fluorescence) and displaying a trogocytic event (enlarged in the left panel) involving the formation of two tubular structures (orange arrows and white dashed line) engaging between a macrophage (green fluorescence) and a cancer cell. These tubular structures evolve with the invagination of the cancer cell membrane. Yellow arrows in the grayscale panels indicate intermediate architectures in the evolution of the tubular structure. Numbers at the bottom left of each black/white panel depict the acquisition time (seconds) for each frame. Scale bars = 5 μm (© Velmurugan et al., 2016; The American Association for Cancer Research).
Antibody engineering to increase FcγR binding affinity augments trogocytosis and cancer cell death (Velmurugan et al., 2016), indicating that prolonged interactions and persistent trogocytosis may result in cell death. In this view, clusterization of trogocytic factors may improve the cell-cell contact stability, thus allowing a sustained contact time interval and then cell death. Moreover, in non-small cell lung cancer, administration of a bispecific antibody targeting EGFR and cMet receptors caused FcγR-mediated monocyte and macrophage interactions and associated trogocytosis of EGFR and cMet from cancer cells, leading to the inhibition of proliferation (Vijayaraghavan et al., 2020). In this model, macrophage-mediated trogocytosis was the major mechanism underlying antibody-mediated anti-tumor efficacy. Thus, although the mechanisms dictating the fate of antibody-opsonized cancer cells following macrophage trogocytosis are unclear, it is likely that cancer cells may not survive persistent trogocytic events and thus undergo cell death.
Monocytes and macrophages can also trogocytose inhibitory molecules that impair their function. For example, monocytes co-cultured with Hodgkin lymphoma cells rapidly (within 1 h) increased their expression of PD-L1/L2 (Kawashima et al., 2020). When monocytes were co-cultured with PD-L1/L2-deficient lymphoma cells, the membrane expression of PD-L1/L2 on monocytes was not increased, suggesting that the augmented expression of PD-L1/L2 occurred via direct membrane transfer from lymphoma cells to monocytes and not by the up-regulation of monocyte endogenous molecules. In vitro, the monocytes acquiring PD-L1 inhibited IFN-γ release by T cells.
Neutrophils express several types of Fcγ receptors (Futosi et al., 2013) relevant for the induction of antibody-dependent cellular cytotoxicity (ADCC) (Vanderven et al., 2017). Several studies indicate that neutrophils, like macrophages, employ FcγR engagement for trogocytosis (Masuda et al., 2013). The first evidence of trogocytosis in neutrophils comes from a study showing that during contact between neutrophils and HER-2/neu expressing tumor cells there was a mutual exchange of fluorescent membrane lipid dyes that was strongly increased in the presence of antibodies targeting HER-2/neu (Horner et al., 2007). Subsequently, Masuda et al. showed that peripheral blood neutrophils acquire T-cell-derived surface molecules in the presence of serum and cell-cell direct interaction as a result of FcγR-mediated trogocytosis, explaining the odd phenomenon of false positives appearing in flow cytometry analyses (Masuda et al., 2012).
Recently, van den Berg group showed that neutrophils employ trogocytosis to kill antibody-opsonized cancer cells in a process termed trogoptosis (Matlung et al., 2018). Trogoptosis is a mechanical process ensuing trogocytosis that involves the disruption of the plasma membrane of target cancer cells leading to lysis and necrotic death. Neutrophil trogoptosis is dependent on both FcγR engagement and cell-cell interactions via the neutrophil β2 integrin CD11b/CD18 that mediates the formation of conjugates with cancer cells (Martinez Sanz et al., 2021; Matlung et al., 2018; van Spriel et al., 2001, 2003). Of note, CD47-SIRPα checkpoint blockade strongly enhances trogoptosis by enabling the integrin-associated protein kindlin-3 to bind talin-2 and the β chain of the integrin in order to stabilize both the interaction of CD11b/CD18 with the cancer target and the anchorage to the cytoskeleton (Bouti et al., 2021; Matlung et al., 2018). This molecular conformation fits with the tubular structures observed in macrophages (Velmurugan et al., 2016) and supports the concept of biomechanical forces driving the initial phases of trogocytosis (Figure 5). In neutrophils, this process allows the formation of an IS that results in trogocytosis and tumor killing (Bouti et al., 2021). Strategies aimed at blocking or disrupting the CD47-SIRPα axis were reported to enable neutrophils to trogocytosis (Cendrowicz et al., 2022) and trogoptotic killing (van Rees et al., 2022) of B-cell lymphoma cells. Furthermore, the activation of neutrophils with Galectin-9 down-modulated CD47, while it up-regulated integrin expression and tumor cell adhesion, resulting in enhanced neutrophil trogocytosis and tumor killing (Ustyanovska Avtenyuk et al., 2021). These studies underline the importance of integrin activation and the role of CD47 as a negative regulator of neutrophil-mediated trogocytosis.
Although trogocytosis and trogoptosis are strictly related mechanical events, this does not necessarily imply that every single “bite”, caused by a trogocytic event, results in the lytic disruption of the cell membrane (i.e., trogoptosis). This is the case of rituximab-opsonized B cell lymphoma cells, where neutrophils can bite off CD20, but are incapable to kill lymphoma cells. Newer studies from van den Berg group demonstrated that a combination of CD47-SIRPα checkpoint blockade and the anti-leishmaniasis drug sodium stibogluconate overcomes the resistance of B-cell lymphoma cells toward neutrophil trogoptotic killing (van Rees et al., 2022).
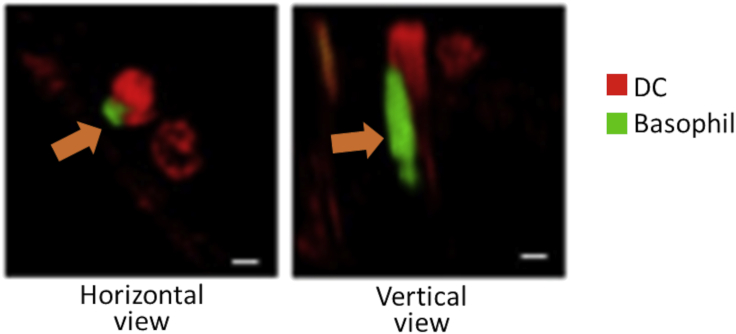
Basophils represent the rarest population of granulocytes playing key roles in allergic inflammation and parasitic infections. Evidence that basophils can carry out trogocytosis comes from one study demonstrating that basophils acquire peptide-MHC-II complexes from DCs through cell contact-dependent trogocytosis (Miyake et al., 2017). These peptide-MHC-II-dressed basophils gained APC function and induced the priming of peptide-specific T cells, as reported for other cross-dressed immune cells including CD4+ T cells, NK cells, macrophages, and mast cells (Dudeck et al., 2017). Blocking of either CD11a or ICAM-1 (but not CD11b) abrogated MHC-II transfer from DCs to basophils, indicating a role for these adhesion molecules in basophil trogocytosis. Of note, Miyake et al. also evidenced the formation of the peculiar tubular architectures during in vivo trogocytosis between DCs and basophils, occurring in mouse-draining lymph nodes (Figure 6) (Miyake et al., 2017). These findings indicate that actin network formation and polymerization could be associated with these tubulation processes and may represent a common early event of trogocytosis, independently of the cell type engaging trogocytosis. Whether trogocytosis between basophils and DCs affects immune system homeostasis in the TME and whether this process occurs among basophils and cancer cells is currently unknown.
Figure 6.
Role of tubular structures in basophil trogocytosis
Fluorescence image showing an in vivo trogocytic event between a basophil (green fluorescence) and a DC (red fluorescence) within a mouse-draining lymph node. Orange arrows indicate the presence of a tubular architecture during trogocytosis (© Miyake et al., 2017; National Academy of Sciences).
Innate immune cell trogocytosis via stable CD11b/CD18 immunological synapse: data from in silico modeling analysis
Despite trogocytosis being a conserved biological process in and between cellular species, it can be triggered by different molecular signals. Innate immune cells express different patterns of membrane receptors, some of which are shared among lineages, whereas others are cell type-specific. As a consequence, the molecular mechanisms mediating trogocytosis in these cells may depend on the cell type with some shared similarities. Given the dependence on cell-cell contact, trogocytosis in immune cells crucially depends on the formation of the IS. However, the links bridging IS to trogocytic event and the underlying molecular mechanisms are still unclear.
During TCR/MHC interaction between T cells and APCs, the IS directs the actin network close to the clusters of TCR complexes (Dopfer et al., 2011) via the PI3K and two small GTPases RhoG and RRas (Uribe-Querol and Rosales, 2021). As Rho compounds are well recognized for their key role in F-actin contraction and polymerization (Lou et al., 2019), it is likely that Rho molecules are recruited near the clustering TCR molecules and then bind actin filaments, triggering synchronized contraction that results in an engulfment-like rearrangement of the membrane. The contractile force derives from the tropomyosin-mediated shortening of the multiple actin fibers (Dogterom and Koenderink, 2019) and leads to the engulfment of the T cell membrane close to the TCR/pMHC clusters. The process then culminates with the internalization of the vesicles containing the TCR/pMHC complexes. These assumptions are consolidated by studies demonstrating the central role of RhoG and RRas GTPases for T cell receptor internalization concomitantly with the formation of IS (Martínez-Martín et al., 2011), whereas the initial formation of the IS requires the recruitment of F-actin polymers (Calvo and Izquierdo, 2021).
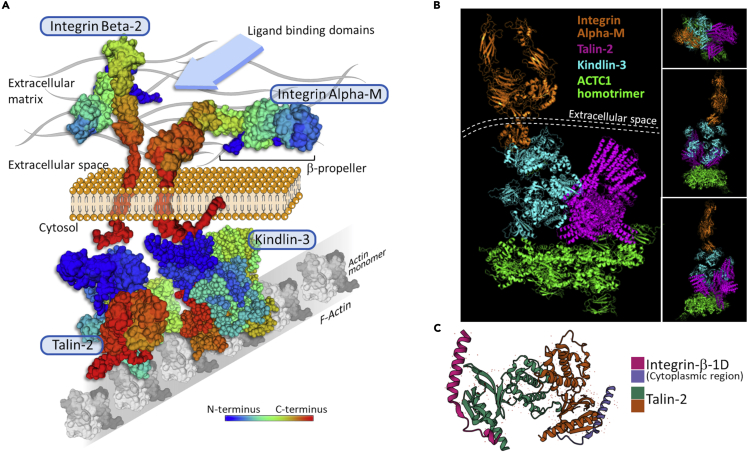
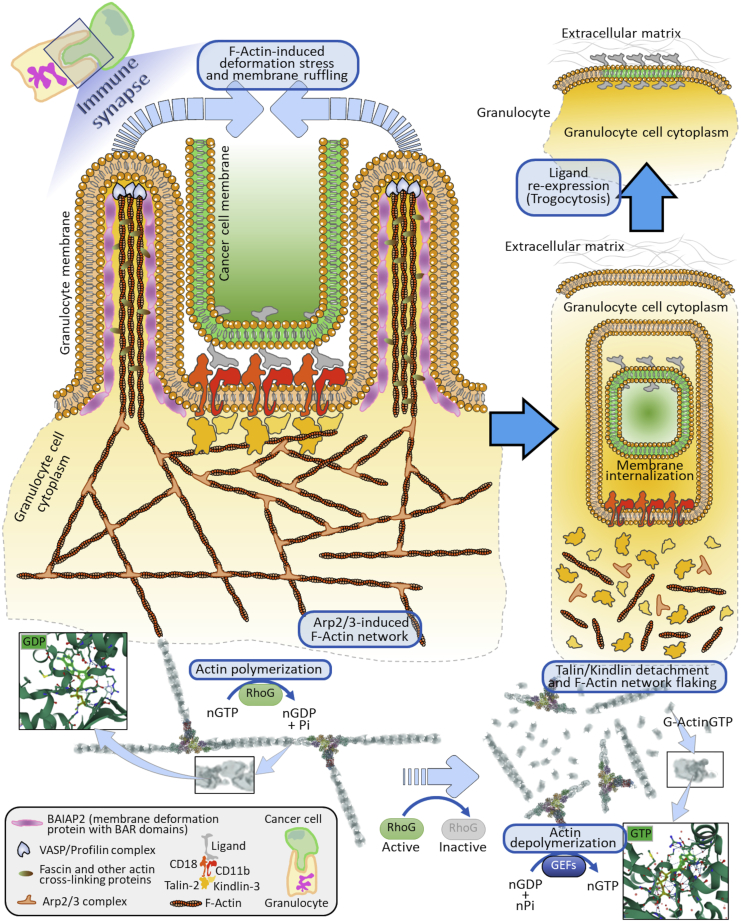
As mentioned above, NK cells, macrophages, and neutrophils express FcγR that allows the killing of target cells by ADCC, which classically occurs through phagocytosis (in the case of macrophages) or degranulation-mediated apoptosis (in the case of NK cells and neutrophils) of the opsonized target cell. Matlung et al. showed that neutrophils employ trogocytosis as a means for ADCC of cancer cells, namely trogoptosis, whereas NK cell-mediated ADCC is independent of trogocytosis and is strictly degranulation-mediated (Matlung et al., 2018). This suggests that different immune effector cells employ trogocytosis for distinct purposes. Neutrophils and antibody-opsonized cancer cells associate through high-affinity interactions that involve the integrins CD11b/CD18. Of note, these integrins are known to be capable to bind specific focal adhesion molecules, including talins and kindlins, as depicted in our model (Figure 7A and Data S1) and reported previously (Sun et al., 2020). For example, kindlin-3, the only kindlin expressed in hematopoietic cells (Fagerholm et al., 2014), is another focal adhesion factor with similar properties shared by talin-2 (Sun et al., 2020). On the other hand, kindlin-3 and talin-2 are required for the binding to and activation of integrins, including CD11b/CD18 (Bouti et al., 2021; Critchley et al., 1999). These considerations enforce the role of talins and kindlins as key adapter molecules permitting integrins association with F-actin. Indeed, our computational model processed by the protein-protein docking platform ClusPro (Kozakov et al., 2017; Comeau et al 2004; Kozakov et al., 2006) suggests that talin-2 and kindlin-3 not only bind to actin homotrimers but also associate to form a stable talin/kindlin complex (Figure 7B and Data S1). Talins also display binding affinity to the cytosolic region of integrins β1-D (Figure 7C). These observations consolidate the notion that the CD11b/CD18 complex can bind to actin through a stable association to talin/kindlin dimers, which in turn allows the clusterization of integrins onto the granulocyte membrane and a concomitant local accumulation of F-actin segments. Preserving F-actin architecture inside the granulocyte membrane is pivotal for the generation of membrane tubules around the target cell membrane.
Figure 7.
Association of integrins to F-actin through talin-2 and kindlin-3 in granulocytes
(A) Hypothetical surface model of human integrins CD18 and CD11b associated with F-actin through focal adhesion molecules (Talin-2, Kindlin-3) as an early signal for integrin/ligand stabilization and clusterization within the granulocyte cell membrane. Talin-2 markedly activates Integrin-beta-2 thus increasing the affinity binding of the CD18/CD11b integrins to its ligand on cancer cells (cyan arrow). Actin (ACTC1) monomers (Uniprot code, P68032); talin-2 (Uniprot code, Q9Y4G6); kindlin-3 (Uniprot code, Q86UX7); integrin beta-2 (Uniprot code, P05107); integrin alpha-M (Uniprot code, P11215).
(B) 3D stereoscopic computational model showing integrin Alpha-M (RCSB-PDB code: 7P2D) with its C-terminal sequence (cytoplasmic region in panel A) binding the kindlin-3 (RCSB-PDB code: 7C3M) and talin-2 (RCSB-PDB code: 6R9T). These two focal adhesion molecules are in turn stably complexed to an actin homotrimer composed of ACTC1 monomers (RCSB-PDB code: 7LRG). Dashed lines represent the cell membrane separating the extracellular space from the cell cytosol. Upper right box depicts the integrin side view of the model referred to as the actin homotrimer axis. The intermediate and lower right boxes represent the kindlin-3 and talin-2 side views of the model referred to as the same trimer axis. The docking model was obtained via ClusPro processing of the indicated RCSB-PDB codes (© Kozakov et al., 2017).
(C) Particular of the integrin-talin association mediated by the cytoplasmic regions of the integrin molecule (pink & purple colors) demonstrating the ability of talin-2 to act as a focal adhesion factor by binding to integrins (RCSB-PDB code: 3G9W).
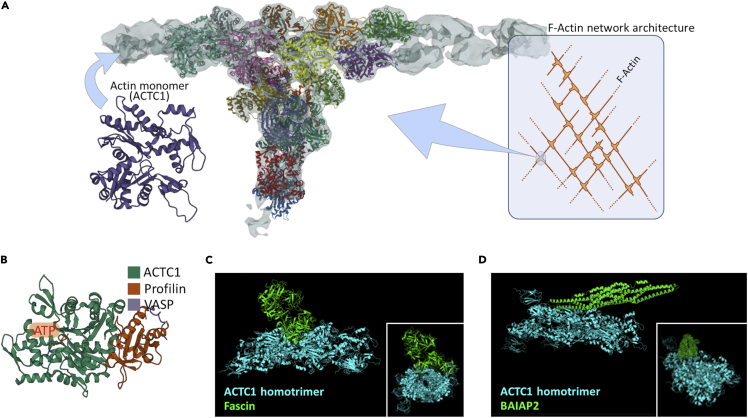
The maintenance of the cytoskeletal F-actin network can in part occur by a fine modulation of local polymerization/depolymerization of F-actin filaments. Therefore, all relevant molecules known to stably bind actin monomers or F-actin could play a key role in these processes. In this regard, the Arp2/3 complex with actin monomers (i.e., ACTC1) constitutes a key molecule to generate and maintain the F-actin network architecture (Figure 8A and Data S1) (Lee and Dominguez, 2010). Actin monomers also bind to profilin and VASP (Figure 8B), which are relevant factors for the elongation and formation of the aforementioned tubular units (Critchley et al., 1999). Fascin, a F-actin crosslinker, further increases the stability of these structures by cross-linking F-actin filaments as shown by our docking analysis (Figure 8C and Data S1) and by other reports (Lee and Dominguez, 2010). BAR/IMD Domain Containing Adaptor Protein 2 (BAIAP2) contributes to membrane ruffling via F-actin linking close to the membrane, as reported by Lee and co-workers (Lee and Dominguez, 2010) and by our protein-protein docking model (Figure 8D and Data S1). These observations and other studies (Critchley et al., 1999; Pinon et al., 2014) strongly suggest that these actin-binding molecules, together with focal adhesion molecules (including talin-2 and kindlin-3), serve as intermediate factors connecting F-actin with membrane integrins.
Figure 8.
Models of relevant actin-binding molecules mediating the cytoskeleton architecture maintenance and organization
(A) Details of the Arp2/3 complex (colored protein subunits) associated with F-actin filaments (gray color), composed by ACTC1 monomers. Light cyan box shows the role of the Arp2/3 complex (light cyan circle) in F-actin network architecture. RCSB-PDB code, 7AQK.
(B) Actin (ACTC1) monomer associated with two elongation elements (profilin and VASP), needed for the formation and elongation of the F-actin fibers. Details on ATP/GTP (orange box) binding region are depicted. RCSB-PDB code, 2PAV.
(C) 3D stereoscopic computational model delineating fascin (RCSB-PDB code, 1DFC) complexed to an ACTC1 homotrimer (RCSB-PDB code, 7LRG). Fascin binds actin polymers to generate F-actin fibers. Box shows a front view referring to the actin homotrimer axis underlining the strict association between the two proteins.
(D) 3D stereoscopic computational model showing BAIAP2 (RCSB-PDB code, 1Y2O), a membrane deformation element, associated with an ACTC1 homotrimer (RCSB-PDB code 7LRG), which elicits membrane ruffling. Box illustrates a front view of the actin homotrimer axis highlighting the firm binding between the two proteins.
Proposed model for a role of the actin network in trogocytosis via talin/kindlin switchers
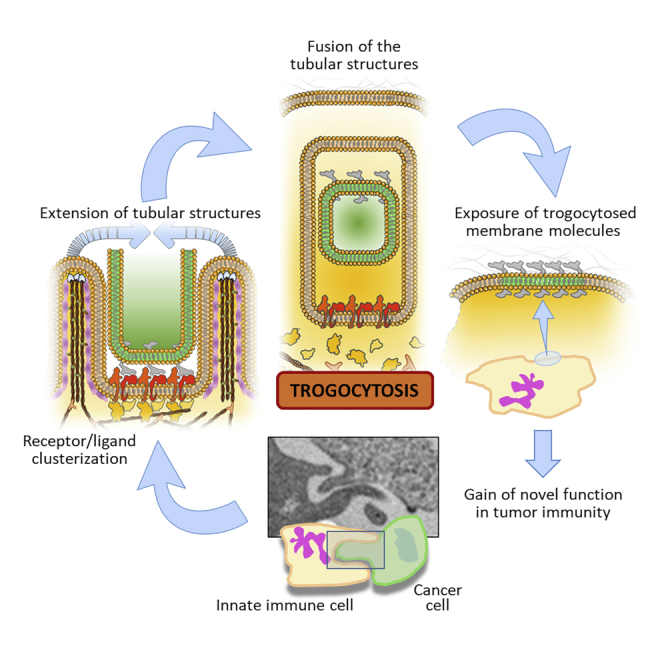
Based on these observations and with the support of the aforementioned literature, we here propose a hypothetical model delineating the relevant role of integrins and focal adhesion molecules for the association to the F-actin network, as a main driving force to sustain the trogocytosis process in neutrophils and possibly other innate immune cells during direct contact with cancer cells (Figure 9). Given the shared adhesion molecules and integrins expressed with neutrophils, it may be speculated that eosinophils employ this process for facilitating the formation of the IS. In our model, the granulocyte starts to engage an IS with a tumor cell, by the integrins CD11b/CD18 firmly associated with their specific ligand on cancer cells. This allows the formation of integrin clusters in the granulocyte membrane surface and the recruitment of talin-2 and kindlin-3.
Figure 9.
Hypothetic molecular model for the role of F-actin network reorganization as a main biomechanical driver for granulocyte trogocytosis of cancer cells
The generation and maintenance of an immune synapse starts with CD18/CD11b integrins-ligand complex clusterization inside the granulocyte membrane surface (orange-color membrane). These clusters elicit the recruitment of talin-2 and kindlin-3, which bind to the cytosolic regions of integrins. Talin-2 and kindlin-3 also associate with the F-actin fibers, thus activating the accumulation of F-actin filaments tight to the clusters. Through the Arp2/3 complexes, the F-actin network extends locally and at the sides of the clusters leading to the formation of F-actin fibers. VASP, Fascins, and BAIAP2 elicit the generation of peculiar tubular membrane structures, whose formation and stability are sustained by actin polymerization via active RhoG, which also leads to the local extension of the actin network by the degradation of GTP into GDP and phosphate (Pi). GDP then interacts with actin monomers (upper left box). At this point, these tubular architectures, guided by the actin network diffusion force, will entrap the cancer cell membrane (green color) and ligands. In parallel, the ligands on the cancer cell membrane detach from the integrins located on the granulocyte membrane. This leads to the spontaneous dissociation of talin-2 and kindlin-3 from integrins and in turn to F-actin decomplexation, with subsequent actin network disaggregation. Concomitantly, the prevalence of inactive RhoG stimulates the GEFs-dependent F-Actin depolymerization and GEFs-guided GTP formation (lower right box), culminating with the exposition of ligand molecules into the granulocyte membrane by sequential membrane fusion events, thus terminating the process of trogocytosis.
Such molecular architecture is central in intermediating a stable binding to the F-actin fibers close to the integrin clusters. In parallel, this molecular binding cascade will sustain RhoG-induced F-actin polymerization (through Arp2/3 and fascins) and actin elongation close to the IS region. This has two parallel effects: (i) the formation of an F-actin network locally to the IS, and (ii) the generation and extension of F-actin-guided tubular membrane structures via the VASP and BAIAP2 proteins (Figure 9). At this point, the tubular structures continue to extend until they encounter another similar structure to assemble with, via membrane-to-membrane fusion, thus leading to cancer cell membrane patch internalization. This activates a cascade of events: (i) integrins on the immune cell membrane detach from ligands on the cancer cell surface, (ii) integrins de-clusterize, (iii) de-clusterization favors the dissociation of talins and kindlins from the integrins, and the subsequent F-actin depolymerization by guanine nucleotide exchange factors (GEFs) and RhoG inactivation, and (iv) termination of trogocytic process by the exposition of integrin ligands on granulocyte membrane. The latter occurs through sequential cholesterol-based membrane fusion events (Witt et al., 2021; Martens and McMahon, 2008; Yang et al., 2016), leading to the final exposure of the ligands to the granulocyte surface membrane. Overall, this model highlights that the stability of IS is pivotal for the initiation and termination of trogocytosis (Figure 9). We may speculate that the stability and time duration of IS, together with immune checkpoint blockade (such as CD47-SIRPα), can determine prolonged trogocytic events that may result in trogoptosis in neutrophils and possibly in other innate immune cells. The present model, corroborated by our in silico docking analyses, may be theoretically applied to other integrins that bind focal adhesion molecules, such as talins and kindlins.
Concluding remarks
Trogocytosis allows immune cells to expose on their membrane surface molecules they do not genetically express, acquiring specific signaling features provided by the captured molecule. As trogocytosis implies the acquisition of molecules not intrinsically expressed by the receiving cell, it may be regarded as a transitory process depending on the mean half-life of the receiving protein and thus associated with its biophysical features. Most studies have evaluated trogocytosis by flow cytometry and in some cases the percentages of reported trogocytic cells are remarkably high. If trogocytosis was such a frequent event this would question the parameters employed for decades by immunologists to distinguish immune cell types based on membrane receptors expressed. Given the artifacts often produced by autofluorescence, we recommend coupling flow cytometry to fluorescence microscopy when studying trogocytosis.
In the TME, trogocytosis may be regarded both as a feedback mechanism of immune regulation and as an opportunistic strategy for cancer cells to escape immunity following the deprivation of cognate antigens. The latter aspect worryingly challenges the success of cancer immunotherapies in patients and strategies aimed at circumventing this side effect have been proposed, including alternative dosing (Taylor and Lindorfer, 2015) and combinatorial targeting (Hamieh et al., 2019). On the other hand, trogocytosis can find therapeutic applications as a tool to transiently expose molecules of interest to immune cells without genetic manipulation, with the aim to enhance their functions in vivo in adoptive immunotherapy of cancer and other diseases. This strategy was first employed to engineer CCR7 expression on expanded human NK cells to improve lymph node homing after adoptive transfer (Somanchi et al., 2012). A thorough understanding of the mechanisms regulating trogocytosis in immune cells, including the complex dynamics behind the IS formation and stabilization, will be instrumental for the development of strategies to appropriately harness this process for therapeutic purposes.
Limitations of the study
One limitation of this study is that trogocytosis in eosinophils was demonstrated only after stimulation with IL-33, thus other activation stimuli capable of promoting cell-cell adhesion via CD11b/CD18 should be tested. Moreover, our 3D models obtained by ClusPro protein-protein docking analysis are in silico representations. These protein complexes need future experimental confirmations to evaluate their real feasibility.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rat anti-mouse CD18 | Biolegend | Cat#101417; RRID:AB_2832273 |
| Rat anti-mouse Siglec-F | BD Biosciences | Cat# 562681, RRID:AB_2722581 |
| Chemicals, peptides, and recombinant proteins | ||
| PKH67 | Sigma-Aldrich | Cat#S-MINI67 |
| PKH26 | Sigma-Aldrich | Cat#S-MINI26 |
| DAPI | Thermo Fisher Scientific | Cat#D1306 |
| Recombinant mouse IL-33 | Biolegend | Cat# 580506 |
| Recombinant mouse IL-5 | Peprotech | Cat#215-15 |
| Recombinant mouse SCF | Cell Guidance Systems | Cat#GFM9-10 |
| Recombinant mouse FLT3-L | Cell Guidance Systems | Cat# GFM6-100 |
| Recombinant mouse GM-CSF | Cell Guidance Systems | Cat# GFM15-20 |
| Experimental models: Cell lines | ||
| EG7.OVA | American Type Culture Collection | CRL-2113 |
| B16.F10 | American Type Culture Collection | CRL-6475 |
| MCA205 | Merck Millipore | SCC173 |
| MC38 | Provided by Dr. Carlos Alfaro | RRID:CVCL_B288 |
| TC-1 | Provided by Dr. Guido Kroemer | RRID:CVCL_4699 |
| Experimental models: Organisms/strains | ||
| C57BL/6 mice | Charles River Laboratories | C57BL/6NCrl |
| Software and algorithms | ||
| GraphPad Prism 7 | GraphPad Software Inc. | https://www.graphpad.com/ |
| Kaluza Analysis | Beckman Coulter | https://www.beckman.com/ |
| Adobe Photoshop CS5 | Adobe | https://www.adobe.com/ |
| PyMol | Schrödinger LLC | https://pymol.org/ |
| ClusPro (via PIPER algorithm) | Boston University and Stony Brook University | https://cluspro.org/ |
| NGL Viewer | The Center of Molecular Life Sciences – University of Basel | https://swissmodelwxpasy.org/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dr. Giovanna Schiavoni (giovanna.schiavoni@iss.it).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Generation of bone marrow-derived eosinophils
Mice were housed in the animal facilities at the Istituto Superiore di Sanità (Rome, Italy). All experiments and procedures were approved by the Italian Ministry of Health (Permit No. 243/2016-PR to protocol No. D9997.13) and the Ethical Committee for Animal Experimentation of the Istituto Superiore di Sanità, in accordance with the Guide for the Care and Use of Laboratory Animals of the European Community Council Directives. Bone marrow-derived murine eosinophils were obtained following a protocol already described (Andreone et al., 2019), with some modifications. Briefly, bone marrow cells from naïve C57Bl/6 mice were cultured in RPMI 1640 containing 20% FBS, 1% glutamine, 25 mM Hepes, 1X NEAA, 1 mM sodium pyruvate, supplemented with 100 ng/mL SCF and 100 ng/mL FLT3-L (all from Cell Guidance Systems). From day 4, 10 ng/mL IL-5 (Peprotech) was added to the culture every other day. At day 14, eosinophils were harvested and re-plated at the concentration of 1,5 × 106/mL in the presence of either 100 ng/mL IL-33 (Biolegend; activated EOS) or 10 ng/mL IL-5 (resting EOS). GM-CSF (10 ng/mL; Cell Guidance Systems) was added to the cultures 24 h prior to use (day 15 or 16). Eosinophil purity (>80%) was determined by flow cytometry (CD11b+Siglec-F+Ly6G−CD11c−).
Tumor cell lines
Murine EG7.OVA lymphoma cells (EG7, CRL-2113; American Type Culture Collection), B16.F10 metastatic melanoma cells (ATCC, CRL-6475), MC38 colon carcinoma cells (kindly provided by Dr. Carlos Alfaro, University of Navarra, Pamplona, Spain), TC-1 lung carcinoma cells (kindly provided by Dr. Guido Kroemer, Gustave Roussy Cancer Institute, Villejuif, France) and MCA205 fibrosarcoma cells (Merck Millipore, Burlington, MA, USA, SCC173) were used.
Method details
Trogocytosis assays
Tumor cells cells were labelled with either PKH67 Green or PKH26 Red fluorescent Cell Linker (Sigma) according to the manufacturer instructions and then co-cultured for 60 min with resting or activated EOS at 4:1 EO:tumor cells ratio. In experiments with anti-CD18 antagonist, activated EOS were pre-incubated with 10 μg/mL anti-mouse CD18 mAb (clone M18/2, Biolegend) for 25 min at 4°C prior to co-culture with PKH-labelled tumor cells. For trogocytosis assay by confocal microscopy, cells were transferred onto poly-L-lysine-coated coverslips and the cover glasses were mounted on microscope slides with Vectashield antifade mounting medium containing DAPI (Vector Laboratories). CLSM observations were performed with a Leica TCS SP2 AOBS apparatus, using a 63X/1.40 NA oil objective and excitation spectral laser lines at 405, 488 and 594 nm. Image acquisition and processing were carried out using the Leica Confocal Software 2.6 rel 1537 (Leica Microsystems) and Adobe Photoshop CS5 software programs. Signals from different fluorescent probes were taken in sequential scan settings. Several cell conjugates for each condition were analyzed for both quantitative analysis and representative images of PKH+ eosinophils. For trogocytosis assay by flow cytometry, at the end of the co-culture, cells were labelled with BV421 anti-mouse Siglec-F mAb (BD Biosciences) in the presence of 0.5 mM EDTA to disrupt cell conjugates and analyzed by flow cytometry. The percentage of trogocytosis in eosinophils was evaluated as percentage of PKH+ cells in a population gated on Siglec-F+ and doublets exclusion. For analysis of trogocytosis of eosinophils by time-lapse video microscopy, MC38 cells were labelled with PKH26 Red fluorescent Cell Linker and plated on Cellvis 6 Well glass bottom plate with high performance #1.5 cover glass (Fisher scientific). Resting or activated eosinophils were added to the culture at a 2:1 EOS: tumor cell ratio. Time lapse recordings were performed over a period of 1 h at a 30 seconds interval with a 20X objective in a Zeiss LSM 900 confocal microscope.
Ultrastructural analysis by transmission electron microscopy (TEM)
Resting and IL-33 activated eosinophils were co-cultured with B16 melanoma cells (10:1 ratio) for 60 min at 37°C and then fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4. Following washes in cacodylate buffer, cells were post-fixed in 1% OsO4 in the same buffer and further washed with 0.1 M cacodylate. Cells were then dehydrated in ethanol gradient from 50% to 100% (v/v) and embedded in Agar 100 resin (Agar Scientific, Essex, UK) at 65°C for 48 h. Ultra thin sections were obtained using an ultra-microtome and collected on 200-mesh grids, counterstained with uranyl acetate for 10 min and lead citrate for further 10 min. Samples were observed in a Philips 208s transmission electron microscope at 100 kW (Philips, Amsterdam, The Netherlands).
Quantification and statistical analysis
All statistical analysis were performed using GraphPad Prism Software (GraphPad, La Jolla, CA). One-way analysis of variance was performed to compare means among groups, followed by post hoc testing (Tukey). Values were considered as significant when the probability was below the 5% confidence level (p < 0.05).
Acknowledgments
Dr. Giovanna Schiavoni is supported by Italian Association for Cancer Research [AIRC IG-2018 21366]. Dr. Silvia Piconese is supported by AIRC [IG-2017 19784], Ministry of Education, University and Research (MIUR) [Progetti di Ricerca di Interesse Nazionale (PRIN) Grant 2017 Prot. 2017K7FSYB] and Italian Ministry of Health [GR-2019-12368551].
Author contributions
Conceptualization, data research, and contributions to discussions of the content, GS, FM, CA, SP, SA, and FS; data collection and analysis, SA, FS, FN, AT, MF, GS, and FM; Writing original draft, GS and FM; Reviewing original draft, CA, SP, SA, and FS.
Declaration of interests
The authors declare no competing interests.
Published: October 21, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.105110.
Supplemental information
Data and code availability
Data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Alari-Pahissa E., Ataya M., Moraitis I., Campos-Ruiz M., Altadill M., Muntasell A., Moles A., López-Botet M. NK cells eliminate Epstein-Barr virus bound to B cells through a specific antibody-mediated uptake. PLoS Pathog. 2021;17:e1009868. doi: 10.1371/journal.ppat.1009868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreone S., Spadaro F., Buccione C., Mancini J., Tinari A., Sestili P., Gambardella A.R., Lucarini V., Ziccheddu G., Parolini I., et al. IL-33 promotes CD11b/CD18-mediated adhesion of eosinophils to cancer cells and synapse-polarized degranulation leading to tumor cell killing. Cancers. 2019;11:E1664. doi: 10.3390/cancers11111664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel S.R., Johansson M.W., McNamee D.M., Mosher D.F. Roles of integrin activation in eosinophil function and the eosinophilic inflammation of asthma. J. Leukoc. Biol. 2008;83:1–12. doi: 10.1189/jlb.0607344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beum P.V., Kennedy A.D., Williams M.E., Lindorfer M.A., Taylor R.P. The shaving reaction: rituximab/CD20 complexes are removed from mantle cell lymphoma and chronic lymphocytic leukemia cells by THP-1 monocytes. J. Immunol. 2006;176:2600–2609. doi: 10.4049/jimmunol.176.4.2600. [DOI] [PubMed] [Google Scholar]
- Beum P.V., Mack D.A., Pawluczkowycz A.W., Lindorfer M.A., Taylor R.P. Binding of rituximab, trastuzumab, cetuximab, or mAb T101 to cancer cells promotes trogocytosis mediated by THP-1 cells and monocytes. J. Immunol. 2008;181:8120–8132. doi: 10.4049/jimmunol.181.11.8120. [DOI] [PubMed] [Google Scholar]
- Bonaccorsi I., Morandi B., Antsiferova O., Costa G., Oliveri D., Conte R., Pezzino G., Vermiglio G., Anastasi G.P., Navarra G., et al. Membrane transfer from tumor cells overcomes deficient phagocytic ability of plasmacytoid dendritic cells for the acquisition and presentation of tumor antigens. J. Immunol. 2014;192:824–832. doi: 10.4049/jimmunol.1301039. [DOI] [PubMed] [Google Scholar]
- Bouti P., Zhao X.W., Verkuijlen P.J.J.H., Tool A.T.J., van Houdt M., Köker N., Köker M.Y., Keskin O., Akbayram S., van Bruggen R., et al. Kindlin3-Dependent CD11b/CD18-integrin activation is required for potentiation of neutrophil cytotoxicity by CD47-SIRPα checkpoint disruption. Cancer Immunol. Res. 2021;9:147–155. doi: 10.1158/2326-6066.CIR-20-0491. [DOI] [PubMed] [Google Scholar]
- Calvo V., Izquierdo M. Role of actin cytoskeleton reorganization in polarized secretory traffic at the immunological synapse. Front. Cell Dev. Biol. 2021;9:629097. doi: 10.3389/fcell.2021.629097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin L.M., Eleme K., McCann F.E., Davis D.M. Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. J. Exp. Med. 2001;194:1507–1517. doi: 10.1084/jem.194.10.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caumartin J., Favier B., Daouya M., Guillard C., Moreau P., Carosella E.D., LeMaoult J. Trogocytosis-based generation of suppressive NK cells. EMBO J. 2007;26:1423–1433. doi: 10.1038/sj.emboj.7601570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cendrowicz E., Jacob L., Greenwald S., Tamir A., Pecker I., Tabakman R., Ghantous L., Tamir L., Kahn R., Avichzer J., et al. DSP107 combines inhibition of CD47/SIRPα axis with activation of 4-1BB to trigger anticancer immunity. J. Exp. Clin. Cancer Res. 2022;41:97. doi: 10.1186/s13046-022-02256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau S.R., Gatchell D.W., Vajda S., Camacho C.J. ClusPro: a fully automated algorithm for protein-protein docking. Nucleic Acids Res. 2004;32:W96–W99. doi: 10.1093/nar/gkh354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R.E., Sprent J., Marchalonis J.J. Antigen-binding specificity of isolated cell-surface immunoglobulin from thymus cells activated to histocompatibility antigens. Proc. Natl. Acad. Sci. USA. 1972;69:2556–2560. doi: 10.1073/pnas.69.9.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A.C., Santos J.M.O., Gil da Costa R.M., Medeiros R. Impact of immune cells on the hallmarks of cancer: a literature review. Crit. Rev. Oncol. Hematol. 2021;168:103541. doi: 10.1016/j.critrevonc.2021.103541. [DOI] [PubMed] [Google Scholar]
- Critchley D.R., Holt M.R., Barry S.T., Priddle H., Hemmings L., Norman J. Integrin-mediated cell adhesion: the cytoskeletal connection. Biochem. Soc. Symp. 1999;65:79–99. [PubMed] [Google Scholar]
- Das Mohapatra A., Tirrell I., Bénéchet A.P., Pattnayak S., Khanna K.M., Srivastava P.K. Cross-dressing of CD8α + dendritic cells with antigens from live mouse tumor cells is a major mechanism of cross-priming. Cancer Immunol. Res. 2020;8:1287–1299. doi: 10.1158/2326-6066.CIR-20-0248. [DOI] [PubMed] [Google Scholar]
- Dhainaut M., Coquerelle C., Uzureau S., Denoeud J., Acolty V., Oldenhove G., Galuppo A., Sparwasser T., Thielemans K., Pays E., et al. Thymus-derived regulatory T cells restrain pro-inflammatory Th1 responses by downregulating CD70 on dendritic cells. EMBO J. 2015;34:1336–1348. doi: 10.15252/embj.201490312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogterom M., Koenderink G.H. Actin-microtubule crosstalk in cell biology. Nat. Rev. Mol. Cell Biol. 2019;20:38–54. doi: 10.1038/s41580-018-0067-1. [DOI] [PubMed] [Google Scholar]
- Dolan B.P., Gibbs K.D., Ostrand-Rosenberg S. Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J. Immunol. 2006;177:6018–6024. doi: 10.4049/jimmunol.177.9.6018. [DOI] [PubMed] [Google Scholar]
- Dopfer E.P., Minguet S., Schamel W.W.A. A new vampire saga: the molecular mechanism of T cell trogocytosis. Immunity. 2011;35:151–153. doi: 10.1016/j.immuni.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Dudeck J., Medyukhina A., Fröbel J., Svensson C.M., Kotrba J., Gerlach M., Gradtke A.C., Schröder B., Speier S., Figge M.T., Dudeck A. Mast cells acquire MHCII from dendritic cells during skin inflammation. J. Exp. Med. 2017;214:3791–3811. doi: 10.1084/jem.20160783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerholm S.C., Lek H.S., Morrison V.L. Kindlin-3 in the immune system. Am. J. Clin. Exp. Immunol. 2014;3:37–42. [PMC free article] [PubMed] [Google Scholar]
- Futosi K., Fodor S., Mócsai A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 2013;17:638–650. doi: 10.1016/j.intimp.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary R., Voelkl S., Palmisano R., Ullrich E., Bosch J.J., Mackensen A. Antigen-specific transfer of functional programmed death ligand 1 from human APCs onto CD8+ T cells via trogocytosis. J. Immunol. 2012;188:744–752. doi: 10.4049/jimmunol.1101412. [DOI] [PubMed] [Google Scholar]
- Gatault S., Delbeke M., Driss V., Sarazin A., Dendooven A., Kahn J.E., Lefèvre G., Capron M. IL-18 is involved in eosinophil-mediated tumoricidal activity against a colon carcinoma cell line by upregulating LFA-1 and ICAM-1. J. Immunol. 2015;195:2483–2492. doi: 10.4049/jimmunol.1402914. [DOI] [PubMed] [Google Scholar]
- Gonzalez V.D., Huang Y.W., Delgado-Gonzalez A., Chen S.Y., Donoso K., Sachs K., Gentles A.J., Allard G.M., Kolahi K.S., Howitt B.E., et al. High-grade serous ovarian tumor cells modulate NK cell function to create an immune-tolerant microenvironment. Cell Rep. 2021;36:109632. doi: 10.1016/j.celrep.2021.109632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu P., Gao J.F., D'Souza C.A., Kowalczyk A., Chou K.Y., Zhang L. Trogocytosis of CD80 and CD86 by induced regulatory T cells. Cell. Mol. Immunol. 2012;9:136–146. doi: 10.1038/cmi.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamieh M., Dobrin A., Cabriolu A., van der Stegen S.J.C., Giavridis T., Mansilla-Soto J., Eyquem J., Zhao Z., Whitlock B.M., Miele M.M., et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568:112–116. doi: 10.1038/s41586-019-1054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasim M.S., Marotel M., Hodgins J.J., Vulpis E., Makinson O.J., Asif S., Shih H.Y., Scheer A.K., MacMillan O., Alonso F.G., et al. When killers become thieves: trogocytosed PD-1 inhibits NK cells in cancer. Sci. Adv. 2022;8:eabj3286. doi: 10.1126/sciadv.abj3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner H., Frank C., Dechant C., Repp R., Glennie M., Herrmann M., Stockmeyer B. Intimate cell conjugate formation and exchange of membrane lipids precede apoptosis induction in target cells during antibody-dependent, granulocyte-mediated cytotoxicity. J. Immunol. 2007;179:337–345. doi: 10.4049/jimmunol.179.1.337. [DOI] [PubMed] [Google Scholar]
- Hudrisier D., Bongrand P. Intercellular transfer of antigen-presenting cell determinants onto T cells: molecular mechanisms and biological significance. FASEB J. 2002;16:477–486. doi: 10.1096/fj.01-0933rev. [DOI] [PubMed] [Google Scholar]
- Joly E., Hudrisier D. What is trogocytosis and what is its purpose? Nat. Immunol. 2003;4:815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- Kawashima M., Carreras J., Higuchi H., Kotaki R., Hoshina T., Okuyama K., Suzuki N., Kakizaki M., Miyatake Y., Ando K., et al. PD-L1/L2 protein levels rapidly increase on monocytes via trogocytosis from tumor cells in classical Hodgkin lymphoma. Leukemia. 2020;34:2405–2417. doi: 10.1038/s41375-020-0737-9. [DOI] [PubMed] [Google Scholar]
- Klueg K.M., Muskavitch M.A. Ligand-receptor interactions and trans-endocytosis of Delta, Serrate and Notch: members of the Notch signalling pathway in Drosophila. J. Cell Sci. 1999;112:3289–3297. doi: 10.1242/jcs.112.19.3289. [DOI] [PubMed] [Google Scholar]
- Kozakov D., Hall D.R., Xia B., Porter K.A., Padhorny D., Yueh C., Beglov D., Vajda S. The ClusPro web server for protein-protein docking. Nat. Protoc. 2017;12:255–278. doi: 10.1038/nprot.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozakov D., Brenke R., Comeau S.R., Vajda S. PIPER: an FFT-based protein docking program with pairwise potentials. Proteins. 2006;65:392–406. doi: 10.1002/prot.21117. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Dominguez R. Regulation of actin cytoskeleton dynamics in cells. Mol. Cells. 2010;29:311–325. doi: 10.1007/s10059-010-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K.J., Wu C.H., Lu C.H., Shen C.Y., Kuo Y.M., Tsai C.Y., Hsieh S.C., Yu C.L. Trogocytosis between non-immune cells for cell clearance, and among immune-related cells for modulating immune responses and autoimmunity. Int. J. Mol. Sci. 2021;22:2236. doi: 10.3390/ijms22052236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H.Y., Zhao W., Li X., Duan L., Powers A., Akamatsu M., Santoro F., McGuire A.F., Cui Y., Drubin D.G., Cui B. Membrane curvature underlies actin reorganization in response to nanoscale surface topography. Proc. Natl. Acad. Sci. USA. 2019;116:23143–23151. doi: 10.1073/pnas.1910166116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T., Ma R., Li Z., Mansour A.G., Teng K.Y., Chen L., Zhang J., Barr T., Caligiuri M.A., Yu J. Hijacking TYRO3 from tumor cells via trogocytosis enhances NK-cell effector functions and proliferation. Cancer Immunol. Res. 2021;9:1229–1241. doi: 10.1158/2326-6066.CIR-20-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlenkin A., Uzana R., Frankenburg S., Eisenberg G., Eisenbach L., Pitcovski J., Gorodetsky R., Nissan A., Peretz T., Lotem M. Capture of tumor cell membranes by trogocytosis facilitates detection and isolation of tumor-specific functional CTLs. Cancer Res. 2008;68:2006–2013. doi: 10.1158/0008-5472.CAN-07-3119. [DOI] [PubMed] [Google Scholar]
- MacNabb B.W., Tumuluru S., Chen X., Godfrey J., Kasal D.N., Yu J., Jongsma M.L.M., Spaapen R.M., Kline D.E., Kline J. Dendritic cells can prime anti-tumor CD8. Immunity. 2022;55:982–997.e8. doi: 10.1016/j.immuni.2022.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcenaro E., Cantoni C., Pesce S., Prato C., Pende D., Agaugué S., Moretta L., Moretta A. Uptake of CCR7 and acquisition of migratory properties by human KIR+ NK cells interacting with monocyte-derived DC or EBV cell lines: regulation by KIR/HLA-class I interaction. Blood. 2009;114:4108–4116. doi: 10.1182/blood-2009-05-222265. [DOI] [PubMed] [Google Scholar]
- Marciano-Cabral F., Zoghby K.L., Bradley S.G. Cytopathic action of Naegleria fowleri amoebae on rat neuroblastoma target cells. J. Protozool. 1990;37:138–144. doi: 10.1111/j.1550-7408.1990.tb05884.x. [DOI] [PubMed] [Google Scholar]
- Martens S., McMahon H.T. Mechanisms of membrane fusion: disparate players and common principles. Nat. Rev. Mol. Cell Biol. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- Martinez Sanz P., van Rees D.J., van Zogchel L.M.J., Klein B., Bouti P., Olsman H., Schornagel K., Kok I., Sunak A., Leeuwenburg K., et al. G-CSF as a suitable alternative to GM-CSF to boost dinutuximab-mediated neutrophil cytotoxicity in neuroblastoma treatment. J. Immunother. Cancer. 2021;9:e002259. doi: 10.1136/jitc-2020-002259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Martín N., Fernández-Arenas E., Cemerski S., Delgado P., Turner M., Heuser J., Irvine D.J., Huang B., Bustelo X.R., Shaw A., Alarcón B. T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity. 2011;35:208–222. doi: 10.1016/j.immuni.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda S., Iwasaki S., Tomaru U., Baba T., Katsumata K., Ishizu A. Possible implication of Fc γ receptor-mediated trogocytosis in susceptibility to systemic autoimmune disease. Clin. Dev. Immunol. 2013;2013:345745. doi: 10.1155/2013/345745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda S., Iwasaki S., Tomaru U., Sato J., Kawakami A., Ichijo K., Sogo S., Baba T., Katsumata K., Kasahara M., Ishizu A. Mechanism of Fcγ receptor-mediated trogocytosis-based false-positive results in flow cytometry. PLoS One. 2012;7:e52918. doi: 10.1371/journal.pone.0052918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlung H.L., Babes L., Zhao X.W., van Houdt M., Treffers L.W., van Rees D.J., Franke K., Schornagel K., Verkuijlen P., Janssen H., et al. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 2018;23:3946–3959.e6. doi: 10.1016/j.celrep.2018.05.082. [DOI] [PubMed] [Google Scholar]
- Mattei F., Andreone S., Marone G., Gambardella A.R., Loffredo S., Varricchi G., Schiavoni G. Eosinophils in the tumor microenvironment. Adv. Exp. Med. Biol. 2020;1273:1–28. doi: 10.1007/978-3-030-49270-0_1. [DOI] [PubMed] [Google Scholar]
- Miyake K., Karasuyama H. The role of trogocytosis in the modulation of immune cell functions. Cells. 2021;10:1255. doi: 10.3390/cells10051255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Shiozawa N., Nagao T., Yoshikawa S., Yamanishi Y., Karasuyama H. Trogocytosis of peptide-MHC class II complexes from dendritic cells confers antigen-presenting ability on basophils. Proc. Natl. Acad. Sci. USA. 2017;114:1111–1116. doi: 10.1073/pnas.1615973114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Nakayama M., Kawano M., Amagai R., Ishii T., Harigae H., Ogasawara K. Fratricide of natural killer cells dressed with tumor-derived NKG2D ligand. Proc. Natl. Acad. Sci. USA. 2013;110:9421–9426. doi: 10.1073/pnas.1300140110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M. Antigen presentation by MHC-dressed cells. Front. Immunol. 2014;5:672. doi: 10.3389/fimmu.2014.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliano O., Morrison R.M., Chauvin J.M., Banerjee H., Davar D., Ding Q., Tanegashima T., Gao W., Chakka S.R., DeBlasio R., et al. Tim-3 mediates T cell trogocytosis to limit antitumor immunity. J. Clin. Invest. 2022;132:e152864. doi: 10.1172/JCI152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinon P., Pärssinen J., Vazquez P., Bachmann M., Rahikainen R., Jacquier M.C., Azizi L., Määttä J.A., Bastmeyer M., Hytönen V.P., Wehrle-Haller B. Talin-bound NPLY motif recruits integrin-signaling adapters to regulate cell spreading and mechanosensing. J. Cell Biol. 2014;205:265–281. doi: 10.1083/jcb.201308136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi O.S., Zheng Y., Nakamura K., Attridge K., Manzotti C., Schmidt E.M., Baker J., Jeffery L.E., Kaur S., Briggs Z., et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J., Reichelt M., Wetzel S.A. Lymphocytes and trogocytosis-mediated signaling. Cells. 2021;10:1478. doi: 10.3390/cells10061478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström A., Eriksson M., Cerboni C., Johansson M.H., Sentman C.L., Kärre K., Höglund P. Acquisition of external major histocompatibility complex class I molecules by natural killer cells expressing inhibitory Ly49 receptors. J. Exp. Med. 2001;194:1519–1530. doi: 10.1084/jem.194.10.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somanchi S.S., Somanchi A., Cooper L.J.N., Lee D.A. Engineering lymph node homing of ex vivo-expanded human natural killer cells via trogocytosis of the chemokine receptor CCR7. Blood. 2012;119:5164–5172. doi: 10.1182/blood-2011-11-389924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Xiao D., Ni Y., Zhang T., Cao Z., Xu Z., Nguyen H., Zhang J., White G.C., Ding J., et al. Structure basis of the FERM domain of kindlin-3 in supporting integrin αIIbβ3 activation in platelets. Blood Adv. 2020;4:3128–3135. doi: 10.1182/bloodadvances.2020001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki E., Kataoka T.R., Hirata M., Kawaguchi K., Nishie M., Haga H., Toi M. Trogocytosis-mediated expression of HER2 on immune cells may be associated with a pathological complete response to trastuzumab-based primary systemic therapy in HER2-overexpressing breast cancer patients. BMC Cancer. 2015;15:39. doi: 10.1186/s12885-015-1041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabiasco J., Espinosa E., Hudrisier D., Joly E., Fournié J.J., Vercellone A. Active trans-synaptic capture of membrane fragments by natural killer cells. Eur. J. Immunol. 2002;32:1502–1508. doi: 10.1002/1521-4141(200205)32:5<1502::AID-IMMU1502>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Taylor R.P., Lindorfer M.A. Fcγ-receptor-mediated trogocytosis impacts mAb-based therapies: historical precedence and recent developments. Blood. 2015;125:762–766. doi: 10.1182/blood-2014-10-569244. [DOI] [PubMed] [Google Scholar]
- Tekguc M., Wing J.B., Osaki M., Long J., Sakaguchi S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2023739118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe-Querol E., Rosales C. The multiple roles of trogocytosis in immunity, the nervous system, and development. BioMed Res. Int. 2021;2021:1601565. doi: 10.1155/2021/1601565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustyanovska Avtenyuk N., Choukrani G., Ammatuna E., Niki T., Cendrowicz E., Lourens H.J., Huls G., Wiersma V.R., Bremer E. Galectin-9 triggers neutrophil-mediated anticancer immunity. Biomedicines. 2021;10:66. doi: 10.3390/biomedicines10010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rees D.J., Brinkhaus M., Klein B., Verkuijlen P., Tool A.T.J., Schornagel K., Treffers L.W., van Houdt M., Kater A.P., Vidarsson G., et al. Sodium stibogluconate and CD47-SIRPα blockade overcome resistance of anti-CD20-opsonized B cells to neutrophil killing. Blood Adv. 2022;6:2156–2166. doi: 10.1182/bloodadvances.2021005367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Spriel A.B., Leusen J.H., van Egmond M., Dijkman H.B., Assmann K.J., Mayadas T.N., van de Winkel J.G. Mac-1 (CD11b/CD18) is essential for Fc receptor-mediated neutrophil cytotoxicity and immunologic synapse formation. Blood. 2001;97:2478–2486. doi: 10.1182/blood.v97.8.2478. [DOI] [PubMed] [Google Scholar]
- van Spriel A.B., van Ojik H.H., Bakker A., Jansen M.J.H., van de Winkel J.G.J. Mac-1 (CD11b/CD18) is crucial for effective Fc receptor-mediated immunity to melanoma. Blood. 2003;101:253–258. doi: 10.1182/blood.V101.1.253. [DOI] [PubMed] [Google Scholar]
- Vanderven H.A., Jegaskanda S., Wheatley A.K., Kent S.J. Antibody-dependent cellular cytotoxicity and influenza virus. Curr. Opin. Virol. 2017;22:89–96. doi: 10.1016/j.coviro.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Vanherberghen B., Andersson K., Carlin L.M., Nolte-'t Hoen E.N.M., Williams G.S., Höglund P., Davis D.M. Human and murine inhibitory natural killer cell receptors transfer from natural killer cells to target cells. Proc. Natl. Acad. Sci. USA. 2004;101:16873–16878. doi: 10.1073/pnas.0406240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varricchi G., Galdiero M.R., Loffredo S., Lucarini V., Marone G., Mattei F., Marone G., Schiavoni G. Eosinophils: the unsung heroes in cancer? Oncoimmunology. 2018;7:e1393134. doi: 10.1080/2162402X.2017.1393134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velmurugan R., Challa D.K., Ram S., Ober R.J., Ward E.S. Macrophage-mediated trogocytosis leads to death of antibody-opsonized tumor cells. Mol. Cancer Ther. 2016;15:1879–1889. doi: 10.1158/1535-7163.MCT-15-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraghavan S., Lipfert L., Chevalier K., Bushey B.S., Henley B., Lenhart R., Sendecki J., Beqiri M., Millar H.J., Packman K., et al. Amivantamab (JNJ-61186372), an Fc enhanced EGFR/cMet bispecific antibody, induces receptor downmodulation and antitumor activity by monocyte/macrophage trogocytosis. Mol. Cancer Ther. 2020;19:2044–2056. doi: 10.1158/1535-7163.MCT-20-0071. [DOI] [PubMed] [Google Scholar]
- Wakim L.M., Bevan M.J. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471:629–632. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt H., Savić F., Verbeek S., Dietz J., Tarantola G., Oelkers M., Geil B., Janshoff A. Membrane fusion studied by colloidal probes. Eur. Biophys. J. 2021;50:223–237. doi: 10.1007/s00249-020-01490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.T., Kreutzberger A.J.B., Lee J., Kiessling V., Tamm L.K. The role of cholesterol in membrane fusion. Chem. Phys. Lipids. 2016;199:136–143. doi: 10.1016/j.chemphyslip.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.J., Li X.L., Wang D., Huang X.C., Mathis J.M., Duan W.M., Knight D., Shi R., Glass J., Zhang D.Q., et al. Trogocytosis of MHC-I/peptide complexes derived from tumors and infected cells enhances dendritic cell cross-priming and promotes adaptive T cell responses. PLoS One. 2008;3:e3097. doi: 10.1371/journal.pone.0003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Zhang L., Xiang S., Hu Y., Wu Z., Shen J. Gnawing between cells and cells in the immune system: friend or foe? A review of trogocytosis. Front. Immunol. 2022;13:791006. doi: 10.3389/fimmu.2022.791006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer J., Ioannidis V., Held W. H-2D ligand expression by Ly49A+ natural killer (NK) cells precludes ligand uptake from environmental cells: implications for NK cell function. J. Exp. Med. 2001;194:1531–1539. doi: 10.1084/jem.194.10.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.