Abstract
Aim
Characterize use and efficacy/effectiveness of virtual, augmented, or mixed reality (VR/AR/MR) technology as non-pharmacological therapy for chronic pain.
Methods
Systematic search of 12 databases to identify empirical studies, of individuals who experience chronic pain or illness involving chronic pain, published between 1990 and 2021. JBI Critical Appraisal Checklists assessed study bias and a narrative synthesis was provided.
Results
46 studies, investigating a total of 1456 participants and including 19 randomized controlled trials (RCT), were reviewed. VR/AR/MR was associated with improved pain-related outcomes in 78% of the RCTs.
Conclusion
While most studies showed effects immediately or up to one month post treatment, RCTs are needed to further evaluate VR/AR/MR, establish long-term benefits, and assess accessibility, especially among individuals who experience pain management disparities.
Keywords: : chronic conditions, chronic pain, disparities, pain management, technology, virtual reality
Plain language summary
Virtual, augmented and mixed reality (VR/AR/MR) are technologies that can be used to manage chronic pain. The use and effectiveness of VR/AR/MR were examined during a review of 46 research studies, which included 1456 participants and 19 randomized controlled trials (RCTs). In 78% of the RCTs, VR/AR/MR improved pain or pain-related outcomes. While most studies showed a benefit on pain immediately or up to 1 month after treatment, more research is needed to assess the long-term benefits of VR/AR/MR on pain and understand how these technologies provide pain relief in the body. Additionally, the accessibility and cost–effectiveness of VR/AR/MR must be evaluated. These areas for future research must consider individuals who experience disparities in the treatment of chronic pain.
Tweetable abstract
A systematic review of 46 studies, including 1456 participants and 19 RCTs, finds that virtual/augmented/mixed reality can have short-term benefits for individuals experiencing chronic pain. #VR/AR/MR #chronicpain
Chronic pain is a multidimensional health problem associated with reduced activity and productivity, disability, decreased quality of life, worsening chronic disease, psychological effects such as depression and anxiety and potential side effects and complications that may result from pain medications [1,2]. The International Association for the Study of Pain defines chronic pain as pain lasting or recurring for over 3 months [3]. In USA, approximately 50 million adults are affected by chronic pain and approximately 20 million experience high-impact chronic pain that often limits life or work activities [4]. The highest prevalence of chronic pain and high-impact chronic pain has been reported among women, individuals who live in rural areas, and older adults who were previously but not currently employed, experience financial instability and receive public health insurance [4]. Annually, chronic pain contributes to approximately US$560 to $635 billion in economic costs because of direct medical expenses, lost productivity and disability programs [5,6]. A multi-modal, multidisciplinary approach, such as the biopsychosocial care model, is required to manage chronic pain. This approach may include psychotherapy, complementary and integrative medicine, physical rehabilitation, interventional treatment and pharmacology [7]. Virtual reality (VR), augmented reality (AR) and mixed reality (MR) have emerged as promising, multi-modal, non-pharmacological approaches to pain management that are available to clinicians and individuals living with chronic pain.
The term ‘virtual reality’ was introduced in the late 1980's by computer scientist Jaron Lanier and it was popularized in the 1990's [8]. Virtual reality integrates computer graphics, body tracking and sensory input devices, visual displays, sounds and other sensations to create an immersive virtual environment [9]. People can engage with this computer-generated, simulated environment in several ways – such as by wearing a headset or head-mounted display (HMD), wearing goggles, or watching images projected onto a screen – and the degree of immersion varies with the type of equipment used to enter the environment. In the virtual environment, individuals can access various software programs (known as applications), including virtual gaming, exercise-based therapies, guided meditation and hypnosis. These applications can be operated via an increasing list of platforms, such as smartphones, computers, Microsoft's Xbox 360, Sony's PlayStation® VR and headsets, including Meta Quest's Oculus devices (such as the Oculus Quest) and HTC devices (such as the HTC VIVE) [10]. Augmented reality involves the real-time overlay of digital content on what a person sees in the real, physical world [11]. For example, a smartphone can be used on a city street to obtain information about buildings in one's field of vision [12] or individuals can play virtual games wherein they race toy cars on top of a table [13]. Augmented reality applications can be operated via smartphones, computers and projectors and AR glasses or headsets such as the Google Glass Enterprise Edition 2 and Oculus Quest 2. Mixed reality, a combination of VR and AR, allows individuals to see the real, physical world while also seeing virtual objects [11]. Applications for MR can be operated on similar platforms as VR and AR applications, and MR glasses such as the Microsoft HoloLens 2.
These technologies are hypothesized to work via various pathways to decrease chronic pain [14–16]. They promote distraction from chronic pain by diverting attention away from noxious stimuli and toward more pleasant or engaging stimuli [17]. They also provide a sense of control and can lead to possible cortical re-patterning, thereby producing analgesia [17,18]. In addition, VR/AR/MR-based approaches may serve to address factors that can exacerbate chronic pain by promoting behavioral skills for self-management and coping with pain. Because of these benefits, coupled with the creation of an immersive and engaging virtual environment, VR/AR/MR may be appealing, accessible, effective and scalable methods of implementing customized pain management for individuals at home, particularly for long-term chronic pain management.
Although several studies have demonstrated positive effects of VR/AR/MR on pain and pain-related outcomes, others have produced inconclusive evidence [19,20]. This systematic review was necessary because no comprehensive appraisal of the evidence has been published to date, and there are gaps in the literature regarding the use and efficacy/effectiveness of these technologies. A preliminary search of PROSPERO, MEDLINE, Cochrane Database of Systematic Reviews and JBI Database of Systematic Reviews and Implementation Reports revealed published reviews of VR effectiveness on musculoskeletal pain conditions, mental health and acute pain management. There are also ongoing reviews focused on VR effects in the context of rehabilitation programs (e.g., stroke, phantom limb pain and chronic pain), inpatient settings, cancer pain, burn injury and procedural pain. Yet, no current or in-progress systematic reviews specific to chronic pain across the pediatric and adult lifespan were identified. In addition, VR/AR/MR applications for pain have typically been used in clinic or hospital settings, but cost reductions and advances in the technology have created the potential for use at home [21]. A systematic review of the available VR/AR/MR studies for chronic pain will provide evidence for improving research and practice by informing the future development of VR/AR/MR-based interventions for chronic pain.
The overarching objective of this review is to evaluate the use and efficacy/effectiveness of VR/AR/MR technology, versus usual care or control (where possible), for chronic pain and pain-related outcomes. The following review questions were addressed among children, adolescents, and adults with chronic pain conditions:
What are the types of VR/AR/MR applications or software that are used for pain management?
What are the characteristics of VR/AR/MR applications or software that are used for pain management?
How are VR/AR/MR applications or software used for pain management?
What is the mechanism of action of VR/AR/MR interventions for reducing pain?
Are VR/AR/MR interventions efficacious and cost-effective for pain management?
Methods
We conducted this systematic review by following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [22] and the Joanna Briggs Institute (JBI) methodology for systematic reviews of effectiveness evidence [23]. An a priori protocol was registered at PROSPERO 2019 (CRD42019117469).
Inclusion & exclusion criteria
This review considered experimental, quasi-experimental and non-experimental studies of children, adolescents, and adults of all ages and genders who experience a chronic condition or illness involving chronic pain, persistent pain, or recurrent pain that lasted for more than 3 months. Non-cancer chronic pain (primary) and chronic cancer pain (secondary) were included. Because of the focus on chronic pain, this review did not include studies wherein participants experienced acute, procedural, experimental, burn or postoperative pain. We considered studies that compared the intervention to usual care or a control condition, and evaluated VR, AR and/or MR technology for chronic pain and any pain-related outcomes. Pain-related outcomes include physical functionality, activities of daily living and quality of life. Among the methods of outcome measurement were validated instruments, observation and self-report.
Search strategy
A comprehensive literature search was undertaken to identify relevant, published studies. Search strategies were developed and conducted by an experienced medical librarian with input from the research team in accordance with the PRISMA guidelines [22] and were peer-reviewed by another medical librarian. Pre-identified sentinel articles were hand searched for keywords relating to the study objectives. The searches combined controlled vocabulary supplemented with keywords related to the concepts of chronic pain (e.g., intractable pain, persistent pain and recurrent pain), pain management (e.g., decreased pain, increased physical functioning and improved quality of life) and the intervention of VR (e.g., AR, virtual environment and immersive display). The search terms were then translated for each additional literature database and grey literature resource appropriate for the study topic. Searches were undertaken 3 October 2018, and rerun on 14 June 2021 and 23 November 2021. The searches were limited to English language and year of publication between 1 January 1990 and 31 December 2021. Prior to 1990, VR was used as a computer and gaming interface and its utilization in healthcare became popularized during the 1990's [8]. Reference lists in selected articles were also screened for additional studies.
12 bibliographic databases were searched: EBSCO's Business Source Complete, CINAHL, PsycInfo and Science and Technology Collection, Cochrane Database of Systematic Reviews, Embase.com, IEEE Xplore, JBI EBP Database, ProQuest Dissertations and Theses Global, MEDLINE via PubMed, Scopus.com and Web of Science Core Collection. The five grey literature sources searched were National Technical Reports Library, Open Grey, Papers First, Proceedings First, PROSPERO and REHABDATA. Clinical trials registries searched were Cochrane Central Register of Controlled Trials and ClinicalTrials.gov. The full electronic search strategies for all sources are provided in Supplementary Table 1. After the searches, all identified citations were collated and uploaded into EndNote X9 (Clarivate Analytics, PA, USA) and duplicates were manually removed.
Assessment of methodological quality
First, the primary reviewer screened the articles selected for retrieval. Eligible studies were then critically appraised independently by all clinical authors and non-author reviewers for methodological quality using JBI standardized critical appraisal instruments for randomized controlled trials (RCTs), quasi-experimental studies, analytical cross-sectional studies, case reports and case series [24]. The certainty of the evidence was subsequently assessed with the Grading of Recommendations, Assessment, Development, and Evaluation approach [25]. Lastly, the primary reviewer examined all the articles and critical appraisal instruments completed by the other reviewers. Any disagreements among the independent reviews were resolved by the decision of the primary reviewer.
Selected studies were included in the review if they met the minimum criteria: seven out of 13 items on the JBI Critical Appraisal Checklist for Randomized Controlled Trials, five out of nine items on the JBI Critical Appraisal Checklist for Quasi-Experimental Studies (non-randomized experimental studies), five out of eight items on the JBI Critical Appraisal Checklist for Analytical Cross-Sectional Studies, five out of eight items on the JBI Critical Appraisal Checklist for Case Reports and six out of 10 items on the JBI Critical Appraisal Checklist for Case Series [24]. Minimum criteria were checklist items identified as the most important methodological criteria based on each study design. For example, minimum criteria for RCTs included randomization, similarity of treatment groups at baseline, similar treatment of groups except for the intervention of interest, intent-to-treat analysis, reliable measurement of outcomes, appropriate statistical analysis and appropriate trial design.
Data extraction
Data were independently extracted from included studies by all clinical authors and non-author reviewers using a researcher-developed tool that is provided in Supplementary Table 2. This tool, which expanded on the standardized JBI Data Extraction Form [26], was used to collect data specifically related to the review's purpose and objectives. Extracted data included specific details about the study populations, methods, interventions and outcomes of significance for the review objectives. To minimize errors after data extraction, the primary reviewer checked the data and clarified any discrepancies by reviewing the respective articles.
Data synthesis
A statistical meta-analysis of the data was not possible due to the heterogeneity of the study populations, interventions and comparators, outcome measurements and data analysis across the studies. Therefore, we utilized a vote-counting approach based on the direction of the effect reported in each RCT. A sign test was conducted, and a 95% confidence interval (CI) was computed for the RCTs included [27]. Statistical significance was p < 0.05. Additionally, characteristics of all included studies have been presented and discussed in narrative form, including tables (see Table 1) where appropriate.
Table 1. . Characteristics of included studies.
| Study (year), country | Study design, study duration, and post-intervention follow-up | Sample size and population | Interventions (I) and control condition or comparator (C) included in the study | Outcomes reported | Ref. |
|---|---|---|---|---|---|
| Randomized controlled trials | |||||
| Austin et al. Australia | Randomized, cross-over trial; 1 day; no follow-up | 16 adults (≥18 years old) with spinal cord injury and chronic neuropathic pain | I: 3D, head-mounted delivery of virtual environment C: 2D screen application of virtual environment |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[28] |
| Darnall et al. USA | Pilot RCT investigating feasibility and efficacy; 21 days; follow-up at 1 day post intervention | 74 adults (ages 25–74 years old) with chronic back pain and fibromyalgia | I: 21-day, skills-based, self-management program based on principles of CBT, biofeedback, and mindfulness delivered via VR C: Audio delivery of 21-day, skills-based, self-management program |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: Yes Efficacy: Yes Cost–effectiveness: No |
[29] |
| Garcia et al. USA | Randomized, placebo-controlled trial; 56 days; no follow-up | 179 community-dwelling adults (ages 18–81 years old) with chronic low back pain | I: 8-week, 3D, immersive, VR pain self-management program that incorporates principles of CBT, mindfulness, and pain neuroscience education C: 8-week, non-immersive delivery of 2D nature footage and neutral music via Sham VR headset |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[30] |
| Garcia-Palacios et al. Spain | Pilot RCT investigating feasibility, acceptability, and preliminary efficacy; 3 weeks; follow-up at 3 weeks post intervention | 61 adults (ages 23–70 years old) with fibromyalgia syndrome | I: Group CBT program with VR as an addition to activity pacing C: Treatment as usual (follow-up sessions with a rheumatologist for review of medication treatment) |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[19] |
| Jeon et al. Korea | Pilot pre-test and post-test study; 1 day; no follow-up | 10 adults (ages 28–50 years old) with complex regional pain syndrome type I | I: Body swapping training video presented via VR, with mental rehearsal C: Body swapping training video presented via VR, without mental rehearsal |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[31] |
| Jin et al. Canada | Randomized, controlled crossover study; 1 day; no follow-up | 20 adults (ages 30–75 years old) with chronic pain | I: Immersive VR game C: Self-mediated control with typical pain distraction activities used daily (e.g., reading, meditating, and playing a mobile game) |
Pain: Yes Pain-related outcomes: No Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[32] |
| Lewis et al. United Kingdom | RCT; 6 weeks; follow-up at 2 weeks post intervention | 45 adults (ages 18–78 years old) with complex regional pain syndrome and body perception disturbance | I: Visual illusions with digital manipulation of participants' hands using a mediated VR device C: Display of visual images, via a mediated VR device, without digital manipulation of participants' hands |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[33] |
| Matheve et al. Belgium | RCT; 1 day; no follow-up | 48 adults (ages 18–65 years old) with chronic, nonspecific low back pain | I: Non-immersive VR games controlled by performing pelvic tilt exercises C: Performing pelvic tilt exercises, without VR games, according to a beep tone |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[34] |
| Nambi et al. Saudi Arabi | RCT; 4 weeks; follow-up at 6 months post intervention | 60 adult university football players (ages 18–25 years old) with chronic low back pain | I #1: VR training (physical therapy using VR) with a VR game controlled by trunk movements I #2: Isokinetic training performed in an isokinetic dynamometer C: Conventional training of core muscles of the trunk, with stretching |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[35] |
| Nambi et al. Saudi Arabi | RCT; 4 weeks; follow-up at 6 months post intervention | 54 adult university soccer players (ages 18–25 years old) with chronic low back pain | I #1: VR balance training, focused on core stability muscles, with a VR game controlled by trunk movements I #2: Combined physical rehabilitation using a Swiss ball for balance training of core stability muscles C: Conventional balance training (isotonic and isometric exercises) for core muscles, with stretching |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[36] |
| Nambi et al. Saudi Arabia | RCT; 4 weeks; follow-up at 8 weeks post intervention and 6 months post intervention | 45 adult university football players (ages 18–45 years old) with chronic low back pain | I #1: VR balance training, focused on core stability muscles, with a VR game controlled by trunk movements I #2: Isokinetic training performed in an isokinetic dynamometer C: Conventional balance training (isotonic and isometric exercises) for core muscles, with stretching |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[37] |
| Nusser et al. Germany | RCT; 3 weeks; no follow-up | 55 adults (≥18 years old) with non-traumatic chronic neck pain | I #1: Standard rehabilitation program (involving individual and group, general and neck-specific exercise therapy) and individual neck-specific sensorimotor training using a VR device I #2: Standard rehabilitation program and general sensorimotor training (skill exercises, balance exercises, and games) C: Standard rehabilitation program |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[38] |
| Rezaei et al. Iran | RCT; 4 weeks; follow-up at 5 weeks post intervention | 42 adults (ages 22–46 years old) with non-specific chronic neck pain | I: VR video game, with increasing stages of difficulty, controlled by participants' head movements C: Conventional proprioceptive training (exercises included eye-follow, gaze stability, eye–head coordination and position sense, and movement sense) |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[39] |
| Rothgangel et al. The Netherlands | RCT; 10 weeks; follow-up at 6-months post intervention | 75 adults (ages 44–74 years old) with a unilateral lower limb amputation who experience phantom limb pain | I #1: Traditional mirror therapy followed by tele-treatment at home with AR mirror therapy I #2: Traditional mirror therapy followed by self-delivered mirror therapy C: Sensorimotor exercises without mirror therapy followed by self-delivered sensorimotor exercises |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[20] |
| Sarig Bahat et al. Australia | RCT; 4 weeks; follow-up at 3 months post intervention | 90 adults (≥18 years old) with chronic neck pain | I #1: VR kinematic training, with activity in the virtual environment controlled by participants' head movements I #2: Kinematic training using a head-mounted laser beam and wall poster C: Wait-list control |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[40] |
| Sarig Bahat et al. Australia | Pilot RCT; 5 weeks; follow-up at 3 months post intervention | 32 adults (ages 26–55 years old) with chronic neck pain | I: Kinematic and VR training, with activity in the virtual environment controlled by participants' head movements C: Kinematic training using a head-mounted laser beam and wall poster |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[41] |
| Tejera et al. Spain | RCT; 4 weeks; Follow-up at 1 month post intervention and at 3 months post intervention | 44 adults (ages 18–65 years old) with non-specific chronic neck pain | I: VR treatment, with activity in the virtual environment controlled by participants' neck movements C: Exercise treatment, with flexion, extension, rotation, and tilt exercises |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[42] |
| Venuturupalli et al. USA | Pilot, randomized, cross-over study investigating feasibility; 1 day; no follow-up | 17 adults (≥18 years old) with physician-diagnosed autoimmune disorders and chronic pain | I: VR respiratory biofeedback environment C: VR guided mediation environment |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[43] |
| Yilmaz Yelvar et al. Turkey | RCT; 2 weeks; no follow-up | 44 adults (ages 35–64 years old) with subacute and chronic, non-specific low back pain | I: Traditional physical therapy program (involving hot pack, TENS, deep heat with ultrasound, and therapeutic exercises) with integration of a 15-minute VR walking video C: Traditional physical therapy program |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[44] |
| Quasi-experimental studies | |||||
| Alemanno et al. Italy | Pre-test and post-test study; 4–6 weeks; no follow-up | 20 adults (ages 19–72 years old) with chronic low back pain | I: VR-based sensorimotor rehabilitation using an avatar C: None |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[45] |
| Botella et al. Spain | Pre-test and post-test study; 7 weeks; follow-up at 6 months post intervention | 6 adults (47–65 years old) with fibromyalgia | I: Group CBT program with VR-based relaxation and mindfulness C: None |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[46] |
| Fowler et al. USA | Implementation-effectiveness, pre-test and post-test study; 3 weeks; no follow-up | 16 adult veterans (ages 28–63 years old) with chronic pain | I: VR distraction and exposure therapy, with increasing intensity of stimulation and movement C: None |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[47] |
| Glavare et al. Sweden | Pre-test and post-test study; 6 weeks; no follow-up | 12 adults (ages 18–65 years old) with chronic neck pain |
I: Neck range of motion exercises using VR, with increasing levels of difficulty C: None |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[48] |
| Hennessy et al. USA | Pilot study investigating content, usability, safety, and acceptance; 1 week; follow-up at 3–5 days post-intervention | 12 adults (ages 43–60 years old) with chronic low back pain | I: VR walking modules with progressive movement exposure C: None |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[49] |
| House et al. USA | Feasibility study; 8 weeks; follow-up at 8 weeks post intervention | 6 adults (ages 22–78 years old), with upper body chronic pain post breast cancer surgery | I: Integrative VR rehabilitation games, with increasing stages of difficulty C: None |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[50] |
| Igna et al. Romania | Pre-test and post-test study; 3 weeks; no follow-up | 68 adults (ages 24–74 years old) with chronic back pain | I #1: Physiotherapy, medication, and mindfulness-based CBT I #2: Physiotherapy, medication, and VR-enhanced CBT C: Usual pharmacological and physiotherapy treatment |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[51] |
| Jones et al. USA | Pre-test and post-test study; 1 day; no follow-up | 30 adults (ages 35–79 years old) with various chronic pain conditions | I: Immersive, 360-degree, VR fantasy landscape C: None |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[52] |
| Liu et al. USA | Preliminary study investigating efficacy; 1 day; no follow-up | 31 adults (ages 20–81 years old) with migraines, headaches, or other forms of chronic pain (not specified) | I: VR-guided meditation C: None |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[53] |
| Matamala-Gomez et al. Spain | Pre-test and post-test study; 1 day; no follow-up | 19 adults (ages 40–55 years old) with complex regional pain syndrome type 1 or type 2 | I: Observation of virtual arm, with varying levels of transparency and size C: None |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[54] |
| Mouraux et al. Belgium | Preliminary, pre-test and post-test study; 1 week; follow-up at 24 hours post intervention | 22 adults (ages 18–75 years old) with chronic neuropathic pain | I: 3D, AR, mirror visual feedback therapy, with training exercises and virtual games of increasing levels of difficulty C: None |
Pain: Yes Pain-related outcomes: No Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[55] |
| Ortiz-Catalan et al. Sweden and Slovenia | Pre-test and post-test study; 6 weeks; follow-up at 1 month post intervention, 3 months post intervention, and 6 months post intervention | 14 adults (ages 26–74 years old) with chronic, intractable phantom limb pain | I: Phantom motor execution using myoelectric pattern recognition, AR, and VR, with virtual games controlled by phantom movements C: None |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[56] |
| Putrino et al. USA | Pilot study; duration was not reported; no follow-up | 8 adults (ages 44–71 years old) with neuropathic pain | I: Exposure to a scenic VR environment and a somatic VR environment (involving upper and lower extremity movements) C: None |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[57] |
| Roosink et al. Canada | Proof-of-principle and feasibility study; 2 weeks; no follow-up | 9 adults (ages 25–72 years old) with spinal cord injury and neuropathic pain |
I: Interactive VR walking using an avatar, with virtual feedback C: Static presentation of a virtual scene |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[58] |
| Rutledge et al. USA | Feasibility study; duration was not reported; no follow-up | 14 adult veterans (ages 37–76 years old) with an upper or lower extremity amputation, who experience phantom limb pain | I: Bicycling through a VR environment, as an avatar, using a bicycle pedaler and a customized pedal for prosthesis C: None |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[59] |
| Shiri et al. Israel | Pre-test and post-test study; duration was not reported; follow-up at 1 month post intervention and 3 months post intervention | 10 adolescents (ages 10–17 years old) with chronic headache | I: VR relaxation combined with biofeedback (tracking of galvanic skin response) C: None |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[60] |
| Solcà et al. Switzerland | Pre-test and post-test, crossover study; 1 day; no follow-up | 48 adults (ages 23–80 years old) with complex regional pain syndrome | I: Mirror therapy using synchronous heartbeat-enhanced VR (virtual hand flashing in synchrony with heartbeat) C: Mirror therapy using asynchronous heartbeat-enhanced VR |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[61] |
| Trost et al. USA | Pilot study investigating feasibility and preliminary efficacy; 2 weeks; follow-up at 7 days post intervention and at 2 weeks post intervention | 27 adults (ages 23–70 years old) with complete paraplegia after spinal cord injury and neuropathic pain | I: Immersive, spatially tracked, VR walking (using an avatar), with virtual games C: View of avatar in 360-degree virtual scene with no control over virtual walking |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[62] |
| Villiger et al. Switzerland | Pre-test and post-test study; 4 weeks; follow-up at 12–16 weeks post intervention | 14 adults (ages 28–71 years old) with neuropathic pain from chronic, incomplete spinal cord injury | I: VR-augmented neurorehabilitation, with VR tasks (of increasing stages of difficulty) for muscle training C: None |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[63] |
| Won et al. USA | Pilot study investigating usability, acceptance, ease of use, and engagement; duration was not reported; follow-up at 1 month post intervention | 9 adults (ages 19–60 years old) with complex regional pain syndrome | I: VR mirror visual feedback module, with avatar hands C: None |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[64] |
| Zauderer et al. France | Pilot and feasibility study; 3 months; no follow-up | 18 adults (≥18 years old) with non-specific chronic neck pain | I: Standardized, immersive, VR exercise therapy (including active cervical spine range of motion and eye-neck coordination exercises) and non-immersive VR exercise therapy (aerobic, mobility, and muscle strengthening exercises, and a personalized, home-based exercise program) C: None |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[65] |
| Analytical cross-sectional study | |||||
| Solcà et al. USA | Cross-sectional, prospective, intervention study; 2 days; no follow-up | 15 adults (ages 33–61 years old) with chronic leg pain | I #1: Personalized, visual, VR feedback of perceived SCS-induced paresthesia displayed on patient's virtual body I #2: Personalized, visual, VR feedback with rotation of the virtual body and spatial misalignment between visual VR feedback and SCS-induced paresthesia C: VR illumination of body with no SCS-induced paresthesia |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[66] |
| Case reports | |||||
| Ambron et al. USA | Pre-test and post-test study; 6 weeks; no follow-up | 2 adults (specific ages were not provided) with unilateral transtibial amputation who experience phantom limb pain | I: VR games, of increasing levels of difficulty, using robot avatar legs controlled by participants' lower limb movements C: None |
Pain: Yes Pain-related outcomes: No Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[67] |
| Oneal et al. USA | Pre-test and post-test study; 6 months; follow-up at 1 month post intervention | 1 adult (age 36 years old) with chronic neuropathic pain from spinal cord injury | I: VR hypnosis and self-hypnosis at home between VR sessions C: Previous trial of standard hypnosis conducted with participant |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[68] |
| Ortiz-Catalan et al. Sweden | Pre-test and post-test study; 18 weeks; no follow-up | 1 adult (age 72 years old) with an amputated limb who experiences phantom limb pain | I: AR, with the use of a virtual limb to play a game controlled by phantom motions C: None |
Pain: Yes Pain-related outcomes: No Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[69] |
| Case series | |||||
| Garrett et al. Canada | Exploratory, mixed-methods, pre-test and post-test study; 4 weeks; follow-up at 6 hours post intervention and 24 hours post intervention | 8 adults (ages 31–71 years old) with chronic pain | I: VR-based mindfulness and meditation, exposure to a VR fantasy landscape and a scenic VR environment, and virtual problem-solving games C: None |
Pain: Yes Pain-related outcomes: Yes Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[14] |
| Sato et al. Japan | Pre-test and post-test study; duration was not reported; no follow-up | 5 adults (ages 46–74 years old), with complex regional pain syndrome | I: Non-immersive, VR mirror visual feedback therapy, using an avatar hand, with hand exercises C: None |
Pain: Yes Pain-related outcomes: No Mechanism of action: No Efficacy: Yes Cost–effectiveness: No |
[70] |
AR: Augmented reality; C: Control condition or comparator; CBT: Cognitive behavioral therapy; I: Interventions; RCT: Randomized controlled trials; SCSVR: Virtual reality.
Results
Study inclusion
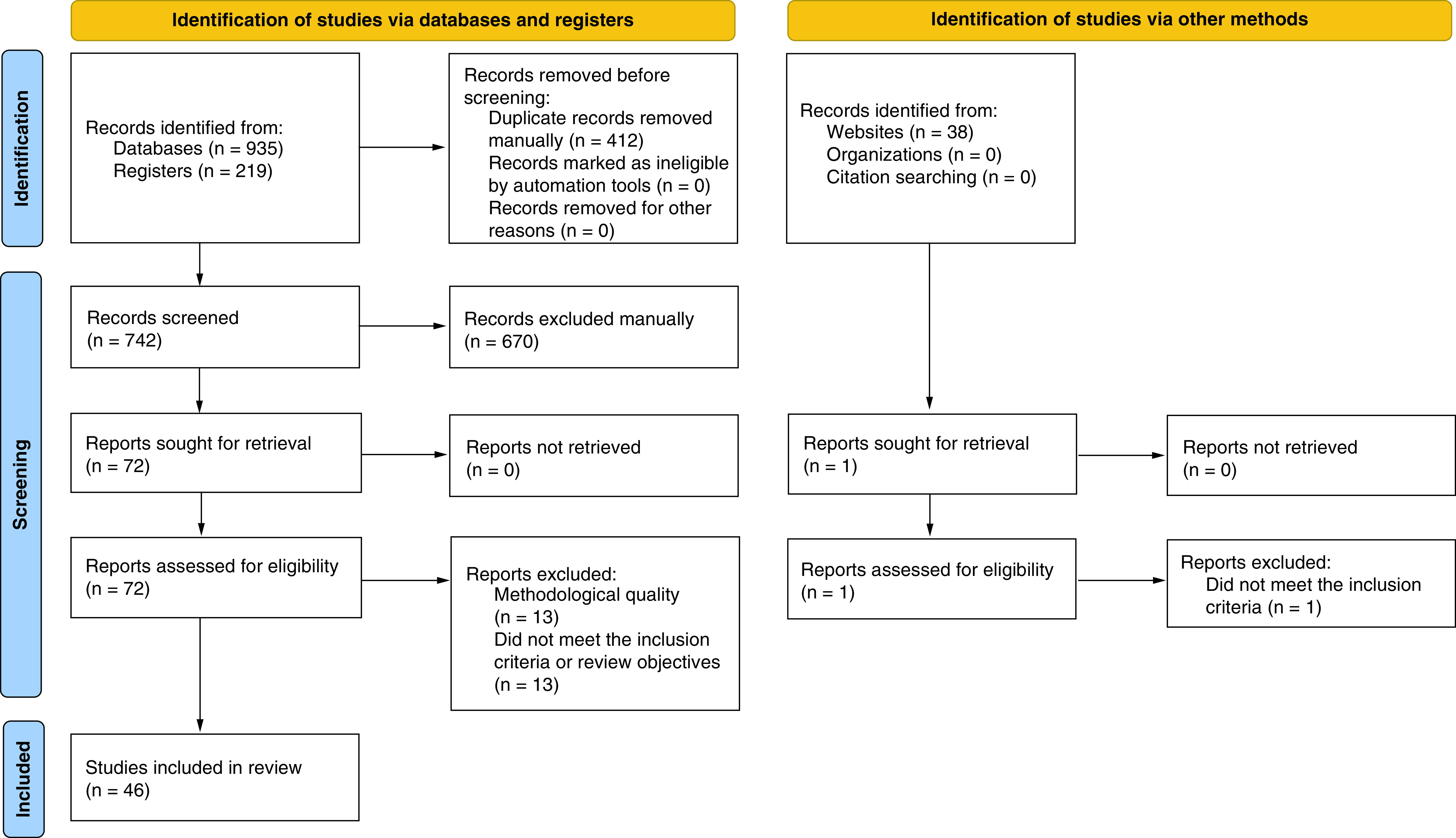
A total of 1192 articles were identified through the searches. Duplicates (412) were excluded, leaving 780 articles to be screened in the initial title and abstract screening phase. The results were exported to an EndNote library and reviewed by the clinical authors. After excluding 707 articles based on the title and abstract because of unmet inclusion criteria or review objectives, 73 articles were eligible for full-text review and critical appraisal. An additional 14 articles were excluded during the full-text review phase, leaving 59 articles that met all the eligibility criteria for inclusion. After assessing the articles for methodological quality using the JBI standardized critical appraisal instruments [24], 46 were retained for inclusion in this review. Figure 1 shows the PRISMA flow diagram [71].
Figure 1. . PRISMA flow diagram of study selection process.
Characteristics of included studies
The 46 studies that were reviewed include 19 RCTs [19,20,28–44], 21 quasi-experimental studies [45–65] one analytical cross-sectional study [66] three case reports [67–69] and two pilot case series [14,70]. The total sample size for these studies was 1456 and the number of participants in the individual studies ranged from one [68,69] to 179 [30]. Characteristics of the studies are summarized in Table 1.
Of the studies, 42 included virtual reality, two included augmented reality and two included mixed reality. Among the 19 RCTs included in this review, the type of VR/AR/MR intervention, intervention duration and the control condition varied widely, including interventions without VR/AR/MR and treatment as usual. For example, four RCTs examined VR-based physical therapy approaches in comparison to in-person approaches [39–41,44], three compared virtual behavioral therapies to in-person therapies (e.g., cognitive behavioral therapy [CBT] and mirror therapy) [17,19,20,27,31,45] and one study compared the use of immersive VR gaming for distraction to self-mediated distraction interventions [32]. The follow-up period varied across the studies and ranged from 6 hours to 6 months. In 24 studies, there was no follow-up beyond the immediate post-intervention period.
All the studies, except one [60], involved adult participants who were ages 18 years and older. Chronic pain conditions were not mutually exclusive and were listed as: chronic back pain (n = 10), neuropathic pain (n = 8), chronic neck pain (n = 7), phantom limb pain (n = 6), complex regional pain syndrome (n = 5), fibromyalgia (n = 4), chronic pain (n = 2), various chronic pain conditions including headaches (n = 2), chronic pain syndrome (n = 1), rheumatoid arthritis and systemic lupus erythematosus (n = 1), chronic leg pain (n = 1), and upper body chronic pain post cancer surgery (n = 1). In the study involving adolescents, participants were ages 10–17 and they experienced chronic headache [60].
Of the participants in the included studies, 708 (48.6%) were females and 650 (44.6%) were males. It was unclear what genders were involved in four studies [34,38,53,65] because participants were either reported as females or males with no other gender categories specified. In another study, the gender for one participant was reported as ‘Other’ [30]. Four studies had 100% females and six studies had 100% males. In two articles, data was not reported regarding gender [57,68].
Most of the articles (n = 32) did not provide data on race, ethnicity, or other sociodemographic factors (e.g., education, employment, and income). In eight studies, most of the participants were White [19,29,30,43,47,50,52,64] and in two studies, most of the participants were Black [49,62]. In one article, five participants were described as non-White and no data on race were reported for the remaining participants [59]. In another article, 96% of the participants were reported as White and no data were reported regarding the other participants' race [52].
Outcomes of interest and data collection instruments varied across the studies. All the studies included the reduction of pain and/or improvement of pain-related outcomes as study outcomes. Of the studies, 46 investigated the reduction of pain, 41 investigated the improvement of pain-related outcomes and 24 studies also evaluated the feasibility and/or acceptability of the technologies. Across the studies, pain-related outcomes included fear of movement, range of motion and kinematics, pain-related functional limitations or interference, emotional distress (such as depression), health status, daily functioning, functional disability, coping skills and quality of life. Outcomes related to feasibility and/or acceptability included acceptability of and satisfaction with VR, adverse effects or side effects and preferences in the type of VR experience.
Methodological quality
The overall quality of the RCTs was moderate, with a low risk of bias for most of the studies. Low risk of bias (or bias not serious) was related to having few study limitations such as the lack of blinding, a control group or follow-up. There was one RCT with a moderate risk of bias that was related to a lack of blinding of participants to treatment assignment, lack of blinding of those delivering treatment and lack of follow-up [44]. However, the authors reported that the participants and therapists were not blinded because of the nature of the intervention [44]. It was unclear whether at least one criterion was met in five RCTs because the information was not reported. These criteria included concealment of allocation to treatment groups, blinding of participants to treatment assignment, blinding of treatment assignment among those delivering treatment and blinding of outcome assessors to treatment assignment [29,31,32,39,43]. In two of these RCTs, it was unclear whether true randomization was used because the process of random assignment was not described [31,32]. Results of the critical appraisal assessments are provided in Supplementary Tables 3–7.
Results of the included studies consisted of both positive and negative findings; thus, publication bias was undetected. However, given the limitations of the included studies that were described above, the certainty of the evidence ranged from low to high with most of the studies demonstrating low certainty. This classification indicates that further research is highly likely to influence the confidence in the estimate of effect and is likely to change the estimate [25].
Review findings
The characteristics of the included studies are presented in Table 1 and a summary that addresses the sub-questions of this review are provided below.
Types of VR/AR/MR applications or software used for chronic pain management
The types of technology varied across the studies. Of the VR studies, 13 utilized immersive HMDs or headsets [28–31,42,43,47,48,53,57,59,61,65], 13 utilized immersive HMDs or headsets that were either tethered to computers and external cameras or required a computer to operate the software [14,32,38,40,41,49,52,54,59,62,64,66,67] and 14 utilized a desktop or laptop and displayed the non-immersive virtual environment on a desktop or laptop monitor, projector screen, or other screen [19,33–37,39,45,46,50,58,60,63,70]. One study utilized a device that was described as a VR helmet [68]; however, it was unclear whether the device was tethered or not. One study utilized a tethered, immersive HMD then transitioned to a portable VR headset and smartphone, when they became available, for participants' use at home if desired [59]. In one study, participants used video glasses to watch a virtual video on an iPod [44]. In another study, the VR device was not described [51].
Two studies used a non-immersive AR or MR system, consisting of a desktop computer and camera and presented the environment on a computer screen [55,56]. In one study, a VR and myoelectrically-controlled AR environment was presented on a computer screen [69]. Another study utilized a tablet with a built-in camera to display the AR environment [20].
Characteristics of VR/AR/MR applications or software used for chronic pain management
Several devices and systems were used in the studies. The most utilized hardware to deliver VR across studies was the HMD, predominantly the Oculus Rift® (n = 12). Other HMDs included the HTC VIVE and Samsung Oculus Gear VR, among others. These devices have built-in stereoscopic screens, which display separate images for each eye and sound and motion tracking sensors [72]. Other hardware used in the studies, such as the Wrap™1200VR and the Wrap920, are digital video eyewear products typically designed for AR applications [73]. Head-mounted displays and eyewear devices provide immersive video experiences for users. Systems included the Virtual Reality Rehabilitation System and the BrightArm Duo Rehabilitation System – an experimental robotic platform that modulates gravity loading on the upper extremities [50]. These types of systems are like the Microsoft Kinect and Nintendo Wii because they integrate haptics and projected images or avatars on screens so that users' motions are mimicked.
Various VR/AR/MR environments were used across the studies. In the context of this review, VR environment is a broad term that refers to a digital setting capable of arousing feelings of presence and immersion in VR/AR/MR users. Environments included VR or AR games, rehabilitation games or training exercises, VR programs or applications (such as a guided meditation application), VR experiences with and without gaming elements, software (such as a reality substitution software) and environments (such as a simulator for chronic pain treatment). While often designed for pleasure, VR games can also have therapeutic applications, such as distraction to mitigate painful experiences [74]. When VR/AR applications are used with sensors and haptics in rehabilitative settings to improve users' physical or cognitive functioning, they may be referred to as rehabilitation games, rehabilitation training, or exergaming [75]. As seen in the studies included in this review, the level of immersion in the VR environment can range from the projection of images on desktops or across screens in entire rooms to the use of avatars via HMDs.
Approaches for using VR/AR/MR applications or software for chronic pain management
The included studies applied a variety of approaches to using VR/AR/MR technology for chronic pain management. The approaches were not mutually exclusive and included: coping with chronic pain and/or associated psychosocial correlates (n = 14); rehabilitation therapy (physical or neuro rehabilitation) (n = 10); mirror therapy (n = 7); adjunct/enhancement to CBT (n = 4) or to replace guided imagery (n = 1) in the psychological treatment of pain; gaming (n = 3); virtual feedback or biofeedback (n = 3); prediction of motion intent (n = 2); visual feedback therapy or visual representation of spinal cord stimulation-induced paresthesia to enhance analgesia (n = 2); meditation and relaxation to reduce chronic pain and/or stress (n = 2); adjunct to activity management (n = 1) or an adjunct home therapy in chronic pain management (n = 1); graded exposure therapy for kinesiophobia (n = 2) and hypnosis (n = 1). Of the studies, 84.8% (n = 39) were conducted within a healthcare or research setting, such as a clinic or laboratory, while 15.2% (n = 7) were home-based. A group format was used to deliver the intervention in two studies [19,46].
Types of experiences that were provided by the VR/AR/MR applications or software were active (n = 25), passive (n = 14), or both (n = 7). Active experiences enabled participants to engage with interactive elements in the VR/AR/MR environment by completing specific tasks, such as shooting snowballs at targets. In contrast, passive or relaxing experiences allowed for immersion in the VR/AR/MR environment without active interaction, such as ‘traveling’ through the environment on a boat ride. The frequency or timing of VR/AR/MR delivery was two or more times in approximately 93.5% (n = 43) of the studies, with exposure to the VR/AR/MR environments, or dose, during each period of use ranging from one minute [33] to 2 hours [46]. The 2-hour experience was a group session in which a computer display, not an HMD, was used. In one study, participants were free to use the AR tele-treatment at home for their desired length and frequency [20]. However, participants used a tablet, not an HMD, to complete the tele-treatment. In another study, there was no set time limit for use of the technology, but the virtual environment was presented on a desktop monitor instead of an HMD [70]. Although the study duration was reported in three of the articles, the specific duration of VR/AR/MR use was not reported [42,56,58]. In five articles, the study duration was not reported (see Table 1). In another article, neither the study duration nor the specific duration of VR use was reported [64].
Mechanism of action of VR/AR/MR interventions for reducing chronic pain
Of the included studies, only one directly investigated the mechanism of action of VR/AR/MR for reducing chronic pain. In this study, the proposed mechanisms were mastery of behavioral skills for pain coping and enhanced self-efficacy for pain self-management and treatment effects were attributed to the didactic and skills-based components of the immersive behavioral therapy [29]. In the remaining 45 articles, mechanisms of VR/AR/MR action were presented as the basis for the study or were discussed in support of study findings. These mechanisms were not mutually exclusive and included: cognitive and or/attentional distraction (n = 26); mechanisms of mirror therapy such as activation of the mirror neuron system, promotion of cortical reorganization, and provision of normalized visual feedback of movements to reduce pain perception (n = 7); activation of motor control mechanisms, function and movement execution, and/or coordination (n = 4); reversal of maladaptive changes in central neuroplasticity (n = 4); interactivity for motivation and enjoyment or training (n = 4); pain modulation mechanisms (n = 3); relaxation (n = 3); immersion (n = 2); cognitive-emotional mechanisms or emotional engagement (n = 2); modulation of the central body representation (n = 2); sensory feedback and activation of neurons to enhance motor activity (n = 2); promotion of self-efficacy for pain coping behaviors (n = 1); endorphin release (n = 1); alterations in the inflammatory process (n = 1); psychoneuromuscular theory (n = 1), activation of cortical and subcortical neuronal circuits to stimulate learning and recovery (n = 1) and visuotactile or visuomotor stimulations (n = 1).
Efficacy/effectiveness & cost-effectiveness of using VR/AR/MR interventions for chronic pain management
All 46 included studies investigated the efficacy/effectiveness of using VR/AR/MR for addressing pain and/or pain related outcomes as primary and/or secondary study objectives. However, the cost–effectiveness of using these technologies was not investigated. The efficacy/effectiveness findings provided here are not mutually exclusive.
There was a statistically significant reduction in pain intensity, phantom sensations, or pain unpleasantness in 29 (63%) of the 46 included studies. 19 of these 29 studies were RCTs, of which 78% (n = 15) demonstrated statistically significant benefits associated with the use of VR/AR/MR technology for pain (95% CI: 54%, 94%; p = 0.019) relative to the control group. Of these 15 RCTs, only one study utilized a sham VR headset as the control condition [30]. The remaining 14 RCTs utilized active control conditions without VR/AR/MR as the comparison, including an audio version of the content from the VR intervention program, mirror therapy, physical therapy, a rehabilitation program, and typical pain distraction activities. One of these studies also included a wait-list control as a second comparator [40]. In 82.7% (n = 24) of the 29 studies, effects on pain were found in the short-term (up to four weeks post-treatment) or immediate post-treatment period. Two studies found both short-term and long-term effects, with long-term effects at five weeks post intervention (n = 1) and 12–16 weeks post treatment (n = 1). Long-term effects were found in three studies, at 8 weeks post intervention (n = 1) and 6 months post intervention (n = 2). Although findings were not statistically significant in the remaining included studies (n = 17), some studies had clinically significant findings. For example, in one study, eight of 12 participants experienced an improvement in pain scores, with an average decrease of 7.8 points (SD = 5.1) [49]. In another study, VR conditions resulted in a 50% decrease in pain ratings [54].
In 52.2% (n = 24) of the included studies, there was a statistically significant improvement in various pain-related outcomes. These outcomes included: psychological correlates of pain such as affect, depression, anxiety, mood, or stress (n = 12); functional status, daily functioning, or mobility (n = 9); pain interference in activities of daily living and/or sleep (n = 6), kinesiophobia (fear of pain due to movement; n = 5), quality of life (n = 3), disability (n = 3), limb/joint range or strength (n = 2), cognitive functions (n = 2), coping skills (n = 1) and time spent thinking of pain (n = 1). In 75% (n = 18) of the 24 studies, effects on pain-related outcomes were found immediately post-treatment. Long-term effects were found in six of the studies, at 5 weeks post intervention (n = 1), 3 months post treatment (n = 1), 8 weeks and 6 months post intervention (n = 1) and 6 months post intervention (n = 3).
Other outcomes of interest
24 studies evaluated the feasibility and/or acceptability of using VR for pain and/or pain-related outcomes. In half of these studies, most participants reported satisfaction or high satisfaction with the VR experience or found VR to be an acceptable intervention for chronic pain. Participants described the experience as logical, useful, helpful and/or immersive [19,58,59]. They also reported high levels of enjoyment, motivation, attention [63] and engagement during the VR intervention [52]. In one study, two of 10 participants did not perceive the VR treatment as helpful [60]. However, there was an improvement in their pre-post treatment quality of life scores. In a few studies, some participants provided comments regarding limitations of the VR technology and practicality of its use as an adjunctive therapy. These participants reported frustration with using complex or cumbersome control systems, inability to use VR equipment during periods of severe pain and short-term duration of treatment effects [14]; an unpleasant weight of the study device – a helmet with an integrated HMD and sensors for head-movement tracking [38]; heaviness or bulkiness of the VR glasses or headset [43,48,65]; and discomfort in using corrective glasses with the headset [65].
Adverse effects or negative side effects were reported in 33.3% (n = 8) of these 24 studies. These effects included: nausea or motion sickness (4%, n = 4 to 24%, n = 6) [29,30,47]; mild nausea, rated at a level of 3 out of 10 (62.5%, n = 5 and 3.3%, n = 1) [14,52]; discomfort of device (5.9%, n = 1) [43]; dizziness in two of 98 study sessions [47]; transient musculoskeletal pain, physical fatigue and difficulties in maintaining attention (77.8%, n = 7) [58]; and ‘slight’ cybersickness (22.2%, n = 2) [64]. In one study, the presence or absence of adverse effects or negative side effects was not reported [47]. Some of these effects resolved with slowing the experience or taking a break from the device. Despite experiencing these effects, many participants either remained in the study because their ability to participate was not affected, expressed interest in using VR at home, and/or purchased a VR device to use at home.
Discussion
Effective pain management requires multifaceted interventions that employ pharmacological and non-pharmacological strategies. However, chronic pain management has posited a significant challenge for healthcare providers because a multidisciplinary treatment approach is lacking [19]. This systematic review of 46 studies suggests that VR/AR/MR can aid in providing patients with relief from chronic pain and improving pain-related outcomes.
Although several types of VR/AR/MR applications or software were utilized in several ways according to numerous mechanisms of action across the included studies, VR/AR/MR demonstrated statistically significant or potential clinical benefits for chronic pain and chronic pain-related outcomes. In the majority of the RCTs, the statistically significant benefits were demonstrated in comparison to active control conditions. The limited use of sham interventions and wait-listed control conditions inhibits our understanding of whether these findings, which were primarily short-term effects, are therapy-specific effects. For included studies in which the primary outcome measure was pain reduction, most of the studies reported high levels of pain reduction among study participants and benefits such as reduction of pain intensity, phantom sensations and pain unpleasantness. In studies that measured pain-related outcomes, the use of VR/AR/MR technology was also associated with substantial improvements. Benefits were demonstrated for outcomes such as pain interference, health status, fear of movement, functional capacity, perceived quality of life and coping strategies. In addition, some of the studies demonstrated the feasibility of VR/AR/MR use and high levels of acceptability among users and healthcare providers.
The VR/AR/MR interventions utilized among included studies were diverse, with VR being the most common technological approach employed. Few studies (n = 7) were home-based and only three of these studies included the option for use of a wireless device [20,30,59]. Additionally, participants in a few studies (n = 6) raised concerns regarding the convenience of the technology. These findings may help to improve the design, uptake and effectiveness of VR/AR/MR interventions; thus, they have important implications for long-term use of these technologies. There remain many barriers for patients seeking to access care at pain clinics or via integrative pain management clinicians, including costs and prohibitive distances to travel [76,77]. In addition, the coronavirus disease 2019 pandemic has further hastened the urgency to deliver effective nonpharmacological pain management interventions remotely to patients in the safety and comfort of their homes. The advancements in VR/AR/MR technology in recent years create the potential for increased accessibility and use of the technology in the patient's home environment as a part of their daily activities. Accordingly, utilizing VR/AR/MR modalities to manage chronic pain at home may be of interest to patients unable to travel or access in-person care [78]. Moreover, use of home-based interventions creates the opportunity for long-term evaluation of chronic pain and identification of patterns over time.
In studies that evaluated the acceptability and/or feasibility of VR/AR/MR, participants reported high satisfaction levels with the technology along with minimal, if any, adverse effects, or negative side effects. User satisfaction was specifically high in areas such as immersion, realism, helpfulness and usefulness of VR/AR/MR [19,58–60]. This underlines the fact that researchers must consider the nature of the virtual environments they design for VR/AR/MR interventions because the development of sophisticated VR technology may potentially be for naught if it does not appeal to the user [79]. The review finding reinforces the need for researchers to evaluate the level of immersion of their virtual environments and conduct analyses of how factors, such as immersion, affect pain and treatment outcomes [79].
Although this review focused on chronic pain management, our findings are consistent with current literature that has assessed the use of VR for various types of pain, including acute pain and found significant improvement in pain levels [79–81]. Most of the included studies did not directly address the mechanism of action for VR/AR/MR, but over half of the studies cited the benefits of distraction in pain management and alluded to the benefits of pain reduction because of distraction. Changing the way that the brain physically registers pain through a complex combination of immersion, emotional engagement and cognitive distraction that is imbedded into the current experience draws attention away from the amount of pain being consciously experienced [32,74]. Stimulating the visual cortex while simultaneously engaging other senses, through features that allow users' minds to engage in an immersive experience, may have a substantial effect on moderating the processing of nociceptive stimuli and improving pain outcomes [17]. We infer that this process may be key in addressing and relieving chronic pain. Future research should characterize treatment mechanisms and duration of treatment effects across diverse patient populations living with chronic pain conditions. Addressing this gap will require investigations that capture both patient-reported outcomes and objective metrics, such as brain imaging, blood-based biomarkers and quantitative sensory testing.
Some of the included studies incorporated VR/AR/MR into evidence-based clinical interventions, such as hypnosis, biofeedback and physical therapy, resulting in significant improvements in pain and functional capacity [38,60,68]. Aligning VR/AR/MR with other modalities has become an emerging line of research, with some evidence that coupling of VR/AR/MR with methods such as hypnosis may be more effective for chronic pain management than either intervention alone [17]. One advantage of VR/AR/MR-based pain management interventions is the unique opportunity for managing chronic pain while also reducing biopsychosocial distress, anxiety and depression among patients [17,18,50,51,53]. Because pain-related outcomes can be triggered by psychosocial factors such as stress, the reduction of biopsychosocial stress may also include a potential effect of pain reduction [82].
We also aimed to assess the cost–effectiveness of VR/AR/MR interventions, but the included studies did not investigate cost-related outcomes. Interventions that involve VR/AR/MR could potentially be an affordable alternative for patients suffering from uncontrolled pain, especially as the cost of such technology, particularly VR, continues to decrease [21,81]. As the VR/AR/MR market continues to evolve, future studies are needed to assess the cost–effectiveness of such interventions for hospital, in-clinic and home use in addition to assessing feasibility of access to such interventions [81]. The combination of decreased technology costs, flexibility and customizability of immersive features and improvements in software and hardware design result in numerous potential applications for patients who are suffering from a wide array of acute and chronic pain conditions ranging from visceral to somatic pain [17]. These factors increase the potential and necessity for widespread dissemination of technology-based interventions throughout health systems [17,43], with the capability to continue treatment post-discharge. Therefore, VR/AR/MR technologies may be used to support individual and customized pain self-management, which can contribute to a decrease in healthcare expenses and expenditure of clinical resources.
Notably, most studies did not report data regarding race, ethnicity, or other sociodemographic factors. This may have been because most studies were conducted outside of the USA. While race is a socially constructed concept, it is paramount that future researchers assess and analyze socioeconomic and sociocultural contexts as well as the availability of resources and quality of infrastructure for persons with chronic pain. Addressing social determinants of health (SDOH) is at the forefront of achieving health equity. However, there was a paucity of attention to SDOH in the included studies, with demographics often limited to male/female gender, age, disease state, type of chronic pain and level of education. Attention to social-environmental-cultural context in future studies is particularly important given documented biases in healthcare. Such attention is also required when testing and refining intervention strategies for populations that have been historically marginalized because of race, ethnicity, or geographic location. Because pain is influenced by biological, psychological and social factors [83] and quality of life is a multidimensional concept often considered in investigations of pain, not examining social factors may contribute to further marginalization. Moreover, the acceptability and utility, access, mechanism of action, potential efficacy and customizability of VR/AR/MR technologies to individual needs may be affected by these factors [84].
Limitations of this review
There are some limitations to this systematic review. The specific inclusion criteria for this review may have limited the number of available studies. Despite conducting a comprehensive literature search, the final number of included studies may have been limited because the use of VR/AR/MR technology for chronic pain is still a developing area of research with few published studies. As a result, the number of RCTs and studies involving children and adolescents was also limited. Furthermore, this review only included studies published in the English language, potentially excluding studies otherwise eligible.
The heterogeneity of the study populations, interventions and comparators, outcome measurements and data analysis across the studies posed a challenge for synthesizing the results. Most of the studies included small sample sizes and in 45.6% (n = 21) of the studies, a comparison condition or comparator was not included. Because of these factors, the generalizability of the study results and the power of the findings are limited. Despite the heterogeneity in RCT outcomes, a count synthesis was conducted. However, a limitation of this approach is the inability to capture the magnitude of effect sizes. In addition, half of the studies did not include pain relief follow-up beyond the immediate post-intervention period. In a few of the studies that included a follow-up (n = 9), follow-up occurred within 1 month of treatment, resulting in insufficient data for determining VR's efficacy/effectiveness for long-term pain relief. Thus, there is a need for RCTs with larger sample sizes that are designed to provide high-quality evidence on the long-term efficacy of VR/AR/MR interventions. The RCTs included in this review tested a diverse set of VR/AR/MR interventions, of varying immersion and duration, with differing control groups, and were conducted on patients with a spectrum of chronic pain conditions, thus inhibiting our ability to inferentially ascertain the impact of these therapies. Nonetheless, there were significant findings that can be used to inform the future development of VR/AR/MR-based interventions for chronic pain. As the body of VR/AR/MR research grows, future systematic reviews may benefit from examining RCTs focused on comparing improvements in physical health functioning (e.g., physical therapy) and behavioral health functioning (e.g., CBT and mirror therapy) among patients with chronic pain conditions. Future studies examining the impact of VR/AR/MR compared with other pain management approaches would benefit from improved data reporting and interpretation as outlined by pain-focused international research groups, specifically when reporting group differences on patient-reported outcomes and pain medication utilization [85,86].
Conclusion
This review supports findings of current literature regarding the efficacy/effectiveness of VR/AR/MR in reducing pain and improving pain-related outcomes among patients living with chronic pain. The potential that innovative, non-pharmacological technologies, such as VR/AR/MR, offer individuals to cope with chronic pain is significant. While the efficacy/effectiveness of VR/AR/MR technology varied across studies, most studies showed short-term effects on reducing pain and improving pain-related outcomes. These pain-related outcomes included coping skills, daily functioning or functional capacity and perceived quality of life. Based on the findings of this review, there is no available evidence on the cost–effectiveness of using these technologies for home-based chronic pain management. However, the portability of VR/AR/MR enables use of these technologies in the delivery of home-based, pain self-management interventions to decrease chronic pain and its negative effects.
VR/AR/MR technologies can serve as efficacious methods of delivering non-pharmacological interventions for addressing treatment gaps in chronic pain management. Effective pain management must address psychosocial and behavioral factors while promoting self-management in conjunction with pharmacological and physical approaches [79,87]. VR/AR/MR technologies hold promise in addressing the various challenges that healthcare providers and patients have experienced in achieving effective pain management. As more rigorous research is conducted to evaluate the effectiveness of these technologies, data from such research can be used in support of their widespread dissemination throughout healthcare systems and in patients' homes.
Recommendations for practice
The following preliminary practice recommendations can be made:
VR/AR/MR technologies can be effective methods for delivering interventions for chronic pain.
VR/AR/MR-based interventions may be considered as a strategy to support home-based chronic pain management. This strategy may benefit historically marginalized individuals and those who live in locations where access to in-person interventions is limited.
Recommendations for research
The following recommendations can be made for future research:
-
RCTs are required to evaluate VR/AR/MR technologies, particularly for home-based chronic pain management.
There is a need for conducting more RCTs, with larger sample sizes, to generate data on a larger scale that can inform health systems in adopting VR/AR/MR interventions.
-
Research should be conducted to evaluate the mechanisms of action of VR/AR/MR interventions for achieving pain relief.
Further research is needed to identify and test specific mechanisms that result in pain relief from VR/AR/MR use and how specific factors, such as the type of equipment, intervention dose, along with the level of immersion and enjoyment of the VR/AR/MR environment, affect pain relief. This will require capturing patient-reported outcomes and objective pain-related measures (e.g., imaging, blood-based biomarkers, and quantitative sensory testing).
Research should be conducted to explore the accessibility and cost-effectiveness of implementing VR/AR/MR-based interventions, especially in the home setting.
Research of VR/AR/MR technologies should be conducted in partnership with members of historically marginalized groups, such as Black adults who experience chronic pain.
-
Future VR/AR/MR programs should be tailored to the characteristics and needs of different patient groups.
Although extensive research has demonstrated the effects of distraction for reducing pain, there is a need for further research that investigates tailored distraction techniques via VR/AR/MR in addressing different types and subtypes of pain that encompass individual, procedural, interventional, contextual, and social factors [88].
Future studies should also assess the effects of combining VR/AR/MR with evidence-based pain management approaches such as CBT, mindfulness, and biofeedback.
Future RCTs comparing VR/AR/MR with evidence-based pain management interventions should adhere to best data reporting and evaluation practices, including those outlined by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT).
Summary points.
Although the use of virtual, augmented, or mixed reality (VR/AR/MR) technology for chronic pain has increased, there is a dearth of literature regarding the use and efficacy/effectiveness of these technologies.
This review of 46 empirical studies included 19 randomized controlled trials (RCTs) (n = 1011 participants), 21 quasi-experimental studies (n = 413), 1 analytical cross-sectional study (n = 15), three case reports (n = 4), and two pilot case series (n = 13), with a total of 1456 participants across all studies.
Most of the included studies investigated VR, utilized immersive head-mounted displays, and did not include a follow-up beyond the immediate post-intervention period.
In most studies, VR was utilized to cope with chronic pain and associated psychosocial correlates or was integrated into rehabilitation therapy.
Efficacy/effectiveness outcomes included pain (46 studies) and pain-related outcomes (41 studies), such as functional status, psychological correlates of pain, and pain interference in activities of daily living.
VR/AR/MR technology was associated with a statistically significant reduction in pain intensity, phantom sensations, or pain unpleasantness in 63% of the studies and a statistically significant improvement in various pain-related outcomes in 52.2% of the studies. Among these studies, 78% of the 19 RCTs had improved pain-related outcomes, with small to large effect sizes.
In half of the 24 studies that evaluated the feasibility and/or acceptability of using VR for pain and/or pain-related outcomes, most participants reported satisfaction or high satisfaction with the VR experience or found VR to be an acceptable intervention for chronic pain.
Adverse effects or negative side effects were reported in 33.3% of 24 studies and these effects were primarily mild.
The overall quality of the studies was moderate, with a low risk of bias for most studies. Of the 19 RCTs, one study exhibited a moderate risk for bias, it was unclear if at least one criterion was met in 5 studies, and two studies did not utilize true randomization. In the RCTs, there was a wide range of results of high to low certainty, with overall low certainty reported.
VR/AR/MR technology can be an effective method for delivering interventions for chronic pain.
Clinical trials are needed to further evaluate VR/AR/MR technology for home-based chronic pain management, mechanisms of action of VR/AR/MR interventions for achieving pain relief, and accessibility and cost-effectiveness of implementing VR/AR/MR-based interventions, especially among members of historically marginalized groups.
Supplementary Material
Acknowledgments
The authors thank R Chandler, H Ross, A-T Ayuk-Arrey, A Gibson, A Landers, J Bai and A Long for their assistance with this systematic review. Acquisition of data for the work: R Chandler, H Ross, A-T Ayuk-Arrey, A Gibson, A Landers, J Bai and A Long. Analysis and interpretation of data, and drafting and revising the work: R Chandler.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/pmt-2022-0030
Author contributions
Conception and design of the work: N Matthie. Acquisition, analysis or interpretation of data for the work: All authors. Drafting the work or revising it critically for important intellectual content: All authors. Final approval of the version to be published: All authors. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: All authors.
Financial & competing interests disclosure
This work was supported in part by the National Heart, Lung and Blood Institute (3U01HL128566-02S1 and K23HL133457) and the National Institute of Nursing Research (R21NR019872-01 and R01NR02012-01) of the National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.Treede R-D, Rief W, Barke A et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 160(1), 19–27 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Dydyk AM, Conermann T. Chronic pain. StatPearls. (2022). www.ncbi.nlm.nih.gov/books/NBK553030/ [PubMed] [Google Scholar]
- 3.International Association for the Study of Pain. Chronic pain has arrived in the ICD-11 (2019). www.iasp-pain.org/publications/iasp-news/new-diagnostic-codes-for-chronic-pain-approved-under-icd-11/
- 4.Dahlhamer J, Lucas J, Zelaya C et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb. Mortal. Wkly Rep. 67(36), 1001–1006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 13(8), 715–724 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. www.ncbi.nlm.nih.gov/books/NBK91497/
- 7.Institute for Clinical Systems Improvement. Pain: assessment, non-opioid treatment approaches and opioid management (2019). www.icsi.org/wp-content/uploads/2019/10/Pain-Interactive-7th-V2-Ed-8.17.pdf
- 8.Virtual Reality Society. When was virtual reality invented? (2017). www.vrs.org.uk/virtual-reality/who-coined-the-term.html
- 9.Rothbaum BO, Hodges LF, Kooper R, Opdyke D, Williford JS, North M. Effectiveness of computer-generated (virtual reality) graded exposure in the treatment of acrophobia. Am. J. Psychiatry 152(4), 626–628 (1995). [DOI] [PubMed] [Google Scholar]
- 10.Joe Bardi, 3D Cloud by Marxent. What is virtual reality? [Definition and Examples] (2019). www.marxentlabs.com/what-is-virtual-reality/
- 11.Brigham TJ. Reality check: basics of augmented, virtual, and mixed reality. Med Ref Serv Q. 36(2), 171–178 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Market Business News. What is virtual reality or VR? Definition and examples (2021). https://marketbusinessnews.com/financial-glossary/virtual-reality-vr/
- 13.Virtual Reality Society. Virtual reality apps (2017). www.vrs.org.uk/virtual-reality/apps.html
- 14.Garrett B, Taverner T, McDade P. Virtual reality as an adjunct home therapy in chronic pain management: an exploratory study. JMIR Med Inform. 5(2), e11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones T, Skadberg R, Moore T. A pilot study of the impact of repeated sessions of virtual reality on chronic neuropathic pain. Int J Virtual Real. 18(1), 19–34 (2018). [Google Scholar]
- 16.Pourmand A, Davis S, Marchak A, Whiteside T, Sikka N. Virtual reality as a clinical tool for pain management. Curr Pain Headache Rep. 22(8), 53 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Li A, Montaño Z, Chen VJ, Gold JI. Virtual reality and pain management: current trends and future directions. Pain Manag. 1(2), 147–157 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gold JI, Belmont KA, Thomas DA. The neurobiology of virtual reality pain attenuation. Cyberpsychol Behav. 10(4), 536–544 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Palacios A, Herrero R, Vizcaíno Y et al. Integrating virtual reality with activity management for the treatment of fibromyalgia: acceptability and preliminary efficacy. Clin. J. Pain 31(6), 564–572 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Rothgangel A, Braun S, Winkens B, Beurskens A, Smeets R. Traditional and augmented reality mirror therapy for patients with chronic phantom limb pain (PACT study): results of a three-group, multicentre single-blind randomized controlled trial. Clin. Rehabil. 32(12), 1591–1608 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Schroeder D, Korsakov F, Jolton J, Keefe FJ, Haley A, Keefe DF. Creating widely accessible spatial interfaces: mobile VR for managing persistent pain. IEEE Comput. Grap. Appl. 33(3), 82–88 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med. 6(7), e1000097 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tufanaru C, Campbell J, Hopp L. Chapter 3: systematic reviews of effectiveness. In: JBI Manual for Evidence Synthesis. Munn Z, Aromataris E (Eds). The Joanna Briggs Institute; (2020). https://synthesismanual.jbi.global [Google Scholar]
- 24.The Joanna Briggs Institute. Critical appraisal tools (2019). https://jbi.global/critical-appraisal-tools
- 25. The GRADE Working Group, GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. Schünemann H, Brożek J, Guyatt G, Oxman A (Eds). The GRADE Working Group, 2013 (2020). https://gdt.gradepro.org/app/handbook/handbook.html#h.1i2bwkm8zpjo [Google Scholar]
- 26.The Joanna Briggs Institute. Appendix 10.3 JBI Data Extraction Form for Review for Systematic Reviews and Research Syntheses. In: JBI Manual for Evidence Synthesis. Aromataris E, Munn Z (Eds) (2020). https://jbi-global-wiki.refined.site/space/MANUAL/3283910857 [Google Scholar]
- 27.McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Renea V, Johnston RV. Chapter 9: summarizing study characteristics and preparing for synthesis. In: Higgins JPT, Thomas J, Chandler Jet al.. et al. (Eds). Cochrane Handbook for Systematic Reviews of Interventions; (2019). https://training.cochrane.org/handbook/current/chapter-09#section-9-5 [Google Scholar]
- 28.Austin PD, Craig A, Middleton JW et al. The short-term effects of head-mounted virtual-reality on neuropathic pain intensity in people with spinal cord injury pain: a randomised cross-over pilot study. Spinal Cord 59(7), 738–746 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Darnall BD, Krishnamurthy P, Tsuei J, Minor JD. Self-administered skills-based virtual reality intervention for chronic pain: randomized controlled pilot study. JMIR Form Res. 4(7), e17293 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Only included study that directly investigated the mechanism of action of virtual reality
- 30.Garcia LM, Birckhead BJ, Krishnamurthy P et al. An 8-week self-administered at-home behavioral skills-based virtual reality program for chronic low back pain: double-blind, randomized, placebo-controlled trial conducted during COVID-19. J Med Internet Res. 23(2), e26292 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Included study with the largest sample size
- 31.Jeon B, Cho S, Lee J-H. Application of virtual body swapping to patients with complex regional pain syndrome: a pilot study. Cyberpsychol Behav Soc Netw. 17(6), 366–370 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Jin W, Choo A, Gromala D, Shaw C, Squire P. A virtual reality game for chronic pain management: a randomized, controlled clinical study. Stud Health Technol Inform. 220, 154–160 (2016). [PubMed] [Google Scholar]
- 33.Lewis JS, Newport R, Taylor G, Smith M, McCabe CS. Visual illusions modulate body perception disturbance and pain in Complex Regional Pain Syndrome: a randomized trial. Eur. J. Pain. 25(7), 1551–1563 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Matheve T, Bogaerts K, Timmermans A. Virtual reality distraction induces hypoalgesia in patients with chronic low back pain: a randomized controlled trial. J NeuroEngineering Rehabil. 17(1), 55 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nambi G, Abdelbasset WK, Alrawaili SM, Alsubaie SF, Abodonya AM, Saleh AK. Virtual reality or isokinetic training; its effect on pain, kinesiophobia and serum stress hormones in chronic low back pain: a randomized controlled trial. Technol. Health Care 29(1), 155–166 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Nambi G, Abdelbasset WK, Alsubaie SF et al. Short-term psychological and hormonal effects of virtual reality training on chronic low back pain in soccer players. J Sport Rehab. 30(6), 884–893 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Nambi G, Abdelbasset WK, Elsayed SH et al. Comparative effects of isokinetic training and virtual reality training on sports performances in university football players with chronic low back pain-randomized controlled study. Evid Based Complement Alternat Med. 2020, 2981273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nusser M, Knapp S, Kramer M, Krischak G. Effects of virtual reality-based neck-specific sensorimotor training in patients with chronic neck pain: a randomized controlled pilot trial. J Rehabil Med. 53(2), jrm00151 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rezaei I, Razeghi M, Ebrahimi S, Kayedi S, Rezaeian Zadeh A. A novel virtual reality technique (Cervigame®) compared to conventional proprioceptive training to treat neck pain: a randomized controlled trial. J Biomed Phys Eng. 9(3), 355–366 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarig Bahat H, Croft K, Carter C, Hoddinott A, Sprecher E, Treleaven J. Remote kinematic training for patients with chronic neck pain: a randomised controlled trial. Eur. Spine J. 27(6), 1309–1323 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Sarig Bahat H, Takasaki H, Chen X, Bet-Or Y, Treleaven J. Cervical kinematic training with and without interactive VR training for chronic neck pain – A randomized clinical trial. Man Ther. 20(1), 68–78 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Tejera DM, Beltran-Alacreu H, Cano-de-la-Cuerda R et al. Effects of virtual reality versus exercise on pain, functional, somatosensory and psychosocial outcomes in patients with non-specific chronic neck pain: a randomized clinical trial. Int J Environ Res Public Health. 17(16), E5950 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venuturupalli RS, Chu T, Vicari M, Kumar A, Fortune N, Spielberg B. Virtual reality–based biofeedback and guided meditation in rheumatology: a pilot study. ACR Open Rheuma. 1(10), 667–675 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yilmaz Yelvar GD, Çırak Y, Dalkılınç M, Parlak Demir Y, Guner Z, Boydak A. Is physiotherapy integrated virtual walking effective on pain, function, and kinesiophobia in patients with non-specific low-back pain? Randomised controlled trial. Eur. Spine J. 26(2), 538–545 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Alemanno F, Houdayer E, Emedoli D et al. Efficacy of virtual reality to reduce chronic low back pain: proof-of-concept of a non-pharmacological approach on pain, quality of life, neuropsychological and functional outcome. PLOS ONE. 14(5), e0216858 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Botella C, Garcia-Palacios A, Vizcaíno Y, Herrero R, Baños RM, Belmonte MA. Virtual reality in the treatment of fibromyalgia: a pilot study. Cyberpsychol Behav Soc Netw. 16(3), 215–223 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Fowler CA, Ballistrea LM, Mazzone KE et al. Virtual reality as a therapy adjunct for fear of movement in veterans with chronic pain: single-arm feasibility study. JMIR Form Res. 3(4), e11266 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glavare M, Stålnacke B-M, Häger CK, Löfgren M. Virtual reality exercises in an interdisciplinary rehabilitation programme for persons with chronic neck pain: a feasibility study. J Rehabil Med Clin Commun. 4, 1000067 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hennessy RW, Rumble D, Christian M, Brown DA, Trost Z. A graded exposure, locomotion-enabled virtual reality app during walking and reaching for individuals with chronic low back pain: cohort gaming design. JMIR Serious Games. 8(3), e17799 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.House G, Burdea G, Grampurohit N et al. A feasibility study to determine the benefits of upper extremity virtual rehabilitation therapy for coping with chronic pain post-cancer surgery. Br J Pain. 10(4), 186–197 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Igna R, Stefan S, Onac I, Onac I, Ungur RA, Szentagotai Tatar A. mindfulness-based cognitive-behavior therapy (MCBT) versus virtual reality (VR) enhanced CBT, versus treatment as usual for chronic back pain. A clinical trial. J Evid-Based Psychother. 14(2), 229–247 (2014). [Google Scholar]
- 52.Jones T, Moore T, Choo J. The impact of virtual reality on chronic pain. PLOS ONE. 11(12), e0167523 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu K, Madrigal E, Chung JS et al. Preliminary study of virtual-reality-guided meditation for veterans with stress and chronic pain. Altern. Ther. Health Med. AT6861 (2021). [PubMed] [Google Scholar]
- 54.Matamala-Gomez M, Diaz Gonzalez AM, Slater M, Sanchez-Vives MV. Decreasing pain ratings in chronic arm pain through changing a virtual body: different strategies for different pain types. J Pain. 20(6), 685–697 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Mouraux D, Brassinne E, Sobczak S et al. 3D augmented reality mirror visual feedback therapy applied to the treatment of persistent, unilateral upper extremity neuropathic pain: a preliminary study. J Man Manip Ther. 25(3), 137–143 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ortiz-Catalan M, Guðmundsdóttir RA, Kristoffersen MB et al. Phantom motor execution facilitated by machine learning and augmented reality as treatment for phantom limb pain: a single group, clinical trial in patients with chronic intractable phantom limb pain. Lancet 388(10062), 2885–2894 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Putrino D, Tabacof L, Breyman E et al. Pain reduction after short exposure to virtual reality environments in people with spinal cord injury. Int J Environ Res Public Health. 18(17), 8923 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roosink M, Robitaille N, Jackson PL, Bouyer LJ, Mercier C. Interactive virtual feedback improves gait motor imagery after spinal cord injury: an exploratory study. Restor Neurol Neurosci. 34(2), 227–235 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rutledge T, Velez D, Depp C et al. A virtual reality intervention for the treatment of phantom limb pain: development and feasibility results. Pain Med. 20(10), 2051–2059 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Shiri S, Feintuch U, Weiss N et al. A virtual reality system combined with biofeedback for treating pediatric chronic headache—A pilot study. Pain Med. 14(5), 621–627 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Solcà M, Ronchi R, Bello-Ruiz J et al. Heartbeat-enhanced immersive virtual reality to treat complex regional pain syndrome. Neurol. 91(5), e479–e489 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Trost Z, Anam M, Seward J et al. Immersive interactive virtual walking reduces neuropathic pain in spinal cord injury: findings from a preliminary investigation of feasibility and clinical efficacy. Pain 163(2), 350–361 (2022). [DOI] [PubMed] [Google Scholar]
- 63.Villiger M, Bohli D, Kiper D et al. Virtual reality–augmented neurorehabilitation improves motor function and reduces neuropathic pain in patients with incomplete spinal cord injury. Neurorehabil Neural Repair. 27(8), 675–683 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Won AS, Barreau AC, Gaertner M et al. Assessing the feasibility of an open-source virtual reality mirror visual feedback module for complex regional pain syndrome: pilot usability study. J Med Internet Res. 23(5), e16536 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zauderer J, Lefèvre-Colau M-M, Davoine É et al. Exercise therapy program using immersive virtual reality for people with non-specific chronic neck pain: a 3-month retrospective open pilot and feasibility study. Ann Phys Rehabil Med. 65(2), 101527 (2022). [DOI] [PubMed] [Google Scholar]
- 66.Solcà M, Krishna V, Young N et al. Enhancing analgesic spinal cord stimulation for chronic pain with personalized immersive virtual reality. Pain 162(6), 1641–1649 (2021). [DOI] [PubMed] [Google Scholar]
- 67.Ambron E, Miller A, Kuchenbecker KJ, Buxbaum LJ, Coslett HB. Immersive low-cost virtual reality treatment for phantom limb pain: evidence from two cases. Front Neurol. 9, 67 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oneal BJ, Patterson DR, Soltani M, Teeley A, Jensen MP. Virtual reality hypnosis in the treatment of chronic neuropathic pain: a case report. Int J Clin Exp Hypn. 56(4), 451–462 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ortiz-Catalan M, Sander N, Kristoffersen MB, Håkansson B, Brånemark R. Treatment of phantom limb pain (PLP) based on augmented reality and gaming controlled by myoelectric pattern recognition: a case study of a chronic PLP patient. Front Neurosci. 8(24), 1–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sato K, Fukumori S, Matsusaki T et al. Nonimmersive virtual reality mirror visual feedback therapy and its application for the treatment of complex regional pain syndrome: an open-label pilot study. Pain Med. 11(4), 622–629 (2010). [DOI] [PubMed] [Google Scholar]
- 71.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 372, n71 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Angelov V, Petkov E, Shipkovenski G, Kalushkov T. Modern virtual reality headsets. In: 2020 International Congress on Human-Computer Interaction, Optimization and Robotic Applications (HORA). IEEE, 1–5 (2020). 10.1109/HORA49412.2020.9152604 [DOI] [Google Scholar]
- 73.Yu M, Zhou R, Wang H, Zhao W. An evaluation for VR glasses system user experience: the influence factors of interactive operation and motion sickness. Appl Ergon 74, 206–213 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Wiederhold BK, Gao K, Sulea C, Wiederhold MD. Virtual reality as a distraction technique in chronic pain patients. Cyberpsychol Behav Soc Netw. 17(6), 346–352 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Donath L, Rössler R, Faude O. Effects of Virtual Reality Training (Exergaming) Compared to Alternative Exercise Training and Passive Control on Standing Balance and Functional Mobility in Healthy Community-Dwelling Seniors: A Meta-Analytical Review. Sports Med. 46(9), 1293–1309 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Lagisetty P, Slat S, Thomas J, Macleod C, Golmirzaie G, Bohnert AS. Access to Multimodal Pain Management for Patients with Chronic Pain: An Audit Study. J. Gen. Intern. Med. 36(3), 818–820 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes access to pain management services for patients receiving opioid therapy for chronic pain.
- 77.Scott-Richardson M, Giordano NA, Highland KB. The Public Health Need to Estimate State Readiness in Pain Management Policy Implementation and Equity. Pain Med (2021). [DOI] [PubMed] [Google Scholar]
- 78.Eccleston C, Blyth FM, Dear BF et al. Managing patients with chronic pain during the COVID-19 outbreak: considerations for the rapid introduction of remotely supported (eHealth) pain management services. Pain 161(5), 889–893 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Discusses clinical evidence supporting remote therapies for patients with chronic pain.
- 79.Malloy KM, Milling LS. The effectiveness of virtual reality distraction for pain reduction: a systematic review. Clin Psyc Rev. 30(8), 1011–1018 (2010). [DOI] [PubMed] [Google Scholar]
- 80.Chan E, Foster S, Sambell R, Leong P. Clinical efficacy of virtual reality for acute procedural pain management: a systematic review and meta-analysis. PLOS ONE. 13(7), e0200987 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mallari B, Spaeth EK, Goh H, Boyd BS. Virtual reality as an analgesic for acute and chronic pain in adults: a systematic review and meta-analysis. J Pain Res. 12, 2053–2085 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wippert P-M, Wiebking C. Stress and alterations in the pain matrix: a biopsychosocial perspective on back pain and its prevention and treatment. Int J Environ Res Public Health. 15(4), E785 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raja S, Carr DB, Cohen M et al. The revised IASP definition of pain: concepts, challenges, and compromises. Pain 161(9), 1976–1982 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trost Z, Zielke M, Guck A et al. The promise and challenge of virtual gaming technologies for chronic pain: the case of graded exposure for low back pain. Pain Manag. 5(3), 197–206 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Gewandter JS, Smith SM, Dworkin RH et al. Research approaches for evaluating opioid sparing in clinical trials of acute and chronic pain treatments: Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials recommendations. Pain 162(11), 2669–2681 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dworkin RH, Turk DC, Mcdermott MP et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 146(3), 238–244 (2009). [DOI] [PubMed] [Google Scholar]
- 87.Turk DC, Swanson KS, Tunks ER. Psychological approaches in the treatment of chronic pain patients–when pills, scalpels, and needles are not enough. Can. J. Psychiatry 53(4), 213–223 (2008). [DOI] [PubMed] [Google Scholar]
- 88.Birnie KA, Chambers CT, Spellman CM. Mechanisms of distraction in acute pain perception and modulation. Pain 158(6), 1012–1013 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


