Abstract
A “Tet(L)-12” version of Tet(L), a tetracycline efflux protein with 14 transmembrane segments (TMS), was constructed by deletion of two central TMS. Tet(L)-12 catalyzed Na+/H+ antiport and antiport with K+ as a coupling ion as well as or better than wild-type Tet(L) but exhibited no tetracycline-Me2+/H+ antiport in Escherichia coli vesicles.
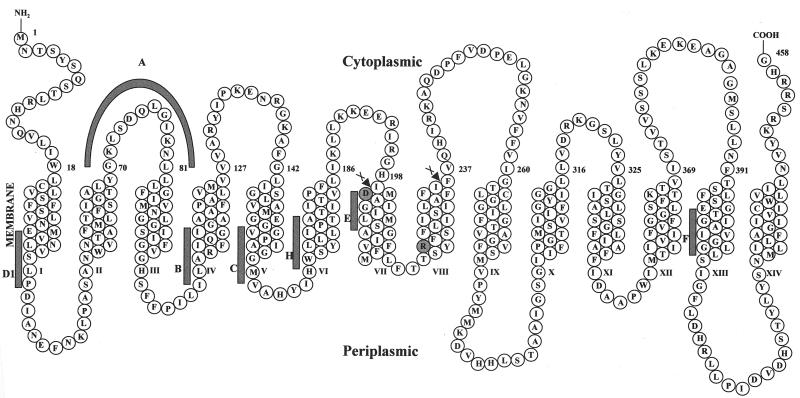
The majority of the prokaryotic tetracycline (Tet) efflux proteins fall within structurally related families of the major facilitator superfamily (MFS) of transporters that have either 12 transmembrane segments (TMS) or 14 TMS (21, 24). The 12-TMS Tet proteins include most of the tetracycline efflux proteins found in gram-negative bacteria, while the 14-TMS tetracycline efflux proteins are predominantly found in gram-positive bacteria (17). These two groups of Tet proteins are structurally similar to a larger group of drug and multidrug efflux proteins (DHAs) that also function by a secondary antiporter mechanism (16, 21, 22). The major and closely related examples of the 14-TMS Tet proteins are Tet(L), including the chromosomally encoded Tet(L) of Bacillus subtilis, and Tet(K), including that encoded in Staphylococcus aureus plasmids (17). Both Tet(L) and Tet(K) catalyze the exchange of extracellular H+ for a cytoplasmic complex of Tet and a divalent metal ion that is optimally Co2+ (10, 27), as do 12-TMS Tet proteins (28). Because the reactions catalyzed by the 12-TMS and 14-TMS tetracycline efflux proteins are so similar, it has been suggested that the three-dimensional structures of the catalytically active “cores” of these two types of Tet proteins will also be similar (12). Moreover, there is symmetry within structures of individual 12-TMS tetracycline efflux proteins, and complementation between mutants in different halves of the molecule has been shown (23). This led to the proposal that the 12-TMS tetracycline efflux proteins arose from gene duplication of an ancestral 6-TMS-encoding gene (7, 16, 22, 23). It was further suggested that the origin of the 14-TMS DHA family was the incorporation of two TMS from another source into the middle of a 12-TMS precursor (7). In possible support of this proposal, it is interesting to note the displacement in 14-TMS proteins of a large, central cytoplasmic loop that is typically found in 12-TMS MFS proteins (Fig. 1). This loop has recently been demonstrated to promote membrane insertion of 12-TMS LacY (26).
FIG. 1.
Topological diagram of Tet(L) highlighting the region deleted in construction of Tet(L)-12. The gray bars show the positions of motifs that were noted by Paulsen et al. (21) in 12- and/or 14-TMS drug/H+ antiporters. The two MslI sites used in construction of Tet(L)-12 are indicated by the scissors arrowheads.
From a functional point of view, there are reasons to hypothesize that, as with eukaryotic multidrug efflux proteins, Tet proteins and other prokaryotic drug exporters possess (or evolved from proteins that possessed) physiological functions that are unrelated to their current drug substrates. First, chromosomes of several prokaryotes contain numerous genes with significant sequence similarity to established DHA-12 or DHA-14 members whose products do not show comparable activities (19). Also, the chromosomally encoded DHA-12 or DHA-14 proteins are often expressed at levels that would confer little if any drug resistance, suggesting that they may have other roles that are well served by that expression level (24). Studies from our laboratory have shown that Tet(L) and Tet(K) are indeed multifunctional, having the capacity to confer resistance to low levels of tetracycline (Tcr) and also having two other modes of antiport activity (1–4, 11, 25). They catalyze electrogenic Na+(K+)/H+ antiport (with H+/Na+ or K+ of >1), which is physiologically important for Na+ resistance and for Na+- and K+-dependent pH homeostasis in B. subtilis (1–4, 25). Tet(L) and Tet(K) also catalyze an electrogenic antiport of the cytoplasmic solutes in exchange for K+ rather than H+, such that net K+ uptake occurs (11, 14). This mode has a physiological role in K+ acquisition in B. subtilis (14, 25). These findings raise the possibility that some tetracycline efflux proteins may have evolved from housekeeping antiporters that catalyze monovalent cation/H+(K+) antiport. It was thus of interest to take advantage of a pair of strategically located restriction sites that facilitated removal, from the 14-TMS Tet(L) protein, of the middle two TMS, VII and VIII: i.e., the ones proposed to have been inserted late in the evolution of the Tet(L) and Tet(K) family.
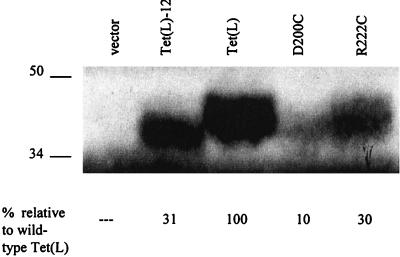
Figure 1 provides a diagrammatic representation of the Tet(L) protein from B. subtilis drawn by using topological data that were obtained for Tet(K) by others (6, 12). The diagram shows the cleavage sites, a pair of MslI sites, that were used to cleave and religate to form the Tet(L)-12 construct. Shaded in the diagram are the two charged amino acids, D200 and R222, that are in TMS VII and VIII but are deleted in the Tet(L)-12 construct. D200, but not R222, is conserved among the 14-TMS Tet proteins. The deletion construct was fully sequenced to confirm that it was correctly constructed and contained no additional mutations. Separate individual site-directed mutations were made in tet(L) to change the two charged residues D200 and R222 to cysteines. This was achieved by the method described by Kunkel et al. (15), followed by complete sequencing. The three mutated tet(L) forms, tet(L)-12, the D200C mutant, and the R222C mutant, were each cloned into pGEM3Zf(+) (Promega) under control of the T7 promoter; this gives low-level expression that is optimal for Na+-related assays that were carried out with transformants of the antiporter-deficient Escherichia coli NM81 strain (ΔnhaA) (20). The three tet(L) forms were also cloned into the shuttle vector pBK15 (obtained from K. Zen) under the control of the ermC promoter; this gives a higher level of expression that is optimal for tetracycline-related assays carried out in transformants of E. coli DH5α and K+-related assays that were carried out in an E. coli mutant, TK2420, that has mutations in each of three K+ uptake systems (5). Tetracycline-Co2+/H+ antiport and Na+/H+ antiport activities were assessed by energy (Tris d-lactate)-dependent uptake of the radioactive solute into everted membrane vesicles of the appropriate E. coli transformant. Everted vesicles were prepared and assayed as described previously (10), except that 5 mM dithiothreitol was used during preparation, and instead of a potassium phosphate buffer, the preparation and assay buffer was 10 mM BTP {1, 3-bis [tris (hydroxymethyl) methylamino] propane}. Data are corrected for a control experiment in which the uncoupler carbonyl cyanide-m-chlorophenylhydrazone (CCCP) was present at 10 μM. The net K+ uptake mode was assayed in right-side-out membrane vesicles that were prepared by the method of Kaback (13). The assay, described previously (11), compared energy (Tris d-lactate)-dependent, net 86Rb+ uptake by vesicles loaded with 100 μM KCl, as opposed to choline Cl, under conditions in which there was no chemical gradient of K+(Rb+) across the membrane. The data presented are averages of assays conducted in duplicate in at least two independent experiments. For determinations of the amounts of different mutant Tet(L) proteins that were incorporated into the membrane relative to wild-type levels, Western analyses were conducted with membrane vesicles from E. coli DH5α transformants with an antibody that had been raised to a peptide corresponding to the Tet(L) N terminus (4). Detection by chemiluminescence was accomplished with the Amersham ECL (enhanced chemiluminescence) kit (Amersham), and quantification was carried out with ImageQuant software (Molecular Dynamics). As shown in Fig. 2, Tet(L)-12 and the R222C mutant protein were incorporated into the membrane at approximately one-third the level of the wild-type Tet(L), whereas the incorporation of the D200C mutant was significant but lower.
FIG. 2.
Western analyses of everted membrane vesicles from E. coli DH5α transformed with either a vector control or recombinant pBK15 plasmids bearing genes encoding the indicated forms of Tet(L). Mr values are indicated to the left, and the percentage of protein incorporation relative to wild-type Tet(L) is shown for Tet(L)-12 and the two site-directed mutant forms at the bottom of the figure.
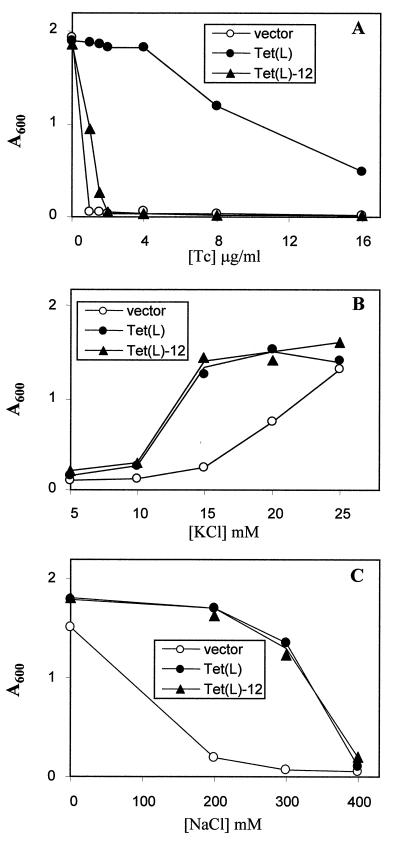
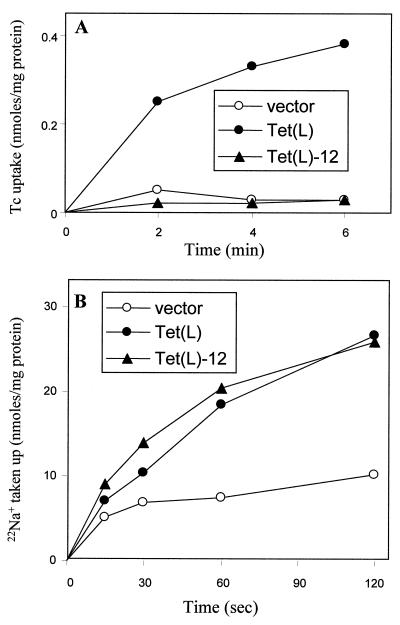
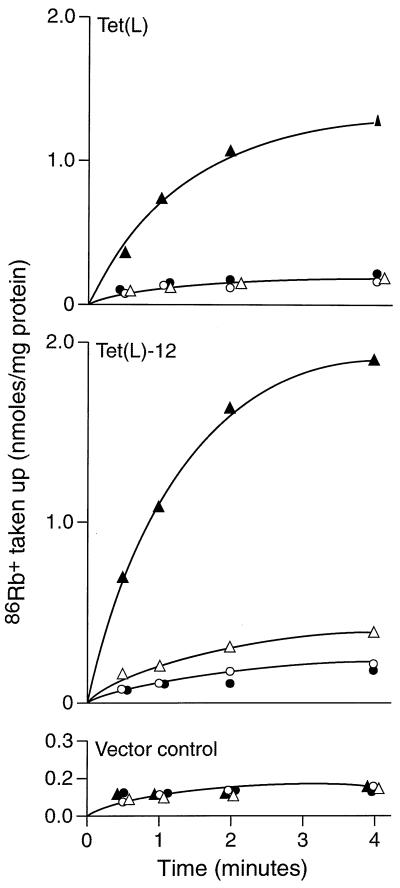
As shown in Fig. 3, Tet(L)-12 supported almost no Tcr in a direct comparison with wild-type Tet(L) and a vector control (Fig. 3A) but exhibited a wild-type capacity to complement the K+ uptake-deficient E. coli strain TK2420 (Fig. 3B) and to restore Na+ resistance to Na+-sensitive E. coli NM81. Transport data correlated with these findings. As shown in Fig. 4, everted vesicles from Tet(L)-12 exhibited no energy-dependent Tet uptake (Fig. 4A), whereas it exhibited wild-type levels of Na+ uptake (Fig. 4B). Presumably the modest Tcr conferred by Tet(L)-12 results from retention of some tetracycline binding capacity. Net K+ uptake activity was assayed in a protocol in which energy-dependent Rb+ uptake was monitored in right-side-out membrane vesicles; net K+ accumulation was dependent upon the presence of intravesicular K+ (as opposed to choline). As shown in Fig. 5, Tet(L)-12 exhibits more rapid and extensive Rb+ uptake. Since the results are normalized to total vesicle protein and the Tet(L)-12 vesicles contain less Tet protein than found in the wild-type vesicles, the difference is even greater than drawn had it been it normalized to Tet content.
FIG. 3.
Complementation capacities of Tet(L)-12 compared to that of wild-type Tet(L). The growth of the indicated transformants of E. coli DH5α on different concentrations of tetracycline (A), E. coli TK2420 on different concentrations of added KCl (B), and E. coli NM81 on different concentrations of added NaCl (C) are shown as the A600 measured after 15 h of growth. The results are the mean values of at least three independent experiments conducted in duplicate.
FIG. 4.
Transport of tetracycline (Tc) and Na+ by everted vesicles of Tet(L)-12 compared with that of wild-type Tet(L). (A) Everted vesicles from the indicated transformants of E. coli DH5α were assayed for tetracycline-Co2+/H+ antiport via the energy-dependent accumulation of radiolabeled tetracycline (25 μM) in the presence of 100 μM CoCl2. (B) Everted vesicles from the indicated transformants of E. coli NM81 were assayed for energy-dependent Na+/H+ antiport via 22Na+ uptake.
FIG. 5.
Capacity of Tet(L)-12 and wild-type Tet(L) to support energy-dependent accumulation of Rb+ by right-side-out vesicles of TK2420. Vesicles loaded with either choline Cl (circles) or KCl (triangles) were diluted into buffer containing the labeled Rb+ alone (open symbols) or together with the electron donor, d-lactate (closed symbols). The uptake observed in the vector controls under all conditions (lower panel) represents the level of equilibration of Rb+ as opposed to accumulation.
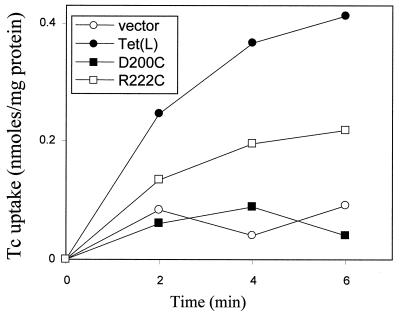
The small periplasmic loop between TMS VII and VIII that is deleted does not contain any residues of known importance in the transport mechanism. Therefore, in identifying candidate residues whose loss in Tet(L)-12 might relate to the loss of tetracycline transport capacity, we focused upon the two charged amino acids that are predicted by topology studies to be in TMS regions, D200 and R222. As shown in Fig. 6, the R222C mutant Tet(L) exhibited tetracycline transport activity that was less than that of the wild type but significant, whereas the D200C mutant protein exhibited no tetracycline transport activity. Even though less of the Tet(L) D200C protein was incorporated into the membrane, activity would have been detected were the protein active. Indeed, the D200C mutant protein was found to bind tetracycline well, as evidenced by much higher tetracycline binding controls than were observed with wild-type Tet(L) or R222C. Transport assays were consistent with the growth experiments, with the R222C mutant exhibiting all three transport modes but with D200C exhibiting only Na+/H+ antiport and net K+ (Rb+) uptake capacity. It is possible that D200C is an essential residue for tetracycline-Co2+/H+ antiport but not for any of the other activities of Tet(L). Either its alteration or its deletion together with the central two TMS might then account for the absence of that activity in Tet(L)-12. However, the excision of two entire TMS is likely to have had global effects on the protein, and even the single mutagenic change of D200 might affect activity indirectly. The basis for the change in the activity spectrum upon formation of Tet(L)-12 is thus likely to be complex. Whatever the details of how the change develops, the retention of robust Na+/H+ activity and net K+ uptake activity by Tet(L)-12 resonates with suggestions that ancestral forms of current antibiotic efflux proteins may have had different topologies and may have had a different spectrum of activities that included physiologically important transport capacities.
FIG. 6.
Tetracycline-Co2+/H+ antiport activity of D200C and R222C mutants of Tet(L) assayed in everted vesicles via energy-dependent uptake of tetracycline. The uptake activity of wild-type Tet(L) vesicles of E. coli DH5α is shown in comparison with that for vesicles from cells with the vector control or the D200C or R222C mutant.
Finally, two truncated forms of the wild-type Tet(L) were prepared as part of this study in view of the finding of others that approximately the N-terminal one-quarter to one-half of the Tet(B) and Tet(K) proteins, respectively, was sufficient to complement K+-deficient E. coli strains (8, 9,18). An earlier construct that we had prepared to resemble the reported truncated Tet(K) had not shown K+ uptake activity, but we had not assessed membrane incorporation of that protein (11). New constructs were made of wild-type Tet(L) that retained either six or eight full N-terminal TMS; incorporation of these proteins into the membrane was shown to be close to wild-type levels, but neither Tcr nor complementation of E. coli K+ uptake was observed. Thus, Tet(L)-12 is the only smaller Tet(L) form for which we have so far been able to confirm activity.
Acknowledgments
This work was supported by research grant GM52837 from the National Institute of General Medical Sciences.
REFERENCES
- 1.Cheng J, Baldwin K, Guffanti A A, Krulwich T A. The Na+/H+ antiport activity conferred by Bacillus subtilis tetA(L), a 5′ truncation product of tetA(L), and related plasmid genes upon Escherichia coli. Antimicrob Agents Chemother. 1996;40:852–857. doi: 10.1128/aac.40.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng J, Guffanti A A, Krulwich T A. The chromosomal tetracycline resistance locus of Bacillus subtilis encodes a Na+/H+ antiporter that is physiologically important at elevated pH. J Biol Chem. 1994;269:27365–27371. [PubMed] [Google Scholar]
- 3.Cheng J, Guffanti A A, Wang W, Krulwich T A, Bechhofer D H. Chromosomal tetA(L) gene of Bacillus subtilis: regulation of expression and physiology of a tetA(L) deletion strain. J Bacteriol. 1996;178:2853–2860. doi: 10.1128/jb.178.10.2853-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng J, Hicks D B, Krulwich T A. The purified Bacillus subtilis tetracycline efflux protein TetA(L) reconstitutes both tetracycline-cobalt/H+ and Na+/H+ exchange. Proc Natl Acad Sci USA. 1996;93:14446–14451. doi: 10.1073/pnas.93.25.14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein W, Kim B S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971;108:639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginn S L, Brown M H, Skurray R A. Membrane topology of the metal-tetracycline/H+ antiporter TetA(K) from Staphylococcus aureus. J Bacteriol. 1997;179:3786–3789. doi: 10.1128/jb.179.11.3786-3789.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffith J K, Baker M E, Rouch D A, Page M G, Skurray R A, Paulsen I T, Chater K F, Baldwin S A, Henderson P J. Membrane transport proteins: implications of sequence comparisons. Curr Opin Cell Biol. 1992;4:684–695. doi: 10.1016/0955-0674(92)90090-y. [DOI] [PubMed] [Google Scholar]
- 8.Griffith J K, Kogoma T, Corvo D L, Anderson W L, Kazim A L. An N-terminal domain of the tetracycline resistance protein increases susceptibility to aminoglycosides and complements potassium uptake defects in Escherichia coli. J Bacteriol. 1988;170:598–604. doi: 10.1128/jb.170.2.598-604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guay G G, Tuckman M, McNicholas P, Rothstein D M. The tet(K) gene from Staphylococcus aureus mediates the transport of potassium in Escherichia coli. J Bacteriol. 1993;175:4927–4929. doi: 10.1128/jb.175.15.4927-4929.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guffanti A A, Krulwich T A. Tetracycline/H+ antiport and Na+/H+ antiport catalyzed by the Bacillus subtilis TetA(L) transporter expressed in Escherichia coli. J Bacteriol. 1995;177:4557–4561. doi: 10.1128/jb.177.15.4557-4561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guffanti A A, Cheng J, Krulwich T A. Electrogenic antiport activities of the Gram-positive Tet proteins include a Na+(K+)/K+ mode that mediates net K+ uptake. J Biol Chem. 1998;273:26447–26454. doi: 10.1074/jbc.273.41.26447. [DOI] [PubMed] [Google Scholar]
- 12.Hirata T, Fujihira E, Kimura-Someya T, Yamaguchi A. Membrane topology of the staphylococcal tetracycline efflux protein Tet(K) determined by antibacterial resistance gene fusion. J Biochem. 1998;124:1206–1211. doi: 10.1093/oxfordjournals.jbchem.a022239. [DOI] [PubMed] [Google Scholar]
- 13.Kaback H R. Bacterial membranes. Methods Enzymol. 1971;22:99–120. [Google Scholar]
- 14.Krulwich, T. A., J. Jin, A. A. Guffanti, and D. H. Bechhofer. Functions of tetracycline efflux proteins that do not involve tetracycline. J. Mol. Microbiol. Biotechnol., in press. [PubMed]
- 15.Kunkel T A, Benenek K, McClary J. Efficient site-directed mutagenesis using uracil-containing DNA. Methods Enzymol. 1991;204:125–139. doi: 10.1016/0076-6879(91)04008-c. [DOI] [PubMed] [Google Scholar]
- 16.Levy S B. Active efflux mechanisms for antimicrobial resistance. Antimicrob Agents Chemother. 1992;36:695–703. doi: 10.1128/aac.36.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMurry L M, Levy S B. Tetracycline resistance in gram-positive bacteria. In: Fischetti V A, Novick R P, Ferretti J J, Portnoy D A, Rood J I, editors. Gram-positive pathogens. Washington, D.C.: ASM Press; 2000. pp. 660–677. [Google Scholar]
- 18.Nakamura T, Matsura Y, Ishihara A, Kitagawa T, Suzuki T, Unemoto T. N-terminal quarter part of tetracycline transporter from pACYC184 complements K+ uptake activity in K+ uptake-deficient mutants of Escherichia coli and Vibrio alginolyticus. Biol Pharm Bull. 1995;18:1189–1193. doi: 10.1248/bpb.18.1189. [DOI] [PubMed] [Google Scholar]
- 19.Okstad O A, Gronstad A, Lindback T, Kolsto A B. Insertional inactivation of a Tet(K)/Tet(L) like transporter does not eliminate tetracycline resistance in Bacillus cereus. FEMS Microbiol Lett. 1997;154:181–186. doi: 10.1111/j.1574-6968.1997.tb12641.x. [DOI] [PubMed] [Google Scholar]
- 20.Padan E, Maisler N, Taglicht D, Karpel R, Schuldiner S. Deletion of ant in Escherichia coli reveals its function in adaptation to high salinity and an alternative Na+/H+ antiporter system(s) J Biol Chem. 1989;264:20297–20302. [PubMed] [Google Scholar]
- 21.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulsen I T, Skurray R A. Topology, structure and evolution of two families of proteins involved in antibiotic and antiseptic resistance in eukaryotes and prokaryotes—an analysis. Gene. 1993;124:1–11. doi: 10.1016/0378-1119(93)90755-r. [DOI] [PubMed] [Google Scholar]
- 23.Rubin R A, Levy S B, Jeinrikson R L, Kezdy F J. Gene duplication in the evolution of the two complementing domains of Gram-negative bacterial tetracycline efflux proteins. Gene. 1990;87:7–13. doi: 10.1016/0378-1119(90)90489-e. [DOI] [PubMed] [Google Scholar]
- 24.Saier H M, Jr, Paulsen I T, Sliwinski M K, Pao S S, Skurray R A, Nikaido H. Evolutionary origins of multidrug and drug-specific efflux pumps in bacteria. FASEB J. 1998;12:265–274. doi: 10.1096/fasebj.12.3.265. [DOI] [PubMed] [Google Scholar]
- 25.Wang W, Guffanti A A, Wei Y, Ito M, Krulwich T A. Two types of Bacillus subtilis tetA(L) deletion strains reveal the physiological importance of TetA(L) in K+ acquisition as well as in Na+, alkali, and tetracycline resistance. J Bacteriol. 2000;182:2088–2095. doi: 10.1128/jb.182.8.2088-2095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinglass A B, Kaback H R. The central cytoplasmic loop of the major facilitator superfamily of transport proteins governs efficient membrane insertion. Proc Natl Acad Sci USA. 2000;97:8938–8943. doi: 10.1073/pnas.140224497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi A, Shiina Y, Fujihira E, Sawai T, Noguchi N, Sasatsu M. The tetracycline efflux protein encoded by the tet(K) gene from Staphylococcus aureus is a metal-tetracycline/H+ antiporter. FEBS Lett. 1995;365:193–197. doi: 10.1016/0014-5793(95)00455-i. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi A, Udagawa T, Sawai T. Transport of divalent cations with tetracycline as mediated by the transposon Tn10-encoded tetracycline resistance protein. J Biol Chem. 1990;265:4809–4813. [PubMed] [Google Scholar]