Abstract
Perioperative immune function, postoperative cognitive function and prognosis are momentous issues for patients undergoing digestive tract cancer surgery. Studies have investigated the efficacy of dexmedetomidine (DEX) administration on these issues, but the results are inconsistent. Therefore, this meta-analysis aimed to summarize all the existing evidence and draw a conclusion more accurately on these associations. Trials were located through electronic searches of the PubMed, Embase, the Cochrane Library and Web of Science databases sources (from the establishment date of databases to April 2022). Bibliographies of the retrieved articles were checked. A total of 17 RCTs involving 1619 patients were included. The results showed that DEX decreased the level of C-reactive protein (SMD = -4.26, 95%CI: -6.16, -2.36), TNF-α (SMD = -4.22, 95%CI: -5.91, -2.54) and IL-6 (SMD = -2.71, 95%CI: -4.46, -0.97), and increased the level of IL-10 (SMD = 1.74, 95%CI: 0.25, 3.24). DEX also increased CD4+ T cells (SMD = 0.55, 95%CI: 0.29, 0.82) and CD4+/CD8+ ratio (SMD = 0.62, 95%CI: 0.24, 1.01). Thus, DEX was associated with alleviation of postoperative systemic inflammatory response and immune dysfunction. Furthermore, DEX increased mini-mental state examination scores at 12h (SMD = 1.10, 95%CI: 0.74,1.45), 24h (SMD = 0.85, 95%CI: 0.59, 1.11), 48h (SMD = 0.89, 95%CI: 0.50, 1.28) and 72h (SMD = 0.75, 95%CI: 0.38, 1.11) after surgery. DEX decreased the occurrence of postoperative cognitive dysfunction (POCD) at 24h (OR = 0.22, 95%CI: 0.11, 0.46) and 72h (OR = 0.39, 95%CI: 0.22, 0.68) after surgery. DEX decreased first flatus time (SMD = -1.55, 95%CI: -2.82, -0.27) and hospital stay (SMD = -1.23, 95%CI: -1.88, -0.59). Therefore, based on perioperative immune dysfunction alleviation, DEX attenuated POCD and potential neuroinflammation, improved postoperative recovery and clinical prognosis of patients undergoing digest tract cancer surgery. Further studies are necessary to elucidate the clinical application of DEX from an immunological perspective.
Keywords: dexmedetomidine, immune function, postoperative cognitive dysfunction, digestive tract cancer, prognosis, meta-analysis
Introduction
Systemic immune perturbations occur with cancer development (1). Tumor-burdened microenvironment affects the quantity and differentiation of T cells, neutrophils, and monocyte, especially for the elderly with underlying diseases and impaired immune function (2, 3). Radical surgery is the preferred treatment for most patients with early-stage cancer (4). However, the incidence of systemic inflammatory response syndrome (SIRS) increased during the perioperative period due to anesthesia, surgical trauma, and pre-existing comorbidities. The release of damage-associated molecular patterns (DAMPs) or alarmins following the surgical injury is the important involved mechanism (5, 6). DAMPs could activate immune cells including neutrophils and lymphocytes, and trigger the release of pro-inflammatory mediators including IL-6, IL-1, and TNF-α (7, 8). High mobility group box 1 protein (HMGB1) is a DAMP molecule. It affects the activation and differentiation of Treg and is associated with cancer recurrence and metastasis (9). Meanwhile, perioperative factors and peripheral inflammation are associated with central nervous system (CNS) neuroinflammation and pathologies (10, 11). Elevated inflammatory cytokines in the CNS are concentrated in the hippocampus, where the receptors of pro-inflammatory cytokines were highly expressed, leading to postoperative cognitive dysfunction (POCD), especially for the elderly with an impaired blood-brain barrier (BBB) (12, 13). Therefore, perioperative immune dysfunction and CNS neuroinflammation have momentous clinical implications in postoperative recovery, tumor recurrence, and metastasis, etc.
Dexmedetomidine (DEX) is a highly selective α2 adrenergic receptor agonist, especially for the α2A adrenergic receptor located in the locus coeruleus nucleus (14). DEX has been frequently used in the perioperative period because of its sedative pharmacology. DEX could attenuate stress responses and emotional disorders, and create stable hemodynamic profiles during stressful events such as surgery or anesthetic induction (15). DEX could resemble natural sleep, increase physiological sleep-wake cycle for ICU patients, and reduce the risk of delirium (16). DEX could also reduce the level of postoperative inflammatory factors through PI3K-Akt signaling (17), and inhibit cancer development through the upregulation of miR-185 and inactivation of SOX9-Wnt-β-catenin signaling (18). Moreover, there is growing evidence that DEX has a potential role during perioperative period for the prevention and alleviation of inflammation and immune dysfunction (19).
Multiple RCTs have been conducted to determine whether perioperative intravenous DEX could alleviate postoperative SIRS and POCD in patients undergoing radical surgery (20, 21). However, due to the methodology and small sample size, interpretation of these studies has limitations and the results are inconsistent. Meanwhile, the mechanism by which DEX interferes with cellular and humoral immunity is still unclear. Therefore, this meta-analysis aimed to summarize all existing evidence and systematically review the impact of DEX on perioperative immune dysfunction, POCD, and postoperative recovery, to provide guidance for clinical treatment and prognosis.
Methods
This meta-analysis was conducted based on the criteria of the Cochrane Handbook for Systematic Reviews of Interventions (version 6.2). The results were presented according to the preferred reporting items declared by Systematic Review and Meta-Analysis (PRISMA) 2020. Ethical approval was not required, as this study only included articles of published data in the public domain.
Literature search
Two reviewers performed the literature search, systematically searching the PubMed, Embase, the Cochrane Library, and Web of Science databases sources until April 2022 for studies exploring the application of perioperative DEX in patients with digestive tract cancer. The following search terms were used: (1) “Dexmedetomidine”, “MPV-1440”, “Precedex” or “Dexmedetomidine Hydrochloride”, (2) “Esophageal Neoplasms”, “Stomach Neoplasms”, “Gastrointestinal Neoplasms”, “Colorectal Neoplasms”, “Colonic Neoplasms”, “Rectal Neoplasms”, “Intestinal Neoplasms”, “Esophageal Cancer”, “Gastric Cancer”, “Intestinal Cancer”, “Colorectal Cancer”, “Colon Cancer”, “Rectal Cancer”, “Gastrointestinal Cancer” or “Digestive Tract Cancer”. The above two categories of search terms were combined using the Boolean operator “and”. The search strategies are shown in Table 1 , and the detailed electronic search strategies for PubMed, Cochrane Library, EMBASE and Web of Science databases are shown in Supplementary Table 1 . In addition, the reference lists of the retrieved articles and prior reviews were manually checked for additional eligible studies. We applied no linguistic restrictions in the literature search.
Table 1.
The search strategies until February 2022.
| Search terms | PubMed | Embase | Web of science | Cochrane | |
|---|---|---|---|---|---|
| #1 | Dexmedetomidine | 7555 | 15170 | 9929 | 6184 |
| #2 | MPV-1440 | 7556 | 4 | 1 | 3 |
| #3 | Precedex | 7558 | 523 | 54 | 82 |
| #4 | Dexmedetomidine Hydrochloride | 7555 | 130 | 223 | 123 |
| #5 | #1 OR #2 OR #3 OR #4 | 7559 | 15170 | 9931 | 6181 |
| #6 | Esophageal Neoplasms | 63836 | 2205 | 3419 | 2395 |
| #7 | Stomach Neoplasms | 119232 | 5731 | 5952 | 3896 |
| #8 | Gastrointestinal Neoplasms | 443191 | 1149 | 12158 | 5394 |
| #9 | Colorectal Neoplasms | 240215 | 5798 | 13841 | 8733 |
| #10 | Colonic Neoplasms | 92777 | 1677 | 3429 | 4402 |
| #11 | Rectal Neoplasms | 69204 | 1662 | 4772 | 3351 |
| #12 | Intestinal Neoplasms | 270675 | 383 | 3813 | 2216 |
| #13 | Esophageal Cancer | 73668 | 35427 | 53390 | 4858 |
| #14 | Gastric Cancer | 152596 | 102860 | 128855 | 8623 |
| #15 | Intestinal Cancer | 285551 | 1648 | 49274 | 4676 |
| #16 | Colorectal Cancer | 271702 | 233478 | 251089 | 17298 |
| #17 | Colon Cancer | 152047 | 113537 | 158616 | 8164 |
| #18 | Rectal Cancer | 76673 | 42414 | 59070 | 6221 |
| #19 | Gastrointestinal Cancer | 469120 | 18366 | 118013 | 11785 |
| #20 | Digestive Tract Cancer | 159,200 | 516 | 30,874 | 621 |
| #21 | #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 | 651,377 | 492021 | 964,761 | 56828 |
| #22 | #5 AND #21 | 92 | 126 | 97 | 137 |
Inclusion and exclusion criteria
RCTs conducted to compare DEX with placebo in patients undergoing digest tract tumor surgery were all enrolled. Included studies need to report at least one of the outcomes, including inflammatory factors, cellular immunity, cognitive function, and prognosis. Exclusion criteria were as follows: (1) reviews, letters, editorials, or observational (prospective or retrospective cohort) study; (2) comparisons of DEX with other sedatives(midazolam, fentanyl, propofol, etc.); (3) no intravenous administration; (4) no target outcomes; (5) data was unable to obtain or insufficient. If there were overlapping data among two or more studies, we included the one with the largest sample size.
Study selection and data abstraction
Two reviewers independently screened the titles and abstracts of the retrieved studies from the electronic databases. Subsequently, eligible studies were selected after full-text screenings according to the pre-defined criteria. Disagreements were resolved by discussion between two reviewers or consultation with the corresponding authors. The following data of the included studies were abstracted: study characteristics (first author, year of publication, and study design), study population, baseline characteristics (age, sample size, interventions, and anesthesia method), outcomes (inflammatory factors, cellular immunity, cognitive function, and prognosis), and outcome data (sample size and the number of events between groups).
Study quality assessment
The bias risks of RCTs were assessed using the revised Cochrane risk-of-bias tool for randomized trials (RoB2), which is more detailed and comprehensive than RoB1, and includes five domains: randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results. The level of the bias risk in each domain and overall were scored as ‘low risk’, ‘some concerns’, or ‘high risk’. We used the funnel plots to assess the publication bias, and there was no significant asymmetry in the funnel plots of the present data ( Supplementary Figures 1 – 4 ). Thus, there was no significant publication bias. Supplementary Table 2 presents the GRADE analysis of the variables examined in this meta-analysis. The certainty was high for POCD and MMSE; moderate for CRP, IL-6, IL-10, lymphocyte subsets, first flatus time, hospital stay and extubation time, and low for TNF-α.
Statistical analysis
Statistical analysis for this meta-analysis was This meta-analysis’s statistical analyses were conducted using the Review Manager version 5.4 software (the Cochrane Collaboration 2014, Nordic Cochrane Centre Copenhagen, Denmark; https://community.cochrane.org/). The pooled effects were calculated and described by standard mean difference (SMD) with a 95% Confidence Interval (the Confidence Interval, 95% CI) and the risk ratio with 95% CI. The significant heterogeneity was indicated by a P-value of < 0.10 in the Cochrane Q test or an I² value of > 50%, which led to the use of random-effects models and the exploration of a potential source of heterogeneity. Otherwise, the fixed-effects model was selected.
Results
Study selection outcome
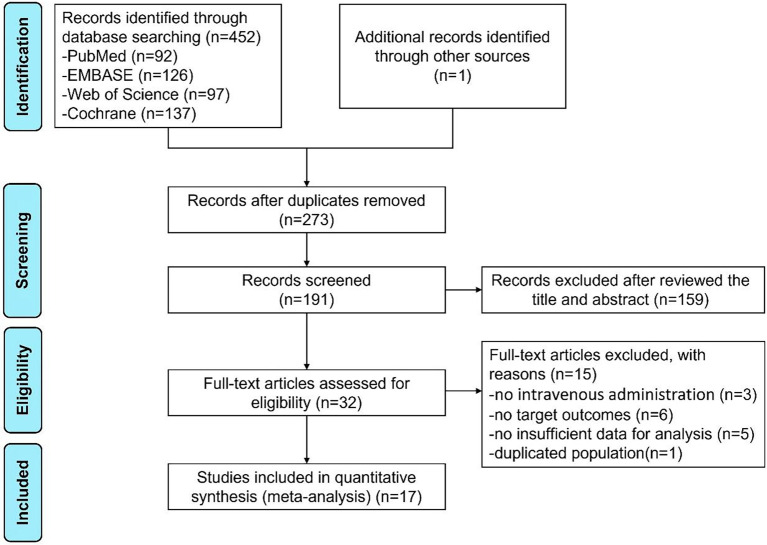
The flow chart of literature retrieval is shown in Figure 1 . Through searching the PubMed, Embase, Cochrane Library, and Web of Science databases, the initial search yielded 452 articles. We also identified the potential studies by searching the reference lists of published reviews, in this way, we found a relevant article from Chinese biological and medical database. Duplicate articles were removed. After screening the records, reviewing the title and abstract, and the full-text screenings with the pre-defined criteria to exclude 15 studies, a total of 17 studies were included in this meta-analysis.
Figure 1.
The flow chart of literature retrieval of this meta-analysis.
Study characteristics
17 RCTs involving 1619 patients undergoing surgical resection of digest tract tumors were finally included. Baseline characteristics and detailed administration of included studies are shown in Table 2 . The mean age of patients ranged from 36.3 to 74.1 years, and the sample size was from 48 to 180. The detailed usage and dosage of DEX are shown in Table 2 . DEX was administered intravenously in all studies. 14 studies administered a loading dose before induction and followed by continuous infusion. The other 3 studies only administered a loading dose before induction. The control group was injected intravenously with the same volume of normal saline.
Table 2.
Characteristics of included studies.
| Author -Year | Trial type | Simple size | Mean Age(Years) | Male ratio (%) | Intervention study(DEX dose/administration mode) | Neoplasm’s type | Comparators | Anesthesia method | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | DEX | CON | DEX | CON | DEX | ||||||
| Mao Y-2020 (22) | RCT | 29 | 29 | 63.5 | 65.2 | 82.8 | 79.3 | Bolus (0.5 ug/kg) before induction, and then continuous infusion (0.2-0.4 ug/kg/h) during operation | Esophageal Cancer | Saline | I.V. |
| Dong W-2017 (23) | RCT | 37 | 37 | 38.7 | 36.3 | 54.1 | 62.2 | Bolus (1 ug/kg; 15 min) before induction, and then continuous infusion (0.2 ug/kg/h) during operation | Gastric Cancer | Saline | Combined |
| Niu Y-2022 | RCT | 30 | 30 | 69.0 | 68.0 | 60.0 | 56.7 | Bolus (0.6 ug/kg; 15 min) before induction, and then continuous infusion (0.2 ug/kg/h) during operation | Gastric Cancer | Saline | I.V. |
| Wang Z-2020 (17) | RCT | 50 | 60 | 68.3 | 68.4 | 60.0 | 63.3 | Bolus (0.5 ug/kg) before induction, and then continuous infusion (0.4 ug/kg/h) during operation | Gastric Cancer | Saline | Combined |
| Zhu Z-2017 (24) | RCT | 45 | 45 | 51.5 | 51.8 | 55.6 | 48.9 | Bolus (0.6 ug/kg) before induction | Gastric Cancer | Saline | Combined |
| Huai Q-2022 (25) | RCT | 40 | 40 | 63.5 | 61.7 | 65.0 | 60.0 | Bolus (0.5 ug/kg; 10 min) before induction, and then continuous infusion (0.4 ug/kg/h) during operation | Colon Cancer | Saline | Inhalation |
| Wang K-2018 (26) | RCT | 69 | 72 | 45.3 | 42.5 | 47.8 | 50.0 | Bolus (1 ug/kg; 10-15 min) before induction, and then continuous infusion (1 ug/kg/h) during operation | Colon Cancer | Saline | Combined |
| Zhao L-2020 (27) | RCT | 84 | 92 | 53.1 | 52.5 | 56.0 | 45.7 | Bolus (200ug) | Colon Cancer | Saline | I.V. |
| Ben Z-2016 (28) | RCT | 44 | 44 | 68.6 | 59.1 | Bolus (0.5 ug/kg) before induction, and then continuous infusion (0.1 ug/kg/h) during operation | Rectal Cancer | Saline | I.V. | ||
| Chen C-2016 (29) | RCT | 30 | 30 | 60.1 | 56.7 | 50.0 | 46.7 | Bolus (1 ug/kg; 10 min) before induction, and then continuous infusion (0.3 ug/kg/h) during operation | Colorectal Cancer | Saline | Inhalation |
| Kong Y-2021 (30) | RCT | 60 | 120 | 65.0 | 65.0 | NA | NA | Bolus (0.5 ug/kg) before induction, and then continuous infusion (0.4/0.8 ug/kg/h) during operation | Colorectal Cancer | Saline | I.V. |
| Liu X-2017 (31) | RCT | 48 | 48 | 69.1 | 68.4 | 54.2 | 52.1 | Bolus (1.5 ug/kg;30min) | Colorectal Cancer | Saline | I.V. |
| Liu Y-2020 (32) | RCT | 24 | 24 | 68.6 | 69.6 | 54.2 | 62.5 | Bolus (0.5 ug/kg; 15 min) before induction, and then continuous infusion (0.6 ug/kg/h) during operation | Colorectal Cancer | Saline | I.V. |
| Pan C-2016 (33) | RCT | 41 | 41 | 73.9 | 71.9 | NA | 48.8 | Bolus (0.5 ug/kg) before induction, and then continuous infusion (0.3 ug/kg/h) during operation | Colorectal Cancer | Saline | NA |
| Sun W-2021 (34) | RCT | 28 | 28 | 59.0 | 60.0 | 60.7 | 67.9 | Bolus (1 ug/kg; 10 min) before induction, and then continuous infusion (0.5 ug/kg/h) during operation | Colorectal Cancer | Saline | Combined |
| Zhang J-2019 (35) | RCT | 60 | 80 | 74.1 | 73.8 | 66.7 | 63.8 | Bolus (1 ug/kg; 15 min) before induction, and then continuous infusion (0.2-0.7 ug/kg/h) during operation | Colorectal Cancer | Saline | I.V. |
| Zhang Y-2014 (36) | RCT | 20 | 60 | 71.5 | 72.0 | 55.2 | 60.0 | Bolus (0.5 ug/kg; 15 min) before induction, and then continuous infusion (0.2/0.5/0.8 ug/kg/h) during operation | Colorectal Cancer | Saline | I.V. |
Risk of bias assessment
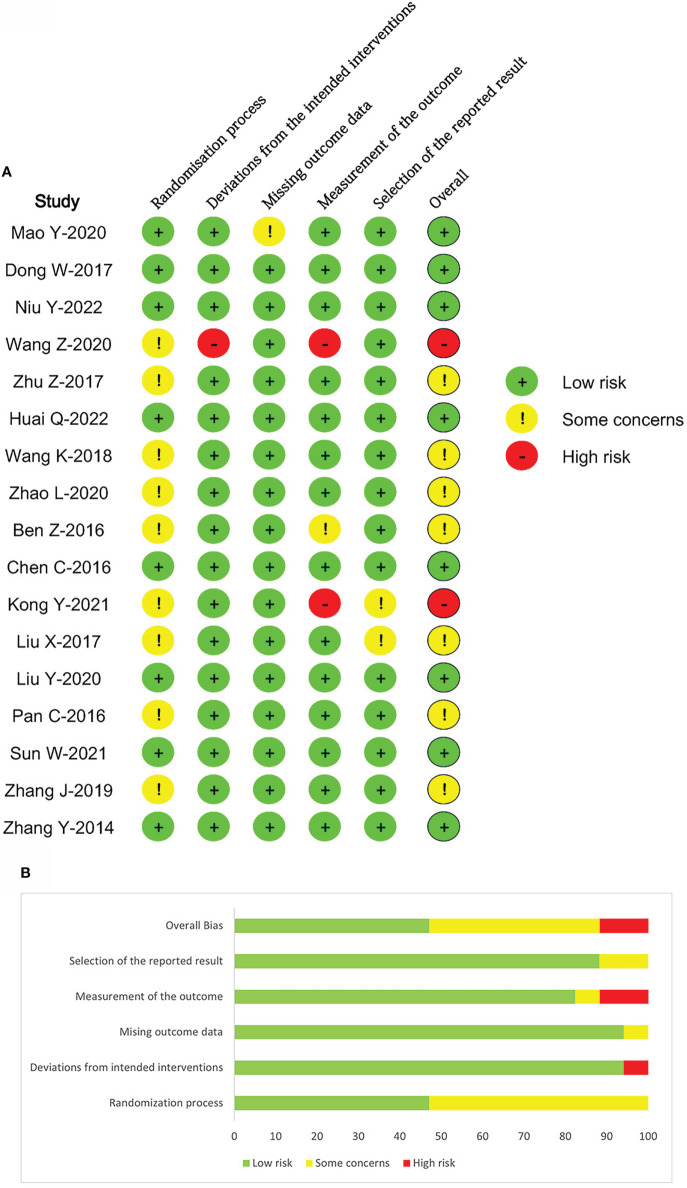
The risk of bias assessment for individual studies is shown in Figure 2 . Of the included trials, nine studies did not give a specific randomization process, which was categorized as “some concerns”. One study deviated from the intended intervention, and two studies were judged high risk in terms of the measurement of the outcomes. The remaining eight studies were identified as low risk.
Figure 2.
Methodological quality graph and summary of the included studies: Risk of bias summary (A), Risk of bias graph (B).
Synthesis of results
The different outcomes of 17 studies were synthesized. First, the changes of inflammatory mediators were concerned, as surgical and anesthetic factors can lead to systemic inflammatory responses that further exacerbate central inflammation. Then, T lymphocyte subsets were observed, which has been proven to be an important indicator of immune dysfunction, The systemic inflammatory response and immune dysfunction in the perioperative period will further aggravate the CNS neuroinflammation and cognitive dysfunction of patients, thus affecting postoperative recovery and clinical prognosis. Therefore, our study comprehensively considered the effects of DEX on inflammatory mediators, T lymphocytes, POCD, and postoperative recovery.
Effects of DEX on postoperative inflammatory mediators
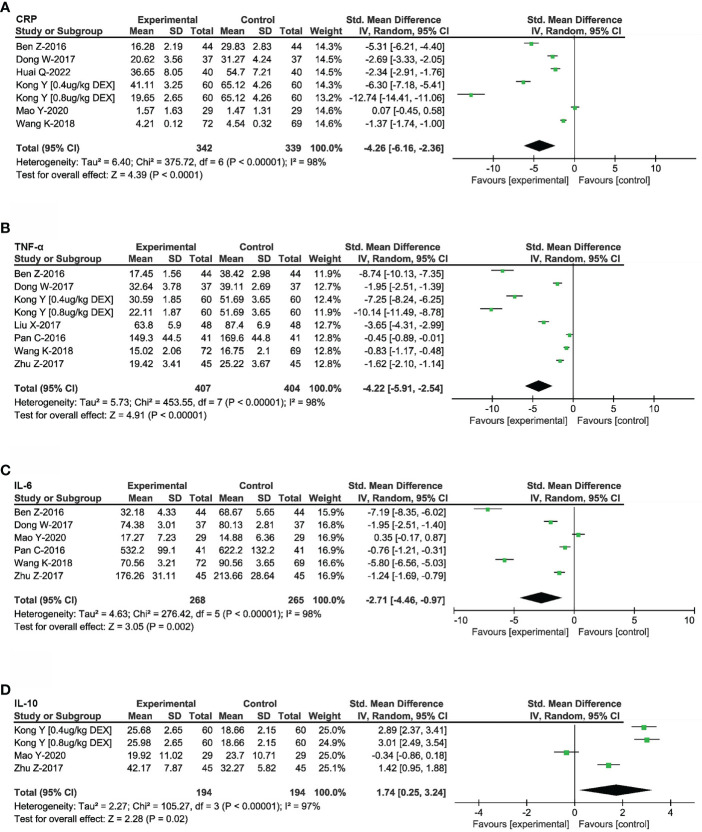
9 RCTs that reported the postoperative levels of inflammatory mediators in 889 patients with digest tract tumors were included (22–26, 28, 30, 31, 33). CRP has been used as a marker of acute inflammatory responses in a variety of psychiatric and physical conditions. As shown in Figure 3A , the pooled results based on the random-effects model indicated that the use of DEX was significantly associated with reduced CRP release (SMD = -4.26, 95%CI: -6.16, -2.36). Meanwhile, in order to illustrate the effects of DEX on inflammation, several inflammatory cytokines were investigated based on the involved studies. The results indicated that DEX decreased the release of TNF-α (SMD = -4.22, 95%CI: -5.91, -2.54, Figure 3B ) and IL-6 (SMD = -2.71, 95%CI: -4.46, -0.97, Figure 3C ), but increased the release of IL-10 (SMD = 1.74, 95%CI: 0.25, 3.24, Figure 3D ). These release changes could improve cellular immunosuppression and attenuate the progress of digest tract cancer.
Figure 3.
Effects of DEX on inflammatory mediators. Forest plot of odds ratio, analyzed by Mantel-Haenszel statistics in the random-effect model. Meta-analysis of the DEX effect on CRP (A), TNF-a (B), IL-6 (C) and IL-10 (D) respectively.
Effects of DEX on postoperative T lymphocytes
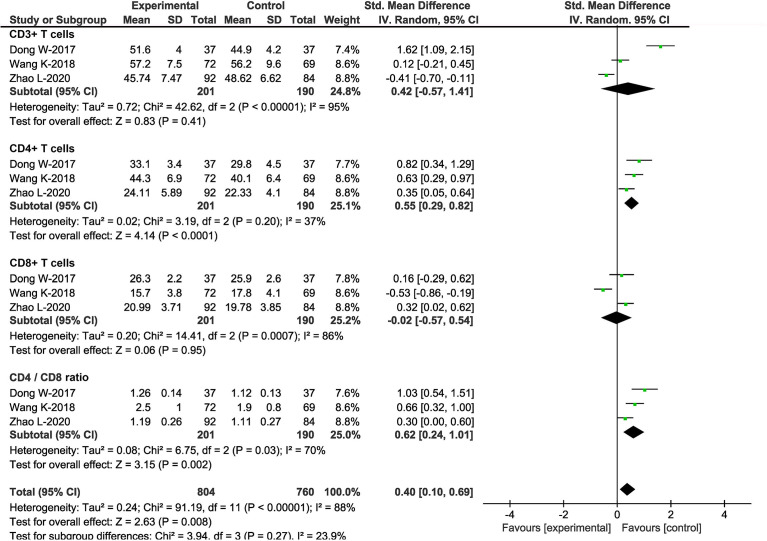
T lymphocytes play roles in perioperative immune homeostasis and tumor resistance within the digestive tract; thus, the state and subsets of T lymphocytes were investigated. Three studies, including 391 patients, investigated the effects of DEX on T lymphocytes (23, 26, 27). T lymphocyte subsets included were CD3+, CD4+, and CD8+ T cells. At the same time, we calculated the CD4+/CD8+ ratio, which decreased and indicated impaired immune functions and poor prognosis. Separately, there was no significant difference in the counts of CD3+ T cells (SMD = 0.42, 95%CI: -0.57, 1.41) and CD8+ T cells (SMD = -0.02, 95%CI: -0.57, 0.54) between patients treated with and without DEX at 24h postoperatively. In contrast, CD4+ T cell counts (SMD = 0.55, 95%CI: 0.29, 0.82) and CD4+/CD8+ ratio (SMD = 0.62, 95%CI: 0.24, 1.01) increased in patients with DEX ( Figure 4 ). Therefore, DEX attenuated the variation of cellular immune functions caused by surgical trauma, stress responses and other perioperative factors.
Figure 4.
Effects of DEX on T lymphocytes. Forest plot of odds ratio, analyzed by Mantel-Haenszel statistics in the random -effect model. Meta-analysis of the DEX effect on CD3+ T cells, CD4+ T cells, CD8+ T cells and CD4+ /CD8+ ratio respectively.
Effects of DEX on cognitive function
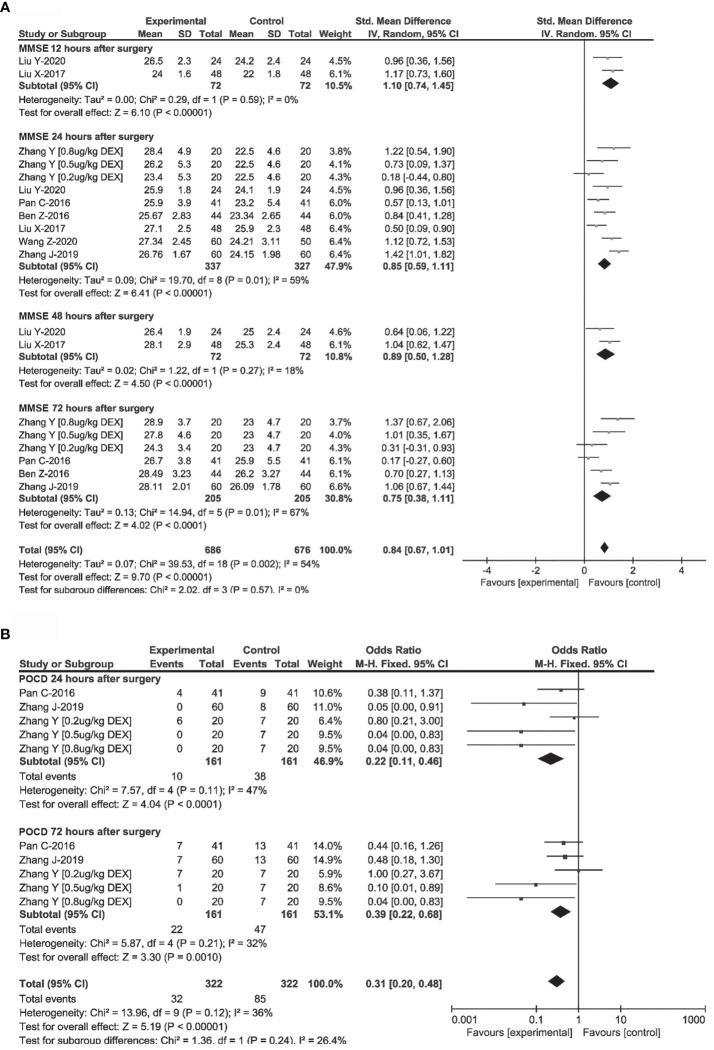
Random-effects model and fixed-effect model were used to synthesize the MMSE scores and the incidence of POCD at different time points after surgery in 7 RCTs to consider the effect of DEX on postoperative cognitive function comprehensively (17, 28, 31–33, 35, 36). As shown in Figure 5 , in digest tract tumor patients, DEX administration was associated with higher MMSE scores at 12h (SMD = 1.10, 95%CI: 0.74, 1.45), 24h (SMD = 0.85, 95%CI: 0.59, 1.11), 48h (SMD = 0.89, 95%CI: 0.50, 1.28) and 72h (SMD = 0.75, 95%CI: 0.38, 1.11) after surgery. DEX administration was also associated with a significant reduction in the occurrence of POCD at 24h (OR = 0.22, 95%CI: 0.11, 0.46) and 72h (OR = 0.39, 95%CI: 0.22, 0.68) after surgery. As previous studies indicated, the changes of cognitive function could be associated with perioperative immune function and neuroinflammation (37).
Figure 5.
Effects of DEX on postoperative cognitive function. Forest plot of odds ratio, analyzed by Mantel-Haenszel statistics in the random-effect model. Meta-analysis of the DEX effect on MMSE at 24h, 48h, 72h after surgery (A), and the occurrence of POCD at 24h, 72h after surgery (B) respectively.
Effects of DEX on postoperative recovery
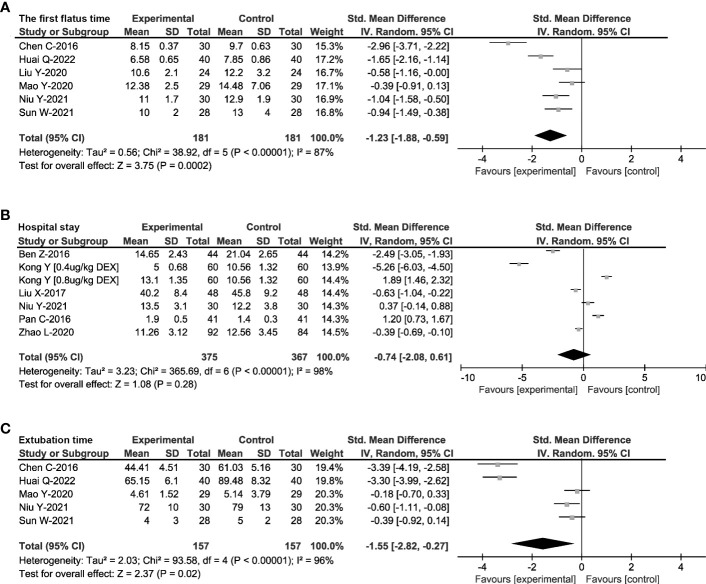
The perioperative stress and inflammation could affect gastrointestinal motility, extubation time and hospital stay, which are considered as the indicators for patient recovery and clinical prognosis. Therefore, a random-effects model was used to synthesize the postoperative extubation time, first flatus time and hospital stay of 808 patients in 11 RCTs (22, 25, 27–34, 38). As shown in Figure 6 , DEX administration decreased first flatus time (SMD = -1.55, 95%CI: -2.82, -0.27), and the length of hospital stay (SMD = -1.23, 95%CI: -1.88, -0.59). However, there was no significant difference in postoperative extubation time (SMD = -0.74, 95%CI: -2.08, 0.61).
Figure 6.
Effects of DEX on prognosis. Forest plot of odds ratio, analyzed by Mantel-Haenszel statistics in the random-effect model. Meta-analysis of the effect on the first flatus time (A), hospital stay (B) and postoperative extubation time (C) respectively.
Discussion
This study investigated the effects of DEX administration on postoperative immune dysfunction, cognitive function, and recovery. The results indicated that DEX decreased the level of CRP, TNF-α, and IL-6, and increased the level of IL-10. DEX increased CD4+ T cell counts and CD4+/CD8+ ratio. Furthermore, DEX led to higher MMSE scores during postoperative periods and a significant reduction in the occurrence of POCD. DEX decreased the first flatus time and the length of hospital stay, but not postoperative extubation time. Therefore, DEX administration attenuated postoperative systemic inflammatory response and immune dysfunction, improved cognitive function, and recovery in patients undergoing digest tract cancer surgery.
Previous studies showed that cancer could lead to systemic immune perturbations and affect responses to new immune challenges (1). Meanwhile, surgery could put body in a stress state, which leads to the imbalance of the neuro-endocrine-immune network, resulting in low cellular and humoral immune functions (39). The present results indicated that DEX could decrease postoperative levels of CRP, TNF-α and IL-6, and increase the level of IL-10. CRP is widely used as a marker of inflammation, infection, and tissue damage (40), and plays an important role in tumor development. The prognostic value of CRP has already been shown for digestive tract cancer (41). As a major cytokine in the acute phase, IL-6 is associated with the pathological progress of digest tract cancer (42). Low serum IL-6 level has been shown to be an independent prognostic factor for disease-free survival of patients with hepatitis B virus-related hepatocellular carcinoma who underwent hepatic resection (43). IL-10 is an anti-inflammatory mediator and plays a dual role in immune modulation, depending on cell type and environment (44). The immunosuppressive role of IL-10 has led to the general view that its presence during the development of cancer would facilitate tumor immune escape (45). However, A recent study showed that IL-10 potentiated the antitumor activity of CD8+ T cells by increasing its tissue infiltration, inducing IFN-γ, and favoring effective T cell memory responses (46, 47). Previous studies showed that surgery triggered a central response via afferent nerves to activate the sympathetic-adrenal-medullary (SAM) axis, and increased blood leukocyte counts (48, 49). Dexmedetomidine could affect SAM, reduce the release of epinephrine and norepinephrine, and decrease inflammatory factors (50, 51). DEX could also reverse HMGB1 related systemic and hippocampal inflammatory responses through the following mechanisms: 1) stimulation of the vagus nerve, 2) elimination of DAMP molecules and damaged mitochondria through PINK1-mediated mitophagy, 3) promotion the resolution of inflammation through TGF-31 secreted by F4/80Ly6G (52–54). Therefore, DEX could attenuate the negative effects of DAMP and inflammatory responses during digest tract cancer surgery.
T-lymphocyte could maintain the homeostasis within digestive tract mucosa and play an important role in anti-tumor immunity. The present results indicated that DEX could increase postoperative CD4+ T cells and CD4+/CD8+ ratio. CD4+ T cells play an important role in anti-tumor response. Recent studies have shown that CD4+ T cells not only enhanced the tumoricidal activity of other anti-tumor effector cells, but also blocked tumor growth through directly affecting the progression of tumor cell cycle (55). All mature peripheral T-lymphocytes, labeled by CD3+, represent the general level of immunity, and reduction of CD4+/CD8+ ratio indicates decreased immune function and poor prognosis (56, 57). Therefore, DEX could attenuate perioperative cellular immune function suppression, and further alleviate inflammatory response.
The patients with cancer are more vulnerable to postoperative systemic immune dysfunction and the peripheral environment is connected with CNS (58). Pro-inflammatory signals from peripheral immune system could enter CNS and cause neurotoxic symptoms (59). During radical surgery, intracellular substances released from damaged tissues and organs will be recognized by immune cells (60). Immune cells affect the expression of inflammatory factors, which can trigger the CNS response and amplify neuroinflammation through vagal afferents or BBB (61, 62). The inflammatory cells in CNS release more inflammatory cytokines which concentrate in specific brain regions, leading to the occurrence of POCD (37, 63, 64) and resulting in the development of neurodegenerative diseases (65). The present results indicated that DEX decreased the occurrence of POCD, which could be related to the effect of DEX on perioperative immune function and potential neuroinflammation.
Surgery is a common treatment for gastrointestinal tumors. The digestive tract is an important immune organ, and surgery could cause irreversible damage to it. Resection of digest tract cancer leads to anatomical abnormality and deficient intestinal function, and pneumoperitoneum induces ischemia and hypoxia in intestinal mucosa, which impair intestinal mucosa barrier function and result in intestinal bacterial translocation and inflammatory responses (56, 66). Meanwhile, advanced age, malnutrition, co-morbidities, and the occurrence of POCD could also affect the recovery of gastrointestinal motility, length of hospital stay and even the prognosis of patients. The present result indicated that DEX could decrease first flatus time and the length of hospital stay. The potential mechanisms for flatus time reduction include: 1) DEX reduces surgical stress and pain, then improves hemodynamics stability and intestinal microcirculation alteration (67), 2) DEX improves cognitive dysfunction and early postoperative activity; 3) DEX accelerates intestinal wound healing through increasing intestinal epithelial cell proliferation (68). Postoperative systemic immune dysfunction, POCD and gastrointestinal dysfunction lead to an array of symptoms, and other physiological/psychological diseases. They could affect the length of hospital stay, and the standard of living after discharge. Therefore, DEX administration could be valuable strategy for the patients with tumors to improve postoperative gastrointestinal function and prognosis.
This meta-analysis describes the effect of dexmedetomidine on postoperative systemic inflammation and recovery from the levels of immunomodulators, cellular immunity, cognitive function and prognosis. Hence, the coverage is more comprehensive. Meanwhile, the practical and precise strategies used for comprehensive searches of four databases, inclusion and exclusion criteria, and consideration of study quality indicated the stability and robustness of the present meta-analysis. At the same time, the meta-analysis has some limitations. Firstly, variations in the types and duration of surgery, inconsistent baseline data, concentration and duration of DEX administration may contribute the heterogeneity among studies. However, the funnel plots showed no significant asymmetry, indicating acceptable heterogeneity, as well as the stability and robustness of this meta-analysis. Secondly, the two included studies didn’t describe the detailed blinding process in the methods, leading to the suspicion that patients and investigators were aware of the experimental groups. Then the two studies were identified as high risk in the bias assessment. Thirdly, the RCTs included in this meta-analysis covered a long-time span, in which the surgical methods and equipment may have changed. All these factors may lead to instability in the present analysis. Finally, this meta-analysis has not been pre-registered in a protocol (eg. in PROSPERO), which may result in potential bias. Thus, more prospective studies with larger samples sizes and standardized protocols are required in the future to accurately determine the effects of DEX in postoperative systemic inflammation.
In conclusion, the present study found that DEX administration attenuated postoperative systemic inflammatory response and immune dysfunction. Then, DEX decreased the occurrence of POCD, the first flatus time and length of hospital stay of the patients undergoing digest tract cancer surgery. These results provided a potential therapeutic strategy to improve perioperative immune function, CNS function and clinical prognosis of digest tract cancer. Further studies are necessary to elucidate the clinical application of DEX from an immunological perspective.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author contributions
WX and YuZ collected and performed the data extraction, and wrote the manuscript. ZS and SL contributed to data analysis. KF and YeZ contributed to study design and data analysis. YW, CL, and MZ coordinated data collection and manuscript revision. HZ and ZZ contributed to study design and manuscript revision. CN conceptualized and designed the study, supervised data collection, drafted and revised the manuscript. All authors approved the submitted version.
Funding
This work was supported by Beijing Hope Run Special Fund of Cancer Foundation of China (No. LC2020A01), the National Natural Science Foundation of China (Nos. 82171195, 81771146, 82101281), and Talent Project of National Cancer Center/Cancer Hospital Chinese Academy of Medical Sciences (For CN).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.970557/full#supplementary-material
GRADE Summary of findings for inflammatory mediators, T lymphocytes, cognitive function and prognosis. a. Most information from studies at low or moderate risk of bias (one study at high risk of bias). 1. rate down for inconsistency due to high heterogeneity. 2. rate down for imprecision due to wide CIs.
Detailed search strategy terms for PubMed, Cochrane Library, EMBASE and Web of Science databases.
The funnel plots were used to assess the publication bias for the STD Mean Difference of the DEX effects on CRP (A), TNF-a (B), IL-6 (C) and IL-10 (D).
The funnel plots were used to assess the publication bias for the STD Mean Difference of the DEX effects on CD3+ T cells, CD4+ T cells, CD8+ T cells and CD4/CD8 ratio respectively.
The funnel plots were used to assess the publication bias for the STD Mean Difference of the DEX effects on MMSE at 24h, 48h, 72h after surgery (A), and the occurrence of POCD at 24h, 72h after surgery (B).
The funnel plots were used to assess the publication bias for the STD Mean Difference of the DEX effects on the first flatus time (A), hospital stay (B) and postoperative extubation time (C).
References
- 1. Allen BM, Hiam KJ, Burnett CE, Venida A, DeBarge R, Tenvooren I, et al. Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nat Med (2020) 26:1125–34. doi: 10.1038/s41591-020-0892-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Binnewies M, Mujal AM, Pollack JL, Combes AJ, Hardison EA, Barry KC, et al. Unleashing type-2 dendritic cells to drive protective antitumor cd4(+) t cell immunity. Cell (2019) 177:556–71.e16. doi: 10.1016/j.cell.2019.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer (2021) 21:345–59. doi: 10.1038/s41568-021-00347-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gómez-España MA, Gallego J, González-Flores E, Maurel J, Páez D, Sastre J, et al. SEOM clinical guidelines for diagnosis and treatment of metastatic colorectal cancer (2018). Clin Trans Oncol (2019) 21:46–54. doi: 10.1007/s12094-018-02002-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature (2010) 464:104–7. doi: 10.1038/nature08780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manson J, Thiemermann C, Brohi K. Trauma alarmins as activators of damage-induced inflammation. Br J Surg (2012) 99 Suppl 1:12–20. doi: 10.1002/bjs.7717 [DOI] [PubMed] [Google Scholar]
- 7. Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, et al. A genomic storm in critically injured humans. J Exp Med (2011) 208:2581–90. doi: 10.1084/jem.20111354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pelham CJ, Agrawal DK. Emerging roles for triggering receptor expressed on myeloid cells receptor family signaling in inflammatory diseases. Expert Rev Clin Immunol (2014) 10:243–56. doi: 10.1586/1744666X.2014.866519 [DOI] [PubMed] [Google Scholar]
- 9. Gorgulho CM, Romagnoli GG, Bharthi R, Lotze MT. Johnny on the spot-chronic inflammation is driven by hmgb1. Front Immunol (2019) 10:1561. doi: 10.3389/fimmu.2019.01561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang J, Zhang J, Yu P, Chen M, Peng Q, Wang Z, et al. Remote ischaemic preconditioning and sevoflurane postconditioning synergistically protect rats from myocardial injury induced by ischemia and reperfusion partly via inhibition tlr4/myd88/nf-κb signaling pathway. Cell Physiol Biochem (2017) 41:22–32. doi: 10.1159/000455815 [DOI] [PubMed] [Google Scholar]
- 11. Eckenhoff RG, Maze M, Xie Z, Culley DJ, Goodlin SJ, Zuo Z, et al. Perioperative neurocognitive disorder: state of the preclinical science. Anesthesiology (2020) 132:55–68. doi: 10.1097/ALN.0000000000002956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging (2017) 49:60–8. doi: 10.1016/j.neurobiolaging.2016.08.019 [DOI] [PubMed] [Google Scholar]
- 13. Marottoli FM, Katsumata Y, Koster KP, Thomas R, Fardo DW, Tai LM. Peripheral inflammation, apolipoprotein E4, and amyloid-β interact to induce cognitive and cerebrovascular dysfunction. ASN Neuro (2017) 9:1759091417719201. doi: 10.1177/1759091417719201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chiu KM, Lin TY, Lee MY, Lu CW, Wang MJ, Wang SJ. Dexmedetomidine protects neurons from kainic acid-induced excitotoxicity by activating BDNF signaling. Neurochem Int (2019) 129:104493. doi: 10.1016/j.neuint.2019.104493 [DOI] [PubMed] [Google Scholar]
- 15. Sulaiman S, Karthekeyan RB, Vakamudi M, Sundar AS, Ravullapalli H, Gandham R. The effects of dexmedetomidine on attenuation of stress response to endotracheal intubation in patients undergoing elective off-pump coronary artery bypass grafting. Ann Card Anaesth (2012) 15:39–43. doi: 10.4103/0971-9784.91480 [DOI] [PubMed] [Google Scholar]
- 16. Zhang Z, Ferretti V, Güntan İ., Moro A, Steinberg EA, Ye Z, et al. Neuronal ensembles sufficient for recovery sleep and the sedative actions of α2 adrenergic agonists. Nat Neurosci (2015) 18:553–61. doi: 10.1038/nn.3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Z, Shen Z, Wang H, Zhang L, Dong R. Effect of dexmedetomidine on the cognitive function of patients undergoing gastric cancer surgery by regulating the PI3K/AKT signaling pathway. Oncol Lett (2020) 19:1151–6. doi: 10.3892/ol.2019.11224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tian H, Hou L, Xiong Y, Cheng Q. Dexmedetomidine upregulates microRNA-185 to suppress ovarian cancer growth via inhibiting the SOX9/Wnt/β-catenin signaling pathway. Cell Cycle (Georgetown Tex.) (2021) 20:765–80. doi: 10.1080/15384101.2021.1897270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Minnella EM, Carli F. Prehabilitation and functional recovery for colorectal cancer patients. Eur J Surg Oncol (2018) 44:919–26. doi: 10.1016/j.ejso.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 20. Hu J, Vacas S, Feng X, Lutrin D, Uchida Y, Lai IK, et al. Dexmedetomidine prevents cognitive decline by enhancing resolution of high mobility group box 1 protein-induced inflammation through a vagomimetic action in mice. Anesthesiology (2018) 128:921–31. doi: 10.1097/ALN.0000000000002038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu J, Vogel T, Gao X, Lin B, Kulwin C, Chen J. Neuroprotective effect of dexmedetomidine in a murine model of traumatic brain injury. Sci Rep (2018) 8:4935. doi: 10.1038/s41598-018-23003-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mao Y, Sun XM, Si L, Chen LJ, Liu XS, Zhang Z, et al. Perioperative dexmedetomidine fails to improve postoperative analgesic consumption and postoperative recovery in patients undergoing lateral thoracotomy for thoracic esophageal cancer: a randomized, double-blind, placebo-controlled trial. Pain Res Manage (2020) 2020:12. doi: 10.1155/2020/4145893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dong W, Chen MH, Yang YH, Zhang X, Huang MJ, Yang XJ, et al. The effect of dexmedetomidine on expressions of inflammatory factors in patients with radical resection of gastric cancer. Eur Rev Med Pharmacol Sci (2017) 21:3510–5. [PubMed] [Google Scholar]
- 24. Zhu Z, Li J, Zhu W, Chen T, Li J, Zhang H. Effects of dexmedetomidine on inflammatory factors of patients with radical gastrectomy. Anti-Tumor Pharm (2017) 7:455–9. doi: 10.3969/j.issn.2095-1264.2017.04.15 [DOI] [Google Scholar]
- 25. Huai Q, Yang YC, Cui SZ, Jiang CX, Gao BL. Dexmedetomidine promotes rapid recovery of patients undergoing radial colon cancer resection through regulating cytokine expression: a randomized, controlled, prospective study. Acta Med Mediterr (2022) 38:299–306. doi: 10.19193/0393-6384_2022_1_48 [DOI] [Google Scholar]
- 26. Wang K, Li CW. Effects of dexmedetomidine on inflammatory factors, T lymphocyte subsets and expression of NF-kappa b in peripheral blood mononuclear cells in patients receiving radical surgery of colon carcinoma. Oncol Lett (2018) 15:7153–7. doi: 10.3892/ol.2018.8205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao LQ, Li YL. Application of dexmedetomidine combined with sufentanil in colon cancer resection and its effect on immune and coagulation function of patients. Oncol Lett (2020) 20:1288–94. doi: 10.3892/ol.2020.11643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ben Z, Zheng J, Zhang H. Study on the effects of continuous infusion of dexmedetomidine on inflammatory factors and early postoperative cognitive function of elderly patients receiving laparoscopic radical resection of rectal cancer. Pract Geriatrics (2016) 30:683–6. doi: 10.3969/j.issn.1003-9198.2016.08.021 [DOI] [Google Scholar]
- 29. Chen C, Huang P, Lai L, Luo C, Ge M, Hei Z, et al. Dexmedetomidine improves gastrointestinal motility after laparoscopic resection of colorectal cancer: A randomized clinical trial. Medicine (2016) 95:e4295. doi: 10.1097/MD.0000000000004295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kong YH, Bai ZY, Chen L, Yao YL. Effects of different doses of dexmedetomidine in patients with colorectal cancer. Acta Med Mediterr (2021) 37:3641–6. doi: 10.19193/0393-6384_2021_6_572 [DOI] [Google Scholar]
- 31. Liu X, Zhang J, Xie G, Gao Y. The application of dexmetomidine hydrochloride inanaes the ticprocess of laparoscopic colorectal cancer in elderly patients. Anti-Tumor Pharm (2017) 7:739–42. doi: 10.3969/j.issn.2095-1264.2017.06.21 [DOI] [Google Scholar]
- 32. Liu Y, Zhu XQ, He ZY, Sun ZR, Wu X, Zhong J. Protective effect of dexmedetomidine infusion combined with epidural blockade on postoperative complications after surgery: A prospective randomized controlled clinical trial. J Int Med Res (2020) 48:11. doi: 10.1177/0300060520930168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pan CL, Chen Y, Wu YH. Dexmedetomidine combined with ulinastatin improves postoperative cognitive dysfunction in elderly patients after laparoscopic colorectal cancer surgery by regulating pro-inflammatory/antiinflammatory system. World Chin J Digestol (2016) 24:2755–61. doi: 10.11569/wcjd.v24.i17.2755 [DOI] [Google Scholar]
- 34. Sun WQ, Li FL, Wang XX, Liu HB, Mo H, Pan DB, et al. Effects of dexmedetomidine on patients undergoing laparoscopic surgery for colorectal cancer. J Surg Res (2021) 267:687–94. doi: 10.1016/j.jss.2021.06.043 [DOI] [PubMed] [Google Scholar]
- 35. Zhang JC, Liu GQ, Zhang FX, Fang H, Zhang DW, Liu SC, et al. Analysis of postoperative cognitive dysfunction and influencing factors of dexmedetomidine anesthesia in elderly patients with colorectal cancer. Oncol Lett (2019) 18:3058–64. doi: 10.3892/ol.2019.10611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Y, Xing Z, Xu Y, Xu S. Effects of different doses of dexmedetomidine on cognitive dysfunction in elderly patients early after laparoscopic surgery for colorectal cancer. Nan fang yi ke da xue xue bao = J South Med Univ (2014) 34:743–6. [PubMed] [Google Scholar]
- 37. Feinkohl I, Winterer G, Spies CD, Pischon T. Cognitive reserve and the risk of postoperative cognitive dysfunction. Deutsches Arzteblatt Int (2017) 114:110–7. doi: 10.3238/arztebl.2017.0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Niu Y, Zhang L, Chang J. Effects of dexmedetomidine on short-term prognosis of elderly patients with gastric cancer after laparoscopic radical gastrectomy. Cancer Res Clinic (2021) 33:134–7. doi: 10.3760/cma.j.cn115355-20200326-00149 [DOI] [Google Scholar]
- 39. Pittman QJ. A neuro-endocrine-immune symphony. J Neuroendocrinol (2011) 23:1296–7. doi: 10.1111/j.1365-2826.2011.02176.x [DOI] [PubMed] [Google Scholar]
- 40. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest (2003) 111:1805–12. doi: 10.1172/JCI200318921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Szkandera J, Stotz M, Absenger G, Stojakovic T, Samonigg H, Kornprat P, et al. Validation of c-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br J Cancer (2014) 110:183–8. doi: 10.1038/bjc.2013.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Greene SA. Chronic pain: pathophysiology and treatment implications. Topics Companion Anim Med (2010) 25:5–9. doi: 10.1053/j.tcam.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 43. Cho HJ, Kim SS, Ahn SJ, Park SY, Park JH, Kim JK, et al. Low serum interleukin-6 levels as a predictive marker of recurrence in patients with hepatitis b virus related hepatocellular carcinoma who underwent curative treatment. Cytokine (2015) 73:245–52. doi: 10.1016/j.cyto.2015.02.027 [DOI] [PubMed] [Google Scholar]
- 44. Saraiva M, Vieira P, O’Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med 217 (2020) 217(1):e20190418. doi: 10.1084/jem.20190418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity (2004) 21:137–48. doi: 10.1016/j.immuni.2004.07.017 [DOI] [PubMed] [Google Scholar]
- 46. Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, et al. IL-10 elicits IFNγ-dependent tumor immune surveillance. Cancer Cell (2011) 20:781–96. doi: 10.1016/j.ccr.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 47. Autio K, Oft M. Pegylated interleukin-10: clinical development of an immunoregulatory cytokine for use in cancer therapeutics. Curr Oncol Rep (2019) 21:19. doi: 10.1007/s11912-019-0760-z [DOI] [PubMed] [Google Scholar]
- 48. Wang XW, Cao JB, Lv BS, Mi WD, Wang ZQ, Zhang C, et al. Effect of perioperative dexmedetomidine on the endocrine modulators of stress response: a meta-analysis. Clin Exp Pharmacol Physiol (2015) 42:828–36. doi: 10.1111/1440-1681.12431 [DOI] [PubMed] [Google Scholar]
- 49. Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells–from barracks to boulevards to battlefields: a tale of three hormones–curt Richter award winner. Psychoneuroendocrinology (2012) 37:1345–68. doi: 10.1016/j.psyneuen.2012.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jia ZM, Hao HN, Huang ML, Ma DF, Jia XL, Ma B. Influence of dexmedetomidine to cognitive function during recovery period for children with general anesthesia. Eur Rev Med Pharmacol Sci (2017) 21:1106–11. [PubMed] [Google Scholar]
- 51. Yuki K. The immunomodulatory mechanism of dexmedetomidine. Int Immunopharmacol (2021) 97:107709. doi: 10.1016/j.intimp.2021.107709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li LC, Tian Y, Xiao J, Yang Y, Wu JN, Chen Y, et al. Dexmedetomidine promotes inflammation resolving through TGF-β1 secreted by F4/80(+)Ly6G(+) macrophage. Int Immunopharmacol (2021) 95:107480. doi: 10.1016/j.intimp.2021.107480 [DOI] [PubMed] [Google Scholar]
- 53. Wang Y, Mao X, Chen H, Feng J, Yan M, Wang Y, et al. Dexmedetomidine alleviates LPS-induced apoptosis and inflammation in macrophages by eliminating damaged mitochondria via PINK1 mediated mitophagy. Int Immunopharmacol (2019) 73:471–81. doi: 10.1016/j.intimp.2019.05.027 [DOI] [PubMed] [Google Scholar]
- 54. Hu J, Vacas S, Feng X, Lutrin D, Uchida Y, Lai IK, et al. Dexmedetomidine prevents cognitive decline by enhancing resolution of high mobility group box 1 protein–induced inflammation through a vagomimetic action in mice. Anesthesiology (2018) 128:921–31. doi: 10.1097/ALN.0000000000002038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Seung E, Xing Z, Wu L, Rao E, Cortez-Retamozo V, Ospina B, et al. A trispecific antibody targeting HER2 and T cells inhibits breast cancer growth via CD4 cells. Nature (2022) 603:328–34. doi: 10.1038/s41586-022-04439-0 [DOI] [PubMed] [Google Scholar]
- 56. Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clinics (2005) 21:177–96. doi: 10.1016/j.ccc.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 57. Chu KS, Wang FY, Hsu HT, Lu IC, Wang HM, Tsai CJ. The effectiveness of dexmedetomidine infusion for sedating oral cancer patients undergoing awake fibreoptic nasal intubation. Eur J Anaesthesiol (2010) 27:36–40. doi: 10.1097/EJA.0b013e32832e0d2b [DOI] [PubMed] [Google Scholar]
- 58. Pape K, Tamouza R, Leboyer M, Zipp F. Immunoneuropsychiatry - novel perspectives on brain disorders. Nat Rev Neurol (2019) 15:317–28. doi: 10.1038/s41582-019-0174-4 [DOI] [PubMed] [Google Scholar]
- 59. Balusu S, Van Wonterghem E, De Rycke R, Raemdonck K, Stremersch S, Gevaert K, et al. Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol Med (2016) 8:1162–83. doi: 10.15252/emmm.201606271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Noll F, Behnke J, Leiting S, Troidl K, Alves GT, Müller-Redetzky H, et al. Self-extracellular RNA acts in synergy with exogenous danger signals to promote inflammation. PloS One (2017) 12:e0190002. doi: 10.1371/journal.pone.0190002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li JJ, Wang B, Kodali MC, Chen C, Kim E, Patters BJ, et al. In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J Neuroinflamm (2018) 15:8. doi: 10.1186/s12974-017-1038-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ma G, Chen C, Jiang H, Qiu Y, Li Y, Li X, et al. Ribonuclease attenuates hepatic ischemia reperfusion induced cognitive impairment through the inhibition of inflammatory cytokines in aged mice. Biomed Pharmacother = Biomedecine pharmacotherapie (2017) 90:62–8. doi: 10.1016/j.biopha.2017.02.094 [DOI] [PubMed] [Google Scholar]
- 63. Yin L, Chen X, Ji H, Gao S. Dexmedetomidine protects against sepsis−associated encephalopathy through Hsp90/AKT signaling. Mol Med Rep (2019) 20:4731–40. doi: 10.3892/mmr.2019.10718 [DOI] [PubMed] [Google Scholar]
- 64. Liu Y, Yin Y. Emerging roles of immune cells in postoperative cognitive dysfunction. Mediators Inflammation (2018) 2018:6215350. doi: 10.1155/2018/6215350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Berger M, Nadler JW, Browndyke J, Terrando N, Ponnusamy V, Cohen HJ, et al. Postoperative cognitive dysfunction: minding the gaps in our knowledge of a common postoperative complication in the elderly. Anesthesiol Clinics (2015) 33:517–50. doi: 10.1016/j.anclin.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol (2003) 18:479–97. doi: 10.1046/j.1440-1746.2003.03032.x [DOI] [PubMed] [Google Scholar]
- 67. Yeh YC, Sun WZ, Ko WJ, Chan WS, Fan SZ, Tsai JC, et al. Dexmedetomidine prevents alterations of intestinal microcirculation that are induced by surgical stress and pain in a novel rat model. Anesth Analg (2012) 115:46–53. doi: 10.1213/ANE.0b013e318253631c [DOI] [PubMed] [Google Scholar]
- 68. Schaak S, Cussac D, Cayla C, Devedjian JC, Guyot R, Paris H, et al. Alpha(2) adrenoceptors regulate proliferation of human intestinal epithelial cells. Gut (2000) 47:242–50. doi: 10.1136/gut.47.2.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GRADE Summary of findings for inflammatory mediators, T lymphocytes, cognitive function and prognosis. a. Most information from studies at low or moderate risk of bias (one study at high risk of bias). 1. rate down for inconsistency due to high heterogeneity. 2. rate down for imprecision due to wide CIs.
Detailed search strategy terms for PubMed, Cochrane Library, EMBASE and Web of Science databases.
The funnel plots were used to assess the publication bias for the STD Mean Difference of the DEX effects on CRP (A), TNF-a (B), IL-6 (C) and IL-10 (D).
The funnel plots were used to assess the publication bias for the STD Mean Difference of the DEX effects on CD3+ T cells, CD4+ T cells, CD8+ T cells and CD4/CD8 ratio respectively.
The funnel plots were used to assess the publication bias for the STD Mean Difference of the DEX effects on MMSE at 24h, 48h, 72h after surgery (A), and the occurrence of POCD at 24h, 72h after surgery (B).
The funnel plots were used to assess the publication bias for the STD Mean Difference of the DEX effects on the first flatus time (A), hospital stay (B) and postoperative extubation time (C).
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.