Abstract
Introduction
This study aimed to test the efficacy of a nutritional blend (NB) in improving nutritional biomarkers and preventing cognitive decline among older adults.
Methods
A 1‐year randomized, double‐blind, multicenter, placebo‐controlled trial with 362 adults (58.6% female, mean 78.3 years, SD = 4.8) receiving an NB or placebo. Erythrocyte ω‐3 index and homocysteine concentrations were primary outcomes. Other outcomes included Patient‐Reported Outcomes Measurement Information System (PROMIS) Applied Cognition‐Abilities, composite cognitive score (CCS), Cognitive Function Instrument (CFI) self‐assessment and study partner, hippocampal volume (HV), and Alzheimer's disease signature cortical thickness (CT).
Results
A total of 305 subjects completed the follow‐up. Supplementation increased ω‐3 index and decreased homocysteine, but did not affect CCS, CFI self‐assessment, HV, and CT. Placebo improved and treatment did not change PROMIS at 1 month. Intervention showed a positive effect on CFI study partner.
Discussion
Although improving nutritional biomarkers, this 1‐year trial with a multi‐nutrient novel approach was not able to show effects on cognitive outcomes among older adults.
Keywords: clinical trials, cognition, homocysteine, nutrition, older adults, omega‐3
1. INTRODUCTION
Cognitive decline (CD) has been reported consistently reported with aging across a range of cognitive domains including processing speed, attention, memory, and executive function. 1 Furthermore, CD is an early predictor of Alzheimer's disease (AD) and begins before the onset of dementia, 2 being associated with increased health care costs. 3 Therefore, interventions aiming at preventing CD may have major public health implications. In this sense, increased interest in identifying early predictors of CD has brought attention to potential biomarkers associated with cognitive outcomes. Among these candidates, elevated homocysteine (a marker of insufficient B‐vitamin intake) 4 and low erythrocyte ω‐3 polyunsaturated fatty acids (PUFA) have been considered modifiable risk factors for dementia and AD. 5 , 6 , 7
Elevated homocysteine has been associated independently with CD, white matter damage, brain atrophy, and dementia. 5 It has been shown experimentally that, in young rats, elevated plasma homocysteine induced by deficient diets in dams directly stimulated apoptosis in selected brain areas and persistently affected neurobehavioral capacities. 8 Accordingly, proposed mechanisms for the deleterious effects of homocysteine on cognitive function include cerebrovascular effects, inhibition of methylation reactions, and activation of tau kinases, which may lead to regional brain atrophy, impaired synaptic function, and amyloid beta (Aβ) accumulation. 5 The neuroprotective properties of ω‐3 PUFA also involve multiple pathways, such as favoring cerebral blood flow through improving vascular endothelial function and arterial stiffness, reducing oxidative stress in the brain, and modulating neuronal membrane fluidity. 9
Preclinical and epidemiological evidence in favor of nutritional factors protecting cognitive function has suggested nutrition as a possible intervention pathway to prevent AD and CD, with several nutrients potentially affecting cognition. 4 , 9 , 10 , 11 , 12 , 13 , 14 However, trials supplementing individual nutrients yielded controversial results. 15 Considering multifactorial mechanisms involved in aging and the synergistic effect of nutrients and bioactive compounds, more recent evidence in human 16 , 17 , 18 and animal 19 , 20 studies, on the other hand, have suggested that combining nutrients may be a more promising strategy to prevent CD. In this sense, the Nolan Study was designed to test this novel approach, through a nutritional blend (NB) developed to serve as a source of multiple nutrients. This trial aimed to investigate the efficacy of a 1‐year intervention with a NB on levels of erythrocyte ω‐3 index and plasma homocysteine, as well as its effects on subjective and objective measures of cognitive function and on neuroimaging markers among community‐dwelling older adults without dementia, but with subjective memory complaints. Effects were further assessed in exploratory analyses within sub‐cohorts, stratified based on nutritional deficits and on apolipoprotein E (APOE) ε4 status.
2. MATERIALS AND METHODS
2.1. Study design and population
This double‐blind, multicenter, randomized, placebo‐controlled trial (RCT) was conducted in 18 centers in France with community‐dwelling older adults. Recruitment of participants started in December 2016 and ended in January 2018. Follow‐up ended in February 2019.
Major inclusion criteria for the Nolan Study comprised age ≥70 years; self‐reporting subjective memory complaints; and having a study partner to participate as a source of information. Major exclusion criteria were taking ω‐3 PUFA supplements containing >200 mg of docosahexaenoic acid (DHA)/day over 6 months before inclusion; taking vitamin‐B supplementation in the preceding 3 months; basic activities of daily living (ADL) score <4; Mini‐Mental State Examination (MMSE) score <24; and a diagnosis of dementia. Complete inclusion and exclusion criteria are listed in Table S1. Treatment with anticoagulants or platelet aggregation inhibitors was not prohibited, but was carefully monitored, given that ω‐3 PUFA can affect platelet function.
2.2. Ethical disclosure
Subjects signed an informed consent. The trial protocol (NCT03080675) was approved by the Advisory Committee for Protection of Persons South West and Overseas II (CPP SOOM II) and the French Agency for the Safety of Medicines and Health Products (ANSM).
2.3. Randomization and masking
Participants were randomly assigned (1:1) to one of the following groups: intervention (active supplementation with the NB) or control (placebo). (Details are provided in the Supplementary materials.)
RESEARCH IN CONTEXT
Systematic review: Preclinical and epidemiological evidence in favor of nutritional factors protecting cognition has suggested nutrition as a possible intervention pathway to prevent cognitive decline. However, trials supplementing single nutrients yielded controversial results. Recent evidence on human and animal studies has suggested that combining nutrients may be a more promising strategy.
Interpretation: Our study assessed the efficacy of 1‐year nutritional blend supplementation on erythrocyte ω‐3 index, homocysteine levels, and cognitive outcomes among older adults, and showed a positive effect of intervention in these biomarkers presumed to underlie an attenuation of cognitive decline during aging. Despite some observed effects in secondary outcomes, intervention was not able to prevent cognitive decline as measured by neuropsychological tests.
Future directions: Findings may help development of nutritional strategies for optimizing brain health, protecting cognition, and identifying individuals for whom supplementation may be more effective. Additional investigations are needed, especially trials with longer follow‐up duration targeting individuals who are apolipoprotein E (APOE) ε4 carriers and those with nutritional deficiencies.
2.4. Intervention
The intervention consisted in taking daily an NB characterized by two soft gel capsules (775 mg each) and one powdered sachet of ≈15 g (to be mixed in 120 mL of cold water), or by placebo equivalent doses. Total composition of a daily dose of the NB was established via an in‐depth evaluation of available clinical data on each ingredient, and of previous studies using the NB in aged dogs 19 and cats, 20 and consisted of: thiamin (50 mg), riboflavin (15 mg), niacin (25 mg), pantothenic acid (23 mg), pyridoxine (18 mg), biotin (0.15 mg), folic acid (0.4 mg), cobalamin (0.5 mg), vitamin E (82.6 mg), vitamin C (500 mg), vitamin D (15 µg), choline (85 mg), selenium (80 µg), citrulline (3 g), eicosapentaenoic acid (EPA) (700 mg), and DHA (770 mg). (Additional information about capsules and powered drink composition is presented in the Supplementary materials.)
Subjects attended six in‐person visits: screening, practice (when non‐validated versions of cognitive tests, except MMSE, were administered to familiarize participants with procedures), baseline (beginning of the intervention), 1 month, 6 months, and 12 months. Additional phone calls were set up between visits at 3‐month intervals to follow‐up adverse events, product compliance (defined as good if taking ≥80% of scheduled product intake), and concomitant medication intake. No dietary restrictions were made, but participants were not allowed to take additional supplements containing B vitamins, DHA, and/or EPA.
2.5. Outcomes: Nutritional biomarkers
Erythrocyte ω‐3 index and plasma homocysteine concentrations were designated as the main outcomes based on evidence showing their capacity in underlying an attenuation in CD during aging. 5 , 6 , 7 They were measured at baseline, 6 months, and 1 year. Erythrocyte ω‐3 PUFA was measured by capillary fast gas chromatography–Flame Ionization Detector (GC‐FID) method. The ω‐3 index was calculated as the sum of %DHA and %EPA, expressed as the percentage of total erythrocyte membrane fatty acids. Total plasma homocysteine was measured in µmol/L using a commercially available immunoassay kit (Architect 1L71, Abbott). Measurements were performed by trained professionals. (Further details are given in the Supplementary materials.)
2.6. Outcomes: Cognitive tests
Secondary outcomes included the composite cognitive score (CCS); the Patient‐Reported Outcomes Measurement Information System (PROMIS) Applied Cognition‐Abilities instrument version 1.0; and the Cognitive Function Instrument (CFI) self‐assessment test. The CCS was based on the following neuropsychological outcomes: sum of free recall and of total recall from the Free and Cued Selective Reminding Test (FCSRT); MMSE orientation score (10 items); Wechsler Adult Intelligence Scale (WAIS)‐IV coding test (Digit Symbol Substitution); 2‐minute Category Naming Test (CNT). Combined, these four tests assess timed executive function, episodic memory and global cognition. A Z‐score was calculated for each test and the CCS was defined as the sum of Z‐scores divided by four (higher scores indicating better function). 21 (Further information about the cognitive outcomes is displayed in Supplementary materials.)
Besides these a priori defined secondary outcomes, the CFI study partner questionnaire was included as an exploratory outcome. It includes the same 14 questions as the CFI self‐assessment, but answered by the participant's partner. The CFI instruments and the CCS were assessed at baseline, 6 months, and 1 year.
2.7. Outcomes: Brain imaging
Secondary outcomes included brain measures assessed by magnetic resonance imaging (MRI): hippocampal volume (HV) (right, left and total) and AD signature cortical thickness (CT). The AD signature CT was composed of four different regions of interest (ROIs): entorhinal, inferior temporal, middle temporal, and fusiform, and calculated as the sum of CT multiplied by volume estimates of each hemisphere and each ROI, divided by the sum of volume of all ROIs. MRI was performed on a voluntary basis among a subgroup at baseline (n = 284) and 1 year (n = 183), using the same imaging protocols and processing pipelines from the Alzheimer's Disease Neuroimaging Initiative (ADNI) (http://adni.loni.usc.edu/methods/mri‐tool/mri‐analysis). (Details about MRI are available in Supplementary materials.)
2.8. APOE ε4 status and nutritional deficits
The effect of intervention was also studied in exploratory analyses according to apolipoprotein E (APOE) ε4 status (carrier vs non‐carrier) and to the presence of nutritional deficits at baseline: low ω‐3 index (≤5.69%, lowest quartile) 22 , 23 ; high homocysteine (>14 µmol/L) 23 , 24 ; and low 25 hydroxyvitamin D concentrations (25(OH)D; <20 ng/mL). 23 , 25 For this purpose, a whole blood sample ≥1 mL was collected at baseline for DNA analyses including APOE ε4 status. 25(OH)D concentrations were determined using a commercially available immunoassay kit (Architect 5P02, Abbott). Intervention was also tested by combining the nutritional deficits (≥2 vs <2).
2.9. Statistical analysis
Descriptive statistics (means and SD, frequencies, and percentages) were used. Efficacy analyses were done on a modified intention‐to‐treat (mITT) basis (i.e., including all randomly assigned participants with outcomes measured at baseline who completed at least one post‐baseline visit). Linear mixed‐effects regression (with a random subject intercept and, if significant, a random linear slope and a random center intercept) adjusted on baseline data were performed in the mITT population to determine the effect of intervention compared to placebo on outcomes. For each model, the following fixed effects were included: baseline value, intervention group, time (continuous variable), and interaction between group and time. All available data were included in the models (baseline, 6 months, and 12 months). Analyses on HV were adjusted additionally for baseline total intracranial volume. For CFI, an imputation by the worst modality (“1”) was made in case of one missing item, what allowed to recover between 10% and 13% of the subjects at each visit. Exploratory analyses according to APOE ε4 status and nutritional deficits were performed. The Statistical Analysis Software (SAS) version 9.4 (Cary, NC, USA) was used. Statistical significance was set as 5%.
The Nolan Study was designed originally to last for 4 years but was restricted posteriorly to 1 year because of funding limitations. Due to the limited duration, the primary outcome of the trial was changed from CD measured by a CCS to the aforementioned nutritional biomarkers. (Sample size calculation is available in the Supplementary materials.)
3. RESULTS
3.1. Characterization of the sample and adherence to intervention
Baseline characteristics of participants according to randomized groups are presented in Table 1. From the 362 participants originally enrolled (mean 78.3 years, SD = 4.8; 58.6% female), 57 dropped out during follow‐up (n = 26 intervention, n = 31 control), corresponding to a mean drop‐out rate of 15.7%. Thus 305 participants completed the trial (n = 154 intervention, n = 151 control) (Figure 1).
TABLE 1.
Baseline characteristics of participants of the Nolan Study (modified intention‐to‐treat population)
| Total | Nutritional blend | Placebo | ||
|---|---|---|---|---|
| n = 362 | n = 180 | n = 182 | ||
| mean (SD)* | mean (SD)* | mean (SD)* | P‐value | |
| Female sex | 212 (58.6%) | 104 (57.8%) | 108 (59.3%) | .763 d |
| Age (years) | 78.3 (4.8) | 78.4 (4.9) | 78.2 (4.8) | .724 e |
| Education (n = 361) | ||||
| <4 years | 4 (1.1%) | 2 (1.1%) | 2 (1.1%) | .836 f |
| 4 to 7 years | 28 (7.8%) | 16 (8.9%) | 12 (6.6%) | |
| 8 to 9 years | 61 (16.9%) | 28 (15.6%) | 33 (18.2%) | |
| ≥10 years | 268 (74.2%) | 134 (74.4%) | 134 (74.0%) | |
| Weight (kg) | 69.5 (13.1) | 69.5 (12.6) | 69.4 (13.6) | .917 g |
| Body mass index (kg/m2) | 26.1 (4.0) | 26.1 (4.1) | 26.2 (4.0) | .910 g |
| APOE ε4 status (n = 341) | ||||
| ε4 carrier | 83 (24.3%) | 38 (22.2%) | 45 (26.5%) | .361 d |
| ε4 non‐carrier | 258 (75.7%) | 133 (77.8%) | 125 (73.5%) | |
| Biomarkers | ||||
| Plasma homocysteine (µmol/L) (n = 356) | 12.3 (4.1) | 12.4 (4.5) | 12.2 (3.7) | .833 g,h |
| Erythrocyte ω‐3 index** (%) (n = 321) | 6.5 (1.3) | 6.5 (1.2) | 6.5 (1.3) | .726 g |
| 25 hydroxyvitamin D (ng/mL) (n = 356) | 26.2 (10.9) | 26.6 (11.6) | 25.9 (10.3) | .559 g |
| Cognitive tests | ||||
| CFI self‐assessment score (n = 342) | 4.4 (2.6) | 4.5 (2.6) | 4.4 (2.6) | .689 g |
| CFI study partner score (n = 330) | 3.4 (2.7) | 3.6 (2.7) | 3.2 (2.7) | .067 e |
| PROMIS T‐score (n = 337) | 50.8 (6.1) | 50.9 (6.1) | 50.7 (6.1) | .749 g |
| Composite cognitive score*** (n = 361) | 0.01 (0.70) | 0.00 (0.72) | 0.01 (0.69) | .936 g |
| MMSE score | 28.1 (1.6) | 28.1 (1.7) | 28.1 (1.6) | .964 e |
| CDR status | ||||
| Cognitively normal (CDR = 0) | 144 (39.8%) | 72 (40.0%) | 72 (39.6%) | .932 d |
| Mild cognitive impairment (CDR = 0.5) | 218 (60.2%) | 108 (60.0%) | 110 (60.4%) | |
| Brain imaging a | ||||
| Hippocampal volume (left) (mm3) (n = 280) b | 3477.7 (448.6) | 3465.4 (435.9) | 3490.4 (462.9) | .642 g |
| Hippocampal volume (right) (mm3) (n = 280) b | 3595.2 (466.2) | 3591.8 (439.0) | 3598.7 (494.2) | .903 g |
| Total hippocampal volume (mm3) (n = 280) b | 7072.9 (878.3) | 7057.2 (836.9) | 7089.0 (921.7) | .762 g |
| AD signature cortical thickness**** (mm) (n = 238) c | 2.8 (0.2) | 2.8 (0.2) | 2.8 (0.1) | .262 g |
Abbreviations: AD, Alzheimer's disease; CDR, Clinical Dementia Rating; CFI, Cognitive Function Instrument; MMSE, Mini‐Mental State Examination (score varies from 0 to 30); PROMIS, Patient‐Reported Outcomes Measurement Information System Applied Cognition‐Abilities.
Except where indicated otherwise. **ω‐3 index was calculated as the sum of % docosahexaenoic acid and % eicosapentaenoic acid, expressed as the percentage of total erythrocyte membrane fatty acids. ***Based on four cognitive tests (free and total recall of the Free and Cued Selective Reminding test, 10 Mini‐Mental State Examination orientation items, Digit Symbol Substitution Test, and Category Naming Test. ****Alzheimer's disease signature cortical thickness was composed of the mean cortical thickness of left and right hemispheres of four different regions of interest: entorhinal, inferior temporal, middle temporal, and fusiform weighted by each region's volume.
Imaging cohort was not different for baseline characteristics presented in this table compared to participants who did not perform MRI, except for age (77.9 vs. 80.0 years; P = .001).
n = 142 in the nutritional blend group and n = 138 in the placebo group.
n = 117 in the nutritional blend group and n = 121 in the placebo group.
Chi‐square test.
Kruskal‐Wallis test.
Fisher exact test.
Equal variance two sample t‐test.
P‐value with log transformation.
FIGURE 1.
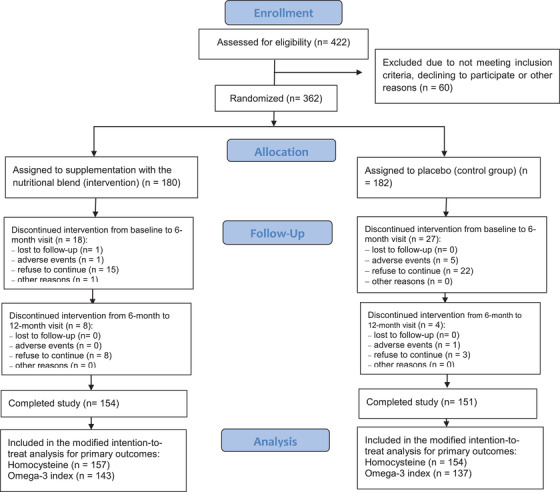
Flow diagram describing the Nolan Study population
Mean compliance to intervention for sachet and capsule consumption after 1 year was, respectively, 79.0% and 81.4% (active group), and 78.5% and 81.8% (control group). Good compliance (≥80%) was presented by 72.1% of participants in the intervention group and 74.2% in placebo group at the end of follow‐up.
3.2. Effect of intervention on nutritional biomarkers
After 1 year, supplementation positively affected the nutritional biomarkers, providing an increase in ω‐3 index (between‐group differences: 2.7%, 95% confidence interval [CI] 2.3 to 3.0; P < .0001) and a decrease in homocysteine levels (−3.2 µmol/L, 95% CI −4.0 to −2.4; P < .0001) (Table 2, Figure S1A,B). From subjects with low ω‐3 index at baseline (n = 36 intervention, n = 36 control), 72.2% in the active group and 2.8% in the placebo group changed to the normal range after 1 year. For those with high homocysteine (n = 39 intervention, n = 34 control), 74.4% of participants in the active group and 35.3% in the placebo group changed to normal range.
TABLE 2.
Mixed‐effects linear regression analysis for change from baseline in primary, secondary, and exploratory outcome measures according to randomized intervention groups among the modified intention‐to‐treat population of non‐demented, community‐dwelling older adults
| Estimated mean within‐group change from baseline a (95% CI); P‐value | Between‐group difference over time (95% CI); P‐value | ||
|---|---|---|---|
| Nutritional blend | Placebo | Nutritional blend vs. placebo | |
| Biomarkers | |||
| Homocysteine (µmol/L) | −2.24 (−2.81, −1.68; <0.0001 |
0.96 (0.38, 1.53); .001 |
−3.20 (−4.01, −2.39); <.0001 |
| ω‐3 index b (%) | 1.53 (1.25, 1.80); <.0001 | −1.13 (−1.41, −0.85); <.0001 | 2.66 (2.27, 3.05); <.0001 |
| Cognitive tests | |||
| PROMIS T‐score (8 items)* | −0.03 (−0.75, 0.70); .945 |
1.15 (0.41, 1.88); .002 |
−1.17 (−2.20, −0.14); .026 |
| Composite cognitive score c | −0.10 (−0.18, −0.02); .016 | −0.10 (−0.18, −0.01); .022 | 0.00 (−0.12, 0.11); .953 |
| CFI self‐assessment score | −0.49 (−0.79, −0.19); .001 | −0.38 (−0.69, −0.07); .015 | −0.11 (−0.54, 0.31); .606 |
| CFI study partner total score | −0.34 (−0.80, 0.12); .134 |
0.14 (−0.34, 0.62); .544 |
−0.48 (−0.95, −0.01); .044 |
| Brain imaging d | |||
| Hippocampal volume left (mm3) | −36.02 (−65.98, −6.06); .019 | −78.39 (−108.31, −48.46); <.0001 | 42.37 (−0.10, 84.83); .051 |
| Hippocampal volume right (mm3) | −40.93 (−71.86, −10.01); .010 | −55.71 (−86.63, −24.79); .001 | 14.78 (−29.04, 58.60); .507 |
| Total hippocampal volume (mm3) | −75.47 (−126.45, −24.49); .004 | −135.00 (−185.95, −84.04); <.0001 | 59.53 (−12.72, 131.78); .106 |
| AD signature CT e (mm) |
−0.01 (−0.02, 0.01); .357 |
−0.03 (−0.04, −0.01); 0.001 | 0.02 (0.00, 0.04); .088 |
Abbreviations: AD, Alzheimer's disease; CFI, Cognitive Function Instrument; CT, cortical thickness; PROMIS, Patient‐Reported Outcomes Measurement Information System Applied Cognition‐Abilities.
*Change after 1 month, and all other outcomes after 12 months of follow‐up.
Estimated with the mean at baseline.
ω‐3 index was calculated as the sum of % docosahexaenoic acid and % eicosapentaenoic acid, expressed as the percentage of total erythrocyte membrane fatty acids.
Based on four cognitive tests (free and total recall of the Free and Cued Selective Reminding test, ten Mini‐Mental State Examination orientation items, Digit Symbol Substitution Test and Category Naming Test.
Magnetic resonance imaging was performed in a subsample (in the present analysis, n = 177 for hippocampal volume and n = 145 for AD signature cortical thickness). Analyses on hippocampal volume and AD signature CT were adjusted for baseline total intracranial volume.
Alzheimer's disease signature cortical thickness was composed by the mean cortical thickness of left and right hemispheres of four different regions of interest: entorhinal, inferior temporal, middle temporal, and fusiform weighted by each region's volume.
3.3. Effect of intervention on cognitive tests
Despite the beneficial effects on nutritional biomarkers related to cognitive function, supplementation did not show an effect on the CCS or the CFI self‐assessment. On the other hand, for the CFI study partner score a significant between‐group difference (−0.48, 95% CI −0.95 to −0.01; P = .044) favoring the intervention group was found. The PROMIS T‐score (weekly measured during the first month of follow‐up), in contrast, provided distinct findings. It did not change among participants receiving the NB after 1 month, but significantly increased among participants in the placebo group (indicating improvement) (between‐group difference: −1.17, 95% CI −2.20 to −0.14; P = .026) (Table 2, Figure S1C–F).
3.4. Effect of intervention on brain imaging measures
Intervention did not have a significant effect on AD signature CT or on HV (Table 2). A trend was observed in between‐group difference in the left HV (42.4 mm3, 95% CI −0.1 to 84.8; P = .051), suggesting higher annual rate of atrophy in the placebo group.
3.5. Effect of intervention according to APOE ε4 status and to nutritional deficits
Analyses according to APOE ε4 status and to presenting low ω‐3 index, high homocysteine, or low 25(OH)D at baseline are shown in Tables 3, 4, 5 and Table S2, respectively. The effect of intervention within individuals with and without combined nutritional deficits are reported in Table S3. In general, the positive effect on biomarkers was similar to the main analyses, and no effect was observed for the CCS and the CFI self‐assessment, regardless of the APOE ε4 status or the presence of deficits. The positive effect of intervention on the CFI study partner was observed only among those with high homocysteine (Table 5). The unexpected finding with the PROMIS T‐score in the main analysis was observed only among APOE ε4 non‐carriers (Table 3), those with normal 25(OH)D (Table S2), and those with one or no nutritional deficit (Table S3). No difference was observed in the effect of treatment between the subgroups.
TABLE 3.
Mixed‐effects linear regression analysis for change from baseline in outcome measures according to randomized intervention groups and APOE ε4 status among the modified intention‐to‐treat population of non‐demented, community‐dwelling older adults
| Estimated mean within‐group change from baseline a (95% CI); P‐value | Between‐group difference over time (95% CI); P‐value | Difference in between‐group differences over time for APOE ε4 carrier vs. non‐carrier | |||||
|---|---|---|---|---|---|---|---|
| Nutritional blend | Placebo | Nutritional blend vs. placebo | |||||
| APOE ε4 non‐carrier | APOE ε4 carrier b | APOE ε4 non‐carrier | APOE ε4 carrier b | APOE ε4 non‐carrier | APOE ε4 carrier b | ||
| Biomarkers | |||||||
| Plasma homocysteine (µmol/L) | −2.40 (−3.05, −1.75); <.0001 | −2.01 (−3.23, −0.79); .001 | 1.11 (0.43, 1.79); .001 | 0.15 (−0.97, 1.28); .789 | −3.51 (−4.45, −2.57); <.0001 | −2.16 (−3.82, −0.50); .011 | 1.35 (−0.56, 3.26); .164 |
| ω‐3 index c (%) | 1.51 (1.20, 1.83); <.0001 | 1.87 (1.25, 2.49); <.0001 | −1.15 (−1.47, −0.82); <.0001 | −1.04 (−1.60, −0.48); .0003 | 2.66 (2.21, 3.11); <.0001 | 2.91 (2.08, 3.75); <.0001 | 0.25 (−0.70, 1.20); 0.601 |
| Cognitive tests | |||||||
| PROMIS T‐score d (8 items) | 0.13 (−0.73, 0.98); .770 | −0.26 (−1.76, 1.25); .737 | 1.37 (0.48, 2.26); .003 | 0.78 (−0.66, 2.21); .287 | −1.25 (−2.48, −0.01); .048 | −1.03 (−3.11, 1.04); .328 | 0.21 (−2.20, 2.63); .861 |
| Composite cognitive score e | −0.09 (−0.19, 0.01); .069 | −0.15 (−0.33, 0.04); .114 | −0.11 (−0.21, −0.01); .035 | −0.10 (−0.27, 0.07); .234 | 0.02 (−0.12, 0.16); .782 | −0.05 (−0.29, 0.20); .719 | −0.07 (−0.35, 0.22); .653 |
| CFI self‐assessment score | −0.58 (−0.92, −0.24); .001 | −0.25 (−0.90, 0.40); .449 | −0.35 (−0.71, 0.01); .055 | −0.61 (−1.24, 0.02); .056 | −0.23 (−0.72, 0.27); .369 | 0.36 (−0.54, 1.27); .430 | 0.59 (−0.44, 1.62); .262 |
| CFI study partner score | −0.42 (−0.79, −0.04); .030 | −0.77 (−1.49, −0.04); .038 | 0.10 (−0.30, 0.50); .624 | −0.28 (−0.98, 0.42); .431 | −0.52 (−1.07, 0.03); .066 | −0.49 (−1.49, 0.52); .343 | 0.03 (−1.11, 1.18); .959 |
Abbreviations: CFI, Cognitive Function Instrument; PROMIS, Patient‐Reported Outcomes Measurement Information System.
Estimated with the mean at baseline.
Defined as presenting at least one ε4 allele.
Calculated as the sum of % docosahexaenoic acid and % eicosapentaenoic acid, expressed as the percentage of total erythrocyte membrane fatty acids.
After 1 month, and all other outcomes after 12 months.
Based on four cognitive tests (free and total recall of the Free and Cued Selective Reminding test, 10 Mini‐Mental State Examination orientation items, Digit Symbol Substitution Test and Category Naming Test.
TABLE 4.
Mixed‐effects linear regression analysis for change from baseline in outcome measures according to randomized intervention groups and omega‐3 status among the modified intention‐to‐treat population of non‐demented, community‐dwelling older adults
| Estimated mean within‐group change from baseline a (95% CI); P‐value | Between‐group difference over time (95% CI); P‐value | Difference in between‐group differences over time for low vs. normal ω‐3 | |||||
|---|---|---|---|---|---|---|---|
| Nutritional blend | Placebo | Nutritional blend vs. placebo | |||||
| Normal ω‐3 index | Low ω‐3 index b | Normal ω‐3 index | Low ω‐3 index b | Normal ω‐3 index | Low ω‐3 index b | ||
| Biomarkers | |||||||
| Plasma homocysteine (µmol/L) | −2.34 (−3.03, −1.66); <.0001 | −1.65 (−2.82, −0.48); .006 | 0.68 (−0.03, 1.39); .060 | 1.93 (0.79, 3.07); .001 | −3.03 (−4.01, −2.04); <.0001 | −3.58 (−5.21, −1.94); <.0001 | −0.55 (−2.46, 1.36); .572 |
| ω‐3 index c (%) | 1.47 (1.14, 1.80); <.0001 | 1.69 (1.10, 2.29); <.0001 | −1.04 (−1.38, −0.70); <.0001 | −1.37 (−1.96, −0.78); <.0001 | 2.51 (2.06, 2.96); <.0001 | 3.06 (2.32, 3.81); <.0001 | 0.56 (−0.32, 1.43); .213 |
| Cognitive tests | |||||||
| PROMIS T‐score d (8 items) | −0.30 (−1.19, 0.59); .509 | 0.81 (−0.73, 2.36); .301 | 0.97 (0.04, 1.89); .040 | 1.65 (0.09, 3.21); .038 | −1.27 (−2.55, 0.02); .053 | −0.84 (−3.03, 1.36); .453 | 0.43 (−2.11, 2.97); .740 |
| Composite cognitive score e | −0.06 (−0.16, 0.04); .235 | −0.18 (−0.35, −0.01); .041 | −0.11 (−0.21, 0.00); .041 | −0.04 (−0.20, 0.13); .653 | 0.05 (−0.10, 0.19); .513 | −0.14 (−0.38, 0.10); .248 | −0.19 (−0.47, 0.09); .184 |
| CFI self‐assessment score | −0.54 (−0.90, −0.18); .003 | −0.47(−1.09, 0.15); .136 | −0.34 (−0.72, 0.04); .075 | −0.43 (−1.03, 0.18); .168 | −0.20 (−0.72, 0.32); .456 | −0.05 (−0.91, 0.82); .916 | 0.15 (−0.86, 1.16); .770 |
| CFI study partner score | −0.48 (−0.89, −0.07); .021 | −0.66(−1.34, 0.02); .058 | 0.00 (−0.43, 0.43); .998 | −0.20 (−0.88, 0.48); .566 | −0.48 (−1.07, 0.11); .112 | −0.46 (−1.42, 0.50); .349 | 0.02 (−1.11, 1.15); .970 |
Abbreviations: CFI, Cognitive Function Instrument; PROMIS, Patient‐Reported Outcomes Measurement Information System.
Estimated with the mean at baseline.
ω‐3 index ≤5.69%.
Calculated as the sum of % docosahexaenoic acid and % eicosapentaenoic acid, expressed as the percentage of total erythrocyte membrane fatty acids.
After 1 month, and all other outcomes after 12 months.
Based on four cognitive tests (free and total recall of the Free and Cued Selective Reminding test, 10 Mini‐Mental State Examination orientation items, Digit Symbol Substitution Test and Category Naming Test.
TABLE 5.
Mixed‐effects linear regression analysis for change from baseline in outcome measures according to randomized intervention groups and homocysteine status among the modified intention‐to‐treat population of non‐demented, community‐dwelling older adults
| Estimated mean within‐group change from baseline a (95% CI); P‐value | Between‐group difference over time (95% CI); P‐value | Difference in between‐group differences over time for high vs. normal homocysteine | |||||
|---|---|---|---|---|---|---|---|
| Nutritional blend | Placebo | Nutritional blend vs. placebo | |||||
| Normal homocysteine | High homocysteine b | Normal homocysteine | High homocysteine b | Normal homocysteine | High homocysteine b | ||
| Biomarkers | |||||||
| Plasma homocysteine (µmol/L) | −2.37 (−3.06, −1.68); <.0001 | −1.82 (−3.18, −0.47); .008 | 0.44 (−0.24, 1.13); .207 | 2.64 (1.24, 4.03); .0002 | −2.82 (−3.73, −1.90); <.0001 | −4.46 (−6.10, −2.83); <.0001 | −1.65 (−3.52, 0.23); .085 |
| Omega‐3 index c (%) | 1.47 (1.16, 1.78); <.0001 | 1.71 (1.14, 2.29); <.0001 | −1.11 (−1.42, −0.79); <.0001 | −1.21 (−1.80, −0.62); <.0001 | 2.58 (2.14, 3.02); <.0001 | 2.92 (2.10, 3.75); <.0001 | 0.34 (−0.60, 1.28); .474 |
| Cognitive tests | |||||||
| PROMIS T‐score d (8 items) | 0.05 (−0.78, 0.89); .897 | −0.42 (−1.95, 1.12); .591 | 1.09 (0.25, 1.93); .011 | 1.31 (−0.28, 2.91); .106 | −1.03 (−2.22, 0.15); .086 | −1.73 (−3.95, 0.48); .125 | −0.70 (−3.21, 1.81); .584 |
| Composite cognitive score e | −0.04 (−0.13, 0.06); .437 | −0.30 (−0.45, −0.14); .0003 | −0.06 (−0.15, 0.04); .245 | −0.23 (−0.40, −0.06); .009 | 0.02 (−0.12, 0.15); .790 | −0.06 (−0.30, 0.17); .587 | −0.08 (−0.35, 0.19); .545 |
| CFI self‐assessment score | −0.59 (−0.93, −0.25); .001 | −0.26 (−0.85, 0.33); .385 | −0.58 (−0.92, −0.24); .001 | 0.42 (−0.24, 1.07); .210 | −0.01 (−0.49, 0.47); .970 | −0.68 (−1.56, 0.20); .131 | −0.67 (−1.67, 0.33); .190 |
| CFI study partner score | −0.37 (−0.76, 0.01); .057 | −0.81 (−1.46, −0.16); .014 | −0.10 (−0.49, 0.29); .612 | 0.34 (−0.40, 1.09); .362 | −0.27 (−0.82, 0.27); .328 | −1.16 (−2.15, −0.17); .022 | −0.88 (−2.01, 0.25); .125 |
Abbreviations: CFI, Cognitive Function Instrument; PROMIS, Patient‐Reported Outcomes Measurement Information System.
Estimated with the mean at baseline.
Homocysteine concentrations >14 µmol/L.
Calculated as the sum of % docosahexaenoic acid and % eicosapentaenoic acid, expressed as the percentage of total erythrocyte membrane fatty acids.
After 1 month, and all other outcomes after 12 months.
Based on four cognitive tests (free and total recall of the Free and Cued Selective Reminding test, 10 Mini‐Mental State Examination orientation items, Digit Symbol Substitution Test and Category Naming Test.
4. DISCUSSION
This study assessed the efficacy of a 1‐year NB supplementation on erythrocyte ω‐3 index and homocysteine levels, as well as on cognitive outcomes among community‐dwelling older adults, and successfully showed a positive effect of intervention in increasing ω‐3 index and decreasing homocysteine concentrations. Some effects were observed in secondary outcomes, but intervention was not able to prevent CD as measured by neuropsychological tests.
The NB provided to participants was composed of several nutrients known to be linked to cognitive function through multiple mechanisms. DHA and EPA have been associated with a multitude of neurobiological effects related to brain aging, including the modulation of neuronal membrane fluidity, stimulation of neuroplasticity, anti‐neuroinflammatory effects, and the reduction of brain oxidative stress. 9 B vitamins (specifically B6, B12, and folate), 4 as well as choline 10 have shown an ability to decrease homocysteine concentrations. Antioxidants such as vitamin C, 11 vitamin E, 12 and selenium 13 were shown to protect against oxidative damage and inflammation in brain. Vitamin D deficiency, in turn, is associated with all‐cause dementia. 14 Finally, the non‐essential amino acid citrulline (a nitric oxide precursor) has shown beneficial effects on the cardiovascular system, specifically in terms of improving blood flow, endothelial function, and blood pressure, 26 and might also be involved in the biological mechanisms of neurodegeneration. 27 Altogether, the aforementioned nutrients particularly benefited the biomarkers after 1 year. Notwithstanding, and in accordance with our findings, some meta‐analyses have concluded that solely lowering homocysteine does not guarantee preventing CD or dementia. 4 , 6 On the other hand, a more recent meta‐analysis has identified homocysteine‐lowering treatments (based on folic acid, vitamin B12, and vitamin B6 supplementation) as promising interventions for AD prevention. 28
Similarly, benefits of ω‐3 PUFA are not warranted by a simple increase in its concentrations. In this sense, a systematic review and meta‐analysis evaluating trials of ω‐3 supplementation was not able to observe a positive effect on global cognitive function, despite some studies showing positive effects in raising erythrocyte or plasma ω‐3. 29 Thus a sufficient decrease in homocysteine and increase in ω‐3 index that would lead to identifiable positive effects in cognitive function still remains to be defined.
Although the present trial was able to impact its primary outcomes, results should be interpreted in the context of the study design. The duration of follow‐up may have been insufficient to verify a positive effect of nutrients in cognitive tests and brain imaging outcomes. Similarly, no overall cognitive improvement was found after a 6‐month intervention with a supplement (composed of ω‐3 PUFA, vitamin D, resveratrol, and whey protein) among Irish older adults. 30 Indeed, a meta‐analysis of animal studies specifically evaluating ω‐3 supplementation on cognitive function, brain Aβ deposition, and hippocampal neuron loss suggested that exposure to nutrient supplementation in intervention studies with humans may be too short (around 0.5% to 1.8% of the lifespan of participants). 31 However, matching the proportional duration of effective supplementation given to rodents (10%–50% of their lifespan) would be expensive and complex. 31 It is possible that the characteristics of our participants may also have exalted this relatively short‐time condition: the population was composed of globally healthy individuals, for whom a 1‐year time frame may not have been sufficient to detect important declines in cognitive function and brain imaging outcomes. Third, the composition of the daily dose may also be mentioned as another potential factor influencing the results. Despite promising findings from previous tests in animals, 19 , 20 it is possible that for some nutrients a higher dose would be needed to detect a effects among older adults. This may be the case of DHA and citrulline. A recent trial demonstrated a 28% increase in cerebrospinal fluid (CSF) DHA levels and a 200% increase in plasma DHA after a 6‐month DHA supplementation of 2152 mg/day among cognitively unimpaired individuals at risk of dementia, and suggested that lower DHA doses (≤1 g/day) would less likely lead to meaningful DHA brain delivery. 32 Although no study to date has investigated the effect of citrulline supplementation on cognitive function, trials targeting physical function among adults ≥50 years of age showed an efficacy with doses varying from 5 g to 10 g/day, mainly among obese, dynapenic‐obese, hypertensive, or malnourished individuals 33 (for comparison, Nolan's blend provided 770 mg/day of DHA and 3 g/day of citrulline).
Despite no effect on the CFI self‐assessment or on the CCS, a positive effect of intervention on the exploratory CFI study partner was observed. Despite the modest, non‐clinically relevant difference between groups, we might speculate whether such changes may precede objective cognitive changes, in a hypothesis that demands further exploring. About the unexpected finding with the PROMIS questionnaire at 1 month, it should be considered that, unlike the other questionnaires, the PROMIS was individually self‐answered in the absence of a clinician or interviewer. Unfortunately, other outcomes were not measured at 1 month, preventing the investigation of whether this small but statistically significant result would translate into other measurable cognitive changes. Taken together, the findings from the cognitive tests indicate that the potential effect of NB remains to be explored in other human populations.
Of interest, analyses of imaging outcomes showed a trend in the left HV, suggesting a potential benefit of the blend in slowing hippocampal atrophy. This finding is consistent with the notion that brain changes precede changes in cognitive function. Despite the lack of studies evaluating HV changes after nutritional supplementation among cognitively normal older adults, some studies were performed with subjects presenting mild cognitive impairment (MCI). 34 , 35 , 36 The RCT Homocysteine and B Vitamins in Cognitive Impairment (VITACOG), providing B‐vitamin supplementation to elderly subjects with MCI, observed a positive effect of treatment in bilateral hippocampal gray matter volume after 2 years, what authors credited to lowering mean plasma homocysteine concentrations by 29% after performing a voxel‐based analysis. 34 Notably, after dividing participants according to their overall baseline median homocysteine, authors further showed that treatment was significantly beneficial (for whole gray matter) only for participants with higher homocysteine levels. 34 This group also presented slower CD over time. 35 In another study, positive findings in both cognitive function and HV were seen after 1‐year of DHA supplementation in older adults with MCI. 36
The effect of intervention was also tested in an exploratory manner according to the presence of nutritional deficits (low ω‐3 index, high homocysteine, low 25(OH)D), to investigate if individuals with one or multiple nutritional deficits should be specific targets for nutritional interventions. It is also possible that the minimum duration of effective nutritional interventions may vary according to the number and type of nutritional deficits, which could be explored in future investigations. This idea is reinforced by the findings of the VITACOG trial, which observed that higher concentrations of DHA significantly enhanced the effects of B‐vitamin supplementation in cognitive outcomes and brain atrophy rates among older adults with MCI, whereas treatment had no effect on individuals with low ω‐3 PUFA. 17 , 37 In addition, a trial with patients with AD found that ω‐3 supplementation improved cognitive performance (measured by MMSE) only among participants with lower homocysteine concentrations, suggesting that adequate B‐vitamin status is required to obtain beneficial effects of ω‐3 PUFA on cognition. 38 These important results highlight the need for fighting nutritional deficits for optimizing possible interventions. In this context, the multitarget nutritional intervention is a key difference of our trial compared to previous studies.
The originality of this trial using a combined approach targeting nutritional biomarkers primarily, neuropsychological tests, cognitive patient‐reported outcomes, and brain imaging outcomes among older adults should be noted as a strength of this study, and also its randomized, controlled design. Moreover, many other secondary and exploratory outcomes of cognitive function (biomarkers, clinical tests, subjective tests, and brain imaging) were assessed and will support further analyses to help build evidence concerning the nutritional aspects associated with cognitive aging. However, some limitations must be noted. As mentioned previously, the 1‐year duration of intervention was not adequate to evidence clinically meaningful results. Given that our sample size calculation was based on primary outcomes (biomarkers), it is possible that it did not achieve adequate statistical power for identifying changes in cognitive tests. As an intrinsic limitation of RCT, adherence decreased with time. The MRI was only performed in a subgroup of participants. Finally, as expected in a preventive trial, education level was particularly high, implicating a high level of cognitive reserve that might have possibly contributed to a slower CD in this sample.
5. CONCLUSIONS
This 1‐year trial found positive effects of a daily NB supplementation on nutritional biomarkers, but was not able to show improvements in cognitive function among dementia‐free, community‐dwelling older adults. Given the evidence on the ability of high homocysteine and low ω‐3 index in predicting impairments in cognitive function, enhancing both biomarkers in a 1‐year range can be possibly considered an initial step on protecting cognition over aging. Findings may help to set nutritional strategies for optimizing brain health and preventing or slowing CD, as also identifying individuals to whom specific nutrient supplementation may be more effective. Additional investigations are needed, especially trials with longer follow‐up periods that target subjects who are APOE ε4 carriers and with nutritional deficiencies.
6. Nolan/DSA Study group
6.1. Nolan Study group
Principal investigator: Bruno Vellas (Toulouse); Co‐principal investigator: Sophie Guyonnet; Study coordinator: Lauréane Brigitte; CRA: Céline Cluzan.
Methodology and statistical analysis: Sandrine Andrieu, Christelle Cantet.
Investigators in coordinating centers: Pierre‐Jean Ousset, Catherine Faisant, Françoise Lala, Julien Delrieu, Nathalie Sastre, Yves Rolland, Sandrine Sourdet, Marie Champarnaud, Philippe Girard; Psychologists: Emeline Combrouze, Audrey Casaux (Toulouse).
Investigators in associated center: Sébastien Laborie, Carine Chiffre, Marc Alonso (Albi); Jean‐François Dartigues, Sophie Auriacombe, Isabelle Bourdel‐Marchasson, Muriel Rainfray, Nathalie Salles, Claire Roubaud Baudron, Sandrine Harston (Bordeaux); Véronique Kostek, Frédérique Olivier, Albine Louchet, Inga Couffignal (Cahors); Marie‐Noëlle‐Cuffi, Amandine Lefort, Sophie Dardenne, Magali Fleury, Corine Costes (Castres); Yannick Bejot, Olivier Rouaud, Cécile Chazalon, Benoit Delpont, Agnès Jacquin‐Piques, Catia Khoumri (Dijon); Lawrence Bories, Evelyne Campistron, Marie‐Laure Pader (Foix); Anne Hustache, Dominique Penchenat (Gourdon); Aurianne Ravaud, Jean Bernard Chauderon (Labastide‐Murat); Françoise Desclaux (Lavaur); Florence Pasquier, Thibaud Lebouvier, Vincent Deramecourt, Pascaline Cassagnaud, Marie‐Anne Mackowiak, Adeline Rollin, Stéphanie Bombois, Catherine Adnet‐Bonte (Lille); Thierry Dantoine (Limoges); Marc Bonnefoy, Thomas Gilbert (Lyon Sud Pierre Bénite); Pierre Krolak Salmon, Jing Xie, Denis Federico, Isabelle Rouch, Christophe Magnier, Marie Hélène Coste, Zaza Makaroff, Keren Danaila Kenny David, Julien Vernaudon (Lyon Villeurbanne); Hélène Mollion, Isabelle Roullet‐Solignac, Maïté Formaglio (Lyon Est); Mathieu Ceccaldi, Claude Gueriot, Radka Gantchev Kletchkova (Marseille); Aurélie Roustan, Kristel Scheirlinckx‐Sudres, Carole Cervera (Montauban); Laurin Brignol (Moissac); Audrey Gabelle, Karim Bennys, Aude Metzger, Cecilia Marelli, Claire Hourregue (Montpellier); Michael Li Yung Tong, Cynthia Laurent, Rosanne Ufkes (Muret); Michel Dutech, Patrick Gervais, Vincent Courrière (Nailloux); Gilles Berrut, Laure De Decker, Sophie Pichierri, Pascal Chevalet, Typhaine Riaudel, Marie‐Hélène Fix, Fanny Pesle, Catherine Couturier (Nantes); Philippe Robert, Renaud David, Aurélie Mouton, Georges Niewiadomski (Nice); Olivier Guerin, Guillaume Sacco, Cyprien Arlaud, Cyrielle Rambaud, Marine Sanchez, Emilie Ferrer (Nice Hôpital Cimiez); Olivier Hanon, Marie Laure Seux, Clémence Boully, Emmanuelle Duron, Lena Joffredo, Anne Chahwakilian, Jean‐Sébastien Vidal, Galdric Orvoen, Edouard Chaussade, Florian Labourée, Laure Caillard, Hermine Lenoir, Sophie Chauvelier, Yasmina Boudali (Paris); Jacques Hugon, Claire Paquet, Emmanuel Cognat, Julien Dumurgier (Paris Lariboisière); Bruno Dubois, Katia Andrade, Stéphane Epelbaum (Paris La Pitié Salpétrière); Frédéric Blanc (Strasbourg); Yves Gasnier, Kai Ostendorf, Jean‐Pierre Salles, Samira Misbah El Idrissi, Isabelle Zagar, Thérèse Uguen, Stéphanie Sallaberry‐Benech, Vincent Dodier, KhaledKhales (Tarbes); Stéphane Oustric, Marie‐Eve Rouge Bugat, Marc Viguier, Emile Escourrou, Laetitia Gimenez, Michel Combier (Toulouse).
Nestlé project team: Jeroen Schmitt, Corina Boschat, Julie Hudry, Dominik Grathwohl, Cecilia Fumero, Emilie Darcillon, Julia Mauger, Ludivine Feraille‐Naze, John Corthesy (Lausanne).
MRI and PET scans group: Pierre Payoux, Anne‐Sophie Salabert (Toulouse); Stéphane Lehéricy, Marie Chupin, Jean‐François Mangin, Ali Bouhayia (Paris); Michèle Allard (Bordeaux); Frédéric Ricolfi (Dijon); Dominique Dubois (Foix); Marie Paule Bonceour Martel (Limoges); François Cotton (Lyon); Alain Bonafé (Montpellier); Stéphane Chanalet (Nice); Françoise Hugon (Tarbes); Fabrice Bonneville, Christophe Cognard, François Chollet (Toulouse).
Biological sample collection: Bertrand Perret, Bénédicte Razat (Toulouse).
Safety management: Pascale Olivier‐Abbal (Toulouse, France).
6.2. DSA Group Sandrine Andrieu, Christelle Cantet, Nicola Coley
CONFLICT OF INTEREST
C.B., J.H., and J.A.J.S. work at the Société des Produits Nestlé SA, Nestlé Research, Lausanne, Switzerland. M.W.W. and B.V. received funds from Nestlé. Other authors declare no conflict of interest. Author disclosures are available in the supporting information.
AUTHOR CONTRIBUTIONS
Sandrine Andrieu, Bruno Vellas, and Jeroen A. J. Schmitt conceived the Nolan Study and designed the research; Duygu Tosun conducted the research; Christelle Cantet analyzed the data; Kelly V. Giudici wrote the paper; Kelly V. Giudici and Christelle Cantet had primary responsibility for the final content. Kelly V. Giudici, Sophie Guyonnet, Christelle Cantet, Philipe de Souto Barreto, Michael W. Weiner, Duygu Tosun, Corina Boschat, Julie Hudry, Sandrine Andrieu, Bruno Vellas, and Jeroen A. J. Schmitt interpreted the data and revised the draft of the paper critically for intellectual content. All authors read and approved the final manuscript.
Supporting information
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
The global sponsor of the Nolan Study is Société des Produits Nestlé SA, Nestlé Research, Lausanne, Switzerland. The sponsor for France is the University Hospital Center of Toulouse. The study sponsor was involved in study center set‐up and monitoring, preparation and distribution of BPB and placebo, data collection and monitoring working closely with the investigators. Initial training sessions and yearly investigator's meeting were organized by both the University Hospital Center of Toulouse and Nestlé. The data sharing activity was supported by the Association Monegasque pour la Recherche sur la maladie d'Alzheimer (AMPA) and the INSERM‐University of Toulouse III UMR 1027 research unit.
Giudicia KV, Guyonnet S, Cantet C, et al. A 1‐year randomized controlled trial of a nutritional blend to improve nutritional biomarkers and prevent cognitive decline among community‐dwelling older adults: The Nolan Study. Alzheimer's Dement. 2022;8:e12314. 10.1002/trc2.12314
Clinical Trial Registry number: NCT03080675 registered at www.clinicaltrials.gov
REFERENCES
- 1. Klimova B, Valis M, Kuca K. Cognitive decline in normal aging and its prevention: a review on non‐pharmacological lifestyle strategies. Clin Interv Aging. 2017;12:903–910. 10.2147/CIA.S132963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu‐Seifert H, Siemers E, Price K, et al. Cognitive impairment precedes and predicts functional impairment in mild Alzheimer's disease. J Alzheimers Dis JAD. 2015;47(1):205–214. 10.3233/JAD-142508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rapp T, Andrieu S, Chartier F, et al. Resource use and cost of Alzheimer's disease in France: 18‐month results from the GERAS observational study. Value Health J Int Soc Pharmacoeconomics Outcomes Res. 2018;21(3):295–303. 10.1016/j.jval.2017.09.019 [DOI] [PubMed] [Google Scholar]
- 4. Clarke R, Bennett D, Parish S, et al. Effects of homocysteine lowering with B vitamins on cognitive aging: meta‐analysis of 11 trials with cognitive data on 22,000 individuals. Am J Clin Nutr. 2014;100(2):657–666. 10.3945/ajcn.113.076349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith AD, Homocysteine Refsum H., B vitamins, and cognitive impairment. Annu Rev Nutr. 2016;36:211–239. 10.1146/annurev-nutr-071715-050947 [DOI] [PubMed] [Google Scholar]
- 6. Setién‐Suero E, Suárez‐Pinilla M, Suárez‐Pinilla P, Crespo‐Facorro B, Ayesa‐Arriola R. Homocysteine and cognition: a systematic review of 111 studies. Neurosci Biobehav Rev. 2016;69:280–298. 10.1016/j.neubiorev.2016.08.014 [DOI] [PubMed] [Google Scholar]
- 7. Hooper C, De Souto Barreto P, Pahor M, Weiner M, Vellas B. The relationship of omega 3 polyunsaturated fatty acids in red blood cell membranes with cognitive function and brain structure: a review focussed on Alzheimer's disease. J Prev Alzheimers Dis. 2018;5(1):78–84. [DOI] [PubMed] [Google Scholar]
- 8. Blaise SA, Nédélec E, Schroeder H, et al. Gestational vitamin B deficiency leads to homocysteine‐associated brain apoptosis and alters neurobehavioral development in rats. Am J Pathol. 2007;170(2):667–679. 10.14283/jpad.2017.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dyall SC. Long‐chain omega‐3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52. 10.3389/fnagi.2015.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. da Costa K‐A, Gaffney CE, Fischer LM, Zeisel SH. Choline deficiency in mice and humans is associated with increased plasma homocysteine concentration after a methionine load. Am J Clin Nutr. 2005;81(2):440–444. 10.1093/ajcn.81.2.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moretti M, Fraga DB, Rodrigues ALS. Preventive and therapeutic potential of ascorbic acid in neurodegenerative diseases. CNS Neurosci Ther. 2017;23(12):921–929. 10.1111/cns.12767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mocchegiani E, Costarelli L, Giacconi R, et al. Vitamin E‐gene interactions in aging and inflammatory age‐related diseases: implications for treatment. A systematic review. Ageing Res Rev. 2014;14:81–101. 10.1016/j.arr.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 13. Solovyev ND. Importance of selenium and selenoprotein for brain function: from antioxidant protection to neuronal signalling. J Inorg Biochem. 2015;153:1–12. [DOI] [PubMed] [Google Scholar]
- 14. Kalra A, Teixeira AL, Diniz BS. Association of vitamin D levels with incident all‐cause dementia in longitudinal observational studies: a systematic review and meta‐analysis. J Prev Alzheimers Dis. 2020;7(1):14–20. 10.1016/j.jinorgbio.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 15. Vauzour D, Camprubi‐Robles M, Miquel‐Kergoat S, et al. Nutrition for the ageing brain: towards evidence for an optimal diet. Ageing Res Rev. 2017;35:222–240. 10.1016/j.arr.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 16. Smith AD, Smith SM, de Jager CA, et al. Homocysteine‐lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PloS One. 2010;5(9):e12244. 10.1371/journal.pone.0012244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oulhaj A, Jernerén F, Refsum H, Smith AD, de Jager CA. Omega‐3 fatty acid status enhances the prevention of cognitive decline by B vitamins in mild cognitive impairment. J Alzheimers Dis JAD. 2016;50(2):547–557. 10.3233/JAD-150777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li M, Li W, Gao Y, et al. Effect of folic acid combined with docosahexaenoic acid intervention on mild cognitive impairment in elderly: a randomized double‐blind, placebo‐controlled trial. Eur J Nutr. 2021;60(4):1795–1808. 10.1007/s00394-020-02373-3 [DOI] [PubMed] [Google Scholar]
- 19. Pan Y, Kennedy AD, Jönsson TJ, Milgram NW. Cognitive enhancement in old dogs from dietary supplementation with a nutrient blend containing arginine, antioxidants, B vitamins and fish oil. Br J Nutr. 2018;119(3):349–358. 10.1017/S0007114517003464 [DOI] [PubMed] [Google Scholar]
- 20. Pan Y, Araujo JA, Burrows J, et al. Cognitive enhancement in middle‐aged and old cats with dietary supplementation with a nutrient blend containing fish oil, B vitamins, antioxidants and arginine. Br J Nutr. 2013;110(1):40–49. 10.1017/S0007114512004771 [DOI] [PubMed] [Google Scholar]
- 21. Andrieu S, Guyonnet S, Coley N et al. Effect of long‐term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo‐controlled trial. Lancet Neurol. 2017;16(5):377–389. 10.1016/S1474-4422(17)30040-6 [DOI] [PubMed] [Google Scholar]
- 22. Bowman GL, Dodge HH, Guyonnet S, et al. . A blood‐based nutritional risk index explains cognitive enhancement and decline in the multidomain Alzheimer prevention trial. Alzheimer's Dement Transl Res Clin Interv. 2019;5:953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lu WH, Giudici KV, Rolland Y, et al. Associations between nutritional deficits and physical performance in community‐dwelling older adults. Front Nutr. 2021;8:771470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346:476–483. [DOI] [PubMed] [Google Scholar]
- 25. Holick MF, Binkley NC, Bischoff‐Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96:1911–30. [DOI] [PubMed] [Google Scholar]
- 26. Allerton TD, Proctor DN, Stephens JM, Dugas TR, Spielmann G, Irving BA. l‐Citrulline Supplementation: impact on Cardiometabolic Health. Nutrients. 2018;10(7):921. 10.3390/nu10070921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marquet‐de Rougé P, Clamagirand C, Facchinetti P, et al. Citrulline diet supplementation improves specific age‐related raft changes in wild‐type rodent hippocampus. Age Dordr Neth. 2013;35(5):1589–1606. 10.1007/s11357-012-9462-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu JT, Xu W, Tan CC, et al. Evidence‐based prevention of Alzheimer's disease: systematic review and meta‐analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry. 2020;91(11):1201–1209. 10.1136/jnnp-2019-321913.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alex A, Abbott KA, McEvoy M, Schofield PW, Garg ML. Long‐chain omega‐3 polyunsaturated fatty acids and cognitive decline in non‐demented adults: a systematic review and meta‐analysis. Nutr Rev. 2020;78:563–578. 10.1093/nutrit/nuz073 [DOI] [PubMed] [Google Scholar]
- 30. Moran C, Scotto di Palumbo A, Bramham J, et al. Effects of a six‐month multi‐ingredient nutrition supplement intervention of omega‐3 polyunsaturated fatty acids, vitamin D, resveratrol, and whey protein on cognitive function in older adults: a randomised, double‐blind, controlled trial. J Prev Alzheimers Dis. 2018;5(3):175–183. 10.14283/jpad.2018.11 [DOI] [PubMed] [Google Scholar]
- 31. Hooijmans CR, Pasker‐de Jong PCM, de Vries RBM, Ritskes‐Hoitinga M. The effects of long‐term omega‐3 fatty acid supplementation on cognition and Alzheimer's pathology in animal models of Alzheimer's disease: a systematic review and meta‐analysis. J Alzheimers Dis JAD. 2012;28(1):191–209. 10.3233/JAD-2011-111217 [DOI] [PubMed] [Google Scholar]
- 32. Arellanes IC, Choe N, Solomon V, et al. Brain delivery of supplemental docosahexaenoic acid (DHA): a randomized placebo‐controlled clinical trial. EBioMedicine. 2020;59:102883. 10.1016/j.ebiom.2020.102883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aubertin‐Leheudre M, Buckinx F. Effects of Citrulline alone or combined with exercise on muscle mass, muscle strength, and physical performance among older adults: a systematic review. Curr Opin Clin Nutr Metab Care. 2020;23(1):8–16. 10.1097/MCO.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 34. Douaud G, Refsum H, de Jager CA, et al. Preventing Alzheimer's disease‐related gray matter atrophy by B‐vitamin treatment. Proc Natl Acad Sci U S A. 2013;110(23):9523–9528. 10.1073/pnas.1301816110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Jager CA, Oulhaj A, Jacoby R, Refsum H, Smith AD. Cognitive and clinical outcomes of homocysteine‐lowering B‐vitamin treatment in mild cognitive impairment: a randomized controlled trial. Int J Geriatr Psychiatry. 2012;27:592–600. 10.1002/gps.2758 [DOI] [PubMed] [Google Scholar]
- 36. Zhang Y‐P, Miao R, Li Q, Wu T, Ma F. Effects of DHA supplementation on hippocampal volume and cognitive function in older adults with mild cognitive impairment: a 12‐month randomized, double‐blind, placebo‐controlled trial. J Alzheimers Dis. 2017;55(2):497–507. 10.3233/JAD-160439 [DOI] [PubMed] [Google Scholar]
- 37. Jernerén F, Elshorbagy AK, Oulhaj A, Smith SM, Refsum H, Smith AD. Brain atrophy in cognitively impaired elderly: the importance of long‐chain ω‐3 fatty acids and B vitamin status in a randomized controlled trial. Am J Clin Nutr. 2015;102(1):215–221. 10.3945/ajcn.114.103283 [DOI] [PubMed] [Google Scholar]
- 38. Jernerén F, Cederholm T, Refsum H, et al. Homocysteine status modifies the treatment effect of omega‐3 fatty acids on cognition in a randomized clinical trial in mild to moderate Alzheimer's disease: the OmegAD Study. J Alzheimers Dis. 2019;69:189–197. 10.3233/JAD-181148 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION


