Summary
Background
Hemoglobin A1c (HbA1c) is used for diabetes diagnosis and management. HbA1c also represents iron-related erythrocyte properties which differ by sex. We investigated erythrocyte properties on HbA1c and glucose, and whether corresponding consequences for mortality differed by sex.
Methods
In this two-sample Mendelian randomization study using the largest publicly available European descent summary statistics, we assessed sex-specific associations of iron (n=163,511) and hemoglobin (188,076 women/162,398 men) with HbA1c (185,022 women/159,160 men) and fasting glucose (73,089 women/67,506 men), of fasting glucose with HbA1c and diabetes (cases=6,589 women/10,686 men, controls=187,137 women/155,780 men), and of fasting glucose (n=140,595), HbA1c (n=146,806) and liability to diabetes (74,124 cases/824,006 controls) with parental attained age (412,937 mothers/415,311 fathers).
Findings
Iron and hemoglobin were inversely associated with HbA1c but not fasting glucose. Fasting glucose was more strongly associated with HbA1c and diabetes in women (1.65 standard deviation (SD) per mmol/L [95% confidence interval 1.58, 1.72]; odds ratio (OR) 7.36 per mmol/L [4.12, 10.98]) than men (0.89 [0.81, 0.98]; OR 2.79 [1.96, 4.98]). The inverse associations of HbA1c and liability to diabetes with lifespan were possibly stronger in men (-1.80 years per percentage [-2.77, -0.42]; -0.93 years per logOR [-1.23, -0.59]) than women (-0.80 [-2.69, 0.66]; -0.44 [-0.62, -0.26]).
Interpretation
HbA1c underestimates fasting glucose in men compared with women, possibly due to erythrocyte properties. Whether HbA1c and liability to diabetes reduce lifespan more in men than women because diagnostic and management criteria involving HbA1c mean that glycemia in men is under-treated compared to women needs urgent investigation.
Funding
None.
Keywords: Diabetes, Fasting glucose, HbA1c, Mortality, Sex difference
Research in context.
Evidence before this study
Hemoglobin A1c (HbA1c) is widely used in the diagnosis and management of diabetes. HbA1c is not only determined by glycemia, but is also affected by erythrocyte properties which differ by sex. Erythrocyte properties affecting HbA1c might lead to HbA1c representing different levels of glycemia in men and women, and thereby contribute to sex differences in the diagnosis, treatment and management of diabetes, and thereby its consequence for mortality. Few studies have assessed sex differences in the association of glucose with HbA1c. Previous Mendelian randomization (MR) studies have suggested type 2 diabetes reduces lifespan, but they did not consider sex-specific associations, and the associations of glucose and HbA1c with mortality are less clear.
Added value of this study
This MR study suggests that HbA1c underestimates fasting glucose in men compared with women, possibly driven by higher iron in men. Using parental attained age as a measure of mortality, we showed that fasting glucose, HbA1c and liability to diabetes were associated with shorter lives. The associations of HbA1c and liability to diabetes with mortality are possibly stronger in men than women.
Implications of all the available evidence
HbA1c, a currently accepted clinical measure of glycemia, underestimates fasting glucose in men compared with women. Diagnostic and management criteria based on HbA1c may result in HbA1c and liability to diabetes reducing lifespan more in men than women. These insights highlight the importance of using measures that reflect the same level of glycemia equitably in different populations in the diagnosis, treatment and management of diabetes.
Alt-text: Unlabelled box
Introduction
Hemoglobin A1c (HbA1c) is one of the criteria for diabetes diagnosis and management, along with plasma glucose.1 HbA1c reflects average blood glucose within the past two to three months and is a key target for glycemic control.2 HbA1c is not only determined by glycemia but is also affected by the rate of hemoglobin glycation. The hemoglobin glycation rate varies between individuals,3 and depends on erythrocyte properties4 and its determinants, such as iron.5
Previous studies have found that the association of glycemia with HbA1c varies by ethnicity.6,7 However, few studies have considered the possibility of differences by sex in the association of glucose with HbA1c, although erythrocyte properties differ by sex. Hemoglobin is higher in men than women,8 because iron levels tend to be higher in men than women.9 Erythrocyte properties affecting HbA1c might lead to HbA1c representing different levels of glycemia in men and women, and thereby contribute to sex differences in the diagnosis, treatment and management of diabetes, and ultimately its consequences.
To assess sex-specifically the level of glycemia reflected by HbA1c and their consequences for mortality, we used Mendelian randomization (MR) to obtain less confounded estimates.10 Previous MR studies have suggested type 2 diabetes reduces lifespan,11,12 whereas HbA1c and glucose have little impact on mortality.12,13 However, these previous studies lacked power through the use of dichotomous outcome,12 did not assess sex-specific associations,12 or measured mortality from age at death13 which excludes people remaining alive and generates selection bias.14 To address these gaps, we first investigated how a key driver, serum iron, and a marker, hemoglobin, of erythrocyte properties affected HbA1c and fasting glucose. Second, we examined sex-specific associations of fasting glucose with HbA1c and diabetes. Third, we assessed sex-specific associations of fasting glucose, HbA1c and liability to diabetes with all-cause mortality. To disentangle the effect of HbA1c from erythrocyte properties, we also used multivariable MR to assess the role of HbA1c independent of hemoglobin as previously,15 as both the glycemic and erythrocytic properties of HbA1c contribute to its consequences.16
Methods
Study design
To examine the different research questions as described in the introduction, we conducted three MR analyses, taking advantage of the largest relevant publicly available genetic summary statistics in people of European ancestry, overall and where possible by sex (Figure 1). Data sources for genetic associations are summarized in Supplemental Table S1. MR relies on the instrumental variable assumptions of relevance, independence and exclusion restriction, that is genetic instruments should be strongly related to the exposure, share no common cause with the outcome, and only influence the outcome via affecting the exposure.10
Figure 1.
Flowchart of this two-sample Mendelian randomization (MR) study.
Genetic predictors for serum iron and hemoglobin
We obtained sex-combined genetic associations for serum iron (n=163,511) from a meta-analysis of genome-wide association studies (GWAS) of clinically measured serum iron from Iceland, and the INTERVAL study in the UK.17 The INTERVAL study excluded samples with sex mismatch, low call rates, duplication, extreme heterozygosity, and excluded people of non-European descent.17 Estimates were adjusted for age, sex and additionally menopausal status, ABO blood group, body mass index (BMI), smoking, alcohol use, and iron supplementation status for the INTERVAL study.17 We obtained sex-combined and sex-specific genetic associations with hemoglobin concentration from the UK Biobank (188,076 women/162,398 men). The UK Biobank recruited approximately 500,000 individuals (intended age 40-69 years, 45.6% men, 94% self-reported European ancestry) from 2006 to 2010 across Great Britain.18 The GWAS was restricted to people of white British ancestry to reduce confounding by population stratification, and excluded participants with excess relatedness or sex chromosome aneuploidy. Summary quality controlled genetic associations for 13.7 million variants were adjusted for age, age2, inferred sex, age × inferred sex, age2 × inferred sex, and the first 20 principal components (http://www.nealelab.is/uk-biobank/).
Genetic predictors for fasting glucose, HbA1c and liability to diabetes
We obtained sex-combined and sex-specific genetic associations with fasting glucose from the Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) in people of European descent without diabetes (73,089 women/67,506 men).19 Summary genetic associations were adjusted for age, study site and principal components.19
We obtained sex-combined genetic associations with HbA1c from MAGIC, including 281,416 individuals without diabetes (70% Europeans).20 We used genetic summary statistics for people of European ancestry (n=146,806), adjusted for study-specific covariates and principal components.20 We obtained sex-specific genetic associations with HbA1c from the UK Biobank (185,022 women/159,160 men), adjusted for age, age2 and 20 principal components (http://www.nealelab.is/uk-biobank/).
We obtained sex-combined genetic associations with liability to diabetes from a meta-analysis of GWAS specific to European ancestry (74,124 type 2 diabetes cases and 824,006 controls) in the DIAbetes Meta-ANalysis of Trans-Ethnic association studies (DIAMANTE) consortium.21 Summary genetic associations were adjusted for study-specific covariates and principal components.21 We obtained sex-specific genetic associations with diagnosed diabetes from the UK Biobank (cases=6,589 women/10,686 men, controls=187,137 women/155,780 men), adjusted for age, age2 and 20 principal components (http://www.nealelab.is/uk-biobank/). Genetic associations for all-or-none traits obtained using linear regression were transformed into log odds ratio (OR) using an established approximation.22
We extracted independent (r2<0.01) genome wide significant (P value <5×10–8) genetic instruments for serum iron, hemoglobin, fasting glucose, HbA1c and liability to diabetes from each GWAS, overall and where possible, by sex. Where effect allele frequency was not given and strand direction was uncertain, we excluded palindromic SNPs before removing correlated SNPs.
Genetic associations with all-cause mortality
We used parental attained age (current age or age at death) from the UK Biobank as a measure of all-cause mortality, because the UK Biobank participants were relatively young at recruitment (∼57 years), which reduces selection bias from selection of survivors and has greater power as parental current age or age at death than participant's mortality status. Genetic associations with fathers’ attained age (n=415,311) and mothers’ attained age (n=412,937) were from a GWAS of European descent UK Biobank participants, adjusted for age, sex, array type and assessment center.23 Estimates are presented in terms of life years longer (positive) or shorter (negative), as previously.24
Statistical analysis
We used the F-statistic to assess instrument strength, obtained from the mean of the square of each SNP-exposure association divided by the square of its standard error.25 An F-statistic larger than 10 suggests weak instrument bias is unlikely.
We aligned the genetic variants based on alleles and/or allele frequency and excluded palindromic SNPs with intermediate effect allele frequency (i.e., 0.42-0.58) when the strand direction was uncertain. We used proxy SNPs (r2≥0.8), where possible, when SNPs were not in the outcome GWAS.
We obtained MR estimates from genetic variant specific Wald estimates (genetic association with outcome divided by genetic association with exposure). To assess the validity of MR analyses, we used methods based on different assumptions, i.e., inverse variance weighted (IVW) with multiplicative random effects,26 the weighted median,27 MR Egger28 and the contamination mixture method.29 IVW assumes all genetic variants are valid or pleiotropy is balanced,26 which is not always plausible. The weighted median is valid when more than half of the information comes from valid SNPs.27 MR Egger is an extension of IVW, assuming no consequence of the instruments confounds exposure on the outcome.28 We used the MR Egger intercept to assess whether the IVW estimate might be affected by violation of the exclusion-restriction assumption.28 The contamination mixture method is robust to outliers and horizontal pleiotropy, with well-controlled type 1 error rates.29,30 As such, we used the contamination mixture method as the main analysis.
We used multivariable IVW and multivariable Lasso to assess the association of genetically predicted HbA1c with mortality adjusted for hemoglobin. We combined genetic variants for HbA1c and hemoglobin and removed duplicated or correlated (r2≥0.01) SNPs. We extracted the associations of the remaining SNPs with HbA1c, hemoglobin and parental attained age, aligned the SNPs based on alleles and/or allele frequency, and fitted one multivariable model. Given that multivariable IVW estimates could be biased when the exclusion-restriction assumption is violated, we used multivariable Lasso as the main analysis. Multivariable Lasso identifies valid genetic variants and fits a standard multivariable IVW model using only the valid instruments, which is robust to pleiotropy.31 We used the conditional F-statistic FTS to examine the instrument strength for each exposure conditional on the other exposure, and the Q-statistic to assess pleiotropy.32
We checked for the associations of all genetic variants with possible confounders (e.g., socioeconomic position, alcohol drinking, smoking and physical activity) (P value <5×10–8) using PhenoScanner (http://www.phenoscanner.medschl.cam.ac.uk/), a comprehensive curated database of publicly available results from large-scale GWAS. We also checked whether genetic variants were located on ABO, a well-known pleiotropic gene.33 To address potential pleiotropy, we excluded these genetic variants in the main analysis and included them in sensitivity analysis.
Sex differences in the estimates were assessed using a two-sided z-test.34 A statistical significance level of 0.05 was used. As the confidence interval (CI) of contamination mixture estimate is not constrained to be symmetric,29 its standard error was estimated as the 95% CI divided by (1.96*2). All statistical analyses were conducted using R version 4.1.1 and the packages “TwoSampleMR” for harmonizing data, “MendelianRandomization” for univariable and multivariable MR, “MVMR” for conditional F-statistics and Q-statistics, and “ieugwasr” for removing correlated SNPs. Results were visualized using the package “forestplot”.
Ethics
All analyses were based on publicly available summary statistics, which does not require ethical approval.
Role of funders
This study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Results
Genetic instruments
We extracted 50 independent (r2<0.01) genome wide significant (P value <5×10–8) genetic instruments for serum iron, 470 (overall), 186 (men) and 220 (women) for hemoglobin, 51 (overall), 18 (men) and 24 (women) for fasting glucose, 98 for HbA1c, and 269 for liability to diabetes. We identified 39 genetic variants associated with possible confounders (P value <5×10–8) or located on ABO gene (Supplemental Table S2). We excluded these SNPs in the main analysis, and included them in sensitivity analysis.
Sex-specific associations of serum iron and hemoglobin with HbA1c and fasting glucose
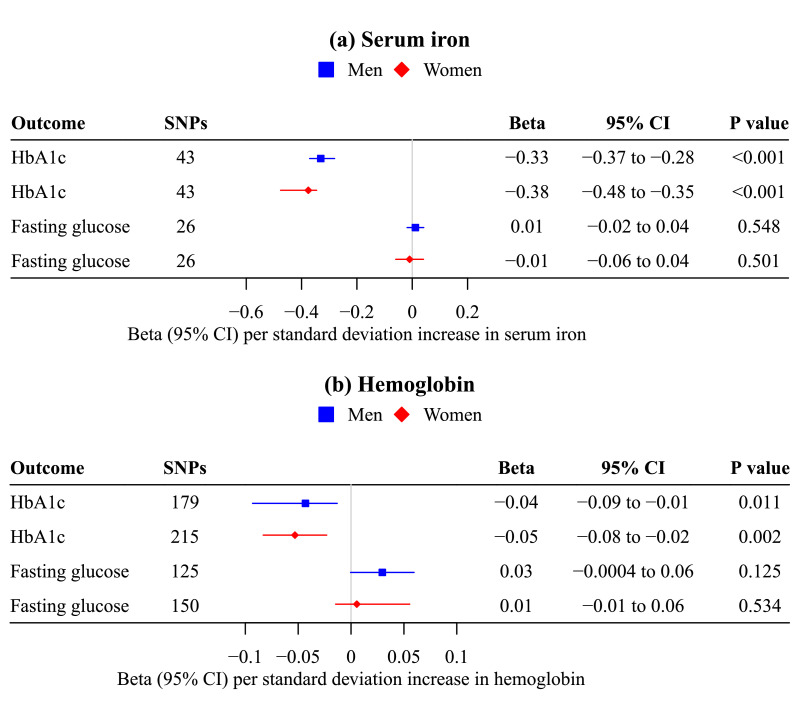
Genetically predicted serum iron and hemoglobin were inversely associated with HbA1c, but were not associated with fasting glucose (Figure 2), suggesting erythrocyte properties affect HbA1c independent of glucose. These associations were similar in men and women (Figure 2), and were robust to other analytic methods and the inclusion of potentially pleiotropic SNPs (Supplemental Table S3, S6).
Figure 2.
Mendelian randomization estimates for sex-specific associations of genetically predicted (a) serum iron (n=163,511; Bell S, et al.) and (b) hemoglobin (188,076 women/162,398 men; UK Biobank) with HbA1c (185,022 women/159,160 men; UK Biobank) and fasting glucose (73,089 women/67,506 men; MAGIC).
a. The contamination mixture method was used.
b. Estimates are expressed in standard deviation for serum iron, hemoglobin and HbA1c, and in mmol/L for fasting glucose.
Sex-specific associations of fasting glucose with HbA1c and diabetes
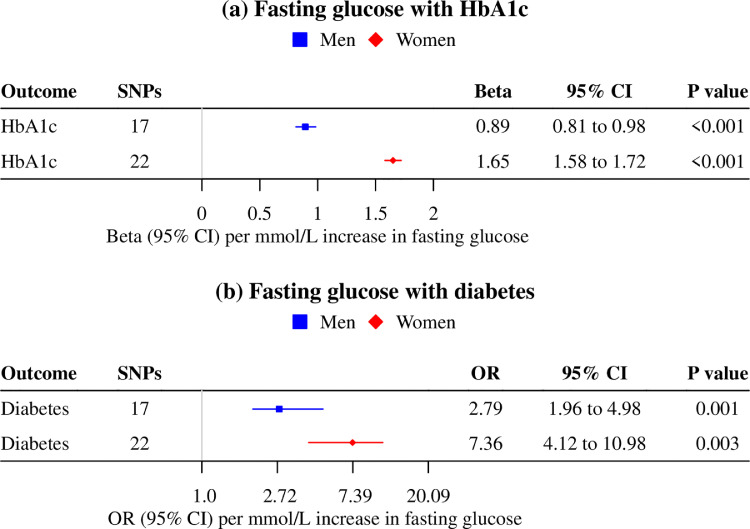
Genetically predicted fasting glucose was more strongly associated with HbA1c and risk of diabetes in women (1.65 standard deviation (SD) per mmol/L [95% confidence interval (CI) 1.58 to 1.72; P value<0.001]; odds ratio (OR) 7.36 per mmol/L [95% CI 4.12 to 10.98; P value 0.003]) than men (0.89 SD per mmol/L [95% CI 0.81 to 0.98; P value<0.001]; OR 2.79 per mmol/L [95% CI 1.96 to 4.98; P value<0.001]) (Figure 3, P value for sex difference <0.001 for HbA1c and 0.005 for diabetes), suggesting HbA1c might underestimate glycemia in men compared with women. Findings were similar when using other analytic methods and including potentially pleiotropic SNPs, despite wider confidence intervals (Supplemental Table S4, S7).
Figure 3.
Mendelian randomization estimates for sex-specific associations of genetically predicted fasting glucose (73,089 women/67,506 men; MAGIC) with (a) HbA1c (185,022 women/159,160 men; UK Biobank) and (b) diabetes (cases=6,589 women/10,686 men, controls=187,137 women/155,780 men; UK Biobank).
a. The contamination mixture method was used.
b. Estimates are expressed in mmol/L for fasting glucose, in standard deviation for HbA1c, and in odds ratio for risk of diabetes.
Sex-specific associations of fasting glucose, HbA1c and liability to diabetes with life years
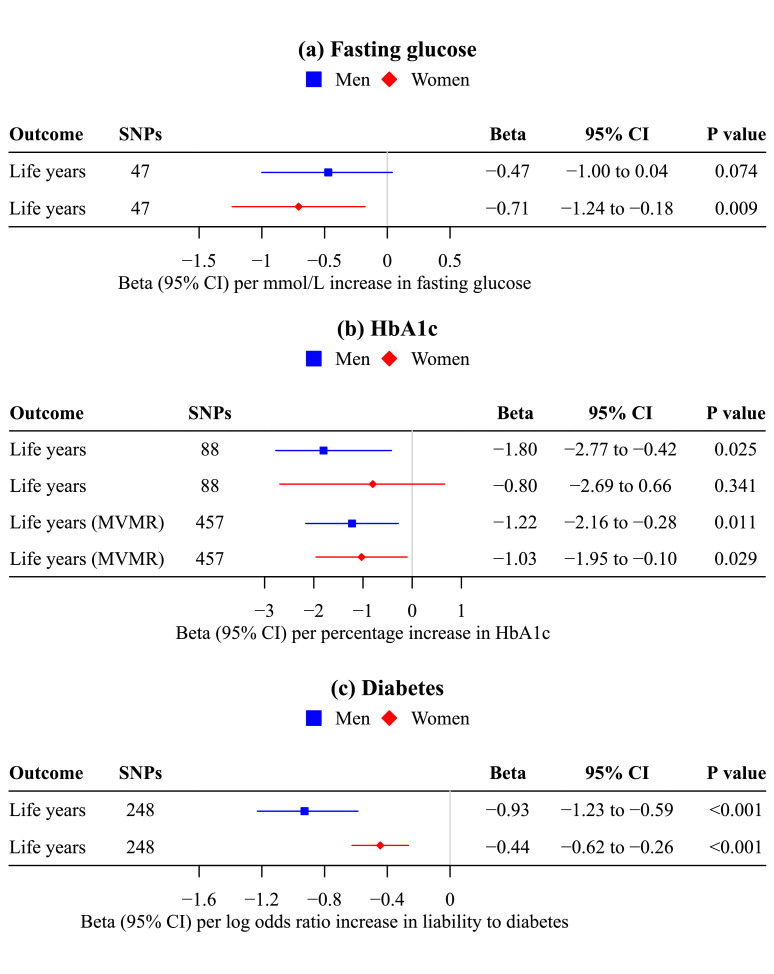
Genetically predicted fasting glucose was inversely associated with life years for women (-0.71 years per mmol/L [95% CI -1.24 to -0.18; P value 0.009]), and possibly men (-0.47 years per mmol/L [95% CI -1.00 to 0.04; P value 0.074]). The associations did not differ by sex (Figure 4, P value for sex difference 0.53), with a pooled estimate of -0.59 life years per mmol/L [95% CI -0.96 to -0.22; P value 0.002].
Figure 4.
Mendelian randomization estimates for sex-specific associations of genetically predicted (a) fasting glucose (n=140,595; MAGIC), (b) HbA1c (n=146,806; MAGIC) and (c) liability to diabetes (74,124 cases/824,006 controls; DIAMANTE) with life years (412,937 women/415,311 men; Pilling LC, et al.).
a. The contamination mixture method was used in univariable Mendelian randomization; multivariable Lasso was used to assess the direct effect of HbA1c adjusted for hemoglobin.
b. Estimates are expressed in mmol/L for fasting glucose, in percentage for HbA1c, in log odds ratio for liability to diabetes, and in life years. Life years gained were estimated by multiplying the Martingale residuals by -2.2869*10/-2.5863*10 in fathers/mothers.24
In univariable MR, genetically predicted HbA1c was inversely associated with life years for men (-1.80 years per percentage [95% CI -2.77 to -0.42, P value 0.025]) but not women (-0.80 years per percentage [95% CI -2.69 to 0.66; P value 0.341]). After adjusting for hemoglobin, the direct effect of HbA1c was similarly associated with fewer life years for men and women (Figure 4), which confirmed the role of glucose in life years. The conditional F-statistics were 16.4 and 38.4 for HbA1c and hemoglobin, respectively. The Q-statistics suggested possible pleiotropy (P value <0.05), substantiating the use of multivariable Lasso.
Genetic liability to diabetes was more strongly associated with fewer life years for men (-0.93 years per logOR [95% CI -1.23 to -0.59; P value <0.001]) than women (-0.44 years per logOR [95% CI -0.62 to -0.26; P value <0.001]) (Figure 4, P value for sex difference 0.01). These associations were robust to sensitivity analyses using other analytic methods and including potentially pleiotropic SNPs, but had wider confidence intervals (Supplemental Tables S5, S8).
Discussion
Our MR study confirmed previous studies suggesting erythrocyte properties affect HbA1c independent of glucose.4,5 We added by showing that HbA1c underestimates fasting glucose in men compared with women, possibly driven by higher iron in men.9 Using parental attained age as a measure of mortality, which reduces selection bias and increases statistical power, we showed that fasting glucose and HbA1c were associated with shorter lives, in contrast to previous MR studies.12,13 Moreover, HbA1c and liability to diabetes were possibly more strongly associated with mortality in men than women.
Iron and hemoglobin were inversely associated with HbA1c, but were not associated with fasting glucose. Consistently, iron deficiency anemia spuriously increases HbA1c at the same level of glucose.5 Iron altering erythrocyte properties and thereby HbA1c moderates the association of glucose with HbA1c. At the same level of glucose, HbA1c levels are higher in groups with lower iron, and lower in groups with higher iron, which means that HbA1c reflects different levels of glycemia in different groups.
We found stronger associations of fasting glucose with HbA1c and diagnosed diabetes in women than men, suggesting HbA1c represents a higher level of glycemia in men than women. Our findings are consistent with previous studies showing men have a lower hemoglobin glycation index (HGI) than women, which indicates lower HbA1c than that predicted by glucose.35,36 This difference could be due to higher iron levels in men,9 and thereby lower HbA1c than women for a given level of glycemia. HbA1c underestimating fasting glucose in men might lead to underdiagnosis and undertreatment of diabetes in men compared with women, because men diagnosed with diabetes based on HbA1c have a substantially higher level of glycemia, and thereby could be more vulnerable to complications than women. Consistently, men with type 2 diabetes are more likely to have solitary high fasting glucose (fasting glucose ≥140 mg/dL and HbA1c <7%),37 and microvascular complications38 than women.
Fasting glucose was similarly associated with fewer life years in men and women, which is inconsistent with previous MR studies suggesting little impact of glucose on mortality.12,13 However, these previous studies had a smaller sample size,12 or assessed mortality from age at death,13 which excludes people remaining alive and generates selection bias.14 HbA1c was inversely associated with life years in men but not women, possibly due to different effects of glycemia by sex, or HbA1c representing different levels of glycemia by sex. After adjusting for hemoglobin, a marker of erythrocyte properties, the direct effect of HbA1c reduced life years similarly in men and women, suggesting that glucose has similar effects in women and men, and the issue is differing levels of hemoglobin by sex affecting the level of glycemia represented by HbA1c, and thereby consequences for mortality.
Our finding that liability to diabetes was more strongly associated with years of life lost in men than women is inconsistent with an observational study showing women with diabetes have a higher risk of all-cause mortality than men, particularly from coronary heart disease (CHD).39 However, a recent MR study suggested that the causal effect of liability to diabetes on CHD is not stronger in women than men.40 Furthermore, comparing relative risks without considering the underlying absolute risk is invalid. Diabetes confers a substantially higher absolute risk of CHD in men than women even at the same relative risk, given the higher prevalence of CHD in men. As such, HbA1c underestimating fasting glucose in men may be affecting the diagnosis and treatment of diabetes, and thereby resulting in the stronger association of liability to diabetes with mortality in men than women. Such differences could be offset by undertreatment of hyperglycemia and other cardiovascular risk factors in women.41
Iron levels moderating the association of fasting glucose with HbA1c are relevant to the use of HbA1c as diagnostic and treatment criteria for diabetes in different populations. Iron levels vary not only by sex, but also by ethnicity and age.9 HbA1c overestimates glycemia in African Americans, Hispanics and Asians compared with non-Hispanic whites,6,7 which could be explained by higher prevalence of iron deficiency anemia in these populations.42 HbA1c may overestimate glycemia in children, given their lower iron levels than adults.9 However, HbA1c criteria used to test for prediabetes or type 2 diabetes in children and adolescents are extrapolated from those in adults.43 We cannot exclude the possibility that other factors influencing erythrocyte lifespan4 also moderate the relation of fasting glucose with HbA1c. HbA1c underestimates glycemia in people with sickle cell traits or hemoglobin E.44,45 Notably, sickle cell disease and hemoglobin E result in iron overload.46,47
HbA1c representing different levels of glycemia by population might lead to different consequences of glycemic control based on managing HbA1c. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, intensive treatment targeting lower HbA1c decreased major cardiovascular events in low and moderate HGI participants with type 2 diabetes, but not in the high HGI participants.35 The risk of hypoglycemia was greatest in high HGI subgroup,35 which may indicate possible overtreatment.
There are several limitations of this study. First of all, MR should fulfill three rigorous assumptions, that is relevance, independence and exclusion restriction.10 To satisfy the relevance assumption, we checked the F-statistics and conditional F-statistics were >10, suggesting little weak instrument bias. To address the independence and exclusion-restriction assumption, we checked whether genetic variants were associated with potential confounders or located on ABO, a well-known pleiotropic gene. We assessed violation of the exclusion-restriction assumption using the MR Egger intercept in univariable MR and the Q-statistic in multivariable MR. The Q-statistic detects potential pleiotropy in the form of excessive heterogeneity in multivariable MR, and has inflated type 1 error in the presence of weak instruments,32 which was not the case here. We used analytic methods robust to pleiotropy as the main analyses, and conducted sensitivity analyses with different underlying assumptions. Second, we obtained genetic predictors for hemoglobin from the same study (i.e., the UK Biobank) as sex-specific genetic associations with HbA1c. We obtained genetic predictors for liability to diabetes from DIAMANTE which overlaps with the UK Biobank from which the GWAS of all-cause mortality was obtained. Nevertheless, two-sample MR methods in a one-sample setting perform well in terms of bias and precision in large biobanks, except for MR Egger which can be biased in the direction and magnitude of the confounding if the variability of instrument strength indicated by I2GX is less than about 97%, which was the case here.48 Third, we extracted sex-combined genetic instruments for serum iron and applied them to derive sex-specific MR estimates for the association with HbA1c, which might limit the detection of sex differences. Fourth, MR can be open to selection bias, particularly from recruiting survivors.49 However, the participants were relatively young likely obviating selective survival to recruitment on genetic endowment and glycemic indicators. We obtained genetic associations with fasting glucose and HbA1c from MAGIC including only individuals without diabetes,19,20 which might underestimate these associations. Fifth, population stratification might affect MR estimates. The studies used were undertaken in people of European descent with genomic control. As such, these associations might not apply to other populations. Although causes should be consistent across settings,50 the effect sizes might vary by population. For example, the association of fasting glucose with HbA1c varies by group according to iron level. Thus, replication in other populations, such as Asians, Hispanics and African American would be worthwhile. Sixth, we obtained sex-specific genetic associations with diagnosed diabetes rather than specifically type 2 diabetes from the UK Biobank, which could result in imprecision but is unlikely to change the estimates substantially. Seventh, although we used the largest available GWAS giving sex-specific genetic associations, the number of cases of diabetes was relatively low. Larger samples are necessary to confirm these sex-specific associations in the future. We did not consider non-linear associations in this MR study, because a majority of observational studies suggested linear associations of glucose with HbA1c,51,52 and of HbA1c with all-cause mortality,53,54 although some previous studies suggested U- or J-shaped relations,55,56 which could be an indicator of confounding or selection bias.57 A recent MR study suggested a linear causal association of HbA1c with coronary artery disease,15 the leading cause of mortality. However, we cannot exclude the possibility that causal associations between these variables might be non-linear by gender.
Our MR study suggested that HbA1c, a currently accepted clinical measure of glycemia, underestimates fasting glucose in men compared with women, possibly due to erythrocyte properties. Diagnostic and management criteria based on HbA1c may result in HbA1c and liability to diabetes reducing lifespan more in men than women. These insights highlight the importance of using measures that reflect the same level of glycemia equitably in different populations in the diagnosis, treatment and management of diabetes.
Contributors
C.M.S. identified the study question and contributed to the study design. G.Y.Y contributed to the study design, analyzed the data and wrote the manuscript. C.M.S and S.L.A.Y reviewed and edited the manuscript. G.Y.Y is the guarantor of this work, and takes responsibility for the integrity of the data analysis. C.M.S and G.Y.Y have verified the underlying data. All authors read and approved the final version of the manuscript.
Data sharing statement
Summary-level data analyzed during the current study are available in the website https://www.decode.com/summarydata/ for GWAS of serum iron, http://www.nealelab.is/uk-biobank/ for UK Biobank (Neale lab), https://magicinvestigators.org/downloads/ for MAGIC, and http://diagram-consortium.org/downloads.html for DIAMANTE, and https://www.ebi.ac.uk/gwas/ for GWAS of parent's attained age.
Declaration of interests
The authors declared no conflict of interest.
Acknowledgements
The authors acknowledge the UK Biobank, MAGIC, DIAMANTE, Bell S, et al. and Pilling LC, et al. for their publicly available summary data. No funding was obtained.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104259.
Appendix. Supplementary materials
References
- 1.American Diabetes Association Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S15–s33. doi: 10.2337/dc21-S002. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Glycemic targets: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S73–s84. doi: 10.2337/dc21-S006. [DOI] [PubMed] [Google Scholar]
- 3.Wilson DM, Xing D, Cheng J, et al. Persistence of individual variations in glycated hemoglobin: analysis of data from the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Randomized Trial. Diabetes Care. 2011;34(6):1315–1317. doi: 10.2337/dc10-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen RM, Franco RS, Khera PK, et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;112(10):4284–4291. doi: 10.1182/blood-2008-04-154112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.English E, Idris I, Smith G, Dhatariya K, Kilpatrick ES, John WG. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia. 2015;58(7):1409–1421. doi: 10.1007/s00125-015-3599-3. [DOI] [PubMed] [Google Scholar]
- 6.Bergenstal RM, Gal RL, Connor CG, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95–102. doi: 10.7326/M16-2596. [DOI] [PubMed] [Google Scholar]
- 7.Wolffenbuttel BH, Herman WH, Gross JL, Dharmalingam M, Jiang HH, Hardin DS. Ethnic differences in glycemic markers in patients with type 2 diabetes. Diabetes Care. 2013;36(10):2931–2936. doi: 10.2337/dc12-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarallo P, Humbert JC, Mahassen P, Fournier B, Henny J. Reticulocytes: biological variations and reference limits. Eur J Haematol. 1994;53(1):11–15. doi: 10.1111/j.1600-0609.1994.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 9.Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372(19):1832–1843. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- 10.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 11.Huang SY, Yang YX, Chen SD, et al. Investigating causal relationships between exposome and human longevity: a Mendelian randomization analysis. BMC Med. 2021;19(1):150. doi: 10.1186/s12916-021-02030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Oort S, Beulens JWJ, van Ballegooijen AJ, Burgess S, Larsson SC. Cardiovascular risk factors and lifestyle behaviours in relation to longevity: a Mendelian randomization study. J Intern Med. 2021;289(2):232–243. doi: 10.1111/joim.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaue S, Kanai M, Karjalainen J, et al. Trans-biobank analysis with 676,000 individuals elucidates the association of polygenic risk scores of complex traits with human lifespan. Nat Med. 2020;26(4):542–548. doi: 10.1038/s41591-020-0785-8. [DOI] [PubMed] [Google Scholar]
- 14.Sanderson E, Glymour MM, Holmes MV, et al. Mendelian randomization. Nat Rev Methods Prim. 2022;2(1):6. doi: 10.1038/s43586-021-00092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo S, Au Yeung SL, Schooling CM. Assessing the linear and non-linear association of HbA(1c) with cardiovascular disease: a Mendelian randomisation study. Diabetologia. 2021;64(11):2502–2510. doi: 10.1007/s00125-021-05537-w. [DOI] [PubMed] [Google Scholar]
- 16.Leong A, Chen J, Wheeler E, et al. Mendelian randomization analysis of hemoglobin A(1c) as a risk factor for coronary artery disease. Diabetes Care. 2019;42(7):1202–1208. doi: 10.2337/dc18-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell S, Rigas AS, Magnusson MK, et al. A genome-wide meta-analysis yields 46 new loci associating with biomarkers of iron homeostasis. Commun Biol. 2021;4(1):156. doi: 10.1038/s42003-020-01575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bycroft C, Freeman C, Petkova D, et al. The UK biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagou V, Mägi R, Hottenga JJ, et al. Sex-dimorphic genetic effects and novel loci for fasting glucose and insulin variability. Nat Commun. 2021;12(1):24. doi: 10.1038/s41467-020-19366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Spracklen CN, Marenne G, et al. The trans-ancestral genomic architecture of glycemic traits. Nat Genet. 2021;53(6):840–860. doi: 10.1038/s41588-021-00852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahajan A, Taliun D, Thurner M, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50(11):1505–1513. doi: 10.1038/s41588-018-0241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lloyd-Jones LR, Robinson MR, Yang J, Visscher PM. Transformation of summary statistics from linear mixed model association on all-or-none traits to odds ratio. Genetics. 2018;208(4):1397–1408. doi: 10.1534/genetics.117.300360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilling LC, Kuo CL, Sicinski K, et al. Human longevity: 25 genetic loci associated in 389,166 UK biobank participants. Aging. 2017;9(12):2504–2520. doi: 10.18632/aging.101334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timmers PR, Mounier N, Lall K, et al. Genomics of 1 million parent lifespans implicates novel pathways and common diseases and distinguishes survival chances. eLife. 2019;8 doi: 10.7554/eLife.39856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45(6):1961–1974. doi: 10.1093/ije/dyw220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted Median estimator. Genet Epidemiol. 2016;40(4):304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgess S, Foley CN, Allara E, Staley JR, Howson JMM. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat Commun. 2020;11(1):376. doi: 10.1038/s41467-019-14156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slob EAW, Burgess S. A comparison of robust Mendelian randomization methods using summary data. Genet Epidemiol. 2020;44(4):313–329. doi: 10.1002/gepi.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grant AJ, Burgess S. Pleiotropy robust methods for multivariable Mendelian randomization. Stat Med. 2021;40(26):5813–5830. doi: 10.1002/sim.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanderson E, Spiller W, Bowden J. Testing and correcting for weak and pleiotropic instruments in two-sample multivariable Mendelian randomization. Stat Med. 2021;40(25):5434–5452. doi: 10.1002/sim.9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chesmore K, Bartlett J, Williams SM. The ubiquity of pleiotropy in human disease. Hum Genet. 2018;137(1):39–44. doi: 10.1007/s00439-017-1854-z. [DOI] [PubMed] [Google Scholar]
- 34.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hempe JM, Liu S, Myers L, McCarter RJ, Buse JB, Fonseca V. The hemoglobin glycation index identifies subpopulations with harms or benefits from intensive treatment in the ACCORD trial. Diabetes Care. 2015;38(6):1067–1074. doi: 10.2337/dc14-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsia DS, Rasouli N, Pittas AG, et al. Implications of the hemoglobin glycation index on the diagnosis of prediabetes and diabetes. J Clin Endocrinol Metab. 2020;105(3):e130–e138. doi: 10.1210/clinem/dgaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rathmann W, Bongaerts B, Kostev K. Association of characteristics of people with type 2 diabetes mellitus with discordant values of fasting glucose and HbA1c. J Diabetes. 2018;10(12):934–941. doi: 10.1111/1753-0407.12823. [DOI] [PubMed] [Google Scholar]
- 38.Maric-Bilkan C. Sex differences in micro- and macro-vascular complications of diabetes mellitus. Clin Sci. 2017;131(9):833–846. doi: 10.1042/CS20160998. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, O'Neil A, Jiao Y, et al. Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: a systematic review and meta-analysis of 5,162,654 participants. BMC Med. 2019;17(1):136. doi: 10.1186/s12916-019-1355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters TM, Holmes MV, Richards JB, et al. Sex differences in the risk of coronary heart disease associated with type 2 diabetes: a mendelian randomization analysis. Diabetes Care. 2021;44(2):556–562. doi: 10.2337/dc20-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao M, Vaartjes I, Graham I, et al. Sex differences in risk factor management of coronary heart disease across three regions. Heart. 2017;103(20):1587–1594. doi: 10.1136/heartjnl-2017-311429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615–624. doi: 10.1182/blood-2013-06-508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care. 2010;33(3):562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lacy ME, Wellenius GA, Sumner AE, et al. Association of sickle cell trait with hemoglobin A1c in African Americans. JAMA. 2017;317(5):507–515. doi: 10.1001/jama.2016.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem. 2001;47(2):153–163. [PubMed] [Google Scholar]
- 46.Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390(10091):311–323. doi: 10.1016/S0140-6736(17)30193-9. [DOI] [PubMed] [Google Scholar]
- 47.Taher AT, Weatherall DJ, Cappellini MD. Thalassaemia. Lancet. 2018;391(10116):155–167. doi: 10.1016/S0140-6736(17)31822-6. [DOI] [PubMed] [Google Scholar]
- 48.Minelli C, Del Greco MF, van der Plaat DA, Bowden J, Sheehan NA, Thompson J. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int J Epidemiol. 2021;50:1651–1659. doi: 10.1093/ije/dyab084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schooling CM, Lopez PM, Yang Z, Zhao JV, Au Yeung SL, Huang JV. Use of multivariable Mendelian randomization to address biases due to competing risk before recruitment. Front Genet. 2020;11 doi: 10.3389/fgene.2020.610852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez PM, Subramanian SV, Schooling CM. Effect measure modification conceptualized using selection diagrams as mediation by mechanisms of varying population-level relevance. J Clin Epidemiol. 2019;113:123–128. doi: 10.1016/j.jclinepi.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the diabetes control and complications trial. Diabetes Care. 2002;25(2):275–278. doi: 10.2337/diacare.25.2.275. [DOI] [PubMed] [Google Scholar]
- 53.Khaw KT, Wareham N, Luben R, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of european prospective investigation of cancer and nutrition (EPIC-Norfolk) BMJ. 2001;322(7277):15–18. doi: 10.1136/bmj.322.7277.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levitan EB, Liu S, Stampfer MJ, et al. HbA1c measured in stored erythrocytes and mortality rate among middle-aged and older women. Diabetologia. 2008;51(2):267–275. doi: 10.1007/s00125-007-0882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carson AP, Fox CS, McGuire DK, et al. Low hemoglobin A1c and risk of all-cause mortality among US adults without diabetes. Circul Cardiovasc Qual Outcomes. 2010;3(6):661–667. doi: 10.1161/CIRCOUTCOMES.110.957936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362(9):800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rutter MK. Low HbA1c and mortality: causation and confounding. Diabetologia. 2012;55(9):2307–2311. doi: 10.1007/s00125-012-2620-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.