Abstract
Purpose: This research determines the effect of sodium lauryl sulfate (SLS) as a surfactant, croscarmellose sodium (CS) as a disintegrating agent, and SLS–CS combinations on 2-((3-(chloromethyl)benzoyl)oxy)benzoic acid (3CH2Cl) (log P = 3.73) tablet formulations. In addition, this study aims to determine the optimum of the 3CH2Cl tablet formula. Methods: The tablets are manufactured through direct compression according to the simplex lattice design. The optimal SLS and CS concentration was determined in vitro using linear and quadratic models to achieve better tablet disintegration and dissolution. Results: The same linear and quadratic coefficient profiles of SLS and CS indicate that the combined coefficient of SLS–CS with a quadratic model can be used to predict the effect of the SLS–CS combination. Based on the linear model coefficients, SLS and CS increase the value of flow time (9.35; 7.65), Carr index (26.17; 21.17), hardness (9.84; 7.44), friability (0.38; 0.31), disintegrating time (5.74; 2.62), and drug release (84.28; 58.65). The quadratic model coefficient shows that SLS–CS combinations increase flow time (0.60), Carr index (2.00), hardness (1.00), and disintegrating time (1.04). Meanwhile, they decrease friability (−0.02) and drug release (−9.10). Conclusions: SLS, CS, and SLS–CS combinations affect the quality of tablet mass and tablets. The optimum tablet formula was 3CH2Cl (300 mg), Ne (9.38%), SLS (0.92%), CS (2.33%), MCC (5%), and SDL (ad 800 mg). 3CH2Cl has analgesic activity despite the presence of tablet excipients. The 3CH2Cl tablet is an innovative formulation and a new alternative for future analgesic drugs.
Introduction
2-((3-(Chloromethyl)benzoyl)oxy)benzoic acid (3CH2Cl) is a new compound synthesized from acetylsalicylic acid and 3-chloromethyl benzoyl chloride.1,2 3CH2Cl has very good potential as an analgesic, an antiplatelet aggregation, and an anti-inflammation drug.1,2 A previous study reported that the active compound of 3CH2Cl could significantly reduce the nociceptive response in mice.2 The Cmax value of 3CH2Cl is 0.57 μg/mL. It indicates the ability of 3CH2Cl to distribute and perfuse widely into the very deep interstitial and intracellular parts of the tissue. However, the lipophilic value of 3CH2Cl (log P) is 3.73.3 While the optimal log P value of the water-soluble compound during tablet formulation is theoretically between 2 and 3,4 the reported log P value of 3CH2Cl indicates the difficulty of solubility in water.
The tablet form is one of the candidates in 3CH2Cl formulation. Tablets may inhibit the hygroscopic character of 3CH2Cl when stored. Tablet formulation requires the addition of several compounds such as surfactants to overcome the lipophilic character of 3CH2Cl. Tablet formulation also needs a disintegrant to accelerate the dissociation of 3CH2Cl. The surfactants and disintegrating agents commonly used in the tablet formulation are sodium lauryl sulfate (SLS) and croscarmellose sodium (CS). SLS has several characteristics, such as the hollow surface of the particles, ease of solubility, and slight fatty.5 The surface of CS has several characteristics such as a thread-root-like form, water insolubility, and rapid swelling when hydrated.5 SLS is expected to accelerate the hydration of the tablet surface and increase the solubility of 3CH2Cl and excipient particles. CS is expected to accelerate the disintegration of tablets. However, the effect and the optimal amount of SLS and CS for the tablet formulation of 3CH2Cl remain unclear. The novelty of this experiment is the tablet formulation of 3CH2Cl using SLS as a surfactant and CS as a disintegrating agent in tablets. SLS and CS overcome 3CH2Cl lipophilic problems on tablet disintegration and dissolution. This formulation is an innovation of 3CH2Cl made in tablets and new alternatives to future analgesic drugs.
This experiment aimed to determine the effect of SLS, CS, and SLS–CS combinations for the formulation of 3CH2Cl tablets. In addition, this study aims to determine the optimum of the 3CH2Cl tablet formula. The effect of SLS, CS, and SLS–CS combinations was analyzed using linear and quadratic models following the simplex lattice design. It is believed that CS can accelerate the disintegration of tablets, while SLS increases hydrated tablets and the solubility of 3CH2Cl. This manuscript demonstrated that the tablet form of 3CH2Cl using CS and SLS exerts an analgesic activity in mice writhing tests. The 3CH2Cl tablet provides a new form of drug, which has a potential for an analgesic drug.
Material and Methods
Raw Materials and Chemicals
Experiments using quality materials such as pro-analytical (p.a.), pharmaceutical grade (p.g.), and food grade (f.g.): salicylic acid (p.g.) (PT. Brataco, Indonesia), 3-chloromethyl benzoyl chloride (p.a.) (Sigma-Aldrich, GmbH, USA), pyridine (p.a.) (Merck KgaA, Darmstadt, Germany), ethanol (p.a.) (Merck KgaA, Darmstadt, Germany), magnesium aluminometasilicate (Neusilin) (p.g.) (Gangwal Chemicals, India), croscarmellose sodium (p.g.) (FMC Biopolymer, USA), microcrystalline cellulose (p.g.) (Flocel 102, Gujarat Microwax Pvt. Ltd, India), spray-dried lactose (p.g.) (Foremost Farms, USA), sodium hydroxide (p.a.) (Merck KgaA, Darmstadt, Germany), potassium dihydrogen phosphate (p.a.) (Merck KgaA, Darmstadt, Germany), and distilled water (f.g.) (Brataco Chemical, Indonesia).
Synthesis and Characterization of 3CH2Cl
Salicylic acid (1.8 mmol), 3-chloromethyl benzoyl chloride (7.2 mmol), pyridine (1.7 × 10–6 mmol), and acetone (14.8 × 10–6 mmol) were mixed homogeneously in an Erlenmeyer flask. The mixture was microwave-irradiated for 5 min with a Millstone Organic Synthesis Unit (MicroSYNTH). The mixture was then placed in a microwave oven (600 Watt, 1 min). Afterward, the mixture was evaluated with ferric chloride (FeCl3) and thin-layer chromatography (TLC) (silica gel F254 stationary phase and n-hexane:ethanol (1:2) mobile phase). This test is to identify the salicylic acid in the mixture. At the beginning, pasta was prepared and then turned into a solution when irradiated by microwaves, and the final product was solid. The synthesis procedure followed the previous experiment, and the stability of 3CH2Cl was proven.1,2 Based on these reasons, the compound 3CH2Cl can be used for tablet formulation.
Preparation of Tablets
The tablet ingredients were weighted using the formula (Table 1) and the direct compression method. The process began by mixing 3CH2Cl with Ne using a mortar and stamper until homogeneous. The mixture was transferred to a cubic mixer and added with SLS, CS, MCC, and SDL to rotate for 2 min at 100 rpm (Erweka). The homogeneous tablet mass was tested for flowability and compressibility. The homogeneous tablet mass was compressed to form tablets (800 mg) with a single punch machine (Jenn Chian Machinery, Taiwan). Tablets were evaluated for hardness, friability, disintegration time, and drug dissolution.
Table 1. Detailed of Experimental Formula and Prediction of the Optimum Formula.
| component | unit | formula |
|||
|---|---|---|---|---|---|
| TA | TB | TC | T Opt. | ||
| 3CH2Cl | mg | 300.00 | 300.00 | 300.00 | 300.00 |
| Ne | % | 9.38 | 9.38 | 9.38 | 9.38 |
| mg | 75.00 | 75.00 | 75.00 | 75.00 | |
| SLS | % | 0.50 | 0.75 | 1.00 | 0.92 |
| mg | 4.00 | 6.00 | 8.00 | 7.36 | |
| CS | % | 4.00 | 3.00 | 2.00 | 2.33 |
| mg | 32.00 | 24.00 | 16.00 | 18.64 | |
| MCC | % | 5.00 | 5.00 | 5.00 | 5.00 |
| mg | 20.00 | 20.00 | 20.00 | 20.00 | |
| SDL ad | mg | 800.00 | 800.00 | 800.00 | 800.00 |
Flow Time
The mass of the tablet was weighed at 100 g and placed on a flowability tester funnel (Erweka, Germany). The funnel valve opened to drain the tablet mass and determine the flow time parameter. The cone of the tablet mass was scanned by infrared to determine the parameter of the angle of repose.
Compressibility
A glass measuring tube (100 mL) was weighted and recorded. Then, the tablet mass was inserted to a glass measuring tube (100 mL) inclined (35°–40°). The glass measuring tube filled with the tablet mass was weighed and recorded. The glass measuring tube loaded with the tablet mass was placed on a density tap volumeter (Erweka, Germany) and tapped 500 times. The initial and final volumes of the tablet mass were recorded to determine the bulk density and tap density. Bulk density is the ratio of the tablet mass to the initial volume, while tap density is the ratio between the tablet mass and volume. Determination of the Carr index value follows eq 1.6
| 1 |
Hardness
Tablets (6) were randomly selected from all tablets7,8 and placed in a hardness tester (Schleuniger, Netherlands). The tablet was pressed by a metal rod until the tablet cracked or broke. The hardness of the tablet can be read on a monitor hardness tester.
Friability
Tablets were randomly selected up to a total weight of more than 6500 mg.7,8 All tablets were dust-free for careful weighing (W0). The tablets were rotated on a drum friability tester (Erweka, Germany) for 4 min at 25 rpm. The tablets were dust-free and carefully reweighed (W1). The value of tablet friability is the difference between the total weight of the initial tablet and the total weight of the final tablet compared to the total weight of the initial tablet. Determination of the friability value follows eq 2.
| 2 |
Disintegration Time
Tablets (18) were selected, and six of which were randomly selected.7,8 Tablets were placed in each tube of the disintegration tester (Erweka Z3, Germany). The cylinder moved up and down in the chamber containing distilled water at 37 °C and 900 mL. Disintegration time is the time required by six tablets for no particles/fragments to remain in the mesh in each tube.
Dissolution
Each tablet was placed in a vessel of a dissolution tester (Electrolab TDT-08L, India) containing phosphate buffer medium pH 6.8 (37 ± 0.5 C; 50 rpm; 900 mL) using the basket method for 60 min.9,10 A sampling of the release of 3CH2Cl (5 mL) was done at 10, 20, 30, 45, and 60 min. The concentration of the dissolved active compound was analyzed using a UV–vis spectrophotometer (Hitachi U-1900, Japan) at the maximum wavelength.
Optimization
The optimization of the tablet formula was generated using the simplex lattice design with a two-factor method. The working concentration of SLS is 0.5–1% and CS is 2–4%. The experiment used three formulas (Table 1) with a proportion of 0.50:4.00 (called TA), TB 0.75:3.00 (called TB), TC 1.00:2.00 (called TC), 0.92:2.33 (T Opt.), flow time, Carr index, hardness, disintegrating time, and drug release were used as the optimization parameters. The optimization response was analyzed in silico (Design Expert ver.10) to predict the tablet formula of 3CH2Cl.
Release Kinetics of 3CH2Cl from the Tablet
The release kinetics of 3CH2Cl from each tablet formula was analyzed using the following eqs 3456:11−14
| 3 |
Qt is the amount of drug dissolved at time (t) [mg], Qo is the initial drug [mg], and Ko is the constant drug release [mg/min–1].
| 4 |
Qt is the amount of drug [mg], KH is the Higuchi constant [mg/min1/2], and t is time [min].
| 5 |
Qt/Q∞ is the fraction of drug released [mg], Kk is the Korsmeyer–Peppas constant [mg/min–1], and n is the diffusion exponential.
| 6 |
(1 – m) is the fraction of insoluble drug [mg], Ti is the lag time before dissolution, a is the initial fraction of drug [mg], b is the shape parameter obtained from the slope of the obtained curve.
The release kinetics of 3CH2Cl from each tablet formula was analyzed using DDSolver software.
Analgesic Activity by the Writhing Test
In this study, 2–3 months old male mice (Mus musculus) weighing about 20–25 g were used to measure the analgesic activity. The writhing test consisted of control groups, active compound groups, and comparator groups. Each group consisted of six mice. Pain was generated using intraperitoneal injection (0.01 mL/g body weight) of 0.6% acetic acid.15 The successful induction of pain was characterized by writhing reactions in mice, such as stretching, the extension of the hind legs, and stomach contraction. For the negative control, mice were given a mixture of excipient and 3% PGA orally, followed by intraperitoneal acetic acid injection after 30 min. The active compound (1.23 mg/20 g body weight) or the comparator (2.05 mg/20 g body weight) was given in another group. The writhing behavior was observed within 10 min.
Results and Discussion
Characterization of 3CH2Cl
Infrared spectra show the ester peak C=O at 1732.10 cm–1, while the peak C–O at 1298.22; 1279.16; and 1262.18 cm–1. The carboxylate peak C=O appeared at 1694.90 cm–1, while C–O at 1262.18 cm–1. The peak C=C was aromatic at 1606.29 cm–1, and the peak C–Cl at 704.24 cm–1. The Rf value of the thin-layer chromatography 3CH2Cl compound in the mobile phase of ethyl acetate:ethanol (1:2) is 0.91; n-hexane:ethanol (1:2) is 0.82; and chloroform:ethanol (4:1) is 0.87. The melting point value of 3CH2Cl is at 109–111 °C.
Formulation of 3CH2Cl Tablets
The 3CH2Cl tablet formula used excipients Ne, SLS, CS, MCC, and SDL. Ne was used to prevent the coagulation of 3CH2Cl.16−18 The SLS–CS combination improved the flowability of 3CH2Cl. SLS can accelerate the tablet hydration through disintegration or dissolution media. SLS also lowered the surface tension of 3CH2Cl particles with a hydrating medium, thereby accelerating the solubility of the particles.19−21 CS can swell when interacting with a hydrating medium so that the surrounding particles were pushed, resulting in tablet disintegration.22−24 The MCC was used as a tablet filler for excellent tablet compactibility, while SDL was used as a high-density filler to adjust the tablet with optimal thickness.25−27 Both MCC and SDL were ideal excipients for the direct compress method.
Determining the Flow Time Value of the 3CH2Cl Tablet Mass
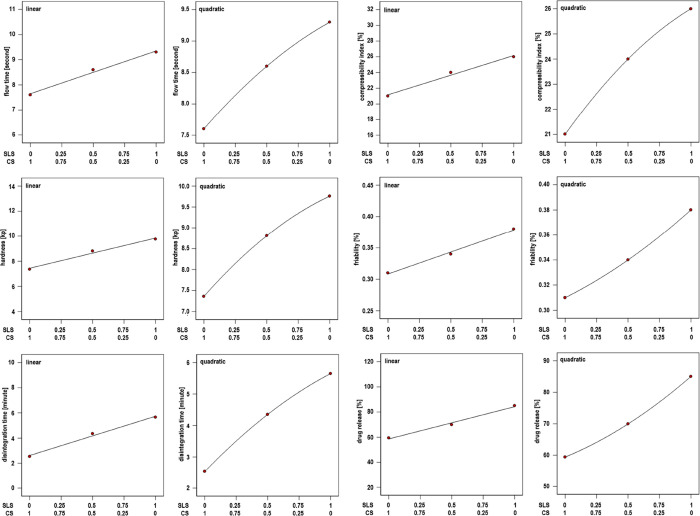
The flow time values of the three tablet mass formulas are shown in Table 2. All formulas have a flow time value of less than 10 s. It means the tablet mass can move freely and fill the tablet machine dies.6 The TA (7.63 s) formula has the fastest flow time, followed by TB (8.60 s) and TC (9.33 s) formulas. The coefficient value (Table 3) from the simplex lattice design method with a linear model shows that SLS (9.35) was dominant in increasing tablet mass flow time, followed by CS (7.65). The linear model coefficient is acceptable based on statistical analysis (see Supporting Information Table S1). The ANOVA results from the quadratic model show a coefficient profile similar to the linear model, where the coefficients of SLS (9.30) and CS (7.60) increased the tablet mass flow time. Through a quadratic model, the combination coefficient of SLS–CS (0.60) shows that the SLS–CS combinations increased the flow time, but the SLS–CS combinations were not as dominant as SLS and CS.
Table 2. Evaluation of Tablet Mass, Tablets, and Dissolution on Formulations of 3CH2Cla.
| tablet | SLS | CS | flow
time |
Carr index | hardness |
friability | disintegrating time | drug
release |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| code | [mg] | [mg] | [s] | SD | [%] | [kp] | SD | [%] | [min] | [%] | SD |
| TA | 4.00 | 32.00 | 7.63 | 0.06 | 21.00 | 7.36 | 0.77 | 0.31 | 2.53 | 59.41 | 0.95 |
| TB | 6.00 | 24.00 | 8.60 | 0.10 | 24.00 | 8.81 | 0.97 | 0.34 | 4.35 | 69.95 | 1.00 |
| TC | 8.00 | 16.00 | 9.33 | 0.06 | 26.00 | 9.76 | 0.59 | 0.38 | 5.65 | 85.04 | 1.05 |
| T opt. | 7.36 | 18.64 | 9.10 | 0.10 | 25.00 | 9.48 | 0.52 | 0.36 | 5.25 | 81.53 | 0.86 |
| P opt. | 7.36 | 18.64 | 9.07 | 25.33 | 9.44 | 0.37 | 5.22 | 80.00 | |||
The quality evaluation of tablets mass, tablets, and dissolution of each formula containing SLS [%] and CS [%]: TA (0.50:4.00), TB (0.75:3.00), TC (1.00:2.00), and T Opt. (0.92:2.33).
Table 3. Polynomial Coefficient of Each Parameter Quality of Tablet Mass and Tabletsa.
| component | flow
time |
Carr
index |
hardness |
friability |
disintegrating
time |
drug
release |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| linear | quadratic | linear | quadratic | linear | quadratic | linear | quadratic | linear | quadratic | linear | quadratic | |
| SLS | 9.35 | 9.30 | 26.17 | 26.00 | 9.84 | 9.76 | 0.38 | 0.38 | 5.74 | 5.65 | 84.28 | 85.04 |
| CS | 7.65 | 7.60 | 21.17 | 21.00 | 7.44 | 7.36 | 0.31 | 0.31 | 2.62 | 2.53 | 58.65 | 59.41 |
| SLS–CS | 0.60 | 2.00 | 1.00 | –0.02 | 1.04 | –9.10 | ||||||
Polynomial coefficients according to the simplex lattice design with the linear and quadratic system. The tablet formula used contains SLS [%] and CS [%]: TA (0.50:4.00), TB (0.75:3.00), and TC (1.00:2.00).
The hollow form of SLS particles caused the surface of the particles to become rough, which might inhibit the movement and increase the flow time of the tablet mass. The shape of CS particles, such as thread roots, made the particles difficult to move and increases the flow time. In addition, this character can inhibit the movement of other particles of the tablet mass component. The screw root shape of the CS particles can fill the hollow of the SLS particles so that the combination particles have a flatter surface and reduce the resistance to movement of the tablet mass.
Determining the Carr Index Value of the 3CH2Cl Tablet Mass
The Carr index values of the three tablet mass formulas are shown in Table 2. The TA and TB formulas have a Carr index value of less than 25%, indicating that the tablet mass was good enough to flow and move slightly and to achieve a stable arrangement in the die chamber of the tablet machine. The TC formula has a Carr index value of more than 25%, indicating that the tablet mass can flow. The particles required more movement to achieve a stable arrangement in the tablet machine die space. The simplex lattice design-method linear model could generate the coefficient values as presented in Table 3. Meanwhile, SLS (26.17) was the most dominant in increasing the Carr index, followed by CS (21.17). The linear model coefficient was acceptable based on statistical analysis (see Supporting Information Table S1). Quadratic model ANOVA had a coefficient profile similar to the linear model. The coefficients of SLS (26.00) and CS (21.00) increased the Carr index of the tablet mass. The quadratic model resulted in the SLS–CS combination coefficient (2.00), showing that SLS–CS increases the Carr index. The SLS–CS combinations were less dominant than SLS and CS.
The hollow SLS particles caused brittle particles. Therefore, when particles were subjected to mechanical stress, the particles could break into smaller sizes. The small SLS particles were difficult to flow while producing much porosity in a stable arrangement. The screw root shape of CS particles caused the tablet mass to be difficult to move and have large porosity in a sturdy structure. The CS particles that fill the cavity of SLS particles can improve the surface morphology of the particles. Still, the remaining part of the CS particles outside the hollow can break into fine particles. Smaller CS particle size can inhibit tablet mass flow.
Determining the Hardness of 3CH2Cl Tablets
The tablet hardness of each formula is shown in Table 2. The TC tablets were the hardest than TA and TB tablets. TC formula tablets had the strongest interlocking between particles among other formula tablets. The simplex lattice design method with a linear model produced the coefficient values (Table 3), while SLS (9.84) was the most dominant in increasing the tablet hardness, followed by CS (7.44). The linear model coefficient was acceptable based on statistical analysis (see Supporting Information Table S1). The quadratic ANOVA model had a coefficient profile similar to the linear model. SLS (9.76) and CS (7.36) coefficients increased tablet hardness. The quadratic model produced an SLS–CS combination coefficient (1.00), indicating the SLS–CS combinations increased tablet hardness.
The 3CH2Cl, Ne, SLS, and CS particles filled random porosity between MCC and SDL particles. When the tablet mass was compressed, a tablet with solid interlocking and little porosity was formed. The cavity of SLS particles broke when compressed into tablets. Tablets had strong interlocking between particles and little porosity. The screw root shape of the CS particles caused the interlocking between the particles in the tablet to become elastic and withstand mechanical stress. The CS particles that filled the SLS particle cavity caused the combination particles to become stronger and more elastic. The resulting tablet had strong interlocking and can withstand mechanical stress.
Determining the Friability of 3CH2Cl Tablets
The tablet friability of each formula is shown in Table 2. The tablet orders of the most friable were TC, TB, and TA tablets. Although the tablet formula TC was the hardest, the tablet TC was the most brittle because the interlocking between the particles on the tablet surface cannot withstand mechanical movements. The simplex lattice design method with a linear model produced the coefficient values presented in Table 3, where SLS (0.38) was the most dominant in increasing tablet friability, followed by CS (0.31). The linear model coefficient was acceptable based on statistical analysis (see Supporting Information Table S1). The quadratic ANOVA model had a coefficient profile similar to the linear model. The coefficients of SLS (0.38) and CS (0.31) increased tablet friability. The quadratic model resulted in an SLS–CS combination coefficient (−0.02), indicating that the SLS–CS combinations decreased tablet friability.
The tablet constituent particles on the tablet surface and the interlocking which were not strong can be released when subjected to mechanical movement. SLS particles were at risk of breaking and forming fine particles when compressed because SLS particles are hollow. If the fine particles are on the tablet surface, the fine particles are released when receiving mechanical movement. Because of the screw shape of the CS particles on the tablet surface, it was difficult for the particles to maintain interlocking when receiving mechanical movements. Particle combination between SLS and CS particles on the tablet surface can support interlocking with other particles that make up the tablet to withstand mechanical movements.
Determining the Disintegration Time of 3CH2Cl Tablets
The tablet disintegration time for each formula is shown in Table 2. The TA tablets were the fastest to disintegrate than TB and TC tablets. TA formula tablets contained the highest CS so that more CS particles hydrate and swell, causing the tablet to disintegrate quickly. The simplex lattice design method with a linear model produced the coefficient values (Table 3). SLS (5.74) was the most dominant ingredient in increasing tablet friability, followed by CS (2.62). The linear model coefficient was acceptable based on statistical analysis (see Supporting Information Table S1). The quadratic ANOVA model had a coefficient profile similar to the linear model. The coefficients of SLS (5.65) and CS (2.53) increased tablet disintegration time. The quadratic model resulted in an SLS–CS combination coefficient (1.04), indicating that the SLS–CS combination increased the tablet disintegration time.
Changes in SLS particle size and the formation of fine particles when the tablet mass was compressed caused the tablet to have a dense porosity. The disintegrating medium had difficulty in penetrating the tablet and slowed down the disintegration. CS particles can function as disintegrants if particles are hydrated and swollen. CS particles needed time to hydrate and swell all the particles so that the tablet disintegration time is longer. The SLS–CS combination particles narrowed the porosity of the tablet so that there was less passage for the disintegrating medium. In addition, tablet hardness increased the disintegration time because the hard tablet had narrow porosity, so the disintegrating medium had difficulty in penetrating the tablet.
Determining the Drug Release of 3CH2Cl Tablets
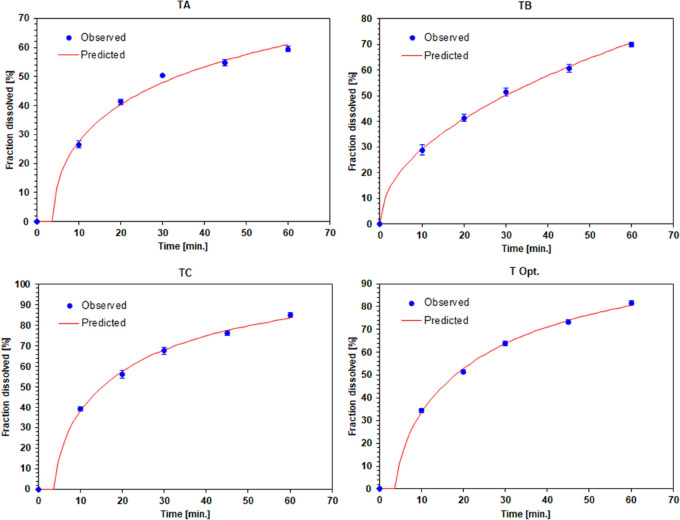
Drug release from each tablet is shown in Table 2 and a detailed profile is shown in Figure 1 (for details see Supporting Information Table S2). Tablets TC with the release of 3CH2Cl were the highest, followed by tablets with TB and TA formulas. The TC formula tablets contained the highest SLS, reducing the surface tension between the 3CH2Cl particles and the dissolution medium. The simplex lattice design method with a linear model resulted in the coefficient values as presented in Table 3, where SLS (84.28) was the most dominant in increasing drug release, followed by CS (58.65). The linear model coefficient was acceptable based on statistical analysis (see Supporting Information Table S1). The quadratic ANOVA model had a coefficient profile similar to the linear model. The coefficients of SLS (85.04) and CS (59.41) increased the release of 3CH2Cl. The quadratic model resulted in an SLS–CS combination coefficient (−9.10), indicating that the SLS–CS combinations decreased the solubility of 3CH2Cl.
Figure 1.
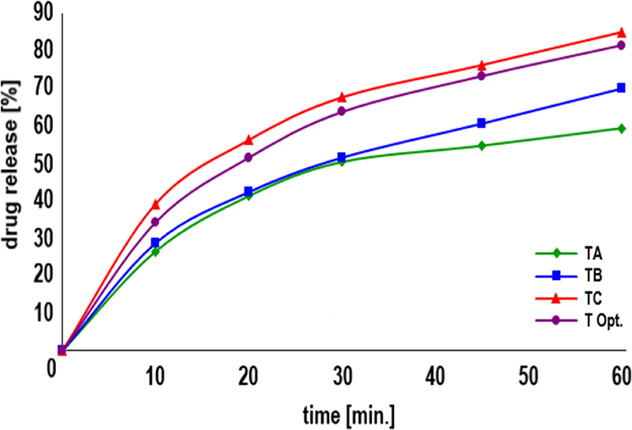
Dissolution profile of 3CH2Cl from tablets containing SLS [%] and CS [%]: TA (0.50:4.00), TB (0.75:3.00), TC (1.00:2.00), and T Opt. (0.92:2.33).
Hollow SLS particles can accelerate the solubility of SLS. The dissolved SLS particles reduced the surface tension of the 3CH2Cl particles with the dissolution medium. Swelling CS particles forced the tablet to disintegrate into tiny particles, thereby increasing the surface area of the 3CH2Cl particles in contact with the dissolution medium. SLS–CS combination particles have a narrow porosity, so the medium had difficulty hydrating other particles and inhibits the solubility of 3CH2Cl particles.
Simplex Lattice Design and ANOVA of 3CH2Cl Tablets
This experiment used the simplex lattice design because the optimization factor for the concentration of SLS and CS is an internal factor of the tablet formula, without any external factors. Linear and quadratic models were used to support each other in predicting the effect of SLS, CS, and a combination of SLS–CS (Table 3 and Figure 2). ANOVA from a linear model can provide R-Squared, Adj R-Squared, Pred R-Squared, and Adeq Precision values to evaluate the model’s acceptability. The coefficient of the polynomial equation of the linear model is accepted if the difference between R-Square and Pred R-Square is less than 0.2 and Adeq Precision is more than 4. Thus, the polynomial coefficients can be used to predict the effect of SLS and CS. The weakness of the linear model is that it cannot predict the impact of the combination of SLS with CS. The quadratic model can produce polynomial coefficients for the influence of SLS, CS, and SLS–CS combinations. However, the quadratic model cannot represent ANOVA parameters like a linear model because of the limited experimental formulas. The effort to maximize these two models in predicting the effect of SLS, CS, and SLS–CS combinations by analyzing the similarity of SLS and CS coefficients is critical. The profiles of the SLS and CS coefficients from the two models are similar. In this case, the coefficient values of the SLS–CS combination in the quadratic model can be used to predict the effect of the SLS–CS combination. The profiles of SLS and CS were similar so that the coefficient values of SLS–CS combined in the quadratic model can be used to predict the effect of the SLS–CS combination on the tablet formulation parameter of 3CH2Cl. Both models are beneficial for experiments using a limited number of formulas due to the availability of 3CH2Cl synthesized by laboratory capacity. Prediction of the optimum formula in this experiment was done numerically according to a linear model. Predicted (P Opt.) and verified (T Opt.) optimum tablet formulas are presented in Table 2.
Figure 2.
Linear and quadratic system profiles of each tablet mass parameter, tablet, and dissolution on the formulation of 3CH2Cl tablets.
Release Kinetics of 3CH2Cl Tablets
The release kinetics models of 3CH2Cl from tablets were analyzed using DDSolver. Rsqr_adj shows the correlation between dissolution time and release of 3CH2Cl. MSE_root determinates the correlation analysis correction, while the Akaike Information Criterion (AIC) demonstrated the suitability of the equation for determining the release kinetics model.28−31 The results of the DDSolver analysis are shown in Table 4 and Figure 3 (for details see Supporting Information Figures S1–S4).
Table 4. Evaluation of the Release Kinetics of 3CH2Cla.
| formula code | parameter | first
order |
Higuchi |
Korsmeyer–Peppas |
Weibull |
kinetics model | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| average | SD | average | SD | average | SD | average | SD | |||
| TA | Rsqr_adj | 0.8545 | 0.02 | 0.9721 | 0.01 | 0.9779 | 0.01 | 0.9907 | 0.01 | Weibull |
| MSE_root | 8.4740 | 0.47 | 3.6791 | 0.57 | 3.2431 | 0.75 | 2.0408 | 0.80 | ||
| AIC | 37.2882 | 0.67 | 27.1910 | 1.89 | 26.2323 | 2.67 | 20.5635 | 4.56 | ||
| TB | Rsqr_adj | 0.9475 | 0.01 | 0.9966 | 0.00 | 0.9961 | 0.00 | 0.9917 | 0.00 | Higuchi |
| MSE_root | 5.7427 | 0.56 | 1.4227 | 0.47 | 1.5214 | 0.53 | 2.2621 | 0.46 | ||
| AIC | 32.5953 | 1.14 | 15.3644 | 4.53 | 16.8557 | 4.27 | 22.229 | 2.35 | ||
| TC | Rsqr_adj | 0.9578 | 0.01 | 0.9885 | 0.00 | 0.9949 | 0.00 | 0.9963 | 0.00 | Weibull |
| MSE_root | 6.3475 | 0.45 | 3.3264 | 0.47 | 2.221 | 0.13 | 1.8553 | 0.28 | ||
| AIC | 33.8125 | 0.87 | 26.0793 | 1.49 | 21.8932 | 0.65 | 19.9178 | 1.81 | ||
| T Opt. | Rsqr_adj | 0.9686 | 0.01 | 0.9926 | 0.00 | 0.9925 | 0.01 | 0.9979 | 0.00 | Weibull |
| MSE_root | 5.2892 | 0.42 | 2.5394 | 0.52 | 2.5334 | 0.39 | 1.2986 | 0.53 | ||
| AIC | 31.6188 | 0.97 | 22.6583 | 2.61 | 23.2554 | 0.57 | 14.8858 | 5.82 | ||
The release kinetics of 3CH2Cl from each tablet formula containing SLS [%] and CS [%]: TA (0.50:4.00), TB (0.75:3.00), TC (1.00:2.00), and T Opt. (0.92:2.33). The model selection was high Rsqr_adj, low mean square error root (MSE_root), and low Akaike Information Criterion (AIC).
Figure 3.
Kinetics profile of the release of 3CH2Cl from tablets containing SLS [%] and CS [%]: TA (0.50:4.00) (Weibull), TB (0.75:3.00) (Higuchi), TC (1.00:2.00) (Weibull), and T Opt. (0.92:2.33) (Weibull).
The TA and TC formulas following the Weibull release kinetics model show that 3CH2Cl was released from the tablet without any delay. The presence of SLS lowered the surface tension of 3CH2Cl with the dissolution medium so that the particles dissolved quickly. This was also supported by the presence of CS, which accelerates the disintegration of tablets into granules or particles, thereby expanding the surface of the particles to dissolve.
The Higuchi release kinetics model of the TB formula shows that the release was influenced by the diffusion mechanism of 3CH2Cl out of the tablet. SLS on the tablet surface accelerated hydrating and was followed by the formation of a hydration layer so that the particles dissolve and leave the tablet. CS served as a disintegrating agent after hydrating and swelling. This condition took time, so 3CH2Cl was allowed to dissolve and diffuse before CS can function.
3CH2Cl-Tablets Showed Analgesic Activity in the Mice Writhing Test
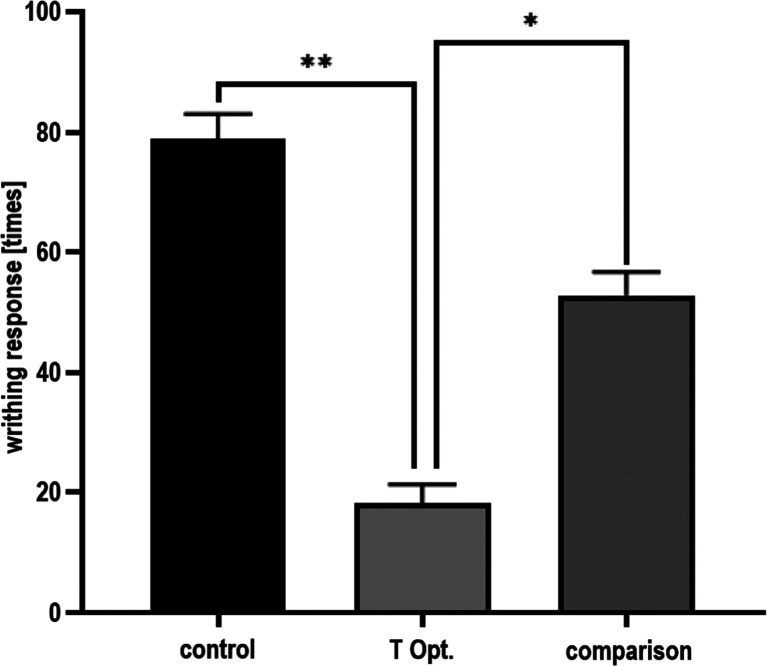
This experiment was conducted using a T Opt. tablet to determine the effect of excipients on the analgesic activity of 3CH2Cl. The results of the analgesic activity test of 3CH2Cl are presented in Figure 4. The control group produced a very high amount of writhing response (78.83 ± 4.17), indicating the success of pain induction using 0.6% acetic acid (dose of 0.01 mL/g body weight). The number of writhes of the T Opt. group (18.17 ± 3.19) was less than that in the control group, showing that 3CH2Cl can suppress pain. The analgesic activity of 3CH2Cl was more effective than that of the comparison compound because 3CH2Cl had less amount of writhe than the comparison compound (52.83 ± 3.87). The significant difference in the amount of writhe of the three groups (P < 0.05) shows that 3CH2Cl had analgesic activity despite the presence of tablet excipients.
Figure 4.
Analgesic activity of 3CH2Cl.
The control group is mice given excipient tablets. The active compound group in mice was assigned 3CH2Cl tablets. The comparison group was mice given acetylsalicylic acid tablets. The three groups induced pain using 0.01 mL/g body weight of 0.6% acetic acid. The significant difference in the amount of writhe of the three groups (P < 0.05) indicated that 3CH2Cl still has analgesic activity despite the presence of tablet excipients.
Conclusions
The polynomial coefficient values of the two models show that the SLS, CS, and SLS–CS combinations increased the parameter values of flow time, Carr index, hardness, and disintegration time. The SLS–CS combination decreased the friability value and the drug release parameters. The optimum tablet formulas of the 3CH2Cl tablet were 3CH2Cl (300 mg), Ne (9.38%), SLS (0.92%), CS (2.33%), MCC (5%), and adjusted with SDL until 800 mg of total weight. Quality predictions of tablet mass were flow time (9.07 s); Carr index (25.33%). Quality tablet predictions are hardness (9.44 kp), friability (0.37%), disintegration time (5.22 min), and drug released in 60 min (80%). SLS was to increase the solubility particles of 3CH2Cl and the excipient. CS had accelerated the disintegration of tablets into particles. The tablet dosage form of 3CH2Cl is an innovative formulation and a new alternative for future analgesic drugs.
Acknowledgments
The authors thank the Research and Community Service Institute of Widya Mandala Catholic University, Surabaya, Indonesia, for supporting grants (5230/WM01/N/2021). The authors also thank Khaterine Irene Phuk, Sherlilyta Stiara Dewi, Angela Tiffany, and Meidelin Ribka Abiati for their assistance during the experiment.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c03147.
Tablet dosage calculation; statistical analysis of 3CH2Cl tablets; the release of 3CH2Cl from the tablets; and the kinetics profile of the release of 3CH2Cl from TA, TB, TC, and T Opt. tablets (PDF)
Author Contributions
W.H. designed the experiments, performed the experiments, analyzed and interpreted the data, and wrote the manuscript. K.F., Y.T., C.C., S.Y.E., and M.A.J. performed the experiments and analyzed and interpreted the data. H.W. analyzed and interpreted the data.
The authors declare no competing financial interest.
Notes
Experiments using experimental animals (mice) have been declared to meet the ethical requirements from the Research Ethics Commission of the Faculty of Veterinary Medicine, Gadjah Mada University, Yogyakarta, Indonesia, with No. 001/EC-FKH/Ex./2022 dated January 14, 2022.
Supplementary Material
References
- Tjahjono Y.; Karnati S.; Foe K.; Anggara E.; Gunawan N. Y.; Wijaya H.; Steven; Suyono H.; Esar S. Y.; Hadinugroho W.; Wihadmadyatami H.; Ergün S.; Widharna R. M.; Caroline Anti-inflammatory activity of 2-((3-(chloromethyl)benzoyl)oxy)benzoic acid in LPS-induced rat model. Prostaglandins Other Lipid Mediators 2021, 154, 1–9. 10.1016/j.prostaglandins.2021.106549. [DOI] [PubMed] [Google Scholar]
- Caroline; Foe K.; Esar S. Y.; Soewandi A.; Wihadmadyatami H.; Megawati R.; Tamayanti W. D.; Kasih E.; Tjahjono Y. Evaluation of analgesic and antiplatelet activity of 2-((3-(chloromethyl)benzoyl)oxy)benzoic acid. Prostaglandins Other Lipid Mediators 2019, 145, 1–8. 10.1016/j.prostaglandins.2019.106364. [DOI] [PubMed] [Google Scholar]
- Caroline N.; Foe K.; Esar S. Y.; Jessica M. A. Characterization of pharmacokinetics of 2-((3-(chloromethyl)benzoyl)oxy) benzoic acid in rats by using hplc-dad method. Int. J. Appl. Pharm. 2019, 11, 279–283. 10.22159/ijap.2019v11i5.34536. [DOI] [Google Scholar]
- Bergström C. A. S.; Larsson P. Computational prediction of drug solubility in water-based systems: Qualitative and quantitative approaches used in the current drug discovery and development setting. Int. J. Pharm. 2018, 540, 185–193. 10.1016/j.ijpharm.2018.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheske P.J.; Cook W.G.; Cable C.G.. Handbook of Pharmaceutical Excipients 8th; Pharmaceutical Press and American Pharmacists Association: London-Washington DC, 2017; pp 282–284. [Google Scholar]
- Aulton E.; Taylor K.M.G.. Aulton’s Pharmaceutics The Design and Manufacture of Medicines; Churchill Livingstone Elsevier: New York, 2017; pp 187–199. [Google Scholar]
- The United States Pharmacopeial Convention . Pharmacopeia 41-National Formulary 36; Twinbrook Parkway: Rockville, 2018; pp 7634–7635. [Google Scholar]
- Hadinugroho W.; Martodihardjo S.; Fudholi A.; Riyanto S. Preparation of Citric Acid-Locust Bean Gum (CA-LBG) for the Disintegrating Agent of Tablet Dosage Forms. J. Pharm. Innov. 2021, 1–16. 10.1007/s12247-021-09591-0. [DOI] [Google Scholar]
- Bertocchi P.; Antoniella E.; Valvo L.; Alimonti S.; Memoli A. Diclofenac sodium multisource prolonged release tablets - A comparative study on the dissolution profiles. J. Pharm. Biomed. Anal. 2005, 37, 679–685. 10.1016/j.jpba.2004.11.041. [DOI] [PubMed] [Google Scholar]
- Bozič D. Z.; Vrečer F.; Kozjek F. Optimization of diclofenac sodium dissolution from sustained release formulations using an artificial neural network. Eur. J. Pharm. Sci. 1997, 5, 163–169. 10.1016/S0928-0987(97)00273-X. [DOI] [Google Scholar]
- Kaleemullah M.; Jiyauddin K.; Thiban E.; Rasha S.; Al-Dhalli S.; Budiasih S.; Gamal O. E.; Fadli A.; Eddy Y. Development and evaluation of Ketoprofen sustained release matrix tablet using Hibiscus rosa-sinensis leaves mucilage. Saudi Pharm. J. 2017, 25, 770–779. 10.1016/j.jsps.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab A.; Khan G. M.; Akhlaq M.; Khan N. R.; Hussain A.; Khan M. F.; Ur-Rehman N.; Khan A. Formulation and evaluation of controlled release matrices of ketoprofen and influence of different co-excipients on the release mechanism. Pharmazie 2011, 66, 677–683. 10.1691/ph.2011.1040. [DOI] [PubMed] [Google Scholar]
- Craciun A. M.; Barhalescu M. L.; Agop M.; Ochiuz L. Theoretical Modeling of Long-Time Drug Release from Nitrosalicyl-Imine-Chitosan Hydrogels through Multifractal Logistic Type Laws. Comput. Math. Methods Med. 2019, 2019, 1–10. 10.1155/2019/4091464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panotopoulos G. P.; Haidar Z. S. Mathematical modeling for pharmacokinetic predictions from controlled drug release nano systems: A comparative parametric study. Biomed. Pharmacol. J. 2018, 11, 1801–1806. 10.13005/bpj/1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.; Majumdar D. K. Analgesic activity of ocimum sanctum and its possible mechanism of action. Pharm. Biol. 1995, 33, 188–192. 10.3109/13880209509065361. [DOI] [Google Scholar]
- Shete A.; Salunkhe A.; Yadav A.; Sakhare S.; Doijad R. Neusilin based liquisolid compacts of albendazole: Design, development, characterization and in vitro anthelmintic activity. Marmara Pharm. J. 2019, 23, 441–456. 10.12991/jrp.2019.151. [DOI] [Google Scholar]
- Lou H.; Liu M.; Wang L.; Mishra S. R.; Qu W.; Johnson J.; Brunson E.; Almoazen H. Development of a mini-tablet of co-grinded prednisone-neusilin complex for pediatric use. AAPS PharmSciTech 2013, 14, 950–958. 10.1208/s12249-013-9981-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneja P.; Kaur B.; Odeku O. A.; Singh I. Development of Corn Starch-Neusilin UFL2 Conjugate as Tablet Superdisintegrant: Formulation and Evaluation of Fast Disintegrating Tablets. J. Drug Delivery 2014, 2014, 1–13. 10.1155/2014/827035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.; Wang C.; Dun J.; Du L.; Hawley M.; Sun C. C. Mechanism for the Reduced Dissolution of Ritonavir Tablets by Sodium Lauryl Sulfate. J. Pharm. Sci. 2019, 108, 516–524. 10.1016/j.xphs.2018.10.047. [DOI] [PubMed] [Google Scholar]
- Li M.; Qiao N.; Wang K. Influence of sodium lauryl sulfate and Tween 80 on carbamazepine-nicotinamide cocrystal solubility and dissolution behaviour. Pharmaceutics 2013, 5, 508–524. 10.3390/pharmaceutics5040508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhmoud H. A.; Akkam Y. H. Combination of surfactants with other excipients: Effects on drug release and dimensional changes in matrices. Trop. J. Pharm. Res. 2019, 8, 2241–2246. 10.4314/tjpr.v18i11.2. [DOI] [Google Scholar]
- Parfati N.; Rani K. C. The effects of croscarmellose sodium concentration on the physicochemical characteristics of orodispersible tablets of atenolol. Pharmaciana 2018, 8, 87–95. 10.12928/pharmaciana.v8i1.7619. [DOI] [Google Scholar]
- Kumar A.; Saharan V. A. Salbutamol Sülfatın Oral Dağılan Tabletlerinin Formülasyonu ve Değerlendirilmesi: Süper Dağıtıcıların Farklı Oranlarının Karşılaştırmalı Çalışması. Turkish J. Pharm. Sci. 2017, 14, 40–48. [Google Scholar]
- Desai P. M.; Er P. X. H.; Liew C. V.; Heng P. W. S. Functionality of disintegrants and their mixtures in enabling fast disintegration of tablets by a quality by design approach. AAPS PharmSciTech 2014, 15, 1093–1104. 10.1208/s12249-014-0137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmin R.; Shoaib M. H.; Ahmed F. R.; Qazi F.; Ali H.; Zafar F. Aceclofenac fast dispersible tablet formulations: Effect of different concentration levels of Avicel PH102 on the compactional, mechanical and drug release characteristics. PLoS One 2020, 15, 1–16. 10.1371/journal.pone.0223201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoorens G.; Krier F.; Leclercq B.; Carlin B.; Evrard B. Microcrystalline cellulose, a direct compression binder in a quality by design environment - A review. Int. J. Pharm. 2014, 473, 64–72. 10.1016/j.ijpharm.2014.06.055. [DOI] [PubMed] [Google Scholar]
- Chaerunisaa A.Y.; Sriwidodo S.; Abdassah M. Microcrystalline Cellulose as Pharmaceutical Excipient. In Pharmaceutical Formulation Design-Recent Practices; Ahmad U., Akhtar J., Eds.; Intechopen: London, 2020; pp 1–21. 10.5772/intechopen.88092 [DOI] [Google Scholar]
- Gu Y.; Wei H. L.; Balikhin M. M. Nonlinear predictive model selection and model averaging using information criteria. Syst. Sci. Control Eng. 2018, 6, 319–328. 10.1080/21642583.2018.1496042. [DOI] [Google Scholar]
- Mircioiu C.; Voicu V.; Anuta V.; Tudose A.; Celia C.; Paolino D.; Fresta M.; Sandulovici R.; Mircioiu I. Mathematical modeling of release kinetics from supramolecular drug delivery systems. Pharmaceutics 2019, 11, 1–45. 10.3390/pharmaceutics11030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siswanto A.; Fudholi A.; Nugroho A. K.; Martono S. in Vitro Release Modeling of Aspirin Floating Tablets Using DDSolver. Indones. J. Pharm. 2015, 26, 94–102. 10.14499/indonesianjpharm26iss2pp94. [DOI] [Google Scholar]
- Zhang Y.; Huo M.; Zhou J.; Zou A.; Li W.; Yao C.; Xie S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. 10.1208/s12248-010-9185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.