Abstract
Malnutrition is a liver cirrhosis complication affecting more than 20%-50% of patients. Although the term can refer to either nutrient deficiency or excess, it usually relates to undernutrition in cirrhosis settings. Frailty is defined as limited physical function due to muscle weakness, whereas sarcopenia is defined as muscle mass loss and an advanced malnutrition stage. The pathogenesis of malnutrition in liver cirrhosis is multifactorial, including decreased oral intake, maldigestion/malabsorption, physical inactivity, hyperammonemia, hypermetabolism, altered macronutrient metabolism and gut microbiome dysbiosis. Patients with chronic liver disease with a Body Mass Index of < 18.5 kg/m2 and/or decompensated cirrhosis or Child-Pugh class C are at the highest risk of malnutrition. For patients at risk of malnutrition, a detailed nutritional assessment is required, typically including a history and physical examination, laboratory testing, global assessment tools and body composition testing. The latter can be done using anthropometry, cross-sectional imaging including computed tomography or magnetic resonance, bioelectrical impedance analysis and dual-energy X-ray absorptiometry. A multidisciplinary team should screen for and treat malnutrition in patients with cirrhosis. Malnutrition and sarcopenia are associated with an increased risk of complications and a poor prognosis in patients with liver cirrhosis; thus, it is critical to diagnose these conditions early and initiate the appropriate nutritional therapy. In this review, we describe the prevalence and pathogenesis of malnutrition in liver cirrhosis patients and discuss the best diagnostic approach to nutritional assessment for them.
Keywords: Malnutrition, Cirrhosis, Nutritional assessment, Sarcopenia, Nutrition, Frailty
Core Tip: Malnutrition is a common complication of liver cirrhosis that is not often addressed by physicians. Due to its association with poor outcomes, it is important to identify patients at risk of malnutrition in order to treat them early. We herein describe the mechanism of malnutrition in cirrhosis and discuss the best diagnostic approach.
INTRODUCTION
Malnutrition is defined as nutrient imbalance (deficiency or excess) with adverse effects on the body’s form, function or outcome. According to the European Association for the Study of Liver Disease (EASL), the term “malnutrition” refers to “undernutrition”[1]. Frailty is defined as limited physical function due to muscle weakness and diminished muscle contractility, while sarcopenia is defined as the generalized loss of muscle mass. Malnutrition is a common complication of liver cirrhosis, with a prevalence rate of 5-92%[2]. The prevalence of malnutrition increases with worsening liver disease[3]. It has been reported that one-fifth of patients with compensated cirrhosis and more than half the patients with decompensated cirrhosis have malnutrition[4]. Additionally, even patients with chronic liver disease who are not cirrhotic can have malnutrition. In this group of patients, malnutrition may be masked by obesity[5]. Due to the increasing prevalence of non-alcoholic fatty liver disease, overweight and obesity are becoming more common in cirrhotic patients. In this review, we describe the pathophysiology of malnutrition in liver cirrhosis and discuss the best diagnostic approach to assess the nutritional status of patients in clinical practice.
METHODS
A PubMed web-based search was conducted to review the literature published from its inception until January 1, 2022, using the keywords ‘malnutrition’, ‘nutritional assessment,’ ‘liver cirrhosis,’ and ‘sarcopenia.’ All relevant articles published in the English language were reviewed, and data on epidemiology, pathogenesis, diagnosis and prognosis were extracted.
PATHOGENESIS
There are multiple factors that contribute to the development of malnutrition and sarcopenia in liver cirrhosis (Figure 1). First, the principal cause of malnutrition is reduced oral intake, and this can be due to anorexia, early satiety, nausea and cognitive impairment in the setting of hepatic encephalopathy. Patients with liver cirrhosis often have altered taste and smell, which can cause anorexia due to changes in the oral flora, use of antibiotics, dry mouth, zinc or magnesium deficiency[6]. Additionally, imbalances between orexigenic and anorexigenic hormones and chronic elevations in cytokines like tumor necrosis factor (TNF)-α can also trigger anorexia[7,8]. Early satiety can be explained by abdominal distension secondary to ascites or altered intestinal motility, which is common in cirrhosis[9]. Furthermore, unpalatable low-salt diets followed by the patients with ascites, alcohol abuse, and frequent tests requiring fasting for hours can all contribute to decreased oral intake[8]. Second, nutrient maldigestion and malabsorption can occur due to reduced bile production, altered intestinal motility with subsequent small bowel bacterial overgrowth, portal hypertensive gastropathy/ enteropathy and long-term lactulose use[8]. Furthermore, pancreatic insufficiency frequently coexists with alcoholic liver cirrhosis, contributing to decreased nutrient uptake. Third, alteration in macronutrient metabolism is an important factor affecting nutritional status in cirrhosis. Carbohydrate metabolism is characterized by increased gluconeogenesis, elevated fasting serum insulin levels, insulin resistance, decreased glycogen synthesis and storage and the early use of lipids and proteins as substrates for energy production and gluconeogenesis[7]. It has been observed that the rate of fat and protein catabolism after a short overnight fast in patients with liver cirrhosis is similar to that of healthy individuals who underwent 2-3 d of starvation[10]. Abnormal protein metabolism manifests itself as more protein catabolism and less synthesis, low levels of branched-chain amino acids (BCAA), and higher levels of aromatic amino acids (AAA), resulting in a lower Fischer’s ratio (BCAA/AAA ratio) which has been associated with complications such as hepatic encephalopathy[11]. Hyperammonemia promotes muscle breakdown and sarcopenia by upregulating myostatin which inhibits protein synthesis[12]. Testosterone levels are decreased in cirrhotic males and this further contributes to decreased protein synthesis and loss of muscle mass[13].
Figure 1.

Factors contributing to malnutrition and sarcopenia in liver cirrhosis.
Lipid metabolism exhibits increased lipolysis, lipid oxidation and ketogenesis[14]. Fourth, hypermetabolism affecting one-third of cirrhotic patients, contributes to malnutrition. It is defined as having a resting energy expenditure > 120% of the predictive value, and it can be caused by infections or chronic inflammation and is not associated with sex, underlying cause or severity of liver disease[15]. Fifth, an imbalance of gut microbiota (dysbiosis) in liver cirrhosis has been suggested as a contributing factor in malnutrition. Short-chain fatty acid-producing bacteria such as Bacteroides are reduced in patients with cirrhosis, and there is a higher abundance of Campylobacterales in moderately malnourished cirrhotics; findings have been associated with malnutrition in children[16-19]. The alteration in gut microbiome composition leads to increased intestinal permeability, bacterial translocation and infectious complications like spontaneous bacterial peritonitis[20]. This results in increased protein catabolism and muscle mass loss mediated by inflammation. Finally, physical inactivity, which is common in patients with significant ascites or hepatic encephalopathy may contribute to reduced muscle mass[21].
Beta blockers have been suggested as a possible external factor contributing to malnutrition in cirrhosis. However, a recent study found that patients who received non-selective beta blockers had actually better skeletal muscle index and improvement in sarcopenia[22].
The role of portal hypertension in malnutrition and sarcopenia is not clear. There is very limited literature about the prevalence of malnutrition and sarcopenia in non-cirrhotic portal hypertension. A study by Lattanzi et al[23] found that the prevalence of sarcopenia in non-cirrhotic portal hypertension was similar to that in patients with compensated cirrhosis. This could suggest that portal hypertension per se may play a role in the development of malnutrition and sarcopenia given the fact that those patients have less liver damage compared to cirrhotic patients. This theory could be supported by the fact that nutritional status improves after transjugular intrahepatic portosystemic shunt (TIPS) and resolution of portal hypertension[24,25].
DIAGNOSIS
Malnutrition screening tools
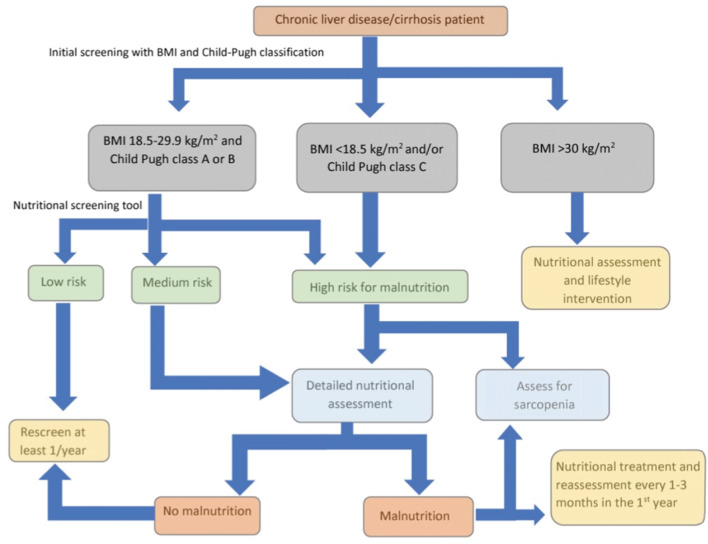
The EASL released clinical practice guidelines in 2019 on nutritional assessment and management in chronic liver disease patients[1]. They recommended the screening of all patients with chronic liver disease for the risk of malnutrition using two tests: The body mass index (BMI) and Child-Pugh classification. Patients with a BMI < 18.5 kg/m2 and/or those with Child-Pugh class C or decompensated cirrhosis are considered at higher risk for malnutrition. On the other hand, patients with BMI 18.5-29.9 kg/m2 and are Child-Pugh class A or B should undergo nutritional screening using one of the following liver disease-specific malnutrition screening tools: The Royal Free Hospital-nutritional prioritizing tool (RFH-NPT) or the liver disease undernutrition screening tool. Those who are at low risk for malnutrition need follow-up and re-assessment every year, while patients with moderate or high risk for malnutrition should have a detailed nutritional assessment. In addition, patients with a high risk for malnutrition need to be assessed for sarcopenia as well (Figure 2).
Figure 2.
Algorithm for nutritional screening and assessment in liver cirrhosis. Adapted from the European Association for the Study of the Liver (EASL) clinical practice guidelines (with permission from Elsevier). Citation: European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172-193. Copyright© 2018 European Association for the Study of the Liver. Published by Elsevier. BMI: Body mass index (Supplementary material).
RFH-NPT uses simple clinical questions that take less than 3 minutes to complete and can be used by non-specialist staff. It classifies patients into low (0 points), medium (1 point), or high risk (2-7 points) for malnutrition. It considers the patient’s nutritional history (unplanned weight loss, dietary intake, BMI) and the presence or absence of fluid overload (ascites and/or peripheral edema). Although it has been validated in a multicenter trial in the United Kingdom, it requires further testing[26]. RFH-NPT was reported to correlate with clinical deterioration, the severity of liver disease, and complications of liver cirrhosis and was found to be an independent predictor of clinical deterioration and transplant-free survival. Furthermore, improvement in RFH-NPT score was associated with improved survival[27]. RFH-NPT is recommended by the European Society of Parenteral Enteral Nutrition guidelines as the best available tool for malnutrition screening in liver disease[28].
The liver disease undernutrition screening tool uses six patient-directed questions about nutritional intake, weight loss, subcutaneous fat loss, muscle mass loss, fluid accumulation and decline in functional status. Its limitation is that it is entirely dependent on the patient’s subjective judgment and has a low negative predictive value[29]. As with RFH-NPT, it needs further validation.
Detailed nutritional assessment
Patients who are at risk of malnutrition during screening should undergo comprehensive nutritional evaluation for confirmation of malnutrition and characterization of their nutritional status. This should ideally be done by a registered dietician or nutritionist. The evaluation process includes history taking, physical examination, laboratory tests, subjective global assessment and specialized methods for body composition assessment.
HISTORY
Patients should be asked about their dietary intake; recent weight loss; use of supplements; alcohol consumption; any eating barriers such as anorexia, nausea, altered taste or smell, abdominal distension or pain, or any socioeconomic barrier; and symptoms of nutritional deficiency such as dermatitis (zinc, niacin, vitamin A), sore tongue (folate, vitamin B12), or paresthesia (thiamine, pyridoxine, vitamin B12). Dietary intake can be assessed using 24-h dietary recall, which is simple to use and does not require a high level of literacy. However, one significant disadvantage is that it is dependent on the patient’s recall skills and may not be representative of daily meal selection or eating behavior[30]. Another option is a 3-d food diary, which requires patients to cooperate and follow standardized instructions; however, it may be burdensome for patients and difficult to implement in those with advanced disease. It is the preferred method because it relies the least on patient recall[31]. Repeated 24-h dietary recalls are another option[32]. At a minimum, the patients should be asked if their relative food intake has changed over time and, if so, how much.
PHYSICAL EXAMINATION
It should include measuring the BMI; examination for ascites and edema; muscle wasting, which is usually done by assessing the temporalis muscle, quadriceps and deltoids; and loss of subcutaneous fat which can be assessed in the chest, eye sockets and triceps areas. The BMI divides patients into four categories: Underweight, normal weight, overweight, and obese. In cirrhotic patients, it can be used to diagnose obesity in the absence of fluid retention. In the case of fluid retention, the patient's dry weight should be used, which can be estimated using the documented patient’s weight prior to the development of fluid retention if available, the patient’s weight post paracentesis, or by subtracting a percentage of weight based on the severity of ascites (5% for mild, 10% for moderate and 15% for severe) with an additional 5% subtracted if bilateral lower limb edema is present[33,34]. This has not been validated yet but has demonstrated excellent inter-observer agreement.
LABORATORY TESTS
The use of serum biomarkers for the diagnosis of malnutrition is controversial and currently they only complement the nutritional assessment[35]. Complete blood count; serum creatinine; serum albumin, C-reactive protein (CRP); levels of vitamins and minerals like zinc, phosphorus, magnesium and iron are included in laboratory tests. Serum protein measurements may be limited in patients with advanced liver cirrhosis and synthetic dysfunction because they do not always reflect nutritional status. CRP may be useful in assessing catabolism and interpreting the results of nutrient levels. It is important to tailor testing according to the patient’s underlying liver disease and comorbidities.
GLOBAL ASSESSMENT TOOLS
Subjective global assessment
It consists of five historical parameters (weight loss, dietary changes, gastrointestinal symptoms, functional capacity and metabolic demand associated with the underlying disease) and three physical examination parameters (loss of subcutaneous fat, muscle wasting and edema/ascites)[36]. Based on the results of these parameters, the patient gets a rating of A (well-nourished), B (moderately malnourished) or C (severely malnourished). Although subjective global assessment (SGA) is simple to administer, has fair to good interobserver reproducibility[37] and correlates with post-operative outcomes in patients without liver cirrhosis, it underestimates the prevalence of sarcopenia and has a low agreement with other methods of nutritional assessment[34,38].
Royal free hospital-SGA
Due to the limitations of the SGA, the royal free hospital-SGA (RFH-SGA) was developed[39]. It consists of dietary intake, BMI based on dry weight and mid-arm muscle circumference. Patients are stratified into three groups: adequately nourished, moderately malnourished and severely malnourished. The RFH-SGA is reproducible, correlates with other measurements of body composition and has shown promise in predicting survival and post-transplant outcomes[40,41]. However, it takes a longer time than SGA and requires additional validation.
Assess for frailty
There are currently no standardized criteria for diagnosing frailty in cirrhosis. There are several geriatric measures that have been used to assess frailty in cirrhotic patients. The Liver Frailty Index measures hand grip strength, balance, and timed chair stands and has been found to be correlated with mortality[42]. The Fried frailty criteria include unintentional weight loss, self-reported exhaustion, grip strength, slow walking speed and low physical activity. An increase in the Fried frailty score was found to be associated with an increased risk of waiting list mortality[43]. The short physical performance battery measures repeated chair stands, balance, and timed 13-foot walk and has been shown to predict transplant waiting list mortality[43].
BODY COMPOSITION TESTING
Body composition testing is summarized in Table 1.
Table 1.
Comparison between body composition testing modalities
|
Modality
|
Accuracy
|
Advantage
|
Disadvantage
|
| Anthropometry | Low | Simple, rapid, not affected by fluid retention | Interobserver variability |
| BIA | Moderate | Easy, portable, relatively inexpensive | Influenced by volume status, requires special equipment |
| Ultrasound | Moderate to high | Inexpensive, radiation-free, bedside | Interobserver variability |
| DEXA scan | High | Suitable for repeat testing | Radiation exposure & high cost (but less than CT scan) |
| CT scan | High | Allows direct assessment of muscle mass | Radiation and contrast exposure, high cost |
| MRI | High | No radiation exposure, allows direct assessment of muscle mass | Expensive, lacks cut-off values |
BIA: Bioelectrical impedance analysis; DEXA: Dual energy X-ray absorptiometry; CT: Computed tomography; MRI: Magnetic resonance imaging.
Anthropometry
These are simple and quick bedside methods for determining body fat and muscle mass that are unaffected by fluid retention. Triceps skin fold (TSF) and mid-arm muscle circumference (MAMC) are the most commonly used measurements [MAMC = mid-arm circumference - (TSF × 0.314)]. Both MAMC and TSF have been found to correlate with survival in cirrhotic patients, with MAMC having higher prognostic power than TSF[44]. These tests have interobserver variability and low accuracy.
Bioelectrical impedance analysis
It determines the water content of the body by measuring the resistance to electrical current flow within the body which is then used to estimate muscle mass. Bioelectrical impedance analysis (BIA) is measured with a special scale or by attaching electrodes to an arm and a leg. It is inexpensive, portable and simple to use; however, the results are influenced by the patient’s volume status which can change in cirrhosis[45].
Computed tomography
The gold standard for sarcopenia assessment is the quantification of muscle mass using cross-sectional imaging[46]. The skeletal muscle index (cm2/m2) is calculated by analyzing the abdominal skeletal muscles at the L3 vertebral level. Cut-off values based on an American study (50 cm2/m2 in males and 39 cm2/m2 in females) that correlated best with outcomes have been proposed, though ethnicity-specific criteria may be required given the fact that Asians have lower lean body mass compared to Western populations[47]. A meta-analysis of the impact of computed tomography (CT-assessed muscle mass on clinical outcomes in liver transplant patients showed an association between low muscle mass and mortality that was independent of the Model for End-Stage Liver Disease Score[48]. Obviously, the routine and multiple CT scans to diagnose sarcopenia are limited by the cost, availability, radiation and contrast exposures; however, since it is often used for other purposes in liver cirrhosis like evaluation of hepatocellular carcinoma and liver transplantation assessment, thus it can be used at least once for assessment of sarcopenia.
Magnetic resonance imaging
The use of magnetic resonance imaging for the assessment of sarcopenia has been suggested with the advantages of high accuracy and lack of ionizing radiation. It is only used for research purposes due to limitations of high cost and lack of cut-off values.
Ultrasonography
It has been more than two decades that the use of ultrasound for skeletal muscle mass estimation in the context of fluid retention has been proposed[49]. The biceps, anterior forearm flexors and quadriceps muscles correlated best with lean body mass. The test is radiation-free and allows bedside assessment at a low cost. A previous study showed that combining BMI with thigh muscle thickness measured by ultrasound is significantly correlated with sarcopenia diagnosed via cross-sectional imaging[34]. However, a more recent study found that ultrasound muscle thickness had no advantage over other bedside techniques (namely MAMC and BIA)[50].
Dual-energy X-ray absorptiometry
It allows regional and whole-body assessment of bone mineral density, fat mass and lean mass. Even though it is less precise compared to a CT scan, it has a lower cost and radiation exposure which makes it more suitable for repeat testing during follow-up[51]. The major limitation is its validity in the case of fluid retention which can lead to the underestimation of sarcopenia. To overcome the confounding effect of ascites, use of appendicular lean mass that excludes the abdominal compartment has been proposed[52]. Other studies proposed the use of arm lean mass to further reduce the effect of lower limb edema and it was found to be superior to appendicular lean mass in terms of mortality[53,54].
IMPACT OF MALNUTRITION, SARCOPENIA AND FRAILTY ON LIVER CIRRHOSIS
Malnutrition has a negative impact on cirrhosis progression and outcome[55]. For example, patients with cirrhosis who are malnourished were found to have twice the rates of hospitalizations and mortality as compared to well-nourished patients[56]. It has also been shown that malnutrition is a predictor of other complications of cirrhosis, such as infections, hepatic encephalopathy and ascites[57-59]. Malnutrition and sarcopenia are independent predictors of poor outcomes in patients with liver cirrhosis and in those undergoing liver transplantation[60-62]. In addition, sarcopenic obesity and myosteatosis are independently associated with long-term mortality in liver cirrhosis[63]. Furthermore, it has been demonstrated that the diagnosis of frailty in cirrhosis is associated with an increase in mortality[64]. Given the significant impact on morbidity and mortality, it is critical to screen all patients with liver cirrhosis for malnutrition and provide nutritional therapy to those who require it in order to improve their quality of life and survival. A multidisciplinary approach is the best way to accomplish this.
CONCLUSION
Malnutrition is a common complication of liver cirrhosis with complex pathophysiology that adversely affects the clinical outcome. A stepwise diagnostic approach should be followed for early recognition and management.
Footnotes
Conflict-of-interest statement: All authors report no relevant conflict of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: July 14, 2022
First decision: July 27, 2022
Article in press: September 9, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Jordan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kitamura K, Japan; Kreisel W, Germany S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
Contributor Information
Sara Haj Ali, Department of Internal Medicine, Faculty of Medicine, Al-Balqa Applied University, Salt 19117, Jordan. sara.hajali@bau.edu.jo.
Awni Abu Sneineh, Department of Gastroenterology and Hepatology, University of Jordan, Faculty of Medicine, Amman 11942, Jordan.
Reem Hasweh, Department of Internal Medicine, Faculty of Medicine, Al-Balqa Applied University, Salt 19117, Jordan.
References
- 1.European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172–193. doi: 10.1016/j.jhep.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Traub J, Reiss L, Aliwa B, Stadlbauer V. Malnutrition in Patients with Liver Cirrhosis. Nutrients. 2021;13 doi: 10.3390/nu13020540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naqvi IH, Mahmood K, Salekeen S, Akhter ST. Determining the frequency and severity of malnutrition and correlating it with the severity of liver cirrhosis. Turk J Gastroenterol. 2013;24:415–422. doi: 10.4318/tjg.2013.0637. [DOI] [PubMed] [Google Scholar]
- 4.Nutritional status in cirrhosis. Italian Multicentre Cooperative Project on Nutrition in Liver Cirrhosis. J Hepatol. 1994;21:317–325. [PubMed] [Google Scholar]
- 5.Bruch JP, Álvares-DA-Silva MR, Alves BC, Dall'alba V. Reduced hand grip strength in overweight and obese chronic hepatitis c patients. Arq Gastroenterol. 2016;53:31–35. doi: 10.1590/S0004-28032016000100007. [DOI] [PubMed] [Google Scholar]
- 6.Normatov I, Kaplan S, Azzam RK. Nutrition in Pediatric Chronic Liver Disease. Pediatr Ann. 2018;47:e445–e451. doi: 10.3928/19382359-20181022-03. [DOI] [PubMed] [Google Scholar]
- 7.Kalaitzakis E, Bosaeus I, Ohman L, Björnsson E. Altered postprandial glucose, insulin, leptin, and ghrelin in liver cirrhosis: correlations with energy intake and resting energy expenditure. Am J Clin Nutr. 2007;85:808–815. doi: 10.1093/ajcn/85.3.808. [DOI] [PubMed] [Google Scholar]
- 8.Plauth M, Schütz ET. Cachexia in liver cirrhosis. Int J Cardiol. 2002;85:83–87. doi: 10.1016/s0167-5273(02)00236-x. [DOI] [PubMed] [Google Scholar]
- 9.Gunnarsdottir SA, Sadik R, Shev S, Simrén M, Sjövall H, Stotzer PO, Abrahamsson H, Olsson R, Björnsson ES. Small intestinal motility disturbances and bacterial overgrowth in patients with liver cirrhosis and portal hypertension. Am J Gastroenterol. 2003;98:1362–1370. doi: 10.1111/j.1572-0241.2003.07475.x. [DOI] [PubMed] [Google Scholar]
- 10.Owen OE, Reichle FA, Mozzoli MA, Kreulen T, Patel MS, Elfenbein IB, Golsorkhi M, Chang KH, Rao NS, Sue HS, Boden G. Hepatic, gut, and renal substrate flux rates in patients with hepatic cirrhosis. J Clin Invest. 1981;68:240–252. doi: 10.1172/JCI110240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ooi PH, Gilmour SM, Yap J, Mager DR. Effects of branched chain amino acid supplementation on patient care outcomes in adults and children with liver cirrhosis: A systematic review. Clin Nutr ESPEN. 2018;28:41–51. doi: 10.1016/j.clnesp.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65:1232–1244. doi: 10.1016/j.jhep.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grossmann M, Hoermann R, Gani L, Chan I, Cheung A, Gow PJ, Li A, Zajac JD, Angus P. Low testosterone levels as an independent predictor of mortality in men with chronic liver disease. Clin Endocrinol (Oxf) 2012;77:323–328. doi: 10.1111/j.1365-2265.2012.04347.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhao VM, Ziegler TR. Nutrition support in end-stage liver disease. Crit Care Nurs Clin North Am. 2010;22:369–380. doi: 10.1016/j.ccell.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Peng S, Plank LD, McCall JL, Gillanders LK, McIlroy K, Gane EJ. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85:1257–1266. doi: 10.1093/ajcn/85.5.1257. [DOI] [PubMed] [Google Scholar]
- 16.Li D, Li Y, Dai W, Wang H, Qiu C, Feng S, Zhou Q, Wang W, Feng X, Yao K, Liu Y, Yang Y, Yang Z, Xu X, Li S, Wei J, Zhou K. Intestinal Bacteroides sp. Imbalance Associated With the Occurrence of Childhood Undernutrition in China. Front Microbiol. 2019;10:2635. doi: 10.3389/fmicb.2019.02635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinh DM, Ramadass B, Kattula D, Sarkar R, Braunstein P, Tai A, Wanke CA, Hassoun S, Kane AV, Naumova EN, Kang G, Ward HD. Longitudinal Analysis of the Intestinal Microbiota in Persistently Stunted Young Children in South India. PLoS One. 2016;11:e0155405. doi: 10.1371/journal.pone.0155405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace JM. Comment on "The ultrastructure of type I collagen at nanoscale: large or small D-spacing distribution? Nanoscale. 2015;7:1233–1234. doi: 10.1039/c4nr03160a. [DOI] [PubMed] [Google Scholar]
- 19.Stadlbauer V, Komarova I, Klymiuk I, Durdevic M, Reisinger A, Blesl A, Rainer F, Horvath A. Disease severity and proton pump inhibitor use impact strongest on faecal microbiome composition in liver cirrhosis. Liver Int. 2020;40:866–877. doi: 10.1111/liv.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horvath A, Rainer F, Bashir M, Leber B, Schmerboeck B, Klymiuk I, Groselj-Strele A, Durdevic M, Freedberg DE, Abrams JA, Fickert P, Stiegler P, Stadlbauer V. Biomarkers for oralization during long-term proton pump inhibitor therapy predict survival in cirrhosis. Sci Rep. 2019;9:12000. doi: 10.1038/s41598-019-48352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, Ma M, Abraldes JG, Paterson I, Haykowsky MJ, Tandon P. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1920–6.e2. doi: 10.1016/j.cgh.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Li TH, Liu CW, Huang CC, Tsai YL, Huang SF, Yang YY, Tsai CY, Hou MC, Lin HC. Non-Selective Beta-Blockers Decrease Infection, Acute Kidney Injury Episodes, and Ameliorate Sarcopenic Changes in Patients with Cirrhosis: A Propensity-Score Matching Tertiary-Center Cohort Study. J Clin Med. 2021;10 doi: 10.3390/jcm10112244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lattanzi B, Gioia S, Di Cola S, D'Ambrosio D, Nardelli S, Tavano D, Farcomeni A, Merli M, Riggio O. Prevalence and impact of sarcopenia in non-cirrhotic portal hypertension. Liver Int. 2019;39:1937–1942. doi: 10.1111/liv.14160. [DOI] [PubMed] [Google Scholar]
- 24.Plauth M, Schütz T, Buckendahl DP, Kreymann G, Pirlich M, Grüngreiff S, Romaniuk P, Ertl S, Weiss ML, Lochs H. Weight gain after transjugular intrahepatic portosystemic shunt is associated with improvement in body composition in malnourished patients with cirrhosis and hypermetabolism. J Hepatol. 2004;40:228–233. doi: 10.1016/j.jhep.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Allard JP, Chau J, Sandokji K, Blendis LM, Wong F. Effects of ascites resolution after successful TIPS on nutrition in cirrhotic patients with refractory ascites. Am J Gastroenterol. 2001;96:2442–2447. doi: 10.1111/j.1572-0241.2001.04051.x. [DOI] [PubMed] [Google Scholar]
- 26.Arora S, Mattina C, McAnenny C, O’Sullivan N, McGeeney L, Calder N, Gatiss G, Davidson B, Morgan M. 608 The development and validation of a nutritional prioritising tool for use in patients with chronic liver disease. J Hepatol 2012;56 Suppl 2, S241. [Google Scholar]
- 27.Borhofen SM, Gerner C, Lehmann J, Fimmers R, Görtzen J, Hey B, Geiser F, Strassburg CP, Trebicka J. The Royal Free Hospital-Nutritional Prioritizing Tool Is an Independent Predictor of Deterioration of Liver Function and Survival in Cirrhosis. Dig Dis Sci. 2016;61:1735–1743. doi: 10.1007/s10620-015-4015-z. [DOI] [PubMed] [Google Scholar]
- 28.Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485–521. doi: 10.1016/j.clnu.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White JV, Guenter P, Jensen G, Malone A, Schofield M Academy Malnutrition Work Group; A. S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) JPEN J Parenter Enteral Nutr. 2012;36:275–283. doi: 10.1177/0148607112440285. [DOI] [PubMed] [Google Scholar]
- 30.Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv Nutr. 2016;7:121–134. doi: 10.3945/an.115.009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tandon P, Raman M, Mourtzakis M, Merli M. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology. 2017;65:1044–1057. doi: 10.1002/hep.29003. [DOI] [PubMed] [Google Scholar]
- 32.De Keyzer W, Huybrechts I, De Vriendt V, Vandevijvere S, Slimani N, Van Oyen H, De Henauw S. Repeated 24-hour recalls versus dietary records for estimating nutrient intakes in a national food consumption survey. Food Nutr Res. 2011;55 doi: 10.3402/fnr.v55i0.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, Esfandiari N, Baracos V, Montano-Loza AJ, Myers RP. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209–1216. doi: 10.1002/lt.23495. [DOI] [PubMed] [Google Scholar]
- 34.Tandon P, Low G, Mourtzakis M, Zenith L, Myers RP, Abraldes JG, Shaheen AA, Qamar H, Mansoor N, Carbonneau M, Ismond K, Mann S, Alaboudy A, Ma M. A Model to Identify Sarcopenia in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2016;14:1473–1480.e3. doi: 10.1016/j.cgh.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 35.Keller U. Nutritional Laboratory Markers in Malnutrition. J Clin Med. 2019;8 doi: 10.3390/jcm8060775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11:8–13. doi: 10.1177/014860718701100108. [DOI] [PubMed] [Google Scholar]
- 37.Hasse J, Strong S, Gorman MA, Liepa G. Subjective global assessment: alternative nutrition-assessment technique for liver-transplant candidates. Nutrition. 1993;9:339–343. [PubMed] [Google Scholar]
- 38.Ferreira LG, Anastácio LR, Lima AS, Correia MI. Assessment of nutritional status of patients waiting for liver transplantation. Clin Transplant. 2011;25:248–254. doi: 10.1111/j.1399-0012.2010.01228.x. [DOI] [PubMed] [Google Scholar]
- 39.Morgan MY, Madden AM, Soulsby CT, Morris RW. Derivation and validation of a new global method for assessing nutritional status in patients with cirrhosis. Hepatology. 2006;44:823–835. doi: 10.1002/hep.21358. [DOI] [PubMed] [Google Scholar]
- 40.Sasidharan M, Nistala S, Narendhran RT, Murugesh M, Bhatia SJ, Rathi PM. Nutritional status and prognosis in cirrhotic patients. Trop Gastroenterol. 2012;33:257–264. [PubMed] [Google Scholar]
- 41.Kalafateli M, Mantzoukis K, Choi Yau Y, Mohammad AO, Arora S, Rodrigues S, de Vos M, Papadimitriou K, Thorburn D, O'Beirne J, Patch D, Pinzani M, Morgan MY, Agarwal B, Yu D, Burroughs AK, Tsochatzis EA. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J Cachexia Sarcopenia Muscle. 2017;8:113–121. doi: 10.1002/jcsm.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai JC, Rahimi RS, Verna EC, Kappus MR, Dunn MA, McAdams-DeMarco M, Haugen CE, Volk ML, Duarte-Rojo A, Ganger DR, O'Leary JG, Dodge JL, Ladner D, Segev DL. Frailty Associated With Waitlist Mortality Independent of Ascites and Hepatic Encephalopathy in a Multicenter Study. Gastroenterology. 2019;156:1675–1682. doi: 10.1053/j.gastro.2019.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14:1870–1879. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alberino F, Gatta A, Amodio P, Merkel C, Di Pascoli L, Boffo G, Caregaro L. Nutrition and survival in patients with liver cirrhosis. Nutrition. 2001;17:445–450. doi: 10.1016/s0899-9007(01)00521-4. [DOI] [PubMed] [Google Scholar]
- 45.Morgan MY, Madden AM, Jennings G, Elia M, Fuller NJ. Two-component models are of limited value for the assessment of body composition in patients with cirrhosis. Am J Clin Nutr. 2006;84:1151–1162. doi: 10.1093/ajcn/84.5.1151. [DOI] [PubMed] [Google Scholar]
- 46.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 48.van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ, IJzermans JN. Systematic Review and Meta-Analysis of the Impact of Computed Tomography-Assessed Skeletal Muscle Mass on Outcome in Patients Awaiting or Undergoing Liver Transplantation. Am J Transplant. 2016;16:2277–2292. doi: 10.1111/ajt.13732. [DOI] [PubMed] [Google Scholar]
- 49.Campbell IT, Watt T, Withers D, England R, Sukumar S, Keegan MA, Faragher B, Martin DF. Muscle thickness, measured with ultrasound, may be an indicator of lean tissue wasting in multiple organ failure in the presence of edema. Am J Clin Nutr. 1995;62:533–539. doi: 10.1093/ajcn/62.3.533. [DOI] [PubMed] [Google Scholar]
- 50.Woodward AJ, Wallen MP, Ryan J, Ward LC, Coombes JS, Macdonald GA. Evaluation of techniques used to assess skeletal muscle quantity in patients with cirrhosis. Clin Nutr ESPEN. 2021;44:287–296. doi: 10.1016/j.clnesp.2021.05.029. [DOI] [PubMed] [Google Scholar]
- 51.Strauss BJ, Gibson PR, Stroud DB, Borovnicar DJ, Xiong DW, Keogh J. Total body dual X-ray absorptiometry is a good measure of both fat mass and fat-free mass in liver cirrhosis compared to "gold-standard" techniques. Melbourne Liver Group. Ann N Y Acad Sci. 2000;904:55–62. doi: 10.1111/j.1749-6632.2000.tb06421.x. [DOI] [PubMed] [Google Scholar]
- 52.Belarmino G, Gonzalez MC, Sala P, Torrinhas RS, Andraus W, D'Albuquerque LAC, Pereira RMR, Caparbo VF, Ferrioli E, Pfrimer K, Damiani L, Heymsfield SB, Waitzberg DL. Diagnosing Sarcopenia in Male Patients With Cirrhosis by Dual-Energy X-Ray Absorptiometry Estimates of Appendicular Skeletal Muscle Mass. JPEN J Parenter Enteral Nutr. 2018;42:24–36. doi: 10.1177/0148607117701400. [DOI] [PubMed] [Google Scholar]
- 53.Eriksen CS, Kimer N, Suetta C, Møller S. Arm lean mass determined by dual-energy X-ray absorptiometry is superior to characterize skeletal muscle and predict sarcopenia-related mortality in cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2021;320:G729–G740. doi: 10.1152/ajpgi.00478.2020. [DOI] [PubMed] [Google Scholar]
- 54.Sinclair M, Hoermann R, Peterson A, Testro A, Angus PW, Hey P, Chapman B, Gow PJ. Use of Dual X-ray Absorptiometry in men with advanced cirrhosis to predict sarcopenia-associated mortality risk. Liver Int. 2019;39:1089–1097. doi: 10.1111/liv.14071. [DOI] [PubMed] [Google Scholar]
- 55.Bunchorntavakul C, Supanun R, Atsawarungruangkit A. Nutritional Status and its Impact on Clinical Outcomes for Patients Admitted to Hospital with Cirrhosis. J Med Assoc Thai. 2016;99 Suppl 2:S47–S55. [PubMed] [Google Scholar]
- 56.Maharshi S, Sharma BC, Srivastava S. Malnutrition in cirrhosis increases morbidity and mortality. J Gastroenterol Hepatol. 2015;30:1507–1513. doi: 10.1111/jgh.12999. [DOI] [PubMed] [Google Scholar]
- 57.Huisman EJ, Trip EJ, Siersema PD, van Hoek B, van Erpecum KJ. Protein energy malnutrition predicts complications in liver cirrhosis. Eur J Gastroenterol Hepatol. 2011;23:982–989. doi: 10.1097/MEG.0b013e32834aa4bb. [DOI] [PubMed] [Google Scholar]
- 58.Lindqvist C, Majeed A, Wahlin S. Body composition assessed by dual-energy X-ray absorptiometry predicts early infectious complications after liver transplantation. J Hum Nutr Diet. 2017;30:284–291. doi: 10.1111/jhn.12417. [DOI] [PubMed] [Google Scholar]
- 59.Ruiz-Margáin A, Macías-Rodríguez RU, Ampuero J, Cubero FJ, Chi-Cervera L, Ríos-Torres SL, Duarte-Rojo A, Espinosa-Cuevas Á, Romero-Gómez M, Torre A. Low phase angle is associated with the development of hepatic encephalopathy in patients with cirrhosis. World J Gastroenterol. 2016;22:10064–10070. doi: 10.3748/wjg.v22.i45.10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gunsar F, Raimondo ML, Jones S, Terreni N, Wong C, Patch D, Sabin C, Burroughs AK. Nutritional status and prognosis in cirrhotic patients. Aliment Pharmacol Ther. 2006;24:563–572. doi: 10.1111/j.1365-2036.2006.03003.x. [DOI] [PubMed] [Google Scholar]
- 61.Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–173, 173.e1. doi: 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 62.Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, Holcombe SA, Wang SC, Segev DL, Sonnenday CJ. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montano-Loza AJ, Angulo P, Meza-Junco J, Prado CM, Sawyer MB, Beaumont C, Esfandiari N, Ma M, Baracos VE. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7:126–135. doi: 10.1002/jcsm.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tandon P, Montano-Loza AJ, Lai JC, Dasarathy S, Merli M. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol. 2021;75 Suppl 1:S147–S162. doi: 10.1016/j.jhep.2021.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]