Abstract
In response to a wide variety of environmental stimuli, the opportunistic fungal pathogen Candida albicans exits the budding cycle, producing germ tubes and hyphae concomitant with expression of virulence genes, such as that encoding hyphal wall protein 1 (HWP1). Biochemical studies implicate cyclic AMP (cAMP) increases in promoting bud-hypha transitions, but genetic evidence relating genes that control cAMP levels to bud-hypha transitions has not been reported. Adenylate cyclase-associated proteins (CAPs) of nonpathogenic fungi interact with Ras and adenylate cyclase to increase cAMP levels under specific environmental conditions. To initiate studies on the relationship between cAMP signaling and bud-hypha transitions in C. albicans, we identified, cloned, characterized, and disrupted the C. albicans CAP1 gene. C. albicans strains with inactivated CAP1 budded in conditions that led to germ tube formation in isogenic strains with CAP1. The addition of 10 mM cAMP and dibutyryl cAMP promoted bud-hypha transitions and filamentous growth in the cap1/cap1 mutant in liquid and solid media, respectively, showing clearly that cAMP promotes hypha formation in C. albicans. Increases in cytoplasmic cAMP preceding germ tube emergence in strains having CAP1 were markedly diminished in the budding cap1/cap1 mutant. C. albicans strains with deletions of both alleles of CAP1 were avirulent in a mouse model of systemic candidiasis. The avirulence of a germ tube-deficient cap1/cap1 mutant coupled with the role of Cap1 in regulating cAMP levels shows that the Cap1-mediated cAMP signaling pathway is required for bud-hypha transitions, filamentous growth, and the pathogenesis of candidiasis.
For many pathogenic fungi, interconversions between morphological growth forms, particularly between yeast growth and filamentous growth, coincide with adaptation to a host environment followed by tissue destruction. Morphological interconversions in fungi are dependent upon signal transduction pathways, including the cyclic AMP (cAMP)-dependent protein kinase A (PKA) pathway (8, 10, 28, 40, 46). For the plant pathogens Ustilago maydis and Magnaporthe grisea, cAMP signaling is important for the establishment of filamentous growth in the former and for formation of the infecting appressorium structure of the latter (40, 46). Knowledge about how cAMP signaling mediates morphological interconversion is best understood for Saccharomyces cerevisiae, a budding yeast that produces elongated pseudohyphal cells and forms filamentous colonies in the presence of limiting nitrogen (28, 46). Pseudohyphae exhibit unipolar budding, do not separate, and invade agar (31). Recent experiments involving gene disruption and epistasis analyses have elucidated both upstream and downstream elements of the cAMP-dependent pseudohyphal growth pathway in S. cerevisiae (28, 40, 46). Adenylate cyclase is activated either through a receptor (Gpr1) that is coupled to a G protein (Gpa2) or by Ras2 (31, 41, 52, 53, 58, 80). The subsequent activation of PKA then results in activation of the Flo8 transcription factor to produce a mucin-like protein, Flo11, that is localized to the cell surface and is required for pseudohyphal growth (44, 50, 63, 67). Although cross-talk between mitogen-activated protein kinase (MAPK) and cAMP signaling pathways is evident (58), transcription factor targets important for filamentous growth appear not to be shared by the two pathways (28, 46). Pseudohyphal defects caused by mutations in STE12 of the MAPK pathway and PHD1 are suppressed by the constitutive activation of PKA through deletion of the regulatory subunit gene (BCY1) (63).
Candida albicans is a common, opportunistic fungal pathogen that exhibits both budding and filamentous growth when proliferating in host tissues. Filamentous growth of C. albicans includes not only the pseudohyphal, elongated yeast-like forms described for S. cerevisiae but true hyphae as well. Compared to that of most pathogenic fungi, the morphological response of C. albicans to environmental conditions is rapid. Germ tubes are produced within 1 h of placing cells in appropriate conditions. The mechanisms employed by C. albicans to quickly achieve this apparently advantageous spectrum of growth morphologies along with optimized metabolic activities are poorly understood.
The relative contribution of yeast and filamentous forms to the pathogenesis of candidiasis is an unresolved issue. However, mutants that do not produce hyphae in vitro have reduced virulence in animal models (29, 49, 73). Expression of hypha-specific virulence factors, such as the hyphal wall protein (HWP1) adhesin gene (75, 76) and secreted aspartyl proteinase (SAP) genes (68, 77), are correlated with the virulence of hyphal forms. Research into the mechanisms that lead to the production of these virulence factors is important for developing strategies to interfere with candidiasis. Studies of the role of cAMP-dependent signaling in morphogenesis may also bring to light common virulence pathways for distantly related fungal pathogens.
Biochemical studies implicate cAMP increases in promoting bud-hypha transitions of C. albicans. Intracellular levels of cAMP increase and, under nutrient limitation, exogenous cAMP or dibutyryl cAMP (dbcAMP) increases the frequency of bud-hypha transitions (14, 61, 62, 86). Inhibitors of cAMP phosphodiesterase or cAMP-dependent protein kinase induce or block germ tube formation, respectively (13, 14). However, genetic studies involving mutational analysis of genes that control cAMP levels and assessment of their role(s) in regulating bud-hypha transitions and filamentous growth have not been reported.
In S. cerevisiae, Ras activation of adenylate cyclase also involves the adenylate cyclase-associated protein (CAP, also known as Srv2p) (20, 24, 72). The CAP gene was identified in a genetic screen for mutants that suppressed defective growth of a strain carrying an inducible hyperactive RAS2val19 gene (20). The CAP gene was also isolated by screening a yeast cDNA expression library with antisera to a 70-kDa protein that copurified with adenylate cyclase (24). CAP is required for normal budding morphology and growth rates in nutrient-rich media (20, 24). Interestingly, the S. cerevisiae CAP gene has been shown to be involved in pseudohyphal differentiation using transposon mutagenesis to screen for mutant strains defective for filamentous growth (57). CAPs of mice (82) and humans (55) are 34% identical and 35% similar to S. cerevisiae CAP, showing that CAP genes are conserved throughout evolution. Although CAPs from different organisms have similar primary and secondary structures, the function of CAPs in developmental programs has diverged among fungi. CAP mutants of Schizosaccharomyces pombe but not S. cerevisiae conjugate and sporulate in inappropriate conditions (35).
Modulation of adenylate cyclase activity by CAP in S. cerevisiae (24, 85) suggested that the CAP gene of C. albicans might affect intracellular cAMP levels, allowing assessment of the role of cAMP in the filamentous growth and virulence of C. albicans. The C. albicans CAP1 gene was cloned, and its identity was established by the presence of sequence similarities to CAP gene products of other organisms, by the reduction in cAMP levels in cap1/cap1 mutants, and by the ability of exogenous cAMP or dbcAMP to promote bud-hypha transitions and filamentous growth in cap1/cap1 mutants. cap1/cap1 mutants were unconditionally deficient in forming bud-hypha transitions and filamentous growth in rich and minimal, liquid and agar-based culture media as well as in serum and saliva at 37°C. Predictably, cap1/cap1 mutants also showed reduced virulence in a systemic model of candidiasis. This is the first report to provide genetic evidence showing that increases in cAMP promote true hypha formation in C. albicans. Interference with CAP1 function has potential for providing novel strategies for interfering with candidiasis.
MATERIALS AND METHODS
C. albicans strains and growth conditions.
C. albicans strains are listed in Table 1. Yeast forms were grown in yeast extract peptone dextrose (YPD) or a yeast nitrogen base containing 50 mM glucose (YNB) (66). Mass conversion of stationary-phase yeasts (grown at 30°C for 48 h) to germ tubes was induced at 37°C in the following prewarmed media: Lee's (pH 6.8) (45), medium 199 (Gibco-BRL) with 150 mM HEPES (pH 7.0) (M199), M199 containing 5% bovine calf serum (Sigma) (M199+serum), 50 mM potassium phosphate (pH 6.0) plus 10% bovine calf serum (23), and 10 mM imidazole-HCl buffer (pH 7.0) containing 0.2 mM MnCl2 (with the following inducing agents: 4 mM N-acetylglucosamine, 10 mM l-proline plus 10 mM glucose, or 2.5 mM glutamine plus 2.5 mM glucose) (18, 49, 71). Whole human saliva was collected on ice and clarified by centrifugation at 10,000 × g for 15 min at 4°C (37). Tetracycline was added to clarified saliva at a concentration of 50 μg/ml to inhibit bacterial growth.
TABLE 1.
C. albicans strains used in this study
| Strain | Genotype | Parent strain | Reference |
|---|---|---|---|
| SC5314 | Wild type | 30 | |
| CAI4 | Δura3::imm434/Δura3::imm434 | SC5314 | 25 |
| UnoPP-1a | Δura3::imm434/Δura3::imm434 Δeno1::URA3/ENO1 | CAI4 | 65 |
| CAC1 | Δura3::imm434/Δura3::imm434 CAP1/cap1::hisG-URA3-hisG | CAI4 | This study |
| CAC1-1 | Δura3::imm434/Δura3::imm434 CAP1/cap1::hisG | CAC1 | This study |
| CAC1-1A | Δura3::imm434/Δura3::imm434 cap1::hisG/cap1::hisG-URA3-hisG | CAC1-1 | This study |
| CAC1-1A1 | Δura3::imm434/Δura3::imm434 cap1::hisG/cap1::hisG | CAC1-1A | This study |
| CACRE1 | Δura3::imm434/Δura3::imm434 CAP1/cap1::hisG ENO1/eno1::URA3 | CAC1-1A1 | This study |
A CAI4 derivative made Ura+ by disruption of an enolase gene with URA3 (65).
For growth analysis in agar-containing media, stationary-phase yeasts were mixed (100 cells/20 ml of medium) with liquefied agar containing M199 adjusted to a neutral pH with 7.5% sodium bicarbonate, Spider medium (48), 2% agar containing 4% bovine calf serum (49), and synthetic low ammonium dextrose (SLAD) containing 50 μM ammonium sulfate (17). Filamentous growth on YPD agar was assessed by streaking strains on YPD plates followed by incubation at room temperature for 2 weeks. Each plate was examined daily for the presence of filamentous growth.
To determine the effect of exogenous cAMP on bud-hypha transitions and filamentous growth of cap1/cap1 mutants, stationary-phase yeasts were induced to form germ tubes and hyphae in liquid M199+serum (106 cells/ml) or in SLAD agar plates containing 10 mM cAMP or dbcAMP (Sigma). M199+serum containing cAMP or dbcAMP was incubated at 37°C for 20 h, and the frequency of germ tube formation was measured at various time points. SLAD plates containing cAMP or dbcAMP were incubated at 37°C for 5 days, and filamentous growth was monitored daily.
Isolation and DNA sequencing of cDNA and genomic clones for CAP1.
CAP1 cDNA clones were found while attempting to identify germ tube-specific surface antigens by screening a C. albicans germ tube cDNA library (78), but cDNAs encoding cell wall surface proteins were not found. Five of the thirteen cDNA clones isolated encoded proteins with homology to adenylate cyclase-associated proteins. pBluescript SK(−) phagemids of the five clones were rescued by in vivo excision (Stratagene) according to the manufacturer's directions. pCAP1, with a 1,655-bp CAP1 cDNA insert, was analyzed further.
Three λ genomic CAP1 clones (CAP2, CAP3, and CAP5) were isolated by screening a λGEM12 genomic library of C. albicans SC5314 (6) with CAP1 cDNA excised from pCAP1 with XbaI and XhoI. pGHCP17 was constructed by subcloning the 3.7-kbp CAP1 genomic HindIII fragment of CAP5 into pBluescript SK(−) and transforming Escherichia coli HB 101 (9). DNA sequences of cDNA and genomic clones were determined by automated cycle sequencing using an automated DNA sequencer (ABI Prism, model 377 and 373; Perkin-Elmer Co.).
The complete genomic DNA sequence of CAP1 was compared with the sequence of SRV2 in the current assembly 6 of the C. albicans genomic sequences from the Stanford DNA Sequencing and Technology Center website (http://www-sequence.stanford.edu/group/candida).
Disruption of CAP1.
To disrupt CAP1 in C. albicans, plasmid pCAPURA3 was constructed by replacing a 132-bp StyI-BsmI segment of CAP1 cDNA in pCAP1 with the 4.0-kbp BamHI-BglII hisG-URA3-hisG cassette from p5921 (25) after generating blunt ends using T4 DNA polymerase (Gibco-BRL) and the Klenow fragment of E. coli DNA polymerase I. E. coli HB101 served as the host strain for transformation and propagation of pCAPURA3.
CAI4 (CAP1/CAP1 ura3/ura3) was transformed using spheroplast transformation (42) with 10 μg of pCAPURA3 digested with PstI to release the CAP1 disruption cassette. Ura+ transformants with a CAP1/cap1::hisG-URA3-hisG genotype were identified by Southern blotting using HindIII-digested genomic DNA prepared by the method of Scherer and Stevens (69). Southern blots were probed with hisG-URA3-hisG from p5921 and PCR-1.2 (Fig. 1A). PCR-1.2 (nucleotides 98 to 1318) was generated by PCR using pGHCP17 as a template and oligonucleotides CAP-R4 (5′-CCATTTTCCAAGAGGAAGCA-3′) and CAP-F4 (5′-CCGACACTGCATTTGCTTTA-3′). Probes were labeled using the enhanced chemiluminescence (ECL) Direct Nucleic Acid Labeling and Detection System (Amersham). CAC1-1 (ura3/ura3 CAP1/cap1::hisG) was selected on YNB media (0.002% uridine) containing 0.05% 5-fluoroorotic acid (7) and used in a second round of transformation to disrupt the remaining copy of CAP1. Colony PCR (81), using the TaqPlus Long PCR system (Stratagene) with primers CAP-R4 and CAP-F4, and Southern blotting were used to determine genotypes. Gene inactivation was confirmed by Northern blot analysis and reverse transcription-PCR (RT-PCR).
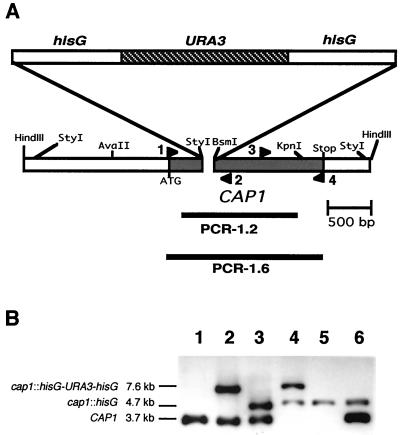
FIG. 1.
Disruption of C. albicans CAP1. (A) Genetic organization of the CAP1 locus. The CAP1 open reading frame (shaded bar) and PCR products (solid line) (PCR-1.2 and PCR-1.6) are indicated. Each arrowhead indicates primers used for RT-PCR to confirm the disruption of CAP1 (1, CAP-NRT1; 2, CAP-F1; 3, CAP-R3; 4, CAP-3F1). (B) Southern blot analysis of HindIII-digested C. albicans genomic DNA probed with PCR-1.2 as described in Materials and Methods. Lanes: 1, parental strain CAI4; 2 and 3, CAP1/cap1 strains CAC1 and CAC1-1, Ura+ and Ura−, respectively; 4 and 5, homozygous cap1/cap1 strains CAC1-1A and CAC1-1A1, Ura+ and Ura−, respectively; 6, CAP1-complemented strain CACRE1.
Complementation of cap1/cap1 mutants at the CAP1 genomic locus was accomplished by cotransformation of a ura3 homozygous cap1/cap1 mutant strain, CAC1-1A1, with eno::URA3 (75) and PCR-1.2, creating CACRE1. DNA sequencing of genomic DNA clones from CACRE1 confirmed that mutations were not inadvertently introduced from PCR-1.2 into the CAP1 locus in the revertant (data not shown).
Cell morphologies were examined using a 40× or 20× objective and differential interference contrast microscopy (OLYMPUS B×60) and photographed (OLYMPUS Magnafire, model S99806). Colonial morphologies were examined using a stereomicroscope (OLYMPUS SZX12) (1.6× objective) with a transmitted light console base or OLYMPUS BX60 microscope (4× objective), and cellular morphologies at colony rims were examined with bright-field illumination using a light microscope (LABOPHOT-2; Nikon) (10× objective) equipped with a charge-coupled device video camera system (OPTRONICS). Photographed images were processed using Adobe PhotoShop 2.5.
Northern blot analysis.
Total RNA was isolated (76) from middle-logarithmic-phase yeasts cultured in 250 ml of YNB at 27°C or in germ tubes (yeasts for the cap1/cap1 mutant) cultured for 3 h in M199 at 37°C and treated with RNase-free DNase I (Gibco-BRL). Probes were PCR-1.2 (Fig. 1A) and a 687-bp PCR product amplified from the 18S rRNA gene of C. albicans SC5314 using primers (5′-ACTTTCGATGGTAGGATAG-3′ and 5′-TGATCATCTTCGATCCCCTA-3′) (54). Electrophoresis, radiolabeling of probes using the random primer method (21, 22), hybridization, and molecular size determination were performed as previously described (76), except that blots were hybridized first with the CAP1 probe (107 cpm), autoradiographed, and then hybridized with the 18S rRNA probe (106 cpm).
RT-PCR.
The first-strand cDNA was synthesized using 1 μg of total RNA according to the manufacturer's directions (Reverse Transcription System; Promega) and was diluted in a final 100-μl volume of nuclease-free water. Two PCR products, representing the 5′ (1 to 605) and 3′ (922 to 1634) portions of CAP1 message (Fig. 1A), were amplified from the first-strand cDNA (10 μl) using oligonucleotides CAP-NRT1 (5′-ATGTCAACCGAGGAGAGTCA-3′) and CAP-F1 (5′-ATGTACGAGATTGGTGTAGG-3′) and CAP-R3 (5′-AGTGAAAATCCATCTCCAGC-3′) and CAP-3F1 (5′-CCAGCATGTTCAACAATTTGAG-3′), respectively. ACT1 cDNA (304 bp), amplified using two ACT1-specific primers, ACT-3R (5′-GGAGTTGAAAGTGGTTTGGTCAATAC-3′) and ACT-5L (5′-GGCTGGTAGAGACTTGACCAACCATTTG-3′) (59), served as a control. PCR products were detected by Southern blotting using PCR-1.6, which spanned the entire CAP1 coding region, as a probe (Fig. 1A). PCR-1.6 (nucleotides 1 to 1634) was generated by PCR using pGHCP17 and oligonucleotides CAP-NRT1 and CAP-3F1. Probe PCR-1.6 was labeled with [α-32P]dCTP (Amersham) as for the Northern blot except that 2 × 106 cpm was added to the membrane.
cAMP assay.
Intracellular cAMP in M199 was extracted as previously described (20) and measured using the cAMP enzyme immunoassay (Amersham). Strains (UnoPP-1, CAC1, CAC1-1A, and CACRE1) were grown to middle-logarithmic phase (optical density at 600 nm [OD600] = 0.6 to 0.7) in M199 at 27°C and then inoculated (4 × 106 cells/ml) into M199 prewarmed to 37°C to induce germ tubes or fresh M199 at 27°C for budding growth. At each time point during germ tube formation (or budding in the case of the cap1/cap1 mutant), 27- and 1.5-ml portions were withdrawn for measurement of cAMP levels and protein concentrations, respectively.
Protein concentrations (Coomassie protein assay; Pierce) were determined on cell extracts from 1.5 ml of culture lysed by boiling for 5 min in 50 μl of 2 N NaOH. Bovine serum albumin (5 to 25 μg/ml) was used to generate a standard curve.
Virulence studies.
The role of the CAP1 gene in the pathogenesis of systemic candidiasis was investigated using male CBA/J mice (5 to 6 weeks old) as previously described (75). C. albicans strains (SC5314 [CAP1/CAP1], CAC1 [CAP1/cap1], CAC1-1A [cap1/cap1] and CACRE1 [CAP1/cap1, revertant]) were grown to stationary phase in peptone-dextrose media. Cells were then harvested, washed, and resuspended in pyrogen-free 0.9% NaCl at a concentration of 106 cells/ml. Four groups of mice (six per group) were injected via the lateral tail vein with 2 × 105 cells in a final volume of 200 μl in two independent studies. Survival was monitored daily. Kidney tissues were cultured on YPD plates to determine the numbers of CFU per gram of tissue and to verify germ tube formation phenotypes. Survival curves were illustrated by the Kaplan-Meier method using the PRISM program 2.0b (GraphPad Software, San Diego, Calif.), and statistical differences between paired groups were compared using the log-rank test.
Nucleotide sequence accession number.
The C. albicans CAP1 genomic sequence has been submitted to GenBank under accession no. AF163838.
RESULTS
Screening and DNA sequence analysis of genomic CAP1.
C. albicans cDNAs homologous to CAP (also called SRV2) genes (see Materials and Methods) were used to isolate three independent genomic clones, each containing a 3.7-kb HindIII fragment (diagrammed in Fig. 1A) found in C. albicans genomic DNA (Fig. 1B, lane 1). A gene encoding an open reading frame identical to that found in the cDNA was named CAP1 because of similarities to CAP genes from other organisms, as described below. The protein product of CAP1 was designated Cap1. Two silent nucleotide differences were found between C. albicans CAP1 and C. albicans SRV2 (reported by the Stanford DNA Sequencing and Technology Center) (assembly 6).
The predicted C. albicans Cap1 protein was 28 to 44% identical in overall primary amino acid sequence to CAPs from other organisms. The conserved RLE/RLE motif important for monomer association, protein localization, and Ras/cAMP-dependent signaling (72, 85, 87), the universally conserved and centrally located stretch of proline residues of unknown function, and two consensus SH3-binding motifs (PXXP) were found in C. albicans Cap1 (Fig. 2). Interestingly, the first 100 amino acids of C. albicans Cap1 showed more dissimilarities to CAPs from other organisms than did the remainder of the protein. The first 100 amino acids of C. albicans Cap1 showed only 28.2 and 26.5% identity to the corresponding regions of S. cerevisiae and S. pombe CAPs, respectively, compared with 45.1 and 41.1% identity in carboxy-terminal regions, respectively.
FIG. 2.
Primary structure alignment of C. albicans Cap1 with CAPs of other organisms. Multiple sequence alignments of CAPs from C. albicans (CaCAP1), S. cerevisiae (ScCAP), S. pombe (SpCAP), mouse (MouseCAP1), and human (HumanCAP1) were performed with ClustalW (79) and illustrated with MacVector 6.5.3 (Oxford Molecular Company). Solid lines indicate residues for the conserved RLE/RLE motif (21 to 30), the polyproline region (289 to 297), and two consensus SH3-binding motifs (358 to 361 and 364 to 367) in C. albicans.
Predicted secondary structures of C. albicans Cap1 and CAP of S. cerevisiae were strikingly conserved, with amino-terminal halves consisting of α-helices separated by loops with small regions of β-sheet, and carboxy-terminal thirds consisting of β-sheets and loops (not shown). The central domain containing prolines was predicted to be a loop in both proteins. Hydrophobicity profiles (43) of the two proteins were also similar.
Expression of the CAP1 gene.
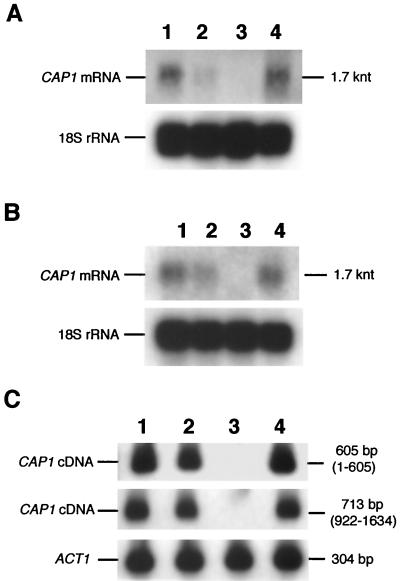
CAP1 was neither a highly expressed nor a developmentally regulated gene (Fig. 3). Detection of the 1.7-kb CAP1 transcript in yeast (Fig. 3A) and germ tube RNA (Fig. 3B) by Northern blotting required long exposure times. Low mRNA levels were consistent with unbiased codon usage in that the effective number of codons (83), 43.1, was typical of genes that are expressed at low levels, such as those for protein kinase C (PKC1) (64) and MAPK (MKC1) (60), with values of 45 and 54.8, respectively.
FIG. 3.
Northern blot and RT-PCR analysis of cap1/cap1 mutants. CAP1 mRNA is absent in the cap1/cap1 strain and present at equivalent low levels in other strains during yeast growth (A) or germ tube induction (B). Total RNA (7 μg/lane), isolated as described in Materials and Methods, was separated in a formaldehyde agarose gel transferred to a nitrocellulose membrane and probed with radiolabeled PCR-1.2 to detect CAP1 mRNA and 18S rRNA as a control. The membrane was exposed to X-ray film for 7 days for detection of CAP1 mRNA and for 4 h for detection of 18S rRNA. (C) Amplification of 5′ (605 bp, 1 to 605) and 3′ (713 bp, 922 to 1634) portions of CAP1 mRNA using RT-PCR followed by Southern blotting using radiolabeled PCR-1.6 as probe. ACT1 mRNA (304 bp) was amplified as a positive control. Lanes 1 to 4, strains UnoPP-1, CAC1, CAC1-1A, and CACRE1.
Construction of the cap1/cap1 mutant and CAP1-complemented strains of C. albicans.
Reiterative site-specific disruption of genomic CAP1 DNA sequences with hisG-URA3-hisG or hisG produced HindIII fragments of 7.6 and 4.7 kb in size, respectively, that hybridized to probes for CAP1 (Fig. 1B) and hisG-URA3-hisG DNA (not shown). To verify that phenotypes of the cap1/cap1 mutant were caused by disruption of CAP1 genes, a complemented strain, CACRE1, was constructed by reintroducing the wild-type CAP1 DNA into one of the cap1::hisG loci of the Ura− cap1/cap1 mutant using cotransformation (75). CAP1 disruption was confirmed by the absence of CAP1 mRNA in the cap1/cap1 mutant CAC1-1A in Northern blot analysis (Fig. 3A and B). To show that read-through or truncated CAP1 mRNA was not present in the cap1/cap1 mutant, RT-PCRs were performed using CAP1-specific primers. CAP1 mRNA could not be detected using a probe (PCR-1.6) which spans the entire coding region of CAP1 (Fig. 3C). Equivalent levels of ACT1 cDNA (304 bp) were present in all strains (Fig. 3C). The cap1/cap1 mutant does not have CAP1 mRNA and cannot produce full or truncated Cap1 proteins.
Analysis of cap1/cap1 mutants.
Growth rates of the cap1/cap1 mutant were equivalent to that of the other strains in rich medium (YPD) but were reduced in minimal medium (YNB) (Table 2). Budding appeared morphologically normal in both media (not shown).
TABLE 2.
Doubling times
| Strain | Doubling time (h)a in:
|
|||||
|---|---|---|---|---|---|---|
| Rich medium (YPD)
|
Minimal medium (YNB)
|
|||||
| 27°C | 30°C | 37°C | 27°C | 30°C | 37°C | |
| UnoPP-1 | 2.2 | 1.6 | 2.0 | 2.9 | 2.9 | 3.0 |
| CAC1 | 2.2 | 1.7 | 2.0 | 2.9 | 2.9 | 3.0 |
| CAC1-1A | 2.3 | 1.7 | 2.2 | 3.9 | 3.7 | 3.8 |
| CACRE1 | 2.2 | 1.7 | 2.1 | 2.8 | 2.9 | 2.9 |
Mean value from two independent experiments whose results differed by less than 20%.
Mass conversion of yeasts to germ tubes (bud-hypha transitions) was induced in liquid media. cap1/cap1 mutants were unconditionally deficient in producing germ tubes in liquid suspension compared to CAP1/cap1 and CAP1/CAP1 strains. For the latter strains, the percentages of yeasts with germ tubes approached 100% in Lee's medium (pH 6.8), M199, M199 with 5% bovine serum albumin, and saliva (Fig. 4). Media containing simple inducers also did not support germ tube production by cap1/cap1 yeasts (not shown). cap1/cap1 yeast cells in M199 with or without serum appeared elongated or pseudohyphal, but germ tubes were not seen. cap1/cap1 mutant cells budded in all conditions, as determined by cell counting and differential labeling of parent yeasts with anti-C. albicans antiserum, permitting unlabeled nascent buds and yeasts produced during the incubation period to be distinguished from inoculum yeasts (not shown).
FIG. 4.
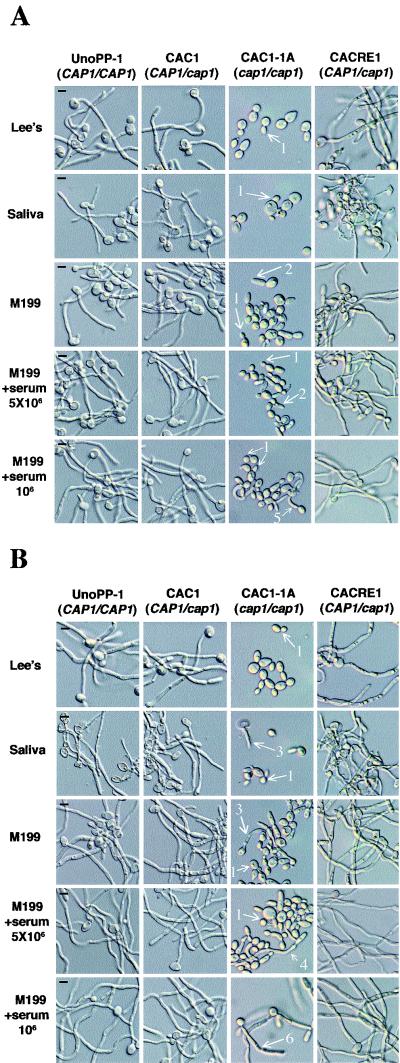
cap1/cap1 strains are defective in bud-hypha transitions. Germ tubes were induced at cell concentrations of 5 × 106 cells/ml (A and B, first four rows) or 106 cells/ml (A and B, bottom rows) in prewarmed Lee's medium, saliva, M199, or M199+serum for 5 h (A) and 20 h (B). cap1/cap1 mutant cells formed buds (arrows 1) or pseudohyphae at low frequency (arrows 2), whereas strains having CAP1 (UnoPP-1, CAC1, and CACRE1) produced typical germ tubes (A and B, first two and fourth columns). At 20 h a few cap1/cap1 mutant yeasts (<10%) produced germ tubes in saliva or M199 (arrows 3). In the presence of serum the frequency of germ tube formation was higher (20 to 30%) (arrow 4). Reducing the inoculum concentration in the presence of serum led to production of germ tubes by 40% of cap1/cap1 mutant yeasts at 5 h (arrow 5), and at 20 h the majority of yeasts had formed germ tubes that were shorter than those of the other strains (arrow 6). Bars, 5 μm.
Upon prolonged incubation, germ tubes were found at low frequencies in cultures of the cap1/cap1 mutant (Fig. 4B). After 20 h of incubation in M199 and in saliva, a few (<10%) cap1/cap1 yeast cells had germ tubes. In M199 containing 5% serum, the percentage was higher (approximately 20 to 30%), resembling cultures of wild-type strains inoculated at cell concentrations that exceed the threshold for germ tube formation (34). Reducing the inoculum led to the emergence of germ tubes in approximately 40% of the cells after 5 h of incubation in M199+serum. By 9 h, most cap1/cap1 mutant cells (>80%) had formed germ tubes (not shown). Germ tubes of cap1/cap1 mutant cells were shorter in length than wild-type germ tubes at 20 h. Further reductions in inoculum concentration did not lead to a higher frequency of germ tube formation. Germ tube formation in the cap1/cap1 mutant in the presence of serum was deficient in that the time required to form germ tubes averaged four to five times longer and average frequencies of germ tube-forming cells were reduced for cap1/cap1 mutant cells compared to strains with CAP1. Similar results were found in 10% serum with 50 mM potassium phosphate buffer (pH 6.0) (not shown).
The ability of cap1/cap1 mutant cells to form germ tubes upon prolonged incubation was limited to media containing serum. Lowering the cell concentration did not enhance germ tube formation in any of the other media tested, including saliva or M199 without serum.
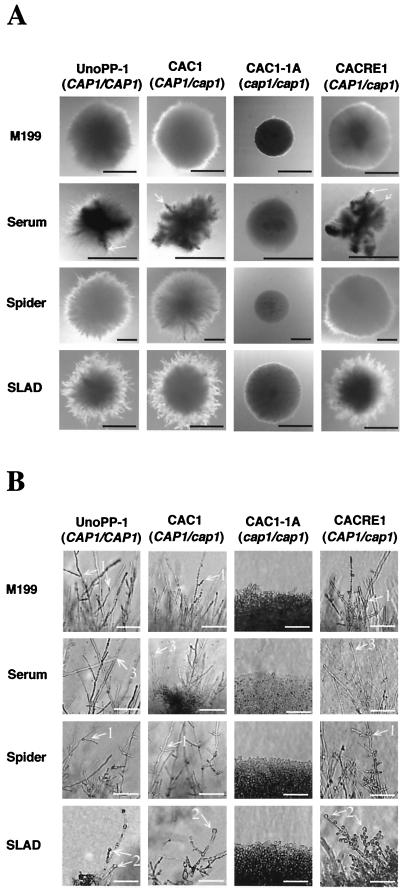
The cap1/cap1 mutant was also unconditionally deficient in producing filamentous growth on agar-containing media (Fig. 5). CAP1 strains grew predominantly as hyphae, but in some cases, pseudohyphae were also seen. The term “filamentous growth” refers collectively to the production of pseudohyphae as well as true hyphae. The periphery of colonies with circular symmetry of CAP1 strains in Spider or M199 medium consisted of extended hyphae with short branches, whereas hyphae in SLAD were septate, with numerous buds and thick-walled terminal buds resembling chlamydospores at hyphal tips. Characteristics of CAP1 strains in asymmetric colonies in serum media were mixed, consisting primarily of numerous branched hyphae bereft of buds and infrequent filaments coated with buds. The spectrum of morphological responses exhibited by strains with CAP1 was absent in colonies produced by cap1/cap1 mutant cells that consisted of budding yeasts independent of medium composition. Strains with CAP1 formed filamentous growth on YPD agar at as early as 1 week, but cap1/cap1 mutant colonies were devoid of filamentous growth even after 2 weeks of culture (not shown).
FIG. 5.
cap1/cap1 strains are defective in filamentous growth. Colonial appearances (A) and cellular morphologies at colony rims (B), respectively, in each agar medium condition are shown. (A) Colonies of the cap1/cap1 mutant consisted of budding yeasts (A and B, third columns), whereas strains with CAP1 (UnoPP-1, CAC1, and CACRE1) produced filamentous growths of differing characteristics depending on the media. The asymmetric colonies formed by strains with CAP1 in serum contained infrequent thick plumes composed of filaments covered with buds radiating from the colony center (arrow). (B) Strains with CAP1 produced uniform hyphae with short branches in M199 and Spider plates (arrows 1) or hyphae with thick-walled terminal buds in SLAD medium (arrow 2). In media with serum, colonies of strains with CAP1 were composed primarily of hyphae bereft of buds (arrow 3). M199 plates were incubated first at 30°C for 48 h and transferred to 37°C for another 48 h, whereas the other plates were incubated for 6 days at 37°C. Black (A) and white (B) bars, 1 mm and 50 μm, respectively.
A single allele of CAP1 was sufficient for normal bud-hypha transitions and filamentous growth of C. albicans. Differences in the timing of germ tube emergence, in the length of hyphae in liquid media, or in colonial morphologies in agar media between strains with one or two copies of the CAP1 gene were not observed.
Measurement of intracellular cAMP levels during germ tube induction.
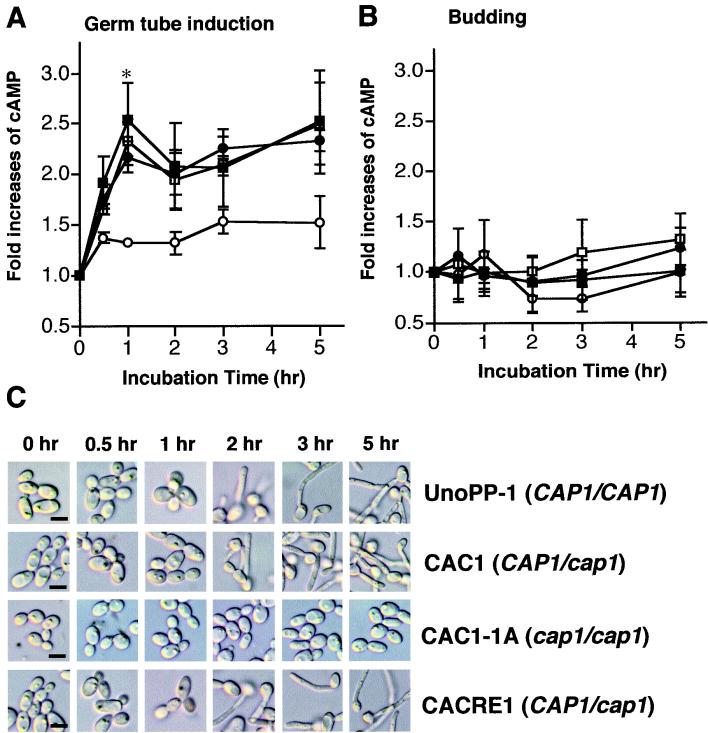
Cytoplasmic cAMP levels were measured under conditions that induce germ tubes (M199 at 37°C) or lead to budding (M199 at 27°C) in wild-type strains. Yeasts grown to middle-logarithmic phase in M199 at 27°C were used as the inoculum. Under germ tube-inducing conditions, the majority of the cells (>95%) in strains with CAP1 had germ tubes by 3 h, whereas cap1/cap1 cells produced buds (Fig. 6C).
FIG. 6.
Reduced cAMP levels of the C. albicans cap1/cap1 mutant in germ tube-inducing conditions compared to those of strains with CAP1. Intracellular cAMP levels for each strain (UnoPP-1 [CAP1/CAP1] [□], CAC1 [CAP1/cap1] [■], CACRE1 [CAP1/cap1] [●], and CAC1-1A [cap1/cap1] [○]) were measured as described in Materials and Methods. Each value in the y axis indicates the fold increase in cAMP over the basal level in each strain at time zero. Error bars indicate the standard deviation of each value from three independent experiments performed in triplicate. (A) Germ tube-inducing conditions (M199 at 37°C). cAMP levels (picomoles per milligram of protein) at time zero for UnoPP-1, CAC1, CAC1-1A, and CACRE1 were 45.3 ± 4.6, 55.1 ± 6.9, 61.8 ± 6.5, and 51.4 ± 6.7 (mean value ± standard deviation), respectively. The decreased cAMP level in the CAP1/cap1 mutant compared to results for strains with CAP1 at 1 h was statistically significant (asterisk, P < 0.01 [UnoPP-1 or CAC1 versus CAC1-1A] and P < 0.05 [CACRE1 versus CAC1-1A] using Bonferroni's multiple comparison test performed with Prism 2.0b [GraphPad Software]). (B) Budding growth in M199 at 27°C. cAMP levels (picomoles per milligram of protein) at time zero for UnoPP-1, CAC1, CAC1-1A, and CACRE1 were 50.9 ± 22.4, 58.1 ± 8.4, 37.4 ± 2.9, and 52.6 ± 6.6, respectively. (C) Morphological changes of UnoPP-1 (CAP1/CAP1), CAP1/cap1 strain (CAC1 and CACRE1), and cap1/cap1 strain (CAC1-1A) were monitored during germ tube induction. Bars, 5 μm.
Intracellular cAMP levels of strains with CAP1 increased sharply after placement in induction conditions, peaking at levels that were 2- to 2.5-fold higher than initial concentrations at 1 h (Fig. 6A). After a small decrease at 2 h, cAMP levels gradually increased over the 5-h incubation period. Consistent with the results for germ tube induction described above, copy number effects were not seen for CAP1 in regulating cAMP levels prior to germ tube emergence. Significant differences in cAMP levels between CAP1/CAP1 and CAP1/cap1 strains were not observed. The cap1/cap1 mutant exhibited a small increase in cAMP at 30 min that plateaued and achieved only a 1.5-fold increase over the 5-h period.
The increase in the cAMP level for CAP1 strains was not seen under conditions where germ tubes were not induced (Fig. 6B).
The effect of cAMP or dbcAMP on colonial morphologies and bud-hypha transitions of the cap1/cap1 mutant.
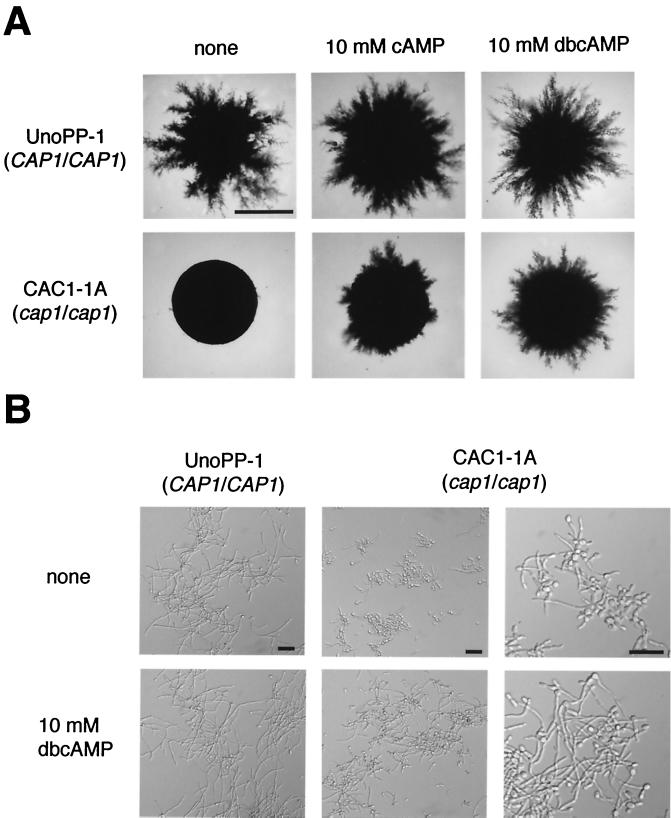
If the reduced cAMP levels were responsible for the defective bud-hypha transitions and colonial morphologies of the cap1/cap1 mutant, then exogenous addition of cAMP should reverse the defects. Both cAMP and dbcAMP dramatically altered the colony morphology of the cap1/cap1 mutant (Fig. 7A). Filamentous growth that closely resembled that of the positive control CAP1 strain was induced. The timing of the onset of filamentous growth for CAP1 strains and for the cap1/cap1 mutant induced by cAMP and dbcAMP was the same, 2 days. dbcAMP was more dramatic in restoring filamentous growth to the cap1/cap1 mutant strain than cAMP (Fig. 7A), indicating that dbcAMP may be taken up by cells more efficiently than cAMP. Filamentous growth of the wild-type strain also appeared to be slightly enhanced in the presence of cAMP and dbcAMP (Fig. 7A).
FIG. 7.
Suppression of defective bud-hypha transitions and filamentous growth in the cap1/cap1 mutant by exogenous cAMP or its derivative, dbcAMP. (A) The wild-type CAP1/CAP1 strain, UnoPP-1, and the cap1/cap1 mutant strain, CAC1-1A, were grown in SLAD medium with or without 10 mM cAMP or dbcAMP for 5 days at 37°C. Bars, 1 mm. (B) Bud-hypha transitions were induced at cell concentrations of 106 cells/ml in prewarmed M199+serum with or without 10 mM dbcAMP for 13 h (first [UnoPP-1] and second [CAC1-1A] columns, 20× objective; third [CAC1-1A] column, 40× objective). Bars, 30 μm.
Hypha formation of the cap1/cap1 mutant in liquid media (M199+serum) was also enhanced by the addition of dbcAMP (10 mM). Hyphae of the cap1/cap1 mutant were much longer, and more hyphae and pseudohyphae were seen, if the media contained dbcAMP. The results appeared most dramatic at 13 h (Fig. 7B). At 3 h twice as many pseudohyphae were detected and the pseudohyphae were longer in the presence of dbcAMP (not shown). Thus, dbcAMP decreased the time required for the emergence of filamentous structures. It was difficult to estimate the effect of exogenous dbcAMP on enhancement of hyphal formation of the wild-type strain because of extensive hypha formation produced independently of the presence of dbcAMP (Fig. 7B). Exogenous cAMP (10 mM) produced similar but less dramatic effects on hyphal formation of the cap1/cap1 mutant (not shown).
These results are consistent with CAP1 regulation of bud-hypha transitions of C. albicans by modulation of cAMP levels.
Virulence studies.
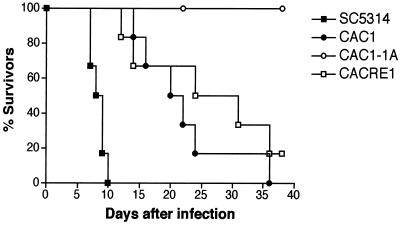
Mice injected with the wild-type C. albicans strain (SC5314) expired within 10 days of injection (Fig. 8). C. albicans strains with a single copy of the CAP1 gene (CAC1 and CACRE1) showed reduced virulence compared with the parental CAP1/CAP1 strain (P = 0.0006); however, 80% of the mice became ill and were sacrificed by 35 days (Fig. 8). In contrast, six mice given the cap1/cap1 mutant survived and behaved normally during the entire period of observation. The survival rate of mice injected with the cap1/cap1 mutant was significantly different from that of control strains (SC5314 versus CAC1-1A, P = 0.0006; CAC1 versus CAC1-1A, P = 0.0007; CACRE1 versus CAC1-1A, P = 0.0069). No statistically significant difference was found between results for the heterozygous CAP1/cap1 mutant (CAC1) and the revertant (CACRE1) (P = 0.3661). CFU of C. albicans were detected in sacrificed mice injected with CAP1 strains (107 CFU per g of kidney). Of the six mice injected with the cap1/cap1 mutant, three had infected kidneys (1.9 × 108 CFU per g of kidney) and three cleared the infection. Yeasts isolated from kidneys of mice that received the cap1/cap1 mutant showed the same defects in forming germ tubes as those used for intravenous injection, verifying the authenticity of strains and the importance of normal kinetics of germ tube formation in virulence.
FIG. 8.
Survival curves of mice (CBA/J, 5 to 6 weeks old, six mice per group) infected with 2 × 105 cells of C. albicans strains SC5314 (CAP1/CAP1), CAC1 (CAP1/cap1), CAC1-1A (CAP1/cap1), and CACRE1 (CAP1/cap1, revertant). Similar results were obtained in two independent experiments. Survival curves were illustrated according to the Kaplan-Meier method using the PRISM program and compared using the log-rank test. A P value of <0.05 was considered significant.
DISCUSSION
Identification and molecular cloning of the CAP1 gene of C. albicans.
Structural features of C. albicans Cap1 conformed closely to those of adenylate cyclase-associated proteins from other organisms (24, 35, 55, 82, 87). Amino- and carboxy-terminal halves rich in alpha-helices and beta-sheets, respectively, separated by a central loop containing a stretch of prolines, are typical of CAPs that have two domains with separable functions. The SH3 binding motifs and the conserved actin-binding region at the carboxy terminus may interact with an Abp1 homologue and actin monomers in C. albicans, as has been shown for similar regions of S. cerevisiae CAP (26, 27, 47, 85). An Abpl homologue was found in the C. albicans genome. Differences in cAMP responses of the cap1/cap1 mutant from those of isogenic CAP1 strains indicate that Cap1 regulates adenylate cyclase activity. cAMP or its membrane-permeable derivative, dbcAMP, partially restored filamentation and enhanced hypha production of the cap1/cap1 mutant strain, further confirming that Cap1 acts through regulation of cAMP levels. CAP1 encodes the adenylate cyclase-associated protein of C. albicans.
The role of cAMP in hypha production and filamentous growth of C. albicans.
Increases in cAMP levels under conditions used in this study were directly correlated with bud-hypha transitions and were not simply a response to the presence of fresh media. Comparable cultures placed under conditions supporting budding growth did not show increases in the cAMP level. These results agree with earlier reports of increases in cAMP levels prior to and accompanying germ tube formation (14, 16, 62). In accord with our findings, cAMP levels are generally found to be low in budding yeasts that are used to induce germ tubes, except in one study (19), which reported basal cAMP levels to be threefold higher than in the other studies at time zero. But cAMP levels dropped within 15 min to levels that were consistent with the time zero values of the other studies, prior to rising. Reasons for the differences are unknown, but the use of late-stationary-phase yeasts (96 h) to induce germ tubes might have contributed to the high cAMP levels at time zero.
The ability of the majority of cap1/cap1 mutant cells to produce hyphae upon prolonged incubation in serum is consistent with a role for cAMP in germ tube formation. An increased length of time may be required for accumulation of threshold levels of cAMP in cap1/cap1 mutant cells that are unable to generate pulses of cAMP but are able to generate cAMP at reduced rates independently of Cap1. The presence of mechanisms independent of Cap1 with lesser effects on cAMP levels is shown by the small increase in cAMP in the cap1/cap1 mutant under germ tube induction conditions. Also, cAMP levels in middle-logarithmic-phase cultures of cap1/cap1 and CAP1 strains were similar, indicating that, as is found for S. cerevisiae (20), basal levels of cAMP are not under Cap1 control in C. albicans. Steroid hormones (38) and unidentified factors of low molecular weight in serum and seminal fluid that promote hyphal formation (4, 23) may interact with C. albicans G protein-coupled receptors, leading to Cap1-independent cAMP responses in C. albicans. Even cAMP itself, which is present in serum at low levels (36), may work in combination with other factors to promote delayed hypha formation in serum in cap1/cap1 mutant cells. Superior hypha-inducing properties of serum relative to other conditions have been noted by others (13, 23, 49). Reasons for the formation of hyphae, albeit at low frequencies, upon prolonged incubation in saliva and M199 without serum are also unknown but may reflect cell cycle influences on the bud-hypha transition (51).
The availability of the cap1/cap1 mutant that grows in yeast forms under hypha-inducing conditions permitted us to clearly show for the first time that cAMP profoundly affects bud-hypha transitions and filamentous growth in C. albicans. For strains with CAP1 genes, the role of cAMP was difficult to detect because of the filamentous appearance of wild-type colonies. Addition of cAMP or its membrane-permeable derivative, dbcAMP, to the cap1/cap1 mutant in agar media promoted growth as filamentous rather than yeast colonies. Filamentous growth of the cap1/cap1 mutant in the presence of dbcAMP was not quite as extensive as for CAP1 strains. Insufficient uptake or rapid degradation of exogenous cAMP or dbcAMP of cap1/cap1 cells might have led to an incomplete restoration of filamentous growth. For S. cerevisiae, the ability to take up cAMP is greatly enhanced by the presence of at least one cam mutation. Without at least one cam mutation, strains having mutations in the gene encoding adenylate cyclase, CYR1, cannot survive. One of the cam mutations causes a loss of PDE function, whereas the others are uncharacterized (32, 33; Warren Heideman, personal communication). By analogy with S. cerevisiae, disruption of the C. albicans PDE2 gene would be predicted to generate strains with enhanced filamentous growth properties (41, 52, 63).
The cAMP-dependent signaling pathway in C. albicans.
The positive correlation between addition of cAMP and filamentous growth in both S. cerevisiae and C. albicans (52, 53, 61, 86; this study) along with the requirement of CAP for filamentous growth of S. cerevisiae (57) suggest that the cAMP-dependent signaling pathway of S. cerevisiae during pseudohyphal growth is a good working model for the C. albicans cAMP-dependent signaling pathway during bud-hypha transitions. Gpr1-Gpa2 regulation of cAMP signaling may be also conserved in C. albicans. A Gpr1 homologue with 43% identity in the first five transmembrane regions and an overall identity of 19% to S. cerevisiae Gpr1 was found in the C. albicans genome, as was a Gpa2 homologue (CAG99) with an overall identity of 43% to S. cerevisiae Gpa2. C. albicans Ras1 is strongly implicated in cAMP signaling by its 50% identity to Ras2 of S. cerevisiae, which interacts with CAP and affects cAMP levels. Importantly, the phenotype of ras1/ras1 null mutants of C. albicans is very similar to that of the cap1/cap1 mutant, with defective bud-hypha transitions and filamentous growth in all hypha-inducing conditions investigated, including both liquid and solid media containing serum at 37°C. The similarity in phenotypes between C. albicans ras1/ras1 mutants and cap1/cap1 mutants strongly suggests that C. albicans RAS1 acts in the same signal transduction pathway as CAP1, the cAMP-dependent signaling pathway (23). Phenotypic similarities also potentially connect a recently identified Cdc2-related kinase, CRK1 (15), to CAP1 and RAS1. CRK1 gene null mutants have a profound defect in hyphal development in all media tested and express reduced amounts of hypha-specific genes under germ tube-inducing conditions. We have also found reduced amounts of HWP1 expression in cap1/cap1 mutants (not shown). Crk1 has been suggested to be one of the downstream targets of Ras1 in hyphal development of C. albicans. The transcription factors in C. albicans targeted by cAMP signaling are less clear. Crk1 and Ras1V13 suppress the defects in hypha production of C. albicans cph1/cph1 efg1/efg1, pointing to the presence of an unknown transcription factor(s) that serves as a downstream target of cAMP signaling. Expression of the C. albicans CRK1 gene in S. cerevisiae led to enhanced filamentous growth that was dependent on Flo8, a PKA-dependent transcription factor. But a homologue of Flo8 has not been found in the C. albicans genome. Another part of the cAMP signaling pathway that is poorly understood involves PKA. Unlike the case with cap1/cap1 and ras1/ras1 mutants, defective germ tube formation is not seen at 37°C on solid media in C. albicans strains lacking TPK2, which encodes a catalytic subunit of PKA (74). Whether additional TPK genes with differing effects on filamentous growth, as is found in S. cerevisiae (63), are present in C. albicans is unknown. A gene encoding the regulatory subunit of PKA has been identified in the C. albicans genome. The role of the PKA regulatory subunit gene in bud-hypha transitions and filamentous growth is currently under investigation in this laboratory.
Defects in hypha formation have been reported for a growing list of null mutants in signal transduction pathway genes; however, the media and temperatures that are required to detect the phenotype for most genes are limited compared to the case with the cap1/cap1 mutant. Null mutants devoid of any one of many other signal transduction pathway genes, such as COS1, SSK1, or MAPK cascade genes (CST20, HST7, CEK1, and CPH1), have medium-conditional deficiencies in filamentous growth (1, 11, 17, 39, 48). Strains with mutations of both alleles of the MAPK genes are unable to produce filamentous growth in solid Spider medium but make normal hyphae in all other solid or liquid media tested (17, 39, 48). The COS1 and SSK1 genes, encoding proteins involved in a two-component signaling pathway, are required for hyphal development in solid media but not in liquid media (1, 11). The phenotypes of cap1/cap1, ras1/ras1, and crk1/crk1 mutants suggest that the presence of defective hypha formation in serum-containing medium at 37°C provides a means for identifying proteins involved in the cAMP-dependent pathway.
The role of Cap1 in hypha production.
Cap1 is required for normal hyphal development under all conditions examined. The ability of cap1/cap1 mutants to form germ tubes after a delay and correction of the phenotype by exogenous cAMP and dbcAMP indicate that modulation of cAMP levels, and not cytoskeletal interactions, is probably responsible for the hypha-promoting effect of Cap1 in C. albicans. This result is consistent with studies in S. cerevisiae showing that neither targeting of CAP to actin cortical patches through the SH3 binding domain nor interaction of CAP with actin monomers is necessary for CAP to transduce cAMP signals (85, 87).
The absence of the growth defects and aberrant budding phenotypes in C. albicans cap1/cap1 mutants compared to results with S. cerevisiae and S. pombe cap null mutants points to possible differences in Cap protein-actin interactions that may relate to the capacity of C. albicans, but not the other yeasts, to form germ tubes and true hyphae (24, 35). Although related, pseudohyphal formation and true hyphal formation are distinct processes that are characterized both by morphological differences and by differences in gene expression patterns in C. albicans. Cap1 may be in part responsible for the morphological differences between germ tubes and pseudohyphae. In S. cerevisiae, the interaction of CAP with actin monomers through the 27 carboxy-terminal amino acids (87) may prevent the hyperpolarization and accentuated concentration of actin filaments seen in buds of cap null mutants (5). However, filamentous actin is highly concentrated at the hyphal tip in C. albicans germ tubes and true hyphae (3). Growth from hyphal tips may require weaker interactions between Cap1 and actin for C. albicans than for S. cerevisiae to facilitate polarized growth during germ tube and hypha formation. The results suggest that CAP function is not required for cytoskeletal organization in C. albicans, as it is in S. cerevisiae.
The mechanism of Cap1-mediated modulation of bud-hypha transitions and filamentous growth is unknown. Studies with S. cerevisiae suggest that the cAMP-dependent pathway causes cells to undergo unipolar budding, a process that, when coupled with elongated growth controlled by the MAPK pathway, produces pseudohyphal cells (63). Mösch and Fink reported that the S. cerevisiae CAP/SRV2 mutant constructed by transposon mutagenesis is defective in pseudohyphal growth and undergoes random budding (57). These reports prompt the idea that C. albicans Cap1 may function to interrupt processes important for budding and that interruption of budding processes is required for bud-hypha transitions and filamentous growth.
Role of Cap1 in virulence.
The avirulence of the cap1/cap1 mutant extends the findings of other studies (11, 12, 49, 70, 84) in showing that the ability to produce hyphae with normal kinetics, as well as the absolute ability to produce hyphae, is important for candidiasis. The avirulence of cap1/cap1 mutants is also supportive of an important role for the cAMP signaling pathway in the growth of C. albicans in host tissue. The rapid production of hypha-specific factors, such as Hwp1 adhesin (75) and others (68, 77), coincident with germ tube formation is likely to be important for systemic candidiasis in mice. The virulence study shows that C. albicans joins other pathogenic fungi in the involvement of the cAMP signaling pathway in pathogenesis. Disruption of the gene encoding the catalytic subunit of cAMP-dependent PKA and disruption of the GPA1 gene affect the virulence of M. grisea (56) and Cryptococcus neoformans (2), respectively.
The divergent phenotypes of cap mutants in S. cerevisiae and S. pombe illustrate that CAP genes play a key role in the variable responses of different fungi to similar environmental conditions. The primary role of CAP1 in C. albicans appears to be to mediate a rapid induction of bud-hypha transitions and filamentous growth in response to a variety of environmental conditions, a hallmark of C. albicans growth. The finding that cap1/cap1 mutants are avirulent in a murine model of systemic candidiasis suggests that antifungal strategies interfering with CAP1-mediated signaling will be important for preventing or inhibiting candidiasis.
ACKNOWLEDGMENTS
Support for this research was provided from grant 1 R01 DE11375-05A2 from the National Institute of Dental and Craniofacial Research. P. Sundstrom is a Scholar of the Burroughs Wellcome Fund.
We thank Janet F. Staab and Paul F. Cook for helpful comments on the manuscript, Mary Lloyd for histological procedures, and Robert S. Munson for providing assistance with automated DNA sequencing at Children's Research Institute.
REFERENCES
- 1.Alex L A, Korch C, Selitrennikoff C P, Simon M I. COS1, a two-component histidine kinase that is involved in hyphal development in the opportunistic pathogen Candida albicans. Proc Natl Acad Sci USA. 1998;95:7069–7073. doi: 10.1073/pnas.95.12.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alspaugh J A, Perfect J R, Heitman J. Cryptococcus neoformans mating and virulence are regulated by the G-protein α subunit GPA1 and cAMP. Genes Dev. 1997;11:3206–3217. doi: 10.1101/gad.11.23.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson J M, Soll D R. Differences in actin localization during bud and hypha formation in the yeast Candida albicans. J Gen Microbiol. 1986;132:2035–2047. doi: 10.1099/00221287-132-7-2035. [DOI] [PubMed] [Google Scholar]
- 4.Barlow A J, Aldersley T, Chattaway F W. Factors present in serum and seminal plasma which promote germ-tube formation and mycelial growth of Candida albicans. J Gen Microbiol. 1974;82:261–272. doi: 10.1099/00221287-82-2-261. [DOI] [PubMed] [Google Scholar]
- 5.Baum B, Li W, Perrimon N. A cyclase-associated protein regulates actin and cell polarity during Drosophila oogenesis and in yeast. Curr Biol. 2000;10:964–973. doi: 10.1016/s0960-9822(00)00640-0. [DOI] [PubMed] [Google Scholar]
- 6.Birse C E, Irwin M Y, Fonzi W A, Sypherd P S. Cloning and characterization of ECE1, a gene expressed in association with cell elongation of the dimorphic pathogen Candida albicans. Infect Immun. 1993;61:3648–3655. doi: 10.1128/iai.61.9.3648-3655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boeke J D, LaCroute F, Fink G R. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 8.Borges-Walmsley M I, Walmsley A R. cAMP signalling in pathogenic fungi: control of dimorphic switching and pathogenicity. Trends Microbiol. 2000;8:133–141. doi: 10.1016/s0966-842x(00)01698-x. [DOI] [PubMed] [Google Scholar]
- 9.Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 10.Bruno K S, Aramayo R, Minke P F, Metzenberg R L, Plamann M. Loss of growth polarity and mislocalization of septa in a Neurospora mutant altered in the regulatory subunit of cAMP-dependent protein kinase. EMBO J. 1996;15:5772–5782. [PMC free article] [PubMed] [Google Scholar]
- 11.Calera J A, Zhao X J, Calderone R. Defective hyphal development and avirulence caused by a deletion of the SSK1 response regulator gene in Candida albicans. Infect Immun. 2000;68:518–525. doi: 10.1128/iai.68.2.518-525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calera J A, Zhao X J, De Bernardis F, Sheridan M, Calderone R. Avirulence of Candida albicans CaHK1 mutants in a murine model of hematogenously disseminated candidiasis. Infect Immun. 1999;67:4280–4284. doi: 10.1128/iai.67.8.4280-4284.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castilla R, Passeron S, Cantore M L. N-acetyl-D-glucosamine induces germination in Candida albicans through a mechanism sensitive to inhibitors of cAMP-dependent protein kinase. Cell Signal. 1998;10:713–719. doi: 10.1016/s0898-6568(98)00015-1. [DOI] [PubMed] [Google Scholar]
- 14.Chattaway F W, Wheeler P R, O'Reilly J. Involvement of adenosine 3′:5′-cyclic monophosphate in the germination of blastospores of Candida albicans. J Gen Microbiol. 1981;123:233–240. doi: 10.1099/00221287-123-2-233. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Zhou S, Wang Q, Chen X, Pan T, Liu H. Crk1, a novel Cdc2-related protein kinase, is required for hyphal development and virulence in Candida albicans. Mol Cell Biol. 2000;20:8696–8708. doi: 10.1128/mcb.20.23.8696-8708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho T, Hamatake H, Kaminishi H, Hagihara Y, Watanabe K. The relationship between cyclic adenosine 3′,5′-monophosphate and morphology in exponential phase Candida albicans. J Med Vet Mycol. 1992;30:35–42. [PubMed] [Google Scholar]
- 17.Csank C, Schröppel K, Leberer E, Harcus D, Mohamed O, Meloche S, Thomas D Y, Whiteway M. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect Immun. 1998;66:2713–2721. doi: 10.1128/iai.66.6.2713-2721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dabrowa N, Howard D H. Proline uptake in Candida albicans. J Gen Microbiol. 1981;127:391–397. doi: 10.1099/00221287-127-2-391. [DOI] [PubMed] [Google Scholar]
- 19.Egidy G A, Paveto M C, Passeron S, Galvagno M. Relationship between cyclic adenosine 3′:5′-monophosphate and germination in Candida albicans. Exp Mycol. 1989;13:428–432. [Google Scholar]
- 20.Fedor-Chaiken M, Deschenes R J, Broach J R. SRV2, a gene required for RAS activation of adenylate cyclase in yeast. Cell. 1990;61:329–340. doi: 10.1016/0092-8674(90)90813-t. [DOI] [PubMed] [Google Scholar]
- 21.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 22.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Addendum. Anal Biochem. 1984;137:266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- 23.Feng Q, Summers E, Guo B, Fink G. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J Bacteriol. 1999;181:6339–6346. doi: 10.1128/jb.181.20.6339-6346.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Field J, Vojtek A, Ballester R, Bolger G, Colicelli J, Ferguson K, Gerst J, Kataoka T, Michaeli T, Powers S, et al. Cloning and characterization of CAP, the S. cerevisiae gene encoding the 70 kd adenylyl cyclase-associated protein. Cell. 1990;61:319–327. doi: 10.1016/0092-8674(90)90812-s. [DOI] [PubMed] [Google Scholar]
- 25.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freeman N L, Chen Z, Horenstein J, Weber A, Field J. An actin monomer binding activity localizes to the carboxyl-terminal half of the Saccharomyces cerevisiae cyclase-associated protein. J Biol Chem. 1995;270:5680–5685. doi: 10.1074/jbc.270.10.5680. [DOI] [PubMed] [Google Scholar]
- 27.Freeman N L, Lila T, Mintzer K A, Chen Z, Pahk A J, Ren R, Drubin D G, Field J. A conserved proline-rich region of the Saccharomyces cerevisiae cyclase-associated protein binds SH3 domains and modulates cytoskeletal localization. Mol Cell Biol. 1996;16:548–556. doi: 10.1128/mcb.16.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gancedo J M. Control of pseudohyphae formation in Saccharomyces cerevisiae. FEMS Microbiol Rev. 2001;25:107–123. doi: 10.1111/j.1574-6976.2001.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 29.Ghannoum M A, Spellberg B, Saporito-Irwin S M, Fonzi W A. Reduced virulence of Candida albicans PHR1 mutants. Infect Immun. 1995;63:4528–4530. doi: 10.1128/iai.63.11.4528-4530.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillum A M, Tsay E Y H, Kirsch D R. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 31.Gimeno C J, Ljungdahl P O, Styles C A, Fink G R. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68:1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 32.Griffioen G, Anghileri P, Imre E, Baroni M D, Ruis H. Nutritional control of nucleocytoplasmic localization of cAMP-dependent protein kinase catalytic and regulatory subunits in Saccharomyces cerevisiae. J Biol Chem. 2000;275:1449–1456. doi: 10.1074/jbc.275.2.1449. [DOI] [PubMed] [Google Scholar]
- 33.Hall D D, Markwardt D D, Parviz F, Heideman W. Regulation of the Cln3-Cdc28 kinase by cAMP in Saccharomyces cerevisiae. EMBO J. 1998;17:4370–4378. doi: 10.1093/emboj/17.15.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hazen K C, Cutler J E. Autoregulation of germ tube formation by Candida albicans. Infect Immun. 1979;24:661–666. doi: 10.1128/iai.24.3.661-666.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawamukai M, Gerst J, Field J, Riggs M, Rodgers L, Wigler M, Young D. Genetic and biochemical analysis of the adenylyl cyclase-associated protein, cap, in Schizosaccharomyces pombe. Mol Biol Cell. 1992;3:167–180. doi: 10.1091/mbc.3.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawarabayashi T, Tsukamoto T, Kishikawa T, Sugimori H. Changes in serum calcium, magnesium, cyclic AMP and monoamine oxidase levels during pregnancy and under prolonged ritodrine treatment for preterm labor. Gynecol Obstet Investig. 1989;28:132–137. doi: 10.1159/000293548. [DOI] [PubMed] [Google Scholar]
- 37.Kimura L H, Pearsall N N. Adherence of Candida albicans to human buccal epithelial cells. Infect Immun. 1978;21:64–68. doi: 10.1128/iai.21.1.64-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinsman O S, Pitblado K, Coulson C J. Effect of mammalian steroid hormones and luteinizing hormone on the germination of Candida albicans and implications for vaginal candidosis. Mycoses. 1988;31:617–626. doi: 10.1111/j.1439-0507.1988.tb04416.x. [DOI] [PubMed] [Google Scholar]
- 39.Köhler J R, Fink G R. Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc Natl Acad Sci USA. 1996;93:13223–13228. doi: 10.1073/pnas.93.23.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kronstad J, De Maria A, Funnell D, Laidlaw R D, Lee N, de Sá M M, Ramesh M. Signaling via cAMP in fungi: interconnections with mitogen-activated protein kinase pathways. Arch Microbiol. 1998;170:395–404. doi: 10.1007/s002030050659. [DOI] [PubMed] [Google Scholar]
- 41.Kübler E, Mösch H U, Rupp S, Lisanti M P. Gpa2p, a G-protein alpha-subunit, regulates growth and pseudohyphal development in Saccharomyces cerevisiae via a cAMP-dependent mechanism. J Biol Chem. 1997;272:20321–20323. doi: 10.1074/jbc.272.33.20321. [DOI] [PubMed] [Google Scholar]
- 42.Kurtz M B, Cortelyou M W, Kirsch D R. Integrative transformation of Candida albicans, using a cloned Candida ADE2 gene. Mol Cell Biol. 1986;6:142–149. doi: 10.1128/mcb.6.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 44.Lambrechts M G, Bauer F F, Marmur J, Pretorius I S. Mucl, a mucin-like protein that is regulated by Mss10, is critical for pseudohyphal differentiation in yeast. Proc Natl Acad Sci USA. 1996;93:8419–8424. doi: 10.1073/pnas.93.16.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee K L, Buckley H R, Campbell C C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia. 1975;13:148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- 46.Lengeler K B, Davidson R C, D'Souza C, Harashima T, Shen W C, Wang P, Pan X, Waugh M, Heitman J. Signal transduction cascades regulating fungal development and virulence. Microbiol Mol Biol Rev. 2000;64:746–785. doi: 10.1128/mmbr.64.4.746-785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lila T, Drubin D G. Evidence for physical and functional interactions among two Saccharomyces cerevisiae SH3 domain proteins, an adenylyl cyclase-associated protein and the actin cytoskeleton. Mol Biol Cell. 1997;8:367–385. doi: 10.1091/mbc.8.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu H, Köhler J, Fink G R. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. . (Erratum, 267:17, 1995.) [DOI] [PubMed] [Google Scholar]
- 49.Lo H J, Köhler J R, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink G R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 50.Lo W S, Dranginis A M. The cell surface flocculin Flo11 is required for pseudohyphae formation and invasion by Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:161–171. doi: 10.1091/mbc.9.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loeb J D J, Sepulveda-Becerra M, Hazan I, Liu H. A G1 cyclin is necessary for maintenance of filamentous growth in Candida albicans. Mol Cell Biol. 1999;19:4019–4027. doi: 10.1128/mcb.19.6.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lorenz M C, Heitman J. Yeast pseudohyphal growth is regulated by GPA2, a G protein α homolog. EMBO J. 1997;16:7008–7018. doi: 10.1093/emboj/16.23.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lorenz M C, Pan X, Harashima T, Cardenas M E, Xue Y, Hirsch J P, Heitman J. The G protein-coupled receptor Gpr1 is a nutrient sensor that regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Genetics. 2000;154:609–622. doi: 10.1093/genetics/154.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Makimura K, Murayama S Y, Yamaguchi H. Detection of a wide range of medically important fungi by the polymerase chain reaction. J Med Microbiol. 1994;40:358–364. doi: 10.1099/00222615-40-5-358. [DOI] [PubMed] [Google Scholar]
- 55.Matviw H, Yu G, Young D. Identification of a human cDNA encoding a protein that is structurally and functionally related to the yeast adenylyl cyclase-associated CAP proteins. Mol Cell Biol. 1992;12:5033–5040. doi: 10.1128/mcb.12.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitchell T K, Dean R A. The cAMP-dependent protein kinase catalytic subunit is required for appressorium formation and pathogenesis by the rice blast pathogen Magnaporthe grisea. Plant Cell. 1995;7:1869–1878. doi: 10.1105/tpc.7.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mösch H U, Fink G R. Dissection of filamentous growth by transposon mutagenesis in Saccharomyces cerevisiae. Genetics. 1997;145:671–684. doi: 10.1093/genetics/145.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mösch H U, Kübler E, Krappmann S, Fink G R, Braus G H. Crosstalk between the Ras2p-controlled mitogen-activated protein kinase and cAMP pathways during invasive growth of Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:1325–1335. doi: 10.1091/mbc.10.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Naglik J R, Newport G, White T C, Fernandes-Naglik L L, Greenspan J S, Greenspan D, Sweet S P, Challacombe S J, Agabian N. In vivo analysis of secreted aspartyl proteinase expression in human oral candidiasis. Infect Immun. 1999;67:2482–2490. doi: 10.1128/iai.67.5.2482-2490.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Navarro-Garcia F, Sanchez M, Pla J, Nombela C. Functional characterization of the MKC1 gene of Candida albicans, which encodes a mitogen-activated protein kinase homolog related to cell integrity. Mol Cell Biol. 1995;15:2197–2206. doi: 10.1128/mcb.15.4.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niimi M. Dibutyryl cyclic AMP-enhanced germ tube formation in exponentially growing Candida albicans cells. Fungal Genet Biol. 1996;20:79–83. doi: 10.1006/fgbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 62.Niimi M, Niimi K, Tokunaga J, Nakayama H. Changes in cyclic nucleotide levels and dimorphic transition in Candida albicans. J Bacteriol. 1980;142:1010–1014. doi: 10.1128/jb.142.3.1010-1014.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan X, Heitman J. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:4874–4887. doi: 10.1128/mcb.19.7.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paravicini G, Mendoza A, Antonsson B, Cooper M, Losberger C, Payton M A. The Candida albicans PKC1 gene encodes a protein kinase C homolog necessary for cellular integrity but not dimorphism. Yeast. 1996;12:741–756. doi: 10.1002/(sici)1097-0061(19960630)12:8<741::aid-yea967>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 65.Postlethwait P, Sundstrom P. Genetic organization and mRNA expression of enolase genes of Candida albicans. J Bacteriol. 1995;177:1772–1779. doi: 10.1128/jb.177.7.1772-1779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rose M D, Winston F, Hieter P. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- 67.Rupp S, Summers E, Lo H J, Madhani H, Fink G. MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J. 1999;18:1257–1269. doi: 10.1093/emboj/18.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schaller M, Korting H C, Schafer W, Bastert J, Chen W, Hube B. Secreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosis. Mol Microbiol. 1999;34:169–180. doi: 10.1046/j.1365-2958.1999.01590.x. [DOI] [PubMed] [Google Scholar]
- 69.Scherer S, Stevens D A. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J Clin Microbiol. 1987;25:675–679. doi: 10.1128/jcm.25.4.675-679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schweizer A, Rupp S, Taylor B N, Rollinghoff M, Schroppel K. The TEA/ATTS transcription factor CaTec1p regulates hyphal development and virulence in Candida albicans. Mol Microbiol. 2000;38:435–445. doi: 10.1046/j.1365-2958.2000.02132.x. [DOI] [PubMed] [Google Scholar]
- 71.Shepherd M G, Yin C Y, Ram S P, Sullivan P A. Germ tube induction in Candida albicans. Can J Microbiol. 1980;26:21–26. doi: 10.1139/m80-004. [DOI] [PubMed] [Google Scholar]
- 72.Shima F, Okada T, Kido M, Sen H, Tanaka Y, Tamada M, Hu C D, Yamawaki-Kataoka Y, Kariya K, Kataoka T. Association of yeast adenylyl cyclase with cyclase-associated protein CAP forms a second Ras-binding site which mediates its Ras-dependent activation. Mol Cell Biol. 2000;20:26–33. doi: 10.1128/mcb.20.1.26-33.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sobel J D, Muller G, Buckley H R. Critical role of germ tube formation in the pathogenesis of candidal vaginitis. Infect Immun. 1984;44:576–580. doi: 10.1128/iai.44.3.576-580.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sonneborn A, Bockmühl D P, Gerads M, Kurpanek K, Sanglard D, Ernst J F. Protein kinase A encoded by TPK2 regulates dimorphism of Candida albicans. Mol Microbiol. 2000;35:386–396. doi: 10.1046/j.1365-2958.2000.01705.x. [DOI] [PubMed] [Google Scholar]
- 75.Staab J F, Bradway S D, Fidel P L, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283:1535–1538. doi: 10.1126/science.283.5407.1535. [DOI] [PubMed] [Google Scholar]
- 76.Staab J F, Ferrer C A, Sundstrom P. Developmental expression of a tandemly repeated, proline- and glutamine-rich amino acid motif on hyphal surfaces on Candida albicans. J Biol Chem. 1996;271:6298–6305. doi: 10.1074/jbc.271.11.6298. [DOI] [PubMed] [Google Scholar]
- 77.Staib P, Kretschmar M, Nichterlein T, Hof H, Morschhäuser J. Differential activation of a Candida albicans virulence gene family during infection. Proc Natl Acad Sci USA. 2000;97:6102–6107. doi: 10.1073/pnas.110031497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sundstrom P, Aliaga G R. Molecular cloning of cDNA and analysis of protein secondary structure of Candida albicans enolase, an abundant, immunodominant glycolytic enzyme. J Bacteriol. 1992;174:6789–6799. doi: 10.1128/jb.174.21.6789-6799.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Toda T, Uno I, Ishikawa T, Powers S, Kataoka T, Broek D, Cameron S, Broach J, Matsumoto K, Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985;40:27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- 81.van Zeijl C M J, van de Kamp E H M, Punt P J, Selten G C M, Hauer B, van Gorcom R F M, van den Hondel C A M J J. An improved colony-PCR method for filamentous fungi for amplification of PCR-fragments of several kilobases. J Biotechnol. 1998;59:221–224. doi: 10.1016/s0168-1656(97)00170-3. [DOI] [PubMed] [Google Scholar]
- 82.Vojtek A B, Cooper J A. Identification and characterization of a cDNA encoding mouse CAP: a homolog of the yeast adenylyl cyclase associated protein. J Cell Sci. 1993;105:777–785. doi: 10.1242/jcs.105.3.777. [DOI] [PubMed] [Google Scholar]
- 83.Wright F. The ‘effective number of codons’ used in a gene. Gene. 1990;87:23–29. doi: 10.1016/0378-1119(90)90491-9. [DOI] [PubMed] [Google Scholar]
- 84.Yamada-Okabe T, Mio T, Ono N, Kashima Y, Matsui M, Arisawa M, Yamada-Okabe H. Roles of three histidine kinase genes in hyphal development and virulence of the pathogenic fungus Candida albicans. J Bacteriol. 1999;181:7243–7247. doi: 10.1128/jb.181.23.7243-7247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu J, Wang C, Palmieri S J, Haarer B K, Field J. A cytoskeletal localizing domain in the cyclase-associated protein, CAP/Srv2p, regulates access to a distant SH3-binding site. J Biol Chem. 1999;274:19985–19991. doi: 10.1074/jbc.274.28.19985. [DOI] [PubMed] [Google Scholar]
- 86.Zelada A, Castilla R, Passeron S, Cantore M L. Reassessment of the effect of glucagon and nucleotides on Candida albicans germ tube formation. Cell Mol Biol (Noisy-le-grand) 1996;42:567–576. [PubMed] [Google Scholar]
- 87.Zelicof A, Protopopov V, David D, Lin X Y, Lustgarten V, Gerst J E. Two separate functions are encoded by the carboxyl-terminal domains of the yeast cyclase-associated protein and its mammalian homologs. Dimerization and actin binding. J Biol Chem. 1996;271:18243–18252. doi: 10.1074/jbc.271.30.18243. [DOI] [PubMed] [Google Scholar]