Abstract
Aims:
To assess whether associations of cardiopulmonary conditions and markers with glaucoma differ by background genetic risk for primary open angle glaucoma (POAG).
Methods:
We constructed a POAG polygenic risk score (PRS) using genome-wide association study summary statistics from a large cross-ancestry meta-analysis. History of glaucoma (including self-report and codes for POAG, “other glaucoma,” or unspecified glaucoma), history of common cardiopulmonary conditions, and cardiopulmonary measures were assessed in the UK Biobank. Stratifying by PRS decile 1 (lowest risk) vs 10 (highest risk), separate multivariable models were estimated to assess associations of cardiopulmonary diseases or factors with glaucoma, adjusting for age, sex, smoking, and medication use. A Bonferroni correction was used to adjust P-values for multiple comparisons.
Results:
Individuals in POAG PRS deciles 1 (417 cases; 44,458 controls; mean age 56.8 years) and 10 (2135 cases; 42,413 controls; mean age 56.7 years) were included. Within decile 1, glaucoma cases had significantly higher glycated hemoglobin (38.5 vs 35.9 mmol/mol) and higher prevalence of diabetes (17.5% vs 6.5%), dyslipidemia (31.2% vs 18.3%), and chronic kidney disease (CKD) (6.7% vs 2.0%), than controls (adjusted P<0.0013 for each). Within decile 10, glaucoma was associated with higher prevalence of dyslipidemia (27.7% vs 17.3%; P=6.9E-05). The magnitude of association between glaucoma and diabetes, CKD, and glycated hemoglobin differed between decile 1 and 10 (contrast test p-for-difference <0.05).
Conclusion:
The relation between systemic conditions and glaucoma vary by underlying genetic predisposition to POAG, with larger associations among those who developed glaucoma despite low genetic risk.
Keywords: Glaucoma, Genetic Risk, Cardiopulmonary Disease, Polygenic Risk Score
Precis:
Stratifying by primary open angle glaucoma polygenic risk score, glaucoma was associated with several cardiopulmonary diseases and measures, with greater magnitudes of association among those who developed glaucoma despite low genetic risk.
INTRODUCTION
Glaucoma, the leading global cause of irreversible blindness, accounts for 9-12% of vision impairment cases and 23% of follow-up eye care appointments in the United Kingdom (UK).[1,2] The pathogenesis of primary open angle glaucoma (POAG), the most common form of glaucoma, is not fully understood.[1,3] While the heritability of POAG is estimated to be relatively high at 70%, the genetic architecture for this disease is incompletely understood.[4-6] For complex diseases such as POAG, polygenic risk scores (PRSs) can be used to account for the cumulative contribution of risk variants, each of which may have a small effect, to provide a single summary measure of an individual’s overall genetic risk for a given disease.[7] Multiple PRSs for POAG have been created using genome wide association data from large case-control studies.[8-10] Higher POAG PRS genetic risk scores are associated with higher risk of POAG, earlier age at diagnosis, and higher intra-ocular pressure, among others.[5,10]
Prior population-based studies have suggested an association between glaucoma and a number of cardiopulmonary diseases.[11-18] Individuals with glaucoma may have a greater burden of type 2 diabetes mellitus (T2DM),[12] hypertension,[13] heart disease,[14] chronic kidney disease (CKD),[15] and stroke,[16] although these associations are complex and inconsistent. Similarly, studies of the association of several cardiometabolic measures, including higher serum glucose,[12] serum triglycerides,[17] body mass index,[18] and blood pressure,[18] with glaucoma have also yielded mixed results. These inconsistencies may be related to failure to account for subjects’ genotypes. Individual’s genetic risk for glaucoma may modulate the relative impact of environmental or other genetic risk factors for cardiopulmonary disease.
We used data from the UK Biobank cohort to test the hypothesis that the associations between glaucoma and cardiopulmonary diseases or factors will differ in those with low glaucoma genetic risk, compared to those with high genetic risk, as assessed by POAG PRS. These findings will contribute to a better understanding of the mechanisms underlying the association between glaucoma and cardiopulmonary diseases.
MATERIALS AND METHODS
Study Sample:
United Kingdom Biobank
This analysis uses data from the Caucasian subset of the prospective, cross-sectional UK Biobank (UKBB), which recruited 502,656 volunteers enrolled in the UK National Health Service (ages 40-69 years) from 22 study sites in the UK, between 2006-2010. Detailed phenotypic and genotypic information was collected via surveys, physical examination, laboratory biological sample testing, and medical records. Eye examination including intraocular pressure (IOP) testing was conducted in 133,668 individuals and spectral domain ocular coherence tomography (OCT) was conducted in a subset of over 84,000 individuals. The National Research Ethics Service Committee NorthWest–Haydock approved the study, and it was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent. This research was conducted using the UK Biobank Resource under application number 50211.
Glaucoma Cases and Controls
Individuals with glaucoma were identified if they self-reported glaucoma on eye problems/disorders (UKBB data field 6148) or noncancer illness (UKBB data field 20002) or had an International Classification of Diseases, Ninth or Tenth Revision (ICD-9/10) diagnosis code (UKBB data fields 41210 and 41271) for POAG (ICD-9 code 365.11, ICD-10 code H40.1), other glaucoma (ICD-10 code H40.8), or glaucoma, unspecified (ICD-9 code 365.9, ICD-10 code H40.9). This definition was chosen based on its use in prior research in population-based and registry-based studies of genetic risk for glaucoma.[9,19] Among those not eligible for the glaucoma case cohort, individuals were included in the control cohort if they did not have an ICD diagnosis of any type of glaucoma (ICD-10 codes H40.XX or ICD-9 codes 365.XX). To attempt to exclude potential undiagnosed cases of glaucoma from our control cohort, in the subset of individuals with available IOP data, those with measured IOP >24 mmHg were also excluded from the control cohort (this criteria was not applied to the glaucoma case cohort).
Outcomes: Cardiopulmonary Diseases and Measures
Medical history of T2DM, hypertension, dyslipidemia (“Disorders of lipoprotein metabolism and other lipidemias”), cardiovascular disease (CVD), stroke, CKD, and chronic obstructive pulmonary disease (COPD) were defined using a composite of self-report (UKBB data field 20002) and ICD-9/10 diagnoses (UKBB data fields 41210 and 41271, ICD codes are listed in Supplement 1). Participants underwent physical examination, and body mass index, systolic and diastolic blood pressure, pulse rate, waist-to-hip ratio, and forced expiratory volume in 1 second to forced vital capacity ratio (FEV1/FVC) were measured. Participants gave blood specimen, and levels of glycated hemoglobin (HbA1c), total cholesterol, high- and low-density lipoprotein cholesterol, vitamin D, and creatinine were measured using standard laboratory chemical procedures.[20]
Stratification Factor: Primary Open Angle Glaucoma Polygenic Risk
To provide a single numeric summary measure of background genetic risk for POAG, data from genome-wide associate study (GWAS) summary statistics from the Caucasian subset of a large cross-ancestry meta-analysis (with the exclusion of the UKBB cohort) were used to create a POAG PRS.[21] See Supplement 2 for further technical details.[9] The ranges for POAG PRS by decile are: Decile 1 <0.19; Decile 2 0.19-0.26, Decile 3 0.26-0.31; Decile 4 o.31-0.36; Decile 5 0.36-0.39; Decile 6 0.39-0.44; Decile 7 0.44-0.48; Decile 8 0.48-0.54; Decile 9 0.54-0.61; Decile 10 >0.61.
Ganglion Cell Complex Thickness:
Spectral domain OCT scans of the macula were obtained on a subset of 67,321 participants using Topcon 3D OCT 1000 Mk2 (Topcon, Inc, Japan).[22,23] Ganglion cell complex (GCC) thickness was defined as the total thickness of the retinal nerve fiber layer, retinal ganglion cell layer, and inner plexiform layer. Data from each participant’s eye with thinner GCC was used. See Supplement 2 for further technical details.
Statistical Analysis:
For glaucoma cases and controls, participant demographic, covariate, and cardiopulmonary characteristics were characterized descriptively and compared using logistic regression models. Prevalence of cardiopulmonary diseases and levels of cardiopulmonary factors were calculated separately for each POAG PRS decile. The number of glaucoma cases and controls in each POAG PRS decile was visualized graphically.
Models assessing the association between glaucoma and cardiopulmonary outcomes were adjusted for age, sex, self-reported smoking history (data field 6119), and self-reported use of beta-blockers, calcium channel blockers, and statins (data field 20003). Separate logistic (for disease outcomes) or linear (for laboratory or examination measure outcomes) regression models were used to assess associations between each cardiopulmonary factor and glaucoma. To detect whether associations between glaucoma and cardiopulmonary factors persisted when stratifying by POAG PRS, similar models were constructed separately for individuals within POAG PRS decile 1 and within decile 10.
For outcomes that were significantly associated with glaucoma within decile 1 or within decile 10, we used a contrast test to assess whether associations differed between decile 1 vs 10 (https://www.hsph.harvard.edu/donna-spiegelman/software/contrasttest/).[24] Trends across POAG PRS decile for prevalence or mean level of these outcomes were visualized graphically, and compared using multivariable models with POAG PRS decile as a continuous variable predictor of each outcome for cases and controls separately.
As a sensitivity analysis, for each outcome that was significantly associated with glaucoma within POAG PRS decile 1 or decile 10, we constructed a logistic (for binary outcomes) or linear (for continuous outcomes) regression model with ganglion cell complex (GCC) thickness (a marker for glaucomatous optic neuropathy) as a predictor of the given outcome, adjusting for age, sex, and refractive error (spherical equivalents).
Data analysis was performed using SAS Enterprise Guide statistical software version 9.4 (SAS Institute Inc., Cary, NC) and RStudio Version 1.1.456 (RStudio Inc., Boston, MA). All statistical tests were 2-sided, and a significance threshold of alpha=0.05 was used. Residuals in regression models had p-values for normality >0.05 in a Shapiro-Wilk tests. Associations that were robust to Bonferroni adjustment for 38 multiple comparisons (alpha=0.0013) were noted.
RESULTS
Of the 453,179 individuals of European descent with available genomics data in the UKBB cohort, 9,779 cases and 437,418 controls were included in this analysis. Mean (standard deviation [SD]) POAG PRS score was 0.48 (0.2) for glaucoma cases and 0.40 (0.2) for controls (p=1E-20); possible scores ranged form −0.1 to 1.1. Demographic and cardiopulmonary characteristics for cases and controls are presented in Table 1. Prevalence or means and standard deviations for cardiopulmonary factors stratified by glaucoma status and POAG PRS decile for all 10 deciles are presented in Supplement 3.
Table 1:
Demographic and Cardiopulmonary Characteristics of 9,779 Glaucoma Cases and 437,418 Controls
| Cases (n=9,779) | Controls (n=437,418) | ||||
|---|---|---|---|---|---|
| Characteristic | n with available data |
Mean (SD) or % |
n with available data |
Mean (SD) or % |
p-value* (cases vs controls) |
| POAG PRS | 9,779 | 0.48 (0.2) | 437,418 | 0.40 (0.2) | <0.0001 |
| Male | 9,779 | 52% | 437,418 | 45.7% | - |
| Age (Years) | 9,779 | 61.4 (6.3) | 437,418 | 56.6 (8.0) | - |
| Beta Blocker Use | 9,779 | 12.5% | 437,418 | 6.7% | - |
| Calcium Channel Blocker Use | 9,779 | 9.1% | 437,418 | 5.6% | - |
| Statin Use | 9,779 | 23.6% | 437,418 | 15.3% | - |
| Medical History |
n with available data |
Prevalence | n with available data |
Prevalence | |
| Diabetes | 9,779 | 12.3% | 437,418 | 6.5% | <0.0001 |
| Hypertension | 9,779 | 47.0% | 437,418 | 32.8% | <0.0001 |
| Dyslipidemia | 9,779 | 28.7% | 437,418 | 17.9% | <0.0001 |
| Cardiovascular Disease | 9,779 | 15.4% | 437,418 | 9.2% | 0.0005 |
| Stroke | 9,779 | 2.5% | 437,418 | 1.3% | <0.0001 |
| Chronic Kidney Disease | 9,779 | 4.4% | 437,418 | 2.0% | <0.0001 |
| Chronic Obstructive Pulmonary Disease | 9,779 | 6.9% | 437,418 | 3.9% | <0.0001 |
| Examination Measures |
n with available data |
Mean (SD) | n with available data |
Mean (SD) | |
| BMI (kg/m^2) | 9,277 | 27.1 (3.9) | 417,540 | 26.9 (3.9) | 0.0002 |
| Systolic BP (mmHg) | 8,704 | 140.6 (16.6) | 390,297 | 136.7 (17) | 0.067 |
| Diastolic BP (mmHg) | 8,702 | 82.0 (8.8) | 384,684 | 81.7 (8.8) | 0.667 |
| Pulse Rate | 8,487 | 68.4 (9.8) | 380,790 | 68.4 (9.5) | 0.025 |
| Waist to Hip Ratio | 9,351 | 0.88 (0.1) | 422,605 | 0.87 (0.1) | 0.2959 |
| FEV1/FVC Ratio | 6,684 | 0.37 (0.7) | 331,930 | 0.35 (0.7) | 0.012 |
| Laboratory Measures |
n with available data |
Mean (SD) | n with available data |
Mean (SD) | |
| Hemoglobin A1C (mmol/mol) | 9,328 | 37.5 (8.0) | 418,178 | 35.9 (6.5) | <0.0001 |
| Cholesterol (mmol/L) | 9,292 | 20.9 (5.8) | 416,009 | 21.4 (5.7) | <0.0001 |
| HDL (mmol/L) | 8,596 | 1.4 (0.4) | 383,593 | 1.5 (0.4) | 0.98 |
| LDL (mmol/L) | 9,319 | 3.5 (0.9) | 417,447 | 3.6 (0.9) | 0.0029 |
| Vitamin D (nmol/L) | 8,899 | 49.5 (20.7) | 400,227 | 49.4 (21.0) | 0.0003 |
| Creatinine (umol/L) | 9,334 | 73.7 (22.7) | 417,979 | 72.2 (17.9) | 0.015 |
p values are from separate logistic regression models of the association between each characteristic and glaucoma, adjusted for age, sex, smoking history, beta blocker, calcium channel blocker, and statin use.
Abbreviations: BMI: body mass index; BP: blood pressure, HDL: high density lipoprotein; LDL: low density lipoprotein.
The prevalence of glaucoma in each decile of POAG PRS is visualized in Figure 1. As expected, the number of cases increased as POAG PRS decile increased, ranging from 417 cases (0.93% prevalence) in decile 1 to 2,135 cases (4.79% prevalence) in decile 10.
Figure 1: Prevalence of Glaucoma Cases by Primary Open Angle Glaucoma Polygenic Risk Decile.
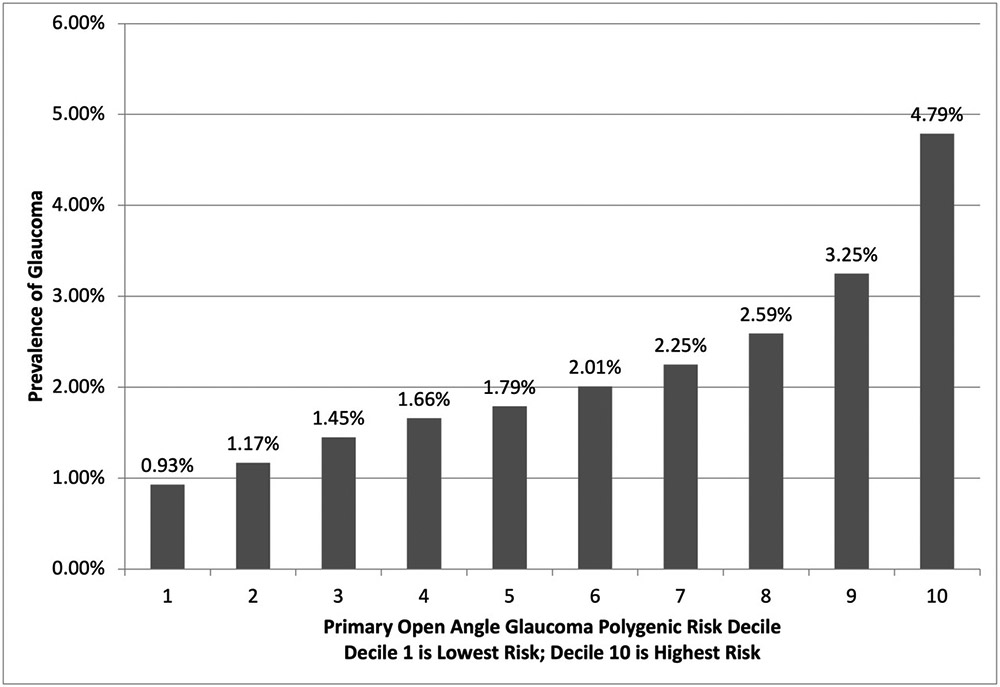
Glaucoma was defined as self-reported glaucoma or diagnosis code for primary open angle glaucoma, other glaucoma, or unspecified glaucoma. The ranges for POAG PRS by decile are: Decile 1 <0.19; Decile 2 0.19-0.26, Decile 3 0.26-0.31; Decile 4 o.31-0.36; Decile 5 0.36-0.39; Decile 6 0.39-0.44; Decile 7 0.44-0.48; Decile 8 0.48-0.54; Decile 9 0.54-0.61; Decile 10 >0.61. POAG: primary open angle glaucoma. Decile 1 indicates lowest background genetic risk for POAG. Decile 10 indicates highest background genetic risk for POAG.
In logistic regression models adjusting for age, sex, smoking history, beta blocker, calcium channel blocker, and statin use, glaucoma cases had higher odds of T2DM, hypertension, dyslipidemia, CVD, stroke, CKD, and COPD compared to controls (p<0.001 for all, Table 1). Compared to controls, glaucoma cases had higher BMI (27.1 vs 26.9 kg/m^2; p=0.0002), lower pulse rate (68.36 vs 68.38 beats per minute; p=0.025), higher HbA1c (37.5 vs 35.9 mmol/mol; p<0.0001), lower cholesterol (20.9 vs 21.4 mmol/L; p<0.0001), lower LDL (3.52 vs 3.57 mmol/L; p=0.003), lower vitamin D (49.45 vs 49.46 nmol/L; p=0.0003), and higher creatinine (73.7 vs 72.2 umol/L; p=0.015) (Table 1).
Using covariate adjusted logistic (for binary outcomes) or linear (for continuous outcomes) regression models, we assessed the association between glaucoma and cardiopulmonary factors, with separate models for individuals in POAG PRS decile 1 (417 cases, 44,458 controls, lowest genetic risk) and decile 10 (2135 cases, 42,413 controls, highest genetic risk). Prevalence or means and standard deviations for each cardiopulmonary factor for cases and controls, within POAG PRS deciles 1 and 10 are presented in Table 2.
Table 2:
Cardiopulmonary Characteristics by Glaucoma Status for Individuals in Primary Open Angle Glaucoma Polygenic Risk Score Deciles 1 (lowest genetic risk) and 10 (highest genetic risk)
| POAG PRS Decile 1 | POAG PRS Decile 10 | |||||
|---|---|---|---|---|---|---|
| Characteristic (2) |
Cases n=417 |
Controls n=44,453 |
p-value (1) (cases vs controls) |
Cases n=2,135 |
Controls n=42,413 |
p-value (1) (cases vs controls) |
| Medical History |
Prevalence | Prevalence | Prevalence | Prevalence | ||
| Diabetes | 17.5% | 6.5% | 9.7E-07** | 9.9% | 6.4% | 0.039* |
| Hypertension | 50.1% | 33.4% | 0.16 | 43.8% | 32.4% | 0.26 |
| Dyslipidemia | 31.2% | 18.3% | 0.028 | 27.7% | 17.3% | 6.9E-05** |
| Cardiovascular Disease | 18.9% | 9.2% | 0.051 | 15.1% | 9.2% | 0.08 |
| Stroke | 1.9% | 1.3% | 0.80 | 2.1% | 1.2% | 0.27 |
| Chronic Kidney Disease | 6.7% | 2.0% | 0.0009** | 4.0% | 2.1% | 0.07 |
| Chronic Obstructive Pulmonary Disease | 9.4% | 4.1% | 0.041* | 5.4% | 4.0% | 0.71 |
| Examination Measures |
Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| BMI (kg/m^2) | 27.2 (3.7) | 26.9 (3.9) | 0.91 | 27.0 (3.8) | 26.8 (3.9) | 0.089 |
| Systolic BP (mmHg) | 140.9 (17.5) | 136.9 (17.0) | 0.67 | 140.1 (16.3) | 136.6 (17.0) | 0.81 |
| Diastolic BP (mmHg) | 81.5 (8.8) | 81.8 (8.9) | 0.19 | 81.9 (8.8) | 81.69 (8.8) | 0.36 |
| Pulse Rate | 69.1 (9.8) | 68.3 (9.5) | 0.11 | 68.0 (9.6) | 68.4 (9.5) | 0.19 |
| Waist to Hip Ratio | 0.89 (0.08) | 0.87 (0.08) | 0.39 | 0.88 (0.08) | 0.87 (0.08) | 0.40 |
| FEV1/FVC Ratio | 0.4 (0.7) | 0.35 (0.72) | 0.11 | 0.4 (0.7) | 0.36 (0.7) | 0.021* |
| Laboratory Measures |
Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Hemoglobin A1C (mmol/mol) | 38.5 (8.5) | 35.9 (6.4) | 8.5E-06** | 36.9 (6.7) | 35.9 (6.4) | 0.80 |
| Cholesterol (mmol/L) | 20.4 (5.6) | 21.2 (5.6) | 0.87 | 21.1 (5.4) | 21.6 (5.7) | 0.015* |
| HDL (mmol/L) | 1.4 (0.4) | 1.5 (0.4) | 0.57 | 1.4 (0.4) | 1.5 (0.4) | 0.34 |
| LDL (mmol/L) | 3.5 (0.9) | 3.6 (0.9) | 0.77 | 3.6 (0.9) | 3.6(0.9) | 0.54 |
| Vitamin D (nmol/L) | 47.8 (19.9) | 49.5 (21.0) | 0.074 | 49.9 (20.3) | 49.3 (21.0) | 0.59 |
| Creatinine (umol/L) | 74.2 (18.0) | 72.3 (17.1) | 0.79 | 73.3 (26.3) | 72.1 (18.0) | 0.18 |
(1) p values are from logistic regression models adjusted for age, sex, smoking history, beta blocker, calcium channel blocker, and statin use.
Indicates p<0.05.
Indicates p<0.0013 (Bonferroni adjusted threshold).
Abbreviations: BMI: body mass index; BP: blood pressure, HDL: high density lipoprotein; LDL: low density lipoprotein.
Within decile 1 (lowest genetic risk for POAG), glaucoma was associated with higher HbA1c (38.51 vs 35.9 mmol/mol; p=8.5E-06) and higher prevalence of T2DM (17.5% vs 6.5%; p=9.7E-7), dyslipidemia (31.2% vs 18.3%; p=0.028), CKD (6.7% vs 2.0%; p=0.0009), and COPD (9.4% vs 4.1%; p=0.041). Within decile 10 (highest genetic risk for POAG), glaucoma was associated with higher forced expiratory volume in one second to forced vital capacity ratio (FEV1/FVC; 0.40 vs 0.36; p=0.021), lower cholesterol (21.1 vs 21.6 mmol/L; p=0.015), and higher prevalence of T2DM (9.9% vs 6.4%; p=0.039) and dyslipidemia (27.7% vs 17.3%; p=7.0E-5).
Overall, the magnitudes of differences in cardiopulmonary disease prevalence and cardiopulmonary factor levels between glaucoma cases and controls were greater within decile 1 compared to within decile 10 (Table 2). Using a contrast test, the magnitude of association of glaucoma with higher prevalence of diabetes (p-for-difference=0.001), higher prevalence of CKD (p=0.046), and higher HbA1c (p<0.0001) were greater in decile 1 compared to decile 10. Associations of glaucoma with dyslipidemia (p=0.73), COPD (p=0.11), FEV1/FVC (p=0.62), or cholesterol level (p=0.25) did not significantly differ between deciles 1 and 10.
Prevalence of T2DM, dyslipidemia, CKD, and COPD, but not dyslipidemia, tended to decrease among glaucoma cases (but not among controls) as POAG PRS decile increased (Figure 2). In covariate adjusted logistic regression models, odds of prevalent T2DM (odds ratio [OR]: 0.956; 95% CI: 0.933, 0.980), CKD (OR: 0.956; 95% CI: 0.921, 0.992), and COPD (OR: 0.962; 95% CI: 0.931, 0.993) decreased with each incremental increase in POAG PRS decile among glaucoma cases.
Figure 2: Prevalence of Type II Diabetes Mellitus, Dyslipidemia, Chronic Kidney Disease, and Chronic Obstructive Pulmonary Disease by Primary Open Angle Glaucoma Polygenic Risk Score Decile, Among Glaucoma Cases and Controls.
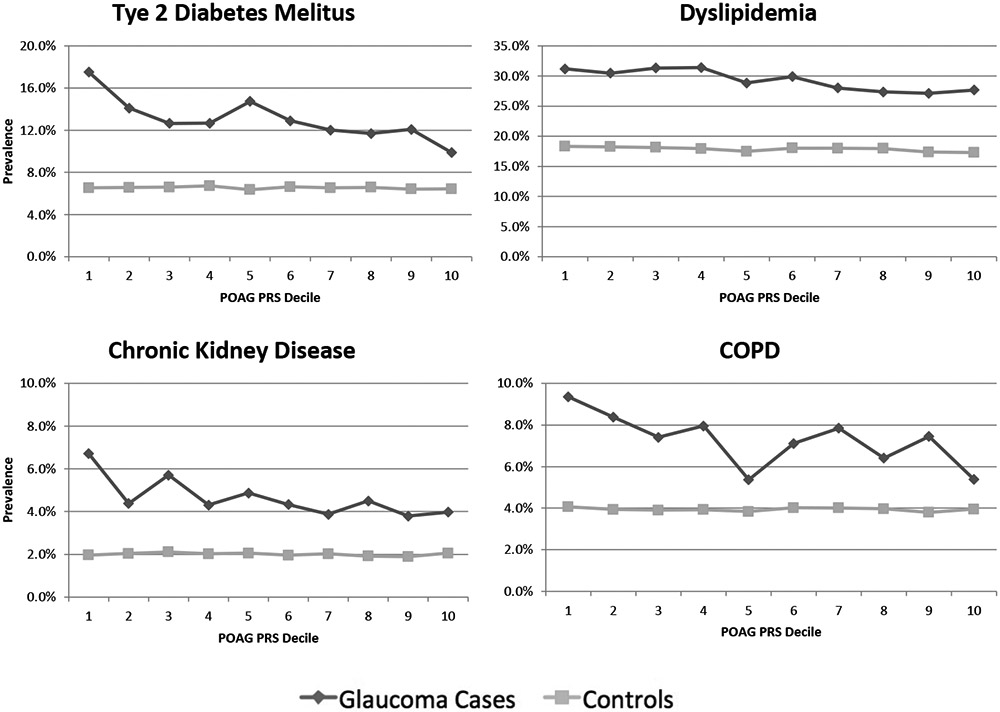
Abbreviations: COPD: Chronic Obstructive Pulmonary Disease. POAG PRS: Primary Open Angle Glaucoma Polygenic Risk Score. Decile 1 indicates lowest background genetic risk for POAG. Decile 10 indicates highest background genetic risk for POAG.
Mean HbA1c decreased as POAG PRS decile increased for glaucoma cases, but remained constant across deciles for controls (Figure 3). Mean cholesterol levels increased as POAG PRS decile increased for both glaucoma cases and controls (Figure 3). In covariate adjusted linear regression models, mean estimated HbA1c decreased (Beta: −0.138 mmol/mol; 95% CI: −0.194, −0.082) and mean estimated cholesterol increased (Beta: 0.073 mmol/L; 95% CI: 0.030, 0.116) with each incremental increase in POAG PRS decile among glaucoma cases.
Figure 3: Blood Levels of Glycated Hemoglobin and Cholesterol by Primary Open Angle Glaucoma Polygenic Risk Score Decile, Among Glaucoma Cases and Controls.
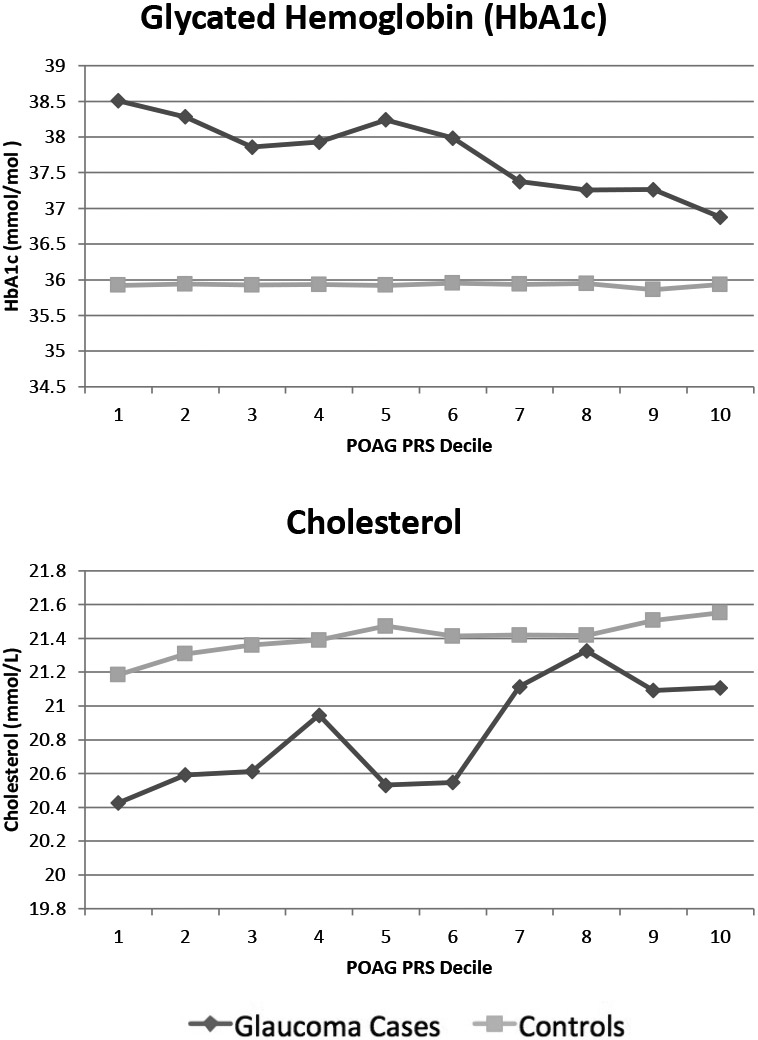
POAG PRS: Primary Open Angle Glaucoma Polygenic Risk Score. Decile 1 indicates lowest background genetic risk for POAG. Decile 10 indicates highest background genetic risk for POAG.
The sensitivity analysis included 739 glaucoma cases and 38,730 controls. Thinner GCC was associated with lower cholesterol in PRS decile 1 (p=0.004). In decile 10, thinner GCC thickness was associated with higher odds of diabetes (p=0.0002) and dyslipidemia (p=0.002), and with lower cholesterol levels (p=0.002) and higher HbA1c (p=0.006). Full sensitivity analysis results are presented in Supplement 4.
DISCUSSION
In this large cross-sectional study of UK Biobank subjects, we found a higher likelihood of prevalent diabetes, hypertension, dyslipidemia, CVD, stroke, CKD, and COPD among glaucoma cases compared to those without glaucoma. Overall, the magnitudes of differences in cardiopulmonary disease prevalence or mean levels of examination or laboratory measures between glaucoma cases and controls was greater among those with lowest background genetic risk for glaucoma relative to those with highest genetic risk.
Our findings corroborate previously reported associations between glaucoma and increased risk of cardiopulmonary diseases.[11-17] Several clinical and epidemiological studies have explored this association, with strong evidence of associations of glaucoma with higher burden of cardiopulmonary diseases such as cardiovascular disease, T2DM, and hypertension.[11-17] Similarly, in the present study, those with glaucoma had higher BMI, HbA1c, and creatinine; and lower pulse rate, cholesterol, LDL, and vitamin D. In contrast with the present study, a prior study of 16,939 Korean individuals reported no association between glaucoma and dyslipidemia or cholesterol levels [17]. Our larger sample size may have allowed for the detection of statistically significant (but perhaps not clinically significant) associations between serum lipids and glaucoma. Others have reported an associated with both LDL and HDL with retinal nerve fiber bundle geometry, [25] consistent with our findings that thinner GCC thickness is associated with greater odds of dyslipidemia in PRS decile 10.
While several studies have assessed the association between glaucoma and cardiopulmonary diseases or factors,[11-17] no prior study has considered that this relationship may differ by underlying glaucoma genetic risk. To better understand the interaction between background genetic risk and metabolic factors, we stratified our analysis by first (lowest genetic risk) vs. tenth (highest genetic risk) decile of POAG PRS. In particular, glaucoma was statistically significantly associated with CKD, COPD, and HbA1c in decile 1, but not in decile 10. While glaucoma was associated with T2DM in both deciles, the magnitude of association was statistically significantly greater in decile 1. Further research with more robust sub-categorization of glaucoma and more repeated assessment of IOP will be needed to consider whether these associations are more prominent in a specific glaucoma subtype (e.g. high vs normal tension glaucoma).
Both genetic and environmental factors drive the development of chronic diseases.[26] In those within POAG PRS decile 10, genetic factors likely play a larger role in the development of glaucoma overwhelming most environmental factors. Genetics likely play a lesser role for those in POAG PRS decile 1; for these individuals, environmental factors (e.g. systemic cardiopulmonary risk factors or interaction between genes that produce these systemic conditions and genes that comprise the POAG PRS) may be more important in determining who develops glaucoma.[27-29] This is consistent with our findings that the associations between glaucoma and cardiopulmonary diseases were greater in decile 1 compared to decile 10. Moreover, prevalence of T2DM, dyslipidemia, CKD, and COPD tended to decrease among glaucoma cases as POAG PRS decile increased.
It is possible that certain genetic loci may exert opposite effects on risk for glaucoma vs. other diseases. For example, the APOE ε4 genotype is associated with increased risk for Alzheimer’s, but may be protective for glaucoma.[30] Similarly, there may be genotypes that are simultaneously risk factors for glaucoma (contributing to high PRS score), but are protective for some cardiopulmonary diseases. This may account for the observation that dyslipidemia was slightly more prevalent among those in POAG PRS decile 1 (8.3%) compared to decile 10 (7.3%). Alternatively, it is possible that glaucoma cases with low genetic risk may have developed glaucoma due to exposure to external environmental risk factors, which may also contribute to cardiopulmonary disease. For example, cigarette smoking might be associated with increased risk of glaucoma and is associated with most cardiopulmonary diseases.[31-33] While our analyses adjusted for cigarette smoking, it is possible that the adjustment was incomplete or that other unmeasured environmental factors contributed to the observed patterns of association.
This study has several important limitations to consider. First, this study is cross-sectional and observational and causality cannot be demonstrated. Second, this analysis utilizes ICD codes and self-reporting to identify glaucoma and cardiopulmonary diseases, which likely resulted in over- or under-reporting of disease outcomes. However, prior studies have suggested high accuracy of ICD code diagnosis of glaucoma.[34-36] Those with diagnosed glaucoma may be more plugged into healthcare and may have a higher overall prevalence of diagnosed disease simply on the basis of being evaluated more often. Third, while those with ICD codes for secondary glaucoma were excluded from our analyses, it is possible that some individuals with secondary glaucoma were inadvertently included in our analysis. If, for example, an individual had glaucoma secondary to diabetic eye disease, this could have contributed to the observed associations between glaucoma and both diabetes and HbA1c. However, we expect this effect to be negligible given our large control group and our sampling strategy. Additionally, our definition of glaucoma has been used in prior research[9,19] and there is evidence for an association between diabetes and POAG, warranting the inclusion of those with diabetes and diabetic eye disease in our study cohort.[37] Fourth, this analysis only includes Caucasian participants; therefore the generalizability to other racial or ethnic groups requires further evaluation. Fifth, the large size of the UKBB cohort allowed us to detect differences that were statistically significant, but may not be clinically meaningful. Lastly, the sensitivity analysis, while perhaps underpowered, did not completely support the main findings. Further research measuring OCT parameters in a larger sample would be needed to characterize associations between cardiopulmonary diseases and differences in retinal nerve fiber layer morphology. Because many individuals with glaucoma are undiagnosed, such studies using OCT parameters as an objective outcome will be important in identifying associations between glaucoma and systemic diseases.
This study also has several noteworthy strengths. First, this is the first study to consider that the association between glaucoma and cardiopulmonary factors may vary by glaucoma genetic risk. Second, the study was large enough to assess the relation between systemic factors and glaucoma stratified by POAG PRS. Third, this study utilizes data from a large cohort with well characterized genotypic and phenotypic data, yielding precise and stable estimates. Moreover, the POAG PRS was created using data from the largest GWAS metanalysis to date, and excluded UK Biobank participants. Fourth, our models include adjustment for potentially important confounders, including age, sex, cigarette use, and potentially confounding medication usage, namely beta-blockers and statins.
In conclusion, associations between glaucoma and cardiopulmonary diseases were larger in magnitude among those who developed glaucoma despite low genetic risk, particularly for diabetes, CKD, COPD, and cholesterol levels. Gene-environment interactions and gene-gene interactions may account for this observed pattern. Further research will be needed to investigate which environmental factors may mediate the observed differences in associations.
Supplementary Material
Key Messages:
What is already known on this topic: Glaucoma has been associated with several cardiopulmonary diseases.
What this study adds: This study found that the association between glaucoma and cardiometabolic diseases differed by background genetic risk for glaucoma. Those who developed glaucoma despite having low genetic risk tended to have a higher prevalence of cardiometabolic disease, particularly for diabetes, CKD, COPD, and cholesterol levels.
How this study might affect research, practice or policy: Individual’s genetic risk for glaucoma may modulate the relative impact of environmental or other genetic risk factors for cardiopulmonary disease. These findings may have implications for glaucoma or cardiometabolic disease screening as the use of genotyping becomes more common in the clinical setting.
Financial support:
This work was supported the MEE Institutional Startup Fund (no grant number) and NIH K23 career development award (K23EY032634) (NZ), NIH R01 EY032559 (JLW, LRP), and NIH R01 EY015473 (LRP). Dr. Pasquale is also supported by an unrestricted Challenge Grant from Research to Prevent Blindness (NYC; no grant number) and The Glaucoma Foundation (no grant number; LRP). The funding organization had no role in the design or conduct of this research.
Footnotes
Conflict of Interest: AK maintains consultancy for Ocuphire Pharma, Inc. LRP is a consultant to Twenty Twenty, Skye Biosciences, and Eyenovia. No conflicting relationship exists for the other authors.
Meeting Presentations: Presented by Ajay Kolli at the 9th World Glaucoma E-Congress, June 2021
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006;90:262–7. doi: 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King A, Azuara-Blanco A, Tuulonen A. Glaucoma. BMJ 2013;346:f3518. doi: 10.1136/bmj.f3518 [DOI] [PubMed] [Google Scholar]
- 3.Greco A, Rizzo MI, De Virgilio A, et al. Emerging Concepts in Glaucoma and Review of the Literature. The American Journal of Medicine 2016;129:1000.e7–1000.e13. doi: 10.1016/j.amjmed.2016.03.038 [DOI] [PubMed] [Google Scholar]
- 4.Blumberg D, Skaat A, Liebmann JM. Chapter 5 - Emerging risk factors for glaucoma onset and progression. In: Bagetta G, Nucci C, eds. Progress in Brain Research. Elsevier; 2015. 81–101. doi: 10.1016/bs.pbr.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 5.Gao XR, Huang H, Kim H. Polygenic Risk Score Is Associated With Intraocular Pressure and Improves Glaucoma Prediction in the UK Biobank Cohort. Trans Vis Sci Tech 2019;8:10–10. doi: 10.1167/tvst.8.2.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang K, Gaitsch H, Poon H, et al. Classification of common human diseases derived from shared genetic and environmental determinants. Nat Genet 2017;49:1319–25. doi: 10.1038/ng.3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugrue LP, Desikan RS. What Are Polygenic Scores and Why Are They Important? JAMA 2019;321:1820–1. doi: 10.1001/jama.2019.3893 [DOI] [PubMed] [Google Scholar]
- 8.Craig JE, Han X, Qassim A, et al. Multitrait analysis of glaucoma identifies new risk loci and enables polygenic prediction of disease susceptibility and progression. Nat Genet 2020;52:160–6. doi: 10.1038/s41588-019-0556-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zebardast N, Sekimitsu S, Wang J, et al. Characteristics of Gln368Ter Myocilin Variant and Influence of Polygenic Risk on Glaucoma Penetrance in the UK Biobank. Ophthalmology Published Online First: 10 March 2021. doi: 10.1016/j.ophtha.2021.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan BJ, Bailey JC, Igo RP Jr, et al. Association of a Primary Open-Angle Glaucoma Genetic Risk Score With Earlier Age at Diagnosis. JAMA Ophthalmology 2019;137:1190–4. doi: 10.1001/jamaophthalmol.2019.3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salim S, Shields MB. Glaucoma and Systemic Diseases. Survey of Ophthalmology 2010;55:64–77. doi: 10.1016/j.survophthal.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 12.Zhao D, Cho J, Kim MH, et al. Diabetes, Fasting Glucose, and the Risk of Glaucoma: A Meta-analysis. Ophthalmology 2015;122:72–8. doi: 10.1016/j.ophtha.2014.07.051 [DOI] [PubMed] [Google Scholar]
- 13.Langman MJS, Lancashire RJ, Cheng KK, et al. Systemic hypertension and glaucoma: mechanisms in common and co-occurrence. British Journal of Ophthalmology 2005;89:960–3. doi: 10.1136/bjo.2004.053397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y-Y, Hu H-Y, Chu D, et al. Patients with Primary Open-Angle Glaucoma May Develop Ischemic Heart Disease More Often than Those without Glaucoma: An 11-Year Population-Based Cohort Study. PLOS ONE 2016;11:e0163210. doi: 10.1371/journal.pone.0163210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djordjevic-Jocic J, Cukuranovic R, Mitic B, et al. Ocular and systemic factors associated with glaucoma in chronic kidney disease patients. Int Urol Nephrol 2014;46:2191–8. doi: 10.1007/s11255-014-0804-0 [DOI] [PubMed] [Google Scholar]
- 16.Rim TH, Lee SY, Bae HW, et al. Increased stroke risk among patients with open-angle glaucoma: a 10-year follow-up cohort study. British Journal of Ophthalmology 2018;102:338–43. doi: 10.1136/bjophthalmol-2017-310415 [DOI] [PubMed] [Google Scholar]
- 17.Shon K, Sung KR. Dyslipidemia, Dyslipidemia Treatment, and Open-angle Glaucoma in the Korean National Health and Nutrition Examination Survey. Journal of Glaucoma 2019;28:550–6. doi: 10.1097/IJG.0000000000001237 [DOI] [PubMed] [Google Scholar]
- 18.Kim H-A, Han K, Lee Y-A, et al. Differential Association of Metabolic Risk Factors with Open Angle Glaucoma according to Obesity in a Korean Population. Scientific Reports 2016;6:38283. doi: 10.1038/srep38283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han X, Souzeau E, Ong J-S, et al. Myocilin Gene Gln368Ter Variant Penetrance and Association With Glaucoma in Population-Based and Registry-Based Studies. JAMA Ophthalmol 2019;137:28–35. doi: 10.1001/jamaophthalmol.2018.4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biomarker data. https://www.ukbiobank.ac.uk/enable-your-research/about-our-data/biomarker-data (accessed 19 Jul 2021).
- 21.Gharahkhani P, Jorgenson E, Hysi P, et al. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat Commun 2021;12:1258. doi: 10.1038/s41467-020-20851-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keane PA, Grossi CM, Foster PJ, et al. Optical Coherence Tomography in the UK Biobank Study - Rapid Automated Analysis of Retinal Thickness for Large Population-Based Studies. PLoS One 2016;11:e0164095. doi: 10.1371/journal.pone.0164095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Currant H, Hysi P, Fitzgerald TW, et al. Genetic variation affects morphological retinal phenotypes extracted from UK Biobank optical coherence tomography images. PLOS Genetics 2021;17:e1009497. doi: 10.1371/journal.pgen.1009497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M, Spiegelman D, Kuchiba A, et al. Statistical Methods for Studying Disease Subtype Heterogeneity. Stat Med 2016;35:782–800. doi: 10.1002/sim.6793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin Q, Ebert T, Tönjes A, et al. Association between serum lipid parameters and retinal nerve fiber layer characteristics. Invest Ophthalmol Vis Sci 2019;60:5579–5579. [Google Scholar]
- 26.Rappaport SM. Genetic Factors Are Not the Major Causes of Chronic Diseases. PLoS One 2016;11. doi: 10.1371/journal.pone.0154387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aschard H, Kang JH, Iglesias AI, et al. Genetic correlations between intraocular pressure, blood pressure and primary open-angle glaucoma: a multi-cohort analysis. Eur J Hum Genet 2017;25:1261–7. doi: 10.1038/ejhg.2017.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laville V, Kang JH, Cousins CC, et al. Genetic correlations between diabetes and glaucoma: an analysis of continuous and dichotomous phenotypes. Am J Ophthalmol 2019;206:245–55. doi: 10.1016/j.ajo.2019.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang JH, Wiggs JL, Rosner BA, et al. Endothelial Nitric Oxide Synthase Gene Variants and Primary Open-Angle Glaucoma: Interactions with Hypertension, Alcohol, and Cigarette Smoking. Arch Ophthalmol 2011;129:773–80. doi: 10.1001/archophthalmol.2011.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margeta MA, Letcher SM, Igo RP, et al. Association of APOE With Primary Open-Angle Glaucoma Suggests a Protective Effect for APOE ε4. Invest Ophthalmol Vis Sci 2020;61:3–3. doi: 10.1167/iovs.61.8.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pérez-de-Arcelus M, Toledo E, Martínez-González MÁ, et al. Smoking and incidence of glaucoma. Medicine (Baltimore) 2017;96. doi: 10.1097/MD.0000000000005761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards R, Thornton J, Ajit R, et al. Cigarette smoking and primary open angle glaucoma: a systematic review. J Glaucoma 2008;17:558–66. doi: 10.1097/IJG.0b013e31815f530c [DOI] [PubMed] [Google Scholar]
- 33.Mallampalli A, Guntupalli KK. Smoking and systemic disease. Clin Occup Environ Med 2006;5:173–92, x. doi: 10.1016/j.coem.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 34.Biggerstaff KS, Frankfort BJ, Orengo-Nania S, et al. Validity of code based algorithms to identify primary open angle glaucoma (POAG) in Veterans Affairs (VA) administrative databases. Ophthalmic Epidemiology 2018;25:162–8. doi: 10.1080/09286586.2017.1378688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muir KW, Gupta C, Gill P, et al. Accuracy of International Classification of Diseases, Ninth Revision, Clinical Modification Billing Codes for Common Ophthalmic Conditions. JAMA Ophthalmol 2013;131:119. doi: 10.1001/jamaophthalmol.2013.577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai CX, Michalak SM, Stinnett SS, et al. Effect of ICD-9 to ICD-10 Transition on Accuracy of Codes for Stage of Diabetic Retinopathy and Related Complications: Results from the CODER Study. Ophthalmol Retina 2021;5:374–80. doi: 10.1016/j.oret.2020.08.004 [DOI] [PubMed] [Google Scholar]
- 37.Song BJ, Aiello LP, Pasquale LR. Presence and Risk Factors for Glaucoma in Patients with Diabetes. Curr Diab Rep 2016;16:124. doi: 10.1007/s11892-016-0815-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


