Abstract
ToxR, the transmembrane regulatory protein required for expression of virulence factors in the human diarrheal pathogen Vibrio cholerae, directly activates and represses the transcription of two outer membrane porins, OmpU and OmpT, respectively. In an attempt to dissect the role of the OmpU and OmpT porins in viability and virulence factor expression, in-frame chromosomal deletions were constructed in the coding sequences of ompU and ompT of V. cholerae. Two separate deletions were introduced into ompU; the first (small) deletion, ΔompU1, removed the coding sequence for 84 internal amino acids (aa), while the second (large) deletion, ΔompU2, removed the coding sequence for the entire amino-terminal 274 aa. The ΔompU1 strain had a growth defect that could not be complemented by episomal expression of full-length ompU. In contrast, a strain with ΔompU2 displayed wild-type growth kinetics in rich media, suggesting that this is the true phenotype of a strain lacking OmpU and that the truncated OmpU protein, rather than the absence of OmpU, may be the cause for the ΔompU1 phenotype. A large deletion removing the coding sequence for the entire N-terminal 273 aa of OmpT (ΔompT) was also constructed in wild-type as well as ΔtoxR and ΔompU2 strains, and these strains displayed wild-type growth kinetics in rich media. However, the ΔompU2 strain was deficient for growth in deoxycholate compared to wild-type, ΔompT, and ΔompU2 ΔompT strains, reinforcing a positive role for the OmpU porin and a negative role for the OmpT porin in V. cholerae resistance to anionic detergents. The ΔompU2, ΔompT, and ΔompU2 ΔompT strains exhibited wild-type levels of in vitro virulence factor expression and resistance to polymyxin B and serum and in vivo colonization levels similar to a wild-type strain in the infant mouse intestine. Our results demonstrate that (i) OmpU and OmpT are not essential proteins, as was previously thought; (ii) these porins contribute to V. cholerae resistance to anionic detergents; and (iii) OmpU and OmpT are not essential for virulence factor expression in vitro or intestinal colonization in vivo.
Vibrio cholerae, a motile gram-negative bacterium, lives primarily in aquatic environments. Upon entry into a human host, V. cholerae can cause the devastating and potentially lethal form of diarrhea known as cholera. The pathogens synthesize virulence factors, including cholera toxin (CT) and toxin coregulated pilus (TCP), in the small intestine, presumably in response to yet-undefined environmental signals (for a review, see reference 48). ToxR, a transmembrane transcriptional activator, initiates these events in association with TcpP, a second transmembrane transcriptional activator, by stimulating the transcription of toxT (13, 18, 20, 26). The ToxT protein directly activates transcription of the ctx and tcp genes, which encode CT and TCP (14). This virulence cascade can be induced in vitro by specific growth conditions (13). The ctx, tcp, and toxT genes are located on mobile genetic elements associated primarily with epidemic V. cholerae (21, 25, 41, 56), while the toxR gene is found in the ancestral Vibrio genome (23, 40, 58), suggesting that ToxR has recently been recruited into this virulence cascade.
The ancestral role of ToxR appears to be as a modulator of outer membrane (OM) porins, because ToxR, independently of TcpP and ToxT, activates and represses transcription of two genes encoding major OM porins, OmpU and OmpT, respectively (8, 31). This regulation results in virtually exclusive expression (in vitro) of OmpU in wild-type (toxR+) strains, while toxR mutant strains express OmpT exclusively. However, even though ompU is transcribed at relatively high levels in toxR+ strains, certain laboratory conditions (e.g., the addition of bile) can stimulate increased levels of ToxR-dependent ompU transcription, indicating that ToxR transcriptional activity is modulated by environmental signals (43). ToxR also regulates expression of OM proteins, potentially porins, in other Vibrio and/or Photobacterium species, including V. parahaemolyticus, V. fluvialis, V. mimicus, and Photobacterium profundum (23, 43, 58).
OM porins of gram-negative bacteria, such as OmpU and OmpT, function primarily as channels for entry and exit of hydrophilic, low-molecular-weight molecules. Porins form hydrophilic channels composed of trimeric β-barrels with pore sizes ranging from 1 to 2 nm in diameter (for a review, see reference 37). The porin channels are small enough to prevent large molecules, including hydrolytic enzymes and binding proteins, from leaving the periplasmic space. Diffusion rates across porins are near maximal for small molecules; however, solute-discriminating properties are based on the amino acid residues that protrude toward the lumen of the porin channel (10). For example, PhoE of Escherichia coli is preferentially permeable toward anions, while OmpF and OmpC show preference toward cations (5). Porins are important constituents of the OM, comprising up to 2% of the entire protein content of the cell (38).
Up to 10 major OM proteins have been identified in V. cholerae (22). Porin activity has been demonstrated for OmpU, OmpT (4, 7), and OmpS, a 43-kDa maltoporin sharing homology with LamB of E. coli (27). Liposome swelling assays predicted a pore size of 1.6 nm for OmpU trimers, while that of OmpT trimers was smaller but not quantitated (7). V. cholerae also has an OmpA homologue (2), as well as the highly immunogenic proteins OmpV, OmpW, and OmpX (12, 51); no known biological function has been demonstrated for these OM proteins. However, porin activity is reportedly absent for OmpV (4) and OmpX (7). It is probable that additional porin activities will be identified in the OM of V. cholerae; at least one additional putative porin (VC0972) was identified within the recently sequenced V. cholerae genome (19). Correct insertion of the OmpU and OmpT porins into the OM requires the type II extracellular protein secretion (EPS) pathway, which is also required for secretion of CT (47).
Porins of gram-negative organisms have been surmised to play roles in the pathogenesis of Neisseria meningitidis (33), Shigella flexneri (6), and Salmonella enterica serovar Typhimurium (35). The OmpC porin of E. coli and serovar Typhimurium is less permeable to bile (and therefore more protective) than OmpF (54). Nikaido (37) hypothesized that enteric pathogens express OmpC in the mammalian host to reduce permeability toward anionic detergents in the high-osmolarity conditions within the intestine, while still permitting permeability of smaller nutrients such as glucose. The OmpF porin, expressed in growth conditions of low osmolarity, is speculated to be beneficial when the bacteria are outside the host in nutrient-poor, aqueous environments.
Understanding the role of the ToxR-regulated OmpU and OmpT porins in V. cholerae pathogenesis has been hindered by difficulties encountered in the construction of V. cholerae strains with mutations in ompU and ompT, leading to the hypothesis that these are essential genes (28, 49). An initial report utilizing a tissue culture model suggested that OmpU acts as an adhesin (50), but this finding has remained unconfirmed (34, 42). We have presented evidence that ToxR mediates increased resistance to anionic detergents in enteropathogenic Vibrio species and that this enhanced resistance correlated with increased OmpU expression in V. cholerae (43). Recently, we succeeded in demonstrating a direct role for OmpU and OmpT in relative resistance to bile by reversing the ToxR-dependent modulation of these porins (i.e., expressing OmpT in place of OmpU in a toxR+ strain and expressing OmpU in place of OmpT in a toxR mutant strain). Our results demonstrated that V. cholerae expressing OmpT is less resistant to anionic detergents than V. cholerae expressing OmpU. Moreover, a strain expressing OmpT in place of OmpU shows significantly reduced virulence factor expression in vitro and intestinal colonization in vivo (42). These results suggest a role for OmpU and OmpT not only in bile resistance but also in the signal transduction cascade that leads to virulence factor expression and intestinal colonization.
In the present study we investigated the phenotype(s) of V. cholerae strains lacking OmpU and/or OmpT. Our results demonstrate that ompU and ompT are not essential genes of V. cholerae, although the design of the gene disruption is important to eliminate any negative effects associated with expression of the remaining porin coding sequence. V. cholerae lacking OmpU was more sensitive to anionic detergents than a wild-type strain, corroborating a protective role for OmpU in bile resistance. V. cholerae strains lacking OmpU, OmpT, or both resembled wild-type strains with respect to virulence factor expression in vitro and intestinal colonization in vivo, indicating that neither OmpU nor OmpT is required for these pathogenic properties.
MATERIALS AND METHODS
Growth conditions and media.
All experiments, except where noted, were performed with classical V. cholerae 0395 grown in Luria broth (LB) supplemented with appropriate antibiotics. Inducing conditions for expression of virulence factors were growth in LB at pH 6.5 at 30°C, and noninducing conditions were growth in LB at pH 8.5 at 30°C. Growth rate experiments with LB supplemented with deoxycholate (DOC; Sigma) were performed at 37°C as described previously (43). Growth experiments in minimal medium were performed at 37°C in M9 medium utilizing glucose (0.2%) as the sole carbon source (55). Resistance to polymyxin B was determined utilizing cultures grown overnight at 37°C in Mueller-Hinton broth (MHB) as described previously (39).
Plasmid construction.
Oligonucleotides used in the construction of the porin deletions are listed in Table 1. Primers contain restriction sites (underlined in Table 1) utilized in cloning. The ΔompU1 deletion construct was generated by PCR amplification of the 3′ end of ompU (11) with primers OMPU2XBI and OMPU1BHI. The resulting 521-bp fragment was digested with XbaI and BamHI and ligated into pWKS30 (57) that had been similarly digested to yield pKEK188. Then, a 5′ sequence of ompU was PCR amplified with primers OMPUP1ERI and OMPUP2BGLII, which resulted in a fragment of 847 bp containing the entire ompU promoter and also the first 24 bp of the coding sequence. This fragment was digested with BglII and EcoRI and ligated into pKEK188 that had been digested with the same restriction enzymes, yielding pKEK189. This ΔompU1 in-frame deletion removes the coding sequence for aa 9 to 90 of the nascent OmpU protein (i.e., including the leader peptide). The entire ΔompU1 construct from pKEK189 was digested with SalI and NotI and then ligated into pKEK229 (9), a derivative of pCVD442 (15), yielding pKEK235, which was used to recombine the mutation into the V. cholerae chromosome.
TABLE 1.
Bacterial strains and oligonucleotides used in this study
| Strain or oligonucleotide | Genotype or sequence | Source or reference |
|---|---|---|
| Strains | ||
| V. cholerae | ||
| O395 | Classical O1, Ogawa, Smr | 30 |
| KKV61 | ΔtoxR | 24 |
| KKV598 | ΔlacZ | 16 |
| KKV669 | ΔompU1 | This study |
| KKV780 | ΔompU2 | This study |
| KKV804 | ΔtoxR ΔompT | This study |
| KKV809 | ΔompT | This study |
| KKV884 | ΔompU2 ΔompT | This study |
| KKV1216 | ΔepsD::kan | This study |
| KKV1232 | ΔepsE::kan ΔompU2 | This study |
| KKV1234 | ΔepsE::kan | This study |
| E. coli | ||
| DH5α | F− deoR endA1 hsdR17 supE44 thi-1 recA1 gyrA96 relA1 Δ(argF-lacZYA)U169 (φ80dlacZΔM15) | 17 |
| SM10λpir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu λpirR6K; Kmr | 31 |
| Oligonucleotides | ||
| OMPTP1HDIII | 5′-GGCAAGCTTAATCTTGAATGTTTTTTGTCAGCAT-3′ | |
| OMPT1NDEI | 5′-AGTGGATTCATATGAAAAAACTCTATTAGCA-3′ | |
| OMPT2SACII | 5′-TCCCCGCGGTTACCAGTAGATACGAGCGCCGATA-3′ | |
| OMPTCTERM1 | 5′-TATGAGTATCATATGCAAGAAGCGAACACTGGC-3′ | |
| OMPTCTERM2 | 5′-GCGGATCCATCGTTTTATCACCAAATAATCC-3′ | |
| OMPTP2NDEI | 5′-GCGGATCCTCATATGAATCCACTGCCTTTTCTT-3′ | |
| OMPU1BHI | 5′-GCGGATCCAATGATCAAGGTAAAAACGCGTC-3′ | |
| OMPU1NDEI | 5′-GACTTAATCATATGAACAAGACTCTGATTGCT-3′ | |
| OMPU2SACII | 5′-CGCCGCGGTTAGAAGTCGTAACGTAGACCGATAGC-3′ | |
| OMPU2XBI | 5′-GCTCTAGACTCGTAGCCAGTGTAGTCAACGT-3′ | |
| OMPUDEL1 | 5′-CAAACTAGGTCATATGGCGTTTACTGCGACATACAA-3′ | |
| OMPUDEL2 | 5′-GCGCCGCGGAGATGAGTATTGAGTTCGAGA-3′ | |
| OMPUP1ERI | 5′-GCGAATTCGTGGCACCAAAAATGACTTTGCC-3′ | |
| OMPUP2BGLII | 5′-GCAGATCTAAGAGCAATCTGAGTCTTGTTCAT-3′ | |
| OMPUP2NDEI | 5′-GCGGATCCTCATATGATTAAGTCCTAATTTATT-3′ |
The ΔompU2 deletion was constructed in several steps. First, the promoter region of ompU was amplified by PCR with primers OMPUPERI and OMPUP2NDEI, and the resulting 828-bp fragment was digested with BamHI and EcoRI, and ligated into pWKS30 digested similarly, to yield pKEK242. A fragment containing the 3′ end of ompU was PCR amplified utilizing primers OMPUDEL1 and OMPUDEL2, and the resulting 476-bp fragment was digested with NdeI and SacII and ligated into pKEK242 that had been similarly digested to yield pKEK252. This ΔompU2 in-frame deletion removes the coding sequence for aa 1 to 273 of OmpU, leaving only the final 68 aa of the protein. The entire ΔompU2 construct from pKEK252 was then digested with SacI and XhoI and ligated into pKEK229 (9), digested similarly, to form pKEK276, which was used to recombine the mutation onto the V. cholerae chromosome.
The ΔompT deletion was obtained by first PCR amplifying the ompT promoter, using primers OMPTP1HDIII and OMPTP2NDEI, digesting the resulting 528-bp fragment with HindIII and BamHI, and then ligating it with pWKS30 digested similarly, to yield pKEK243. Next, the 3′ end of ompT was PCR amplified with primers OMPTCTERM1 and OMPTCTERM2, and the resulting 490-bp fragment was digested with NdeI and BamHI and ligated with pKEK243 digested similarly, yielding pKEK306. This ΔompT in-frame deletion removes the coding sequence for aa 1 to 273 of OmpT, leaving only the final 71 aa of the protein. The entire ΔompT construct from pKEK306 was then digested with NotI and XhoI and ligated with pKEK229 (9), digested similarly, yielding pKEK309, which was used to recombine the mutation onto the V. cholerae chromosome.
Plasmids expressing ompU and ompT from their natural promoters were constructed in the following manner. The entire coding sequence of ompU was PCR amplified using primers OMPU1NDEI and OMPU2SACII. The resulting 1,026-bp fragment was then digested with NdeI and SacII and ligated into pKEK242 digested similarly, yielding PKEK253. Because the NdeI site engineered into the primers is incorporated into the initiating methionine of the OmpU coding sequence, this plasmid reconstructs ompU downstream of its natural ompU promoter. The entire ompT coding sequence was PCR amplified with primers OMPTINDEI and OMPT2SACII, and the resulting 1,035-bp fragment was digested with NdeI and SacII and ligated into pKEK243, similarly digested, to yield pKEK255. As with the ompU plasmid, this plasmid reconstructs ompT downstream of its natural ompT promoter.
Plasmids pAA35 and pAA48, which were used to construct ΔepsD::Kan and ΔepsE::Kan V. cholerae strains, were kindly provided by S. Sozhamannan (1). Plasmid pJS752-3 (46), used to express CT-B for secretion experiments, was a considerate gift of C. Lopez Macias.
Bacterial strains.
Bacterial strains utilized in this study are listed in Table 1. Escherichia coli strain SM10λpir (31) was employed to transfer deletion constructs into the V. cholerae chromosome by conjugation, while strain DH5α (17) was utilized for all cloning experiments. All V. cholerae strains are isogenic with the classical Ogawa strain O395 (30). V. cholerae strains KKV61 (ΔtoxR) (24) and KKV598 (ΔlacZ) (16) have been described previously.
Plasmids pKEK235, pKEK276, pKEK309, pAA35, and pAA48 were used to recombine mutations into the chromosome of V. cholerae O395, KKV61, or KKV780 as described before (15), yielding strains KKV669 (ΔompU1), KKV780 (ΔompU2), KKV809 (ΔompT), KKV1234 (ΔepsE::Kan), KKV804 (ΔtoxR ΔompT), KKV884 (ΔompU2 ΔompT), KKV1216 (ΔepsD::Kan), and KKV1232 (ΔepsE::Kan ΔompU2). Chromosomal mutations were confirmed by PCR with specific primers. The deletion strains were further verified by SDS-PAGE and Western blots probed with either α-OmpU or α-OmpT antisera.
Detection of protein expression.
The isolation of OM proteins from V. cholerae was accomplished using the method of Miller and Mekalanos (31). Whole-cell lysates or OM preparations were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% polyacrylamide gel and stained with Coomassie brilliant blue for visualization. Protein gels were transferred to nitrocellulose for Western blotting using a transblotter (Bio-Rad). The blots were probed with rabbit polyclonal antisera against V. cholerae OmpU, OmpT, and outer membrane proteins (OMPs) (12) (the kind gift of J. Peterson) and developed utilizing the ECL Detection System (Amersham). Culture supernatants were assayed for CT by GM1–enzyme-linked immunosorbent assay (ELISA) with rabbit polyclonal antiserum against the purified B subunit of CT (53). Intracellular CT was determined similarly from V. cholerae strains carrying plasmid pJS752-3 (46) grown in LB overnight. One milliliter of culture was centrifuged, and the pellet was resuspended in sonication buffer (10 mM Tris-HCl, 1 mM EDTA, 20% glucose), sonicated twice for 15 s, and then assayed for CT-B. TCP expression was determined by transduction with CTXΦ-Cm as described previously (56). The CTXΦ-Cm was constructed by replacing the kanamycin cassette in CTXΦ-Kan (kindly provided by M. Waldor) with the Cmr gene from pACYC184 (44). OM integrity was measured using a periplasmic leakage assay, which was determined by Western blots of supernatants from overnight grown cultures carrying plasmid pBR322 (32) with antiserum to β-lactamase (5Prime-3Prime, Inc.).
Resistance to serum and polymyxin B.
For serum resistance assays, overnight cultures (∼107 bacteria) were added to a final concentration of 20% normal human serum (NHS) or 20% heat-inactivated NHS in phosphate-buffered saline (PBS) with 0.1% peptone and incubated for 1 h at 37°C (36). Serum resistance was determined by quantitating the CFU in both NHS and heat-inactivated NHS. MIC determinations for polymyxin B were carried out with bacteria grown overnight at 37°C in MHB as described elsewhere (36).
In vivo colonization assay.
Mixtures of wild-type strain KKV598 (O395 ΔlacZ) with either KKV780 (ΔompU2), KKV809 (ΔompT), or KKV884 (ΔompU2 ΔompT) were coinoculated into 5-day-old CD-1 suckling mice in a peroral inoculum ratio of approximately 105 mutant to 105 wild-type organisms. After 22 h of colonization, the isolated small intestines were homogenized, and the mutant/wild-type ratio was determined by plating dilutions on LB agar containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). In vitro competition was caried out in 5 ml of LB inoculated with the same mixtures and grown at 37°C overnight.
RESULTS
Construction of viable ΔompU V. cholerae strains.
Our initial strategy to generate mutations in both porin genes ompU and ompT involved generating the deletions in V. cholerae carrying an additional episomal copy of the porin gene because it had been suggested that these genes are essential for the viability of V. cholerae (28, 49). With this strategy, the porin gene carried on the plasmid would complement a lethal deletion introduced into the porin gene in the chromosome, allowing for viability in an otherwise nonviable strain. This strategy was used to construct a V. cholerae strain with a small (252-bp) in-frame internal chromosomal deletion within the ompU gene (ΔompU1). This strain (KKV669) was then cured of the episomal copy of ompU and, to our surprise, was viable, although it formed colonies distinctly smaller than those of the wild type on LB agar (data not shown). These results demonstrate that a deletion of ompU does not convey a lethal phenotype to V. cholerae.
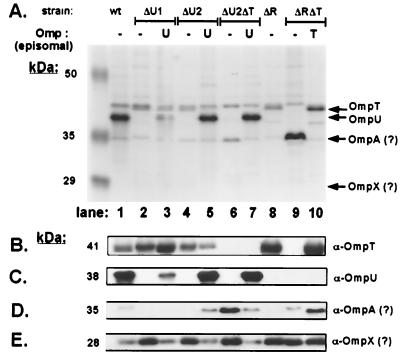
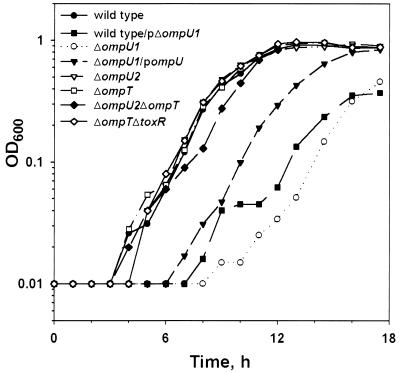
OmpU could not be detected in OM fractions of the ΔompU1 strain, as determined by Western blots probed with OmpU antiserum (Fig. 1A and C, lane 2, compared to wild-type OM, lane 1). The ΔompU1 strain demonstrates a growth defect in minimal M9 medium compared to the isogenic wild-type strain (Fig. 2). Noticeably, complementation of ΔompU1 with the wild-type ompU gene on a plasmid, which restores detectable full-length OmpU to the outer membrane (Fig. 1C, lane 3), did not fully restore the defective growth phenotype of the ΔompU1 strain (Fig. 2), suggesting a dominant-negative phenotype associated with the deleted gene. In fact, ΔompU1 carried on a plasmid (pKEK344) confers a slow-growth phenotype to a wild-type strain (Fig. 2), a result consistent with a negative effect of the internally deleted OmpU polypeptide on cell growth. The predicted ΔompU1 product has a truncated signal peptide of 8 aa fused to the remaining 249 aa of the protein, which is likely not secreted into the periplasm. Although we cannot detect the internally deleted ΔompU1 polypeptide by Western blot even from whole-cell lysates (data not shown), retarded growth kinetics suggest its presence within the cell. The presence of OmpU polypeptide that has not been incorporated into the OM is predicted to be one of the causes of the slow-growth phenotype of strains with mutations in the EPS pathway (47; see also below).
FIG. 1.
Expression of OMPs in ompU and ompT mutant V. cholerae strains. (A) OM fractions were prepared (see Materials and Methods) from O395 (wt, wild type, lane 1), KKV669 (ΔU1, ΔompU1, lanes 2 and 3), KKV780 (ΔU2, ΔompU2, lanes 4 and 5), KKV884 (ΔU2ΔT, ΔompU2 ΔompT, lanes 6 and 7), KKV61 (ΔR, ΔtoxR, lane 8), and KKV804 (ΔRΔT, ΔtoxR ΔompT, lanes 9 and 10). Lanes 3, 5, and 7 contain the OM fractions of KKV669, KKV780, and KKV884, respectively, carrying plasmid pKEK253, which expresses ompU from its natural promoter (episomal U). Lane 10 contains the OM fraction of KKV804 carrying plasmid pKEK255, which expresses ompT from its natural promoter (episomal T). Samples were matched by equivalent optical density at 600 nm (OD600) units, separated by SDS–10% PAGE, and stained with Coomassie blue. The mobilities of molecular mass markers are noted in kilodaltons on the left. Arrows on the right indicate the relative migration rates of OmpU, OmpT, OmpA, and OmpX. (B to E) The OM fractions from the wild type and omp mutants (above) were subjected to Western analysis (see Materials and Methods) utilizing rabbit polyclonal antisera against OmpT (B), OmpU (C), and V. cholerae OMPs that cross-reacted with OM protein bands at 35 kDa (D) and 28 kDa (F) (12). V. cholerae OmpA and OmpX are known to migrate at ∼35 and ∼28 kDa (2, 7), and thus the OM proteins seen here may represent OmpA and OmpX (denoted by a question mark).
FIG. 2.
ΔompU1 strain displays a growth defect. Strains O395 (●), O395 carrying plasmid pKEK433 expressing ΔompU1 (■), KKV669 (○), KKV669 carrying plasmid pKEK253, which expresses ompU from its natural promoter (▾), KKV780 (▿), KKV809 (□), KKV884 (⧫), and KKV804 (◊), were monitored for growth by measuring the OD600. Strains were grown in minimal M9 medium at 37°C with glucose (0.2%) as a carbon source.
Since the remaining ompU coding sequence present in the ΔompU1 strain appears to have a negative effect on growth, we designed a larger in-frame deletion of ompU which removes the entire coding sequence for the amino-terminal 274 aa, leaving only the coding sequence for 67 aa of the C terminus (ΔompU2). Because we knew that a deletion of ompU was not a lethal mutation, the introduction of the ΔompU2 mutation into the V. cholerae chromosome was carried out in strains without an episomal copy of the wild-type ompU gene.
Importantly, the resulting ΔompU2 V. cholerae strain KKV780 formed colonies the same size as those formed by the wild type on LB agar (data not shown). OmpU could not be detected in OM fractions of this strain, as determined by Western blot utilizing OmpU antiserum (Fig. 1A and C, lane 4), and the introduction of wild-type ompU on a plasmid to the ΔompU2 strain restores OmpU expression (Fig. 1A and C, lane 5). The ΔompU2 strain grew identical to the wild type in minimal M9 medium (Fig. 2). Introduction of a plasmid containing ΔompU2 had no deleterious effect on the growth of a wild-type strain (data not shown), indicating that, unlike the ΔompU1 polypeptide, no dominant-negative phenotype is associated with the ΔompU2 polypeptide. Thus, the ΔompU2 strain more accurately represents the true phenotype of a strain lacking OmpU. Our results suggest that the design of the mutation in ompU is critical to obtaining the desired mutant V. cholerae strain, perhaps explaining previous difficulties in obtaining these mutations (28, 49)
Construction of viable ΔompT and ΔompU ΔompT V. cholerae strains.
Under laboratory conditions, toxR wild-type V. cholerae expresses almost exclusively OmpU and very little OmpT, while toxR mutant V. cholerae expresses OmpT exclusively. This is caused by ToxR-dependent activation of the ompU promoter and ToxR repression of the ompT promoter (11, 28). A large in-frame deletion of ompT (ΔompT), which removes the coding sequence for the first 273 aa, leaving only the C-terminal 71 aa, was recombined onto the chromosomes of both wild-type (toxR+) and ΔtoxR V. cholerae strains, resulting in strains KKV809 (ΔompT) and KKV804 (ΔtoxR ΔompT). In contrast to a ΔtoxR strain, the ΔtoxR ΔompT strain had no detectable OmpT in its OM, as determined by Western blot probed with OmpT antiserum (Fig. 1A and B, compare lanes 8 and 9). The introduction of a wild-type copy of ompT onto a plasmid to the ΔtoxR ΔompT strain restores OmpT expression (Fig. 1A and B, lane 10). Both ΔompT and ΔtoxR ΔompT strains have growth rates indistinguishable from that of a wild-type strain in minimal M9 medium (Fig. 2), demonstrating that OmpT is not essential for viability in either toxR+ or toxR mutant V. cholerae.
Finally, the ΔompT mutation was introduced into the chromosome of the ΔompU2 strain KKV780, resulting in strain KKV884 (ΔompU2 ΔompT). There was no detectable OmpU protein in the OM of this strain (Fig. 1A and C, compare lanes 1 and 6). In concentrated OM preparations, OmpT can be detected with specific antiserum in a wild-type (toxR+) strain (Fig. 1B, lane 1), but no OmpT was detected in the OM of the ΔompU2 ΔompT strain (Fig. 1B, lane 6). This double-mutant strain grew almost identically to the wild type in M9 minimal medium (Fig. 2). Evidently, both ToxR-modulated porin genes can be deleted within the same strain without a loss of viability.
Additional OM proteins are present in ΔompU and ΔompT OM.
Porins allow nutrients and other solutes to pass through the OM, and thus the loss of major OM porin(s) may induce compensatory expression of alternate porins to maintain both OM structure and permeability. In OM preparations of several V. cholerae porin deletion strains, expression of alternate OM proteins could be observed (Fig. 1A). A protein band migrating at ∼35 kDa (the known migration rate of OmpA [2]) was noticeably overexpressed in the strains lacking both OmpU and OmpT in their OM, ΔompU2 ΔompT and ΔtoxR ΔompT (lanes 6 and 9). This band was not visible in the same strains complemented with either OmpU- or OmpT-expressing plasmids (lanes 7 and 10). Western blot analysis utilizing antiserum directed against V. cholerae OM proteins (12) (Fig. 1D) revealed that the ∼35-kDa OM protein induced prominently in the ΔtoxR ΔompT strain is probably not the same OM protein induced in the ΔompU2 ΔompT strain (compare Fig. 1A and D, lanes 6 and 9). These results suggest that two separate proteins migrating at ∼35 kDa are overexpressed in the OMs of ΔompU ΔompT and ΔtoxR ΔompT strains.
Another OM protein of ∼28 kDa (the approximate migration rate of OmpX [7]) not visible in the Coomassie blue-stained gel was apparent in Western blots when using the antiserum raised against V. cholerae OM proteins (12) (Fig. 1E). This ∼28-kDa OM protein was more prominently expressed in all strains lacking OmpU in the OM, regardless of the presence or absence of ToxR (lanes 2, 4, 6, 8, 9, and 10). These results suggest that the OM of V. cholerae is a dynamic structure that compensates for the absence of specific porins by increasing the expression of other OM proteins, presumably in an effort to retain structural integrity and/or permeability.
Growth defect of epsE mutant is suppressed by ΔompU2 mutation.
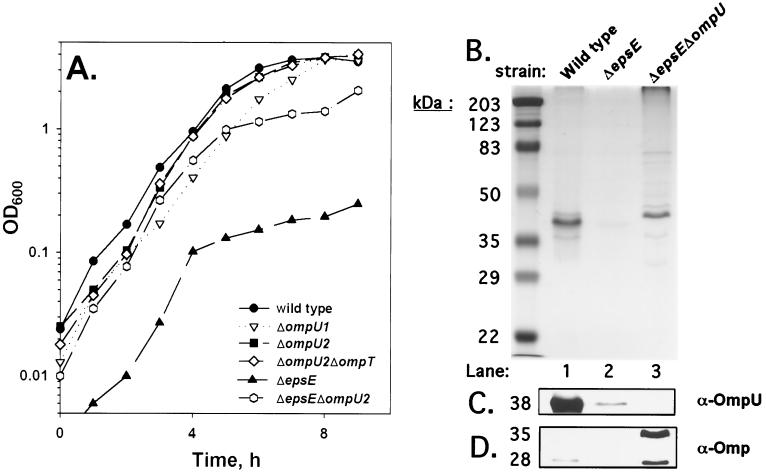
The type II EPS machinery of V. cholerae recognizes proteins in the periplasm targeted for secretion or insertion in the OM (e.g., CT and OmpU or OmpT) and translocates them to their destination (for a review, see reference 45). Recent reports have suggested that a lack of OmpU and OmpT in the OM contribute to the growth defect observed in V. cholerae eps mutant strains (29, 47). Sandkvist and colleagues (47) showed decreased OmpU and OmpT in the OM of eps strains and speculated that a decrease of these two porins in the OM may correlate with the observed growth defect. However, our results demonstrate that a lack of OmpU or OmpT in the OM per se does not cause a growth defect (ΔompU2 and ΔtoxR ΔompT) (Fig. 2), but the growth defect caused by the dominant-negative ΔompU1 allele suggests that OmpU protein that fails to be inserted in the OM may retard growth. To test whether OmpU plays a role in the growth defect reported for an epsE strain, we generated a double-mutant ΔepsE ΔompU2 strain and compared its growth rate in LB to isogenic ΔepsE and ΔompU2 strains (Fig. 3A). As had been noted before (47), an ΔepsE mutant strain displays a severe growth defect, while a ΔompU2 mutant strain has a growth rate similar to that of the wild type and the ΔompU2 ΔompT strains. The ΔompU1 strain displays a slight growth defect in this rich medium, although not as severe as was previously shown in minimal medium (Fig. 2). Interestingly, the introduction of the ΔompU2 mutation into the ΔepsE strain (ΔepsE ΔompU2) greatly ameliorated the growth defect conferred by the ΔepsE mutation. These results are consistent with the hypothesis that the presence of OmpU that is not inserted into the OM contributes (at least in part) to the growth defect of eps strains.
FIG. 3.
Deletion of ompU alters the growth and OM of a ΔepsE strain. (A) Strains O395 (●), KKV669 (▿), KKV780 (■), KKV884 (◊), KKV1234 (▴), and KKV1232 (○) were monitored for growth by measuring the OD600. Strains were grown in LB medium at 37°C. (B) OM fractions were prepared (see Materials and Methods) from O395 (wild type, lane 1), KKV1234 (ΔepsE, lane 2), and KKV1232 (epsE ΔompU, lane 3). Samples were matched by equivalent OD600 units, separated by SDS–10% PAGE, and stained with Coomassie blue. The mobilities of molecular mass markers are noted in kilodaltons on the left. The OM fractions were subjected to Western analysis as described in the text utilizing rabbit polyclonal antisera against OmpU (C) and V. cholerae OM (D) proteins that cross-reacted with OM protein bands at 35 and 28 kDa (12).
The OM protein profiles of wild-type, ΔepsE, and ΔepsE ΔompU2 strains (Fig. 3B and C) revealed that very little OmpU could be detected by Western blot with OmpU antiserum in the OM of the ΔepsE strain (lane 2, compared to the wild type in lane 1), as described previously (47). The OM of the ΔepsE ΔompU2 strain (lane 3) contains no OmpU, as anticipated (Fig. 3C), but showed the presence of OM proteins absent in the ΔepsE strain. Most noticeable is a prominent ∼43-kDa band evident in the OM profile of ΔepsE ΔompU2 that may represent the maltoporin OmpS (27). Also, a Western blot probed with antiserum against V. cholerae OM proteins revealed an ∼28-kDa band in the ΔepsE ΔompU2 strain (Fig. 3D, lane 3) that is absent in the ΔepsE strain (lane 2) but present at lower levels in the wild-type strain (lane 1). Interestingly, the same antiserum revealed the presence of an ∼35-kDa OMP in the ΔepsE ΔompU2 strain that is absent in the OM of both the Δeps and wild-type strains. Thus, the removal of OmpU from the epsE strain allows for the expression and/or insertion of alternate proteins into the OM, which may in turn permit a faster growth rate.
Effect of ompU and ompT deletions on virulence factor expression.
Our previous studies have shown that a ToxR+ strain expressing OmpT in place of OmpU has reduced levels of CT secretion and TCP expression in vitro (42), indicating that the presence of OmpT has a negative effect on the V. cholerae virulence regulatory cascade. To determine if the absence (rather than the presence) of OmpU and/or OmpT affects in vitro virulence factor expression, the various ompU and ompT mutant strains were measured for CT and TCP expression under laboratory inducing conditions. CT secreted into the supernatant was measured by GM1-ELISA assay, while TCP expression was measured both by autoagglutination (a function of TCP expression) and CTXφ transduction frequency (CTXφ utilizes TCP as its receptor [56]). The ΔompU2 and ΔompT strains, as well as the ΔompU2 ΔompT double-mutant strain, secreted CT and expressed TCP similar to a wild-type strain (Table 2), indicating that neither porin is required for CT and TCP expression in vitro. Interestingly, the ΔompU1 strain showed an ∼30-fold decrease in CT secretion compared to wild type, which was accompanied by lack of autoagglutination and a decrease in TCP production as determined by CTXφ-Cm transduction. Because the ΔompU2 mutant had no defect in CT and TCP expression, the defect in virulence factor expression of the ΔompU1 strain must be due to the dominant-negative phenotype associated with the truncated OmpU polypeptide rather than the lack of OmpU. As expected, the ΔtoxR ΔompT strain produced no virulence factors in vitro, due to lack of ToxR, as is seen with a strain containing the ΔtoxR mutation alone (Table 2). None of the porin-deficient strains exhibited virulence factor expression under noninducing, control growth conditions at pH 8.5 and 30°C.
TABLE 2.
Effect of ompU and ompT mutations on virulence factor expression and/or secretion and antimicrobial resistance
| Relevant genotypea | Autoagglutination | CT secretion (ng/ml of supernatant/OD600) | TCP (CTX φ-Cm transductants/ml/OD600) | Polymyxin B MIC (μg/ml) | Serum resistance (no. of survivors relative to wild type/OD600) | CT-B localization (% total)
|
|
|---|---|---|---|---|---|---|---|
| Cells | Medium | ||||||
| Wild type | + | 594 | 3.8 × 105 | 0.30 | 1.0 | 0.3 | 99.7 |
| ΔompU1 | − | 17.8 | 5.9 × 104 | 0.15 | 0.97 | 2.9 | 97.1 |
| ΔompU2 | + | 307 | 1.4 × 105 | 0.30 | 0.84 | 0.3 | 99.7 |
| ΔompU2 ΔompT | + | 104 | 1.1 × 105 | 0.30 | 1.36 | 1.1 | 98.9 |
| ΔompT | + | 211 | 1.6 × 105 | 0.30 | 1.06 | 0.6 | 99.4 |
| ΔtoxR | − | 0 | 0 | 0.60 | 0.54 | 0.1 | 99.9 |
| ΔtoxR ΔompT | − | 0 | 0 | 0.30 | 1.27 | 1.6 | 98.4 |
| ΔepsD | + | 43.8 | 2.7 × 105 | NDb | ND | 70.5 | 29.5 |
| ΔepsE | + | NDb | 1.5 × 105 | ND | ND | 74.9 | 25.1 |
The actual strains used (described in the text) were O395, KKV669, KKV780, KKV884, KKV809, KKV61, KKV804, KKV1216, and KKV1234.
ND, not determined.
Because eps mutants are defective for CT secretion (but not expression) and because ΔompU1 shares a slow-growth phenotype with eps mutants that may depend on the inability to insert OmpU in the OM, we sought to determine if the observed defect in secreted CT by the ΔompU1 strain is related to a defect in CT secretion (vs. CT expression). Strains were transformed with plasmid pJS752-3 (46), which expresses CT-B subunit and then assayed for CT-B present in cell lysates compared to culture supernatants. The ΔompU1 strain retained little intracellular CT-B (2.9%), compared to either epsE or epsD strains, which retained much higher levels of intracellular CT-B (74.9 and 70.5%, respectively). These results suggest that the lower levels of secreted CT by the ΔompU1 strain are not due to a gross defect in protein secretion. As expected, none of the other V. cholerae strains with deletions in ompU and ompT demonstrated defects in CT-B secretion.
Effect of ompU and ompT deletions on resistance to antimicrobial substances.
Next, we tested whether removal of major OM porins can affect the integrity of the OM in V. cholerae, leading to altered permeability or sensitivity to antimicrobial agents acting on the cell surface. To identify any OM defects associated with the lack of OmpU and/or OmpT, we assayed the ΔompU and ΔompT strains for sensitivity to polymyxin B, a polycationic agent that disrupts the OM, and to NHS. The MICs for polymyxin B were similar for all strains (Table 2). Likewise, serum resistance was largely unaffected by the lack of OmpU or OmpT in the OM. These results suggest that the absence of OmpU and OmpT does not substantially affect V. cholerae sensitivity to these antimicrobial agents.
The structural integrity of the OM can also be ascertained by measuring leakage of β-lactamase, which is normally localized in the periplasm (32). We transformed the porin-deficient strains with pBR322 (52) and examined cell-free supernatants by Western blot with antiserum to β-lactamase (36). No significant differences were found in the amount of β-lactamase from the supernatants of the porin deletion strains (data not shown). Additionally, visualization of the lipopolysaccharide (LPS) of the various strains in silver-stained gels did not reveal any obvious structural defects (Jutta Nesper, personal communication). These results show that the absence of the V. cholerae ToxR-modulated porins does not adversely affect OM integrity or LPS structure.
Roles for OmpU and OmpT in resistance to DOC.
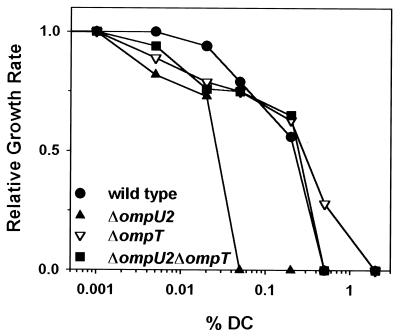
We previously demonstrated that a ToxR+ strain that expresses OmpT in place of OmpU has decreased levels of resistance to DOC, suggesting that OmpU-containing cells are more resistant and that OmpT-containing cells are more sensitive to anionic detergents (42). In order to determine if strains lacking OmpU and/or OmpT have altered levels of resistance to DOC, we tested the ΔompU2, ΔompT, and ΔompU2 ΔompT strains for relative growth rates in DOC. The ΔompU2 strain displays retarded growth kinetics over a wide DOC concentration range in comparison to wild-type, ΔompT, and ΔompU2 ΔompT strains (Fig. 4), corroborating a protective role for OmpU in V. cholerae bile resistance. Interestingly, the introduction of the ΔompT mutation into the ΔompU2 strain (ΔompU2 ΔompT double mutant) increased the level of relative DOC resistance. Also, the single-mutant ΔompT strain had growth kinetics comparable to those of the wild type at lower DOC concentrations, but it consistently grew even better than the wild type at higher concentrations (0.5% DOC). These results are consistent with OmpT playing a negative role in bile resistance.
FIG. 4.
The ΔompU2 mutant strain displays slower growth rates in DOC. V. cholerae strains O395 (●), KKV780 (▴), KKV809 (▾), and KKV884 (■) were grown in LB at 37°C containing the concentrations of DOC indicated (note the logarithmic scale). Growth rates shown are relative to the growth rate in LB alone, as described previously (43). DC, deoxycholate.
Colonization of the infant mouse small intestine.
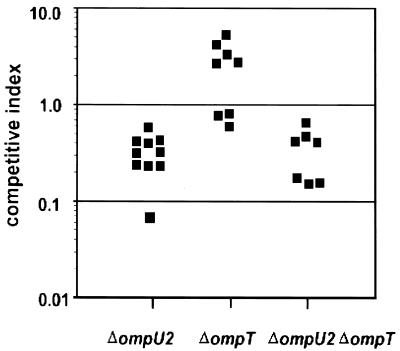
A ToxR+ strain that expresses OmpT in place of OmpU is defective for colonization of the infant mouse small intestine, demonstrating that the presence of the ToxR-repressed porin can inhibit V. cholerae virulence (42). To determine if the absence, rather than the presence, of OmpU and/or OmpT affects colonization of the suckling mouse small intestine, in vivo competition experiments were performed with the ΔompU2, ΔompT, and ΔompU2 ΔompT strains (Fig. 5). The competitive indices for ΔompU2 and ΔompU2 ΔompT strains were similar, if slightly less than those for the wild type (0.32 ± 0.16 and 0.40 ± 0.23, respectively), while that of the ΔompT strain was slightly better than that of the wild type (2.57 ± 1.86). The same trend was seen in an in vitro competition with these strains (ΔompU2, 0.56; ΔompT, 1.20; ΔompU2 ΔompT, 0.36), suggesting that the mild defects or advantages of these strains for colonization in vivo are due to competitive growth differences. Thus, the ToxR-regulated porins OmpU and OmpT are not essential for colonization of the infant mouse small intestine, indicating that OmpU most probably plays no role in adherence in vivo.
FIG. 5.
OmpU and/or OmpT are not required for V. cholerae intestinal colonization. The assay was performed as described in the text. V. cholerae strains KKV780 (ΔompU2), KKV809 (ΔompT), or KKV884 (ΔompU2 ΔompT) were coinoculated with isogenic strain KKV598 (O395 ΔlacZ). The competitive index is the ratio of output mutant/wild type (recovered from the small intestine) divided by the ratio of input mutant/wild type (inoculated into the mouse). Datum points represent individual mice. The values for the ΔompT strain are significantly different (P < 0.01) than those of the ΔompU2 and ΔompU2 ΔompT strains, as determined by using a Student's two-tailed t test.
DISCUSSION
The results of our study underscore the importance of the design of the ompU mutation in obtaining cells that reflect the true phenotype of a strain lacking OmpU without additional adverse phenotypes. We constructed two different in-frame deletions in the ompU gene, and strains containing these mutations had very different phenotypes. The ΔompU1 V. cholerae strain has a small in-frame deletion (84 aa) and displayed a considerable growth defect. This phenotype is dominant negative, because ΔompU1 can confer a slow-growth phenotype on a wild-type (ompU+) strain in trans. In contrast, a strain containing a large in-frame deletion (274 aa), ΔompU2, which removes the majority of the coding sequence at the amino terminus, grows like a wild-type strain in both minimal and rich media.
We suspect that the slow-growth phenotype associated with the ΔompU1 mutation is caused by the internally deleted OmpU polypeptide, although truncated peptide could not be detected by OmpU antisera. Because the ΔompU1 mutation removes 13 aa of the 21-aa leader peptide, the resultant OmpU polypeptide is likely not secreted into the periplasm, thus suggesting that it exerts its effect from within the cytoplasm. The ΔompU2 mutation was designed to remove the entire leader peptide, as well as the majority of the coding sequence for the mature protein, and this deletion eliminated the negative effects on growth seen with ΔompU1. Therefore, it is reasonable to conclude that the ΔompU2 strain represents the true phenotype of a V. cholerae strain lacking OmpU. Likewise, the ΔompT mutation, which removed the majority of the amino terminus of OmpT, does not cause any growth defect in toxR+ or toxR mutant strains in either minimal or rich medium, and therefore this mutation truly represents an ompT null mutation. Other researchers have reported difficulty in the disruption of ompU or ompT (28, 49), which had led to the hypothesis that these genes are essential for V. cholerae viability. We suggest that the design of the ompU or ompT mutations attempted could have led to a slow-growth phenotype which made it difficult to isolate these mutant strains.
Porins constitute a major portion of the OM proteins, and it has been estimated that OmpU represents ca. 30 to 60% of the total OMPs of V. cholerae (7). Given the functional as well as the likely structural roles of OmpU, it is perhaps not surprising to find at least one alternate OM protein expressed in its absence. An OM protein migrating at 28 kDa was consistently overexpressed in strains without OmpU, regardless of the presence or absence of ToxR or OmpT. The relative migration rate of this protein approximates the reported size for OmpX, a 29-kDa OMP that was reported not to have porin activity (7). Likewise, OM proteins migrating at 35 kDa were overexpressed in the OM of two double-mutant strains, one lacking both OmpU and OmpT (ΔompU2 ΔompT) and the other lacking OmpT in a ΔtoxR background (ΔompT ΔtoxR). OmpT is the major OM protein expressed in a ΔtoxR strain, so again it is not surprising that its absence leads to expression of alternate OMPs to maintain structural integrity and/or permeability. Interestingly, the two ∼35-kDa OMPs overexpressed in the double-mutant strains appear to be two different OM proteins. An ∼35-kDa OMP has been identified in V. cholerae that shares antigenic epitopes with OmpA of E. coli (2), but it remains to be determined whether one of the ∼35-kDa OMPs represents OmpA or some other porin or protein.
The type II EPS pathway of V. cholerae is required for secretion of CT and other factors, as well as insertion of OmpU and OmpT into the OM (47). Sandkvist et al. suggested that the growth defect of an eps strain might be related to a lack of OmpU and OmpT in the OM, causing a destabilizing effect on OM integrity. However, our results have demonstrated that strains lacking OmpU in the OM grow similar to the wild type. Notably, deletion of ompU (ΔompU2) in an epsE strain significantly increases the growth rate of this strain, suggesting that the presence of non-OM localized OmpU in a strain lacking a functional EPS leads to the reported growth defect. In other words, it is apparently not the absence of OmpU in the OM but rather the presence of OmpU within the cell that contributes to the growth defect of an eps strain. It is therefore tempting to speculate that the growth defect associated with the ΔompU1 deletion is likewise related to the presence of OmpU polypeptide not localized to the OM. Still, the ΔompU2 epsE strain does not demonstrate a wild-type growth rate and maintains the filamentous cellular morphology previously noted for the epsE strain (47). Clearly, the presence of non-OM localized OmpU is only partially responsible for the multiple phenotypes associated with eps mutations. Notably, both the ΔompU2 epsE and the epsE strains synthesized flagella and were motile, in contrast to what was recently reported (1).
Our studies have previously demonstrated that V. cholerae strains expressing OmpU are more resistant to bile salts and other anionic detergents, and strains expressing OmpT are less resistant (42, 43). Consistent with our hypothesis that OmpU plays a protective role in enhanced bile resistance, the strain lacking OmpU (ΔompU2) was markedly more sensitive to the bile salt DOC than the wild-type strain. Our present data also support a negative role for OmpT in bile resistance because the ΔompT strain was more resistant to high concentrations of DOC than the wild-type strain. Interestingly, a strain lacking both OmpU and OmpT (ΔompU2 ΔompT) displayed levels of DOC resistance similar to that of the wild type. toxR+ strains express little OmpT under laboratory conditions, but apparently this low level of expression is sufficient to render the ΔompU2 strain more sensitive to DOC than a ΔompU2 ΔompT strain and to render a wild-type strain more sensitive than a ΔompT strain.
Our findings corroborate the overall hypothesis that the relationship between OmpU and OmpT of V. cholerae is similar to that postulated to exist between OmpC and OmpF in serovar Typhimurium. Like other intestinal bacteria (37), V. cholerae must persist in an environment that is full of bile salts, where porin properties are ideal for excluding these large, negatively charged hydrophobic compounds. In serovar Typhimurium, OmpC, which has a small pore size and is more cationic selective than OmpF, is less permeable to bile salts and is predicted to be preferentially expressed within the intestinal environment. On the other hand, in nutrient-poor, low-osmolarity environments, such as in ponds and streams, OmpF, which has a larger pore size and is less cationic selective (and more permeable to bile) than OmpC, is presumed to be preferentially expressed (37). We hypothesize an analogous scenario for V. cholerae, with the expression of OmpU being preferred within the intestine and OmpT being preferentially expressed within the aquatic environment. Although OmpU has been reported previously to have a larger pore size than OmpT utilizing carbohydrate solutes (7), this does not necessarily reflect the relative permeabilities of these porins toward anionic detergents. Studies aimed at determining the relative ionic specificities for OmpU and OmpT are in progress.
Strains lacking either OmpU or OmpT or both expressed and secreted wild-type levels of CT and produced wild-type levels of TCP under laboratory inducing conditions, indicating that the lack of either porin does not adversely affect the ToxR/TcpP/ToxT virulence signaling cascade. However, we have recently shown that expression of OmpT in place of OmpU negatively affects the virulence cascade at the level of toxT transcription (42). These results are consistent with a negative role for OmpT, but the lack of a positive role for either OmpU or OmpT, in virulence factor expression. However, in contrast to the strain truly lacking OmpU (ΔompU2), a strain with the ΔompU1 mutation had a defect in CT and TCP production under laboratory inducing conditions. This mutation confers a dominant-negative effect similar to those conferred by mutations in the EPS apparatus (see above), but its defect in expression of extracellular CT could not be attributed to lack of secretion. It is therefore conceivable that the ΔompU1 mutation affects the ToxR/TcpP/ToxT signaling cascade, either indirectly by altering growth or directly by interfering with inducing signals.
Deep-rough serovar Typhimurium mutants have been described as having drastically less porins in the OM compared to the wild type (3), which leads to increased phospholipid in the OM and rapid transmembrane diffusion of lipophilic solutes like bile and contributing to instability of the OM (38). We were unable to identify any similar perturbations to the integrity of the OM of V. cholerae omp mutants by several criteria. None of the ompU and/or ompT deletion strains displayed any increase in serum or polymyxin B sensitivity, leakage of β-lactamase from the periplasm, or structural abnormality in the LPS, indicating that the lack of OmpU and/or OmpT in the OM does not result in a defective OM or abnormal LPS.
OmpU has been hypothesized to serve as an adhesin during V. cholerae intestinal colonization (50). Due to difficulties in obtaining ompU mutations, however, the previous studies were not performed in strains lacking OmpU. Our experiments with porin-deficient strains demonstrate that neither OmpU nor OmpT are essential for colonization of the mouse small intestine. The mild colonization defect associated with the ΔompU2 strain (<10-fold) may be attributed to an inability of this strain to compete with the wild type in regard to ultimate growth yield, because a similar competitive defect was seen during growth in vitro. The ΔompU2 strain was noticeably more sensitive to DOC than was the wild type, and therefore we expected that it would be defective for colonization in the presence of bile salts within the intestine. However, it colonized similarly to the double-mutant ΔompU2 ΔompT strain, which has levels of bile resistance similar to those of the wild type. It appears that OmpU-dependent bile resistance is not a critical factor in colonization of the infant mouse intestine. This may not be an ideal model to study the interaction between the pathogens and the anionic detergents in vivo.
Our previous study demonstrated that a strain expressing OmpT in place of OmpU is significantly reduced for intestinal colonization (>100-fold); in combination with the experiments presented here, it is clear that this colonization defect is due to the presence of OmpT rather than the absence of OmpU. One hypothesis that accounts for this observation is that the presence of OmpT in the OM alters the flux of signaling molecules into the periplasm, thus reducing the expression of TCP and other colonization factors via the ToxR/TcpP/ToxT virulence cascade. This could be corroborated in vitro, because a strain expressing OmpT in place of OmpU expresses considerably less CT and TCP under laboratory inducing conditions. As shown here, strains lacking either OmpU and/or OmpT express CT and TCP in a way similar to that of wild type in vitro and colonize similar to the wild type in vivo. Taken together, this suggests that the most important role for ToxR in porin modulation during colonization is the repression of ompT rather than the activation of ompU. However, it should be noted that the current studies were performed in V. cholerae strains of the classical biotype. Because the classical and El Tor biotypes differ significantly in the laboratory conditions that induce CT and TCP expression, ompU and ompT mutations may have different effects on the virulence cascade in the two biotypes.
ACKNOWLEDGMENTS
We thank J. Peterson for his generous gift of antisera to V. cholerae OM proteins, S. Sozhamannan for providing plasmids pAA35 and pAA48, C. Lopez-Macias for plasmid pJS752-3, M. Waldor for CTXΦ-Kan, and J. Nesper for assistance with the LPS analysis.
This study was supported by an institutional new faculty award of the Howard Hughes Medical Institute to K.E.K. and National Institutes of Health Microbial Pathogenesis training grant AI07271-15 to D.P.
REFERENCES
- 1.Ali A, Johnson J A, Franco A A, Metzger D J, Connell T D, Morris J G, Jr, Sozhamannan S. Mutations in the extracellular protein secretion pathway genes (EPS) interfere with rugose polysaccharide production in and motility of Vibrio cholerae. Infect Immun. 2000;68:1967–1974. doi: 10.1128/iai.68.4.1967-1974.2000. . (Erratum, 68:3792.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alm R, Braun G, Morona R, Manning P. Detection of an OmpA-like protein in Vibrio cholerae. FEMS Microbiol Lett. 1986;37:99–104. [Google Scholar]
- 3.Ames G F, Spudich E N, Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974;117:406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benz R, Maier E, Chakraborty T. Purification of OmpU from Vibrio cholerae classical strain 569B: evidence for the formation of large cation-selective ion-permeable channels by OmpU. Microbiologia. 1997;13:321–330. [PubMed] [Google Scholar]
- 5.Benz R, Schmid A, Van der Ley P, Tommassen J. Molecular basis of porin selectivity: membrane experiments with OmpC-PhoE and OmpF-PhoE hybrid proteins of Escherichia coli K-12. Biochim Biophys Acta. 1989;981:8–14. doi: 10.1016/0005-2736(89)90075-8. [DOI] [PubMed] [Google Scholar]
- 6.Bernardini M L, Sanna M G, Fontaine A, Sansonetti P J. OmpC is involved in invasion of epithelial cells by Shigella flexneri. Infect Immun. 1993;61:3625–3635. doi: 10.1128/iai.61.9.3625-3635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakrabarti S R, Chaudhuri K, Sen K, Das J. Porins of Vibrio cholerae: purification and characterization of OmpU. J Bacteriol. 1996;178:524–530. doi: 10.1128/jb.178.2.524-530.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champion G A, Neely M N, Brennan M A, DiRita V J. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol Microbiol. 1997;23:323–331. doi: 10.1046/j.1365-2958.1997.2191585.x. [DOI] [PubMed] [Google Scholar]
- 9.Correa N E, Lauriano C M, McGee R, Klose K E. Phosphorylation of the flagellar regulatory protein FlrC is necessary for Vibrio cholerae motility and enhanced colonization. Mol Microbiol. 2000;35:743–755. doi: 10.1046/j.1365-2958.2000.01745.x. [DOI] [PubMed] [Google Scholar]
- 10.Cowan S W, Schirmer T, Rummel G, Steiert M, Ghosh R, Pauptit R A, Jansonius J N, Rosenbusch J P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992;358:727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- 11.Crawford J A, Kaper J B, DiRita V J. Analysis of ToxR-dependent transcription activation of ompU, the gene encoding a major envelope protein in Vibrio cholerae. Mol Microbiol. 1998;29:235–246. doi: 10.1046/j.1365-2958.1998.00925.x. [DOI] [PubMed] [Google Scholar]
- 12.Das M, Chopra A K, Cantu J M, Peterson J W. Antisera to selected outer membrane proteins of Vibrio cholerae protect against challenge with homologous and heterologous strains of V. cholerae. FEMS Immunol Med Microbiol. 1998;22:303–308. doi: 10.1111/j.1574-695X.1998.tb01219.x. [DOI] [PubMed] [Google Scholar]
- 13.DiRita V J, Neely M, Taylor R K, Bruss P M. Differential expression of the ToxR regulon in classical and El Tor biotypes of Vibrio cholerae is due to biotype-specific control over toxT expression. Proc Natl Acad Sci USA. 1996;93:7991–7995. doi: 10.1073/pnas.93.15.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiRita V J, Parsot C, Jander G, Mekalanos J J. Regulatory cascade controls virulence in Vibrio cholerae. Proc Natl Acad Sci USA. 1991;88:5403–5407. doi: 10.1073/pnas.88.12.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardel C L, Mekalanos J J. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect Immun. 1996;64:2246–2255. doi: 10.1128/iai.64.6.2246-2255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:577–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 18.Hase C C, Mekalanos J J. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci USA. 1998;95:730–734. doi: 10.1073/pnas.95.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidelberg J F, Eisen J A, Nelson W C, Clayton R A, Gwinn M L, Dodson R J, Haft D H, Hickey E K, Peterson J D, Umayam L, Gill S R, Nelson K E, Read T D, Tettelin H, Richardson D, Ermolaeva M D, Vamathevan J, Bass S, Qin H, Dragoi I, Sellers P, McDonald L, Utterback T, Fleishmann R D, Nierman W C, White O. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature. 2000;406:477–483. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins D E, DiRita V J. Transcriptional control of toxT, a regulatory gene in the ToxR regulon of Vibrio cholerae. Mol Microbiol. 1994;14:17–29. doi: 10.1111/j.1365-2958.1994.tb01263.x. [DOI] [PubMed] [Google Scholar]
- 21.Karaolis D K, Johnson J A, Bailey C C, Boedeker E C, Kaper J B, Reeves P R. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc Natl Acad Sci USA. 1998;95:3134–3139. doi: 10.1073/pnas.95.6.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley J T, Parker C D. Identification and preliminary characterization of Vibrio cholerae outer membrane proteins. J Bacteriol. 1981;145:1018–1024. doi: 10.1128/jb.145.2.1018-1024.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y B, Okuda J, Matsumoto C, Takahashi N, Hashimoto S, Nishibuchi M. Identification of Vibrio parahaemolyticus strains at the species level by PCR targeted to the toxR gene. J Clin Microbiol. 1999;37:1173–1177. doi: 10.1128/jcm.37.4.1173-1177.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klose K E, Mekalanos J J. Differential regulation of multiple flagellins in Vibrio cholerae. J Bacteriol. 1998;180:303–316. doi: 10.1128/jb.180.2.303-316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovach M E, Shaffer M D, Peterson K M. A putative integrase gene defines the distal end of a large cluster of ToxR-regulated colonization genes in Vibrio cholerae. Microbiology. 1996;142(Pt. 8):2165–2174. doi: 10.1099/13500872-142-8-2165. [DOI] [PubMed] [Google Scholar]
- 26.Krukonis E S, Yu R R, Dirita V J. The Vibrio cholerae ToxR/TcpP/ToxT virulence cascade: distinct roles for two membrane-localized transcriptional activators on a single promoter. Mol Microbiol. 2000;38:67–84. doi: 10.1046/j.1365-2958.2000.02111.x. [DOI] [PubMed] [Google Scholar]
- 27.Lang H, Palva E T. The ompS gene of Vibrio cholerae encodes a growth-phase-dependent maltoporin. Mol Microbiol. 1993;10:891–901. doi: 10.1111/j.1365-2958.1993.tb00960.x. [DOI] [PubMed] [Google Scholar]
- 28.Li C C, Crawford J A, DiRita V J, Kaper J B. Molecular cloning and transcriptional regulation of ompT, a ToxR-repressed gene in Vibrio cholerae. Mol Microbiol. 2000;35:189–203. doi: 10.1046/j.1365-2958.2000.01699.x. [DOI] [PubMed] [Google Scholar]
- 29.Marsh J W, Taylor R K. Identification of the Vibrio cholerae type 4 prepilin peptidase required for cholera toxin secretion and pilus formation. Mol Microbiol. 1998;29:1481–1492. doi: 10.1046/j.1365-2958.1998.01031.x. [DOI] [PubMed] [Google Scholar]
- 30.Mekalanos J J, Swartz D J, Pearson G D, Harford N, Groyne F, de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983;306:551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- 31.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minsky A, Summers R G, Knowles J R. Secretion of beta-lactamase into the periplasm of Escherichia coli: evidence for a distinct release step associated with a conformational change. Proc Natl Acad Sci USA. 1986;83:4180–4184. doi: 10.1073/pnas.83.12.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller A, Gunther D, Brinkmann V, Hurwitz R, Meyer T F, Rudel T. Targeting of the pro-apoptotic VDAC-like porin (PorB) of Neisseria gonorrhoeae to mitochondria of infected cells. EMBO J. 2000;19:5332–5343. doi: 10.1093/emboj/19.20.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakasone N, Iwanaga M. Characterization of outer membrane protein OmpU of Vibrio cholerae O1. Infect Immun. 1998;66:4726–4728. doi: 10.1128/iai.66.10.4726-4728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Negm R S, Pistole T G. The porin OmpC of Salmonella typhimurium mediates adherence to macrophages. Can J Microbiol. 1999;45:658–669. [PubMed] [Google Scholar]
- 36.Nesper J, Kapfhammer D, Klose K E, Merkert H, Reidl J. Characterization of Vibrio cholerae O1 antigen as the bacteriophage K139 receptor and identification of IS1004 insertions aborting O1 antigen biosynthesis. J Bacteriol. 2000;182:5097–5104. doi: 10.1128/jb.182.18.5097-5104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikaido H. Outer membrane. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 29–47. [Google Scholar]
- 38.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Hare M D, Felmingham D, Ridgway G L, Gruneberg R N. The comparative in vitro activity of twelve 4-quinolone antimicrobials against enteric pathogens. Drugs Exp Clin Res. 1985;11:253–257. [PubMed] [Google Scholar]
- 40.Osorio C R, Klose K E. A region of the transmembrane regulatory protein ToxR that tethers the transcriptional activation domain to the cytoplasmic membrane displays wide divergence among Vibrio species. J Bacteriol. 2000;182:526–528. doi: 10.1128/jb.182.2.526-528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson K M, Mekalanos J J. Characterization of the Vibrio cholerae ToxR regulon: identification of novel genes involved in intestinal colonization. Infect Immun. 1988;56:2822–2829. doi: 10.1128/iai.56.11.2822-2829.1988. . (Erratum, 57:660, 1989.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Provenzano D, Klose K E. Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance, virulence factor expression, and intestinal colonization. Proc Natl Acad Sci USA. 2000;97:10220–10224. doi: 10.1073/pnas.170219997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Provenzano D, Schuhmacher D A, Barker J L, Klose K E. The virulence regulatory protein ToxR mediates enhanced bile resistance in Vibrio cholerae and other pathogenic Vibrio species. Infect Immun. 2000;68:1491–1497. doi: 10.1128/iai.68.3.1491-1497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rose R E. The nucleotide sequence of pACYC184. Nucleic Acids Res. 1988;16:355. doi: 10.1093/nar/16.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russel M. Macromolecular assembly and secretion across the bacterial cell envelope: type II protein secretion systems. J Mol Biol. 1998;279:485–499. doi: 10.1006/jmbi.1998.1791. [DOI] [PubMed] [Google Scholar]
- 46.Sanchez J, Holmgren J. Recombinant system for overexpression of cholera toxin B subunit in Vibrio cholerae as a basis for vaccine development. Proc Natl Acad Sci USA. 1989;86:481–485. doi: 10.1073/pnas.86.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandkvist M, Michel L O, Hough L P, Morales V M, Bagdasarian M, Koomey M, DiRita V J. General secretion pathway (eps) genes required for toxin secretion and outer membrane biogenesis in Vibrio cholerae. J Bacteriol. 1997;179:6994–7003. doi: 10.1128/jb.179.22.6994-7003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skorupski K, Taylor R K. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol Microbiol. 1997;25:1003–1009. doi: 10.1046/j.1365-2958.1997.5481909.x. [DOI] [PubMed] [Google Scholar]
- 49.Sperandio V, Bailey C, Giron J A, DiRita V J, Silveira W D, Vettore A L, Kaper J B. Cloning and characterization of the gene encoding the OmpU outer membrane protein of Vibrio cholerae. Infect Immun. 1996;64:5406–5409. doi: 10.1128/iai.64.12.5406-5409.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sperandio V, Giron J A, Silveira W D, Kaper J B. The OmpU outer membrane protein, a potential adherence factor of Vibrio cholerae. Infect Immun. 1995;63:4433–4438. doi: 10.1128/iai.63.11.4433-4438.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stevenson G, Leavesley D I, Lagnado C A, Heuzenroeder M W, Manning P A. Purification of the 25-kDa Vibrio cholerae major outer-membrane protein and the molecular cloning of its gene: ompV. Eur J Biochem. 1985;148:385–390. doi: 10.1111/j.1432-1033.1985.tb08850.x. [DOI] [PubMed] [Google Scholar]
- 52.Sutcliffe J G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci USA. 1978;75:3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Svennerholm A, Lange S, Holmgren J. Correlation between intestinal synthesis of specific immunoglobulin A and protection against experimental cholera in mice. Infect Immun. 1978;21:1–6. doi: 10.1128/iai.21.1.1-6.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thanassi D G, Cheng L W, Nikaido H. Active efflux of bile salts by Escherichia coli. J Bacteriol. 1997;179:2512–2518. doi: 10.1128/jb.179.8.2512-2518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wai S N, Moriya T, Kondo K, Misumi H, Amako K. Resuscitation of Vibrio cholerae O1 strain TSI-4 from a viable but nonculturable state by heat shock. FEMS Microbiol Lett. 1996;136:187–191. doi: 10.1111/j.1574-6968.1996.tb08047.x. [DOI] [PubMed] [Google Scholar]
- 56.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 57.Wang R F, Kushner S R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- 58.Welch T J, Bartlett D H. Identification of a regulatory protein required for pressure-responsive gene expression in the deep-sea bacterium Photobacterium species strain SS9. Mol Microbiol. 1998;27:977–985. doi: 10.1046/j.1365-2958.1998.00742.x. [DOI] [PubMed] [Google Scholar]