Abstract
Potatoes (Solanum tuberosum) are one of the most important crops worldwide. However, its production and nutrient content are endangered by both biotic and abiotic stresses. The main yield losses are caused by pest damage (e.g., Colorado potato beetle and aphids), virus disease (e.g., Potato leafroll virus and Potato viruses Y and X), or oomycete pathogens (like Phytophthora infestans), which also significantly affect the production of antinutrients and toxic metabolites of plants. Therefore, the use of genetic engineering could be an efficient tool, not harmful to the environment, and beneficial to the consumer. In this review, we focus on the main sources of problems in the field of potato production according to approved genetic modifications, their traditional solution and positive impact of gene transfection reducing economic losses, use of insecticides, and improving the nutritional properties of potatoes. We summarize all transgenic events that have been performed on potatoes and have been approved for cultivation and/or direct use or processing as feed or food.
Keywords: potatoes, GMO, Colorado potato beetle, Potato virus Y, Phytophthora infestans, acrylamide
1. Introduction
A perennial plant Solanum tuberosum from the family Solanaceae produces starchy tubers called potatoes. With more than 5000 varieties, it is the fourth most productive crop after rice, maize, and wheat.11 The main producers are China, Russia, and India. In 2018, potato production reached 4% of the total world food production.2 In comparison to other crops, potatoes are less demanding to grow and can also be grown at higher altitudes.3 Moreover, they need less input than other vegetables. In 2019, more than 17 million hectares of potatoes were harvested worldwide.4
The dominant nutrients in potatoes are carbohydrates, mainly starch.5 However, potatoes are also an important source of vitamins B6, B3, B9, and C, fiber, magnesium, potassium, phosphorus, and iron.6 As a result of their composition, they are important in preventing micronutrient deficiencies, especially in poorer regions of the world, or preventing problems with high blood pressure.7 However, the composition of nutrients and antinutrients is directly proportional to the potato variety, growing conditions, method of storage, and last but not least, processing of tubers for food or feed.
To make starch and some other nutrients more bioavailable, potatoes must be processed.1 However, boiling or other treatment at high temperatures for a long period causes losses of some of the nutrients. Potatoes are one of the important sources of vitamin C in the human diet, but cooking without their skin causes a loss of vitamin C by up to 45%.8 The most favorite method of potato preparation is frying. This requires higher temperatures (more than 170 °C) that could cause the formation of acrylamide.9,10. In addition to acrylamide, other antinutrients, such as glycoalkaloids or malondialdehyde, may be present in potatoes as the secondary metabolites of the plant immune system synthesized as a defense against different pests or diseases.
Climate change could have a significant impact on world potato production as a result of the higher probability of disease occurrence.11 Higher temperatures can help spread insect pests, such as the Colorado potato beetle, into areas that are too cold for them today; similarly, some diseases (such as potato late blight) could benefit from these conditions and, therefore, may become a greater threat.12,13 Another factor that directly affects potato production is poorer water availability. The solution of these problems could be in shifting of growing areas, improving water management, or creating new potato cultivars. The creation of new cultivars can be a long-term solution. Traditional plant breeding techniques are very time-consuming and often unsuccessful. In contrast, genetic modification (GM) is targeted more precisely; thus, genetically modified organisms (GMOs) have a given property, and currently, the adverse effects of natural long-term breeding are eliminated. In recent years, the view of GMOs has changed because these crops help address some of the nutritional deficiencies of consumers.
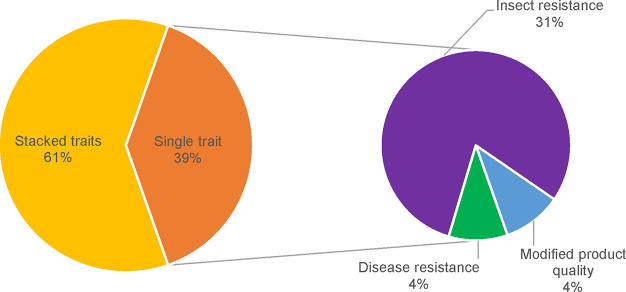
In 1996, Argentina, Australia, Canada, China, Mexico, and the United States have begun planting GM crops. Since then, GM crop cultivation has spread to 26 countries.14 GM potatoes occupied 2265 ha in United States and Canada during 2019.15 Up to date, 51 GM events in potatoes have been approved.16 All of these GM potatoes are prepared by Agrobacterium tumefaciens-mediated plant transformation. Most GMOs are connected with insect resistance (60%), disease resistance (38%) and modification of potato quality (34%). These properties are usually stacked in one potato (Figure 1), which is especially beneficial for farmers, who can then increase their productivity even in difficult agricultural conditions.17 Of the 51 approved GM potatoes, 37 are approved for cultivation, 43 are approved for feed and 47 are approved for food.16 Approved transgenic potatoes usually contain antibiotic resistance genes, which allowed transformed plants to metabolize/modify antibiotics during selection. Today’s research is trying to avoid the usage of selectable markers18 as a result of the antibiotic resistance spread. Current research is also focused on usage of new breeding techniques.19
Figure 1.
Traits affected by genetic modifications in approved potato cultivars.
In this review, we focus on possible consumer health risks connected with the main adverse condition cultivation of potato that cause economic loss. We present individual issues with their scope and traditional solution, together with a simple solution offered by GM. In conclusion, we want to clarify the approved genetic modifications in potatoes and show their benefits for not only the farmers but also the consumers.
2. Potato Insect Pests
Potatoes are endangered by various insect pests, which could destroy potato production directly (defoliation and tuber damage) or indirectly (expansion of viruses). Extensive damage depends upon insect, season, and vegetative conditions. Some insect pests are just minor (aphids), and some of them are very serious and worldwide.
Colorado potato beetle (Leptinotarsa decemlineata, CPB) is a well-known pest, which belongs to coleopteran insects. This pest causes extensive economical losses mainly on potatoes.20 The CPB causes the loss of around a third of the global potato crop each year.21 Adult CPB can overwinter in the soil for a period of at least 30 days. After this period (in spring), they are ready to disperse, preferring to walk over flight to the host.22 Females can lay up to 800 eggs during their lifetime. The eggs deposit in masses of 20–60 eggs on the lower surface of the foliage of the host plant.23 CPB larvae and adults feed on the leaves of the host plant, resulting in its defoliation and, unless controlled, eventual loss. As a defense, the infested potato plant produces glycoalkaloids (GAs).24
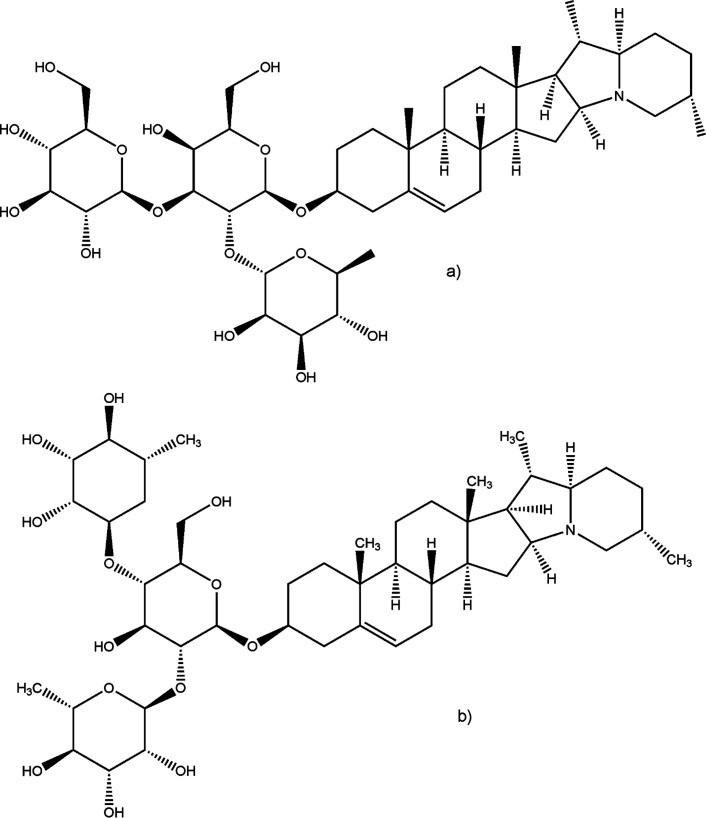
GAs, nitrogen-containing steroidal glycosides, are typical alkaloids found in the Solaneceae family. More than 80 different GAs have been identified, and the most common GAs found in potatoes are α-chaconine and α-solanine (Figure 2).25 These metabolites are highly stable and are found throughout the plant. A higher level of these metabolites is found in actively growing tissues (flowers, berries, etc.), whereas in tubers, they are lower and predominantly under their skin.26 A higher concentration of these substances could be found in parts turned green and also in sprouting or rotting parts of the tubers.27 According to the Implementing Regulation of the European Commission (2017/2470), the accepted safety limit is less than 150 mg of total GAs/kg of fresh weight of unpeeled raw potato tuber as a result of the potential toxicity of these metabolites.28 The lowest adverse effect observed was at a dose of 1 mg of GAs/kg of body weight per day.29 However, the concentrations of these metabolites also depend upon potato cultivar, extent of damage, and exposure to light.26 The mechanism of GA metabolism is very complex, and the toxicity is higher for humans than for animals. There have been reported inhibition of acetylcholinesterase and affection of the digestive system through the disruption of the cell membrane. High concentrations could cause nausea or vomiting in humans.25,29
Figure 2.
Structures of (a) α-solanine and (b) α-chaconine.
The most commonly used method for the control of CPB is the application of insecticides. In fact, the CPB is responsible for creating the modern insecticide industry.20 As is well-known, the overuse of synthetic substances is a major factor in environmental pollution. Many organic contaminants, such as organochlorine pesticides, polycyclic aromatic hydrocarbons, phthalate esters, and polychlorinated biphenyls, are known for their persistence, relatively high toxicity, and bioaccumulation in the environment. Some of these chemicals enter the soil and act as a secondary source of emissions.30 The residues of these substances in the soils pose a potential threat to animal and human health when entering the food chains.31 Another danger of insecticides lies in their metabolites that could be more toxic than the original insecticide.32 They can later cause health problems, such as cancer or interference with the endocrine system. Another challenge is a selective pressure on the pest, resulting in growing insecticide resistances. The ability of insect pests to form a resistant subpopulation has been reported against 56 major insecticides.20 However, there still exist some alternatives to synthetic insecticides that could be gentler to the environment.33
One such alternative is related to the CPB pathogen Bacillus thuringiensis (Bt). Bt is a Gram-positive soil bacterium known mainly for the production of proteinateous insecticidal δ-endotoxins. These insecticidal crystal toxins are divided into two main groups, namely, Cry and Cyt protein toxins. These toxins are encoded by multigenic family cry or cyt genes and form parasporal inclusions during sporulation. Cry proteins are toxic to Lepidoptera, Diptera, Hymenoptera, and Coleoptera orders of insects, whereas the Cyt proteins are active only against Diptera and, therefore, are not widely used.34,35 Cry proteins are produced as protoxins, and they must be ingested by CPB larvae. Protoxins are solubilized under alkaline conditions. The activation is performed by trypsin- and chymotrypsin-like proteases forming toxic fragments. Fragments are capable of specific interaction with the midgut cell receptors and create a pore in the epithelium.35,36 These toxins in combination with Bt spores are used as biological insecticides. The main advantage is the specificity of the toxins. Bt insecticides are gentle to humans, non-target wildlife, and beneficial arthropods.21 On the other hand, the toxins are specific only to young larval stages, are not active against borer insects, and are very sensitive to ultraviolet (UV) radiation.37 Today, several biopesticides are commercially available and are used mainly in ecological agriculture. However, these products still need to improve their effectivity and stability.38 With the development of biotechnologies, transgenic plants expressing Cry toxin genes started to be developed. Previously, transgenic potatoes containing the cry3A toxin encoded by the cry3A gene have been shown to possess specific resistance to CPB.39 To date, approximately 30 potato events with the integrated cry3A gene have been approved.40 These GM plants are able to produce Cry toxins evenly throughout the plant as a result of the constitutive, tissue non-specific promoter of the Cauliflower mosaic virus (CaMV 35S). The genetic modification of potato solves the main problems: protein is produced inside the plant and, thus, is protected against UV, and thanks to stable production, there is no need to look for the correct larval stage. The majority of the approved GM potatoes were developed by the Monsanto Company. Atlantic NewLeaf and New Leaf Russet Burbank potatoes are approved for cultivation in Canada and the United States. Hi-Lite NewLeaf Y, New Leaf Y Russet Burbank, and Shepody NewLeaf Y potatoes have also included genes for resistance to Potato virus Y. All of these three GM potatoes are approved for cultivation in Canada and the U.S.A.40 Following doubts and public opposition to products containing GM potatoes, the market for processed potatoes was closed, and thus, the cultivation of these potatoes was reduced. In addition, processors began to label their products (especially french fries) as GM-free. On the basis of these facts, there was a significant drop in sales, and Monsanto stopped selling these potatoes in the spring of 2001.41 The Russian Academy of Sciences developed two GM potatoes with the trade names Elizaveta plus and Lugovskoi plus. These potatoes have been approved since 2005 and 2007, respectively, in Russia for direct use or processed in food.42 During 2016, the Federation Council of the Russian Federation adopted a law that banned the usage of GMO for food production. This law includes new registration procedures for GMO and necessary permissions to work in this field. Implementation of this law should support the Russian strategy “to produce the cleanest agricultural products in the world”.43
The safety of the Cry toxins is still discussed. Some studies showed the potential of Cry proteins to activate the human immune system and cause the allergic response.36,44,45 However, these results are obtained from purified Cry toxins, and in the case of potatoes, an analysis of these proteins should be performed after heat treatment. A possible solution of this problem lies in the usage of specific promoters. For example, the ST-LS1 promoter, which is a potato promoter active only in photosynthetic tissues (leaf/stem), caused higher concentrations of Cry toxins in potato leaves, and almost no Cry toxins were detected in the roots.39 For the specific expression of the toxin in place where the larvae started to consume the plant, the wound-inducible promoter isolated from Asparagus officinalis (AoPR1) could also be used effectively.46
3. Potato Virus Diseases (Potato Virus Y and Potato Leafroll Virus)
Potatoes are a vegetatively propagated crop. Without proper control of tubers for the presence of a virus particles, it can spread quickly and could cause a reduction in plant vitality and tuber yield.47 The virus infection can also spread easily by insects and cause huge losses. For periodic plant controls and virus infection testing, the double antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) or polymerase chain reaction (PCR) is used.48 Sufficient testing for virus detection is a problem mainly in the developing world. Higher temperatures are associated with an increased number of insect vectors and the next spread of the virus from the inoculated potato.12 There are about 50 different viruses infecting the Solanaceae plants, but only a few of them are responsible for major losses globally.49 The most important and well-known viruses that affect potato production are the Potato virus Y (PVY) and Potato leafroll virus (PLRV).
PVY is a member of the family Potyviridae, a group of viruses with single-stranded positive-sense RNA (+ssRNA) genomes. These viruses are characterized by high mutation rates and extremely high genetic variability.50 PVY could be classified, on the basis of the presence/absence of recombination events in their genome, into several strain groups (PVYO, PVYC, PVYN, PVYNTN, PVYN-Wi, PVYE, and PVYZ). These groups vary in their symptomology in different potato cultivars.12,51,52 Symptoms are also related to environmental conditions or whether the infection is primary or secondary.12,52,53 Symptoms may occur in the foliage or tubers. Different types of mosaic, necrotic reactions (from limited localized necrotic lesions to systemic yellowing and vein necrosis) could be induced in foliage that could result in growth retardation.12 Some strains can cause so-called potato tuber necrotic ringspot disease in susceptible cultivars, making these tubers unmarketable.12 The PLRV belongs to the Luteoviridae family (+ssRNA virus).48 Primary infection causes chlorosis, necrosis, and leaf curling. The leaves of some cultivars may turn red. Seed-borne infection results in stunted, severely damaged plants with reduced yield in both tuber size and number, as in the case of PVY infection.54 Tubers with typical net necrosis may be the result of infection in the current season. During infection, there is an excessive accumulation of carbohydrates in the leaves, corresponding to a reduction in the tubers. This may be related to necrosis of phloem cells or blocking the photoassimilation movement from the chloroplast to the cytosol by the triosephosphate translocator. Tuber symptoms are rare and depend upon the cultivar.
In response to virus infection, the potatoes change their sugar balance. Soluble sugars accumulate in non-infected leaves of the infected plants.55 For example, callose, a plant polysaccharide produced in response to viral infection, is used to detect the viral infection by staining with resorcinol blue. The accumulation of reactive oxygen species scavengers is rapid in PVYN-infected leaves, which is related to the lower damage seen on the leaves.55 Another response is the upregulation of proteins during the virus infection; it could be calreticulin, salt tolerance protein, acidic endochitinase, and patatin.56 Some of these proteins may trigger an allergic reaction in consumers. The typical response of plants to biotic stress is the synthesis and accumulation of phenylpropanoids. Significant accumulation was observed mainly for 3-trans-caffeoylquinic acid and 4-trans-caffeoylquinic acid. These substances are also related to the resistance of plants to these viruses.55 They are stored under the peel of the potato.57 Caffeoylquinic acids are well-known for their antioxidant, antibacterial, neuroprotective, and other activities;58−60 therefore, the consumer should not be afraid of these substances.
PVY is not specific only to potato plants but could cause serious diseases to several other members of the Solanaceae family, including tobacco, tomato, or pepper.61 PVY is transmitted mainly in non-persistent form by aphids.12,50,62 Acquisition and transmission of virus particles occur when winged aphids briefly test the plants. The virus stays in the aphid stylet, and aphids are viruliferous only for a short period of time (minutes to tens of minutes).50 For this type of virus transmission, the usage of pesticides is inefficient because the aphid can spread the virus to a healthy plant before it dies.63,64 On the other hand, the transmission of PLRV is more complicated. The infection could be introduced by planting infected potatoes or an insect vector. Here, the primary insect vectors are only a few species of aphids (mainly Myzus persicae). The acquisition of the virus by the aphid is by feeding on infected plants. The virus enters the hemocoel by crossing the intestinal membrane of the aphid digestive system. From this place, the virus can easily cross another membrane and enter the salivary glands. From that moment on, the aphid transmits the virus for its remaining life.54 This is called persistent transmission.65 Aphids can also infect tubers in storage. The reduction of insect vectors could help to regulate virus spread.
The essential way to reduce the virus spreading is the crop screening programs and destruction of infected plants.64 When PVYO was the dominant strain, potato tuber certification was mainly based on visual inspections before 2000, because this strain induces visible mosaic foliar disease in many cultivars.47 Today, the situation is completely different as a result of new recombinant strains that show only mild and often transient foliar symptoms. Therefore, detection of this infection during field inspection is difficult.47 In addition, the infection could be spread unnoticed by infected aphids. In the case of PLRV, the use of insecticides is very effective compared to PVY.54 Mineral oils have been used to limit the spread of PVY. These oils are capable of interfering with aphid feeding and virus transmission; however, phytotoxic activity was observed.66 There are also substances that are able to make a physical barrier to stylet penetration.64 To minimize PVY infection, different planting strategies can be used (such as planting a non-host border crop around the potato crop).66
Plant virus resistance is divided into two types. The hypersensitive reaction (HR) involves programmed cell death, conferred by Ny genes.63 The replication of a virus is limited and leads to local necrotic lesions. These lesions prevent further spread of the virus.12 The second type of resistance is called extreme resistance (ER) and involves the R genes. These genes could possibly encode specific inhibitors of a specific virus strain accumulation.67 During virus inoculation, potatoes are without or with very little visible necrosis.68 ER to PVY has been found in the Solanum tuberosum L. group Andigena (Ryadg), Solanum stoloniferum (Rysto), Solanum chacoense (Rychc), Solanum demissum, and Solanum hougassi.69 These genes are used during breeding programs.63,70 Breeding resistant cultivars could prevent infection and spreading of the virus in the plant.63 On the other hand, these breeding strategies are very difficult, long-term, and sometimes very expensive. The other plant response to virus invasion is antiviral RNA silencing [RNA interference (RNAi)]. It is a host response triggered by viral double-stranded RNA. The process involves a RNA-induced silencing complex (RISC), a multiprotein complex that contains one strand of small-interfering RNA (siRNA) or micro RNA (miRNA). RISC uses this RNA to guide the complex to recognize complementary viral mRNA. Upon recognition, the RNase component of RISC called Slicer is activated and cleaves the viral mRNA. The initial RNA material for the activation of the process is provided by RNase type-III-like enzyme called Dicer by sensing and subsequent cleavage of the double-stranded RNA intermediates of viral replication. This resistant strategy is effective against all DNA and RNA viruses, but it is relatively slow and does not lead to complete removal of the virus.71 This defense strategy is also known as pathogen-derived resistance.
There are five different approved GM potatoes with resistance to viral infection. Their trade names are Hi-Lite NewLeaf Y potato, New Leaf Y Russet Burbank potato, New Leaf Plus Russet Burbank potato, Shepody NewLeaf Y potato, and a new potato SPT TICAR (potato event TIC-AR233-5). Except for the last potato, the GM potatoes were developed by the Monsanto Company and were commented on in the previous section. The last GM potato was developed by Technoplant Argentina and has been approved for food, feed, and cultivation in Argentina since 2018.72 During 2019, there is a formal registration of this GM potato in the National Seed Institute’s register.73 The main advantage for the farmers is the cost reduction; less insecticides have to be used, and the tubers do not need any crop screening before planting for the next year. The TICAR potatoes should be available on the Argentinian market in the near future. The rest of the GM potatoes are approved for cultivation only in the United States and Canada. Countries with approval for the food use of these potatoes are Australia, Canada, Japan, Mexico, New Zealand, South Korea, and the United States.74,75 All of these approved GM potatoes are using the gene silencing strategy that is a part of a plant active defense mechanism.76 The gene inserted for PVY resistance encodes the PVY coat protein (CP). CP plays an important role in viral translation and activation of host R genes and helps cell-to-cell virus propagation.77 Resistance against PLRV is achieved by inserting and expressing the replicase gene.78 In the case of resistance to both viruses, GM potato plants do not express a detectable amount of transgenic proteins inserted. This confirms the functional mechanism of gene silencing and proves the safety of these GM plants.79−81
4. Oomycete Pathogenes
One of the oldest potato diseases is the late blight (LB) disease caused by the fungus Phytophthora infestans. This disease caused a huge famine in Ireland during the 19th century. To date, LB is one of the most devastating and economically important diseases that affects potato and tomato crops.82 Costs associated with crop damage and costs located for the control of P. infestans could cross a billion euros annually.83
P. infestans belongs to a group of fungi-like organisms. The Oomycetes include the genus Phythophtora with mainly plant pathogens widely spread around the world,84 affecting tubers and foliage. Foliar infection (water-soaked lesions) reduces the tuber production; thus, the effect is indirect.85 The lesions are surrounded by sporangia that are easily dispersed on other leaves and can cause new lesions in a short time.86 During watering, the sporangia are often washed from the leaves and can infect the potato tubers, which usually results in complete yield loss. Furthermore, tuber lesions of the LB could easily be colonized by soft-rotting bacteria.85,86 Pathogen infection causes oxidative damage: when the potato plant is attacked by the pathogen, the cell membrane peroxidation increases with the production of peroxidase, hydrogen peroxide, and malondialdehyde [CH2(CHO)2].87 Malondialdehyde is a highly toxic aldehyde and potential mutagen and artherogen interacting with DNA or proteins.88 As a response to biotic stress, the plant could synthesize chitinase, a potential allergen for sensitive consumers.
To control the disease, fungicides are used in increased amounts; therefore, potatoes become one of the most fungicide-treated crops.86 However, P. infestans can easily develop resistance to fungicides. Nowadays, farmers apply about 10 or more different fungicides during the season to protect the yield.86,89 This burdens the environment and endangers public health through chemical accumulation in food chains, as mentioned above. Several pesticides are known as resistance inducers. For example, application of potassium phosphite increased levels of antioxidant enzymes, phenolics, flavonoids, and anthocyanin and activated plant defense responses to LB disease.87 Additionally, exogenous application of ethylene supported the induction of defense responses in resistant potato type.90 A haustorium grows from the mycelium of P. infestans into the intercellular space but is not able to penetrate the cell membrane. The haustorium produces avirulence (Avr) factors that could be recognized by the host plant when they cross the cell membrane. If the host plant holds the corresponding R gene, it responds with HR.91 Rapid evolution of the Avr genes is necessary for successful breakdown of resistance in the host plant. Therefore, the ER genes are also under pressure to recognize these new Avr to be able to trigger HR and stop the spread of the pathogen. Most cultivated potatoes are susceptible to LB disease, but wild relatives are a perfect source of resistance.92 By conventional breeding, a resistant gene from Solanum deemissum was bred into a new potato variety, but over time, the resistance broke down. Introgression (hybridization between two species and repeated backcrossing) of R genes from wild species is time-consuming and efficient just until the new mutation of the Avr genes is developed.93 The sexual production of spores (oospores) could help develop new mutations in the Avr gene. The oospores can survive in the soil for several years and infect disease-free tubers.86 As a result of the sexual reproduction of this oomycete, there is a fast and easy genetic diversification of this organism.
The Rpi-vnt1 resistance gene encoding coiled-coil nucleotide-binding leucine-rich repeat protein is used for commercial genetic transformation. This gene originates from the American Solanum venturii species and belongs to the leucine zipper/NBS/LRR class of plant resistance genes, which used the leucine zipper part for the protein–protein interaction or dimerization.94 It is generally considered a promising source of resistance to LB disease because it was shown to provide resistance to several highly virulent European strains of P. infestans.95 There are four approved GM potatoes with this gene insertion. Their trade names are Simplot Innate, Innate Acclimate, Innate Hibernate, and event name W8.96 All of these potatoes were developed by J.R. Simplot Co. Except for Simplot Innate, all other variants are approved for cultivation at least in the United States and Canada. Simplot Innate was accepted for food in Canada in 2020.97 These potatoes were also evaluated as safe and nutritionally the same as conventional potato varieties.98,99
5. Modified Product Quality
Modification of potato quality involves a reduction in the formation of acrylamide. This is achieved mainly by the reduction of amino acid asparagine or reducing sugars. Potato starch is used in the textile industry as well as a binder. Strains suitable for industrial applications produce more potato starch than potatoes suitable for food and feed.
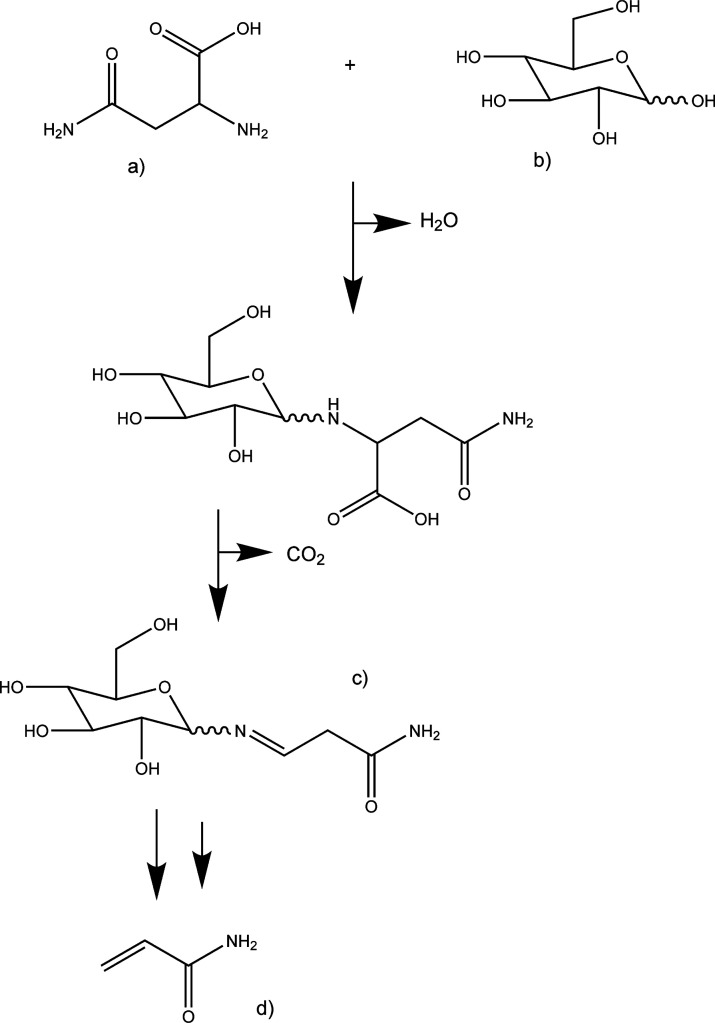
Potatoes do not contain fat; thus, potato products usually need the addition of fat and salt to be more desirable and tasty to customers. These products are responsible for obesity and health problems mainly in developed countries. Potato products require high temperatures during preparation, which are achieved by frying (>170 °C). This method of preparation is dangerous because it can lead to the Maillard reaction (reaction between sugar and amino acids), which can produce acrylamide (Figure 3). Acrylamide production depends upon a high asparagine content, a high reducing sugar content (fructose and glucose), humidity below 30%, and temperatures above 100 °C.11
Figure 3.
Scheme of the Maillard reaction: (a) asparagine, (b) glucose, (c) Schiff base, and (d) acrylamide.
Acrylamide is a colorless compound widely used for the production of polyacrylamide, which is applied in soil conditioning, wastewater treatment, cosmetics, and textile industry. However, acrylamide is degradable by various microorganisms; thus, higher concentrations in the environment are not expected. According to the European Commission Regulation (2017/2158), the acrylamide limit in fried potato products is approximately 750 μg/kg. Acrylamide could induce oxidative DNA damage, leading to cell death.100,101
The concentration of acrylamide precursors can be affected by potato variety, growing conditions, or storage conditions. Drought has a significant effect on the content of reducing sugars (glucose and fructose) in tubers. The concentration of the reducing sugars increases with a low temperature102 and depends mainly upon the potato variety.57,103 However, the acrylamide concentration depends mostly upon the frying time and temperature. Acrylamide levels in chips processed at high temperatures for short frying times were lower than in those processed at low temperatures for long frying times.104 Treatments of potatoes before frying, such as blanching in distilled water or adding yeast Aureobasidium pullulans, reduce the acrylamide content without affecting sensoric properties.104,105 Black spots are a postharvest physiological phenomenon resulting from polyphenol oxidase activity. Their formation is associated with potato dropping during the handling of potato tubers at harvest, transport, storage, and processing, and it is more or less a cosmetic defect in the potato industry.94,103
To reduce the precursors of acrylamide formation, the gene silencing cassette is introduced to reduce the expression of the asparagine synthetase 1 gene (Asn1). Another cassette reduces the expression of the α-glucan, water dikinase gene (also called the starch-associated R1 gene) and the phosphorylase L gene (PhL). This combination of genes significantly reduced acrylamide levels in fried potatoes.99,106,107,108 The reduction of the number of black spots is achieved by silencing of the polyphenol oxidase 5 gene (Ppo5). There are 16 approved GM potatoes with genes inserted to reduce asparagine production, levels of reducing sugars, and formation of black spots. All of these potatoes were developed by the J.R. Simplot Company. This company started to develop and advertise GM potatoes using only potato genes,41 with the focus on the requirements of the consumer; in this way, they evaded the failure that Monsanto had to face in 2001. Seven approved GM potatoes have the trade names: Innate Cultivate, Innate Generate, Simplot Innate, Innate Accelerate, Innate Invigorate, Innate Acclimate, and Innate Hibernate.109 These GM potatoes are still planted, with about 40 ha in Canada in 2019.15 A total of 4 out of 16 approved GM potatoes also have integrated genes for foliar resistance to LB marked as the second generation of Simplot Innate.41 Some of them have been successfully tested for their safety.99
Potatoes with a high starch content composed exclusively of amylopectin are important for the starch industry. These potatoes are useful in textile sizing or papermaking. BASF Plant Science developed two approved GM potatoes (Amflora and Starch Potato), but none of them are approved for cultivation and are no longer marketed. The inserted gene minimizes the amylose content by lowering the level of granule-bound starch synthase. This enzyme is responsible for amylose synthesis in many plants.110 The Amflora potato was first accepted by the European Food Safety Authority,111 but the acceptance was canceled by the General Court of the European Union with the justification that Amflora potato presents a potential risk to human and animal health.
Potatoes are exposed to a number of stressors during their life, which reduce their yields and worsen the nutritional quality of tubers. Because potatoes are an important commodity in the food and feed industry, there is growing pressure to adopt genetically modified plants that are tolerant to adverse conditions. Great development is also expected thanks to the new efficient genome editing tool (CRISPR/Cas9), which will make genetic transfection faster and more efficient than conventional breeding and Agrobacterium-mediated transformation. As many years of use have shown, the use of GM plants is not harmful to the environment (unlike chemical pesticides), and GM crops are one of the most studied crops in terms of their potential toxicities and adverse effects. As a result of the spread of antibiotic resistance, the use of antibiotic resistance as a selection marker should also be considered in the future. The priority of future research in the field of genetic modification of the potato should also be the adaptation of the plants to the effects of climate change. Potatoes are usually grown in higher altitudes because they require lower temperatures during the season. The rise of the temperature caused by climate change and global warming could therefore significantly reduce their crop area. Thus far, no GM potato cultivar prepared for this purpose has been engineered.
From all of the above, it can be concluded that many potato threats are elegantly addressed by genetic modification and transgenic plants could produce less metabolites that are harmful for the consumers (malondialdehyde, calreticulin, glycoalkaloids, and others). Even though the adoption of GM crops is the fastest growing agricultural technology in the world, the biggest weakness of their promising applications remains the legislation limitation. However, it could be expected that legislative limits are likely to be breached soon as a result of the current food crisis in the world. In addition, the scientific community has been given a clear and consistent signal for a long time, especially recently by awarding the Nobel Prize for the use of CRISPR/Cas9, of which the use refutes all of the standard arguments of GM crop deniers used thus far.
This work was supported by the mobility project from the Czech Ministry of Education, Youth and Sports INTER-COST (Grant LTC20015) and the UCT Prague Specific University Research (Grant A2_FPBT_2021_036). This review is based on work from COST Action CA18111 (PlantEd), supported by the European Cooperation in Science and Technology (COST, http://www.cost.eu).
The authors declare no competing financial interest.
References
- Zaheer K.; Akhtar M. H. Potato production, usage, and nutrition—A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 711–721. 10.1080/10408398.2012.724479. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO) . FAO Statistical Yearbook; FAO: Rome, Italy, 2020; 10.4060/cb1329en. [DOI]
- Food and Agriculture Organization of the United Nations (FAO) . Potato Production, 2018; FAO: Rome, Italy, 2018; https://Ourworldindata.Org/Grapher/Potato-Production (accessed April 21, 2022).
- Shahbandeh M.Global Potato Production 2002–2019; Statista: New York, 2021; https://www.statista.com/statistics/382174/global-potato-production/ (accessed Dec 20, 2021).
- Górska-Warsewicz H.; Rejman K.; Kaczorowska J.; Laskowski W. Vegetables, Potatoes and Their Products as Sources of Energy and Nutrients to the Average Diet in Poland. Int. J. Environ. Res. Public Health 2021, 18, 3217. 10.3390/ijerph18063217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torabian S.; Farhangi-Abriz S.; Qin R.; Noulas C.; Sathuvalli V.; Charlton B.; Loka D. A. Potassium: A Vital Macronutrient in Potato Production—A Review. Agronomy 2021, 11, 543. 10.3390/agronomy11030543. [DOI] [Google Scholar]
- Stone M. S.; Martin B. R.; Weaver C. M. Short-Term RCT of Increased Dietary Potassium from Potato or Potassium Gluconate: Effect on Blood Pressure, Microcirculation, and Potassium and Sodium Retention in Pre-Hypertensive-to-Hypertensive Adults. Nutrients 2021, 13, 1610. 10.3390/nu13051610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essodolom P.; Ekpetsi Chantal B.; Mamatchi M.; Kousanta A. Effect of temperature on the degradation of ascorbic acid (vitamin c) contained in infant supplement flours during the preparation of porridges. Int. J. Adv. Res. 2020, 8, 116–121. 10.21474/IJAR01/10605. [DOI] [Google Scholar]
- Bethke P. C.; Bussan A. J. Acrylamide in processed potato products. Am. J. Potato Res. 2013, 90, 403–424. 10.1007/s12230-013-9321-4. [DOI] [Google Scholar]
- Dite Hunjek D.; Pelaić Z.; Čošić Z.; Pedisić S.; Repajić M.; Levaj B. Chemical constituents of fresh-cut potato as affected by cultivar, age, storage, and cooking. J. Food Sci. 2021, 86, 1656–1671. 10.1111/1750-3841.15712. [DOI] [PubMed] [Google Scholar]
- Hijmans R. J. The effect of climate change on global potato production. Am. J. Potato Res. 2003, 80, 271–279. 10.1007/BF02855363. [DOI] [Google Scholar]
- Karasev A. v; Gray S. M. Continuous and emerging challenges of Potato virus Y in potato. Annu. Rev. Phytopathol. 2013, 51, 571–586. 10.1146/annurev-phyto-082712-102332. [DOI] [PubMed] [Google Scholar]
- Skendžić S.; Zovko M.; Pajač Živković I.; Lešić V.; Lemić D. Effect of Climate Change on Introduced and Native Agricultural Invasive Insect Pests in Europe. Insects 2021, 12, 985. 10.3390/insects12110985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- gmo answers . GMOs around the World; CropLife International: Brussels, Belgium, 2022; https://gmoanswers.com/gmos-around-world (accessed April 21, 2022).
- International Service for the Acquisition of Agri-biotech Applications (ISAAA) . Global Status of Commercialized Biotech/GM Crops in 2019: Biotech Crops Drive Socio-Economic Development and Sustainable Environment in the New Frontier; ISAAA, Ithaca, NY, 2019.
- International Service for the Acquisition of Agri-biotech Applications (ISAAA) . Potato (Solanum tuberosum L.) GM Events (51 Events); ISAAA, Ithaca, NY, 2022; https://www.isaaa.org/gmapprovaldatabase/crop/default.asp?CropID=16&Crop=Potato (accessed April 21, 2022).
- International Service for the Acquisition of Agri-biotech Applications (ISAAA) . Stacked Traits in Biotech Crops, Pocket K No. 42; ISAAA, Ithaca, NY, 2022; https://www.isaaa.org/resources/publications/pocketk/42/default.asp (accessed April 21, 2022).
- Bukovinszki A.; Divéki Z.; Csányi M.; Palkovics L.; Balázs E. Engineering resistance to PVY in different potato cultivars in a marker-free transformation system using a ‘shooter mutant’ A. tumefaciens. Plant Cell Rep. 2007, 26, 459–465. 10.1007/s00299-006-0257-8. [DOI] [PubMed] [Google Scholar]
- Colley M. R.; Dawson J. C.; McCluskey C.; Myers J. R.; Tracy W. F.; Lammerts van Bueren E. T. Exploring the emergence of participatory plant breeding in countries of the Global North—A review. J. Agric. Sci. 2021, 159, 320–338. 10.1017/S0021859621000782. [DOI] [Google Scholar]
- Alyokhin A.; Baker M.; Mota-Sanchez D.; Dively G.; Grafius E. Colorado potato beetle resistance to insecticides. Am. J. Potato Res. 2008, 85, 395–413. 10.1007/s12230-008-9052-0. [DOI] [Google Scholar]
- Kadoić Balaško M.; Mikac K. M.; Bažok R.; Lemic D. Modern techniques in Colorado potato beetle (Leptinotarsa decemlineata Say) control and resistance management: History review and future perspectives. Insects 2020, 11, 581. 10.3390/insects11090581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F.; Liu N.; Crossley M. S.; Wang P.; Ma Z.; Guo J.; Zhang R. Cropland connectivity affects genetic divergence of Colorado potato beetle along an invasion front. Evol. Appl. 2021, 14, 553–565. 10.1111/eva.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharijaya A.; Vosman B. Managing the Colorado potato beetle; the need for resistance breeding. Euphytica 2015, 204, 487–501. 10.1007/s10681-015-1467-3. [DOI] [Google Scholar]
- Davidson-Lowe E.; Szendrei Z.; Ali J. G. Asymmetric effects of a leaf-chewing herbivore on aphid population growth. Ecol. Entomol. 2019, 44, 81–92. 10.1111/een.12681. [DOI] [Google Scholar]
- Benkeblia N. Potato glycoalkaloids: Occurrence, biological activities and extraction for biovalorisation—A review. Int. J. Food Sci. Technol. 2020, 55, 2305–2313. 10.1111/ijfs.14330. [DOI] [Google Scholar]
- Petersson E. v; Arif U.; Schulzova V.; Krtková V.; Hajšlová J.; Meijer J.; Andersson H. C.; Jonsson L.; Sitbon F. Glycoalkaloid and calystegine levels in table potato cultivars subjected to wounding, light, and heat treatments. J. Agric. Food Chem. 2013, 61, 5893–5902. 10.1021/jf400318p. [DOI] [PubMed] [Google Scholar]
- Koffi G. Y.; Remaud-Simeon M.; Due A. E.; Combes D. Isolation and chemoenzymatic treatment of glycoalkaloids from green, sprouting and rotting Solanum tuberosum potatoes for solanidine recovery. Food Chem. 2017, 220, 257–265. 10.1016/j.foodchem.2016.10.014. [DOI] [PubMed] [Google Scholar]
- Matthews D.; Jones H.; Gans P.; Coates S.; Smith L. M. J. Toxic secondary metabolite production in genetically modified potatoes in response to stress. J. Agric. Food Chem. 2005, 53, 7766–7776. 10.1021/jf050589r. [DOI] [PubMed] [Google Scholar]
- Schrenk D.; Bignami M.; Bodin L.; Chipman J. K.; del Mazo J.; Hogstrand C.; Hoogenboom L.; Leblanc J.-C.; Nebbia C. S.; Nielsen E.; Ntzani E.; Petersen A.; Sand S.; Schwerdtle T.; Vleminckx C.; Wallace H.; Brimer L.; Cottrill B.; Dusemund B.; Mulder P.; Vollmer G.; Binaglia M.; Ramos Bordajandi L.; Riolo F.; Roldán-Torres R.; Grasl-Kraupp B. Risk assessment of glycoalkaloids in feed and food, in particular in potatoes and potato-derived products. EFSA J. 2020, 18, e06222 10.2903/j.efsa.2020.6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberemok V. V.; Laikova K. V.; Zaitsev A. S.; Temirova Z. Z.; Gal’chinsky N. V.; Nyadar P. M.; Shumskykh M. N.; Zubarev I. V. The need for the application of modern chemical insecticides and environmental consequences of their use: A mini review. J. Plant Protect. Res. 2017, 57, 427–432. 10.1515/jppr-2017-0044. [DOI] [Google Scholar]
- Sun J.; Pan L.; Tsang D. C. W.; Zhan Y.; Zhu L.; Li X. Organic contamination and remediation in the agricultural soils of China: A critical review. Sci. Total Environ. 2018, 615, 724–740. 10.1016/j.scitotenv.2017.09.271. [DOI] [PubMed] [Google Scholar]
- Wołejko E.; Jabłońska-Trypuć A.; Wydro U.; Butarewicz A.; Łozowicka B. Soil biological activity as an indicator of soil pollution with pesticides-a review. Appl. Soil Ecol. 2020, 147, 103356. 10.1016/j.apsoil.2019.09.006. [DOI] [Google Scholar]
- Göldel B.; Lemic D.; Bažok R. Alternatives to Synthetic Insecticides in the Control of the Colorado Potato Beetle (Leptinotarsa decemlineata Say) and Their Environmental Benefits. Agriculture 2020, 10, 611. 10.3390/agriculture10120611. [DOI] [Google Scholar]
- de Maagd R. A.; Bravo A.; Crickmore N. How Bacillus thuringiensis has evolved specific toxins to colonize the insect world. Trends Genet. 2001, 17, 193–199. 10.1016/S0168-9525(01)02237-5. [DOI] [PubMed] [Google Scholar]
- Paul S.; Das S. Natural insecticidal proteins, the promising bio-control compounds for future crop protection. Nucleus 2021, 64, 7–20. 10.1007/s13237-020-00316-1. [DOI] [Google Scholar]
- Rubio-Infante N.; Moreno-Fierros L. An overview of the safety and biological effects of Bacillus thuringiensis Cry toxins in mammals. J. Appl. Toxicol. 2016, 36, 630–648. 10.1002/jat.3252. [DOI] [PubMed] [Google Scholar]
- Bravo A.; Likitvivatanavong S.; Gill S. S.; Soberón M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431. 10.1016/j.ibmb.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira J. L.; Fraceto L. F.; Bravo A.; Polanczyk R. A. Encapsulation Strategies for Bacillus thuringiensis: From Now to the Future. J. Agric. Food Chem. 2021, 69, 4564–4577. 10.1021/acs.jafc.0c07118. [DOI] [PubMed] [Google Scholar]
- Mi X. X.; Ji X. Z.; Yang J. W.; Liang L. N.; Si H. J.; Wu J. H.; Zhang N.; Wang D. Transgenic potato plants expressing cry3A gene confer resistance to Colorado potato beetle. C. R. Biol. 2015, 338, 443–450. 10.1016/j.crvi.2015.04.005. [DOI] [PubMed] [Google Scholar]
- International Service for the Acquisition of Agri-biotech Applications (ISAAA) . Events with Gene cry3A; ISAAA: Ithaca, NY, 2022; https://www.isaaa.org/gmapprovaldatabase/gene/default.asp?GeneID=66&Gene=cry3A (accessed April 21, 2022).
- Halterman D.; Guenthner J.; Collinge S.; Butler N.; Douches D. Biotech potatoes in the 21st century: 20 years since the first biotech potato. Am. J. Potato Res. 2016, 93, 1–20. 10.1007/s12230-015-9485-1. [DOI] [Google Scholar]
- CIS Alliance for Biosafety . First “Russian” Transgenic Potato Approved in Russia As Food Source; bio verlag gmbh: Aschaffenburg, Germany, 2006; https://organic-market.info/news/First_Russian_transgenic_potato_approved_in_Russia_as_food_source.html (accessed April 21, 2022).
- Roudik P.Russia: Full Ban on Food with GMOs; Library of Congress: Washington, D.C., 2016; https://www.loc.gov/item/global-legal-monitor/2015-10-06/russia-ban-introduced-on-use-of-gmos-in-food/ (accessed April 21, 2022).
- Torres-Martínez M.; Rubio-Infante N.; García-Hernández A. L.; Nava-Acosta R.; Ilhuicatzi-Alvarado D.; Moreno-Fierros L. Cry1Ac toxin induces macrophage activation via ERK1/2, JNK and p38 mitogen-activated protein kinases. Int. J. Biochem. Cell Biol. 2016, 78, 106–115. 10.1016/j.biocel.2016.06.022. [DOI] [PubMed] [Google Scholar]
- Santos-Vigil K. I.; Ilhuicatzi-Alvarado D.; García-Hernández A. L.; Herrera-García J. S.; Moreno-Fierros L. Study of the allergenic potential of Bacillus thuringiensis Cry1Ac toxin following intra-gastric administration in a murine model of food-allergy. Int. Immunopharmacol. 2018, 61, 185–196. 10.1016/j.intimp.2018.05.029. [DOI] [PubMed] [Google Scholar]
- Ahmed H. A. A.; Onarıcı S.; Bakhsh A.; Akdoǧan G.; Karakoç O. C.; Özcan S. F.; Aydın G.; Aasım M.; Ünlü L.; Sancak C.; Naimov S.; Özcan S. Targeted expression of insecticidal hybrid SN19 gene in potato leads to enhanced resistance against Colorado potato beetle (Leptinotarsa decemlineata Say) and tomato leafminer (Tuta absoluta Meyrick). Plant Biotechnol. Rep. 2017, 11, 315–329. 10.1007/s11816-017-0453-8. [DOI] [Google Scholar]
- Gray S. M.; Power A. G. Anthropogenic influences on emergence of vector-borne plant viruses: The persistent problem of Potato virus Y. Curr. Opin Virol. 2018, 33, 177–183. 10.1016/j.coviro.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Stammler J.; Oberneder A.; Kellermann A.; Hadersdorfer J. Detecting potato viruses using direct reverse transcription quantitative PCR (DiRT-qPCR) without RNA purification: An alternative to DAS-ELISA. Eur. J. Plant Pathol. 2018, 152, 237–248. 10.1007/s10658-018-1468-x. [DOI] [Google Scholar]
- Kreuze J. F.; Souza-Dias J. A. C.; Jeevalatha A.; Figueira A. R.; Valkonen J. P. T.; Jones R. A. C.. Viral diseases in potato. In The Potato Crop, 1st ed.; Campos H., Ortiz O., Eds.; Springer, Cham, Switzerland, 2020: pp 389–430, 10.1007/978-3-030-28683-5_11. [DOI] [Google Scholar]
- Xu Y.; Gray S. M. Aphids and their transmitted potato viruses: A continuous challenges in potato crops. J. Integr. Agric. 2020, 19, 367–375. 10.1016/S2095-3119(19)62842-X. [DOI] [Google Scholar]
- Radcliffe E. B.; Ragsdale D. W. Aphid-transmitted potato viruses: The importance of understanding vector biology. Am. J. Potato Res. 2002, 79, 353–386. 10.1007/BF02870173. [DOI] [Google Scholar]
- Baebler Š.; Coll A.; Gruden K. Plant molecular responses to Potato Virus Y: A continuum of outcomes from sensitivity and tolerance to resistance. Viruses 2020, 12, 217. 10.3390/v12020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd E.; Carpenter E.; Ross B. T.; Zidack N.; Flenniken M. L. Potato cultivar and seed type affect the development of systemic potato virus Y (PVY N-Wi) Infection. Am. J. Potato Res. 2018, 95, 183–190. 10.1007/s12230-017-9625-x. [DOI] [Google Scholar]
- Loebenstein G.; Gaba V.. Viruses of Potato. In Viruses and Virus Diseases of Vegetables in the Mediterranean Basin, 1st ed.; Loebenstein G., Lecoq H., Eds.; Academic Press: San Diego, CA, 2012: Vol. 84, Chapter 6, pp 209–246, 10.1016/B978-0-12-394314-9.00006-3. [DOI] [Google Scholar]
- Kogovšek P.; Pompe-Novak M.; Petek M.; Fragner L.; Weckwerth W.; Gruden K. Primary metabolism, phenylpropanoids and antioxidant pathways are regulated in potato as a response to Potato virus Y infection. PLoS One 2016, 11, e0146135 10.1371/journal.pone.0146135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko I.; Spechenkova N.; Mamaeva A.; Makhotenko A. v; Love A. J.; Kalinina N. O.; Taliansky M. Role of the methionine cycle in the temperature-sensitive responses of potato plants to potato virus Y. Mol. Plant Pathol. 2021, 22, 77–91. 10.1111/mpp.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio S.L.; Petropoulos S.A.; Dias M.I.; Pereira C.; Calhelha R.C.; Fernandes A.; Leme C. M. M.; Alexopoulos A.; Santos-Buelga C.; Ferreira I.; Barros L. Phenolic composition and cell-based biological activities of ten coloured potato peels (Solanum tuberosum L.). Food Chem. 2021, 363, 130360. 10.1016/j.foodchem.2021.130360. [DOI] [PubMed] [Google Scholar]
- Zhang X. D.; Liu X. Q.; Kim Y. H.; Whang W. K. Chemical constituents and their acetyl cholinesterase inhibitory and antioxidant activities from leaves of Acanthopanax henryi: Potential complementary source against Alzheimer’s disease. Arch. Pharm. Res. 2014, 37, 606–616. 10.1007/s12272-013-0252-x. [DOI] [PubMed] [Google Scholar]
- Liu W. W.; Li J. D.; Zhang X. M.; Zu Y. X.; Yang Y.; Liu W. J.; Xu Z. H.; Gao H.; Sun X.; Jiang X. W.; Zhao Q. C. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. 10.1021/acs.jafc.0c03804. [DOI] [PubMed] [Google Scholar]
- Kamarauskaite J.; Baniene R.; Raudone L.; Vilkickyte G.; Vainoriene R.; Motiekaityte V.; Trumbeckaite S. Antioxidant and Mitochondria-Targeted Activity of Caffeoylquinic-Acid-Rich Fractions of Wormwood (Artemisia absinthium L.) and Silver Wormwood (Artemisia ludoviciana Nutt.). Antioxidants 2021, 10, 1405. 10.3390/antiox10091405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. A. C. Global plant virus disease pandemics and epidemics. Plants 2021, 10, 233. 10.3390/plants10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S.; Ghanim M.; Roberts A.; Gray S. M. Different potato virus Y strains frequently co-localize in single epidermal leaf cells and in the aphid stylet. J. Gen. Virol. 2021, 102, 1576. 10.1099/jgv.0.001576. [DOI] [PubMed] [Google Scholar]
- Valkonen J. P. T. Elucidation of virus-host interactions to enhance resistance breeding for control of virus diseases in potato. Breed. Sci. 2015, 65, 69–76. 10.1270/jsbbs.65.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller K. B.; McIntosh C.; Zidack N. Valuing disease prevention in a vegetatively propagated annual crop: Benefits from the Montana Seed Potato Certification Program. Plant Dis. 2020, 104, 2060–2067. 10.1094/PDIS-03-19-0443-SR. [DOI] [PubMed] [Google Scholar]
- DeBlasio S. L.; Wilson J. R.; Tamborindeguy C.; Johnson R. S.; Pinheiro P. v; MacCoss M. J.; Gray S. M.; Heck M. Affinity Purification-Mass Spectrometry Identifies a Novel Interaction between a Polerovirus and a Conserved Innate Immunity Aphid Protein that Regulates Transmission Efficiency. J. Proteome Res. 2021, 20, 3365–3387. 10.1021/acs.jproteome.1c00313. [DOI] [PubMed] [Google Scholar]
- Coutts B.Potato Virus Y in Potato Crops; Agriculture and Food, Department of Primary Industries and Regional Development, Government of Western Australia: South Perth, Western Australia, Australia, 2018; https://www.agric.wa.gov.au/potatoes/potato-virus-y-potato-crops (accessed Dec 20, 2021).
- Kang B.-C.; Yeam I.; Jahn M. M. Genetics of plant virus resistance. Annu. Rev. Phytopathol. 2005, 43, 581–621. 10.1146/annurev.phyto.43.011205.141140. [DOI] [PubMed] [Google Scholar]
- Torrance L.; Cowan G. H.; McLean K.; MacFarlane S.; Al-Abedy A. N.; Armstrong M.; Lim T.-Y.; Hein I.; Bryan G. J. Natural resistance to Potato virus Y in Solanum tuberosum Group Phureja. Theor. Appl. Genet. 2020, 133, 967–980. 10.1007/s00122-019-03521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera M. R.; Vidalon L. J.; Montenegro J. D.; Riccio C.; Guzman F.; Bartolini I.; Ghislain M. Molecular and genetic characterization of the Ryadg locus on chromosome XI from Andigena potatoes conferring extreme resistance to potato virus Y. Theor. Appl. Genet. 2018, 131, 1925–1938. 10.1007/s00122-018-3123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki T.; Sano M.; Asano K.; Nakayama T.; Maoka T. Effect of temperature on resistance to Potato virus Y in potato cultivars carrying the resistance gene Rychc. Plant Pathol. 2018, 67, 1629–1635. 10.1111/ppa.12862. [DOI] [Google Scholar]
- de Ronde D.; Butterbach P.; Kormelink R. Dominant resistance against plant viruses. Front. Plant Sci. 2014, 5, 307. 10.3389/fpls.2014.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Service for the Acquisition of Agri-biotech Applications (ISAAA) . Event Name: TIC-AR233-5; ISAAA: Ithaca, NY, 2018; https://www.isaaa.org/gmapprovaldatabase/event/default.asp?EventID=404&Event=TIC-AR233-5 (accessed April 21, 2022).
- eFarmNews . The First Argentine GMO Potato Almost Ready To Be Launched; eFarmNews: Buenos Aires, Argentina, 2020. http://efarmnewsar.com/2019-10-15/the-first-argentine-gmo-potato-almost-ready-to-be-launched-in-2020.html (accessed 21 April 2022).
- Australia New Zealand Food Authority (ANZFA) . Food Derived from Insect and Potato Virus Y-Protected Potato Lines RBMT15-101, SEMT15-02 and SEMT15-15; ANZFA: Kingston, Australia and Wellington, New Zealand, 2001.
- Canadian Food Inspection Agency (CFIA) . Determination of Environmental Safety of SEMT15-02, SEMT15-15, and RBMT15-101 Colorado Potato Beetle and Potato Virus Y Resistant Potato Lines Developed by Monsanto; CFIA: Ottawa, Ontario, Canada, 2017.
- Khalid A.; Zhang Q.; Yasir M.; Li F. Small RNA Based Genetic Engineering for Plant Viral Resistance: Application in Crop Protection. Front. Microbiol. 2017, 8, 43. 10.3389/fmicb.2017.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilal M.; Tabassum B.; Ali Q.; Nasir I. A. Down Regulation of Potato Virus Y (PVY) Coat Protein (CP) Expression by Iberis gibraltarica Protein Extract. Cytol. Genet. 2021, 55, 80–86. 10.3103/S0095452721010102. [DOI] [Google Scholar]
- Lawson E. C.; Weiss J. D.; Thomas P. E.; Kaniewski W. K. NewLeaf Plus® Russet Burbank potatoes: Replicase-mediated resistance to potato leafroll virus. Mol. Breed. 2001, 7, 1–12. 10.1023/A:1009637325028. [DOI] [Google Scholar]
- Ehrenfeld N.; Romano E.; Serrano C.; Arce-Johnson P. Replicase mediated resistance against Potato Leafroll Virus in potato Desirée plants. Biol. Res. 2004, 37, 71–82. 10.4067/S0716-97602004000100008. [DOI] [PubMed] [Google Scholar]
- Collinge D. B.; Jørgensen H. J. L.; Lund O. S.; Lyngkjær M. F. Engineering pathogen resistance in crop plants: Current trends and future prospects. Annu. Rev. Phytopathol. 2010, 48, 269–291. 10.1146/annurev-phyto-073009-114430. [DOI] [PubMed] [Google Scholar]
- Rashid M.-O.; Zhang X.-Y.; Wang Y.; Li D.-W.; Yu J.-L.; Han C.-G. The Three Essential Motifs in P0 for Suppression of RNA Silencing Activity of Potato leafroll virus Are Required for Virus Systemic Infection. Viruses 2019, 11, 170. 10.3390/v11020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.; Ospina-Giraldo M. D. Relative Disease Susceptibility of Cultivated Varieties of Potato to Different Isolates of Phytophthora infestans. J. Pa. Acad. Sci. 2011, 85, 140–146. 10.5325/jpennacadscie.85.4.0140. [DOI] [Google Scholar]
- Haverkort A. J.; Boonekamp P. M.; Hutten R.; Jacobsen E.; Lotz L. A. P.; Kessel G. J. T.; Visser R. G. F.; van der Vossen E. A. G. Societal costs of late blight in potato and prospects of durable resistance through cisgenic modification. Potato Res. 2008, 51, 47–57. 10.1007/s11540-008-9089-y. [DOI] [Google Scholar]
- de Andrade Lourenço D.; Branco I.; Choupina A. Phytopathogenic oomycetes: A review focusing on Phytophthora cinnamomi and biotechnological approaches. Mol. Biol. Rep. 2020, 47, 9179–9188. 10.1007/s11033-020-05911-8. [DOI] [PubMed] [Google Scholar]
- Mayton H.; Rauscher G.; Simko I.; Fry W. E. Evaluation of the RPi-ber late blight resistance gene for tuber resistance in the field and laboratory. Plant Breed. 2011, 130, 464–468. 10.1111/j.1439-0523.2010.01836.x. [DOI] [Google Scholar]
- Yuen J. Pathogens which threaten food security: Phytophthora infestans, the potato late blight pathogen. Food Secur. 2021, 13, 247–253. 10.1007/s12571-021-01141-3. [DOI] [Google Scholar]
- Mohammadi M. A.; Han X.; Zhang Z.; Xi Y.; Boorboori M.; Wang-Pruski G. Phosphite application alleviates Pythophthora infestans by modulation of photosynthetic and physio-biochemical metabolites in potato leaves. Pathogens 2020, 9, 170. 10.3390/pathogens9030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio D.; Stewart A. J.; Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr., Metab. Cardiovasc. Dis. 2005, 15, 316–328. 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Appeltans S.; Pieters J. G.; Mouazen A. M. Potential of laboratory hyperspectral data for in-field detection of Phytophthora infestans on potato. Precis. Agric. 2022, 23, 876–893. 10.1007/s11119-021-09865-0. [DOI] [Google Scholar]
- Yang X. H.; Chen L.; Yang Y.; Guo X.; Chen G. X.; Xiong X. Y.; Dong D. F.; Li G. C. Transcriptome analysis reveals that exogenous ethylene activates immune and defense responses in a high late blight resistant potato genotype. Sci. Rep. 2020, 10, 14. 10.1038/s41598-020-78027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkort A. J.; Struik P. C.; Visser R. G. F.; Jacobsen E. Applied biotechnology to combat late blight in potato caused by Phytophthora infestans. Potato Res. 2009, 52, 249–264. 10.1007/s11540-009-9136-3. [DOI] [Google Scholar]
- Karki H. S.; Jansky S. H.; Halterman D. A. Screening of Wild Potatoes Identifies New Sources of Late Blight Resistance. Plant Dis. 2021, 105, 368–376. 10.1094/PDIS-06-20-1367-RE. [DOI] [PubMed] [Google Scholar]
- Mayton H.; Griffiths H.; Simko I.; Cheng S.; Lorenzen J.; de Jong W.; Fry W. E. Foliar and tuber late blight resistance in a Solanum tuberosum breeding population. Plant Breed. 2010, 129, 197–201. 10.1111/j.1439-0523.2009.01671.x. [DOI] [Google Scholar]
- Ballvora A.; Ercolano M. R.; Weiss J.; Meksem K.; Bormann C. A.; Oberhagemann P.; Salamini F.; Gebhardt C. The R1 gene for potato resistance to late blight (Phytophthora infestans) belongs to the leucine zipper/NBS/LRR class of plant resistance genes. Plant J. 2002, 30, 361–371. 10.1046/j.1365-313X.2001.01292.x. [DOI] [PubMed] [Google Scholar]
- Roman M. L.; Izarra M.; Lindqvist-Kreuze H.; Rivera C.; Gamboa S.; Tovar J. C.; Forbes G. A.; Kreuze J. F.; Ghislain M. R/Avr gene expression study of Rpi-vnt1.1 transgenic potato resistant to the Phytophthora infestans clonal lineage EC-1. Plant Cell, Tissue Organ Cult. 2017, 131, 259–268. 10.1007/s11240-017-1281-9. [DOI] [Google Scholar]
- International Service for the Acquisition of Agri-biotech Applications (ISAAA) . GM Events with Foliar Late Blight Resistance; ISAAA: Ithaca, NY, 2022; https://www.isaaa.org/gmapprovaldatabase/gmtrait/default.asp?TraitID=39&GMTrait=Foliar%20Late%20Blight%20Resistance (accessed April 21, 2022).
- Health Canada . Novel Food Information: Simplot Innate Potato Event Gen2-Z6; Health Canada: Ottawa, Ontario, Canada, 2021.
- Habig J. W.; Rowland A.; Pence M. G.; Zhong C. X. Food safety evaluation for R-proteins introduced by biotechnology: A case study of VNT1 in late blight protected potatoes. Regul. Toxicol. Pharmacol. 2018, 95, 66–74. 10.1016/j.yrtph.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Mukerji P.; Rudgers G. W.; Gibson C.; Roper J. M. Safety evaluation of E12, W8, X17, and Y9 potatoes: Nutritional evaluation and 90-day subchronic feeding study in rats. Regul. Toxicol. Pharmacol. 2020, 115, 104712. 10.1016/j.yrtph.2020.104712. [DOI] [PubMed] [Google Scholar]
- Nowak A.; Zakłos-Szyda M.; Żyżelewicz D.; Koszucka A.; Motyl I. Acrylamide Decreases Cell Viability, and Provides Oxidative Stress, DNA Damage, and Apoptosis in Human Colon Adenocarcinoma Cell Line Caco-2. Molecules 2020, 25, 368. 10.3390/molecules25020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J.; Zheng J.; Huang J.; Ho C.-T.; Ou S. Interaction of acrylamide, acrolein, and 5-hydroxymethylfurfural with amino acids and DNA. J. Agric. Food Chem. 2020, 68, 5039–5048. 10.1021/acs.jafc.0c01345. [DOI] [PubMed] [Google Scholar]
- Grudzińska M.; Boguszewska-Mańkowska D.; Zarzyńska K. Drought stress during the growing season: Changes in reducing sugars, starch content and respiration rate during storage of two potato cultivars differing in drought sensitivity. J. Agron. Crop Sci. 2021, 10.1111/jac.12498. [DOI] [Google Scholar]
- Plata-Guerrero R.; Guerra-Hernandez E.; Garcia-Villanova B. Determination of Reducing Sugar and Asparagine in Potatoes. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 2556–2568. 10.1080/10826070903249732. [DOI] [Google Scholar]
- Liyanage D. W. K.; Yevtushenko D. P.; Konschuh M.; Bizimungu B.; Lu Z.-X. Processing strategies to decrease acrylamide formation, reducing sugars and free asparagine content in potato chips from three commercial cultivars. Food Control 2021, 119, 107452. 10.1016/j.foodcont.2020.107452. [DOI] [Google Scholar]
- Emadi A.; Yousefi B.; Eslami M.; Abdolshahi A. Reduction of acrylamide formation in bread and fried potato products using probiotic microorganisms: A systematic review and dose-response meta-analysis. J. Food Meas. Charact. 2021, 15, 4277–4287. 10.1007/s11694-021-00997-5. [DOI] [Google Scholar]
- Baritelle A.; Hyde G.; Thornton R.; Bajema R. A classification system for impact-related defects in potato tubers. Am. J. Potato Res. 2000, 77, 143–148. 10.1007/BF02853938. [DOI] [Google Scholar]
- Nashilevitz S.; Melamed-Bessudo C.; Aharoni A.; Kossmann J.; Wolf S.; Levy A. A. The legwd mutant uncovers the role of starch phosphorylation in pollen development and germination in tomato. Plant J. 2009, 57, 1–13. 10.1111/j.1365-313X.2008.03664.x. [DOI] [PubMed] [Google Scholar]
- Tran N. L.; Barraj L. M.; Collinge S. Reduction in Dietary Acrylamide Exposure—Impact of Potatoes with Low Acrylamide Potential. Risk Anal. 2017, 37, 1754–1767. 10.1111/risa.12709. [DOI] [PubMed] [Google Scholar]
- International Service for the Acquisition of Agri-biotech Applications (ISAAA) . GM Events with Lowered Free Asparagine; ISAAA: Ithaca, NY, 2022; https://www.isaaa.org/gmapprovaldatabase/gmtrait/default.asp?TraitID=32&GMTrait=Lowered%20Free%20Asparagine (accessed April 21, 2022).
- Hassan-Hauser C.; Mayer W.; Hörtner H. Detection of the starch modifying gbss-antisense construct in transgenic potatoes. Z. Lebensm.-Unters. -Forsch. A 1998, 206, 83–87. 10.1007/s002170050219. [DOI] [Google Scholar]
- Scientific Opinion on the annual Post-Market Environmental Monitoring (PMEM) report from BASF Plant Science Company GmbH on the cultivation of genetically modified potato EH92-527-1 in 2010. EFSA J. 2012, 10, 2558. 10.2903/j.efsa.2012.2558. [DOI] [Google Scholar]