Abstract
Objectives
To investigate the appropriateness of antibiotic prescribing among GPs in the private primary healthcare sector in South Africa.
Methods
An anonymized national database of claims for antibiotic prescriptions was obtained from a large medical insurer. Antibiotic prescriptions were categorized based on International Classification of Diseases (ICD-10) codes as ‘appropriate’, ‘potentially appropriate’ and ‘inappropriate’ using a classification scheme developed by Chua et al. (BMJ 2019; 364: k5092). Further assessments of antibiotic choice, dosage and duration of treatment were carried out to determine the appropriateness of ‘appropriate’ and ‘potentially appropriate’ prescriptions in comparison with treatment guidelines.
Results
In February 2018, 188 141 antibiotics were prescribed for 174 889 patients who consulted GPs in the private sector. Penicillins were the most frequently prescribed antibiotic class, making up 40.7% of all antibiotics prescribed. Amoxicillin/clavulanic acid was the most frequently prescribed antibiotic, making up 28.6% of all antibiotics prescribed. Diseases of the respiratory system generated the highest number of prescriptions, making up 46.1% of all diagnoses. Of all prescriptions, 8.8% were appropriate, 32.0% were potentially appropriate, 45.4% were inappropriate and 13.8% could not be assessed. Of the appropriately and potentially appropriately prescribed antibiotics, 30.8% were correct antibiotic selections. Of the correctly selected antibiotics for adults, 57.7% had correct doses. Of the antibiotics prescribed with correct doses for adults, 76.7% had correct dosage frequencies and durations of treatment.
Conclusions
The study revealed that antibiotics were frequently prescribed inappropriately by GPs in the private primary healthcare sector. There is thus a need to develop stewardship interventions in the sector.
Introduction
Antibiotics are one of the most frequently prescribed medicines globally,1 and there is evidence that inappropriate antibiotic use is especially prevalent in low- and middle-income countries (LMICs).1,2 A key driver of antibiotic resistance is antibiotic use.3–5 The development of antibiotic resistance is a global public health challenge,1,3,6–8 compounded by the reduction in the discovery of novel antibiotics.1,4,9,10 Antibiotic use can, however, be modified11–13 by ensuring appropriate prescribing. Judicious antibiotic prescribing includes avoiding unnecessary prescribing,3 selecting efficacious narrow-spectrum agents,3,14,15 choosing optimum dosages and deferring prescribing when possible.3
Approximately 85%–95% of antibiotics used in humans are consumed by outpatients.16 Several studies have demonstrated that inappropriate antibiotic prescribing is more common in the private sector compared with the public sector.17–19 National estimates of inappropriate antibiotic use are lacking in LMICs,20 and in South Africa, there is limited published research and assessment of antibiotic prescribing in primary care.21 There is also minimal information about the appropriateness of antibiotic prescribing in the private sector, where the utilization of treatment guidelines is not mandatory.22,23 We describe the antibiotic prescribing patterns amongst GPs in the private primary healthcare sector in South Africa.
Methods
Ethics
Ethical approval for the study was granted by the Humanities and Social Sciences Research Ethics Committee of the University of KwaZulu-Natal (reference number: HSSREC/00000966/2020). Patient consent was not needed as this was a retrospective study of de-identified data from the claims database of a medical insurer.
Study population
We undertook a descriptive analysis of the claims database of a health insurer. The anonymized antibiotic claims database included all enrollees and their beneficiaries, nationwide. About 15.0% of South Africans have health insurance. The insurance schemes are either open schemes (free for anyone to join) or restricted schemes (serving particular industries). The data analysed was from one of the largest open schemes, making up >30% of all health insurance beneficiaries in South Africa.24
Data extraction and analysis
Claims data for all systemic antibiotic prescriptions by GPs for February 2018 was provided by the insurer. Antimicrobials prescribed for HIV and tuberculosis were excluded.
Prescriptions were stratified by antibiotic classes, disease classes and age groups25 (0–17, 18–64 and ≥65 years, i.e. children, adults and older adults, respectively).7 The numbers of patients receiving one, two and three or more antibiotics per encounter were also noted.4,7,26
Identifying diagnosis codes
Diseases were identified using the recorded International Classification of Diseases (ICD-10) codes.27 Antibiotic claims (claims for dispensing antibiotics) were linked to medical claims (claims by GPs for health services) if the latter were received on the same day or within 7 days before the receipt of the antibiotic claims.28 Both antibiotic claims and medical claims reported ICD codes, but the codes from the medical claims were given priority as the codes from the antibiotic claims were often vague (e.g. Z76.9 described as, ‘Person encountering health services in unspecified circumstances’). Where no link could be made between an antibiotic claim and a medical claim, the code recorded by the antibiotic claim was used (noting that roughly 66% of the antibiotic claims without related medical claims came directly from the GPs who prescribed and dispensed the antibiotics and provided precise diagnosis codes).
Appropriateness of prescriptions
Prescriptions were categorized into three groups: ‘appropriate’ (tier 1); ‘potentially appropriate’ (tier 2); and ‘inappropriate prescriptions’ (tier 0); i.e. prescriptions generated for diagnoses that almost always warrant; sometimes warrant; or never warrant antibiotics, respectively, according to the scheme developed by Chua et al.7 in 2019. We added a fourth group—‘unknown’ (tier 3) —for claims without clear diagnosis codes.7,20,28
Like previous studies,7,11,20 if multiple diagnoses were linked to an antibiotic claim, the diagnosis in tier 1 was given priority. If no tier 1 diagnosis was recorded, the diagnosis in the tier 2 category was given priority. If only tier 0 and tier 3 diagnoses were recorded, then the diagnosis was classified as ‘unknown’ (tier 3). Since an objective of the study was also to stratify all prescriptions by disease classes, if multiple diagnoses (from different disease classes) for a patient fell in a particular tier, the code from the medical claim that matched the code reported by the antibiotic claim was given priority. In cases where none of the codes matched the code from the antibiotic claim, the diagnosis was classified as ‘unknown’ but still assigned to an appropriateness tier.
For example, if prescription A had J02.9 (acute pharyngitis, unspecified) and H66.0 (acute suppurative otitis media), reported by the medical claim (both of which are considered diseases that sometimes warrant a prescription—tier 2) and Z76.9 reported by the antibiotic claim, the prescription was categorized into tier 2 (potentially appropriate). However, the diagnosis was neither classified as a ‘J’ (diseases of the respiratory system) nor an ‘H’ (diseases of the eye and adnexa/diseases of the ear and mastoid process) disease class but categorized as an ‘unknown disease class’. Conversely, if the antibiotic claim for prescription A reported J02.9, then the diagnosis was classified as a ‘J’ disease class.
Some disease codes were not in the list of ICD codes provided by Chua et al.7 (which was based on the coding system used in the USA and was slightly different from the South African system). The disease descriptions were thus used for the classification into the tiers (examples are provided in Table S1, available as Supplementary data at JAC Online). If an unlisted code had no description, then the appropriateness of antibiotic prescription was classified as ‘unknown’. Unlisted codes that could not be assigned to any ICD code description were also classified as ‘unknown’.
Appropriateness of antibiotic prescribing was determined with reference to the South African Standard Treatment Guidelines and Essential Medicines List (STGs/EMLs)29 alongside the classification by Chua et al.7 with the STGs given priority where there was any discordance.
Prescriptions for diseases that always or sometimes warrant antibiotic prescribing were assessed for appropriateness in terms of antibiotic selection and dosage as contained in the STGs/EMLs (2018 version) and the South African Antibiotic Stewardship Programme (SAASP) Pocket Guide to Antibiotic Prescribing in Adults.30 However, the adherence to treatment guidelines was only carried out for antibiotic choice (and not dosing and duration of treatment) for children and older adults since these groups of patients would often require dosages to be calculated based on body weight, and this information was not provided.
The doses, dosage frequencies and durations of treatment were inferred from the database as it contained information on the dosage forms, the strengths and the total quantities of the antibiotics dispensed, e.g. if amoxicillin was recommended at a dose of 1000 mg twice daily for 10 days and 40 capsules of 500 mg of amoxicillin were dispensed, then the dose, dosage frequency and duration of treatment were considered correct.
If multiple antibiotics were prescribed for a diagnosis for which the guidelines only recommended one antibiotic, if one of the antibiotics was guideline concordant, the guideline-concordant antibiotic was considered an appropriate choice while the others were considered wrong choices. If an antibiotic was recommended by the guidelines for only a subset of patients, the antibiotic was considered a correct choice for all patients, since it was not always possible to tell which patients fell into a particular subset, e.g. it was impossible to tell if a patient had a penicillin allergy.
Where appropriateness of selection and dosage could not be assessed, either because the information from the guidelines was absent or ambiguous, or the guidelines recommended that referrals be made to secondary or tertiary institutions, appropriateness of prescribing was considered unknown.
Results
Descriptive statistics
In February 2018, there were 174 889 patient encounters with GPs that led to the prescribing of 188 141 antibiotics. Females made up 99 446 (56.9%) of all patients, 53 413 (30.5%) were children, 106 125 (60.7%) were adults and 15 351 (8.8%) were older adults. A total of 162 424 (92.9%) patients were prescribed one antibiotic, 11 741 (6.7%) patients were prescribed two antibiotics and 724 (0.4%) patients were prescribed three or more antibiotics. The highest number of antibiotics prescribed was six. Over half (52.5%) of the consultations took place in Gauteng (91 800), while 15.0% (26 241) and 13.7% (23 890) took place in Western Cape and KwaZulu-Natal, respectively, the three most populated provinces in South Africa.
Types of antibiotics prescribed
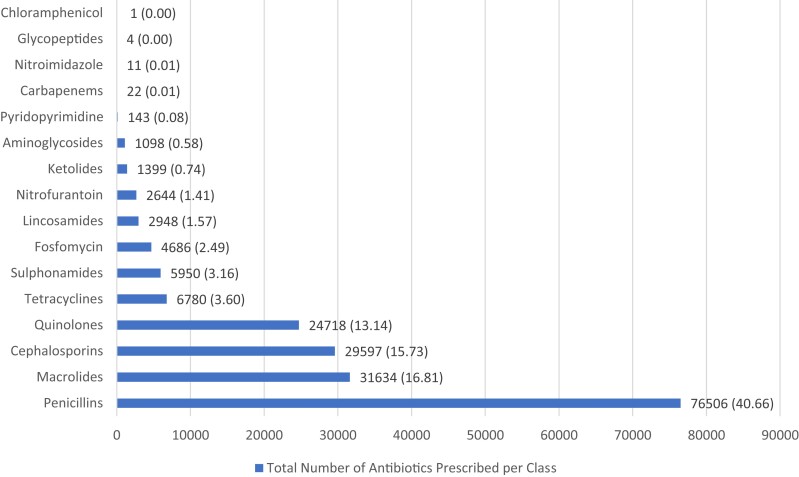
Penicillins were the most frequently prescribed antibiotic class, making up 40.7% (76 506) of all antibiotics prescribed. Macrolides, cephalosporins and quinolones were the second, third and fourth most prescribed antibiotic classes, respectively, making up 16.8% (31 634), 15.7% (29 597) and 13.1% (24 718) of all antibiotics prescribed (Figure 1).
Figure 1.
Number of antibiotics prescribed per antibiotic class (percentages in parentheses).
Amoxicillin/clavulanic acid was the most frequently prescribed antibiotic, making up 28.6% (53 837) of all antibiotics prescribed. Amoxicillin and azithromycin were the second and third most prescribed antibiotics, respectively, making up 9.8% (18 374) and 9.3% (17 519) of all antibiotics prescribed. Cefpodoxime was the most frequently prescribed cephalosporin, making up 6.7% (12 529) of all antibiotics prescribed, while ciprofloxacin was the most frequently prescribed quinolone, making up 8.5% (16 078) of all antibiotics prescribed. Ceftriaxone was the most common injectable prescribed, making up 2.4% (4564) of all antibiotics prescribed.
Indications for antibiotics
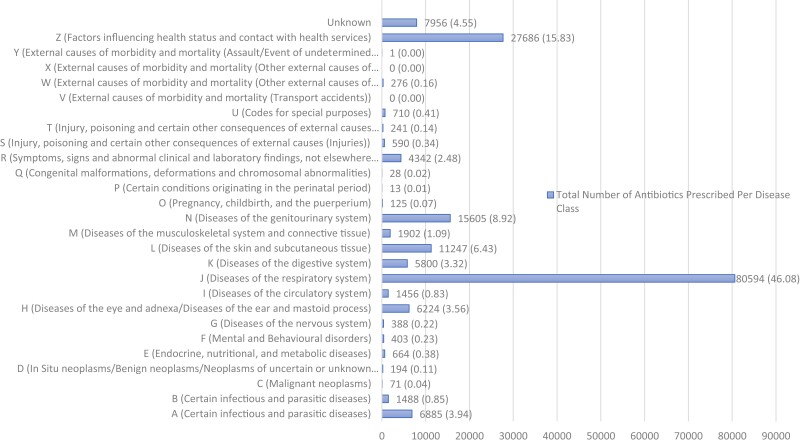
Diseases of the respiratory system (J00–J99) generated the highest number of prescriptions, making up 46.1% (80 594) of all diagnoses. Factors influencing health status and contact with health services (Z00–Z99) were the diagnoses that generated the second highest antibiotic prescriptions, making up 15.8% (27 686) of all diagnoses. However, 84.0% (23 263) of the diagnoses in this class were Z76.9, i.e. the code recorded for claims in which diagnoses were unknown. There were also ICD-10 codes, U98.0 and U98.1, described as ‘non-disclosure’ and ‘service provider refusal to disclose clinical information’, respectively. These codes, together with the Z76.9 codes made up 13.7% (23 973) of all diagnoses, i.e. 13.7% of all encounters had unspecified diagnoses. The disease classes with the third and fourth highest number of prescriptions were diseases of the genitourinary system (N00–N99) and diseases of the skin and subcutaneous tissue (L00–L99), respectively, and made up 8.9% (15 605) and 6.4% (11 247) of all diagnoses (Figure 2).
Figure 2.
Number of antibiotics prescribed per disease class (percentages in parenthesis). ‘Unknown’ diagnosis class represents encounters for which multiple diagnoses (from different classes) were recorded per appropriateness tier and, therefore, diagnoses could not be categorized into any specific class. (A–B) Certain infectious and parasitic diseases; (C) Malignant neoplasms; (D) In Situ neoplasms/Benign neoplasms/Neoplasms of uncertain or unknown behaviour/Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism; (E) Endocrine, nutritional, and metabolic diseases; (F) Mental and Behavioural disorders; (G) Diseases of the nervous system; (H) Diseases of the eye and adnexa/Diseases of the ear and mastoid process; (I) Diseases of the circulatory system; (J) Diseases of the respiratory system; (K) Diseases of the digestive system; (L) Diseases of the skin and subcutaneous tissue; (M) Diseases of the musculoskeletal system and connective tissue; (N) Diseases of the genitourinary system; (O) Pregnancy, childbirth, and the puerperium; (P) Certain conditions originating in the perinatal period; (Q) Congenital malformations, deformations and chromosomal abnormalities; (R) Symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified; (S) Injury, poisoning and certain other consequences of external causes (Injuries); (T) Injury, poisoning and certain other consequences of external causes (Injuries/Effects of foreign body entering through natural orifice/Burns and corrosions/Frostbite, et cetera); (U) Codes for special purposes; (V) External causes of morbidity and mortality (Transport accidents); (W) External causes of morbidity and mortality (Other external causes of accidental injury); (X) External causes of morbidity and mortality (Other external causes of accidental injury/Intentional self-harm/Assault); (Y) External causes of morbidity and mortality (Assault/Event of undetermined intent/Legal intervention and operations of war/Complications of medical and surgical care, et cetera); (Z) Factors influencing health status and contact with health services.
Acute upper respiratory infections of multiple sites and unspecified (J06.8 and J06.9) were the diseases that generated the highest number of prescriptions and made up 13.7% (24 030) of all diagnoses. Acute sinusitis (J01), acute pharyngitis (J02) and acute tonsilitis (J03) together made up 16.3% (28 492) of all diagnoses, while acute bronchitis (J20) and bronchitis, unspecified (J40) together made up 5.6% (9784) of all diagnoses. Cystitis (N30) and urinary tract infection (UTI), unspecified (N39.0) together made up 7.0% (12 216) of all diagnoses, while impetigo (L01), cutaneous abscess (L02) and cellulitis (L03) together made up 4.5% (7874) of all diagnoses. Gastroenteritis and colitis also commonly generated antibiotic prescriptions and made up 2.5% (4359) of all diagnoses. Details of prescribing stratified by age groups are provided in Tables S2–S4.
Appropriateness of antibiotic prescriptions based on indications
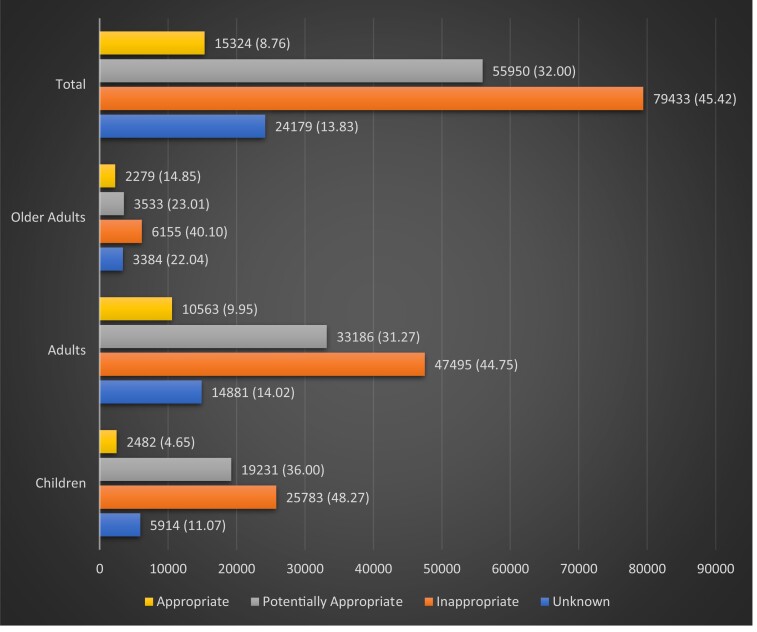
Only 8.8% (15 324) of all the prescriptions were appropriate; while 32.0% (55 950) were potentially appropriate; 45.4% (79 433) were inappropriate, and 13.8% (24 182) could not be assessed for appropriateness due to a lack of specified diagnosis code or because they contained unlisted codes without disease description or with unclear descriptions (Figure 3).
Figure 3.
Number of antibiotics prescribed per appropriateness (based on indication) tier (percentages in parentheses).
Appropriateness of antibiotic selection, dosage, and duration of treatment
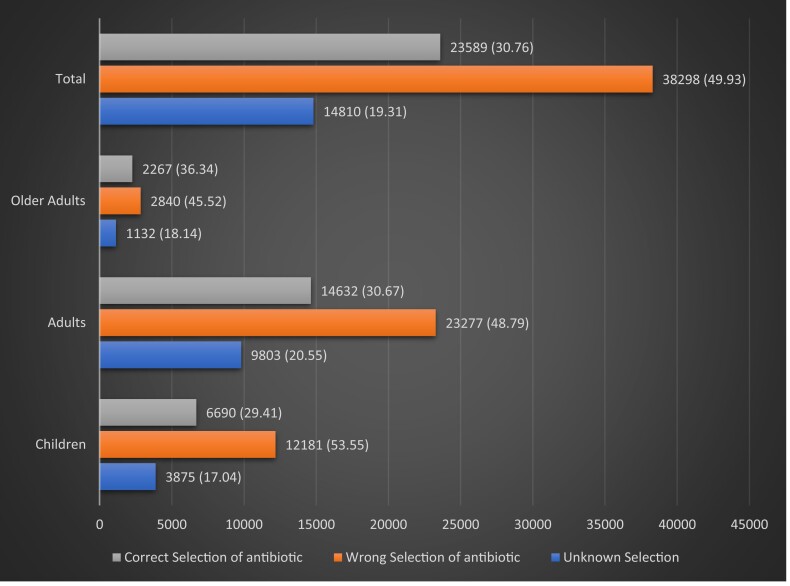
A total of 71 274 (40.8%) patients had appropriate or potentially appropriate prescriptions based on indication, for which a total of 76 697 individual antibiotics were prescribed. Of these antibiotics, 30.8% (23 589) were correct selections, 49.9% (38 298) were incorrect selections and 19.3% (14 810) could not be assessed (Figure 4).
Figure 4.
Number of prescriptions with correct, wrong or unknown antibiotic selections (percentages are in parentheses).
For adults, of all the appropriately and potentially appropriately prescribed antibiotics, 14 632 were correct choices, of which 57.7% (8445) were prescribed at the correct doses, 27.4% (4009) were prescribed with the wrong doses and 14.9% (2178) could not be assessed.
Of the antibiotics prescribed with the correct dose, 76.7% (6479) were prescribed with the correct dosage frequency and duration of treatment, 20.6% (1737) were prescribed with the wrong dosing frequency and/or duration of treatment, while 2.7% (229) could not be assessed.
Discussion
This was a nationwide study that assessed 174 889 patient encounters with GPs in the private primary healthcare sector that led to antibiotic prescription. Amoxicillin/clavulanic acid was markedly the most frequently prescribed antibiotic; this is noteworthy because this broad-spectrum agent was rarely recommended in the treatment guidelines. It was mostly recommended for special cases such as the treatment of UTIs in young children and pregnancy; however, for most diseases where amoxicillin was recommended, amoxicillin/clavulanic acid was frequently prescribed instead. Also, azithromycin (macrolides were mostly recommended for patients with penicillin allergies) was often prescribed for diseases for which amoxicillin was recommended. Another commonly prescribed antibiotic that stood out as a wrong choice was cefpodoxime, which was not recommended for the treatment of any disease, except in the case of pyelonephritis where β-lactams, in general, were recommended. Ciprofloxacin was also commonly prescribed; however, ciprofloxacin was frequently correctly prescribed for the treatment of cystitis and unspecified UTIs, which were common diseases among adults and older adults. Nevertheless, the narrow-spectrum nitrofurantoin should have been more frequently used for these conditions. These four antibiotics alone—amoxicillin/clavulanic acid, azithromycin, ciprofloxacin and cefpodoxime—made up 53.1% (99 963) of all the antibiotics prescribed. Other broad-spectrum antibiotics like levofloxacin and clarithromycin were also frequently prescribed, indicating the extensive prescribing of broad-spectrum agents in the private primary healthcare sector. Apart from being incorrectly selected in several cases, these agents were also commonly prescribed for conditions that never require antibiotics. The results of a 2018 study analysing antibiotic prescribing in English primary care showed that quinolones made up only 2.2%, while combination penicillins (99% of which were amoxicillin/clavulanic acid) made up only 10.4% of all the antibiotics prescribed.3
Diseases of the respiratory system generated the highest number of prescriptions, making up 46.1% of all diagnoses. Of concern was that acute upper respiratory infections of multiple sites and unspecified (J06.8 and J06.9)—infections that never warrant antibiotic prescribing—were the most common infections for which antibiotics were prescribed, making up 13.7% of all diagnoses. Acute bronchitis and bronchitis, unspecified, for which antibiotics are inappropriate, also generated a significant number of antibiotic prescriptions, especially amongst older adults.
Diseases like gastroenteritis and colitis and acute pharyngitis and sinusitis, which are categorized as conditions that sometimes require an antibiotic, were also frequently encountered. These conditions may have also been associated with over-prescribing because many of these diseases are self-limiting and/or of viral origin. For example, group A Streptococcus is the most common cause of bacterial pharyngitis but only accounts for 5%–18% and 20%–37% of all pharyngitis cases in adults and children, respectively,11,31 indicating that over 60% of pharyngitis infections are of viral origin. The scope of this study, however, did not cover the proportion of encounters for diagnoses that sometimes require a prescription, but for which antibiotics were not prescribed. It is, therefore, impossible to estimate the level of inappropriate prescribing that may have occurred for these diagnoses. The use of point-of-care tests to distinguish between viral and bacterial infections is thus crucial.32
As many as 45.4% of the antibiotic prescriptions were inappropriate, i.e. the prescriptions were for diagnoses that never require antibiotics, and multiple antibiotics were also prescribed for these diagnoses. Approximately 40% (4958 of 12 465) of all encounters with multiple antibiotics prescribed had prescriptions for diagnoses that never warrant antibiotics. In some of these cases, as many as four individual antibiotics were prescribed, revealing a high proportion of inappropriate prescribing.
A US study7 in 2019 assessing the appropriateness of outpatient prescribing among privately insured patients revealed that 23.2% of all prescriptions were inappropriate, 12.8% were appropriate, 35.5% were potentially appropriate and 28.5% could not be assessed. A 2021 Chinese study20 also assessing the appropriateness of antibiotic prescribing among outpatients revealed a higher level of inappropriate prescribing compared with this study. The study identified inappropriate prescribing in 51.4% of all prescriptions, while 15.3% and 28.5% of all prescriptions were appropriate and potentially appropriate, respectively. In only 4.8% of encounters could antibiotic prescribing not be assessed—a possible reason why higher amounts of inappropriateness could be identified compared with this study.
Where antibiotics were appropriately prescribed in terms of indication, approximately 50% of the choices were incorrect. This occurred though there was leniency in deciding that an antibiotic choice was correct. For instance, for UTIs, unspecified, amoxicillin/clavulanic acid was the drug of choice for only pregnant women and young children. Since it was impossible to tell which patients were pregnant, for all unspecified UTI cases, amoxicillin/clavulanic acid was considered a correct choice. Therefore, appropriateness based on antibiotic selection was likely overestimated.
Inappropriate prescribing in terms of indication was most prominent in children (48.3% of all prescriptions) compared with adults (44.8%) and older adults (40.1%). Incorrect antibiotic choices were also the most evident in children (53.6% of all antibiotics prescribed) compared with adults (48.8%) and older adults (45.5%).
For adults where antibiotic selections were correct, only 57.7% of the doses selected were correct, while 76.7% of all antibiotics with the correct dose had the right dosage frequency and duration of treatment. This is of concern as under-dosing and over-dosing, even when the antibiotic choices are correct, puts patients at risk of treatment failure and at risk of developing adverse effects, respectively. Under-dosing, like over-dosing, is also a cause of antibiotic resistance because apart from selecting for resistant strains,33 treatment may either need to be restarted or alternative antibiotic treatment may be required, and all antibiotic use, appropriate or not, contributes to the development of resistance.34 Re-treatment should, therefore, be avoided by ensuring initial treatments are appropriate. A 2018 study in South Africa21 assessing adherence to guidelines in public primary care revealed that of all cases for which an antibiotic was prescribed, 17.1% were prescribed unnecessarily. Wrong antibiotic selections were made in 11.5% of records, while 12.9% and 9.5% of prescriptions had incorrect doses and durations of therapy, respectively. The most frequently prescribed antibiotics were amoxicillin (37.9%) and flucloxacillin (12.7%). This is a better performance than what was obtained in this private sector assessment. However, the study was limited to Cape Town.
There were a few limitations to this study. Like previous studies,7,11,20 the appropriateness of antibiotic prescribing in this study relied on recorded diagnosis codes and not the examination of patient files. It is, therefore, possible that wrong codes were recorded for some diagnoses. For example, UTIs were the recorded diagnoses for several young adult males, where this is rare as the reported incidence is 5–8 per 10 000 per year in men aged <50 years.35 Also, some of these encounters coded as UTIs had the typical treatments for sexually transmitted diseases. There is, therefore, a likelihood that some sexually transmitted diseases were coded as UTIs. Further, a few antibiotics were prescribed for conditions like hypertension and diabetes. This may indicate that the primary diagnoses were these chronic conditions but the secondary diagnosis for which the antibiotics may have been prescribed were likely omitted. Prescribers may also code diagnoses that do not require antibiotics with codes for diseases that require antibiotics to justify the prescribing of antibiotics.7,28,36 Further, inappropriate use of antibiotics could be because of wrong indications.37
In total, 13.7% of all diagnoses could not be assessed for appropriateness of antibiotic prescribing due to the lack of specific diagnosis codes. Further, where guideline recommendations were ambiguous or lacking, assessments for appropriateness in terms of antibiotic selection and dosage could not be carried out. In this case, a similar study used expert opinions for the assessments of appropriateness.38 This was not done in this study.
Correctness of selections and dosages were based on only the recommendations of the South African STGs/EMLs and the SAASP Pocket Guidelines for Antibiotic Prescribing in Adults. However, some prescribers may have chosen to use other guidelines, and clinical cure may have been achieved using some antibiotics considered by this study as wrong choices. Some antibiotics that were not guideline recommendations could also have been selected based on the results of laboratory tests and were, therefore, appropriate.
This study revealed that a substantial number of antibiotics are inappropriately prescribed by GPs in the private primary healthcare sector in South Africa, necessitating urgent stewardship interventions to sustain the efficacy of existing antibiotics, contain antibiotic resistance, improve the standard of care and reduce unnecessary healthcare expenditure by health insurers.
Supplementary Material
Acknowledgements
We are grateful to the members of staff of the health insurance company who provided the database. The data were obtained from Discovery Health Medical Scheme (DHMS), registration number 1125, and with the support and assistance of Discovery Health (Pty) Ltd (DH), an accredited administrator and managed care provider for medical schemes. DHMS and DH had no influence on the methodology used, analysis completed, and conclusions drawn in this research, and this research is entirely independent from DHMS and DH.
Contributor Information
Mobolaji Eniola Alabi, Antimicrobial Research Unit, College of Health Sciences, University of Kwa-Zulu, Natal, Durban, South Africa.
Sabiha Yusuf Essack, Antimicrobial Research Unit, College of Health Sciences, University of Kwa-Zulu, Natal, Durban, South Africa.
Funding
This study was funded by the South African Medical Research Council and Swedish FORTE as well as the College of Health Sciences, University of KwaZulu-Natal. All opinions, findings, conclusions, or recommendations expressed in the article are those of the authors and do not reflect the views of the organizations that funded the research. The funders also played no role in designing the study, nor in the decision to submit the work for publication.
Transparency declarations
S.Y.E. is the chairperson of the Global Respiratory Infection Partnership and a member of the Global Hygiene Council, both sponsored by unrestricted educational grants from Reckitt and Benckiser (Ltd.) UK. M.E.A has none to declare.
Supplementary data
Tables S1 –S4 are available as Supplementary data at JAC Online.
References
- 1. Gebeyehu E, Bantie L, Azage M. Inappropriate use of antibiotics and its associated factors among urban and rural communities of Bahir Dar City administration, Northwest Ethiopia. PLOS One 2015; 10: e0138179. 10.1371/journal.pone.0138179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Godman B, Haque M, McKimm Jet al. . Ongoing strategies to improve the management of upper respiratory tract infections and reduce inappropriate antibiotic use particularly among lower and middle-income countries: findings and implications for the future. Curr Med Res Opin 2020; 36: 301–27. 10.1080/03007995.2019.1700947 [DOI] [PubMed] [Google Scholar]
- 3. Dolk FCK, Pouwels KB, Smith DRMet al. . Antibiotics in primary care in England: which antibiotics are prescribed and for which conditions? J Antimicrob Chemother 2018; 73Suppl 2: ii2–10. 10.1093/jac/dkx504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hicks LA, Bartoces MG, Roberts RMet al. . US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clinical Infect Dis 2015; 60: 1308–16. [DOI] [PubMed] [Google Scholar]
- 5. Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 2010. 74: 417–33. 10.1128/MMBR.00016-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gualano MR, Gili R, Scaioli Get al. . General population’s knowledge and attitudes about antibiotics: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf 2015; 24: 2–10. 10.1002/pds.3716 [DOI] [PubMed] [Google Scholar]
- 7. Chua K-P, Fischer MA, Linder JA. Appropriateness of outpatient antibiotic prescribing among privately insured US patients: ICD-10-CM based cross sectional study. BMJ 2019; 364: k5092. 10.1136/bmj.k5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang CN, Huttner BD, Magrini Net al. . Pediatric antibiotic prescribing in China according to the 2019 World Health Organization Access, Watch, and Reserve (AWaRe) antibiotic categories. J Pediatr 2020; 220: 125–31. [DOI] [PubMed] [Google Scholar]
- 9. Suda KJ, Hicks LA, Roberts RMet al. . Trends and seasonal variation in outpatient antibiotic prescription rates in the United States, 2006 to 2010. Antimicrob Agents Chemother 2014; 58: 2763–66. 10.1128/AAC.02239-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T 2015; 40: 277–83. [PMC free article] [PubMed] [Google Scholar]
- 11. Fleming-Dutra KE, Hersh AL, Shapiro DJet al. . Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA 2016; 315: 1864–73. 10.1001/jama.2016.4151 [DOI] [PubMed] [Google Scholar]
- 12. Fleming-Dutra KE, Bartoces M, Roberts RMet al. . Characteristics of primary care physicians associated with high outpatient antibiotic prescribing volume. Open Forum Infect Dis 2018; 5: ofx279. 10.1093/ofid/ofx279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. King LM, Bartoces M, Fleming-Dutra KEet al. . Changes in US outpatient antibiotic prescriptions from 2011–2016. Clin Infect Dis 2020; 70: 370–77. 10.1093/cid/ciz225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hersh AL, Fleming-Dutra KE, Shapiro DJet al. . Frequency of first-line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med 2016; 176: 1870–2. 10.1001/jamainternmed.2016.6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Katende-Kyenda NL, Lubbe MS, Serfontein JHPet al. . Antimicrobial prescribing patterns in a group of private primary health care clinics in South Africa. Health SA Gesondheid 2007; 12: 21–9. 10.4102/hsag.v12i1.240 [DOI] [Google Scholar]
- 16. Duffy E, Ritchie S, Metcalfe Bet al. . Antibacterials dispensed in the community comprise 85%-95% of total human antibacterial consumption. J Clin Pharm Ther 2018; 43: 59–64. 10.1111/jcpt.12610 [DOI] [PubMed] [Google Scholar]
- 17. Siddiqi S, Hamid S, Rafique Get al. . Prescription practices of public and private health care providers in Attock District of Pakistan. Int J Health Plann Manage 2002; 17: 23–40. 10.1002/hpm.650 [DOI] [PubMed] [Google Scholar]
- 18. Rahman N A, Teng CL, Sivasampu S. Antibiotic prescribing in public and private practice: a cross-sectional study in primary care clinics in Malaysia. BMC Infect Dis 2016; 16: 208. 10.1186/s12879-016-1530-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Farooqui HH, Mehta A, Selvaraj S. Outpatient antibiotic prescription rate and pattern in the private sector in India: evidence from medical audit data. PLoS One 2019; 14: e0224848. 10.1371/journal.pone.0224848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao H, Wei L, Li Het al. . Appropriateness of antibiotic prescriptions in ambulatory care in China: a nationwide descriptive database study. Lancet Infect Dis 2021; 21: 847–57. 10.1016/S1473-3099(20)30596-X [DOI] [PubMed] [Google Scholar]
- 21. Gasson J, Blockman M, Willems B. Antibiotic prescribing practice and adherence to guidelines in primary care in the Cape Town Metro District, South Africa. S Afr Med J 2018; 108: 304–10. 10.7196/SAMJ.2017.v108i4.12564 [DOI] [PubMed] [Google Scholar]
- 22. Chunnilall D, Peer AK, Naidoo Iet al. . An evaluation of antibiotic prescribing patterns in adult intensive care units in a private hospital in KwaZulu-Natal. S Afr J Infect Dis 2015; 30: 17–22. https://hdl.handle.net/10520/EJC169849 [Google Scholar]
- 23. Chetty S, Reddy M, Ramsamy Yet al. . Antimicrobial stewardship in South Africa: a scoping review of the published literature. JAC Antimicrob Resist 2019; 1: dlz060. 10.1093/jacamr/dlz060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. BusinessTech . These Are the Biggest Medical Aid Schemes in South Africa. 2019. https://businesstech.co.za/news/lifestyle/346434/these-are-the-biggest-medical-aid-schemes-in-south-africa/. [Google Scholar]
- 25. Havers FP, Hicks LA, Chung JRet al. . Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Netw Open 2018; 1: e180243. 10.1001/jamanetworkopen.2018.0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Systems for Improved Access to Pharmaceuticals and Services (SIAPS). How to Investigate Antimicrobial Use in Hospitals: Selected Indicators. 2012. https://siapsprogram.org/wp-content/uploads/2012/12/12-096-AMR-Hospital-Indicator-Manual.English.final-11.13.12.pdf. [Google Scholar]
- 27. WHO . ICD-10 Version:2016. 2016. https://icd.who.int/browse10/2016/en. [Google Scholar]
- 28. Olesen SW, Barnett ML, MacFadden DRet al. . Trends in outpatient antibiotic use and prescribing practice among US older adults, 2011-15: observational study. BMJ 2018; 362: k3155. 10.1136/bmj.k3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Department of Health, South Africa . Standard Treatment Guidelines and Essential Medicines List for South Africa, Primary Healthcare Level, 2018 edition. http://www.kznhealth.gov.za/pharmacy/phc-stg-2018v1.pdf.
- 30. Wasserman S, Boyles T, Mendelson M. A Pocket Guide to Antibiotic Prescribing for Adults in South Africa. 2015. http://www.medicine.uct.ac.za/sites/default/files/image_tool/images/55/SAASP%20Antibiotic%20Gudidelines_2015.pdf.
- 31. Dobson EL, Klepser ME, Pogue JMet al. . Outpatient antibiotic stewardship: interventions and opportunities. J Amer Pharm Assoc (2003) 2017; 57: 464–73. 10.1016/j.japh.2017.03.014 [DOI] [PubMed] [Google Scholar]
- 32. Mendelson M, Rottingen JA, Gopinathan Uet al. . Maximising access to achieve appropriate human antimicrobial use in low-income and middle-income countries. Lancet 2016; 387: 188–98. 10.1016/S0140-6736(15)00547-4 [DOI] [PubMed] [Google Scholar]
- 33. Huttner A, Harbarth S, Carlet JAet al. . Antimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob Resist Infect Control 2013; 2: 31. 10.1186/2047-2994-2-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. English BK, Gaur AH. The use and abuse of antibiotics and the development of antibiotic resistance. Adv Exp Med Biol 2010; 659: 73–82. 10.1007/978-1-4419-0981-7_6 [DOI] [PubMed] [Google Scholar]
- 35. Seminerio JL, Aggarwal G, Sweetser S. 26-Year-old man with recurrent urinary tract infections. Mayo Clin Proc 2011; 86: 557–60. 10.4065/mcp.2010.0600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pinder R, Berry D, Sallis Aet al. . Antibiotic Prescribing and Behaviour Change in Healthcare Settings: Literature Review and Behavioural Analysis. 2015. https://spiral.imperial.ac.uk/bitstream/10044/1/22194/2/Behaviour_Change_for_Antibiotic_Prescribing_-_FINAL.pdf. [Google Scholar]
- 37. Al-Sayyed B, Le J, Al-Tabbaa MMet al. . Uncomplicated urinary tract infection in ambulatory primary care pediatrics: are we using antibiotics appropriately? J Pediatr Pharmacol Ther 2019; 24: 39–44. 10.5863/1551-6776-24.1.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith DR, Dolk FC, Pouwels KBet al. . Defining the appropriateness and inappropriateness of antibiotic prescribing in primary care. J Antimicrob Chemother 2018; 73Suppl 2: ii11–18. 10.1093/jac/dkx503 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.