Background:
The use of extracorporeal cardiopulmonary resuscitation (ECPR) has improved survival in patients with cardiac arrest; however, factors predicting survival remain poorly characterized. A systematic review and meta-analysis was conducted to examine the predictors of survival of ECPR in pediatric patients.
Methods:
We searched EMBASE, PubMed, SCOPUS, and the Cochrane Library from 2010 to 2021 for pediatric ECPR studies comparing survivors and non-survivors. Thirty outcomes were analyzed and classified into 5 categories: demographics, pre-ECPR laboratory measurements, pre-ECPR co-morbidities, intra-ECPR characteristics, and post-ECPR complications.
Results:
Thirty studies (n = 3794) were included. Pooled survival to hospital discharge (SHD) was 44% (95% CI: 40%–47%, I2 = 67%). Significant predictors of survival for pediatric ECPR include the pre-ECPR lab measurements of PaO2, pH, lactate, PaCO2, and creatinine, pre-ECPR comorbidities of single ventricle (SV) physiology, renal failure, sepsis, ECPR characteristics of extracorporeal membrane oxygenation (ECMO) duration, ECMO flow rate at 24 hours, cardiopulmonary resuscitation (CPR) duration, shockable rhythm, intra-ECPR neurological complications, and post-ECPR complications of pulmonary hemorrhage, renal failure, and sepsis.
Conclusion:
Prior to ECPR initiation, increased CPR duration and lactate levels had among the highest associations with mortality, followed by pH. After ECPR initiation, pulmonary hemorrhage and neurological complications were most predictive for survival. Clinicians should focus on these factors to better inform potential prognosis of patients, advise appropriate patient selection, and improve ECPR program effectiveness.
Keywords: cardiac arrest, extracorporeal cardiopulmonary resuscitation, extracorporeal membrane oxygenation, pediatric
Key Points:
Predictors of survival in pediatric ECPR are poorly understood, and no randomized controlled trials exist on this topic.
This meta-analysis is the largest study to date examining these factors, investigating 30 possible predictors and including 30 studies (n = 3794).
This study found that the factors most associated with mortality prior to ECPR initiation were increased CPR duration, decreased lactate levels, and decreased pH.
1. Introduction
While cardiopulmonary resuscitation (CPR) has been shown to dramatically improve survival rates for pediatric in-hospital cardiac arrest patients, overall survival to hospital discharge (SHD) for these patients after prolonged CPR remains low at approximately 28%.[1–3] The adoption of extracorporeal membrane oxygenation (ECMO) since 1976 has greatly increased survival rates for this patient population.[4–7]
The use of ECMO for cardiopulmonary resuscitation (ECPR) has been increasing rapidly in pediatric and adult populations.[8,9] Recent analysis of the extracorporeal life support organization database (ELSO) estimated SHD rates for pediatric ECPR patients to be 42%.[9] Lasa et al demonstrated not only increased survival but also increased favorable neurological outcomes in ECPR compared to conventional CPR.[10]
Variability in survival outcomes and limited data on associated risks including severe neurological, renal, and cardiac complications have led to a lack of consensus on implementation guidelines and patient selection.[7,11–13] These factors place the decision to cannulate onto the provider’s clinical judgement.
This variability, coupled with rapidly improving technological developments and increased adoption of ECPR within the last 10 years, has made a meta-analysis covering the most recent literature a necessity.[14,15] This literature review and meta-analysis analyzed predictors of survival in the most recent studies on pediatric ECPR to identify risk factors for mortality, allowing providers to make more informed decisions on patient selection and improve ECPR program effectiveness.
2. Methods
2.1. Data source and search strategy
Database searches were performed by 2 independent researchers in EMBASE, PubMed, SCOPUS, and the Cochrane Library with individual search strategies for each database (Table S1, Supplemental Digital Content, http://links.lww.com/MD/H424). Text-word searches and standardized medical subject heading were included in the search terms. References cited in eligible reviews were also examined. Studies between January 1, 2010 and February 5th, 2021 were searched without language restrictions. No methodology filters or document filters were used.
2.2. Study eligibility
Studies were included if humans enrolled were under 21 years of age, ECPR was performed during cardiac arrest, stratification between ECPR survivors and non survivors was present, data was present with a minimum of 2 metrics reported with measures of central tendency and variability, and the publication date was between 2010 and February 2021.
Animal trials, conference abstracts, reviews, trial protocols, simulations, editorials, letters, comments, practice guidelines, book chapters, and duplicate studies were excluded. Any studies including fewer than 10 patients who underwent ECPR, outcomes that did not include SHD, and studies with incomplete data were excluded.
2.3. Review process and data collection
Studies were reviewed by 2 independent authors. Abstracts agreed by both reviewers were identified for detailed review of the full manuscript. Duplicate publications were identified through comparison of reports for author names, enrollment date, setting, intervention, participant number, or baseline data. Disagreements between authors over the inclusion or exclusion of studies were resolved independently by a third author. Articles were identified and data was extracted from included studies. Methodological quality was reviewed utilizing the Newcastle–Ottawa Quality Assessment scale for case-control studies or cohort studies.[16]
All outcomes examined in this meta-analysis were documented in 2 or more studies. As such, 30 predictors of survival were examined, classified into 5 main categories: demographics, pre-ECPR laboratory measurements, pre-ECPR co-morbidities, intra-ECPR characteristics, and post-ECPR complications. Demographic information included age, gender, race, and weight. Laboratory measurements included baseline creatinine, bicarbonate, lactate, PaCO2, PaO2, and arterial pH. Preexisting co-morbidities studied were single ventricle (SV) physiology, primary myocardial disease, pulmonary hypertension, renal failure, and sepsis. Intra-ECPR characteristics included details on the ECPR treatment itself, specifically CPR duration, ECMO duration, ECMO flow rates at 4 hours and 24 hours, cannulation sites, shockable rhythm, and neurological complications. Post-ECPR complications comprised of pulmonary hemorrhage, renal failure, and sepsis. Survival rates across studies were additionally analyzed.
2.4. Statistical analysis
Data was analyzed using statistical software R 4.1.3. Studies that reported median and interquartile range or median and range were converted into mean and standard deviation using the methodology in Wan et al 2014.[17] Heterogeneity of pooled data was calculated using I2.[18,19] Random-effects models were used due to the heterogeneity of study protocols unless included studies numbered fewer than 5, as recommended in the Cochrane Handbook.[20,21] Pooled risk ratios (RR) and standardized mean differences (SMD) were calculated for binary and continuous data respectively. Publication bias was assessed for all outcomes where included studies numbered 10 or more using Egger’s tests and funnel plots.[22–24] All results were considered statistically significant if P < .05.
Using the Grading of Recommendations, Assessment, Development, and Evaluation approach, we evaluated the level of certainty in the data abstracted from the included studies. All predictors of survival were evaluated on risk of bias, inconsistency, indirectness, imprecision, and publication bias, and all were found to be low quality (Table S2, Supplemental Digital Content, http://links.lww.com/MD/H425).
3. Results
3.1. Study selection
The preferred reporting items for systematic reviews and meta-analyses flow diagram & study selection for this systematic review and meta-analysis is depicted in Figure S1, Supplemental Digital Content, http://links.lww.com/MD/H430. The systematic search of articles identified 12,072 results. After title and abstract screening, 124 full-text articles were identified as potentially relevant. Thirty studies were included after full-text review. No randomized controlled trial was found on the subject. Table 1 summarizes the key characteristics of each study included in the meta-analysis.
Table 1.
Characteristics of included studies.
| Author and yr | Region | Yrs | N | Female | Survival (%) | Setting | Type |
|---|---|---|---|---|---|---|---|
| Alsoufi, B., 2014[25] | Saudi Arabia | 2007-2012 | 39 | 14 | 41 | IHCA | R |
| Anton-Martin, P., 2020[26] | USA | 2000-2013 | 73 | 26 | 44 | IHCA | R |
| Bembea, M., 2019[27] | USA | 2000-2014 | 593 | 242 | 41 | IHCA | R |
| Beshish, A., 2018[28] | USA | 2005-2015 | 80 | 40 | 48 | IHCA | R |
| Brunner, A., 2016[29] | Switzerland | 2008-2014 | 19 | NR | 16 | Both | R |
| Chen, G., 2018[30] | Asia Pacific | 1999-2016 | 321 | 142 | 51 | IHCA | R |
| Chrysostomou, C., 2013[31] | USA | 2006-2010 | 40 | 15 | 75 | NR | R |
| De Mul, A., 2019[32] | Switzerland | 2008-2016 | 55 | 20 | 25 | Both | R |
| Erek, E., 2017[33] | Turkey | 2010-2014 | 25 | NR | 20 | IHCA | R |
| Guo, Z., 2019[34] | China | 2017 | 11 | 3 | 36 | IHCA | R |
| Huang, S., 2012[35] | Taiwan | 1999-2009 | 54 | 17 | 46 | IHCA | R |
| Jolley, M., 2014[36] | Multi | 1998-2013 | 293 | 121 | 36 | IHCA | R |
| Kane, D., 2010[37] | USA | 1995-2009 | 172 | 77 | 51 | IHCA | R |
| KendIrlI, T., 2020[38] | Turkey | 2014-2017 | 15 | 8 | 33 | IHCA | R |
| Kramer, P., 2020[39] | Germany | 2005-2016 | 72 | 34 | 36 | Both | R |
| McMullan, D., 2014[40] | Multi | 1998-2010 | 641 | 257 | 39 | NR | R |
| Melvan, J., 2020[41] | USA | 2002-2017 | 184 | 92 | 43 | NR | R |
| Philip, J., 2014[42] | USA | 2005-2012 | 59 | NR | 46 | IHCA | R |
| Polimenakos, A., 2017[43] | USA | 2007-2011 | 21 | NR | 62 | IHCA | R |
| Raymond, T., 2010[44] | USA | 2000-2007 | 199 | 75 | 44 | IHCA | R |
| Shakoor, A., 2019[45] | USA | 2010-2017 | 70 | 28 | 54 | IHCA | R |
| Shin, H., 2016[46] | SK | 2013-2016 | 12 | 6 | 33 | NR | R |
| Sivarajan, V., 2011[47] | Australia | 2002-2006 | 37 | 18 | 38 | NR | R |
| Topjian, A., 2019[48] | Multi | 2009-2015 | 157 | 57 | 46 | IHCA | R |
| Torres-Andres, F., 2018[49] | USA | 2007-2015 | 58 | 23 | 65.5 | Both | R |
| Tsukahara, K., 2014[50] | Japan | 2003-2012 | 21 | 8 | 14 | Both | R |
| Walter, E., 2011[51] | Germany | 1992-2008 | 42 | 19 | 40 | IHCA | R |
| Wolf, M., 2012[52] | USA | 2002-2011 | 90 | NR | 56 | IHCA | R |
| Yates, A., 2019[53] | USA | 2013-2016 | 33 | 14 | 39 | NR | P |
| Zeybek, C., 2017[54] | Turkey | 2009-2016 | 34 | NR | 33 | IHCA | R |
NR = not reported, P = prospective, R = retrospective, SK = South Korea.
3.2. Study characteristics
A total number of 3794 participants from 30 studies were included for analysis in this meta-analysis. The mean age of participants was 397 days, with 42% of participants being female. All studies were published between the years 2010 and 2021.
3.3. Risk of bias
Of the 30 studies included, 26 were assessed to be of good quality, while 4 were assessed to be of poor quality.[16] A summary of the risk of biases in each study is provided in Table S3, Supplemental Digital Content, http://links.lww.com/MD/H426. Publication bias was assessed in all outcomes that included 10 or more studies using Egger’s test and found to be non-significant in all outcomes except for duration of CPR. Funnel plots are shown in Figure S2, Supplemental Digital Content, http://links.lww.com/MD/H431.
3.4. Outcomes
Pooled SHD was 44% (CI 95% = 40%–47%) (Fig. S3A, Supplemental Digital Content, http://links.lww.com/MD/H432). Chrysostomou et al 2013 was identified as a potential outlier using leave-one-out sensitivity analysis with the highest survival rate of 75%.[31] Studies were additionally evaluated for publication bias and visual inspection of the associated funnel plot found no clear asymmetry (Fig. S3B, Supplemental Digital Content, http://links.lww.com/MD/H432). Egger’s test found nonsignificant heterogeneity (P > .11). Meta-regression models did not find any statistically significant association between SHD and year of publication (Table S4, Supplemental Digital Content, http://links.lww.com/MD/H427). Meta-regression also found no significant association between proportion of survivors and number of patients in study, which was used as a surrogate for institutional experience.
3.5. Patient demographics
No significant difference was found between the 1138 survivors and the 1453 non-survivors in terms of age (SMD = 0.04 [-0.14 to 0.21], I2 = 72%, P = .66) or 1018 survivors and the 1343 non-survivors in terms of weight (SMD = 0.12 [-0.06 to 0.30], I2 = 74%, P = .18). Gender was additionally found to be not significantly different between the 1108 survivors than the 1406 non-survivors (RR = 0.93 [0.82–1.06], I2 = 22%, P = .28). Of the 4 races analyzed (White, Black, Hispanic, or Asian), none were significantly associated with an increased risk of mortality (Table 2).
Table 2.
Summary of metrics compared between pediatric survivors and non-survivors of ECPR.
| Metric | Studies | Population | Survivors | Non-survivors | I 2 | P | Risk ratio [95% CI] | Hedge’s g [95% CI] |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age | 24 | 2610 | 343 ± 1045 | 440 ± 1372 | 72% | .66 | 0.04 [-0.14 - 0.21] | |
| Gender-Male | 18 | 2514 | 55% | 59% | 22% | .28 | 0.93 [0.82 - 1.06] | |
| Race-Asian | 5 | 1408 | 11% | 13% | 0% | .43 | 0.73 [0.27 - 1.95] | |
| Race-Black | 5 | 1286 | 15% | 16% | 31% | .78 | 1.05 [0.66 - 1.68] | |
| Race-Hispanic | 4 | 1087 | 14% | 15% | 0% | .50 | 0.9 [0.68 - 1.21] | |
| Race-White | 5 | 1286 | 61% | 56% | 14% | .54 | 1.05 [0.87 - 1.26] | |
| Weight | 19 | 2380 | 6.3 ± 8.4 | 6.3 ± 9.8 | 74% | .18 | 0.12 [-0.06 - 0.3] | |
| Pre-ECPR lab measurements | ||||||||
| Creatinine | 4 | 193 | 0.83 ± 0.56 | 1.1 ± 0.7 | 21% | <.01 | -0.41 [-0.7 - -0.12] | |
| Bicarbonate | 5 | 1328 | 19.8 ± 8.6 | 20 ± 9.4 | 18% | .68 | 0.03 [-0.18 - 0.25] | |
| Lactate | 11 | 616 | 6.9 ± 6.1 | 9.6 ± 7.6 | 41% | .02 | -0.36 [-0.64 - -0.07] | |
| PaCO2 | 5 | 842 | 55.7 ± 25.0 | 60.2 ± 33.0 | 0% | .045 | -0.13 [-0.26 - 0] | |
| PaO2 | 4 | 1061 | 44.1 ± 44.4 | 38.4 ± 37.5 | 8% | <.01 | 0.25 [0.13 - 0.38] | |
| pH | 15 | 2005 | 7.2 ± 0.2 | 7.1 ± 0.3 | 0% | <.01 | 0.21 [0.09 - 0.33] | |
| Pre-ecpr co-morbidities | ||||||||
| Single ventricle physiology | 12 | 1390 | 39% | 45% | 47% | .35 | 0.85 [0.58 - 1.23] | |
| Primary myocardial disease | 12 | 1580 | 12% | 7% | 31% | .13 | 1.5 [0.87 - 2.6] | |
| Pulmonary hypertension | 3 | 270 | 3% | 5% | 0% | .22 | 0.44 [0.12 - 1.61] | |
| Renal failure | 3 | 129 | 24% | 46% | 0% | <.01 | 0.47 [0.28 - 0.81] | |
| Sepsis | 3 | 1020 | 3% | 6% | 24% | .04 | 0.52 [0.28 - 0.97] | |
| Intra-ECPR characteristics | ||||||||
| CPR duration | 17 | 1204 | 37.3 ± 25.2 | 47.9 ± 38.3 | 37% | .00 | -0.36 [-0.54 - -0.18] | |
| ECMO duration | 21 | 2330 | 96.9 ± 120.3 | 118.9 ± 115.7 | 30% | .00 | -0.23 [-0.36 - -0.1] | |
| ECMO Flow Rate at 24 h | 3 | 786 | 118.5 ± 49.2 | 130.8 ± 53.9 | 0% | .03 | -0.15 [-0.3 - -0.01] | |
| ECMO Flow Rate at 4 h | 4 | 826 | 119.1 ± 51.1 | 124.3 ± 58.2 | 0% | .64 | -0.03 [-0.17 - 0.11] | |
| Site-femoral | 2 | 143 | 11% | 15% | 0% | .43 | 0.71 [0.31 - 1.65] | |
| Site-neck | 6 | 343 | 40% | 43% | 0% | .37 | 0.9 [0.68 - 1.19] | |
| Site-thorax | 6 | 399 | 54% | 54% | 12% | .47 | 1.09 [0.82 - 1.45] | |
| Shockable rhythm | 6 | 1205 | 17% | 11% | 0% | .01 | 1.51 [1.14 - 1.98] | |
| Neurological complications | 9 | 2320 | 17% | 37% | 31% | <.01 | 0.43 [0.32 - 0.58] | |
| Post-ECPR complications | ||||||||
| Pulmonary hemorrhage | 4 | 1076 | 2% | 7% | 49% | <.01 | 0.34 [0.17–0.69] | |
| Renal failure | 4 | 540 | 22% | 48% | 0% | 0 | 0.47 [0.36 - 0.61] | |
| Sepsis | 4 | 243 | 15% | 26% | 0% | .033 | 0.57 [0.34 - 0.96] | |
ECPR = extracorporeal cardiopulmonary resuscitation.
3.6. Patient baseline laboratory measurements
On average, patients were in a state of mixed respiratory and metabolic acidosis prior to ECPR commencement, with depressed pH, elevated PaCO2, and decreased bicarbonate levels along with elevated lactate levels with hypoxemia (Table 2).
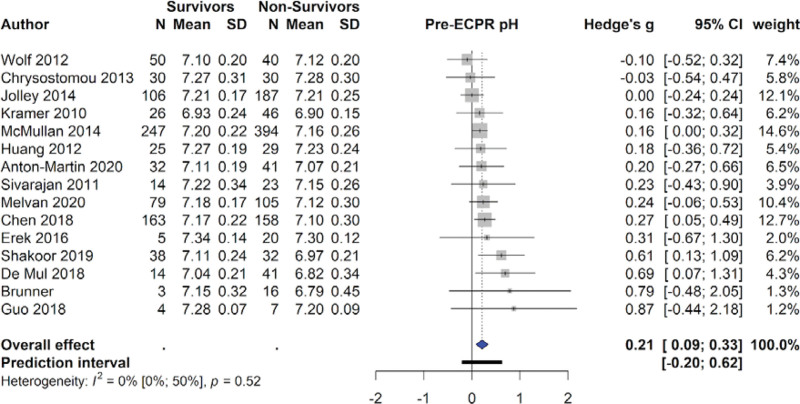
Higher PaO2 levels were predictive of survival (410 survivors & 505 non-survivors, SMD = 0.25 [0.13–0.38], I2 = 8%, P < .01), as were higher pH levels (833 survivors & 1153 non-survivors, SMD = 0.21 [0.09–0.33], I2 = 0%, P < .01). The 291 survivors also had significantly lower lactate levels than the 306 non-survivors (SMD = -0.36 [-0.64 to -0.07], I2 = 46%, P < .001), and significantly lower PaCO2 levels (SMD = -0.13 [-0.26 to 0.004], I2 = 0%, P = .045). However, when leave-one-out sensitivity analysis was conducted, this effect was not robust (Table S5, Supplemental Digital Content, http://links.lww.com/MD/H428). Survivors (n = 90) had significantly lower creatinine levels than non-survivors (n = 103) (SMD = -0.41 [-0.70 to -0.12], I2 = 21%, P < .01) (Table 2). Figure 1 displays the forest plots for pre-ECPR pH.
Figure 1.
Forest plot examining pre-ECPR pH. ECPR = extracorporeal cardiopulmonary resuscitation.
3.7. Patient significant preexisting complications
Renal failure was seen in significantly fewer of the 55 survivors than 74 non-survivors (RR = 0.47 [0.28–0.81], I2 = 0%, P = .01). Pre-ECPR sepsis was also associated with reduced chances of survival (RR = 0.52 [0.28–0.97], I2 = 24%, P = .04) (Table 2).
On the other hand, the odds of primary myocardial disease were not significantly different between the 403 survivors compared to the 508 non-survivors (RR = 1.14 [0.70–1.85], I2 = 0%, P = .60). This pattern was also seen in pulmonary hypertension prior to ECPR (RR = 0.44 [0.12–1.61], I2 = 0%, P = .22). Finally, the odds of SV physiology were not significantly different in the 613 survivors than in the 777 non-survivors (RR = 0.85 [0.58–1.23], I2 = 47%, P = .35) (Table 2).
3.8. Intra-ECPR characteristics
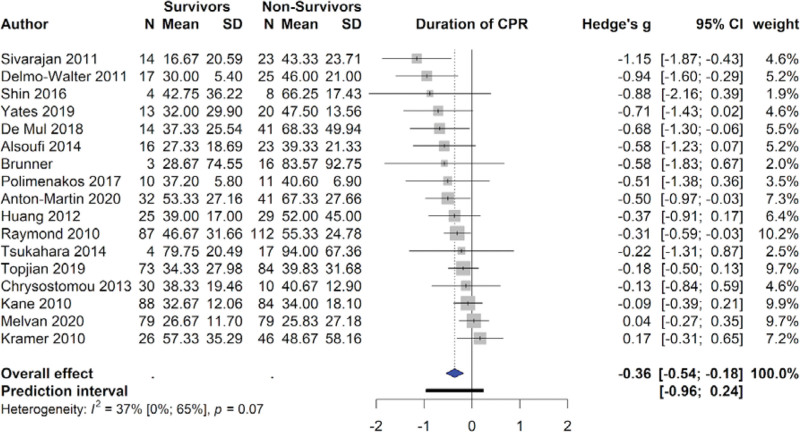
Duration of CPR was negatively associated with survival, with survivors on average receiving 37.3 ± 25.2 minutes and non-survivors receiving 47.9 ± 38.3 minutes of CPR (SMD = -0.36 [-0.54 to -0.18], I2 = 37%, P < .01). Duration of ECMO was also negatively associated with survival, with survivors on average receiving 94.5 ± 117.7 minutes and non-survivors receiving 116.3 ± 115.8 minutes of ECMO (SMD = -0.23 [-0.36 to -0.10], I2 = 30%, P < .01) (Table 2). However, publication bias was detected in the duration of CPR using Egger’s test. Leave-one-out sensitivity analysis was conducted and found the negative significant effect of CPR duration on survival was robust (Table S6, Supplemental Digital Content, http://links.lww.com/MD/H429). ECMO flow rate at 24 hours was also significantly reduced in survivors, with survivors receiving 118.5 ± 49.2 mL/kg/min and non-survivors receiving 130.8 ± 53.9 mL/kg/min (SMD = -0.15 [-0.30 to -0.01], I2 = 0%, P = .03). Patients with shockable rhythms, defined as either ventricular fibrillation or pulseless ventricular tachycardia, were more likely to survive than patients with non-shockable rhythms (RR = 1.51 [1.14–1.98], I2 = 0%, P = .01). Neurological complications during ECMO were associated with a reduced chance of survival (RR 0.43 [0.32–0.58], I2 = 31%, P < .01) (Table 2). A forest plot for CPR duration is provided in Figure 2.
Figure 2.
Forest plot for CPR duration. CPR = cardiopulmonary resuscitation.
3.9. Post-ECPR complications
Post-ECPR pulmonary hemorrhage, renal failure, and sepsis were significantly associated with decreased chance of survival. Pulmonary hemorrhage was seen 3 times as often in non-survivors than in survivors (RR = 0.34 [0.17–0.69], I2 = 49%, P < .001). Renal failure was seen twice as often in non-survivors as survivors (RR = 0.47 [0.36–0.61], I2 = 0%, P < .01). Sepsis was also associated with a reduction in survival (RR = 0.57 [0.34–0.96], I2 = 0%, P = .03) (Table 2).
4. Discussion
This manuscript examined the current literature regarding the use of ECPR in pediatric settings. ECPR SHD rates in pediatric populations is 44%, with an average patient age of 13 months. Meta-regression found that unadjusted survival rates have not improved over the past 10 years. This effect may be confounded by increased indications for ECPR use and expansion of ECPR use into higher-risk patient populations, including patients with non-cardiac illnesses. Meta-regression additionally found that the number of patients in a study did not correlate with survival rates, indicating that institutional experience may play less of a significant role in mortality than previously thought.[55]
We also summarized the predictors of survival to provide helpful information to clinicians responsible for patient selection at institutions with the equipment and expertise to provide ECPR. Previous systematic reviews and meta-analysis exist on the topic of pediatric ECPR; however, none quantitatively synthesize current evidence to rank predictors of survival clinicians can use to predict survival.[11,56–58] To our knowledge, this study examines the greatest breadth of predictors of survival, the most recent data, and the greatest number of patients to date in any meta-analysis of pediatric ECPR.
Prior to ECPR initiation, increased CPR duration and decreased lactate levels had among the highest associations with mortality, followed by decreased pH. After ECPR initiation, pulmonary hemorrhage and neurological complications were most predictive for survival. While the exact ranking of these variables is difficult to determine due to their overlapping confidence intervals, the evidence does suggest that clinicians should pay close attention to these variables specifically when determining patient selection for ECPR.
Thirty studies were analyzed, with survival rates ranging from 16% to 75%. This could be attributed to differing protocols, patient populations, indications and contraindications for treatment, institutional experience and equipment, and the relatively small sample sizes in each study. This wide range illustrates the need for a meta-analysis to effectively synthesize these diverging reports.
Only one study, Chrysostomou et al 2013, demonstrated significantly higher survival rates than the pooled estimates.[31] Of note, patients in this study also had decreased acidosis, increased ECMO flow rates at 4 and 24 hours, and decreased ECMO duration times. These increased survival rates could have been due to a broader indication for ECPR. Alternatively, the preference of clinicians in the study to more quickly wean patients off ECMO may have reduced mortality, as this meta-analysis found that increased ECMO duration is negatively correlated with survival.
Age, gender, race, and weight were not found to be significantly associated with increased risk of mortality. Repeated observations have been drawn in prior literature indicating that pediatric ECPR patients have higher survival rates than adult ECPR patients.[9,59–61] In turn, many have speculated that younger pediatric patients might have higher survival rates than older pediatric patients. This meta-analysis found no evidence for such a correlation. Similar to what prior meta-analyses have found in adults, this meta-analysis found no correlation between gender or weight and mortality.[62] Prior studies have found conflicting results on whether race is associated with mortality, and this meta-analysis found that of Asian, Black, Hispanic, and White race, none were found to be significantly associated with mortality.[8,60,61,63]
Patients’ baseline laboratory values at time of ECPR initiation were explored, and lower lactate levels, lower PaCO2, higher PaO2, and higher pH were found to be significant predictors of survival. Severe respiratory acidosis secondary to hypoxemia has been previously associated with decreased survival, consistent with our findings.[64,65] Elevated creatinine levels was also a significant predictors of mortality.[66,67] However, timing of creatinine measurements varied between studies, which limits the applicability of these findings.
Non-survivors were nearly twice as likely to suffer from pre-ECMO renal failure (46% vs 24%). One proposed mechanism behind this is that the existing renal failure may prevent an effective acid-base buffer response to the mixed respiratory and metabolic acidosis incurred by the cardiac arrest due to decreased bicarbonate production. This finding has not been well-examined in pediatric ECPR patients, but similar results have been shown in adult ECPR patients.[13]
In this meta-analysis, SV physiology was not significantly associated with survival. Extensive research has been done demonstrating that neonates with SV physiology are more likely to suffer from cardiac arrest.[68,69] However, conflicting evidence exists regarding the effect of SV physiology in neonates on ECMO and ECPR survival rates.[70–72] Alsoufi et al 2014 found that the specific anatomic and surgical variants of SV physiology play a large role in survival rates, with patients who had aortopulmonary shunts or Norwood first-stage palliation having higher survival rates.[25,73] These confounding factors may be playing a role in the conflicting reports in the literature.
Both the pediatric and adult ECPR literature have consistently reported that patients with underlying cardiac illnesses are more likely to survive ECPR than patients with underlying non-cardiac illnesses.[74] One hypothesis is that ECMO serves as a supplement for the heart, allowing additional time for the underlying cardiac illness to resolve. Primary myocardial disease was not found to be a significant predictor of survival. This fits well with this hypothesis, as even given the additional time provided by ECMO, this specific underlying cardiac illness cannot be resolved.
One topic of key interest has been the relationship between CPR duration, time-to-ECMO initiation, and survival. This meta-analysis found that prolonged conventional CPR is associated with poor outcomes in pediatric populations.[43] Nevertheless, positive patient outcomes for ECPR have been demonstrated after 30 minutes, between 30 to 50 minutes, and even up to 90 to 220 minutes after cardiac arrest.[13] The most recent guidelines published by the International Consensus on Cardiopulmonary Resuscitation do not indicate an optimal cutoff time after cardiac arrest beyond which return of spontaneous circulation is unlikely.[75]
Conflicting reports have also been published on the efficacy of the duration of ECMO as a predictor of survival, with several studies showing no significant difference in ECMO duration between survivors and non-survivors.[76] This meta-analysis found that ECMO duration was negatively associated with survival. As such, clinicians should continue to make efforts to reduce time spent by patients on ECMO.
ECMO flow rate at 24 hours was also shown to be a significant predictor of survival, with patients with reduced flow rates having increased chances of survival. Reduction in the rate of ECMO flow is usually initiated when the patient is considered to meet eligibility in recovery to be weaned from ECMO.[77–79] The need for a higher ECMO flow rate at the 24th hour suggests a lack of resolution of the underlying cardiac arrest or cardiac dysfunction. These patients are significantly more likely to face mortality. This is likely correlated with ECMO duration, as patients with higher flow rates at 24 hours are more likely to require ECMO for an increased duration. ECMO flow rate at 4 hours was not significantly different between survivors and non-survivors. This non-significance is likely attributable to the lack of adequate time for perfusion and resolution of the underlying cardiac arrest at the 4th hour in comparison to the 24th hour.
While most patients did not present with shockable rhythms, patients presenting with shockable rhythms were more likely to survive. These results are consistent with similar findings reported in adults.[62,80,81] Intra-ECPR neurological complications were found to be associated with a large reduction in survival, with patients presenting with neurological complications while on ECMO having a nearly 60% reduction in survival rates. Neurological complications occurred in 37% of non-survivors and should be monitored closely by clinicians as an effective prognostic factor for mortality.
Renal failure was the most common post-ECPR complication (48% of non-survivors), with sepsis (26%) more common than pulmonary hemorrhage (7%). Interestingly, pulmonary hemorrhage was the most predictive of mortality, with these complications roughly one-third as likely to occur in survivors as non-survivors. Post-ECPR renal failure and post-ECPR sepsis were roughly 50% more likely to occur in non-survivors than survivors. Renal failure, sepsis, and pulmonary hemorrhage post-ECPR have all been previously shown to be significant predictors of survival in pediatric populations.[27]
Five of the thirty included studies were studies examining data from the ELSO registry.[27,30,36,40,44] As such, the possibility of double counting patients from an institution that both published their results and reported the data to the ELSO registry exists. An additional sensitivity analysis was conducted to eliminate this possible bias by removing all ELSO registry studies and comparing these findings with the findings reported in this paper. Of the 5 most predictive variables—increased CPR duration, lactate levels, pH, pulmonary hemorrhage, and neurological complications—all remained statistically significant predictors of survival (Fig. S4, Supplemental Digital Content, http://links.lww.com/MD/H433).
4.1. Limitations
This meta-analysis is limited by a few constraints. First, all but one study included in this meta-analysis were retrospective, and the majority were single-center reports. Chart review studies are more likely to suffer from both confounding and selection bias and cannot be conducted blinded in contrast to randomized controlled trials. However, mortality outcomes of ECPR patients are largely dependent on factors that cannot be randomly assigned, which reduces the benefit of a randomized controlled trial over retrospective observational chart reviews. Second, certain metrics contained substantial between-study heterogeneity, and publication bias was detected for 1 metric - duration of CPR. This heterogeneity was partially accounted for by using a random-effects model whenever substantial heterogeneity was detected and by employing sensitivity analysis to validate results in which publication bias was detected. Third, certain predictor variables may be correlated, causing spurious estimates for those variables. Without access to individual patient data, these variables cannot be placed in a more comprehensive model that can control for other variables. Fourth, this meta-analysis was constrained by the data available in prior reports. Little data has been published regarding time-to-ECMO initiation, neurological outcomes, or long-term survival outcomes. Further research should examine these additional outcomes to provide a more comprehensive overview on how patients fare after ECPR.
5. Conclusion
This meta-analysis is the largest meta-analysis examining the greatest number of studies and greatest number of patients to date in any meta-analysis of pediatric ECPR. Thirty studies (n = 3794) on pediatric ECPR published within the last 10 years were examined, and this analysis found the factors most associated with survival prior to ECPR initiation were increased CPR duration and lactate levels, followed by decreased pH. After ECPR initiation, pulmonary hemorrhage and neurological complications were the most associated with survival. ECPR protocols and guidelines that are adjusted to better monitor these metrics may lead to improved survival.
Author contributions
NS and AS designed the study. AS and AG identified studies included and performed data collection. NS provided statistical analysis. JAC supervised the manuscript. All authors drafted the manuscript and have read and approved the final manuscript.
Conceptualization: Nitish Sood, Anish Sangari.
Data curation: Nitish Sood, Anish Sangari, Arnav Goyal.
Formal analysis: Nitish Sood.
Investigation: Nitish Sood, Anish Sangari, Arnav Goyal, J. Arden S. Conway.
Methodology: Anish Sangari, Arnav Goyal, J. Arden S. Conway.
Project administration: Anish Sangari.
Software: Nitish Sood, Anish Sangari.
Supervision: J. Arden S. Conway.
Writing – original draft: Nitish Sood, Anish Sangari, Arnav Goyal.
Writing – review & editing: Nitish Sood, Anish Sangari, Arnav Goyal, J. Arden S. Conway.
Supplementary Material
Abbreviations:
- CPR =
- cardiopulmonary resuscitation
- ECPR =
- extracorporeal cardiopulmonary resuscitation
- ECMO =
- extracorporeal membrane oxygenation
- ELSO =
- extracorporeal life support organization
- RR =
- risk ratio
- SHD =
- survival to hospital discharge
- SMD =
- standardized mean differences
- SV =
- single ventricle
NS and AS contributed equally to this work.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The data used for this meta-analysis were obtained from articles listed within the list of references corresponding to Table 1. All supplementary tables and figures are attached under the supplemental materials file, along with the PRISMA Checklist and PRISMA Flow Diagram. The corresponding author (N.S.) can be contacted for further information.
Supplemental Digital Content is available for this article.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Sood N, Sangari A, Goyal A, Conway JAS. Predictors of survival for pediatric extracorporeal cardiopulmonary resuscitation: A systematic review and meta-analysis. Medicine 2022;101:39(e30860).
Contributor Information
Anish Sangari, Email: ASangari@augusta.edu.
Arnav Goyal, Email: AGoyal@augusta.edu.
J. Arden S. Conway, Email: JConway@augusta.edu.
References
- [1].Matos RI, Watson RS, Nadkarni VM, et al. Duration of cardiopulmonary resuscitation and illness category impact survival and neurologic outcomes for in-hospital pediatric cardiac arrests. Circulation. 2013;127:442–51. [DOI] [PubMed] [Google Scholar]
- [2].Talikowska M, Tohira H, Finn J. Cardiopulmonary resuscitation quality and patient survival outcome in cardiac arrest: a systematic review and meta-analysis. Resuscitation. 2015;96:66–77. [DOI] [PubMed] [Google Scholar]
- [3].Garcia-Jorda D, Walker A, Camphaug J, et al. Bedside chest compression skills: performance and skills retention in in-hospital trained pediatric providers. A simulation study. J Crit Care. 2019;50:132–7. [DOI] [PubMed] [Google Scholar]
- [4].Chauhan S, Malik M, Malik V, et al. Extra corporeal membrane oxygenation after pediatric cardiac surgery: a 10 year experience. Ann Card Anaesth. 2011;14:19–24. [DOI] [PubMed] [Google Scholar]
- [5].Del Nido PJ, Dalton HJ, Thompson AE, et al. Extracorporeal membrane oxygenator rescue in children during cardiac arrest after cardiac surgery. Circulation. 1992;86(5 SUPPLII300–4. [PubMed] [Google Scholar]
- [6].Mattox KL, Beall AC, Jr. Resuscitation of the moribund patient using portable cardiopulmonary bypass. Annal Thoracic Surg. 1976;22:436–42. [DOI] [PubMed] [Google Scholar]
- [7].Farhat A, Bowens CD, Thiagarajan R, et al. Extracorporeal cardiopulmonary resuscitation. In: Advances in Extracorporeal Membrane Oxygenation. London: IntechOpen, 2019:3. [Google Scholar]
- [8].Chan T, Thiagarajan RR, Frank D, et al. Survival after extracorporeal cardiopulmonary resuscitation in infants and children with heart disease. J Thoracic Cardiovascular Surg. 2008;136:984–92. [DOI] [PubMed] [Google Scholar]
- [9].Organization ELS. ECLS registry report: international summary. Available at: https://www.elso.org/Registry/Statistics/InternationalSummary.aspx [access date March 1, 2021].
- [10].Lasa JJ, Rogers RS, Localio R, et al. Extracorporeal cardiopulmonary resuscitation (E-CPR) during Pediatric in-hospital cardiopulmonary arrest is associated with improved survival to discharge. Circulation. 2016;133:165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Holmberg MJ, Geri G, Wiberg S, et al. Extracorporeal cardiopulmonary resuscitation for cardiac arrest: a systematic review. Resuscitation. 2018;131:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Panchal AR, Berg KM, Hirsch KG, et al. 2019 American heart association focused update on advanced cardiovascular life support: use of advanced airways, vasopressors, and extracorporeal cardiopulmonary resuscitation during cardiac arrest: an update to the American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2019;140:E881–94. [DOI] [PubMed] [Google Scholar]
- [13].Ryan J. Extracorporeal membrane oxygenation for pediatric cardiac arrest. Crit Care Nurse. 2015;35:60–9. [DOI] [PubMed] [Google Scholar]
- [14].Richardson ASC, Schmidt M, Bailey M, et al. ECMO cardio-pulmonary resuscitation (ECPR), trends in survival from an international multicentre cohort study over 12-years. Resuscitation. 2017;112:34–40. [DOI] [PubMed] [Google Scholar]
- [15].Sangari A, Sood N, Hauger J. Abstract 266: quantifying the effect of technological advancements and guidelines on pediatric extracorporeal cardiopulmonary resuscitation survival outcomes. Circulation. 2020;142(Suppl_4):A266–A266. [Google Scholar]
- [16].Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [access date March 1, 2021].
- [17].Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Deeks JJ, Higgins JPT, Altman DG, et al. Chapter 10: analysing data and undertaking meta-analyses. Cochrane Training. Published 2021. Available at: https://training.cochrane.org/handbook/current/chapter-10. [Access date March 01, 2021]. [Google Scholar]
- [19].Sood N, Goyal A, Grogan D, et al. Abstract 9115: determining optimal hemoglobin transfusion threshold for patients with severe traumatic brain injury: a systematic review and meta-analysis. Circulation. 2021;144(Suppl_2):A9115–A9115. [Google Scholar]
- [20].Tufanaru C, Munn Z, Stephenson M, et al. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evidence-Based Healthcare. 2015;13:196–207. [DOI] [PubMed] [Google Scholar]
- [21].Murad MH, Montori VM, Ioannidis JPA, et al. Fixed-effects and random-effects models. Guyatt G, Rennie D, Meade MO, Cook DJ. et al. In: Users’ Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice. 3rd ed. New York: McGraw Hill Education, 2015. [Google Scholar]
- [22].Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Online). 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- [23].Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Alsoufi B, Awan A, Manlhiot C, et al. Results of rapid-response extracorporeal cardiopulmonary resuscitation in children with refractory cardiac arrest following cardiac surgery. Eur J Cardiothorac Surg. 2014;45:268–75. [DOI] [PubMed] [Google Scholar]
- [26].Anton-Martin P, Moreira A, Kang P, et al. Outcomes of paediatric cardiac patients after 30 minutes of cardiopulmonary resuscitation prior to extracorporeal support. Cardiol Young. 2020;30:607–16. [DOI] [PubMed] [Google Scholar]
- [27].Bembea MM, Ng DK, Rizkalla N, et al. Outcomes after extracorporeal cardiopulmonary resuscitation of pediatric in-hospital cardiac arrest: a report from the get with the guidelines-resuscitation and the extracorporeal life support organization registries. Crit Care Med. 2019;47:e278–85. [DOI] [PubMed] [Google Scholar]
- [28].Beshish AG, Baginski MR, Johnson TJ, et al. Functional status change among children with extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in a pediatric cardiac ICU: a single institution report. Pediatr Crit Care Med. 2018;19:665–71. [DOI] [PubMed] [Google Scholar]
- [29].Brunner A, Dubois N, Rimensberger PC, et al. Identifying prognostic criteria for survival after resuscitation assisted by extracorporeal membrane oxygenation. Critical Care Res Pract. 2016;2016:9521091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen GL, Qiao YR, Ma JH, et al. Extracorporeal cardiopulmonary resuscitation in children of Asia Pacific: a retrospective analysis of extracorporeal life support organization registry. Chin Med J (Engl). 2018;131:1436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chrysostomou C, Morell VO, Kuch BA, et al. Short- and intermediate-term survival after extracorporeal membrane oxygenation in children with cardiac disease. J Thoracic Cardiovascular Surg. 2013;146:317–25. [DOI] [PubMed] [Google Scholar]
- [32].De Mul A, Nguyen DA, Doell C, et al. Prognostic evaluation of mortality after pediatric resuscitation assisted by extracorporeal life support. J Pediatr Intensive Care. 2019;8:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Erek E, Aydin S, Suzan D, et al. Extracorporeal cardiopulmonary resuscitation for refractory cardiac arrest in children after cardiac surgery. Anatolian J Cardiol. 2017;17:328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Guo Z, Yang Y, Zhang W, et al. Extracorporeal cardiopulmonary resuscitation in children after open heart surgery. Artif Organs. 2019;43:633–40. [DOI] [PubMed] [Google Scholar]
- [35].Huang SC, Wu ET, Wang CC, et al. Eleven years of experience with extracorporeal cardiopulmonary resuscitation for paediatric patients with in-hospital cardiac arrest. Resuscitation. 2012;83:710–4. [DOI] [PubMed] [Google Scholar]
- [36].Jolley M, Yarlagadda VV, Rajagopal SK, et al. Extracorporeal membrane oxygenation-supported cardiopulmonary resuscitation following stage 1 palliation for hypoplastic left heart syndrome. Pediatr Crit Care Med. 2014;15:538–45. [DOI] [PubMed] [Google Scholar]
- [37].Kane DA, Thiagarajan RR, Wypij D, et al. Rapid-response extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in children with cardiac disease. Circulation. 2010;122(11 SUPPL. 1):S241–8. [DOI] [PubMed] [Google Scholar]
- [38].KendIrlI T, Ozcan S, Havan M, et al. Pediatric extracorporeal cardiopulmonary resuscitation: single-center study. Turkish J Med Sci. 2020;51:1733–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kramer P, Mommsen A, Miera O, et al. Survival and mid-term neurologic outcome after extracorporeal cardiopulmonary resuscitation in children. Pediatr Crit Care Med. 2020;21:e316–24. [DOI] [PubMed] [Google Scholar]
- [40].McMullan DM, Thiagarajan RR, Smith KM, et al. Extracorporeal cardiopulmonary resuscitation outcomes in term and premature neonates. Pediatr Crit Care Med. 2014;15:e9–e16. [DOI] [PubMed] [Google Scholar]
- [41].Melvan JN, Davis J, Heard M, et al. Factors associated with survival following extracorporeal cardiopulmonary resuscitation in children. World J Pediatr Congenital Heart Surg. 2020;11:265–74. [DOI] [PubMed] [Google Scholar]
- [42].Philip J, Burgman C, Bavare A, et al. Nature of the underlying heart disease affects survival in pediatric patients undergoing extracorporeal cardiopulmonary resuscitation. J Thoracic Cardiovascular Surg. 2014;148:2367–72. [DOI] [PubMed] [Google Scholar]
- [43].Polimenakos AC, Rizzo V, El-Zein CF, et al. Post-cardiotomy rescue extracorporeal cardiopulmonary resuscitation in neonates with single ventricle after intractable cardiac arrest: attrition after hospital discharge and predictors of outcome. Pediatr Cardiol. 2017;38:314–23. [DOI] [PubMed] [Google Scholar]
- [44].Raymond TT, Cunnyngham CB, Thompson MT, et al. Outcomes among neonates, infants, and children after extracorporeal cardiopulmonary resuscitation for refractory inhospital pediatric cardiac arrest: a report from the national registry of cardiopulmonary resuscitation. Pediatr Crit Care Med. 2010;11:362–71. [DOI] [PubMed] [Google Scholar]
- [45].Shakoor A, Pedroso FE, Jacobs SE, et al. Extracorporeal cardiopulmonary resuscitation (ECPR) in infants and children: a single-center retrospective Study. World J Pediatric Congenital Heart Surg. 2019;10:582–9. [DOI] [PubMed] [Google Scholar]
- [46].Shin HJ, Song S, Park HK, et al. Results of extracorporeal cardiopulmonary resuscitation in children. J Chest Surg. 2016;49:151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sivarajan VB, Best D, Brizard CP, et al. Duration of resuscitation prior to rescue extracorporeal membrane oxygenation impacts outcome in children with heart disease. Intensive Care Med. 2011;37:853–60. [DOI] [PubMed] [Google Scholar]
- [48].Topjian AA, Telford R, Holubkov R, et al. The association of early post-resuscitation hypotension with discharge survival following targeted temperature management for pediatric in-hospital cardiac arrest. Resuscitation. 2019;141:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Torres-Andres F, Fink EL, Bell MJ, et al. Survival and long-term functional outcomes for children with cardiac arrest treated with extracorporeal cardiopulmonary resuscitation. Pediatr Crit Care Med. 2018;19:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tsukahara K, Toida C, Muguruma T. Current experience and limitations of extracorporeal cardiopulmonary resuscitation for cardiac arrest in children: a single-center retrospective study. J Intensive Care. 2014;2:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Walter EMD, Alexi-Meskishvili V, Huebler M, et al. Rescue extracorporeal membrane oxygenation in children with refractory cardiac arrest. Interact Cardiovasc Thorac Surg. 2011;12:929–34. [DOI] [PubMed] [Google Scholar]
- [52].Wolf MJ, Kanter KR, Kirshbom PM, et al. Extracorporeal cardiopulmonary resuscitation for pediatric cardiac patients. Annal Thoracic Surg. 2012;94:874–80. [DOI] [PubMed] [Google Scholar]
- [53].Yates AR, Sutton RM, Reeder RW, et al. Survival and cardiopulmonary resuscitation hemodynamics following cardiac arrest in children with surgical compared to medical heart disease. Pediatr Crit Care Med. 2019;20:1126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zeybek C, Avsar MK, Yildirim O, et al. Utilization of extracorporeal membrane oxygenation in pediatric cardiac surgery: a single center experience, 34 cases in 8 years. Iranian J Pediatrics. 2017;27:e14402. [Google Scholar]
- [55].Turek JW, Andersen ND, Lawson DS, et al. Outcomes before and after implementation of a pediatric rapid-response extracorporeal membrane oxygenation program. Annal Thoracic Surg. 2013;95:2140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Farhat A, Ling RR, Jenks CL, et al. Outcomes of pediatric extracorporeal cardiopulmonary resuscitation: a systematic review and meta-analysis. Crit Care Med. 2021;49:682–92. [DOI] [PubMed] [Google Scholar]
- [57].Esangbedo ID, Brunetti MA, Campbell FM, et al. Pediatric extracorporeal cardiopulmonary resuscitation: a systematic review*. Pediatr Crit Care Med. 2020;21:e934–43. [DOI] [PubMed] [Google Scholar]
- [58].Joffe AR, Lequier L, Robertson CMT. Pediatric outcomes after extracorporeal membrane oxygenation for cardiac disease and for cardiac arrest: a review. ASAIO J. 2012;58:297–310. [DOI] [PubMed] [Google Scholar]
- [59].Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal life support organization registry international report 2016. ASAIO J. 2017;63:60–7. [DOI] [PubMed] [Google Scholar]
- [60].Thiagarajan RR, Laussen PC, Rycus PT, et al. Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation. 2007;116:1693–700. [DOI] [PubMed] [Google Scholar]
- [61].Thiagarajan RR, Brogan TV, Scheurer MA, et al. Extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in adults. Annal Thoracic Surg. 2009;87:778–85. [DOI] [PubMed] [Google Scholar]
- [62].Wang J, Ma Q, Zhang H, et al. Predictors of survival and neurologic outcome for adults with extracorporeal cardiopulmonary resuscitation: a systemic review and meta-analysis. Medicine (Baltim). 2018;97:e13257–e13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Brogan TV, Thiagarajan RR, Rycus PT, et al. Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med. 2009;35:2105–14. [DOI] [PubMed] [Google Scholar]
- [64].Jamme M, Ben Hadj Salem O, Guillemet L, et al. Severe metabolic acidosis after out-of-hospital cardiac arrest: risk factors and association with outcome. Annal Intensive Care. 2018;8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kim YJ, Lee YJ, Ryoo SM, et al. Role of blood gas analysis during cardiopulmonary resuscitation in out-of-hospital cardiac arrest patients. Medicine (United States). 2016;95:e3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gounden V, Bhatt H, Jialal I. Renal function tests. In: StatPearls. FL: Treasure Island. 2021. [PubMed] [Google Scholar]
- [67].Kelly RB, Harrison RE. Outcome predictors of pediatric extracorporeal cardiopulmonary resuscitation. Pediatr Cardiol. 2010;31:626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Marino BS, Tabbutt S, MacLaren G, et al. Cardiopulmonary resuscitation in infants and children with cardiac disease: a scientific statement from the American heart association. Circulation. 2018;137:e691–782. [DOI] [PubMed] [Google Scholar]
- [69].Lowry AW, Knudson JD, Cabrera AG, et al. Cardiopulmonary resuscitation in hospitalized children with cardiovascular disease: estimated prevalence and outcomes from the Kids’ Inpatient database. Pediatr Crit Care Med. 2013;14:248–55. [DOI] [PubMed] [Google Scholar]
- [70].Kulik TJ, Moler FW, Palmisano JM, et al. Outcome-associated factors in pediatric patients treated with extracorporeal membrane oxygenator after cardiac surgery. Circulation. 1996;94(9 SUPPLII63–8. [PubMed] [Google Scholar]
- [71].Alsoufi B, Al-Radi OO, Gruenwald C, et al. Extra-corporeal life support following cardiac surgery in children: analysis of risk factors and survival in a single institution. Eur J Cardiothorac Surg. 2009;35:1004–11. [DOI] [PubMed] [Google Scholar]
- [72].Aharon AS, Drinkwater DC, Jr, Churchwell KB, et al. Extracorporeal membrane oxygenation in children after repair of congenital cardiac lesions. Annals Thoracic Surg. 2001;72:2095–102. [DOI] [PubMed] [Google Scholar]
- [73].Booth KL, Roth SJ, Thiagarajan RR, et al. Extracorporeal membrane oxygenation support of the Fontan and bidirectional Glenn circulations. Annal Thoracic Surg. 2004;77:1341–8. [DOI] [PubMed] [Google Scholar]
- [74].Alsoufi B, Al-Radi OO, Nazer RI, et al. Survival outcomes after rescue extracorporeal cardiopulmonary resuscitation in pediatric patients with refractory cardiac arrest. J Thoracic Cardiovascular Surg. 2007;134:952–959.e952. [DOI] [PubMed] [Google Scholar]
- [75].de Caen AR, Kleinman ME, Chameides L, et al. Part 10: Paediatric basic and advanced life support: 2010 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2010;81(1 SUPPL.1):e213–e259. [DOI] [PubMed] [Google Scholar]
- [76].Sangari A, Sood N, Sood A, et al. 42 Improving patient selection in international pediatric ECPR cohorts. Ann Emerg Med. 2021;78:S18. [Google Scholar]
- [77].Rao P, Khalpey Z, Smith R, et al. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest. Circulation Heart Failure. 2018;11:e004905. [DOI] [PubMed] [Google Scholar]
- [78].Ki KK, Passmore MR, Chan CHH, et al. Low flow rate alters haemostatic parameters in an ex-vivo extracorporeal membrane oxygenation circuit. Intensive Care Med Exp. 2019;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Malfertheiner MV, Broman LM, Belliato M, et al. Management strategies in venovenous extracorporeal membrane oxygenation: a retrospective comparison from five European centres. Critic Care Resuscitation. 2017;19:76–81. [PubMed] [Google Scholar]
- [80].D’Arrigo S, Cacciola S, Dennis M, et al. Predictors of favourable outcome after in-hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation: a systematic review and meta-analysis. Resuscitation. 2017;121:62–70. [DOI] [PubMed] [Google Scholar]
- [81].Debaty G, Babaz V, Durand M, et al. Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis. Resuscitation. 2017;112:1–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.