Abstract
Rationale:
Chronic intermittent ethanol (CIE) vapor inhalation is a widely used model of alcohol dependence, but the impact of CIE on cue-elicited alcohol seeking is poorly understood.
Objective:
Here, we assessed the effects of CIE on alcohol-seeking elicited by cues paired with alcohol before or after CIE vapor inhalation.
Methods:
In Experiment 1, male and female Long Evans rats were trained in a discriminative stimulus (DS) task, in which one auditory cue (the DS) predicts the availability of 15% ethanol and a control cue (the NS) predicts no ethanol. Rats then underwent CIE or served as controls. Subsets of each group received access to oral ethanol twice a week during acute withdrawal. After CIE, rats were presented with the DS and NS cues under extinction and retraining conditions to determine whether they would alter their responses to these cues. In Experiment 2, rats underwent CIE prior to training in the DS task.
Results:
CIE enhanced behavioral responses to cues previously paired with alcohol, but only in rats that received access to alcohol during acute withdrawal. When CIE occurred before task training, male rats were slower to develop cue responses and less likely to enter the alcohol port, even though they had received alcohol during acute withdrawal.
Conclusions:
These results suggest that CIE vapor inhalation alone does not potentiate the motivational value of alcohol cues, but that an increase in cue responses requires alcohol experience during acute withdrawal. Further, under some conditions CIE may disrupt responses to alcohol-paired cues.
Keywords: cues, alcohol dependence, withdrawal, addiction, rat, sex differences
Introduction
Environmental cues that predict the availability of drugs like alcohol can drive several aspects of addiction, including escalation of alcohol use and the propensity to relapse even after long periods of abstinence (Courtney et al. 2015; Preston et al. 2018). Chronic intermittent ethanol (CIE) vapor inhalation is a commonly used model of alcohol dependence which has been shown to drive increased alcohol consumption and self-administration, including habitual and compulsive alcohol seeking behaviors (Gilpin et al. 2008; Griffin et al. 2009; Radke et al. 2017; Renteria et al. 2018; Xie et al. 2019). Yet we know relatively little about the impact of CIE vapor inhalation on reactivity to alcohol cues, including in models of relapse.
The existing literature on the impact of CIE vapor inhalation on behaviors related to alcohol cues is mixed. For instance, CIE vapor inhalation appears to enhance cue-induced reinstatement of alcohol seeking under some conditions (Liu and Weiss 2002, 2003), but not others (Ciccocioppo et al. 2003; Eisenhardt et al. 2015). One salient difference between the methods used in these studies is the availability of alcohol during acute withdrawal: studies showing enhanced cue-induced reinstatement have included periods of access to alcohol during the vapor phase when animals are in acute withdrawal (Liu and Weiss 2002, 2003). Importantly, one caveat of the cue-induced reinstatement model of relapse is that it involves response-contingent presentations of cues. This makes the model, essentially, a test of conditioned reinforcement (i.e., the degree to which these cues can reinforce behaviors), but not necessarily the conditioned motivational properties of cues (i.e., the degree to which cue presentations can invigorate behavior). Therefore, cue-induced reinstatement may fail to capture some aspects of cue-driven relapse in humans, which is frequently precipitated by cues outside of the individual’s control (Epstein et al. 2009; Preston et al. 2009, 2018). Additionally, reinstatement of alcohol seeking by response-contingent presentation of cues is often less robust than reinstatement induced by non-contingent contextual cues signaling alcohol availability (Tsiang and Janak 2006). This may be why many studies investigating alcohol relapse use a combination of contingent and non-contingent cue presentations (Ciccocioppo et al. 2002, 2003; Williams and Schimmel 2008), limiting precise interpretation of their results.
Given the limitations of response-contingent cue presentation as a model of relapse to alcohol seeking, it is important to understand the effect of CIE vapor inhalation on responses to non-contingent alcohol cues. Work in this area thus far has been limited: Kufahl et al. (2011) found that CIE vapor inhalation did not alter alcohol-seeking behavior in the presence of non-contingent (olfactory and auditory) contextual cues predicting the availability of alcohol. Importantly, animals in that study did not have the opportunity to consume or self-administer alcohol in acute withdrawal during the vapor phase of this experiment. Therefore, it remains unknown whether CIE vapor inhalation can enhance behavioral responses to contextual alcohol cues, if subjects are given access to alcohol during acute withdrawal. Additionally, the effects of CIE vapor inhalation on the invigoration of alcohol-seeking behaviors by discrete cues signaling alcohol availability have not been reported.
To address these open questions, we examined the effects of CIE vapor inhalation on behavioral responses to cues that were previously paired with alcohol availability, and the effects of CIE on subsequent alcohol cue learning and responsivity. In Experiment 1 we trained rats in a discriminative stimulus (DS) task with alcohol reward (Richard et al. 2018; Ottenheimer et al. 2019) prior to CIE vapor inhalation. We then assessed the effects of CIE on alcohol consumption, somatic withdrawal signs, behavior in an open field, and alcohol cues responses under both extinction and retraining conditions. In Experiment 2 we assessed the effects of CIE on subsequent cue learning in the DS task, as well as aversion-resistant drinking. We found that the effects of CIE on alcohol cue responses depend on both the timing of CIE relative to cue learning, and on access to alcohol during acute withdrawal. Additionally, we found sex-specific effects of CIE on alcohol consumption and aversion-resistant drinking.
Materials and Methods
Subjects
Long-Evans rats (n= 101, 59 females and 60 males; Envigo) weighing 225–275 grams at arrival were used in this study. Rats were individually housed with ad libitum access to food and water and were maintained on a 14-hr/10-hr light/dark cycle (lights on at 6am; lights off at 8pm). All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Minnesota and were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (NIH).
Experiment 1: Impact of CIE vapor inhalation on previously learned cue responses
Rats (n=80; 40 males, 40 females) received 8 weeks of intermittent access (Hopf et al. 2010) to 15% ethanol in their home cages to acclimate them to its taste and pharmacological effects, and to ensure they were sufficiently motivated to learn the DS task for alcohol reward. Rats that failed to drink at least 2 g/kg/day during the final 2 weeks of pre-exposure were excluded from further testing (n=20; 13 males; 7 females). Rats that met drinking criteria (n=60; 33 females, 27 males) then underwent DS task training. They were trained for 15 days with the DS only, followed by 12 sessions that included the NS for a total of 27 training sessions. At this point, rats that failed to meet learning criteria (DS response probability > 0.5, NS response probability < 0.5, DS/NS response ratio > 1.5) were designated as “non-learners” and excluded from further assessment of cue-related behavior (n=17; 7 males; 10 females) but were assessed for the effects of CIE vapor inhalation on alcohol consumption. Rats were then assigned to either the CIE or control groups, matched and counterbalanced based on port entry probability during the DS cue. Rats then underwent 3 weeks of CIE as described below. Rats that were over-exposed to ethanol vapor during this phase (BECs > 500 mg/dl or intoxication rating >4) were excluded from further assessment (1 female, 2 males). To assess the impact of access to ethanol during acute withdrawal, some rats (n=28; 7 male controls, 6 male CIE, 8 female controls, 8 female CIE) received access to 15% ethanol in the home cage for 2-hr periods, 2 days a week, 6–8 hours after CIE rats were removed from the vapor chambers, whereas the remaining rats did not receive access to oral alcohol during the vapor exposure phase (n=30; 5 male controls, 8 male CIE, 7 female control, 10 female CIE). Finally, rats underwent final cue testing 2–3 weeks after CIE as described below.
Experiment 2: Impact of CIE vapor inhalation on subsequent alcohol cue learning and responsivity
Rats (n=39; 20 males, 19 females) were given 2 weeks of intermittent access to 15% ethanol in their home cages and were then assigned to matched CIE and control groups based on their baseline alcohol consumption (n=9–10 per group). Rats underwent 3 weeks of CIE as described below; all rats received access to 15% ethanol in the home cage for 2-hr periods, 2 days a week, 6–8 hours after CIE rats were removed from the vapor chambers. Rats that were over-exposed to ethanol vapor were excluded from further assessment (3 females). They were then assessed for quinine-adulterated alcohol consumption. Approximately 2 weeks following the cessation of CIE, rats underwent training in the DS task: rats were trained with the DS cue alone for 10 sessions, followed by 12 sessions with both the DS and the NS. Following an additional assessment of quinine-adulterated alcohol drinking they underwent final cue testing as described below.
Discriminative Stimulus (DS) Task Training
Rats were trained in operant chambers (29.53 × 23.5 × 27.31 cm) equipped with tone and white noise generators, and a reward port with a head entry detector (Med Associates Inc., Fairfax, VT). Prior to DS task training we conducted port training sessions in which 15% ethanol (.1 mL) was delivered to the reward receptacle via syringe pump. Sessions terminated when rats entered the port after 30 successive alcohol deliveries or 120 minutes had elapsed, whichever occurred first. A second supplemental port entry training session was provided for rats that did not enter the port 30 times in the first session. The third and final port entry session included all rats. After port training, rats were trained in a DS task, as described previously (Richard et al. 2016, 2018; Ottenheimer et al. 2019). In this task, entry into the port during one auditory cue (the DS) resulted in delivery of 15% ethanol (0.1mL) to the port. Port entry in the absence of the DS or during the non-rewarded stimulus (NS) had no programmed consequences. Auditory cues were a combination of 2700 and 4100kHz tones (DS) and white noise (NS). Training consisted of five stages. In stages 1–4 30 DS cues were presented with decreasing lengths (60s, 30s, 20s, 10s). Rats advanced to the next stage when they had entered the port on at least 60% of the DS cues. The NS cue (10s) was introduced in the fifth stage and was also presented 30 times for a total of 60 cues. For all stages, cues were presented on a pseudorandom variable interval schedule with varying mean intertrial intervals to keep the sessions at 90 minutes. At the end of each session leftover ethanol was collected from the reward port to assess overall consumption.
Chronic Intermittent Ethanol (CIE) Vapor Exposure
Rats underwent chronic intermittent exposure (CIE) to ethanol vapor in a passive vapor inhalation system (La Jolla Alcohol Research, Inc., San Diego, CA) that is described in detail elsewhere (Gilpin et al. 2008). During vapor exposure, rats remained in their individual housing cages with ad libitum access to food and water. Shortly before the start of vapor exposure, cages were inserted into the vapor chamber apparatus, with filter tops removed. Control rats were either placed into vapor chambers for exposure to room air or placed on adjacent shelving in the same room due to space constraints. CIE was administered for 14-hours per session, 4 sequential nights a week, for 3 weeks. After exposure sessions intoxication levels were rated according to behavioral markers for both CIE and control rats, as described elsewhere (Barker et al. 2017). BECs were assessed once a week after the first vapor exposure cycle in both CIE and control rats. Blood was collected from the tail vein using a 70μL heparinized capillary tube. Plasma was separated from the red blood cells, and injected into an alcohol analyser (Model AM1, Analox Instruments Ltd., Stourbridge, United Kingdom). Vapor chamber settings were adjusted according to daily intoxication ratings and weekly blood ethanol concentrations (BECs). Somatic withdrawal signs were assessed during the final week of vapor exposure in both CIE and control rats, 6–8 hours after rats were removed from the vapor chambers (Majchrowicz 1975).
Cue Test: DS Extinction and Retraining
Approximately 2.5 weeks after the completion of CIE (Experiment 1) or the end of DS task training (Experiment 2), rats underwent four cue tests in the same behavioral chambers used for DS task training. During the first cue test rats were presented with 10 probe cues (5 DS and 5 NS) under extinction conditions (no ethanol reward). Cue testing under extinction was performed to differentiate behavioral responding to the cue from behavioral changes driven directly by ethanol reinforcement. Immediately afterwards, 60 cues (30 DS and 30 NS) were presented under retraining conditions, where port entry during the DS once again resulted in an alcohol reward. Rats underwent three additional testing sessions, spaced 48 hrs apart, with increasing number of trials or amounts of ethanol delivery in the following order: 1) 80 cues (40 DS and 40 NS), 2) 120 cues (60 DS and 60 NS), and 3) 120 cues with double the amount of ethanol per reward (0.2ml), to identify maximal responding for ethanol.
Quinine-Adulterated Alcohol Consumption Tests
To assess the degree to which the rats’ consumption of alcohol was compulsive rats underwent quinine-adulterated alcohol consumption tests before and after vapor exposure or task training (Seif et al. 2013; Hopf and Lesscher 2014). Rats were given home cage access to 15% ethanol with 0, 10, 30 and 90 mg/L quinine hydrochloride (Seif et al. 2015; Sneddon et al. 2018), for 24-hr periods, with at least 24 hrs in between tests.
Statistical Analysis
Statistical analyses were conducted using MATLAB (Mathworks). The effects of CIE vapor exposure, sex, and access to ethanol during acute withdrawal were assessed using linear mixed models, with random effects included for subject. Fixed effects for session, test number, and/or quinine concentration were included as appropriate for each behavioral test.
Results
Female rats consumed more ethanol during intermittent access and DS task training
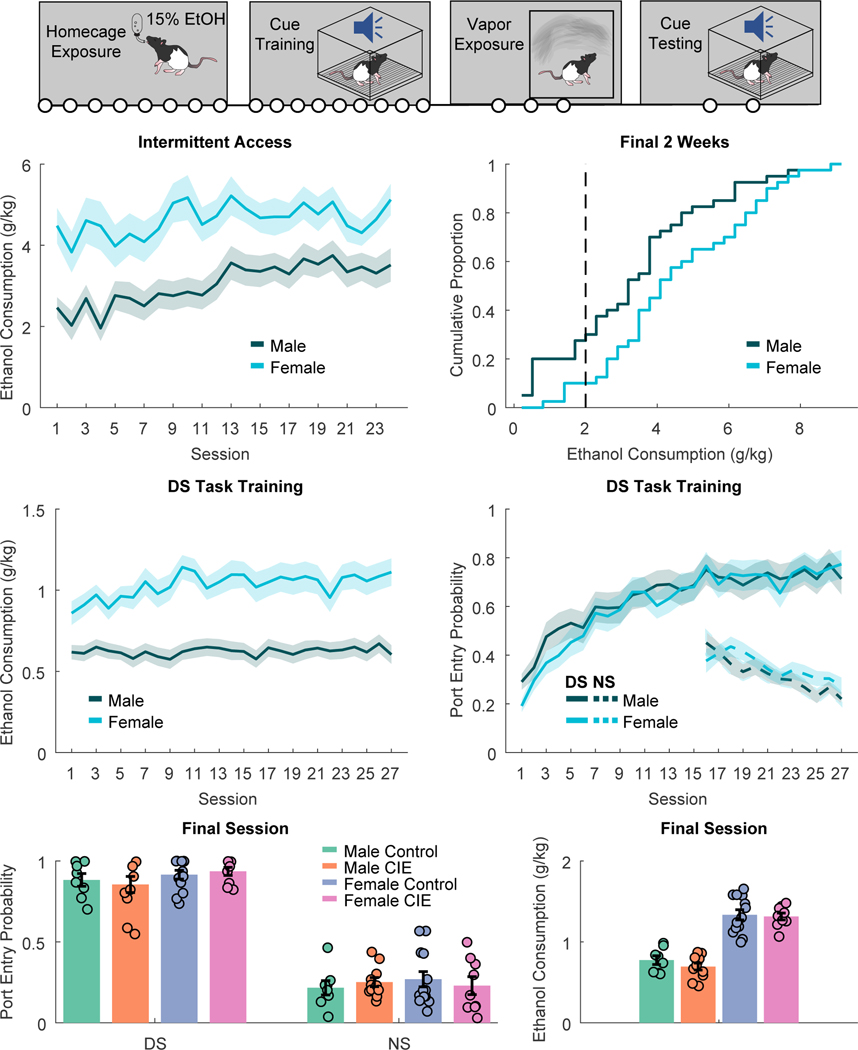
In our first experiment rats received 8 weeks of homecage intermittent access to 15% ethanol prior to training the DS task (Fig. 1A). We found that female rats consumed significantly more g/kg ethanol throughout intermittent access (Fig 1B; main effect of sex, F(1,1895)=36.798, p < 0.001), including during the final 2 weeks (Fig. 1C; t(78)=2.6386, p = 0.01). Males escalated their consumption across intermittent access, but females did not (main effect of session: F(1,1844)=62.146, p < 0.001; session X sex interaction: (F(1,1844)=20.605, p < 0.001; main effect of session, males: F(1,938)=85.757, p < 0.001; females: F(1,906)=1.4385, p = 0.23). Female rats also consumed more g/kg ethanol across training in the DS task (Fig. 1D; main effect of sex: F(1,1615)=19.411, p < 0.001). Increased g/kg consumption in the task may be partially due to the limit on maximal alcohol consumption during each session (30 trials x 0.1 mL 15% ethanol) and the consistent weight differences between males and females in this study (F(1,1615)=704.23, p < 0.001). Across training rats increased their port entry response probability during the DS (Fig. 1E; F(1,1611)=33.237, p < 0.001) and decreased their port entry probability during the NS (F(1,703)=29.687, p < 0.001), with no differences observed between male and female rats (F values of 0.344 to 1.56). After 27 sessions rats differed in their responses to the DS and NS in both response probability (Fig. 1F; F(1,80)=704.65, p < 0.001) and response latency (F(1,57)=13.3, p < 0.001). Rats were then assigned to matched CIE or control groups based on DS task performance.
Figure 1. DS task training prior to vapor exposure.
A) Timeline of experiments. Rats first underwent home cage intermittent access to alcohol and training in the DS task, prior to ethanol vapor exposure. Circles indicate the number of weeks in each experimental phase. B) Ethanol consumption (g/kg) during homecage intermittent access, prior to task training, C) Cumulative distribution of mean ethanol consumption during the final 2 weeks of intermittent access in males (red) versus females (blue). The dotted line marks the minimum consumption required during this period to move on to training in the DS task. D) Ethanol consumption during training in the DS task. E) Port entry probability during the DS ethanol cue (solid line) and NS control cue (dotted line) across training. F) Final port entry probability during the DS (left) and NS (right) in rats assigned to vapor exposure (CIE) or control conditions after training. G) Ethanol consumption during the final DS task training session. Lines with shading, and bar plots with error bars indicated mean +/− SEM. Circles represent data from individual animals.
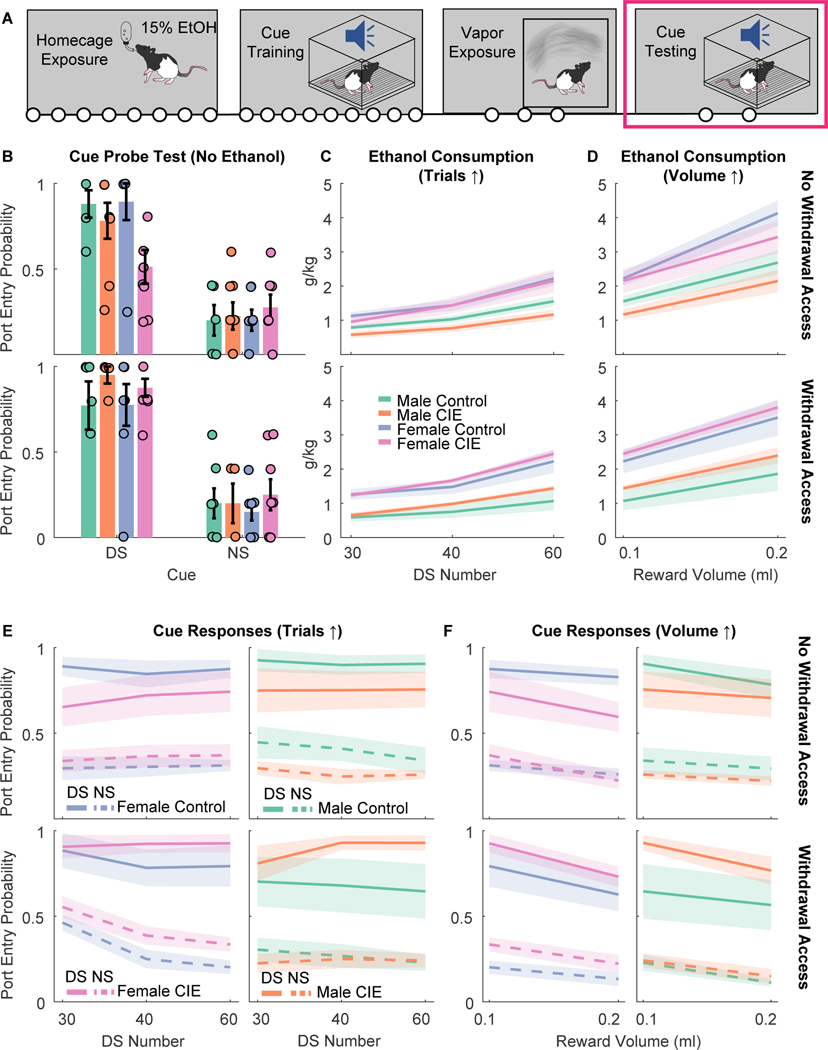
CIE vapor inhalation increased behavioral responses to alcohol cues, but only in rats that received access to ethanol during acute withdrawal
Approximately 2.5 weeks after the end of CIE rats (Fig. 2A) were tested for their responses to the DS and NS cues under extinction conditions (Fig. 2B). We found that the effects of CIE on port entry probability during the DS depended on whether rats received access to alcohol during acute withdrawal (CIE X alcohol access interaction: F(1,47)=6.01, p = 0.018): CIE rats that received alcohol access were more likely to enter the port during the DS than controls on average, whereas CIE rats that did not receive alcohol access had a lower port entry probability, though no pairwise comparisons were significantly different after corrections for multiple comparisons (Fig. 2B). We found no effect of sex on DS port entry probability (main effect of sex: F(1,47)=1.12, p = 0.295; CIE X sex interaction: F(1,47)=1.36, p = 0.25; CIE X alcohol access X sex interaction: F(1,47)=0.43, p = 0.51). We also found no effect of sex, CIE or alcohol access on port entry probability during the NS (F values of 0.05 to 0.73). We next assessed whether CIE would alter response probability or latency under retraining conditions (with ethanol delivery). Because we were concerned about potential ceiling effects with the standard number of trials, we also tested rats with increasing numbers of trials (Fig. 2C and 2E; from 30 to 60) or ethanol reward per trial (Fig. 2D and 2F; increasing the maximal ethanol reward per session from 0.1 ml to 0.2ml). While females consumed more ethanol during these sessions with increasing trial numbers (Fig. 2C; F(1,166)=38.414, p < 0.001) there was no interaction between sex and CIE vapor inhalation of alcohol access during withdrawal (F values ranging from 0.039 to 0.995), and female and male rats did not significantly differ in their response probability (Fig. 2E; main effect of sex: F(1,166)=0.416, p = 0.52). Additionally, because adding sex as a variable did not improve the fit for our linear mixed model analysis of DS port entry probability (AIC = −164.18 without sex versus −159,98 with sex) we combined males and female for subsequent analyses. Overall, we found that increasing the trial number reduced port entry probability (Fig. 2E; F(1,157)=5.11, p = 0.025), but that there was no interaction between this factor and CIE or alcohol access (F values 1.17 to 1.70). When we pooled data across the tests, we found that CIE had opposing effects on DS port entry probability, depending on whether the rats had alcohol access during acute withdrawal (CIE x alcohol access interaction, F(1,161) =5.13, p = 0.025), increasing port entry probability in rats that received access, and decreasing port entry probability in rats that did not (similar to the test results under extinction conditions). While we saw a non-significant decrease in port entry probability during the NS as trial number increased (F(1,157) = 3.48, p = 0.064), we observed no effect of CIE or interactions with alcohol access for port entry probability during the NS (F values 0.052 to 1.37).
Figure 2. Cue responses and ethanol consumption in the DS task after vapor exposure.
A) Timeline of experiments. Following homecage ethanol exposure, cue training and ethanol vapor exposure, rats underwent cue testing. Circles indicate the number of weeks in each experimental phase. B) Port entry probability during the DS (left) or NS (right) in the cue probe test (no ethanol delivered) after being exposed to vapor (CIE) or control conditions. Rats are split into those that did not receive alcohol access during acute withdrawal in the vapor phase (top) or rats that did receive access in acute withdrawal (bottom). Circles represent data from individual animals. Bar plots with error bars indicated mean +/− SEM. C) Ethanol consumption during cue “retraining” and testing in which ethanol was re-introduced, and the number of trials was increased across sessions, in rats that received vapor exposure (CIE) or control conditions after task training. Lines with shading indicate mean +/− SEM. D) Ethanol consumption during cue testing in which the amount of 15% ethanol delivered on each trial was increased from 0.1 to 0.2 ml. E) Port entry probability during the DS (solid lines) or NS (dotted lines) during cue “retraining” and testing in which ethanol was re-introduced, and the number of trials was increased across sessions. F) Port entry probability during the DS and NS during cue testing in which the amount of 15% ethanol delivered on each trial was increased from 0.1 to 0.2 ml.
Increasing the reward volume resulted in similar effects (Fig. 2D and 2F). Increasing the reward volume per trial increased overall consumption (F(1,108) = 55.466, p < 0.001), in a manner that interacted with sex (F(1,108) = 7.98, p < 0.005), but we found no interaction between reward volume, sex, and either CIE or withdrawal access (F values 0.079 to 1.19). Increasing reward volume also decreased overall DS port entry probably, presumably due to satiation (F(1,108) = 12.258, p < 0.001), though we observed no interaction between reward volume and CIE or withdrawal access for DS port entry probability (F values 0.88 to 2.84). When we pooled data across the tests, we found that CIE had opposing effects on DS port entry probability, depending on whether the rats had alcohol access during acute withdrawal (Fig. 2F; CIE x alcohol access interaction, F(1,106) =6.10, p = 0.015), increasing port entry probability in rats that received access, and decreasing port entry probability in rats that did not (similar to the test results under extinction conditions). This is consistent with what we observed during reacquisition with increasing trial numbers. Overall, CIE vapor inhalation increased responding to the DS in rats that received access to alcohol during acute withdrawal, and decreased responding in rats that did not receive access during acute withdrawal.
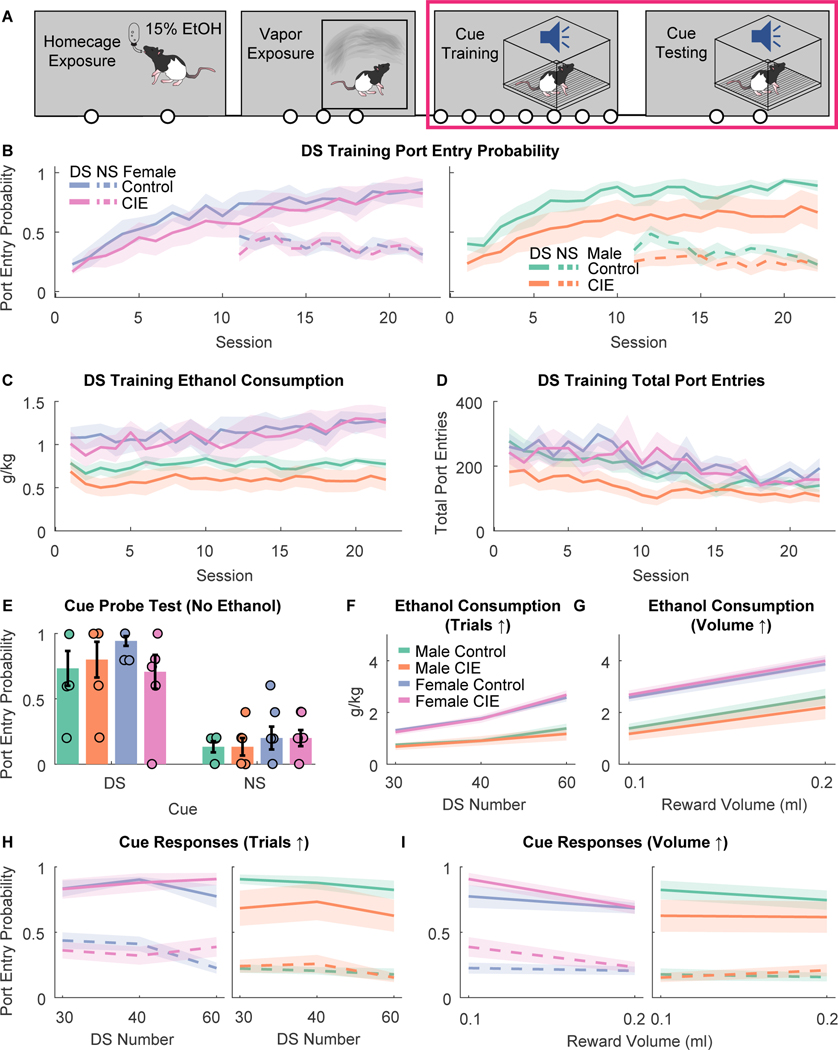
CIE vapor inhalation reduced entries into the alcohol port during subsequent cue learning in male rats
Next, we sought to determine whether the induction of alcohol dependence with CIE vapor inhalation would enhance learning for or responses to subsequently learned alcohol cues (Fig. 3A). Rats underwent 2 weeks of homecage intermittent access to 15% ethanol and were assigned to matched CIE versus control groups. Rats underwent 3 weeks of CIE vapor inhalation as in Experiment 1 and were trained in the DS task (Fig. 3B–D). Similar to Experiment 1, females consumed more g/kg ethanol during training (Fig. 3B; main effect of sex: F(1,739)=4.85, p = 0.028; session X sex interaction, F(1,739)=16.641, p < 0.001). We found no effect of CIE or interaction with session or sex for ethanol consumption during training (F values 0.085 to 1.69). As expected, port entry probability increased across sessions (Figure 3A; F(1,739)=75.134, p < 0.001), but this effect depended on sex (session X sex interaction, F(1,739)=18.059, p < 0.001). We found a trend towards an interaction between session, sex and CIE vapor inhalation (F(1,739)=3.29, p = 0.070), though this interaction did not reach statistical significance. Overall, CIE rats appeared to have blunted port entry probability during the DS compared to controls. This decrease was not specific to the DS period. Port entry probability during the NS was blunted by CIE (main effect of CIE, F(1,400)=5.3685, p = 0.021; session X CIE interaction, F(1,400)=2.9211, p = 0.088). We found no effect of sex or interaction with CIE or session for NS port entry probability (F values 0.0071 to 1.49). Additionally, total port entries during DS training differed based on CIE vapor exposure (Figure 3C; CIE, F(1,739)=8.2778, p = 0.0041), sex (F(1,739)=9.0935, p = 0.00265) and potentially their interaction (CIE X Sex, F(1,739)=2.844, p = 0.092). Male CIE animals had blunted port entries overall relative to controls (main effect of CIE, F(1,391)=8.9437, p = 0.00296; session X CIE interaction, F(1,391)=10.29, p = 0.0014), but female CIE animals did not (main effect of CIE, F(1,348)=0.13, p = 0.71; session X CIE interaction, F(1,348)=0.022, p = 0.88). Overall, CIE exposure did not enhance cue learning or responsivity during training and appeared to generally reduce entries into the alcohol port, especially in male rats.
Figure 3. DS task training after vapor exposure.
A) Timeline of experiments. Rats first underwent home cage intermittent access to alcohol and ethanol vapor exposure prior to training in the DS task and subsequent cue testing. Circles indicate the number of weeks in each experimental phase. B) Port entry probability during the DS (solid lines) or NS (dotted lines) during DS task training after exposure to ethanol vapor (CIE) or control conditions. Lines with shading indicate mean +/− SEM. C) Ethanol consumption (g/kg) during DS task training after CIE or control conditions. D) Total port entries during DS task training after CIE or control conditions. E) Port entry probability during the DS (left) or NS (right) in the cue probe test (no ethanol delivered) that occurred after DS task training. Bar plots with error bars indicate mean +/− SEM. Circles represent data from individual animals. F) Ethanol consumption during cue “retraining” and testing in which ethanol was re-introduced, and the number of trials was increased across sessions, in rats that received vapor exposure (CIE) or control conditions prior to task training. Lines with shading indicate mean +/− SEM. G) Ethanol consumption during cue testing in which the amount of 15% ethanol delivered on each trial was increased from 0.1 to 0.2 ml. H) Port entry probability during the DS (solid lines) or NS (dotted lines) during cue “retraining” and testing in which ethanol was re-introduced, and the number of trials was increased across sessions. I) Port entry probability during the DS and NS during cue testing in which the amount of 15% ethanol delivered on each trial was increased from 0.1 to 0.2 ml. Lines with shading, and bar plots with error bars indicated mean +/− SEM.
After another set of drinking tests, rats underwent a cue probe test and retraining testing as conducted in Experiment 1 (Fig. 3E–I). During the cue probe test (Fig. 3E) we found no effect of CIE or sex, or any interaction for DS response probability (Fs 0.23 to 0.69), DS response latency (Fs 0.09 to 0.32), or NS response probability (Fs 0.61 to 2.38). We observed a trend towards an effect of sex on total port entries (F(1,25)=3.43, p = 0.078), but no effect of CIE or interaction (Fs 0.28 to 0.1). During retraining with increasing trial numbers (Fig 3. F and 3H) we found a trend towards an interaction between sex, CIE vapor inhalation, and test number for ethanol consumption (F(1,70)=3.844, p = 0.053), and a significant interaction of sex and CIE for DS response probability (F(1,70)=6.5379, p = 0.0127), but no effect of CIE on NS response probability (F(1,70)=0.905, p = 0.344), or interactions of CIE with other factors for this behavioral measure (Fs 0.047 to 0.37). In male rats CIE significantly reduced DS response probability across the retraining sessions (main effect of CIE, F(1,32)=5.78, p = 0.022), but did not significantly alter NS response probability (F(1,32)=2.52, p = 0.12) or total port entries (F(1,32)=0.25, p = 0.61). In contrast, in females we found no significant effect of CIE on DS response probability (main effect of CIE, F(1,38)=0.43, p = 0.51; CIE x test interaction, F(1,38)=0.009, p = 0.92). Finally, during the tests with increasing ethanol reward, pre-training CIE produced similar effects on DS port entry probability (Fig. 3G and 3I): CIE had sex-dependent effects (interaction of CIE and sex, F(1,44) = 4.14, p = 0.047) that did not depend on reward volume (interaction of CIE, sex and reward volume, F(1,44) = 1.17, p = 0.29). Prior CIE vapor inhalation reduced DS port entry probability in males (F(1,20) = 8.35, p = 0.009) but not females (F(1,24) = 0.001, p = 0.97). We found no effect of CIE on NS port entry probability (F(1,44) = 0.27, p = 0.60) or interaction of CIE with other factors (F values 0.13 to 0.32). Overall, CIE vapor inhalation prior to DS task training reduced alcohol port entry behavior during training, as well as DS-evoked port entry behavior during retraining with ethanol, in male but not female rats. Prior CIE vapor inhalation had no effect on cue-evoked behavior in the absence of alcohol delivery.
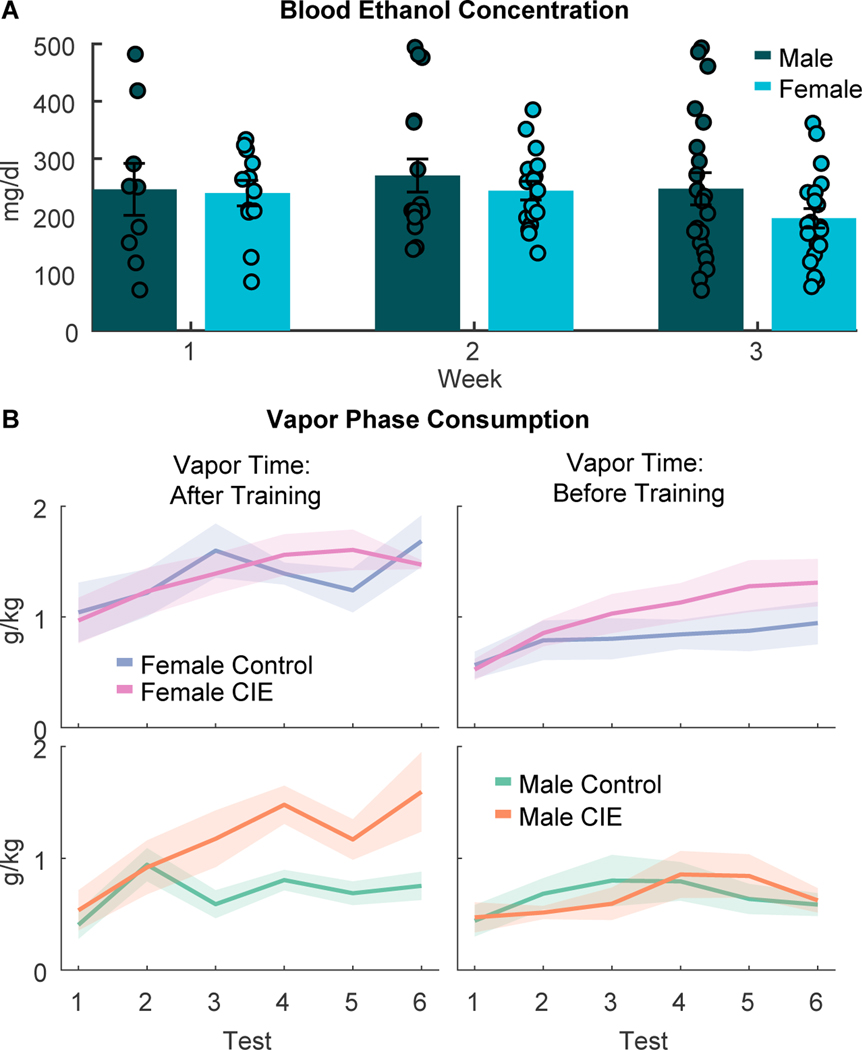
CIE vapor inhalation produced high blood ethanol concentrations in males and females, but only increased alcohol consumption in male rats
During the 3-week CIE vapor inhalation period, blood ethanol concentrations (BECs) were assessed immediately after the first vapor exposure session of the week (Fig. 4A). As expected, we observed high BECs (mean values 200–300 mg/dl) that did not differ between male and females (F(1,93)=0.587, p = 0.44), though we did see a trend towards an effect of week (F(1,93)=9.8357, p = 0.053). During the CIE period, we also assessed ethanol drinking behavior 2-times a week in 2-hr sessions occurring 6–8 hours after CIE rats were removed from vapor chambers. Overall, CIE vapor inhalation drove increased consumption across test sessions (CIE x test interaction, F(1,347)=7.85, p = 0.0054). Because we observed an interaction of sex with test session and the timing of vapor exposure (Experiment 1 versus Experiment 2; F(1,347)=4.65, p=0.032) and a trend towards an interaction of these factors with CIE (F(1,347)=3.18, p = 0.075) we split males and females for follow-up analyses. Overall, CIE male rats increased alcohol consumption relative to controls (Fig. 4B, bottom; test X CIE interaction, F(1,166)=6.87, p = 0.0096), but female CIE rats did not (Fig. 4B, top; main effect of CIE and interactions with other factors, Fs <0.58), consistent with some prior reports (Morales et al. 2015). CIE vapor inhalation also increased signs of somatic withdrawal (main effect of CIE, F(1,69)=5.6197, p = 0.02). We found no significant interaction of CIE and sex (F(1,69)=2.554, p = 0.1145) or any other main effects or interactions of other factors (Fs < 0.01 to .834) for assessment of somatic withdrawal.
Figure 4. Blood ethanol levels and ethanol consumption during the vapor exposure phase.
A) Blood ethanol concentrations (BECs) measured after the first vapor exposure of the week (Tuesday mornings) across three weeks of vapor exposure. B) Ethanol consumption (g/kg) during the twice weekly ethanol consumption tests 6–8 hours after CIE rats were removed from the vapor chambers. Rats on the left received vapor exposure (CIE) or control conditions after DS task training. Lines with shading, and bar plots with error bars indicated mean +/− SEM. Circles represent data from individual animals.
Sex-dependent effects of CIE vapor inhalation on aversion-resistant drinking
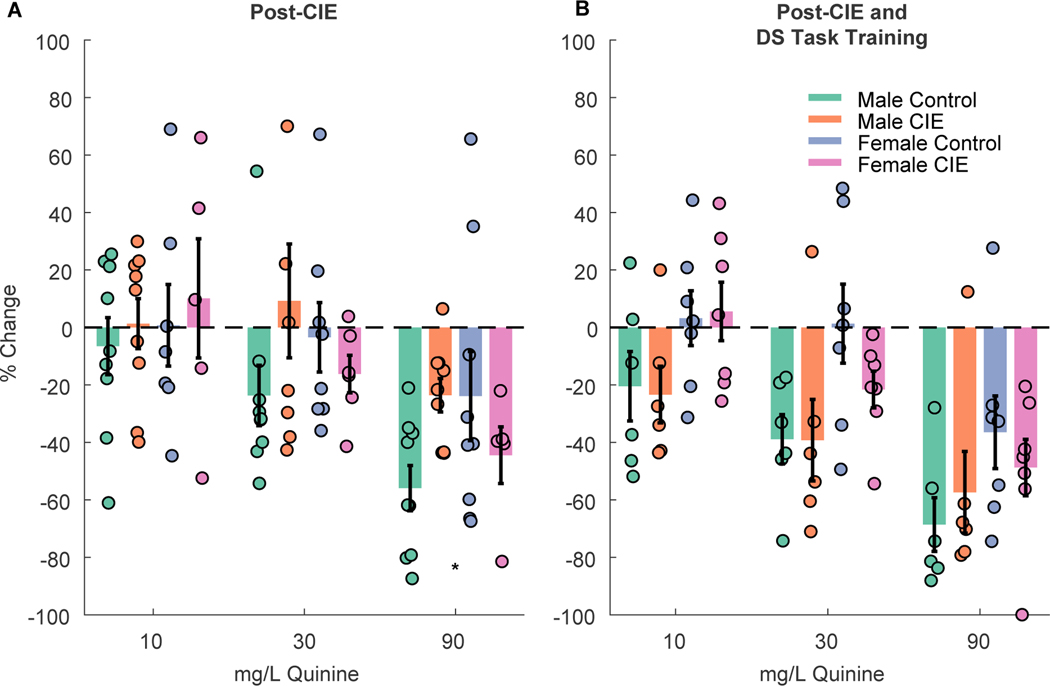
To assess “aversion-resistant” drinking after CIE vapor inhalation we measured consumption of 15% alcohol with and without quinine adulteration (Fig 5). Overall, we found that CIE vapor inhalation reduced the impact of quinine on alcohol consumption in male, but not female rats (Fig. 5A; CIE x sex interaction: F(1,151)=5.5964, p = 0.0193). When we split males and females for separate analyses we found a significant effect of quinine concentration (F(1,110)=31.63, p < 0.001) and interaction of concentration and CIE (F(1,110)= 4.32, p = 0.04) in males, but only an effect of quinine concentration in females (main effect of quinine; F(1,97)=10.91, p = 0.001; quinine X CIE, F(1,97)=1.66, p = 0.20), suggesting that CIE vapor inhalation increased aversion-resistant drinking in males but not females. When rats were retested after training in the DS task, we found significant effects of quinine concentration (Fig. 5B; F(1,96)=9.60, p = 0.0026), CIE vapor inhalation (F(1,96)=4.09, p = 0.046) and sex (F(1,96)=13.66, p < 0.001) on g/kg ethanol consumption, but no interactions between any of these factors (Fs 0.10 to 2.58), indicating that the male-specific effects of CIE on aversion resistance did not persist after task training.
Figure 5. Quinine-adulterated alcohol consumption.
Bar graphs show the % change in consumption from ethanol alone at 10, 30 and 90 mg/L quinine after exposure to ethanol vapor (CIE) or control conditions (A) and after subsequent DS task training (B). Bar plots with error bars indicated mean +/− SEM. Circles represent data from individual animals. * = p < 0.05 interaction between CIE vapor inhalation and sex.
Discussion
Here, we demonstrate that CIE vapor inhalation can enhance cue-elicited alcohol-seeking behavior, but only under specific circumstances. When rats were exposed to CIE after cue learning and were allowed to repeatedly access oral alcohol while in acute withdrawal during the vapor phase, we later saw an increase in the probability of alcohol port entries during the cue, under extinction and retraining conditions. In contrast, rats that did not receive access to alcohol during acute withdrawal did not show an increase in their responses to alcohol cues. Additionally, rats that received CIE vapor inhalation prior to cue learning did not show enhanced responses to cues, and male rats in particular engaged in less alcohol-seeking behavior during the task. While CIE increased responses to previously learned alcohol cues in both male and female rats that received acute withdrawal access, we observed sex-specific effects on some other measures. Specifically, CIE vapor inhalation increased alcohol consumption and aversion-resistant drinking selectively in male rats.
Sex differences in ethanol consumption and the effects of CIE vapor inhalation
In the current studies we found that females consumed more g/kg ethanol during homecage intermittent access and DS task training, and that CIE vapor inhalation increased ethanol consumption and “aversion-resistant” drinking in male, but not female rats. Elevated ethanol consumption in female rats is consistent with a rich prior literature documenting sex differences in alcohol consumption in rodents (Becker and Koob 2016). Female rats, including Long Evans, have been shown to consume more g/kg ethanol under both continuous (Li and Lumeng 1984; Lancaster and Spiegel 1992; Juárez and De Tomasi 1999; Lorrai et al. 2019) and intermittent access conditions (Priddy et al. 2017; Aguirre et al. 2020), as well as during self-administration (Blanchard et al. 1993; Randall et al. 2017). Whether these differences in voluntary consumption result in different blood ethanol concentrations in female and male rats remains unclear. Some studies have reported that males have higher blood ethanol concentrations after receiving identical doses of injected ethanol (Rachamin et al. 1980; Mankes et al. 1991; Morales et al. 2015), but others have reported similar blood ethanol levels (Randall et al. 2017). Blood ethanol levels have been reported to be similar at the end of a 4 hour drinking session (Marco et al. 2017), but these values likely do not correspond to peak levels during these sessions. Whether female rats are consuming more g/kg to reach similar or greater blood ethanol concentrations in comparison to male rats remains an important open question.
Our finding that CIE vapor inhalation increases alcohol consumption in male but not female rats is also consistent with some prior work (Morales et al., 2015; but see Priddy et al., 2017). This may be due in part to the heightened consumption in control females; CIE vapor inhalation drives consumption in male rats that is similar to consumption levels in control females. We also found that CIE vapor inhalation selectively impacted aversion-resistant drinking in male, but not female rats. Prior reports examining the impact of CIE vapor inhalation on aversion-resistant drinking have not, to our knowledge, included female subjects (Vendruscolo et al. 2012), though differences in other measures of compulsive alcohol seeking have been reported (Xie et al. 2019). We also found that females were generally more aversion-resistant than males, consistent with some prior reports (Fulenwider et al. 2019; Sneddon et al. 2020), but not others (Randall et al. 2017; Sneddon et al. 2018; DeBaker et al. 2020). If anything, CIE vapor inhalation made female rats more sensitive to quinine, which parallels previous findings from female mice tested in a footshock conflict procedure (Xie et al. 2019; Richard 2019). Interestingly, the effects of CIE vapor inhalation and sex were not apparent after the completion of DS task training. The reduced impact of CIE vapor inhalation could simply be due to the time since the manipulation, but the lack of sex difference at this time point is more surprising. Whether this is due to the impact of DS task training, the increasing age of the rats, or some other factor, remains to be determined. Despite our observed sex differences in baseline intake and the effects of CIE vapor inhalation on intake and aversion-resistant drinking, we saw similar effects of CIE vapor inhalation on behavioral responses to cues previously paired with alcohol in males and females. Yet, given the other sex differences we found, and the long history of male-only CIE vapor inhalation studies, it is important to consider whether prior effects reported for CIE vapor inhalation are generalizable to female rodents. Additionally, even when similar behavioral effects are reported, these effects may be driven by different psychological and neurobiological mechanisms.
Psychological mechanisms underlying CIE vapor inhalation effects on cue responsivity
The importance of access to alcohol during acute withdrawal for the cue effects reported here may shed some light on the potential psychological mechanisms underlying these CIE vapor inhalation effects. Increased alcohol consumption and seeking after CIE vapor inhalation has generally been attributed to activation of stress systems and/or negative reinforcement mechanisms (Gilpin and Koob 2010; Vendruscolo et al. 2012; Walker 2012; Koob et al. 2014; de Guglielmo et al. 2016; Tunstall et al. 2017; Somkuwar et al. 2017). While these explanations are frequently discussed in combination, they are not synonymous. Stress can also potentiate cue salience and responsivity without the opportunity for new learning via negative reinforcement (Sinha and Li 2007; Glynn et al. 2018). Our results suggest that new learning about alcohol is necessary for potentiated cue responding following CIE vapor inhalation, but we cannot rule out that a shorter delay between CIE vapor inhalation and cue testing might yield different results. Access to alcohol during acute withdrawal may have also acted independently of new learning by reducing the general severity of withdrawal symptoms, though we did not observe any differences in somatic withdrawal signs in rats that did or did not have access to oral alcohol in this phase. Access to alcohol during protracted withdrawal prior to DS cue testing or training may also have blunted the impact of CIE vapor inhalation. While our observed changes in cue-elicited behavior were gated by access to alcohol during acute withdrawal, they were not dependent on re-experiencing the cue-alcohol association, either during acute or protracted withdrawal. Instead, the patterns we observed during retraining and extended cue testing were already present during our initial cue probe test. This suggests that reappraisal of the value of the alcohol during acute withdrawal may transfer to the cue itself without additional cue-alcohol pairings.
Learning and responsivity to non-alcohol cues is impacted by CIE vapor inhalation
Under some conditions, rather than observing increases in cue responsivity, we actually saw decreases in cue reactivity and/or alcohol seeking. These findings are less surprising in the context of prior work on behavioral responses to non-alcohol cues. Deficits in learning, discrimination or reactivity to non-alcohol cues have been reported after CIE vapor inhalation (Barker et al. 2016), especially after more prolonged CIE procedures in both rats and mice (DePoy et al. 2015; Natividad et al. 2018). Yet, CIE vapor inhalation has also been reported to enhance subsequent responding to non-alcohol cues by mice, including in tests of Pavlovian-to-instrumental transfer (Shields and Gremel 2021), as well as pairwise visual discrimination and reversal learning (DePoy et al. 2013). At this point it is difficult to reconcile these disparate findings, but it is likely that both the duration and time since CIE vapor inhalation impact the direction of the effects, with longer and/or more recent exposure being more likely to produce deficits in cue-guided behavior and memory.
Conclusions
Here we found that CIE vapor inhalation potentiated responding to previously learned alcohol cues when rats had the opportunity to consume alcohol during acute withdrawal, but that it failed to enhance cue responding either in the absence of this access or when cue-alcohol training occurred after CIE. This suggests that high levels of alcohol exposure alone are not enough to produce the types of behavioral changes that result in heightened risk of relapse and craving even after long periods of abstinence. Instead, the learning that occurs during voluntary alcohol consumption and self-administration is a critical factor in the degree to which alcohol cues can control behavior. Additionally, sex differences in the ability of CIE to alter drinking, but not cue-evoked responding, indicate that altered consumption is not a prerequisite for altered alcohol cue reactivity. Together these results highlight the importance of selecting appropriate alcohol seeking and exposure models, tested in both male and female subjects, to better understand the neural and psychological mechanisms underlying alcohol addiction.
Acknowledgements:
This work was supported in part by National Institutes of Health grants R00AA025384 and R01AA028770 to JMR.
Footnotes
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Aguirre CG, Stolyarova A, Das K, et al. (2020) Sex-dependent effects of chronic intermittent voluntary alcohol consumption on attentional, not motivational, measures during probabilistic learning and reversal. PLoS One 15:e0234729. 10.1371/JOURNAL.PONE.0234729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Bryant KG, Osborne JI, Chandler LJ (2017) Age and Sex Interact to Mediate the Effects of Intermittent, High-Dose Ethanol Exposure on Behavioral Flexibility. Front Pharmacol 8:. 10.3389/FPHAR.2017.00450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Lench DH, Chandler LJ (2016) Reversal of alcohol dependence-induced deficits in cue-guided behavior via mGluR2/3 signaling in mice. Psychopharmacology (Berl) 233:235–242. 10.1007/s00213-015-4101-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF (2016) Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev 68:242–263. 10.1124/pr.115.011163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard BA, Steindorf S, Wang S, Glick SD (1993) Sex Differences in Ethanol-induced Dopamine Release in Nucleus Accumbens and in Ethanol Consumption in Rats. Alcohol Clin Exp Res 17:968–973. 10.1111/j.1530-0277.1993.tb05650.x [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F (2003) Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology (Berl) 168:208–15. 10.1007/s00213-002-1380-z [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F (2002) Effect of selective blockade of mu(1) or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology 27:391–9. 10.1016/S0893-133X(02)00302-0 [DOI] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, Ray LA (2015) The effect of alcohol priming on neural markers of alcohol cue-reactivity. Am J Drug Alcohol Abuse 41:300–8. 10.3109/00952990.2015.1044608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Crawford E, Kim S, et al. (2016) Recruitment of a Neuronal Ensemble in the Central Nucleus of the Amygdala Is Required for Alcohol Dependence. J Neurosci 36:9446–53. 10.1523/JNEUROSCI.1395-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaker MC, Moen JK, Robinson JM, et al. (2020) Unequal interactions between alcohol and nicotine co-consumption: suppression and enhancement of concurrent drug intake. Psychopharmacology (Berl) 237:967–978. 10.1007/s00213-019-05426-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, et al. (2013) Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci 110:14783–14788. 10.1073/PNAS.1308198110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Wright T, et al. (2015) Chronic alcohol alters rewarded behaviors and striatal plasticity. Addict Biol 20:345–348. 10.1111/ADB.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhardt M, Hansson AC, Spanagel R, Bilbao A (2015) Chronic intermittent ethanol exposure in mice leads to an up-regulation of CRH/CRHR1 signaling. Alcohol Clin Exp Res 39:752–62. 10.1111/acer.12686 [DOI] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, et al. (2009) Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry 66:88–94. 10.1001/archgenpsychiatry.2008.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulenwider HD, Nennig SE, Price ME, et al. (2019) Sex Differences in Aversion-Resistant Ethanol Intake in Mice. Alcohol Alcohol. 10.1093/alcalc/agz022 [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF (2010) Effects of ?-adrenoceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology (Berl) 212:431–439. 10.1007/s00213-010-1967-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Lumeng L, Koob GF (2008) Dependence-induced alcohol drinking by alcohol-preferring (P) rats and outbred Wistar rats. Alcohol Clin Exp Res 32:1688–96. 10.1111/j.1530-0277.2008.00678.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn RM, Rosenkranz JA, Wolf ME, et al. (2018) Repeated restraint stress exposure during early withdrawal accelerates incubation of cue-induced cocaine craving. Addict Biol 23:80–89. 10.1111/ADB.12475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, Lopez MF, Yanke AB, et al. (2009) Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 201:569–580. 10.1007/s00213-008-1324-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Chang S-J, Sparta DR, et al. (2010) Motivation for Alcohol Becomes Resistant to Quinine Adulteration After 3 to 4 Months of Intermittent Alcohol Self-Administration. Alcohol Clin Exp Res 34:1565–1573. 10.1111/j.1530-0277.2010.01241.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Lesscher HMB (2014) Rodent models for compulsive alcohol intake. Alcohol 48:253–64. 10.1016/j.alcohol.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez J, De Tomasi EB (1999) Sex Differences in Alcohol Drinking Patterns During Forced and Voluntary Consumption in Rats. Alcohol 19:15–22. 10.1016/S0741-8329(99)00010-5 [DOI] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, et al. (2014) Addiction as a stress surfeit disorder. Neuropharmacology 76 Pt B:370–82. 10.1016/j.neuropharm.2013.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Martin-Fardon R, Weiss F (2011) Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR 2/3 agonist LY379268 and increased functional activity of mGluR 2/3 in rats with a history of ethanol dependence. Neuropsychopharmacology 36:2762–73. 10.1038/npp.2011.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster FE, Spiegel KS (1992) Sex differences in pattern of drinking. Alcohol 9:415–420. 10.1016/0741-8329(92)90041-8 [DOI] [PubMed] [Google Scholar]
- Li T-K, Lumeng L (1984) Alcohol Preference and Voluntary Alcohol Intakes of Inbred Rat Strains and the National Institutes of Health Heterogeneous Stock of Rats. Alcohol Clin Exp Res 8:485–486. 10.1111/j.1530-0277.1984.tb05708.x [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F (2002) Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci 22:7856–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F (2003) Stimulus conditioned to foot-shock stress reinstates alcohol-seeking behavior in an animal model of relapse. Psychopharmacology (Berl) 168:184–91. 10.1007/s00213-002-1267-z [DOI] [PubMed] [Google Scholar]
- Lorrai I, Contini A, Gessa GL, et al. (2019) Operant, oral alcohol self-administration: Sex differences in Sardinian alcohol-preferring rats. Alcohol 79:147–162. 10.1016/j.alcohol.2019.04.003 [DOI] [PubMed] [Google Scholar]
- Majchrowicz E (1975) Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia 43:245–254. 10.1007/BF00429258 [DOI] [PubMed] [Google Scholar]
- Mankes RF, Glick SD, Hoeven T, LeFevre R (1991) Alcohol Preference and Hepatic Alcohol Dehydrogenase Activity in Adult Long-Evans Rats is Affected by Intrauterine Sibling Contiguity. Alcohol Clin Exp Res 15:80–85. 10.1111/j.1530-0277.1991.tb00521.x [DOI] [PubMed] [Google Scholar]
- Marco EM, Peñasco S, Hernández M-D, et al. (2017) Long-Term Effects of Intermittent Adolescent Alcohol Exposure in Male and Female Rats. Front Behav Neurosci 0:233. 10.3389/FNBEH.2017.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, McGinnis MM, McCool BA (2015) Chronic ethanol exposure increases voluntary home cage intake in adult male, but not female, Long-Evans rats. Pharmacol Biochem Behav 139:67–76. 10.1016/j.pbb.2015.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Steinman MQ, Laredo SA, et al. (2018) Phosphorylation of calcium/calmodulin-dependent protein kinase II in the rat dorsal medial prefrontal cortex is associated with alcohol-induced cognitive inflexibility. Addict Biol 23:1117–1129. 10.1111/adb.12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenheimer DJ, Wang K, Haimbaugh A, et al. (2019) Recruitment and disruption of ventral pallidal cue encoding during alcohol seeking. Eur J Neurosci 50:3428–3444. 10.1111/ejn.14527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Kowalczyk WJ, Phillips KA, et al. (2018) Exacerbated Craving in the Presence of Stress and Drug Cues in Drug-Dependent Patients. Neuropsychopharmacology 43:859–867. 10.1038/npp.2017.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J, et al. (2009) Cocaine craving and use during daily life. Psychopharmacology (Berl) 207:291–301. 10.1007/s00213-009-1655-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priddy BM, Carmack SA, Thomas LC, et al. (2017) Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav 152:61–67. 10.1016/j.pbb.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachamin G, Macdonald JA, Wahid S, et al. (1980) Modulation of alcohol dehydrogenase and ethanol metabolism by sex hormones in the spontaneously hypertensive rat. Effect of chronic ethanol administration. Biochem J 186:483–490. 10.1042/bj1860483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Jury NJ, Kocharian A, et al. (2017) Chronic EtOH effects on putative measures of compulsive behavior in mice. Addict Biol 22:423–434. 10.1111/adb.12342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Stewart RT, Besheer J (2017) Sex differences in alcohol self-administration and relapse-like behavior in Long-Evans rats. Pharmacol Biochem Behav 156:1–9. 10.1016/j.pbb.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria R, Baltz ET, Gremel CM (2018) Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nat Commun 2018 91 9:1–11. 10.1038/s41467-017-02615-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM (2019) Female Rodents Yield New Insights into Compulsive Alcohol Use and the Impact of Dependence. Alcohol Clin Exp Res acer.14129. 10.1111/acer.14129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Ambroggi F, Janak PH, Fields HL (2016) Ventral Pallidum Neurons Encode Incentive Value and Promote Cue-Elicited Instrumental Actions. Neuron 90:1165–73. 10.1016/j.neuron.2016.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Stout N, Acs D, Janak PH (2018) Ventral pallidal encoding of reward-seeking behavior depends on the underlying associative structure. Elife 7:e33107. 10.7554/eLife.33107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Chang S-J, Simms JA, et al. (2013) Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci 16:1094–100. 10.1038/nn.3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Simms JA, Lei K, et al. (2015) D-Serine and D-Cycloserine Reduce Compulsive Alcohol Intake in Rats. Neuropsychopharmacology 40:2357–2367. 10.1038/npp.2015.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields CN, Gremel CM (2021) Prior Chronic Alcohol Exposure Enhances Pavlovian-to-Instrumental Transfer. Alcohol. 10.1016/J.ALCOHOL.2021.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CSR (2007) Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev 26:25–31. 10.1080/09595230601036960 [DOI] [PubMed] [Google Scholar]
- Sneddon EA, Ramsey OR, Thomas A, Radke AK (2020) Increased Responding for Alcohol and Resistance to Aversion in Female Mice. Alcohol Clin Exp Res acer.14384. 10.1111/acer.14384 [DOI] [PubMed] [Google Scholar]
- Sneddon EA, White RD, Radke AK (2018) Sex Differences in Binge-Like and Aversion-Resistant Alcohol Drinking in C57 BL /6J Mice. Alcohol Clin Exp Res 43:acer.13923. 10.1111/acer.13923 [DOI] [PubMed] [Google Scholar]
- Somkuwar SS, Vendruscolo LF, Fannon MJ, et al. (2017) Abstinence from prolonged ethanol exposure affects plasma corticosterone, glucocorticoid receptor signaling and stress-related behaviors. Psychoneuroendocrinology 84:17–31. 10.1016/J.PSYNEUEN.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiang MT, Janak PH (2006) Alcohol seeking in C57BL/6 mice induced by conditioned cues and contexts in the extinction-reinstatement model. Alcohol 38:81–8. 10.1016/j.alcohol.2006.05.004 [DOI] [PubMed] [Google Scholar]
- Tunstall BJ, Carmack SA, Koob GF, Vendruscolo LF (2017) Dysregulation of brain stress systems mediates compulsive alcohol drinking. Curr Opin Behav Sci 13:85–90. 10.1016/j.cobeha.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, et al. (2012) Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci 32:7563–71. 10.1523/JNEUROSCI.0069-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM (2012) Conceptualizing withdrawal-induced escalation of alcohol self-administration as a learned, plasticity-dependent process. Alcohol 46:339–348. 10.1016/j.alcohol.2012.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KL, Schimmel JS (2008) Effect of naltrexone during extinction of alcohol-reinforced responding and during repeated cue-conditioned reinstatement sessions in a cue exposure style treatment. Alcohol 42:553–63. 10.1016/j.alcohol.2008.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Buck LA, Bryant KG, Barker JM (2019) Sex differences in ethanol reward seeking under conflict in mice. Alcohol Clin Exp Res acer.14070. 10.1111/acer.14070 [DOI] [PMC free article] [PubMed] [Google Scholar]