Abstract
PilQ is a member of the secretin family of outer membrane proteins and is specifically involved in secretion of type IV pili in Neisseria meningitidis, Neisseria gonorrhoeae, and Pseudomonas aeruginosa. The quaternary structure of PilQ from N. meningitidis was analyzed by transmission electron microscopy by using a negative stain. Single particle averaging was carried out with a total data set of 650 individual particles, which produced a projection map generated from 296 particles at an estimated resolution of 2.6 nm. Oligomeric PilQ adopts a donut-like structure with an external ring that is 16.5 nm in diameter surrounding a central cavity that is 6.5 nm in diameter. Self-rotation and power spectrum analysis demonstrated the presence of 12-fold rotational symmetry, showing that PilQ is organized as a ring of 12 identical subunits. A model of the type IV meningococcal pilus fiber, based on the X-ray crystal structure of the N. gonorrhoeae pilin subunit, fitted neatly into the cavity, demonstrating how PilQ could serve as a channel for the growing pilus fiber.
Infection of humans by pathogenic bacteria initially requires attachment of the bacteria to specific host cell surfaces. Among the many virulence factors employed by the gram-negative bacterium Neisseria meningitidis and the closely related organism Neisseria gonorrhoeae, type IV pili are most important in the early stages of infection of human hosts (36). Type IV pili form filaments of regularly ordered protein (length, 1,000 to 4,000 nm; diameter, 5 to 6 nm) which emanate from the surface of the bacterium and mediate attachment to specific epithelial tissue receptors during tissue colonization (18, 56). In addition to this adhesin role, type IV pili are also involved in a variation of target tissue specificity (22), natural competence for transformation (16, 58), twitching motility on solid and mucosal surfaces (50), and bacterial autoagglutination (51).
Type IV pili are expressed by many pathogenic organisms, including Pseudomonas aeruginosa, Vibrio cholerae, and enteropathogenic Escherichia coli (17, 19, 48). The pilin subunits of type IV pili from different species display a high degree of identity in the N-terminal domain that is predicted to form the central helical core of the pilus filament (34). Many of the accessory genes involved in prepilin processing, inner membrane transport, assembly, and outer membrane translocation are homologous to genes involved in the general type II secretory pathway, also termed the secreton (37). Homologous systems mediate DNA uptake in Bacillus subtilis (1) and filamentous phage morphogenesis (40, 41). Biogenesis of type IV bundle-forming pili in enteropathogenic E. coli depends on a cluster of 14 bfp genes located on a large plasmid found in many enteropathogenic E. coli strains (45, 46). Expression of these 14 genes in a K-12 laboratory strain of E. coli that is normally nonpiliated is sufficient to elicit bundle-forming pilus formation (46). Type IV pilus biogenesis and type II secretion in P. aeruginosa are regulated by approximately 30 chromosomal genes (2). The exoproteins secreted via this pathway include diverse proteins, such as exotoxin A, elastase, and lipase, as well as type IV pili (4). In addition to the pilin subunit, the only P. aeruginosa pilus biogenesis molecule whose function is known is PilD. This protease cleaves the conserved hydrophobic leader peptide from prepilin subunits (49).
Very little is known about neisserial pilus biogenesis, and the genes encoding the component proteins are scattered throughout the N. meningitidis and N. gonorrhoeae genomes (55). The only pilus biogenesis proteins that are associated with the outer membrane and have been characterized to date are PilC, PilP, and PilQ. Neisserial PilC− mutants show a dramatic reduction in piliation (23, 31). Furthermore, PilC has been implicated as a putative adhesin and is also found on the tip of the pilus fiber (22, 39). Mutants that do not express PilP are also nonpiliated, and it seems that this lipoprotein is required for stable expression of the multimeric form of PilQ (6).
PilQ of pathogenic Neisseria strains is an antigenically conserved, abundant outer membrane protein which forms a large multimer composed of 10 to 12 subunits (6, 7, 55). It is resistant to sodium dodecyl sulfate (SDS) treatment or heating, and the conserved C-terminal residues are necessary for functioning and expression of the multimeric form (6, 53). Mutants that express defective forms of the protein lack pili and do not have pilus-associated phenotypes. The N. meningitidis PilQ monomer has a polymorphic region in its N terminus; an octapeptide repeat occurs four to seven times, and this is a type of variation that is unprecedented in bacteria. The C terminus of PilQ exhibits identity with members of the secretin family of proteins required for translocation of macromolecules across the outer membrane (40). These proteins include Klebsiella oxytoca PulD (32, 33), Yersinia enterocolitica YscC (25), Salmonella InvG (5), Erwinia chrysanthemi OutD (43), and P. aeruginosa XcpQ (3) and PilQ (30), which secrete a variety of proteins. All of these secretins oligomerize to form detergent-stable homocomplexes consisting of 10 to 14 subunits (21, 24, 28, 43) around a central channel through which the secreted macromolecules are thought to be translocated. The PilQ proteins are specifically required for biogenesis of type IV pili in N. meningitidis, N. gonorrhoeae, and P. aeruginosa (6, 30, 53). PilQ− and PilP− mutants release PilC, indicating that PilQ, PilP, and PilC may interact during the terminal stages of pilus biogenesis (6).
With the exception of the K. oxytoca PulD-PulS system (32, 33), previous studies of secretins by electron microscopy (EM) have been limited to descriptions of the morphology of secretin particles in negatively stained preparations (3–5, 8, 25, 28, 52). Although the observations made have been useful in establishing that secretins adopt a ring-like shape in projection, there has been no attempt to assign symmetry to the secretin complexes concerned. It is tempting to assume that these complexes have some form of rotational symmetry around the central cavity through which the secreted molecules pass. One way of acquiring evidence for this hypothesis is to collect data for a large number of different particles and calculate an averaged projection map for the secretin complex. The averaged map can then be subjected to analysis to determine what (if any) symmetry terms are present. A power spectrum of the PulD-PulS complex derived from single particle analysis established that it possessed 12-fold rotational symmetry, which was subsequently used to generate a low-resolution three-dimensional model (32). Given that the secretins are a large family of proteins from different organisms and mediate secretion of distinct macromolecular substrates, there is no reason to suppose that other members of the family necessarily have the same quaternary structure. Quaternary structure is the first level of detail that can be assigned to a macromolecular assembly and is important because it places limitations on the types of models that can be constructed for pilus biogenesis.
We therefore conducted an analysis of the N. meningitidis PilQ complex by using single particle averaging methods which enabled us to calculate a projection structure at a defined resolution and assign a quaternary structure to it. There are several factors that favor secretin characterization in N. meningitidis. First, this bacterium contains only one secretin, which makes it a simpler model system than, for example, P. aeruginosa, which has genes that encode at least five secretins (www.pseudomonas.com). Second, meningococcal PilQ is naturally expressed at high levels, and so no surrogate host is required for overexpression and purification. Another key advantage of studying the Neisseria type IV pilus biogenesis system is that there is a model for the pilus fiber, which was constructed from the crystal structure of the N. gonorrhoeae pilin (9, 10, 34). Combining the derived N. meningitidis pilus model with the PilQ projection map presented here revealed that the PilQ oligomeric ring has internal dimensions that can accommodate the pilus fiber.
MATERIALS AND METHODS
Bacterial strains.
Meningococcal strain M1080 was grown overnight on 5% blood agar in an atmosphere containing 10% CO2 before harvesting. Knockout strain M1080 with a transposon mutant disrupting pilQ in the ATG start codon (53) was used as a negative control in the immunoblotting analysis.
Purification of meningococcal PilQ.
Meningococcal cells were ultrasonically disrupted on ice in the presence of the protease inhibitors phenylmethylsulfonyl fluoride, leupeptin, pepstatin A, and 2-mercaptoethanol (Sigma). The cell envelope fraction was extracted with 4% (wt/vol) deoxycholate in buffer A (100 mM NaCl, 20 mM KH2PO4, 50 mM KCl, 5 mM EDTA, 10 mM Tris; pH 7.5) and pelleted. The pellet was subsequently dissolved in a buffer containing 4% (wt/vol) SDS and 20% (vol/vol) glycerol and dialyzed in buffer A with 0.1% (wt/vol) SDS. After concentration in Microsep300 columns (Filtron), the preparation was loaded on a continuous 15 to 40% (wt/vol) sucrose gradient and ultracentrifuged at 106,000 × g and 4°C for 24 h in an SW41 rotor (Beckman). PilQ-containing fractions were monitored by immunoblotting and were dialyzed in buffer A with no detergent. PilQ complexes were concentrated again in Microsep300 columns before they were used.
Immunoblotting.
PilQ in whole-cell lysates and fractions during purification was detected by immunoblotting by using rabbit polyclonal antibodies raised against purified N. meningitidis PilQ from strain M1080 at a dilution of 1:4,000 (53). The conditions used for sample preparation, SDS-polyacrylamide gel electrophoresis (PAGE), electroblotting, and antigen detection have been described previously (54).
Sample purity.
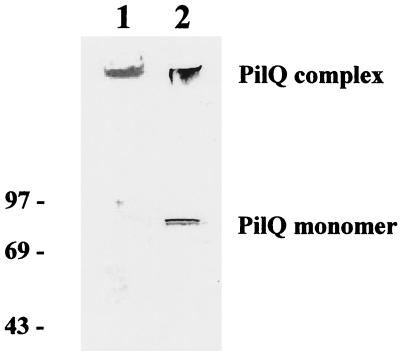
A sample SDS-PAGE–immunoblot gel indicating the quality and purity of the preparation used for single-particle analysis is shown in Fig. 1. Gels were also silver stained and were found to be negative for proteins other than PilQ, including PilP (∼20 kDa). The results of immunoblotting experiments performed with antibodies against PilE, PilC, and lipopolysaccharide were also all negative, demonstrating that the sample used contained isolated PilQ complex.
FIG. 1.
Detection of purified PilQ by SDS-PAGE and immunoblotting. Lane 1 shows the results of SDS-PAGE and Coomassie blue staining of purified PilQ; the purified PilQ complex is located at the well-stacking gel interface. For lane 2, after SDS-PAGE and electroblotting the filter was reacted with anti-meningococcal PilQ rabbit antiserum; the reactive material immediately below the number in this lane was the PilQ complex that was retained in the well and in the upper part of the stacking gel. The locations of the PilQ complex and monomer (∼80 kDa) are indicated.
EM sample preparation and image scanning.
Samples of PilQ were adsorbed to freshly glow-discharged carbon-coated grids (no. 300–400) essentially as previously described (20). However, the grids were incubated in samples containing PilQ (1 mg/ml) for 2 to 3 min; then they were washed for 15 s in water and finally incubated for 30 s in 2% (wt/vol) uranyl acetate. Micrographs (calibrated magnification, ×37,500) were obtained with a Philips CM100 operating at acceleration voltage of 100 keV. Images were recorded in transmission EM low-dose mode. Micrographs were subsequently scanned with a Zeiss SCAI densitometer in 14-μm increments, corresponding to a pixel size of 3.7 Å at the specimen level.
Single particle alignment.
Image analysis and single particle alignment were performed by using the interactive SPIDER and WEB packages (13, 14). PilQ complexes were manually selected (15) by using a fixed square box size of 65 by 65 pixels (240 by 240 Å), and the densities were normalized and Fourier filtered. Particles were then aligned by using two stages of cross-correlation: reference-free alignment based on a global average for all selected particles and subsequent circular rotation and translational shifts (15). Aligned particles were then classified by correspondence analysis (26, 27) and hierarchical ascendant clustering (12, 35). Dendrograms were created at defined thresholds and viewed in the graphical interface WEB; particles were grouped together, and averages were created. Higher-level symmetry was determined by using self-orientational analysis, in which multiple correlation peaks were found by comparing a rotated image with the same nonrotated reference image. The resolution of the final PilQ average was determined to be 2.6 nm by using cross-resolution comparisons of two sets of data subaverages and phase residual analysis (14).
Pilin modelling.
A three-dimensional homology model for the N. meningitidis pilin subunit was constructed based on the X-ray crystal structure of the homologous N. gonorrhoeae pilin subunit (PDB accession code 2PIL), using the program Modeller (42). A model of the N. meningitidis pilus fiber was then constructed by applying the transformations given in the 2PIL PDB header, based on the pilus fiber model described previously (9, 10, 34).
RESULTS
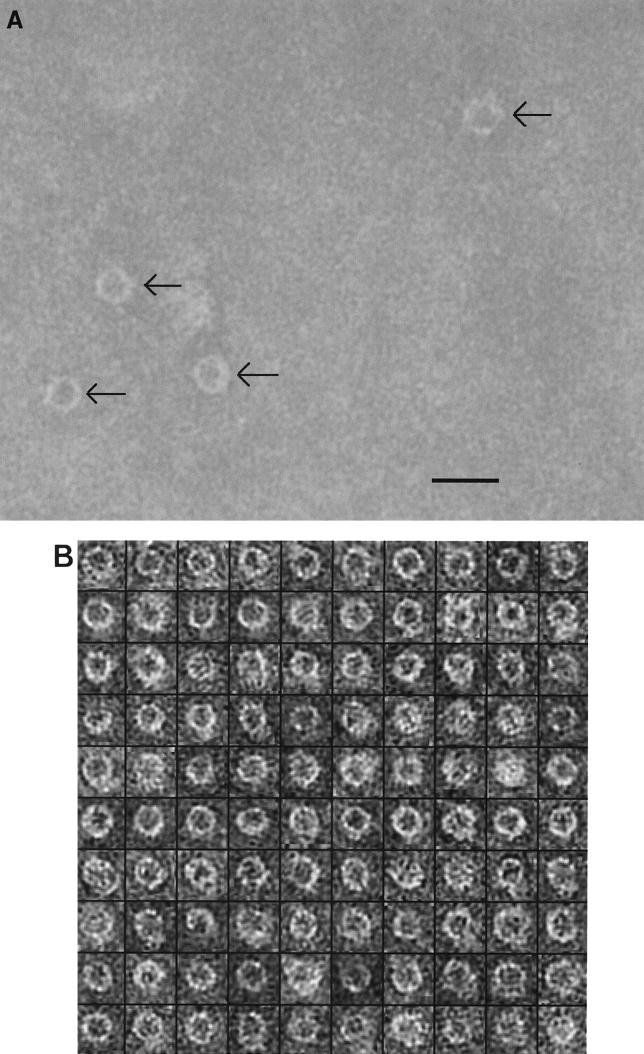
An electron micrograph of a negatively stained PilQ sample is shown in Fig. 2A. The detergent-solubilized PilQ complexes formed ring-shaped particles, each of which was approximately circular and contained a central cavity; thus, they were similar in appearance to secretin preparations observed previously (3). Although the particles may be crudely described as circular, around the circumference of each particle a ring of high-contrast stain with some depth and protruding structures could be seen. From the unprocessed micrograph data, the PilQ complexes could be estimated to have diameters of approximately 15.5 to 16.5 nm and 6.0- to 6.5-nm-diameter cavities in the central region. We found that PilQ consistently adsorbed to grids with low affinity, and therefore comparatively high protein concentrations were required. Protease-treated XcpQ also adheres poorly to EM grids (4), and a previous study of P. aeruginosa PilQ recorded only a few particles (3), so this may be a general property of secretins. Figure 2B shows a montage of selected and oriented particles; the majority of the particles were oriented with the cavity face-on, indicating that the meningococcal PilQ complex preferentially bound to the grids via one face. In the 40 micrographs recorded, no particles with a side view of PilQ were seen. It should be noted that binding via a preferred face is a commonly observed phenomenon in EM studies and is a consequence of favored electrostatic and steric interactions between the specimen and the grid. In several particles some detail could be seen in the central cavity region. It was difficult, however, to ascertain whether this was a consequence of the presence of extraneous material in the cavity, particle skewing relative to the support film, or an occasional staining artifact. Including these particles diminished the quality of alignments and averaging, so ambiguous particles were not included in our analysis.
FIG. 2.
(A) Electron micrograph of negatively stained, detergent-solubilized PilQ complexes (arrows). Scale bar = 20 nm. (B) Montage of 100 selected PilQ particles after Fourier filtering and contrast normalization. The montage is representative of the total sample population (n = 650).
Single-particle analysis is a method that was developed for studying macromolecular complexes by EM. Each particle contains projection information about the three-dimensional structure of the object, but for individual particles the signal/noise ratio is low and so fine structural detail cannot be discerned. By averaging the information for many hundred particles, the signal/noise ratio and hence the resolution of the image are improved. This procedure takes account of the different orientation of each particle on the grid. Single-particle analysis was performed with an initial data set consisting of 650 individual PilQ particles that were clearly and evenly stained, and visual inspection of a montage of all of the particles after rotational and translational alignments showed that the procedure worked excellently. Using reference-free alignment, reference-based alignment (11), or a combination of the two methods, we obtained very similar results, probably because the regular size of the central stain-excluding cavity and the sharply defined particle border aided correct alignment. Correspondence analysis of the data set supported the assumption that the particles examined had the same orientation with respect to the specimen film. This assumption was confirmed by repeating the analysis with the same data set but using an inverted reference particle (either a global average or a selected reference) and comparing the classification hierarchy following realignment. Only one orientation was present since the pattern of classifications for the inverted data sets was identical to that of the original data set.
Single-particle analysis effectively classifies particles into different groups depending on their projection shapes; in the case of the meningococcal PilQ complex, the 650-particle ensemble was remarkably uniform, with all main groups having a similar appearance. An averaged projection for the major group, generated by using 296 closely clustered particles, is shown in Fig. 3A. A closely spaced contour was found to delineate the perimeter of the complex, which had a maximum diameter of 16.5 nm. The central cavity was also sharply defined and had a regular diameter of 6.5 nm. The ring also contained features around the circumference that could plausibly correspond to individual PilQ subunits. Examination of the average stain distribution across the diameter of the complex showed that for each feature the distance from the outside of the complex to the inside face of the cavity was at most 5.0 to 5.5 nm (Fig. 3B). Given that the molecular mass of the PilQ monomer is approximately 80 kDa, it is reasonable to infer that each feature corresponded to an individual subunit.
FIG. 3.
(A) Nonsymmetry averaged projection of PilQ (n= 296; ascendant classification threshold, 0.04; sampling interval, 0.47 nm). Scale bar = 6 nm. (B) Stain density across the diameter of the averaged PilQ projection.
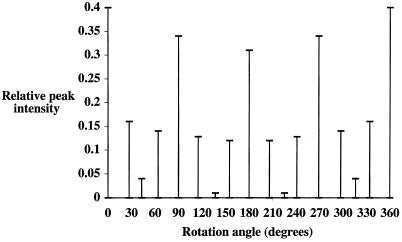
The projection map in Fig. 3A was examined for internal symmetry and provided clues about assembly of the complex. One analytical method is to use self-orientational analysis, in which two copies of the map are rotated relative to each other and compared for peak correlation. If higher-order symmetry is present in the map, this is apparent from peaks at certain angles in the multiple-peak orientation search. In this analysis, large peaks were readily apparent at 90° intervals, and there was a subset of smaller but significant peaks at 30° intervals (Fig. 4). These data demonstrated that the PilQ complex possesses 12-fold symmetry since periodic repeats were seen at 180°, 90°, and 30° intervals, reflecting strong 2- and 4-fold correlations and relatively weaker 12-fold correlations. The somewhat stronger signal intensities of the 2- and 4-fold symmetries were probably due to two factors. First, the spatial resolution (2.6 nm) limited the finer detail of each domain which could be identified; for example, the divisions between the 12 5-nm domains were not exactly delineated. This allowed the stronger 2- and 4-fold symmetries to dominate the projection and was apparent when 12-fold symmetry averaging was applied to the PilQ complex; the peak densities of the 12 discrete domains were averaged out, and the fine detail of individual domains was lost (data not shown). Second, the PilQ complex also exhibited some conformational distortion (some particles were not aligned perfectly face-on), which led to partial squaring of the edges of the ring, which in turn biased the 4-fold periodicity.
FIG. 4.
Multiple-peak orientation search for the PilQ average (see Fig. 3A). A full ring search detecting 360 maximum peaks was employed. For relative peak intensities of >0.04, the variation from exact 30° repeats was no greater than ± 5°.
The preliminary assignment of 12-fold rotational symmetry to the meningococcal PilQ complex was confirmed by several additional assessments of the data. The 12-fold repeat was found consistently by using orientational analysis with projection data created from a variety of averages, including less and more data (n = 230, n = 296, and n = 540). Reclassification of the original data set based on novel global averages rotated through 30° intervals (which should be structurally equivalent for a dodecamer) showed in each case that the structures of the classification hierarchies at a variety of thresholds were identical. Finally, rotational power spectrum analysis of the projection in Fourier space showed the presence of 12-fold symmetry with additional 2-, 3-, 4-, and 6-fold symmetries (data not shown).
A model has been proposed for the N. gonorrhoeae type IV pilus, based on the pilin subunit crystal structure (34). Although there are regions of sequence variability, the overall folds of N. meningitidis and N. gonorrhoeae pilin structures are likely to be very similar. This allowed us to construct a homology model for the N. meningitidis pilin subunit, based on the N. gonorrhoeae pilin crystal structure. From this structure, we generated a model for the N. meningitidis pilus fiber and superimposed it onto the PilQ complex projection map (data not shown). The pilus helix fits tightly in the central cavity, and it seems that the PilQ multimer can accommodate the emerging pilus fiber without serious structural rearrangement.
DISCUSSION
The results presented here established that the N. meningitidis PilQ complex is a dodecamer with 12-fold rotational symmetry. The quaternary structure of only one other secretin, K. oxytoca PulD, has been established to date (32), and the results have important ramifications for secretin assembly, secretion, and pilus biogenesis. Our assignment of quaternary structure to PilQ was based on the presence of strong multiples contributing to 12-fold symmetry peaks in our projection map. Given that PilQ and PulD show much higher sequence homology in the C-terminal portions of their amino acid sequences, this suggests that at least some of the conservation arises from formation of a quaternary structure.
It should be appreciated that there are significant differences between types of secretins involved in the general secretory pathway type II secretion process (e.g., PulD and XcpQ) and the homologous systems that are required for type IV pilus biogenesis; these molecules translocate different substrates, are present in different organisms, and exhibit substantial sequence divergence, particularly in the N terminus but also in the C terminus. Although structural and functional analyses have been performed for two secretins of the general secretory pathway, XcpQ (4) and PulD (32, 33), only one study has addressed the structure of a secretin engaged in type IV pilus biogenesis, Pseudomonas PilQ (3). In this case, no conclusions regarding the quaternary structure of the oligomeric complexes were reached, although it was clear that the PilQ complex possessed a ring-like structure. The original estimates of the P. aeruginosa PilQ complex average diameter (18.3 ± 1.2 nm) and internal cavity diameter (5.3 ± 0.8 nm) (n = 17) are comparable to the values presented here. It should also be appreciated that in Pseudomonas studies, it is impossible to know which of the five or more secretin homologues is actually observed in each image since they are simultaneously expressed and also copurify. The data presented here for the meningococcal PilQ complex are based on an analysis of the secretin expressed in situ in a host bacterium in which only one such protein complex exists. In addition to these considerations, type IV pilus expression is quite different in Neisseria and Pseudomonas species; the pilus-associated competence for transformation observed throughout the life cycles of neisserial strains is not found in P. aeruginosa.
Although the central cavity in the N. meningitidis PilQ projection map was stained deeply, it was difficult to determine in projection whether it represented a continuous hole through the center of the complex or a partial depression in one face. For example, photosystem II is a higher plant thylakoid membrane protein complex which has a cavity that is localized to the lumenal membrane face (47). The multi-drug-resistance protein P-glycoprotein also has a cavity localized to one side of the membrane (38). Crystallographic examination of small pseudocrystals of the secretin XcpQ from P. aeruginosa (4) suggested that the central cavity may contain significant protein mass, although a complete three-dimensional model would be required to determine the detailed nature of the cavity. The projection data and image analysis results show that the meningococcal PilQ complex adhered to the support film predominantly in one orientation, a feature commonly observed in many single-particle analysis studies (11). A recent study of the Pseudomonas PilQ homologue XcpQ showed that this secretin adopted either a closed ring or horseshoe conformation (4), and preparations of the E. coli PapC usher exhibit similar conformational variability (52). By contrast, the PilQ preparations examined here were structurally homogeneous.
It is unclear how the specificity for individual secreted macromolecules is conferred; in an attempt to obtain some insight into this problem, a model for the assembled pilus fiber was superimposed on the projection model for PilQ (data not shown). It is clear that an emerging pilus fiber can be accommodated by PilQ without serious structural rearrangement. A recent study by Wolfgang et al. (57) provided evidence that type IV pili are indeed translocated in their assembled state and therefore provides some justification for this hypothesis.
The question of the gated nature of secretins has been a matter of some debate (40) and is related to the nature of the cavity in PilQ. Recent work (29, 32, 33) indicates that secretins are electrophysiologically gated channels, although this does not exclude the possibility that they can be closed by physical interactions, such as those enforced by a pilus fiber. Clearly, more information on the three-dimensional structure of PilQ and the other secretins is needed in order to establish whether gating occurs by blockage of the pore and what mechanisms might exist for reversing this process to allow passage of the pilus.
The recently described low-resolution crystal structure of the bacteriophage phi29 DNA packaging motor has some interesting structural parallels with the secretins (44). Both structures exhibit 12-fold rotational symmetry around a central cavity through which substrate passage apparently occurs. The secretins may therefore be members of an even larger family of proteins which have a common quaternary organization and mediate passage of macromolecular substrates.
ACKNOWLEDGMENTS
This work was supported in part by the North of England Structural Biology Consortium (NESBIC) funded by the BBSRC and the Norwegian Research Council. J.P.D. is a Fellow of the Lister Institute of Preventive Medicine.
We thank P. Bullough, A. Kitmitto, M. Rosenberg, and G. Verlarde for useful discussions.
REFERENCES
- 1.Albano M, Breitling R, Dubnau D A. Nucleotide sequence and genetic organization of the Bacillus subtilis comG operon. J Bacteriol. 1989;171:5386–5405. doi: 10.1128/jb.171.10.5386-5404.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alm R A, Mattick J S. Identification of a gene PilV required for type 4 fimbrial biogenesis in P. aeruginosa whose product possesses a pre-pilin-like leader sequence. Gene. 1997;192:89–98. doi: 10.1111/j.1365-2958.1995.tb02413.x. [DOI] [PubMed] [Google Scholar]
- 3.Bitter W, Koster M, Latjinhouwers M, de Cock H, Tommassen J. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol Microbiol. 1998;27:209–219. doi: 10.1046/j.1365-2958.1998.00677.x. [DOI] [PubMed] [Google Scholar]
- 4.Brok R, Van Gelder P, Winterhalter M, Ziese U, Koster A J, de Cock H, Koster M, Tommassen J, Bitter W. The C-terminal domain of the Pseudomonas secretin XcpQ forms oligomeric rings with pore activity. J Mol Biol. 1999;294:1169–1179. doi: 10.1006/jmbi.1999.3340. [DOI] [PubMed] [Google Scholar]
- 5.Crago A M, Koronakis V. Salmonella InvG forms a ring-like multimer that requires the InvH lipoprotein for outer membrane localization. Mol Microbiol. 1998;30:47–56. doi: 10.1046/j.1365-2958.1998.01036.x. [DOI] [PubMed] [Google Scholar]
- 6.Drake S, Sandstedt S A, Koomey M. PilP, a pilus biogenesis lipoprotein in Neisseria gonorrhoeae, affects expression of PilQ as a high molecular mass multimer. Mol Microbiol. 1997;23:657–668. doi: 10.1046/j.1365-2958.1997.2511618.x. [DOI] [PubMed] [Google Scholar]
- 7.Drake S L, Koomey M. The product of the PilQ gene is essential for the biogenesis of type IV pilus in N. gonorrhoeae. Mol Microbiol. 1995;18:975–986. doi: 10.1111/j.1365-2958.1995.18050975.x. [DOI] [PubMed] [Google Scholar]
- 8.Drummelsmith J, Whitfield C. Translocation of group 1 capsular polysaccharide to the surface of Escherichia coli requires a multimeric complex in the outer membrane. EMBO J. 2000;19:57–66. doi: 10.1093/emboj/19.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forest K T, Bernstein S L, Getzoff E D, So M, Tribbick G, Geysen H M, Deal C D, Tainer J A. Assembly and antigenicity of the Neisseria gonorrhoeae pilus mapped with antibodies. Infect Immun. 1996;64:644–652. doi: 10.1128/iai.64.2.644-652.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forest K T, Tainer J A. Type-4 pilus-structure: outside to inside and top to bottom—a minireview. Gene. 1997;192:165–169. doi: 10.1016/s0378-1119(97)00008-5. [DOI] [PubMed] [Google Scholar]
- 11.Frank J. Electron tomography: 3-D imaging with the transmission electron microscope. New York, N.Y: Plenum Press; 1992. [Google Scholar]
- 12.Frank J, Penczek P, Grassucci R, Srivastav S. 3-D reconstruction of the 70S ribosome in ice: the distribution of ribosomal RNA. J Cell Biol. 1991;115:597–605. doi: 10.1083/jcb.115.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank J, Radermacher M, Penczek P, Zhu J, Ladjadj M, Leith A. SPIDER and WEB: processing and visualisation of images in 3-D electron microscopy and related fields. J Struct Biol. 1996;116:190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 14.Frank J, Verschoor A, Boublik M. Computer averaging of electron micrographs of 40S ribosomal sub-units. Science. 1981;214:1353–1355. doi: 10.1126/science.7313694. [DOI] [PubMed] [Google Scholar]
- 15.Frank J, Wagenknecht T. Automatic selection of molecular images from electron micrographs. Ultramicroscopy. 1984;12:169–176. [Google Scholar]
- 16.Frøholm L O, Jyssum K, Bøvre M. Electron microscopical and cultural features of Neisseria meningitidis competence variants. Acta Pathol Microbiol Scand. 1973;81:525–537. doi: 10.1111/j.1699-0463.1973.tb02238.x. [DOI] [PubMed] [Google Scholar]
- 17.Giron J A, Ho A S Y, Schoolnik G K. An inducible bundle forming pilus of enteropathogenic E. coli. Science. 1991;254:710–713. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- 18.Heckels J E. Structure and function of pili of pathogenic Neisseria species. Clin Microbiol Rev. 1989;2:S66–S73. doi: 10.1128/cmr.2.suppl.s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hobbs M, Mattick J S. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein secretion apparatus: a general system for the assembly of surface associated protein complexes. Mol Microbiol. 1993;10:233–243. doi: 10.1111/j.1365-2958.1993.tb01949.x. [DOI] [PubMed] [Google Scholar]
- 20.Holzenburg A, Shephard F H, Ford R C. Localisation of the oxygen evolving complex of photosystem II by Fourier difference analysis. Micron. 1994;25:447–451. [Google Scholar]
- 21.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jönsson A, Ilver D, Falk P, Normark S. Sequence changes in the pilus subunit lead to tropism variation of Neisseria gonorrhoeae to human tissue. Mol Microbiol. 1994;13:403–416. doi: 10.1111/j.1365-2958.1994.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 23.Jönsson A, Normark S. Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly. EMBO J. 1991;10:477–488. doi: 10.1002/j.1460-2075.1991.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazmierczak B I, Mielke D L, Russel M, Model P. PIV, a filamentous phage protein that mediates phage export across the bacterial-cell envelope, forms a multimer. J Mol Biol. 1994;238:187–198. doi: 10.1006/jmbi.1994.1280. [DOI] [PubMed] [Google Scholar]
- 25.Koster M, Bitter W, De Cock H, Allaoui A, Cornelis G R, Tommassen J. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol Microbiol. 1997;26:789–797. doi: 10.1046/j.1365-2958.1997.6141981.x. [DOI] [PubMed] [Google Scholar]
- 26.Lebart L, Maurineau A, Warwick K M. Multi-variate descriptive statistical analysis. New York, N.Y: Wiley; 1984. [Google Scholar]
- 27.Lebart L, Morineau A, Tabard N. Techniques de la description statisque. Paris, France: Dunod; 1977. [Google Scholar]
- 28.Linderoth N A, Simon M N, Russel M. The filamentous phage pIV multimer visualised by scanning transmission electron microscopy. Science. 1997;278:1635–1638. doi: 10.1126/science.278.5343.1635. [DOI] [PubMed] [Google Scholar]
- 29.Marciano D K, Russel M, Simon S M. An aqueous channel for filamentous phage export. Science. 1999;284:1516–1519. doi: 10.1126/science.284.5419.1516. [DOI] [PubMed] [Google Scholar]
- 30.Martin P R, Hobbs M, Free P D, Jeske Y, Mattick J S. Characterization of PilQ, a new gene required for the biogenesis of type 4 fimbriae in Pseudomonas aeruginosa. Mol Microbiol. 1993;9:857–868. doi: 10.1111/j.1365-2958.1993.tb01744.x. [DOI] [PubMed] [Google Scholar]
- 31.Nassif X, Beretti J L, Lowy J, Stenberg P, Ogaora P, Pfeifer J, Normark S, So M. Roles of pilin and PilC in adhesion of N. meningitidis to human epithelial and endothelial cells. Proc Natl Acad Sci USA. 1994;91:3769–3773. doi: 10.1073/pnas.91.9.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nouwen N, Ranson N, Saibil H, Wolpensinger B, Engel A, Ghazi A, Pugsley A P. Secretin PulD: association with pilot PulS, structure, and ion-conducting channel formation. Proc Natl Acad Sci USA. 1999;96:8173–8177. doi: 10.1073/pnas.96.14.8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nouwen N, Stahleberg H, Pugsley A P, Engel A. Domain structure of secretin PulD revealed by limited proteolysis and electron microscopy. EMBO J. 2000;19:2229–2236. doi: 10.1093/emboj/19.10.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parge H E, Forest K T, Hickey M J, Christensen D A, Getzoff E D, Tainer J A. Structure of the fibre forming protein pilin at 2.6 Angstrom resolution. Nature. 1995;378:32–38. doi: 10.1038/378032a0. [DOI] [PubMed] [Google Scholar]
- 35.Penczek P, Radermacher M, Frank J. 3-D reconstruction of single particles embedded in ice. Ultramicroscopy. 1992;40:33–53. [PubMed] [Google Scholar]
- 36.Potts W J, Saunders J R. Nucleotide-sequence of the structural gene for class-1 pilin from Neisseria meningitidis—homologies with the pilE locus of Neisseria gonorrhoeae. Mol Microbiol. 1988;2:647–653. doi: 10.1111/j.1365-2958.1988.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 37.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenberg M F, Callaghan R, Ford R C, Higgins C F. Structure of the multidrug resistance P-glycoprotein to 2.5nm resolution determined by electron microscopy and image analysis. J Biol Chem. 1997;272:10685–10694. doi: 10.1074/jbc.272.16.10685. [DOI] [PubMed] [Google Scholar]
- 39.Rudel T, van Putten J P M, Gibbs C P, Haas R, Meyer T F. PilC neisserial protein identified as a type-4 tip pilus located adhesin. Nature. 1995;373:357–359. doi: 10.1038/373357a0. [DOI] [PubMed] [Google Scholar]
- 40.Russel M. Macromolecular assembly and secretion across the bacterial cell envelope: type II protein secretion systems. J Mol Biol. 1998;279:485–499. doi: 10.1006/jmbi.1998.1791. [DOI] [PubMed] [Google Scholar]
- 41.Russel M. Phage assembly—a paradigm for bacterial virulence factor export. Science. 1994;265:612–614. doi: 10.1126/science.8036510. [DOI] [PubMed] [Google Scholar]
- 42.Sali A, Blundell T A. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 43.Shevchick V E, Robert-Baudouy J, Condemine G. Specific interaction between OutD, an Erwinia chrysanthemi OMP of the general secretory pathway and secreted proteins. EMBO J. 1997;16:3007–3016. doi: 10.1093/emboj/16.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simpson A A, Tao Y Z, Leiman P G, Badasso M O, He Y N, Jardine P J, Olson N H, Morais M C, Grimes S, Anderson D L, Baker T S, Rossmann M G. Structure of the bacteriophage phi 29 DNA packaging motor. Nature. 2000;408:745–750. doi: 10.1038/35047129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sohel I, Puente J L, Ramer S W, Bieber D, Wu C Y, Schoolnik G K. Enteropathogenic Escherichia coli: identification of a gene cluster coding for bundle-forming pilus morphogenesis. J Bacteriol. 1996;178:2613–2628. doi: 10.1128/jb.178.9.2613-2628.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stone K D, Zhang H Z, Carlson L K, Donnenberg M S. A cluster of fourteen genes from enteropathogenic Escherichia coli is sufficient for biogenesis of a type IV pilus. Mol Microbiol. 1996;20:325–337. doi: 10.1111/j.1365-2958.1996.tb02620.x. [DOI] [PubMed] [Google Scholar]
- 47.Stoylova S, Flint T D, Ford R C, Holzenburg A. Comparison of photosystem II 3D structure as determined by electron crystallography of frozen-hydrated and negatively stained specimens. Micron. 1998;29:341–348. [Google Scholar]
- 48.Strom M S, Lory S. Structure-function and biogenesis of the type IV pili. Annu Rev Microbiol. 1993;47:565–596. doi: 10.1146/annurev.mi.47.100193.003025. [DOI] [PubMed] [Google Scholar]
- 49.Strom M S, Nunn D N, Lory S. Multiple roles of the pilus biogenesis protein PilD: involvement of PilD in excretion of enzymes from P. aeruginosa. J Bacteriol. 1991;173:1175–1180. doi: 10.1128/jb.173.3.1175-1180.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swanson J. Studies on gonococcus infection. XII. Colony color and opacity variants of gonococci. Infect Immun. 1978;19:320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swanson J, Kraus S J, Gotschlich E C. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1971;134:886–906. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thanassi D G, Saulino E T, Lombardo M J, Roth R, Heuser J, Hultgren S J. The PapC usher forms an oligomeric channel: implications for pilus assembly across the outer membrane. Proc Natl Acad Sci USA. 1998;95:3146–3151. doi: 10.1073/pnas.95.6.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tønjum T, Caugant D A, Dunham S A, Koomey M. Structure and function of repetitive sequence elements associated with a polymorphic domain of the N. meningitidis PilQ protein. Mol Microbiol. 1998;29:975–986. doi: 10.1046/j.1365-2958.1998.00910.x. [DOI] [PubMed] [Google Scholar]
- 54.Tønjum T, Freitag N E, Namork E, Koomey M. Identification and characterisation of PilG, a highly conserved pilus assembly gene in pathogenic Neisseria. Mol Microbiol. 1995;16:451–464. doi: 10.1111/j.1365-2958.1995.tb02410.x. [DOI] [PubMed] [Google Scholar]
- 55.Tønjum T, Koomey M. The pilus colonisation factor of pathogenic Neisseria species: organelle biogenesis and structure/function relationships. Gene. 1997;192:155–163. doi: 10.1016/s0378-1119(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 56.Virji M, Alexandrescu C, Ferguson D J, Saunders J R, Moxon E R. Variations in the expression of pili—the effect on adherence of Neisseria meningitidis to human epithelial and endothelial cells. Mol Microbiol. 1992;6:1271–1279. doi: 10.1111/j.1365-2958.1992.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 57.Wolfgang M, van Putten J P M, Hayes S F, Dorward D, Koomey M. Components and dynamics of fiber formation define a ubiquitous biogenesis pathway for bacterial pili. EMBO J. 2000;19:6408–6418. doi: 10.1093/emboj/19.23.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Q Y, Deryckere D, Lauer P, Koomey M. Gene conversion in Neisseria gonorrhoeae—evidence for its role in pilus antigenic variation. Proc Natl Acad Sci USA. 1992;89:5366–5370. doi: 10.1073/pnas.89.12.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]