ABSTRACT
The immune system is key to host defense against pathogenic organisms. Aging is associated with changes in the immune system, with a decline in protective components (immunosenescence), increasing susceptibility to infectious disease, and a chronic elevation in low-grade inflammation (inflammaging), increasing the risk of multiple noncommunicable diseases. Nutrition is a determinant of immune cell function and of the gut microbiota. In turn, the gut microbiota shapes and controls the immune and inflammatory responses. Many older people show changes in the gut microbiota. Age-related changes in immune competence, low-grade inflammation, and gut dysbiosis may be interlinked and may relate, at least in part, to age-related changes in nutrition. A number of micronutrients (vitamins C, D, and E and zinc and selenium) play roles in supporting the function of many immune cell types. Some trials report that providing these micronutrients as individual supplements can reverse immune deficits in older people and/or in those with insufficient intakes. There is inconsistent evidence that this will reduce the risk or severity of infections including respiratory infections. Probiotic, prebiotic, or synbiotic strategies that modulate the gut microbiota, especially by promoting the colonization of lactobacilli and bifidobacteria, have been demonstrated to modulate some immune and inflammatory biomarkers in older people and, in some cases, to reduce the risk and severity of gastrointestinal and respiratory infections, although, again, the evidence is inconsistent. Further research with well-designed and well-powered trials in at-risk older populations is required to be more certain about the role of micronutrients and of strategies that modify the gut microbiota–host relationship in protecting against infection, especially respiratory infection.
Keywords: immunity, inflammation, infection, aging, gut microbiota, vitamin C, vitamin D, vitamin E, zinc, selenium
Statement of Significance: The article integrates the current state of knowledge around the impacts of aging, nutrition, and the gut microbiota on the immune system and then comprehensively reviews the influence of selected micronutrients (vitamins C, D, and E and zinc and selenium) and pro- and prebiotics on immunity, inflammation, and infection, particularly respiratory tract infection, focusing on trials conducted in older humans.
Introduction
The immune system is key to host defense against pathogenic organisms. It is dispersed throughout the body, with cells moving between body compartments via the bloodstream and the lymph. The immune system is highly sophisticated and has barrier, recognition, elimination, and memory components. These are achieved as a result of the multiple cell types, cellular interactions, and chemical mediators that together form the immune response. Individuals with weakened immunity are at increased risk of infections and of infections becoming more severe. Thus, there is interest in those factors that support the immune system and those factors that weaken it. The diet and the gut microbiota are 2 interrelated factors that influence the immune response; among dietary components, a range of micronutrients have vital roles in the immune system. The immune system also changes through the life course and many older people show a decline in immune responses. This has been termed “immunosenescence” and predisposes older people to infections and also to weaker vaccination responses than seen in young and middle-aged adults. Older people can also show an elevation in inflammation, termed “inflammaging.” This can be seen with the development of some chronic inflammatory conditions with age, but also with chronic low-grade inflammation which increases risks of the common noncommunicable diseases of aging. This review describes the effects of selected key micronutrients (vitamins C, D, and E and zinc and selenium) and strategies to beneficially alter the gut microbiota on the immune system and on infection risk and severity, with a focus on older people. The review starts with an overview of the immune system and the general effects of aging, the gut microbiota, and nutrition on it.
Overview of the Immune System and Its Components
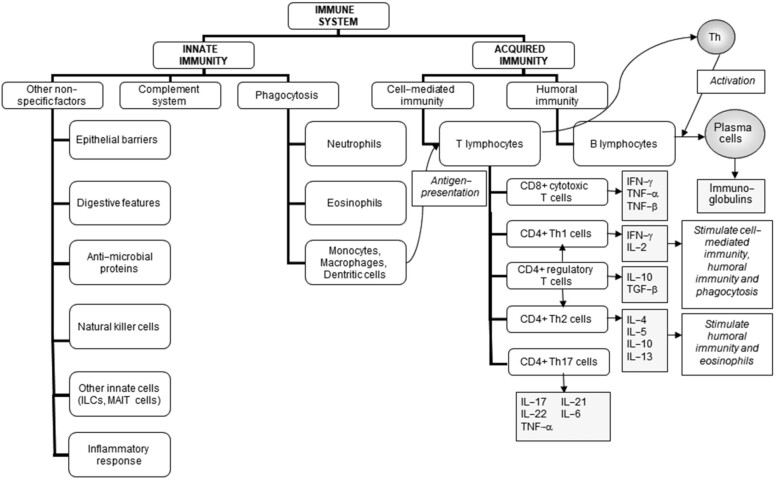
The immune system acts to protect the host individual from infectious agents that occur in the environment (pathogenic bacteria, viruses, fungi, parasites) and from other noxious insults. It also plays a role in surveillance and destruction of tumor cells, in clearing dead and dying cells and cellular debris, in wound healing, and in enabling tolerance to harmless environmental constituents like food and commensal bacteria and to the host. The immune response involves various cell types distributed in many locations throughout the body. Cells move between these locations in the bloodstream and the lymph. Immune cells are organized into discrete lymphoid organs in some places in the body. Immune cells arise and mature in the primary lymphoid organs (bone marrow and thymus) and interact with one another and with antigens in the secondary lymphoid organs, which include lymph nodes and the spleen. The immune system has 2 general functional divisions. These are the innate (also termed “natural”) immune system and the acquired (also termed “specific” or “adaptive”) immune system (Table 1, Figure 1) (1, 2). These 2 parts of the immune system are functionally interlinked.
TABLE 1.
The components of the immune system and their classification into innate and acquired immunity1
| Innate (natural) immunity | Acquired (adaptive) immunity | ||
|---|---|---|---|
| Barriers | Cellular components | Cell-mediated immunity | Humoral immunity |
| SkinMucosal surfacesMucusAntimicrobial proteins in secretionsAcid pH of stomach | Granulocytes (neutrophils, basophils,eosinophils, mast cells)Phagocytes (neutrophils, macrophages,monocytes, dendritic cells)Inflammatory responseNK cellsOther innate cells (includes innate lymphoid cells, mucosal associated invariant T cells) |
T lymphocytes (helper, cytotoxic, regulatory, others)Cytokines | B lymphocytesAntibodies |
| Memory response | |||
Adapted from reference 1.
FIGURE 1.
The components of the immune system and their division into innate and acquired immunity. ILC, innate lymphoid cell; MAIT, mucosal associated invariant T; TGF, transforming growth factor; Th, T helper. Reproduced from reference 2 .
Physical barriers, soluble factors, and phagocytic cells contribute to innate immunity; indigenous commensal bacteria within the gastrointestinal tract may also be considered part of innate immunity (see section “The role of the gut microbiota in shaping and supporting the immune system”). The phagocytic cells are the granulocytes (also known as polymorphonuclear leukocytes and including neutrophils, basophils, eosinophils), monocytes, and macrophages. Inflammation is part of innate immunity. In general, inflammation acts to create an environment that is hostile to pathogens, it initiates pathogen killing, and it causes changes in the metabolism of the host. Many cell types play roles in the inflammatory response, which involves the production of, and responses to, a number of chemical mediators. The cardinal signs of inflammation are redness, swelling, heat, pain, and loss of function. These are all caused by the cellular activation and chemical mediator release that occur during the initiation and perpetuation of the inflammatory response. Although the inflammatory response is designed to be damaging to pathogens, the cellular activities and the chemical mediators that are involved in inflammation can also cause damage to host tissues. Fortunately, inflammation is normally self-limiting and resolves, often rapidly. This is because various inhibitory mechanisms are activated as inflammation runs its course. Loss of these regulatory processes can result in excessive, inappropriate, or ongoing inflammation that can cause irreparable damage to host tissues, leading to pathology and disease.
Although it has been traditionally considered that innate immunity has no memory and is therefore not influenced by prior exposure to a pathogenic organism, it is now thought that innate immunity can be primed or trained through exposure to general structural features of organisms, termed “microbe-associated molecular patterns.” Phagocytic cells, the main effectors of innate immunity, express receptors that recognize microbe-associated molecular patterns; these receptors are termed “pattern recognition receptors” and include the Toll-like receptors (TLRs). Binding of bacteria to surface receptors on phagocytes triggers phagocytosis (engulfing) and subsequent destruction of the bacteria by toxic chemicals, such as superoxide radicals and hydrogen peroxide. Natural killer (NK) cells also possess surface receptors and destroy their target cells (virally infected cells, tumor cells) by the release of cytotoxic proteins. In this way, innate immunity provides a rapid first line of defense against invading pathogens. However, an immune response often requires the coordinated actions of both innate immunity and the more powerful and flexible acquired immunity.
Acquired immunity involves the specific recognition of molecules (termed “antigens”) on an invading pathogen, which distinguish it as being foreign to the host. Lymphocytes are the main effector cells of acquired immunity. These are classified as T and B lymphocytes (also called T cells and B cells). B lymphocytes develop and mature in the bone marrow before being released into the circulation, while T lymphocytes develop in the bone marrow but mature in the thymus. Each individual lymphocyte carries surface receptors for a single antigen, meaning that the acquired immune system is highly specific. However, acquired immunity is extremely diverse; the lymphocyte repertoire in humans has been estimated to be able to recognize approximately 1011 antigens. The high degree of specificity, combined with the huge lymphocyte repertoire, means that only a relatively small number of lymphocytes will be able to recognize any given antigen. Therefore, acquired immunity involves proliferation of antigen-specific cells (called clonal expansion) to increase the number of lymphocytes that have the ability to recognize the antigen causing the initial response. This process takes time, meaning that the acquired immune response becomes effective over several days after the initial activation. It also persists for some time after the removal of the initiating antigen. This persistence gives rise to immunological memory, which is also a characteristic feature of acquired immunity. Memory is the basis for a stronger, more effective immune response upon re-exposure to an antigen (i.e., reinfection with the same pathogen) and is the basis of vaccination.
B lymphocytes are the immune cells that produce antibodies, which are antigen-specific immunoglobulins (Igs). This form of protection is called humoral immunity. B lymphocytes also carry Igs on their surface that are capable of binding an antigen. Binding of these surface Igs with antigen causes proliferation of the B lymphocyte and subsequent transformation into plasma cells, which secrete large amounts of antibody with the same specificity as the parent cell. There are 5 major classes of Igs (IgA, IgD, IgG, IgM, and IgE), each of which elicits different aspects of the humoral immune response. Antibodies can “neutralize” toxins or microorganisms by binding to them and preventing their attachment to host cells, and they can activate complement proteins in plasma, which, in turn, promote the destruction of bacteria by phagocytes.
Humoral immunity deals with extracellular pathogens (e.g., many bacteria). However, some pathogens, particularly viruses, but also certain bacteria, enter host cells, meaning they can escape humoral immunity. Instead, they are dealt with by cell-mediated immunity, which involves T lymphocytes. T lymphocytes express antigen-specific T-cell receptors (TCRs) on their surface. However, unlike B lymphocytes, T lymphocytes are only able to recognize antigens that are presented to them on a cell surface (the cell presenting the antigen to the T lymphocyte is termed an “antigen-presenting cell”). Dendritic cells are professional antigen-presenting cells, but other phagocytes also act in this way. Activation of the TCR results in T-lymphocyte proliferation. Activated T lymphocytes also synthesize and secrete the cytokine IL-2, which further promotes proliferation and differentiation. There are several types of T lymphocytes, including cytotoxic T cells, helper T cells, and regulatory T cells. Cytotoxic T lymphocytes carry the surface protein marker CD8 and kill infected cells and tumor cells by secretion of cytotoxic enzymes, which cause lysis of the target cell. Helper T lymphocytes carry the surface protein marker CD4 and eliminate pathogens by stimulating the phagocytic activity of macrophages and the proliferation of, and antibody secretion by, B lymphocytes. Helper T cells that have not previously encountered antigen produce mainly IL-2 upon the initial encounter with an antigen. These cells can differentiate into either T helper type (Th) 1 or Th2 cells. This differentiation is regulated by cytokines: IL-12 and IFN-γ promote the development of Th1 cells, while IL-4 promotes the development of Th2 cells. Th1 cells produce IL-2 and IFN-γ, which activate macrophages, NK cells, and cytotoxic T lymphocytes, which are the principal effectors of cell-mediated immunity against bacteria, viruses, and fungi. Th2 cells produce IL-4, which stimulates IgE production, and IL-5, an eosinophil-activating factor. Th2 cells are responsible for defense against helminthic parasites, which is due to IgE-mediated activation of mast cells and basophils. More recently characterized classes of helper T cells include Th17 cells, which are involved in inflammation and autoimmunity, and regulatory T cells, which produce IL-10 and transforming growth factor-β and suppress the activities of other T cells and B cells, so preventing inappropriate activation.
Factors That Influence Immunity
Overview
Any measurement of an immune or inflammatory biomarker in a group of individuals reveals significant heterogeneity (3–6). This heterogeneity relates to between-individual differences in the factors that influence the immune response. These include many unmodifiable factors such as genetics, sex, stage of the life course, and time of day, but many modifiable factors also influence the immune response. These include stress, physical fitness, frailty, body fatness, diet, and gut microbiota composition. Here, we focus on aging, the gut microbiota, and nutrition as factors influencing the immune response.
The effect of aging on the immune system: the dual burdens of immunosenescence and inflammaging
In comparison to younger adults, older adults are more susceptible to infectious diseases [e.g., influenza, pneumonia, tuberculosis, coronavirus disease 2019 (COVID-19)] and account for a greater proportion of the total infectious disease burden in high-income countries (7–13) and for higher use of antibiotics (14). It is increasingly recognized that older individuals experience prolonged infection periods, which are associated with an increased risk of morbidity and mortality (9, 13, 15). Vaccination is considered the optimal preventative measure against infections and mortality caused by them, yet vaccines often have reduced efficacy in older adults (9, 16–19). Thus, the aging population poses a unique challenge, requiring the identification and implementation of new preventative and therapeutic approaches to reduce infectious disease burden and enhance vaccine efficacy.
One important factor contributing to increased susceptibility to infection in older people is age-related immune decline, termed “immunosenescence” (20–24). Multiple changes to immune cell development, numbers, and function occur as part of immunosenescence (Table 2). First, there is decreased output of immune cells from bone marrow (25, 26), the site of origin of all immune cells. In addition, involution of the thymus with age decreases output of naive T lymphocytes with a loss in TCR diversity and an accumulation of memory T lymphocytes (27). The overall result of these changes is lowered numbers of T lymphocytes in the blood, changes in the ratio of different T lymphocytes (e.g., fewer naive and relatively more memory T cells), and impaired T-lymphocyte responsiveness. Immunosenescence also affects B lymphocyte numbers and function and the function of antigen-presenting cells and some components of innate immunity, including impairment of several fundamental aspects of neutrophil, macrophage, and NK-cell function (Table 2). Thus, aging can be associated with a reduced ability to mount an effective and appropriate immune response to both novel and previously encountered pathogens, which increases an individual's risk of severe disease and mortality. Additionally, loss of T-cell– and NK-cell–mediated immunity can increase an individual's risk for developing cancerous lesions and metastasis. Age-related changes in the immune response are exaggerated with frailty (28) and in those with poorer micronutrient status (29–31), suggesting that poor nutrition is one contributor to the immune decline seen in many older people.
TABLE 2.
Summary of the key features of age-related immune decline (immunosenescence)1
| Cell type | Effect seen in immunosenescence |
|---|---|
| T lymphocyte |
|
| B lymphocytes |
|
| Dendritic cells |
|
| Neutrophils |
|
| Monocytes |
|
| Macrophages |
|
| NK cells |
|
TLR, Toll-like receptor.
While inflammation is necessary to orchestrate an appropriate immune response to an infection (see earlier), aging introduces a paradox: chronically raised low-grade inflammation, termed “inflammaging” (32–36). Inflammaging is seen as an increase in blood plasma or serum concentrations of the acute-phase protein C-reactive protein (CRP) and of inflammatory cytokines like IL-6 (32–36). This may reflect sensitized proinflammatory signaling pathways in older people. One of the current prevailing theories for the cause of chronic inflammation is the decreased turnover of “senescent cells” (37). In younger individuals, senescent cells are identified, eliminated, and replaced quickly. However, with age, the number of senescent cells can rapidly accumulate. In part, the accumulation of senescent cells occurs because there is a decrease in immune surveillance mechanisms, which are meant to remove damaged or necrotic host cells and maintain tissue homeostasis. Senescent cells are characterized by a chronic secretion of pro-tumorigenic and proinflammatory molecules, collectively referred to as the “senescence-associated secretory phenotype” (37). This array of secreted factors is considered to be a key contributor to inflammaging and is suggested to increase an older individual's risk for chronic disease (32–38) (Figure 2). Inflammation has been identified as a strong predictor of all-cause mortality in the elderly (39).
FIGURE 2.
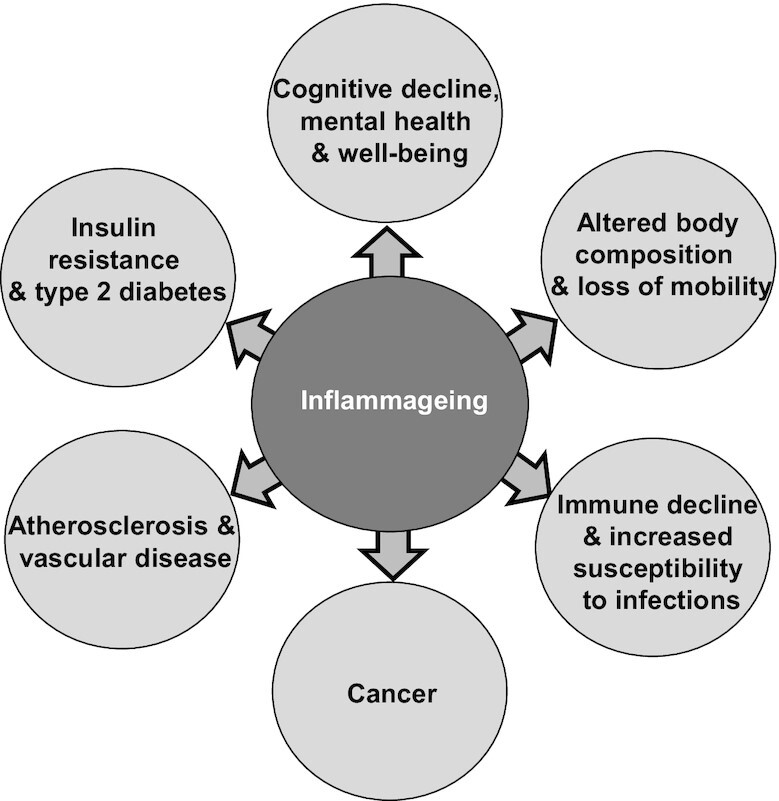
The central role of inflammaging in chronic conditions of aging. Adapted (color changes) from reference 33.
The role of the gut microbiota in shaping and supporting the immune system
The human gut is host to a significant number of bacteria and other microorganisms, which are collectively referred to as the gut microbiota. The large intestine has the greatest number and diversity of bacterial species, estimated at 1011 bacteria/g of colonic contents (40). In addition to hosting the largest quantity of microbes in the human body, the gut wall is also the largest site of immune tissue, known as the gut-associated lymphoid tissue (GALT). It is estimated that, in humans, 70% of immune cells are associated with the GALT, signifying its importance. The key roles of the GALT are surveillance of microorganisms and other sources of antigens (e.g., components of food) in the gut lumen and mounting either active or tolerogenic responses to these. Thus, the host immune system plays a role in regulating the composition of the gut microbiota. In turn, the gut microbiota plays a role in both the development and proper functioning of the host immune system (41–43) (Figure 3). Commensal bacteria of the microbiota seem to contribute to the development of a normally functioning immune system: in germ-free animal models, there are observable reductions in the size of all lymphoid organs, Ig production, and lymphocyte populations (44). The microbial communities that make up the gut microbiota have direct and indirect effects on host immune function (41–44). Indigenous commensal bacteria within the gastrointestinal tract play a role in host immune defense by creating a barrier against colonization by pathogens and they also compete with pathogenic bacteria for available nutrients. In addition to creating a physical barrier, many products of the metabolism of commensal bacteria, including lactic acid and antimicrobial proteins, can directly inhibit the growth of pathogens. As well as these direct interactions between commensal and pathogenic bacteria, they can interact with the host's gut epithelium and GALT (41–43). These communications with the host may occur through metabolites released from the bacteria or through direct cell-to-cell contact. Among the most important products of oligosaccharide metabolism by gut bacteria are the SCFAs (acetate, propionate, and butyrate), with butyrate being an important fuel for the gut epithelium and also regulating epithelial gene expression through histone modification. SCFAs increase gut mucosal barrier integrity by increasing mucus secretion, IgA production, and tight junction proteins (45, 46). Some lactobacilli convert tryptophan into kynurenine metabolites, which directly activate the aryl hydrocarbon receptor (47), which is necessary for proper gut barrier functionality and the development of specific immunoregulatory T cells (48, 49). Polysaccharide A produced by Bacteroides fragilis drives the differentiation of regulatory T cells, which are essential for keeping immune responses in check and for the development of oral tolerance (50). Thus, overall, the gut microbiota contributes to gut and immune development, to host immune defense, to maintenance of tolerance, and to control of inflammation.
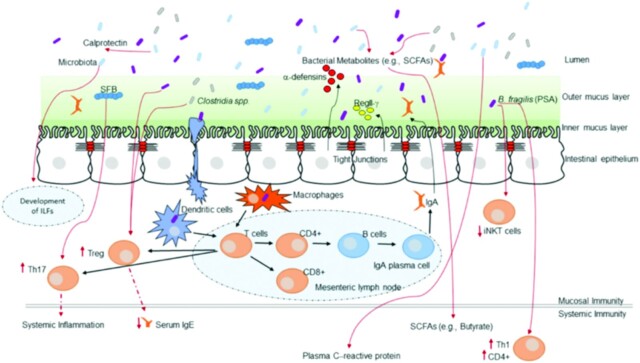
FIGURE 3.
How the gut microbiota shapes host immunity. Multiple immune effectors function together to minimize bacterial-epithelial invasion. These include the mucus layer, epithelial antibacterial proteins, and IgA secreted by lamina propria plasma cells. Compartmentalization is accomplished by unique anatomic adaptations that limit commensal bacterial exposure to the immune system. Some microbes are sampled by intestinal dendritic cells. The loaded dendritic cells traffic to the mesenteric lymph nodes through the intestinal lymphatic but do not migrate to distal tissues. This compartmentalizes live bacteria and induction of immune responses to the mucosal immune system. Induced B cells and T-cell subsets recirculate through the lymphatic and the bloodstream back to mucosal sites, where B cells differentiate into IgA-secreting plasma cells. Thus, the intestinal microbiota shapes host mucosal as well as systemic immunity. ILF, isolated lymphoid follicle; iNKT, invariant natural killer T; PSA, polysaccharide A; SFB, segmented filamentous bacteria; Th, T helper. Treg, regulatory T cell. Reproduced from reference 43 with permission.
The human gut microbiota demonstrates a high degree of variability among individuals (51), reflecting differing exposures to environmental factors and the influence of the host phenotype. The human gut microbiota is strongly influenced by habitual diet (52–55). Furthermore, aging and the presence or absence of diseases significantly influence the composition of the microbiota (56). For example, with aging, the abundance and diversity of bifidobacteria decline (57), while bacteria including streptococci, staphylococci, enterococci, and enterobacteria increase (58). There are significant differences in the microbiota of free-living older adults and those residing in residential care (59, 60), as shown in Figure 4. Aging also affects the GALT. In murine models, aging reduced gut mucosal secretory IgA responses, impaired oral tolerance to new antigens, and impaired mucosal dendritic cell function (61), as reviewed elsewhere (62–65). Given the role of the gut microbiota in supporting the host immune system, it is likely that age-related changes in the microbiota are linked with immunosenescence and inflammaging.
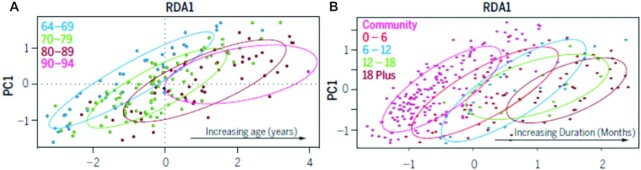
FIGURE 4.
Changes to the gut microbiota with age and with duration of residential care. Redundancy analysis plot of microbiota composition (log-transformed OTU dataset) of (A) community-dwelling individuals by age (years) (n = 176; P < 0.002). (B) Full dataset of community and long-term residential care individuals by duration in care (months) (n = 282; P < 0.001). OTU, operational taxonomic unit. Reproduced from reference 59 with permission from American Association for the Advancement of Science (AAAS). PC, principal component; RDA, redundancy analysis.
The role of nutrition in supporting the immune system
Foods and beverages provide macro- and micronutrients, as well as other bioactive components, that contribute to the normal functioning of the immune system, including supporting barrier function (66–69). Components of the diet act in a variety of ways to influence the immune response:
Macronutrients act as fuels for energy generation by immune cells;
Macronutrients provide substrate (“building blocks”) for the biosynthesis that is involved in the immune response (e.g., amino acids for Igs, cytokines, new receptors, acute-phase proteins);
Many micronutrients are regulators of molecular and cellular aspects of the immune response (e.g., iron, zinc, vitamin A, vitamin D);
Some nutrients are substrates for the synthesis of chemicals involved in the immune response (e.g., arginine and nitric oxide; arachidonic acid and eicosanoids);
Some micronutrients have specific anti-infection roles (e.g., zinc, vitamin D);
Many nutrients and plant bioactives are involved in protection of the host from the oxidative and inflammatory stress imposed by the immune response (e.g., vitamin C, vitamin E, cysteine, zinc, copper, selenium, flavonoids);
Many food components contribute to creating a diverse gut microbiota that supports the immune response (e.g., plant-derived fibers and nondigestible polysaccharides, prebiotic oligosaccharides).
These considerations suggest multiple sites of interaction of food components with the immune system. First, absorbed food components can act systemically to target the different components of the immune system (e.g., in bone marrow, the thymus, the bloodstream, secondary lymphoid organs, and other organs). Second, multiple food components can act to influence the immune system without being absorbed systemically. For example, they could have local actions on epithelial barrier function or on the GALT; they could modulate the gut microbiota composition, so influencing gut microbiota–immune system cross-talk; they could be fermented by the microbiota resulting in metabolites (e.g., SCFAs), which can act locally on epithelial and immune cells or be absorbed and act systemically; or they could train or prime immune cells involved in surveillance of the luminal contents of the gastrointestinal tract. Although these latter actions are primarily focused on the gastrointestinal tract, because of recirculation of cells from the GALT to other sites, including the respiratory tract, effects initiated at the gut level can have actions elsewhere, including the airways.
Micronutrients, Immunity, and Infection with an Emphasis on Older People
Micronutrients to support the immune system in older people
Nutritional status is considered to be a major contributor to immune system development (70) and function (66–69). It follows that nutrient status can be a determinant of susceptibility to, or severity of, infection (71, 72). Furthermore, the relation between nutrition, immunity, and infection suggests that specific nutrients and other dietary components could be used to favorably modulate immune cell function, inflammatory responses, and infectious disease susceptibility and prognosis (2, 66–69, 73, 74). This is particularly important in older people, because many have generally poor nutritional status and, in particular, habitual intakes of a number of vitamins and minerals that are below those that are recommended (75–79). Since micronutrients are important to supporting the immune response and controlling inflammation (2, 66, 67, 80, 81), insufficient intake may deleteriously impact immune competence, contributing to increasing the susceptibility of older people to infection. Hence, it has been suggested that micronutrient supplementation at or above recommended intakes may be beneficial in older individuals (82, 83). Among the micronutrients, the roles of vitamins A, C, D, and E and the minerals zinc, copper, iron, and selenium are well explored, but B vitamins, vitamin K, magnesium, and others all have roles. Here we review the roles of vitamins C, D, and E and zinc and selenium on immunity and infection with a focus on older people.
Vitamin C
Vitamin C contributes to supporting innate and adaptive immune defenses, as well as to the physical barriers that limit entry to pathogens (84, 85). Vitamin C helps to maintain the integrity of the internal and external barriers acting as a cofactor of enzymes required in collagen synthesis and cross-linking and in connective tissue healing (86). Many innate immune cells, particularly granulocytes and macrophages, produce reactive oxidative species (ROS) to lyse bacteria or promote apoptosis of infected host cells. However, ROS can cause unintended damage to both the cell producing them and to neighboring, uninfected host cells. Vitamin C, as a potent antioxidant, helps to protect against such damage. Neutrophils have specific transport systems for vitamin C uptake and accumulation (87, 88) and to recycle oxidized vitamin C (89) in order to protect against the damage caused by ROS. During infection, granulocytes such as neutrophils produce a high concentration of ROS for microbial killing, which, in turn, increases vitamin C uptake in a nonspecific manner and increases the host requirement for vitamin C to protect itself (90). Vitamin C is required for apoptosis and macrophage-mediated clearance of dead immune cells and can promote chemotaxis of neutrophils (84). Vitamin C supports differentiation, proliferation, and function of T and B cells (80, 81, 84, 91). Incubation of mononuclear cells isolated from the blood of older adults with severe community-acquired pneumonia with vitamin C decreased oxidative stress, DNA damage, and inflammatory cytokine production (92). A vitamin C–deficient diet in healthy young-adult humans decreased mononuclear cell vitamin C content by 50% and decreased the T-lymphocyte–mediated immune responses to recall antigens (93).
The ability of supplemental vitamin C to enhance immune function has been investigated in adults, including older adults, in several studies. One study reported that 6 wk of vitamin C supplementation (1000 mg/d) in elderly women (mean age: 72.8 y) resulted in no significant changes in leukocyte expression of genes encoding the proinflammatory markers IL-1, IL-6, and CRP or the anti-inflammatory marker IL-10 (94). However, a clear trend of decreasing IL-6 and increasing in IL-10 mRNA was observed (94). A 6-wk randomized controlled trial (RCT) of vitamin C (1000 mg/d) in patients aged 45 to 60 y with diagnosed type 2 diabetes and poor glycemic control reported increased phagocytosis and oxidative burst by granulocytes (95). Vitamin C (500 mg/d) restored multiple indices of immune function in both older men and women (mean age: 74 y), bringing them closer to the levels seen in young adults (mean age: 38 y) (96), effects that were maintained 6 mo postsupplementation. The combination of vitamin C (1000 mg/d) and vitamin E (200 mg/d) resulted in a significant increase in lymphocyte proliferation and in granulocyte phagocytosis in both healthy older women and in older women (mean age: 72 y) with major depressive disorders or coronary artery disease (97).
If vitamin C is needed to support the immune system, then vitamin C deficiency would lead to increased susceptibility to, and severity of, infectious disease. In fact, recognition of the connection between vitamin C and infectious disease dates to the early 20th century when it was found that vitamin C deficiency was associated with scurvy and its related infections, including pneumonia (98). In the 1970s, the Nobel Laureate Linus Pauling suggested that high doses of vitamin C might decrease the incidence and duration of the common cold (99). Thereafter, a number of trials investigating vitamin C supplementation for the prevention and treatment of the common cold were conducted, but comparisons of the different studies have been complicated because of the variations in the dosage administered and the participants studied and inadequate control groups (98). A meta-analysis of 24 RCTs involving children and adults was conducted to determine whether vitamin C has an effect on the incidence, duration, and severity of the common cold at a dosage of at least 200 mg/d (100). Overall, there was little benefit in consuming vitamin C to decrease the incidence of the common cold for the general population of participants who used vitamin C regularly for a period of 2 wk to 5 y (risk ratio: 0.95; 95% CI: 0.92, 0.98). However, when the trials were subgrouped into whether participants had “no physical stress” or “heavy acute stress,” vitamin C supplementation was found to benefit those who experienced heavy acute stress (i.e., physical activity). The risk ratio of the “no physical stress” trials was 0.97 (95% CI: 0.94, 1.00) while the risk ratio of the “heavy acute stress” trials was 0.48 (95% CI: 0.35, 0.64). Participants undertaking acute physical activity included marathon runners, skiers, and soldiers conducting subarctic exercises. Those who engage in short-term exposure to extreme physical stress may have higher antioxidant intake requirements because of the increased ROS production compared with the general population. In the same meta-analysis, regular supplementation with vitamin C appeared to decrease the duration of colds: high-dose (>200 mg/d), regular intake of vitamin C significantly reduced the duration of cold symptoms by 8% in adults and 14% in children (100). These high doses also prevented the onset of symptoms in extreme athletes (100). Vitamin C supplementation had a modest effect on the severity of colds, defined as days confined to the home (100). It is possible that granulocytes and other immune cells can better respond to an infection and the related increased metabolic requirements for vitamin C in those who have consumed these higher doses of vitamin C, resulting in more rapid resolution of symptoms.
Vitamin C supplementation (200 mg/d) for 4 wk in hospitalized older adults with acute respiratory tract infections who were identified to have very low vitamin C concentrations reduced respiratory symptom scores (101). Several studies report an association between low vitamin C status and increased susceptibility to, and severity of, COVID-19 [e.g., (102)]. However, such studies cannot be used to infer cause and effect. A small retrospective case study in patients with severe (mean age: 63 y) or critical (mean age: 55 y) COVID-19 pneumonia reported that a high dose of intravenous vitamin C (∼170 mg/kg body weight daily) significantly decreased CRP concentrations at day 3 and 7, and found that blood lymphocyte and T-helper cell counts in severe patients reached normal levels at day 3 (103). There was a trend to improved airway function and reduced Sequential Organ Failure Assessment (SOFA) score (103). In contrast, a small open-label RCT in patients (mean age: 57.5 y for cases and 61 y for controls) with severe COVID-19 infection did not find significantly better outcomes at discharge in the group treated with a high dose of vitamin C (6 g/d) in addition to the standard treatment regimen (104). Trials of vitamin C in patients with COVID-19 have been reviewed recently (105).
In summary, vitamin C supports many aspects of innate and acquired immunity and vitamin C deficiency results in immune impairments. Supplemental vitamin C seems to enhance immune cell functions at the doses tested and in older adults. Trials of vitamin C in relation to respiratory infection are inconsistent, perhaps relating to dose and the characteristics of the participants studied. Despite conflicting results, the effects of vitamin C may be useful to prevent or reduce the severity of respiratory diseases in groups most at risk for severe disease, such as older adults and/or those who have low concentrations of vitamin C (98). Future work should aim to confirm or refute the proposed benefits of vitamin C on respiratory disease in older adults through conduct of well-designed and adequately powered studies.
Vitamin D
The precursor to the active form of vitamin D can be acquired from the diet or be produced via UVB irradiation of the skin. Subsequent hydroxylation reactions involving the enzymes 25-hydroxylase and 1-α-hydroxylase, located in the liver and kidney, respectively, produce the active form, 1-α,25-dihydroxyvitamin D, also known as calcitriol. Some immune cells, including macrophages and dendritic cells, also express 1-α-hydroxylase activity, and so can produce calcitriol (106, 107). Calcitriol binds to the vitamin D receptor, which is a transcription factor acting to regulate cellular gene expression. Many immune cell types express the vitamin D receptor and respond to vitamin D, including dendritic cells, monocytes, macrophages, T cells, and B cells, and so vitamin D is now considered to also be an important regulator of immune function and inflammation (108–115).
Vitamin D enhances epithelial integrity and induces antimicrobial peptide (e.g., cathelicidin) synthesis in epithelial cells and macrophages (108, 116), directly enhancing host defense. The effects of vitamin D on the cellular components of immunity are rather complex. Vitamin D promotes differentiation of monocytes to macrophages and increases phagocytosis, superoxide production, and bacterial killing by innate immune cells (81). It also promotes antigen processing by dendritic cells, although antigen presentation may be impaired (117–119); this has been interpreted as a pro-tolerogenic role for vitamin D. Vitamin D is also reported to inhibit CD4+ and CD8+ T-cell proliferation and production of cytokines by Th1 lymphocytes and of antibodies by B lymphocytes (120–122), highlighting the paradoxical nature of its effects. Effects on Th2 responses are not clear, but vitamin D seems to increase the number of regulatory T lymphocytes (119, 123, 124). Incubation of human memory T cells with vitamin D promoted a switch from a proinflammatory to an anti-inflammatory phenotype (125). Incubation of blood mononuclear cells from older people with vitamin D resulted in higher IL-10 production in response to LPS (126). An RCT of a vitamin D analog in older adults (mean age: 73 y) reported an increase in LPS-induced production of IL-10 by isolated blood mononuclear cells, an increased ratio of CD4+ to CD8+ cells in the blood, and a decrease in the number of CD8+CD28– cells (127); LPS-induced production of IL-6 and IFN-γ was not affected (127). A cross-sectional study identified that older men (but not women) who were replete in vitamin D had a lower risk of having very low NK activity, in comparison to older men with low vitamin D (128). Low-dose vitamin D (10 μg/d) in healthy adults (40–55 y) did not affect the blood regulatory T-cell population but attenuated the seasonal increase in IFN-γ production by T cells (129). Several studies have investigated the association between vitamin D status and response to seasonal influenza vaccination, with inconsistent findings: a meta-analysis of 4 studies identified that vitamin D deficiency reduces seroprotection to the H3N2 and B components, but not to the H1N1 component, but has no effect on seroconversion to any of the components (130).
If vitamin D is needed to support the immune system, then vitamin D deficiency would lead to increased susceptibility to, and severity of, infectious disease. In the 19th and early 20th centuries it was found that cod liver oil and exposure to the sun helped treat tuberculosis (131), although the role of vitamin D itself was not immediately evident. Niels Ryberg Finsen was awarded the Nobel Prize for Medicine in 1903 for demonstrating the benefits of UV light to patients with tuberculosis of the skin—lupus vulgaris—which either cured or improved the disease in approximately 95% of patients (132), and by 1920, phototherapy was routinely used to treat pulmonary tuberculosis. While UV therapy can affect immune function in the skin, independent of the effect on vitamin D synthesis, these early studies had findings that are consistent with the later-demonstrated effects of vitamin D on immune function. Research in the early 21st century showed that TLR activation of macrophages upregulates the vitamin D receptor and vitamin D-1-α-hydroxylase genes, which, in turn, induce the expression of the antimicrobial peptide cathelicidin, which has activity against Mycobacterium tuberculosis (108, 116, 133). Furthermore, there is a positive relation between circulating vitamin D and cathelicidin, consistent with the idea that sufficient vitamin D status supports this antibacterial mechanism (134). Clinical trials have examined the efficacy of vitamin D intervention on treatment of tuberculosis, often using the rate of sputum conversion from a positive to a negative culture result as the key outcome, during the lengthy antimicrobial treatment period for patients with active pulmonary tuberculosis. In a meta-analysis of RCTs (135), high-dose vitamin D supplementation given after the initiation of antimicrobial treatment did not speed the recovery in patients infected with tuberculosis, but recovery was more rapid in those who were infected with the multidrug-resistant strains of M. tuberculosis where drug therapy would have been ineffective, thus allowing the benefit of vitamin D treatment to, perhaps, become more apparent.
Observational findings have linked low concentrations of vitamin D to increased risk of viral acute respiratory infection. For example, Berry et al. (136) described an inverse linear relation between serum 25-hydroxyvitamin D concentrations and respiratory tract infections in a cross-sectional study of 6789 British adults. Similarly, data from the US Third NHANES, which included 18,883 adults, showed an independent inverse association between serum 25-hydroxyvitamin D and recent upper respiratory tract infection (URTI) (137). Other studies also reported that individuals with low vitamin D status have a higher risk of viral respiratory tract infections (138, 139). A recent meta-analysis of RCTs involving 48,488 participants (140) examined the relation between vitamin D supplementation and the prevention of acute respiratory infections and found a small, but significant, protective effect of daily administration of 400 to 1000 IU vitamin D taken for 12 mo or less against 1 or more acute respiratory infection compared with the placebo (OR: 0.70; 95% CI: 0.55, 0.89). Baseline vitamin D status was not associated with a protective benefit, although the authors speculated that the heterogeneity of the studies examined may have masked a potentially greater benefit for supplement use in those with vitamin D insufficiency or deficiency at baseline (140).
There has been significant interest in vitamin D and COVID-19 (141, 142). Numerous trials report associations between low vitamin D status and increased susceptibility to, and severity of, COVID-19 [e.g., (143)] and meta-analyses of such studies report that vitamin D deficiency is associated with increased risk of severe COVID-19, hospitalization with COVID-19, and mortality from COVID-19 [e.g., (144, 145)]. A prospective study from the UK Biobank involving 8297 adults who had COVID-19 test results and records of their use of vitamin D supplements, serum 25-hydroxyvitamin D, and a number of covariates found a 34% lower risk of COVID-19 infection associated with the habitual use of vitamin D supplements, although there was no association with baseline vitamin D status (146). One large-scale retrospective study suggests a possible role of vitamin D in suppressing CRP and proinflammatory cytokine production implicated during the cytokine storm in COVID-19 infections, thus reducing COVID-19 severity (147). A study in an Italian residential care home reported that a bolus of vitamin D reduced mortality from COVID-19 (148). Some studies report that vitamin D supplementation in patients hospitalized with COVID-19 reduced COVID-19 severity (e.g., need for intensive care unit admission, mortality) (149, 150), although not all such studies reported benefits from vitamin D (151). Further studies are needed to determine whether inadequate vitamin D status is associated with a higher risk of more severe COVID-19 and whether vitamin D can be used to reduce disease severity.
In summary, vitamin D has pleiotropic effects on immunity, but it does support many aspects of both innate and acquired immunity, promoting antibacterial and antiviral defenses while also promoting a pro-tolerogenic environment. Supplemental vitamin D seems to reduce the risk, and perhaps severity, of respiratory tract infections. The effects of vitamin D may be useful to prevent or reduce the severity of respiratory diseases in groups most at risk for severe disease, such as older adults, and/or those who have low concentrations of vitamin D; this is important since, globally, vitamin D intake and status are low, especially in older people (78, 79). Future work should aim to confirm or refute the proposed benefits of vitamin D on respiratory disease in older adults through conduct of well-designed and adequately powered studies. In terms of understanding the effects of supplemental vitamin D and in the design of trials, consideration should be given to the genetic polymorphisms found in the vitamin D binding protein and the vitamin D receptor, as they have been associated with respiratory disease outcomes (152–154), as well as sex-related differences in how vitamin D might affect immune responses (128, 155).
Vitamin E
Vitamin E, or tocopherol, is a potent lipid-soluble antioxidant that can prevent oxidative stress-induced damage to cellular lipids (156). Vitamin E has also been demonstrated to have anti-inflammatory activities independent of its antioxidant properties (157, 158). In animal models, vitamin E has anti-inflammatory, anti-atherosclerotic, and antitumor properties (159, 160). In immune cells, vitamin E modulates lipid microdomain formation in membranes (161), signal transduction (162), and prostaglandin (PG) E2 (PGE2) synthesis (163). In laboratory animals, vitamin E deficiency decreases lymphocyte proliferation, NK-cell activity, specific antibody production following vaccination, and phagocytosis by neutrophils (159, 164, 165). Vitamin E has been shown to support T-cell differentiation in the thymus of rats (166) and to enhance the immune response of aged mice (163). In healthy adults aged over 60 y a positive association between plasma vitamin E and cell-mediated immune responses and a negative association between plasma vitamin E and the risk of infections were reported (167).
In general, vitamin E has been demonstrated to improve age-associated impairments in immune function in the elderly (164, 168). A study conducted in healthy older men and women (mean age: 70.4 y) found that a variety of immune functions (e.g., neutrophil chemotaxis and phagocytosis, lymphocyte proliferation and IL-2 production, NK-cell activity) were enhanced after 3 mo of vitamin E supplementation (200 mg/d) (169). However, some of these effects were not maintained 6 mo postsupplementation (169), suggesting that continual supplementation may be required in older adults and/or that dietary vitamin E intake habits in this population were inadequate. The combination of vitamin E and vitamin C (200 and 1000 mg/d, respectively) could restore several blood neutrophil and lymphocyte functions in healthy older men and women to closer to those observed in young adults (96). High-dose vitamin E (800 mg/d) for 30 d increased lymphocyte proliferation and delayed-type hypersensitivity in older participants (170). A follow-up RCT using 3 doses of vitamin E (60, 200, and 800 mg/d) showed dose-dependent increases in lymphocyte proliferation, IL-2 production, delayed-type hypersensitivity, and responses to some vaccinations including to hepatitis B virus in older adults (171). The effect of vitamin E may be mediated by a decrease in PGE2 and/or other lipid-peroxidation products (170).
In aged mice, vitamin E supplementation has been reported to decrease influenza viral titer, which was linked to an enhancement of Th1 cytokines and reduced PGE2 production (172, 173). Similarly, in mice, vitamin E has been shown to modulate neutrophil responses and enhance resistance to Streptococcus pneumoniae (174). S. pneumoniae infection is a key driver of morbidity and mortality in older patients and is typically associated with an uncontrolled pulmonary infiltration of neutrophils and neutrophil-driven pulmonary inflammation. Surprisingly, baseline levels of pneumococcal-induced transepithelial migration by granulocytes from young or elderly individuals are reported to be indistinguishable (175). Furthermore, granulocytes from older individuals are reported to be more efficient at killing bacteria ex vivo than those from younger individuals (175). Interestingly, the higher antimicrobial activity found for granulocytes from older adults correlated with increased activity of neutrophil elastase, a protease that is required to kill S. pneumoniae (175). Incubation with vitamin E increased elastase activity in granulocytes from young individuals and boosted their ability to kill S. pneumoniae (175), while incubation with granulocytes from older individuals diminished their migration, which was associated with reduced inflammation (175). Supporting the use of vitamin E to help combat pneumonia, a secondary analysis of the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study in Finland reported that the incidence of pneumonia was significantly lower in the group receiving vitamin E (176). However, the older adults who participated in this study were smokers, and thus these results may not be applicable to nonsmoking older adults. One RCT reported that vitamin E supplementation (200 IU or ∼135 mg/d) for 1 y decreased the risk of URTIs and the common cold in older people in nursing homes (177), but another study did not see an effect of supplemental vitamin E (200 mg/d) on the incidence, duration, or severity of respiratory tract infections in an elderly population (178). Interestingly, polymorphisms for genes encoding various cytokines, including IL-2 and IL-10, have been associated with respiratory tract infections (179), while polymorphisms for genes encoding various cytokines have been shown to influence the effect of vitamin E on production of those cytokines (180). Thus, genetic polymorphisms may, in part, determine the ability of vitamin E on lower respiratory tract infection risk in older people, and this should be considered when testing the efficacy of vitamin E on respiratory disease outcome.
AS03A is an α-tocopherol oil-in-water emulsion-based adjuvant system that has recently been used in combination with a H1N1 and H9N2 influenza vaccine. AS03A was reported to enhance the longevity of B- and T-cell–mediated responses to vaccination, mitigating the negative effects of age-associated impairments in adaptive immunity (181–183). However, the exact underlying mechanisms for the α-tocopherol–induced protection are not clear (184). Similar studies had been previously conducted in farm animals, in which vitamin E has been utilized as an adjuvant to augment humoral immunity (185).
In summary, vitamin E supports many aspects of innate and acquired immunity and has anti-inflammatory effects including reduction in PGE2 production. Animal studies clearly demonstrate the ability of vitamin E to reverse age-related immune impairments. Supplemental vitamin E seems to enhance immune cell functions at the doses tested and in older adults, but these intakes are high compared with those that are recommended. Trials of vitamin E in relation to respiratory infection are inconsistent, perhaps relating to dose and the characteristics of the participants studied, including genotype. Future work should aim to confirm or refute the proposed benefits of vitamin E on respiratory disease in older adults considering confounding factors.
Zinc
Zinc is required for the activity of enzymes involved in a number of processes such as cell proliferation and differentiation, cell membrane integrity, and DNA and RNA synthesis. Systemic and intracellular concentrations of zinc are tightly regulated, with the vast majority of the body's zinc being closely bound to intracellular metallothioneins (MTs), enzymes, transcription factors, and other proteins. The expression of MT genes is highly responsive to zinc concentrations, viral infections, and to a lesser extent to oxidative stress (186, 187). Adequate zinc status is needed for a well-functioning immune system (188–191). Zinc participates in regulating intracellular signaling pathways in innate and adaptive immune cells, and has been demonstrated to have anti-inflammatory, antioxidant, and antiviral properties (192, 193). Zinc status, as determined by serum or plasma zinc concentrations, has been correlated with alterations in total immune cell numbers, particularly helper T cells, and in immune cell function (31,188–191), which together are associated with increased susceptibility to infections (194, 195). Zinc deficiency has a marked impact on bone marrow, decreasing the number immune precursor cells, with reduced output of naive B lymphocytes, and causes thymic atrophy, reducing output of naive T lymphocytes (196). Therefore, zinc is important in maintaining T- and B-lymphocyte numbers. Zinc deficiency impairs many aspects of innate immunity, including phagocytosis, respiratory burst, and NK-cell activity (188–191). Zinc also supports the release of neutrophil extracellular traps that capture microbes (197). There are marked effects of zinc deficiency on acquired immunity. Circulating CD4+ T-lymphocyte numbers and function (e.g., IL-2 and IFN-γ production) are decreased and there is a disturbance in favor of Th2 cells (188–191). Likewise, B-lymphocyte numbers and antibody production are decreased in zinc deficiency (188–191). Zinc supports proliferation of CD8+ cytotoxic T lymphocytes (188–191), key cells in antiviral defense. Moderate or mild zinc deficiency or experimental zinc deficiency in humans results in decreased NK-cell activity, T-lymphocyte proliferation, IL-2 production, and cell-mediated immune responses, which can all be corrected by zinc repletion (198–200).
Zinc deficiency is prevalent in older individuals (201). For example, in 1 study in the United States, 30% of nursing home residents were zinc deficient (202). Interestingly, many of the perturbations observed in zinc deficiency parallel those that occur in aging, such as a reduction in thymic activity, an imbalance in T-helper cell numbers, blunted immune response to vaccination, and general impairments in the functions of immune cells (31, 203, 204). Zinc supplementation has been associated with general improvements in age-related impairments of immune function, reductions in inflammatory markers, and, in some instances, with reduced incidence of infection. For example, zinc supplementation (45 mg/d) for 3 mo increased the number of helper T cells and cytotoxic T cells in the blood of older participants residing in nursing homes (mean age: 79.5 y) and there was a strong trend to increased lymphocyte proliferation (205). In another study, older participants (>70 y of age) received zinc (50 mg/d) for 1 mo: there was an increase in the number of blood T cells, in the delated-type hypersensitivity response, and in the antibody response to tetanus vaccination (206). In that study there was no effect of zinc on lymphocyte proliferation (206). In a more recent study, zinc supplementation (30 mg/d) for 3 mo in elderly nursing home residents with low zinc status enhanced the proliferative capacity of T cells (207). Further, there was a strong positive correlation between zinc concentrations and T-cell proliferation and total number of T cells in the blood (207).
Aside from its potential roles in enhancing immunity and decreasing inflammation (see above), free zinc ions can act on the buccal membranes of the oral cavity and nasopharyngeal tissues by interfering with the capsid assembly of rhinoviruses (208), suggesting a nonimmunologic mechanism by which zinc supplementation above that required to maintain adequate zinc status might affect the incidence or severity of the common cold. A meta-analysis of RCTs of zinc supplementation in children and adults (209) found that the overall duration of the cold was 1.03 d shorter with zinc than in the placebo group (95% CI: −1.72, −0.34 d) when given within 24 to 48 h after the onset of cold symptoms. When studies were subgrouped by zinc dosage, administered in the form of zinc acetate or zinc gluconate lozenges, a zinc intake of more than 75 mg/d reduced the duration of the cold by 1.97 d (95% CI: −3.09, −0.85), with significant heterogeneity in findings. There was no significant reduction in the duration of cold symptoms with a zinc intake of less than 75 mg/d (mean difference: 0.13 d; 95% CI: −0.54, 0.79). The high heterogeneity seen in the >75-mg/d subgroup may be explained by a placebo effect (i.e., the taste of zinc lozenges is difficult to mask) and/or the amount of zinc released in the oral cavity. Zinc lozenges that use additives such as citrate, tartrate, or glycine bind zinc tightly, thus releasing less free zinc ions in the oral cavity. In addition, other variable components of lozenges, such as oils, and the processing temperature used, may impact the solubility of zinc from its complex and deliver a lower zinc dose than indicated (210). When adults and children were studied as subgroups (209), adults had a reduced duration of cold symptoms with oral zinc supplementation by 1.12 d (95% CI: −2.17, −0.06) while children had a reduced duration by only 0.62 d (95% CI: −0.82, −0.42). This difference may be explained by the different zinc formulations given to the adults and children; adults received zinc acetate or zinc gluconate in the form of lozenges while children received either zinc sulfate syrup or zinc gluconate lozenges. Zinc lozenges would dissolve slowly in the oral cavity, thus giving more time for zinc to exert its effects on the buccal membranes than syrup, which is immediately swallowed. The meta-analysis (209) also examined whether prophylactic administration of zinc decreases the incidence of the common cold. Two trials involving children who were given RDA levels of zinc sulfate in the form of syrup or tablets for 5 to 7 mo showed significantly decreased incidence of the cold by 36% compared with the placebo group (incident rate ratio: 0.64; 95% CI: 0.70, 0.88) with high heterogeneity. In addition, children missed fewer days of school when supplemented with zinc compared with the placebo group (mean difference: −0.66 d; 95% CI: −0.99, −0.33).
Zinc deficiency is suggested to be a risk factor for pneumonia in older adults (211). Low serum zinc concentrations were linked to a higher risk of pneumonia and longer duration of pneumonia episodes as well as increased antibiotic use in older adults in nursing homes (202). In a murine model of S. pneumoniae infection, dietary zinc restriction resulted in a reduced ability to control bacterial growth, leading to increased virulence and infection, and a much greater inflammatory response (212). Interestingly, the activation and infiltration of phagocytic cells into the infected region were not affected by zinc restriction. Rather, zinc-supplemented phagocytic cells had higher concentrations of intracellular zinc, which promoted lysis of phagocytized bacteria, demonstrating that zinc can act as an antimicrobial agent. While these findings are promising, a study in hospitalized adults (>50 y of age) with community-acquired pneumonia found that zinc (25 mg twice daily for 4 d in combination with standard treatment) did not improve outcomes (213). It is possible that the duration of zinc supplementation was too short to have a benefit. However, a meta-analysis revealed that, in children, zinc supplementation significantly reduced mortality caused by severe pneumonia (214). Given the data linking zinc deficiency and pneumonia susceptibility and health outcomes, and the rising rate of resistance of S. pneumoniae to conventional antibiotics (215), further research is warranted to determine the efficacy of supplemental zinc in attenuating S. pneumoniae infection and disease-associated mortality in older adults.
In vitro models of influenza infection have demonstrated that zinc can inhibit influenza virus RNA polymerase activity and significantly reduced viral titers (216, 217). Similarly, in vitro studies have demonstrated that zinc can inhibit RNA-dependent RNA polymerases of severe acute respiratory syndrome coronavirus (SARS-CoV)-1 (218), which has high homology with SARS-CoV-2, the virus responsible for COVID-19. Multiple studies report an association between low zinc status and increased susceptibility to, and severity of, COVID-19 [e.g. (219)], although such studies cannot demonstrate cause and effect. Zinc supplementation in patients hospitalized with COVID-19 is reported to reduce the risk of poor outcome, including mortality in some studies (220, 221) but not others (222). Clearly more, and better, studies are needed in this area.
Centenarians (those aged 90–100 y) are a useful model for understanding healthy aging, and studies comparing changes in immunity between older individuals (65–80 y) and centenarians may yield mechanistic insights, including about the role of zinc. One study reported differences in NK activity, MT and cytokine concentrations, and DNA-repair enzyme activity between older adults and healthy centenarians (223). The authors suggest that centenarians have lower MT mRNA, resulting in greater zinc ion bioavailability, supporting NK-cell activity and a higher capacity for DNA repair. MT polymorphisms have also been demonstrated to be involved in maintaining innate immune responses and intracellular zinc availability in the aged, suggesting that mechanisms that maintain adequate zinc homeostasis/metabolism in immune cells are associated with healthy aging and longevity (224). Given that responses to nutritional interventions are highly variable between participants despite similarities in anthropometrics, age, and sex, personalized nutrition may be leveraged in the future to address differences in response to dietary interventions and immune/inflammatory responses associated with specific gene polymorphisms.
It is important to note that, while low zinc status is associated with a weakened immune system, long-term and high-dose supplementation of zinc can lead to copper deficiency (225), highlighting a potential adverse effect of high-dose or prolonged zinc supplementation.
In summary, zinc supports many aspects of innate and acquired immunity and has anti-inflammatory and antioxidant effects. Supplemental zinc seems to promote immune cell functions at the doses tested and in older adults, but these intakes are sometimes high compared with those that are recommended. Trials of zinc in relation to respiratory infection are inconsistent, perhaps relating to dose and formulation and the characteristics of the participants studied, including genotype, and there are few such studies in older adults. Future work should aim to confirm or refute the proposed benefits of zinc on respiratory disease in older adults through conduct of well-designed and adequately powered studies.
Selenium
Selenium, most often ingested as selenomethionine, is an essential micronutrient that is incorporated into selenoproteins as selenocysteine. Selenoproteins, such as glutathione peroxidases and iodothyronine deiodinase, are involved in multiple biological processes, such as redox signaling, antioxidant defenses, thyroid hormone production, and both immune and inflammatory responses (226). Thus, unsurprisingly, selenium deficiency has been linked to increased mortality and cancer risk, as well as impaired immune defenses (227). Mutations in the selenocysteine insertion sequence have been associated with impaired lymphocyte proliferation, abnormal cytokine secretion, and telomere shortening, highlighting the importance of selenoproteins in immune cells (228). While selenium deficiency is rare in the United States and Canada, people in other countries such as those living in parts of China, Europe, and Russia, are at risk for selenium deficiency. Selenium intake in these countries is inadequate due to low soil selenium concentrations, resulting in variable levels of selenium incorporation into food and, thus, an inadequate intake to support the optimal expression of the selenoproteins (229).
In mice, selenium has been shown to be vital for both adaptive and innate immune cells, particularly for NK- and T-cell function (230, 231). Experimental data in aged mice suggest that selenium supplementation may augment immune function: splenocytes from aged mice that were supplemented with selenium for 8 wk had increased proliferation capacity after stimulation with mitogens (232). Additionally, in vivo alloantigen-activated lymphocytes from selenium-supplemented aged mice contained significantly higher numbers of cytotoxic T lymphocytes, which resulted in an enhanced capacity to destroy tumor cells (232). This effect occurred in the absence of changes in the ability of the cells to produce IL-2, suggesting that selenium restored the age-related defect in cell proliferation through an increase in the number of IL-2 receptors (232). Selenium deficiency in mice increases susceptibility to viral infection and permits viral mutation, including of influenza viruses, and so allowing normally weak viruses to become more virulent (233–236). The permissive effect of selenium deficiency on viral mutation and virulence seems to relate to the higher oxidative stress that exists in the absence of selenium. The effects of selenium on antiviral immunity have been comprehensively reviewed recently (237–239).
A study in older women (90–106 y), linked both zinc and selenium deficiency to a reduced percentage of NK cells in the blood (240). Selenium supplementation (100–400 μg/d depending on the study) has been shown to improve various aspects of immune function in humans (241–243), including in the elderly. Older people in nursing homes received 100 μg selenium daily as selenium-enriched yeast for 6 mo: lymphocyte proliferation in response to mitogens increased (244). An RCT in older people (57–84 y), demonstrated that 6 mo of selenium supplementation (400 μg/d) increased the number of T cells, CD4+ T cells, and NK cells in blood and increased NK-cell activity (245). However, the impact of supplementation was not sustained postsupplementation. Two other RCTs of selenium in younger adults are also of particular interest. Broome et al. (246) conducted a 15-wk RCT of 2 doses of selenium (50 or 100 μg/d) in adults (20–47 y of age) with marginal selenium status. After 6 wk, participants received the live attenuated oral poliomyelitis vaccine. Selenium resulted in a dose-dependent increase in blood T-cell numbers and in ex vivo mononuclear cell responses (proliferation, IFN-γ production) to the vaccine. Selenium-supplemented individuals also showed more rapid clearance of the poliovirus and had a lower number of virus mutants appearing in their feces (246). A more recent RCT compared 3 doses of selenium (50, 100, and 200 μg/d) from selenium-enriched yeast over 12 wk in adults (mean age: 56 y); participants received the seasonal influenza vaccine after 10 wk (247). Selenium (100 μg/d) increased blood cytotoxic T-cell numbers before influenza vaccination. Two weeks postvaccination, blood mononuclear cells were stimulated ex vivo with the influenza vaccine. Selenium supplementation increased T-cell proliferation and production of IL-8 and IL-10. However, the highest dose of selenium decreased the granzyme B content of cytotoxic T cells (247). There was no effect of selenium on mucosal influenza-specific antibodies.
An observational study found that selenium concentrations were significantly lower in patients with tuberculosis than in controls (248, 249). Similarly, low redox status, high oxidative stress, and low serum selenium status were observed in patients with pulmonary tuberculosis and anti-tuberculosis treatment steadily increased the concentration of selenium (250). Selenium and vitamin E supplementation, in combination with standard anti-tuberculosis treatment, reduced malondialdehyde, a marker of oxidative damage, in patients with newly diagnosed tuberculosis, in comparison to controls who only received standard treatment (251). However, it should be noted that M. tuberculosis requires selenium for its own survival and replication (252). No studies have assessed the impact of selenium on immune responses during tuberculosis infection in older individuals. Some studies report an association between low selenium status and increased susceptibility to, and severity of, COVID-19 [e.g., (219)].
In summary, selenium supports many aspects of innate and acquired immunity and has anti-inflammatory effects. Animal studies clearly demonstrate the importance of selenium to antiviral immunity. Supplemental selenium seems to enhance immune cell functions at the doses tested and in older adults. There are few trials of selenium in relation to respiratory infection. Future work should aim to identify whether selenium has benefits on respiratory disease, especially in older adults, through conduct of well-designed and adequately powered studies.
Immune Enhancement through Targeting the Gut Microbiota
Modulation of gut microbiota and immunity
There are a variety of foods, food components, and supplements that modulate the gut microbiota, supporting the growth of bacteria that are considered favorable to health and well-being (53–55, 253). The proposed health benefits include favorably modulating the immune response and helping to control inflammation.
Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (254). Commonly used probiotics include different lactobacilli and bifidobacteria, although other bacterial genera and some yeasts are also used as probiotics (254). Whether many probiotics survive stomach acid and populate the intestine is still under debate (255), although live probiotic organisms can be cultured from fecal samples from individuals consuming those organisms. Even though they are defined as “live” microbes (254), there is increasing evidence that many probiotic preparations may not contain high numbers of live organisms but instead contain dead organisms, bacterial cell walls, bacterial metabolites, or bacteriophages that could all be biologically active (256). Indeed, organisms do not have to be alive to elicit immunological benefits (257), giving rise to the concepts of parabiotics and postbiotics (258, 259).
Prebiotics were previously largely restricted to fructo-oligosaccharides and inulins that promote the growth of bifidobacteria [see discussion in (260)]; however, the definition has been broadened to include “a substrate that is selectively utilized by host microorganisms conferring a health benefit” (260). Oligo- and polysaccharides remain the most commonly used prebiotics, and foods such as onions, Jerusalem artichokes, leeks, garlic, bananas, and asparagus have some of the highest potential to provide these prebiotics. Mushrooms, some species of yeast, and some seaweeds may exert prebiotic effects through B-glucans (261, 262), although B-glucans appear to also act through immune-training mechanisms (263, 264). Polyphenols in spices have also been shown to affect gut microbiota and may be considered as prebiotics (260, 265). The culinary herbs, black pepper, cayenne pepper, cinnamon, ginger, Mediterranean oregano, and rosemary demonstrated prebiotic-like activity by promoting the growth of beneficial bacteria and suppressing the growth of pathogenic bacteria in vitro. Synbiotics are combinations of at least 1 prebiotic with at least 1 probiotic (266).
Many studies have examined the effect of various probiotic organisms, either alone or in combination, on immune function, infection, and inflammatory conditions in humans (267), including in older people (268). Probiotic organisms increased NK-cell activity in older people in several studies (269–272), and increased phagocytosis by monocytes (269) and granulocytes (273) and monocyte oxidative burst (274). The effects of probiotics on NK-cell activity, phagocytosis, and oxidative burst were seen with live but not dead organisms. Probiotics increased production of IFN-α (273) and of IFN-γ, IL-5, and IL-10 (275) by blood mononuclear cell cultures stimulated with a T-cell agonist. However, T-cell proliferation was not affected by probiotics in several studies (269, 276, 277). Most studies of prebiotics and immunity in older people have used either fructo-oligosaccharides or galacto-oligosaccharides (278). There are reports that prebiotics increase NK-cell activity (279, 280) and granulocyte phagocytosis of bacteria (279).
The effect of probiotics and prebiotics on seasonal influenza vaccination in older people has been investigated in a number of studies, with variable findings (281–290) according to the probiotic organism or prebiotic used. Recent systematic reviews and meta-analyses confirm that probiotics or oligosaccharide-type prebiotics enhance the response to seasonal influenza vaccination in adults (291, 292). Antibody titers to the H1N1 strain of the influenza virus were higher in individuals receiving probiotics, prebiotics, and either pro- or prebiotics than in those in placebo groups (291). Antibody titers to the H3N2 strain of the influenza virus were higher in individuals receiving probiotics and either pro- or prebiotics, while antibody titers to the B strain were higher in individuals receiving either pro- or prebiotics (291). This meta-analysis did not perform any subgroup analysis by age, so the overall effect in older people is not clear. However, a separate meta-analysis of RCTs reported effects on seroprotection and seroconversion post-seasonal influenza vaccination in adults and included subgroup analysis according to age (292). This meta-analysis identified higher seroprotection to the H1N1 and H3N2 strains in individuals receiving pre- or probiotics than in those in placebo groups (OR, H1NI: 1.83; 95% CI: 1.19, 2.82; OR, H3N2: 2.85; 95% CI: 1.59, 5.10). Seroprotection to the B strain was not affected by pre- or probiotics. There was higher seroconversion to the B strain in individuals receiving pre- or probiotics than in placebo groups (OR: 2.11; 95% CI: 1.38, 3.21) with a trend (both P = 0.07) for higher seroconversion to the H1N1 and H3N2 strains (OR, H1N1: 1.52; 95% CI: 0.75, 3.09; OR, H3N2: 2.54; 95% CI: 0.93, 6.91).
Subgroup analysis according to whether probiotics or prebiotics were used indicated that probiotics increased seroconversion to the H3N2 and B components and increased seroprotection to the H3N2 component (292). Prebiotics increased seroprotection to the H1N1 and H3N2 components (292).
Subgroup analysis according to age indicated that an effect of pre- or probiotics on seroconversion and seroprotection to H1N1 was seen in healthy older adults (OR seroconversion: 2.93; 95% CI: 1.47, 5.87; OR seroprotection: 2.46; 95% CI: 1.15, 5.26) but not in healthy younger/middle-aged adults (292). There was also an effect of pre- or probiotics on seroprotection to H1N1 in hospitalized older adults (OR: 2.06; 95% CI: 1.11, 3.82), but there was no effect on seroconversion. Greater seroconversion to H3N2 was seen with pre- or probiotics than with placebo in healthy older adults (OR: 3.68; 95% CI: 1.11, 12.25) and there was a trend to higher seroprotection in both healthy older adults (OR: 2.27; 95% CI: 0.94, 5.47) and hospitalized older adults (OR: 2.83; 95% CI: 0.97, 8.21). Both seroprotection and seroconversion were higher with pre- or probiotics than with placebo in healthy younger/middle-aged adults. Seroconversion to the B component was greater with pre- or probiotics than placebo in healthy older adults (OR: 2.69; 95% CI: 1.51, 4.78) and there was a trend to greater seroconversion with pre- or probiotics in hospitalized older adults (OR: 2.05; 95% CI: 0.92, 4.58). These findings indicate that pre- or probiotics increase responses to the seasonal influenza vaccine in adults, including in older adults. However, the number of studies included in the subgroup analyses was small (e.g., only 1 study in hospitalized older adults).
Modulation of gut microbiota and infections
As a result of their dual influence on the gut microbiota and on the host's gut epithelium and immune system, probiotics are proposed to reduce infections. This would be most obvious for gastrointestinal infections. In accordance with this, several lactobacilli strains have been shown to reduce the incidence, duration, and severity of diarrhea in children (293–297). Recent studies have identified that heat-killed lactobacilli decreased the risk and duration of diarrhea in children (298). In adults, there is evidence that probiotics protect against antibiotic-associated diarrhea (299–303). Systematic reviews and meta-analyses identify that probiotics (again, especially some lactobacilli stains) reduce the incidence and duration of antibiotic-associated diarrhea and of Clostridium difficile–associated diarrhea in adults (304–306) and may be effective in treating these conditions (307). However, although probiotics are effective in preventing and treating diarrhea in both children and adults, there are considerable differences in the effects of different probiotic species and strains and the effects observed with 1 type of probiotic cannot be extrapolated to another.
The gut microbiota seems to play a role in determining infections at sites distant from the gut, including in the respiratory tract (308, 309), suggesting a gut–lung axis of some importance in maintaining respiratory health that may be amenable to modulation by probiotics. There are a number of studies of probiotics in respiratory disease, mainly in children, and mainly using different lactobacilli and bifidobacteria. Many of these studies report that probiotics reduce the incidence or severity of respiratory tract infections, as indicated by systematic reviews and meta-analyses (310–318). A meta-analysis of 20 RCTs in children and adults (all adults were young or middle-aged) that used a variety of probiotic organisms found that, compared with placebo, probiotics reduced the number of days of illness and the number of days absent from daycare, school, or work (313). A second meta-analysis of 12 RCTs in children and adults identified that probiotics reduced the risk of URTIs compared with placebo (OR: 0.53; 95% CI: 0.37, 0.76) and reduced the duration of URTIs (mean duration: −1.89 d; 95% CI: −2.03, −1.75 d) and the need for antibiotic prescription to treat the URTI (314). While probiotics were superior to placebo in this meta-analysis, the quality of the included studies was considered to be low. These meta-analyses combined trials in children and adults and did not include trials in older people. However, a recent meta-analysis of 22 RCTs investigated effects of probiotic fermented dairy products on respiratory tract infections in children, adults, and older people (319). Compared with placebo, consumption of probiotics reduced the risk of respiratory tract infections in all age groups combined (RR: 0.81; 95% CI: 0.74, 0.89) and separately in children (RR: 0.82; 95% CI: 0.73, 0.93), in adults (RR: 0.81; 95% CI: 0.66, 1.00), and in the elderly population (RR: 0.78; 95% CI: 0.61, 0.98). Disease-specific analysis identified that probiotics decreased the risk of URTIs (RR: 0.83; 95% CI: 0.73, 0.93), pneumonia (RR: 0.76; 95% CI: 0.61, 0.95), and the common cold (RR: 0.68; 95% CI: 0.49, 0.96) and tended (P = 0.06) to decrease the risk of lower respiratory tract infections (RR: 0.78; 95% CI: 0.60, 1.01). Taken together, these findings provide evidence that probiotics, in particular some lactobacilli and bifidobacteria, reduce the incidence, and improve the outcomes, of respiratory infections in humans, including the elderly.
Another recent meta-analysis investigated the effect of synbiotics on respiratory tract infections (320). It included 16 RCTs conducted in infants, children, and adults. The synbiotics mostly included lactobacilli or bifidobacteria as probiotics and fructo-oligosaccharides, galacto-oligosaccharides, or inulin as prebiotics. Compared with placebo, synbiotics decreased the incidence of respiratory tract infection (rate ratio: 0.84; 95% CI: 0.73, 0.96) and the risk of developing a respiratory tract infection (risk ratio: 0.84; 95% CI: 0.74, 0.95). Subgroup analysis demonstrated that synbiotics decreased the incidence of respiratory tract infection in adults (rate ratio: 0.68; 95% CI: 0.57, 0.81) but not in infants and children.
Modulation of gut microbiota and inflammation
There is some evidence that probiotics may reduce the inflammation associated with aging, as discussed elsewhere recently (321). Probiotics decrease the proinflammatory cytokines IL-1, IL-6, and TNF-α (322) and increase transforming growth factor-β made by regulatory T cells (323). Furthermore, probiotics have been shown to increase antioxidant activity (glutathione) in a meta-analysis of 16 clinical studies (324).
Conclusions and Perspectives
Aging is associated with changes in the immune system, with a decline in protective components (immunosenescence), so increasing susceptibility to infectious disease (and also contributing to the development of autoimmunity and malignancy, which are not discussed herein), and a chronic elevation in low-grade inflammation, so increasing the risk of multiple noncommunicable diseases. Thus, the age-related changes in the immune system increase the risk of both communicable and noncommunicable diseases in the population, contributing to stress on health care and social care systems. Age-related immune decline also contributes to poor vaccine efficacy, resulting in wasted resource and creating a false sense of security in recipients. The higher rate of infections among older people means they are significant users of antibiotics, contributing to the development of antibiotic resistance. For these reasons, age-related immune decline (the combination of immunosenescence and inflammaging) is a major public health challenge that requires solutions. This is especially important because the number of older people is increasing (325–327) and also because changes in the environment and weather conditions, due to climate change, may alter the dynamics and geographic range of existing, and favor the emergence of, novel infectious diseases (328–330).
Nutrition is a determinant of immune cell development and function and of the gut microbiota. In turn, the gut microbiota shapes and controls the immune and inflammatory responses. Many older people show changes in food intake, with many not meeting micronutrient recommendations or consuming enough protein. Older people also show changes in the gut microbiota, which can be accelerated by moving into residential care. Hence it seems likely that the age-related changes in immune competence, low-grade inflammation, and gut dysbiosis are interlinked and all relate, at least in part, to the age-related changes in nutrition.
Multiple micronutrients play multiple roles within the immune system (Table 3). There is evidence that, through restoring status, micronutrient supplements can reverse immune deficits in older people and/or in those with insufficient intakes. Nevertheless, there are inconsistencies in the literature reporting on micronutrient trials and immune outcomes in humans. These inconsistencies relate in part to the large between-individual differences in immune markers that exist (3–5), meaning that sample sizes of human trials have sometimes been too small to identify the hypothesized effect. This is particularly important because the effect size of nutritional interventions may be modest. Despite the evidence that individual micronutrients can improve immune outcomes, the evidence that this will reduce the risk or severity of infections is inconsistent. Therefore, further research with well-designed and well-powered trials in at-risk older populations is required to be more certain about the role of micronutrients on infection, especially respiratory infection. In this regard, rationally designed micronutrient combinations using appropriate levels of intake (there are many trials of medium to high doses of single micronutrients, some showing benefit and some not) should be examined and there should be more studies of the effects on responses to common vaccinations, including both immune responses (e.g., antibody titers) and clinical protection. It is clear that a sizeable fraction of older adults fall short in the intake of many micronutrients, particularly of those with known immunomodulatory effects (i.e., vitamins C, D, and E and zinc and, in some countries, also selenium). Thus, promoting the intake of these micronutrients to correct marginal deficiencies may have a considerable impact on the aged immune system and help in the battle against infectious disease. With regard to individual micronutrients, most studies have investigated single doses at 1 or only a few time points; more needs to be known about the dose-response effects in older people of all the micronutrients discussed herein. It is known that very high intakes of some micronutrients (e.g., zinc) impair the immune response and therefore more clarity is needed on upper limits of intake from the immune system perspective. Other areas to be explored include sex-related differences in immunosenescence and inflammaging and how these relate to nutrient intake and micronutrient supplementation and how micronutrients interact with genetic polymorphisms to determine immune and infectious outcomes.
TABLE 3.
Summary of the effects of selected micronutrients on different aspects of immunity1
| Micronutrient | Role in barrier function | Role in cellular aspects of innate immunity | Role in T-cell–mediated immunity | Role in B-cell–mediated immunity |
|---|---|---|---|---|
| Vitamin C | Promotes collagen synthesis and connective tissue healing; protects against oxidative damage; promotes wound healing | Supports function of neutrophils, monocytes and macrophages including phagocytosis; promotes neutrophil chemotaxis; supports NK-cell activity | Promotes production, differentiation and proliferation of T cells, especially cytotoxic T cells; regulates IFN-γ production | Promotes production and proliferation of B cells; promotes antibody production |
| Vitamin D | Promotes epithelial integrity; promotes production of antimicrobial proteins (cathelicidin, β-defensin); promotes homing of T cells to the skin | Promotes differentiation of monocytes to macrophages; promotes macrophage phagocytosis, oxidative burst and bacterial killing; supports NK-cell activity | Promotes antigen processing but can inhibit antigen presentation; can inhibit T-cell proliferation, Th1-cell function, and cytotoxic T-cell function; promotes the development of regulatory T cells; inhibits differentiation and maturation of dendritic cells; regulates IFN-γ production | Can decrease antibody production |
| Vitamin E | Protects against oxidative damage | Reduces inflammation; supports NK-cell activity; promotes neutrophil phagocytosis | Promotes interaction between dendritic cells and T cells; promotes T-cell proliferation and function, especially Th1 cells; regulates (promotes) IL-2 production | Supports antibody production |
| Zinc | Maintains integrity of the skin and mucosal membranes; Promotes complement activity | Reduces inflammation; supports monocyte and macrophage phagocytosis; promotes formation of neutrophil extracellular traps; supports NK-cell activity | Promotes Th1-cell response; promotes proliferation of cytotoxic T cells; promotes development of regulatory T cells; regulates (promotes) IL-2 and IFN-γ production; reduces development of Th9 and Th17 cells | Supports antibody production, IgG |
| Selenium | — | Reduces inflammation; supports NK-cell activity | Regulates differentiation and proliferation of T cells; regulates (promotes) IFN-γ production | Supports antibody production |
Adapted from reference 2. Th, T-helper;
Probiotic, prebiotic, or synbiotic strategies that modulate the gut microbiota, especially by promoting the colonization of some strains of lactobacilli and bifidobacteria, have been demonstrated to modulate some immune and inflammatory biomarkers in older people and, in some cases, to reduce the risk and severity of gastrointestinal and respiratory infections. However, it is important to note that the evidence is again inconsistent and, in some cases, considered to be of low quality. One reason for the inconsistency is likely to be that the effects of probiotic organisms are species and strain specific. Better definitions of those organisms that have immune benefits should be a focus for future research, which should use well-designed and well-powered studies and appropriate outcomes. It is now evident that some effects of probiotics do not require them to be alive. This has given rise to the concepts of postbiotics and parabiotics, which include dead organisms, bacterial extracts, bacterial cell wall components, and metabolic products of bacteria such as SCFAs. Another consideration is the matrix used to deliver probiotics, which might be important in determining their effects, and the matrix and exact chemical form of micronutrients, which might affect their bioavailability and function. Clearly, this is an area that is ripe for research in the context of immunity, inflammation, and infection, but to generate high-quality evidence (for or against the food component being investigated), serious consideration needs to be given to the number of factors that could influence the outcome of human trials.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—PCC, SNM, CBS, and HZ: conceptualization; PCC, EFO, SNM, YA, CBS, BT, and HZ: drafting of the manuscript; PCC, SNM, CBS, and HZ: review of the manuscript; PCC: overall responsibility; and all authors: read and approved the final manuscript.
Notes
Based on a symposium (“Nutrition, Immunosenescence, and Infectious Disease: An Overview of the Scientific Evidence on Micronutrients and Bioactives”) sponsored by HALEON held at Nutrition 2021 (American Nutrition Society), June 2021.
Author disclosures: PCC has received research funding from Bayer Consumer Care; acts as an advisor/consultant to BASF AS, DSM, Cargill, Danone/Nutricia, Smartfish, Nutrileads, Bayer Consumer Care, HALEON, and Kemin; and has received speaking fees from BASF AS, DSM, Danone/Nutricia, Bayer Consumer Care, HALEON, Kemin, and Proctor and Gamble. SNM currently has research support from USDA, NIH, and Gates Foundation and acts as an advisor to Nestle Research and Development, Danone Essential Dairy and Plant-based, Cargill Healthy Aging Group, Pharmavit, Arthritis Foundation, and Barilla Health and Wellness. HZ has received research funding from National Center for Complementary and Integrative Health (NCCIH) grant 2 R90AT008924-01 and Thaena, Inc.; acts as an advisor or member of scientific advisory board of Burt's Bees, Clorox, HALEON, and Nutranext; and has received speaking fees from Metagenics, Mymee, and Doctor's Data. YA and CBS report no conflicts of interest but report research support from USDA project number 2032-51530-026-000-D. EFO and BT report no conflicts of interest.
Abbreviations used: COVID-19, coronavirus disease 2019; CRP, C-reactive protein; GALT, gut-associated lymphoid tissue; MT, metallothionein; PG, prostaglandin; PGE2, prostaglandin E2; RCT, randomized controlled trial; ROS, reactive oxygen species; SARS-CoV, severe acute respiratory syndrome coronavirus; TCR, T-cell receptor; Th, T-helper type; TLR, Toll-like receptor; URTI, upper respiratory tract infection.
Contributor Information
Philip C Calder, School of Human Development and Health, Faculty of Medicine, University of Southampton, Southampton, United Kingdom; NIHR Southampton Biomedical Research Centre, University Hospital Southampton NHS Foundation Trust and University of Southampton, Southampton, United Kingdom.
Edwin Frank Ortega, Nutritional Immunology Laboratory, Jean Mayer–USDA Human Nutrition Research on Aging at Tufts University, Boston, MA, USA.
Simin N Meydani, Nutritional Immunology Laboratory, Jean Mayer–USDA Human Nutrition Research on Aging at Tufts University, Boston, MA, USA.
Yuriko Adkins, USDA Western Human Nutrition Research Center, Davis, CA, USA; Nutrition Department, University of California, Davis, CA, USA.
Charles B Stephensen, USDA Western Human Nutrition Research Center, Davis, CA, USA; Nutrition Department, University of California, Davis, CA, USA.
Brice Thompson, Department of Pharmaceutics, University of Washington, Seattle, WA, USA.
Heather Zwickey, Helfgott Research Institute, National University of Natural Medicine, Portland, OR, USA.
References
- 1. Miles EA, Childs CE, Calder PC. Long-chain polyunsaturated fatty acids (LCUFAs) and the developing immune system: a narrative review. Nutrients. 2021;13:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calder PC. Nutrition and immunity: lessons for COVID-19. Eur J Clin Nutr. 2021;75:1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calder PC, Kew S. The immune system: a target for functional foods?. Br J Nutr. 2002;88:S165–76. [DOI] [PubMed] [Google Scholar]
- 4. Cummings JH, Antoine J-M, Aspiroz F, Bourdet-Sicard R, Brandtzaeg P, Calder PCet al. PASSCLAIM—gut health and immunity. Eur J Nutr. 2004;43(Suppl 2):118–73. [DOI] [PubMed] [Google Scholar]
- 5. Albers R, Antoine J-M, Bourdet-Sicard R, Calder PC, Gleeson M, Lesourd Bet al. Markers to measure immunomodulation in human nutrition intervention studies. Br J Nutr. 2005;94:452–81. [DOI] [PubMed] [Google Scholar]
- 6. Calder PC, Ahluwalia N, Albers R, Bosco N, Bourdet-Sicard R, Haller Det al. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br J Nutr. 2013;109(Suppl 1):S1–34. [DOI] [PubMed] [Google Scholar]
- 7. Yoshikawa TT. Epidemiology and unique aspects of aging and infectious diseases. Clin Infect Dis. 2000;30:931–3. [DOI] [PubMed] [Google Scholar]
- 8. Pera A, Campos C, López N, Hassouneh F, Alonso C, Tarazona Ret al. Immunosenescence: implications for response to infection and vaccination in older people. Maturitas. 2015;82:50–5. [DOI] [PubMed] [Google Scholar]
- 9. Watson A, Wilkinson TMA. Respiratory viral infections in the elderly. Ther Adv Resp Dis. 2021;15:175346662199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi T, Denouel A, Tietjen AK, Campbell I, Moran E, Li Xet al. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis. 2020;222:S577–83. [DOI] [PubMed] [Google Scholar]
- 11. Ishifuji T, Sando E, Kaneko N, Suzuki M, Kilgore PE, Ariyoshi Ket al. Adult Pneumonia Study Group—Japan (APSG-J). Recurrent pneumonia among Japanese adults: disease burden and risk factors. BMC Pulmon Med. 2017;17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bloom BR, Atun R, Cohen T, Dye C, Fraser H, Gomez GBet al. Tuberculosis. In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major infectious diseases. Washington (DC): The International Bank for Reconstruction and Development/The World Bank; 2018. pp. 233–313. [Google Scholar]
- 13. CDC COVID-19 Response Team . Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12-March 28, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gillespie D, Hood K, Bayer A, Carter B, Duncan D, Espinasse Aet al. Antibiotic prescribing and associated diarrhoea: a prospective cohort study of care home residents. Age Ageing. 2015;44:853–60. [DOI] [PubMed] [Google Scholar]
- 15. Bartoszko J, Loeb M. The burden of influenza in older adults: meeting the challenge. Aging Clin Exp Res. 2021;33:711–7. [DOI] [PubMed] [Google Scholar]
- 16. Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–69. [DOI] [PubMed] [Google Scholar]
- 17. Trzonkowski P, My´sliwska J, Pawelec G, My´sliwski A. From bench to bedside and back: the SENIEUR Protocol and the efficacy of influenza vaccination in the elderly. Biogerontology. 2009;10:83–94. [DOI] [PubMed] [Google Scholar]
- 18. Derhovanessian E, Pawelec G. Vaccination in the elderly. Microb Biotechnol. 2012;5:226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ciabattini A, Nardini C, Santoro F, Garagnani P, Franceschi C, Medaglini D. Vaccination in the elderly: the challenge of immune changes with aging. Semin Immunol. 2018;40:83–94. [DOI] [PubMed] [Google Scholar]
- 20. De Martinis M, Modesti M, Ginaldi L. Phenotypic and functional changes of circulating monocytes and polymorphonuclear leucocytes from elderly persons. Immunol Cell Biol. 2004;82:415–20. [DOI] [PubMed] [Google Scholar]
- 21. Agarwal S, Busse PJ. Innate and adaptive immunosenescence. Ann Allergy Asthma Immunol. 2010;104:183–90. [DOI] [PubMed] [Google Scholar]
- 22. Pawelec G, Larbi A, Derhovanessian E. Senescence of the human immune system. J Comp Pathol. 2010;142(Suppl 1):S39–44. [DOI] [PubMed] [Google Scholar]
- 23. Castelo-Branco C, Soveral I. The immune system and aging: a review. Gynecol Endocrinol. 2014;30:16–22. [DOI] [PubMed] [Google Scholar]
- 24. Bektas A, Schurman SH, Sen R, Ferrucci L. Human T cell immunosenescence and inflammation in aging. J Leukocyte Biol. 2017;102:977–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pritz T, Weinberger B, Grubeck-Loebenstein B. The aging bone marrow and its impact on immune responses in old age. Immunol Lett. 2014;162:310–5. [DOI] [PubMed] [Google Scholar]
- 26. Bulati M, Caruso C, Colonna-Romano G. From lymphopoiesis to plasma cells differentiation, the age-related modifications of B cell compartment are influenced by “inflamm-ageing”. Ageing Res Rev. 2017;36:125–36. [DOI] [PubMed] [Google Scholar]
- 27. Palmer DB. The effect of age on thymic function. Front Immunol. 2013;4:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yao X, Hamilton RG, Weng N-P, Xue Q-L, Bream JH, Li Het al. Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine. 2011;29:5015–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahluwalia N. Aging, nutrition and immune function. J Nutr Health Aging. 2004;8:2–6. [PubMed] [Google Scholar]
- 30. Ahluwalia N, Sun J, Krause D, Mastro A, Handte G. Immune function is impaired in iron-deficient, homebound, older women. Am J Clin Nutr. 2004;79:516–21. [DOI] [PubMed] [Google Scholar]
- 31. Haase H, Mocchegiani E, Rink L. Correlation between zinc status and immune function in the elderly. Biogerontology. 2006;7:421–8. [DOI] [PubMed] [Google Scholar]
- 32. Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured?. Nutr Rev. 2007;65:S173–6. [DOI] [PubMed] [Google Scholar]
- 33. Calder PC, Bosco N, Bourdet-Sicard R, Capuron L, Delzenne N, Doré Jet al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev. 2017;40:95–119. [DOI] [PubMed] [Google Scholar]
- 34. Ventura MT, Casciaro M, Gangemi S, Buquicchio R. Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin Mol Allergy. 2017;15:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Atienza M, Ziontz J, Cantero JL. Low-grade inflammation in the relationship between sleep disruption, dysfunctional adiposity, and cognitive decline in aging. Sleep Med Rev. 2018;42:171–83. [DOI] [PubMed] [Google Scholar]
- 36. Fülöp T, Larbi A, Witkowski JM. Human inflammaging. Gerontology. 2019;65:495–504. [DOI] [PubMed] [Google Scholar]
- 37. Burton DGA, Stolzing A. Cellular senescence: immunosurveillance and future immunotherapy. Ageing Res Rev. 2018;43:17–25. [DOI] [PubMed] [Google Scholar]
- 38. Fulop T, Larbi A, Pawelec G, Khalil A, Cohen AA, Hirokawa Ket al. Immunology of aging: the birth of inflammaging. Clin Rev Allergy Immunol. 2021;18:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arai Y, Martin-Ruiz CM, Takayama M, Abe Y, Takebayashi T, Koyasu Set al. Inflammation, but not telomere length, predicts successful ageing at extreme old age: a longitudinal study of semi-supercentenarians. EBioMedicine. 2015;2:1549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thomas CM, Versalovic J. Probiotics-host communication: modulation of signaling pathways in the intestine. Gut Microbes. 2010;1:148–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ahern PP, Maloy KJ. Understanding immune–microbiota interactions in the intestine. Immunology. 2020;159:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Samuelson DR, Welsh DA, Shellito JE. Regulation of lung immunity and host defense by the intestinal microbiota. Front Microbiol. 2015;6:1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Belkaid Y, Harrison OJ. Homeostatic immunity and the microbiota. Immunity. 2017;46:562–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Macia L, Thorburn AN, Binge LC, Marino E, Rogers KE, Maslowski KMet al. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunol Rev. 2012;245:164–76. [DOI] [PubMed] [Google Scholar]
- 47. Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini Get al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–85. [DOI] [PubMed] [Google Scholar]
- 48. Cervantes-Barragan L, Chai JN, Tianero MD, Di Luccia B, Ahern PP, Merriman Jet al. Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T cells. Science. 2017;357:806–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Scott SA, Fu J, Chang PV. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc Natl Acad Sci. 2020;117:19376–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci. 2010;107:12204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Human Microbiome Project Consortium . Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SAet al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science. 2018;362:776–80. [DOI] [PubMed] [Google Scholar]
- 54. Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:35–56. [DOI] [PubMed] [Google Scholar]
- 56. Hopkins MJ, Sharp R, Macfarlane GT. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut. 2001;48:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arboleya S, Watkins C, Stanton C, Ross RP. Gut bifidobacteria populations in human health and aging. Front Microbiol. 2016;7:1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Biagi E, Candela M, Turroni S, Garagnani P, Franceschi C, Brigidi P. Ageing and gut microbes: perspectives for health maintenance and longevity. Pharmacol Res. 2013;69:11–20. [DOI] [PubMed] [Google Scholar]
- 59. O'Toole PW, Jeffery IB. Gut microbiota and aging. Science. 2015;350:1214–5. [DOI] [PubMed] [Google Scholar]
- 60. Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack Set al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–84. [DOI] [PubMed] [Google Scholar]
- 61. Fujihashi K, Kiyono H. Mucosal immunosenescence: new developments and vaccines to control infectious diseases. Trends Immunol. 2009;30:334–43. [DOI] [PubMed] [Google Scholar]
- 62. Ogra PL. Ageing and its possible impact on mucosal immune responses. Ageing Res Rev. 2010;9:101–6. [DOI] [PubMed] [Google Scholar]
- 63. Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh Ket al. Gut microbiome and aging: physiological and mechanistic insights. Nutr Healthy Aging. 2018;4:267–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Badal VD, Vaccariello ED, Murray ER, Yu KE, Knight R, Jeste DVet al. The gut microbiome, aging, and longevity: a systematic review. Nutrients. 2020;12:3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. DeJong EN, Surette MG, Bowdish DME. The gut microbiota and unhealthy aging: disentangling cause from consequence. Cell Host Microbe. 2020;28:180–9. [DOI] [PubMed] [Google Scholar]
- 66. Calder PC. Feeding the immune system. Proc Nutr Soc. 2013;72:299–309. [DOI] [PubMed] [Google Scholar]
- 67. Calder PC. Nutrition, immunity and COVID-19. BMJ Nutr Prev Health. 2020;3:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Childs CE, Calder PC, Miles EA. Diet and immune function. Nutrients. 2019;11:1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wu D, Lewis ED, Pae M, Meydani SN. Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance. Front Immunol. 2019;9:3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kelly D, Coutts AG. Early nutrition and the development of immune function in the neonate. Proc Nutr Soc. 2000;59:177–85. [DOI] [PubMed] [Google Scholar]
- 71. Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66:464S–77S. [DOI] [PubMed] [Google Scholar]
- 72. Calder PC, Jackson AA. Undernutrition, infection and immune function. Nutr Res Rev. 2000;13:3–29. [DOI] [PubMed] [Google Scholar]
- 73. Poles J, Karhu E, McGill M, McDaniel HR, Lewis JE. The effects of twenty-four nutrients and phytonutrients on immune system function and inflammation: a narrative review. Transl Res. 2021;7:333–76. [PMC free article] [PubMed] [Google Scholar]
- 74. Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal nutritional status for a well functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12:1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Eggersdorfer M, Akobundu U, Bailey RL, Shlisky J, Beaudreault AR, Bergeron Get al. Hidden hunger: solutions for America's aging populations. Nutrients. 2018;10:1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Reider CA, Chung R-Y, Devarshi PP, Grant RW, Hazels Mitmesser S. Inadequacy of immune health nutrients: intakes in US adults, the 2005-2016 NHANES. Nutrients. 2020;12:1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. ter Borg S, Verlaan S, Hemsworth J, Mijnarends DM, Schols J, Luiking YCet al. Micronutrient intakes and potential inadequacies of community-dwelling older adults: a systematic review. Br J Nutr. 2015;113:1195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Amrein K, Scherkl M, Hoffmann M, Neuwersch-Sommeregger S, Köstenberger M, Tmava Berisha Aet al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. van der Wielen RP, Löwik MR, van den Berg H, de Groot LC, Haller J, Moreiras Oet al. Serum vitamin D concentrations among elderly people in Europe. Lancet North Am Ed. 1995;346:207–10. [DOI] [PubMed] [Google Scholar]
- 80. Maggini S, Pierre A, Calder PC. Immune function and micronutrient requirements change over the life course. Nutrients. 2018;10:1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system—working in harmony to reduce the risk of infection. Nutrients. 2020;12:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lesourd B. Nutritional factors and immunological ageing. Proc. Nutr. Soc. 2006;65:319–325. [DOI] [PubMed] [Google Scholar]
- 83. Pae M, Meydani SN, Wu D. The role of nutrition in enhancing immunity in aging. Aging Dis. 2012;3:91–129. [PMC free article] [PubMed] [Google Scholar]
- 84. Carr A, Maggini S. Vitamin C and immune function. Nutrients. 2017;9:1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Abobaker A, Alzwi A, Alraied AHA. Overview of the possible role of vitamin C in management of COVID-19. Pharmacol Rep. 2020;72:1517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Maggini S, Wintergerst ES, Beveridge S, Hornig DH. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. 2007;98:S29–35. [DOI] [PubMed] [Google Scholar]
- 87. Corpe CP, Lee JH, Kwon O, Eck P, Narayanan J, Kirk KLet al. 6-Bromo-6-deoxy-L-ascorbic acid: an ascorbate analog specific for Na+-dependent vitamin C transporter but not glucose transporter pathways. J Biol Chem. 2005;280:5211–20. [DOI] [PubMed] [Google Scholar]
- 88. Washko P, Rotrosen D, Levine M. Ascorbic acid transport and accumulation in human neutrophils. J Biol Chem. 1989;264:18996–9002. [PubMed] [Google Scholar]
- 89. Wang Y, Russo TA, Kwon O, Chanock S, Rumsey SC, Levine M. Ascorbate recycling in human neutrophils: induction by bacteria. Proc Natl Acad Sci. 1997;94:13816–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Washko PW, Wang Y, Levine M. Ascorbic acid recycling in human neutrophils. J Biol Chem. 1993;268:15531–5. [PubMed] [Google Scholar]
- 91. Manning J, Mitchell B, Appadurai DA, Shakya A, Pierce LJ, Wang Het al. Vitamin C promotes maturation of T-cells. Antioxid Redox Signaling. 2013;19:2054–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chen Y, Luo G, Yuan J, Wang Y, Yang X, Wang Xet al. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediators Inflamm. 2014;2014:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jacob RA, Kelley DS, Pianalto FS, Swendseid ME, Henning SM, Zhang JZet al. Immunocompetence and oxidant defense during ascorbate depletion of healthy men. Am J Clin Nutr. 1991;54:1302S–9S. [DOI] [PubMed] [Google Scholar]
- 94. Żychowska M, Grzybkowska A, Zasada M, Piotrowska A, Dworakowska D, Czerwińska-Ledwig Oet al. Effect of six weeks 1000 mg/day vitamin C supplementation and healthy training in elderly women on genes expression associated with the immune response—a randomized controlled trial. J Int Soc Sports Nutr. 2021;18:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chuangchot N, Boonthongkaew C, Phoksawat W, Jumnainsong A, Leelayuwat C, Leelayuwat N. Oral vitam:in C treatment increases polymorphonuclear cell functions in type 2 diabetes mellitus patients with poor glycemic control. Nutr Res. 2020;79:50–9. [DOI] [PubMed] [Google Scholar]
- 96. de la Fuente M, Sánchez C, Vallejo C, Díaz-Del Cerro E, Arnalich F, Hernanz Á. Vitamin C and vitamin C plus E improve the immune function in the elderly. Exp Gerontol. 2020;142:111118. [DOI] [PubMed] [Google Scholar]
- 97. de la Fuente M, Ferrández MD, Burgos MS, Soler A, Prieto A, Miquel J. Immune function in aged women is improved by ingestion of vitamins C and E. Can J Physiol Pharmacol. 1998;76:373–80. [PubMed] [Google Scholar]
- 98. Hemilä H. Vitamin C and infections. Nutrients. 2017;9:339–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Pauling L. Vitamin C and the common cold. San Francisco (CA): WH Freeman; 1970. [Google Scholar]
- 100. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev(1):2013:CD000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hunt C, Chakravorty NK, Annan G, Habibzadeh N, Schorah CJ. The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. Int J Vitam Nutr Res. 1994;64:212–9. [PubMed] [Google Scholar]
- 102. Chiscano-Camón L, Ruiz-Rodriguez JC, Ruiz-Sanmartin A, Roca O, Ferrer R. Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit Care. 2020;24:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhao B, Ling Y, Li J, Peng Y, Huang J, Wang Yet al. Beneficial aspects of high dose intravenous vitamin C on patients with COVID-19 pneumonia in severe condition: a retrospective case series study. Ann Palliat Med. 2021;10:1599–609. [DOI] [PubMed] [Google Scholar]
- 104. Jamalimoghadamsiahkali S, Zarezade B, Koolaji S, SeyedAlinaghi S, Zendehdel A, Tabarestani Met al. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial. Eur J Med Res. 2021;26:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Holford P, Carr AC, Jovic TH, Ali SR, Whitaker IS, Marik PEet al. Vitamin C-an adjunctive therapy for respiratory infection, sepsis and COVID-19. Nutrients. 2020;12:3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003;102:3314–6. [DOI] [PubMed] [Google Scholar]
- 107. Gottfried E, Rehli M, Hahn J, Holler E, Andreesen R, Kreutz M. Monocyte-derived cells express CYP27A1 and convert vitamin D3 into its active metabolite. Biochem Biophys Res Commun. 2006;349:209–13. [DOI] [PubMed] [Google Scholar]
- 108. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SRet al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. [DOI] [PubMed] [Google Scholar]
- 109. Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–96. [DOI] [PubMed] [Google Scholar]
- 110. Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. 2011;134:123–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cantorna MT, Snyder L, Lin Y-D, Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015;7:3011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Medrano M, Carrillo-Cruz E, Montero I, Perez-Simon JA. Vitamin D: effect on haematopoiesis and immune system and clinical applications. Int J Mol Sci. 2018;19:2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Prietl B, Treiber G, Pieber T, Amrein K. Vitamin D and immune function. Nutrients. 2013;5:2502–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sassi F, Tamone C, D'Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10:1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf). 2012;76:315–25. [DOI] [PubMed] [Google Scholar]
- 116. Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–77. [DOI] [PubMed] [Google Scholar]
- 117. Ferreira GB, Vanherwegen A-S, Eelen G, Gutiérrez ACF, Van Lommel L, Marchal Ket al. Vitamin D3 induces tolerance in human dendritic cells by activation of intracellular metabolic pathways. Cell Rep. 2015;10:711–25. [DOI] [PubMed] [Google Scholar]
- 118. Lee W-P, Willekens B, Cras P, Goossens H, Martínez-Cáceres E, Berneman ZNet al. Immunomodulatory effects of 1,25-dihydroxyvitamin D on dendritic cells promote induction of T cell hyporesponsiveness to myelin-derived antigens. J Immunol Res. 2016;2016:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ferreira GB, Gysemans CA, Demengeot J, da Cunha J, Vanherwegen A-S, Overbergh Let al. 1,25-Dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. J Immunol. 2014;192:4210–20. [DOI] [PubMed] [Google Scholar]
- 120. Lemire JM, Adams JS, Sakai R, Jordan SC. 1alpha,25-Dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J Clin Invest. 1984;74:657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Rigby WF, Yirinec B, Oldershaw RL, Fanger MW. Comparison of the effects of 1,25-dihydroxyvitamin D3 on T lymphocyte subpopulations. Eur J Immunol. 1987;17:563–6. [DOI] [PubMed] [Google Scholar]
- 122. Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–47. [DOI] [PubMed] [Google Scholar]
- 123. Jeffery LE, Wood AM, Qureshi OS, Hou TZ, Gardner D, Briggs Zet al. Availability of 25-hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J Immunol. 2012;189:5155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Huang Y, Zhao Y, Ran X, Wang C. Increased expression of herpesvirus entry mediator in 1,25-dihydroxyvitamin D3-treated mouse bone marrow-derived dendritic cells promotes the generation of CD4+CD25+Foxp3+ regulatory T cells. Mol Med Rep. 2014;9:813–8. [DOI] [PubMed] [Google Scholar]
- 125. Dankers W, Davelaar N, van Hamburg JP, van de Peppel J, Colin EM, Lubberts E. Human memory Th17 cell populations change into anti-inflammatory cells with regulatory capacity upon exposure to active vitamin D. Front Immunol. 2019;10:1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rizka A, Setiati S, Sadikin M, Mansur IG. Immunomodulatory effect of in vitro calcitriol in fit and frail elderly. Int Immunopharmacol. 2021;96:107737. [DOI] [PubMed] [Google Scholar]
- 127. Rizka A, Setiati S, Harimurti K, Sadikin M, Mansur IG. Effect of alfacalcidol on inflammatory markers and T cell subsets in elderly with frailty syndrome: a double blind randomized controlled trial. Acta Med Indones. 2018;50:215–21. [PubMed] [Google Scholar]
- 128. Oh S, Chun S, Hwang S, Kim J, Cho Y, Lee Jet al. Vitamin D and exercise are major determinants of natural killer cell activity, which is age- and gender-specific. Front Immunol. 2021;12:594356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Maboshe W, Macdonald HM, Wassall H, Fraser WD, Tang JCY, Fielding Set al. Low-dose vitamin D supplementation does not affect natural regulatory T cell population but attenuates seasonal changes in T cell-produced IFN-γ: results from the D-SIRe2 randomized controlled trial. Front Immunol. 2021;12:623087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lee MD, Lin CH, Lei WT, Chang HY, Lee HC, Yeung CYet al. Does vitamin D deficiency affect the immunogenic responses to influenza vaccination? A systematic review and meta-analysis. Nutrients. 2018;10:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chocano-Bedoya P, Ronnenberg AG. Vitamin D and tuberculosis. Nutr Rev. 2009;67:289–93. [DOI] [PubMed] [Google Scholar]
- 132. Zasloff M. Fighting infections with vitamin D. Nat Med. 2006;12:388–90. [DOI] [PubMed] [Google Scholar]
- 133. Wang T-T, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao Jet al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12. [DOI] [PubMed] [Google Scholar]
- 134. Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TRet al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Jolliffe DA, Ganmaa D, Wejse C, Raqib R, Haq MA, Salahuddin Net al. Adjunctive vitamin D in tuberculosis treatment: meta-analysis of individual participant data. Eur Respir J. 2019;53:4–13. [DOI] [PubMed] [Google Scholar]
- 136. Berry DJ, Hesketh K, Power C, Hypponen E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr. 2011;106:1433–40. [DOI] [PubMed] [Google Scholar]
- 137. Ginde AA, Mansbach JM, Camargo CA. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kuwabara A, Tsugawa N, Ao M, Ohta J, Tanaka K. Vitamin D deficiency as the risk of respiratory tract infections in the institutionalized elderly: a prospective 1-year cohort study. Clin Nutr ESPEN. 2020;40:309–13. [DOI] [PubMed] [Google Scholar]
- 139. Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin D and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5:e11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Jolliffe DA, Camargo CA Jr., Sluyter JD, Aglipay M, Aloia JF, Ganmaa Det al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021;9:276–92. [DOI] [PubMed] [Google Scholar]
- 141. Mercola J, Grant WB, Wagner CL. Evidence regarding vitamin D and risk of COVID-19 and its severity. Nutrients. 2020;12:3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Griffin G, Hewison M, Hopkin J, Kenny R, Quinton R, Rhodes Jet al. Vitamin D and COVID-19: evidence and recommendations for supplementation. R Soc Open Sci. 2020;7:201912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Merzon E, Tworowski D, Gorohovski A, Vinker S, Golan Cohen A, Green Iet al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287:3693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Pereira M, Dantas Damascena A, Galvão Azevedo LM, de Almeida Oliveira T, da Mota Santana J. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2022;62:1308–16. [DOI] [PubMed] [Google Scholar]
- 145. Petrelli F, Luciani A, Perego G, Dognini G, Colombelli PL, Ghidini A. Therapeutic and prognostic role of vitamin D for COVID-19 infection: a systematic review and meta-analysis of 43 observational studies. J Steroid Biochem Mol Biol. 2021;211:105883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Ma H, Zhou T, Heianza Y, Qi L. Habitual use of vitamin D supplements and risk of coronavirus disease 2019 (COVID-19) infection: a prospective study in UK Biobank. Am J Clin Nutr. 2021;113:1275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clin Exp Res. 2020;32:2141–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Cangiano B, Fatti LM, Danesi L, Gazzano G, Croci M, Vitale Get al. Mortality in an Italian nursing home during COVID-19 pandemic: correlation with gender, age, ADL, vitamin D supplementation, and limitations of the diagnostic tests. Aging. 2020;12:24522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Shah K, Saxena D, Mavalankar D. Vitamin D supplementation, COVID-19 and disease severity: a meta-analysis. QJM. 2021;114:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ghasemian R, Shamshirian A, Heydari K, Malekan M, Alizadeh-Navaei R, Ebrahimzadeh MAet al. The role of vitamin D in the age of COVID-19: a systematic review and meta-analysis. Int J Clin Pract. 2021;75:e14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CSCet al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325:1053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. McNally JD, Sampson M, Matheson LA, Hutton B, Little J. Vitamin D receptor (VDR) polymorphisms and severe RSV bronchiolitis: a systematic review and meta-analysis. Pediatr Pulmonol. 2014;49:790–9. [DOI] [PubMed] [Google Scholar]
- 153. de Albuquerque Borborema ME, de Souza Pereira JJ, Dos Santos Peixoto A, Crovella S, Schindler HC, da Silva Rabello MCet al. Differential distribution in vitamin D receptor gene variants and expression profile in Northeast Brazil influences upon active pulmonary tuberculosis. Mol Biol Rep. 2020;47:7317–22. [DOI] [PubMed] [Google Scholar]
- 154. Randolph AG, Yip W-K, Falkenstein-Hagander K, Weiss ST, Janssen R, Keisling Set al. Vitamin D-binding protein haplotype is associated with hospitalization for RSV bronchiolitis. Clin Exp Allergy. 2014;44:231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Jales Neto LH, Hounkpe BW, Fernandes GH, Takayama L, Caparbo VF, Lopes NHMet al. Transcriptomic analysis of elderly women with low muscle mass: association with immune system pathway. Aging. 2021;13:20992–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Miyazawa T, Burdeos GC, Itaya M, Nakagawa K, Miyazawa T. Vitamin E: regulatory redox interactions. IUBMB Life. 2019;71:430–41. [DOI] [PubMed] [Google Scholar]
- 157. Galli F, Azzi A, Birringer M, Cook-Mills JM, Eggersdorfer M, Frank Jet al. Vitamin E: emerging aspects and new directions. Free Radical Biol Med. 2017;102:16–36. [DOI] [PubMed] [Google Scholar]
- 158. Meydani M. Vitamin E. Lancet North Am Ed. 1995;345:170–5. [DOI] [PubMed] [Google Scholar]
- 159. Lewis ED, Meydani SN, Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. 2019;71:487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Pein H, Ville A, Pace S, Temml V, Garscha U, Raasch Met al. Endogenous metabolites of vitamin E limit inflammation by targeting 5-lipoxygenase. Nat Commun. 2018;9:3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Wang X, Quinn PJ. Vitamin E and its function in membranes. Prog Lipid Res. 1999;38:309–36. [DOI] [PubMed] [Google Scholar]
- 162. Marko MG, Ahmed T, Bunnell SC, Wu D, Chung H, Huber BTet al. Age-associated decline in effective immune synapse formation of CD4+ T cells is reversed by vitamin E supplementation. J Immunol. 2007;178:1443–9. [DOI] [PubMed] [Google Scholar]
- 163. Meydani SN, Meydani M, Verdon CP, Shapiro AA, Blumberg JB, Hayes KC. Vitamin E supplementation suppresses prostaglandin E1(2) synthesis and enhances the immune response of aged mice. Mech Ageing Dev. 1986;34:191–201. [DOI] [PubMed] [Google Scholar]
- 164. Wu D, Meydani SN. Age-associated changes in immune function: impact of vitamin E intervention and the underlying mechanisms. Endocr Metab Immune Disord Drug Targets. 2014;14:283–9. [DOI] [PubMed] [Google Scholar]
- 165. Lee G, Han S. The role of vitamin E in immunity. Nutrients. 2018;10:1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Moriguchi S, Miwa H, Okamura M, Maekawa K, Kishino Y, Maeda K. Vitamin E is an important factor in T cell differentiation in thymus of F344 rats. J Nutr Sci Vitaminol (Tokyo). 1993;39:451–63. [DOI] [PubMed] [Google Scholar]
- 167. Chavance M, Herbeth B, Fournier C, Janot C, Vernhes G. Vitamin status, immunity and infections in an elderly population. Eur J Clin Nutr. 1989;43:827–35. [PubMed] [Google Scholar]
- 168. Meydani SN, Han SN, Wu D. Vitamin E and immune response in the aged: molecular mechanisms and clinical implications. Immunol Rev. 2005;205:269–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. de la Fuente M, Hernanz A, Guayerbas N, Victor VM, Arnalich F. Vitamin E ingestion improves several immune functions in elderly men and women. Free Radical Res. 2008;42:272–80. [DOI] [PubMed] [Google Scholar]
- 170. Meydani SN, Barklund MP, Liu S, Meydani M, Miller RA, Cannon JGet al. Vitamin E supplementation enhances cell-mediated immunity in healthy elderly subjects. Am J Clin Nutr. 1990;52:557–63. [DOI] [PubMed] [Google Scholar]
- 171. Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski Ret al. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA. 1997;277:1380–6. [DOI] [PubMed] [Google Scholar]
- 172. Han SN, Wu D, Ha WK, Beharka A, Smith DE, Bender BSet al. Vitamin E supplementation increases T helper 1 cytokine production in old mice infected with influenza virus. Immunology. 2000;100:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Hayek MG, Taylor SF, Bender BS, Han SN, Meydani Met al. Vitamin E supplementation decreases lung virus titers in mice infected with influenza. J Infect Dis. 1997;176:273–6. [DOI] [PubMed] [Google Scholar]
- 174. Bou Ghanem EN, Clark S, Du X, Wu D, Camilli A, Leong JMet al. The α-tocopherol form of vitamin E reverses age-associated susceptibility to Streptococcus pneumoniae lung infection by modulating pulmonary neutrophil recruitment. J Immunol. 2015;194:1090–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Bou Ghanem EN, Lee JN, Joma BH, Meydani SN, Leong JMet al. The alpha-tocopherol form of vitamin E boosts elastase activity of human PMNs and their ability to kill. Front Cell Infection Microbiol. 2017;7:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Hemilä H. Vitamin E administration may decrease the incidence of pneumonia in elderly males. Clin Intervent Aging. 2016;11:1379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Meydani SN, Leka LS, Fine BC, Dallal GE, Keusch GT, Singh MFet al. Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA. 2004;292:828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Graat JM, Schouten EG, Kok FJ. Effect of daily vitamin E and multivitamin-mineral supplementation on acute respiratory tract infections in elderly persons: a randomized controlled trial. JAMA. 2002;288:715–21. [DOI] [PubMed] [Google Scholar]
- 179. Belisle SE, Hamer DH, Leka LS, Dallal GE, Delgado-Lista J, Fine BCet al. IL-2 and IL-10 gene polymorphisms are associated with respiratory tract infection and may modulate the effect of vitamin E on lower respiratory tract infections in elderly nursing home residents. Am J Clin Nutr. 2010;92:106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. England A, Valdes AM, Slater-Jefferies JL, Gill R, Howell WM, Calder PCet al. Variants in the genes encoding TNF-α, IL-10, and GSTP1 influence the effect of α-tocopherol on inflammatory cell responses in healthy men. Am J Clin Nutr. 2012;95:1461–7. [DOI] [PubMed] [Google Scholar]
- 181. Dewé W, Durand C, Marion S, Oostvogels L, Devaster J-M, Fourneau M. A multi-criteria decision making approach to identify a vaccine formulation. J Biopharm Stat. 2016;26:352–64. [DOI] [PubMed] [Google Scholar]
- 182. Madan A, Collins H, Sheldon E, Frenette L, Chu L, Friel D, Drame Met al. Evaluation of a primary course of H9N2 vaccine with or without AS03 adjuvant in adults: a phase I/II randomized trial. Vaccine. 2017;35:4621–8. [DOI] [PubMed] [Google Scholar]
- 183. van der Most RG, Clément F, Willekens J, Dewé W, Walravens K, Vaughn DWet al. Long-term persistence of cell-mediated and humoral responses to A(H1N1)pdm09 influenza virus vaccines and the role of the AS03 adjuvant system in adults during two randomized controlled trials. Clin Vaccin Immunol. 2017;24:e00553–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Lartey S, Pathirana RD, Zhou F, Jul-Larsen Å, Montomoli E, Wood Jet al. Single dose vaccination of the ASO3-adjuvanted A(H1N1)pdm09 monovalent vaccine in health care workers elicits homologous and cross-reactive cellular and humoral responses to H1N1 strains. Hum Vaccin Immunother. 2015;11:1654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Tengerdy RP. Vitamin E, immune response, and disease resistance. Ann NY Acad Sci. 1989;570:335–44. [DOI] [PubMed] [Google Scholar]
- 186. Sadeghsoltani F, Mohammadzadeh I, Safari M-M, Hassanpour P, Izadpanah M, Qujeq Det al. Zinc and respiratory viral infections: important trace element in anti-viral response and immune regulation. Biol Trace Elem Res. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Subramanian Vignesh K, Deepe GS Jr.. Metallothioneins: emerging modulators in immunity and infection. Int J Mol Sci. 2017;18:2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Wessels I, Maywald M, Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017;9:1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Maywald M, Wessels I, Rink L. Zinc signals and immunity. Int J Mol Sci. 2017;18:2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Hojyo S, Fukada T. Roles of zinc signaling in the immune system. J Immunol Res. 2016;2016:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Maares M, Haase H. Zinc and immunity: an essential interrelation. Arch Biochem Biophys. 2016;611:58–65. [DOI] [PubMed] [Google Scholar]
- 192. Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G. The role of zinc in antiviral immunity. Adv Nutr. 2019;10:696–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Prasad AS, Beck FWJ, Bao B, Fitzgerald JT, Snell DC, Steinberg JDet al. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85:837–44. [DOI] [PubMed] [Google Scholar]
- 194. Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. 2017;9:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Walker CF, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr. 2004;24:255–75. [DOI] [PubMed] [Google Scholar]
- 196. Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277–98. [DOI] [PubMed] [Google Scholar]
- 197. Hasan R, Rink L, Haase H. Zinc signals in neutrophil granulocytes are required for the formation of neutrophil extracellular traps. Innate Immunity. 2013;19:253–64. [DOI] [PubMed] [Google Scholar]
- 198. Beck FW, Prasad AS, Kaplan J, Fitzgerald JT, Brewer GJ. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am J Physiol. 1997;272:E1002–7. [DOI] [PubMed] [Google Scholar]
- 199. Tapazoglou E, Prasad AS, Hill G, Brewer GJ, Kaplan J. Decreased natural killer cell activity in patients with zinc deficiency with sickle cell disease. J Lab Clin Med. 1985;105:19–22. [PubMed] [Google Scholar]
- 200. Sandström B, Cederblad A, Lindblad BS, Lönnerdal B. Acrodermatitis enteropathica, zinc metabolism, copper status, and immune function. Arch Pediatr Adolesc Med. 1994;148:980–5. [DOI] [PubMed] [Google Scholar]
- 201. Yasuda H, Tsutsui T. Infants and elderlies are susceptible to zinc deficiency. Sci Rep. 2016;6:21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Meydani SN, Barnett JB, Dallal GE, Fine BC, Jacques PF, Leka LSet al. Serum zinc and pneumonia in nursing home elderly. Am J Clin Nut. 2007;86:1167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Haase H, Rink L. The immune system and the impact of zinc during aging. Immun Ageing. 2009;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Mocchegiani E, Malavolta M, Costarelli L, Giacconi R, Piacenza F, Lattanzio Fet al. Is there a possible single mediator in modulating neuroendocrine-thymus interaction in ageing?. Curr Aging Sci. 2013;6:99–107. [DOI] [PubMed] [Google Scholar]
- 205. Fortes C, Forastiere F, Agabiti N, Fano V, Pacifici R, Virgili Fet al. The effect of zinc and vitamin A supplementation on immune response in an older population. J Am Geriatr Soc. 1998;46:19–26. [DOI] [PubMed] [Google Scholar]
- 206. Duchateau J, Delepesse G, Vrijens R, Collet H. Beneficial effects of oral zinc supplementation on the immune response of old people. Am J Med. 1981;70:1001–4. [DOI] [PubMed] [Google Scholar]
- 207. Barnett JB, Dao MC, Hamer DH, Kandel R, Brandeis G, Wu Det al. Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2016;103:942–51. [DOI] [PubMed] [Google Scholar]
- 208. Novick SG, Godfrey JC, Godfrey NJ, Wilder HR. How does zinc modify the common cold? Clinical observations and implications regarding mechanisms of action. Med Hypotheses. 1996;46:295–302. [DOI] [PubMed] [Google Scholar]
- 209. Singh M, Das RR. Zinc for the common cold. Cochrane Database Syst Rev. 2013;(6):CD001364. [DOI] [PubMed] [Google Scholar]
- 210. Eby GA. Elimination of efficacy by additives in zinc acetate lozenges for common colds. Clin Infect Dis. 2001;32:1520. [DOI] [PubMed] [Google Scholar]
- 211. Barnett JB, Hamer DH, Meydani SN. Low zinc status: a new risk factor for pneumonia in the elderly?. Nutr Rev. 2010;68:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Eijkelkamp BA, Morey JR, Neville SL, Tan A, Pederick VG, Cole Net al. Dietary zinc and the control of Streptococcus pneumoniae infection. PLoS Pathog. 2019;15:e1007957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Sharafi S, Allami A. Efficacy of zinc sulphate on in-hospital outcome of community-acquired pneumonia in people aged 50 years and over. Int J Tuberc Lung Dis. 2016;20:685–8. [DOI] [PubMed] [Google Scholar]
- 214. Wang L, Song Y. Efficacy of zinc given as an adjunct to the treatment of severe pneumonia: a meta-analysis of randomized, double-blind and placebo-controlled trials. Clin Respir J. 2018;12:857–64. [DOI] [PubMed] [Google Scholar]
- 215. Cornick JE, Bentley SD. Streptococcus pneumoniae: the evolution of antimicrobial resistance to beta-lactams, fluoroquinolones and macrolides. Microbes Infect. 2012;14:573–83. [DOI] [PubMed] [Google Scholar]
- 216. Ghaffari H, Tavakoli A, Moradi A, Tabarraei A, Bokharaei-Salim F, Zahmatkeshan Met al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J Biomed Sci. 2019;26:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Oxford JS, Perrin DD. Inhibition of the particle-associated RNA-dependent RNA polymerase activity of influenza viruses by chelating agents. J Gen Virol. 1974;23:59–71. [DOI] [PubMed] [Google Scholar]
- 218.te Velthuis AJW, van den Worm SHE, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6:e1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Heller RA, Sun Q, Hackler J, Seelig J, Seibert L, Cherkezov Aet al. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021;38:101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Carlucci PM, Ahuja T, Petrilli C, Rajagopalan H, Jones S, Rahimian J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J Med Microbiol. 2020;69:1228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Frontera JA, Rahimian JO, Yaghi S, Liu M, Lewis A, de Havenon Aet al. Treatment with zinc is associated with reduced in-hospital mortality among COVID-19 patients: a multi-center cohort study. Research Square. 26 Oct 2020. doi: 10.21203/rs.3.rs-94509/v1. [DOI] [Google Scholar]
- 222. Thomas S, Patel D, Bittel B, Wolski K, Wang Q, Kumar Aet al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Network Open. 2021;4:e210369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Mocchegiani E, Muzzioli M, Giacconi R, Cipriano C, Gasparini N, Franceschi Cet al. Metallothioneins/PARP-1/IL-6 interplay on natural killer cell activity in elderly: parallelism with nonagenarians and old infected humans. Effect of zinc supply. Mech Ageing Dev. 2003;124:459–68. [DOI] [PubMed] [Google Scholar]
- 224. Mocchegiani E, Giacconi R, Cipriano C, Malavolta M. NK and NKT cells in aging and longevity: role of zinc and metallothioneins. J Clin Immunol. 2009;29:416–25. [DOI] [PubMed] [Google Scholar]
- 225. Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ. 2010;7:1342–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Lu J, Holmgren A. Selenoproteins. J Biol Chem. 2009;284:723–7. [DOI] [PubMed] [Google Scholar]
- 227. Rayman MP. Selenium and human health. Lancet North Am Ed. 2012;379:1256–68. [DOI] [PubMed] [Google Scholar]
- 228. Schoenmakers E, Agostini M, Mitchell C, Schoenmakers N, Papp L, Rajanayagam Oet al. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J Clin Invest. 2010;120:4220–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Combs GF Jr. Selenium in global food systems. Br J Nutr. 2001;85:517–47. [DOI] [PubMed] [Google Scholar]
- 230. Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signaling. 2012;16:705–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Avery JC, Hoffmann PR. Selenium, selenoproteins, and immunity. Nutrients. 2018;10:1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Roy M, Kiremidjian-Schumacher L, Wishe HI, Cohen MW, Stotzky G. Supplementation with selenium restores age-related decline in immune cell function. Exp Biol Med. 1995;209:369–75. [DOI] [PubMed] [Google Scholar]
- 233. Beck MA, Levander OA. Host nutritional status and its effect on a viral pathogen. J Infect Dis. 2000;182:S93–6. [DOI] [PubMed] [Google Scholar]
- 234. Beck M, Handy J, Levander O. Host nutritional status: the neglected virulence factor. Trends Microbiol. 2004;12:417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Beck MA, Nelson HK, Shi Q, Van Dael P, Schiffrin EJ, Blum Set al. Selenium deficiency increases the pathology of an influenza virus infection. FASEB J. 2001;15:1481–3. [PubMed] [Google Scholar]
- 236. Nelson HK, Shi Q, Van Dael P, Schiffrin EJ, Blum S, Barclay Det al. Host nutritional selenium status as a driving force for influenza virus mutations. FASEB J. 2001;15:1727–38. [PubMed] [Google Scholar]
- 237. Guillin OM, Vindry C, Ohlmann T, Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;11:2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Zhang J, Saad R, Taylor EW, Rayman MP. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020;37:101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Hiffler L, Rakotoambinina B. Selenium and RNA virus interactions: potential implications for SARS-CoV-2 infection (COVID-19). Front Nutr. 2020;7:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Ravaglia G, Forti P, Maioli F, Bastagli L, Facchini A, Mariani Eet al. Effect of micronutrient status on natural killer cell immune function in healthy free-living subjects aged >/=90 y. Am J Clin Nutr. 2000;71:590–8. [DOI] [PubMed] [Google Scholar]
- 241. Roy M, Kiremidjian-Schumacher L, Wishe HI, Cohen MW, Stotzky G. Supplementation with selenium and human immune cell functions. I. Effect on lymphocyte proliferation and interleukin 2 receptor expression. Biol Trace Elem Res. 1994;41:103–14. [DOI] [PubMed] [Google Scholar]
- 242. Hawkes WC, Kelley DS, Taylor PC. The effects of dietary selenium on the immune system in healthy men. Biol Trace Elem Res. 2001;81:189–213. [DOI] [PubMed] [Google Scholar]
- 243. Kiremidjian-Schumacher L, Roy M, Wishe HI, Cohen MW, Stotzky G. Supplementation with selenium and human immune cell functions. II. Effect on cytotoxic lymphocytes and natural killer cells. Biol Trace Elem Res. 1994;41:115–27. [DOI] [PubMed] [Google Scholar]
- 244. Peretz A, Nève J, Desmedt J, Duchateau J, Dramaix M, Famaey JP. Lymphocyte response is enhanced by supplementation of elderly subjects with selenium-enriched yeast. Am J Clin Nutr. 1991;53:1323–8. [DOI] [PubMed] [Google Scholar]
- 245. Wood SM, Beckham1 C, Yosioka A, Darban H, Watson RR. beta-Carotene and selenium supplementation enhances immune response in aged humans. Integr Med. 2000;2:85–92. [DOI] [PubMed] [Google Scholar]
- 246. Broome CS, McArdle F, Kyle JAM, Andrews F, Lowe NM, Hart CAet al. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr. 2004;80:154–62. [DOI] [PubMed] [Google Scholar]
- 247. Ivory K, Prieto E, Spinks C, Armah CN, Goldson AJ, Dainty JRet al. Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults. Clin Nutr. 2017;36:407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Choi R, Kim H-T, Lim Y, Kim M-J, Kwon OJ, Jeon Ket al. Serum concentrations of trace elements in patients with tuberculosis and its association with treatment outcome. Nutrients. 2015;7:5969–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Ramakrishnan K, Shenbagarathai R, Kavitha K, Thirumalaikolundusubramanian P, Rathinasabapati R. Selenium levels in persons with HIV/tuberculosis in India, Madurai City. Clin Lab. 2012;58:165–8. [PubMed] [Google Scholar]
- 250. Qi C, Wang H, Liu Z, Yang H. Oxidative stress and trace elements in pulmonary tuberculosis patients during 6 months anti-tuberculosis treatment. Biol Trace Elem Res. 2021;199:1259–67. [DOI] [PubMed] [Google Scholar]
- 251. Seyedrezazadeh E, Ostadrahimi A, Mahboob S, Assadi Y, Ghaemmagami J, Pourmogaddam M. Effect of vitamin E and selenium supplementation on oxidative stress status in pulmonary tuberculosis patients. Respirology. 2008;13:294–8. [DOI] [PubMed] [Google Scholar]
- 252. Jaquess PA, Smalley DL, Duckworth JK. Enhanced growth of Mycobacterium tuberculosis in the presence of selenium. Am J Clin Pathol. 1981;75:209–10. [DOI] [PubMed] [Google Scholar]
- 253. Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong Ket al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot Bet al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14. [DOI] [PubMed] [Google Scholar]
- 255. Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25:716–29. [DOI] [PubMed] [Google Scholar]
- 256. Vitetta L, Vitetta G, Hall S. Immunological tolerance and function: associations between intestinal bacteria, probiotics, prebiotics, and phages. Front Immunol. 2018;9:2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Wang S, Ahmadi S, Nagpal R, Jain S, Mishra SP, Kavanagh Ket al. Lipoteichoic acid from the cell wall of a heat killed Lactobacillus paracasei D3-5 ameliorates aging-related leaky gut, inflammation and improves physical and cognitive functions: from C. elegans to mice. GeroScience. 2020;42:333–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Nataraj BH, Ali SA, Behare PV, Yadav H. Postbiotics-parabiotics: the new horizons in microbial biotherapy and functional foods. Microb Cell Fact. 2020;19:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMMet al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021;18:649–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJet al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. [DOI] [PubMed] [Google Scholar]
- 261. Ejima R, Akiyama M, Sato H, Tomioka S, Yakabe K, Kimizuka Tet al. Seaweed dietary fiber sodium alginate suppresses the migration of colonic inflammatory monocytes and diet-induced metabolic syndrome via the gut microbiota. Nutrients. 2021;13:2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Jayachandran M, Chen J, Chung SSM, Xu B. A critical review on the impacts of β-glucans on gut microbiota and human health. J Nutr Biochem. 2018;61:101–10. [DOI] [PubMed] [Google Scholar]
- 263. Han B, Baruah K, Cox E, Vanrompay D, Bossier P. Structure-functional activity relationship of B-glucans from the perspective of immunomodulation: a mini-review. Front Immunol. 2020;11:658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. de Marco Castro E, Calder PC, Roche HM. β-1,3/1,6-Glucans and immunity: state of the art and future directions. Mol Nutr Food Res. 2021;65:1901071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Lu Q-Y, Summanen PH, Lee R-P, Huang J, Henning SM, Heber Det al. Prebiotic potential and chemical composition of seven culinary spice extracts. J Food Sci. 2017;82:1807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke Ket al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17:687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Lomax AR, Calder PC. Probiotics, immune function, infection and inflammation: a review of the evidence from studies conducted in humans. Curr. Pharm Des. 2009;15:1428–518. [DOI] [PubMed] [Google Scholar]
- 268. Childs CE, Calder PC. Modifying the gut microbiome through diet: effects on the immune system of elderly subjects. In: Fulop T, Franceschi C, Hirokawa K, Pawelec Geditors. Handbook of immunosensecence. Springer Publishing; 2018. pp. 31, https://link.springer.com/referencework/10.1007/978-3-319-64597-1#bibliographic-information. [Google Scholar]
- 269. Dong H, Rowland I, Thomas LV, Yaqoob P. Immunomodulatory effects of a probiotic drink containing Lactobacillus casei Shirota in healthy older volunteers. Eur J Nutr. 2013;52:1853–63. [DOI] [PubMed] [Google Scholar]
- 270. Takeda K, Okumura K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the human NK-cell activity. J Nutr. 2007;137:791S–3S. [DOI] [PubMed] [Google Scholar]
- 271. Gill HS, Rutherfurd KJ, Cross ML. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: an investigation of age-related immunological changes. J Clin Immunol. 2001;21:264–71. [DOI] [PubMed] [Google Scholar]
- 272. Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr. 2001;74:833–9. [DOI] [PubMed] [Google Scholar]
- 273. Arunachalam K, Gill HS, Chandra RK. Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis (HN019). Eur J Clin Nutr. 2000;54:263–7. [DOI] [PubMed] [Google Scholar]
- 274. Parra D, de Morentin BM, Cobo JM, Mateos A, Martinez JA. Monocyte function in healthy middle-aged people receiving fermented milk containing Lactobacillus casei. J Nutr Health Aging. 2004;8:208–11. [PubMed] [Google Scholar]
- 275. Spaiser SJ, Culpepper T, Nieves C Jr, Ukhanova M, Mai V, Percival SSet al. Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9-1, and Bifidobacterium longum MM-2 ingestion induces a less inflammatory cytokine profile and a potentially beneficial shift in gut microbiota in older adults: a randomized, double-blind, placebo-controlled, crossover study. J Am Coll Nutr. 2015;34:459–69. [DOI] [PubMed] [Google Scholar]
- 276. Nyangale EP, Farmer S, Cash HA, Keller D, Chernoff D, Gibson GR. Bacillus coagulans GBI-30, 6086 modulates Faecalibacterium prausnitzii in older men and women. J Nutr. 2015;145:1446–52. [DOI] [PubMed] [Google Scholar]
- 277. Miyazawa K, Kawase M, Kubota A, Yoda K, Harata G, Hosoda M, He F. Heat-killed Lactobacillus gasseri can enhance immunity in the elderly in a double-blind, placebo-controlled clinical study. Beneficial Microbes. 2015;6:441–9. [DOI] [PubMed] [Google Scholar]
- 278. Lomax AR, Calder PC. Prebiotics, immune function, infection and inflammation: a review of the evidence. Br J Nutr. 2009;101:633–58. [DOI] [PubMed] [Google Scholar]
- 279. Vulevic J, Drakoularakou A, Yaqoob P, Tzortzis G, Gibson GR. Modulation of the fecalmicroflora profile and immune function by a novel trans-mixture (B-GOS) in healthy elderly volunteers. Am J Clin Nutr. 2008;88:1438–46. [DOI] [PubMed] [Google Scholar]
- 280. Vulevic J, Juric A, Walton GE, Claus SP, Tzortzis G, Toward REet al. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br J Nutr. 2015;114:586–95. [DOI] [PubMed] [Google Scholar]
- 281. van Puyenbroeck K, Hens N, Coenen S, Michiels B, Beunckens C, Molenberghs Get al. Efficacy of daily intake of Lactobacillus casei Shirota on respiratory symptoms and influenza vaccination immune response: a randomized, double-blind, placebo controlled trial in healthy elderly nursing home residents. Am J Clin Nutr. 2012;95:1165–71. [DOI] [PubMed] [Google Scholar]
- 282. Bosch M, Mendez M, Perez M, Farran A, Fuentes MC, Cune J. Lactobacillus plantarum CECT7315 and CECT7316 stimulate immunoglobulin production after influenza vaccination in elderly. Nutr Hosp. 2012;27:504–9. [DOI] [PubMed] [Google Scholar]
- 283. Akatsu H, Arakawa K, Yamamoto T, Kanematsu T, Matsukawa N, Ohara Het al. Lactobacillus in jelly enhances the effect of influenza vaccination in elderly individuals. J Am Geriatr Soc. 2013;61:1828–30. [DOI] [PubMed] [Google Scholar]
- 284. Maruyama M, Abe R, Shimono T, Iwabuchi N, Abe F, Xiao JZ. The effects of non-viable Lactobacillus on immune function in the elderly: a randomised, double-blind, placebo-controlled study. Int J Food Sci Nutr. 2016;67:67–73. [DOI] [PubMed] [Google Scholar]
- 285. Enani S, Przemska-Kosicka A, Childs CE, Maidens C, Dong H, Conterno Let al. Impact of ageing and a synbiotic on the immune response to seasonal influenza vaccination; a randomised controlled trial. Clin Nutr. 2018;37:443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Boge T, Remigy M, Vaudaine S, Tanguy J, Bourdet-Sicard R, van der Werf S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine. 2009;27:5677–84. [DOI] [PubMed] [Google Scholar]
- 287. Castro-Herrera VM, Fisk HL, Wootton M, Lown M, Owen-Jones E, Lau Met al. Combination of the probiotics Lacticaseibacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis, BB-12 has limited effect on biomarkers of immunity and inflammation in older people resident in care homes: results from the Probiotics to Reduce Infections iN CarE home reSidentS randomized, controlled trial. Front Immunol. 2021;12:643321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Akatsu H, Nagafuchi S, Kurihara R, Okuda K, Kanesaka T, Ogawa Net al. Enhanced vaccination effect against influenza by prebiotics in elderly patients receiving enteral nutrition. Geriatr Gerontol Int. 2016;16:205–13. [DOI] [PubMed] [Google Scholar]
- 289. Bunout D, Hirsch S, Pia De La Maza M, Munoz C, Haschke F, Steenhout Pet al. Effects of prebiotics on the immune response to vaccination in the elderly. J Parenter Enter Nutr. 2002;26:372–6. [DOI] [PubMed] [Google Scholar]
- 290. Przemska-Kosicka A, Childs CE, Enani S, Maidens C, Dong H, Dayel IBet al. Effect of a synbiotic on the response to seasonal influenza vaccination is strongly influenced by degree of immunosenescence. Immun Ageing. 2016;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Yeh TL, Shih PC, Liu SJ, Lin CH, Liu JM, Lei WTet al. The influence of prebiotic or probiotic supplementation on antibody titers after influenza vaccination: a systematic review and meta-analysis of randomized controlled trials. Drug Des Dev Ther. 2018;12:217–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Lei WT, Shih PC, Liu SJ, Lin CY, Yeh TL. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2017;9:1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Yang B, Lu P, Li MX, Cai XL, Xiong WY, Hou HJet al. A meta-analysis of the effects of probiotics and synbiotics in children with acute diarrhea. Medicine (Baltimore). 2019;98:e16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Li YT, Xu H, Ye JZ, Wu WR, Shi D, Fang DQet al. Efficacy of Lactobacillus rhamnosus GG in treatment of acute pediatric diarrhea: a systematic review with meta-analysis. World J Gastroenterol. 2019;25:4999–5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Urbańska M, Gieruszczak-Białek D, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment Pharmacol Ther. 2016;43:1025–34. [DOI] [PubMed] [Google Scholar]
- 296. Patro-Gołąb B, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for treating acute gastroenteritis in children. An update. Nutrients. 2019;11:2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Ianiro G, Rizzatti G, Plomer M, Lopetuso L, Scaldaferri F, Franceschi Fet al. Bacillus clausii for the treatment of acute diarrhea in children: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2018;10:1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Malagón-Rojas JN, Mantziari A, Salminen S, Szajewska H. Postbiotics for preventing and treating common infectious diseases in children: a systematic review. Nutrients. 2020;12:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Hickson M, D'Souza AL, Muthu N, Rogers TR, Want S, Rajkumar Cet al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. McFarland LV. Meta-Analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101:812–22. [DOI] [PubMed] [Google Scholar]
- 301. Hempel S, Newberry SJ, Maher AR, Wang Z, Miles JN, Shanman Ret al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea. JAMA. 2012;307:1959–69. [DOI] [PubMed] [Google Scholar]
- 302. Jafarnejad S, Shab-Bidar S, Speakman JR, Parastui K, Daneshi-Maskooni M, Djafarian K. Probiotics reduce the risk of antibiotic-associated diarrhea in adults (18–64 years) but not the elderly (>65 Years). Nutr Clin Pract. 2016;31:502–13. [DOI] [PubMed] [Google Scholar]
- 303. Calder P, Hall V. Understanding gut-immune interactions in management of acute infectious diarrhoea. Nurs Older People. 2012;24:29–39. [DOI] [PubMed] [Google Scholar]
- 304. Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst. Rev. 2010;(11):CD003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016;9:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Ma Y, Yang JY, Peng X, Xiao KY, Xu Q, Wang C. Which probiotic has the best effect on preventing Clostridium difficile-associated diarrhea? A systematic review and network meta-analysis. J Digest Dis. 2020;21:69–80. [DOI] [PubMed] [Google Scholar]
- 307. Cai J, Zhao C, Du Y, Zhang Y, Zhao M, Zhao Q. Comparative efficacy and tolerability of probiotics for antibiotic-associated diarrhea: systematic review with network meta-analysis. United Eur Gastroenterol J. 2018;6:169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Clarke TB. Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via NOD-like receptor ligands. Infect Immun. 2014;82:4596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Zhang N, He Q-S. Commensal microbiome promotes resistance to local and systemic infections. Chin Med J (Engl). 2015;128:2250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Vouloumanou EK, Makris GC, Karageorgopoulos DE, Falagas ME. Probiotics for the prevention of respiratory tract infections: a systematic review. Int J Antimicrob Agents. 2009;34:197.e1–e10. [DOI] [PubMed] [Google Scholar]
- 311. Liu KX, Zhu YG, Zhang J, Tao LL, Lee JW, Wang XDet al. Probiotics' effects on the incidence of nosocomial pneumonia in critically ill patients: a systematic review and meta-analysis. Crit Care. 2012;16:R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Liu S, Hu P, Du X, Zhou T, Pei X. Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: a meta-analysis of randomized, placebo-controlled trials. Indian Pediatr. 2013;50:377–81. [DOI] [PubMed] [Google Scholar]
- 313. King S, Glanville J, Sanders ME, Fitzgerald A, Varley D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Hao Q, Lu Z, Dong BR, Huang CQ, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2011;(9):CD006895. [DOI] [PubMed] [Google Scholar]
- 315. Ozen M, Kocabas Sandal G, Dinleyici EC. Probiotics for the prevention of pediatric upper respiratory tract infections: a systematic review. Expert Opin Biol Ther. 2015;15:9–20. [DOI] [PubMed] [Google Scholar]
- 316. Araujo GV, Oliveira Junior MH, Peixoto DM, Sarinho ES. Probiotics for the treatment of upper and lower respiratory-tract infections in children: systematic review based on randomized clinical trials. J Pediatr (Rio J). 2015;91:413–27. [DOI] [PubMed] [Google Scholar]
- 317. Wang Y, Li X, Ge T, Xiao Y, Liao Y, Cui Yet al. Probiotics for prevention and treatment of respiratory tract infections in children: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016;95:e4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Laursen RP, Hojsak I. Probiotics for respiratory tract infections in children attending day care centers—a systematic review. Eur J Pediatr. 2018;177:979–94. [DOI] [PubMed] [Google Scholar]
- 319. Rashidi K, Razi B, Darand M, Dehghani A, Janmohammadi P, Alizadeh S. Effect of probiotic fermented dairy products on incidence of respiratory tract infections: a systematic review and meta-analysis of randomized clinical trials. Nutr J. 2021;20:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Chan CKY, Tao J, Chan OS, Li HB, Pang H. Preventing respiratory tract infections by synbiotic interventions: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2020;11:979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Jukic Peladic N, Dell'Aquila G, Carrieri B, Maggio M, Cherubini A, Orlandoni P. Potential role of probiotics for inflammaging: a narrative review. Nutrients. 2021;13:2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Park C, Brietzke E, Rosenblat JD, Musial N, Zuckerman H, Ragguett R-Met al. Probiotics for the treatment of depressive symptoms: an anti-inflammatory mechanism?. Brain Behav Immun. 2018;73:115–24. [DOI] [PubMed] [Google Scholar]
- 323. Eslami M, Bahar A, Keikha M, Karbalaei M, Kobyliak NM, Yousefi B. Probiotics function and modulation of the immune system in allergic diseases. Allergol Immunopathol (Madr). 2020;48:771–88. [DOI] [PubMed] [Google Scholar]
- 324. Roshan H, Ghaedi E, Rahmani J, Barati M, Najafi M, Karimzedeh Met al. Effects of probiotics and synbiotic supplementation on antioxidant status: a meta-analysis of randomized clinical trials. Clin Nutr ESPEN. 2019;30:81–8. [DOI] [PubMed] [Google Scholar]
- 325. Crimmins EM. Lifespan and healthspan: past, present, and promise. Gerontologist. 2015;55:901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Vaupel JW. Biodemography of human ageing. Nature. 2010;464:536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet North Am Ed. 2017;389:1323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Dash SP, Dipankar P, Burange PS, Rouse BT, Sarangi PP. Climate change: how it impacts the emergence, transmission, resistance and consequences of viral infections in animals and plants. Crit Rev Microbiol. 2021;47:307–22. [DOI] [PubMed] [Google Scholar]
- 329. Drexler M, Institute of Medicine (US) . Global challenges. what you need to know about infectious disease. Washington (DC): National Academies Press; 2010. [PubMed] [Google Scholar]
- 330. Gibb R, Franklinos LHV, Redding DW, Jones KE. Ecosystem perspectives are needed to manage zoonotic risks in a changing climate. BMJ. 2020;371:m3389. [DOI] [PMC free article] [PubMed] [Google Scholar]