ABSTRACT
The aim of the current review was to explore the association between various dietary antioxidants and the risk of developing Parkinson's disease (PD). PubMed, Scopus, Web of Science, and Google Scholar were searched up to March 2021. Prospective, observational cohort studies, nested case-control, and case-control designs that investigated the association between antioxidants and PD risk were included. A random-effects model was used to pool the RRs. The certainty of the evidence was rated using the GRADE (Grading of Recommendations Assessment, Development, and Evaluations) scoring system. In addition, a dose–response relation was examined between antioxidant intake and PD risk. Six prospective cohort studies and 2 nested case-control (total n = 448,737 with 4654 cases), as well as 6 case-control (1948 controls, 1273 cases) studies were eligible. The pooled RR was significantly lower for the highest compared with the lowest intake categories of vitamin E (n = 7; 0.84; 95% CI: 0.71, 0.99) and anthocyanins (n = 2; 0.76; 95% CI: 0.61, 0.96) in cohort studies. Conversely, a significantly higher risk of PD was observed for higher lutein intake (n = 3; 1.86; 95% CI: 1.20, 2.88) among case-control studies. Dose–response meta-analyses indicated a significant association between a 50-mg/d increase in vitamin C (n = 6; RR: 0.94; 95% CI: 0.88, 0.99), a 5-mg/d increment in vitamin E (n = 7; RR: 0.84; 95% CI: 0.70, 0.99), a 2-mg/d increment in β-carotene (n = 6; RR: 0.94, 95% CI: 0.89, 0.99), and a 1-mg/d increment in zinc (n = 1; OR: 0.65; 95% CI: 0.49, 0.86) and a reduced risk of PD. Overall, higher intake of antioxidant-rich foods may be associated with a lower risk of PD. Future well-designed prospective studies are needed to validate the present findings. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (https://www.crd.york.ac.uk/PROSPERO, CRD42021242511).
Keywords: antioxidants, Parkinson's, meta-analysis, observational studies, ascorbic acid, carotenoids
Statement of Significance: Based on the literature, although previous meta-analyses have reviewed the association between specific types of dietary antioxidants and Parkinson's disease (PD) risk, several restrictions may distort these results. Notably, this is the first study to assess whether there is a dose–response relation between the amount of consumed dietary antioxidants and the risk of PD.
Introduction
Parkinson's disease (PD) is a chronic and progressive neurodegenerative disorder characterized by rigidity, bradykinesia, slowness of movement, and tremors (1). It has been estimated that 0.3% of the general population in industrialized countries, and 1% of those above the age of 60 y, are prone to the degeneration of dopaminergic neurons, the hallmark of PD (2). The exact underlying cause of this neurodegenerative disorder is still unknown. Nonetheless, it has been suggested that oxidative stress, neuroinflammation, and mitochondrial dysfunction are involved in PD pathogenesis (3).
Given that oxidative stress is involved in dopaminergic neurotoxicity (4), it has been hypothesized that the consumption of antioxidant-rich foods may represent a promising approach to protect against neuronal damage by scavenging reactive oxygen species (ROS) (5). Accordingly, attention has been placed on the neuroprotective effects of dietary antioxidants on PD outcomes. A large Swedish cohort that followed up participants for 17 y showed that higher dietary vitamin E and C consumption was associated with a lower risk of PD (6). Moreover, several epidemiological studies have reported an inverse relation between dietary carotenoids and the risk of PD (7, 8). However, results from the Nurses’ Health Study (NHS) and Health Professionals Follow-Up Study did not support this association (9). Consequently, it remains inconclusive which specific antioxidants are related to the risk of PD.
Two previous meta-analyses have reviewed the association between specific types of dietary antioxidants and PD risk (10, 11). However, several major limitations may have distorted the generated outcomes. The reviews comprised a limited number of studies and included both a cross-sectional study (12) and a study that assessed whole foods containing vitamins (13). In addition, both meta-analyses failed to assess whether there may be a dose–response relation between the amount of consumed dietary antioxidants and the risk of PD. Expanding from these previous reviews, we identified 6 new relevant population-based cohort studies (6, 8, 9, 14, 15). This systematic review and dose–response meta-analysis of observational studies summarizes the available findings relating to the potential associations between dietary intake of numerous antioxidants and the risk of developing PD. These antioxidants included vitamin C, vitamin E, vitamin A, α-carotene, β-carotene, lycopene, β-cryptoxanthin, lutein, flavonoids, selenium, zinc, and overall antioxidant capacity. Where possible, the dose–response relation was also examined.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (16) were used to conduct this systematic review and meta-analysis. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (https://www.crd.york.ac.uk/PROSPERO, CRD42021242511).
Search strategy
Electronic databases, including PubMed, Scopus, Web of Science, and Google Scholar, were systematically searched up to March 2021. No filters or restrictions were applied to publication time or language. Detailed information relating to the search strategy of databases as well as keywords relating to dietary intake of various antioxidants, PD, and study design are described in Supplemental Table 1. The reference lists of selected publications were also searched manually in an effort to avoid missing any relevant articles.
Eligibility and study selection
Two reviewers (ST and HM) independently selected eligible articles that met the following criteria: 1) observational studies with a prospective cohort, nested case-control, or case-control design; 2) conducted in adults (≥18 y); 3) reported the consumption of the dietary antioxidants vitamin C, vitamin E, vitamin A, selenium, zinc, α-carotene, β-carotene, lycopene, β-cryptoxanthin, lutein, flavonoids, and antioxidant capacity; 4) reported the risk estimate of PD as an outcome variable; and 5) reported ORs, RRs, or HRs along with 95% CIs. Studies with a cross-sectional design, intervention studies, review articles, case reports, letters to the editor, and those conducted in children or patients with specific diseases were not included. When multiple publications used duplicate or overlapping data, the most recent publication with the longest follow-up time was included.
Data extraction
The following characteristics from selected eligible studies were recorded: first author's name, the country where the study was conducted, publication year, gender, age range and/or mean age (year), study follow-up duration, number of participants/cases, dietary assessment method, type of exposure, method of outcome assessment, dietary intake categories (high vs. low), and adjusted covariates. Reported effect sizes in the form of ORs, RRs, or HRs and the 95% CIs of risk of PD were also recorded. The process of data extraction was performed individually by 3 authors (ST, AJ, and HM).
Quality assessment
The quality of included studies was assessed by 2 independent researchers (ST and AJ) using the Cochrane Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool (17). This checklist examined whether study bias related to 7 potential domains: confounding, selection of participants, exposure assessment, misclassification of exposure during follow-up, missing data, measurement of the outcome, and selective reporting of the results. According to this scale, the overall quality of studies was categorized as low, moderate, or serious risk of bias (Supplemental Table 2).
Data synthesis and statistical analysis
RRs and their 95% CIs constituted the effect sizes of the pooled cohort studies. The reported ORs from nested case-control and HRs from cohort studies were considered the equivalent to RRs (18). Furthermore, ORs (and 95% CIs) were used as the effect sizes in the analysis of case-control studies. Separate analyses were carried out for cohort and case-control studies.
Reported risk estimates of PD for the highest compared with the lowest category of dietary antioxidant intake were pooled using the DerSimonian and Laird random-effects model (19). Cochrane's Q test of heterogeneity and the I2 statistic (P < 0.05) were conducted to evaluate heterogeneity across studies (20). To detect potential heterogeneity, subgroup analyses stratified by sex, follow-up duration, number of participants/cases, country of study, and adjustment for confounding variables including vitamin and mineral supplement use, physical activity, and alcohol intake were used. A sensitivity analysis was also performed to determine the influence of each individual study on the overall effect size. Publication bias was evaluated using Egger's regression test (21) and Begg's test (22).
Second, the linear dose–response relation was tested using generalized least-squares trend estimation, according to the methods developed by Greenland and Longnecker and colleagues (23, 24). Finally, the shape of the dose–response relations was examined using studies that reported sufficient information (25). The correlation within each category of relative risk was taken into account and the study-specific estimates were combined using a 1-stage linear mixed-effects meta-analysis (26). This method estimates the study-specific slope lines and combines them to find an overall average slope (27, 28). This single-stage approach has been shown to be more precise, flexible, and efficient than the traditional 2-stage method (26). The best fitting, second-order fractional polynomial was used to perform the dose–response meta-analysis when only 2 studies were available. Statistical analyses were conducted using STATA version 14 software (StataCorp). In addition, Grading of Recommendations Assessment, Development, and Evaluations (GRADE) was applied to evaluate the quality of the evidence for each relation (29).
A priori power analyses were performed using recently validated methods (30, 31). We found matching RR and average sample sizes across all outcomes in the previous meta-analyses (10, 11). In line with this, we anticipated to detect a small statistically significant effect size with an ɑ of 5% and a statistical power of 80%. The present analyses included the minimum number of studies required to obtain power using a random-effects model (Supplemental Table 3).
Results
Literature search
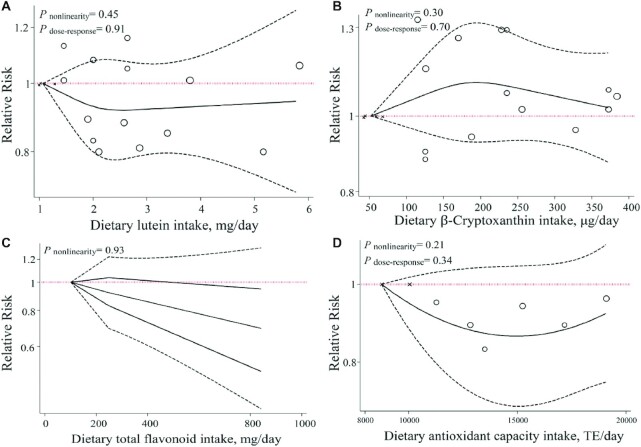
We identified 2230 publications through initial electronic searches. Of those, 622 articles were eliminated based on duplicates, animal studies, and nonoriginal articles. A total of 1566 records were removed following the title and abstract screening. Of 42 full-text publications, 29 were excluded for either of the following reasons: irrelevant outcomes (n = 3), insufficient information (n = 11), irrelevant exposure (n = 11), cross-sectional design (n = 2), and duplicated reports (n = 2). Detailed reasons for study exclusion are described in Supplemental Table 4. Ultimately, 6 prospective cohort studies (5 publications) (6, 8, 9, 14, 15), 2 nested case-control (32, 33), and 6 case-control studies (7, 34–38) met the inclusion criteria. The flow diagram of the study selection process is provided in Figure 1.
FIGURE 1.
Flow diagram of study selection.
Study characteristics
In total, 448,737 participants, consisting of 4654 cases with PD, formed the analyses of prospective cohort studies (6, 8, 9, 14, 15, 32, 33). A total of 1948 controls and 1273 cases made up the analysis for the case-control studies (7, 34–38). These studies were conducted from 1996 to 2021 in Sweden (6, 8), Singapore (14), the United States (9, 15, 33, 35–37), Japan (7, 32, 38), and Germany (34). Four studies (3 prospective cohort and 1 case-control) included only men (8, 9, 15, 32, 35), 2 prospective cohort studies enrolled only women (8, 9, 15), whereas other studies were conducted in both genders (6, 7, 14, 33, 34, 36–38). The follow-up duration among cohort studies ranged from 14 (8) to 28 (32) y. All cohort studies measured dietary antioxidant intakes using a validated FFQ (6, 8, 9, 14, 15, 32, 33). Among case-control studies, 4 used FFQs (34–37) and 2 used a diet history questionnaire to assess dietary intakes (7, 38). All effect sizes were adjusted for by smoking status. Some studies controlled for additional confounding variables, which included age (n = 8), BMI (n = 7), alcohol consumption (n = 6), coffee consumption (n = 9), energy intake (n = 9), and vitamin and mineral supplement use (n = 3). The general characteristics of cohort and case-control studies are summarized in Supplemental Tables 5 and 6, respectively.
Among 13 studies, the dietary intakes of various antioxidants were evaluated. These included the following: vitamin C (cohort, n = 7; case-control, n = 5), vitamin E (cohort, n = 7; case-control, n = 5), vitamin A (cohort, n = 2; case-control, n = 1), α-carotene (cohort, n = 3; case-control, n = 2), β-carotene (cohort, n = 6; case-control, n = 5), β-cryptoxanthin (cohort, n = 3; case-control, n = 2), total carotenoids (cohort, n = 3; case-control, n = 1), lycopene (cohort, n = 3; case-control, n = 2), lutein (cohort, n = 3; case-control, n = 3), total flavonoids (cohort, n = 2), flavonols (cohort, n = 2), flavones (cohort, n = 2), flavanones (cohort, n = 2), flavan-3-ols (cohort, n = 2), polymers (cohort, n = 2), anthocyanins (cohort, n = 2), total antioxidant capacity (TAC; cohort, n = 2), nonenzymatic antioxidant capacity (NEAC; cohort, n = 1), zinc (case-control, n = 2), selenium (case-control, n = 1), and total xanthophylls (case-control, n = 1). These antioxidants were included into the highest versus lowest category meta-analysis (6–9, 14, 15, 32–38). Among these studies, 8 had sufficient information to be included into the linear dose–response meta-analysis (6–9, 14, 15, 32, 38). However, the nonlinear dose–response meta-analysis was conducted only on 6 prospective cohort studies (6, 8, 9, 14, 15) (Table 1).
TABLE 1.
Dietary antioxidants and risk of Parkinson's disease1
| Highest vs. lowest category meta-analysis | Dose-response meta-analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Studies, n | RR (95% CI) | I2, % (95% CI) | P-heterogeneity | Dose, unit | Studies, n | RR (95% CI) | I2, % | P-heterogeneity | |
| Cohort studies | |||||||||
| Vitamin C | 7 | 0.95 (0.77, 1.18) | 75.9 (49, 89) | <0.001 | 50 mg/d | 6 | 0.94 (0.88, 0.99) | 55.2 | 0.06 |
| Vitamin E | 7 | 0.84 (0.71, 0.99) | 51.9 (0, 80) | 0.05 | 5 mg/d | 7 | 0.84 (0.70, 0.99) | 58.3 | 0.02 |
| Vitamin A | 2 | 1.11 (0.92, 1.33) | 0.0 (0, 90) | 0.33 | 1000 IU/d | 1 | 1.00 (0.94, 1.06) | — | — |
| α-Carotene | 3 | 1.06 (0.89, 1.25) | 0.0 (0, 90) | 0.46 | 0.5 mg/d | 3 | 0.98 (0.92, 1.04) | 0.4 | 0.36 |
| β-Carotene | 6 | 0.88 (0.76, 1.03) | 45.2 (0, 78) | 0.10 | 2 mg/d | 6 | 0.94 (0.89, 0.99) | 58 | 0.03 |
| β-Cryptoxanthin | 3 | 1.03 (0.88, 1.22) | 0.0 (0, 90) | 0.87 | 100 μg/d | 3 | 1.01 (0.96, 1.06) | 0.0 | 0.47 |
| Total carotenoids | 3 | 0.98 (0.81, 1.19) | 32.1 (0, 93) | 0.22 | 5 mg/d | 3 | 0.99 (0.91, 1.08) | 48.9 | 0.14 |
| Lycopene | 3 | 1.04 (0.88, 1.24) | 15.9 (0, 91) | 0.30 | 2 mg/d | 3 | 1.01 (0.97, 1.04) | 0.0 | 0.44 |
| Lutein | 3 | 1.00 (0.82, 1.21) | 34.5 (0, 79) | 0.21 | 1 mg/d | 3 | 1.00 (0.94, 1.06) | 44.2 | 0.16 |
| Total flavonoids | 2 | 0.77 (0.46, 1.29) | 77.1 | 0.03 | 100 mg/d | 2 | 0.95 (0.86, 1.04) | 83.4 | 0.01 |
| Flavonols | 2 | 0.80 (0.63, 1.00) | 0.0 | 0.44 | — | — | — | — | — |
| Anthocyanins | 2 | 0.76 (0.61, 0.96) | 0.0 | 0.54 | — | — | — | — | — |
| Flavones | 2 | 0.86 (0.68, 1.07) | 0.0 | 0.95 | — | — | — | — | — |
| Flavanones | 2 | 0.88 (0.63, 1.24) | 52.8 | 0.14 | — | — | — | — | — |
| Flavan-3-ols | 2 | 0.89 (0.61, 1.30) | 61.2 | 0.10 | — | — | — | — | — |
| Polymers | 2 | 0.79 (0.48, 1.31) | 77.2 | 0.03 | — | — | — | — | — |
| TAC | 2 | 0.93 (0.78, 1.11) | 0.0 | 0.67 | 2000 TE/d | 2 | 0.98 (0.94, 1.02) | 0.0 | 0.47 |
| NEAC | 1 | 0.79 (0.60, 1.04) | — | — | 5 mg/d | 1 | 0.96 (0.90, 1.03) | — | — |
| Case-control studies | |||||||||
| Vitamin C | 5 | 0.92 (0.72, 1.18) | 0.0 (0, 79) | 0.41 | 50 mg/d | 1 | 0.99 (0.76, 1.29) | — | — |
| Vitamin E | 5 | 0.80 (0.57, 1.12) | 23.4 (0, 69) | 0.26 | 5 mg/d | 1 | 0.34 (0.16, 0.69) | — | — |
| Vitamin A | 1 | 1.15 (0.62, 2.11) | — | — | — | — | — | — | — |
| α-Carotene | 2 | 0.82 (0.38, 1.78) | 51 | 0.15 | 0.5 mg/d | 1 | 0.65 (0.41, 1.02) | — | — |
| β-Carotene | 5 | 0.92 (0.64, 1.33) | 47.8 (0, 81) | 0.10 | 2 mg/d | 1 | 0.70 (0.51, 0.94) | — | — |
| β-Cryptoxanthin | 2 | 1.22 (0.80, 1.85) | 0.0 | 0.71 | 100 μg/d | 1 | 1.05 (0.95, 1.17) | — | — |
| Total carotenoids | 1 | 1.17 (0.49, 2.81) | — | — | — | — | — | — | — |
| Lycopene | 2 | 1.13 (0.50, 2.54) | 66.6 | 0.08 | — | — | — | — | — |
| Lutein | 3 | 1.86 (1.20, 2.88) | 23.4 (0, 92) | 0.27 | — | — | — | — | — |
| Zinc | 2 | 0.64 (0.31, 1.31) | 68.8 | 0.07 | 1 mg/d | 1 | 0.65 (0.49, 0.86) | — | — |
| Selenium | 1 | 1.01 (0.60, 1.70) | — | — | — | — | — | — | — |
| Total xanthophylls | 1 | 3.16 (1.08, 9.30) | — | — | — | — | — | — | — |
NEAC, nonenzymatic antioxidant capacity; TAC, total antioxidant capacity; TE, Trolox equivalents.
Risk-of-bias assessment
Based on the ROBINS-I tool, 9 studies were categorized as being at moderate risk of bias (6–9, 14, 15, 35, 36, 38) and 4 studies had a serious risk of bias due to the possibility of residual confounding or insufficient information regarding the selection of participants (32–34, 37) (Supplemental Table 2).
Findings from the meta-analysis
Association between dietary vitamin C intake and risk of PD
A total of 7 prospective cohort studies (total n = 318,784) with 4570 cases (6, 8, 9, 14, 33), and 5 case-control studies (7, 34–37) were included in the analysis of dietary vitamin C intake. The risk estimate of PD was similar for the highest compared with the lowest category of dietary vitamin C intake (RR: 0.95; 95% CI: 0.77, 1.18; I2 = 75.9%; 95% CI: 49, 89; P-heterogeneity < 0.001; Supplemental Figure 1) among cohort studies and for the vitamin C intake category (OR: 0.92; 95% CI: 0.72, 1.18; I2 = 0.0%; 95% CI: 0, 79; P-heterogeneity = 0.41; Supplemental Figure 2) among case-control studies (Table 1).
The subgroup analysis of dietary vitamin C intake and risk of PD for cohort studies showed that the association was significant only in studies with women participants (RR: 0.77; 95% CI: 0.62, 0.95; n = 2), those that controlled for physical activity (RR: 0.75; 95% CI: 0.63, 0.79; n = 3), and those not controlling for alcohol intake (RR: 1.31, 95% CI: 1.10, 1.58; n = 2) (Supplemental Table 7).
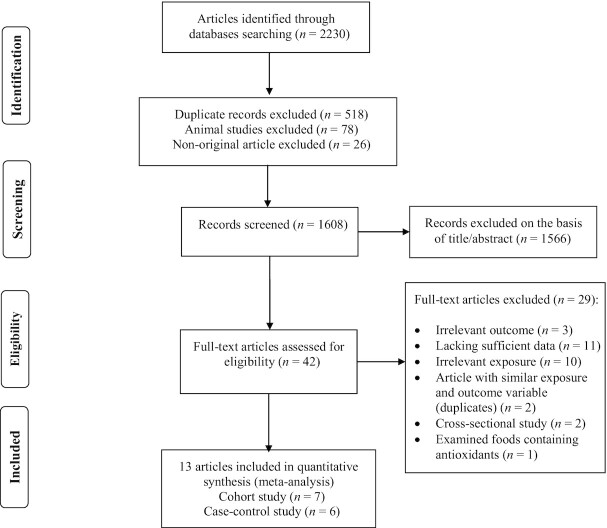
Six prospective cohort studies (4 publications) presented sufficient data for vitamin C dose–response meta-analyses (6, 8, 9, 14). The results demonstrated that each 50-mg/d increment in dietary vitamin C consumption was associated with a 6% lower risk of PD (RR: 0.94; 95% CI: 0.88, 0.99; I2 = 55.2%, P-heterogeneity = 0.06; n = 6) (Supplemental Figure 3, Table 1). However, no association was observed in the analysis of 1 case-control study (7) (OR: 0.99; 95% CI: 0.76, 1.29; I2 = 0.0%, P-heterogeneity = 0.47) (Table 1). There was evidence of departure from linearity between dietary vitamin C intake and risk of PD (P-nonlinearity = 0.01, P-dose–response = 0.23; n = 7; Figure 2A).
FIGURE 2.
Dose–response associations of dietary vitamin intake and risk of Parkinson's disease. (A) Vitamin C; (B) vitamin E in random-effects models. Solid lines represent the RR of the association between dietary vitamin intake and PD; dashed lines represent 95% CIs.
Association between dietary vitamin E intake and risk of PD
Seven prospective cohort studies (6, 8, 9, 14, 32) with 316,405 participants, comprising 3444 cases of PD and 5 case-control studies (1024 cases, 1580 controls) (7, 34–37), were considered eligible for the analysis of dietary vitamin E intake and risk of PD. The highest compared with the lowest category of dietary vitamin E intake was associated with a 16% lower risk of PD in the analysis of cohort studies (RR: 0.84; 95% CI: 0.71, 0.99; I2 = 51.9%; 95% CI: 0, 80; P-heterogeneity = 0.05; Supplemental Figure 4) but not in case-control studies (OR: 0.80; 95% CI: 0.57, 1.12; I2 = 23.4%; 95% CI: 0, 69; P-heterogeneity = 0.26; Supplemental Figure 5) (Table 1).
Findings from the subgroup analyses of cohort studies suggested that follow-up duration, number of participants, geographical region, and adjustment for vitamin and mineral supplement use and alcohol intake could explain the observed heterogeneity (Supplemental Table 8).
A linear dose–response meta-analysis of prospective cohort studies indicated that each 5-mg/d increment in vitamin E consumption was associated with a 16% lower risk of PD (RR: 0.84; 95% CI: 0.70, 0.99; P = 0.049, I2 = 58.3%, P-heterogeneity = 0.02; n = 7) (Supplemental Figure 6, Table 1), with no evidence of departure from linearity (P-nonlinearity = 0.48, P-dose–response = 0.11; n = 7; Figure 2B).
Association between dietary vitamin A intake and risk of PD
Two cohort studies (14, 33) (939 cases, 62,964 participants) and 1 case-control study (36) analyzed the association between dietary vitamin A intake and risk of PD. The highest category of vitamin A intake did not significantly reduce the risk of PD in cohort studies (RR: 1.11; 95% CI: 0.92, 1.33; I2 = 0.0%; 95% CI: 0, 90; P-heterogeneity = 0.33; n = 3; Supplemental Figure 7) or in 1 case-control study (OR: 1.15; 95% CI: 0.62, 2.11) (Table 1).
Based on the dose–response analysis on 1 prospective cohort study (14), a 1000-IU/d increment in dietary vitamin A intake was not associated with a reduced risk of PD (RR: 1.00; 95% CI: 0.94, 1.06) (Table 1). Due to only 1 of the studies reporting categorical data, we could not perform a nonlinear dose–response meta-analysis.
Association between dietary α-carotene intake and risk of PD
Three cohort studies (total n = 189,671) with 1580 cases (9, 14) and 2 case-control studies (controls n = 418) with 306 cases (7, 35) were included in the α-carotene analyses. Higher dietary α-carotene intake was not significantly associated with the risk of PD in the analysis of 3 cohort studies (RR: 1.06; 95% CI: 0.89, 1.25; I2 = 0.0%; 95% CI: 0, 90; P-heterogeneity = 0.46; Supplemental Figure 8) and 2 case-control studies (OR: 0.82; 95% CI: 0.38, 1.78; I2 = 55%, P-heterogeneity = 0.15; Supplemental Figure 9) (Table 1).
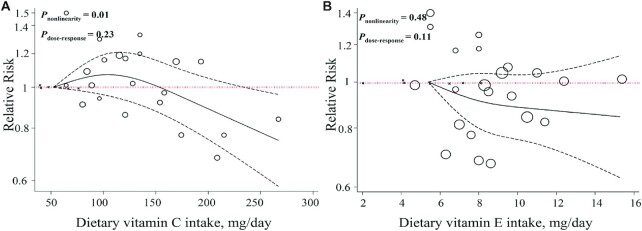
This was confirmed by a linear dose–response meta-analysis, which suggested that each 0.5-mg/d increase in α-carotene intake was not associated with the risk of PD in cohort (9, 14) (RR: 0.98; 95% CI: 0.92, 1.04; I2 = 0.4%, P-heterogeneity = 0.36; n = 3) and case-control (7) (OR: 0.65; 95% CI: 0.41, 1.02; n = 1) studies (Supplemental Figure 10, Table 1), with no evidence of departure from linearity (P-nonlinearity = 0.14, P-dose–response = 0.60; n = 4; Figure 3A).
FIGURE 3.
Dose–response associations of dietary carotenoid intake and risk of Parkinson's disease. (A) α-Carotene; (B) β-carotene; (C) lycopene; (D) total carotenoids in random-effects models. Solid lines represent the RR of the association between dietary carotenoid intake and PD; dashed lines represent 95% CIs.
Association between dietary β-carotene intake and risk of PD
Six prospective cohort studies with 3370 cases of PD among a total of 316,069 participants (6, 8, 9, 14) and 5 case-control studies (1024 cases, 1580 controls) (7, 34–37) were included in the analysis of dietary β-carotene intake. The highest category of β-carotene intake compared with the lowest category was not associated with the risk of PD in the analysis of cohort studies (RR: 0.88; 95% CI: 0.76, 1.03; I2 = 45.2%, P=heterogeneity = 0.10; 95% CI: 0, 78; n = 6; Supplemental Figure 11) and analysis of case-control studies (OR: 0.92; 95% CI: 0.64, 1.33; I2 = 47.8%; 95% CI: 0, 81; P-heterogeneity = 0.10; n = 5; Supplemental Figure 12) (Table 1).
Moreover, each 2-mg/d increase in dietary β-carotene intake was weakly associated with a 6% lower risk of PD based on 6 prospective cohort studies (6, 8, 9, 14) (RR: 0.94; 95% CI: 0.89, 0.99; P = 0.049, I2 = 58%, P-heterogeneity = 0.03; n = 6) and 30% lower risk from 1 case-control study (7) (OR: 0.70, 95% CI: 0.51, 0.94; n = 1) (Supplemental Figure 13, Table 1). Evidence of nonlinearity (P-nonlinearity = 0.43, P-dose–response = 0.09; n = 7; Figure 3B) was not observed in the analysis of cohort studies that presented sufficient information for the nonlinear dose–response meta-analysis (6, 8, 9, 14).
Association between dietary lycopene intake and risk of PD
A total of 3 prospective cohort studies, derived from 2 publications (total n = 189,671) with 1580 cases (9, 14), and 2 case-control studies (35, 37) were considered eligible for the analysis that examined the association between dietary lycopene intake and risk of PD. In comparison to the lowest category of dietary lycopene intake, the highest intake category was not associated with a reduced risk of PD in cohort (RR: 1.04; 95% CI: 0.88, 1.24; I2 = 15.9%; 95% CI: 0, 91; P-heterogeneity = 0.30; Supplemental Figure 14) and case-control (OR: 1.13; 95% CI: 0.50, 2.54; I2 = 66.6%, P-heterogeneity = 0.08; Supplemental Figure 15) studies (Table 1).
Three prospective cohort studies were included in the dose–response meta-analyses (9, 14). Findings revealed no significant association between an increase of 2 mg dietary lycopene intake/d and risk of PD (RR: 1.01; 95% CI: 0.97, 1.04; I2 = 0.0%, P-heterogeneity = 0.44; n = 3) (Supplemental Figure 16, Table 1). There was no evidence of nonlinearity from cohort studies (P-nonlinearity = 0.52, P-dose–response = 0.72; n = 4; Figure 3C).
Association between dietary total carotenoid intake and risk of PD
Three cohort studies (total n = 189,671) with 1580 cases (9, 14) and 1 case-control study (35) were included in the analysis of total dietary carotenoid intake. There was no significant difference in the risk of developing PD between high and low categories of total dietary carotenoid intake in cohort studies (RR: 0.98; 95% CI: 0.81, 1.19; I2 = 32.1%, P-heterogeneity = 0.22; n = 3) (Supplemental Figure 17, Table 1).
Findings from a linear dose–response meta-analysis of cohort studies (9, 14) indicated that a 5-mg/d increase in the dietary total carotenoid intake was not associated with a reduced risk of PD (RR: 0.99; 95% CI: 0.91, 1.08; I2 = 48.9%, P-heterogeneity = 0.14; n = 3) (Supplemental Figure 18, Table 1), with no evidence of departure from linearity (P-nonlinearity = 0.95, P-dose–response = 0.93; n = 4; Figure 3D).
Association between dietary lutein intake and risk of PD
The association between dietary lutein intake and risk of PD was estimated by 3 prospective cohort studies (total n = 189,671) with 1580 cases (9, 14) and 3 case-control studies (870 controls, 433 cases) (35–37). The overall effect size indicated that higher lutein intake was associated with increased risk of PD in case-control studies (OR: 1.86; 95% CI: 1.20, 2.88; I2 = 23.4%; 95% CI: 0, 79; P-heterogeneity = 0.27; Supplemental Figure 19) but not in cohort studies (RR: 1.00; 95% CI: 0.82, 1.21; I2 = 34.5%; 95% CI: 0, 92; P-heterogeneity = 0.21; Supplemental Figure 20) (Table 1).
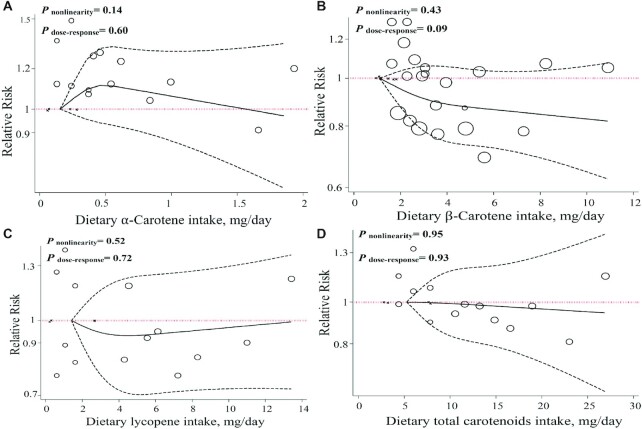
There was no significant association for a 1-mg/d increment in lutein consumption and risk of PD (RR: 1.00; 95% CI: 0.94, 1.06; I2 = 44.2%; P-heterogeneity = 0.16; n = 3) (Supplemental Figure 21, Table 1). We found no evidence of departure from linearity between dietary lutein intake and the risk of PD (P-nonlinearity = 0.45, P-dose–response = 0.91; n = 4; Figure 4A).
FIGURE 4.
Dose–response associations of dietary antioxidant intake and risk of Parkinson's disease. (A) lutein; (B) β-cryptoxanthin; (C) total flavonoids; (D) total antioxidant capacity in random-effects models. Solid lines represent the RR of the association between dietary carotenoid intake and PD; dashed lines represent 95% CIs.
Association between dietary β-cryptoxanthin intake and risk of PD
Three cohort studies (1580 cases, 189,671 participants) (9, 14) and 2 case-control studies (306 cases, 418 controls) (7, 35) focused on β-cryptoxanthin and the risk of PD. The relative risk of PD was lower for the highest compared with the lowest category of dietary β-cryptoxanthin intake among both cohort studies (RR: 1.03; 95% CI: 0.88, 1.22; I2 = 0.0%; 95% CI: 0, 90; P-heterogeneity = 0.87; n = 3; Supplemental Figure 22) and case-control studies (OR: 1.22; 95% CI: 0.80, 1.85; I2 = 0.0%, P-heterogeneity = 0.71; n = 2; Supplemental Figure 23) (Table 1).
There was no linear association between dietary β-cryptoxanthin intake and risk of PD in cohort studies (9, 14) (RR: 1.01; 95% CI: 0.96, 1.06; I2 = 0.0%, P-heterogeneity = 0.47; n = 3) as well as case-control studies (7) (OR: 1.05; 95% CI: 0.95, 1.17; n = 1) (Supplemental Figure 24, Table 1), with no evidence of departure from linearity (P-nonlinearity = 0.30, P-dose–response = 0.70; n = 4; Figure 4B).
Association between dietary flavonoid intake and risk of PD
Two prospective cohort studies from 1 publication, which consisted of 805 PD cases among a total of 129,617 participants, were included in the pooled analysis of total dietary flavonoid intake and their subclasses (flavanones, anthocyanins, flavan-3-ols, flavonols, flavones, and polymers) (15). The summary RR of PD for the highest flavonoid intake was lower in comparison to the lowest intake (RR: 0.77; 95% CI: 0.46, 1.29; I2 = 77.1%, P-heterogeneity = 0.03) (Supplemental Figure 25, Table 1). Comparing the highest and lowest categories for the subclasses showed that high dietary intake of anthocyanins was significantly associated with a lower risk of PD (RR-anthocyanins: 0.76; 95% CI: 0.61, 0.96; I2 = 0.0%, P-heterogeneity = 0.54) (Table 1).
In the linear dose–response meta-analysis based on 2 cohorts, no significant association was found between a 100-mg/d increment of dietary total flavonoid intake and risk of PD (RR: 0.95; 95% CI: 0.86, 1.04; I2 = 83.4%, P-heterogeneity = 0.01; n = 2) (Supplemental Figure 26, Table 1). No evidence of departure from linearity was seen between dietary total flavonoid intake and risk of PD (P-nonlinearity = 0.93; n = 2; Figure 4C).
Association between dietary antioxidant capacity intake and risk of PD
Two cohort studies (1329 cases, 84,837 participants) (8) and 1 cohort study (461 cases, 41,624 participants) (6) were included in the analyses of TAC and NEAC, respectively. No significant differences in the risk of PD were observed between the highest and lowest intake categories of both TAC (RR: 0.93; 95% CI: 0.78, 1.11; I2 = 0.0%, P-heterogeneity = 0.67) and NEAC (RR: 0.79; 95% CI: 0.60, 1.04) (Table 1).
The results of the linear dose–response meta-analysis illustrated that a 2000–Trolox equivalents/d increase in TAC (RR: 0.98; 95% CI: 0.94, 1.02; I2 = 0.0%, P-heterogeneity = 0.47; n = 2) was not associated with a lower risk of PD (Table 1), with no evidence of departure from linearity (P-nonlinearity = 0.21, P-dose–response = 0.34; n = 2; Figure 4D).
Association between dietary zinc intake and risk of PD
Two case-control studies (37, 38) analyzed the association between dietary zinc intake and risk of PD. The relative risk of PD was lower for the highest compared with the lowest category of dietary zinc intake (OR: 0.64; 95% CI: 0.31, 1.31; I2 = 68.8%, P-heterogeneity = 0.07). In addition, a 1-mg/d increment in dietary zinc intake was associated with a significantly lower risk of PD (OR: 0.65; 95% CI: 0.49, 0.86; n = 1) (Table 1).
Sensitivity analyses and publication bias
The sensitivity analysis for the association between dietary vitamin E intake and risk of PD showed that the exclusion of various studies changed the pooled RRs. These studies included the Hantikainen et al. study (6) (RR: 0.87; 95% CI: 0.73, 1.04), the SMC study by Yang et al. (8) (RR: 0.87; 95% CI: 0.73, 1.04), the COSM study by Yang et al. (8) (RR: 0.83; 95% CI: 0.68, 1.03), the NHS study by Hughes et al. (9) (RR: 0.84; 95% CI: 0.69, 1.02), and the Morens et al. study (32) (RR: 0.85; 95% CI: 0.72, 1.01). Moreover, excluding the study by Hughes et al. (9) altered the overall effect of dietary β-carotene intake and risk of PD (RR: 0.85; 95% CI: 0.72, 0.99). Moreover, findings from the sensitivity analysis for the association between dietary total flavonoid intake and zinc intake with risk of PD indicated that excluding the NHS study by Gao et al. (15) (RR: 0.59; 95% CI: 0.43, 0.83) and the Powers et al. study (37) (RR: 0.43; 95% CI: 0.23, 0.80) altered the pooled effect size. However, other effect sizes were not influenced by 1 particular study. No evidence of publication bias, based on Begg's and Egger's tests, was found in the analyses of cohort and case-control studies.
Grading the evidence
The certainty of the evidence was rated using the GRADE approach. GRADE evidence tables for cohort and case-control studies are presented in Supplemental Tables 9 and 10, respectively. The quality of the assessed evidence was rated as very low or low for all outcomes, with various downgrades for serious risk of bias, inconsistency, indirectness, and imprecision.
Discussion
The present meta-analysis indicated that dietary intake of vitamin E and anthocyanins can significantly reduce the risk of PD. Furthermore, higher lutein intake was associated with an increased risk of PD in case-control studies, a finding that was not confirmed by cohort studies. Dose–response meta-analyses showed that an increased daily consumption of 50 mg vitamin C, 5 mg vitamin E, 2 mg β-carotene, and 1 mg zinc could be associated with lower risk of PD. The certainty of the evidence was graded between very low and low for all outcomes.
Several possible mechanisms have been suggested to explain the relation between the aforementioned antioxidants and PD. Increased consumption of antioxidants is associated with a decreased risk of chronic diseases, including cardiovascular diseases (39), diabetes (40), Alzheimer disease (41), and cancer (42). The brains of patients with PD have low concentrations of endogenous antioxidants [glutathione and coenzyme Q10 (CoQ10)] (43), increased dopamine oxidation (44), and high iron concentrations (45). Based on the evidence, oxidative stress can be an important factor in the neurodegeneration associated with PD (46, 47). Thus, free radicals are believed to be involved in neuronal loss (48). According to several epidemiological studies, antioxidant-rich diets can prevent and protect from oxidative damage and neurodegeneration (49, 50).
Vitamin E has displayed neuroprotective actions against free radical–mediated injury. This is exemplified by vitamin E protecting neurons in the locus coeruleus (the principal site for norepinephrine synthesis) from death in an early model of PD (51, 52), preventing the toxin-induced destruction of striatal dopaminergic terminals (53), and controlling the concentrations of antioxidant defenses such as glutathione and superoxide dismutase (SOD) (54, 55). Like vitamin E, vitamin A and C have also demonstrated neuroprotective effects alone or in combination with CoQ10 (56, 57). The antioxidant activity of these vitamins leads to their neuroprotective roles in neurodegenerative diseases. Additionally, vitamin A and β-carotene prevent the constitution of fibrillar ɑ-synuclein aggregates and destabilize the generated ɑ-synuclein aggregates. As a result, vitamin A and β-carotene can prevent synucleinopathies like PD (56).
Polyphenols are a group of chemical compounds that have demonstrated the ability to reduce oxidative stress (58, 59). The cellular and molecular mechanisms by which polyphenols, particularly flavonoids, protect neurons from oxidative damage and degeneration are poorly understood. However, it has been suggested that flavonoids operate by scavenging reactive nitrogen species (RNS) and ROS (58, 60). This is achieved through the control of signaling pathways associated with cell survival (61–63).
Previous studies have reported conflicting findings regarding the association between dietary antioxidants and PD risk. A meta-analysis of 6 observational studies found that a moderate intake of vitamin E was inversely associated with PD, whereas no association was seen for vitamin C and β-carotene (11). Another meta-analysis indicated a nonsignificant, inverse association between both α and β-carotene and PD risk (10). These previous nonsignificant associations are in line with a number of nonsignificant findings generated from the present review. Extending these previous findings is the observed significant relation between higher lutein intake and increased risk of PD in case-control studies.
Findings from a number of previous studies that assessed the association between antioxidants and other neurodegenerative diseases contradict the finding from the current review. One case-control study demonstrated no associations between vitamin E, lycopene, and CoQ10 with dementia, while compromised vitamin C and β-carotene intake was associated with dementia (64). This may be due to certain antioxidants having no effect on cerebrospinal fluid biomarkers related to amyloid or tau pathology in patients with Alzheimer disease (65).
Based on intervention studies, the progression of PD may be slowed by vitamin E and vitamin C (66). Due to the increasing incidence rates of worldwide PD (67), raising the consumption of antioxidant-rich diets seems to be a viable recommendation to prevent PD. Furthermore, multiple cohort studies revealed a mean daily intake well below the RDA for different types of antioxidants (68, 69), confirming the need to increase the consumption of antioxidant-rich foods.
The present meta-analysis has numerous strengths. The inclusion of 6 new cohort studies in addition to performing dose–response analyses are key strengths of the present review relative to previous meta-analyses. A nonlinear dose–response association between dietary antioxidants and the risk of PD was assessed for the first time. Notably, the GRADE system was adopted to evaluate the overall quality of the evidence. Given that the included studies consisted of long, prospective cohort designs, this minimized the risk of recall and selection biases. Most of the included studies adjusted for important confounders. The large study sample sizes, as confirmed by a power analysis, ensure high statistical power and increase the generalizability of results. In an effort to examine sources of potential heterogeneity, a range of subgroup analyses were performed. Moreover, the most recently developed statistical method was applied to conduct nonlinear dose–response analyses.
There are a number of limitations that need to be considered. Due to the observational nature of studies, causality cannot be elicited from the results. During long-term follow-up periods within cohort studies, possible changes in dietary intakes may occur, which were not taken into account among a majority of included studies. A high degree of heterogeneity between studies and significant study bias reduces the confidence in the estimated effect sizes. Higher antioxidant intakes are associated with higher consumption of other neuroprotective nutrients, greater compliance with dietary guidelines, and lower intakes of unhealthy foods. Most of the included studies did not assess the dietary intake of these other nutrients/foods, failing to adequately adjustment for this potential confounding factor. Moreover, the majority of included studies have been conducted in developed countries. Thus, the obtained results may not be applicable to a range of countries/societies. Due to the use of FFQs to assess the dietary antioxidant intake, the reported values may not be precise and account for antioxidant absorption. FFQs can lead to recall bias and the overestimated intake of fruits, vegetables, and consequently, water-soluble antioxidants. They may further underestimate the intake of fats and oils, which are linked to fat-soluble antioxidants (70).
In conclusion, the present dose–response meta-analysis revealed that higher consumption of dietary antioxidants, specifically vitamin E, vitamin C, and polyphenols such as anthocyanins, is associated with a lower risk of PD. Importantly, the quality rating of the meta-evidence indicated that there is low confidence in the generated effect size estimates across a number of examined dietary antioxidants. Future well-designed prospective cohort studies may be needed to reliably determine whether the dietary consumption of antioxidants may be a plausible option for the prevention of PD.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—ST, AJ, and HM: contributed to the conception of research, searched databases, and performed the data extraction and statistical analysis; ST and SMG: wrote the manuscript; HM, AJ, and NT: critically revised the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by the Students’ Scientific Research Center (SSRC) of Tehran University of Medical Sciences, IR.TUMS.MEDICINE.REC.1400.1048 (code: 1400-2-125-54068).
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1–26 and Supplemental Tables 1–10 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: CoQ10, coenzyme Q10; GRADE, Grading of Recommendations Assessment, Development, and Evaluations; NEAC, nonenzymatic antioxidant capacity; NHS, Nurses’ Health Study; PD, Parkinson's disease; ROBINS-I, Risk of Bias in Non-randomized Studies of Interventions; ROS, reactive oxygen species; TAC, total antioxidant capacity.
Contributor Information
Sepide Talebi, Students’ Scientific Research Center (SSRC), Tehran University of Medical Sciences, Tehran, Iran; Department of Clinical Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
Seyed Mojtaba Ghoreishy, Department of Clinical Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
Ahmad Jayedi, Social Determinant of Health Research Center, Semnan University of Medical Sciences, Semnan, Iran.
Nikolaj Travica, Deakin University, IMPACT–the Institute for Mental and Physical Health and Clinical Translation, Food & Mood Centre, School of Medicine, Barwon Health, Geelong, Australia.
Hamed Mohammadi, Department of Clinical Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
References
- 1. Tysnes O-B, Storstein A. Epidemiology of Parkinson's disease. J Neural Transm. 2017;124(8):901–5. [DOI] [PubMed] [Google Scholar]
- 2. De Rijk MC, Launer LJ, Berger K, Breteler M, Dartigues JF, Baldereschi M, Fratiglioni L, Lobo A, Martinez-Lage J, Trenkwalder Cet al. . Prevalence of Parkinson's disease in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl 5):S21–3. [PubMed] [Google Scholar]
- 3. He J, Zhu G, Wang G, Zhang F. Oxidative stress and neuroinflammation potentiate each other to promote progression of dopamine neurodegeneration. Oxid Med Cell Longev. 2020;2020:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR. Oxidative stress and Parkinson's disease. Front Neuroanatomy. 2015;9:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duarte-Jurado AP, Gopar-Cuevas Y, Saucedo-Cardenas O, Loera-Arias MdJ, Montes-de-Oca-Luna R, Garcia-Garcia A, Rodriguez-Rocha H. Antioxidant therapeutics in Parkinson's disease: current challenges and opportunities. Antioxidants. 2021;10(3):453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hantikainen E, Trolle Lagerros Y, Ye W, Serafini M, Adami HO, Bellocco R, Bonn S. Dietary antioxidants and the risk of Parkinson disease: the Swedish National March Cohort. Neurology. 2021;96(6):e895–903. [DOI] [PubMed] [Google Scholar]
- 7. Miyake Y, Fukushima W, Tanaka K, Sasaki S, Kiyohara C, Tsuboi Y, Yamada T, Oeda T, Miki T, Kawamura Net al. . Dietary intake of antioxidant vitamins and risk of Parkinson's disease: a case-control study in Japan. Eur J Neurol. 2011;18(1):106–13. [DOI] [PubMed] [Google Scholar]
- 8. Yang F, Wolk A, Hakansson N, Pedersen NL, Wirdefeldt K. Dietary antioxidants and risk of Parkinson's disease in two population-based cohorts. Mov Disord. 2017;32(11):1631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hughes KC, Gao X, Kim IY, Rimm EB, Wang M, Weisskopf MG, Schwarzschild MA, Ascherio A. Intake of antioxidant vitamins and risk of Parkinson's disease. Mov Disord. 2016;31(12):1909–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takeda A, Nyssen OP, Syed A, Jansen E, Bueno-de-Mesquita B, Gallo V. Vitamin A and carotenoids and the risk of Parkinson's disease: a systematic review and meta-analysis. Neuroepidemiology. 2014;42(1):25–38. [DOI] [PubMed] [Google Scholar]
- 11. Etminan M, Gill SS, Samii A. Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson's disease: a meta-analysis. Lancet Neurol. 2005;4(6):362–5. [DOI] [PubMed] [Google Scholar]
- 12. de Rijk MC, Breteler MM, den Breeijen JH, Launer LJ, Grobbee DE, van der Meché FG, Hofman A. Dietary antioxidants and Parkinson disease: the Rotterdam Study. Arch Neurol. 1997;54(6):762–5. [DOI] [PubMed] [Google Scholar]
- 13. Anderson C, Checkoway LH, Franklin GM, Beresford S, Smith-Weller T, Swanson PD. Dietary factors in Parkinson's disease: the role of food groups and specific foods. Mov Disord. 1999;14(1):21–7. [DOI] [PubMed] [Google Scholar]
- 14. Ying AF, Khan S, Wu Y, Jin AZ, Wong ASY, Tan EK, Yuan JM, Koh WP, Tan LCS. Dietary antioxidants and risk of Parkinson's disease in the Singapore Chinese Health Study. Mov Disord. 2020;35(10):1765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao X, Cassidy A, Schwarzschild MA, Rimm EB, Ascherio A. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology. 2012;78(15):1138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moher D, Liberati AA, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(jul21 1):b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jüni P, Loke Y, Pigott T, Ramsay C, Regidor D, Rothstein H, Sandhu L, Santaguida P, Schünemann H, Shea B. Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I): detailed guidance. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Symons M, Moore D. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol. 2002;55(9):893–9. [DOI] [PubMed] [Google Scholar]
- 19. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 23. Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4(3):218–28. [DOI] [PubMed] [Google Scholar]
- 24. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. 2006;6(1):40–57. [Google Scholar]
- 25. Harrell FE Jr. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. Switzerland: Springer; 2015. [Google Scholar]
- 26. Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. One-stage dose-response meta-analysis for aggregated data. Stat Methods Med Res. 2019;28(5):1579–96. [DOI] [PubMed] [Google Scholar]
- 27. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. [DOI] [PubMed] [Google Scholar]
- 28. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hedges LV, Pigott TD. The power of statistical tests in meta-analysis. Psychol Methods. 2001;6(3):203. [PubMed] [Google Scholar]
- 31. Valentine JC, Pigott TD, Rothstein HR. How many studies do you need? A primer on statistical power for meta-analysis. J Educ Behav Stat. 2010;35(2):215–47. [Google Scholar]
- 32. Morens DM, Grandinetti A, Waslien CI, Park CB, Ross GW, White LR. Case-control study of idiopathic Parkinson's disease and dietary vitamin E intake. Neurology. 1996;46(5):1270–4. [DOI] [PubMed] [Google Scholar]
- 33. Paganini-Hill A. Risk factors for Parkinson's disease: the leisure world cohort study. Neuroepidemiology. 2001;20(2):118–24. [DOI] [PubMed] [Google Scholar]
- 34. Hellenbrand W, Boeing H, Robra BP, Seidler A, Vieregge P, Nischan P, Joerg J, Oertel WH, Schneider E, Ulm G. Diet and Parkinson's disease. II: a possible role for the past intake of specific nutrients. Results from a self-administered food-frequency questionnaire in a case-control study. Neurology. 1996;47(3):644–50. [DOI] [PubMed] [Google Scholar]
- 35. Scheider WL, Hershey LA, Vena JE, Holmlund T, Marshall JR, . Dietary antioxidants and other dietary factors in the etiology of Parkinson's disease. Mov Disord. 1997;12(2):190–6. [DOI] [PubMed] [Google Scholar]
- 36. Johnson CC, Gorell JM, Rybicki BA, Sanders K, Peterson EL. Adult nutrient intake as a risk factor for Parkinson's disease. Int J Epidemiol. 1999;28(6):1102–9. [DOI] [PubMed] [Google Scholar]
- 37. Powers KM, Smith-Weller T, Franklin GM, Longstreth WT Jr, Swanson PD, Checkoway H. Parkinson's disease risks associated with dietary iron, manganese, and other nutrient intakes. Neurology. 2003;60(11):1761–6. [DOI] [PubMed] [Google Scholar]
- 38. Miyake Y, Tanaka K, Fukushima W, Sasaki S, Kiyohara C, Tsuboi Y, Yamada T, Oeda T, Miki T, Kawamura Net al. . Dietary intake of metals and risk of Parkinson's disease: a case-control study in Japan. J Neurol Sci. 2011;306(1-2):98–102. [DOI] [PubMed] [Google Scholar]
- 39. Farbstein D, Kozak-Blickstein A, Levy AP. Antioxidant vitamins and their use in preventing cardiovascular disease. Molecules. 2010;15(11):8098–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scott JA, King GL. Oxidative stress and antioxidant treatment in diabetes. Ann NY Acad Sci. 2004;1031(1):204–13. [DOI] [PubMed] [Google Scholar]
- 41. Chang Y-T, Chang W-N, Tsai N-W, Huang C-C, Kung C-T, Su Y-J, Lin W-C, Cheng B-C, Su C-M, Chiang Y-F. The roles of biomarkers of oxidative stress and antioxidant in Alzheimer's disease: a systematic review. Biomed Res Int. 2014;2014:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Collins AR. Antioxidant intervention as a route to cancer prevention. Eur J Cancer. 2005;41(13):1923–30. [DOI] [PubMed] [Google Scholar]
- 43. Götz M, Gerstner A, Harth R, Dirr A, Janetzky B, Kuhn W, Riederer P, Gerlach M. Altered redox state of platelet coenzyme Q 10 in Parkinson's disease. J Neural Transm. 2000;107(1):41–8. [DOI] [PubMed] [Google Scholar]
- 44. Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson's disease. J Neurochem. 1999;73(3):1127–37. [DOI] [PubMed] [Google Scholar]
- 45. Lan J, Jiang D. Excessive iron accumulation in the brain: a possible potential risk of neurodegeneration in Parkinson's disease. J Neural Transm. 1997;104(6-7):649–60. [DOI] [PubMed] [Google Scholar]
- 46. Prasad KN, Cole WC, Hovland AR, Prasad KC, Nahreini P, Kumar B, Edwards-Prasad J, Andreatta CP. Multiple antioxidants in the prevention and treatment of neurodegenerative disease: analysis of biologic rationale. Curr Opin Neurol. 1999;12(6):761–70. [DOI] [PubMed] [Google Scholar]
- 47. Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson's disease. Ann NY Acad Sci. 2003;991(1):120–31. [DOI] [PubMed] [Google Scholar]
- 48. Jesberger JA, Richardson JS. Oxygen free radicals and brain dysfunction. Int J Neurosci. 1991;57(1-2):1–17. [DOI] [PubMed] [Google Scholar]
- 49. Obrenovich ME, Nair NG, Beyaz A, Aliev G, Reddy VP. The role of polyphenolic antioxidants in health, disease, and aging. Rejuvenation Res. 2010;13(6):631–43. [DOI] [PubMed] [Google Scholar]
- 50. Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51(3):609–14. [DOI] [PubMed] [Google Scholar]
- 51. Cadet JL, Katz M, Jackson-Lewis V, Fahn S. Vitamin E attenuates the toxic effects of intrastriatal injection of 6-hydroxydopamine (6-OHDA) in rats: behavioral and biochemical evidence. Brain Res. 1989;476(1):10–15. [DOI] [PubMed] [Google Scholar]
- 52. Pasbakhsh P, Omidi N, Mehrannia K, Gholi SA, Ragerdi KI, Abasi M, Kord VA. The protective effect of vitamin E on locus coeruleus in early model of Parkinson's disease in rat: immunoreactivity evidence. Iran Biomed J. 2008;12(4):217–22. [PubMed] [Google Scholar]
- 53. Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand. 1971;82(S367):69–93. [DOI] [PubMed] [Google Scholar]
- 54. Perumal A, Gopal V, Tordzro W, Cooper T, Cadet J. Vitamin E attenuates the toxic effects of 6-hydroxydopamine on free radical scavenging systems in rat brain. Brain Res Bull. 1992;29(5):699–701. [DOI] [PubMed] [Google Scholar]
- 55. Allan Butterfield D, Castegna A, Drake J, Scapagnini G, Calabrese V. Vitamin E and neurodegenerative disorders associated with oxidative stress. Nutr Neurosci. 2002;5(4):229–39. [DOI] [PubMed] [Google Scholar]
- 56. Ono K, Yamada M. Vitamin A potently destabilizes preformed α-synuclein fibrils in vitro: implications for Lewy body diseases. Neurobiol Dis. 2007;25(2):446–54. [DOI] [PubMed] [Google Scholar]
- 57. Shults CW, Oakes D, Kieburtz K, Beal MF, Haas R, Plumb S, Juncos JL, Nutt J, Shoulson I, Carter J. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59(10):1541–50. [DOI] [PubMed] [Google Scholar]
- 58. Jimenez-Del-Rio M, Guzman-Martinez C, Velez-Pardo C. The effects of polyphenols on survival and locomotor activity in Drosophila melanogaster exposed to iron and paraquat. Neurochem Res. 2010;35(2):227–38. [DOI] [PubMed] [Google Scholar]
- 59. Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JP. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr. 2008;3(3-4):115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vauzour D, Ravaioli G, Vafeiadou K, Rodriguez-Mateos A, Angeloni C, Spencer JP. Peroxynitrite induced formation of the neurotoxins 5-S-cysteinyl-dopamine and DHBT-1: implications for Parkinson's disease and protection by polyphenols. Arch Biochem Biophys. 2008;476(2):145–51. [DOI] [PubMed] [Google Scholar]
- 61. Levites Y, Youdim MB, Maor G, Mandel S. Attenuation of 6-hydroxydopamine (6-OHDA)-induced nuclear factor-kappaB (NF-κB) activation and cell death by tea extracts in neuronal cultures. Biochem Pharmacol. 2002;63(1):21–9. [DOI] [PubMed] [Google Scholar]
- 62. Levites Y, Amit T, Youdim MB, Mandel S. Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (−)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem. 2002;277(34):30574–80. [DOI] [PubMed] [Google Scholar]
- 63. Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006;545(1):51–64. [DOI] [PubMed] [Google Scholar]
- 64. von Arnim CA, Herbolsheimer F, Nikolaus T, Peter R, Biesalski HK, Ludolph AC, Riepe M, Nagel G, ActiFE Ulm Study Group . Dietary antioxidants and dementia in a population-based case-control study among older people in South Germany. J Alzheimers Dis. 2012;31(4):717–24. [DOI] [PubMed] [Google Scholar]
- 65. Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, Cotman C, Cottrell B, Montine TJ, Thomas RG. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch Neurol. 2012;69(7):836–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fahn S. An open trial of high-dosage antioxidants in early Parkinson's disease. Am J Clin Nutr. 1991;53(1):380S–2S. [DOI] [PubMed] [Google Scholar]
- 67. Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2014;29(13):1583–90. [DOI] [PubMed] [Google Scholar]
- 68. Carr AC, Lykkesfeldt J. Discrepancies in global vitamin C recommendations: a review of RDA criteria and underlying health perspectives. Crit Rev Food Sci Nutr. 2021;61(5):742–55. [DOI] [PubMed] [Google Scholar]
- 69. Hemilä H. Vitamin E and mortality in male smokers of the ATBC study: implications for nutritional recommendations. Front Nutr. 2020;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stepaniak U, Micek A, Grosso G, Stefler D, Topor-Madry R, Kubinova R, Malyutina S, Peasey A, Pikhart H, Nikitin Y. Antioxidant vitamin intake and mortality in three Central and Eastern European urban populations: the HAPIEE study. Eur J Nutr. 2016;55(2):547–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.