Abstract
Background & Objective
The COVID-19 pandemic imposed global concern and became one of the deadliest pandemics of the twenty-first century. Several vaccines were developed against SARS-CoV-2 to counteract the effects of this virus. This study aims to determine the post-vaccination side effects of the most common COVID-19 vaccines used in the Eastern province of Saudi Arabia.
Methods
This is a cross-sectional study using an online questionnaire distributed randomly through social media. Frequencies were calculated to determine participants’ demographic information, vaccination details, and post-vaccination side effects. Univariate and multiple regression analysis were applied to test the association between individuals’ willingness to receive a booster dose and different categorical variables.
Results
A total of 1004 participants were included in the survey, of which 0.6%, 85.3% and 14.1% completed either one, two or three doses of the Pfizer mRNA vaccine and Oxford AstraZeneca vaccines, respectively. The similar common side effects between the first and the second doses were significantly associated with the type of vaccine received; these included fatigue (Pfizer 54.4%, Oxford 73.2%; p < 0.001), headache (Pfizer 33.2%, Oxford 44.7%; p = 0.002), and fever (Pfizer 25.1%, Oxford 57.6%; p < 0.001). Additionally, unusual side effects were also reported (palpitations and menstrual abnormalities). Getting SARS-CoV2 infection after vaccination was significantly associated with the type of vaccine received at the first dose (Chi-Square=5.496, p = 0.019). A statistically significant association was found between the individuals’ willingness to receive a booster dose and their gender (Chi-Square = 39.493, p < 0.001), age (Chi-Square = 11.668, p = 0.02), presence of allergies (Chi-Square = 5.602, p = 0.018), and previous COVID-19 infection (Chi-Square = 9.495, p = 0.002).
Conclusion
Despite the described side effects, further studies should be done to investigate the unusual and rare side effects to assess COVID-19 vaccines effectiveness and safety over longer period of time within a more diverse population.
Keywords: SARS-CoV-2, Pfizer-BioNTech vaccine, Oxford-AstraZeneca vaccine, side effects
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is, by far, one of the most concerning pandemics humans have witnessed in the twenty-first century.1 It first emerged as several pneumonia cases from an unknown origin in Wuhan, China. By the beginning of January 2020, 41 pneumonia cases in Wuhan were admitted into a hospital, and all were confirmed as carrying a novel coronavirus (2019-nCoV). Afterwards, the virus was identified to be genetically similar to the Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), and was officially named SARS-CoV-2.2,3 Subsequently and despite all the efforts exerted by the Chinese government to confine the virus, it started to spread all over the globe.4 Up to March 2022, the number of confirmed cases globally has reached more than 490 million, with approximately 6.15 million deaths.5
After the World Health Organization (WHO) declared COVID-19 a pandemic in March 2020, the number of infected cases increased swiftly.6 This high surge of cases sparked an international response. Reflecting on the lessons learned from other coronavirus outbreaks, such as the Middle Eastern Respiratory Syndrome Coronavirus (MERS-CoV) and the Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), the WHO recommended the public practice frequent hand hygiene, wear masks, and keep a social distance of at least one meter.7,8 Since the first cases were all travel related in Saudi Arabia, strict preventive strategies were applied immediately to minimize the spread of virus, such as cancelling all international flights, closing the borders, and lockdown.
Despite all the preventive measures, the coronavirus pandemic continued to challenge the health care and economic systems internationally, provoking the implementation of more effective measures to help eradicate this deadly virus.9 Across the globe, work was directed towards developing new vaccines that would gain more control over the virus. Different companies in developed countries competed to develop a vaccine, but only some of them were approved for use.3
In December 2020, the vaccination program in Saudi Arabia was initiated when the mRNA-based vaccine from Pfizer-BioNTech was approved for use. However, at first it was only targeted at individuals with a high risk of severe COVID-19 complications, such as the elderly and people with chronic diseases. Later in February 2021, the viral vector-based vaccine from Oxford-AstraZeneca was approved for use, increasing the number of vaccine doses administrated all over the country.10 Initially, two doses of vaccine were required for all Saudi citizens and residents to be considered immune, but by the end of 2021 a third booster dose was mandated to enhance immunity against all SARS-CoV2 variants (eg, alpha, beta, gamma, delta and omicron).11 As of March 2022, the number of vaccine doses administered in Saudi Arabia reached over 60 million.12
A few international and local studies have been published reporting some of the side effects of COVID-19 vaccines experienced by recipients. The most common side effects include fatigue, fever, and injection site pain with slight variations depending on the type of vaccine (ie, mRNA or viral vector).13,14
Since the COVID-19 vaccines are relatively new, closely monitoring the side effects is necessary to ensure the safety of vaccinated individuals. Therefore, vaccine recipients were required to report any side effects they experienced during the first week post-vaccination either through the Sehaty (my health) Application (ie, the same App where people register for the vaccine) or through the Ministry of Health (MOH)’s hotline (937), especially further onset of side effects or the development of severe side effects.10
This study conducted an online-based questionnaire to explore the common and rare side effects of the COVID-19 vaccines available in Saudi Arabia. We discuss their effectiveness, as well as the willingness of individuals to receive a third booster dose.
Methods
This cross-sectional retrospective study was conducted in the Eastern province of Saudi Arabia. An online questionnaire (both Arabic and English versions) was prepared using Google Forms. It was randomly distributed targeting all age groups included in the Saudi’s MOH vaccination program through social media (WhatsApp). Saudi Arabia’s MOH vaccination program was launched on the 17th of December 2020 and this study was conducted from the 4th of December 2021 to the 28th of December 2021. Participants from all age groups were required to give their consent in the first page of the questionnaire by answering a question that includes their agreement to participate in the study to move into the next section of the questionnaire, so all data collection was conducted after informed consent was obtained.
The study questionnaire was divided into four main sections. The first section collected general information about the participants, such as age, gender, place of residence, chronic diseases, allergies, whether they have been infected previously with COVID-19, number of vaccine doses received, and their willingness to take the third (booster) dose. The last three sections were structured similarly, and collected information about the first, second and third doses of COVID-19 vaccine. This included the type of vaccine administrated, any side effects that occurred and their time of onset, the development of COVID-19 infection post-vaccination, and if hospitalization was required. In the side effects subsection of the questionnaire, 21 side effects were included because they were reported by other studies.13 These included pain at site of injection, swelling at site of injection, redness at site of injection, fatigue, headache, fever, among other things. An additional subsection was included to allow the participants to report other unlisted side effects that they may have experienced.
Ethical approval was obtained before starting the study from the Institutional Research Review and Ethics committee at Imam Abdulrahman Bin Faisal University on November 21, 2021 (UGS-2021-01-430).
Inclusion Criteria
This study included participants from all cities of Eastern province who were aged 12 years and above.
Exclusion Criteria
All participants who were not from the Eastern province of Saudi Arabia were excluded from the study.
Sample Size
Given that the actual percentage of vaccinated individuals in Saudi Arabia is 75%, we calculated that a sample size of 288 would be sufficient to give a 95% confidence interval with a 5% margin of error. The sample size was double checked and confirmed with the online sample size calculator Raosoft. After the questionnaire distribution, 1051 responses were received, all the 1051 participants were willing to take part in the study. However, to reduce sampling bias and to increase the statistical power of the study, this number was lowered to 1018 after the exclusion of responses based on the above-mentioned exclusion criteria.
Statistical Analysis
Participants’ responses were cross-checked for errors. Afterwards, the data were analyzed using IBM Statistical Package for the Social Sciences (SPSS, version 28) with a significance level of p ≤ 0.05. Various descriptive analysis tests were used to analyze and present the data.
Frequencies and percentages were calculated to determine participants’ demographics and the reported side effects of each dose of COVID-19 vaccine. Pearson’s Chi-square test was used to test for statistically significant associations between categorical variables, and the likelihood ratio was applied to variables with an expected cell count less than 5. Furthermore, a multiple regression analysis was performed to identify significant predictors of willingness among participants to receive the third (booster) vaccine dose.
Results
The raw data included a total of 1018 participants answering the survey. Females accounted for 61.7% of the participants. Participants were 12 years old or older, and placed into different age group categories: 12–17 (3.2%), 18–29 (23.5%), 30–49 (41.7%), 50–64 (29.6%), and older than 65 years (2.1%). A majority of subjects (75.8%) declared not having any chronic illness, while remaining participants disclosed having diabetes (6%), hypertension (8%), and asthma (2.4%). Nearly 11% of the participants had allergies. Out of the 1018 subjects; 227 (22.3%) were previously infected with SARS-CoV-2 before vaccination, 866 (85.1%) received two doses of COVID-19 vaccine, and 143 (14%) completed three doses (Table 1).
Table 1.
Participants Demographic Information
| Variable | Frequency | % | |
|---|---|---|---|
| Age Groups | 12–17 | 33 | 3.2 |
| 18–29 | 239 | 23.5 | |
| 30–49 | 424 | 41.7 | |
| 50–65 | 301 | 29.6 | |
| Older than 65 | 21 | 2.1 | |
| Sex | Male | 390 | 38.3 |
| Female | 628 | 61.7 | |
| Chronic disease | None | 772 | 75.8 |
| Diabetes | 59 | 6 | |
| Hypertension | 79 | 8 | |
| Immunodeficiency | 11 | 11.2 | |
| Asthma | 24 | 2.4 | |
| Heart disease | 13 | 1.3 | |
| Obesity | 33 | 3.3 | |
| Cancer | 7 | 0.7 | |
| Renal disease | 2 | 0.2 | |
| Allergies | No allergy | 904 | 88.8 |
| Food | 48 | 4.7 | |
| Drug | 23 | 2.3 | |
| Skin | 13 | 1.3 | |
| Other | 6 | 0.6 | |
| Infected with SARS-CoV-2 before vaccination | Yes | 227 | 22.3 |
| No | 791 | 77.7 | |
| Doses of COVID-19 vaccine | 0 | 3 | 0.3 |
| 1 | 6 | 0.6 | |
| 2 | 866 | 85.1 | |
| 3 | 143 | 14 |
Prevalence of Side Effects Associated with COVID-19 Vaccines
A total of 1004 participants were included in the further statistical data analysis. Few participants were excluded since they either did not receive any COVID-19 vaccines (3 participants only) or received Moderna vaccine (11 participants only).
Among the three vaccination doses, Pfizer was the vaccine received by the most participants (Table 2). Participants who completed one, two and three vaccine doses accounted for 0.6%, 85.3%, and 14.1%, respectively.
Table 2.
The Number of Participants Who Completed Different Types and Doses of COVID-19 Vaccine and the Frequency of Their Associated Side Effects
| No. of Participants | Side Effects | |
|---|---|---|
| 1st dose | 989 (answered) | Yes (77.5%) No (22.5%) |
| Pfizer | 772 (78.1%) | Yes 662 (85.8%) No 110 (14.2%) |
| Oxford | 217 (21.9%) | Yes 199 (91.7%) No 18 (8.3%) |
| 2nd dose | 978 (answered) | Yes (87.2%) No (12.8%) |
| Pfizer | 816 (83.4%) | Yes 644 (79%) No 172 (21%) |
| Oxford | 162 (16.6%) | Yes 114 (70.4%) No 48 (29.6%) |
| 3rd dose | 144 | Yes (76.4%) No (23.6%) |
| Pfizer | 142 (98.6%) | Yes 108 (76%) No 34 (24%) |
| Oxford | 2 (1.4%) | Yes 2 (100%) No 0 (0%) |
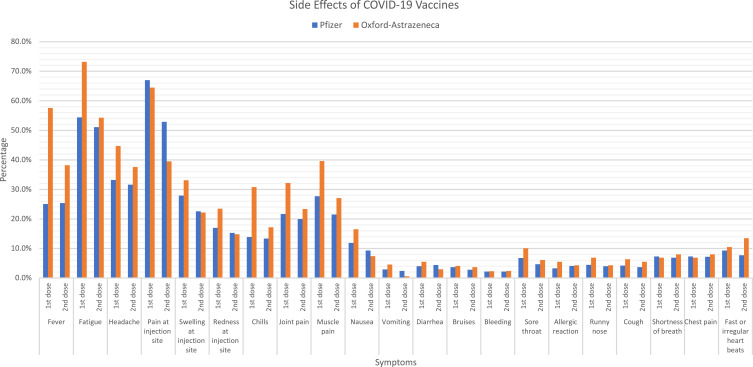
For the first dose of vaccine, 989 participants answered the questions related to the side effects. Of these participants, 772 and 217 received the Pfizer and Oxford vaccines, respectively. Most Pfizer vaccine receivers experienced side effects after the first dose (n = 662, 85.8%) (Table 3, Figure 1). The most reported side effects were side effects local to the inject site, such as pain (n = 518, 67%), redness (n = 132, 17%) and swelling (n = 216, 27.9%), as well as systemic side effects, such as fatigue (n = 420, 54.4%), fever (n = 194, 25.1%) and headache (n = 257, 33.2%). The majority of those who received the Oxford vaccine experienced side effects (n = 199, 91.7%). The most reported side effects were similar to the Pfizer vaccine; these included injection site side effects, such as pain (n = 140, 64.5%), redness (n = 51, 23.5%) and swelling (n = 72, 33.1%), as well as fatigue (n = 159, 73.2%), fever (n = 125, 57.6%) and headache (n = 97, 44.7%). These side effects were mostly experienced within the first three days post-vaccination (data not shown). Moreover, univariate analysis examined the association between each of the side effects declared and the type of vaccine taken. Results showed a significant association between the side effects fatigue (p < 0.001), fever (p < 0.001), headache (p = 0.002), chills (p < 0.001), joint pain (p = 0.001) and muscle pain (p = 0.001) with the type of vaccine received.
Table 3.
The Different Side Effects Reported in All 3 Doses and Their Correlation with the Type of Vaccine After the First and Second Dose
| Side Effects | First Dose | P value | Second Dose | P value | Third Dose | |||
|---|---|---|---|---|---|---|---|---|
| Pfizer | Oxford | Pfizer | Oxford | Pfizer | Oxford | |||
| N=772 | N=217 | N=816 | N=162 | N=142 | N=2 | |||
| Local side effects, n (%) | ||||||||
| Swelling at injection site | 216 (27.9) | 72 (33.1) | 0.136 | 185 (22.6) | 36 (22.2) | 0.901 | 34 (23.9) | 1 (50) |
| Redness at injection site | 132 (17) | 51 (23.5) | 0.032 | 125 (15.3) | 24 (14.8) | 0.871 | 23 (16.1) | 1 (50) |
| Pain at injection site | 518 (67) | 140 (64.5) | 0.476 | 432 (52.9) | 64 (39.5) | 0.002 | 66 (46.4) | 1 (50) |
| Systemic side effects | ||||||||
| Fatigue | 420 (54.4) | 159 (73.2) | 0.000 | 417 (51.1) | 88 (54.3) | 0.454 | 60 (42.2) | 2 (100) |
| Fever | 194 (25.1) | 125 (57.6) | 0.000 | 208 (25.4) | 62 (38.2) | 0.001 | 37 (26) | 2 (100) |
| Headache | 257 (33.2) | 97 (44.7) | 0.002 | 258 (31.6) | 61 (37.6) | 0.134 | 38 (26.7) | 2 (100) |
| Joint pain | 168 (21.7) | 70 (32.2) | 0.001 | 164 (20) | 38 (23.4) | 0.335 | 25 (17.6) | 1 (50) |
| Muscle pain | 214 (27.7) | 86 (39.6) | 0.001 | 176 (21.5) | 44 (27.1) | 0.119 | 30 (21.1) | 1 (50) |
| Nausea | 92 (11.9) | 36 (16.5) | 0.07 | 76 (9.3) | 12 (7.4) | 0.439 | 7 (4.9) | 0 |
| Vomiting | 23 (2.9) | 10 (4.6) | 0.238 | 20 (2.4) | 1 (0.6) | 0.040* | 3 (2.1) | 0 |
| Diarrhea | 31 (4) | 12 (5.5) | 0.334 | 36 (4.4) | 5 (3) | 0.442 | 5 (3.5) | 0 |
| Bruises | 29 (3.7) | 9 (4.1) | 0.791 | 23 (2.8) | 6 (3.7) | 0.556* | 2 (1.4) | 0 |
| Bleeding | 17 (2.2) | 5 (2.3) | 0.929* | 18 (2.2) | 4 (2.4) | 0.838* | 0 | 0 |
| Chills | 108 (13.9) | 67 (30.8) | 0.000 | 110 (13.4) | 28 (17.2) | 0.204 | 13 (9.1) | 1 (50) |
| Sore throat | 53 (6.8) | 22 (10.1) | 0.108 | 39 (4.7) | 10 (6.1) | 0.458 | 2 (1.4) | 0 |
| Allergic reaction | 26 (3.3) | 12 (5.5) | 0.143 | 34 (4.1) | 7 (4.3) | 0.929 | 0 | 0 |
| Runny nose | 35 (4.5) | 15 (6.9) | 0.158 | 33 (4) | 7 (4.3) | 0.871 | 1 (0.7) | 0 |
| Cough | 33 (4.2) | 14 (6.4) | 0.183 | 31 (3.7) | 9 (5.5) | 0.302 | 0 | 0 |
| Shortness of breath | 57 (7.3) | 15 (6.9) | 0.813 | 57 (6.9) | 13 (8) | 0.639 | 2 (1.4) | 0 |
| Chest pain | 57 (7.3) | 15 (6.9) | 0.813 | 59 (7.2) | 13 (8) | 0.724 | 1 (0.7) | 0 |
| Fast or irregular heartbeat | 72 (9.3) | 23 (10.5) | 0.574 | 63 (7.7) | 22 (13.5) | 0.016 | 1 (0.7) | 0 |
Notes: *For this test, the number of expected count was lower than 5 for Pearson’s chi square test. Therefore, likelihood ratio was considered. The numbers in bold indicate the P values for which the side effects are significantly correlated to the type of vaccine.
Figure 1.
The declared side effects of COVID-19 vaccines (Pfizer and Oxford) after the first and second dose.
Focusing on the second dose of vaccine, 978 participants answered the questions related to the side effects. Of these participants, 816 and 162 received the Pfizer and Oxford vaccine, respectively. Side effects were reported by 644 of Pfizer vaccine receivers (79%) and 114 of Oxford vaccine receivers (70.4%) (Table 3, Figure 1). The side effects that were reported, pain at injection site (n = 432, 52.9%), fatigue (n = 417, 51.1%), headache (n = 258, 31.6%) and fever (n = 208, 25.4%) were most common among participants that received the Pfizer vaccine. As for those who received the Oxford vaccine, they commonly reported fatigue (n = 88, 54.3%), pain at the injection site (n = 64, 39.5%), fever (n = 62, 38.2%) and headache (n = 61, 37.6%). Similar to the first dose, the side effects related to the second dose most commonly occurred within the first 3 days post-vaccination (data not shown). Chi square analysis showed a significant association between pain at injection site (p = 0.002), fever (p = 0.001), and vomiting (p = 0.04) with the type of vaccine received for the second dose.
At the time of survey distribution (end of December 2021), only 144 participants received the third vaccine dose. Almost all of them (142) received the Pfizer vaccine, and only 2 received Oxford vaccine (Table 3). Similar side effects to the first and second dose were reported, fatigue (n = 60, 42.2%), fever (n = 37, 26%) and pain at injection site (n = 66, 46.4%).
After receiving the first vaccine dose, infection with SARS-CoV2 was reported by 62 individuals, of which 41 participants received the Pfizer vaccine and 21 participants received the Oxford vaccine. Most of these participants (32 out of 62) were infected 1–3 months post-vaccination, and only two individuals (one Pfizer receiver and one Oxford receiver) were hospitalized with a severe infection (Table 4). Chi-square analysis showed a statistically significant association between the type of vaccine (ie, either Pfizer or Oxford) at the first dose and being infected with SARS-CoV2 afterwards (Chi-Square = 5.496, df = 1, p = 0.019).
Table 4.
Infection Post-Vaccination for the 1st and 2nd Dose (Pfizer and Oxford)
| Type of Vaccine | First Dose | Second Dose | ||
|---|---|---|---|---|
| Pfizer | Oxford | Pfizer | Oxford | |
| Total | n=772 | n=217 | n=816 | n=162 |
| Infection with SARS-CoV2 Post Vaccine | n (%) | |||
| No | 731(94.6) | 196 (90.3) | 786 (96.3) | 153 (94.5) |
| Yes | 41 (5.3) | 21 (9.6) | 30 (3.7) | 9 (5.5) |
| Duration | ||||
| Less than a month | 16 (39) | 7 (33.3) | 15 (50) | 7 (77.8) |
| 1–3 months | 20 (48.8) | 12 (57.2) | 5 (16.7) | 2 (22.2) |
| More than 3 months | 5 (1.22) | 2 (9.5) | 9 (30) | 0 |
| Required hospitalization | ||||
| Yes | 1 (2.4) | 1 (4.8) | 3 (10) | 1 (11.1) |
| No | 40 (97.6) | 20 (95.2) | 27 (90) | 8 (88.9) |
Compared to the first dose, fewer individuals (39 compared to 62) reported getting infected after receiving the second dose. Thirty of these participants received the Pfizer vaccine and 9 of these participants received the Oxford vaccine. The majority (22 out of 39) were infected less than a month after receiving the second dose, and 4 individuals required hospitalization due to a severe infection. The association between the type of vaccine administered for the second dose and post-vaccine infection with SARS-CoV2 was statistically insignificant (Chi-Square = 1.247, df = 1, p = 0.264).
Willingness to Receive Booster Dose
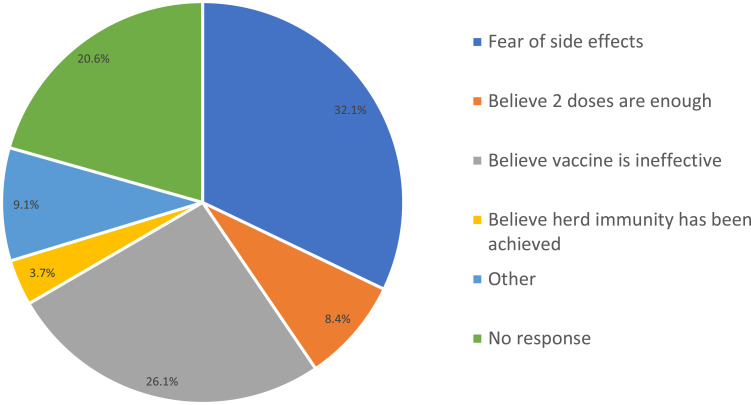
Investigating the acceptance of a booster vaccine dose, 61.7% of participants were willing to receive a booster vaccine dose, while 38.3% of participants were against it. The reported reasons for refusal included fear of side effects (32.1%), fears that the vaccine is ineffective (26.1%), ideology that the two doses is enough (8.4%), and ideology that herd immunity has been achieved (3.7%), as shown in Figure 2.
Figure 2.
Reasons behind the refusal of the booster dose.
Univariate analysis was performed to study the association between the willingness to receive a booster dose and participants’ gender, age, presence of chronic diseases, presence of allergy, and previous COVID-19 infection (Table 5). Results showed a statistically significant association between the willingness to take a booster dose and gender (Chi-Square = 39.493, p < 0.001), age (Chi-Square = 11.668, p = 0.02), presence of allergies (Chi-Square = 5.602, p = 0.018), and previous COVID-19 infection (Chi-Square = 9.495, p = 0.002). A significantly higher percentage of male participants (73.8%) were willing to receive a booster dose than female participants (54%). Focusing on age, most subjects older than 65 years (85.7%) were willing to receive a booster dose, whereas 43% of participants aged 30 to 49 years old refused to take the booster dose. Nearly half of participants with allergies refused booster doses (48.6%), while only 37.1% of participants without allergies refused booster doses. Among the participants who had a previous history of COVID-19 infection, 47.3% refused to take the booster dose compared to 35.8% of participants who had no history of infection. On the other hand, no significant association was found between having a chronic disease and acceptance of the booster dose (p = 0.058).
Table 5.
Correlation Between Willingness to Receive the Booster Dose and Gender, Age, Chronic Diseases, Allergy, Previous COVID-19 Infection
| Variable | Willing to Receive a Booster Dose | Chi-square (X2) | P-value | |
|---|---|---|---|---|
| Yes | No | |||
| n (%) | n (%) | |||
| Gender | ||||
| Female | 332 (54) | 286 (46.3) | 39.494 | <0.001 |
| Male | 287 (73.8) | 102 (26.2) | ||
| Age group (in years) | ||||
| 12–17 | 19 (57.6) | 14 (42.4) | 11.668 | 0.02 |
| 18–29 | 148 (62.7) | 88 (37.3) | ||
| 30–49 | 236 (57) | 178 (43) | ||
| 50–65 | 198 (66) | 102 (34) | ||
| Older than 65 | 18 (85.7) | 3 (14.3) | ||
| Chronic diseases | ||||
| No | 477 (60.2) | 316 (39.8) | 3.601 | 0.058 |
| Yes | 142 (67.3) | 69 (32.7) | ||
| Allergy | ||||
| Allergy present | 57 (51.4) | 54 (48.6) | 5.602 | 0.018 |
| No known allergy | 562 (62.9) | 331 (37.1) | ||
| Previous COVID-19 infection | ||||
| Yes | 116 (52.7) | 104 (47.3) | 9.495 | 0.002 (phi=0.098) |
| No | 503 (64.2) | 281 (35.8) | ||
A multiple regression analysis was conducted to determine if age, gender, presence of allergies, and history of previous COVID-19 infection can predict an individual’s acceptance of a booster vaccine dose. Results showed that 5% of the variance in willingness to receive a booster dose can be explained by the predictors (p < 0.001). Additionally, results showed that gender (p < 0.001) and history of previous COVID-19 infection (p = 0.003) can significantly predict and individuals’ willingness to receive the booster dose. Age and presence of allergies were not significant predictors, with p-values of 0.950 and 0.059, respectively.
Discussion
The vaccination program in Saudi Arabia was launched when the Ministry of Health (MOH) approved the Pfizer vaccine for use in December 2020, which was followed by Oxford-AstraZeneca vaccine approval in February 2021.10 As of March 2022, the number of vaccine doses administered in Saudi Arabia has reached over 60 million.12
Between April and July 2020, there was a large wave of COVID-19 cases that peaked at 4500 cases per day.5 After implementing the vaccination program in December 2020, cases started to decline, ranging from 100 to 300 cases per day between January and March of 2021.5 April through August 2021, the number of cases increased to 1200 per day.5 A second wave took place during January and February, when nearly 5900 cases were reported each day.5 While the number of cases spiked in early 2022, the case fatality rate (ie, the ratio between confirmed deaths and confirmed cases) declined from 1.7% in January 2022 to 1.2% in March 2022.5,15
When discussing the cons of receiving a COVID-19 vaccine, it is crucial to consider the side effects, since this is the most concerning aspect for the community. Thus, this survey was conducted to collect data about the side effects experienced by the residents of the eastern province of Saudi Arabia after administration of the approved COVID-19 vaccines.
In this study, the percentage of participants that experienced side effects after the first dose of Pfizer vaccine (85.8%) was slightly higher than those that experienced side effects following the second dose (79%). In addition, slightly different side effects were experienced after the second dose. Conversely, El-Shitany et al studied the side effects of Pfizer vaccine (in either the first or the second dose, or both) in Saudi Arabia residents and reported that the majority of side effects were experienced after the second dose; 90% of individuals experienced flu-like symptoms such as headache, fever, chills, and muscle pain, and 70–80% of individuals experienced local side effects, such as injection site pain.13
In our study, Oxford-AstraZeneca vaccine recipients primarily reported most side effects after the first dose (91.7%), and relatively less participates reported side effects after the second dose (70.4%). Similar results were found in a study conducted by Alhazmi et al in Jazan, Saudi Arabia. In this study, most of the participants only received the first dose (75% of them received the Oxford-AstraZeneca vaccine), and fatigue was the most reported side effect.14 On the other hand, a study conducted in Bangladesh assessing side effects after receiving at least one dose of Oxford-AstraZeneca vaccine found that the majority of participants experienced local side effects more than systemic ones.15,16
Our report on the side effects after both types, Pfizer and Oxford-AstraZeneca, of COVID-19 vaccine administration occurred concurrently with a study conducted by Almufty et al comparing the side effects of COVID-19 vaccines available in Iraq. This study found that the most common side effects reported by Oxford-AstraZeneca recipients were fatigue and fever, while Pfizer recipients most commonly reported injection site reaction.17,18 Similarly, GRZĄDKO1 et al studied the side effects of COVID-19 vaccines in Poland. The most reported side effects by Pfizer vaccine receivers and by Oxford-AstraZeneca vaccine receivers were related to the injection site and fatigue, respectively. However, in contrast to our findings, this study concluded that more side effects occurred after the second doses of the Pfizer and Oxford-AstraZeneca vaccines compared to the first doses.19
Similarly to our results, Kaura et al conducted a prospective observational study on Oxford-AstraZenca vaccine side effects in Indian health-care workers. That study reported systemic side effects (eg, fever) more than local (eg, injection site pain) with higher rates following the first dose.20 Also, our results are in accordance with what was initially discovered during the clinical trial phases. For the Pfizer vaccine, local side effects at the injection site were more common than systemic ones.21 On the contrary, for the Oxford-AstraZeneca vaccine, systemic side effects (eg, fatigue) were more common.22 Therefore, when comparing the side effects of the Pfizer and Oxford-AstraZeneca vaccines, it can be concluded that local side effects at the injection site were more common for those who received the Pfizer vaccine, whereas systemic side effects such as fatigue and fever were more common in Oxford-AstraZeneca vaccine receivers. This can be explained by the different immune system responses that each vaccine was built upon, mRNA for Pfizer and viral vector for Oxford-AstraZeneca.23
It is important to state that most of the common side effects reported by the participants in our study were the same as the side effects that MOH informed the Saudi citizens and residents about prior to registering for the vaccine through the Sehaty Application. In addition, awareness about possible side effects was raised through different platforms, such as social media and the MOH website. These side effects are also concurrent with the factsheets for recipients and caregivers distributed by the FDA (Food and Drug Administration) for the Pfizer vaccine, as well as the manufacture sheet for the Oxford-AstraZeneca vaccine.
Additionally, uncommon side effects were also reported in our study, which could raise serious warnings about the vaccines and should be investigated in future studies. One of these side effects was palpitations. Palpitations were also observed among participants in a study conducted by Alghamdi et al in Taif, Saudi Arabia. Most of their participants reported palpitations following the second dose of Oxford-AstraZeneca vaccine.24 Another study conducted by Singh et al reported 3 cases (2 females, 1 male) of myocarditis (inflammation of the heart muscle) after receiving Oxford-AstraZeneca vaccine. However, all the 3 cases had other co-morbidities which makes the direct relevance of vaccine to the development of myocarditis unclear.25 Montgomery et al reported a case series of 23 male members of the US military (22 were healthy) who were diagnosed with myocarditis within 4 days of receiving mRNA vaccine (eg, Pfizer) mainly following the second dose.26 It is worth to mention that the FDA did not include palpitations as one of the side effects of Pfizer vaccine when it was first approved for use. However, it was listed after some vaccine recipients, mostly males younger than 40 years of age, reported developing myocarditis and pericarditis (inflammation of the heart’s lining) as side effects of the Pfizer vaccine.27
Another rare but interesting side effect reported by a few female participants was changes in their menstrual cycle. A total of 18 females (1.82%) experienced menstrual changes after receiving the first vaccine dose, of these 15 females received Pfizer vaccine and 3 females received the Oxford vaccine. After the second dose of the Pfizer vaccine, 15 females (1.53%) reported menstrual disturbances. These changes were mainly irregular cycles, increased length of menstrual cycle, and increased menstrual flow. A study conducted by Alghamdi et al also reported participants experiencing abnormal menstruation, and the number of cases was also higher among females that received the Pfizer vaccine (18 cases) compared to the Oxford vaccine (7 cases).24 Menstrual cycles can be influenced by several factors, including hormones, stress, and weight changes.28 Vaccination stimulates an immune response, which may affect the hypothalamus-pituitary-ovarian axis, resulting in menstrual changes.28,29 Most of the menstrual changes reported were usually temporary, and many women reported that their menses returned to normal during the following cycle.29,30 A cohort study found that changes in menstrual cycle length were not associated with COVID-19 vaccination.31 Therefore, these changes cannot be directly attributed to the COVID-19 vaccines because of the relatively low number of reported menstrual changes following COVID-19 vaccine administration compared to the total number of vaccines given and the prevalence of menstrual disorders in general.29,30 Since menstrual disturbances are common and can be influenced by many factors, further research is needed to study its possible association with COVID-19 vaccines.
In the current study, the incidence of SARS-CoV2 infection post-vaccination with either the Pfizer or Oxford-AstraZeneca vaccines was more common after the first dose than the second dose, and only a small percentage required hospitalization due to severe infection. This result is supported by the fact that the efficacy of a vaccine increases with each dose. Each booster dose increases the duration of immunity and strengthens the protection from infection, lowering the risk of hospitalization and death from severe disease.32 Similarly, a study on health-care workers revealed lower rates of COVID-19 infection after receiving two doses of vaccine compared with only one dose (18.9% compared with 41.5%). This study also found that individuals who were fully vaccinated had a reduced infection severity than those who were partially vaccinated.33 Another study showed a significant reduction in the rates of moderate to severe infection after 2 doses of vaccine compared to the unvaccinated group (1.2% vs 3.4%).34 In our study, the rate of COVID-19 infection after 2 doses of vaccine (3.9%) is lower than that reported by Kaur et al (18.9%) and by Satwik et al (12%).33,34 This discrepancy may be explained by the presence of different variants of SARS-CoV-2 in different regions.33 These studies were also conducted on health-care workers, who have higher exposure due to the nature of their jobs.
Regarding the willingness to receive COVID-19 vaccine a few local and international studies were published such as Mahmud et al and Al-Hanawi et al, both were conducted in Saudi Arabia when the vaccination program was just initiated and have not been mandated yet.35,36 In our study, the concern was the willingness to receive a third booster dose since it has not been mandated yet during the time of conducting the study. Out of 1004 participants, 61.7% were willing to receive booster (third) doses, and the remaining 38.3% were hesitant. These percentages were similar to the vaccine acceptance and refusal rates (61.8% and 38.2%, respectively) reported in a study done in the United States, and slightly lower than what was found in Poland (71%) and China (75.2%).37–39 The two most common reasons for refusal among our participants were the fear of side effects and vaccine ineffectiveness, which are similar to those described in other studies.38,40
Gender and previous COVID-19 infection were significant predictors of booster dose acceptance. Participants who had been infected with SARS-CoV-2 were less accepting of the booster dose compared to participants without a history of COVID-19 infection. Similarly, Rzymski et al found that booster dose refusal was significantly higher among subjects with a previous history of COVID-19 infection, especially when the infection occurred after at least one dose of the vaccine.38 Moreover, our survey showed that female participants were more hesitant than males to receive the booster dose, similar to other study results.37,39 However, conversely, Rzymski et al found that booster acceptance was significantly higher in females.38 Whereas, no significant association between gender and acceptance of the booster dose was reported in another study.31
Age was not a significant predictor of willingness to receive the booster dose, according to our results. However, a separate study found that acceptance of the booster dose increases with age (adjusted odds ratio per year: 1.04, 95% confidence interval: 1.02–1.06).31 Our participants within the age range of 30–49 years had the highest refusal percentage (43%), while 85.7% of participants older than 65 years were willing to receive the booster dose. On the other side, another study showed the highest acceptance rate of COVID-19 booster vaccines in adults between 45 and 54 years of age (81.2%), while the lowest acceptance rate was found in adults over 65 years of age (69.6%).29 Finally, we found no significant association between the presence of chronic disease and the acceptance of the booster vaccine, which was contradictory to another study that found that the willingness to receive the booster dose was significantly higher among individuals who had a chronic illnesses.28
Conclusion
This survey study elucidated the common and rare side effects of the available COVID-19 vaccines in the eastern province of Saudi Arabia and discussed the vaccine effectiveness.
COVID-19 vaccines were developed and approved after the initiation of the SARS-CoV-2 pandemic. Although the most common side effects were known and clearly stated by the manufacturers, more research is needed on the side effects and their long-term consequences.
Despite the benefits of this survey and its possible application in further studies, this study can be extended on a longer period of time to get bigger sample size in order to cope with the big percentage of vaccinated population living in Saudi Arabia. Moreover, some of the exclusion criteria and survey tools could be changed to enlarge the number of participants and their diversity. Consequently, a bigger and diversified sample size would avoid low counts in certain categories and would allow a better comparison between different approved vaccines.
Our survey is one of the few studies that explored the side effects of two of the most administrated COVID-19 vaccines in Saudi Arabia (Pfizer mRNA vaccine and Oxford AstraZeneca vaccines). Most of the side effects reported in our study were among the most common and known side effects worldwide. However, our study also reported a few rare side effects that need further investigation to assess the COVID-19 vaccines effectiveness and safety over longer periods of time within a more diverse population.
Acknowledgments
This work was carried out as a non-funded project at Imam Abdulrahman bin Faisal university. The authors acknowledge the University for the support. This paper could not be completed without the supervising of Prof. Maha Farhat and the extreme effort and cooperation of medical students: Rabab Al-Ibrahim, Abrar Almohammedali, Roaa Aljishi, Baneen Alalwan.
Declarations
All authors declare that this study complies with the Declaration of Helsinki.
Ethics Approval and Consent to Participate
All participants above 18 years of age gave informed consent prior to participation in the survey.
All participants under 18 years of age were approved by the ethics committee to provide informed consent on their own behalf.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Roychoudhury S, Das A, Sengupta P, et al. Viral pandemics of twenty-first century. J Microbiol Biotechnol Food Sci. 2021;10:711–716. doi: 10.15414/jmbfs.2021.10.4.711-716 [DOI] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al.Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haque A, Pant AB. Efforts at COVID-19 vaccine development: challenges and successes. Vaccines. 2020;8(4):739. doi: 10.3390/vaccines8040739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Algaissi AA, Alharbi NK, Hassanain M, Hashem AM. Preparedness and response to COVID-19 in Saudi Arabia: building on MERS experience. J Infect Public Health. 2020;13(6):834–838. doi: 10.1016/j.jiph.2020.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Coronavirus (COVID-19) dashboard. Available from: https://covid19.who.int. Accessed September 19, 2021.
- 6.WHO Director-General’s opening remarks at the media briefing on COVID-19-11 March 2020. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020. Accessed September 19, 2021.
- 7.Jee Y. WHO international health regulations emergency committee for the COVID-19 outbreak. Epidemiol Health. 2020;42:e2020013. doi: 10.4178/epih.e2020013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khanna RC, Cicinelli MV, Gilbert SS, Honavar SG, Murthy GVS. COVID-19 pandemic: lessons learned and future directions. Indian J Ophthalmol. 2020;68(5):703–710. doi: 10.4103/ijo.IJO_843_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mofijur M, Fattah IMR, Alam MA, et al. Impact of COVID-19 on the social, economic, environmental and energy domains: lessons learnt from a global pandemic. Sustain Prod Consum. 2021;26:343–359. doi: 10.1016/j.spc.2020.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assiri A, Al-Tawfiq JA, Alkhalifa M, et al. Launching COVID-19 vaccination in Saudi Arabia: lessons learned, and the way forward. Travel Med Infect Dis. 2021;43:102119. doi: 10.1016/j.tmaid.2021.102119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministry of Health Saudi Arabia. الصحة, ف. ب. و. [MOH]. Available from: https://www.moh.gov.sa/en/Pages/Default.aspx. Accessed April 09, 2022.
- 12.covidvax.live: live COVID-19 Vaccination Tracker - See vaccinations in real time! Available from: http://covidvax.live/location/sau. Accessed April 09, 2022.
- 13.El-Shitany NA, Harakeh S, Badr-Eldin SM, et al. Minor to moderate side effects of Pfizer-BioNTech COVID-19 vaccine among Saudi residents: a retrospective cross-sectional study. Int J Gen Med. 2021;14:1389–1401. doi: 10.2147/IJGM.S310497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alhazmi A, Alamer E, Daws D, et al. Evaluation of side effects associated with COVID-19 vaccines in Saudi Arabia. Vaccines. 2021;9(6):674. doi: 10.3390/vaccines9060674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our World Data. 2020.
- 16.Sultana A, Shahriar S, Tahsin MR, et al. A retrospective cross-sectional study assessing self-reported adverse events following immunization (AEFI) of the COVID-19 vaccine in Bangladesh. Vaccines. 2021;9(10):1090. doi: 10.3390/vaccines9101090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almufty, H. B.; Mohammed, S. A.; Abdullah, A. M.; Merza, M. A. Potential Adverse Effects of COVID19 Vaccines among Iraqi Population; a Comparison between the Three Available Vaccines in Iraq; a Retrospective CrossSectional Study. Diabetes Metab. Almufty, H. B.; Mohammed, S. A.; Abdullah, A. M.; Merza, M. A. Potential Adverse Effects of COVID19 Vaccines among Iraqi Population; a Comparison between the Three Available Vaccines in Iraq; a Retrospective CrossSectional Study. Diabetes Metab. Syndr. 2021;15(5):102207. doi: 10.1016/j.dsx.2021.102207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrzejczak-Grządko S, Czudy Z, Donderska M. Side effects after COVID-19 vaccinations among residents of Poland. Eur Rev. 2021;25:4418–4421. [DOI] [PubMed] [Google Scholar]
- 19.Kaur U, Ojha B, Pathak BK, et al. A prospective observational safety study on ChAdOx1 NCoV-19 Corona virus vaccine (recombinant) use in healthcare workers- first results from India. EClinicalMedicine. 2021;38:101038. doi: 10.1016/j.eclinm.2021.101038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polack FP, Thomas SJ, Kitchin N, et al.Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. NEJM. 2020. doi: 10.1056/nejmoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falsey AR, Sobieszczyk ME, Hirsch I, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 NCoV-19) Covid-19 vaccine. N Engl J Med. 2021;385(25):2348–2360. doi: 10.1056/NEJMoa2105290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funk CD, Laferrière C, Ardakani A. Target product profile analysis of COVID-19 vaccines in phase III clinical trials and beyond: an early 2021 perspective. Viruses. 2021;13(3):418. doi: 10.3390/v13030418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alghamdi AN, Alotaibi MI, Alqahtani AS, Al Aboud D, Abdel-Moneim AS. BNT162b2 and ChAdOx1 SARS-CoV-2 post-vaccination side-effects among Saudi vaccinees. Front Med. 2021;8. doi: 10.3389/fmed.2021.760047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh R, Chakrabarti SS, Gambhir IS, et al. Acute cardiac events after ChAdOx1 NCoV-19 Corona virus vaccine: report of three cases. Am J Ther. 2022;29:e579–e585. doi: 10.1097/MJT.0000000000001472 [DOI] [PubMed] [Google Scholar]
- 25.Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with MRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202–1206. doi: 10.1001/jamacardio.2021.2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Food and Drug Administration. Comirnaty and Pfizer-Biontech COVID-19 Vaccine. US Food and Drug Administration; 2022. [Google Scholar]
- 27.Kurdoğlu Z. Do the COVID-19 vaccines cause menstrual irregularities? Int J Womens Health Reprod Sci. 2021;9(3):158–159. doi: 10.15296/ijwhr.2021.29 [DOI] [Google Scholar]
- 28.Male V. Menstrual changes after Covid-19 vaccination. BMJ. 2021;374:n2211. doi: 10.1136/bmj.n2211 [DOI] [PubMed] [Google Scholar]
- 29.Coronavirus vaccine - weekly summary of Yellow Card reporting. GOV.UK. Available from: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting. Accessed March 13, 2022.
- 30.Edelman A, Boniface ER, Benhar E, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a U.S. cohort. Obstet Gynecol. 2022;139:481–489. doi: 10.1097/AOG.0000000000004695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall V, Hopkins S. COV-BOOST: evidence to support rapid booster deployment. Lancet. 2021;398(10318):2209–2211. doi: 10.1016/S0140-6736(21)02799-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaur U, Bala S, Ojha B, Jaiswal S, Kansal S, Chakrabarti SS. Occurrence of COVID-19 in priority groups receiving ChAdOx1 NCoV-19 Coronavirus vaccine (recombinant): a preliminary analysis from North India. J Med Virol. 2022;94(1):407–412. doi: 10.1002/jmv.27320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satwik R, Satwik A, Katoch S, Saluja S. ChAdOx1 NCoV-19 effectiveness during an unprecedented surge in SARS COV-2 infections. Eur J Intern Med. 2021;93:112–113. doi: 10.1016/j.ejim.2021.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Hanawi MK, Ahmad K, Haque R, Keramat SA. Willingness to receive COVID-19 vaccination among adults with chronic diseases in the Kingdom of Saudi Arabia. J Infect Public Health. 2021;14(10):1489–1496. doi: 10.1016/j.jiph.2021.08.002 [DOI] [PubMed] [Google Scholar]
- 35.Kabir R, Mahmud I, Chowdhury MTH, et al. COVID-19 vaccination intent and willingness to pay in Bangladesh: a cross-sectional study. Vaccines. 2021;9(5):416. doi: 10.3390/vaccines9050416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rzymski P, Poniedziałek B, Fal A. Willingness to receive the booster COVID-19 vaccine dose in Poland. Vaccines. 2021;9(11):1286. doi: 10.3390/vaccines9111286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Dean J, Yin Y, et al. Determinants of COVID-19 vaccine acceptance and hesitancy: a health care student-based online survey in Northwest China. Front Public Health. 2022;9. doi: 10.3389/fpubh.2021.777565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yadete T, Batra K, Patros MJ, Patros MJ, Bester JC, Bester JC. Assessing acceptability of COVID-19 vaccine booster dose among adult Americans: a cross-sectional study | HTML. Vaccines. 2021;9. doi: 10.3390/vaccines9121424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Liu L, Pei M, Li X, Li N. Willingness of the general public to receive A COVID-19 vaccine booster — China, April–May 2021. China CDC Wkly. 2022;4(4):66–70. doi: 10.46234/ccdcw2022.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sønderskov KM, Vistisen HT, Dinesen PT, Østergaard SD. COVID-19 booster vaccine willingness. Ugeskriftet.dk. 2021.