Abstract
Intracellular pathogenic bacteria, including Mycobacterium tuberculosis, frequently have multitiered defense mechanisms ensuring their survival in host phagocytic cells. One such defense determinant in M. tuberculosis is the katG gene, which encodes an enzyme with catalase, peroxidase, and peroxynitritase activities. KatG is considered to be important for protection against reactive oxygen and nitrogen intermediates produced by phagocytic cells. However, KatG also activates the front-line antituberculosis drug isoniazid, hence rendering M. tuberculosis exquisitely sensitive to this compound. In this context, katG expression represents a double-edged sword, as it is an important virulence determinant but at the same time its activity levels determine sensitivity to INH. Thus, it is important to delineate the regulation and expression of katG, as this not only can aid understanding of how M. tuberculosis survives and persists in the host but also may provide information of relevance for better management of INH therapy. Here, we report the first extensive analysis of the katG promoter activity examined both in vitro and in vivo. Using S1 nuclease protection analysis, we mapped the katG mRNA 5′ ends and demonstrated that two promoters, P1furA and P1katG, control transcription of katG. The furA and katG genes are cotranscribed from P1furA. Both P1furA and P1katG promoters show induction upon challenge with hydrogen peroxide and cumene hydroperoxide. Studies carried out using the transcriptional fusions P1furA-gfp, P1katG-gfp, and P1furA-P1katG-gfp confirmed the existence of two katG promoters. In addition, we showed that both promoters are expressed in vivo during intracellular growth of virulent M. tuberculosis H37Rv. P1furA is induced early upon infection, and P1katG becomes active only upon extended growth in macrophages. These studies delineate the transcriptional organization of the furA-katG region and indicate differential regulation in vivo of the two katG promoters. These phenomena most likely reflect the differing demands at sequential stages of the infection cycle and may provide information for improved understanding of host-pathogen interactions in tuberculosis and for further optimization of INH chemotherapy.
Mycobacterium tuberculosis is the most common cause of death from a single infectious agent worldwide. It is a facultative, intracellular pathogen capable of surviving and persisting in the highly oxidative environment of phagocytic cells (1, 7). M. tuberculosis can evade the host immune system by preventing phagosome-lysosome fusion and resists killing by reactive oxygen and reactive nitrogen intermediates (2, 5, 8, 16, 25, 31, 33, 34, 37, 44, 45). As is the case with most pathogenic bacteria, M. tuberculosis has evolved mechanisms of protection against oxidative stress by way of specific defenses and global responses. Examples of such defense systems include katG (encoding a catalase-peroxidase), and ahpC (encoding a homolog of alkyl hydroperoxide reductase). The products of these two genes are important in protection against oxidative stress, specifically peroxides, and in macrophage parasitism of pathogenic mycobacteria (3, 6, 15, 20, 22, 24–26, 28, 43).
Unlike other mycobacteria with two catalases (14) and other microorganisms with multiple peroxide-dismutating or -reducing enzymes (27) which are differentially regulated during the growth cycle (17), M. tuberculosis has a single catalase-peroxidase, encoded by the katG gene (21). KatG has also been shown to have peroxynitritase activity (41). KatG activity is necessary for growth and persistence in mice and guinea pigs (24). KatG is also involved in the activation of isonicotinic acid hydrazide (INH) (4, 19, 42, 49) and hence has been implicated in the exquisite sensitivity of M. tuberculosis to INH (9, 48). In this context, one of the mechanisms of M. tuberculosis resistance to INH is via the inactivation of the katG gene (30, 49). As KatG is an important virulence determinant in M. tuberculosis and at the same time plays a critical role in rendering the tubercle bacilli sensitive to INH, its potentially varied levels of expression in vivo during infection and periods of antibiotic treatment may play opposing roles in the survival of the pathogen. In this context, our present knowledge of katG regulation and expression is limited, and KatG levels have not been considered in the majority of studies published to date.
In all mycobacteria, the katG locus is genetically linked to the furA gene (Fig. 1A) (11, 32). In this study we present characterization of the katG promoters and their expression. We show that katG is transcribed from two promoters, one transcript encompassing both the furA and the katG genes and the other corresponding to katG alone. We also show the induction of both promoters by peroxides in vitro and their differential expression during growth of M. tuberculosis in macrophages. These studies indicate the existence of a dual and stage-specific induction of katG promoters, a phenomenon of potential significance for the physiology of the tubercle bacillus. Due to the presence of only one catalase in M. tuberculosis, unlike in other bacteria where at least two enzymes allow balanced H2O2 and peroxide homeostasis (17), the tubercle bacillus may have evolved ways of appropriately tuning katG expression from two promoters to respond to varied environmental inputs and physiological demands.
FIG. 1.
(A) Genetic map of the furA-katG loci in mycobacteria. In all mycobacteria, furA and katG are linked. Both furA and katG are inactivated by multiple mutations in M. leprae (circles, insertions; triangles, deletions). (B) Promoter-gfp reporter fusions. The katG promoters (P1furA and P1katG) were cloned in front of the green fluorescent reporter gene (gfp) either individually or together as described in Materials and Methods. pTZ175 carries P1furA upstream of gfp; pTZ176 carries P1katG upstream of gfp; pTZ177 carries both P1furA and P1katG upstream of katG. pTZ177 also carries an intact furA gene. All three plasmids confer resistance to kanamycin.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and electrotransformation.
Unless otherwise noted, Mycobacterium bovis BCG (strain Pasteur; ATCC 27291) and M. tuberculosis H37Rv (ATCC 27294) were grown in Middlebrook 7H9 medium or on 7H10 agar plates (Difco) supplemented with 0.05% Tween, 0.2% glycerol, and ADC (10% bovine serum albumin fraction V, dextrose, and catalase) enrichment for M. bovis BCG or OADC (which also contains oleic acid) for M. tuberculosis. All manipulations of live M. tuberculosis were carried out under biosafety level 3 conditions. All transformations in Escherichia coli were performed with the strain DH5α. E. coli was grown in Luria broth (Difco) at 37°C. Wherever necessary, 25 μg of kanamycin/ml was added to the medium. The preparation and the transformation of electrocompetent mycobacteria were performed as previously described (23).
Construction of plasmids.
The furA promoter was PCR amplified using primers Mtbfur1 (5′-GCTCATCGGAACATACGAAGG-3′, located at positions −138 to −117 upstream of the furA initiation codon) and katGS1-P22 (5′-TGTGGATGCGCATTCACTGCT-3′, located at +80 to +102 relative to the furA initiation codon). This fragment was cloned into pCR2.1, digested with EcoRI, and ligated to HindIII/EcoRI adapters. This was then digested with HindIII and cloned into pMYGFP2 to give pTZ175. The katG promoter was amplified using primers Mtbfur2 (5′-ACCATCACATCGTCTGCCGGT-3′, located at −215 to −194 relative to the katG initiation codon) and katGS1 (5′-TGGGTGGGTGTTGCTCGGGCACAGCA-3′, located at −4 to +22 relative to the katG initiation codon) and cloned as for pTZ175 to yield pTZ176. The furA and katG promoters were amplified using primers Mtbfur1 and katGS1 and cloned as for pTZ175 to yield pTZ177. All constructs were sequenced to confirm the correct orientation and sequence of nucleotides. pMYGFP2 and pahpC-gfp were constructed elsewhere (10). All plasmids are kanamycin resistant.
RNA isolation and S1 nuclease protection analysis.
Total cellular RNA was isolated by centrifugation through a cushion of 5.7 M CsCl (13). Uniformly 32P-labeled single-stranded hybridization probes were prepared using pJS121, containing a 1,724-bp SmaI-BamHI fragment of pYZ55 (49) that encompasses the furA-katG loci, and primers katGS1, katGS1-P2 (5′-TGGACTCGTAGCGCGCGACGGAG-3′), and katGS1-P22. Equal amounts of RNA (33 μg) were hybridized with aliquots of the radioactively labeled DNA probe. S1 nuclease protection analysis was carried out as described previously (13). S1 nuclease digestion products were analyzed on sequencing gels (7.5% polyacrylamide–8 M urea–100 mM Tris–100 mM boric acid–2 mM EDTA, pH 8.3) along with the sequencing ladder. Because of the uniform labeling of single-stranded DNA, which dramatically improves the sensitivity of the assay, radioactive decay contributes to the presence of multiple bands corresponding to the 5′ end of mRNA, as has been noted (13). Images were quantitated using densitometry (at least three samples). All bands corresponding to the promoter region were included in the quantitation.
Fluorescence microscopy.
M. tuberculosis H37Rv strains harboring promoter-gfp fusion plasmids were grown in static cultures until the cells reached mid-exponential phase. J774A (ATCC TIB-67) macrophage infections and fluorescence microscopy analyses were carried out as described previously (46). Relative fluorescence units (RFU) were previously defined (46).
Flow cytometry.
M. tuberculosis H37Rv, containing the various promoter-gfp fusion plasmids, were grown in static cultures (7H9 plus OADC or Sauton minimal medium, which contains 3.6 mM potassium dihydrogen phosphate, 6 mM citric acid, 6% glycerol, 30 mM l-asparagine, 1 mM magnesium sulfate heptahydrate, 2 μM copper sulfate, 7 μM zinc sulfate heptahydrate, and 70 μM ferric ammonium citrate) until the cells reached mid-exponential phase. Flow cytometric analysis was then carried out, as previously described (10, 38) using a FACStar Plus system (Becton Dickinson Immunocytometry Systems). Illumination was with a 200-mW, 488-nm argon ion laser, and emission light was detected through a 530/30-nm band pass filter. Data were collected for 20,0000 individual particles per sample, with gating for size to eliminate interference due to potential clumping, as published previously (12).
RESULTS
Mapping of the 5′ end of transcripts in the furA-katG region.
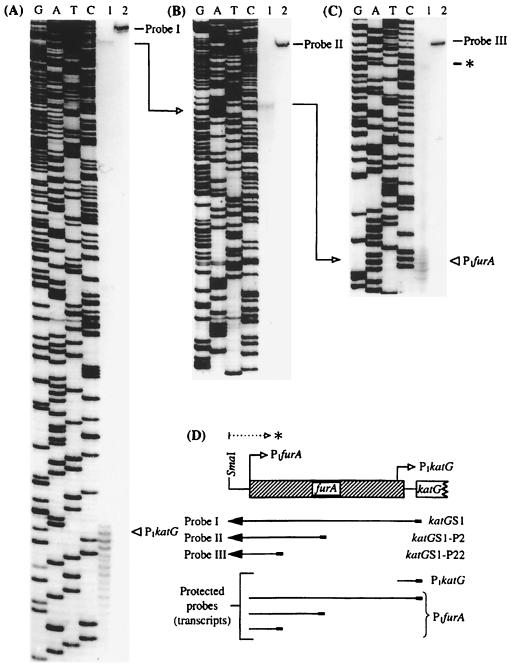
With the long-term goal of investigating expression and regulation of katG in M. tuberculosis, we mapped the katG mRNA 5′ ends using S1 nuclease protection analysis as described in Materials and Methods. First, by using a primer located within the katG coding sequence, a single-stranded DNA probe was generated. This probe (probe I) (Fig. 2), when hybridized with RNA from M. bovis BCG, yielded two products that were protected from digestion by S1 nuclease. The pattern observed suggested the presence of two katG transcripts (Fig. 2A), as the mRNA corresponding to both the upstream and the downstream bands encompassed katG coding sequences. The downstream mRNA 5′ end (termed P1katG) was located 54 nucleotides upstream of the katG coding sequences. The signal from P1katG was much stronger than the upstream signal and was located within the 3′-end portion of the furA gene (13 bp upstream of the furA stop codon). We next asked whether the upstream transcript was located within the coding region of furA or encompassed a complete FurA sequence. The mapping of the 5′ end of the corresponding mRNA was carried out using a series of primers at various positions downstream of the second transcription start site (Fig. 2B and C). The results of these studies indicate that the two detectable transcripts of katG have their 5′ ends at positions 54 and 471 bp upstream of the initiation codon of katG. Significantly, the upstream transcript also coincided with the translational start site of FurA. Thus, we conclude that the katG transcript initiating further upstream also encompasses the FurA coding sequence, and hence, the upstream promoter was termed P1furA (Fig. 2D).
FIG. 2.
Mapping of the 5′ ends of the katG mRNA by S1 nuclease protection analysis. RNA was isolated from M. bovis BCG. S1 nuclease protection assays were carried out as described in Materials and Methods. The protected products (lanes 1) were analyzed on standard sequencing gels alongside the undigested probe (lanes 2) and sequencing ladders (lanes G, A, T, and C) generated by the primer and template used to synthesize the corresponding probes. (A) Mapping of P1katG and detection of a transcript containing katG sequences that initiates further upstream (a detailed mapping of the 5′ end of this transcript is shown in panels B and C) using probe I, generated with the primer katGS1 (at positions −4 to +22 relative to the katG initiation codon). (B) Mapping of the mRNA 5′ end using probe II, generated with the primer katGS1-P2 (at positions +226 to +250 relative to the furA initiation codon). (C) Mapping of the P1furA 5′ end using probe III, generated with the primer katGS1-P22 (at positions +80 to +102 relative to the furA initiation codon). (D) Schematic representation of the probes and the protected fragments in relationship to the furA and katG genes. P1katG and P1furA, mRNA 5′ ends. The P1furA mRNA start site coincides with the furA translation initiation site (GenBank accession number AF002194). Arrows between the panels indicate the stepwise mapping of P1furA. Note that one of the transcripts initiating from P1furA, shown at the bottom of panel D, encompasses both furA and katG coding regions. ∗, a transcript initiating further upstream from P1furA for which the 5′ end has not been mapped.
Induction of furA and katG transcription by hydrogen peroxide and CHP.
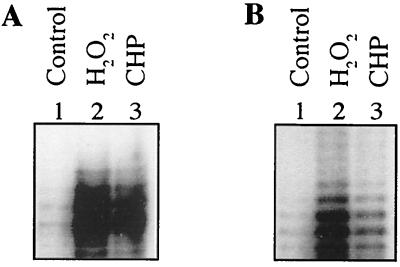
It has been shown previously that katG is induced upon exposure of M. tuberculosis to oxidants (35, 36). We reasoned that if both mRNA 5′ ends were involved in expression of katG, then both transcripts may be induced upon stimulation with peroxides. Thus, the effects of peroxides on the induction of P1katG and P1furA were tested. M. bovis BCG was exposed to either 62.5 μM hydrogen peroxide (H2O2) or 62.5 μM cumene hydroperoxide (CHP) for 2 h. After exposure, the RNA was isolated and S1 nuclease protection analysis was carried out as described above. As can be seen in Fig. 3, the levels of both transcripts were significantly increased. As determined by PhosphorImager analysis, a 5.4-fold increase and a 3.7-fold increase in the proximal katG transcript levels (starting at P1katG) were detected upon exposure to H2O2 and CHP, respectively, compared to untreated controls (Fig. 3A). Similarly, a 7.1-fold increase and a 2.2-fold increase compared to untreated controls were observed in the distal transcript levels (starting at P1furA) (Fig. 3B).
FIG. 3.
Activation of P1furA and P1katG in M. bovis BCG upon exposure to hydrogen peroxide and CHP. Lanes 1, untreated control; lanes 2, 62.5 μM H2O2; lanes 3, 62.5 μM CHP. (A) S1 nuclease mapping using probe I, showing induction from P1katG; (B) S1 nuclease mapping using probe III, showing induction from P1furA. Equal amounts of total RNA (33 μg) were used in each sample.
Two promoters control expression of M. tuberculosis katG.
To rule out the possibility that the two katG mRNA 5′ ends were processing products, we generated transcriptional fusions between P1furA and P1katG with gfp (Fig. 1B) and subjected them to promoter activity analyses using previously established methodologies (13, 38). The results of these experiments are shown in Table 1. After 7 days of growth, both P1furA and P1katG showed promoter activity. Furthermore, the effects of the two promoters were additive, as pTZ177 (with both P1furA and P1katG fused in tandem with gfp) showed the highest transcriptional activity. Similar results were obtained when the strains were tested using a different (Sauton minimal) medium (Table 1).
TABLE 1.
Promoter activity of P1furA and P1katG in M. tuberculosis H37Rv
| Medium | Mean relative fluorescencea (RFU)
|
||||
|---|---|---|---|---|---|
| pTZ175b | pTZ176c | pTZ177d | Positive controle | Negative controlf | |
| 7H9 + OADC | 23.2 | 12.8 | 43.8 | 101.3 | 6.2 |
| Sauton | 16.7 | 17 | 60 | BDL | |
Determined from 20,000 individual particles per sample. The bacteria were grown for 7 days without inducing agents.
P1furA-gfp.
P1katG-gfp.
Transcriptional fusion of both (P1furA and P1katG) promoters in tandem with gfp.
pahpC-gfp with PahpC from M. bovis BCG upstream of gfp. The pahpC from M. bovis BCG was used instead of the one from M. tuberculosis because it is constitutively expressed, while the one from M. tuberculosis is not.
pMYGFP2 containing promoterless gfp. BDL, below detection limit.
In vivo expression of P1furA and P1katG in infected macrophages.
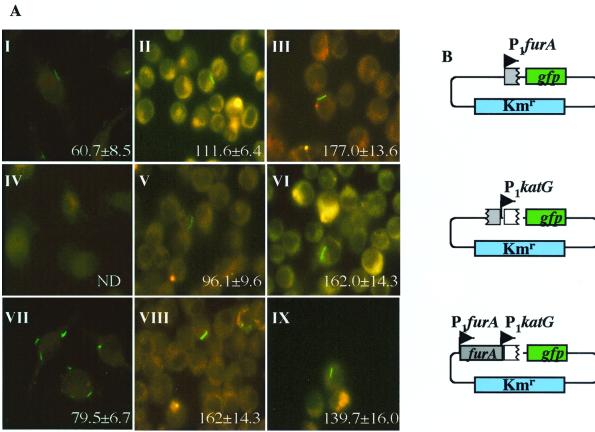
Since our primary interest is in understanding katG regulation and expression in vivo, we used the gfp fusions to study katG promoter activities in infected macrophages. For the in vivo analyses, J774A macrophages were infected with M. tuberculosis H37Rv containing the promoter-gfp fusion plasmids pTZ175, pTZ176, and pTZ177. The results, as visualized by fluorescence microscopy at 2 h, 3 days, and 7 days postinfection, are shown in Fig. 4A. As evidenced by gfp fluorescence, P1furA activity can be seen immediately (2 h) postinfection (Fig. 4A, panel I). In contrast, expression from P1katG was not detectable until the later stages of infection, but by the third day postinfection a high level of activity was observed (Fig. 4A, panels IV and V). At 3 days, the amount of fluorescence detected in the presence of both promoters was significantly greater than the level for P1furA or P1katG alone (Fig. 4A, panels II, V, and VIII). At 7 days following phagocytosis, the levels of expression from both promoters individually were virtually identical (Fig. 4A, panels III and VI). These results show that both katG promoters are active in vivo and reveal the phenomenon of a differential expression of the two katG promoters in vivo.
FIG. 4.
Differential induction of P1furA and P1katG in M. tuberculosis H37Rv in infected macrophages. (A) Fluorescence microscopy of J774A murine macrophages infected with H37Rv carrying the transcriptional fusion constructs shown in panel B. Numbers are RFU, quantitated as previously described (47). Columns correspond to 2 h, 3 days, and 7 days postinfection from left to right, respectively. (B) Graphic representation of the promoter-gfp fusions used (see Fig. 1 for details).
DISCUSSION
Protection against oxidative stress is one of the primary defenses that enable microbial pathogens to survive in the host. Understanding the regulation and expression of virulence determinants is fundamental to deciphering how pathogenic mycobacteria, e.g., M. tuberculosis, survive in host macrophages. The catalase-peroxidase KatG is an important virulence factor in M. tuberculosis, and it is thus significant to determine how it is regulated and expressed. Our results, obtained by using S1 nuclease mapping of M. bovis BCG RNA, indicate that there are two transcriptional start sites and two promoters of katG. One is located immediately upstream of the katG gene. The other transcript starts from P1furA and corresponds to the furA promoter, from which both furA and katG are cotranscribed. We propose a model in which the two katG promoters are differentially expressed with potentially different outcomes, as the transcription from the upstream promoter allows expression of both furA and katG while the downstream promoter supports expression of katG only. Since mycobacterial furA is a regulatory gene (47), expression of katG alone and in combination with furA may lead to different physiological outcomes in M. tuberculosis.
The furA gene and the katG gene are genetically linked in all species of mycobacteria including Mycobacterium smegmatis, M. tuberculosis complex, and Mycobacterium marinum, as shown in Fig. 1A. Interestingly, in Mycobacterium leprae these genes are inactive due to the presence of insertions and deletions (Fig. 1A). A similar linkage of the furA and katG orthologs is also seen in Streptomyces coelicolor (furA-catC) and Streptomyces reticuli (furS-cpeB) (18, 50). S. coelicolor furA is highly homologous to the furA gene from M. tuberculosis. Studies on the S. coelicolor furA-catC loci (18) have revealed promoter activities similar to that which we have reported here, with the existence of two transcripts. One transcript encompasses the furA-catC genes and the other covers only the catC gene. The previously reported studies on the M. tuberculosis katG promoter have suggested the existence of a number of transcriptional start sites near the katG initiation codon (29) that do not coincide with the mRNA start sites reported in our work. However, such studies were performed with a plasmid-borne M. tuberculosis katG gene studied for expression in E. coli and M. smegmatis (29), which could explain the discrepancies between studies carried out in heterologous hosts and our findings derived in an autologous system.
By using a katG-lux construct, Sherman et al. (35) observed a sevenfold induction of katG expression upon exposing BCG to H2O2. Our results are in agreement with these findings and furthermore show that peroxides induce both katG promoters. Both organic and hydrogen peroxides affect katG transcription. Most importantly, our green fluorescent protein fluorescence data show that both promoters are expressed in synthetic media and during infection of mouse macrophages. Hence, both transcriptional starts are utilized in vitro and in vivo. The results reported here also reveal a staged expression from the two promoters. The promoter corresponding to the larger transcript, starting at P1furA, is activated early in infection, while the smaller transcript, starting at P1katG, is produced later in infection. The effects of the two promoters are cumulative.
The genetic linkage of furA and katG in mycobacteria suggests that FurA may control katG. Indeed, recent studies including inactivation of mycobacterial furA have demonstrated that it is a negative regulator of katG (47). A similar role for FurA has also been reported for S. coelicolor (18), albeit based on indirect observations without inactivating the furA gene in this organism. Interestingly, the S. coelicolor FurA is closely related to FurA of M. tuberculosis. From our in vivo data, there is no obvious repression of katG up to 7 days postinfection, although furA is expressed at corresponding time points based on the P1furA-gfp data. This may reflect the possibility that the bacteria are in a highly oxidative environment in macrophages for prolonged periods of time or that additional signals contribute to the activation in vivo. Studies are currently under way to characterize FurA further and to reveal the molecular mechanisms by which it regulates one or both of the katG promoters and possibly additional genes involved in oxidative stress.
The observation that katG expression continues for prolonged periods of time in infected macrophages may explain the exquisite sensitivity of the tubercle bacillus to INH in vivo. However, important variabilities in clinical responses to INH chemotherapy have been noted (39, 40). Often, such phenomena are not associated with mutations in katG or other genes implicated in INH resistance or with host factors such as differences in INH inactivation in the body. We propose that some of these phenomena could be due to phenotypic differences based on expression patterns of M. tuberculosis katG from its two promoters defined in this work, as their regulation may vary depending upon different conditions in anatomically diverse infection sites.
ACKNOWLEDGMENTS
S.M., T.C.Z., and J.S. contributed equally to this paper.
T.C.Z. was supported by an NRSA fellowship from NIH. This work was supported by grant AI42999 from NIAID.
REFERENCES
- 1.Armstrong J A, Hart P D A. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971;134:713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan J, Fan X D, Hunter S W, Brennan P J, Bloom B R. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991;59:1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Xie Q W, Nathan C. Alkyl hydroperoxide reductase subunit C (AhpC) protects bacterial and human cells against reactive nitrogen intermediates. Mol Cell. 1998;1:795–805. doi: 10.1016/s1097-2765(00)80079-9. [DOI] [PubMed] [Google Scholar]
- 4.Chouchane S, Lippai I, Magliozzo R S. Catalase-peroxidase (Mycobacterium tuberculosis KatG) catalysis and isoniazid activation. Biochemistry. 2000;39:9975–9983. doi: 10.1021/bi0005815. [DOI] [PubMed] [Google Scholar]
- 5.Clemens D L, Horwitz M A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper A M, Segal B H, Frank A A, Holland S M, Orme I M. Transient loss of resistance to pulmonary tuberculosis in p47phox−/− mice. Infect Immun. 2000;68:1231–1234. doi: 10.1128/iai.68.3.1231-1234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dannenberg A M, Jr, Rook G A. Pathogenesis of pulmonary tuberculosis: an interplay of tissue-damaging and macrophage-activating immune responses—dual mechanisms that control bacillary multiplication. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C.: American Society for Microbiology; 1994. pp. 459–483. [Google Scholar]
- 8.Deretic V, Fratti R A. Mycobacterium tuberculosis phagosome. Mol Microbiol. 1999;31:1603–1609. doi: 10.1046/j.1365-2958.1999.01279.x. [DOI] [PubMed] [Google Scholar]
- 9.Deretic V, Pagan-Ramos E, Zhang Y, Dhandayuthapani S, Via L E. The extreme sensitivity of Mycobacterium tuberculosis to the front-line antituberculosis drug isoniazid. Nat Biotechnol. 1996;14:1557–1561. doi: 10.1038/nbt1196-1557. [DOI] [PubMed] [Google Scholar]
- 10.Deretic V, Philipp W, Dhandayuthapani S, Mudd M H, Curcic R, Garbe T, Heym B, Via L E, Cole S T. Mycobacterium tuberculosis is a natural mutant with an inactivated oxidative-stress regulatory gene: implications for sensitivity to isoniazid. Mol Microbiol. 1995;17:889–900. doi: 10.1111/j.1365-2958.1995.mmi_17050889.x. [DOI] [PubMed] [Google Scholar]
- 11.Deretic V, Song J, Pagan-Ramos E. Loss of oxyR in Mycobacterium tuberculosis. Trends Microbiol. 1997;5:367–372. doi: 10.1016/S0966-842X(97)01112-8. [DOI] [PubMed] [Google Scholar]
- 12.Dhandayuthapani S, Via L E, Thomas C A, Horowitz P M, Deretic D, Deretic V. Green fluorescent protein as a marker for gene expression and cell biology of mycobacterial interactions with macrophages. Mol Microbiol. 1995;17:901–912. doi: 10.1111/j.1365-2958.1995.mmi_17050901.x. [DOI] [PubMed] [Google Scholar]
- 13.Dhandayuthapani S, Zhang Y, Mudd M H, Deretic V. Oxidative stress response and its role in sensitivity to isoniazid in mycobacteria: characterization and inducibility of ahpC by peroxides in Mycobacterium smegmatis and lack of expression in M. aurum and M. tuberculosis. J Bacteriol. 1996;178:3641–3649. doi: 10.1128/jb.178.12.3641-3649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farr S B, Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991;55:561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fazal N. Influence of Mycobacterium tuberculosis catalase gene (KatG) expression on nitric oxide production and the intracellular growth of transfected Mycobacterium smegmatis strains within murine macrophages. Biochem Mol Biol Int. 1997;42:135–142. doi: 10.1080/15216549700202511. [DOI] [PubMed] [Google Scholar]
- 16.Fratti R A, Vergne I, Chua J, Skidmore J, Deretic V. Regulators of membrane trafficking and Mycobacterium tuberculosis phagosome maturation block. Electrophoresis. 2000;21:3378–3385. doi: 10.1002/1522-2683(20001001)21:16<3378::AID-ELPS3378>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Flecha B, Demple B. Homeostatic regulation of intracellular hydrogen peroxide concentration in aerobically growing Escherichia coli. J Bacteriol. 1997;179:382–388. doi: 10.1128/jb.179.2.382-388.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn J S, Oh S Y, Roe J H. Regulation of the furA and catC operon, encoding a ferric uptake regulator homologue and catalase-peroxidase, respectively, in Streptomyces coelicolor A3(2) J Bacteriol. 2000;182:3767–3774. doi: 10.1128/jb.182.13.3767-3774.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heym B, Alzari P M, Honore N, Cole S T. Missense mutations in the catalase-peroxidase gene, katG, are associated with isoniazid resistance in Mycobacterium tuberculosis. Mol Microbiol. 1995;15:235–245. doi: 10.1111/j.1365-2958.1995.tb02238.x. [DOI] [PubMed] [Google Scholar]
- 20.Heym B, Stavropoulos E, Honore N, Domenech P, Saint-Joanis B, Wilson T M, Collins D M, Colston M J, Cole S T. Effects of overexpression of the alkyl hydroperoxide reductase AhpC on the virulence and isoniazid resistance of Mycobacterium tuberculosis. Infect Immun. 1997;65:1395–1401. doi: 10.1128/iai.65.4.1395-1401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heym B, Zhang Y, Poulet S, Young D, Cole S T. Characterization of the katG gene encoding a catalase-peroxidase required for the isoniazid susceptibility of Mycobacterium tuberculosis. J Bacteriol. 1993;175:4255–4259. doi: 10.1128/jb.175.13.4255-4259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillas P J, del Alba F S, Oyarzabal J, Wilks A, Ortiz De Montellano P R. The AhpC and AhpD antioxidant defense system of Mycobacterium tuberculosis. J Biol Chem. 2000;275:18801–18809. doi: 10.1074/jbc.M001001200. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs W R, Jr, Kalpana G V, Cirillo J D, Pascopella L, Snapper S B, Udani R A, Jones W, Barletta R G, Bloom B R. Genetic systems for mycobacteria. Methods Enzymol. 1991;204:537–555. doi: 10.1016/0076-6879(91)04027-l. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Kelley C, Collins F, Rouse D, Morris S. Expression of katG in Mycobacterium tuberculosis is associated with its growth and persistence in mice and guinea pigs. J Infect Dis. 1998;177:1030–1035. doi: 10.1086/515254. [DOI] [PubMed] [Google Scholar]
- 25.Manca C, Paul S, Barry III E C, Freedman V H, Kaplan G. Mycobacterium tuberculosis catalase and peroxidase activities and resistance to oxidative killing in human monocytes in vitro. Infect Immun. 1999;67:74–79. doi: 10.1128/iai.67.1.74-79.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middlebrook G, Kohn M L. Some observations on the pathogenicity of isoniazid resistant variants of the tubercle bacilli. Science. 1953;118:297–299. doi: 10.1126/science.118.3063.297. [DOI] [PubMed] [Google Scholar]
- 27.Milano A, De Rossi E, Gusberti L, Heym B, Marone P, Riccardi G. The katE gene, which encodes the catalase HPII of Mycobacterium avium. Mol Microbiol. 1996;19:113–123. doi: 10.1046/j.1365-2958.1996.352876.x. [DOI] [PubMed] [Google Scholar]
- 28.Mitchison D A, Selkon J B, Lloyd J. Virulence in the guinea pig, susceptibility to hydrogen peroxide, and catalase activity of isoniazid-sensitive tubercle bacilli from South Indian and British patients. J Pathol Bacteriol. 1963;86:377–386. doi: 10.1002/path.1700860213. [DOI] [PubMed] [Google Scholar]
- 29.Mulder M A, Zappe H, Steyn L M. The Mycobacterium tuberculosis katG promoter region contains a novel upstream activator. Microbiology. 1999;145:2507–2518. doi: 10.1099/00221287-145-9-2507. [DOI] [PubMed] [Google Scholar]
- 30.Musser J M. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin Microbiol Rev. 1995;8:496–514. doi: 10.1128/cmr.8.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mustafa T, Phyu S, Nilsen R, Bjune G, Jonsson R. Increased expression of Fas ligand on Mycobacterium tuberculosis infected macrophages: a potential novel mechanism of immune evasion by Mycobacterium tuberculosis? Inflammation. 1999;23:507–521. doi: 10.1023/a:1020286305950. [DOI] [PubMed] [Google Scholar]
- 32.Pagan-Ramos E, Song J, McFalone M, Mudd M H, Deretic V. Oxidative stress response and characterization of the oxyR-ahpC and furA-katG loci in Mycobacterium marinum. J Bacteriol. 1998;180:4856–4864. doi: 10.1128/jb.180.18.4856-4864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pancholi P, Mirza A, Bhardwaj N, Steinman R M. Sequestration from immune CD4+ T cells of mycobacteria growing in human macrophages. Science. 1993;260:984–986. doi: 10.1126/science.8098550. [DOI] [PubMed] [Google Scholar]
- 34.Russell D G. Mycobacterium and Leishmania: stowaways in the endosomal network. Trends Cell Biol. 1995;5:125–128. doi: 10.1016/s0962-8924(00)88963-1. [DOI] [PubMed] [Google Scholar]
- 35.Sherman D R, Mdluli K, Hickey M J, Arain T M, Morris S L, Barry III C E, Stover C K. Compensatory ahpC gene expression in isoniazid-resistant Mycobacterium tuberculosis. Science. 1996;272:1641–1643. doi: 10.1126/science.272.5268.1641. [DOI] [PubMed] [Google Scholar]
- 36.Sherman D R, Sabo P J, Hickey M J, Arain T M, Mahairas G G, Yuan Y, Barry III C E, Stover C K. Disparate responses to oxidative stress in saprophytic and pathogenic mycobacteria. Proc Natl Acad Sci USA. 1995;92:6625–6629. doi: 10.1073/pnas.92.14.6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stenger S, Niazi K R, Modlin R L. Down-regulation of CD1 on antigen-presenting cells by infection with Mycobacterium tuberculosis. J Immunol. 1998;161:3582–3588. [PubMed] [Google Scholar]
- 38.Via L E, Dhandayuthapani S, Deretic D, Deretic V. Green fluorescent protein. A tool for gene expression and cell biology in mycobacteria. Methods Mol Biol. 1998;101:245–260. doi: 10.1385/0-89603-471-2:245. [DOI] [PubMed] [Google Scholar]
- 39.Wallis R S, Patil S, Cheon S H, Edmonds K, Phillips M, Perkins M D, Joloba M, Namale A, Johnson J L, Teixeira L, Dietze R, Siddiqi S, Mugerwa R D, Eisenach K, Ellner J J. Drug tolerance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1999;43:2600–2606. doi: 10.1128/aac.43.11.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallis R S, Perkins M D, Phillips M, Joloba M, Namale A, Johnson J L, Whalen C C, Teixeira L, Demchuk B, Dietze R, Mugerwa R D, Eisenach K, Ellner J J. Predicting the outcome of therapy for pulmonary tuberculosis. Am J Respir Crit Care Med. 2000;161:1076–1080. doi: 10.1164/ajrccm.161.4.9903087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wengenack N L, Jensen M P, Rusnak F, Stern M K. Mycobacterium tuberculosis KatG is a peroxynitritase. Biochem Biophys Res Commun. 1999;256:485–487. doi: 10.1006/bbrc.1999.0358. [DOI] [PubMed] [Google Scholar]
- 42.Wengenack N L, Lopes H, Kennedy M J, Tavares P, Pereira A S, Moura I, Moura J J, Rusnak F. Redox potential measurements of the Mycobacterium tuberculosis heme protein KatG and the isoniazid-resistant enzyme KatG(S315T): insights into isoniazid activation. Biochemistry. 2000;39:11508–11513. doi: 10.1021/bi001239v. [DOI] [PubMed] [Google Scholar]
- 43.Wilson T, de Lisle G W, Marcinkeviciene J A, Blanchard J S, Collins D M. Antisense RNA to ahpC, an oxidative stress defence gene involved in isoniazid resistance, indicates that AhpC of Mycobacterium bovis has virulence properties. Microbiology. 1998;144:2687–2695. doi: 10.1099/00221287-144-10-2687. [DOI] [PubMed] [Google Scholar]
- 44.Yu K, Mitchell C, Xing Y, Magliozzo R S, Bloom B R, Chan J. Toxicity of nitrogen oxides and related oxidants on mycobacteria: M. tuberculosis is resistant to peroxynitrite anion. Tuber Lung Dis. 1999;79:191–198. doi: 10.1054/tuld.1998.0203. [DOI] [PubMed] [Google Scholar]
- 45.Yuan Y, Lee R E, Besra G S, Belisle J T, Barry C E., III Identification of a gene involved in the biosynthesis of cyclopropanated mycolic acids in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:6630–6634. doi: 10.1073/pnas.92.14.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zahrt T C, Deretic V. An essential two-component signal transduction system in Mycobacterium tuberculosis. J Bacteriol. 2000;182:3832–3838. doi: 10.1128/jb.182.13.3832-3838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zahrt T C, Song J, Siple J, Deretic V. Mycobacterial FurA is a negative regulator of catalase-peroxidase gene katG. Mol Microbiol. 2000;39:1174–1185. doi: 10.1111/j.1365-2958.2001.02321.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Dhandayuthapani S, Deretic V. Molecular basis for the exquisite sensitivity of Mycobacterium tuberculosis to isoniazid. Proc Natl Acad Sci USA. 1996;93:13212–13216. doi: 10.1073/pnas.93.23.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992;358:591–593. doi: 10.1038/358591a0. [DOI] [PubMed] [Google Scholar]
- 50.Zou P, Borovok I, Ortiz de Orue Lucana D, Muller D, Schrempf H. The mycelium-associated Streptomyces reticuli catalase-peroxidase, its gene and regulation by FurS. Microbiology. 1999;145:549–559. doi: 10.1099/13500872-145-3-549. [DOI] [PubMed] [Google Scholar]