Abstract
Objective
This study was aimed to assess the incidence of and risk factors for autism spectrum disorder (ASD) among preterm infants born <29 weeks’ gestational age (GA).
Methods
A retrospective cohort study of infants born <29 weeks’ GA admitted to two tertiary neonatal intensive care units (2009 to 2017) and followed ≥18 months corrected age (CA) at a neonatal follow-up clinic. The primary outcome was ASD, diagnosed using standardized testing or provisional diagnosis at ≥18 months CA. Patient data and 18-month CA developmental outcomes were obtained from the local Canadian Neonatal Follow Up Network database and chart review. Stepwise logistic regression assessed factors associated with ASD.
Results
Among 300 eligible infants, 26 (8.7%) were diagnosed with confirmed and 21 (7.0%) with provisional ASD for a combined incidence of 15.7% (95% confidence interval [CI] 11.7 to 20.3). The mean follow-up duration was 3.9 ± 1.4 years and the mean age of diagnosis was 3.7 ± 1.5 years. Male sex (adjusted odds ratio [aOR] 4.63, 95% CI 2.12 to 10.10), small for gestational age status (aOR 3.03, 95% CI 1.02 to 9.01), maternal age ≥35 years at delivery (aOR 2.22, 95% CI 1.08 to 4.57) and smoking during pregnancy (aOR 5.67, 95% CI 1.86 to 17.29) were significantly associated with ASD. Among ASD infants with a complete 18-month CA developmental assessment, 46% (19/41) had no neurodevelopmental impairment (Bayley-III<70, deafness, blindness, or cerebral palsy).
Conclusions
ASD is common among infants born <29 weeks’ GA and possibly associated with identified risk factors. Such findings emphasize the importance of ASD evaluation among infants <29 weeks’ GA and for continued reporting of developmental outcomes beyond 18-months of corrected age.
Keywords: Autism spectrum disorder, Neurodevelopmental outcomes, Prematurity
The outcomes of extreme preterm infants born <29 weeks’ gestational age (GA) have markedly improved throughout the past decades (1–3), yet over 17% of survivors are diagnosed with significant neurodevelopmental impairment (sNDI) at 18-month corrected age (CA) (4). The standardized 18-month neurodevelopmental evaluation has limitations as it may over diagnose some impairments or prove too early to capture others (5). Autism spectrum disorder (ASD) is an important long-term neurodevelopmental morbidity that is typically diagnosed after 2 years of age and is therefore not recorded in most neonatal follow-up studies. ASD is defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM)-5 as a deficit in social communication as well as repetitive and restrictive patterns of behavior which present early in the development of the child (6). Although the exact etiology of ASD is unclear, a common hypothesis suggests a multifactorial pathogenesis that includes a combination of genetic susceptibilities as well as external stressors from intrauterine and immediate postnatal life (7,8).
In 2015, the prevalence of ASD in Canada was 1 in 66 children (1.5%) among all children aged 5 to 17 years old (9). A recent systematic review estimated that over 7% of preterm infants born <37 weeks’ GA are diagnosed with ASD, which suggests a correlation between prematurity and risk of ASD (7). However, few studies have focused on risk factors among infants born <29 weeks’ GA. Similarly, it remains unclear if the high incidence of ASD in extremely preterm infants is due only to antenatal risk factors or whether postnatal factors, such as neonatal morbidities and therapeutic interventions, also contribute. Therefore, we aimed to assess the incidence of and explore risk factors for ASD among preterm infants born <29 weeks’ GA.
METHODS
Study population and eligibility criteria
This retrospective cohort study included preterm infants born <29 weeks’ GA from January 1, 2009, to December 31, 2017, who were admitted to two tertiary-care neonatal intensive care units (NICUs), survived to NICU discharge and were followed at the Montreal Children’s Hospital – McGill University Health Center neonatal follow-up (NNFU) clinic. We excluded infants with a major congenital anomaly, who died or who did not have at least one follow-up visit at 18 months CA. The study sample size was based on a sample of convenience. This study was approved by the Institutional Research Ethics Board, and parental consent was waived.
Data collection
Data on pregnancy, delivery, infant characteristics, and neonatal morbidities were obtained from the local Canadian Neonatal Network (CNN) database. Information on 18-month CA standardized neurodevelopmental assessments and socio-environmental factors were obtained from the local Canadian Neonatal Follow-Up Network database (CNFUN) (10). The CNN and CNFUN are national standardized databases developed using operation manuals detailing precise definitions for data abstractors (4,10–12). Electronic charts of eligible infants were reviewed to collect information regarding ASD diagnosis at ≥18 months CA. Patient data from the CNN and CNFUN databases were linked to chart review data by unique patient record number.
Variable definitions
Potential risk factors were categorized as perinatal, socioeconomic, and postnatal factors. Perinatal factors included maternal characteristics (maternal age, hypertension, diabetes, chorioamnionitis, antenatal steroids, use of assisted reproduction technologies, and smoking during pregnancy) and infant characteristics (GA, birth weight, sex, multiple births, caesarean delivery, small for GA status <10th percentile (13), Apgar score at 5 minutes, Score for Neonatal Acute Physiology version-II). Socioeconomic factors included maternal factors (education, employment, place of birth, and relationship status), number of adults at home, number of children at home, primary language spoken at home, and the number of languages to which the child was regularly exposed. Postnatal factors included length of stay, postnatal systemic steroid use, and neonatal morbidities (severe neurological injury [grade ≥3 intraventricular hemorrhage or periventricular leukomalacia visible on cranial ultrasound] (14), retinopathy of prematurity stage ≥3 (15), bronchopulmonary dysplasia [supplemental oxygen or respiratory support at 36 weeks’ corrected GA] (16), Bell stage ≥2 necrotizing enterocolitis (17), and late-onset sepsis).
Outcome assessment and definitions
In the NNFU clinic, infants born <29 weeks are evaluated at 4-, 9-, 18, and 36-month CA and at 5 years old, with additional visits planned as needed. Infants are evaluated by an interdisciplinary team, including a paediatrician specialized in neonatal follow-up, an occupational therapist, physiotherapist, psychologist, and speech therapist. Based on 18-month CA assessments, significant NDI was defined as one or more of the following conditions: motor, language, or cognitive Bayley Scales of Infant Development, Third Edition (Bayley-III) score <70, cerebral palsy with Gross Motor Function Classification Scale ≥ 3, bilateral visual impairment, hearing aid, or cochlear implants (10).
In the NNFU clinic, paediatricians specialized in development assessment used the DSM-5 (2013) criteria for autism spectrum disorder or the DSM-IV-TR (2000) criteria for autistic disorder/Asperger’s disorder/pervasive developmental disorder-not otherwise specified (18) to give a provisional diagnosis of ASD (19). Infants with a provisional diagnosis of ASD were then referred to an interdisciplinary ASD clinic for standardized evaluation using the Autism Diagnostic Observation Schedule (ADOS, ADOS-2) (19).
For this study, the primary outcome of ASD was defined as a confirmed ASD diagnosis using standardized testing or a provisional ASD diagnosis made in the NNFU clinic (awaiting formal evaluation) at ≥18 months CA. The inclusion of provisional diagnosis of ASD made in NNFU clinic was based on our preliminary data that indicated a strong positive predictive value of provisional diagnoses for subsequent confirmed ASD in our setting: 26/29 (89.7%) infants with provisional ASD who were formally evaluated had a subsequent confirmed diagnosis. However, we cannot ascertain possible bias due to preconceived diagnostic expectations from the multidisciplinary team and the parents.
Statistical analyses
Unadjusted comparisons between infants with ASD and those without were made using the Chi-square test for categorical variables and the Student’s t-test or Wilcoxon rank-sum test for continuous variables, as appropriate.
To account for the chronological order in which risk factors may contribute to ASD, analyses were conducted in two steps. First, multivariable logistic regression with stepwise selection (P<0.2 for model entry and P<0.05 to stay in the model) was used to identify perinatal factors that were significantly associated with ASD (perinatal model). Second, socioeconomic factors and postnatal factors that were significantly associated with ASD in the univariate analysis (P<0.05) were introduced in the multivariable perinatal model. Crude odds ratios (OR) and adjusted odds ratios (aOR) with corresponding 95% confidence interval (CI) were calculated. Collinearity between variables was evaluated using the variance inflation factor (VIF). We did not make adjustments for multiple comparisons (20). The 18-month outcomes are presented for descriptive purposes but were not integrated into the multivariate analysis as timing of evaluation correlated with ASD diagnosis rather than serving as an independent risk factor.
To evaluate potential biases, we conducted two sensitivity analyses. To assess potential biases due to the length of follow-up, we compared subsets of infants born before 2016 and those born in 2016 or later. To assess potential biases due to differing diagnostic criteria of ASD used in our study, we conducted analyses using confirmed ASD only and a provisional diagnosis of ASD as separate outcomes. Statistical analyses were performed in R version 3.6.1 using Tidyverse version 1.3.0. A two-sided P value <0.05 was considered statistically significant.
RESULTS
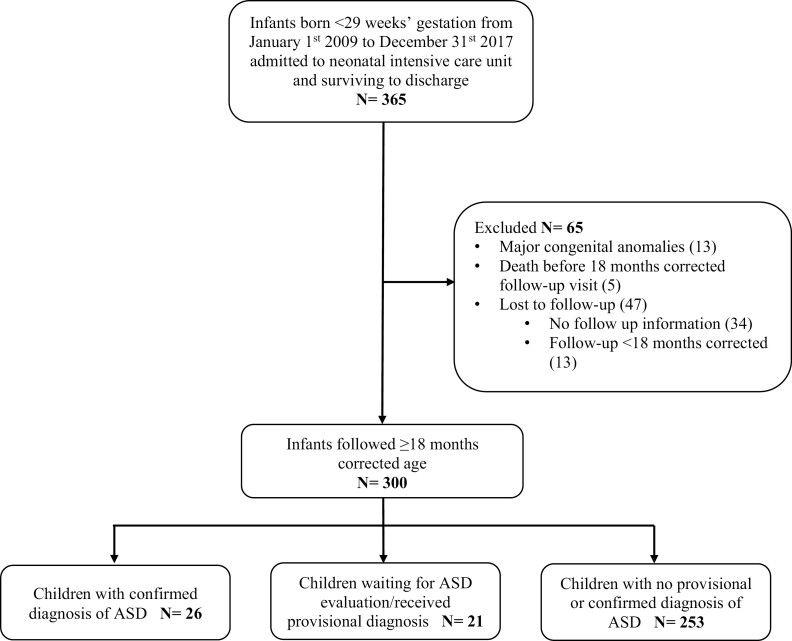
The final study population included 300 infants (Figure 1). Compared with infants followed for ≥18 months CA, infants lost to follow up were born at later GAs, had higher birth weights and had a shorter length of NICU stay (Supplementary Table 1).
Figure 1.
Flow diagram of study population.
Of the 300 infants included in analyses, 47 (15.7%, 95% CI 11.7 to 20.3) had ASD, of whom 26 (8.7%, 95% CI 5.7 to 12.4) had a confirmed diagnosis, and 21 (7.0%, 95% CI 4.4 to 10.5) had a provisional diagnosis. The age at the most recent follow-up visit for children with a confirmed diagnosis was significantly higher than for those with a provisional diagnosis (5 [4;5] versus 3 [2;4] years, P<0.01). Although patient characteristics and the incidences of NDI/ sNDI were similar between groups, children with provisional ASD diagnosis had a higher median Bayley-III cognitive score than children with confirmed ASD (median Bayley-III language and motor scores were also numerically but not statistically significantly different;Supplementary Table 2).
Infants with ASD were more often male, born to mothers ≥35 years old and born to mothers who smoked cigarettes during pregnancy compared to infants without ASD (Table 1). After stepwise selection, four perinatal factors were found to be significantly associated with ASD: male sex, small for GA status, maternal age ≥35 years, and smoking during pregnancy (Table 2). No significant collinearity was observed between these variables (all VIFs < 1.07). Socioeconomic and postnatal factors were similar between infants with and without ASD (Table 3) and were consequently not added to the multivariable perinatal model.
Table 1.
Comparison of perinatal factors among children with and without ASD (confirmed or provisional diagnosis)
| Perinatal factors | Confirmed/provisional ASD N=47 |
N | No ASD N=253 |
N | P value |
|---|---|---|---|---|---|
| Maternal characteristics | |||||
| Maternal age | 34 (8) | 46 | 32 (6) | 251 | 0.09 |
| Maternal age <35 years Maternal age ≥35 years |
26 (57) 20 (43) |
46 | 183 (73) 68 (27) |
251 | 0.04 |
| Hypertension | 8 (17) | 46 | 34 (14) | 251 | 0.65 |
| Diabetes | 3 (7) | 46 | 21 (8) | 251 | 1.00 |
| Chorioamnionitis | 22 (54) | 41 | 118 (54) | 219 | 1.00 |
| Antenatal steroids | 43 (93) | 46 | 231 (92) | 251 | 1.00 |
| Assisted reproduction technologies used | 14 (30) | 47 | 51 (21) | 240 | 0.28 |
| Smoking during pregnancy | 8 (18) | 45 | 11 (5) | 236 | <0.01 |
| Infant characteristics | |||||
| Gestational age (weeks) | 26 [24;27] | 47 | 26 [25;27] | 253 | 0.28 |
| Gestational age, < 26 weeks Gestational age, 26–29 weeks |
18 (38) 29 (62) |
47 | 95 (38) 158 (62) |
253 | 1.00 |
| Birth weight (grams) | 770 [692;970] | 47 | 880 [740;1060] | 251 | 0.11 |
| Male sex | 36 (77) | 47 | 115 (46) | 251 | <0.01 |
| Multiple births | 13 (28) | 46 | 72 (29) | 251 | 1.00 |
| Caesarean delivery | 27 (59) | 46 | 152 (61) | 251 | 0.94 |
| Small for gestational age | 7 (15) | 46 | 15 (6) | 250 | 0.06 |
| Apgar at 5 min <7 | 21 (46) | 46 | 120 (48) | 251 | 0.91 |
| SNAP-II score >20 | 12 (27) | 45 | 79 (31) | 251 | 0.64 |
| Birth year | |||||
| 2009–2013 | 21 (45) | 4 | 121 (48) | 25 | 0.81 |
| 2014–2017 | 26 (55) | 7 | 132 (52) | 3 | |
Data presented as n (%) or median [IQR].
ASD Autism spectrum disorder; SNAP Score for Neonatal Acute Physiology.
Table 2.
Stepwise logistic regression analyses for perinatal risk factors associated with autism spectrum disorder (confirmed or provisional)
| Perinatal risk factors | Adjusted odds ratio (95% CI) |
|---|---|
| Maternal characteristics | |
| Maternal age | |
| <35 years | Reference |
| ≥35 years | 2.22 (1.08–4.57) |
| Smoking during pregnancy | 5.67 (1.86–17.29) |
| Infant characteristics | |
| Male sex | 4.63 (2.12–10.10) |
| Small for gestational age | 3.03 (1.02–9.01) |
Table 3.
Comparison of socioeconomic and postnatal factors among children with and without ASD (confirmed or provisional diagnosis)
| Confirmed/provisional ASD N=47 |
N | No ASD N=253 |
N | P value | |
|---|---|---|---|---|---|
| Socioeconomic factors | |||||
| Maternal post-secondary education completed* | 35 (76) | 46 | 184 (74) | 249 | 0.90 |
| Mother employed or student | 22 (48) | 46 | 141 (57) | 249 | 0.35 |
| Mother born in Canada | 26 (59) | 44 | 132 (53) | 247 | 0.60 |
| Single parent at birth | 3 (7) | 45 | 4 (2) | 233 | 0.09 |
| >1 adult at home at 18 months corrected age | 42 (91) | 46 | 222 (90) | 246 | 1.00 |
| >1 child at home at 18 months corrected age | 29 (63) | 46 | 140 (57) | 249 | 0.52 |
| Primary language English or French | 29 (64) | 45 | 180 (74) | 242 | 0.23 |
| Child exposed to >1 language | 31 (69) | 45 | 178 (74) | 236 | 0.64 |
| Postnatal factors | |||||
| Length of stay (days) | 117 [89;133] | 46 | 100 [76;125] | 251 | 0.08 |
| Postnatal systemic steroids | 22 (48) | 46 | 108 (43) | 250 | 0.67 |
| Severe neurological injury | 10 (22) | 46 | 33 (13) | 251 | 0.20 |
| Severe retinopathy of prematurity | 5 (11) | 44 | 30 (12) | 245 | 1.00 |
| Bronchopulmonary dysplasia | 21 (46) | 46 | 88 (35) | 251 | 0.23 |
| Necrotizing enterocolitis | 6 (13) | 46 | 19 (8) | 251 | 0.25 |
| Late-onset sepsis | 19 (41) | 46 | 78 (31) | 251 | 0.23 |
Data presented as n (%) or median [IQR].
ASD Autism spectrum disorder.
Includes pre-university CÉGEP programs unique to the Quebec education system.
Infants with ASD had higher rates of NDI, sNDI, and lower median Bayley scores at 18-months CA compared to those without (Table 4). Among the 41 infants with ASD and complete 18-month CA neurodevelopmental evaluation, 19 (46%) did not have sNDI at that time. Of note, among infants with sNDI and those without, 35% and 9% had ASD, respectively.
Table 4.
Comparison of 18-month corrected age follow-up outcomes among children with and without ASD (confirmed or provisional diagnosis)
| Confirmed/provisional ASD N=47 |
N | No ASD N=253 |
N | P value | |
|---|---|---|---|---|---|
| Neurodevelopmental impairment* | 29 (69) | 42 | 95 (41) | 229 | <0.01 |
| Significant neurodevelopmental impairment | 22 (54) | 41 | 41 (18) | 228 | <0.01 |
| Cerebral palsy | 4 (10) | 40 | 13 (6) | 229 | 0.29 |
| Bayley III cognitive <70 | 5 (14) | 36 | 8 (5) | 170 | 0.05 |
| Bayley III cognitive score | 85 [70;95] | 36 | 95 [90;105] | 170 | <0.01 |
| Bayley III language <70 | 20 (56) | 36 | 28 (17) | 164 | <0.01 |
| Bayley III language score | 64 [56;81] | 36 | 89 [74;97] | 164 | <0.01 |
| Bayley III motor <70 | 9 (26) | 35 | 11 (7) | 167 | <0.01 |
| Bayley III motor score | 76 [68;91] | 35 | 94 [85;100] | 167 | <0.01 |
| Hearing loss† | 0 (0) | 40 | 4 (2) | 227 | 1.00 |
| Bilateral visual impairment | 0 (0) | 33 | 2 (1) | 155 | 1.00 |
| Age at most recent follow-up visit (years) | 4 [3;5] | 47 | 4 [3;5] | 253 | 0.33 |
Data presented as n (%) or median [IQR].
Bayley-III scores at 18 months corrected age were not available for all infants. Reasons for a missing score in one or more categories include: child was uncooperative during the evaluation, impairment was too severe for evaluation to be performed, child was absent at the 18-month corrected age visit, parents refused their child’s score to be collected in the Canadian Neonatal Follow-Up Network database.
ASD Autism spectrum disorder; Bayley-III Bayley Scales of Infant Development, Third Edition.
Neurodevelopmental impairment: one or more of the following conditions: motor, language or cognitive Bayley-III score <85, cerebral palsy with Gross Motor Function Classification Scale ≥ 1, unilateral or bilateral visual impairment, sensorineural or mixed hearing loss.
Hearing loss requiring hearing aids or cochlear implants.
Age at most recent follow-up for children with and without ASD was similar, which is evidence that the likelihood of developing ASD during the follow-up period was comparable (Table 4).
Sensitivity analyses among infants born before 2016, between 2016 and 2017, among children with confirmed ASD and among children with provisional diagnosis of ASD showed similar effect directions (association between ASD and identified risk factors) (Supplementary Table 3).
DISCUSSION
In this Canadian retrospective cohort study, the confirmed, provisional, and combined incidences of ASD were 8.7%, 7.0%, and 15.7%, respectively among premature infants born <29 weeks’ GA. We identified significant perinatal risk factors for ASD in this population including male sex, maternal age ≥35 years, small for GA, and cigarette smoking during pregnancy. The incidence of ASD in our cohort was higher than that reported in the general population (1.5%) (9), which reinforces previous findings on the increasing incidence of ASD with decreasing GA (21–24). Such an association may be attributable to biological factors, socioeconomic determinants of health, as well as enhanced monitoring of these high-risk infants (25).
Previous cohorts of premature infants, including a systematic review on ASD (median GA 28 weeks) have reported a 7.0% incidence of ASD, which is similar to the confirmed ASD rate in our cohort but below our combined confirmed/provisional rate of ASD (7). On the one hand, the systematic review by Agrawal et al. may underestimate the rate of ASD since it included studies of infants born in the late 1990s and early 2000s, whereas the prevalence of ASD within the general population has increased in the past decades due to increased awareness and broadened criteria/understanding of ASD (7,9,26). On the other hand, our rate of ASD may be overestimated since we included a provisional diagnosis in the primary outcome. Children in our study were evaluated and diagnosed earlier (mean age 3.7 ± 1.5 years) yet may not continue to meet ASD criteria over time (27).
We found that perinatal factors (male sex, small for GA status, maternal age, and cigarette smoking during pregnancy) are associated with higher odds of ASD among our study population. This is consistent with previous studies among different GA groups (28). Male sex has consistently been associated with ASD in the general population, likely due to differences in neuroanatomy, genetic predispositions, and hormonal factors (28,29). Parental ages have also been previously identified as risk factors for ASD (30). Despite controversial previous findings, we report a significant association between cigarette smoking during pregnancy and ASD (28,31). This variability between studies may be due to cigarette use serving as a surrogate for socioeconomic factors and physiological factors, such as intrauterine growth restriction and increased predisposition to preterm delivery (28,31,32). Likewise, small for GA status was associated with ASD. This could indicate that intrauterine growth disruption contributes to the development of ASD through shared genetic mechanisms and may overlap with other prenatal risk factors such as pre-eclampsia and placental pathology (25,33).
We did not find that GA at birth significantly contributes to the risk of ASD in our cohort of infants born <29 weeks. This could be explained by the small sample size or the possibility that GA does not further increase the risk of ASD within such a high-risk population. Neither postnatal steroid exposure nor neonatal morbidities was associated with ASD in our cohort. Similarly, a recent cohort study of nearly 5000 very low birth weight infants with morbidity rates similar to ours did not find an association between ASD and neonatal morbidities (23). While our study may have been underpowered to detect significant associations between postnatal steroid use and ASD, this may also reflect differing clinical practices surrounding steroid administration between sites.
There are conflicting findings on the role of socioeconomic conditions serving as risk factors for ASD (34–36). It has been previously suggested that the association was mitigated by under-diagnosis, lower referral frequency, and hindered access to quality care within minority groups (34,36,37). We did not observe an association between socioeconomic factors and ASD in our cohort of children. This may be explained by Canada’s publicly funded healthcare system that secures access to ASD resources regardless of parental socioeconomic conditions.
The 18-month CA standardized assessment correlated with the risk of ASD as all Bayley-III scores were lower among infants with ASD. This is similar to previous studies that found preterm infants with low Bayley-III scores were more likely to develop ASD than those with a high and stable cognitive performance trajectory but may also be due to enhanced monitoring of infants with lower Bayley-III scores (38). Nevertheless, 19/41 (46%) infants with ASD did not have an sNDI at 18-month CA. Since the majority of follow-up registries of extremely preterm infants focus on 18-month CA outcomes, ASD is not routinely reported in many NNFU neonatal follow-up studies, yet has a profound impact on the patient, family, and educational system (36). Early identification of children with symptoms suggestive of ASD can result in earlier diagnoses which then lead to prompt access to multidisciplinary services (39–41). The 18-month CA outcomes in our cohort were comparable to those reported in larger cohorts of infants born <29 weeks’ GA, which suggests that our findings may be generalizable to other settings (4).
Strengths and limitations
This was one of few studies that assessed the incidence of ASD in a contemporary cohort of infants born <29 weeks (7). We used a validated dataset reflecting current practices and conducted multiple sensitivity analyses that showed consistent results. This study has some limitations. First, the absence of data on children lost to follow up might have increased the incidence of ASD since infants lost to follow up were born less prematurely and in an overall better state of health than their followed counterparts. Second, although the positive predictive value of the paediatricians’ provisional diagnoses of ASD was high, the inclusion of provisional ASD in the primary outcome may overestimate the incidence of ASD. However, misclassifying infants with a provisional diagnosis as non-ASD or excluding them would also lead to biased estimates, and our sensitivity analysis among infants with confirmed ASD showed overall similar results. Third, school-age function could not be evaluated as children were <7 years of age at the time of the study. Since the mean length of follow-up was approximately 4 years, it is possible that mild cases of ASD may diverge from the standard criteria of diagnosis over time.
Preterm infants born <29 weeks’ GA are at high risk of ASD. Perinatal factors (sex, maternal age, small for GA status, and cigarette smoking during pregnancy) were associated with ASD. These findings emphasize the need for enhanced surveillance and evaluation for ASD among preterm infants <29 weeks’ GA. Additional larger cohort studies are required to better understand risk factors, progression, and interventions for ASD among extremely preterm infants.
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the staff at the Maternal-Infant Care (MiCare) Research Centre at Mount Sinai Hospital, Toronto, ON for organizational support of CNN and CNFUN and their support in database management and data preparation.
Contributor Information
Andrée-Anne Busque, McGill University, Montreal, Quebec, Canada.
Elias Jabbour, McGill University Health Center, Research Institute, Montreal, Quebec, Canada.
Sharina Patel, McGill University, Montreal, Quebec, Canada; McGill University Health Center, Research Institute, Montreal, Quebec, Canada.
Élise Couture, Department of Pediatrics, Montreal Children’s Hospital - McGill University Health Centre, Montreal, Quebec, Canada.
Jarred Garfinkle, McGill University Health Center, Research Institute, Montreal, Quebec, Canada; Department of Pediatrics, Montreal Children’s Hospital - McGill University Health Centre, Montreal, Quebec, Canada.
May Khairy, Department of Pediatrics, Montreal Children’s Hospital - McGill University Health Centre, Montreal, Quebec, Canada.
Martine Claveau, Department of Pediatrics, Montreal Children’s Hospital - McGill University Health Centre, Montreal, Quebec, Canada.
Marc Beltempo, McGill University Health Center, Research Institute, Montreal, Quebec, Canada; Department of Pediatrics, Montreal Children’s Hospital - McGill University Health Centre, Montreal, Quebec, Canada.
AUTHOR CONTRIBUTIONS
A-AB participated in the study design, acquisition of data, statistical analyses, and the drafting of the manuscript. EJ and SP assisted in all parts of the aforementioned process. ÉC, MK, JG, and MC helped in the interpretation of the results, reviewed the manuscript, and added significant contributions to the text. MB designed the study, supervised data collection, analysis and interpretation of the results, and critically appraised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FUNDING
MB holds an Early Career Investigator Grant from the CIHR Institute of Human Development, Child and Youth Health (IHDCYH), a research grant funding from the FRSQ Clinical Research Scholar Career Award Junior 1, and an Early Career Investigator Grant from the Montreal Children’s Hospital Foundation. A-AB received the Dr. Barkev and Mrs. Alice Andonian Research Bursary in Paediatrics and the Clarke McLeod Memorial Scholarship from McGill University.
POTENTIAL CONFLICTS OF INTEREST
All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
REFERENCES
- 1. Lee SK, Beltempo M, McMillan DD, et al. Outcomes and care practices for preterm infants born at less than 33 weeks’ gestation: A quality-improvement study. CMAJ. 2020;192:E81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hee Chung E, Chou J, Brown KA, et al. Neurodevelopmental outcomes of preterm infants: A recent literature review. Transl Pediatr 2020;9:S3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 2015;314:1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Synnes A, Luu TM, Moddemann D, et al. Determinants of developmental outcomes in a very preterm Canadian cohort. Arch Dis Child Fetal Neonatal Ed 2017;102:F235–4. [DOI] [PubMed] [Google Scholar]
- 5. Taylor GL, Joseph RM, Kuban KCK, et al. Changes in neurodevelopmental outcomes from age 2 to 10 years for children born extremely preterm. Pediatrics 2021;147:e2020001040. doi: 10.1542/peds.2020-001040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Arlington, VA, Washington, D.C.: American Psychiatric Association; 2013. [Google Scholar]
- 7. Agrawal S, Rao SC, Bulsara MK, Patole SK.. Prevalence of autism spectrum disorder in preterm infants: A meta-analysis. Pediatrics 2018;142:e20180134. [DOI] [PubMed] [Google Scholar]
- 8. Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 2011;68:1095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Public Health Agency of Canada. Autism Spectrum Disorder among Children and Youth in Canada 2018; A Report of the National Autism Spectrum Disorder Surveillance System 2018 [November, 2020]. Available from: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/autism-spectrum-disorder-children-youth-canada-2018.html#a2-6.
- 10. Canadian Neonatal Follow-Up Network Annual Report 2019. Neonatal Follow-Up Network, 2019. [Google Scholar]
- 11. Puthattayil ZB, Luu TM, Beltempo M, et al. Risk factors for re-hospitalization following neonatal discharge of extremely preterm infants in Canada. Paediatr Child Health 2019;26:e96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shah PS, Seidlitz W, Chan P, Yeh S, Musrap N, Lee SK.. Internal audit of the Canadian Neonatal Network data collection system. Am J Perinatol 2017;34:1241–1249. [DOI] [PubMed] [Google Scholar]
- 13. Schlaudecker EP, Munoz FM, Bardají A, et al. Small for gestational age: Case definition & guidelines for data collection, analysis, and presentation of maternal immunisation safety data. Vaccine 2017;35(48 Pt A):6518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Szpecht D, Szymankiewicz M, Nowak I, Gadzinowski J.. Intraventricular hemorrhage in neonates born before 32 weeks of gestation-retrospective analysis of risk factors. Childs Nerv Syst 2016;32:1399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Eye Institute. Retinopathy of Prematurity July 10, 2019 [cited February 19, 2020]. Available from: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/retinopathy-prematurity.
- 16. Davidson LM, Berkelhamer SK.. Bronchopulmonary dysplasia: Chronic lung disease of infancy and long-term pulmonary outcomes. J Clin Med 2017;6:4. doi: 10.3390/jcm6010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thompson AM, Bizzarro MJ.. Necrotizing enterocolitis in newborns: Pathogenesis, prevention and management. Drugs 2008;68:1227–38. [DOI] [PubMed] [Google Scholar]
- 18. Kulage KM, Smaldone AM, Cohn EG, et al. How will DSM-5 affect autism diagnosis? a systematic literature review and meta-analysis. J Autism Dev Disord 2014;44:1918–32. [DOI] [PubMed] [Google Scholar]
- 19. Brian JA, Zwaigenbaum L, Ip A, et al. Standards of diagnostic assessment for autism spectrum disorder. Paediatr Child Health 2019;24:444–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–6. [PubMed] [Google Scholar]
- 21. Brumbaugh JE, Weaver AL, Myers SM, Voigt RG, Katusic SK.. Gestational age, perinatal characteristics, and autism spectrum disorder: A birth cohort study. J Pediatr 2020;220:175–83.e8. doi: 10.1016/j.jpeds.2020.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crump C, Sundquist J, Sundquist K, et al. Preterm or early term birth and risk of autism. Pediatrics 2021;148:e2020032300. doi: 10.1542/peds.2020-032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davidovitch M, Kuint J, Lerner-Geva L, et al. Postnatal steroid therapy is associated with autism spectrum disorder in children and adolescents of very low birth weight infants. Pediatr Res 2020;87:1045–51. [DOI] [PubMed] [Google Scholar]
- 24. Joseph RM, O’Shea TM, Allred EN, et al. Prevalence and associated features of autism spectrum disorder in extremely low gestational age newborns at age 10 years. Autism Res 2017;10:224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Joseph RM, Korzeniewski SJ, Allred EN, et al. Extremely low gestational age and very low birthweight for gestational age are risk factors for autism spectrum disorder in a large cohort study of 10-year-old children born at 23-27 weeks’ gestation. Am J Obstet Gynecol 2017;216:304.e1–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Naarden Braun K, Christensen D, Doernberg N, et al. Trends in the prevalence of autism spectrum disorder, cerebral palsy, hearing loss, intellectual disability, and vision impairment, metropolitan Atlanta, 1991-2010. PLoS One 2015;10:e0124120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zwaigenbaum L, Bryson S, Lord C, et al. Clinical assessment and management of toddlers with suspected autism spectrum disorder: Insights from studies of high-risk infants. Pediatrics. 2009;123:1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang C, Geng H, Liu W, Zhang G.. Prenatal, perinatal, and postnatal factors associated with autism: A meta-analysis. Medicine (Baltim) 2017;96:e66–96-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen C, Van Horn JD.. Developmental neurogenetics and multimodal neuroimaging of sex differences in autism. Brain Imaging Behav. 2017;11:38–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z.. Advanced parental age and autism risk in children: A systematic review and meta-analysis. Acta Psychiatr Scand. 2017;135:29–41. [DOI] [PubMed] [Google Scholar]
- 31. Lee BK, Gardner RM, Dal H, et al. Brief report: Maternal smoking during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2012;42:2000–5. [DOI] [PubMed] [Google Scholar]
- 32. Kiechl-Kohlendorfer U, Ralser E, Pupp Peglow U, Reiter G, Griesmaier E, Trawöger R.. Smoking in pregnancy: A risk factor for adverse neurodevelopmental outcome in preterm infants? Acta Paediatr. 2010;99:1016–9. [DOI] [PubMed] [Google Scholar]
- 33. Lampi KM, Lehtonen L, Tran PL, et al. Risk of autism spectrum disorders in low birth weight and small for gestational age infants. J Pediatr. 2012;161:830–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jo H, Schieve LA, Rice CE, et al. Age at autism spectrum disorder (ASD) diagnosis by race, ethnicity, and primary household language among children with special health care needs, United States, 2009-2010. Matern Child Health J. 2015;19:1687–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rai D, Lewis G, Lundberg M, et al. Parental socioeconomic status and risk of offspring autism spectrum disorders in a Swedish population-based study. J Am Acad Child Adolesc Psychiatry. 2012;51:467–76.e6. [DOI] [PubMed] [Google Scholar]
- 36. Roman-Urrestarazu A, van Kessel R, Allison C, Matthews FE, Brayne C, Baron-Cohen S.. Association of race/ethnicity and social disadvantage with autism prevalence in 7 million school children in England. JAMA Pediatr. 2021;175:e210054. doi: 10.1001/jamapediatrics.2021.0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith KA, Gehricke JG, Iadarola S, Wolfe A, Kuhlthau KA.. Disparities in service use among children with autism: A systematic review. Pediatrics. 2020;145(Suppl 1):S35–46. [DOI] [PubMed] [Google Scholar]
- 38. Chen LW, Wang ST, Wang LW, et al. Early neurodevelopmental trajectories for autism spectrum disorder in children born very preterm. Pediatrics 2020;146:e20200297. doi: 10.1542/peds.2020-0297 [DOI] [PubMed] [Google Scholar]
- 39. Lai M-C, Anagnostou E, Wiznitzer M, Allison C, Baron-Cohen S.. Evidence-based support for autistic people across the lifespan: Maximising potential, minimising barriers, and optimising the person–environment fit. Lancet Neurol 2020;19:434–51. [DOI] [PubMed] [Google Scholar]
- 40. Wallace KS, Rogers SJ.. Intervening in infancy: Implications for autism spectrum disorders. J Child Psychol Psychiatry 2010;51:1300–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zwaigenbaum L, Bauman ML, Stone WL, et al. Early identification of autism spectrum disorder: Recommendations for practice and research. Pediatrics 2015;136Suppl 1:S10–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.